- Department of Pharmacy, The Third Xiangya Hospital, Central South University, Changsha, Hunan, China
Objective: Few real-world studies have shown clear association between interleukin (IL)-17 inhibitors and inflammatory bowel disease (IBD) onset. This study investigated the reporting prevalence and evaluated the clinical features and management of IL-17 inhibitor-related IBD events.
Methods: We used the US FDA Adverse Event Reporting System database and retrieved data, from 2015 to 2022, on IL-17 inhibitors to identify gastrointestinal inflammatory events and conduct disproportionality analyses by estimating the reporting odds ratios (RORs) and corresponding 95% confidence intervals (CIs). Furthermore, case reports and case series, from 2015 to 30 November 2022, on IBD induced by IL-17 inhibitors were collected for retrospective analysis.
Results: A total of 388 cases of primary suspected IL-17 inhibitor-associated gastrointestinal events were reported (268 IBD and 120 colitis), including 348 cases involving secukinumab (SEC), 36 cases involving ixekizumab (IXE), and 4 cases involving brodalumab (BRO). Statistically significant reporting rates of total IBD events were observed for SEC and IXE (ROR = 2.13, 95% CI [1.96-2.30] and ROR = 2.79, 95% CI [2.39-3.27], respectively), whereas BRO did not trigger a safety signal. Twenty-nine studies, which included 34 cases, showed evidence of IBD, following SEC (79.4%) and IXE (20.6%) treatment. The median age was 42 years; typical initial symptoms included diarrhea (90.9%), abdominal pain (57.6%), bloody diarrhea (51.5%), and fever (36.4%). The median time to onset of IBD symptoms was 2.9 months. Some cases were accompanied by elevated white blood cell (WBC) count (87.5%), erythrocyte sedimentation rate (ESR; 85.7%), C-reactive protein (CRP; 100%), and fecal calprotectin (FC; 100%). Cessation of IL-17 inhibitors plus treatment with corticosteroids and TNF antagonists, as either monotherapy or in combination, could lead to complete clinical remission. The median time to remission after IL-17 inhibitor discontinuation was 4 weeks.
Conclusion: IL-17 inhibitor treatment is associated with exacerbation and new onset of IBD and colitis. Obtaining a detailed patient history before initiation of treatment and monitoring gastrointestinal symptoms and intestinal inflammatory biomarkers during IL-17 inhibitor treatment is important for safe use of these drugs.
Introduction
Interleukin (IL)-17 is a significant proinflammatory cytokine and a therapeutic target for rheumatological diseases as blocking IL-17 reduces disease activity and improves patient quality of life (Ouyang et al., 2008). The anti-IL-17 agents secukinumab (SEC), ixekizumab (IXE), and brodalumab (BRO), which were launched in January 2015, April 2016, and July 2017, respectively, are monoclonal IgG4 antibodies directed against IL-17A (SEC and IXE), or against the IL-17 receptor (BRO) (Langley et al., 2014; Griffiths et al., 2015; Lebwohl et al., 2015), and have been used to treat various autoimmune disorders, such as psoriasis (Pso), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and axial spondyloarthritis (ax-Spa) (van der Heijde et al., 2017; Menter et al., 2019; Gossec et al., 2020; Wendling et al., 2022; Ramiro et al., 2023). Promising efficacy results in dermatology and rheumatology prompted the evaluation of these drugs in treatment of inflammatory bowel disease (IBD). Unfortunately, they were ineffective and induced paradoxical events (disease exacerbation after treatment with a theoretically curative drug) that prevented approval for treatment of patients with IBD. Increasing cases of induction or exacerbation of IBD have been reported among patients treated with drugs inhibiting IL-17 (Caron et al., 2022; Burisch et al., 2020), which suggests that rheumatologists and dermatologists should increase awareness of the risk and improve safety measures to prevent drug-induced IBD, as well as evaluate patients carefully to identify any contraindications before the initiation of IL-17 inhibitor therapy for psoriasis and/or rheumatological diseases (Felice et al., 2019).
To date, the pathophysiological mechanisms underlying these paradoxical effects are not well-defined, there is limited real-world data demonstrating the association between IL-17 inhibitor therapy and the onset of IBD, and there are no clear guidelines for the management of patients who experience these adverse events. We aimed to assess differences in safety signals using the FDA Adverse Event Reporting System (FAERS) database, and describe characteristics, management, and prognosis of IBD flare or new onset in patients after anti-IL-17 drug therapy in a real-life setting, which provides valuable information about emerging gastrointestinal safety issues related to IL-17 inhibitors.
Methods
Pharmacovigilance study
In this study, we conducted a retrospective, disproportionality, pharmacovigilance analysis. Data from 2015 Quarter 1 (Q1) to 2022 Q3 were retrieved from the publicly available FAERS database in FDA website to evaluate the risk of gastrointestinal inflammatory conditions with the use of different IL-7 inhibitor therapies.
We assessed the use of IL-17 inhibitors in a large-scale population. Study drugs were IL-17 inhibitors on the market: SEC, IXE, and BRO. To identify IL-17 inhibitor-related records, both brand names and generic names were used. Furthermore, IBD and colitis case reports in FAERS were coded using the preferred term (PT) according to the Medical Dictionary for Regulatory Activities Terminology (MedDRA). Drugs were assigned a role (primary suspect, secondary suspect, concomitant, and interacting) by the person reporting the adverse drug reaction (ADR). ADRs were categorized into two main groups: 1) “IBD,” including reported diagnoses of ulcerative colitis (UC), Crohn’s disease (CD), and undifferentiated IBD (uIBD); 2) “colitis,” including reported diagnoses of colitis (microscopic, ischemic, eosinophilic, and undifferentiated), proctitis, and enteritis. The effects of disproportionality analysis were evaluated using the established pharmacovigilance index reporting odds ratio (ROR), which were calculated as (a*d)/(b*c) (Supplementary Table S1). In our study, an event was defined as significant when the lower limit of the 95% CI of the ROR >1 and there were at least three cases to define a signal (van Puijenbroek et al., 2002).
