
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 15 February 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1102581
This article is part of the Research TopicCurcuminoids: Their Pleiotropism Against Hallmarks of CancerView all 8 articles
 Qingmin He1,2†
Qingmin He1,2† Chuan Liu3†
Chuan Liu3† Xiaohan Wang1,4
Xiaohan Wang1,4 Kang Rong1,4
Kang Rong1,4 Mingyang Zhu1,4
Mingyang Zhu1,4 Liying Duan1,4
Liying Duan1,4 Pengyuan Zheng1,2,4*
Pengyuan Zheng1,2,4* Yang Mi1,2,4*
Yang Mi1,2,4*Objective: Curcumin is a plant polyphenol extracted from the Chinese herb turmeric. It was found that curcumin has good anti-cancer properties in a variety of cancers, but the exact mechanism is not clear. Based on the network pharmacology and molecular docking to deeply investigate the molecular mechanism of curcumin for the treatment of colon cancer, it provides a new research direction for the treatment of colon cancer.
Methods: Curcumin-related targets were collected using PharmMapper, SwissTargetPrediction, Targetnet and SuperPred. Colon cancer related targets were obtained using OMIM, DisGeNET, GeneCards and GEO databases. Drug-disease intersection targets were obtained via Venny 2.1.0. GO and KEGG enrichment analysis of drug-disease common targets were performed using DAVID. Construct PPI network graphs of intersecting targets using STRING database as well as Cytoscape 3.9.0 and filter core targets. Molecular docking via AutoDockTools 1.5.7. The core targets were further analyzed by GEPIA, HPA, cBioPortal and TIMER databases.
Results: A total of 73 potential targets of curcumin for the treatment of colon cancer were obtained. GO function enrichment analysis yielded 256 entries, including BP(Biological Progress):166, CC(celluar component):36 and MF(Molecular Function):54. The KEGG pathway enrichment analysis yielded 34 signaling pathways, mainly involved in Metabolic pathways, Nucleotide metabolism, Nitrogen metabolism, Drug metabolism - other enzymes, Pathways in cancer,PI3K-Akt signaling pathway, etc. CDK2, HSP90AA1, AURKB, CCNA2, TYMS, CHEK1, AURKA, DNMT1, TOP2A, and TK1 were identified as core targets by Cytoscape 3.9.0. Molecular docking results showed that the binding energies of curcumin to the core targets were all less than 0 kJ-mol-1, suggesting that curcumin binds spontaneously to the core targets. These results were further validated in terms of mRNA expression levels, protein expression levels and immune infiltration.
Conclusion: Based on network pharmacology and molecular docking initially revealed that curcumin exerts its therapeutic effects on colon cancer with multi-target, multi-pathway. Curcumin may exert anticancer effects by binding to core targets. Curcumin may interfere with colon cancer cell proliferation and apoptosis by regulating signal transduction pathways such as PI3K-Akt signaling pathway,IL-17 signaling pathway, Cell cycle. This will deepen and enrich our understanding of the potential mechanism of curcumin against colon cancer and provide a theoretical basis for subsequent studies.
Colon cancer (CC) has the third highest incidence of all tumors worldwide and is a common cause of oncologic death (Bray et al., 2018). Meanwhile, the incidence and morbidity and mortality of CC are increasing rapidly. The development of CC is a long-term complex process, and although the diagnosis and treatment measures of CC have made great progress in recent years, the 5-year survival rate of CC is still less than 40%, and most CC patients develop tumor recurrence, metastasis and drug resistance (Riihimäki et al., 2016). With the development of herbal medicine in recent years, the monomeric active ingredients extracted from Chinese herbs have become a hot spot for global research. The Chinese medicinal ingredient curcumin is a biologically active substance extracted from the rhizome of turmeric, which belongs to acidic polyphenolic compounds (Zang et al., 2014). A large number of studies have shown that curcumin has good clinical application value as it inhibits tumor cell activity, reduces migration invasion ability, induces autophagy and promotes apoptosis (Doello et al., 2018; Shakeri et al., 2019). In recent years, the potential anticancer properties of curcumin have attracted more attention. However, the anti-cancer mechanism of curcumin is not fully understood.
Chinese medicine has multi-target and multi-pathway mechanisms of action for treating diseases. Therefore, it is necessary to use big data to mine all existing targets and pathways related to curcumin and CC. Network pharmacology is a method that integrates bioinformatics and pharmacology. Through data integration and computational analysis, it can systematically clarify the relationship between drugs and diseases and explore the mechanism of drug action (Boezio et al., 2017). In this study, we screened and predicted the potential targets and signaling pathways of curcumin for the treatment of CC by means of network pharmacology and molecular docking techniques, and provided scientific basis for later drug development and clinical application.
The databases involved in this study (Table 1) and the research process outline (Figure 1).
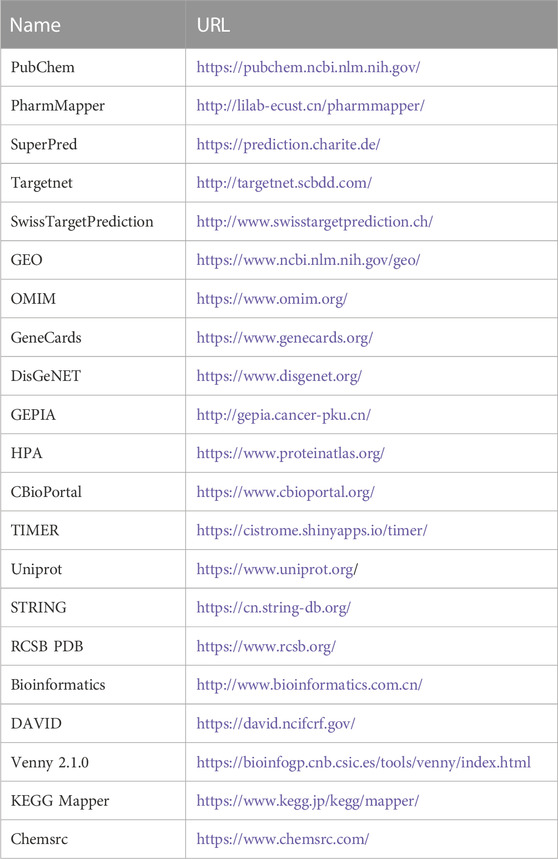
TABLE 1. Basic information of the database used for the screening of curcumin in the treatment of colon cancer.
Details of curcumin are available in PubChem (https://pubchem.ncbi.nlm.nih.gov/) (Kim et al., 2021) using CAS: 458-37-7 as the search term. Obtain the targets of curcumin using curcumin’s SDF file or curcumin’s Canonical SMILES in PharmMapper (http://lilab-ecust.cn/pharmmapper/), SwissTargetPrediction (http://www.swisstargetprediction.ch/), Targetnet (http://targetnet.scbdd.com/) and SuperPred (https://prediction.charite.de/) (Nickel et al., 2014; Yao et al., 2016; Wang et al., 2017; Daina et al., 2019). The obtained targets were translated into gene names through the UniProt database (https://www.uniprot.org/) (UniProt Consortium, 2018). Finally, the obtained target genes were combined and de-duplicated. The results were the potential targets of curcumin.
