
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 25 April 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1093194
This article is part of the Research Topic Research and Development of Small Molecule Anti-cancer Drugs Targeting Immune Checkpoints View all 8 articles
Background: Anti-PD-(L)1 antibody monotherapy or in combination with VEGF(R) blockade has been applied widely for cancer treatment. Whether combination therapy increases irAEs still remains controversial.
Methods: A systematic review and meta-analysis comparing PD-(L)1 and VEGF(R) blockade combination therapy with PD-(L)1 inhibitors alone was performed. Phase II or III randomized clinical trials reporting irAEs or trAEs were included. The protocol was registered with PROSPERO, CRD42021287603.
Results: Overall, 77 articles were included in the meta-analysis. A total of 31 studies involving 8,638 participants were pooled and an incidence for PD-(L)1 inhibitor monotherapy with any grade and grade ≥3 irAEs of 0.25 (0.20, 0.32) and 0.06 (0.05, 0.07), respectively, were reported. Two studies with 863 participants pooled for PD-(L)1 and VEGF(R) blockade showed that an incidence of any grade and grade ≥3 irAEs were 0.47 (0.30, 0.65) and 0.11 (0.08, 0.16), respectively. Regarding pairwise comparisons for irAEs, only one study was included, indicating no significant difference between the two regimens in terms of colitis, hyperthyroidism, and hypothyroidism for any grade and grade ≥3, while there was a trend of higher incidence for any grade hyperthyroidism under the combination therapy. The incidence of reactive cutaneous capillary endothelial proliferation (RCCEP) was as high as 0.80 under camrelizumab monotherapy.
Conclusion: Total incidences of any grade and grade ≥3 irAEs were higher in the combination treatment group. Direct comparisons indicated no significant difference between the two regimens for any grade and grade ≥3 specific irAEs. RCCEP and thyroid disorders need to be paid attention to clinically. Moreover, trials with direct comparisons are needed and the safety profiles of the two regimens should be further explored. Exploration of the mechanism of action and regulatory management of adverse events should be enhanced.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=287603, identifier CRD42021287603
Immune check inhibitors (ICIs) have revolutionized oncology through the approach of blocking intrinsic down-regulators of immunity and increasing antitumor immunity, as well as countering immune suppression in the tumor microenvironment (Postow et al., 2018; Brahmer et al., 2021). Programmed cell death (ligand) 1 (PD-(L)1) inhibitors monotherapy or combination regimens have been approved as first-line or second-line therapies in a range of cancer types. Anti-vascular endothelial growth factor (receptor) (VEGF(R)) agents targeting the VEGF signaling pathway, which may have synergistic effects with PD-(L)1 blockade (Meder et al., 2018; Yi et al., 2019) and potentially reverse the resistance to ICIs (Kim et al., 2019; Yi et al., 2019; Saeed et al., 2021), have been approved in combination with PD-(L)1 inhibitors for the first-line treatment of hepatocellular carcinoma (Finn et al., 2020a) and renal cell carcinoma (Motzer et al., 2020a; Powles et al., 2020a; Choueiri et al., 2021), and second-line treatment and beyond of endometrial cancer (Makker et al., 2019). In several circumstances, studies revealed that the dual combination regimen could significantly improve survival benefits compared with the PD-(L)1 inhibitor alone, representing a promising therapeutic effect (Huang et al., 2021; Chen et al., 2022).
There is accumulating evidence that ICIs are associated with immune-related adverse events (irAEs), which often demand multidisciplinary collaboration from the clinician. Presently, the mechanism of irAEs is not elucidated, which is perhaps related to the off-target effects from the excessively activated immune system as well as the production of inflammatory cytokines resulting from T-cell activation (Martins et al., 2019; Kennedy and Salama, 2020). Since trials exploring the efficacy of combination regimens are increasing, there are urgent concerns about irAEs, especially severe irAEs which are life-threatening.
A systematic review evaluating the incidence of common irAEs of single-agent PD-(L)1 inhibitor found that diarrhea (9.47%) and hypothyroidism (6.07%) were of relatively high risk (Wang et al., 2019). A similar result of hypothyroidism (5.6%) was also reported in the meta-analysis of 13 studies with 3,803 participants (Baxi et al., 2018). With respect to PD-(L)1 and VEGF(R) dual inhibitors, the risk of irAEs reported ranged broadly from 38% to 56% (Motzer et al., 2019; Powles et al., 2020a) while no meta-analysis was performed to reach a consensus. Moreover, a meta-analysis focusing on the direct comparison between PD-(L)1 blockade plus anti-VEGF(R) agents with PD-(L)1 blockade alone has not yet been conducted. Accordingly, we conducted a systematic review and meta-analysis to explore whether the incidence of irAEs increased in combination therapy, compared with anti-PD-(L)1 antibody monotherapy.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was applied in this report (Moher et al., 2009). The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO), number CRD42021287603.