Descriptive study
A comprehensive search of multiple electronic databases, including PubMed, Embase, Wanfang, China National Knowledge Infrastructure (CNKI), and China Biology Medicine disc (CBMdisc), from January 2015 to September 2022, regarding IL-17 inhibitor-induced IBD was conducted, with no language restrictions. The search terms were “secukinumab or Cosentyx”, “Ixekizumab or Taltz,” “Brodalumab or Siliq,” “Ulcerative colitis,” “Crohn’s disease,” and “Inflammatory bowel disease”. Case reports and case series were included, and reviews, mechanistic studies, animal studies, and articles for which the full text was not available were excluded. Two reviewers searched the literature independently and examined the relevant studies for further assessment of inclusion/exclusion criteria and to identify clinical characteristics; collected data included the region of patient location, age, sex, Indication of IL-17 inhibitors, medical history, time to onset, clinical manifestations, laboratory tests, histopathological examinations, treatment, and prognosis of IL-17 inhibitor-induced IBD. The time to onset of target IBD was defined as the time from the start date of IL-17 inhibitor administration to the onset of the associated IBD.
Results
IL-17 inhibitor-associated IBD in the FAERS database
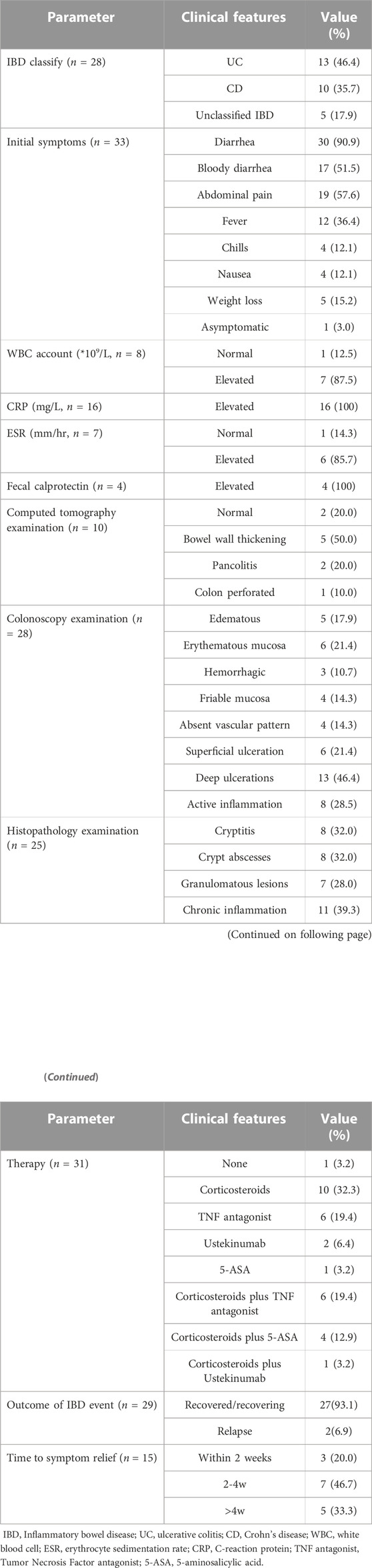
Up to 2022 Q3, the numbers of reported adverse event (ADE) cases in the FAERS database related to SEC, IXE, and BRO were 44,688, 24,406, and 1796, respectively. From screening individual IL-17 inhibitor-related gastrointestinal inflammatory case reports, a total of 388 cases of primary suspect IL-17 inhibitor-associated inflammatory bowel disease and colitis were reported, of which 348 were SEC, 36 were IXE, and 4 were BRO. These cases included 268 IBD diagnoses and 120 colitis diagnoses (Figure 1). The main characteristics of the patients, disease seriousness, delay of onset, and disease outcomes are detailed in Table 1.
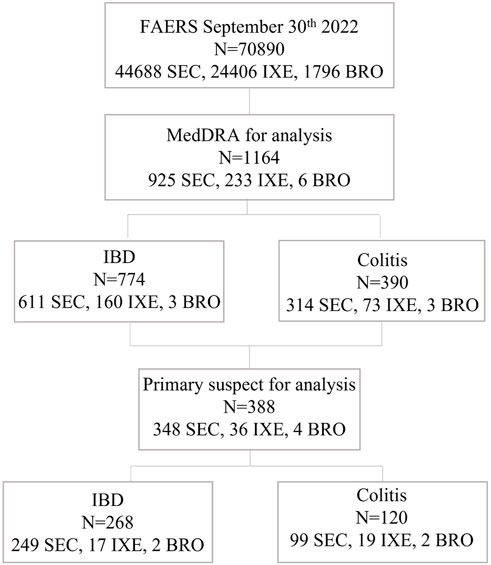
FIGURE 1. Flow chart of the study protocol. FAERS, FDA Adverse Event Reporting System; MedDRA, Medical Dictionary for Regulatory Activities Terminology; IBD, inflammatory bowel diseases; SEC, secukinumab, IXE, ixekizumab IXE; BRO, brodalumab.
Disproportionality analyses
From the entire database, the disproportionality analyses identified three unique preferred terms (PTs) for IBD and 12 PTs for colitis. Globally, all cases of IBD after SEC and IXE treatment accounted for 1.36% and 0.66% of all reported cases, respectively, and there was a statistically significant reporting rate of total IBD events observed among patients treated with SEC and IXE (ROR = 2.13, 95% CI [1.96–2.30] and ROR = 2.79, 95% CI [2.39-3.27], respectively), whereas this proportion was lower for BRO (0.17%; ROR = 1.48, 95% CI [0.48-4.6]) (Table 2). All colitis cases after SEC and IXE treatment accounted for 0.70% and 0.30% of all reported cases, respectively, and statistically significant reporting rates were also observed for total colitis events after SEC and IXE treatment (ROR = 1.60, 95% CI [1.43-1.79] and ROR = 1.96, 95% CI [1.56-2.47], respectively) (Table 3).
