- 1Department of Dermatology, Hunan Key Laboratory of Medical Epigenomics, The Second Xiangya Hospital of Central South University, Changsha, Hunan, China
- 2Department of Nephrology, Hunan Key Laboratory of Kidney Disease and Blood Purification, The Second Xiangya Hospital of Central South University, Changsha, Hunan, China
Aims: To determine the bioactive components of Hedyotis Diffusae Herba (HDH) and the targets in treating lupus nephritis (LN), and so as to elucidate the protective mechanism of HDH against LN.
Methods and results: An aggregate of 147 drug targets and 162 LN targets were obtained from online databases, with 23 overlapped targets being determined as potential therapeutic targets of HDH against LN. Through centrality analysis, TNF, VEGFA and JUN were screened as core targets. And the bindings of TNF with stigmasterol, TNF with quercetin, and VEGFA with quercetin were further validated by molecular docking. By conducting Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses for drug targets, disease targets and the shared targets, TNF signaling pathway, Toll-like receptor signaling pathway, NF-kappa B signaling pathway and HIF-1 signaling pathway, etc., were found in all these three lists, indicating the potential mechanism of HDH in the treatment of LN.
Conclusion: HDH may ameliorate the renal injury in LN by targeting multi-targets and multi-pathways, including TNF signaling pathway, NF-kappa B signaling pathway, HIF-1 signaling pathway and so on, which provided novel insights into further researches of the drug discovery in LN.
Introduction
Systemic lupus erythematosus (SLE) is a multi-system autoimmune disease along with abnormally activated immune system, attacking practically any organ system in the human body (Calle-Botero and Abril, 2020; Kiriakidou and Ching, 2020). In SLE, lupus nephritis (LN) is considered as the major pathogenic and fatal risk contributors (Maria and Davidson, 2020), affecting almost 40% of adults with SLE, with 10% of LN patients having to face the torment of end-stage renal disease ultimately (Bastian et al., 2002; Jakes et al., 2012; Almaani et al., 2017). Recently, the efficacy of corticosteroids, cyclophosphamide, mofetil/mycophenolate, and calcineurin inhibitors in LN was identified (Fanouriakis et al., 2019), yet low complete response rates, risk of flares, side effects and adverse outcome events of these treatments still remain major concerns to physicians (Hobeika et al., 2019). Hence, finding effective and safe alternative medicines to fight against LN is an urgent global issue to be addressed.
Traditional Chinese medicine is one of the oldest healing systems and has been widely used for thousands of years (Tang et al., 2008; Liu et al., 2021). Due to the multi-targets function (Jiang et al., 2021), traditional Chinese medicine is now recognized as one of the prominent alternative therapies for the treatment of various diseases, including SLE and LN (Yuan et al., 2019a; Zhang and Wei, 2020). Tripterygium wilfordii Hook F. (TWHF) is such a typical representative that has been proved to ameliorate LN through its anti-inflammatory and immunosuppressive effects (Tao and Lipsky, 2000; Ma et al., 2017; Song et al., 2020). However, because of its toxicity, the clinical application of TWHF is severely limited, especially in patients with reproductive needs (Ren et al., 2021). Hedyotis Diffusae Herba (HDH) is another traditional Chinese medicine herb that is widely applied in a variety of prescriptions for the treatment of immune-related diseases (Fan et al., 2010; Jiang et al., 2010). In 2010, Jiang et al. (2010) reported that Bizhongxiao Decoction, which contains HDH, could improve rheumatoid arthritis by regulating the protein expression and function of peripheral blood mononuclear cells. In addition, the protective role and mechanism of HDH in cancer was also demonstrated recently (Wang et al., 2020; Liu et al., 2022; Ma et al., 2023). Besides, Fan et al. (2010) found that the application of HDH could significantly inhibit the expression of regulated on activation, normal T cell expressed and secreted (RANTES, also known as C-C motif chemokine 5), a biomarker of LN (Das and Brunner, 2009), in serum and renal tissue of MRL/lpr mice (lupus-prone mice). However, the specific mechanism of HDH as related to LN remains unclear due to limited researches. Hence, studies investigating key molecular targets and mechanism of HDH against LN needs to be carried out.
Network pharmacology is an emerging interdisciplinary science that integrates virtual computing, high-throughput data analyses, network database retrieval, bioinformatic network construction and network topology analyses (Kibble et al., 2015; Wang et al., 2022a), and was developed for the explanation of the relationship between drug components, targets, and diseases (Luo et al., 2020; Jiao et al., 2022). Nowadays, network pharmacology has become a holistic and efficient tool to unveil the pharmacological mechanism of traditional Chinese medicine and is conducive to provide deep insights into new medicine developing from a network perspective (Hopkins, 2008; Sucher, 2013). HDH, as a traditional Chinese herbal medicine widely applied in prescriptions for LN, has a wide range of pharmacological compounds, however, its mechanism for the treatment of LN remains obscure. In this study, analyses based on integrated network pharmacology were applied to tackle this issue and identify active ingredients and pivotal targets of HDH against LN.
Materials and methods
Identifying LN related targets
LN related targets were retrieved from the OMIM database (Hamosh et al., 2000) (https://omim.org/), the DisGeNET database (Piñero et al., 2015; Piñero et al., 2020) (https://www.disgenet.org/) and the DigSee database (Kim et al., 2013; Kim et al., 2017) (http://210.107.182.61/geneSearch/). As a biomedical literature based authoritative database storing information about human genes and genetic phenotypes, OMIM database could provide us with phenotypes related targets. In the OMIM database, the term “Lupus nephritis” was used as a bait to retrieve LN related records and genes, and 75 records containing 35 LN related genes were obtained finally. Distinct with the OMIM database, the DisGeNET database and the DigSee database possess more massive data of human disease-related genes and scoring systems to help screening reliable diseases-related genes. By inputting “Lupus nephritis” into query box and setting “Score_gda” > 0.05 in DisGeNET database or “Evidence Sentence Score” > 0.5 in DigSee database, 51 LN associated targets from the DisGeNET database and 115 targets obtained from the DigSee database were acquired. All genes were then transformed into unified names by UniProt (UniProt Consortium, 2018) (https://www.uniprot.org/). A total of 162 genes were identified as LN targets by removing duplicates.
Bioactive components of HDH
TCMSP database (Ru et al., 2014) (https://tcmspw.com/tcmsp.php) is a platform that captures relationship between Chinese herbal medicines, targets and diseases. And most importantly, it provides physical and chemical characteristics of herbal ingredients. To obtain bioactive chemical components of HDH, “Hedyotis Diffusae Herba” was used as the search term, and the components meeting following criterions were selected for further analyses: 1) oral bioavailability ≥40%; 2) drug-likeness ≥0.18; 3) number of rotatable bond <10; 4) molecular weight: 180–500; and 5) has corresponding Pubchem Cid. Poriferasterol, stigmasterol and quercetin were identified as bioactive chemical components of HDH.
Identifying HDH related targets
To acquire HDH related targets, SMILE strings, InChI strings or names of HDH bioactive components that were acquired from the previous step were then inputted into the query boxes of the STITCH database (Szklarczyk et al., 2016) (http://stitch.embl.de/), the BATMAN-TCM database (Liu et al., 2016) (http://bionet.ncpsb.org/batman-tcm/) and the TCMSP database (Ru et al., 2014) (https://tcmspw.com/tcmsp.php), with the results being limited to homo species. Targets from the BATMAN-TCM database under the criterion of score >15 were selected for subsequent analyses. And for targets predicted from the TCMSP database, those displayed as originating from the DrugBank database were selected. All the returned targets were converted into standard names by UniProt (UniProt Consortium, 2018). Finally, 77 targets of stigmasterol, 40 targets of poriferasterol and 83 targets of quercetin were predicted as HDH corresponding targets.
Functional enrichment analyses and network construction
The Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways enrichment analysis were conducted by online function annotation tool DAVID (Huang et al., 2009a; Huang et al., 2009b) (https://david.ncifcrf.gov/, version 6.8), and further visualized by a data visualization website (http://www.bioinformatics.com.cn/). While the protein-protein interaction (PPI) networks for LN targets, HDH targets and the overlapped targets were constructed by the STRING database (Szklarczyk et al., 2019) (https://string-db.org/, version 11.0), a platform storing known PPIs originating from other databases and predicted PPIs that were obtained through computer algorithms. Further construction of HDH-bioactive components-targets network, LN targets network, and bioactive components-the overlapped targets-enriched KEGG pathways network were conducted by Cytoscape (version 3.7.2).
Screening of key therapeutic targets
The overlapped targets between LN related targets and HDH related targets were got by using TBtools (Chen et al., 2020a) (version 1.0686). PPI network of these overlapped targets was then retrieved by the STRING database (Szklarczyk et al., 2019), with the species being limited to homo species and the confidence level being set to ≥0.900. Based on the PPI network, betweenness centrality plot and degree centrality plot were drawn by using MATLAB software. Finally, pivotal LN related proteins that were targeted by HDH bioactive components were identified by comparing the betweenness centrality score and degree centrality score among the overlapped targets.
