
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 02 May 2023
Sec. Drugs Outcomes Research and Policies
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1096366
This article is part of the Research TopicEffective Methods to Promote Appropriate Use of MedicinesView all 16 articles
Background and objective: Adverse drug reactions (ADRs) are the main safety concerns of clinically used medications. Accumulating evidence has shown that ADRs can affect men and women differently, which suggests sex as a biological predictor in the risk of ADRs. This review aims to summarize the current state of knowledge on sex differences in ADRs with the focus on the commonly used psychotropic, cardiovascular, and analgesic medications, and to aid clinical decision making and future mechanistic investigations on this topic.
Methods: PubMed search was performed with combinations of the following terms: over 1,800 drugs of interests, sex difference (and its related terms), and side effects (and its related terms), which yielded over 400 unique articles. Articles related to psychotropic, cardiovascular, and analgesic medications were included in the subsequent full-text review. Characteristics and the main findings (male-biased, female-biased, or not sex biased ADRs) of each included article were collected, and the results were summarized by drug class and/or individual drug.
Results: Twenty-six articles studying sex differences in ADRs of six psychotropic medications, ten cardiovascular medications, and one analgesic medication were included in this review. The main findings of these articles suggested that more than half of the ADRs being evaluated showed sex difference pattern in occurrence rate. For instance, lithium was found to cause more thyroid dysfunction in women, and amisulpride induced prolactin increase was more pronounced in women than in men. Some serious ADRs were also found to exert sex difference pattern, such as clozapine induced neutropenia was more prevalent in women whereas simvastatin/atorvastatin-related abnormal liver functions were more pronounced in men.
Adverse drug reactions (ADRs), or drug side effects, are defined as harmful, unintended events resulting from the use of medications. For a new drug entity to be approved by the US Food and Drug Administration (FDA), its safety and potential ADRs must be assessed during the investigational stage. According to a recent study, about 17% of the investigational drugs failed in phase 3 or pivotal trials because of safety concerns (Hwang et al., 2016). Even for the drugs that have been approved for clinical use, their ADRs can still be concerning. Serious ADRs were shown to result in over 100,000 deaths per year, making it the fourth leading cause of death in the US (Giacomini et al., 2007). Other less severe ADRs have been associated with drug discontinuation, poor adherence, and suboptimal treatment outcomes (DiBonaventura et al., 2012). Therefore, it is of great translational value to identify the risk factors for common or serious ADRs, so that clinical monitoring or medication change can be applied accordingly.
As an easy-to-use patient characteristic, sex has been identified as an important predictor in both disease incidence and treatment outcomes. For instance, among non-smokers, women are found to have higher risk of developing lung cancer compared to men (Ragavan and Patel, 2022), whereas women tend to respond better to epidermal growth factor receptor (EGFR) inhibitors, a targeted therapy for lung cancer, than men (Chen et al., 2013). Likewise, the role of sex in the likelihood of ADRs has been evaluated in numerous medications. One illustrative example is zolpidem, a medication used to treat insomnia. Twenty years after its approval to the market, FDA issued Drug Safety Communication (U.S. Food and Drug Administration, 2022) to require a decreased initial dose of zolpidem in women, due to the accumulating evidence indicating that women experience more driving impairment than men under the same recommended dose (Verster and Roth, 2012; Farkas et al., 2013). Subsequent pharmacokinetic studies found that the same dose resulted in significantly higher zolpidem plasma concentration in women than in men (Olubodun et al., 2003; Greenblatt et al., 2014; Greenblatt et al., 2022), which might be able to explain the higher incidence of zolpidem-related ADRs in women. Even though sex difference has gained increasing awareness nowadays, many of the existing clinical trials did not provide sex specific data when evaluating drug efficacy and safety (Hayes and Redberg, 2008; Beery and Zucker, 2011), making it challenging to promote sex-aware prescribing for most of the medications.
Here, we systematically review and summarize the existing literature evaluating sex differences in ADRs to address the fundamental question that whether sex should be considered in drug prescription to prevent/minimize ADRs. If so, for which drugs/drug classes. To summarize and discuss the findings of the included literature, we classified the medications into their therapeutic area. We chose to focus on psychotropic, cardiovascular, and analgesic medications because the above three drug classes are the top categories with sex difference studies available from our web scraping results. Furthermore, the above three drug categories yield the largest number of the “most prescribed drugs” in the US (Fuentes et al., 2018), supporting their broad use and clinical impact. It is to note that oncology mediations were not evaluated in this review due to the inherent cytotoxic effects and the different standard in the ADR recordings (Nguyen et al., 2019). By summarizing the main findings of the commonly used medications in the three drug classes, we aim to facilitate clinical decision making by improving the current understanding of sex differences in ADRs. More importantly, this review highlights the need of further research on sex-aware evaluation of ADRs.
To search for evidence of sex difference in ADRs, we performed web scraping in PubMed using a R package “easyPubMed” (Fantini, 2019). The keywords used for searching were drugs of interests, sex difference (and its related terms), and side effects (and its related terms). The full list of searching terms and other restrictions can be found in Supplementary Table S1. For the drugs of interests, we used a list of 1,819 drugs which have established human targets and the corresponding ADRs recorded in clinical trials from a previously published paper by (Nguyen et al., 2019). Web scraping was performed in March 2022.
All studies resulted from web scraping were considered regardless of study design or date of publication. We first performed an initial screening on the title and abstract to exclude unrelated literature. Then, we did full-text review with the focus on psychotropic, cardiovascular, and analgesic medications. To ensure the drugs included in the review belong to the above three categories, we used Anatomical Therapeutic Chemical (ATC) Classification developed by WHO, (2023) as the reference. Studies were excluded during the full-text review if 1. language was not English; 2. the drug of interest was not in the 1,819 drug list; 3. sex difference was evaluated in drug efficacy rather than ADRs; 4. sex difference in ADRs were caused by a combination of drugs rather than a specific drug; 5. significance level was not reported; 6. The ADR being evaluated is not a well-established ADR as endorsed by Micromedex (IBM MICROMEDEX, 2022). Review articles were also inspected to identify additional original studies to be included.
For each of the study included in this review, the following information was collected: 1. study design; 2. race and age (adults or children) of the study population; 3. health status of the participants (healthy volunteers or patients with specific diseases); 4. number of male and female participantsin the study; 5. drug of interest; 6. dosing regimen; 7. ADRs being inspected in the study; 8. results for sex difference study in ADRs (male-biased ADR, female-biased ADR, or ADR with no sex difference); 9. any pharmacokinetic (PK) measurement if applicable.
Literature search for the 1,819 drugs through web scraping retrieved 448 unique publications. Figure 1 summarized the process of study selection, which resulted in a total of 26 studies included in this review. The characteristics of each study such as drug of interest, study design, number of subjects, dosing regimen, etc. were recorded in Table 1. Sex differences in ADRs were summarized for six psychotropic medications, ten cardiovascular medications, and one analgesic medication. The rest of the result session was structured to first briefly introduce the clinical significance and common ADRs of the medications, followed by the evidence of sex difference in common or serious ADRs related to the drug of interest.
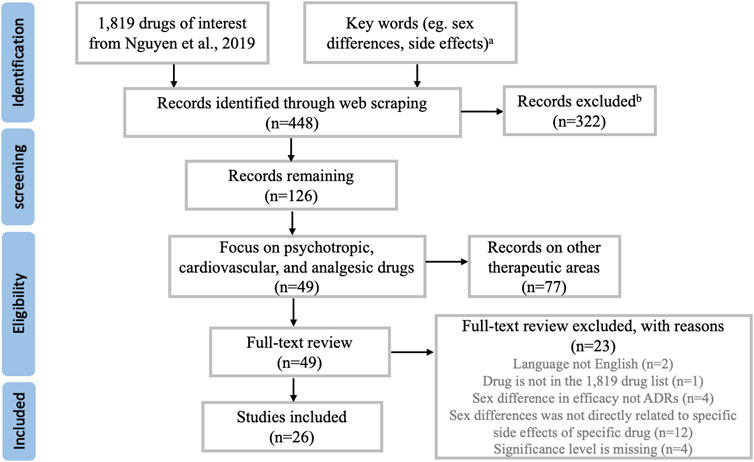
FIGURE 1. Overview of the study selection process. Note (A) A complete list of the keywords used in web scraping can be found in Supplementary Table S1. Note (B) Most studies being excluded here were not related to drug side effects, or not related to human subjects.