GSE74602 was selected from the GEO database (https://www.ncbi.nlm.nih.gov/geo/) as the study subject (Clough and Barrett, 2016). Differentially expressed genes were screened using GEO2R with adj.P.Val < 0.05, |logFC|>1 as screening criteria. The obtained differentially expressed genes were presented in volcano plots by TBtools (Chen et al., 2020). Some CC-related targets were obtained by searching the OMIM (https://www.omim.org/), DisGeNET (https://www.disgenet.org/), and GeneCards databases (https://www.genecards.org/) (Amberger et al., 2015; Stelzer et al., 2016; Piñero et al., 2020) using the search term “colon cancer”. The differentially expressed genes obtained from the GEO database were intersected with the CC-related targets obtained from these three databases to obtain CC disease-related targets.
The intersection of curcumin targets and CC targets was performed using Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/index.html), and the intersecting genes represented the potential targets of curcumin for CC treatment. The protein-protein interaction network of common targets was constructed using STRING database (https://cn.string-db.org/), and the tsv file was downloaded and imported into Cytoscape 3.9.0 software for visualization.
The GO and KEGG enrichment analysis of potential targets of curcumin for CC treatment was performed by “Functional Annotation” in the DAVID website (https://david.ncifcrf.gov/) (Sherman et al., 2022). The obtained data were organized and visualized by Bioinformatics (http://www.bioinformatics.com.cn/).
The drug-target-pathway network was constructed by introducing curcumin, potential targets of curcumin for CC treatment and KEGG pathway into Cytoscape. The nodes represent curcumin, genes or pathways, and the connecting lines represent the relationship of biomolecules.
In “cytohubba” of Cytoscape 3.9.0. Degree, Maximum Neighborhood Component (MNC), Maximal Clique Centrality (MCC) and Closeness were used to filter the top 15 targets respectively. The intersection of the targets obtained by these four calculation methods is the core targets.
We used the original ligand of the target protein as a positive control for subsequent molecular docking. We downloaded the SDF format file of curcumin from PubChem and the SDF format file of the original ligand from the PDB database (https://www.rcsb.org/). We convert the SDF format to mol2 format via OpenBabel-3.1.1. We import the mol2 format of the ligand into AutoDockTools 1.5.7 to set the torsion and output it as a pdbqt format file. The PDB file of the target protein is downloaded and the details of the protein are collected in the PDB database (Table 2). Proteins were dehydrated and de-liganded in PyMOL. Proteins were hydrogenated in AutoDockTools 1.5.7 and output as a file in pdbqt format. AutoDockTools 1.5.7 was restarted. The pdbqt files of the receptor and ligand are imported into AutoDockTools 1.5.7. When the docking box is constructed, the receptor protein is centered, the docking box completely covers the receptor protein and the ligand is located outside the docking box. The parameters of the docking box are collected (Table 3). Molecular docking is performed in AutoDockTools 1.5.7, and the magnitude of the binding energy reflects the possibility of binding between the receptor and ligand. The lower the binding energy, the higher the affinity between the receptor and the ligand. The lower the binding energy, the more stable the conformation of the receptor and the ligand. The binding energy of molecular docking is collected in AutoDockTools 1.5.7. The results of molecular docking are visualized in PyMOL.
In the “Expression DIY” of GEPIA (http://gepia.cancer-pku.cn/), the mRNA expression levels and pathological stages of the core targets were verified (Tang et al., 2017). |log2FC|Cutoff:1, p-value Cutoff:0.01.
To investigate the expression of core targets in CC tissues, we analyzed the core targets in the Human Protein Atlas database (https://www.proteinatlas.org/) (Uhlén et al., 2015). The protein expression levels of the core targets in CC tissues and normal colon tissues were compared.
The colon Cancer (CPTAC-2 Prospective, Cell 2019) dataset containing 110 samples was selected for analysis in cBioPortal (https://www.cbioportal.org/) (Cerami et al., 2012). Information on the genetic alterations of the core targets was obtained.
To elucidate the potential mechanisms of the immune microenvironment in CC, we entered the core targets into the TIMER database (https://cistrome.shinyapps.io/timer/) (Li et al., 2017) to explore the association between core targets and the level of immune infiltration.
A total of 448 curcumin action targets were obtained. A total of 1732 differentially expressed genes were screened from the GSE74602 dataset (Figure 2A). A total of 5102 targets were obtained by OMIM, DisGeNET and GeneCards. 1732 differentially expressed genes and 5102 targets were intersected to finally obtain 704 CC related targets.
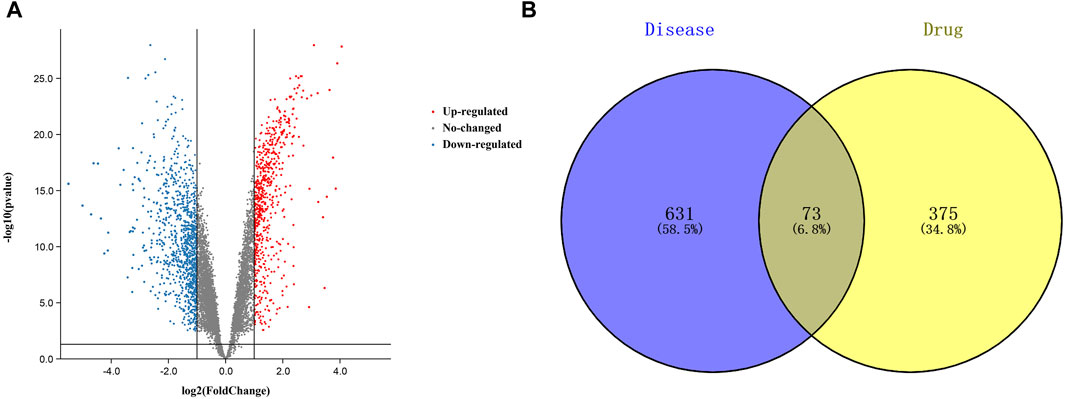
FIGURE 2. Targets relevant to the treatment of colon cancer. ((A). Volcano plot of DEGs associated with colon cancer. (B). Venn diagram showing the common part of curcumin and colon cancer).
The results of Venn diagram showed that 73 common targets were screened by matching 448 drug targets and 704 disease targets (Figure 2B). These 73 common targets were potential targets for curcumin in the treatment of CC. The 73 targets were imported into the STRING database to obtain the PPI network. The PPI network data were organized and visualized by Cytoscape 3.9.0, and 70 nodes and 230 edges were found in the PPI network. When the nodes are larger and darker, the degree of the node is larger (Figure 3A).
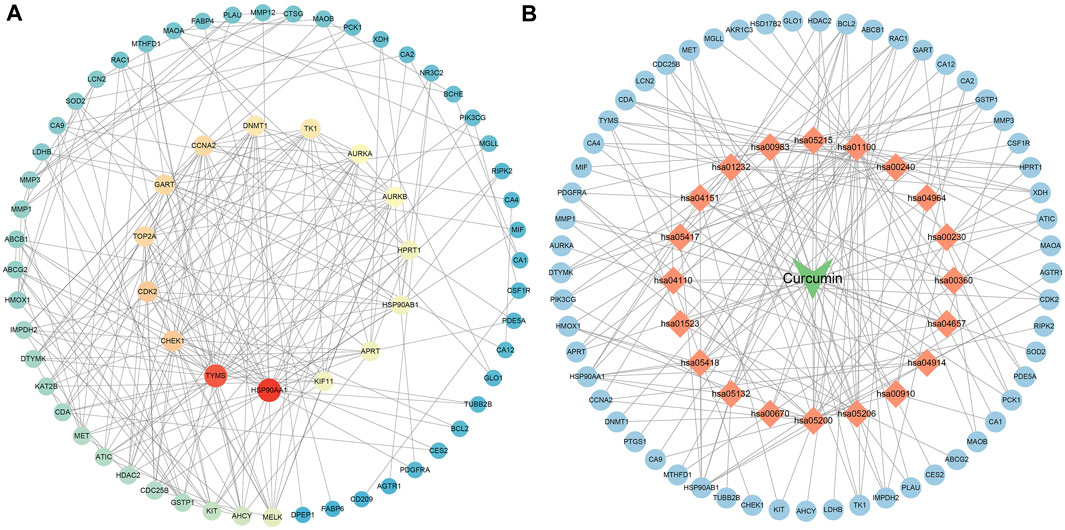
FIGURE 3. PPI network diagram ((A). PPI network of potential targets for curcumin therapy of colon cancer. (B). Drug-target-pathway network diagram. The blue circles represent targets, the orange diamonds are pathways, and the green triangles are curcumin).
