- Department of Gastroenterology, Digestive Disease Hospital, The First Affiliated Hospital of Nanchang University, Nanchang, China
Background: Currently, the management of Helicobacter pylori (H. pylori) infection in elderly patients is controversial. We investigated whether high-dose dual therapy would serve as the first-line therapy in elderly patients.
Methods: This was a single-center, randomized study of 150 elderly patients with H. pylori infection who were randomly assigned to 14-day therapy with pantoprazole 40 mg 3 times daily and either amoxicillin 1,000 mg 3 times daily or amoxicillin 1,000 mg twice daily, clarithromycin 500 mg twice daily and bismuth 220 mg twice daily. H. pylori eradication was evaluated by a 13C-urea breath test 4 weeks after the completion of treatment.
Results: Successful eradication was achieved in 89.3% of the high-dose dual therapy (HT) group in the intention-to-treat (ITT) analysis, 91.7% in the modified intention-to-treat (mITT) analysis, and 93.0% for per-protocol (PP) analysis which was similar to the bismuth-containing quadruple therapy (BQT) group (86.6%, 87.8%, and 90.3%, respectively). There were no significant difference between the HT group and the BQT group in the ITT analysis (p = 0.484), mITT analysis (p = 0.458), or PP analysis (p = 0.403). HT was associated with fewer side effects (10.6% of patients) than BQT (26.6%) (p = 0.026).
Conclusion: In this trial, we found that 14-day HT had a similar eradication rate to BQT but fewer side effects, which may be better for elderly patients.
1 Introduction
Helicobacter pylori (H. pylori) infection and related diseases are a major global health concern. Approximately 44% of the global population is infected with H. pylori (Malfertheiner et al., 2017). The prevalence of upper gastrointestinal diseases is increasing in elderly populations worldwide. Approximately 53%–73% of elderly peptic ulcer patients are infected with H. pylori; however, elderly H. pylori-positive patients are less likely to receive treatment (Pilotto, 2004). Peptic ulcers are currently the main impact of H. pylori on healthcare resources, and older patients are at the greatest risk, as the mortality rate from peptic ulcers increases from approximately 1 in 100 000 at age 20 to 2-3 in 100 000 at age 70 (Bank et al., 1986). The Fifth Chinese National Consensus Report on management highly recommends that patients with peptic ulcers receive eradication of H. pylori (Liu et al., 2018). A study conducted by Leung et al. (Leung et al., 2018) demonstrated that among patients infected with H. pylori who received eradication therapy, the gastric cancer risk was significantly lower in the elderly compared with the matched general population highlighting the importance of eradication therapy for elderly patients.
A proton pump inhibitor (PPI) and the antibiotics clarithromycin and amoxicillin/metronidazole, the so-called standard triple therapy, are used as the first-line eradication therapy for H. pylori infection, worldwide (Salles and Mégraud, 2007). However, this kind of treatment may cause resistance-associated H. pylori eradication failure due to increasing antimicrobial resistance. Bismuth-containing quadruple therapy (BQT) is commonly used in China and is superior to the standard triple therapy (Xie et al., 2018). In general, elderly patients show a low tolerance and compliance to H. pylori eradication therapy (Salles and Mégraud, 2007). In some countries and regions, it is difficult to obtain drugs such as bismuth and tetracycline. Moreover, the risk of adverse effects of H. pylori eradication therapy increases in elderly patients. Therefore, comprehensive benefit and risk assessment for elderly patients undergoing H. pylori eradication treatment are essential.
In the 1990s, some European scholars proposed a dual therapy regimen of PPI plus an antibiotic, but compared with the triple regimen proposed in early guidelines, this regimen has a lower eradication rate, so it is not widely used in clinical treatment (Unge et al., 1989). However, according to some recent literature reports, increasing the dose of PPI and/or amoxicillin in the dual therapy regimen can effectively eradicate H. pylori (Goh et al., 2012; Attumi and Graham, 2014; Yang et al., 2015; Yu et al., 2019). Some studies have shown that the drug resistance of the bismuth quadruple regimen after eradication failure is significantly higher than that of the dual therapy (Ren et al., 2014). In China, the rural population accounts for the main proportion of patients with H. pylori infection. In regard to elderly patients, the instructions for taking two drugs are more concise and easier to understand than the instructions for taking four drugs. Several meta-analyses have compared the efficacy and safety of dual therapy and other regimens, and all of them concluded that PPI-amoxicillin is equally effective as other commonly used therapeutic regimens in the eradication of H. pylori (Gao et al., 2020a; Li et al., 2021). Therefore, high-dose dual therapy (HT) may be an effective eradication regimen for elderly patients who are not allergic to penicillin.