Lithium is recommended as the first-line treatment for both acute mania and maintenance phase in bipolar disorder (Yatham et al., 2018). Recent evidence has also suggested the value of lithium in reducing suicidal rate in patients with bipolar or major depression disorder (Smith and Cipriani, 2017). Despite its significant clinical benefits, lithium has gradually become less widely utilized due to its narrow therapeutic index and requirement for frequent blood tests. Some common ADRs of lithium are tremor, polyuria, hypothyroidism, weight gain, and increased thirst. Other more severe ADRs such as bradycardia, sinus node dysfunction, and seizure might happen at a lower rate.
Sex differences were identified in lithium-related thyroid dysfunction, tremor, weight gain, and oedema. Özerdem et al. (2014) assessed sex differences in lithium associated thyroid dysfunction through a retrospective, naturalistic study. One hundred four men and 136 women taking lithium for bipolar disorder with thyroid-stimulating hormone (TSH) level available were included in the study. Using 0.3–3 µIU/mL as the normal range of TSH, the researchers found that significantly fewer female patients (55.9%) were within the normal range compared to male patients (71.2%) (p = 0.016). Notably, the difference in the proportion of normal TSH between male and female patients was not significant in the non-lithium treated group, which suggested that the observed sex differences in thyroid dysfunction is related to lithium treatment rather than the disease state. The vulnerability to thyroid dysfunction in lithium-treated women has also been observed by Chantal Henry in another retrospective study (Henry, 2002). By interviewing 22 male and 38 female patients about lithium ADRs, the researcher found that more female patients than male patients reported new diagnosis of hypothyroidism during the first year of lithium treatment (37% vs. 9%, p < 0.05). Weight gain was also shown to affect more female than male patients (47% vs. 18%, p < 0.05) in the same study whereas tremor was more pronounced in male than female patients (54% vs. 26%, p < 0.05). There is a more recent retrospective study investigating reasons for lithium discontinuation performed by Öhlund et al. (2018). The results showed that female patients were more likely to discontinue lithium due to weight gain (p < 0.01) and oedema (p < 0.01) compared to male patients. To conclude, current evidence suggested that lithium-associated thyroid dysfunction, weight gain, and oedema affect more female patients, while lithium-associated tremor affect more male patients in the treatment of bipolar disorder.
Amisulpride is an atypical antipsychotic with selective blockade of dopamine 2 and dopamine 3 receptors. It has been reported by multiple studies to be an effective and well-tolerated treatment for schizophrenia (Puech et al., 1998; Leucht et al., 2013). More recently, the clinical significance of amisulpride has been evaluated in combination therapies with other antipsychotics such as olanzapine in treatment-resistant schizophrenia (Schmidt-Kraepelin et al., 2022; Woo et al., 2022). On the safety prospective, amisulpride is associated with increased prolactin level, weight gain, hypotension, sexual dysfunction, and prolonged QT interval.
As one of the well-established adverse events of amisulpride, increased prolactin level was reported to be sex-biased by multiple studies (Düring et al., 2019; Hoekstra et al., 2021). In the BeSt InTro study, 93 men and 51 women with schizophrenia diagnosis were randomized to different antipsychotics including amisulpride (Johnsen et al., 2020). When comparing amisulpride induced ADRs between sexes, the researchers found that women had significantly higher mean prolactin level (1,869 mIU/L) then men (920 mIU/L) under amisulpride treatment (p < 0.001) (Hoekstra et al., 2021). Further evaluations showed that the serum level of amisulpride was higher in women than in men after adjusting for the daily dose (p = 0.019), which might explain the observed female-biased ADR. As a potential consequence of elevated prolactin level (Halbreich et al., 2003), sexual disturbance was also evaluated in this study. Using Udvalg for Kliniske Undersøgelser side effect score (UKU score) as the measurement for sexual disturbance, the researchers found that women had more sexual disturbance compared to men with marginal significance (p = 0.051). Notably, similar findings were observed in a separate study conducted by Düring et al., 2019. By following 35 men and 21 women with schizophrenia taking amisulpride monotherapy, the researchers found that prolactin level was higher in women (p < 0.01) compared to men after 6 weeks of amisulpride treatment. Women also reported higher sexual dysfunction load than men did (p < 0.01). In conclusion, amisulpride related prolactin elevation and sexual dysfunction are more common in women than in men in treating schizophrenia, even though the average daily dose is similar between two sexes.
Clozapine and olanzapine are both atypical antipsychotics with similar molecular structures. Clozapine is known as one of the most effective antipsychotics and it is the gold standard for treatment resistant schizophrenia. However, studies have shown that the use of clozapine in schizophrenia is suboptimal (Warnez and Alessi-Severini, 2014), which might involve several reasons including a range of serious adverse events of this medication. For instance, clozapine is associated with myocarditis, cardiomyopathy, and neutropenia, all of which can be life-threatening. Recently, the use of olanzapine in treatment resistant schizophrenia has been widely discussed, as several studies have shown that olanzapine is non-inferior to clozapine in terms of safety and efficacy in hard-to-treat schizophrenia (Tollefson et al., 2001; Bitter et al., 2004; Naber et al., 2005). In terms of common adverse events, both clozapine and olanzapine are recognized as being high risk for weight gain, hyperglycemia, and dyslipidemia (Rummel-Kluge et al., 2010; Kraal et al., 2017).
Even though clozapine and olanzapine have similar profiles in metabolic ADRs, the impact of sex on some of those ADRs were observed to be different between the two medications. In the BeSt InTro study (Hoekstra et al., 2021), sex differences in BMI increase was evaluated in patients randomized to olanzapine group. BMI increase was found to be more pronounced in men (1.48 kg/m2) than in women (0.24 kg/m2) (p < 0.001). Interestingly, the direction of sex difference in treatment-related weight gain was shown to be opposite in patients taking clozapine. In a retrospective study conducted by Lau et al., 2016, 67 men and 50 women attending the outpatient clozapine clinic were recruited and their weight change from 3 months to 12 months after clozapine initiation was calculated. The percentage weight change (weight change divided by the 3-month weight) was found to be significantly higher in women (+5.5%) than in men (+1.3%) (p = 0.01). To analyze sex differences in more serious ADRs of clozapine, Hollingworth et al. reviewed all reported clozapine related neutropenia, myocarditis, and cardiomyopathy cases in Australia monitoring database from 1993 to 2014 (Hollingworth et al., 2018). Sex differences were observed with neutropenia happening more in women (OR 1.45, CI 1.28–1.67), while cardiomyopathy (OR 2.53, CI 1.9–3.37) and myocarditis (OR 1.58, CI 1.34–1.87) happened more in men. These findings suggest sex as an important factor in clozapine and olanzapine related weight gain as well as in more serious adverse events of clozapine.
Aripiprazole is an atypical antipsychotic with numerous FDA approved indications including schizophrenia, bipolar I disorder, autistic disorder, Tourette’s syndrome, and major depressive disorder. Because of its unique receptor binding profile, aripiprazole has different mechanism of actions from other antipsychotics and is sometimes referred as a third-generation antipsychotic (Freudenreich and Freudenreich, 2020). In addition to its confirmed efficacy in various disease areas, aripiprazole has also been shown to induce less adverse events compared with other antipsychotics (Leucht et al., 2013). Some common ADRs of aripiprazole are weight gain, nausea, vomiting, tremor, and fatigue. More serious ADRs such as prolonged QT interval, myocardial infarction, and neutropenia have been observed at a lower rate.