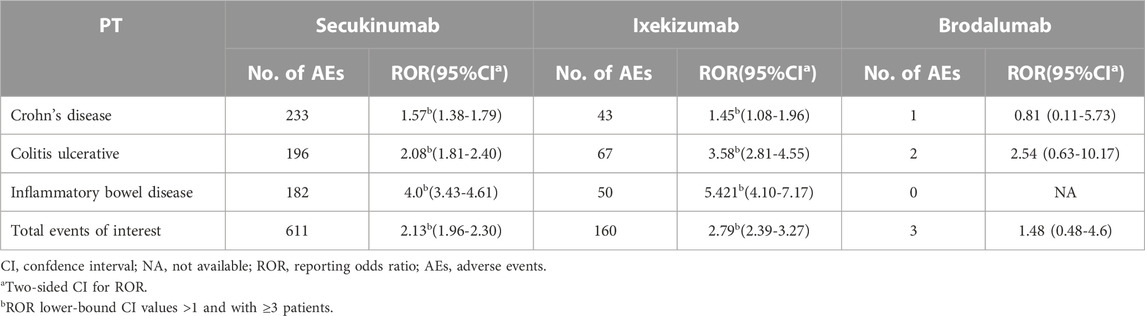
TABLE 2. Interleukin -17 inhibitors associated with Inflammatory bowel disease and the reporting odds ratios in FAERS database.
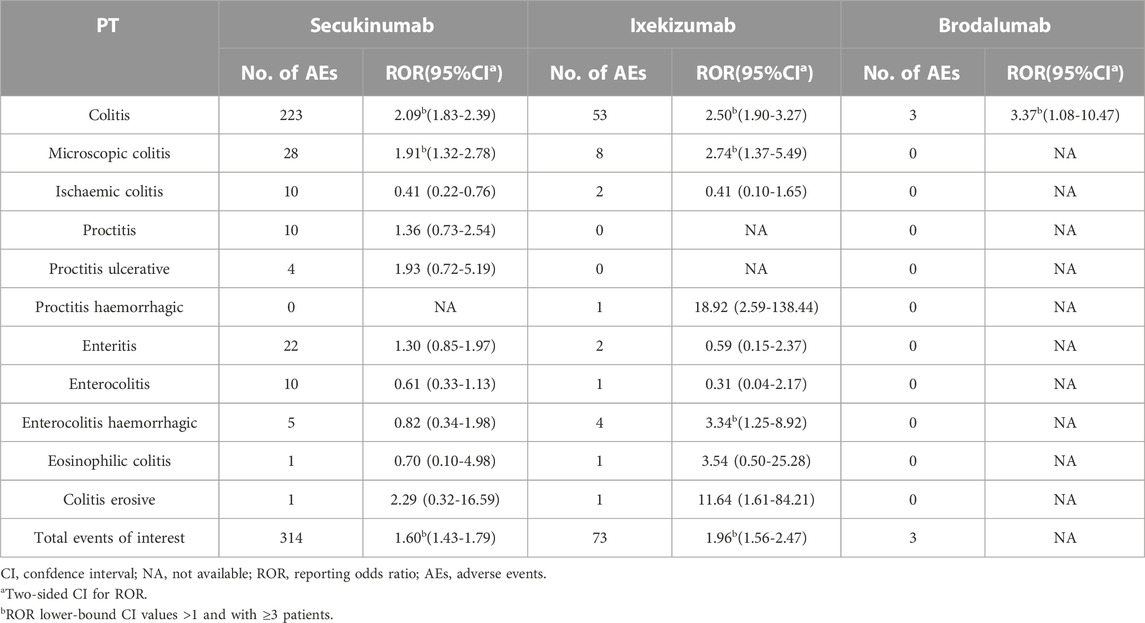
TABLE 3. Interleukin-17 inhibitors associated with Colitis and the reporting odds ratios in FAERS database.
Descriptive analysis
A total of 29 studies were identified in the published literature, including 25 case reports (Ehrlich et al., 2018; Grimaux et al., 2018; Paul et al., 2018; Wang et al., 2018; Achufusi et al., 2019; Grossberg, 2019; Haidari et al., 2019; Johnston and Veettil, 2019; Philipose et al., 2019; Rodriguez et al., 2019; Shimizu et al., 2019; Smith et al., 2019; Darch et al., 2020; Fieldhouse et al., 2020; Marin et al., 2020; Merino Gallego et al., 2020; Nazarian et al., 2020; Ali et al., 2021; Mu et al., 2021; Obeidat and Murakami, 2021; Ma et al., 2022; Sykaras et al., 2022; Kukol et al., 2019; Nallapeta et al., 2019; Sethi et al., 2019) and 4 case series (Fobelo et al., 2018; Shukla et al., 2018; Vernero et al., 2019; Lee et al., 2020); a total of 34 cases raised evidence of IBD occurrence during therapy with SEC (27 cases, 79.4%) and IXE (7 cases, 20.6%). Characteristics of the included cases are presented in Table 4. The patient sex ratio (male/female) was 1, the median age of included patients was 42 years (range 19–76 years), and 23 patients (67.7%) were between 26 and 50 years old. Data on race showed that 93.9% of patients (32 cases) were white, and two were Asian. Three patients (8.8%) had a prior history of IBD, and five patients (14.7%) had a family history of IBD. History of smoking was reported for eight patients (23.5%). The main indication for anti-IL-17 therapy was Pso (20 cases, 58.8%), followed by AS (7 cases, 20.6%), PsA (4 cases, 11.8%), and Pso with PsA (3 cases, 8.8%). Twenty-four patients had prior exposure to a TNF antagonist with inadequate response, of whom 13 received prior adalimumab and 11 received prior etanercept. The time between IL-17 inhibitor treatment start and symptom onset was assessable in 27 cases, showing a median time to onset of 2.9 months (range, 0.47–48 months); onset of symptoms occurred in 5 cases (18.5%) in less than a month, in 9 cases (33.3%) between 1 and 3 months, and in 10 cases (37.1%) between 3 and 12 months.

