- 1Fundació Institut Universitari Per a la Recerca a l’Atenció Primària De Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain
- 2Universitat Autònoma De Barcelona (Cerdanyola del Vallès), Bellaterra, Spain
- 3Marketing Farmacéutico and Investigación Clínica, Barcelona, Spain
- 4Plataforma SCReN, UIC IDIAPJGol, Barcelona, Spain
- 5Departament De Farmacologia, Terapèutica i Toxicologia, Universitat Autònoma De Barcelona (Cerdanyola del Vallès), Bellaterra, Spain
- 6Institut Català De la Salut, Barcelona, Spain
Objectives: To describe the sex and gender differences in the treatment initiation and in the socio-demographic and clinical characteristics of all patients initiating an oral anticoagulant (OAC), and the sex and gender differences in prescribed doses and adherence and persistence to the treatment of those receiving direct oral anticoagulants (DOAC).
Material and methods: Cohort study including patients with non-valvular atrial fibrillation (NVAF) who initiated OAC in 2011–2020. Data proceed from SIDIAP, Information System for Research in Primary Care, in Catalonia, Spain.
Results: 123,250 people initiated OAC, 46.9% women and 53.1% men. Women were older and the clinical characteristics differed between genders. Women had higher risk of stroke than men at baseline, were more frequently underdosed with DOAC and discontinued the DOAC less frequently than men.
Conclusion: We described the dose adequacy of patients receiving DOAC, finding a high frequency of underdosing, and significantly higher in women in comparison with men. Adherence was generally high, only with higher levels in women for rivaroxaban. Persistence during the first year of treatment was also high in general, being significantly more persistent women than men in the case of dabigatran and edoxaban. Dose inadequacy, lack of adherence and of persistence can result in less effective and safe treatments. It is necessary to conduct studies analysing sex and gender differences in health and disease.
Introduction
Atrial fibrillation (AF) is the most common form of chronic arrhythmia, affecting 2–3% of the population in Europe and USA (Kirchhof, 2017; Hindricks et al., 2021) or 4.4% of the Spanish population older than 40 (Gómez-Doblas et al., 2014). It is associated with several cardiovascular conditions, and it increases the risk of stroke. Men are more often affected by AF, although women have a higher risk of experiencing stroke (Lip et al., 2012; Hindricks et al., 2021). To prevent stroke in non-valvular atrial fibrillation (NVAF), oral anticoagulants (OAC) are usually prescribed; vitamin K antagonists (VKA) have been used for years and since 2011 in Spain (Agencia Española de Medicamentos y Productos Sanitarios, 2012) direct oral anticoagulants (DOAC) were introduced.
Several authors have described the trends of use of OAC for stroke prevention in NVAF in the recent years (Basaran et al., 2017; Wilkinson et al., 2021; Calderon et al., 2022). From 2014 to 2017, there were more patients with NVAF initiating OAC in our setting (from 67.9% to 81.9%), with an increase of DOAC over time (Programa d’harmonització farmacoterapèutica et al., 2019; Giner-Soriano et al., 2020).
Also diverse studies, including some conducted by our group (Giner-Soriano et al., 2016; 2020; Gomez-Lumbreras et al., 2018), have described the clinical profile of OAC-users (Giner-Soriano et al., 2016; Rodríguez-Mañero et al., 2017), for instance Rodríguez-Mañero et al. described different clinical characteristics and outcomes in octogenarians with AF to those in younger people; the adequacy of the prescription (Dalmau Llorca et al., 2021), reporting inadequate OAC prescriptions in up to 67.6% of the cases; or the adherence and persistence to the treatment, using different methods and finding different compliance levels (Hurtado-Navarro et al., 2018; Banerjee et al., 2020; Sabaté et al., 2021).
Nevertheless, not many studies have described all these aspects highlighting sex and gender differences (Dagres et al., 2007; Avgil Tsadok et al., 2015; Loikas et al., 2017; Tamargo et al., 2017; Raccah et al., 2018), even knowing that women are less likely to receive OAC despite their higher risk of stroke in comparison with men, as they may respond differently to cardiovascular medications, have different incidence of adverse events, or may require different VKA doses (Raccah et al., 2018).
Differences in dosage in women and men have not been analysed either, even taking into account that dose reductions are required for all DOAC depending on different clinical conditions (See Supplementary file, Supplementary Table S1) (European Medicines Agency, 2012b; European Medicines Agency, 2013c; European Medicines Agency, 2016a; Boehringher Ingelheim International GmbH. London (United Kingdom): European Medicines Agency (EMA); 2008, 2013), that under- and overdosing may impact on the effectiveness and safety of these drugs (Shen et al., 2021), that dose adequation has been scarcely described (Programa d’harmonització farmacoterapèutica. et al., 2019) and that sex and gender analyses need to be routinely conducted in research to help decision-making therapeutic process, harm reduction and health equality promotion (Solans-Domènech et al., 2022), taking into account that “sex” refers to biological aspects, such as pharmacokinetic, pharmacodynamic and clinical differences of AF and OAC treatment; and “gender” refers to the sociocultural ones, as how their stroke risk is perceived by the prescribers and how this impacts on OAC prescription (Madsen et al., 2017; Raccah et al., 2018).
In this context, we conducted a study on the use, effectiveness, and safety of OAC prescribed for stroke prevention in NVAF from 2011–2020 in a Primary Health Care (PHC) cohort in Catalonia, Spain. The objectives of the present manuscript were: 1) To describe sex and gender differences in the treatment initiation and in the socio-demographic and clinical profile for all patients who initiated an OAC prescription; and 2) To describe sex and gender differences in the prescribed doses and the adherence and persistence for those initiating DOAC.
Material and methods
Study design
Population-based cohort study including adults with NVAF who initiated OAC treatment.
Population included
We included all ≥18 years-old individuals with an active diagnosis of NVAF registered in PHC electronic records who initiated treatment with OAC from January 2011 to December 2020. Patients were followed-up from the day of initiation of treatment with OAC up to death, disenrollment from the database, end of treatment or end of study period.
Population excluded
We excluded people with valvular AF and people using DOAC for indications other than NVAF: those diagnosed with pulmonary embolism or deep vein thrombosis during the previous 12 months to the OAC initiation, and those receiving OAC for surgical prophylaxis of hip or knee replacement during the previous 6 months.
