
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 30 January 2023
Sec. Drugs Outcomes Research and Policies
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1097238
This article is part of the Research TopicEffective Methods to Promote Appropriate Use of MedicinesView all 16 articles
Background: Little research addressed deprescribing-focused medication optimization interventions while utilizing implementation science. This study aimed to develop a pharmacist-led medication review service with a deprescribing focus in a care facility serving patients of low income receiving medications for free in Lebanon followed by an assessment of the recommendations’ acceptance by prescribing physicians. As a secondary aim, the study evaluates the impact of this intervention on satisfaction compared to satisfaction associated with receiving routine care.
Methods: The Consolidated Framework for Implementation Research (CFIR) was used to address implementation barriers and facilitators by mapping its constructs to the intervention implementation determinants at the study site. After filling medications and receiving routine pharmacy service at the facility, patients 65 years or older and taking 5 or more medications, were assigned into two groups. Both groups of patients received the intervention. Patient satisfaction was assessed right after receiving the intervention (intervention group) or just before the intervention (control group). The intervention consisted of an assessment of patient medication profiles before addressing recommendations with attending physicians at the facility. Patient satisfaction with the service was assessed using a validated translated version of the Medication Management Patient Satisfaction Survey (MMPSS). Descriptive statistics provided data on drug-related problems, the nature and the number of recommendations as well as physicians’ responses to recommendations. Independent sample t-tests were used to assess the intervention’s impact on patient satisfaction.
Results: Of 157 patients meeting the inclusion criteria, 143 patients were enrolled: 72 in the control group and 71 in the experimental group. Of 143 patients, 83% presented drug-related problems (DRPs). Further, 66% of the screened DRPs met the STOPP/START criteria (77%, and 23% respectively). The intervention pharmacist provided 221 recommendations to physicians, of which 52% were to discontinue one or more medications. Patients in the intervention group showed significantly higher satisfaction compared to the ones in the control group (p < 0.001, effect size = 1.75). Of those recommendations, 30% were accepted by the physicians.
Conclusion: Patients showed significantly higher satisfaction with the intervention they received compared to routine care. Future work should assess how specific CFIR constructs contribute to the outcomes of deprescribing-focused interventions.
Polypharmacy, commonly known as the concomitant daily uptake of five or more medications (Sloane and Zimmerman, 2018; Zechmann et al., 2020), is prevalent among older adults (Khera et al., 2019; Vasilevskis et al., 2019). With some chronic conditions, the use of multiple medications is essential for the improvement of a patient’s health, making polypharmacy appropriate (Duncan et al., 2017; Mair and Fernandez-Llimos, 2017; Halli-Tierny A et al., 2019). In other circumstances, drug-related problems (DRPs) may occur. According to the Pharmaceutical Care Network Europe (PCNE), a DRP is “an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes” (Pharmaceutical Care Network Europe, 2003). When DRPs occur while a patient is using multiple medications, the potential detriment of medications may exceed their projected benefit, deeming polypharmacy inappropriate (Chau et al., 2016; Duncan et al., 2017; Martin et al., 2018). Such DRPs are more likely to occur with Potentially Inappropriate Medications (PIMs). The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication (PIM) use in older adults is an explicit list of PIMs that should be avoided among older adults and patients with certain diseases, prescribed at a lower dose or with caution (Samuel, 2015). When a decision is made to prescribe them, they should be carefully monitored. Beers Criteria PIMs have been found to be associated with poor health outcomes, including confusion, falls, and mortality (Fick et al., 2006; Stockl et al., 2010).
Medication review is often recommended to optimize medication use. Medication review, as an overarching term, is used to describe a review of medicines carried out when a health professional meets a patient and there is a decision to prescribe or stop a medicine following a comprehensive and structured process supported by the patient’s records (National Institute for Health and Care Excellence, 2015). Medication optimization builds on the medication review process and occurs when a patient’s medications have been “optimized” by the health professional care team and, consequently, the patient uses the regimen in an ideal manner to improve health outcomes (McFarland et al., 2021). Thus, a medication review is considered part of the plan for inappropriate therapy resolution aiming for medication optimization (de Oliveira Santos Silva et al., 2019).
One medication optimization strategy that has been gaining momentum in the past few years to address medication safety issues is deprescribing (Bleidt, 2019; Clark et al., 2020; Korenvain et al., 2020). Deprescribing is taken to mean more than simply stopping medicines and is considered to be “a planned, stepwise process, specifying the type of medication in question, detailing explicit goals, and including dose reduction and substitution” (Duncan et al., 2017). Deprescribing is the tapering of medications and has to take place to minimize medication-caused harm and improve outcomes (Bleidt, 2019; Tandun et al., 2019; Farrell et al., 2020). To that end, deprescribing has the potential not only to solve DRPs but also to minimize health costs and improve quality of life, while maintaining or even improving clinical outcomes. Studies addressing deprescribing showed a significant potential for reducing the use of potentially inappropriate medications and subsequent adverse outcomes reduction in various settings (Kojima et al., 2012; Mckean et al., 2016; Cossette et al., 2017; Kimura et al., 2017; Wouters et al., 2017; Vasilevskis et al., 2019; Balsom et al., 2020; Dharmarajan et al., 2020; Kua et al., 2020; Schapira et al., 2020; McCarthy et al., 2022). Deprescribing-focused interventions showed promising results in the available literature. Studies addressing deprescribing in the hospital setting showed a significant decrease in PIMs use among older adults (Mckean et al., 2016; Cossette et al., 2017; Kimura et al., 2017; Vasilevskis et al., 2019; Schapira et al., 2020). Similarly, with five studies conducted in nursing homes, physicians’ or pharmacists-directed reviews led to PIMs and subsequent adverse outcomes reduction (Kojima et al., 2012; Wouters et al., 2017; Balsom et al., 2020; Dharmarajan et al., 2020; Kua et al., 2020). A recent intervention focusing on general practitioners in primary care showed a significant decrease in medications they originally prescribed to patients reducing unnecessary medication use by patients (McCarthy et al., 2022). Deprescribing of specific medications has been investigated in earlier interventions. Many of those showed positive outcomes as those targeting benzodiazepines, antidepressants, antidiabetics, anticholinergics, proton pump inhibitors, and non-steroidal anti-inflammatory medications (Reeve et al., 2017; Maund et al., 2019; Tandun et al., 2019; Martinez et al., 2020; Rashid et al., 2020); while deprescribing of other medications continues to be challenging. For example, the withdrawal of urate-lowering drugs was associated with the recurrence of gout episodes (Beslon et al., 2018). The discontinuation of some preventative medicines, such as warfarin, was found to cause harm (Narayan and Nishtala, 2017). Further, little evidence is known about statins’ maximum duration of use, hence a physician’s clinical judgment is needed to decide whether a patient needs to continue taking a statin (van der Ploeg et al., 2020).
To be successfully implemented in practice, and for this success to be sustainable, some issues should be addressed. Medication reviews successful in achieving medication optimization through deprescribing would involve health professionals of multiple backgrounds. Strategies and interventions that would facilitate this collaboration would include factors such as mutual acceptance and readiness of team members towards collaboration, performing as a team rather than an individual; communication strategies among clinicians and shared decision-making, and care coordination (Mustafa Sirimsi et al., 2022). Further, pharmacist-led deprescribing strategies would need to be conceptualized and guided by implementation science (IS) (Pereira et al., 2021; Ailabouni et al., 2022)—the study of the incorporation of evidence-based findings into practice, to improve healthcare—(Bauer et al., 2015; Ronquillo et al., 2018). One framework that has shown promise in implementing medication regimen optimization services in practice is the Consolidated Framework for Implementation Research (CFIR) (Shoemaker et al., 2017; Baumgartner et al., 2020). The prospect of utilizing CFIR in implementing health services in low- and middle-income countries is promising (Ojo et al., 2021). A recent study applied the CFIR in medication reconciliation implementation in a Brazilian hospital indicating that available resources and communication are key constructs of influence in the implementation process (Fernandes et al., 2022). Further, Shoemaker et.al highlighted the importance of using CFIR in implementing professional services at the community pharmacy level (Shoemaker et al., 2017).
Patients’ attitude toward deprescribing was assessed in multiple studies and a positive attitude was observed in most of these studies (Tegegn et al., 2018; Kua et al., 2019; 2020; Shrestha et al., 2021). For instance, in a study performed in a resource-limited setting in Ethiopia, 82% of patients were willing to have one of their medications stopped if the physician suggested it. Assessing patient satisfaction with pharmacist-led services including medication reviews and medication optimization is increasingly investigated in research (Moczygemba et al., 2010; Kim et al., 2016; Cardosi et al., 2018; Nigussie and Edessa, 2018; Basheti et al., 2019; Jordan et al., 2021; Kabba et al., 2021; Kebede et al., 2021). A recent systematic review showed high patient satisfaction toward pharmacist-led medication review interventions (Bou Malham et al., 2021). In addition, patients showed a positive attitude toward pharmacists’ competencies and involvement in deprescribing, especially the elderly (Bužančić et al., 2021). Further, a study reported that patient satisfaction could be enhanced by deprescribing as patients felt uncomfortable with the use of multiple medications (Reeve et al., 2014). For example, review tackling patient satisfaction with the use of proton-pump inhibitors indicated that patients were more satisfied when taking PPI on-demand rather than chronically, meaning that they showed satisfaction with PPI deprescribing (Boghossian et al., 2017). To that end, assessing patient satisfaction with a pharmacy service after being introduced to a medication review service focusing on deprescribing would be helpful when disseminating and implementing such a service.