TABLE 4. Summary of demographic features of patients with interleukin (IL)-17 inhibitors-induced Inflammatory bowel disease.
Clinical manifestations and laboratory and radiological features
Among the 34 cases included, IBD classification was reported in 28 cases, with 13 cases (46.4%) identified as UC, 10 cases (35.7%) as CD, and 5 cases (17.9%) as unclassified IBD. Symptoms were reported for 33 patients; the most common initial symptoms were diarrhea (30 cases, 90.9%), followed by abdominal pain (19 cases, 57.6%), bloody diarrhea (17 cases, 51.5%), and fever (12 cases, 36.4%). Other accompanying symptoms included weight loss (5 cases, 15.2%), chills (4 cases, 12.1%), and nausea (4 cases, 12.1%). One patient underwent colonoscopy for colorectal cancer screening, which revealed asymptomatic Crohn’s disease (Table 5).
The laboratory results for IL-17 inhibitor-induced IBD cases are summarized in Table 5. Elevated white blood cell count (WBC) was observed in seven patients (87.5%) out of eight reported cases, elevated erythrocyte sedimentation rate (ESR) levels were observed in six patients (85.7%), and increased C-reactive protein was reported in 16 patients (100%). Levels of fecal calprotectin were increased in four patients (100%).
Computed tomography (CT) results were reported for 10 patients. Typical imaging features were bowel wall thickening (5 cases, 50.0%), pancolitis (2 cases, 20.0%), and colon perforation (1 case, 10.0%); two patients showed normal CT results.
Colonoscopy and histopathological examination
Colonoscopy results were reported for 28 patients, with deep ulcerations found in 13 patients (46.4%) and superficial ulcerations found in 6 patients (21.4%); eight patients (28.5%) showed active inflammation, four patients (14.3%) had friable mucosa, and four patients (14.3%) had loss of the normal vascular pattern. Intestinal mucosal biopsies were performed in 25 patients, and the characteristics of histopathological examination presented mainly as chronic inflammation (11 cases, 39.3%), cryptitis (8 cases, 32.0%), crypt abscesses (8 cases, 32.0%), and granulomatous lesions (7 cases, 28.0%).
Treatment and prognosis
A total of 31 patients discontinued IL-17 inhibitor therapy after the onset of IBD. Among the 34 included patients, 19 (61.3%) underwent monotherapy for IBD; corticosteroid therapy was administered in 10 cases (32.3%), TNF antagonist therapy in 6 cases (19.4%), ustekinumab therapy in 2 cases (6.4%), and mesalazine therapy in 1 case (3.2%). A total of 11 patients (35.5%) received combination therapy, and one patient did not receive any treatment. Six patients failed in treatment with corticosteroids alone or in combination with mesalazine; they obtained significant improvement when treatment was changed to infliximab alone or in combination with corticosteroids (Table 5). All patients showed improvement, except for two patients who relapsed after 2 and 3 months of remission, respectively. Symptom improvement occurred between 2 days and 3 months after discontinuation of IL-17 inhibitor therapy, with three patients (20.0%) experiencing improvement within 2 weeks, seven patients (46.7%) between 2 and 4 weeks, and five patients (33.3%) showing improvement after more than 4 weeks. The median time to remission after discontinuation of IL-17 inhibitor treatment was 4 weeks. After discontinuation of IL-17 inhibitors, three patients (17.7%) switched to a TNF antagonist, five patients switched to ustekinumab (35.3%), and four patients (23.5%) selected another type of monoclonal antibody, such as tildrakizumab or guselkumab.
Discussion
To the best of our knowledge, this is the first real-life comparative safety study using data from the FAERS database that assessed onset or exacerbation of IBD and colitis associated with all three IL-17 inhibitors in current use. Our study has provided supportive disproportionality analysis and data on the clinical characteristics of these rare adverse events. We found, from the perspective of pharmacovigilance, that the IL-17 inhibitors SEC (ROR = 2.13, 95% CI [1.96-2.30]) and IXE (ROR = 2.79, 95% CI [2.39-3.27]) can trigger IBD, while BRO did not trigger safety signals. We have also provided practical information about IBD and colitis types, time to symptom onset, and outcomes of treatment with IL-17 inhibitors, which had not been the focus of previous studies. A total of 388 case reports of primary suspect IL-17 inhibitor-related gastrointestinal inflammatory conditions were identified in the FAERS database, which included 268 IBD diagnoses and 120 colitis diagnoses. We detected four cases associated with BRO treatment, whereas the number of IBD cases that occurred after SEC treatment was 10 times greater than that observed after IXE therapy (89.7% vs. 9.3%). In a post-marketing study via VigiBase in 2021, no IBD was detected among patients in the BRO group, and the number of IBD cases associated with SEC treatment was five times that observed in the IXE treatment group (83.3% vs. 16.2%) (Petitpain et al., 2021), which is consistent with our findings. The number of IBD cases among patients treated with SEC, IXE, and BRO could, in part, be traced back to the different launch data (January 2015, April 2016, and July 2017, respectively). It is also worth noting that IL-17 inhibitors are not identical as they differ in pharmacology and target molecules. IXE is a humanized IgG4 antibody that has strong affinity for IL-17A, while SEC and BRO are fully humanized antibodies. Both SEC and IXE work by inhibiting IL-17A homodimers and IL-17A/F heterodimers, while BRO blocks the IL-17 receptor, thereby blocking all IL-17 isoforms and exerting a broader, less targeted effect (Fauny et al., 2020a). These data provide a potential explanation for the lower number cases of IBD associated with IXE or BRO therapy compared to treatment with SEC.