Among aripiprazole-related ADRs, weight gain and some cardiovascular ADRs were shown to impact men and women differently. In a study evaluating sex differences in pharmacokinetics and ADRs of aripiprazole, 89 men and 68 women from multiple aripiprazole bioequivalence clinical trials were recruited (Belmonte et al., 2016). PK parameters were calculated, and physical assessments were performed several times before and after a single dose of 10 mg aripiprazole. The study found that AUC and Cmax of aripiprazole were significantly higher in women (p < 0.05), which indicated a higher aripiprazole exposure in women even under the same dose. In concordance with the observed difference in PK parameters, the blood pressure lowering effects of aripiprazole were found to be more pronounced in women at all measured times (p < 0.01). At 8 h after the dose, the mean systolic blood pressure in women was 105 mmHg versus 116 mmHg in men (p < 0.001). In addition, women were found to have higher heart rate and larger QTc interval compared to men at multiple measured times (p < 0.001). As a well-established ADR of aripiprazole, weight gain has also been shown to impact women and men differently. In the BeSt InTro study (Hoekstra et al., 2021), men were observed to have higher BMI increase compared to women after 52 weeks of aripiprazole use (0.64 kg/m2 vs. −0.04 kg/m2, p = 0.016). In conclusion, sex differences have been observed in multiple aripiprazole related ADRs including weight gain, blood pressure reduction, increased heart rate and QTc. Since some of the conclusions were based on a single dose of aripiprazole, further investigation is warranted to explore the sex difference in long-term aripiprazole use.
Risperidone is a second-generation antipsychotic with serotonin 5-hydroxytryptamine receptor 2 (5-HT2) blocking activities at low doses and dopamine D2 receptor blocking activities at higher doses (Megens et al., 1994). Risperidone is proven to mitigate both positive and negative symptoms of schizophrenia, with less concern about dyskinesia which is a prevalent ADR of most antipsychotics (Labelle et al., 2001). Some common ADRs of risperidone are rash, weight gain, hyperprolactinemia, parkinsonism, and fatigue.
Sex differences in risperidone-associated rash, weight gain, parkinsonism, and dystonia have been evaluated. In a randomized study, 100 men and 90 women taking daily risperidone were followed up for 1 year to assess drug-related ADRs (Pu et al., 2020). At the end of the follow-up period, more female patients reported rash related to risperidone than male patients (p = 0.03). In another post hoc analysis on an open-label study, ADRs in 232 men and 98 women taking risperidone were analyzed for differences between sexes (Labelle et al., 2001). Weight gain was found to happen more in men compared to women with marginal significance (p = 0.085). No sex difference was identified for parkinsonism (p = 0.889) or dystonia (p = 0.512). To conclude, risperidone-related rash is more prevalent in women, whereas no significant sex difference was found in weight gain, parkinsonism, or dystonia related to risperidone treatment in schizophrenia.
Amiodarone is a class III antiarrhythmic drug which is highly effective and widely used in both supraventricular and ventricular arrhythmias (Connolly, 1999). However, amiodarone is also well-known for its potential ADRs on different organs such as thyroid, heart, lung, liver, and eyes. A previous study showed that the prevalence of amiodarone-related ADRs is 15% in the first year, and may increase to 50% in long term use, which would ultimately lead to medication discontinuation in 20%–50% of the patients (van Erven and Schalij, 2010). Some common ADRs of amiodarone are thyroid dysfunction, photosensitivity, and visual disturbance. Amiodarone can also cause more serious adverse events such as bradyarrhythmia, sinus arrest, and hepatotoxicity.
In a prospective cohort study (Essebag et al., 2007), Essebag et al. enrolled 583 men and 390 women with new onset atrial fibrillation (AF) and followed the participants for up to 30 months for amiodarone related ADRs. The researchers found that amiodarone use was associated with increased risk of pacemaker insertion only in women but not in men (HR: 4.69, 95% CI: 1.99–11.05, vs. HR: 1.05, 95% CI: 0.42–2.58, p = 0.02). This significant difference remained after adjusting for daily dose, weight, and the use of other antiarrhythmic medications. In another retrospective study (Roten et al., 2009), Roten et al. reviewed amiodarone associated ADRs in 192 men and 72 women who were referred to clinic for AF management. Their analysis showed that women overall experienced more amiodarone-related ADRs than men (56% vs. 36%, p = 0.046), and there were significant sex differences in the occurrence of phototoxicity under amiodarone treatment (21% in women vs. 8% in men, p = 0.047). The results above suggest that closer monitoring is needed in female population taking amiodarone since they are more likely to experience ADRs such as bradyarrhythmia requiring pacemaker insertion and phototoxicity.
Sotalol is a class III antiarrhythmic agent which is approved for treatment of AF and ventricular arrhythmia. Its efficacy in reducing death and preventing recurrence of arrhythmia has been proven to be superior to other antiarrhythmic drugs (Mason, 1993). However, along with its high efficacy, sotalol can induce some lethal ADRs such as Torsades de pointes (TdP), which may lead to sudden cardiac death. To unveil whether sex is a risk factor for sotalol induced TdP, Lehmann et al. assessed the prevalence of TdP development under sotalol treatment in 3,135 adult patients and compared the results between sexes (Lehmann et al., 1996). TdP was observed in 44 of 2,336 men (1.9%) and in 33 of 799 women (4.1%), and the difference was statistically significant (p < 0.001). Further logistic regression also suggested female sex as a significant risk factor in TdP development (p < 0.0001), even after adjusting for sotalol dose. Since TdP is such a lethal ADR, the results above emphasize the need for closer monitoring of cardiac function in female patients taking sotalol.
Despite the recent advancement in the treatment options for hyperlipidemia and in the prevention of coronary artery disease, statins remain the first line therapy due to their high efficacy, low cost, and relatively safe profile. The pharmacological effects of statins have been proven in lowering the low density lipoprotein cholesterol (LDL-C) by 20%–50%, as well as lowering triglyceride by 10%–20% (Taylor et al., 2013). In terms of safety, statins are well tolerated by the vast majority of patients, but they can still cause some ADRs such as myalgias, urinary tract infection, and increased liver enzymes, which can all lead to treatment interruption or discontinuation. Sex differences in the ADRs of two commonly used statins, simvastatin and atorvastatin, have been evaluated in a prospective cohort study (Smiderle et al., 2014). A total of 164 men and 331 women on simvastatin or atorvastatin treatment participated in the study, and they were evaluated every 3 months for statin related ADRs. The researchers observed higher occurrence of myalgia in women than in men (25.9% vs. 20.3%, p = 0.002), while more creatinine phosphokinase (CPK) increase and/or elevated liver enzymes were observed in men than in women (11.1% vs. 7.6%, p = 0.017) under simvastatin or atorvastatin treatment. These results request more attention on the role of sex in statin associated ADRs, and further studies are warranted to explore the potential mechanism of the observed sex differences.
Angiotensin converting enzyme (ACE) inhibitors are effective antihypertensives working through inhibition of renin-angiotensin system. ACE inhibitors are recommended by multiple guidelines as first-line treatment for hypertension (Williams and Mancia, 2018), and their use has been expanded to other disease areas such as acute myocardial infarction, heart failure, and kidney diseases. While most patients tolerate ACE inhibitors well, some patients can still experience hypotension, dizziness, dry cough, and other more serious ADRs such as angioedema and renal impairment during the treatment.