This study addresses several literature gaps. First, little research has addressed interventions focusing on deprescribing in facilities serving patients of low socioeconomic status, especially in the primary care setting (Milos et al., 2013; Cheong et al., 2018; Hailu et al., 2020). Patients of low income, a class representing a significant proportion of the global patient population, are most likely to benefit from deprescribing in multiple ways. Those include therapeutic as well as economic benefits resulting from the reduced burden of money spent on medications by institutions that subsidize those medications ensuring the sustainability of service provision to those patients. This is particularly relevant in the Lebanese healthcare system where a significant percentage of medication expenses are not covered. Second, few studies have utilized an implementation science conceptual framework to guide the implementation of interventions with a focus on deprescribing. This approach would ensure that different factors influencing implementation are being considered and incorporated as needed. Third, little work has assessed patient satisfaction related to deprescribing, a key desired outcome of deprescribing interventions that would enhance the incorporation of patient’ preferences in the care process. Finally, few studies addressed deprescribing in the Arab region and in developing countries where polypharmacy is suspected to be as high as it is in developed countries (Alsuwaidan et al., 2019; Al-Dahshan et al., 2020; Badawy et al., 2020; Abu Farha et al., 2021).
This study is guided by the Consolidated Framework for Implementation Research (CFIR), a conceptual framework developed to guide the systematic assessment of implementation contexts while addressing factors that might influence intervention implementation and effectiveness (Shoemaker et al., 2017). The CFIR includes five major domains (intervention characteristics, outer setting, inner setting, characteristics of individuals, and process) with underlying constructs that can potentially influence the implementation of interventions. Using tools such as STOPP/START criteria, this study aimed to develop a pharmacist-led medication review service with a deprescribing focus in a healthcare facility serving patients of low-income receiving medications for free in Beirut, Lebanon followed by an assessment of the recommendations’ acceptance by prescribing physicians. As a secondary aim, the study evaluates the impact of this medication review service on the satisfaction of the study participants compared to the routine care they receive.
This research project followed a prospective experimental study design. Ethical approval for the study was obtained from the Institutional Review Board (IRB) at Beirut Arab University (protocol number 2022-H-0076-P-M-0465). Written informed consent was obtained from all participants assuring that any information provided by the patients is confidential.
The research team searched for a site serving patients of low income at subsidized rates or for free in Beirut, Lebanon. This was intended to provide a dual benefit so that in addition to patient benefit from improved health outcomes, which would apply to patients on polypharmacy in different settings, the site might benefit from decreasing the economic burden of unnecessary medication use. Accordingly, a non-governmental charitable association located in Beirut, Lebanon was selected as the intervention site. The healthcare facility provides low-charge consultations and free medications to 1,000 registered patients of low income. The healthcare team consists of 28 physicians, one full-time nurse, and three assistants in addition to a pharmacist, a volunteer nurse, and assistant staffing the pharmacy. In this facility, GPs prescribe medications, but their role is mostly centered around referral to specialty physicians for patients with chronic conditions.
Following two visits by the intervention pharmacist that were intended to introduce the project to the leadership team of the healthcare facility and study the pharmacy setting, a letter was obtained from the facility manager, who is also a general practitioner at the facility, approving the study execution at the facility. The study was then introduced to all specialty physicians and general practitioners who might interact with eligible patients (a total of 17 physicians and general practitioners) to facilitate the endorsement of the project. Those physicians were candidates for following patients in our sample. The rest of the 28 physicians in the facility were of specialties not related to our study focus such as paediatricians for example. The 17 physicians were then provided with a formal letter describing the project plan. Physicians and pharmacy staff were provided with a copy of the STOPP/START criteria that were used as a key tool in the study (Cowan and Riley, 2015; O’mahony et al., 2015).
The study population consisted of patients registered within the facility. Patients were included in the study if they were 65 years or older, met the World Health Organization’s polypharmacy definition of taking five or more medications (World Health Organization, 2019), picked up their medications themselves, and were cognitively capable of participating in the study. A class of patients collects their medications at the facility while being seen by a physician practicing outside the facility. Those patients were not targeted by this work. The intervention focused on patients seen by physicians in the facility so that the clinical pharmacist researcher can follow up on medication review results with physicians practicing at the facility that the clinical pharmacist researcher can access.
The intervention was guided by the adapted CFIR (Shoemaker et al., 2017). The CFIR comprised constructs that were operationalized and adapted into those five domains as follows: 1) Proposed pharmacy service characteristics-This domain addresses relative advantage, adaptability, complexity, and cost of the medication review service; 2) Outer setting-This domain addresses external influences on the intervention’s implementation including patient needs and resources, cosmopolitanism or the level at which the pharmacy is networked with other pharmacies, peer pressure, and external policies and incentives; 3) Inner setting- This domain addresses different characteristics of the implementing facility such as structural characteristics, pharmacy culture, readiness for implementation including leadership engagement, available resources and access to knowledge and information, and the implementation climate including tension for change, compatibility, organization incentives and rewards; 4) Pharmacy staff characteristics- This domain addresses pharmacist’s and pharmacy staff’s beliefs about the intervention, self-efficacy, and other personal attributes that may affect implementation; 5) Process of implementation- This domain addresses stages of implementation for the proposed pharmacy service such as planning, engaging formally appointed internal leaders, champions and external change agents executing, reflecting and evaluating the intervention. A detailed mapping of the intervention setup to the CFIR is described in Table 1. The intervention was first tested on a convenience sample of eight patients at the facility using less than five medications. This was followed by a pilot study of 46 participants meeting inclusion criteria including the use of five or more medications. Findings from pilot study participants meeting the inclusion criteria were included in the final calculations since no changes were made to the employed methods following this step. Data collection for the pilot and the full study took place between June and September 2021.
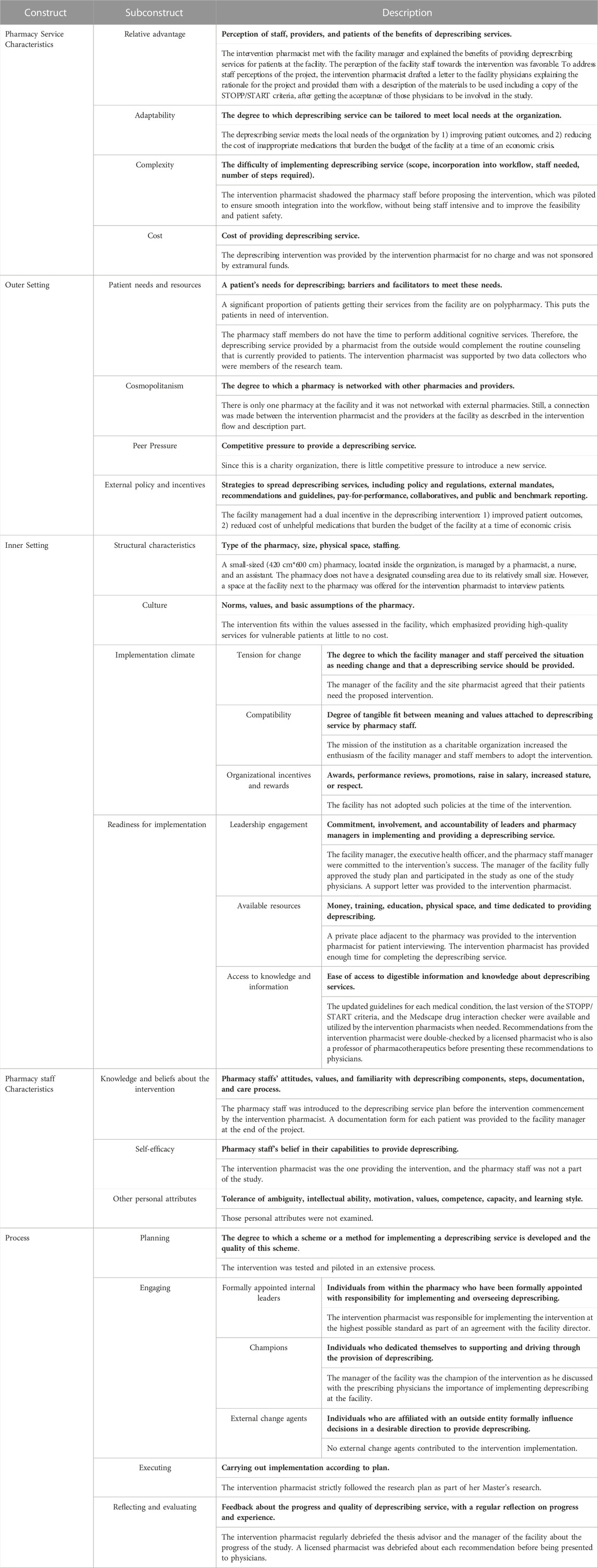
TABLE 1. The deprescribing intervention setup mapped to the adapted Consolidated Framework for Implementation Research.