The widespread presence of H. pylori in elderly patients and the role of H. pylori in peptic ulcers, precancerous lesions of gastric cancer, gastric cancer, and gastric lymphoma (MALT) make a diagnosis and eradication therapy particularly important in this population. However, there is currently no consensus or guideline on H. pylori eradication therapy in elderly patients. Therefore, it is of practical significance to develop a safe and effective H. pylori eradication program for elderly patients, which is worthy of further study.
2 Materials and methods
2.1 Subjects and study design
This single-center, prospective, open-label, randomized controlled trail was conducted at the First Affiliated Hospital of Nanchang University, Nanchang, China. Between February 2020 and February 2022, a total of 162 eligible cases were planned, 8 patients met the exclusion criteria, 4 patients refused to participate, and 150 patients were enrolled in outpatient clinics. Our study followed the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) statement for reporting randomized controlled trials. Written informed consent was obtained from all participants. The study protocol was approved by the Institutional Ethics Board of the First Affiliated Hospital of Nanchang University, Nanchang, China (No. 101.2019). The trial was registered in the Chinese Clinical Trials Registration (chictr.org.cn), registered number is ChiCTR2000029679.
Detailed inclusion criteria were as follows: 1). male or female patients aged ≥60 years; 2) patients diagnosed with H. pylori infection by positive C-urea breath test (UBT) or by positive gastric mucosa biopsy results (UBT results between 4‰ and 6‰ were not included in the group, as well as those who remained above 4‰ after 7 days of reexamination); 3). patients who signed the informed consent form.
The exclusion criteria were as follows: 1). patients who had received standard H. pylori eradication therapy before enrollment; 2). patients who had taken antibiotics, bismuth, and PPIs within 4 weeks before initiating treatment; 3). alcohol abuse (daily alcohol intake exceeding 50 G for more than 6 months); 4). patients with a serious primary diseases of the liver, kidney, heart, brain, lung, endocrine system, and hematopoietic system; 5). clinically significant liver or kidney insufficiency (transaminase greater than 1.5 times the upper limit of normal, or serum creatinine greater than the upper limit of normal; 6). patients who were allergic to drugs related to medications.
2.2 Treatment regiments
The HT consisted of pantoprazole 40 mg 3 times daily and amoxicillin 1,000 mg 3 times daily for 14 days. Patients were advised to take pantoprazole 30 min before breakfast, lunch, and dinner, and amoxicillin after breakfast, lunch, and dinner.
The BQT consisted of pantoprazole 40 mg twice daily, amoxicillin 1,000 mg twice daily, clarithromycin 500 mg twice daily, and bismuth 220 mg twice daily for 14 days. Pantoprazole and bismuth were taken 30 min before breakfast and dinner, and amoxicillin and clarithromycin were taken 30 min after breakfast and dinner.
2.3 Determination of successful eradication
Negative UBT results 4–6 weeks after the end of treatment indicated successful H. pylori eradication. Side effects and compliance were assessed using a questionnaire administered after the end of therapy. Compliance was defined as good when more than 80% of the total pills were taken. Side effects were graded according to their influence on daily life, classified as “mild” (transient and well-tolerated), “moderate” (discomfort that partially affected daily life), or “severe” (severe interruption of daily activities).
2.4 Sample size estimation and statistical analysis
The purpose of this study was verify whether HT could achieve a similar effective eradication rate to BQT in elderly patients. Based on a previous study, we estimated the eradication rate of the BQT groups to be 89.7% (Yang et al., 2019a). We assumed an eradication rate of 90% in both the BQT and HT groups; with a non-inferiority margin delta (δ) = 0.1 (10%), α = 0.05 (two-sided), and 1-β = 0.90, we expected to recruit at least 75 patients per group (assuming a 10% loss to follow-up).
Statistical analyses to identify prediction factors were performed using SPSS 25.0 for Windows (SPSS, Chicago, IL). Continuous variables are expressed as the mean ± standard deviation, and categorical data are presented as absolute numbers and percentages. The chi-square test was used to determine the significance of differences between categorical variables and the t-test were used for continuous variables. The eradication rate of H. pylori was calculated using intention-to-treat (ITT), modified intention-to-treat (mITT), and per-protocol (PP) analyses together with a 95% confidence interval (CI).
3 Results
3.1 Basic patient demographics
A total of 162 patients were assessed for eligibility, and 150 patients were recruited for this study. In the HT group, 2 patients were lost to follow-up, and 1 paytient was not compliant with medications. In the BQT group, 1 patient was lost to follow-up, and 2 patients were not compliant with medications (Figure 1).