Evidence of sex differences in ACE inhibitor induced ADRs was found in lisinopril, enalapril, and captopril. Interestingly, most of the sex difference analysis has been focused on ACE inhibitor induced bronchospasm and cough. In a retrospective study (Wood, 1995), the prevalence of new onset bronchospasm and cough was assessed in 1,013 patients taking captopril, lisinopril, or enalapril. Women were found to experience more bronchospasm (58% vs. 42%) and cough (59% vs. 41%) reactions compared to men; however, the difference was not statistically significant. Notably, patients under the three different treatments were not separated when the prevalence was reported, which means that the rate of bronchospasm and cough in each individual medication group was unknown. In another randomized, double-blind clinical trial investigating sex differences in efficacy and safety of antihypertensives, 3,535 hypertensive patients (1,209 men and 2,326 women) were recruited and followed during 8 weeks of treatment (Fan et al., 2008). In patients randomized to captopril group, the prevalence of cough was found to be significantly higher in women than in men (14.3% vs. 8.4%, p = 0.005). This female-biased ACE inhibitor induced cough was also observed in lisinopril by Os et al. in a randomized, double-blind clinical trial (Os et al., 1994). In this study, 206 men and 206 women were randomized to lisinopril group, and cough was found to happen three times more often in women than in men (12.6% vs. 4.4%, p = 0.0027). Overall, although some non-significant findings exist, more evidence suggests an increased risk of ACE inhibitor induced cough in women.
Both amlodipine and nifedipine are dihydropyridine calcium channel blockers (CCBs) which are widely used for treating hypertension, stable and variant angina. Although structurally similar, amlodipine differs from nifedipine and other dihydropyridine CCBs by its long half-life, enabling once daily dosing (Haria and WagstaffAmlodipine, 1995). In terms of ADRs, both amlodipine and nifedipine are observed to cause hypotension, palpations, edema, and flushing with slightly different occurrence rate.
Sex difference studies are available for amlodipine-related neurological ADRs and nifedipine-related cough and edema. Abad Santos et al. conducted a bioequivalent study in 36 healthy volunteers (18 men and 18 women) to study sex differences in amlodipine induced ADRs as their secondary objective (Abad-Santos et al., 2005). All subjects received a single 10 mg dose of each amlodipine formulation with a 14-day washout period. After statistical analysis, the researchers did not find any significant difference between men and women in amlodipine related headache (44% vs. 28%), dizziness (11% vs. 28%), or tiredness (17% vs. 6%). Sex difference in nifedipine-related edema was studied in a prospective study by Fan et al. (Fan et al., 2008). A total of 327 men and 620 women were randomized to nifedipine sustained release (SR) group and were followed up for 8 weeks to evaluate drug related ADRs. Women were found to be more susceptible to ADRs related to nifedipine SR than their men counterpart (15.8% vs. 9.8%, p = 0.017), with intolerable edema being the main type of ADR observed. In another study assessing the role of sex in nifedipine associated cough, 218 men and 198 women were randomized to nifedipine group and were followed up for 10 weeks (Os et al., 1994). No sex difference was identified by this study in nifedipine related cough (men 3% vs. women 2.8%). To conclude, women were found to experience more intolerable edema from nifedipine SR, while no sex difference was found in nifedipine associated cough or amlodipine associated headache, dizziness, or tiredness.
Atenolol is one of the drugs classified as beta-blocker, and it is used to treat several conditions such as hypertension, cardiac dysrhythmia, angina pectoris, etc. Recently, the effectiveness of atenolol has been assessed in other disease areas including anxiety (Armstrong and Kapolowicz, 2020). In terms of its safety profile, most patients tolerate atenolol well. Bradyarrhythmia, hypotension, dizziness, and fatigue are the most common ADRs observed with atenolol treatment. There is one study evaluating sex difference in ADRs related to atenolol in treating hypertension. After following 191 men and 403 women on atenolol therapy for 8 weeks, the researchers found that fatigue and bradycardia were most common ADRs during treatment period, and there was no sex difference in the occurrence rate of those ADRs (men 15.8% vs. women 11.6%, p = 0.497) (Fan et al., 2008).
Opioids are widely used in the management of moderate to severe pain. As one of the potent opioid analgesia, morphine is recommended for pain management in various disease types such as cancer, acute pulmonary edema, and myocardial infarction (Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology ESC, 2012; Wiffen et al., 2016). However, the use of morphine has been cautioned due to a wide range of ADRs including pruritus, nausea, vomiting, dizziness, urinary retention, and more seriously, drug dependence, respiratory depression, and cardiac arrest.
Sex differences have been investigated in multiple morphine induced ADRs such as gastrointestinal ADRs and respiratory depression. In a prospective observational study undertaken by Sadhasivam et al. (Sadhasivam et al., 2015), 219 children undergoing tonsillectomy or adenotonsillectomy (T/TC) surgery were recruited and the efficacy and safety of morphine were compared between boys and girls. No sex difference was observed in respiratory depression (10% in boys vs. 7% in girls, p = 0.81), postoperative nausea and vomiting (6% in boys vs. 9% in girls, p = 0.2), and pruritus (41% in boys vs. 33% in girls, p = 0.54). Likewise, sex differences in morphine related ADRs were also assessed by Fillingim et al. in healthy adult women (n = 61) and men (n = 39) (Fillingim et al., 2005). All subjects in the study were intravenously administered 0.08 mg/kg single dose of morphine, after which the incidence of pruritus, nausea, and emesis were assessed. Similar to the previously described study, no evidence of sex difference was found in pruritus (8% in men vs. 10% in women). However, the prevalence of nausea and emesis were found to be significantly higher in women than in men (nausea 35% vs. 3%, emesis 18% vs. 0, p < 0.005). The results from the two studies above indicates that the role of sex in morphine related nausea and vomiting might be different in different disease states and/or age groups.
Despite the careful premarketing evaluation and postmarketing surveillance, adverse drug reactions remain a global public health issue leading to morbidity, mortality, and huge financial loss. In the United States, severe ADRs have been estimated to occur more than 2 million times in hospitalized patients every year, which ultimately result in 100,000 deaths (Giacomini et al., 2007). The financial burden caused by ADRs has been calculated to be equivalent to 16% of total healthcare expenditures in the US in 2016 (Watanabe et al., 2018). Although some recent efforts have been invested into ADR prediction (Lounkine et al., 2012; Mohsen et al., 2021; Zhang et al., 2021), it remains challenging to identify patients with high risk to develop certain ADRs clinically, which might be due to lack of data, limited sample size of ADR studies, and the complex nature of ADR generation, etc. As a ready-to-use clinical character, sex has recently been shown to be an influencer in the risk of ADR development (Tharpe, 2011; Nakagawa and Kajiwara, 2015). Here, we systematically reviewed the role of sex in the risk of ADRs caused by commonly used psychotropic, cardiovascular, and analgesic medications. Our findings suggested that several common and/or severe ADRs have difference prevalence in men versus in women as shown in Figure 2.
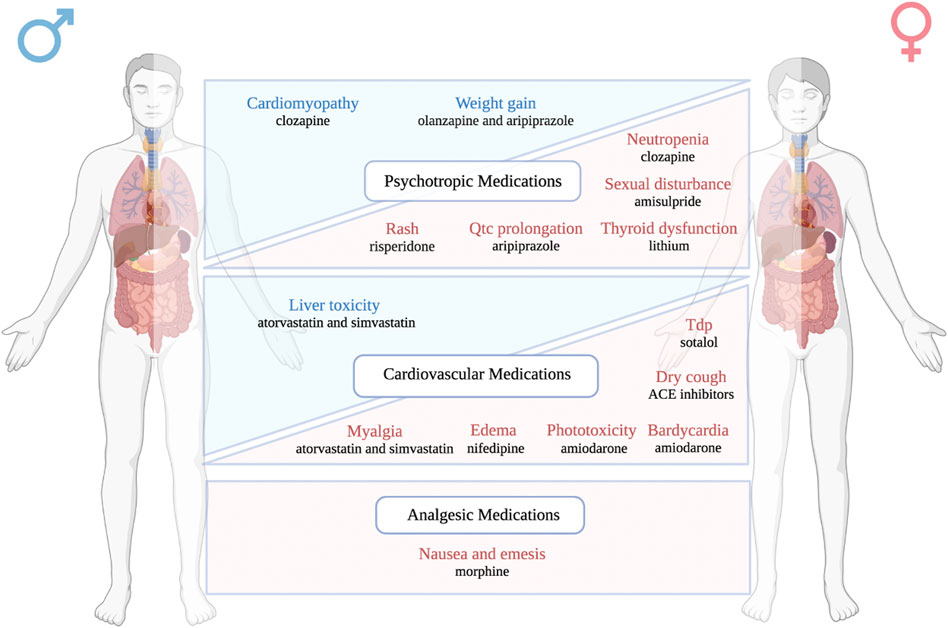
FIGURE 2. A schematic figure listing the main ADRs showing sex differences in occurrence rate. The adverse drug reactions highlighted in blue are male-biased ADRs, while the adverse drug reactions highlighted in red are female-biased ADRs. Drugs and their associated sex biased ADRs are classified into three different therapeutic categories: psychotropic medications, cardiovascular medications, and analgesic medications.