73 potential targets of curcumin for CC treatment were imported into DAVID for GO and KEGG enrichment analysis. The GO enrichment analysis yielded 256 entries, including BP (Biological Progress): 166, CC(celluar component):36 and MF (Molecular Function):54. The top 6 entries of each type of analysis were selected for visualization in Bioinformatics (Figure 4A). 73 targets were involved in biological processes mainly one-carbon metabolic process,G2/M transition of mitotic cell cycle, response to drug, response to xenobiotic stimulus, inflammatory response, purine nucleotide biosynthetic process, etc. It mainly functions in the extracellular exosome, cytosol, secretory granule lumen, cell surface, membrane, ficolin-1-rich granule lumen, etc. The main molecular functions involved are protein homodimerization activity, carbonate dehydratase activity, ATP binding, hydro-lyase activity, identical protein binding, zinc ion binding, etc. KEGG pathway enrichment analysis screened 34 signaling pathways and visualized the top 20 pathways (Figure 4B), which mainly involved Metabolic pathways, Nucleotide metabolism, Nitrogen metabolism, Drug metabolism - other enzymes, Pathways in cancer, PI3K-Akt signaling pathway, etc. The PI3K-Akt signaling pathway is more important and this pathway was selected for mapping (Figure 5). The red markers in the figure represent potential targets for curcumin intervention.
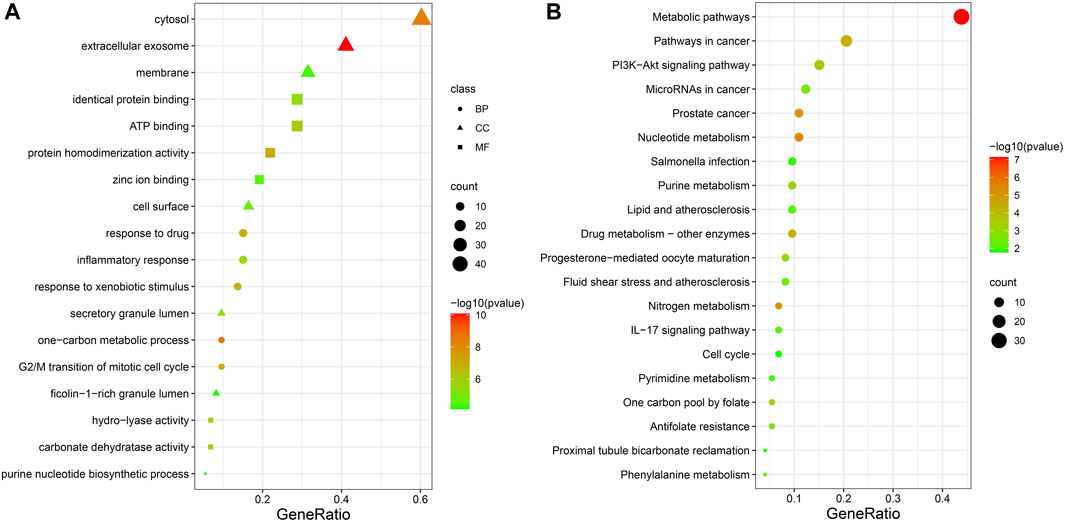
FIGURE 4. Bubble plot of enrichment analysis ((A). GO functional enrichment analysis of curcumin in colon cancer. (B). KEGG pathway enrichment analysis of curcumin in colon cancer).
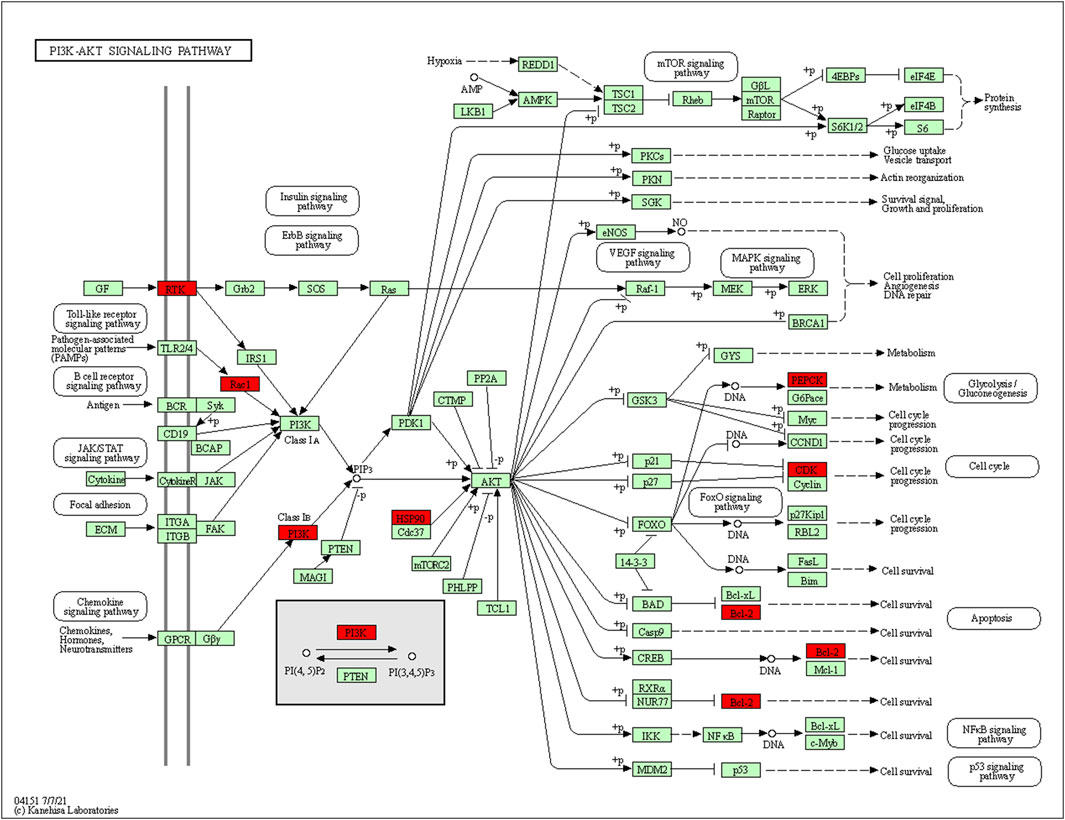
FIGURE 5. PI3K-Akt signaling pathway (red marks represent potential targets for curcumin intervention).
The top 20 KEGG pathways were imported into Cytoscape to construct a drug-target-pathway network (Figure 3B). The blue circles are targets, the orange diamonds are pathways, and the green triangles are curcumin. The results showed that curcumin exerts its effects in treating CC through multiple targets and multiple signaling pathways.