To date, studies based on pooled data from clinical studies, observational studies, retrospective studies, and meta-analyses have not provided substantial detail regarding the clinical features of IBD induced by IL-17 inhibitors (Onac et al., 2021; Schreiber et al., 2019; Caron et al., 2022). Our study has provided separate analyses of initial symptom onset, laboratory features, colonoscopy results, histopathological examination, treatment, and outcome. By collecting medical records from published case reports and case series involving IL-17 inhibitor-associated IBD, we revealed that diarrhea, bloody diarrhea, abdominal pain, and fever were the most common symptoms, which were often accompanied by elevated WBC count, ESR, C-reactive protein, and fecal calprotectin level. A systematic review concluded that gut histological and macroscopic mucosal inflammation occurred in up to 100% of patients with rheumatological disease, and 80% of these patients had increased fecal calprotectin levels (Fauny et al., 2020b), revealing that fecal calprotectin may act as a useful and non-invasive marker to predict IBD in patients with SpA or AS. Therefore, fecal calprotectin should be monitored to guide physicians’ decisions and enable stratification of patients to ensure optimal treatment. We also summarized colonoscopy and histopathological examination data from the included cases. According to the literature, ileocolonoscopy allows an accurate diagnosis of Crohn’s disease or ulcerative colitis in 90% of cases (Passos et al., 2018); therefore, it is recommended that patients with diarrhea, abdominal pain, bloody stools, or fever should be alerted to the possibility of IBD and undergo colonoscopy during IL-17 inhibitor treatment.
In the descriptive analysis, we observed that 91.2% of the cases were new-onset IBD, and about half of the new-onset cases were detected within 3 months of starting anti-IL-17 therapy. A study by Deodhar et al. (2019) reported that 30 of the 41 retrieved IBD cases (73.2%) were new-onset cases (Deodhar et al., 2019). Another analysis based on VigiBase data indicated that 91.8% of cases were new-onset IBD, and approximately three-quarters of IBD relapse cases were detected within 3 months of starting anti-IL-17 therapy (Petitpain et al., 2021). Given the long half-life of IL-17 inhibitors, the relatively short interval between drug use and IBD onset revealed an early impact of the drug on disease symptoms. However, it remains difficult to demonstrate whether anti-IL-17 drugs are coincidentally present or potential causative inducers of new or latent IBD. Previous research had demonstrated that patients with Pso, PsA, or AS may be at higher risk of developing IBD and that populations of patients with these rheumatologic diseases and populations with IBD have considerable overlap (Fieldhouse et al., 2020). In a genome-wide association study, several IBD loci were identified as overlapping with those of other immune-mediated inflammatory diseases (IMIDs), most notably AS and Pso (Jostins et al., 2012). The prevalence of IBD in Pso is estimated to be 1%–2% compared to 0.4% in the general population (Eppinga et al., 2017). Therefore, some Pso patients might have subclinical IBD triggered by IL-17 inhibitors. Since no recommendations are available regarding the management of patients with a history of IBD before starting an anti-IL-17 drug, it is of great importance to complete comprehensive patient history assessments, including the patient’s personal and family history of inflammatory bowel disease and other IMIDs before initiating treatment to help guide selection of the most appropriate management options. In recent years, the number of studies describing the correlation between slow and rapid onset of IBD, following IL-17 inhibitor treatment, has gradually increased. However, the pathogenesis of these paradoxical reactions after IL-17 administration remains unclear. The pathophysiology behind IBD involves upregulation of proinflammatory and immune-regulatory cytokines in the mucosa of the small and large intestines. IL-17 is a cytokine that is thought to contribute to the development of IBD and has strong proinflammatory activity (Magyari et al., 2014), which is significantly elevated in patients with active ulcerative colitis and Crohn’s disease (Seiderer et al., 2008). A previous study in mice has shown that blocking IL-17 leads to worsening of colitis by increasing tumor necrosis factor-α, interferon-γ, IL-6, and cytokines that promote the inflammatory response (Ogawa et al., 2004). Furthermore, IL-17A or IL-17RA inhibition has been associated with severe weakening of the intestinal epithelial barrier and reduction of intestinal epithelial integrity, which causes leakage of microorganisms and inflammation (Maxwell et al., 2015). However, it is important to note that IL-17 might also have beneficial effects within the intestinal tract. In animal models, gut-protective IL-17A was shown to be independently produced in the colonic lamina propria, where it promoted epithelial barrier function by regulating tight junction proteins after acute intestinal injury (Lee et al., 2015). This suggests a delicate balance between potentially favorable effects and detrimental effects orchestrated by IL-17 in the gut.
At present, there is no clinical guidance for the management of patients with disease flare or new-onset IBD after IL-17 inhibitor therapy. Our study suggests that alternative treatments to improve gastrointestinal symptoms and treatment of the underlying pathology have included mainly corticosteroids and TNF antagonists, as either monotherapy or combination therapy; mesalazine and other biological agents (such as ustekinumab) were shown to be effective as well. Corticosteroids were used in 66.7% of cases in our study, and the type, dose, and duration in each case depended on the improvement of symptoms. Discontinuation of IL-17 inhibitors resulted in substantial improvement of symptoms in all patients, except for two who experienced relapse after 2 or 3 months of remission. Generally, the prognosis of IBD related to IL-17 inhibitors was excellent, and we observed that most patients (66.7%) recovered within 4 weeks. However, we still recommend that these patients be followed up for a longer period to monitor for potential recurrence.