Participants were approached by a trained data collector with a clinical pharmacy background after picking up their medications from the pharmacy at the facility, which included their routine interaction with the facility site pharmacist, who provides routine medication dispensing at the pharmacy. Informed patient consent was made by the intervention pharmacist or a trained data collector with a clinical pharmacy background at this point while explaining to patients that the care they receive at the facility would not be impacted in any way if they choose not to participate. Participants were divided into two groups in order of their exit from the pharmacy. The intervention group was first introduced to the study purpose of deprescribing PIMs, received disease and medication counseling, and was then administered the translated Medication Management Patient Satisfaction Survey (MMPSS) (Moon et al., 2016). The MMPSS was developed with the aim of providing a reliable and brief patient satisfaction survey specific to pharmacists providing comprehensive medication management services. It consists of ten questions, nine of which used a scale from 1 to 4 (strongly agree, agree, disagree, strongly disagree) and asks patients to evaluate their experiences with the clinical pharmacist. The final question asks patients to rate their overall quality of care and services on a Likert-scale from 1 to 5 (excellent to poor). An aggregate scale score for MMPSS is calculated by summing the score for each item in the scale. See Supplementary Appendix SA1.
On the other hand, the control group was administered the MMPSS satisfaction survey before taking the intervention. For ethical reasons, control group patients were provided with the same intervention by the same intervention pharmacist following filling out the MMPSS survey. The satisfaction survey was administered verbally by two trained data collectors.
The patient interview lasted for 15–20 min and consisted of gathering data by the intervention pharmacist addressing demographic and full medical profile information with patients. These data included underlying medical conditions, chronic and acute medication lists including non-prescription and herbal products, any problem with medication, previous medical and surgical history, and family history. Those data were used along with data from the patient’s health file at the facility to determine the current medications the patient is taking without reconciliation. Afterward, each patient’s condition, from both groups, was assessed to screen for DRPs including PIMs and potential prescribing omissions (PPOs) using the most updated version of the STOPP/START criteria as well as the newly updated clinical guidelines of relevance to each case. The STOPP/START criteria are organized according to specific physiological body systems, thereby enhancing their useability. STOPP criteria, in particular, were selected for their comprehensiveness and sensitivity in assessing PIMs compared to alternative explicit criteria (e.g., Beers criteria) (Aguiar et al., 2021). The Medscape interaction checker was utilized in the initial assessment for the compatibility of drugs while checking related guidelines as needed including those by the American College of Cardiology/American Heart Association, the European Society of Cardiology, the Global Initiative for Chronic Obstructive Lung Disease, and the American Diabetes Association (O’Gara et al., 2013; Amsterdam et al., 2014; Yancy et al., 2017; Valgimigli et al., 2018; Whelton et al., 2018; Arnett et al., 2019; Grundy et al., 2019; Vogelmeir et al., 2020; American Diabetes Association, 2021; Medscape, 2021). The clinical pharmacist researcher assigned DRPs to one of eleven categories: no or unclear indication, better alternative available, regimen needs simplification, overdosage, overuse of therapy, drug-drug interaction, presence of an adverse drug reaction or drug allergy, unsafe medication, duplication therapy, omission, regimen needs intensification (Pharmaceutical Care Network Europe, 2003; Lim et al., 2018). Later, therapeutic recommendations which include drug discontinuation, dosage or regimen adjustment, drug substitution, or new drug prescription, were double-checked with a licensed pharmacist who is also a professor of pharmacotherapeutics. Final recommendations were then presented and discussed with prescribing physicians at the intervention site. In some instances, multiple DRPs for a patient would have been solved by one proposed recommendation, so the number of DRPs and the number of recommendations were not expected to match. These recommendations were either accepted, deferred (postponed to monitor the current patient’s condition or to order laboratory testing), or rejected. The acceptance rate of DRPs was analyzed and presented according to physician specialty.
At the end of the project, a discussion was conducted with the manager of the facility to highlight all the recommendations and subsequent physicians’ responses, together with the researcher’s suggestions aimed at optimizing patient care. A copy of each patient documentation form was provided to the facility manager for the sustainability of patient care at the facility.
The primary outcome of this study was the impact of the medication optimization deprescribing focused intervention on patient satisfaction. The process of translating, adapting, and validating the Medication Management Patient Satisfaction Survey (MMPSS) into Lebanese Arabic is described in the thesis of the first author (Alaa Eddine N, 2022). A manuscript describing the process is currently under review in this journal. Secondary outcome measures were the number of changes and subcategories of changes proposed by the pharmacist including drug discontinuation, substitution, initiation, or dosage adjustment, and the proportion of changes accepted by the prescribing physicians measured as a percentage of accepted, deferred, or rejected recommendations for each physician.
Descriptive statistics were used to describe the characteristics of the sample. They also provided data on drug-related problems, the nature and the number of recommendations as well as physicians’ responses to recommendations. Descriptive analyses were done using IBM SPSS 24® generating frequencies, as well as means and ranges as relevant.
Descriptive statistics of the summary score (items 1–9) of MMPSS were computed for each experimental group to assess patient satisfaction with the service. Independent sample t-tests were used to check for the difference between control and intervention groups for the summary score of items one to nine and for item ten alone. Responses for items one to nine were coded from 0 to 3 with “strongly disagree” given a code of 0 and “strongly agree” given a code of 3. For item 10, a similar coding was followed with “poor” given a code of 0 and “excellent” given a code of 4.
A pilot study was carried out on 46 patients (23 per group) to calculate the required sample size needed that would ensure that the study is adequately powered. A priori power analysis was done and accordingly, a sample size of 57 patients per group was needed to detect an intervention effect size on patient satisfaction of 0.5 as measured by MMPSS, with a power of 80%. Power analysis and the analysis of the intervention’s impact on patient satisfaction and on were done using the online software Jamovi®.
In total, 157 patients met the inclusion criteria for the study. Of those, 143 patients were enrolled in the study: 72 patients in the control group and 71 patients in the experimental group. See Figure 1. The mean age of the patients was 72 years in both groups. The majority of the patients were female (67% in the control group and 70% in the experimental group). Patients had an average of four comorbidities and were using an average of eight medications daily in both groups. See Table 2.
After assessing all patient profiles (72 control and 71 experimental), DRPs were analyzed for both groups and presented for intervention and control groups according to the Anatomical Therapeutic Classification (ATC) classification of medications indicating comparable rates of medication use in different categories. See Table 3. Overall, 25 patients (18%) had no DRPs and 83% had one or more DRPs. Those DRPs ranged from one DRP (44%) to six DRPs per patient (1%), with a total of 231 DRPs. A drug regimen that needs to be intensified counted for the highest percentage (22%), followed by a drug with no or unclear indication (16%), an unsafe medication (16%), and a drug omission (14%). Further, 66% of the screened DRPs met the STOPP/START criteria (77%, and 23% respectively). The most common DRP in the STOPP category was that Aspirin was not indicated; while in the START category, the need for an Angiotensin-Converting Enzyme Inhibitor (ACEI) was the most common DRP encountered. The remaining DRPs were revealed by matching their use to guideline-based recommendations or by running medications used through a drug-interaction checker. See Table 4.
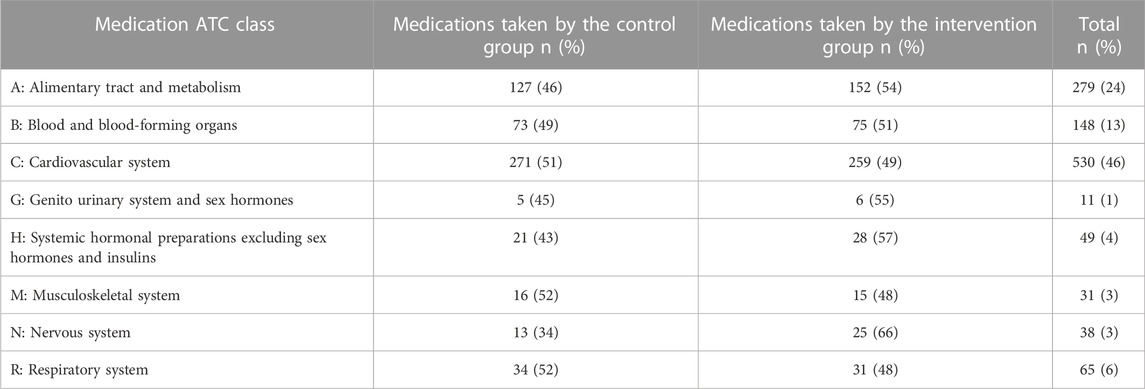
TABLE 3. Anatomical Therapeutic Classification (ATC) classification of medications among intervention and control two groups (World Health Organization, 2022).
In total, 221 recommendations, divided into six categories, were provided to physicians. Of those recommendations, more than half, 52%, were to discontinue one medication, 23% were to intensify a therapeutic regimen, and 13% were to initiate one medication. Added together, deprescribing recommendations; i.e., discontinue a medication, decrease a dose, switch to an alternative, and simplify a regimen; comprised 64% of recommendations, which was almost double the sum of drug initiation and regimen intensification recommendations (36%). See Table 5. The intervention pharmacist discussed the recommendations with three cardiologists, two endocrinologists, two gastroenterologists, one pulmonologist, and one orthopaedist. The majority of the recommendations were provided to cardiologists (72%). More than half of the total recommendations were rejected (56%), 30% were accepted, and the remaining (14%) were deferred. The rate of accepting recommendations was not uniform across physicians. The pulmonologist and the orthopaedist accepted all the recommendations received, while cardiologist-1 and gastroenterologist-1 showed an acceptance rate of 42% followed by endocrinologist-1 (35%). Cardiologist-3, on the other hand, accepted only 5% of the recommendations provided (Table 6).