Data source
The data source is the Information System for Research in Primary Care (SIDIAP) (Recalde et al., 2022; SIDIAP, 2022), which captures clinical information of approximately 5,8 million people from Catalonia, Spain (around 80% of the Catalan population). This information is pseudonymized, originated from different data sources: 1) ECAP (electronic health records in PHC in Catalonia); including socio-demographic characteristics, residents in nursing homes/long-term care facilities (LTCF), comorbidities registered as International Classification of Diseases (ICD)-10 codes (see Supplementary file, Table 1) (WHO, 2019), specialist referrals, clinical parameters, toxic habits, sickness leave, date of death, laboratory test data, and drug prescriptions issued in PHC, registered as Anatomical, Therapeutic, Chemical classification system (ATC) codes (see Supplementary file, Table 2) (WHO Collaborating Centre for Drug Statistics Methodology, 2022); 2) pharmacy invoice data corresponding to the PHC drug prescriptions, which includes the number of packages dispensed each month; and 3) database of diagnoses at hospital discharge (CMBD) (CatSalut. Servei Català de la Salut, 2022).
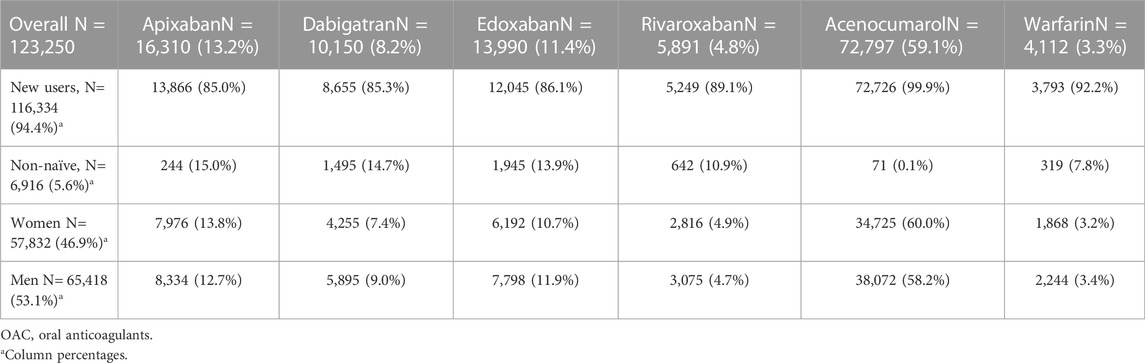
TABLE 1. Population with non-valvular atrial fibrillation initiating treatment with oral anticoagulants in 2011–2020.
Variables
The variables assessed at baseline were: socio-demographic characteristics, toxic habits, comorbidities, body mass index (BMI), CHA2DS2VASc (score accounting for: C; congestive heart failure, H; hypertension, A2; age ≥75, D; diabetes, S2; prior stroke, V; vascular disease, A; age 65–75, Sc: sex category) (Lip et al., 2012), HAS-BLED (score accounting for Hypertension, Abnormal renal/liver function, Stroke, Bleeding history, Labile INR, Elderly, Drugs/alcohol) (Lip et al., 2011), and drug exposure to OAC.
Drug exposure
Patients with NVAF were considered as exposed to any OAC if they started a new prescription during the study period (2011–2020). If they did not have any OAC prescription the prior 12 months, they were considered new users or naive patients. If they had received any other prescription of OAC the prior 12 months, different to the prescription that motivated the inclusion, they were defined as prevalent users or non-naive patients.
The drug exposure-related variables assessed after the DOAC treatment start were:
- Dose of DOAC; in order to ascertain the adequacy of dosing according to the Summary of Product Characteristics (SPC). Those people meeting these conditions but receiving the full dose were defined as “overdosed”, and those not meeting these conditions but receiving the reduced dose were defined as “underdosed”. For the conditions of dose reduction for each DOAC, please see Supplementary file, Supplementary Table S3.
- Treatment switch; when a different OAC is initiated during the study period.
- Discontinuation; defined as no dispensing of OAC during more than 2 months after having initiated treatment. Thus, persistence is defined as no discontinuation of OAC. Data included for discontinuation calculations: 2011–2019, as pharmacy invoice data might be registered after finishing the study period in December 2020.
- Adherence; measured by Medication Possession Ratio (MPR), which is the quotient between the drug amount dispensed and the drug amount prescribed during a defined period of time. MPR was calculated during all the time that a patient is receiving OAC treatment, thus, not discontinued it. A patient is adherent to the treatment when MPR ≥80%. Data included for adherence calculations: 2011–2019.
Statistical analysis
The baseline characteristics of the cohort were described as relative and absolute frequencies for categorical variables and with mean and standard deviation (SD) or median and interquartile range (IQR) for quantitative variables. The results are shown for all the population included and stratified by gender. We performed a bivariate analysis across genders using the Chi-square test for categorical data and the Kaplan-Meier curves to estimate the restricted mean survival time for time to event variables.
The smooth algorithm was used to model the drug exposure. It is an automated method to obtain consistent patterns of drug exposition throughout study period (Ouchi et al., 2022). With the algorithm, we obtained the exact moment of each treatment presentation, combinations, additions, or interactions to other drugs, the time exposed to that treatment, moments of discontinuation, persistence and adherence.
We calculated adherence as an approximation of the medication possession ratio (MPR). It was the rate between the total days covered by the packages dispensed at the pharmacy by the total days of active prescriptions for that treatment. A threshold of 80% classified patients into adherents and non-adherents.
All statistical analyses were conducted with R software (version 4.1 or superior).