The PPI network diagram of potential targets (Figure 3A) was analyzed by CytoHubba, and 10 core targets were selected (Table 4). The molecular docking results showed that the binding energies between curcumin and the target proteins were all less than 0. Curcumin is tightly linked to amino acid residues through hydrogen bonds. The binding energy, amino acid residues and hydrogen bonds are collected (Table 5). The binding energies of curcumin to CDK2, DNMT1 and TK1 were all smaller than those of the positive control, suggesting that curcumin has a stronger binding capacity to these target proteins than the positive control. The binding energies of curcumin with CCNA2, TYMS, TOP2A, HSP90AA1, AURKB, CHEK1, and AURKA were all close to those of the positive control, which indicates that the binding ability between curcumin and these target proteins was close to that of the positive control. In summary, we can know that the results of molecular docking are plausible and true, that curcumin binds strongly to core target proteins, and that curcumin may exert anticancer effects by binding to core target proteins. The results of molecular docking are visualized (Figures 6, 7).
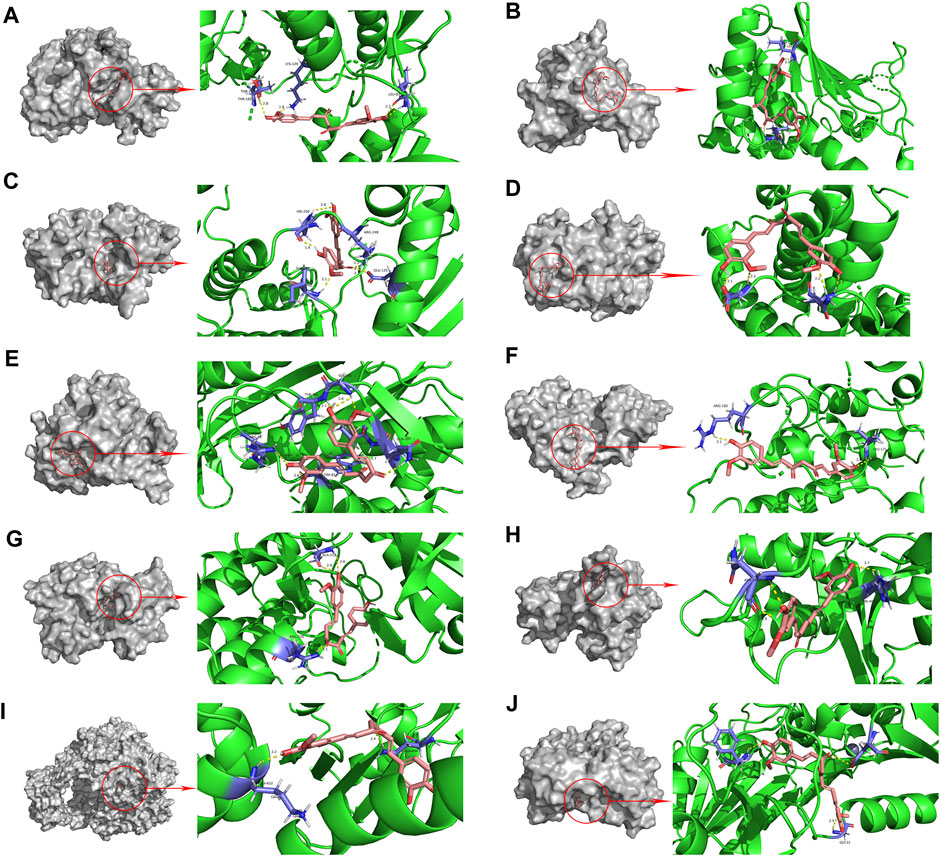
FIGURE 6. Molecular docking pattern of curcumin and core target protein. ((A). Curcumin-CDK2, (B). Curcumin-HSP90AA1, (C). Curcumin-AURKB, (D). Curcumin-CCNA2, (E). Curcumin-TYMS, (F). Curcumin-CHEK1, (G). Curcumin-AURKA, (H). Curcumin-DNMT1, (I). Curcumin-TOP2A, (J). Curcumin-TK1).
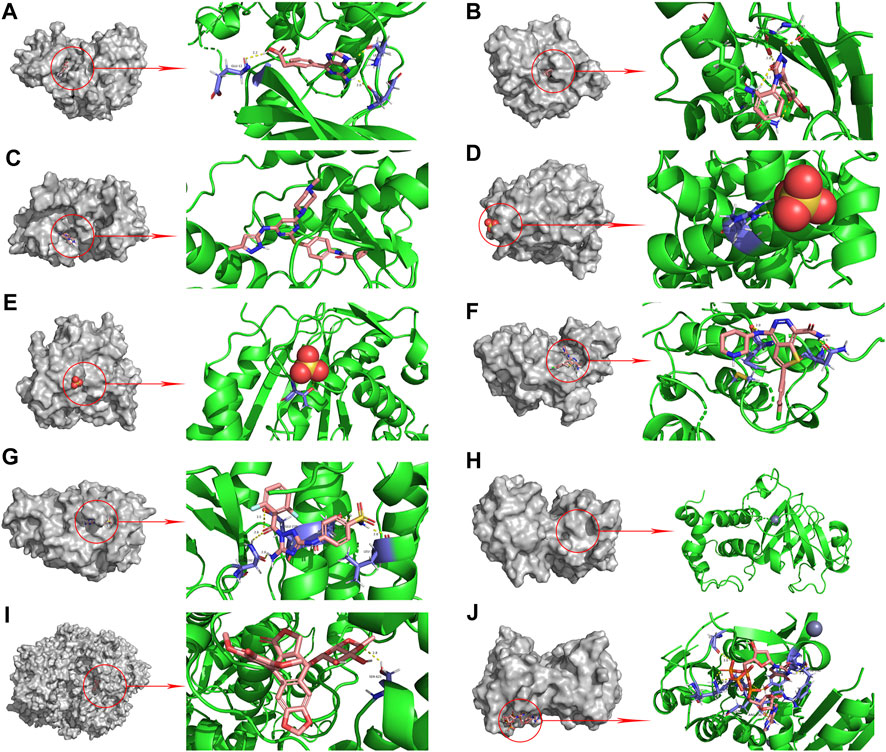
FIGURE 7. Molecular docking pattern of original ligand and core target protein. ((A). HJK-CDK2, (B). 6DL-HSP90AA1, (C). VX6-AURKB, (D). SO4-CCNA2, (E). SO4-TYMS, (F). C70-CHEK1, (G). SKE-AURKA, (H). ZN-DNMT1, (I). EVP-TOP2A, (J). 4TA-TK1).
The expression of the core targets was different in CC tissues and normal tissues. The mRNA levels of CDK2, HSP90AA1, AURKB, CCNA2, TYMS, CHEK1, AURKA, TOP2A, and TK1 were significantly higher in CC than in normal tissues (p <0.01) (Figure 8A). In addition, we analyzed the relationship between the mRNA levels of core targets and the pathological stage of CC. The results showed that the mRNA levels of CCNA2 and TYMS significantly changed with pathological stage (p <0.01) (Figure 8B).