Three electronic databases (PubMed, Embase, and Cochrane CENTRAL) were systematically retrieved from inception to 22 October 2021, with language restricted to English. Keywords such as PD-1, PD-L1, and randomized were used (see Supplementary Table S1). Clinicaltrials.gov and conference proceedings (American Society of Clinical Oncology, European Society for Medical Oncology, and American Association for Cancer Research) were manually searched. References of eligible studies were also manually reviewed.
Phase II or III randomized controlled trials that reported PD-(L)1 inhibitor monotherapy or PD-(L)1 inhibitors plus anti-VEGF(R) agents for the treatment of cancer patients irrespective of solid or hematologic malignancies were included. Trials that only compared different dosages or administration intervals were excluded. Additionally, sequential combination therapy was excluded. The primary outcomes were incidence of any grade irAEs and grade ≥3 irAEs. The secondary outcomes were incidence of any grade treatment-related adverse events (trAEs) and grade ≥3 trAEs. Adverse events were graded according to the National Cancer Institute-Common Terminology Criteria for Adverse Events (CTCAE) (CTCAE, 2017). When duplicate cohorts were reported, the most recent publication with comprehensive data was included. Two authors (TQY and WDW) first independently screened the titles and/or abstracts to identify potential trials and then checked the full-text articles for eligibility. Disagreements were resolved by a third author (LN).
Two authors (TQY and WDW) extracted the following data using a pre-designed form independently: study characteristics (first author, publication year, NCT number, and trial name), methods (trial phase, masking status, and line of treatment), participants (cancer type, performance status, and PD-L1 expression status), interventions (intervention and comparison group regimes), and outcomes (follow-up duration, adverse event type, incidence of irAEs and trAEs (grade 1 to 5 and grade 3 to 5)). Any discrepancies were resolved by a third investigator (LN).
Two reviewers (TQY and WDW) independently evaluated the methodological quality of eligible studies using the Cochrane Collaboration’s tool based on the following items: random sequence generation, allocation concealment, blinding of participants and healthcare providers, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias (Higgins et al., 2011). Any conflict was resolved by a third reviewer (LN).
The effect size of the safety profile was estimated by relative risk (RR) with a corresponding 95% confidence interval (CI). Incidence of PD-(L)1 inhibitor monotherapy and in combination with anti-VEGF(R) agents were pooled, respectively, for there were few direct comparisons. In addition, pooled effects of the direct comparisons between the two regimens were also estimated using the random effects model. R software (version 3.5.3) with meta package (version 4.9–3) was used. The classic half-integer continuity correction (adding 0.5 to each cell) was used when zero adverse events were reported in any arm.
Heterogeneity was examined by Cochran Q and I2 statistic, with significance set at p < 0.10. I2 of greater than 50% was considered as high risk, 25%–50% as moderate risk, and less than 25% as low risk (Higgins et al., 2003). Funnel plot and Egger’s test were employed to explore the potential publication bias and the small-study effect when more than ten studies were included. Sensitivity analysis was performed by omitting eligible studies one by one. Statistical significance was considered when p < 0.05 if not noted.