Results
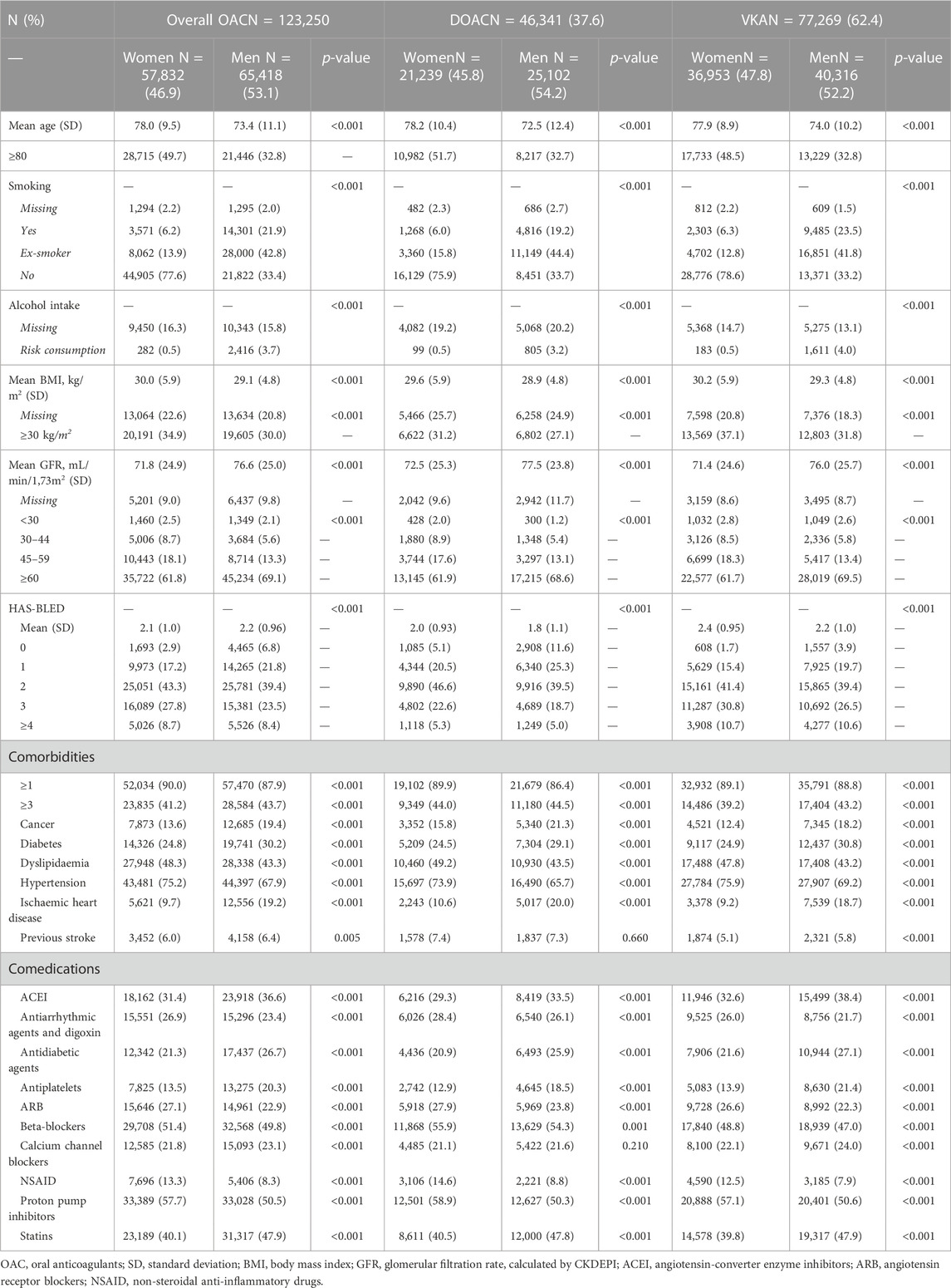
During the study period, 123,250 people with NVAF initiated OAC treatment in our setting; 57,832 (46.9%) of them were women and 65,418 (53.1%) were men (Figure 1). Most patients (94.3% women and 94.5% men) were new users, and the OAC prescribed were VKA in 62.4% of patients (Table 1). The number of initiations with DOAC increased by year and decreased with VKA. Since 2019, DOAC accounted for more than 50% of treatment starts (Supplementary Table S4). Patients were followed-up during a mean of 45.6 months (SD 31.5); 45.7 months for women and 45.4 for men (p = 0.143).
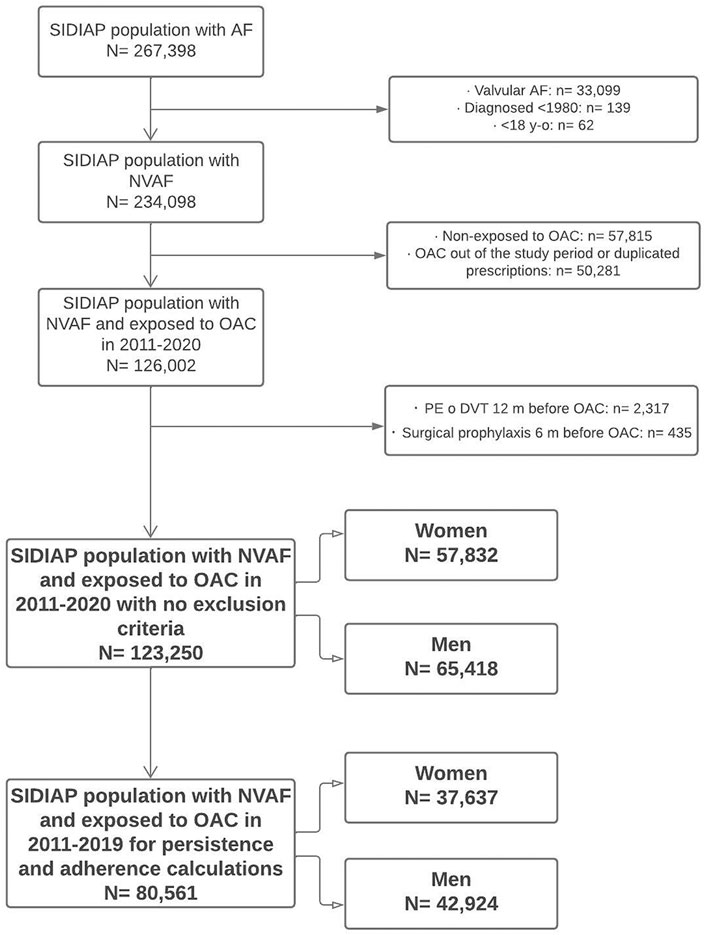
FIGURE 1. Flow diagram of population included. SIDIAP: Information System for Research in Primary Care. AF, atrial fibrillation; NVAF, non-valvular atrial fibrillation; OAC, oral anticoagulants; PE, pulmonary embolism; DVT, deep vein thrombosis.
