
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol. , 25 January 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1109966
This article is part of the Research Topic Natural Products, Derivatives, and Mimics as Immunomodulating Agents for Inflammatory Diseases View all 7 articles
 Seyed Ahmad Hosseini1
Seyed Ahmad Hosseini1 Zainab Shateri2*
Zainab Shateri2* Farhad Abolnezhadian3
Farhad Abolnezhadian3 Elham Maraghi4
Elham Maraghi4 Maryam Haddadzadeh Shoushtari5
Maryam Haddadzadeh Shoushtari5 Marzie Zilaee1
Marzie Zilaee1Background: Asthma essentially represents a chronic inflammatory disease that manifests as a lifelong condition with different severity throughout the life of patients with asthma. Pomegranate holds three times the antioxidant activity compared to other polyphenol-rich food sources like green tea, which may positively impact asthma.
Aim of the study: This research aimed to investigate the pomegranate supplementation influences clinical symptoms, eosinophil, basophil, and neutrophil counts in patients with allergic asthma.
Materials and Methods: Participants (n = 64) suffering from mild to moderate allergic asthma were randomly divided into two groups: The control group received placebo capsules and the intervention group received 250 mg pomegranate extract capsules twice a day (for 8 weeks). To analyze the data, we used SPSS software (version 22). The significance level of p-value was considered less than 0.05.
Results: The findings showed that the pomegranate extract improved patients’ clinical symptoms like daily breath shortness, nocturnal breath shortness, and limitation of asthma-related activity in the intervention group compared to the control group. Furthermore, eosinophil, basophil, and neutrophil counts were significantly decreased in the intervention group. Also, by comparing the two groups, the levels of change in neutrophils and eosinophils were statistically significant.
Conclusion: It appears that the pomegranate extract can ameliorate some clinical symptoms and reduce neutrophils, basophils, and eosinophils in allergic asthma patients.
Clinical Trial Registration: https://www.irct.ir/trial/45612; identifier: IRCT20200205046384N1.
Asthma represents a chronic inflammatory disease that manifests as a lifelong condition with different severity throughout the life of patients with asthma (Nunes et al., 2017). In fact, it is a complex, multifactorial, chronic disease related to the respiratory tract associated with inflammation of the airways, which increases reaction and regeneration in response to other types of physical and chemical stimuli (Kumari et al., 2015).
Research has shown the most common asthma symptoms include airway obstruction or reversibility of obstruction, wheezing, and breath shortness (Gauthier et al., 2015). Asthma symptoms may be intermittent or persistent, displaying as mild, moderate, or severe (Bateman et al., 2008).
In allergic asthma, increased immunoglobulin E (IgE) production in response to environmental allergens represents the most substantial detectable factor for asthma progression, mainly when allergies occur in the early stages of life (Hamelmann, 2007).
According to published statistics, more than 15 million people have asthma yearly (Hasankhani et al., 2013). It is estimated that there are currently 300 million asthma patients worldwide; by 2025, 100 million people might be added to this amount. It has been demonstrated that the prevalence of asthma in adults is 4.3% worldwide (To et al., 2012). According to the National Health Information, the prevalence of asthma in adults aged 20 to 44 in Iran was reported to be 8.9%, which shows an increasing prevalence over the last decade (Fazlollahi et al., 2018). The prevalence of asthma is reported between 5% and 15% in Iran (Hasankhani et al., 2013). The number of people with asthma in Ahvaz is more than the average of the whole of Iran (Raji et al., 2020). Recent research was shown poor control of asthma exists in 53%–58% of patients despite receiving appropriate treatment (Hasankhani et al., 2013), which can justify the high prevalence of asthma disease.
The pomegranate is an edible fruit cultivated in many countries, including Iran (Aviram et al., 2000), the most significant producer of pomegranate in the world (Chandra et al., 2010). The edible part of a pomegranate (approximately 50% of the total weight of the fruit) contains 80% water and 20% seeds (Raji et al., 2020).
In traditional medicine, pomegranate has been used to treat diseases due to its bioactive compounds. It has anti-hepatotoxic, anti-diabetic, anti-tumor, antimicrobial, anti-inflammatory, and anti-viral properties, influencing skin, oral and cardiovascular conditions (Rouhi et al., 2017). The pomegranate is considered a rich source of polyphenols (Mertens-Talcott et al., 2006). It has been indicated that polyphenols can retain antioxidant and anti-inflammatory features in the human body. In addition, it has been shown that pomegranate holds three times the antioxidant activity compared to other polyphenol-rich food sources like green tea (Danesi and Ferguson, 2017).
In asthma, there is continuous airway inflammation with a mucosal influx of T lymphocytes, eosinophils, mast cells, and the release of proinflammatory cytokines and mediators of lipids (Barnig et al., 2018). Eosinophils and neutrophils are the primary cells involved in asthma disease pathology and inflammation (Bloemen et al., 2007; Chandra et al., 2010; Pelaia et al., 2015).
Rogerio et al. evaluated the effect of one of the active ingredients of pomegranate called ellagic acid in mice with asthma. It was found that ellagic acid reduced the number of eosinophils and neutrophils (Rogerio et al., 2008). Also, a study conducted by Bachoual et al. was demonstrated that the extract of pomegranate peel inhibited myeloperoxidase of neutrophils in vitro and reduced pneumonia (Bachoual et al., 2011). Furthermore, Alves et al. assessed the effect of ellagic acid on allergic airway response in mice with asthma. It was shown that ellagic acid accelerates airway clearance by reducing total leukocytes and eosinophils (de Freitas Alves et al., 2013).