Quantitively, we included studies evaluating sex differences in ADR occurrence for 6 psychotropic medications, with 18 drug-specific ADRs showing sex differences, 15 drug-specific ADRs showing no sex difference; 10 cardiovascular medications, with 8 drug-specific ADRs showing sex differences, 4 drug-specific ADRs showing no sex difference; 1 analgesic medication with 3 drug-related ADRs showing no sex difference. The 17 drugs discussed in this review cover a variety of disease areas such as bipolar disorder, schizophrenia, arrhythmia, hypertension, hyperlipidemia, pain, etc. Notably, as an important class of psychotropic medication, the antidepressant medications in our searching list did not result in any study showing sex differences in ADR, which implies that more sex-awareness is needed for this particular drug class. A complete list of the sex difference findings in ADR can be found in Table 2.
Intriguingly, we identified some well-established ADRs which were shown to exert sex difference patterns by multiple studies. For instance, lithium was found to cause more thyroid dysfunction in women than in men (Henry, 2002; Özerdem et al., 2014), and amisulpride was shown to increase prolactin level more in women than in men (Düring et al., 2019; Hoekstra et al., 2021). In addition to the consistent findings on sex biased ADRs, sex difference research in serious ADRs is also worth mentioning. As a rare but life threatening ADR of clozapine, neutropenia was found to happen more in women than in men in a retrospective study (Hollingworth et al., 2018), suggesting that more surveillance is needed for women with long-term clozapine use. Similarly, after reviewing the ADRs in patients treated with sotalol for arrhythmia, researchers found that more women developed TdP, a fatal ADR of sotalol, than men (Lehmann et al., 1996). These clinically observed ADRs should serve as stimulants for both consideration of sex in drug selection and ADR monitoring, as well as future studies to explore the underlying mechanism behind the observed sex differences.
In addition to the findings showing consistent sex differences in certain ADRs, conflicting results also exist, which makes it difficult to draw a certain conclusion. For instance, morphine-associated nausea and vomiting was concluded as female-biased by Fillingim et al. (Fillingim et al., 2005), whereas no sex difference was observed in the same ADR in another study (Sadhasivam et al., 2015). After carefully reviewed the two studies, we found that the former study recruited healthy adult volunteers, while the latter one recruited children undergoing tonsillectomy or adenotonsillectomy (T/TC) surgery. The distinct target populations made it difficult to compare the results between the two studies, since both age and disease state can impact the risk of drug ADRs (Lavan and Gallagher, 2016). Similarly, other discrepancies in the study design (dosing regimen, follow-up time, definition of certain ADR, ethnicity group of the participants, etc.) also introduce complexities when results were compared between studies. Therefore, we suggest that more thorough study design and more robust methods such as meta-analyses are needed to better understand sex differences in the risk of ADR generation.
For all the studies that are included in this review, we searched the article for potential mechanisms that may explain the observed sex differences. Surprisingly, only five out of the twenty-six studies discussed the putative underlying mechanisms, all of which are related to differences in the serum concentration of the medication between men and women. However, in-depth discussion on the reason of the differences in PK profile between sexes is missing in those studies. In fact, there are recent publications summarizing how sex might impact PK and drug response. It is believed that the intracellular and extracellular water volumes, amount of fat mass, expression of drug metabolizing enzymes and transporters, and glomerular filtration might be different between men and women, which can impact every aspect of absorption, distribution, metabolism, and elimination of a medication (Gandhi et al., 2004; Soldin et al., 2011; Yang et al., 2012). More broadly speaking, other factors such as genetics, hormone, immune system, microorganisms, and environment could also contribute to sex differences in drug efficacy and safety by impacting PK and/or pharmacodynamic of medications (Arnold, 2017; Weersma et al., 2020; Cheng et al., 2022; Huang et al., 2023). Therefore, we suggest that future studies need to consider a wider range of potential mechanisms to better understand the observed sex differences in drug ADRs.
Our study has some limitations. Although the 1,819 drug list used for web scraping covers the majority of the most prescribed cardiovascular, psychotropic, and analgesic medications (Fuentes et al., 2018) (22/24 top 100 cardiovascular medications, 15/15 top 100 psychotropic medications, 9/9 top 100 analgesic medications are in drug list), we are missing two commonly prescribed cardiovascular medications which are furosemide and aspirin. We manually searched evidence of sex differences in ADR related to the above two medications using the same criteria as listed in Figure 1, which resulted in one study showing sex differences in reported bleeding events related to aspirin (Rydberg et al., 2014). This retrospective study found that women were at a lower risk of aspirin related bleeding compared to men (RR 0.8, 95% CI 0.66-1.96). Since we did not use an exhaustive list of cardiovascular, psychotropic, and analgesic medications, one limitation of our study is that we might miss evidence of sex differences in ADRs related to some less commonly used medications under the three categories above. Second, the distinct quality and study design (dosage, route of administration, target population, etc.) of the included studies introduce complexities when comparing the results among the studies. For instance, we found that differences in the risk of bias of the included studies may contribute to conflicting results. Using Risk Of Bias In Non-randomized Studies - of Intervention (ROBINS-I) as the tool (Sterne et al., 2016), we found that the study conducted by Müller et al. (2006) has a moderate risk of bias due to confounding because of its naturalistic study design and the different dosage of amisulpride used by participants. In comparison, the study conducted by Hoekstra et al. (2021) has a low risk of bias due to confounding since the patients received the same dose of amisulpride. This difference in risk of bias may be able to explain the conflicting finding of the two studies on sex differences in sexual disfunction related to amisulpride. Therefore, we suggest that the results of this review should be carefully interpreted with the quality and design of the original study. Third, our search results are exclusively generated from PubMed search. A more comprehensive list of relevant studies might be achieved by including other databases such as Cochrane Library and Web of Science.
Overall, sex differences in ADRs have been studied and identified in a handful of psychotropic, cardiovascular, and analgesic medications. However, to better understand the underlying mechanism of the observed sex differences in ADRs, further studies with more comprehensive study design are warranted. Some key factors to consider are clearly documented ADRs in each sex group, collection of PK data, pharmacogenomic data, measurement of microorganism, document of environmental exposure, etc. It is of great clinical significance to understand how sex can impact the risk of ADRs so that more personalized approaches could be applied to minimize the burden caused by ADRs.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
YS, LC, and RSH conceived the idea. YS and YH performed the web scraping. YS, LC, and YZ screened the literature resulted from web scraping, and collected the characteristics and main findings of each paper included in this review. YS wrote the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by NIH/NCI Grant No. R01CA229618 which RSH received.