Women were older than men, had higher BMI, and worse kidney function, whereas men were more frequently smokers and drinkers. HAS-BLED score was different between women and men; there were more men than women with scores 0–1 and more women than men with scores ≥2. Cancer, diabetes, ischaemic heart disease or previous stroke were more frequent in men, while dyslipidaemia or hypertension were more frequent in women. The most frequent comedications used were proton-pump inhibitors, beta-blockers, statins or angiotensin-converter enzyme inhibitors (ACEI), with differences between women and men for most drugs (Table 2).
Regarding the stroke risk, women initiating DOAC had a mean CHA2DS2VASc score of 3.9 (SD 1.4) and men 2.6 (1.6), whereas it was 3.9 (1.2) in women and 2.7 (1.4) in men initiating VKA. In Figure 2 we see that 87.6% of anticoagulated women had a CHA2DS2VASc score ≥3, and there were 12.4% of women with a score ≤2 receiving OAC treatment, which would not be always indicated in this case. Apixaban showed the highest frequency of women with score ≥3 (90.3%) and dabigatran, the lowest (82%). As it is a sex-dependent measure, CHA2DS2VASc score was lower in men; 79.4% of men had CHA2DS2VASc ≥2 and 20.6%, <2. Also apixaban had the highest percentage of men with high stroke risk (82.5%) and dabigatran the lowest (69.5%).
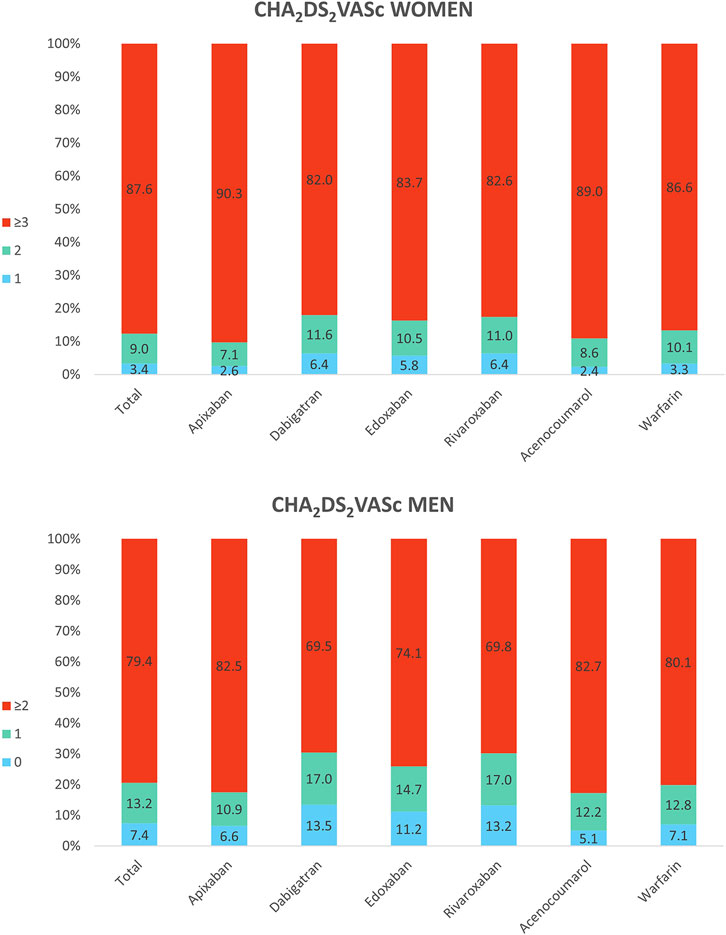
FIGURE 2. Baseline CHA2DS2VASc in women and men treated with oral anticoagulants. The top of Figure 2 shows the CHA2DS2VASc scores in all women receiving anticoagulants and by substance: 1, 2 and ≥3. The bottom shows the CHA2DS2VASc scores in all men receiving anticoagulants and by substance: 0, 1 and ≥2.
Regarding the dose adequacy to the SPC, women were more frequently underdosed than men for all DOAC, except for edoxaban (p = 0.355), being apixaban the DOAC with the highest frequency of underdosing; 39% of women and 27.4% of men (p < 0.001) were receiving a reduced dose despite they did not fulfil the criteria for the reduction. Men were more frequently overdosed than women, being significant only dabigatran (12% vs. 7.6%, p < 0.001). See Figure 3 and Supplementary Table S5.
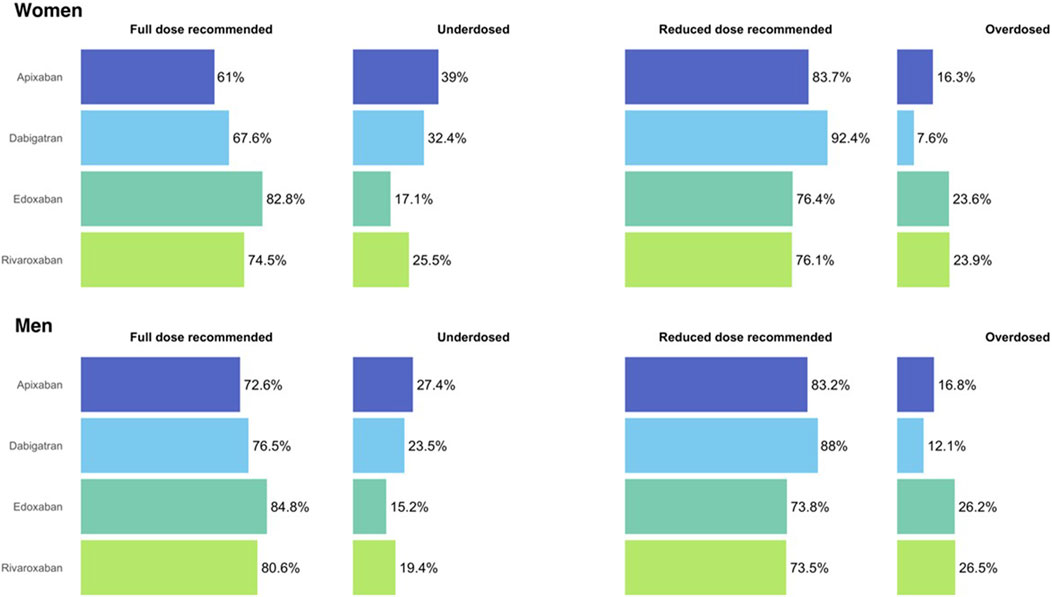
FIGURE 3. Dose adequacy in women and men initiating treatment with direct oral anticoagulants. Figure 3 shows the dose adequacy for all direct oral anticoagulants, women at the upper part and men at the bottom.