Corticosteroids like dexamethasone are currently used as anti-asthmatic. Still, various studies have shown that microbes in the airways can inhibit corticosteroid reactions and potentially affect corticosteroid therapy’s effectiveness in treating asthma (Kumari et al., 2015). Hence, based on the antioxidant and anti-inflammatory features of the pomegranate, it appears pomegranate extract administration can helpfully affect patients with asthma. In contrast, the effect of pomegranate extract on allergic asthma has not been studied in human models. Therefore, this investigation aimed to study the effects of pomegranate extract on complete blood count with differential (CBC-diff) and clinical symptoms in patients with allergic asthma (mild to moderate severity).
The current study used a double-blind randomized controlled trial. Participants were people with allergic asthma who attended the allergy and asthma center. Using the average comparison formula with β = 0.2, α = 0.05, and S = 0.7 based on the previous study, (Zilaee et al., 2019) the sample size was computed for the serum levels of eosinophils. Therefore, at least 31 people were required to participate in each group. Assuming 10% violation of protocols or removal, we allocated 35 patients to each group. The individuals in the present study were ultimately assigned to each group based on the severity of the disease (1:1 ratio) and clinical symptoms according to a permuted block randomization (set by a biostatistician). In order to perform a double-blind study, one person was requested to number the capsule bottles.
After explaining the method to the participants, blood samples were taken to check the serum IgE level to determine allergic asthma (Bateman et al., 2008; Zilaee et al., 2019). Since serum IgE levels are more valid for confirming allergic asthma compared to other tests (such as skin prick test), we applied IgE serum levels to determine allergic asthma (Sunyer et al., 1995). According to previous studies, serum IgE level ≥30 international units (IU) are considered allergic asthma (Bateman et al., 2008; Mason et al., 2015). Therefore, patients with IgE ≥30 IU were studied. At the beginning of the investigation, the consent form was taken from the participants, and then they were requested to complete the research questionnaires. Questionnaires were filled again at the end of the study. Information on the patients’ clinical symptoms was asked, including daily breath shortness, nocturnal breath shortness, limitation of asthma-related activity, salbutamol spray usage, and nocturnal waking up.
One of the valuable tools to evaluate lung function is the spirometry test (Sewa and Ong, 2014). Spirometry is one of the tests of pulmonary function to assess clinical symptoms and the response of patients with asthma to drugs. Forced expiratory volume in 1 s (FEV1) is one of the most important parameters of the spirometry test (Bateman et al., 2008). FEV1 correlates with the severity of airway obstruction and is the most reproducible lung function parameter (Enright et al., 2012). Patients with allergic asthma were divided into two groups (moderate and mild) using clinical symptoms applying the Global Initiative for Asthma (GINA) diagnostic criteria and its severity (FEV1 ≥ 60% by spirometry test). In mild persistent asthma, symptoms occur less than once a day and more than once a week. The disease symptoms may influence the patient’s sleep and activity. FEV1 is ≥80% and asthma symptoms are experienced at night more than twice a month. In moderate persistent asthma, symptoms occur daily and may influence the patient’s sleep and activity. FEV1 is 60%–80% and asthma symptoms occur at night more than once a week (Bateman et al., 2008).
A permutated block randomization method was used to randomly allocate subjects into the supplement and placebo groups, and their consumption and possible side effects were monitored weekly by phone call. Supplements were given to individuals in two stages (in the 1st and 4th week). Patients were asked to stop consuming the pomegranate, pomegranate paste, pomegranate juice, and products containing pomegranate during the research period. It was also requested that the capsule bottles be delivered at the end of the study. Individuals who did not regularly consume the capsules or were sensitive to the supplements were excluded from the study. Anthropometric measurements, including waist circumference (WC), weight, hip circumference, and waist-to-hip ratio (WHR), were measured at the study’s beginning and end. Height was also recorded at the beginning of the research.
Duration of asthma disease, demographic information, history of medication use, level of physical activity using the short-form of international physical activity questionnaire, and blood pressure was evaluated at the beginning and the end of the study.
The researchers, the physician, and all the participants followed a double-blinded scheme using a numbering method for the bottles until the completion of the statistical analysis. To examine the energy intake and macronutrients, researchers used a 24-h dietary recall on 3 days (one weekend day and two weekdays) at the beginning and the end of the research.
Because of the immorality of removing patients’ drugs, asthma and allergy specialist used the same drugs for all the participants. A code was assigned to each patient to maintain the confidentiality of information.
The study participants included 40 women and 30 men ranging from 18 to 65 years old. Based on its severity (FEV1 ≥ 60%) and clinical symptoms by the GINA diagnostic criteria, participants were divided into two groups: allergic asthma with mild and moderate (by asthma and allergy specialist). The participants were asked to take the supplements regularly, and how to take them was fully explained. Social information, drugs, lifestyle, and medical history were completed using a questionnaire. Persistent allergic asthma (mild to moderate), body mass index (BMI) less than 30 kg/m2, serum IgE ≥30 IU, insensitivity to pomegranate, and age of 18–65 years old were involved in the inclusion criteria of the study.