A total of 4,038 records were identified, of which 486 duplicates were excluded. After screening by title and/or abstract, 251 articles were included for full-text screening. Ultimately, 77 articles [(Finn et al., 2020a; Powles et al., 2020a; Choueiri et al., 2021); (Motzer et al., 2019); (Borghaei et al., 2015; Brahmer et al., 2015; Fehrenbacher et al., 2016; Carbone et al., 2017; Hamid et al., 2017; Bang et al., 2018; D'Angelo et al., 2018; Fehrenbacher et al., 2018; Ferris et al., 2018; Larkin et al., 2018; Long et al., 2018; McDermott et al., 2018; Bonomi et al., 2019; Burtness et al., 2019; Cohen et al., 2019; Eng et al., 2019; Fradet et al., 2019; Hellmann et al., 2019; Kato et al., 2019; Larkin et al., 2019; Levy et al., 2019; Long et al., 2019; Mok et al., 2019; Necchi et al., 2019; O'Reilly et al., 2019; Pujol et al., 2019; Rini et al., 2019; Robert et al., 2019; Scherpereel et al., 2019; Siu et al., 2019; Theelen et al., 2019; Andre et al., 2020; Ferris et al., 2020; Finn et al., 2020b; Galsky et al., 2020; Herbst et al., 2020; Huang et al., 2020; Kojima et al., 2020; Motzer et al., 2020b; Planchard et al., 2020; Popat et al., 2020; Powles et al., 2020b; Reardon et al., 2020; Rizvi et al., 2020; Robert et al., 2020; Shitara et al., 2020; Zamarin et al., 2020; Zhang et al., 2020; Boku et al., 2021; Boyer et al., 2021; Gettinger et al., 2021; Gogas et al., 2021; Hamanishi et al., 2021; Heudobler et al., 2021; Jassem et al., 2021; Kuruvilla et al., 2021; Liu et al., 2021; Lu et al., 2021; Mahmood et al., 2021; McBride et al., 2021; Motzer et al., 2021; Nayak et al., 2021; Park et al., 2021; Powles et al., 2021; Pujade-Lauraine et al., 2021; Reck et al., 2021; Sezer et al., 2021; Spigel et al., 2021; van der Heijden et al., 2021; Winer et al., 2021; Chawla et al., 2022)] were included in the meta-analysis. The most frequent reasons for exclusion during full-text screening were duplicate cohorts (77 records) and no outcome data of interest (45 records). (See Figure 1).
Among 77 trials included, 54 (70.1%) studies [(Finn et al., 2020a; Powles et al., 2020a; Choueiri et al., 2021); (Motzer et al., 2019); (Borghaei et al., 2015); (Brahmer et al., 2015); (Carbone et al., 2017); (Bang et al., 2018); (Fehrenbacher et al., 2018; Ferris et al., 2018; Larkin et al., 2018); (Burtness et al., 2019; Cohen et al., 2019; Eng et al., 2019; Fradet et al., 2019; Hellmann et al., 2019; Kato et al., 2019; Larkin et al., 2019); (Long et al., 2019); (Mok et al., 2019); (Rini et al., 2019); (Robert et al., 2019); (Andre et al., 2020; Finn et al., 2020b; Ferris et al., 2020; Galsky et al., 2020); (Motzer et al., 2020b; Powles et al., 2020b; Huang et al., 2020; Kojima et al., 2020; Planchard et al., 2020; Popat et al., 2020; Reardon et al., 2020; Rizvi et al., 2020; Robert et al., 2020; Shitara et al., 2020); (Boku et al., 2021; Boyer et al., 2021; Gettinger et al., 2021; Gogas et al., 2021; Hamanishi et al., 2021); (Jassem et al., 2021); (Kuruvilla et al., 2021); (Lu et al., 2021); (Motzer et al., 2021); (Park et al., 2021; Powles et al., 2021; Pujade-Lauraine et al., 2021; Reck et al., 2021; Sezer et al., 2021; Spigel et al., 2021; van der Heijden et al., 2021; Winer et al., 2021); (Fuchs et al., 2022)] were phase III trials, 22 (28.6%) (Fehrenbacher et al., 2016; Hamid et al., 2017; D'Angelo et al., 2018; Long et al., 2018; McDermott et al., 2018; Bonomi et al., 2019; Levy et al., 2019; Necchi et al., 2019; O'Reilly et al., 2019; Pujol et al., 2019; Scherpereel et al., 2019; Siu et al., 2019; Theelen et al., 2019; Zamarin et al., 2020; Zhang et al., 2020; Heudobler et al., 2021; Liu et al., 