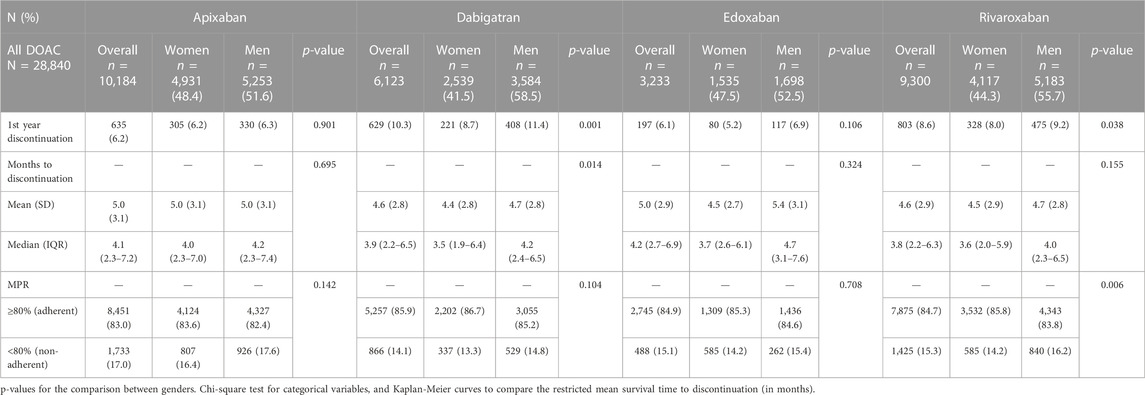
There were 80,561 patients initiating OAC in 2011–2019 (Figure 1) and 28,840 of them initiated DOAC. The discontinuations and adherence to DOAC are shown in Table 3. Men discontinued DOAC treatment more frequently than women during the first year after initiation. Dabigatran showed the highest frequency of discontinuation (8.7% in women and 11.4% in men, p < 0.001). Rivaroxaban also did show differences between genders (8% vs. 9.2%, p = 0.038). Edoxaban demonstrated the lowest discontinuation rate (5.2% in women and 6.9% in men, p = 0.106), with differences in the time to discontinuation (4.5 months in women vs. 5.4 in men, p = 0.034). Discontinuations for apixaban were not different between genders (6.2% in women and 6.3% in men, p = 0.901). Regarding the time to discontinuation, we found differences between women and men for dabigatran (4.4 vs. 4.7 months, p = 0.014), but not for the rest of DOAC.
Adherence to all DOAC was high, as most patients had MPR ≥80%. Women and men showed differences for rivaroxaban (85.8% vs. 83.8%, p = 0.006). The other DOAC showed no differences; 83.6% in women and 82.4% in men for apixaban, 85.9% and 86.7% for dabigatran, and 84.9% and 85.3% for edoxaban.
Discussion
We have analysed 10 years-data on OAC use for stroke prevention in NVAF in a PHC database in Catalonia, Spain. We found an increasing number of NVAF diagnoses over time, which obviously increased the number of people treated with OAC. Despite the criteria and recommendations in Spain positioning the VKA as the first choice in this indication up to the moment of data availability (Agencia Española de Medicamentos y Productos Sanitarios, 2012; Programa d’harmonització farmacoterapèutica, 2018), our study showed a progressive increase in the number of DOAC initiations and a decrease in VKA. In fact, the most updated information available indicates that DOAC account for more than 50% of the OAC initiations in NVAF.
There were more men in our study, as they are more frequently affected by AF (Hindricks et al., 2021). Comorbidities and risk of stroke and bleeding were similar to those described in prior investigations (Basaran et al., 2017; Giner-Soriano et al., 2020; Calderon et al., 2022), also with differences between men and women as previously described, as Loikas et al., who examined sex and gender differences in thromboprophylaxis in patients with NVAF. They reported more men with the disease but women with higher CHA2DS2VASc, as in our study. They also found that from 2011 to 2015 the number of women receiving OAC increased, except in older than 80, who were anticoagulated less frequently than in 2011 (Loikas et al., 2017).
We found that women were more frequently underdosed than men with all DOAC, even though they have a higher risk of stroke and the use of lower doses than recommended can presumably result in an increased rate of events (Raccah et al., 2018; Hindricks et al., 2021). We also found that men receiving dabigatran were more frequently overdosed than women, similar to the findings of Avgil Tsadok et al., where women were more frequently treated with dabigatran low dose than men (Avgil Tsadok et al., 2015). Carbone et al. found 29% of inadequate doses in octogenarian patients but being more frequently men those under- or overdosed (Carbone et al., 2022). Steinberg et al. found 13% of inadequate doses in elderly, women, and those with higher CHA2DS2VASc scores (Steinberg et al., 2016). In the past, we had already found a non-despicable frequency of inadequate doses for apixaban, dabigatran and rivaroxaban, although we had not studied gender differences (Programa d’harmonització farmacoterapèutica. et al., 2019). We believe that underdosing could have been caused by insufficient knowledge or lack of confidence in the appropriate dose (Sato et al., 2018; Cavillon Decaestecker et al., 2021), or by fear of the prescribers of causing harm, such as bleeding, as women present higher frequency and severity of adverse drug reactions than men (Tamargo et al., 2017; Fernández et al., 2020). We also think that prescribers might be more concerned for bleeding risk than for stroke risk, as overdosing was less frequent, and it might have been caused by insufficient knowledge of the criteria for dose reduction for each DOAC (Sato et al., 2018; Cavillon Decaestecker et al., 2021).
With regards to DOAC compliance, although studies used different methods to calculate persistence and adherence to treatment, we found better levels of persistence than other authors, close to 90% during the first year. For instance, Sabaté et al., with data proceeding from eight different databases in Europe, described persistence of 54–66% (Sabaté et al., 2021), or Banerjee et al.; who described persistence of 42.3–50.7%. Banerjee also assessed adherence levels calculating the Proportion of Days Covered (PDC), and approximately 60% of patients were considered adherents (Banerjee et al., 2020). In a comparable setting to ours, Hurtado-Navarro et al. found similar adherence levels to those in our study, also calculated by PDC, when they analysed those patients with at least two dispensings of the DOAC (Hurtado-Navarro et al., 2018). Although different levels of medication compliance have been described in women and men for different diseases (Billimek et al., 2015; Højgaard et al., 2018), none of the studies above analysed the differences in adherence and persistence to OAC treatment between women and men, and we did not find substantial differences between genders in our study.