We would like to thank BioRender as Figure 2 of this manuscript was made in BioRender.com.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1096366/full#supplementary-material
Abad-Santos, F., Novalbos, J., Gálvez-Múgica, M. A., Gallego-Sandín, S., Almeida, S., Vallée, F., et al. (2005). Assessment of sex differences in pharmacokinetics and pharmacodynamics of amlodipine in a bioequivalence study. Pharmacol. Res. 51 (5), 445–452. doi:10.1016/j.phrs.2004.11.006
Armstrong, C., and Kapolowicz, M. R. (2020). A preliminary investigation on the effects of atenolol for treating symptoms of anxiety. Mil. Med. 185 (11–12), e1954–e1960. doi:10.1093/milmed/usaa170
Arnold, A. P. (2017). A general theory of sexual differentiation. J. Neurosci. Res. 95 (1–2), 291–300. doi:10.1002/jnr.23884
Beery, A. K., and Zucker, I. (2011). Sex bias in neuroscience and biomedical research. Neurosci. Biobehav Rev. 35 (3), 565–572. doi:10.1016/j.neubiorev.2010.07.002
Belmonte, C., Ochoa, D., Román, M., Cabaleiro, T., Talegón, M., Sánchez-Rojas, S. D., et al. (2016). Evaluation of the relationship between pharmacokinetics and the safety of aripiprazole and its cardiovascular effects in healthy volunteers. J. Clin. Psychopharmacol. 36 (6), 608–614. doi:10.1097/JCP.0000000000000577
Bijur, P. E., Esses, D., Birnbaum, A., Chang, A. K., Schechter, C., and Gallagher, E. J. (2008). Response to morphine in male and female patients: Analgesia and adverse events. Clin. J. Pain 24 (3), 192–198. doi:10.1097/AJP.0b013e31815d3619
Bitter, I., Dossenbach, M. R. K., Brook, S., Feldman, P. D., Metcalfe, S., Gagiano, C. A., et al. (2004). Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 28 (1), 173–180. doi:10.1016/j.pnpbp.2003.09.033
Chen, X., Liu, Y., Røe, O. D., Qian, Y., Guo, R., Zhu, L., et al. (2013). Gefitinib or erlotinib as maintenance therapy in patients with advanced stage non-small cell lung cancer: A systematic review. PLoS One 8 (3), e59314. doi:10.1371/journal.pone.0059314
Cheng, S., Flora, D. R., Rettie, A. E., Brundage, R. C., and Tracy, T. S. (2022). Pharmacokinetic modeling of warfarin І - model-based analysis of warfarin enantiomers with a target mediated drug disposition model reveals CYP2C9 genotype-dependent drug-drug interactions of S-warfarin. Drug Metab. Dispos. 50 (9), 1287–1301. doi:10.1124/dmd.122.000876
Connolly, S. J. (1999). Evidence-based analysis of amiodarone efficacy and safety. Circulation 100 (19), 2025–2034. doi:10.1161/01.cir.100.19.2025
Coulter, D. M., and Edwards, I. R. (1987). Cough associated with captopril and enalapril. Br. Med. J. Clin. Res. Ed. 294 (6586), 1521–1523. doi:10.1136/bmj.294.6586.1521
DiBonaventura, M., Gabriel, S., Dupclay, L., Gupta, S., and Kim, E. (2012). A patient perspective of the impact of medication side effects on adherence: Results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry 12 (1), 20. doi:10.1186/1471-244X-12-20
Düring, S. W., Mø, N., Bak, N., Glenthøj, B. Y., and Ebdrup, B. H. (2019). Sexual dysfunction and hyperprolactinemia in schizophrenia before and after six weeks of D2/3 receptor blockade - an exploratory study. Psychiatry Res. 274, 58–65. doi:10.1016/j.psychres.2019.02.017
Essebag, V., Reynolds, M. R., Hadjis, T., Lemery, R., Olshansky, B., Buxton, A. E., et al. (2007). Sex differences in the relationship between amiodarone use and the need for permanent pacing in patients with atrial fibrillation. Arch. Intern Med. 167 (15), 1648–1653. doi:10.1001/archinte.167.15.1648
Fan, X., Han, Y., Sun, K., Wang, Y., Xin, Y., Bai, Y., et al. (2008). Sex differences in blood pressure response to antihypertensive therapy in Chinese patients with hypertension. Ann. Pharmacother. 42 (12), 1772–1781. doi:10.1345/aph.1L036
Fantini, D. (2019). easyPubMed: Search and retrieve scientific publication records from PubMed. Available at: https://CRAN.R-project.org/package=easyPubMed (Accessed Feb 15 2023).
Farkas, R. H., Unger, E. F., and Temple, R. (2013). Zolpidem and driving impairment--identifying persons at risk. N. Engl. J. Med. 369 (8), 689–691. doi:10.1056/NEJMp1307972
Fillingim, R. B., Ness, T. J., Glover, T. L., Campbell, C. M., Hastie, B. A., Price, D. D., et al. (2005). Morphine responses and experimental pain: Sex differences in side effects and cardiovascular responses but not analgesia. J. Pain 6 (2), 116–124. doi:10.1016/j.jpain.2004.11.005
Freudenreich, O. (2020). “Antipsychotics: Overview,”. [Internet] in Psychotic disorders: A practical guide. Editor O. Freudenreich (Cham: Springer International Publishing).
Fuentes, A. V., Pineda, M. D., and Venkata, K. C. N. (2018). Comprehension of top 200 prescribed drugs in the US as a resource for pharmacy teaching, training and practice. Pharm. (Basel) 6 (2), 43. doi:10.3390/pharmacy6020043
Gandhi, M., Aweeka, F., Greenblatt, R. M., and Blaschke, T. F. (2004). Sex differences in pharmacokinetics and pharmacodynamics. Annu. Rev. Pharmacol. Toxicol. 44, 499–523. doi:10.1146/annurev.pharmtox.44.101802.121453
Giacomini, K. M., Krauss, R. M., Roden, D. M., Eichelbaum, M., Hayden, M. R., and Nakamura, Y. (2007). When good drugs go bad. Nature 446 (7139), 975–977. doi:10.1038/446975a
Greenblatt, D. J., Harmatz, J. S., Singh, N. N., Steinberg, F., Roth, T., Moline, M. L., et al. (2014). Gender differences in pharmacokinetics and pharmacodynamics of zolpidem following sublingual administration. J. Clin. Pharmacol. 54 (3), 282–290. doi:10.1002/jcph.220
Greenblatt, D. J., Harmatz, J. S., von Moltke, L. L., Wright, C. E., Durol, A. L., Harrel-Joseph, L. M., et al. (2022). Comparative kinetics and response to the benzodiazepine agonists triazolam and zolpidem: Evaluation of sex-dependent differences. Available at: https://pubmed.ncbi.nlm.nih.gov/10773013/(Accessed Sep 14, 2022).
Halbreich, U., Kinon, B. J., Gilmore, J. A., and Kahn, L. S. (2003). Elevated prolactin levels in patients with schizophrenia: Mechanisms and related adverse effects. Psychoneuroendocrinology 1, 53–67. doi:10.1016/s0306-4530(02)00112-9
Haria, M., and WagstaffAmlodipine, A. J. (1995). Amlodipine. A reappraisal of its pharmacological properties and therapeutic use in cardiovascular disease. Drugs 50 (3), 560–586. doi:10.2165/00003495-199550030-00009
Hayes, S. N., and Redberg, R. F. (2008). Dispelling the myths: Calling for sex-specific reporting of trial results. Mayo Clin. Proc. 83 (5), 523–525. doi:10.4065/83.5.523
Henry, C. (2002). Lithium side-effects and predictors of hypothyroidism in patients with bipolar disorder: Sex differences. J. Psychiatry Neurosci. 27 (2), 104–107.