2021; Mahmood et al., 2021; McBride et al., 2021; Nayak et al., 2021; Chawla et al., 2022; Singh et al., 2022) were phase II, and 1 (1.3%) (Herbst et al., 2020) was phase II/III trial. Regimens in 29 (37.7%) studies (Carbone et al., 2017; Hamid et al., 2017; Long et al., 2018; McDermott et al., 2018; Burtness et al., 2019; Hellmann et al., 2019; Larkin et al., 2019; Long et al., 2019; Mok et al., 2019; Motzer et al., 2019; Rini et al., 2019; Finn et al., 2020a; Andre et al., 2020; Powles et al., 2020a; Powles et al., 2020b; Galsky et al., 2020; Rizvi et al., 2020; Robert et al., 2020; Shitara et al., 2020; Boyer et al., 2021; Choueiri et al., 2021; Gogas et al., 2021; Jassem et al., 2021; Mahmood et al., 2021; McBride et al., 2021; Motzer et al., 2021; Powles et al., 2021; Reck et al., 2021; Sezer et al., 2021) were frontline (1 or ≥1 line) therapies and in 32 (41.6%) studies (Brahmer et al., 2015; D'Angelo et al., 2018; Ferris et al., 2018; Larkin et al., 2018; Bonomi et al., 2019; Cohen et al., 2019; Fradet et al., 2019; Kato et al., 2019; Levy et al., 2019; O'Reilly et al., 2019; Pujol et al., 2019; Siu et al., 2019; Theelen et al., 2019; Ferris et al., 2020; Finn et al., 2020b; Herbst et al., 2020; Huang et al., 2020; Kojima et al., 2020; Popat et al., 2020; Reardon et al., 2020; Zhang et al., 2020; Gettinger et al., 2021; Hamanishi et al., 2021; Heudobler et al., 2021; Kuruvilla et al., 2021; Lu et al., 2021; Park et al., 2021; Spigel et al., 2021; van der Heijden et al., 2021; Chawla et al., 2022; Fuchs et al., 2022; Singh et al., 2022) were second-line (2 or ≥2 line) and in 6 (7.8%) studies (Bang et al., 2018; Eng et al., 2019; Necchi et al., 2019; Planchard et al., 2020; Boku et al., 2021; Liu et al., 2021) were third-line setting (3 or ≥3 line). In addition, one (1.3%) study (Robert et al., 2019) focused on first/second-line therapy, seven (9.1%) studies (Borghaei et al., 2015; Fehrenbacher et al., 2016; Fehrenbacher et al., 2018; Scherpereel et al., 2019; Motzer et al., 2020b; Nayak et al., 2021; Winer et al., 2021) on second/third-line, and two (2.6%) studies (Zamarin et al., 2020; Pujade-Lauraine et al., 2021) on second to fourth line therapy. PD-L1 expression status in 54 (70.1%) studies was noted as unselected [(Winer et al., 2021; Chawla et al., 2022; Fuchs et al., 2022; Singh et al., 2022); (Winer et al., 2021; Chawla et al., 2022; Fuchs et al., 2022; Singh et al., 2022); (Winer et al., 2021; Chawla et al., 2022; Fuchs et al., 2022; Singh et al., 2022); (Borghaei et al., 2015; Brahmer et al., 2015; Fehrenbacher et al., 2016); (Borghaei et al., 2015; Brahmer et al., 2015; Fehrenbacher et al., 2016); (Borghaei et al., 2015; Brahmer et al., 2015; Fehrenbacher et al., 2016); (Fehrenbacher et al., 2018; Ferris et al., 2018; Larkin et al., 2018; Long et al., 2018; McDermott et al., 2018); (Burtness et al., 2019; Cohen et al., 2019; Eng et al., 2019; Fradet et al., 2019); (Kato et al., 2019; Larkin et al., 2019; Levy et al., 2019; Long et al., 2019); (O'Reilly et al., 2019; Pujol et al., 2019; Rini et al., 2019; Robert et al., 2019; Scherpereel et al., 2019); (Theelen et al., 2019); (Ferris et al., 2020); (Galsky et al., 2020); (Motzer et al., 2020b; Huang et al., 2020; Kojima et al., 2020); (Powles et al., 2020b; Popat et al., 2020; Reardon et al., 2020; Rizvi et al., 2020; Robert et al., 2020); (Zamarin et al., 2020); (Boku et al., 2021); (Gettinger et al., 2021; Gogas et al., 2021; Hamanishi et al., 2021); (Kuruvilla et al., 2021; Liu et al., 2021; Lu et al., 2021); (McBride et al., 2021; Motzer et al., 2021; Nayak et al., 2021; Park et al., 2021; Powles et al., 