The exclusion criteria of this study included unwillingness, pregnancy, lactation, smoking, malignancy, taking multivitamin/mineral supplements during the last 2 months, diabetes, autoimmune diseases, and other lung diseases. The current study was recorded in the Iranian Registry of Clinical Trials (IRCT20200205046384N1). Also, the Ahvaz Jundishapur University of Medical Sciences Ethics Committee approved the present trial.
The intervention group was given a pomegranate extract capsule containing 250 mg of pomegranate seed extract twice a day (500 mg in total) for 8 weeks. The control group was given placebo capsules (rusk powder). The placebo capsule and the pomegranate extract capsule were similar in terms of size, color, and shape. The placebo and pomegranate extract were provided by Karaj Institute of Medicinal Plants, Alborz Province, Iran. Ghavipour et al. proved that the daily consumption of 500 mg of pomegranate extract for 8 weeks in patients with rheumatoid arthritis reduces inflammation and oxidative stress (Ghavipour et al., 2017). Therefore, in this research, the effect of 500 mg of pomegranate extract was evaluated for 8 weeks. Semi-industrial grinding machines, semi-industrial percolation, vacuum filters, and semi-industrial spray dryers are used to prepare pomegranate extract.
1. Ground sweet pomegranate seeds (powder) and solvent (water) were extracted in a semi-industrial percolation machine at a temperature of 50 °C for 8 h.
2. The liquid extract was filtered by a vacuum filter and concentrated in a spray dryer, and a powder extract was obtained.
3. The powder extract was packed in the form of capsules.
The investigated outcomes included the clinical symptoms of asthma, which were assessed at the beginning and end of the study. The clinical symptoms of asthma consisted of daily breath shortness, nocturnal breath shortness, salbutamol spray usage, nocturnal waking up, and limitation of asthma-related activity.
In addition, the other outcomes were recorded, including basophil, eosinophil, and neutrophil counts at the beginning and end of the study.
As previously mentioned, the severity of asthma was defined based on the FEV1 and clinical symptoms. Patients with FEV1 ≥ 80% were categorized into the mild group, and patients with FEV1 between 60% and 80% were classified into the moderate group. Clinical asthma symptoms were also evaluated at the start and end of the study.
At the start of the research and the end of the eighth week, 6 mL of venous blood was drawn from all patients. After centrifugation, blood samples were kept in the freezer at −80 °C until the end of the study.
The total IgE concentration in patients’ serum was measured using a Roche enzyme-linked immunosorbent assay (ELISA) kit (Germany). CBC-diff was used to examine eosinophils, neutrophils, and basophils. All experiments of this research were performed in the Pasteur laboratory, Ahvaz, Iran.
To evaluate the patients’ diet in terms of total calories and received macronutrients, a 24-h dietary recall was used on a one-day weekend and two weekdays at the beginning and the end of the research. Nutritionist 4 (N4) software was used for patients’ diet analysis. Patients’ calorie intake can influence their weight; therefore, their calorie intake was assessed. In this study, a Mercury sphygmomanometer was applied to evaluate blood pressure based on the standard method after 5–10 min of rest at the beginning and the end of the study.
Ellagic acid is a thermodynamically extremely stable molecule retaining four rings representing its lipophilic domain. It also has four phenolic groups and two lactone groups, which act as donors and acceptors of hydrogen bonding, respectively (Bala et al., 2006). In this study, a high-performance liquid chromatography (HPLC) method was utilized to measure ellagic acid. Punicalagin is a high-weight molecule that is a polyphenol with antioxidant and water-soluble properties that is extracted from pomegranate fruit (Kulkarni et al., 2007). In addition, the punicalagin was measured by the HPLC method. To evaluate the amounts of bioactive compounds in pomegranate extract, some capsules were randomly analyzed by the Medicinal Plants and Drugs Research Institute of Shahid Beheshti University, Tehran, Iran.
Quantitative variables (age, duration of asthma, etc.) were reported as mean ± standard deviation, and qualitative variables (gender, the severity of asthma, etc.) were reported using numbers (percentage). Data with a non-normal distribution were described as the median (mid-quartile range). The Shapiro–Wilk test was used to assess the normality of quantitative variables. The chi-square test (or Fisher’s exact test) was applied to examine the relationship between qualitative variables (intervention group versus control group). In addition, the Mann–Whitney U test and independent T-test were used for non-parametric and parametric data, respectively. The paired T-test and Wilcoxon for parametric and non-parametric variables were respectively applied to evaluate the mean of quantitative variables in each group at the beginning and the end of the study. All research hypotheses were examined at the 5% level using software SPSS 22.
In this research, 70 people participated, from whom 64 completed the study. Out of 70 people who participated in the study, 64 people finished the research. The reasons for the exclusion of other participants are shown in Figure 1.
In Table 1, the patient’s demographic information is depicted. It can be perceived that there was no significant difference based on dietary variables, demographic features, anthropometric indices, clinical characteristics, asthma severity, physical activity, age of asthma onset, and infant feeding between the control and intervention groups (p > 0.05). Also, there was no statistically significant difference between the control and pomegranate extract groups based on the drugs taken by patients (Symbicort, Tiova, Budesonide, and Salbutamol spray) (p > 0.05).
Table 2 compares anthropometric indices, physical activity, dietary intake, and blood pressure in two groups of pomegranate extract and control. There was no statistically significant difference in any of the mentioned variables between the two groups at the beginning and the end of the study (p > 0.05).