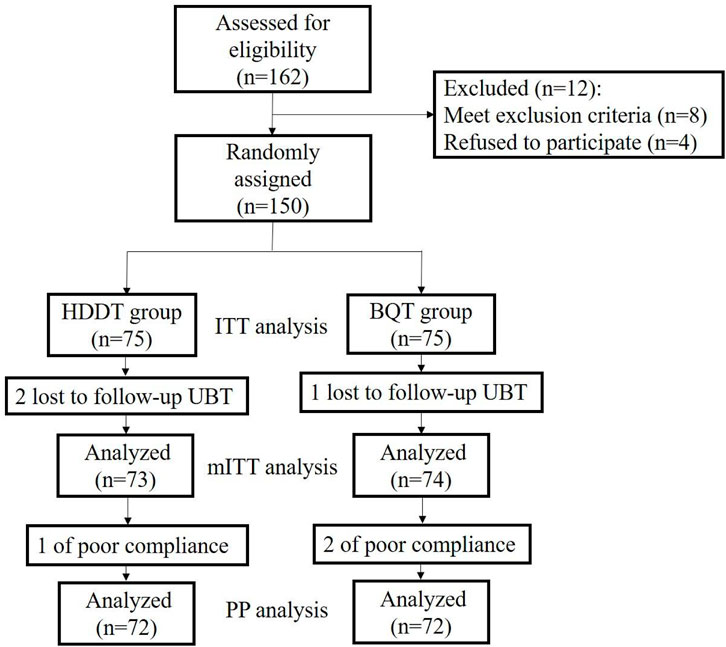
FIGURE 1. Flow diagram of this study. HT, high-dose dual therapy, BQT, bismuth quadruple therapy, ITT, intension-to-treat, PP, pre-protocol.
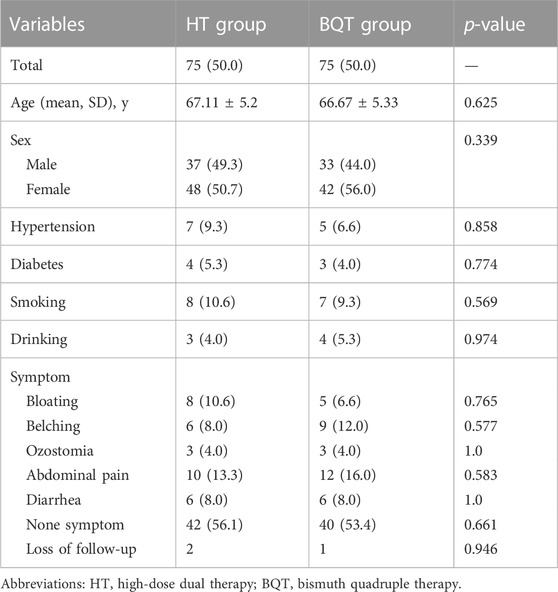
The distribution of patients according to sex, symptoms, and mean age is shown in Table 1. There were no significant difference between the two groups regarding clinical characteristics.
3.2 H. pylori eradication rates
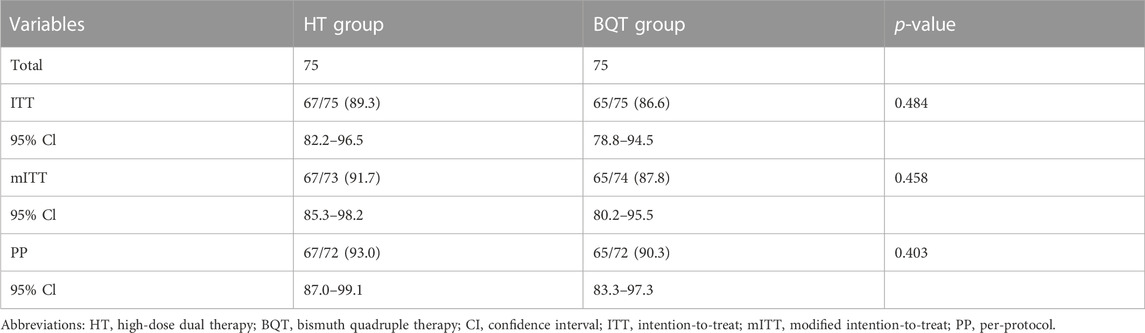
In the ITT analysis, H. pylori was eradicated in 89.3% of patients (67/75) (95% Cl: 82.2–96.5) in the HT group compared with 86.6% (65/75) (95% Cl: 78.8–94.5) in the BQT group (Table 2). In the mITT analysis, H. pylori were eradicated in 91.7% of patients (67/73) (95% Cl:85.3–98.2) in the HT group compared with 87.8% (65/74) (95% Cl:80.2–95.5) in the BQT group (Table 2). In the PP analysis, H. pylori was eradicated in 93.0% of patients (67/72) (95% Cl: 87.0–99.1) in the HT group compared with 90.3% (65/72) (95% Cl: 83.3–97.3) in the BQT group (Table 2).