Hoekstra, S., Bartz-Johannessen, C., Sinkeviciute, I., Reitan, S. K., Kroken, R. A., Løberg, E. M., et al. (2021). Sex differences in antipsychotic efficacy and side effects in schizophrenia spectrum disorder: Results from the BeSt InTro study. NPJ Schizophr. 7 (1), 39. doi:10.1038/s41537-021-00170-3
Hollingworth, S. A., Winckel, K., Saiepour, N., Wheeler, A. J., Myles, N., and Siskind, D. (2018). Clozapine-related neutropenia, myocarditis and cardiomyopathy adverse event reports in Australia 1993-2014. Psychopharmacol. Berl. 235 (7), 1915–1921. doi:10.1007/s00213-018-4881-0
Huang, Y., Shan, Y., Zhang, W., Lee, A. M., Li, F., Stranger, B. E., et al. (2023). Deciphering genetic causes for sex differences in human health through drug metabolism and transporter genes. Nat. Commun. 14 (1), 175. doi:10.1038/s41467-023-35808-6
Hwang, T. J., Carpenter, D., Lauffenburger, J. C., Wang, B., Franklin, J. M., and Kesselheim, A. S. (2016). Failure of investigational drugs in late-stage clinical development and publication of trial results. JAMA Intern Med. 176 (12), 1826–1833. doi:10.1001/jamainternmed.2016.6008
IBM MICROMEDEX (2022). IBM watson health, greenwood village, Colorado, USA. Available at: www.micromedexsolutions.com (Accessed Jun 20 2022).
Ishani, A., Weinhandl, E., Zhao, Z., Gilbertson, D. T., Collins, A. J., Yusuf, S., et al. (2005). Angiotensin-converting enzyme inhibitor as a risk factor for the development of anemia, and the impact of incident anemia on mortality in patients with left ventricular dysfunction. J. Am. Coll. Cardiol. 45 (3), 391–399. doi:10.1016/j.jacc.2004.10.038
Johnsen, E., Kroken, R. A., Løberg, E. M., Rettenbacher, M., Joa, I., Larsen, T. K., et al. (2020). Amisulpride, aripiprazole, and olanzapine in patients with schizophrenia-spectrum disorders (BeSt InTro): A pragmatic, rater-blind, semi-randomised trial. Lancet Psychiatry 7 (11), 945–954. doi:10.1016/S2215-0366(20)30341-2
Kraal, A. Z., Ward, K. M., and Ellingrod, V. L. (2017). Sex differences in antipsychotic related metabolic functioning in schizophrenia spectrum disorders. Psychopharmacol. Bull. 47 (2), 8–21.
Labelle, A., Light, M., and Dunbar, F. (2001). Risperidone treatment of outpatients with schizophrenia: No evidence of sex differences in treatment response. Can. J. Psychiatry 46 (6), 534–541. doi:10.1177/070674370104600608
Lau, S. L., Muir, C., Assur, Y., Beach, R., Tran, B., Bartrop, R., et al. (2016). Predicting weight gain in patients treated with clozapine: The role of sex, body mass index, and smoking. J. Clin. Psychopharmacol. 36 (2), 120–124. doi:10.1097/JCP.0000000000000476
Lavan, A. H., and Gallagher, P. (2016). Predicting risk of adverse drug reactions in older adults. Ther. Adv. Drug Saf. 7 (1), 11–22. doi:10.1177/2042098615615472
Lehmann, M. H., Hardy, S., Archibald, D., MacNeil, D. J., Quart, B., and MacNeil, D. J. (1996). Sex difference in risk of torsade de pointes with d,l-sotalol. Circulation 94 (10), 2535–2541. doi:10.1161/01.cir.94.10.2535,
Leucht, S., Cipriani, A., Spineli, L., Mavridis, D., Orey, D., Richter, F., et al. (2013). Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet 382 (9896), 951–962. doi:10.1016/S0140-6736(13)60733-3
Lounkine, E., Keiser, M. J., Whitebread, S., Mikhailov, D., Hamon, J., Jenkins, J. L., et al. (2012). Large-scale prediction and testing of drug activity on side-effect targets. Nature 486 (7403), 361–367. doi:10.1038/nature11159
Mason, J. W. (1993). A comparison of seven antiarrhythmic drugs in patients with ventricular tachyarrhythmias. Electrophysiologic Study versus Electrocardiographic Monitoring Investigators. N. Engl. J. Med. 329 (7), 452–458. doi:10.1056/NEJM199308123290702
Megens, A. A., Awouters, F. H., Schotte, A., Meert, T. F., Dugovic, C., Niemegeers, C. J., et al. (1994). Survey on the pharmacodynamics of the new antipsychotic risperidone. Psychopharmacol. Berl. 114 (1), 9–23. doi:10.1007/BF02245439
Mohsen, A., Tripathi, L. P., and Mizuguchi, K. (2021). Deep learning prediction of adverse drug reactions in drug discovery using open TG–GATEs and FAERS databases. Front. Drug Discov. 1, 768792. [Internet]. doi:10.3389/fddsv.2021.768792
Müller, M. J., Regenbogen, B., Sachse, J., Eich, F. X., Härtter, S., and Hiemke, C. (2006). Gender aspects in the clinical treatment of schizophrenic inpatients with amisulpride: A therapeutic drug monitoring study. Pharmacopsychiatry 39 (2), 41–46. doi:10.1055/s-2006-931540
Naber, D., Riedel, M., Klimke, A., Vorbach, E. U., Lambert, M., Kühn, K. U., et al. (2005). Randomized double blind comparison of olanzapine vs. clozapine on subjective well-being and clinical outcome in patients with schizophrenia. Acta Psychiatr. Scand. 111 (2), 106–115. doi:10.1111/j.1600-0447.2004.00486.x
Nakagawa, K., and Kajiwara, A. (2015). Female sex as a risk factor for adverse drug reactions. Nihon rinsho Jpn. J. Clin. Med. [Internet] 73 (4), 25936145.
Nguyen, P. A., Born, D. A., Deaton, A. M., Nioi, P., and Ward, L. D. (2019). Phenotypes associated with genes encoding drug targets are predictive of clinical trial side effects. Nat. Commun. 10 (1), 1579. doi:10.1038/s41467-019-09407-3
Öhlund, L., Ott, M., Oja, S., Bergqvist, M., Lundqvist, R., Sandlund, M., et al. (2018). Reasons for lithium discontinuation in men and women with bipolar disorder: A retrospective cohort study. BMC Psychiatry 18 (1), 37. doi:10.1186/s12888-018-1622-1
Olubodun, J. O., Ochs, H. R., von Moltke, L. L., Roubenoff, R., Hesse, L. M., Harmatz, J. S., et al. (2003). Pharmacokinetic properties of zolpidem in elderly and young adults: Possible modulation by testosterone in men. Br. J. Clin. Pharmacol. 56 (3), 297–304. doi:10.1046/j.0306-5251.2003.01852.x
Os, I., Bratland, B., Dahlöf, B., Gisholt, K., Syvertsen, J. O., and Tretli, S. (1994). Female preponderance for lisinopril-induced cough in hypertension. Am. J. Hypertens. 7 (11), 1012–1015. doi:10.1093/ajh/7.11.1012
Özerdem, A., Tunca, Z., Çımrın, D., Hıdıroğlu, C., and Ergör, G. (2014). Female vulnerability for thyroid function abnormality in bipolar disorder: Role of lithium treatment. Bipolar Disord. 16 (1), 72–82. doi:10.1111/bdi.12163
Pu, C., Huang, B., Zhou, T., Cheng, Z., Wang, Y., Shi, C., et al. (2020). Gender differences in the first-year antipsychotic treatment for Chinese first-episode schizophrenia. Neuropsychiatr. Dis. Treat. 16, 3145–3152. doi:10.2147/NDT.S280719
Puech, A., Fleurot, O., and Rein, W. (1998). Amisulpride, and atypical antipsychotic, in the treatment of acute episodes of schizophrenia: A dose-ranging study vs. haloperidol. The amisulpride study group. Acta Psychiatr. Scand. 98 (1), 65–72. doi:10.1111/j.1600-0447.1998.tb10044.x
Ragavan, M. V., and Patel, M. I. (2022). Understanding sex disparities in lung cancer incidence: Are women more at risk? Lung Cancer Manag. 9 (3), LMT34.