Conclusion
Our retrospective analysis demonstrated that use of IL-17 inhibitors is associated with exacerbation or new-onset of IBD and colitis within 6 months of therapy. Rheumatologists and dermatologists should be on alert for gastrointestinal symptoms, such as diarrhea, bloody diarrhea, abdominal pain, and fever, along with elevated WBC count, ESR, C-reactive protein, and fecal calprotectin levels during IL-17 inhibitor therapy. It is essential to complete a comprehensive patient history prior to the initiation of treatment, include appropriate workup to monitor intestinal inflammatory markers, such as fecal calprotectin, and perform colonoscopy during treatment to help identify early symptoms and prevent severe complications. Early detection of adverse events via prompt evaluation, cessation of IL-17 inhibitors, and initiation of alternative treatment (e.g., corticosteroids and anti-TNF therapy) can generally lead to clinical remission of IBD. Further prospective studies are needed to evaluate and confirm risk factors for development of IBD associated with IL-17 inhibitor therapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ChW and ZD conceived of the study; ChW and ZD drafted and revised the manuscript; and CuW and SW made data elaboration and performed statistical analyses. All authors contributed to the final manuscript and approved the submitted version.
Funding
This study was supported by the Natural Science Foundation of Hunan Province in China (2021JJ40911).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1124628/full#supplementary-material
References
Achufusi, T. G., Harnee, P. S., and Rawlins, S. (2019). A rare case of new-onset ulcerative colitis following initiation of secukinumab. Case Rep. Med. 2019, 2975631–2975635. doi:10.1155/2019/2975631
Ali, A. K., Torosian, A., Porter, C., Bloomfeld, R. S., and Feldman, S. R. (2021). New onset inflammatory bowel disease in patient treated with secukinumab: Case report and review of literature. Dermatol Ther. 34, e15151. doi:10.1111/dth.15151
Burisch, J., Eigner, W., Schreiber, S., Aletaha, D., Weninger, W., Trauner, M., et al. (2020). Risk for development of inflammatory bowel disease under inhibition of interleukin 17: A systematic review and meta-analysis. PLOS ONE 15, e0233781. doi:10.1371/journal.pone.0233781
Caron, B., Jouzeau, J. Y., Miossec, P., Petitpain, N., Gillet, P., Netter, P., et al. (2022). Gastroenterological safety of IL-17 inhibitors: A systematic literature review. Expert Opin. Drug Saf. 21, 223–239. doi:10.1080/14740338.2021.1960981
Darch, K. M., Holland, T. L., and Spelman, L. J. (2020). Secukinumab-induced inflammatory bowel disease in a patient treated for chronic plaque psoriasis and psoriatic arthritis: A case report and review of the role of novel biologic agents targeting the p19 subunit of IL-23. Case Rep. Med. 2020, 9404505. doi:10.1155/2020/9404505
Deodhar, A., Mease, P. J., McInnes, I. B., Reich, K., BlAuvelt, A., et al. (2019). Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: Integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res. Ther. 21, 111. doi:10.1186/s13075-019-1882-2
Ehrlich, D., Jamaluddin, N., Pisegna, J., and Padua, D. (2018). A challenging case of severe ulcerative colitis following the initiation of secukinumab for ankylosing spondylitis. Case Rep. Gastrointest. Med. 2018, 9679287–9679294. doi:10.1155/2018/9679287
Eppinga, H., Poortinga, S., Thio, H. B., Nijsten, T. E. C., Nuij, V. J. A. A., van der Woude, C. J., et al. (2017). Prevalence and phenotype of concurrent psoriasis and inflammatory bowel disease. Inflamm. Bowel Dis. 23, 1783–1789. doi:10.1097/MIB.0000000000001169
Fauny, M., D'Amico, F., Bonovas, S., Netter, P., Danese, S., Loeuille, D., et al. (2020). Faecal calprotectin for the diagnosis of bowel inflammation in patients with rheumatological diseases: A systematic review. J. Crohns Colitis 14, 688–693. doi:10.1093/ecco-jcc/jjz205
Fauny, M., Moulin, D., D'Amico, F., Netter, P., Petitpain, N., Arnone, D., et al. (2020). Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann. Rheum. Dis. 79, 1132–1138. doi:10.1136/annrheumdis-2020-217927
Felice, C., Leccese, P., Scudeller, L., Lubrano, E., Cantini, F., Castiglione, F., et al. (2019). Red flags for appropriate referral to the gastroenterologist and the rheumatologist of patients with inflammatory bowel disease and spondyloarthritis. Clin. Exp. Immunol. 196, 123–138. doi:10.1111/cei.13246
Fieldhouse, K. A., Ukaibe, S., Crowley, E. L., Khanna, R., O'Toole, A., and Gooderham, M. J. (2020). Inflammatory bowel disease in patients with psoriasis treated with interleukin-17 inhibitors. Drugs Context 9, 2020. doi:10.7573/dic.2020-2-1
Fobelo, L. M., Serrano, G. R., and Castro, F. M. (2018). Emergence of inflammatory bowel disease during treatment with secukinumab. J. Crohns Colitis 12, 1131–1133. doi:10.1093/ecco-jcc/jjy063
Gossec, L., Baraliakos, X., Kerschbaumer, A., de Wit, M., Mclnnes, I., Dougados, M., et al. (2020). EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 79, 700–712. doi:10.1136/annrheumdis-2020-217159