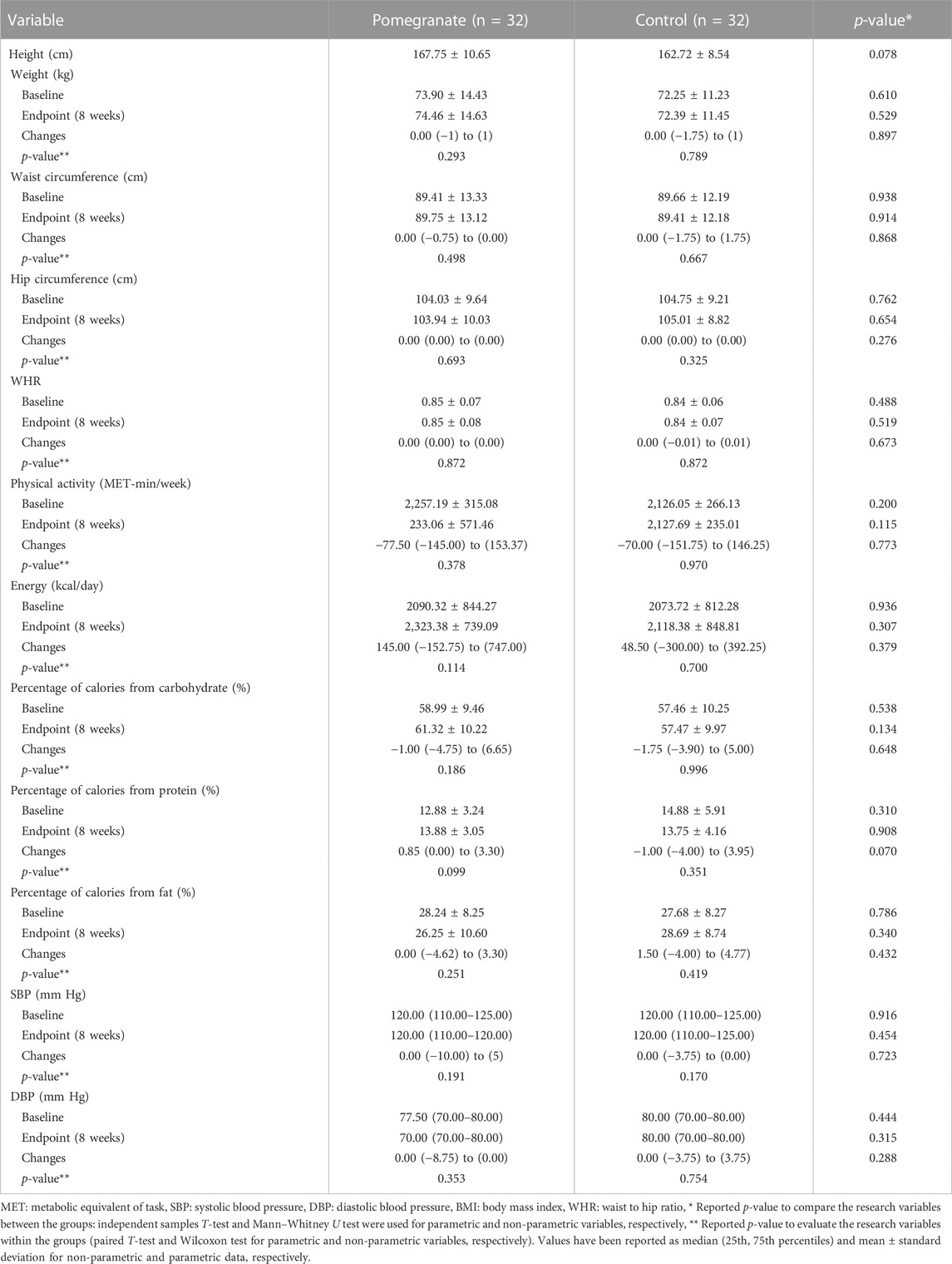
TABLE 2. Comparing anthropometric indices, physical activity, dietary intake, and blood pressure in the intervention and control groups.
At the beginning of the study, there was no statistically significant difference in terms of asthma symptoms and salbutamol spray consumption. At the end of the study, the findings indicated that supplementation with pomegranate extract improved day-and-night shortness of breath and activity limitation due to asthma symptoms in the intervention group versus the control group (p < 0.05). There was no observed a statistically significant difference in terms of salbutamol spray consumption at the end of the study (p > 0.05) (Table 3; Table 4).