Roten, L., Rimoldi, S. F., Schwick, N., Sakata, T., Heimgartner, C., Fuhrer, J., et al. (2009). Gender differences in patients referred for atrial fibrillation management to a tertiary center. Pacing Clin. Electrophysiol. 32 (5), 622–626. doi:10.1111/j.1540-8159.2009.02335.x
Rummel-Kluge, C., Komossa, K., Schwarz, S., Hunger, H., Schmid, F., Lobos, C. A., et al. (2010). Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 123 (2–3), 225–233. doi:10.1016/j.schres.2010.07.012
Rydberg, D. M., Holm, L., Mejyr, S., Loikas, D., Schenck-Gustafsson, K., von Euler, M., et al. (2014). Sex differences in spontaneous reports on adverse bleeding events of antithrombotic treatment. Eur. J. Clin. Pharmacol. 70 (1), 117–126. doi:10.1007/s00228-013-1591-8
Sadanaga, T., Yoshimura, M., Sakamoto, T., Sumida, H., and Ogawa, H. (2009). Enalapril-induced cough is associated with non-severe heart failure. Int. J. Cardiol. 135 (2), 275–276. doi:10.1016/j.ijcard.2008.03.063
Sadhasivam, S., Chidambaran, V., Olbrecht, V. A., Costandi, A., Clay, S., Prows, C. A., et al. (2015). Opioid-related adverse effects in children undergoing surgery: Unequal burden on younger girls with higher doses of opioids. Pain Med. 16 (5), 985–997. doi:10.1111/pme.12660
Schmidt-Kraepelin, C., Feyerabend, S., Engelke, C., Riesbeck, M., Meisenzahl-Lechner, E., Verde, P. E., et al. (2022). Amisulpride and olanzapine combination treatment versus each monotherapy in acutely ill patients with schizophrenia in Germany (COMBINE): A double-blind randomised controlled trial. Lancet Psychiatry 9 (4), 291–306. doi:10.1016/S2215-0366(22)00032-3
Smiderle, L., Lima, L. O., Hutz, M. H., Van der Sand, C. R., Van der Sand, L. C., Ferreira, M. E. W., et al. (2014). Evaluation of sexual dimorphism in the efficacy and safety of simvastatin/atorvastatin therapy in a southern Brazilian cohort. Arq. Bras. Cardiol. 103 (1), 33–40. doi:10.5935/abc.20140085
Smith, K. A., and Cipriani, A. (2017). Lithium and suicide in mood disorders: Updated meta-review of the scientific literature. Bipolar Disord. 19 (7), 575–586. doi:10.1111/bdi.12543
Soldin, O. P., Chung, S. H., and Mattison, D. R. (2011). Sex differences in drug disposition. J. Biomed. Biotechnol. 2011, 187103. doi:10.1155/2011/187103
Sterne, J. A., Hernán, M. A., Reeves, B. C., Savović, J., Berkman, N. D., Viswanathan, M., et al. (2016). ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919. doi:10.1136/bmj.i4919i4919
Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) (2012). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 33 (20), 2569–2619. doi:10.1093/eurheartj/ehs215
Taylor, F., Huffman, M. D., Macedo, A. F., Moore, T. H. M., Burke, M., Davey Smith, G., et al. (2013). Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2021, CD004816. doi:10.1002/14651858.CD004816.pub5
Tharpe, N. (2011). Adverse drug reactions in women’s health care. J. Midwifery Womens Health 56 (3), 205–213. doi:10.1111/j.1542-2011.2010.00050.x
Tollefson, G. D., Birkett, M. A., Kiesler, G. M., and Wood, A. J. (2001). Double-blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biol. Psychiatry 49 (1), 52–63. doi:10.1016/s0006-3223(00)01026-x
U.S. Food and Drug Administration (2022). Drug Safety Communication Risk of next morning impairment after use of insomnia drugs. Available at: https://www.fda.gov/files/drugs/published/Drug-Safety-Communication–Risk-of-next-morning-impairment-after-use-of-insomnia-drugs–FDA-requires-lower-recommended-doses-for-certain-drugs-containing-zolpidem-%28Ambien–Ambien-CR–Edluar–and-Zolpimist%29.pdf (Accessed Sep 14 2022).
van Erven, L., and Schalij, M. J. (2010). Amiodarone: An effective antiarrhythmic drug with unusual side effects. Heart 96 (19), 1593–1600. doi:10.1136/hrt.2008.152652
Verster, J. C., and Roth, T. (2012). Gender differences in highway driving performance after administration of sleep medication: A review of the literature. Traffic Inj. Prev. 13 (3), 286–292. doi:10.1080/15389588.2011.652751
Warnez, S., and Alessi-Severini, S. (2014). Clozapine: A review of clinical practice guidelines and prescribing trends. BMC Psychiatry 14, 102. doi:10.1186/1471-244X-14-102
Watanabe, J. H., McInnis, T., and Hirsch, J. D. (2018). Cost of prescription drug-related morbidity and mortality. Ann. Pharmacother. 52 (9), 829–837. doi:10.1177/1060028018765159
Weersma, R. K., Zhernakova, A., and Fu, J. (2020). Interaction between drugs and the gut microbiome. Gut 69 (8), 1510–1519. doi:10.1136/gutjnl-2019-320204
WHO (2023). Anatomical therapeutic chemical (ATC) classification. Available at: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (Accessed Feb 15 2023).
Wiffen, P. J., Wee, B., and Moore, R. A. (2016). Oral morphine for cancer pain. Cochrane Database Syst. Rev. 4, CD003868. doi:10.1002/14651858.CD003868.pub4
Williams, B., and Mancia, G. (2018). Ten commandments of the 2018 ESC/ESH HTN guidelines on hypertension in adults. Eur. Heart J. 39 (33), 3007–3008. doi:10.1093/eurheartj/ehy439
Woo, Y. S., Park, S. Y., Yoon, B. H., Choi, W. S., Wang, S. M., and Bahk, W. M. (2022). Amisulpride augmentation in schizophrenia patients with poor response to olanzapine: A 4-week, randomized, rater-blind, controlled, pilot study. Pilot Study 20 (3), 567–572. doi:10.9758/cpn.2022.20.3.567
Wood, R. (1995). Bronchospasm and cough as adverse reactions to the ACE inhibitors captopril, enalapril and lisinopril. A controlled retrospective cohort study. Br. J. Clin. Pharmacol. 39 (3), 265–270. doi:10.1111/j.1365-2125.1995.tb04447.x
Yang, L., Li, Y., Hong, H., Chang, C. W., Guo, L. W., Lyn-Cook, B., et al. (2012). Sex differences in the expression of drug-metabolizing and transporter genes in human liver. J. Drug Metab. Toxicol. 3 (3), 1000119. doi:10.4172/2157-7609.1000119
Yatham, L. N., Kennedy, S. H., Parikh, S. V., Schaffer, A., Bond, D. J., Frey, B. N., et al. (2018). Canadian network for mood and anxiety treatments (CANMAT) and international society for bipolar disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 20 (2), 97–170. doi:10.1111/bdi.12609
Keywords: sex differences, adverse drug reactions, psychotropic, cardiovascular, analgesic
Citation: Shan Y, Cheung L, Zhou Y, Huang Y and Huang RS (2023) A systematic review on sex differences in adverse drug reactions related to psychotropic, cardiovascular, and analgesic medications. Front. Pharmacol. 14:1096366. doi: 10.3389/fphar.2023.1096366
Received: 12 November 2022; Accepted: 10 April 2023;
Published: 02 May 2023.
Edited by:
Yen-Ming Huang, National Taiwan University, TaiwanReviewed by:
Renate Grohmann, LMU Munich University Hospital, GermanyCopyright © 2023 Shan, Cheung, Zhou, Huang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. Stephanie Huang, cnNodWFuZ0B1bW4uZWR1
†Present address: Department of Pharmacy, North Memorial Health Hospital, Robbinsdale, MN, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.