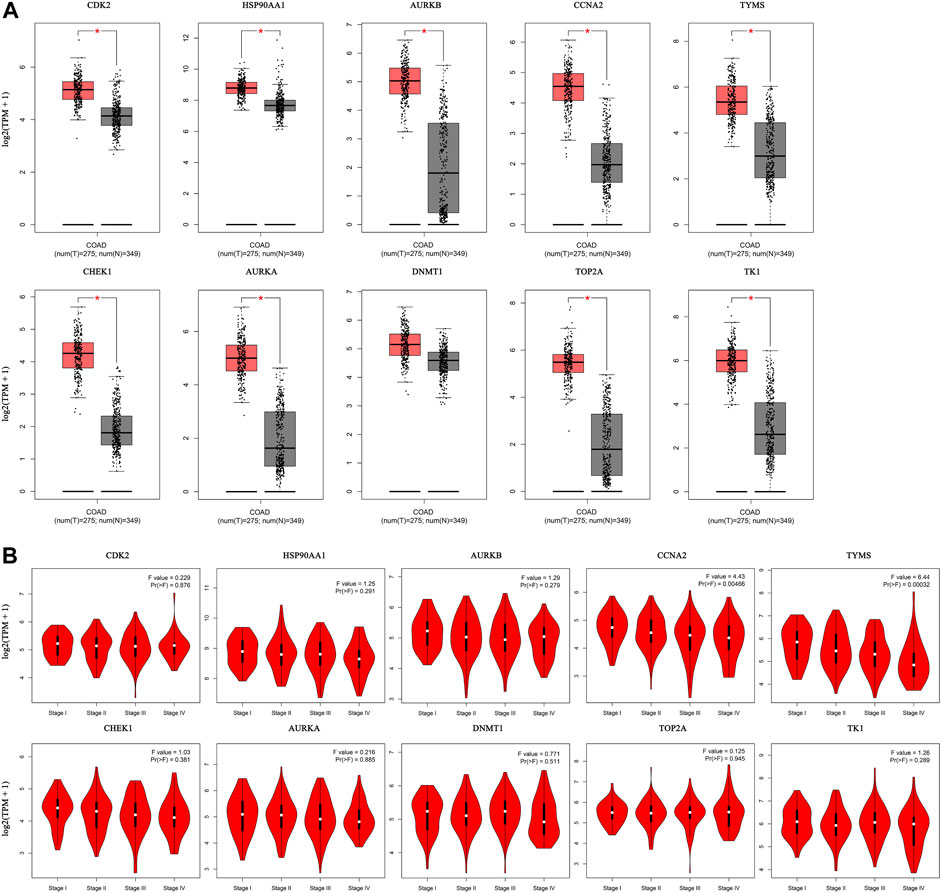
FIGURE 8. Hub gene expression in the GEPIA database. ((A). Box plot of hub gene mRNA expression levels in the GEPIA database. Red represents tumor tissues and gray represents normal tissues. (B). Stage diagram of hub gene mRNA expression levels and pathological stages in the GEPIA database).
Immunohistochemical staining images in the HPA database were analyzed to observe the expression levels of core target proteins in CC. We found elevated expression levels of CDK2, HSP90AA1, AURKB, CCNA2, TYMS, AURKA, DNMT1, TOP2A, and TK1 in CC tissues compared with normal colon tissues (Figure 9). No immunohistochemical data for CHEK1 were found in the HPA database.
We found that 57 of 110 CC patients (52%) had genetic mutations in these targets (Figure 10A). We found a positive correlation between protein expression and mRNA levels of the core targets (Figure 10B), and no correlation data were found for CCNA2 and CHEK1.
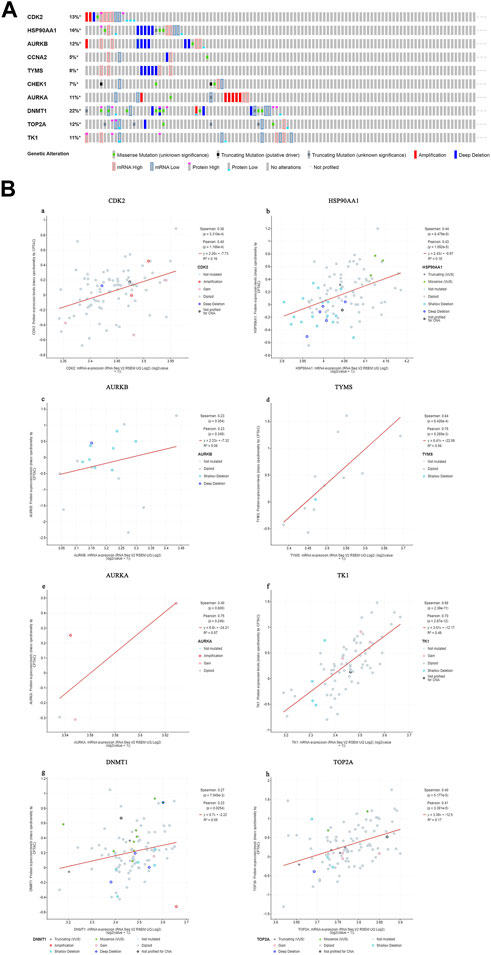
FIGURE 10. Genetic information of hub targets. ((A). Data showed that 57 of 110 patients (52%) had genetic mutations in these targets. (B). The diagram shows the correlation between the mRNA and protein levels of (a) CDK2, (b) HSP90AA1, (c) AURKB, (d) TYMS, (e) AURKA, (f) TK1, (g) DNMT1, (h) TOP2A).
The relationship between core targets and immune cell infiltration was analyzed. The results showed that the expression of HSP90AA1 was positively correlated with the infiltration of B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils and dendritic cells. The expression of HSP90AA1 was negatively correlated with purity. The expression of AURKB and TK1 were positively correlated with infiltration of purity, B cells, neutrophils and dendritic cells. The expression of AURKB and TK1 were negatively correlated with infiltration of CD8+ T cells, CD4+ T cells and macrophages. The expression of CCNA2 was positively correlated with infiltration of purity, B cells, CD8+ T cells, macrophages, neutrophils, and dendritic cells. The expression of CCNA2 was negatively correlated with infiltration of CD4+ T cells. The expression of TYMS was positively correlated with infiltration of B cells, CD8+ T cells, macrophages, neutrophils and dendritic cells. The expression of TYMS was negatively correlated with infiltration of purity and CD4+ T cells. The expression of AURKA was positively correlated with infiltration of purity, B cells, CD8+ T cells, CD4+ T cells, macrophages and neutrophils. The expression of AURKA was negatively correlated with infiltration of dendritic cells. The expression of DNMT1 was positively correlated with infiltration of purity, B cells, CD4+ T cells, macrophages, neutrophils and dendritic cells. The expression of DNMT1 was negatively correlated with infiltration of CD8+ T cells. The expression of CDK2, CHEK1 and TOP2A were positively correlated with the infiltration of purity, B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils and dendritic cells (Figure 11). We analyzed the clinical significance of core targets and immune cell infiltration in CC using a Cox proportional risk model. The results showed that age, CD8+ T cells, CDK2 and CCNA2 were significantly associated with clinical outcomes in patients with CC (Table 6).
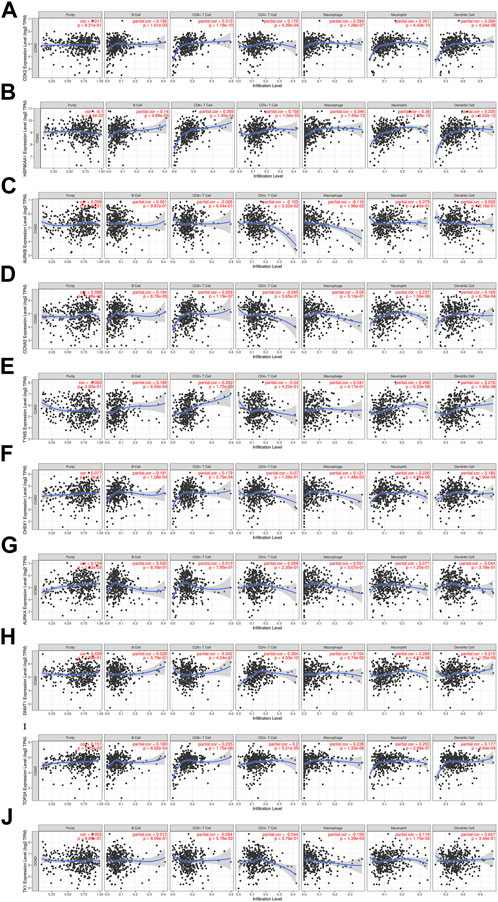
FIGURE 11. Relationship between differentially expressed core targets and immune cell infiltration. ((A). CDK2, (B). HSP90AA1, (C). AURKB, (D). CCNA2, (E). TYMS, (F). CHEK1, (G). AURKA, (H). DNMT1, (I). TOP2A, (J). TK1).