2021; Pujade-Lauraine et al., 2021); (Spigel et al., 2021; van der Heijden et al., 2021; Winer et al., 2021)]. There were 59 (76.6%) trials [(Finn et al., 2020a); (Motzer et al., 2019); (Borghaei et al., 2015; Brahmer et al., 2015; Fehrenbacher et al., 2016; Carbone et al., 2017; Hamid et al., 2017; Bang et al., 2018; D'Angelo et al., 2018; Fehrenbacher et al., 2018; Ferris et al., 2018; Larkin et al., 2018); (Burtness et al., 2019; Cohen et al., 2019; Eng et al., 2019); (Hellmann et al., 2019; Kato et al., 2019; Larkin et al., 2019; Levy et al., 2019; Long et al., 2019; Mok et al., 2019; Necchi et al., 2019; O'Reilly et al., 2019); (Robert et al., 2019; Scherpereel et al., 2019; Siu et al., 2019; Theelen et al., 2019; Andre et al., 2020; Finn et al., 2020b; Ferris et al., 2020); (Herbst et al., 2020; Huang et al., 2020; Kojima et al., 2020); (Powles et al., 2020b; Planchard et al., 2020; Popat et al., 2020); (Rizvi et al., 2020; Robert et al., 2020; Shitara et al., 2020); (Zhang et al., 2020; Boku et al., 2021; Boyer et al., 2021); (Gogas et al., 2021; Hamanishi et al., 2021; Heudobler et al., 2021; Jassem et al., 2021; Kuruvilla et al., 2021; Liu et al., 2021; Lu et al., 2021; Mahmood et al., 2021); (Park et al., 2021); (Pujade-Lauraine et al., 2021; Reck et al., 2021; Sezer et al., 2021; Spigel et al., 2021); (Winer et al., 2021; Chawla et al., 2022; Fuchs et al., 2022; Singh et al., 2022)] with Eastern Cooperative Oncology Group (ECOG) performance status score of 0–1, 7 trials (9.1%) (Long et al., 2018; Fradet et al., 2019; Pujol et al., 2019; Galsky et al., 2020; Zamarin et al., 2020; McBride et al., 2021; Powles et al., 2021) with ECOG score of 0–2, 1 trial (1.3%) (Bonomi et al., 2019) with ECOG score of 2–3, 8 (10.4%) studies (McDermott et al., 2018; Rini et al., 2019; Powles et al., 2020a; Motzer et al., 2020b; Reardon et al., 2020; Choueiri et al., 2021; Motzer et al., 2021; Nayak et al., 2021) with Karnofsky performance score ≥70 and 1 (1.3%) study (Gettinger et al., 2021) with Zubrod performance status score of 0–1. As for dual combination therapy, six regimens were reported in our study: pembrolizumab plus bevacizumab (Nayak et al., 2021), pembrolizumab plus lenvatinib (Motzer et al., 2021), pembrolizumab plus axitinib (Powles et al., 2020a), nivolumab plus cabozantinib (Choueiri et al., 2021), atezolizumab plus bevacizumab (McDermott et al., 2018; Rini et al., 2019; Finn et al., 2020a), avelumab plus axitinib (Motzer et al., 2019). (See Supplementary Table S2).
Bias of 71 (92.2%) studies [(Finn et al., 2020a; Powles et al., 2020a; Choueiri et al., 2021); (Motzer et al., 2019); (Borghaei et al., 2015; Brahmer et al., 2015; Fehrenbacher et al., 2016; Carbone et al., 2017; Hamid et al., 2017; Bang et al., 2018; D'Angelo et al., 2018; Fehrenbacher et al., 2018; Ferris et al., 2018; Larkin et al., 2018; Long et al., 2018; McDermott et al., 2018; Bonomi et al., 2019; Burtness et al., 2019; Cohen et al., 2019; Eng et al., 2019; Fradet et al., 2019; Hellmann et al., 2019; Kato et al., 2019); (Mok et al., 2019; Necchi et al., 2019; O'Reilly et al., 2019; Pujol et al., 2019; Rini et al., 2019; Robert et al., 2019; Scherpereel et al., 2019; Siu et al., 2019; Theelen et al., 2019; Andre et al., 2020; Ferris et al., 2020); (Motzer et al., 2020b; Powles et al., 2020b; Galsky et al., 2020; Herbst et al., 2020; Huang et al., 2020; Kojima et al., 2020; Planchard et al., 2020; Popat et al., 2020; Reardon et al., 2020; Rizvi et al., 2020; Robert et al., 2020; Shitara et al., 2020; Zamarin et al., 2020; Zhang et al., 2020); (Gettinger et al., 2021; Gogas et al., 2021; Hamanishi et