Molecular docking simulation
The crystal structures of these targets were retrieved from PDB database (Berman et al., 2002) (http://www.pdb.org), while 3D-structures of stigmasterol and quercetin were downloaded from Pubchem database (Kim et al., 2023) (https://pubchem.ncbi.nlm.nih.gov/). Molecular pretreatment, molecular docking simulation and the visualization of docking results were performed by PyMOL software (version 2.4.0) and AutoDockTools (Morris et al., 2008; Morris et al., 2009) (version 1.5.6).
Results
HDH targets, functions and PPI network
Flow chart of the integrated network pharmacology research is described as in Figure 1. In brief, HDH therapeutic targets and the LN related targets were attained from online databases firstly, with the overlapped part being recognized as latent therapeutic targets of LN targeted by HDH. Subsequently, functional enrichment analyses and PPI network construction were implemented to elucidate the protective mechanism of HDH against LN. Centrality analysis based on the PPI network of shared targets was then performed to help us identify core targets within the network. At last, the binding between core targets and their corresponding bioactive components of HDH was simulated by molecular docking technique.
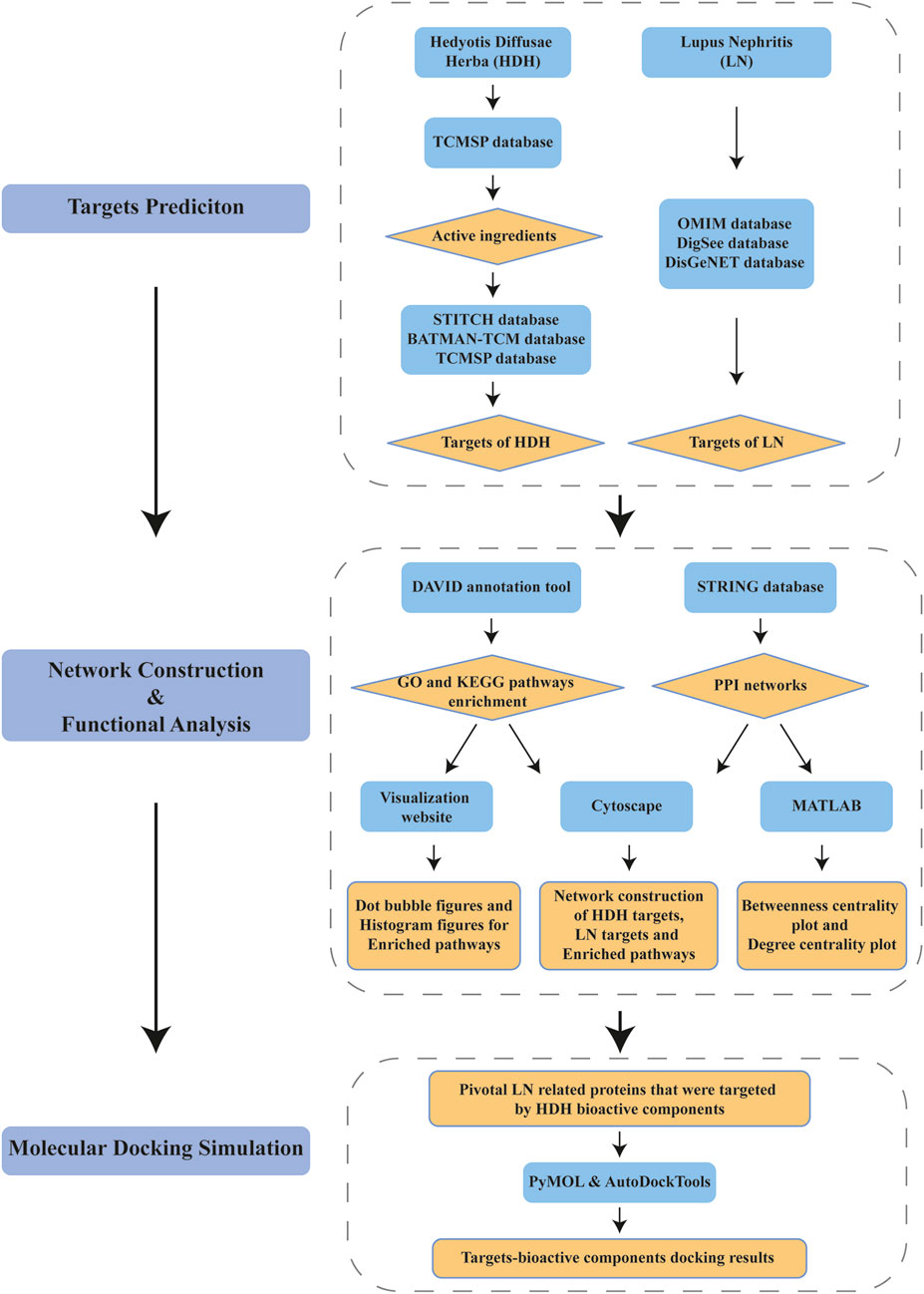
FIGURE 1. Flow chart of network pharmacology method identifying pivotal targets of Hedyotis Diffusae Herba (HDH) for the treatment of lupus nephritis (LN).
Based on the above strategy, three bioactive components of HDH (poriferasterol, stigmasterol, and quercetin) were identified. And an aggregate of 147 targets of these components were predicted by the STITCH database, the BATMAN-TCM database and the TCMSP database (Figure 2A). KEGG enrichment analysis suggested that HDH related targets were mainly involved in HIF-1 signaling pathway, TNF signaling pathway, PI3K-Akt signaling pathway, and NOD-like receptor signaling pathway, etc. (Figure 2B). Besides, as shown in Supplementary Figure S1, HDH corresponding targets were closely related to types of cancers, infectious diseases and autoimmune diseases like rheumatoid arthritis. GO enrichment analysis for cellular component showed that most of these proteins were located in the extracellular space, caveola or plasma membrane, or form receptor complex (Figure 2C). And GO enrichment analysis for biological process indicated that HDH targets might participate in the response to drug, oxidation-reduction process and regulation of transcription (Figure 2C). Besides, the GO enrichment analysis for molecular function implied that targets of HDH active components possessed the ability to bind heme, enzyme and so on; and also had steroid hormone receptor activity, RNA polymerase II transcription factor activity, protein homodimerization activity, oxidoreductase activity, and so on (Figure 2C). Moreover, a PPI network was then constructed for HDH bioactive components related targets (Figure 3). A total of 147 targets nodes and 1,302 protein-protein edges were present in the network of HDH-HDH bioactive components-HDH related targets (Figure 3).
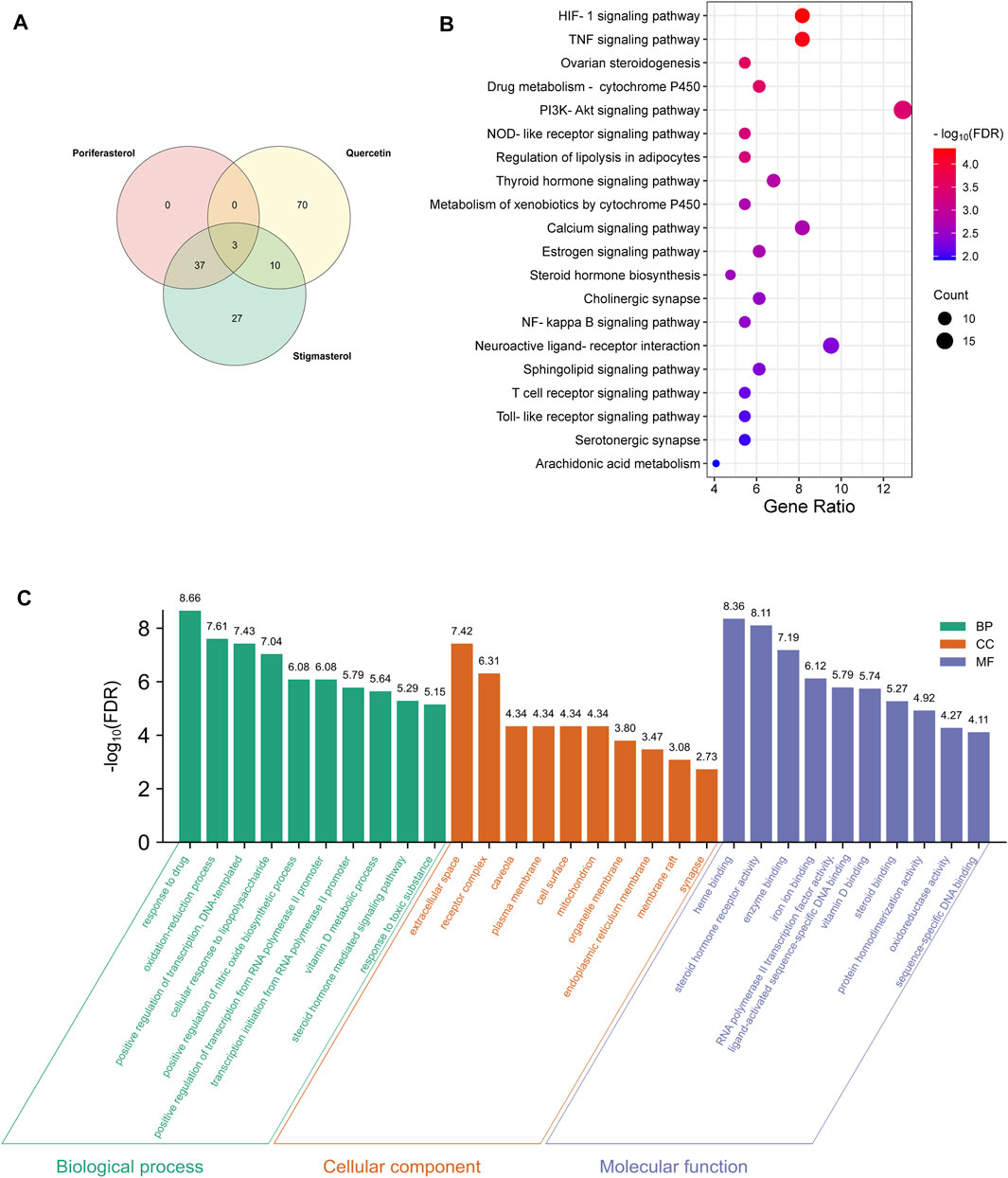
FIGURE 2. HDH targets and functional enrichment analyses. (A) Venn diagram of the predicted HDH targets. (B) Dot bubble plot of the top 20 Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathways enriched by HDH related targets. The size of dots represents the number of enriched proteins, and the color represents −log10 (FDR). (C) Histogram plot of the top 10 enriched biological processes, cellular components and molecular functions of HDH targets by Gene Ontology (GO) enrichment analysis. FDR, false discovery rate; BP, biological process; CC, cellular component; MF, molecular function.