There were no significant difference in the eradication rates in the ITT, mITT, and PP analysis between the 2 treatment groups.
3.3 Side effects and compliance
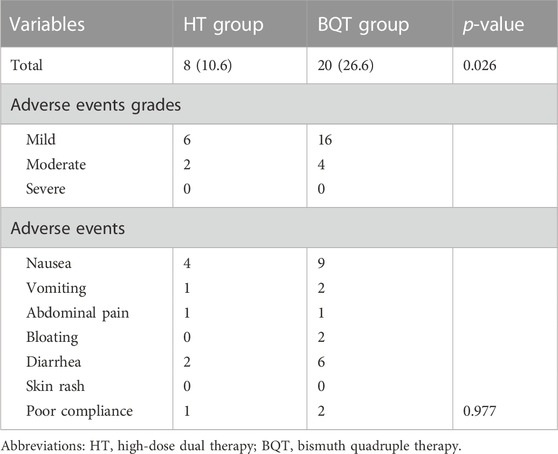
Side effects included nausea, vomiting, bloating, abdominal pain, diarrhea, and skin rash (Table 3). In the HT group, the frequency of side effects was 10.6% (8/75) of patients. In the BQT group, the frequency of side effects was 26.6% (20/75) of patients (p = 0.026). The side effects were all mild and moderate. Treatment was reported as tolerable except for one patient in the HT group and two patients in the BQT group who could not tolerate the treatment regimen due to skin rash or nausea. The compliance rates was 98.7% (74/75) in the HT group and 97.3% (73/75) in the BQT group.
3.4 Previous literature review
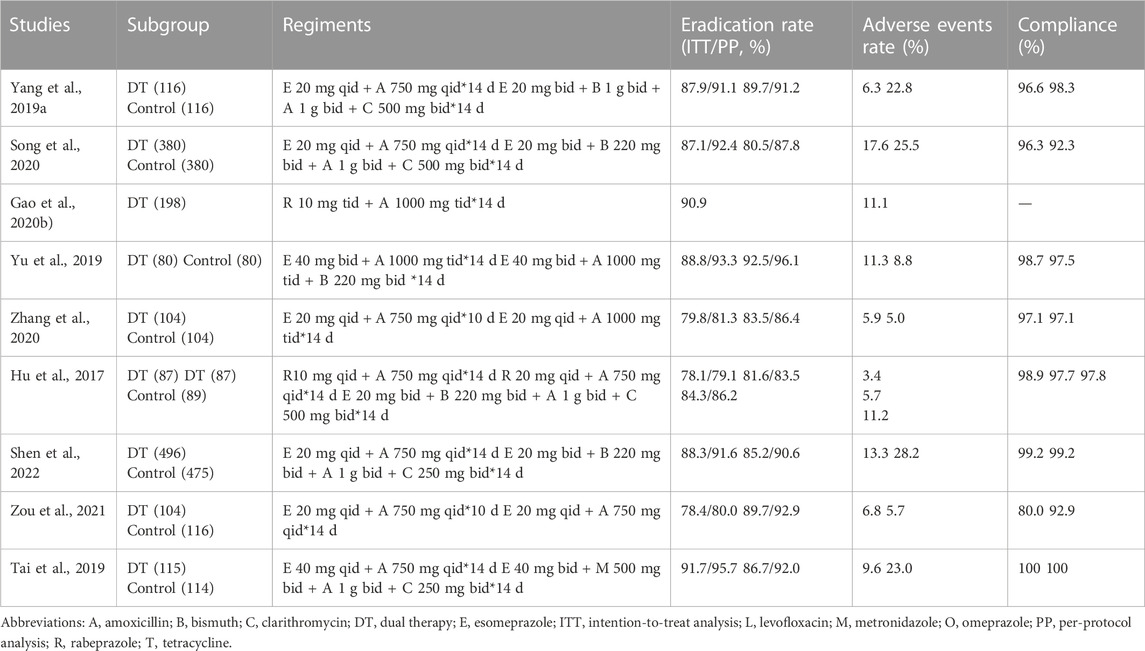
A previous literature review is illustrated in Table 4, which includes. 9 studies conducted in China and published in the last 5 years. Rabeprazole and esomeprazole were the most commonly used PPIs in these studies. Most studies reported a low incidence of adverse reactions to PPI-amoxicillin dual therapy.
4 Discussion
In the current study, we conducted the first, randomized, controlled trial to comparing the efficacy of 14-day hybrid and 14-day bismuth quadruple therapies for the first-line treatment of H. pylori infection in elderly patients. Although dual therapy is not currently recommended as the first-line regimen for treating H pylori infection, many studies have reported an effective eradication rate of this regimen (Ren et al., 2014; Yang et al., 2015; Tai et al., 2019). Our results showed that both regimens achieved a successful eradication rate, and HT with pantoprazole and amoxicillin was efficient with a rate of 93.0% in the PP analysis, 91.7% in the mITT analysis and 89.3% in the ITT analysis. Side effects were few although a minority of patients in the BQT group complained of vomiting due to clarithromycin. The HT was generally well tolerated by patients.