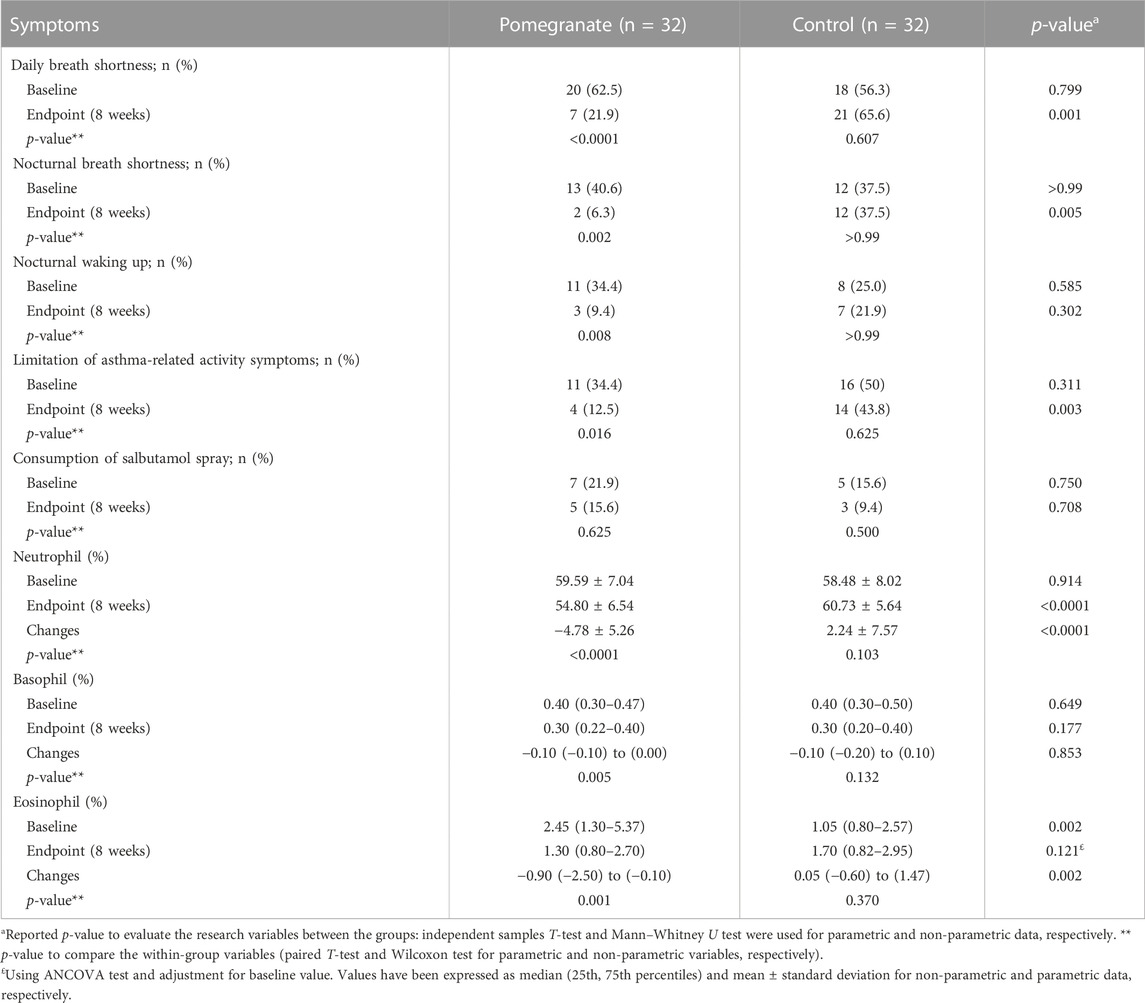
TABLE 3. Comparing clinical changes, basophil, eosinophil, and neutrophil counts between the intervention and control groups.
Mean blood levels of neutrophils, basophils, and eosinophils in blood cell count were compared between the control and intervention groups at the start and the end of the study. In terms of basophils and neutrophils, no statistically significant difference was found between the intervention group and the control group at the beginning of the study (p > 0.05). Still, eosinophils had a statistically significant difference (p = 0.002). The findings showed that within-group differences were statistically significant in eosinophils, neutrophils and basophils in the group receiving pomegranate extract (p = 0.001, p < 0.0001 and p = 0.005, respectively). Also, the results indicated that supplementation with pomegranate extract significantly reduced neutrophils in the intervention group compared to the control group (p < 0.0001). At the end of the study, no statistically significant difference was observed between the intervention group and the control group at the end of the study in eosinophils (p = 0.121). Furthermore, the change levels in neutrophils and eosinophils in the intervention group compared to the control group were statistically significant at the end of the study (p < 0.0001 and p = 0.002, respectively). In terms of basophils, no statistically significant difference was detected between the intervention group and the control group at the end of the study (p > 0.05) (Table 3).
The ellagic acid and punicalagin were measured by the HPLC method. Each 250 mg capsule of pomegranate extract contained 2.1 μg of ellagic acid, 118.4 μg of punicalagin alpha, and 53 μg of punicalagin beta.
The current study used a randomized controlled trial that was performed employing a double-blind design in which the effects of pomegranate (500 mg/day for 8 weeks) in persistent allergic asthma were investigated in terms of clinical symptoms and CBC-diff. The findings showed that pomegranate extract could improve daily breath shortness, nocturnal breath shortness, and limitation of asthma-related activity and can also decrease neutrophils and eosinophils in the pomegranate receiving group in comparison to the control group. Since this research was the first one to investigate the effects of pomegranate extract on allergic asthma, this has made it difficult to evaluate the results of our research with similar studies. In a study conducted on the impact of saffron on the clinical symptoms in patients with allergic asthma, the results showed that it could improve the clinical symptoms (Zilaee et al., 2019) because saffron, like pomegranate, has anti-inflammatory and antioxidant properties.