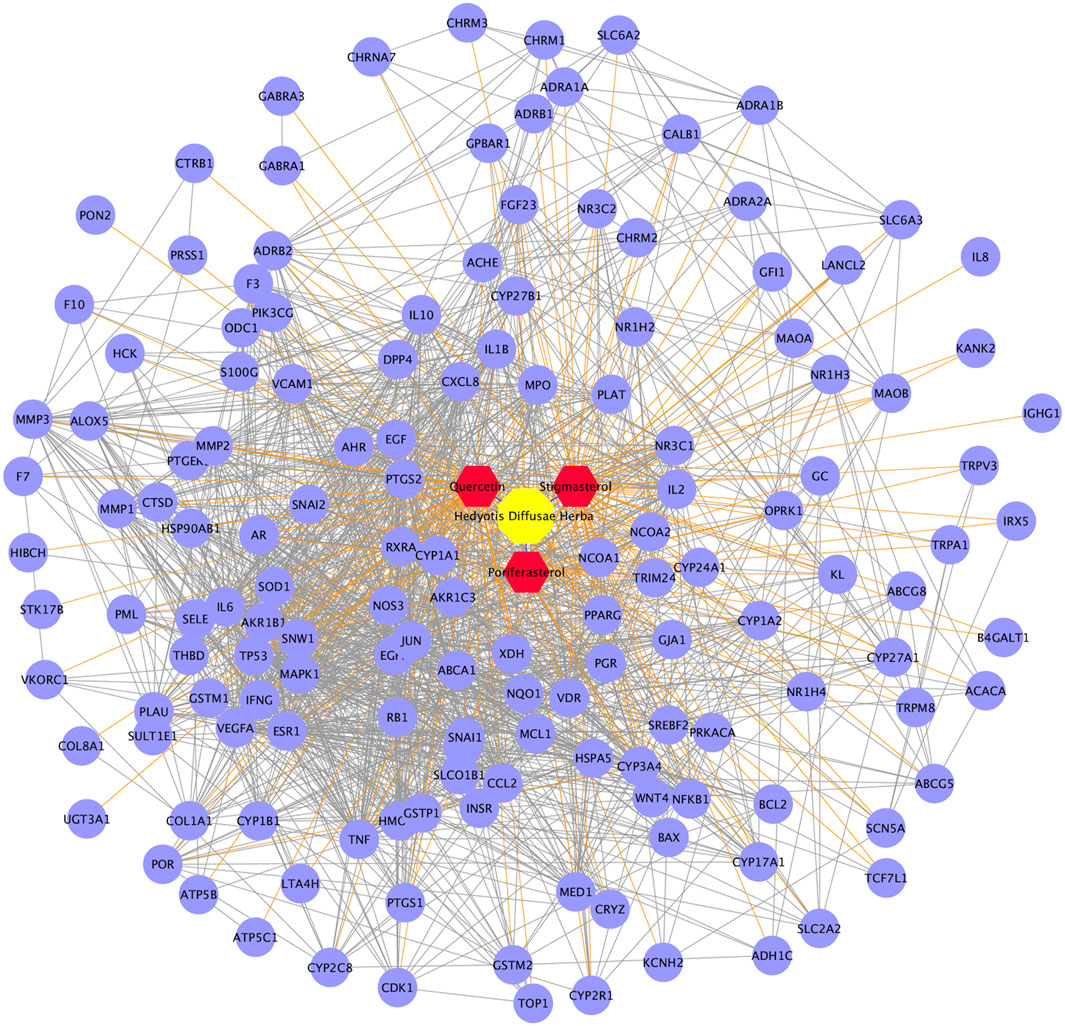
FIGURE 3. Protein-protein interaction (PPI) network of proteins targeted by three bioactive components of HDH. The yellow octagon represents HDH, the red hexagons represent components of HDH, while the purple dots represent the predicted targets of these three chemical components. The blue lines represent interaction between HDH and its bioactive components, the orange lines represent interaction between HDH bioactive components and the predicted targets, while the PPI networks of targets were represented as prey lines.
LN therapeutic targets, functions and PPI network
In total, 162 targets were identified as LN therapeutic targets from the OMIM database, the DisGeNET database and the DigSee database. KEGG pathway enrichment analysis illustrated that cytokine-cytokine receptor interaction, TNF signaling pathway, complement and coagulation cascades, Toll-like receptor signaling pathway participated in the course of LN (Figure 4A). Additionally, HDH enriched pathways such as NOD-like receptor signaling pathway, PI3K-Akt signaling pathway, HIF-1 signaling pathway were also enriched in the analysis of LN targets (Figure 4A). On the other hand, besides SLE, these targets were also enriched in other autoimmune diseases like rheumatoid arthritis and inflammatory bowel disease, or infection diseases like tuberculosis, malaria and leishmaniasis (Supplementary Figure S2). GO enrichment analysis for biological process indicated that LN was highly correlated with inflammatory response and immune response (Figure 4B). And as shown in the analysis for cellular component, most of the potential LN therapeutic targets were located in the extracellular space, the external side of plasma membrane and cell surface (Figure 4B). Furthermore, GO enrichment analysis for molecular functions showed that LN targets were enriched in cytokine activity, IgG binding, protein homodimerization activity and so on (Figure 4B). A PPI network was then constructed for LN targets. As described in Figure 4C, these disease targets formed a complex network with 2,701 edges.