Descriptive statistics of the summary medication management patient satisfaction score (MMPSS items one to nine) per group are presented in Table 7. Patients in the control and experimental groups had a meaningful difference in satisfaction in favor of the provided intervention. Cohen’s d effect size of 1.75 (t (141) = −10.48, p < 0.001). Data in both groups were not normally distributed. Due to the non-normality of the outcome by groups, a Mann-Whitney non-parametric analysis was also completed, providing the same conclusion (Table 8). The control group had a mean score of 15.2 (SD: 3.74), which is statistically significantly lower than that of the experimental group by six points (21.1; SD: 2.9).
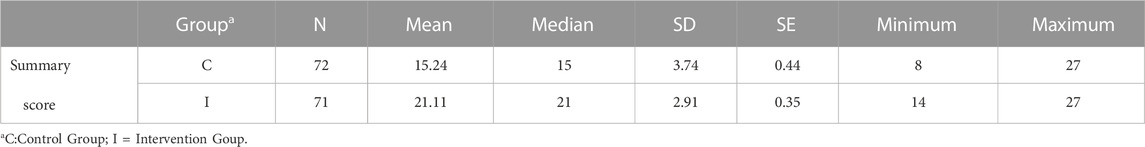
TABLE 7. Descriptive statistics of the summary medication management patient satisfaction score per group.
This study assessed the impact of a pharmacist-led medication optimization service with a deprescribing focus targeting older adults of low income on polypharmacy while utilizing the adapted consolidated framework for implementation research in planning the service (Shoemaker et al., 2017). Results showed that problems related to medications and medication inappropriateness are widespread among the studied population providing an opportunity for a pharmacist-driven intervention. While physician acceptance of provided pharmacist recommendations was not optimal, patients provided with this service showed much greater satisfaction with the provided service compared to the regular medication dispensing service routinely provided by the facility site pharmacist.
Regarding drug-related problems, the findings reported here are in line with other studies that show a high prevalence of DRPs in older adults (68%–93%) (Allard et al., 2001; Sellors et al., 2003; Milos et al., 2013; Chan et al., 2014). Of the reasons that typically cause this high DRP prevalence among the studied population in different settings, the fact that recruited patients were suffering from multiple comorbidities and hence, were followed by multiple prescribers, with insufficient coordination between them might have been a key issue driving DRPs in this patient population (Vinks et al., 2009; Tan et al., 2014; Campbell A et al., 2018; Cheong et al., 2018; Khera et al., 2019). Still, it is important to note that DRPs found in this study at this facility were comparable to the literature as indicated above. This indicates that, despite the limited resources, the quality of patient care in this setting is comparable to others.
Being the main target, PIMs, as defined by the STOPP criteria, counted for half of the DRPs in this study, with the majority of PIMs falling in the cardiovascular drug class (no indication for Aspirin and long-term use of DAPT), followed by the unsafe use of sulfonylurea in elderly. Consistent with our results, studies addressing PIM prevalence among elderly patients indicated a prevalence of PIMs of 45%–60% (Saab et al., 2006; Eze and Olowu, 2011; Zeenny et al., 2017). A study conducted in Ethiopia revealed that the inappropriate use or omission of antithrombotic medications is prevalent in Ethiopian older adults (Getachew et al., 2016). Other studies determined PPIs, antithrombotic, sulfonylurea, and benzodiazepines as the most frequent PIMs screened by STOPP criteria (Dalleur et al., 2014; Chau et al., 2016; Kimura et al., 2017), in addition to NSAIDs, skeletal muscle relaxants, antihistamines, estrogen, and drugs for the central nervous system, as described by Beer’s criteria (Fadare et al., 2013; van Heerden et al., 2016; Ammerman et al., 2019; Cardwell et al., 2020; Deyo et al., 2020) This diversity in reported PIMs is common among studies. In this study, patients were of low income, hence, OTC medications (NSAIDs, antihistamines), and other medications not provided by the facility (CNS drugs) were not likely to be used, to begin with, as they were not typically affordable to patients. Second, patient records that were available at the facility for the intervention pharmacist might have been missing some information on the use of those products.
The recommendations provided by the intervention pharmacist to the specialty physicians at the facility mainly focused on deprescribing rather than initiating new medications, with the most common being discontinuing a medication. Intensification of a regimen, on the other hand, was the second common recommendation. This comes in agreement with literature where drug discontinuation was a frequent recommendation in many pharmacist-led interventions targeting elderly patients on polypharmacy (Vinks et al., 2009; Milos et al., 2013; Chau et al., 2016; Campins et al., 2017; Hurmuz et al., 2018; Cardwell et al., 2020). Some studies described pharmacist recommendations that only focused on providing deprescribing recommendations to physicians without addressing therapy intensification. (Kurt Kroenke and Pinholt, 1990; Dalleur et al., 2014; Morrison and MacRae, 2015; Pruskowski and Handler, 2017; Wouters et al., 2017; Cheong et al., 2018; Clark et al., 2020; Deyo et al., 2020; Kua et al., 2021). On the contrary, drug initiation, patient education, laboratory monitoring, and dose adjustment were more common recommendations in other studies (Tan et al., 2014; Campbell A et al., 2018; Khera et al., 2019). One of the latter studies targeted inappropriate medication use among elderly patients without necessarily being on polypharmacy (Campbell A et al., 2018). In addition, the explicit criteria for screening for PIMs such as the STOPP/START criteria were not used in some of these studies (Tan et al., 2014; Campbell A et al., 2018). These factors could all result in differences in pharmacists’ recommendations between studies.
Thirty percent of the recommendations provided to different specialty physicians were accepted in this study. This acceptance rate is in line with several studies performed in multiple settings (20%–42%) (Vinks et al., 2009; Touchette et al., 2012; Pruskowski and Handler, 2017; Clark et al., 2020). Higher acceptance rates (75%–99%), however, were observed in other studies (Blakey and Hixson-Wallace, 2000; Roth et al., 2013; Campins et al., 2017; Kimura et al., 2017; Cheong et al., 2018; Balsom et al., 2020; Kua et al., 2021). Hailu et.al, a study conducted in a hospital in Ethiopia, showed a similar rate of DRPs (82%), but a high acceptance rate (92%) (Hailu et al., 2020). The fact that many of the drugs that constituted better alternatives for patients were in shortage at the time of the study has likely contributed to a significant proportion of those physician rejections for the intervention pharmacist recommendations. Under conditions of resource scarcity, physicians tend to give patients the available medication, even if it does not provide the most optimal therapeutic effect, rather than keeping the patient without therapy. This issue is often overlooked in research that is carried out in settings where resource scarcity does not represent a significant barrier and would warrant exploration in future work. Even with this factor taken into consideration, it was interesting to note that adopting an implementation science approach in planning this study did not seem to increase the acceptance rate of physicians in this setting above average rates reported in the literature. This suboptimal acceptance rate for recommendations provided by pharmacists should be explored in the future implementation of science-driven interventions building on and complementing this work.
The fact that patients receiving services in the facility welcomed the intervention provided by the intervention pharmacist is a key finding in this study. One would have suspected that patients, in this kind of setting, where medications are provided free of charge may have received an intervention focusing on a reduction in the number of offered medications with suspicion. This did not seem to be the case with the intervention producing a wide margin of increase in patient satisfaction. This finding could be promising with regards to patient satisfaction towards deprescribing in settings providing medications for little to no charge and requires further investigation. It is possible that because START/STOPP criteria were used, patients felt they would get additional medications prescribed as a result of their medication review, not just have medications deprescribed, in case their clinical condition required so. This could have further boosted their satisfaction with the provided service and reduced the likelihood of the misconception that the economic saving from deprescribing medications was the key driver for the work.
Earlier research assessed patient satisfaction with pharmacist-provided care in the community, primary care, and hospital settings (Alhomoud et al., 2016; Soeiro et al., 2017; Nigussie and Edessa, 2018; Jordan et al., 2021; Kabba et al., 2021; Kebede et al., 2021). In most of these studies, research indicated that patient satisfaction with pharmacist services was linked to interpersonal aspects such as communication with and being respectful to patients, as well as disease and therapy management offered by pharmacists. In Sierra Leone and Ethiopia, where health resources are relatively limited, an important factor leading to patient satisfaction was having those services provided by the pharmacist for free (Kabba et al., 2021; Kebede et al., 2021). This is in line with our study, where the intervention pharmacist reviewed therapeutic regimens for patients of low income and completed the service for no charge.
An implementation science approach was used to guide the provision of the pharmacist intervention in this study. The CFIR, which was applied here, was previously used in implementing various health interventions such as the implementation of fall prevention projects, clinical practice guidelines in nursing practice, and the HPV vaccine schedule among adolescents (Shaw et al., 2013; Breimaier et al., 2015; Garbutt et al., 2018). Taken together and in agreement with this study, these findings indicate that the application of CFIR proved to be a helpful framework in organizing the implementation process of those projects being particularly valuable in managing barriers and facilitators behind the success of the implementation.
Further, the literature indicates that patient satisfaction with health interventions guided by implementation science is promising. The application of implementation science in family planning and in providing life narrative interviews for medical inpatients led to high satisfaction and acceptance among participants (Rybarczyk et al., 2019; Weis and Festin, 2020). Moreover, an initiative to implement a measurement program of office and home blood pressure in primary care demonstrated favorable satisfaction among patients and providers towards the service that might decrease the use of unnecessary antihypertensive medications and enhance hypertension control (Doane et al., 2018). These findings are consistent with our study, where patient satisfaction with the provided intervention that was guided by implementation science was high.