The results revealed that pomegranate extract supplementation exerted a statistically significant effect on reducing the neutrophil, basophil, and eosinophil counts. Moreover, pomegranate extract significantly decreased neutrophil and eosinophil counts in the intervention group compared to the control group. Inflammation, increased airway response, and structural changes are the main features of asthma. Eosinophils represent the primary cells involved in inflammation and asthma pathology. In non-allergic and allergic asthma, these cells’ development, maturity, activation, and survival influence the respiratory system (Pelaia et al., 2015). Not only eosinophil-induced inflammation but also neutrophil-induced inflammation is involved in the pathogenesis of asthma. They cause the secretion of a wide range of products, including leukotrienes, cytokines, proteases, and metalloproteins. Also, neutrophil products cause airway obstruction, excessive mucus secretion, and airway over-response (Bloemen et al., 2007). This inflammatory process leads to shortness of breath, coughing, and wheezing. Therefore, the elimination of inflammation in the airways occurs naturally in the host body as an active reaction (Barnig et al., 2018). In the present study, the improvement in the clinical symptoms of the patients could be justified by the decrease in eosinophil and neutrophil counts. Also, improvement in the clinical symptoms of patients led to improvement in lung function parameters (using spirometry tests) (Shateri et al., 2022).
Studies have shown that pomegranate extract reduces inflammatory mediators in different ways, but there is little information about the specific anti-allergic property of pomegranate (Ismail et al., 2012). According to the studies, ellagitannin and ellagic acid represent polyphenols found in some fruits, including pomegranate, are rich in anti-inflammatory, antioxidant and anti-cancer properties. Over the past few years, pomegranate has received much attention as a new treatment approach (Danesi and Ferguson, 2017). Furthermore, punicalagin maintains the highest antioxidant effect compared to the other pomegranate polyphenols. Punicalagin is a complex ellagitannin responsible for more than 50% of pomegranate’s antioxidant activity (Bayat-Chadegani et al., 2015). In addition, based on previous studies conducted on animal models, it has been proven that ellagic acid has an apparent anti-inflammatory effect by inhibiting nuclear factor kappa-B (NF-KB) activation in mice with asthma. So, it can be considered a therapeutic agent for allergic asthma (Zhou et al., 2014).
Also, the current study showed that supplementation with pomegranate extract in asthma patients compared with the control group did not cause any remarkable difference in systolic and diastolic blood pressure. There are inconsistent findings on the impact of pomegranate on blood pressure. Some studies supported the influence of pomegranate on systolic and diastolic blood pressure (Sohrab et al., 2008; Giménez-Bastida et al., 2021). Still, other studies did not support its effectiveness (Esmaillzadeh et al., 2004; Sumner et al., 2005). Various mechanisms have been suggested for the impact of the pomegranate on blood pressure. It has been shown that vitamin C can effectively reduce systolic and diastolic blood pressure by improving and restoring nitric oxide activity through vasodilation of the arteries (Shateri et al., 2016). Pomegranate also contains different amounts of vitamin C in the range of 52.8 mg–72 mg/100 g of fresh pomegranate seeds (Opara et al., 2009). In addition, one of the mechanisms of the pomegranate on blood pressure is its inhibitory effect on the angiotensin-converting enzyme (Sohrab et al., 2008). The possible reason for inconsistent systolic and diastolic blood pressure results in the present study versus previous studies could be caused by the differences in the sample size, population, and the different parts of consumed pomegranate.
This research, like many other studies, contains its limitations. We employed self-report tools to assess asthma symptoms in this study, so participants may not have provided accurate information. In addition, if this intervention is performed for a more prolonged period, more reliable results are likely to be obtained. The strengths of the current study included a double-blind, randomized, placebo-controlled scheme and the first clinical trial to assess the impacts of pomegranate extract in a human model of allergic asthma.
The pomegranate extract seems to decrease neutrophil, basophil, and eosinophil in patients with allergic asthma and improve some of their clinical symptoms. Although the effect of pomegranate extract on asthma has been examined for the first time in the human model, studies with more sample sizes and more extensive periods should be conducted to confirm the results and effectiveness of pomegranate extract in clinical settings.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ahvaz Jundishapur University of Medical Sciences Ethics Committee (No. IR.AJUMS.REC.1398.905). The patients/participants provided their written informed consent to participate in this study.
ZS and SH have contributed substantially to conceptualizing and designing the work. ZS and FA participated in the acquisition of data. SH was the supervisor of this study. Medical experts for the current research were FA and MH. The statistical data were analyzed by EM. The manuscript was written by ZS and SH and edited by MZ. The manuscript was read and approved by the authors.
The Vice Chancellor for Research of Ahvaz Jundishapur University of Medical Sciences supported this study (grant number: NRC-9823). This article is taken from the Ph.D. thesis of the second author of the present study.