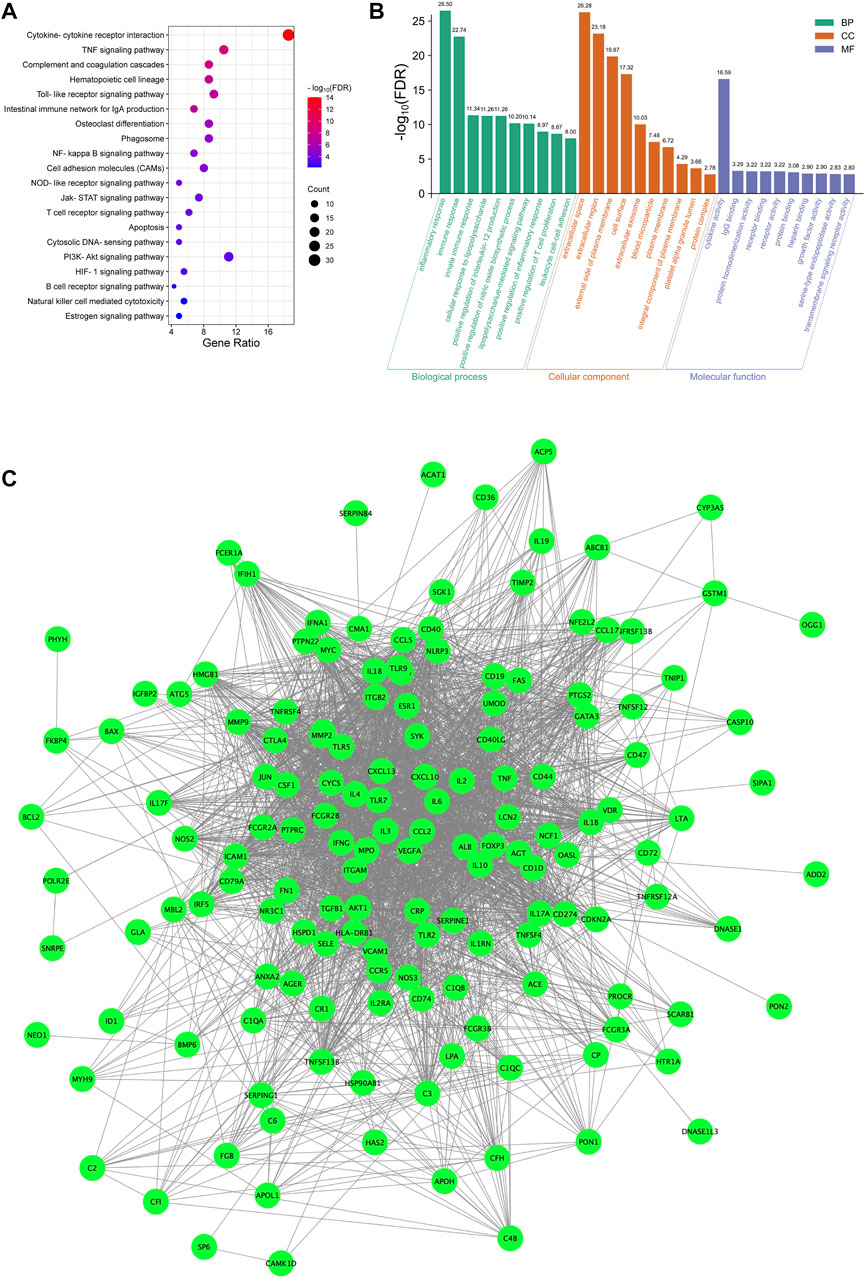
FIGURE 4. Functional enrichment analyses and PPI network of LN targets. (A) Dot bubble plot of the top 20 KEGG signaling pathways enriched by the targets of LN. Size of dots represents the number of enriched proteins, and the color represents −log10 (FDR). (B) Histogram plot of the top 10 biological processes, cellular components and molecular functions of LN targets by GO enrichment analysis. (C) PPI network of LN targets. FDR, false discovery rate; BP, biological process; CC, cellular component; MF, molecular function.
Network and functions of LN-related proteins that were targeted by HDH
As displayed in Figure 5A, 44 pathways were shared between HDH targes and LN targets, including TNF signaling pathway, Toll-like receptor signaling pathway, NF-kappa B signaling pathway and so on. And a total of 23 targets of LN that were also targeted by HDH active components were identified (Figure 5B). Further KEGG enrichment analysis of these shared targets indicated that the functions of these targets were enriched in TNF signaling pathway, NOD-like receptor signaling pathway, cytokine-cytokine receptor interaction, NF-kappa B signaling pathway, and HIF-1 signaling pathway, etc. (Figure 5C). The GO enrichment analysis of these overlapped targets was shown in Figure 5D. The analysis for biological process implied that the overlapped targets were related to the nitric oxide biosynthetic process mostly, and were also associated with humoral immune response (Figure 5D). As for the molecular functions, cytokine activity and identical protein binding were associated with these targets most obviously (Figure 5D). Besides, these targets were located in the extracellular space and external side of plasma membrane mostly (Figure 5D). A network containing the active components of HDH, the overlapped targets and their enriched pathways was displayed in Figure 6.
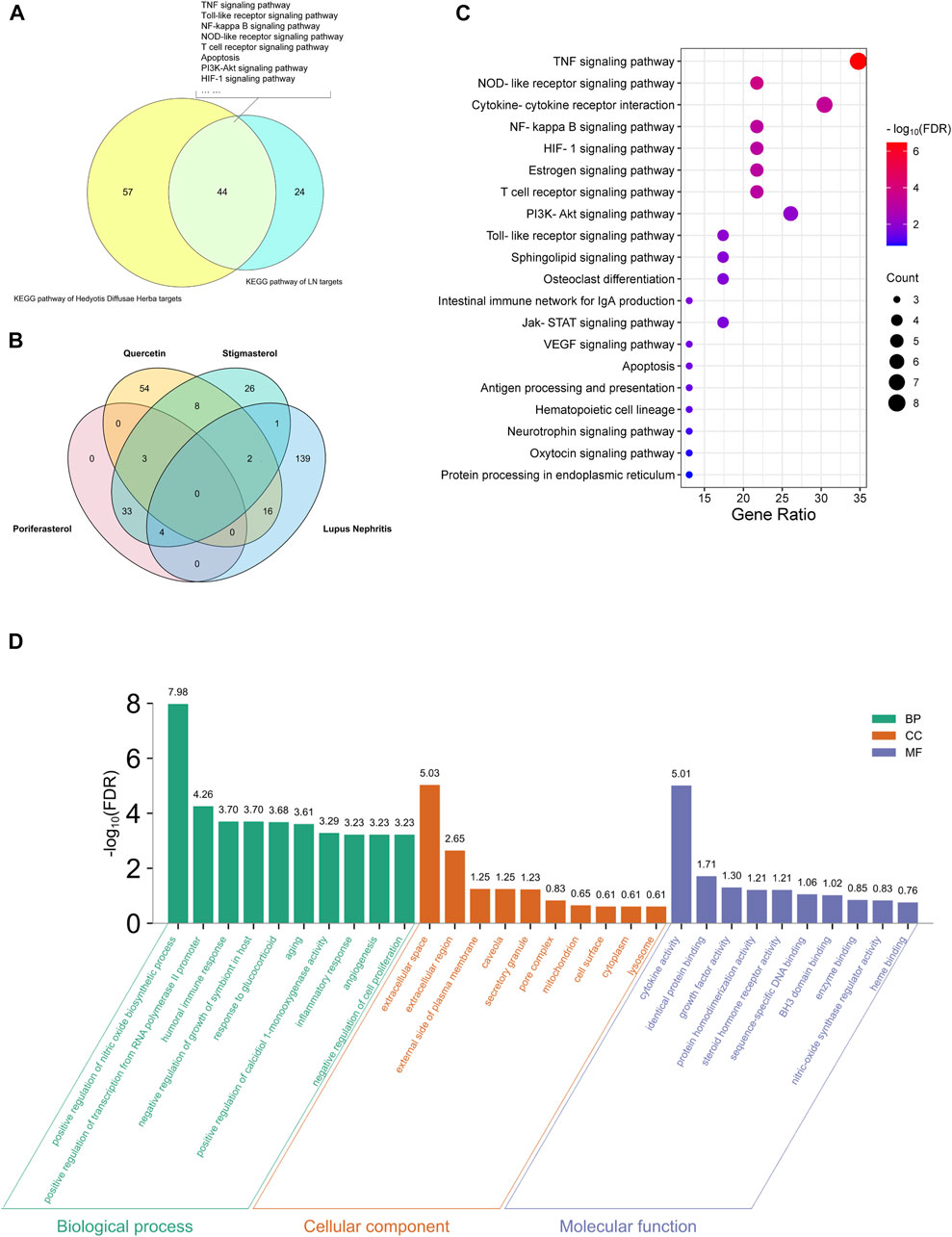
FIGURE 5. Functional enrichment analyses of LN targets that were targeted by HDH. (A) Venn diagram composed of the KEGG pathways of HDH and KEGG pathways of LN targets. (B) Venn diagram composed of the predicted poriferasterol targets, the quercetin targets, the stigmasterol targets and the LN targets. (C) Dot bubble plot of the top 20 enriched KEGG signaling pathways of the overlapped targets of LN and HDH. The size of dots represents the number of enriched genes, and the color represents −log10 (FDR). (D) Histogram plot of the top 10 biological processes, cellular components and molecular functions of the shared targets by GO enrichment analysis. FDR, false discovery rate; BP, biological process; CC, cellular component; MF, molecular function.