This study calls for increased attention to this population in future research and targeted medication optimization interventions. Pharmacists and other health professionals should be proactive in pursuing deprescribing-focused interventions in settings with limited resources noting the benefits of such interventions in these settings including increased patient satisfaction. In countries where deprescribing has not been integrated into practice, as in Lebanon, measures could be taken to facilitate the transition using an implementation science approach that considers as many implementation science considerations as possible. This includes providing facilities with different resources for intervention success; education and training of physicians, pharmacists, and medical staff; together with the incorporation of deprescribing guidelines and tools such as the STOPP/START criteria into routine practice. It is also recommended that mechanisms of financing deprescribing related activities would be established to promote its sustainability. These mechanisms should be informed by cost effectiveness analyses of interventions such as this, which are typically done and presented separately. This would be interesting to pursue and could be investigated in future research.
Another area for future research would consider the benefits vs. risks of deprescribing medication using tools such as STOPP/START criteria accounting for risks such as rebound of conditions as a result of the withdrawal of specific medications. Clinicians would benefit from extra training on managing those risks as part of different aspects to effectively and safely implement deprescribing. This along with the facilitation of interprofessional therapeutic management of medication regimens would lead to better patient satisfaction and outcomes.
This prospective experimental study has specific strengths. It is unique in coupling the deprescribing approach with the use of an implementation science framework in planning the intervention while assessing patients’ satisfaction with the medication management pharmacist’s service. Still, this study had its limitations. It was conducted in a single center in Lebanon, limiting its generalizability. In addition, for logistical reasons, the implementation and outcomes of recommendations were not monitored. Studies spanning those areas could be targeted by future research.
This intervention conducted in a facility serving patients of low income found a high prevalence of inappropriate medications taken by these patients comparable to the literature indicating that, despite the limited resources, the quality of patient care in this facility is comparable to other settings. Further, in a facility where medications are provided free of charge, patients enrolled in the study were highly satisfied with the new service they received from the pharmacist showing promise for future interventions addressing deprescribing in similar settings. The pharmacist performing the intervention provided suggestions to physicians yielding an average acceptance rate, which calls for further research into the best ways of integrating implementation science principles in guiding pharmacist interventions. Future work should assess how specific CFIR constructs contribute to the outcomes of deprescribing interventions.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Beirut Arab University Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
NA: Conducting a research and investigation process, formal analysis, Writing Original Draft JS: Design of methodology; data analysis; Writing—Review & Editing AE-Y: data analysis; Implementation of intervention; Writing—Review & Editing HS: Implementation of intervention; Writing—Review & Editing MA: Conceptualization of research, Development of methodology; Implementation of intervention; data analysis; Writing Original Draft.
The authors appreciate the support of Dr. Jean Moon for the creation of an Arabic-Lebanese version of MMPSS. The authors greatly appreciate the assistance of Mariam Al-Kadi, and Omar Mabsout throughout the data collection period.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1097238/full#supplementary-material
Abu Farha, R. K., Mukattash, T. L., Al-Sakran, L., Abu Hammour, K., and Zawiah, M. (2021). Prevalence and predictors of polypharmacy in Jordanian hospitalised patients: A cross-sectional study. Int. J. Clin. Pract. 75, e13742. doi:10.1111/ijcp.13742
Aguiar, J. P., Gama Marques, J., and Alves da Costa, F. (2021). Utility and limitations of a screening tool of older person’s prescription among psychiatric elder patients: A comprehensive review. Aging Health Res. 1, 100031. doi:10.1016/J.AHR.2021.100031
Ailabouni, N. J., Reeve, E., Helfrich, C. D., Hilmer, S. N., and Wagenaar, B. H. (2022). Leveraging implementation science to increase the translation of deprescribing evidence into practice. Res. Soc. Adm. Pharm. 18, 2550–2555. doi:10.1016/j.sapharm.2021.05.018
Al-Dahshan, A., Al-Kubiasi, N., Al-Zaidan, M., Saeed, W., Kehyayan, V., and Bougmiza, I. (2020). Prevalence of polypharmacy and the association with non-communicable diseases in Qatari elderly patients attending primary healthcare centers: A cross-sectional study. PLoS One 15, e0234386. doi:10.1371/journal.pone.0234386
Alaa Eddine, N. (2022). Development and evaluation of a pharmacist-led deprescribing service in a primary care facility. Beirur Arab University, Lebanon, [master’s thesis]. [Beirut (Lebanon)].
Alhomoud, F. K., Kunbus, A., Ameer, A., and Alhomoud, F. (2016). Quality Assessment of community pharmacy services provided in the United Arab Emirates: Patient experience and satisfaction. J. Appl. Pharm. Sci. 6, 017–023. doi:10.7324/JAPS.2016.60303
Allard, J., Hebert, R., Rioux, M., Asselin, J., and Voyer, L. (2001). Efficacy of clinical medication review on the number of potentially inappropriate prescriptions prescribed for community-dwelling elderly people. CMAJ, 164, 1291–1296.
Alsuwaidan, A., Almedlej, N., Alsabti, S., Daftardar, O., Deaji, F., Amri, A., et al. (2019). A comprehensive overview of polypharmacy in elderly patients in Saudi Arabia. Geriatr. Switz. 4, 36. doi:10.3390/geriatrics4020036
American Diabetes Association (2021). Diabetes care ADA 2021. The journal of clinical and applied research and education 44. Available at: https://care.diabetesjournals.org/content/diacare/suppl/2020/12/09/44.Supplement_1.DC1/DC_44 (Accessed June 1, 2022).
Ammerman, C. A., Simpkins, B. A., Warman, N., and Downs, T. N. (2019). Potentially inappropriate medications in older adults: Deprescribing with a clinical pharmacist. J. Am. Geriatr. Soc. 67, 115–118. doi:10.1111/jgs.15623
Amsterdam, E. A., Wenger, N. K., Brindis, R. G., Casey, D. E., Ganiats, T. G., Holmes, D. R., et al. (2014). 2014 AHA/ACC guideline for the management of patients with non-st-elevation acute coronary syndromes: A report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 130, e344–e426. doi:10.1161/CIR.0000000000000134
Arnett, D. K., Blumenthal, R. S., Albert, M. A., Buroker, A. B., Goldberger, Z. D., Hahn, E. J., et al. (2019). 2019 ACC/AHA guideline on the primary prevention of Cardiovascular Disease: A report of the American college of cardiology/American heart association Task Force on Clinical practice guidelines. J. Am. Coll. Cardiol. 74, e177–e232. doi:10.1016/j.jacc.2019.03.010
Badawy, N. A., Labeeb, S. A., Alsamdan, M. F., and Alazemi, B. F. (2020). Prevalence and risk of polypharmacy among community-dwelling, elderly Kuwaiti patients. Med. Princ. Prac. 29, 166–173. doi:10.1159/000503298
Balsom, C., Pittman, N., King, R., and Kelly, D. (2020). Impact of a pharmacist-administered deprescribing intervention on nursing home residents: A randomized controlled trial. Int. J. Clin. Pharm. 42, 1153–1167. doi:10.1007/s11096-020-01073-6
Basheti, I. A., Tadros, O. K. I., Alnajjar, M. S., and Aburuz, S. (2019). Assessing patient satisfaction with the Medication Management Review service delivered in Jordan. J. Pharm. Health Serv. Res. 10, 49–55. doi:10.1111/jphs.12233
Bauer, M. S., Damschroder, L., Hagedorn, H., Smith, J., and Kilbourne, A. M. (2015). An introduction to implementation science for the non-specialist. BMC Psychol. 3, 32. doi:10.1186/S40359-015-0089-9
Baumgartner, A. D., Clark, C. M., LaValley, S. A., Monte, S. v., Wahler, R. G., and Singh, R. (2020). Interventions to deprescribe potentially inappropriate medications in the elderly: Lost in translation? J. Clin. Pharm. Ther. 45, 453–461. doi:10.1111/jcpt.13103
Beslon, V., Moreau, P., Maruani, A., Maisonneuve, H., Giraudeau, B., and Fournier, J. P. (2018). Effects of discontinuation of urate-lowering therapy: A systematic review. J. Gen. Intern Med. 33, 358–366. doi:10.1007/s11606-017-4233-5
Blakey, S. A., and Hixson-Wallace, J. A. (2000). Clinical and economic effects of pharmacy services in geriatric ambulatory clinic. Pharmacotherapy 20, 1198–1203. doi:10.1592/phco.20.15.1198.34581
Bleidt, B. A. (2019). Deprescribing: An emerging role for pharmacists. J. Pharm. Health Serv. Res. 10, 159–160. doi:10.1111/jphs.12303
Boghossian, T. A., Rashid, F. J., Thompson, W., Welch, V., Moayyedi, P., Rojas-Fernandez, C., et al. (2017). Deprescribing versus continuation of chronic proton pump inhibitor use in adults. Cochrane Database Syst. Rev. 3, CD011969. doi:10.1002/14651858.CD011969.pub2
Bou Malham, C., el Khatib, S., Cestac, P., Andrieu, S., Rouch, L., and Salameh, P. (2021). Impact of pharmacist-led interventions on patient care in ambulatory care settings: A systematic review. Int. J. Clin. Pract. 75, e14864. doi:10.1111/ijcp.14864
Breimaier, H. E., Heckemann, B., Halfens, R. J. G., and Lohrmann, C. (2015). The consolidated framework for implementation research (CFIR): A useful theoretical framework for guiding and evaluating a guideline implementation process in a hospital-based nursing practice. BMC Nurs. 14, 43. doi:10.1186/s12912-015-0088-4
Bužančić, I., Dragović, P., Pejaković, T. I., Markulin, L., and Ortner-Hadžiabdić, M. (2021). Exploring patients’ attitudes toward deprescribing and their perception of pharmacist involvement in a European country: A cross-sectional study. Patient Prefer Adherence 15, 2197–2208. doi:10.2147/PPA.S323846
Campbell, A., Coley, K., Corbo, J., DeLellis, T., Joseph, M., Thorpe, C., et al. (2018). Pharmacist-led drug therapy problem management in an interprofessional geriatric care continuum: A subset of the pivots group - PubMed. Am. Health Drug Benefit 11, 469–478.