CC is a common malignant tumor of the gastrointestinal tract, and most patients have already metastasized at the time of diagnosis (Liu et al., 2019; Taieb and Gallois, 2020), and it is easy to recur even through surgical treatment. Although chemotherapeutic drugs have obvious anti-tumor effects, they can also cause serious adverse effects while killing tumor cells. Curcumin, the active ingredient of turmeric, has been shown to have strong antitumor effects in clinical trials against cancers such as liver, colon, and breast cancers (Lee et al., 2009). However, the mechanism of action of traditional Chinese medicine for disease treatment is multiple targets and multiple pathways, so we need to apply big data to explore the targets and pathways of curcumin and CC. The aim of this study was to explore the potential molecular mechanism of the inhibitory effect of curcumin on CC using network pharmacology combined with bioinformatics, and to provide some theoretical basis for the clinical application of curcumin and the study of CC.
According to the GO enrichment results, curcumin mainly acts in the extracellular exosome, cytosol, secretory granule lumen, cell surface, membrane,ficolin-1-rich granule lumen and other sites. The molecular functions involved are protein homodimerization activity, carbonate dehydratase activity, ATP binding,hydro-lyase activity, identical protein binding, zinc ion binding, etc. In addition, we found that curcumin exerts its effect on the treatment of CC by affecting biological processes such as one-carbon metabolic process,G2/M transition of mitotic cell cycle, response to drug, response to xenobiotic stimulus, inflammatory response, purine nucleotide biosynthetic process, etc.
The KEGG enrichment results showed that many disease pathways that were not relevant to this study were enriched, probably because the same molecular targets exist in the development of different diseases. So we selected signaling pathways that are closely related to CC for analysis. We found that the therapeutic effect of curcumin on CC may be produced by regulating PI3K-Akt signaling pathway, IL-17 signaling pathway and Cell cycle. PI3K-Akt signaling pathway is closely related to the progression of many cancers. During tumor progression, the PI3K-Akt signaling pathway can be activated by multiple types of cellular stimulation or toxic injury, regulating essential cellular functions such as transcription, translation, proliferation, growth and survival. Binding of growth factors to their receptor tyrosine kinase (RTK) stimulates the class la Pl3K subtype, and binding of chemokines, hormones and neurotransmitters to G protein-coupled receptors (GPCR) stimulates the class lb Pl3K subtype. PI3K catalyzes the production of phosphatidylinositol-3,4,5-trisphosphate (PIP3) in cell membranes. PIP3 acts as a second messenger and helps to activate Akt. Akt can regulate a number of key cellular processes by phosphorylating substrates for apoptosis, protein synthesis, metabolism and the cell cycle, promoting cancer cell growth and survival (Chang et al., 2003; Lee et al., 2008).
The interleukin 17 (IL-17) family is a subgroup of cytokines consisting of IL-17A-F that plays a key role in both acute and chronic inflammatory responses. Studies have shown that when IL-17 signaling pathway expression is inhibited, the number of colorectal tumors is reduced and cancer cells have a reduced ability to proliferate (Pan et al., 2022). Cell cycle regulation is inextricably linked to apoptosis. Cell cycle arrest can occur when the cell cycle is depleted or when DNA damage is severe. When cell cycle arrest is irreversible, cells initiate the apoptotic program and apoptosis occurs. Studies have shown that cell cycle arrest can be induced in human CC cells by elevating the expression of cell cycle inhibitory proteins and decreasing the expression of cell cycle progressive proteins, producing an anti-cancer effect (Choi et al., 2019).
The targets of curcumin and CC were taken to intersect and 73 potential targets of curcumin for the treatment of CC were obtained. The top 10 core targets (CDK2, HSP90AA1, AURKB, CCNA2, TYMS, CHEK1, AURKA, DNMT1, TOP2A, and TK1) were further screened. CDK2 is a central factor in the oncogenic signaling pathway and has an important role in the tumor process. When CDK2 is inhibited, cancer cells undergo apoptosis and growth arrest (Barrière et al., 2007). HSP90AA1 is a molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved in cell cycle control and signal transduction. In colorectal cancer, there is a positive correlation between high expression of HSP90AA1 and poorer prognosis of patients (Zhang et al., 2019). Studies have shown that HSP90AA1 enhances the proliferation and invasion of tumor cells, further worsening the disease, and that HSP90AA1 may be a potential target for the treatment of cancer (Wu et al., 2017; Tian et al., 2019).
AURKB is involved in the bipolar attachment of spindle microtubules to kinetochores and is a key regulator of cytokinesis onset during mitosis. Histone H3 on serine 10 and serine 28 can be phosphorylated by AURKB, which is associated with chromosome number stability and chromatin condensation during mitosis (Goldenson and Crispino, 2015). AURKB is highly expressed in tumors, and AURKB overexpression is associated with poor prognosis (Tanaka et al., 2008; Hegyi et al., 2012). CCNA2 is a cell cycle protein that controls the cell cycle by forming specific protein kinase complexes with protein kinases. Overexpression of CCNA2 is associated with poorer OS and DFS in pancreatic ductal adenocarcinoma, and CCNA2 overexpression is associated with disease progression in pancreatic ductal adenocarcinoma (Dong et al., 2019).
TYMS is highly expressed in patients with colorectal cancer and non-small cell lung cancer, and patients with lower TYMS mRNA levels have higher survival rates than those with higher expression (Sun et al., 2015; Jiang et al., 2019). CHEK1 has some anti-apoptotic ability, and a positive correlation between CHEK1 overexpression and tumor malignancy and poorer prognosis has been noted in colorectal cancer (Gali-Muhtasib et al., 2008). AURKA, an oncogene, is highly expressed in cancer patients (Umene et al., 2013; Kivinummi et al., 2017). AURKA exerts its cancer-inducing effects through the Wnt and MAPK signaling pathways (Jacobsen et al., 2018). During DNA replication, DNMT1 is a DNA methyltransferase responsible for maintaining the methylation state of DNA. DNMT1 is highly expressed in cancer (Hino et al., 2009; Wu et al., 2011), and inhibition of DNMT1 slows the progression of cancer (Sun et al., 2017; Wang and Li, 2017; Han et al., 2018).
TOP2A is a key enzyme that alters the topology of DNA by binding to double-stranded DNA molecules. The proliferation and invasion of CC cells can be inhibited and apoptosis can be induced by down-regulating the expression of TOP2A (Zhang et al., 2018). TK1 is a cell cycle regulatory enzyme that plays an important role in nucleotide metabolism. It has been found that TK1 expression is high in cancer patients and high serum TK1 levels are usually associated with cancer stage and increased tumor size (Wu et al., 2013). Serum TK1 expression has been used as a prognostic tool to monitor response to chemotherapy or surgery (Zhang et al., 2006).