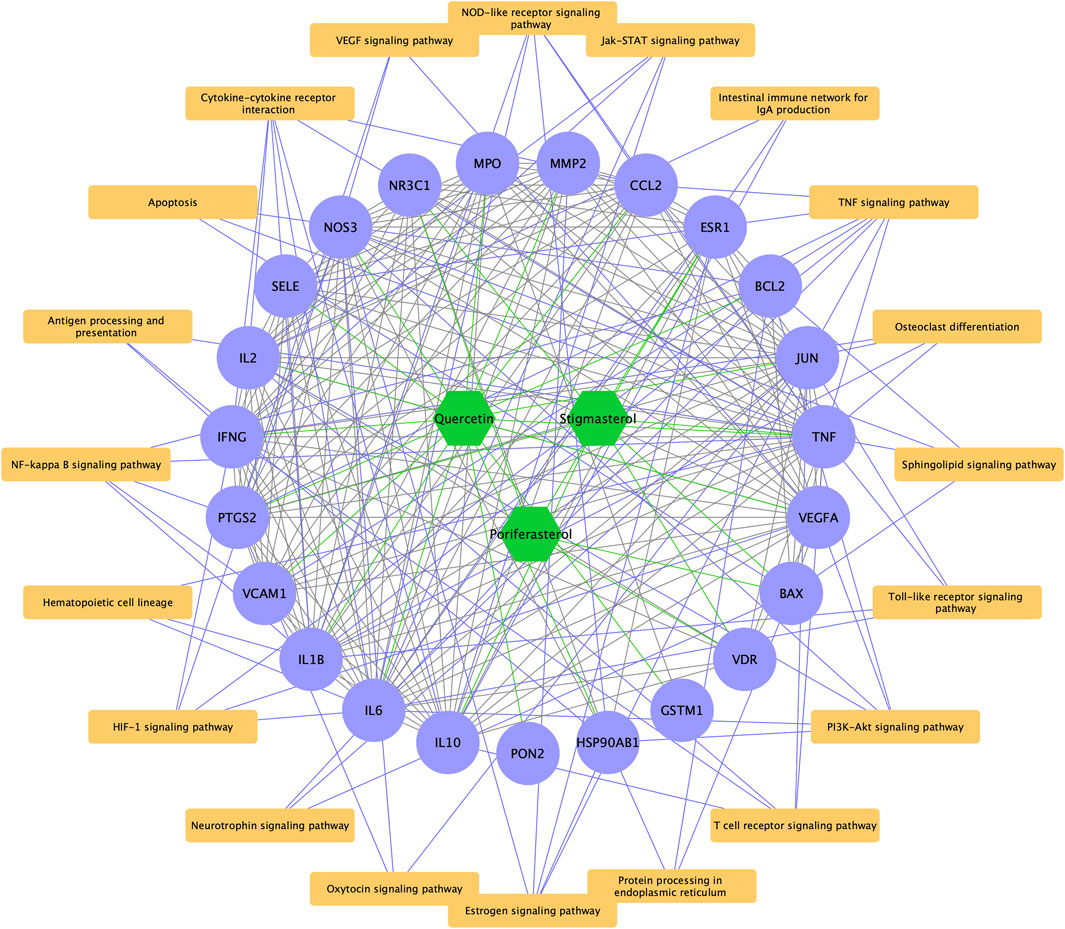
FIGURE 6. Bioactive components-overlapped targets-KEGG signaling pathways network. The green hexagons represent chemical components of HDH, the purple dots represent the overlapped targets of LN and HDH, while the yellow rectangles represent the top 20 KEGG signaling pathways. The green lines represent interaction between chemical components and the overlapped targets, the gray lines represent the PPI networks, while the purple lines are interaction between the overlapped targets and their enriched KEGG signaling pathways.
Screening of pivotal overlapped targets and molecular docking
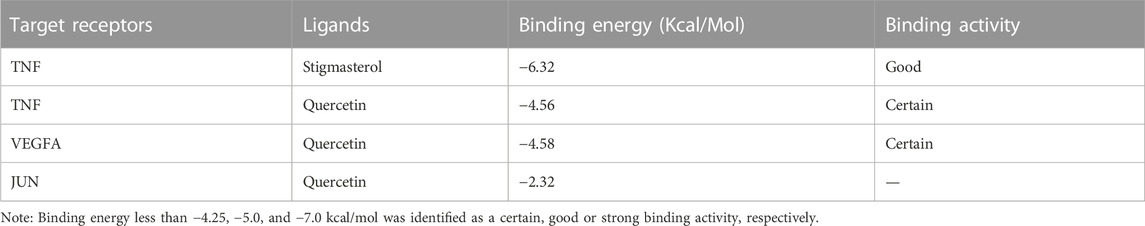
By setting “confidence level” to “≥ 0.900,” a more credible PPI network of the overlapped targets was acquired (Figure 7A). Subsequently, a betweenness centrality map and a degree centrality map based on the predicted PPI network were plotted (Figures 7B, C). According to these maps, TNF, JUN and VEGFA were recognized as the most pivotal targets in the network, as the betweenness centrality score and the degree centrality score of these three targets were highest (Figures 7B, C). Simulation of the binding between these three targets and their corresponding ligands was conducted then, with energy required for binding of each pairs being calculated by AutoDockTools. As summarized in Table 1, binding energy of TNF with stigmasterol was lowest (−6.32 Kcal/Mol), with the binding activity being identified as good. And the free energy required for the binding of TNF with quercetin, and VEGFA with quercetin was −4.56 Kcal/Mol and −4.58 Kcal/Mol, respectively, indicating certain binding activities. While no certain binding activity was found for JUN-quercetin interaction (binding energy =−2.32 Kcal/Mol). Detailed binding modes of macromolecular proteins and their corresponding small ligands were displayed in Figures 7D–G. As shown in Figure 7D, stigmasterol bound with TNF in a groove through 1 hydrogen bond with TYR-191. And Figure 7E implied that quercetin might interact with TNF through six hydrogen bonds with GLY-100, ASP-121, ASN-122, GLN-123 and ILE-212, thus exerting its latent therapeutic effect. Similar results for quercetin with VEGFA and quercetin with JUN could be found in Figures 7F, G.
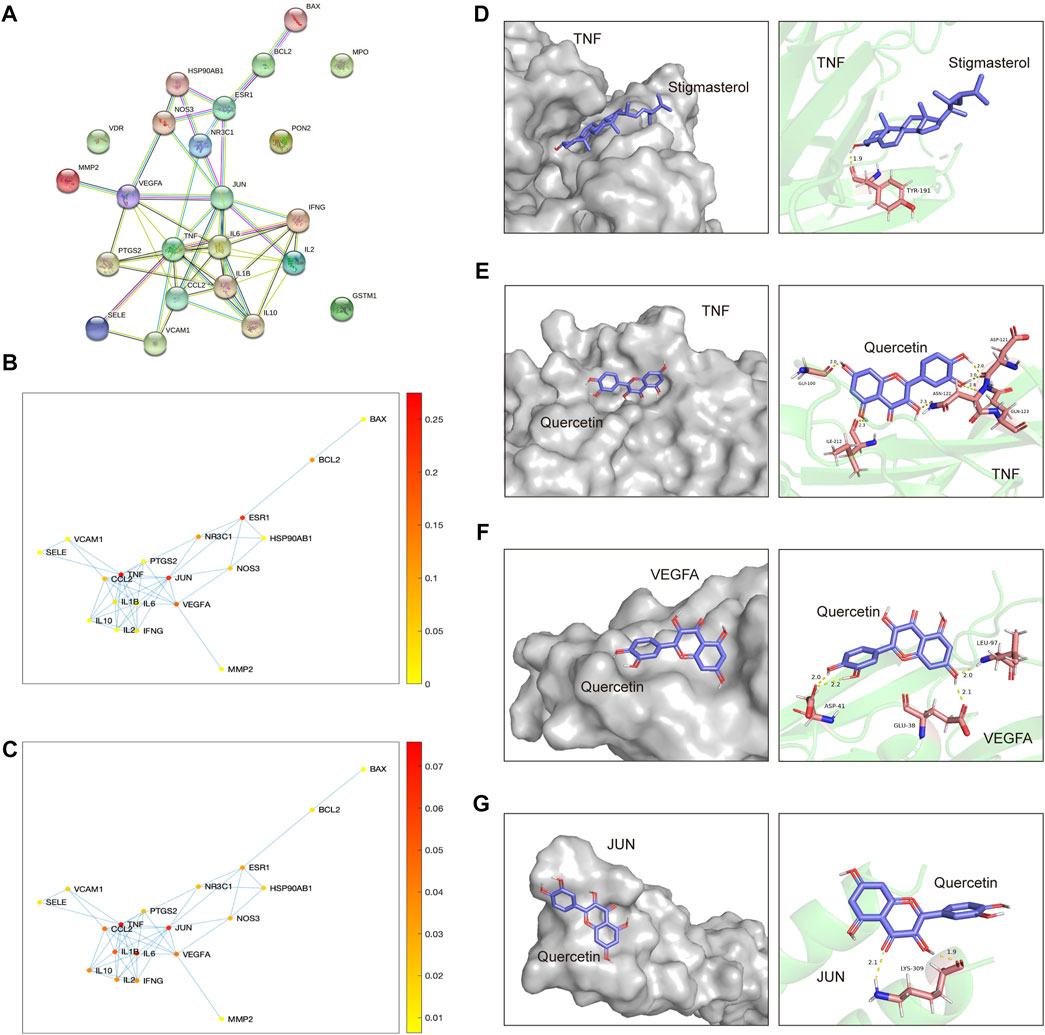
FIGURE 7. Screening of pivotal overlapped targets and molecular docking of HDH components binding to these pivotal targets. (A) PPI network of the overlapped targets with high confidence (interaction score ≥0.900). (B,C) Betweenness centrality plot and Degree centrality plot of the interaction network. The color represents the level of the betweenness centrality score or the degree centrality score of every dots. (D–G) Molecular models of TNF interacting with stigmasterol (D), TNF interacting with quercetin (E), VEGFA interacting with quercetin (F) and JUN interacting with quercetin (G). Small molecule compounds are represented as purple colored, while the gray colored (left panel) and green colored (right panel) macromolecular substances represent the target proteins.