Currently, there are no specific guidelines regarding the management of H. pylori infection in elderly patients. With the increased antibiotic resistance of H. pylori, due to previous multiple exposures to antibiotics, elderly patients fail their intended eradication therapy. Moreover, H. pylori microbiology summarizes the existing evidence for first- and second-line treatment regimens that may be considered for special populations such as the elderly. Elderly patients generally have renal or liver dysfunction, which might affect drug accumulation. As BQT contains more antibiotics than HT, patients tend to experience more side effects. Individual treatment needs to be performed in elderly patients.
The globally increasing antibiotic resistance in recent years is an important cause of first-line H pylori eradication failure (Graham and Fischbach, 2010). To overcome this problem, there are several novel therapies such as sequential therapy, non-bismuth quadruple therapy, and hybrid therapy that have achieved high eradication rates in first-line H pylori eradication (Liou et al., 2013; Liou et al., 2016). Recently, dual therapy has gained attention as both first-line and rescue therapy. However, studies from the different regions show different cure rates, probably due to different dosages of PPI and amoxicillin (Graham et al., 2010; Kwack et al., 2016; Park et al., 2017). Some comments on the heterogeneity of the results with HT should be included. In this respect, some meta-analyses showed higher HT effectiveness in studies in Asian countries than in European countries (Yang et al., 2019b; Gao et al., 2020a; Zhu et al., 2020). There have also been several dual-therapy studies conducted in China, and most of them show successful eradication rates with fewer adverse events (Hu et al., 2017; Tai et al., 2019; Gao et al., 2020b; Song et al., 2020; Zhang et al., 2020; Zou et al., 2021; Shen et al., 2022) (Table 4). Therefore, we questioned whether high-dose and high-frequency amoxicillin-PPI dual therapy could be widely used in elderly patients.
Amoxicillin is widely used in the treatment of H pylori infections; it is one of the most active antimicrobials against H pylori in vivo with a minimum inhibitory concentration of ≤0.01–0.1 mg/L (Hirschl and Rotter, 1996). An improvement in acid inhibition extending into the nighttime hours, maintaining an intragastric pH of 6 or above, would greatly improve the eradication rates of a regiment (Marcus et al., 2012). Several studies have tested the effect of a total daily dose of amoxicillin on the cure rate. There is evidence that a dose of 3 g per day can achieve an effective eradication rate (Kwack et al., 2016; Li et al., 2021).
Sufficient acid inhibition is necessary because intragastric pH is one of the critical determinants and amoxicillin is acid labile. In aition, other factors influence the efficacy of treating H. pylori infection. The PPI potency has a strong ability to reliably maintain a relatively high intragastric pH. A previous study evaluating the efficacy of different PPIs combined with bismuth quadruple regimens for H. pylori eradication demonstrated that the eradication rates between different PPI regimens were similar in treating H. pylori infection (Kan et al., 2020). To achieve a reliable cure rate, a high dose of PPI that is unaffected by CYP2C19 genotypes may be needed. The pantoprazole-based eradication program was less affected by the CYP2C19 polymorphism (Fu et al., 2021). In this study, we used 40 mg pantoprazole which is relatively unaffected by CYP2C19 metabolism. A study conducted by Zullo et al. (2015) showed that a 10-day HT (esomeprazole 40 mg three times daily and amoxicillin 1 g three times daily) was effective and safe as a first-line treatment for H. pylori infection in Italy. However, the eradication rate was below 90%. Ping et al. (Hsu et al., 2018) found that H pylori infection was eradicated in 96.6% of patients who received reverse hybrid therapy (pantoprazole 40 mg plus amoxicillin 1 g twice daily for 14 days, and clarithromycin 500 mg plus metronidazole 500 mg twice daily for the first 7 days). Therefore, the regimen of 40 mg pantoprazole plus 3 g amoxicillin per day may be a favorable choice for elderly patients.
In the HT group, there were 11 patients who concomitantly and continually took medications, such as statins, hypotension, hypoglycemics, and antiplatelet drugs. Successful eradication was achieved in 10 of these patients. Thus, a dual regimen with fewer antibiotics would be an option for older patients or those with multiple comorbidities. Adverse events occurred in 10.6% (8/75) of patients, which was similar to most reports (Tai et al., 2019; Zou et al., 2021). Nausea, diarrhea, and abdominal pain were the most frequently occurring adverse events. All adverse events disappeared spontaneously after treatment.