The molecular docking results showed that curcumin spontaneously bound to core target proteins. It suggested that curcumin could regulate the biological activity of CC-related targets. The reliability of the core targets of curcumin for CC treatment screened by network pharmacology was verified.
In summary, this study systematically illustrated the potential mechanism of curcumin for the treatment of CC through network pharmacology and molecular docking. Curcumin plays an important role in the treatment of CC through multiple targets and pathways after entering the body. The results showed that curcumin could exert anti-cancer effects by binding to CDK2, HSP90AA1, AURKB, CCNA2, TYMS, CHEK1, AURKA, DNMT1, TOP2A, and TK1. Curcumin interferes with tumor cell proliferation and apoptosis by regulating PI3K-Akt signaling pathway,IL-17 signaling pathway, Cell cycle and other signal transduction pathways. These reflect the anti-CC mechanism of curcumin. Due to the poor accuracy and completeness of the database during the network pharmacology study, biological experiments and extensive evidence-based medical validation are still needed at a later stage to ensure the reliability of the study results. Our study provides a new basis for further exploration of the role of curcumin in the treatment of CC and subsequent experimental validation.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Authors and apos; contributions (I) Conception and design: QH and CL; (II) Administrative support: YM and PZ; (III) Provision of study materials or patients: QH and CL; (IV) Collection and assembly of data: QH, CL, XW, KR, MZ, and LD; (V) Data analysis and interpretation: QH and CL; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
The National Natural Science Foundation of China (No. 81970491) funded this manuscript.
The authors would like to thank editors and the reviewers for their valuable comments and suggestions to improve the quality of the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amberger, J. S., Bocchini, C. A., Schiettecatte, F., Scott, A. F., and Hamosh, A. (2015). OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 43, D789–D798. doi:10.1093/nar/gku1205
Barrière, C., Santamaría, D., Cerqueira, A., Galán, J., Martín, A., Ortega, S., et al. (2007). Mice thrive without Cdk4 and Cdk2. Mol. Oncol. 1, 72–83. doi:10.1016/j.molonc.2007.03.001
Boezio, B., Audouze, K., Ducrot, P., and Taboureau, O. (2017). Network-based approaches in pharmacology. Mol. Inf. 36, 1700048. doi:10.1002/minf.201700048
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi:10.3322/caac.21492
Cerami, E., Gao, J., Dogrusoz, U., Gross, B. E., Sumer, S. O., Aksoy, B. A., et al. (2012). The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404. doi:10.1158/2159-8290.Cd-12-0095
Chang, F., Lee, J. T., Navolanic, P. M., Steelman, L. S., Shelton, J. G., Blalock, W. L., et al. (2003). Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia 17, 590–603. doi:10.1038/sj.leu.2402824
Chen, C., Chen, H., Zhang, Y., Thomas, H. R., Frank, M. H., He, Y., et al. (2020). TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13, 1194–1202. doi:10.1016/j.molp.2020.06.009
Choi, Y. J., Lee, C. M., Park, S. H., and Nam, M. J. (2019). Esculetin induces cell cycle arrest and apoptosis in human colon cancer LoVo cells. Environ. Toxicol. 34, 1129–1136. doi:10.1002/tox.22815
Clough, E., and Barrett, T. (2016). The gene expression omnibus database. Methods Mol. Biol. 1418, 93–110. doi:10.1007/978-1-4939-3578-9_5
Daina, A., Michielin, O., and Zoete, V. (2019). SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 47, W357–w364. doi:10.1093/nar/gkz382
Doello, K., Ortiz, R., Alvarez, P. J., Melguizo, C., Cabeza, L., and Prados, J. (2018). Latest in vitro and in vivo assay, clinical trials and patents in cancer treatment using curcumin: A literature review. Nutr. Cancer 70, 569–578. doi:10.1080/01635581.2018.1464347
Dong, S., Huang, F., Zhang, H., and Chen, Q. (2019). Overexpression of BUB1B, CCNA2, CDC20, and CDK1 in tumor tissues predicts poor survival in pancreatic ductal adenocarcinoma. Biosci. Rep. 39. doi:10.1042/bsr20182306
Gali-Muhtasib, H., Kuester, D., Mawrin, C., Bajbouj, K., Diestel, A., Ocker, M., et al. (2008). Thymoquinone triggers inactivation of the stress response pathway sensor CHEK1 and contributes to apoptosis in colorectal cancer cells. Cancer Res. 68, 5609–5618. doi:10.1158/0008-5472.Can-08-0884
Goldenson, B., and Crispino, J. D. (2015). The aurora kinases in cell cycle and leukemia. Oncogene 34, 537–545. doi:10.1038/onc.2014.14
Han, G., Wei, Z., Cui, H., Zhang, W., Wei, X., Lu, Z., et al. (2018). NUSAP1 gene silencing inhibits cell proliferation, migration and invasion through inhibiting DNMT1 gene expression in human colorectal cancer. Exp. Cell Res. 367, 216–221. doi:10.1016/j.yexcr.2018.03.039
Hegyi, K., Egervári, K., Sándor, Z., and Méhes, G. (2012). Aurora kinase B expression in breast carcinoma: Cell kinetic and genetic aspects. Pathobiology 79, 314–322. doi:10.1159/000338082
Hino, R., Uozaki, H., Murakami, N., Ushiku, T., Shinozaki, A., Ishikawa, S., et al. (2009). Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res. 69, 2766–2774. doi:10.1158/0008-5472.Can-08-3070
Jacobsen, A., Bosch, L. J. W., Martens-De Kemp, S. R., Carvalho, B., Sillars-Hardebol, A. H., Dobson, R. J., et al. (2018). Aurora kinase A (AURKA) interaction with Wnt and Ras-MAPK signalling pathways in colorectal cancer. Sci. Rep. 8, 7522. doi:10.1038/s41598-018-24982-z
Jiang, H., Li, B., Wang, F., Ma, C., and Hao, T. (2019). Expression of ERCC1 and TYMS in colorectal cancer patients and the predictive value of chemotherapy efficacy. Oncol. Lett. 18, 1157–1162. doi:10.3892/ol.2019.10395
Kim, S., Chen, J., Cheng, T., Gindulyte, A., He, J., He, S., et al. (2021). PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 49, D1388–d1395. doi:10.1093/nar/gkaa971
Kivinummi, K., Urbanucci, A., Leinonen, K., Tammela, T. L. J., Annala, M., Isaacs, W. B., et al. (2017). The expression of AURKA is androgen regulated in castration-resistant prostate cancer. Sci. Rep. 7, 17978. doi:10.1038/s41598-017-18210-3
Lee, J. T., Lehmann, B. D., Terrian, D. M., Chappell, W. H., Stivala, F., Libra, M., et al. (2008). Targeting prostate cancer based on signal transduction and cell cycle pathways. Cell Cycle 7, 1745–1762. doi:10.4161/cc.7.12.6166
Lee, Y. K., Park, S. Y., Kim, Y. M., and Park, O. J. (2009). Regulatory effect of the AMPK-COX-2 signaling pathway in curcumin-induced apoptosis in HT-29 colon cancer cells. Ann. N. Y. Acad. Sci. 1171, 489–494. doi:10.1111/j.1749-6632.2009.04699.x