The researchers would like to thank Ahvaz Jundishapur University of Medical Sciences and all the participants.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aviram, M., Dornfeld, L., Rosenblat, M., Volkova, N., Kaplan, M., Coleman, R., et al. (2000). Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E–deficient mice. Am. J. Clin. Nutr. 71 (5), 1062–1076. doi:10.1093/ajcn/71.5.1062
Bachoual, R., Talmoudi, W., Boussetta, T., Braut, F., and El-Benna, J. (2011). An aqueous pomegranate peel extract inhibits neutrophil myeloperoxidase in vitro and attenuates lung inflammation in mice. Food Chem. Toxicol. 49 (6), 1224–1228. doi:10.1016/j.fct.2011.02.024
Bala, I., Bhardwaj, V., Hariharan, S., and Kumar, M. R. (2006). Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. analysis 40 (1), 206–210. doi:10.1016/j.jpba.2005.07.006
Barnig, C., Frossard, N., and Levy, B. D. (2018). Towards targeting resolution pathways of airway inflammation in asthma. Pharmacol. Ther. 186, 98–113. doi:10.1016/j.pharmthera.2018.01.004
Bateman, E. D., Hurd, S. S., Barnes, P. J., Bousquet, J., Drazen, J. M., FitzGerald, M., et al. (2008). Global strategy for asthma management and prevention: GINA executive summary. Eur. Respir. J. 31 (1), 143–178. doi:10.1183/09031936.00138707
Bayat-Chadegani, E., Fallahzadeh, H., Gh, A., Rahavi, R., and Maghsoudi, Z. (2015). The effect of pomegranate juice supplementation on muscle damage, oxidative stress and inflammation induced by exercise in healthy young men. J. Isfahan Med. Sch. 32 (320), 2464–2472.
Bloemen, K., Verstraelen, S., Van Den Heuvel, R., Witters, H., Nelissen, I., and Schoeters, G. (2007). The allergic cascade: Review of the most important molecules in the asthmatic lung. Immunol. Lett. 113 (1), 6–18. doi:10.1016/j.imlet.2007.07.010
Chandra, R., Babu, K. D., Jadhav, V. T., Jaime, A., and Silva, T. (2010). Origin, history and domestication of pomegranate. Fruit, Veg. Cereal Sci. Biotechnol. 2, 1–6.
Danesi, F., and Ferguson, L. R. (2017). Could pomegranate juice help in the control of inflammatory diseases? Nutrients 9 (9), 958. doi:10.3390/nu9090958
de Freitas Alves, C., Angeli, G. N., Favarin, D. C., Lemos de Andrade, E., Lazo Chica, J. E., Faccioli, L. H., et al. (2013). The effects of proresolution of ellagic acid in an experimental model of allergic airway inflammation. Mediat. Inflamm. 2013, 863198. doi:10.1155/2013/863198
Enright, P. L., Lebowitz, M. D., and Cockroft, D. W. (2012). Physiologic measures: Pulmonary function tests. Asthma outcome. Am. J. Respir. Crit. care Med. 149 (2), S9–S18. doi:10.1164/ajrccm/149.2_Pt_2.S9
Esmaillzadeh, A., Tahbaz, F., Gaieni, I., Alavi-Majd, H., and Azadbakht, L. (2004). Concentrated pomegranate juice improves lipid profiles in diabetic patients with hyperlipidemia. J. Med. food 7 (3), 305–308. doi:10.1089/jmf.2004.7.305
Fazlollahi, M. R., Najmi, M., Fallahnezhad, M., Sabetkish, N., Kazemnejad, A., Bidad, K., et al. (2018). The prevalence of asthma in Iranian adults: The first national survey and the most recent updates. Clin. Respir. J. 12 (5), 1872–1881. doi:10.1111/crj.12750
Gauthier, M., Ray, A., and Wenzel, S. E. (2015). Evolving concepts of asthma. Am. J. Respir. Crit. care Med. 192 (6), 660–668. doi:10.1164/rccm.201504-0763PP
Ghavipour, M., Sotoudeh, G., Tavakoli, E., Mowla, K., Hasanzadeh, J., and Mazloom, Z. (2017). Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in Rheumatoid Arthritis patients. Eur. J. Clin. Nutr. 71 (1), 92–96. doi:10.1038/ejcn.2016.151
Giménez-Bastida, J. A., Ávila-Gálvez, M. Á., Espín, J. C., and González-Sarrías, A. (2021). Evidence for health properties of pomegranate juices and extracts beyond nutrition: A critical systematic review of human studies. Trends Food Sci. Technol. 114, 410–423. doi:10.1016/j.tifs.2021.06.014
Hamelmann, E. (2007). The rationale for treating allergic asthma with anti-IgE. Eur. Respir. Rev. 16 (104), 61–66. doi:10.1183/09059180.00010401
Hasankhani, H., Gharemohammadlu, R., and Esmaeily, M. (2013). Relation of patients self-efficacy with control of asthma symptoms. J. Gorgan Univ. Med. Sci. 15 (2), 70–76.
Ismail, T., Sestili, P., and Akhtar, S. (2012). Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 143 (2), 397–405. doi:10.1016/j.jep.2012.07.004
Kulkarni, A. P., Mahal, H., Kapoor, S., and Aradhya, S. (2007). In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. J. Agric. food Chem. 55 (4), 1491–1500. doi:10.1021/jf0626720
Kumari, A., Tyagi, N., Dash, D., and Singh, R. (2015). Intranasal curcumin ameliorates lipopolysaccharide-induced acute lung injury in mice. Inflammation 38 (3), 1103–1112. doi:10.1007/s10753-014-0076-y
Mason, R. J., Broaddus, V. C., Martin, T. R., King, T. E., Schraufnagel, D., Murray, J. F., et al. Murray and nadel's textbook of respiratory medicine 2015. 5th ed.