Our study had some limitations. First, we did not perform susceptibility testing which was directly correlated. Second, we did not measure the patients’ intragastric pH value or CYP2C19 genotype. This was also a single-center study. A multicenter study should be conducted to confirm our findings.
In conclusion, our data showed that a 14-day regimen with pantoprazole 40 mg 3 times daily and amoxicillin 1,000 mg 3 times daily achieved an H. pylori eradication rate of 89.3%. This HT had few side effects and avoided the prevalence of antibiotic resistance. Clinicians can choose this treatment when considering H. pylori eradication in elderly patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the First affiliated hospital of Nanchang university. The patients/participants provided their written informed consent to participate in this study.
Author contributions
QY: data collection, data analysis, and manuscript writing; CH: study design and critical revision of the manuscript; YH, ZZ, JH, XS, and YX: material preparation, data collection; YZ: Study design, data interpretation, and critical revision of the manuscript; NL: patient recruitment, study coordination, and study supervision.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 8217033301) and grants from the Double-Thousand Plan of Jiangxi Province (No. jxsq2019201028).
Acknowledgments
We would like to thank YZ provided the funding by grants from the National Natural Science Foundation of China (No. 8217033301) for and grants from the Double-Thousand Plan of Jiangxi Province (No. jxsq2019201028) this writing assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Attumi, T. A., and Graham, D. Y. (2014). High-dose extended-release lansoprazole (dexlansoprazole) and amoxicillin dual therapy for Helicobacter pylori infections. Helicobacter 19 (4), 319–322. doi:10.1111/hel.12126
Bank, L., Wright, J. P., Lucke, W., and Marks, I. N. (1986). Peptic ulcer. A follow-up study. J. Clin. gastroenterology 8 (32), 381–384. doi:10.1097/00004836-198606002-00010
Fu, J., Sun, C. F., He, H. Y., Ojha, S. C., Shi, H., Deng, C. L., et al. (2021). The effect of CYP2C19 gene polymorphism on the eradication rate of Helicobacter pylori by proton pump inhibitors-containing regimens in asian populations: A meta-analysis. Pharmacogenomics 22 (13), 859–879. doi:10.2217/pgs-2020-0127
Gao, C. P., Zhang, D., Zhang, T., Wang, J. X., Han, S. X., Graham, D. Y., et al. (2020). PPI-amoxicillin dual therapy for Helicobacter pylori infection: An update based on a systematic review and meta-analysis. Helicobacter 25 (4), e12692. doi:10.1111/hel.12692
Gao, W., Ye, H., Deng, X., Wang, C., Xu, Y., Li, Y., et al. (2020). Rabeprazole-amoxicillin dual therapy as first-line treatment for H pylori eradication in special patients: A retrospective, real-life study. Helicobacter 25 (5), e12717. doi:10.1111/hel.12717
Goh, K. L., Manikam, J., and Qua, C. S. (2012). High-dose rabeprazole-amoxicillin dual therapy and rabeprazole triple therapy with amoxicillin and levofloxacin for 2 weeks as first and second line rescue therapies for Helicobacter pylori treatment failures. Alimentary Pharmacol. Ther. 35 (9), 1097–1102. doi:10.1111/j.1365-2036.2012.05054.x
Graham, D. Y., and Fischbach, L. (2010). Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 59 (8), 1143–1153. doi:10.1136/gut.2009.192757
Graham, D. Y., Javed, S. U., Keihanian, S., Abudayyeh, S., and Opekun, A. R. (2010). Dual proton pump inhibitor plus amoxicillin as an empiric anti-H. pylori therapy: Studies from the United States. J. Gastroenterol. 45 (8), 816–820. doi:10.1007/s00535-010-0220-x
Hirschl, A. M., and Rotter, M. L. (1996). Amoxicillin for the treatment of Helicobacter pylori infection. J. Gastroenterol. 31 (9), 44–47.