Discussion
Traditional Chinese medicine regards individuals as systems with various states, and has accumulated a large number of traditional Chinese medicine prescriptions (Li and Zhang, 2013). Compounds isolated from Chinese herbal medicine, such as antimalarial drug artemisinin, exhibit similar pharmacological activities as pharmaceutical drugs (Sucher, 2013). The exploration of traditional Chinese medicine ingredients will inject new vitality into the development of new drugs (Wang et al., 2018). Recently, the systems pharmacology method has been widely concerned in the field of Chinese medicine (Zhou et al., 2016), facilitating the transformation of traditional Chinese medicine from empirical medicine to evidence-based medicine (Li and Zhang, 2013). Besides, the application of network pharmacology strategy is also attributing to the elucidation of mechanism of Chinese medicine against diseases and the screening of pivotal components of Chinese medicine (Zhou et al., 2016).
In this study, by applying the systematic integrated network pharmacology method to traditional Chinese medicine research, our analyses identified TNF, VEGFA and JUN as the most pivotal therapeutic targets of HDH against LN (Figures 7B, C), with the binding of the former two proteins with their corresponding HDH components being validated by molecular docking, especially the interaction between stigmasterol and TNF (Figures 7D–G, Table 1).
TNF is a cytokine mainly secreted by macrophages, activating the transcription of several proinflammatory genes and is involved in the regulation of a wide spectrum of biological processes such as cell proliferation, differentiation and apoptosis (Wu et al., 2002). Since Jacob et al elucidated the relationship between TNF and LN in 1998 (Jacob and McDevitt, 1988), there is abundant evidence supporting the pathogenicity of TNF in LN (Boswell et al., 1988; Jacob, 1992; Zhao et al., 2013). By enhancing the expression of numerous cytokines, TNF-alpha mediated the recruitment and adherence of pathogenic inflammatory cells in LN, amplifying kidney injury (Wuthrich, 1992; Davidson, 2016). Besides, TNF was also proved to cause direct injury in podocytes (Pedigo et al., 2016). On the other hand, current clinical data shows that the application of TNF blocker induction therapy may lead to long-term remission in patients with LN, although the safety of TNF blockers still needs long-term observation (Aringer and Smolen, 2012). In the current study, we found that the active components of HDH might exert therapeutic effect on LN through TNF signaling pathway. Besides, TNF was identified as a key target in the network constructed for overlapped targets of LN and HDH.
In the current study, we predicted that stigmasterol and quercetin, two bioactive components of HDH, might target TNF and thus influence the function of TNF. Stigmasterol is a plant sterol exhibiting anti-inflammatory activity (Ahmad Khan et al., 2020; Bakrim et al., 2022). Kangsamaksin et al. (2017) reported that stigmasterol could suppress tumor angiogenesis and cholangiocarcinoma growth by inhibiting TNF-α expression. Such effect was also found in collagen induced arthritis (Ahmad Khan et al., 2020). Besides, some common formulas containing stigmasterol possess apparent properties that suppress the expression of TNF-α (Masola et al., 2018; Wang et al., 2022b). However, no research about the protective role of stigmasterol against LN was found until now. We speculate that stigmasterol may possess the ability to bind to TNF in a groove of TNF and then affect the function of TNF, thus ameliorating LN. This hypothesis is worthy of further validation by experiments.
Quercetin is a flavonol widely distributed in plants (Chen et al., 2020b). The effect of quercetin in down-regulating TNF was reported by abundant researches (Yang et al., 2019; Sul and Ra, 2021; Tsai et al., 2021), too. And Liu et al. (2019) further demonstrated that quercetin could block the expression of pentraxin 3 that was induced by TNF-α and inhibit the proliferation of mesangial cells by inhibiting the activation of NF-kappa B signaling pathway, playing a protective role in LN. Nevertheless, the mechanism of reno-protective role of quercetin in LN still needs to be uncovered.
Our analyses also identified VEGFA as a key target in the course of HDH treatment against LN. It was evidenced that the plasma VEGFA level was reduced when SLE patients were treated with Mycophenolate mofetil (Slight-Webb et al., 2019). And Yuan et al. (2019b) concluded that VEGF-endothelin-1 system was involved in the endothelial cell-podocyte crosstalk in LN. However, rare studies have paid attention to the effect of quercetin on VEGFA. Wang and the colleagues discovered that astragalus membranaceus, a Chinese medicine herb containing quercetin, could alleviate acquired hyperlipidemia through regulating lipid metabolism, in which the upregulated VEGFA might be one of the key targets (Wang et al., 2022c). Yu et al. (2022) also found that quercetin could facilitate the upregulation of the expression of VEGFA and improve fat graft survival. But the mechanism of quercetin in the treatment of LN through VEGFA that was predicted in this study still needs to be further studied.
Besides, this study also elucidated that TNF signaling pathway, Toll-like receptor signaling pathway, NOD-like receptor signaling pathway, NF-kappa B signaling pathway, HIF-1 signaling pathway, and PI3K-Akt signaling pathway might be involved in the pharmacological effect of HDH against LN (Figures 4A, 5A, C). TNF signaling pathway (Aten et al., 2000), Toll-like receptor signaling pathway (Pawar et al., 2007), NF-kappa B signaling pathway (Su et al., 2018), HIF-1 signaling pathway (Chen et al., 2020c), and PI3K-Akt signaling pathway (Stylianou et al., 2011) represent classic signaling pathways of LN. However, limited studies interpreted the effect of HDH from these aspects. In the treatment of cervical cancer, researchers found that a traditional Chinese medicine prescription, Yangshe granule that consists of herbs including HDH, exerted anti-cancer effect through regulating the PI3K-AKT signaling pathway and apoptosis (Ma et al., 2023). The above result was consistent with one of the predicted pathways in our study. While all the predicted mechanism needs to be further validated by experiments.
In general, our study mainly found that the active components of HDH, stigmasterol and quercetin, may play a protective role in LN patients by acting on TNF and VEGFA. Yet the interaction predicted in this study still needs to be tested in vitro and in vivo. However, it should be emphasized that even though the study identified several key targets, the multi-targets effect and multi-pathways effect of traditional Chinese medicine cannot be ignored, and the therapeutic effects of HDH in LN cannot be simply attributed to these binding. Anyway, the research provides us new insights into the therapeutic mechanism of HDH in LN. And the network pharmacology methods used in this study also offered guidance and inspiration for further researches and the development of new drugs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
SL and JY participated in data collection and analyses, and participated in manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Scientific Research Launch Project for new employees of the Second Xiangya Hospital of Central South University to SL.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1118804/full#supplementary-material
References
Ahmad Khan, M., Sarwar, A., Rahat, R., Ahmed, R. S., and Umar, S. (2020). Stigmasterol protects rats from collagen induced arthritis by inhibiting proinflammatory cytokines. Int. Immunopharmacol. 85, 106642. doi:10.1016/j.intimp.2020.106642
Almaani, S., Meara, A., and Rovin, B. H. (2017). Update on lupus nephritis. Clin. J. Am. Soc. Nephrol. 12, 825–835. doi:10.2215/CJN.05780616
Aringer, M., and Smolen, J. S. (2012). Therapeutic blockade of TNF in patients with SLE-promising or crazy? Autoimmun. Rev. 11, 321–325. doi:10.1016/j.autrev.2011.05.001
Aten, J., Roos, A., Claessen, N., Schilder-Tol, E. J. M., Ten Berge, I. J., and Weening, J. J. (2000). Strong and selective glomerular localization of CD134 ligand and TNF receptor-1 in proliferative lupus nephritis. J. Am. Soc. Nephrol. 11, 1426–1438. doi:10.1681/ASN.V1181426
Bakrim, S., Benkhaira, N., Bourais, I., and Benali, T. (2022). Health benefits and pharmacological properties of stigmasterol. Basel: Antioxidants, 11.