Mertens-Talcott, S. U., Jilma-Stohlawetz, P., Rios, J., Hingorani, L., and Derendorf, H. (2006). Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J. Agric. food Chem. 54 (23), 8956–8961. doi:10.1021/jf061674h
Nunes, C., Pereira, A. M., and Morais-Almeida, M. (2017). Asthma costs and social impact. Asthma Res. Pract. 3 (1), 1–11. doi:10.1186/s40733-016-0029-3
Opara, L. U., Al-Ani, M. R., and Al-Shuaibi, Y. S. (2009). Physico-chemical properties, vitamin C content, and antimicrobial properties of pomegranate fruit (Punica granatum L). Food Bioprocess Technol. 2 (3), 315–321. doi:10.1007/s11947-008-0095-5
Pelaia, G., Vatrella, A., Busceti, M. T., Gallelli, L., Calabrese, C., Terracciano, R., et al. (2015). Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediat. Inflamm. 2015, 879783. doi:10.1155/2015/879783
Raji, H., Riahi, A., Borsi, S. H., Masoumi, K., Khanjani, N., AhmadiAngali, K., et al. (2020). Acute effects of air pollution on hospital admissions for asthma, COPD, and bronchiectasis in Ahvaz, Iran. Int. J. Chronic Obstr. Pulm. Dis. 15, 501–514. doi:10.2147/COPD.S231317
Rogerio, A. P., Fontanari, C., É, B., Keller, A. C., Russo, M., Soares, E. G., et al. (2008). Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a murine model of asthma. Eur. J. Pharmacol. 580 (1-2), 262–270. doi:10.1016/j.ejphar.2007.10.034
Rouhi, S. Z. T., Sarker, M. M. R., Rahmat, A., Alkahtani, S. A., and Othman, F. (2017). The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic Sprague–Dawley rats. BMC complementary Altern. Med. 17 (1), 1–13. doi:10.1186/s12906-017-1667-6
Sewa, D. W., and Ong, T. H. (2014). Pulmonary function test: Spirometry. Proc. Singap. Healthc. 23 (1), 57–64. doi:10.1177/201010581402300110
Shateri, Z., Hosseini, S. A., Abolnezhadian, F., Maraghi, E., Haddadzade Shoushtari, M., and Zilaee, M. (2022). Pomegranate extract supplementation improves lung function parameters and IL-35 expression in participants with mild and moderate persistent allergic asthma: A randomized, double-blind, placebo-controlled trial. Front. Nutr. 9, 1026343. doi:10.3389/fnut.2022.1026343
Shateri, Z., Keshavarz, S. A., Hosseini, S., Chamari, M., Hosseini, M., and Nasli, E. (2016). Effect of vitamin C supplementation on blood pressure level in type 2 DiabetesMellitus: A randomized, double-blind, placebo-controlled trial. Biosci. Biotechnol. Res. Asia 13 (1), 279–286. doi:10.13005/bbra/2031
Sohrab, G., Sotoodeh, G., Siasi, F., Neiestani, T., Rahimi, A., and Chamari, M. (2008). Effect of pomegranate juice consumption on blood pressure in type 2 diabetic patients. Iran. J. Endocrinol. Metabolism 9 (4), 399–405.
Sumner, M. D., Elliott-Eller, M., Weidner, G., Daubenmier, J. J., Chew, M. H., Marlin, R., et al. (2005). Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am. J. Cardiol. 96 (6), 810–814. doi:10.1016/j.amjcard.2005.05.026
Sunyer, J., Antó, J. M., Sabrià, J., Roca, J., Morell, F., Rodríguez-Roisin, R., et al. (1995). Relationship between serum IgE and airway responsiveness in adults with asthma. J. allergy Clin. Immunol. 95 (3), 699–706. doi:10.1016/s0091-6749(95)70175-3
To, T., Stanojevic, S., Moores, G., Gershon, A. S., Bateman, E. D., Cruz, A. A., et al. (2012). Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC public health 12 (1), 204–208. doi:10.1186/1471-2458-12-204
Zhou, E., Fu, Y., Wei, Z., and Yang, Z. (2014). Inhibition of allergic airway inflammation through the blockage of NF-κB activation by ellagic acid in an ovalbumin-induced mouse asthma model. Food and Funct. 5 (9), 2106–2112. doi:10.1039/c4fo00384e
Zilaee, M., Hosseini, S. A., Jafarirad, S., Abolnezhadian, F., Cheraghian, B., Namjoyan, F., et al. (2019). An evaluation of the effects of saffron supplementation on the asthma clinical symptoms and asthma severity in patients with mild and moderate persistent allergic asthma: A double-blind, randomized placebo-controlled trial. Respir. Res. 20 (1), 39–11. doi:10.1186/s12931-019-0998-x
Keywords: allergic asthma, blood cell count, complete blood count, CBC, clinical symptoms, clinical trial, pomegranate, Punica granatum
Citation: Hosseini SA, Shateri Z, Abolnezhadian F, Maraghi E, Haddadzadeh Shoushtari M and Zilaee M (2023) Does pomegranate extract supplementation improve the clinical symptoms of patients with allergic asthma? A double-blind, randomized, placebo-controlled trial. Front. Pharmacol. 14:1109966. doi: 10.3389/fphar.2023.1109966
Received: 28 November 2022; Accepted: 10 January 2023;
Published: 25 January 2023.
Edited by:
Leming Sun, Northwestern Polytechnical University, ChinaReviewed by:
Poonam Arora, Shree Guru Gobind Singh Tricentenary University, IndiaCopyright © 2023 Hosseini, Shateri, Abolnezhadian, Maraghi, Haddadzadeh Shoushtari and Zilaee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zainab Shateri, emFpbmFic2hhdGVyaUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.