The meta-analysis conducted by Raccah et al., in 2018 included the pivotal randomized clinical trials of the four DOAC with the purpose of evaluating whether the efficacy and safety of DOAC differed between genders, finding higher risk of stroke and lower risk of major bleeding in women in comparison with men (Raccah et al., 2018). They did not analyse doses prescribed, adherence or persistence.
Considering this lack of information on gender differences in NVAF and generally in medicine, and as sex and gender affect to all aspects of health and disease, it becomes necessary to carry out studies with gender perspective, allowing us to reduce health inequalities (McGregor et al., 2016; Madsen et al., 2017; Mauvais-Jarvis et al., 2020).
Limitations and strengths
This study contains some limitations inherent to database studies, such as missingness for some variables, potential confounders or the lack of register of sex and gender variables and the consequent impossibility to know if they correspond to biological sex at birth or in the moment of register, together with the categorisation of this variable only as binary.
On the other hand, this study has some strengths as the large number of persons included, the representativeness of the general population, complete records, long follow-up periods, or real-world data (Recalde et al., 2022); apart from the fact of adding evidence to the pharmacological management of a very prevalent disease with a gender perspective.
Conclusion
We analysed 10 years-data on OAC in our setting, describing the characteristics and gender differences of NVAF patients at treatment initiation.
We described the dose adequacy of patients receiving DOAC, finding a high frequency of underdosing, and significantly higher in women in comparison with men.
Adherence was generally high, only with higher levels in women for rivaroxaban. Persistence during the first year of treatment was also high in general, being more persistent women than men in the case of dabigatran and rivaroxaban.
Study classification
Study classified by the Spanish Medicines Agency (AEMPS, Agencia Española de Medicamentos y Productos Sanitarios) as EPA-OD, code IDI-ACO-1019–01.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of IDIAPJGol. Study classified by the Spanish Medicines Agency (AEMPS, Agencia Española de Medicamentos y Productos Sanitarios) as EPAOD, code IDI-ACO-1019–01.
Author contributions
MG-S and RM designed the study and wrote the study protocol. MG-S, OP-V, and RM participated in the data acquisition and the operativization of all the necessary variables to be extracted from SIDIAP database. OP-V and DO conducted the statistical analyses. CV-C provided support in the data analysis and in the manuscript writing. All authors participated in the results interpretation. MG-S wrote the first version of the manuscript, which was reviewed and approved by all authors.
Funding
Data were obtained by request of the Pharmacotherapeutic Harmonization Program of the Catalan Health Service.
Acknowledgments
The authors acknowledge Clara Rodríguez and María Aragón from SIDIAP for their work in the data acquisition and operativization of the variables.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1110036/full#supplementary-material
Abbreviations
ACEI, angiotensin-converter enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin receptor blockers; ATC, Anatomical, Therapeutic, Chemical classification system; BMI, body mass index; CMBD, database of diagnoses at hospital discharge; CHA2DS2VASc, Congestive heart failure, Hypertension, Age ≥75, Diabetes, Stroke, Vascular disease, Age 65–75, Sex category; DOAC, direct oral anticoagulants; DVT, deep vein thrombosis; ECAP, electronic health records in Primary Health Care in Catalonia; HAS-BLED, Hypertension, Abnormal renal/liver function, Stroke, Bleeding history, Labile INR, Elderly, Drugs/alcohol; ICD, International Classification of Diseases; INR, International Normalized Ratio; IQR, interquartile range; LTCF, long-term care facilities; MPR, medication possession ratio; NSAID, non-steroidal anti-inflammatory drugs; NVAF, non-valvular atrial fibrillation; OAC, oral anticoagulants; PE, pulmonary embolism; PHC, Primary Health Care; SD, standard deviation; SIDIAP, Information System for Research in Primary Care; SPC, summary of product characteristics; TTR, time in therapeutic range; VKA, vitamin K antagonists.
References
Agencia Española de Medicamentos y Productos Sanitarios (2012). Criterios y recomendaciones generales para el uso de anticoagulantes orales directos (ACOD) en la prevención del ictus y la embolia sistémica en pacientes con fibrilación auricular no valvular. AEMPS (actualización 2016), 1–11. Available at: https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/criterios-anticoagulantes-orales.pdf (Accessed August 5, 2019).
Avgil Tsadok, M., Jackevicius, C. A., Rahme, E., Humphries, K. H., and Pilote, L. (2015). Sex differences in dabigatran use, safety, and effectiveness in a population-based cohort of patients with atrial fibrillation. Circ. Cardiovasc Qual. Outcomes 8, 593–599. doi:10.1161/CIRCOUTCOMES.114.001398
Banerjee, A., Benedetto, V., Gichuru, P., Burnell, J., Antoniou, S., Schilling, R. J., et al. (2020). Adherence and persistence to direct oral anticoagulants in atrial fibrillation: A population-based study. Heart 106, 119–126. doi:10.1136/HEARTJNL-2019-315307
Basaran, O., Filiz Basaran, N., Cekic, E. G., Altun, I., Dogan, V., Mert, G. O., et al. (2017). PRescriptiOn PattERns of oral anticoagulants in nonvalvular atrial fibrillation (PROPER study). Clin. Appl. Thromb. Hemostasis 23, 384–391. doi:10.1177/1076029615614395
Billimek, J., Malik, S., Sorkin, D. H., Schmalbach, P., Ngo-Metzger, Q., Greenfield, S., et al. (2015). Understanding disparities in Lipid management among patients with Type 2 diabetes: Gender differences in medication nonadherence after treatment intensification. Women’s Health Issues 25, 6–12. doi:10.1016/j.whi.2014.09.004
Calderon, J. M., Martinez, F., Diaz, J., Fernandez, A., Sauri, I., Uso, R., et al. (2022). Real-world data of anticoagulant treatment in non-valvular atrial fibrillation. Front. Cardiovasc Med. 8, 733300. doi:10.3389/FCVM.2021.733300
Carbone, A., Santelli, F., Bottino, R., Attena, E., Mazzone, C., Parisi, V., et al. (2022). Prevalence and clinical predictors of inappropriate direct oral anticoagulant dosage in octagenarians with atrial fibrillation. Eur. J. Clin. Pharmacol. 78, 879–886. doi:10.1007/s00228-022-03286-2
CatSalut. Servei Català de la Salut (2022). Conjunt mínim bàsic de dades (CMBD). Available at: http://catsalut.gencat.cat/ca/proveidors-professionals/registres-catalegs/registres/cmbd/ (Accessed November 25, 2022).