Bastian, H. M., Roseman, J. M., McGwin, G., Alarcón, G. S., Friedman, A. W., Fessler, B. J., et al. (2002). Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus 11, 152–160. doi:10.1191/0961203302lu158oa
Berman, H. M., Battistuz, T., Bhat, T. N., Bluhm, W. F., Bourne, P. E., Burkhardt, K., et al. (2002). The protein data bank. Acta Crystallogr. D. Biol. Crystallogr. 58, 899–907. doi:10.1107/s0907444902003451
Boswell, J. M., Yui, M. A., Burt, D. W., and Kelley, V. E. (1988). Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J. Immunol. 141, 3050–3054. doi:10.4049/jimmunol.141.9.3050
Calle-Botero, E., and Abril, A. (2020). Lupus vasculitis. Curr. Rheumatol. Rep. 22, 71. doi:10.1007/s11926-020-00937-0
Chen, C., Chen, H., Zhang, Y., Thomas, H. R., Frank, M. H., He, Y., et al. (2020). TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13, 1194–1202. doi:10.1016/j.molp.2020.06.009
Chen, P. M., Wilson, P. C., Shyer, J. A., Veselits, M., Steach, H. R., Cui, C., et al. (2020). Kidney tissue hypoxia dictates T cell-mediated injury in murine lupus nephritis. Sci. Transl. Med. 12, eaay1620. doi:10.1126/scitranslmed.aay1620
Chen, T., Zhang, X., Zhu, G., Liu, H., Chen, J., Wang, Y., et al. (2020). Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Med. Baltim. 99, e22241. doi:10.1097/MD.0000000000022241
Das, L., and Brunner, H. I. (2009). Biomarkers for renal disease in childhood. Curr. Rheumatol. Rep. 11, 218–225. doi:10.1007/s11926-009-0030-4
Davidson, A. (2016). What is damaging the kidney in lupus nephritis? Nat. Rev. Rheumatol. 12, 143–153. doi:10.1038/nrrheum.2015.159
Fan, Y. S., Wen, C. P., and Xie, Z. J. (2010). Effects of three Chinese herbal antidotes (Herba artemisiae annuae, Herba hedyotis diffusae and Rhizoma cimicifugae) and their different combinations on regulated on activation normal T cell expressed and secreated expression in MRL/lpr mice. Zhongguo Zhong Xi Yi Jie He Za Zhi 30, 1306–1309.
Fanouriakis, A., Kostopoulou, M., Alunno, A., Aringer, M., Bajema, I., Boletis, J. N., et al. (2019). 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 78, 736–745. doi:10.1136/annrheumdis-2019-215089
Hamosh, A., Scott, A. F., Amberger, J., Valle, D., et al. (2000). Online mendelian inheritance in man (OMIM). Hum. Mutat. 15, 57–61.
Hobeika, L., Ng, L., and Lee, I. J. (2019). Moving forward with biologics in lupus nephritis. Adv. Chronic Kidney Dis. 26, 338–350. doi:10.1053/j.ackd.2019.08.008
Hopkins, A. L. (2008). Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 4, 682–690. doi:10.1038/nchembio.118
Huang, da W., Sherman, B. T., and Lempicki, R. A. (2009). Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13. doi:10.1093/nar/gkn923
Huang, da W., Sherman, B. T., and Lempicki, R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57. doi:10.1038/nprot.2008.211
Jacob, C. O. (1992). Genetic variability in tumor necrosis factor production: Relevance to predisposition to autoimmune disease. Reg. Immunol. 4, 298–304.
Jacob, C. O., and McDevitt, H. O. (1988). Tumour necrosis factor-alpha in murine autoimmune 'lupus' nephritis. Nature 331, 356–358. doi:10.1038/331356a0
Jakes, R. W., Bae, S. C., Louthrenoo, W., Mok, C. C., Navarra, S. V., and Kwon, N. (2012). Systematic review of the epidemiology of systemic lupus erythematosus in the asia-pacific region: Prevalence, incidence, clinical features, and mortality. Arthritis Care Res. Hob. 64, 159–168. doi:10.1002/acr.20683
Jiang, N., Li, H., Sun, Y., Zeng, J., Yang, F., Kantawong, F., et al. (2021). Network pharmacology and pharmacological evaluation reveals the mechanism of the sanguisorba officinalis in suppressing hepatocellular carcinoma. Front. Pharmacol. 12, 618522. doi:10.3389/fphar.2021.618522
Jiang, Y. Q., Liang, Q. H., and Xiong, X. G. (2010). Effect of Bizhongxiao decoction on proteomics of peripheral blood mononuclear cells in patients with rheumatoid arthritis. Zhongguo Zhong Xi Yi Jie He Za Zhi 30, 1160–1164.
Jiao, W., Mi, S., Sang, Y., Jin, Q., Chitrakar, B., Wang, X., et al. (2022). Integrated network pharmacology and cellular assay for the investigation of an anti-obesity effect of 6-shogaol. Food Chem. 374, 131755. doi:10.1016/j.foodchem.2021.131755
Kangsamaksin, T., Chaithongyot, S., Wootthichairangsan, C., Hanchaina, R., Tangshewinsirikul, C., and Svasti, J. (2017). Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS One 12, e0189628. doi:10.1371/journal.pone.0189628
Kibble, M., Saarinen, N., Tang, J., Wennerberg, K., Mäkelä, S., and Aittokallio, T. (2015). Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Nat. Prod. Rep. 32, 1249–1266. doi:10.1039/c5np00005j
Kim, J., Kim, J. J., and Lee, H. (2017). An analysis of disease-gene relationship from Medline abstracts by DigSee. Sci. Rep. 7, 40154. doi:10.1038/srep40154
Kim, J., So, S., Lee, H. J., Park, J. C., and Lee, H. (2013). DigSee: Disease gene search engine with evidence sentences (version cancer). Nucleic Acids Res. 41, W510–W517. doi:10.1093/nar/gkt531
Kim, S., Chen, J., Cheng, T., Gindulyte, A., et al. (2023). PubChem 2023 update. Nucleic Acids Res. 51, D1373–d80.
Kiriakidou, M., and Ching, C. L. (2020). Systemic lupus erythematosus. Syst. Lupus Erythematosus. Ann. Intern Med. 172, Itc81–itc96. doi:10.7326/AITC202006020
Li, S., and Zhang, B. (2013). Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 11, 110–120. doi:10.1016/S1875-5364(13)60037-0
Liu, S., Zhu, J. J., and Li, J. C. (2021). The interpretation of human body in traditional Chinese medicine and its influence on the characteristics of TCM theory. Anat. Rec. Hob. 304, 2559–2565. doi:10.1002/ar.24643
Liu, Y., Yu, C., Ji, K., Wang, X., Li, X., Xie, H., et al. (2019). Quercetin reduces TNF-α-induced mesangial cell proliferation and inhibits PTX3 production: Involvement of NF-κB signaling pathway. Phytother. Res. 33, 2401–2408. doi:10.1002/ptr.6430
Liu, Z., Guo, F., Wang, Y., Li, C., Zhang, X., Li, H., et al. (2016). BATMAN-TCM: A bioinformatics analysis tool for molecular mechANism of traditional Chinese medicine. Sci. Rep. 6, 21146. doi:10.1038/srep21146
Liu, Z., Mu, S., Li, S., Liang, J., Deng, Y., Yang, Z., et al. (2022). Hedyotis diffusae Herba-Andrographis Herba inhibits the cellular proliferation of nasopharyngeal carcinoma and triggers DNA damage through activation of p53 and p21. Cancer Gene Ther. 29, 973–983. doi:10.1038/s41417-021-00385-7
Luo, T. T., Lu, Y., Yan, S. K., Xiao, X., Rong, X. L., and Guo, J. (2020). Network pharmacology in research of Chinese medicine formula: Methodology, application and prospective. Chin. J. Integr. Med. 26, 72–80. doi:10.1007/s11655-019-3064-0
Ma, B., Hu, T., Li, P., Yuan, Q., Lin, Z., Tu, Y., et al. (2017). Phylogeographic and phylogenetic analysis for Tripterygium species delimitation. Ecol. Evol. 7, 8612–8623. doi:10.1002/ece3.3344
Ma, C., Gu, Y., Liu, C., Tang, X., Yu, J., Li, D., et al. (2023). Anti-cervical cancer effects of Compound Yangshe granule through the PI3K/AKT pathway based on network pharmacology. J. Ethnopharmacol. 301, 115820. doi:10.1016/j.jep.2022.115820
Maria, N. I., and Davidson, A. (2020). Protecting the kidney in systemic lupus erythematosus: From diagnosis to therapy. Nat. Rev. Rheumatol. 16, 255–267. doi:10.1038/s41584-020-0401-9
Masola, B., Oguntibeju, O. O., and Oyenihi, A. B. (2018). Centella asiatica ameliorates diabetes-induced stress in rat tissues via influences on antioxidants and inflammatory cytokines. Biomed. Pharmacother. 101, 447–457. doi:10.1016/j.biopha.2018.02.115
Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S., et al. (2009). AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791. doi:10.1002/jcc.21256
Morris, G. M., Huey, R., and Olson, A. J. (2008). Using AutoDock for ligand-receptor docking. Curr. Protoc. Bioinforma. Chapter 8, Unit 8.14. doi:10.1002/0471250953.bi0814s24
Pawar, R. D., Ramanjaneyulu, A., Kulkarni, O. P., Lech, M., Segerer, S., and Anders, H. J. (2007). Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J. Am. Soc. Nephrol. 18, 1721–1731. doi:10.1681/ASN.2006101162
Pedigo, C. E., Ducasa, G. M., Leclercq, F., Sloan, A., Mitrofanova, A., Hashmi, T., et al. (2016). Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J. Clin. Investig. 126, 3336–3350. doi:10.1172/JCI85939
Piñero, J., Queralt-Rosinach, N., À, Bravo, Deu-Pons, J., Bauer-Mehren, A., Baron, M., et al. (2015). DisGeNET: A discovery platform for the dynamical exploration of human diseases and their genes. Database (Oxford) 2015, bav028. doi:10.1093/database/bav028
Piñero, J., Ramírez-Anguita, J. M., Saüch-Pitarch, J., Ronzano, F., Centeno, E., Sanz, F., et al. (2020). The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 48, D845-D855–d55. doi:10.1093/nar/gkz1021
Ren, Q., Li, M., Deng, Y., Lu, A., and Lu, J. (2021). Triptolide delivery: Nanotechnology-based carrier systems to enhance efficacy and limit toxicity. Pharmacol. Res. 165, 105377. doi:10.1016/j.phrs.2020.105377
Ru, J., Li, P., Wang, J., Zhou, W., Li, B., Huang, C., et al. (2014). Tcmsp: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform 6, 13. doi:10.1186/1758-2946-6-13
Slight-Webb, S., Guthridge, J. M., Chakravarty, E. F., Chen, H., Lu, R., Macwana, S., et al. (2019). Mycophenolate mofetil reduces STAT3 phosphorylation in systemic lupus erythematosus patients. JCI Insight 4, e124575. doi:10.1172/jci.insight.124575
Song, C. Y., Xu, Y. G., and Lu, Y. Q. (2020). Use of tripterygium wilfordii Hook F for immune-mediated inflammatory diseases: Progress and future prospects. J. Zhejiang Univ. Sci. B 21, 280–290. doi:10.1631/jzus.B1900607
Stylianou, K., Petrakis, I., Mavroeidi, V., Stratakis, S., Vardaki, E., Perakis, K., et al. (2011). The PI3K/Akt/mTOR pathway is activated in murine lupus nephritis and downregulated by rapamycin. Nephrol. Dial. Transpl. 26, 498–508. doi:10.1093/ndt/gfq496
Su, B., Ye, H., You, X., Ni, H., Chen, X., and Li, L. (2018). Icariin alleviates murine lupus nephritis via inhibiting NF-κB activation pathway and NLRP3 inflammasome. Life Sci. 208, 26–32. doi:10.1016/j.lfs.2018.07.009
Sucher, N. J. (2013). The application of Chinese medicine to novel drug discovery. Expert Opin. Drug Discov. 8, 21–34. doi:10.1517/17460441.2013.739602
Sul, O. J., and Ra, S. W. (2021). Quercetin prevents LPS-induced oxidative stress and inflammation by modulating NOX2/ROS/NF-kB in lung epithelial cells. Molecules 26, 6949. doi:10.3390/molecules26226949
Szklarczyk, D., Gable, A. L., Lyon, D., Junge, A., Wyder, S., Huerta-Cepas, J., et al. (2019). STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607-D613–d13. doi:10.1093/nar/gky1131
Szklarczyk, D., Santos, A., von Mering, C., Jensen, L. J., Bork, P., and Kuhn, M. (2016). Stitch 5: Augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 44, D380–D384. doi:10.1093/nar/gkv1277
Tang, J. L., Liu, B. Y., and Ma, K. W. (2008). Traditional Chinese medicine. Lancet 372, 1938–1940. doi:10.1016/S0140-6736(08)61354-9
Tao, X., and Lipsky, P. E. (2000)., 26. viii, 29–50. doi:10.1016/s0889-857x(05)70118-6The Chinese anti-inflammatory and immunosuppressive herbal remedy Tripterygium wilfordii Hook FRheum. Dis. Clin. North Am.
Tsai, C. F., Chen, G. W., Chen, Y. C., Shen, C. K., Lu, D. Y., Yang, L. Y., et al. (2021). Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients 14, 67. doi:10.3390/nu14010067
UniProt Consortium, T. (2018). UniProt: The universal protein knowledgebase. Nucleic Acids Res. 46, 2699. doi:10.1093/nar/gky092
Wang, C. Y., Wang, T. C., Liang, W. M., Hung, C. H., Chiou, J. S., Chen, C. J., et al. (2020). Effect of Chinese herbal medicine therapy on overall and cancer related mortality in patients with advanced nasopharyngeal carcinoma in taiwan. Front. Pharmacol. 11, 607413. doi:10.3389/fphar.2020.607413
Wang, J., Wong, Y. K., and Liao, F. (2018). What has traditional Chinese medicine delivered for modern medicine? Expert Rev. Mol. Med. 20, e4. doi:10.1017/erm.2018.3
Wang, L., Guo, J., Wang, Y., Zhao, P., Liu, B., Zhang, Y., et al. (2022). Anti-inflammatory effects of chaishi tuire granules on influenza A treatment by mediating TRAF6/MAPK14 axis. Front. Med. (Lausanne) 9, 943681. doi:10.3389/fmed.2022.943681
Wang, L., Zheng, W., Yang, J., Ali, A., and Qin, H. (2022). Mechanism of Astragalus membranaceus alleviating acquired hyperlipidemia induced by high-fat diet through regulating lipid metabolism. Nutrients 14, 954. doi:10.3390/nu14050954
Wang, Y., Yuan, Y., Wang, W., He, Y., Zhong, H., Zhou, X., et al. (2022). Mechanisms underlying the therapeutic effects of Qingfeiyin in treating acute lung injury based on GEO datasets, network pharmacology and molecular docking. Comput. Biol. Med. 145, 105454. doi:10.1016/j.compbiomed.2022.105454
Wu, A. J., Hua, H., Munson, S. H., and McDevitt, H. O. (2002). Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc. Natl. Acad. Sci. U. S. A. 99, 12287–12292. doi:10.1073/pnas.172382999
Wuthrich, R. P. (1992). Vascular cell adhesion molecule-1 (VCAM-1) expression in murine lupus nephritis. Kidney Int. 42, 903–914. doi:10.1038/ki.1992.367
Yang, H., Yang, T., Heng, C., Zhou, Y., Jiang, Z., Qian, X., et al. (2019). Quercetin improves nonalcoholic fatty liver by ameliorating inflammation, oxidative stress, and lipid metabolism in db/db mice. Phytother. Res. 33, 3140–3152. doi:10.1002/ptr.6486
Yu, P., Yang, Z., Lu, H., Jin, X., Yang, X., and Qi, Z. (2022). Quercetin may improve fat graft survival by promoting fat browning peripherally. Aesthetic Plast. Surg. 46, 2517–2525. doi:10.1007/s00266-022-02857-z
Yuan, K., Li, X., Lu, Q., Zhu, Q., Jiang, H., Wang, T., et al. (2019). Application and mechanisms of triptolide in the treatment of inflammatory diseases-A review. Front. Pharmacol. 10, 1469. doi:10.3389/fphar.2019.01469
Yuan, M., Tan, Y., Wang, Y., Wang, S. X., Yu, F., and Zhao, M. H. (2019). The associations of endothelial and podocyte injury in proliferative lupus nephritis: From observational analysis to in vitro study. Lupus 28, 347–358. doi:10.1177/0961203319828509
Zhang, L., and Wei, W. (2020). Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 207, 107452. doi:10.1016/j.pharmthera.2019.107452
Zhao, J., Zhang, H., Huang, Y., Wang, H., Wang, S., Zhao, C., et al. (2013). Bay11-7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-κB activation. Int. Immunopharmacol. 17, 116–122. doi:10.1016/j.intimp.2013.05.027
Keywords: Hedyotis Diffusae Herba, network pharmacology, lupus nephritis, TNF, VEGFA, bioactive components
Citation: Yang J and Li S (2023) Molecular mechanism of Hedyotis Diffusae Herba in the treatment of lupus nephritis based on network pharmacology. Front. Pharmacol. 14:1118804. doi: 10.3389/fphar.2023.1118804
Received: 08 December 2022; Accepted: 30 May 2023;
Published: 08 June 2023.
Edited by:
Elham Ahmadian, Tabriz University of Medical Sciences, IranReviewed by:
Dhanachandra Singh Khuraijam, Cleveland Clinic, United StatesJagpreet Singh Nanda, Cedars Sinai Medical Center, United States
Copyright © 2023 Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siying Li, MjIwNDEzMDcxMUBjc3UuZWR1LmNu
 Jinfei Yang1,2
Jinfei Yang1,2 Siying Li
Siying Li