Campins, L., Serra-Prat, M., Gózalo, I., López, D., Palomera, E., Agustí, C., et al. (2017). Randomized controlled trial of an intervention to improve drug appropriateness in communitydwelling polymedicated elderly people. Fam. Pract. 34, 36–42. doi:10.1093/fampra/cmw073
Cardosi, L., Hohmeier, K. C., Fisher, C., and Wasson, M. (2018). Patient satisfaction with a comprehensive medication review provided by a community pharmacist. J. Pharm. Technol. 34, 48–53. doi:10.1177/8755122517752158
Cardwell, K., Smith, S. M., Clyne, B., McCullagh, L., Wallace, E., Kirke, C., et al. (2020). Evaluation of the general practice pharmacist (GPP) intervention to optimise prescribing in Irish primary care: A non-randomised pilot study. BMJ Open 10. e035087, doi:10.1136/bmjopen-2019-035087
Chan, D. C., Chen, J. H., Wen, C. J., Chiu, L. S., and Wu, S. C. (2014). Effectiveness of the medication safety review clinics for older adults prescribed multiple medications. J. Formos. Med. Assoc. 113, 106–113. doi:10.1016/j.jfma.2012.04.013
Chau, S. H., Jansen, A. P. D., van de Ven, P. M., Hoogland, P., Elders, P. J. M., and Hugtenburg, J. G. (2016). Clinical medication reviews in elderly patients with polypharmacy: A cross-sectional study on drug-related problems in The Netherlands. Int. J. Clin. Pharm. 38, 46–53. doi:10.1007/s11096-015-0199-8
Cheong, S. T., Ng, T. M., and Tan, K. T. (2018). Pharmacist-initiated deprescribing in hospitalised elderly: Prevalence and acceptance by physicians. Eur. J. Hosp. Pharm. 25, E35–E39. doi:10.1136/ejhpharm-2017-001251
Clark, C. M., LaValley, S. A., Singh, R., Mustafa, E., Monte, S. v., and Wahler, R. G. (2020). A pharmacist-led pilot program to facilitate deprescribing in a primary care clinic. J. Am. Pharm. Assoc. 60, 105–111. doi:10.1016/j.japh.2019.09.011
Cossette, B., Éthier, J. F., Joly-Mischlich, T., Bergeron, J., Ricard, G., Brazeau, S., et al. (2017). Reduction in targeted potentially inappropriate medication use in elderly inpatients: A pragmatic randomized controlled trial. Eur. J. Clin. Pharmacol. 73, 1237–1245. doi:10.1007/s00228-017-2293-4
Cowan, A., and Riley, S. (2015). STOPP START tool to support medication review. Available at: https://www.nice.org.uk/guidance/cg180 (Accessed June 18, 2014).
Dalleur, O., Boland, B., Losseau, C., Henrard, S., Wouters, D., Speybroeck, N., et al. (2014). Reduction of potentially inappropriate medications using the STOPP criteria in frail older inpatients: A randomised controlled study. Drugs Aging 31, 291–298. doi:10.1007/s40266-014-0157-5
de Oliveira Santos Silva, R., Macêdo, L. A., dos Santos, G. A., Aguiar, P. M., and de Lyra, D. P. (2019). Pharmacist-participated medication review in different practice settings: Service or intervention? An overview of systematic reviews. PLoS One 14, e0210312. doi:10.1371/journal.pone.0210312
Deyo, J. C., Smith, B. H., Biola, H., Ferry, E. M., Orto, V. K., Patel, B., et al. (2020). Reducing high-risk medication use through pharmacist-led interventions in an outpatient setting. J. Am. Pharm. Assoc. 60, e86–e92. doi:10.1016/j.japh.2020.01.013
Dharmarajan, T. S., Choi, H., Hossain, N., Munasinghe, U., Lakhi, F., Lourdusamy, D., et al. (2020). Deprescribing as a clinical improvement focus. J. Am. Med. Dir. Assoc. 21, 355–360. doi:10.1016/j.jamda.2019.08.031
Doane, J., Buu, J., Jason Penrod, M., Bischoff, M., Conroy, M. B., and Stults, B. (2018). Measuring and managing blood pressure in a primary care setting: A pragmatic implementation study. J. Am. Board Fam. Med. 31, 375–388. doi:10.3122/jabfm.2018.03.170450
Duncan, P., Duerden, M., and Payne, R. A. (2017). Deprescribing: A primary care perspective. Eur. J. Hosp. Pharm. 24, 37–42. doi:10.1136/ejhpharm-2016-000967
Eze, U. I., and Olowu, A. O. (2011). Prescribing patterns and inappropriate use of medications in elderly outpatients in a tertiary hospital in Nigeria. Trop. J. Pharm. Res. 10, 1. doi:10.4314/tjpr.v10i1.66536
Fadare, J. O., Agboola, S. M., Opeke, O. A., and Alabi, R. A. (2013). Prescription pattern and prevalence of potentially inappropriate medications among elderly patients in a Nigerian rural tertiary hospital. Ther. Clin. Risk Manag. 9, 115–120. doi:10.2147/TCRM.S40120
Farrell, B., Clarkin, C., Conklin, J., Dolovich, L., Irving, H., McCarthy, L., et al. (2020). Community pharmacists as catalysts for deprescribing: An exploratory study using quality improvement processes. Can. Pharm. J. 153, 37–45. doi:10.1177/1715163519882969
Fernandes, B. D., Foppa, A. A., Ayres, L. R., and Chemello, C. (2022). Implementation of medication reconciliation conducted by hospital pharmacists: A case study guided by the consolidated framework for implementation research. Res. Soc. Adm. Pharm. 18, 3631–3637. doi:10.1016/j.sapharm.2022.01.010
Fick, D. M., Mion, L. C., Beers, M. H., and Waller, J. L. (2006). Health outcomes associated with potentially inappropriate medication use in older adults. Gurwitz 31, 42–51. doi:10.1002/nur.20232
Garbutt, J. M., Dodd, S., Walling, E., Lee, A. A., Kulka, K., and Lobb, R. (2018). Theory-based development of an implementation intervention to increase HPV vaccination in pediatric primary care practices. Implement Sci. 13, 45. doi:10.1186/s13012-018-0729-6
Getachew, H., Bhagavathula, A. S., Abebe, T. B., and Belachew, S. A. (2016). Inappropriate prescribing of antithrombotic therapy in ethiopian elderly population using updated 2015 STOPP/START criteria: A cross-sectional study. Clin. Interv. Aging 11, 819–827. doi:10.2147/CIA.S107394
Grundy, S. M., Stone, N. J., Bailey, A. L., Beam, C., Birtcher, K. K., Blumenthal, R. S., et al. (2019). 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American college of cardiology/American heart association Task Force on Clinical practice guidelines. J. Am. Coll. Cardiol. 73, e285e285–e350. doi:10.1016/j.jacc.2018.11.003
Hailu, B. Y., Berhe, D. F., Gudina, E. K., Gidey, K., and Getachew, M. (2020). Drug related problems in admitted geriatric patients: The impact of clinical pharmacist interventions. BMC Geriatr. 20, 13. doi:10.1186/s12877-020-1413-7
Halli-Tierny, A., Scarbrough, C., and Caroll, D. (2019). Polypharmacy: Evaluating risks and deprescribing. Kansas, United States: Amercian Academy of Family Physician.