Cavillon Decaestecker, M., Ferret, L., Decaestecker, K., Gautier, S., Verdun, S., and Tsogli, E. S. (2021). Direct oral anticoagulants and non-valvular atrial fibrillation: Compliance with dose level guidelines in patients aged 80 Years and over. Drugs Aging 38, 939–950. doi:10.1007/s40266-021-00883-1
Dagres, N., Nieuwlaat, R., Vardas, P. E., Andresen, D., Lévy, S., Cobbe, S., et al. (2007). Gender-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: A Report from the Euro heart Survey on atrial fibrillation. J. Am. Coll. Cardiol. 49, 572–577. doi:10.1016/J.JACC.2006.10.047
Dalmau Llorca, R. M., Martín, C. A., Carrasco-Querol, N., Rojas, Z. H., Drago, E. F., Cumplido, D. R., et al. (2021). Oral anticoagulant adequacy in non-valvular atrial fibrillation in primary care: A cross-sectional study using real-world data (Fantas-TIC study). Int. J. Environ. Res. Public Health 18, 2244. doi:10.3390/IJERPH18052244
Fernández, C. S., Gullón, A., and Formiga, F. (2020). The problem of underdosing with direct-acting oral anticoagulants in elderly patients with nonvalvular atrial fibrillation. J. Comp. Eff. Res. 9, 509–523. doi:10.2217/cer-2019-0197
Giner-Soriano, M., Vedia Urgell, C., Roso-Llorach, A., Morros, R., Capellà, D., Castells, X., et al. (2016). Effectiveness, safety and costs of thromboembolic prevention in patients with non-valvular atrial fibrillation: phase I ESC-FA protocol study and baseline characteristics of a cohort from a primary care electronic database. BMJ Open 6, e010144. doi:10.1136/bmjopen-2015-010144
Giner-Soriano, M., Cortes, J., Gomez-Lumbreras, A., Prat-Vallverdú, O., Quijada-Manuitt, M. A., and Morros, R. (2020). The use and adherence of oral anticoagulants in primary health care in Catalunya, Spain: A real-world data cohort study. Aten. Primaria 52, 529–538. doi:10.1016/j.aprim.2020.05.016
Gómez-Doblas, J. J., Muñiz, J., Alonso Martin, J., Rodríguez-Roca, G., Lobos, J. M., Awamleh, P., et al. (2014). Prevalence of atrial fibrillation in Spain. OFRECE study results. Rev. Esp. Cardiol. Engl. Ed. 67, 259–269. doi:10.1016/j.rec.2013.07.014
Gomez-Lumbreras, A., Cortes, J., Giner-Soriano, M., Quijada-Manuitt, M. A., and Morros, R. (2018). Characteristics of apixaban-treated patients, evaluation of the dose prescribed, and the persistence of treatment: A cohort study in Catalonia. J. Cardiovasc Pharmacol. Ther. 23, 494–501. doi:10.1177/1074248418778544
Hindricks, G., Potpara, T., Dagres, N., Arbelo, E., Bax, J. J., Blomström-Lundqvist, C., et al. (2021). 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS)The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42, 373–498. doi:10.1093/EURHEARTJ/EHAA612
Højgaard, P., Ballegaard, C., Cordtz, R., Zobbe, K., Clausen, M., Glintborg, B., et al. (2018). Gender differences in biologic treatment outcomes—A study of 1750 patients with psoriatic arthritis using Danish health care registers. Rheumatology 57, 1651–1660. doi:10.1093/rheumatology/key140
Hurtado-Navarro, I., García-Sempere, A., Rodríguez-Bernal, C., Santa-Ana-Tellez, Y., Peiró, S., and Sanfélix-Gimeno, G. (2018). Estimating adherence based on prescription or dispensation information: Impact on thresholds and outcomes. A real-world study with atrial fibrillation patients treated with oral anticoagulants in Spain. Front. Pharmacol. 9, 1353. doi:10.3389/fphar.2018.01353
Kirchhof, P. (2017). The future of atrial fibrillation management: Integrated care and stratified therapy. Lancet 390, 1873–1887. doi:10.1016/S0140-6736(17)31072-3
Lip, G. Y. H., Frison, L., Halperin, J. L., and Lane, D. A. (2011). Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: The HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly) score. J. Am. Coll. Cardiol. 57, 173–180. doi:10.1016/j.jacc.2010.09.024
Lip, G. Y. H., Tse, H. F., and Lane, D. A. (2012). Atrial fibrillation. Lancet 379, 648–661. doi:10.1016/S0140-6736(11)61514-6
Loikas, D., Forslund, T., Wettermark, B., Schenck-Gustafsson, K., Hjemdahl, P., and von Euler, M. (2017). Sex and gender differences in thromboprophylactic treatment of patients with atrial fibrillation after the introduction of non-vitamin K oral anticoagulants. Am. J. Cardiol. 120, 1302–1308. doi:10.1016/j.amjcard.2017.07.002
Madsen, T. E., Bourjeily, G., Hasnain, M., Jenkins, M., Morrison, M. F., Sandberg, K., et al. (2017). Article commentary: Sex- and gender-based medicine: The need for Precise terminology. Gend. Genome 1, 122–128. doi:10.1089/gg.2017.0005
Mauvais-Jarvis, F., Bairey Merz, N., Barnes, P. J., Brinton, R. D., Carrero, J. J., DeMeo, D. L., et al. (2020). Sex and gender: Modifiers of health, disease, and medicine. Lancet 396, 565–582. doi:10.1016/S0140-6736(20)31561-0
McGregor, A. J., Hasnain, M., Sandberg, K., Morrison, M. F., Berlin, M., and Trott, J. (2016). How to study the impact of sex and gender in medical research: A review of resources. Biol. Sex. Differ. 7, 46. doi:10.1186/s13293-016-0099-1
Ouchi, D., Giner-Soriano, M., Gómez-Lumbreras, A., Vedia Urgell, C., Torres, F., and Morros, R. (2022). Automatic estimation of the most likely drug combination in electronic health records using the smooth algorithm: Development and validation study. JMIR Med. Inf. 10, e37976. doi:10.2196/37976
Programa d’harmonització farmacoterapèutica Giner-Soriano, M., Vives, R., Molina, A., Morros, R., Mena, D., et al. (2019). Estudi d’utilització d’anticoagulants orals en fibril·lació auricular no valvular. Available at: https://catsalut.gencat.cat/web/.content/minisite/catsalut/proveidors_professionals/medicaments_farmacia/harmonitzacio/pautes/anticoagulants-orals-fibrilacio-auricular/informe-utilitzacio-tractament-anticoagulants-orals-FANV.pdf (Accessed November 25, 2022).