Griffiths, C. E., Reich, K., Lebwohl, M., van de Kerkhof, P., Paul, C., Menter, A., et al. (2015). Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): Results from two phase 3 randomised trials. Lancet 386, 541–551. doi:10.1016/S0140-6736(15)60125-8
Grimaux, X., Leducq, S., Goupille, P., Aubourg, A., Miquelestorena-Standley, E., and Samimi, M. (2018). Aphthous mouth ulcers as an initial manifestation of sécukinumab-induced inflammatory bowel disease. Ann. de Dermatologie de Vénéréologie. 145, 676–682. doi:10.1016/j.annder.2018.07.009
Grossberg, L. B. (2019). A case report of successful treatment of Crohn's disease and psoriasis with guselkumab. Inflamm. Bowel Dis. 25, e84. doi:10.1093/ibd/izz033
Haidari, W., Al-Naqshabandi, S., Ahn, C. S., Bloomfeld, R. S., and Feldman, S. R. (2019). Asymptomatic Crohn’s disease identified in a patient being treated with secukinumab: A case report. SAGE Open Med. Case Rep. 7, 2050313X19893580. doi:10.1177/2050313X19893580
Johnston, D. N., and Veettil, R. (2019). A case of new onset ulcerative colitis following secukinumab treatment. Br. J. Hosp. Med. (Lond). 80, 544–545. doi:10.12968/hmed.2019.80.9.544
Jostins, L., Ripke, S., Weersma, R. K., Duerr, R. H., McGovern, D. P., Hui, K. Y., et al. (2012). Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124. doi:10.1038/nature11582
Kukol, W., Aranez, L., and Marino, D. (2019). P055 development of Crohn’s disease with use of secukinumab. Am. J. Gastroenterol. 114, S15. doi:10.14309/01.ajg.0000578292.95094.87
Langley, R. G., Elewski, B. E., Lebwohl, M., Reich, K., Griffiths, C. E. M., Papp, K., et al. (2014). Secukinumab in plaque psoriasis--results of two phase 3 trials. N. Engl. J. Med. 371, 326–338. doi:10.1056/NEJMoa1314258
Lebwohl, M., Strober, B., Menter, A., Gordon, K., Weglowska, J., Puig, L., et al. (2015). Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N. Engl. J. Med. 373, 1318–1328. doi:10.1056/NEJMoa1503824
Lee, A., Levell, N. J., Shah, S. N., Gaffney, K., and Tremelling, M. (2020). Severe colitis complicating secukinumab (Cosentyx®) therapy. Clin. Exp. Dermatol 45, 344–345. doi:10.1111/ced.14149
Lee, J. S., Tato, C. M., Joyce-Shaikh, B., Gulen, M. F., Gulan, F., Cayatte, C., et al. (2015). Interleukin-23-Independent IL-17 production regulates intestinal epithelial permeability. Immunity 43, 727–738. doi:10.1016/j.immuni.2015.09.003
Ma, J., Liu, M., Liu, Y., and Wang, N. (2022). Ulcerative colitis associatied with Secukinumab. J. adverse drug React. 24, 380–382.
Magyari, L., Kovesdi, E., Sarlos, P., Javorhazy, A., Sumegi, K., and Melegh, B. (2014). Interleukin and interleukin receptor gene polymorphisms in inflammatory bowel diseases susceptibility. World J. Gastroenterol. 20, 3208–3222. doi:10.3748/wjg.v20.i12.3208
Marin, M., Alzueta, N., Pío, M., Gascón, A., and Castresana, M. (2020). Ulcerative colitis induced by ixekizumab: A case report. Eur. J. Hosp. Pharm. 28, 50–52. doi:10.1136/ejhpharm-2019-002016
Maxwell, J. R., Zhang, Y., Brown, W. A., Smith, C. L., Byrne, F. R., Fiorino, M., et al. (2015). Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity 43, 739–750. doi:10.1016/j.immuni.2015.08.019
Menter, A., Strober, B. E., Kaplan, D. H., Kivelevitch, D., Prater, E. F., and Stoff, B. (2019). Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J. Am. Acad. Dermatol 80, 1029–1072. doi:10.1016/j.jaad.2018.11.057
Merino Gallego, E., Gómez Torres, K., and Martínez Amate, E. (2020). Debut of inflammatory bowel disease associated to ixekizumab in patient with moderate, difficult -To-Manage psoriasis. Gastroenterol. Hepatol. 43, 622–623. doi:10.1016/j.gastrohep.2020.04.009
Mu, X., Fardy, J., Reid, S., and Trahey, J. (2021). Severe drug-associated colitis with Crohn’s features in setting of ixekizumab therapy for chronic plaque psoriasis. Bmc Gastroenterol. 21, 361. doi:10.1186/s12876-021-01936-w
Nallapeta, N., Picano, J., and Bou-Abdallah, J. (2019). Delayed onset of inflammatory bowel disease during treatment with secukinumab: 2112. Am. J. Gastroenterol. 114, S1176. doi:10.14309/01.ajg.0000597980.32774.79
Nazarian, A., Grin, A., and Wijeratne, D. T. (2020). Ixekizumab associated new-onset inflammatory bowel disease. ACG Case Rep. J. 7, e00316. doi:10.14309/crj.0000000000000316
Obeidat, A. E., and Murakami, T. T. (2021). New-onset collagenous colitis in a patient with psoriatic arthritis: Can it Be secukinumab? Cureus 13, e16147. doi:10.7759/cureus.16147
Ogawa, A., Andoh, A., Araki, Y., Bamba, T., and Fujiyama, Y. (2004). Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 110, 55–62. doi:10.1016/j.clim.2003.09.013
Onac, I. A., Clarke, B. D., Tacu, C., Lloyd, M., Hajela, V., Batty, T., et al. (2021). Secukinumab as a potential trigger of inflammatory bowel disease in ankylosing spondylitis or psoriatic arthritis patients. Rheumatol. Oxf. 60, 5233–5238. doi:10.1093/rheumatology/keab193
Ouyang, W., Kolls, J. K., and Zheng, Y. (2008). The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467. doi:10.1016/j.immuni.2008.03.004
Passos, M., Chaves, F. C., and Chaves-Junior, N. (2018). The importance of colonoscopy in inflammatory bowel diseases. Arq. Bras. Cir. Dig. 31, e1374. doi:10.1590/0102-672020180001e1374