Hurmuz, M. Z. M., Janus, S. I. M., and van Manen, J. G. (2018). Changes in medicine prescription following a medication review in older high-risk patients with polypharmacy. Int. J. Clin. Pharm. 40, 480–487. doi:10.1007/s11096-018-0602-3
Jordan, J. N., Wadsworth, T. G., Robinson, R., Hruza, H., Paul, A., and O’connor, S. K. (2021). Patient satisfaction with pharmacist-provided health-related services in a primary care clinic. Pharm. (Basel) 9, 187. doi:10.3390/pharmacy9040187
Kabba, J. A., Bah, A. J., James, P. B., Chang, J., Kitchen, C., Jiang, M., et al. (2021). Patients satisfaction with free healthcare pharmaceutical services in Sierra Leone: A national cross-sectional study. Int. J. Clin. Pharm. 43, 556–565. doi:10.1007/s11096-020-01163-5
Kebede, H., Tsehay, T., Necho, M., and Zenebe, Y. (2021). Patient satisfaction towards outpatient pharmacy services and associated factors at dessie town public hospitals, south Wollo, north-east Ethiopia. Patient Prefer Adherence 15, 87–97. doi:10.2147/PPA.S287948
Khera, S., Abbasi, M., Dabravolskaj, J., Sadowski, C. A., Yua, H., and Chevalier, B. (2019). Appropriateness of medications in older adults living with frailty: Impact of a pharmacist-led structured medication review process in primary care. J. Prim. Care Community Health 10, 2150132719890227. doi:10.1177/2150132719890227
Kim, S., Martin, M. T., Pierce, A. L., and Zueger, P. (2016). Satisfaction with medication therapy management services at a university ambulatory care clinic. J. Pharm. Pract. 29, 199–205. doi:10.1177/0897190014550718
Kimura, T., Ogura, F., Yamamoto, K., Uda, A., Nishioka, T., Kume, M., et al. (2017). Potentially inappropriate medications in elderly Japanese patients: Effects of pharmacists’ assessment and intervention based on screening tool of older persons’ potentially inappropriate prescriptions criteria ver.2. J. Clin. Pharm. Ther. 42, 209–214. doi:10.1111/jcpt.12496
Kojima, G., Bell, C., Tamura, B., Inaba, M., Lubimir, K., Blanchette, P. L., et al. (2012). Reducing cost by reducing polypharmacy: The polypharmacy outcomes project. J. Am. Med. Dir. Assoc. 13, 818.e11–e15. doi:10.1016/j.jamda.2012.07.019
Korenvain, C., MacKeigan, L. D., Dainty, K. N., Guilcher, S. J. T., and McCarthy, L. M. (2020). Exploring deprescribing opportunities for community pharmacists using the Behaviour Change Wheel. Res. Soc. Adm. Pharm. 16, 1746–1753. doi:10.1016/j.sapharm.2020.01.019
Kua, C.-H., Reeve, E., Ratnasingam, V., Mak, V. S., Wen Huey Lee, S., Deprescribing Workgroup, S., et al. (2020). Patients' and caregivers' attitudes toward deprescribing in Singapore. J. Gerontol. A Biol. Sci. Med. Sci. 76, 1053–1060. doi:10.1093/gerona/glaa018
Kua, C. H., Yeo, C. Y. Y., Tan, P. C., Char, C. W. T., Tan, C. W. Y., Mak, V., et al. (2021). Association of deprescribing with reduction in mortality and hospitalization: A pragmatic stepped-wedge cluster-randomized controlled trial. J. Am. Med. Dir. Assoc. 22, 82–89.e3. doi:10.1016/j.jamda.2020.03.012
Kua, K. P., Saw, P. S., and Lee, S. W. H. (2019). Attitudes towards deprescribing among multi-ethnic community-dwelling older patients and caregivers in Malaysia: A cross-sectional questionnaire study. Int. J. Clin. Pharm. 41, 793–803. doi:10.1007/s11096-019-00829-z
Kurt Kroenke, L., and Pinholt, E. M. (1990). Reducing polypharmacy in the elderly A controlled trial of physician feedback. J. Am. Geriatr. Soc. 38, 31–36. doi:10.1111/j.1532-5415.1990.tb01593.x
Lim, X., Yeo, Q., Kng, G., Chung, W., and Yap, K. (2018). Validation of a drug-related problem classification system for the intermediate and long-term care setting in Singapore. Pharmacy 6, 109. doi:10.3390/pharmacy6040109
Mair, A., and Fernandez-Llimos, F.SIMPATHY Consortium (2017). Polypharmacy management programmes: The SIMPATHY Project. Eur. J. Hosp. Pharm. 24, 5–6. doi:10.1136/ejhpharm-2016-001044
Martin, P., Tamblyn, R., Benedetti, A., Ahmed, S., and Tannenbaum, C. (2018). Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: The D-PRESCRIBE randomized clinical trial. JAMA 320, 1889–1898. doi:10.1001/jama.2018.16131
Martinez, A. I., Abner, E. L., Jicha, G. A., Rigsby, D. N., Eckmann, L. C., Huffmyer, M. J., et al. (2020). One-year evaluation of a targeted medication therapy management intervention for older adults. J. Mamag Care Spec. Pharm. 26, 520–528. doi:10.18553/jmcp.2020.26.4.520
Maund, E., Stuart, B., Moore, M., Dowrick, C., Geraghty, A. W. A., Dawson, S., et al. (2019). Managing antidepressant discontinuation: A systematic review. Ann. Fam. Med. 17, 52–60. doi:10.1370/afm.2336
McCarthy, C., Clyne, B., Boland, F., Moriarty, F., Flood, M., Wallace, E., et al. (2022). GP-delivered medication review of polypharmacy, deprescribing, and patient priorities in older people with multimorbidity in Irish primary care (SPPiRE study): A cluster randomised controlled trial. PLoS Med. 19, e1003862. doi:10.1371/journal.pmed.1003862
McFarland, M. S., Finks, W., Smith, L., Buck, L., Ourth, H., and Brummel, A. (2021). Integration of CMM into practice PERSPECTIVE. Am. Health Drug Benefits 14, 113–114.
Mckean, M., Pillans, P., and Scott, I. A. (2016). A medication review and deprescribing method for hospitalised older patients receiving multiple medications. Intern Med. J. 46, 35–42. doi:10.1111/imj.12906
Medscape (2021). Drug interactions checker - Medscape drug reference database. Available at: https://reference.medscape.com/drug-interactionchecker (Accessed December 7, 2021).
Milos, V., Rekman, E., Bondesson, Å., Eriksson, T., Jakobsson, U., Westerlund, T., et al. (2013). Improving the quality of pharmacotherapy in elderly primary care patients through medication reviews: A randomised controlled study. Drugs Aging 30, 235–246. doi:10.1007/s40266-013-0057-0
Moczygemba, L. R., Barner, J. C., Brown, C. M., Lawson, K. A., Gabrillo, E. R., Godley, P., et al. (2010). Patient satisfaction with a pharmacist-provided telephone medication therapy management program. Res. Soc. Adm. Pharm. 6, 143–154. doi:10.1016/j.sapharm.2010.03.005
Moon, J., Kolar, C., Brummel, A., Ekstrand, M., Holtan, H., and Rehrauer, D. (2016). Development and validation of a patient satisfaction survey for comprehensive medication management. JMCP J. Manag. Care & Specialty Pharm. 22, 81–86. doi:10.18553/jmcp.2016.22.1.81
Morrison, C., and MacRae, Y. (2015). Promoting safer use of high-risk pharmacotherapy: Impact of pharmacist-led targeted medication reviews. Drugs Real World Outcomes 2, 261–271. doi:10.1007/s40801-015-0031-8
Mustafa Sirimsi, M., de Loof, H., van den Broeck, K., de Vliegher, K., Pype, P., Remmen, R., et al. (2022). Scoping review to identify strategies and interventions improving interprofessional collaboration and integration in primary caree062111. BMJ Open 12. doi:10.1136/bmjopen-2022-062111
Narayan, S. W., and Nishtala, P. S. (2017). Discontinuation of preventive medicines in older people with limited life expectancy: A systematic review. Drugs Aging 34, 767–776. doi:10.1007/s40266-017-0487-1
National Institute for Health and Care Excellence (2015). Medicines optimisation: The safe and effective use of medicines to enable the best possible outcomes NICE guideline. Available at: www.nice.org.uk/guidance/ng5 (Accessed January 6, 2023).
Nigussie, S., and Edessa, D. (2018). The extent and reasons for dissatisfaction from outpatients provided with pharmacy services at two public hospitals in eastern Ethiopia. Front. Pharmacol. 9, 1132. doi:10.3389/fphar.2018.01132
O’Gara, P. T., Kushner, F. G., Ascheim, D. D., Casey, D. E., Chung, M. K., de Lemos, J. A., et al. (2013). 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation 127, e362–e425. doi:10.1161/CIR.0b013e3182742cf6
Ojo, T., Kabasele, L., Boyd, B., Enechukwu, S., Ryan, N., Gyamfi, J., et al. (2021). The role of implementation science in advancing resource generation for health interventions in low- and middle-income countries. Health Serv. Insights 14, 1178632921999652. doi:10.1177/1178632921999652
O’mahony, D., O’sullivan, D., Byrne, S., O’connor, M. N., Ryan, C., and Gallagher, P. (2015). STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 44, 213–218. doi:10.1093/ageing/afu145
Pereira, C. E. O., Bambirra, E. H. F., Fernandes, B. D., Sousa, M. C. V. B., Mendonça, S. A. M., and Chemello, C. (2021). Factors influencing the implementation of pharmaceutical care in outpatient settings: A systematic review applying the consolidated framework for implementation research. Res. Soc. Adm. Pharm. 18, 2579–2592. doi:10.1016/j.sapharm.2021.06.011
Pharmaceutical Care Network Europe (2003). Classification for Drug related problems v9. Available at: https://www.pcne.org/upload/files/417_PCNE_classification_V9-1_final.pdf (Accessed January 7, 2023).