Hsu, P. I., Tsay, F. W., Graham, D. Y., Tsai, T. J., Tsai, K. W., Kao, J. Y., et al. (2018). Equivalent efficacies of reverse hybrid and bismuth quadruple therapies in eradication of Helicobacter pylori infection in a randomized controlled trial. Clin. Gastroenterol. Hepatol. 16 (9), 1427–1433. doi:10.1016/j.cgh.2018.03.031
Hu, J. L., Yang, J., Zhou, Y. B., Li, P., Han, R., and Fang, D. C. (2017). Optimized high-dose amoxicillin-proton-pump inhibitor dual therapies fail to achieve high cure rates in China. Saudi J. gastroenterology official J. Saudi Gastroenterology Assoc. 23 (5), 275–280. doi:10.4103/sjg.SJG_91_17
Kan, L. D., Chen, J., Huang, Y. T., Qiu, Y., Yu, X. L., Fang, H. M., et al. (2020). Evaluation of different proton pump inhibitors combined with bismuth quadruple regimens in Helicobacter pylori eradication. Clin. Exp. Med. 20 (4), 609–614. doi:10.1007/s10238-020-00643-2
Kwack, W., Lim, Y., Lim, C., and Graham, D. Y. (2016). High dose ilaprazole/amoxicillin as first-line regimen for Helicobacter pylori infection in korea. Gastroenterology Res. Pract. 2016, 1648047. doi:10.1155/2016/1648047
Leung, W. K., Wong, I. O. L., Cheung, K. S., Yeung, K. F., Chan, E. W., Wong, A. Y. S., et al. (2018). Effects of Helicobacter pylori treatment on incidence of gastric cancer in older individuals. Gastroenterology 155 (1), 67–75. doi:10.1053/j.gastro.2018.03.028
Li, C., Shi, Y., Suo, B., Tian, X., Zhou, L., and Song, Z. (2021). PPI-Amoxicillin dual therapy four times daily is superior to guidelines recommended regimens in the Helicobacter pylori eradication therapy within asia: A systematic review and meta-analysis. Helicobacter 26 (4), e12816. doi:10.1111/hel.12816
Liou, J. M., Chen, C. C., Chang, C. Y., Chen, M. J., Chen, C. C., Fang, Y. J., et al. (2016). Sequential therapy for 10 days versus triple therapy for 14 days in the eradication of Helicobacter pylori in the community and hospital populations: A randomised trial. Gut 65 (11), 1784–1792. doi:10.1136/gutjnl-2015-310142
Liou, J. M., Chen, C. C., Chen, M. J., Chen, C. C., Chang, C. Y., Fang, Y. J., et al. (2013). Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: A multicentre, open-label, randomised trial. Lancet (London, Engl. 381 (9862), 205–213. doi:10.1016/S0140-6736(12)61579-7
Liu, W. Z., Xie, Y., Lu, H., Cheng, H., Zeng, Z. R., Zhou, L. Y., et al. (2018). Fifth Chinese national consensus report on the management of Helicobacter pylori infection. Helicobacter 23 (2), e12475. doi:10.1111/hel.12475
Malfertheiner, P., Megraud, F., O'Morain, C. A., Gisbert, J. P., Kuipers, E. J., Axon, A. T., et al. (2017). Management of Helicobacter pylori infection-the maastricht V/florence consensus report. Gut 66 (1), 6–30. doi:10.1136/gutjnl-2016-312288
Marcus, E. A., Inatomi, N., Nagami, G. T., Sachs, G., and Scott, D. R. (2012). The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment. Pharmacol. Ther. 36 (10), 972–979. doi:10.1111/apt.12059
Park, H. Y., Kang, E. J., Kim, D. G., Kim, K. J., Choi, J. W., Nam, S. Y., et al. (2017). High and frequent dose of dexlansoprazole and amoxicillin dual therapy for Helicobacter pylori infections: A single arm prospective study. Korean J. Gastroenterol. 70 (4), 176–180. doi:10.4166/kjg.2017.70.4.176
Pilotto, A. (2004). Aging and upper gastrointestinal disorders. Best Pract. Res. Clin. gastroenterology 2004 18, 73–81.
Ren, L., Lu, H., Li, H. Y., Zhu, L. Y., Xu, X. Q., Gu, L. Y., et al. (2014). New dual therapy for primary treatment ofHelicobacter pyloriinfection: A prospective randomized study in shanghai, China. J. Dig. Dis. 15 (11), 622–627. doi:10.1111/1751-2980.12186
Salles, N., and Mégraud, F. (2007). Current management of Helicobacter pylori infections in the elderly. Expert Rev. anti-infective Ther. 5 (5), 845–856. doi:10.1586/14787210.5.5.845
Shen, C., Li, C., Lv, M., Dai, X., Gao, C., Li, L., et al. (2022). The prospective multiple-centre randomized controlled clinical study of high-dose amoxicillin-proton pump inhibitor dual therapy for H. pylori infection in Sichuan areas. Ann. Med. 54 (1), 426–435. doi:10.1080/07853890.2022.2031269