Paul, N., Hektner, K., and Bing, Z. (2018). Secukinumab-induced unmasking of crohn colitis: A case report: 1509. Am. J. Gastroenterol. 113, S866–S867. doi:10.14309/00000434-201810001-01509
Petitpain, N., D'Amico, F., Yelehe-Okouma, M., Jouzeau, J. Y., Netter, P., Peyrin-Biroulet, L., et al. (2021) IL-17 inhibitors and inflammatory bowel diseases: A postmarketing study in Vigibase. Clin. Pharmacol. Ther. 110: 159–168. doi:10.1002/cpt.2155
Philipose, J., Ahmed, M., Idiculla, P. S., Mulrooney, S. M., and Gumaste, V. V. (2019). Severe de novo Ulcerative Colitis following Ixekizumab Therapy. Case Rep. Gastroenterology 12, 617–621. doi:10.1159/000493922
Ramiro, S., Nikiphorou, E., Sepriano, A., Ortolan, A., Webers, C., Baraliakos, X., et al. (2023). ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann. Rheum. Dis. 82, 19–34. doi:10.1136/ard-2022-223296
Rodriguez, M. R., Vazquez, M. J., and Pallares, M. H. (2019). The onset of ulcerative colitis during treatment with secukinumab: Can anti-IL-17a be a trigger for inflammatory bowel disease? Rev. Esp. Enferm. Dig. 111, 720–721. doi:10.17235/reed.2019.5841/2018
Schreiber, S., Colombel, J. F., Feagan, B. G., Reich, K., Deodhar, A. A., McInnes, I. B., et al. (2019). Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: A retrospective analysis of pooled data from 21 clinical trials. Ann. Rheum. Dis. 78, 473–479. doi:10.1136/annrheumdis-2018-214273
Seiderer, J., Elben, I., Diegelmann, J., Glas, J., Stallhofer, J., Tillack, C., et al. (2008). Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): Upregulated colonic IL-17F expression in active Crohn's disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflamm. Bowel Dis. 14, 437–445. doi:10.1002/ibd.20339
Sethi, V., Jacobs, A., and Sethi, A. (2019). P089 secukinumab induced ulcerative colitis in a patient with psoriatic arthritis: A novel approach to refractory cases. Am. J. Gastroenterol. 114, S23. doi:10.14309/01.ajg.0000613324.15639.1f
Shimizu, K., Matsushita, T., Takehara, K., and Hamaguchi, Y. (2019). A case of juvenile localized scleroderma with anti-topoisomerase I antibody. Eur. J. Dermatol 29, 443–444. doi:10.1684/ejd.2018.3426
Shukla, T., McCurdy, J., Fahim, S., and Rostom, A. (2018). A90 three patients with inflammatory bowel disease diagnosed while being treated with secukinumab for psoriasis. J. Can. Assoc. Gastroenterology 1, 135–136. doi:10.1093/jcag/gwy009.090
Smith, M. K., Pai, J., Panaccione, R., Beck, P., Ferraz, J. G., and Jijon, H. (2019). Crohn’s-like disease in a patient exposed to anti-interleukin-17 blockade (ixekizumab) for the treatment of chronic plaque psoriasis: A case report. Bmc Gastroenterol. 19, 162. doi:10.1186/s12876-019-1067-0
Sykaras, A. G., Margellou, E., Vallianou, N. G., Panagopoulos, F., Geladari, E., Kounatidis, D., et al. (2022). Multifaceted secukinumab-induced colitis. Inflamm. Bowel Dis. 28, e47–e48. doi:10.1093/ibd/izab267
van der Heijde, D., Ramiro, S., Landewe, R., Baraliakos, X., Van den Bosch, F., Sepriano, A., et al. (2017). 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann. Rheum. Dis. 76, 978–991. doi:10.1136/annrheumdis-2016-210770
van Puijenbroek, E. P., Bate, A., Leufkens, H. G., Lindquist, M., Orre, R., and Egberts, A. C. (2002). A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 11, 3–10. doi:10.1002/pds.668
Vernero, M., Astegiano, M., and Ribaldone, D. G. (2019). New onset of inflammatory bowel disease in three patients undergoing IL-17a inhibitor secukinumab: A case series. Am. J. Gastroenterol. 114, 179–180. doi:10.1038/s41395-018-0422-z
Wang, J., Bhatia, A., Cleveland, N. K., Gupta, N., Dalal, S., Rubin, D. T., et al. (2018). Rapid onset of inflammatory bowel disease after receiving secukinumab infusion. ACG Case Rep. J. 5, e56. doi:10.14309/crj.2018.56
Wendling, D., Hecquet, S., Fogel, O., Letarouilly, J. G., Verhoeven, F., Pham, T., et al. (2022). 2022 French Society for Rheumatology (SFR) recommendations on the everyday management of patients with spondyloarthritis, including psoriatic arthritis. Jt. Bone Spine. 89, 105344. doi:10.1016/j.jbspin.2022.105344
Keywords: IL-17 inhibitors, inflammatory bowel disease, FAERS database, pharmacovigilance, drug safety
Citation: Deng Z, Wang S, Wu C and Wang C (2023) IL-17 inhibitor-associated inflammatory bowel disease: A study based on literature and database analysis. Front. Pharmacol. 14:1124628. doi: 10.3389/fphar.2023.1124628
Received: 15 December 2022; Accepted: 07 March 2023;
Published: 23 March 2023.
Edited by:
Weihong Sha, Guangdong Academy of Medical Sciences, ChinaReviewed by:
Roberta Ramonda, University of Padua, ItalyYukiko Miyamoto, University of California, San Diego, United States
Manuela Neuman, University of Toronto, Canada
Copyright © 2023 Deng, Wang, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunjiang Wang, d29uZ2NqMjAyMkAxNjMuY29t
†ORCID: Zhenzhen Deng, http://orcid.org/0000-0003-3045-1710; Chunjiang Wang, http://orcid.org/0000-0002-8490-0131
 Zhenzhen Deng
Zhenzhen Deng Shengfeng Wang
Shengfeng Wang Cuifang Wu
Cuifang Wu Chunjiang Wang
Chunjiang Wang