Pruskowski, J., and Handler, S. M. (2017). The DE-PHARM project: A pharmacist-driven deprescribing initiative in a nursing facility. Consult Pharm. 32, 468–478. doi:10.4140/TCP.n.2017.468
Rashid, R., Chang, C., Niu, F., Deguzman, L., Draves, M., Awsare, S., et al. (2020). Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J. Manag. Care Spec. Pharm. 26, 918–924. doi:10.18553/jmcp.2020.26.7.918
Reeve, E., Ong, M., Wu, A., Jansen, J., Petrovic, M., and Gnjidic, D. (2017). A systematic review of interventions to deprescribe benzodiazepines and other hypnotics among older people. Eur. J. Clin. Pharmacol. 73, 927–935. doi:10.1007/s00228-017-2257-8
Reeve, E., Shakib, S., Hendrix, I., Roberts, M. S., and Wiese, M. D. (2014). The benefits and harms of deprescribing. Med. J. Aust. 201, 386–389. doi:10.5694/mja13.00200
Ronquillo, C., Day, J., Warmoth, K., Britten, N., Stein, K., and Lang, I. (2018). An implementation science perspective on deprescribing. Public Policy & Aging Rep. 28, 134–139. doi:10.1093/ppar/pry032
Roth, M. T., Ivey, J. L., Esserman, D. A., Crisp, G., Kurz, J., and Weinberger, M. (2013). Individualized medication assessment and planning: Optimizing medication use in older adults in the primary care setting. Pharmacotherapy 33, 787–797. doi:10.1002/phar.1274
Rybarczyk, B., Shamaskin-Garroway, A., Lanoye, A., Griffin, S., Bellg, A., Stone, R., et al. (2019). Implementation and evaluation of a life narrative interview program for medical inpatients. Clin. Gerontol. 42, 454–460. doi:10.1080/07317115.2018.1470122
Saab, Y. B., Hachem, A., Sinno, S., and El-Moalem, H. (2006). Inappropriate medication use in elderly Lebanese outpatients prevalence and risk factors. Drugs Aging 23, 743–752. doi:10.2165/00002512-200623090-00004
Samuel, M. J. (2015). American Geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 63, 2227–2246. doi:10.1111/JGS.13702
Schapira, M., Calabró, P., Montero-Odasso, M., Osman, A., Guajardo, M. E., Martínez, B., et al. (2020). A multifactorial intervention to lower potentially inappropriate medication use in older adults in Argentina. Aging Clin. Exp. Res. 33, 3313–3320. doi:10.1007/s40520-020-01582-4
Sellors, J., Kaczorowski, J., Sellors, C., Dolovich, L., Woodward, C., Willan, A., et al. (2003). A randomized controlled trial of a pharmacist consultation program for family physicians and their elderly patients. CMAJ 169, 17–22.
Shaw, J., Sidhu, K., Kearney, C., Keeber, M., and McKay, S. (2013). Engaging home health care providers in a fall prevention best practice initiative. Home Health Care Serv. Q. 32, 1–16. doi:10.1080/01621424.2013.757177
Shoemaker, S. J., Curran, G. M., Swan, H., Teeter, B. S., and Thomas, J. (2017). Application of the consolidated framework for implementation research to community pharmacy: A framework for implementation research on pharmacy services. Res. Soc. Adm. Pharm. 13, 905–913. doi:10.1016/j.sapharm.2017.06.001
Shrestha, S., Giri, R., Sapkota, H. P., Danai, S. S., Saleem, A., Devkota, S., et al. (2021). Attitudes of ambulatory care older Nepalese patients towards deprescribing and predictors of their willingness to deprescribe. Ther. Adv. Drug Saf. 12, 20420986211019309. doi:10.1177/20420986211019309
Sloane, P. D., and Zimmerman, S. (2018). Deprescribing in geriatric medicine: Challenges and opportunities. J. Am. Med. Dir. Assoc. 19, 919–922. doi:10.1016/j.jamda.2018.09.018
Soeiro, O. M., Tavares, N. U. L., Júnior, J. M., do, N., Junior, A. A. G., Costa, E. A., et al. (2017). Patient satisfaction with pharmaceutical services in Brazilian primary health care. Rev. Saude Publica 51, 21s. doi:10.11606/S1518-8787.2017051007145
Stockl, K., Le, L., Zhang, S., and Harada, A. S. M. (2010). Clinical and economic outcomes associated with potentially inappropriate prescribing in the elderly. Am J manag care 16. Available at: https://www.ajmc.com/view/ajmc_2010jan_stocklweb_e1_e10 (Accessed January 6, 2023).
Tan, E. C. K., Stewart, K., Elliott, R. A., and George, J. (2014). Pharmacist consultations in general practice clinics: The Pharmacists in Practice Study (PIPS). Res. Soc. Adm. Pharm. 10, 623–632. doi:10.1016/j.sapharm.2013.08.005
Tandun, R., Bubbar, C., and Tejani, A. M. (2019). Who has the guts to deprescribe proton pump inhibitors? A pharmacist-led intervention in a long-term care facility setting. AGING Med. 2, 112–117. doi:10.1002/agm2.12063
Tegegn, H. G., Tefera, Y. G., Erku, D. A., Haile, K. T., Abebe, T. B., Chekol, F., et al. (2018). Older patients’ perception of deprescribing in resource-limited settings: A cross-sectional study in an Ethiopia University hospital. BMJ Open 8, e020590. doi:10.1136/bmjopen-2017-020590
Touchette, D. R., Masica, A. L., Dolor, R. J., Schumock, G. T., Choi, Y. K., Kim, Y., et al. (2012). Safety-focused medication therapy management: A randomized controlled trial. J. Am. Pharm. Assoc. 52, 603–612. doi:10.1331/JAPhA.2012.12036
Valgimigli, M., Bueno, H., Byrne, R. A., Collet, J. P., Costa, F., Jeppson, A., et al. (2018). 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 39, 213–260. doi:10.1093/eurheartj/ehx419
van der Ploeg, M. A., Floriani, C., Achterberg, W. P., Bogaerts, J. M. K., Gussekloo, J., Mooijaart, S. P., et al. (2020). Recommendations for (discontinuation of) statin treatment in older adults: Review of guidelines. J. Am. Geriatr. Soc. 68, 417–425. doi:10.1111/jgs.16219
van Heerden, J. A., Burger, J. R., and Gerber, J. J. (2016). Inappropriate medicine prescribing in older South Africans: A cross-sectional analysis of medicine claims data. S Afr. Med. J. 106, 1010–1016. doi:10.7196/SAMJ.2016.v106i10.10627
Vasilevskis, E. E., Shah, A. S., Hollingsworth, E. K., Shotwell, M. S., Mixon, A. S., Bell, S. P., et al. (2019). A patient-centered deprescribing intervention for hospitalized older patients with polypharmacy: Rationale and design of the Shed-MEDS randomized controlled trial. BMC Health Serv. Res. 19, 165. doi:10.1186/s12913-019-3995-3
Vinks, T. H. A. M., Egberts, T. C. G., de Lange, T. M., and de Koning, F. H. P. (2009). Pharmacist-based medication review reduces potential drug-related problems in the elderly the SMOG controlled trial. Drugs Aging 26, 123–133. doi:10.2165/0002512-200926020-00004
Vogelmeir, C., Agusti, A., Anzueto, A., Barnes, P., Bourbeau, J., Criner, G., et al. (2017). Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Gold executive summary. Am. J. Respir Crit. Care Med. 195, 557–582. doi:10.1164/rccm.201701-0218PP
Weis, J., and Festin, M. (2019). Implementation and scale-up of the standard days method of family planning: A landscape analysis. Glob. Helath Sci. Pract. 8, 114–124. doi:10.9745/GHSP-D-19-00287
Whelton, P. K., Carey, R. M., Aronow, W. S., Casey, D. E., Collins, K. J., Dennison Himmelfarb, C., et al. (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 71, e127–e248. doi:10.1016/j.jacc.2017.11.006
World Health Organization (2022). Anatomical therapeutic chemical (ATC) classification. Available at: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (Accessed January 6, 2023).
World Health Organization (2019). Medication safety in polypharmacy: Technical report. Available at: https://apps.who.int/iris/handle/10665/325454 (Accessed December 4, 2021).
Wouters, H., Scheper, J., Koning, H., Brouwer, C., Twisk, J. W., van der Meer, H., et al. (2017). Discontinuing inappropriate medication use in nursing home residents: A cluster randomized controlled trial. Ann. Intern Med. 167, 609–617. doi:10.7326/M16-2729
Yancy, C. W., Jessup, M., Bozkurt, B., Bulter, J., Casey, D. E., Colvin, M. M., et al. (2017). 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J. Am. Coll. Cardiol. 70, 776–803. doi:10.1016/j.jacc.2017.04.025
Zechmann, S., Senn, O., Valeri, F., Essig, S., Merlo, C., Rosemann, T., et al. (2020). Effect of a patient-centred deprescribing procedure in older multimorbid patients in Swiss primary care - a cluster-randomised clinical trial. BMC Geriatr. 20, 471. doi:10.1186/s12877-020-01870-8
Keywords: implementation science (MeSH), pharmacy, deprescribing, polypharmacy (MeSH), patient satisfaction (MeSH), older adult patients, Lebanon, free medication
Citation: Alaa Eddine N, Schreiber J, El-Yazbi AF, Shmaytilli H and Amin MEK (2023) A pharmacist-led medication review service with a deprescribing focus guided by implementation science. Front. Pharmacol. 14:1097238. doi: 10.3389/fphar.2023.1097238
Received: 13 November 2022; Accepted: 16 January 2023;
Published: 30 January 2023.
Edited by:
Tomoya Tachi, Gifu Pharmaceutical University, JapanReviewed by:
Jérôme Berger, University Center of General Medicine and Public Health, SwitzerlandCopyright © 2023 Alaa Eddine, Schreiber, El-Yazbi, Shmaytilli and Amin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nada Alaa Eddine, bmFkYWFsYWFlZGRpbmVAaG90bWFpbC5jb20=; Mohamed Ezzat Khamis Amin, bW9oYW1lZGV6emF0MjFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.