Li, T., Fan, J., Wang, B., Traugh, N., Chen, Q., Liu, J. S., et al. (2017). Timer: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 77, e108–e110. doi:10.1158/0008-5472.Can-17-0307
Liu, Q., Luo, D., An, H., Zhang, S., Cai, S., Li, Q., et al. (2019). Survival benefit of adjuvant chemotherapy for patients with poorly differentiated stage IIA colon cancer. J. Cancer 10, 1209–1215. doi:10.7150/jca.28917
Nickel, J., Gohlke, B. O., Erehman, J., Banerjee, P., Rong, W. W., Goede, A., et al. (2014). SuperPred: Update on drug classification and target prediction. Nucleic Acids Res. 42, W26–W31. doi:10.1093/nar/gku477
Pan, D., Huang, B., Gan, Y., Gao, C., Liu, Y., and Tang, Z. (2022). Phycocyanin ameliorates colitis-associated colorectal cancer by regulating the gut microbiota and the IL-17 signaling pathway. Mar. Drugs 20, 260. doi:10.3390/md20040260
Piñero, J., Ramírez-Anguita, J. M., Saüch-Pitarch, J., Ronzano, F., Centeno, E., Sanz, F., et al. (2020). The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 48, D845–d855. doi:10.1093/nar/gkz1021
Riihimäki, M., Hemminki, A., Sundquist, J., and Hemminki, K. (2016). Patterns of metastasis in colon and rectal cancer. Sci. Rep. 6, 29765. doi:10.1038/srep29765
Shakeri, A., Cicero, A. F. G., Panahi, Y., Mohajeri, M., and Sahebkar, A. (2019). Curcumin: A naturally occurring autophagy modulator. J. Cell Physiol. 234, 5643–5654. doi:10.1002/jcp.27404
Sherman, B. T., Hao, M., Qiu, J., Jiao, X., Baseler, M. W., Lane, H. C., et al. (2022). David: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50, W216–W221. doi:10.1093/nar/gkac194
Stelzer, G., Rosen, N., Plaschkes, I., Zimmerman, S., Twik, M., Fishilevich, S., et al. (2016). The GeneCards suite: From gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinforma. 54, 1.30.131–1.30.3331. doi:10.1002/cpbi.5
Sun, J., Tian, X., Zhang, J., Huang, Y., Lin, X., Chen, L., et al. (2017). Regulation of human glioma cell apoptosis and invasion by miR-152-3p through targeting DNMT1 and regulating NF2: MiR-152-3p regulate glioma cell apoptosis and invasion. J. Exp. Clin. Cancer Res. 36, 100. doi:10.1186/s13046-017-0567-4
Sun, S., Shi, W., Wu, Z., Zhang, G., Yang, B. O., and Jiao, S. (2015). Prognostic significance of the mRNA expression of ERCC1, RRM1, TUBB3 and TYMS genes in patients with non-small cell lung cancer. Exp. Ther. Med. 10, 937–941. doi:10.3892/etm.2015.2636
Taieb, J., and Gallois, C. (2020). Adjuvant chemotherapy for stage III colon cancer. Cancers (Basel) 12, 2679. doi:10.3390/cancers12092679
Tanaka, S., Arii, S., Yasen, M., Mogushi, K., Su, N. T., Zhao, C., et al. (2008). Aurora kinase B is a predictive factor for the aggressive recurrence of hepatocellular carcinoma after curative hepatectomy. Br. J. Surg. 95, 611–619. doi:10.1002/bjs.6011
Tang, Z., Li, C., Kang, B., Gao, G., Li, C., and Zhang, Z. (2017). Gepia: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45, W98–w102. doi:10.1093/nar/gkx247
Tian, Y., Wang, C., Chen, S., Liu, J., Fu, Y., and Luo, Y. (2019). Extracellular Hsp90α and clusterin synergistically promote breast cancer epithelial-to-mesenchymal transition and metastasis via LRP1. J. Cell Sci. 132, jcs228213. doi:10.1242/jcs.228213
Uhlén, M., Fagerberg, L., Hallström, B. M., Lindskog, C., Oksvold, P., Mardinoglu, A., et al. (2015). Proteomics. Tissue-based map of the human proteome. Science 347, 1260419. doi:10.1126/science.1260419
Umene, K., Banno, K., Kisu, I., Yanokura, M., Nogami, Y., Tsuji, K., et al. (2013). Aurora kinase inhibitors: Potential molecular-targeted drugs for gynecologic malignant tumors. Biomed. Rep. 1, 335–340. doi:10.3892/br.2013.91
Uniprot Consortium, T. (2018). UniProt: The universal protein knowledgebase. Nucleic Acids Res. 46, 2699. doi:10.1093/nar/gky092
Wang, X., and Li, B. (2017). DNMT1 regulates human endometrial carcinoma cell proliferation. Onco Targets Ther. 10, 1865–1873. doi:10.2147/ott.S130022
Wang, X., Shen, Y., Wang, S., Li, S., Zhang, W., Liu, X., et al. (2017). PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 45, W356–w360. doi:10.1093/nar/gkx374
Wu, B. J., Li, W. P., Qian, C., Ding, W., Zhou, Z. W., and Jiang, H. (2013). Increased serum level of thymidine kinase 1 correlates with metastatic site in patients with malignant melanoma. Tumour Biol. 34, 643–648. doi:10.1007/s13277-012-0591-0
Wu, C. T., Wu, C. F., Lu, C. H., Lin, C. C., Chen, W. C., Lin, P. Y., et al. (2011). Expression and function role of DNA methyltransferase 1 in human bladder cancer. Cancer 117, 5221–5233. doi:10.1002/cncr.26150
Wu, J., Liu, T., Rios, Z., Mei, Q., Lin, X., and Cao, S. (2017). Heat shock proteins and cancer. Trends Pharmacol. Sci. 38, 226–256. doi:10.1016/j.tips.2016.11.009
Yao, Z. J., Dong, J., Che, Y. J., Zhu, M. F., Wen, M., Wang, N. N., et al. (2016). TargetNet: A web service for predicting potential drug-target interaction profiling via multi-target SAR models. J. Comput. Aided Mol. Des. 30, 413–424. doi:10.1007/s10822-016-9915-2
Zang, S., Liu, T., Shi, J., and Qiao, L. (2014). Curcumin: A promising agent targeting cancer stem cells. Anticancer Agents Med. Chem. 14, 787–792. doi:10.2174/1871520614666140521114735
Zhang, J., Jia, Q., Zou, S., Zhang, P., Zhang, X., Skog, S., et al. (2006). Thymidine kinase 1: A proliferation marker for determining prognosis and monitoring the surgical outcome of primary bladder carcinoma patients. Oncol. Rep. 15, 455–461. doi:10.3892/or.15.2.455
Zhang, R., Xu, J., Zhao, J., and Bai, J. H. (2018). Proliferation and invasion of colon cancer cells are suppressed by knockdown of TOP2A. J. Cell Biochem. 119, 7256–7263. doi:10.1002/jcb.26916
Keywords: molecular docking, network pharmacology, curcumin, colon cancer, immune infiltration, molecular mechanism
Citation: He Q, Liu C, Wang X, Rong K, Zhu M, Duan L, Zheng P and Mi Y (2023) Exploring the mechanism of curcumin in the treatment of colon cancer based on network pharmacology and molecular docking. Front. Pharmacol. 14:1102581. doi: 10.3389/fphar.2023.1102581
Received: 19 November 2022; Accepted: 07 February 2023;
Published: 15 February 2023.
Edited by:
Daniel Loic Pouliquen, Inserm UMR 1307 (CRCI2NA), ICO., FranceReviewed by:
Salman Akhtar, Integral University, IndiaCopyright © 2023 He, Liu, Wang, Rong, Zhu, Duan, Zheng and Mi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengyuan Zheng, cHl6aGVuZ0B6enUuZWR1LmNu; Yang Mi, eWFuZ21pMTk4QHp6dS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.