Programa d’harmonització farmacoterapèutica (2018). Pautes per a l’harmonització de l’ús d’anticoagulants orals per a la prevenció de l’ictus i l’embòlia sistèmica en pacients amb fibril·lació auricular. Barcelona: Servei Català de la Salut. Departament de Salut. Generalitat de Catalunya. Available at: http://catsalut.gencat.cat/ca/detalls/articles/PautesACO_FA.
Raccah, B. H., Perlman, A., Zwas, D. R., Hochberg-Klein, S., Masarwa, R., Muszkat, M., et al. (2018). Gender differences in efficacy and safety of direct oral anticoagulants in atrial fibrillation: Systematic review and Network meta-analysis. Ann. Pharmacother. 52, 1135–1142. doi:10.1177/1060028018771264
Recalde, M., Rodríguez, C., Burn, E., Far, M., García, D., Carrere-Molina, J., et al. (2022). Data resource profile: The information system for research in primary care (SIDIAP). Int. J. Epidemiol. 51, e324. doi:10.1093/ije/dyac068
Rodríguez-Mañero, M., López-Pardo, E., Cordero, A., Kredieh, O., Pereira-Vazquez, M., Martínez-Sande, J. L., et al. (2017). Clinical profile and outcomes in octogenarians with atrial fibrillation: A community-based study in a specific European health care area. Int. J. Cardiol. 243, 211–215. doi:10.1016/j.ijcard.2017.03.149
Sabaté, M., Vidal, X., Ballarin, E., Rottenkolber, M., Schmiedl, S., Grave, B., et al. (2021). Adherence to direct oral anticoagulants in patients with non-valvular atrial fibrillation: A cross-national comparison in Six European Countries (2008–2015). Front. Pharmacol. 12, 682890. doi:10.3389/fphar.2021.682890
Sato, T., Aizawa, Y., Fuse, K., Fujita, S., Ikeda, Y., Kitazawa, H., et al. (2018). The comparison of inappropriate-low-doses use among 4 direct oral anticoagulants in patients with atrial fibrillation: From the database of a single-center registry. J. Stroke Cerebrovasc. Dis. 27, 3280–3288. doi:10.1016/j.jstrokecerebrovasdis.2018.07.028
Shen, N. N., Zhang, C., Wang, N., Wang, J. L., Gu, Z. C., Han, H., et al. (2021). Real-world prevalence of direct oral anticoagulant off-label doses in atrial fibrillation: An epidemiological meta-analysis. Front. Pharmacol. 12, 581293. doi:10.3389/fphar.2021.581293
SIDIAP (2022). SIDIAP. Information system for research in Primary Care. SIDIAP. Available at: http://www.sidiap.org/index.php/en.
Solans-Domènech, M., and Saborit, S.coordinadores i Grup de la Carta d’Hipàtia d’Alexandria (2022). Eina per incorporar la perspectiva de sexe i gènere en els continguts de recerca. Barcelona: Agència de Qualitat i Avaluació Sanitàries de Catalunya. Departament de Salut. Generalitat de Catalunya. Available at: https://aquas.gencat.cat/web/.content/minisite/aquas/publicacions/2022/eina_perspectiva_sexe_genere_recerca_aquas2022.pdf (Accessed November 15, 2022).
Steinberg, B. A., Shrader, P., Thomas, L., Ansell, J., Fonarow, G. C., Gersh, B. J., et al. (2016). Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: The ORBIT-AF II registry. J. Am. Coll. Cardiol. 68, 2597–2604. doi:10.1016/j.jacc.2016.09.966
Tamargo, J., Rosano, G., Walther, T., Duarte, J., Niessner, A., Kaski, J. C., et al. (2017). Gender differences in the effects of cardiovascular drugs. Eur. Heart J. Cardiovasc Pharmacother. 3, 163–182. doi:10.1093/ehjcvp/pvw042
WHO Collaborating Centre for Drug Statistics Methodology (2022). ATC/DDD index 2022. Available at: https://www.whocc.no/atc_ddd_index/ (Accessed November 25, 2022).
WHO (2019). International statistical classification of diseases and related health problems 10th revision. ICD-10 Version: 2019. Available at: https://icd.who.int/browse10/2019/en (Accessed November 25, 2022).
Keywords: oral anticoagulants (OACs), atrial fibrillation, gender differences, adherence–compliance–persistance, electronic health records–EHR, primary health care (PHC)
Citation: Giner-Soriano M, Prat-Vallverdú O, Ouchi D, Vilaplana-Carnerero C and Morros R (2023) Sex and gender differences in the use of oral anticoagulants for non-valvular atrial fibrillation: A population-based cohort study in primary health care in catalonia. Front. Pharmacol. 14:1110036. doi: 10.3389/fphar.2023.1110036
Received: 28 November 2022; Accepted: 18 January 2023;
Published: 07 February 2023.
Edited by:
Carlos Alves, University of Coimbra, PortugalReviewed by:
Ippazio Cosimo Antonazzo, University of Milan-Bicocca, ItalyMaxim Grymonprez, Ghent University, Belgium
Copyright © 2023 Giner-Soriano, Prat-Vallverdú, Ouchi, Vilaplana-Carnerero and Morros. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Giner-Soriano, bWdpbmVyQGlkaWFwamdvbC5pbmZv
 Maria Giner-Soriano
Maria Giner-Soriano Oriol Prat-Vallverdú3
Oriol Prat-Vallverdú3