Song, Z., Zhou, L., Xue, Y., Suo, B., Tian, X., and Niu, Z. (2020). A comparative study of 14-day dual therapy (esomeprazole and amoxicillin four times daily) and triple plus bismuth therapy for first-line Helicobacter pylori infection eradication: A randomized trial. Helicobacter 25 (6), e12762. doi:10.1111/hel.12762
Tai, W. C., Liang, C. M., Kuo, C. M., Huang, P. Y., Wu, C. K., Yang, S. C., et al. (2019). A 14 day esomeprazole- and amoxicillin-containing high-dose dual therapy regimen achieves a high eradication rate as first-line anti-Helicobacter pylori treatment in taiwan: A prospective randomized trial. J. Antimicrob. Chemother. 74 (6), 1718–1724. doi:10.1093/jac/dkz046
Unge, P., Gad, A., Gnarpe, H., and Olsson, J. (1989). Does omeprazole improve antimicrobial therapy directed towards gastric Campylobacter pylori in patients with antral gastritis? A pilot study. Scand. J. Gastroenterol. Suppl. 167, 49–54. doi:10.3109/00365528909091311
Xie, Y., Zhang, Z., Hong, J., Liu, W., Lu, H., Du, Y., et al. (2018). Furazolidone-containing triple and quadruple eradication therapy for initial treatment for Helicobacter pylori infection: A multicenter randomized controlled trial in China. Helicobacter 23 (5), e12496. doi:10.1111/hel.12496
Yang, J., Zhang, Y., Fan, L., Zhu, Y. J., Wang, T. Y., Wang, X. W., et al. (2019). Eradication efficacy of modified dual therapy compared with bismuth-containing quadruple therapy as a first-line treatment of Helicobacter pylori. Am. J. Gastroenterol. 114 (3), 437–445. doi:10.14309/ajg.0000000000000132
Yang, J. C., Lin, C. J., Wang, H. L., Chen, J. D., Kao, J. Y., Shun, C. T., et al. (2015). High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin. gastroenterology hepatology official Clin. Pract. J. Am. Gastroenterological Assoc. 13 (5), 895–905. doi:10.1016/j.cgh.2014.10.036
Yang, X., Wang, J. X., Han, S. X., and Gao, C. P. (2019). High dose dual therapy versus bismuth quadruple therapy for Helicobacter pylori eradication treatment: A systematic review and meta-analysis. Medicine 98 (7), e14396. doi:10.1097/MD.0000000000014396
Yu, L., Luo, L., Long, X., Liang, X., Ji, Y., Graham, D. Y., et al. (2019). High-dose PPI-amoxicillin dual therapy with or without bismuth for first-line Helicobacter pylori therapy: A randomized trial. Helicobacter 24, e12596. doi:10.1111/hel.12596
Zhang, Y., Zhu, Y. J., Zhao, Z., Zhao, J. T., Wang, T. Y., Yang, J., et al. (2020). Efficacy of modified esomeprazole-amoxicillin dual therapies for Helicobacter pylori infection: An open-label, randomized trial. Eur. J. gastroenterology hepatology 32 (5), 563–568. doi:10.1097/MEG.0000000000001646
Zhu, Y. J., Zhang, Y., Wang, T. Y., Zhao, J. T., Zhao, Z., Zhu, J. R., et al. (2020). High dose PPI-amoxicillin dual therapy for the treatment of Helicobacter pylori infection: A systematic review with meta-analysis. Ther. Adv. gastroenterology 13, 1756284820937115. doi:10.1177/1756284820937115
Zou, P. Y., Hu, J., Zhao, J. T., Zhao, Z., Mei, H., Yang, J., et al. (2021). 10-Day and 14-day high-dose dual therapy for the treatment of Helicobacter pylori: A propensity score matching analysis. Helicobacter 26 (5), e12833. doi:10.1111/hel.12833
Keywords: bismuth, dual therapy, elderly patients, eradication, Helicobacter pylori
Citation: Yang Q, He C, Hu Y, Hong J, Zhu Z, Xie Y, Shu X, Lu N and Zhu Y (2023) 14-day pantoprazole- and amoxicillin-containing high-dose dual therapy for Helicobacter pylori eradication in elderly patients: A prospective, randomized controlled trial. Front. Pharmacol. 14:1096103. doi: 10.3389/fphar.2023.1096103
Received: 11 November 2022; Accepted: 23 January 2023;
Published: 01 February 2023.
Edited by:
Tanveer Ahmed Khan, National Institute of Health, PakistanReviewed by:
Joao Massud, Independent researcher, São Paulo, BrazilStylianos Karatapanis, General Hospital of Rhodes, Greece
Copyright © 2023 Yang, He, Hu, Hong, Zhu, Xie, Shu, Lu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nonghua Lu, bHVub25naHVhQG5jdS5lZHUuY24=; Yin Zhu, emh1eWluMjdAc2luYS5jb20uY24=
†These authors have contributed equally to this work
 Qinyu Yang
Qinyu Yang Cong He
Cong He Yi Hu
Yi Hu Junbo Hong
Junbo Hong Zhenhua Zhu
Zhenhua Zhu Yong Xie
Yong Xie Xu Shu
Xu Shu Nonghua Lu
Nonghua Lu Yin Zhu
Yin Zhu