al., 2021; Heudobler et al., 2021; Jassem et al., 2021; Kuruvilla et al., 2021; Liu et al., 2021; Lu et al., 2021; Mahmood et al., 2021; McBride et al., 2021; Motzer et al., 2021; Nayak et al., 2021; Park et al., 2021; Powles et al., 2021; Pujade-Lauraine et al., 2021; Reck et al., 2021; Sezer et al., 2021; Spigel et al., 2021; van der Heijden et al., 2021; Winer et al., 2021; Chawla et al., 2022; Fuchs et al., 2022; Singh et al., 2022)] were regarded as high risk and 6 (7.8%) (Larkin et al., 2019; Levy et al., 2019; Long et al., 2019; Finn et al., 2020b; Boku et al., 2021; Boyer et al., 2021) were low risk. 67 (87.0%) studies [(Finn et al., 2020a; Powles et al., 2020a; Choueiri et al., 2021); (Motzer et al., 2019); (Borghaei et al., 2015; Brahmer et al., 2015; Fehrenbacher et al., 2016; Carbone et al., 2017); (Bang et al., 2018; D'Angelo et al., 2018; Fehrenbacher et al., 2018; Ferris et al., 2018; Larkin et al., 2018; Long et al., 2018; McDermott et al., 2018; Bonomi et al., 2019; Burtness et al., 2019; Cohen et al., 2019; Eng et al., 2019; Fradet et al., 2019; Hellmann et al., 2019; Kato et al., 2019); (Mok et al., 2019; Necchi et al., 2019; O'Reilly et al., 2019; Pujol et al., 2019; Rini et al., 2019; Robert et al., 2019; Scherpereel et al., 2019; Siu et al., 2019; Theelen et al., 2019; Andre et al., 2020; Ferris et al., 2020); (Motzer et al., 2020b; Powles et al., 2020b; Herbst et al., 2020; Huang et al., 2020; Kojima et al., 2020; Planchard et al., 2020; Popat et al., 2020; Reardon et al., 2020; Rizvi et al., 2020); (Zamarin et al., 2020); (Zhang et al., 2020); (Gettinger et al., 2021; Gogas et al., 2021; Hamanishi et al., 2021; Heudobler et al., 2021; Jassem et al., 2021; Kuruvilla et al., 2021; Liu et al., 2021; Lu et al., 2021; Mahmood et al., 2021; McBride et al., 2021; Motzer et al., 2021; Nayak et al., 2021; Park et al., 2021; Powles et al., 2021; Pujade-Lauraine et al., 2021; Reck et al., 2021; Sezer et al., 2021; Spigel et al., 2021; van der Heijden et al., 2021; Winer et al., 2021; Chawla et al., 2022; Fuchs et al., 2022; Singh et al., 2022)] were open-label and 2 (2.6%) studies (Hamid et al., 2017; Shitara et al., 2020) were partially blinded (patients and investigators masked only to the pembrolizumab dose in one study and patients masked only to the combination therapy groups and unblinded to pembrolizumab monotherapy in the other study), which was regarded as the most common reason for high risk. Only 8 (10.4%) trials (Larkin et al., 2019; Levy et al., 2019; Long et al., 2019; Finn et al., 2020b; Galsky et al., 2020; Robert et al., 2020; Boku et al., 2021; Boyer et al., 2021) were set as double-blind or quadruple-blind. (See Supplementary Table S3).
For PD-(L)1 inhibitor monotherapy, 49 studies [(Borghaei et al., 2015; Brahmer et al., 2015; Fehrenbacher et al., 2016; Carbone et al., 2017; Hamid et al., 2017; Bang et al., 2018); (Fehrenbacher et al., 2018; Ferris et al., 2018; Larkin et al., 2018); (Cohen et al., 2019); (Fradet et al., 2019); (Hellmann et al., 2019); (Larkin et al., 2019); (Long et al., 2019); (Mok et al., 2019); (Pujol et al., 2019); (Robert et al., 2019; Scherpereel et al., 2019; Siu et al., 2019; Theelen et al., 2019; Andre et al., 2020); (Finn et al., 2020b; Motzer et al., 2020b; Galsky et al., 2020; Herbst et al., 2020; Huang et al., 2020; Kojima et al., 2020); (Powles et al., 2020b; Popat et al., 2020; Reardon et al., 2020; Rizvi et al., 2020); (Shitara et al., 2020); (Boyer et al., 2021); (Gettinger et al., 2021); (Hamanishi et al., 2021); (Jassem et al., 2021; Kuruvilla et al., 2021; Liu et al., 2021; Lu et al., 2021); (Nayak et al., 2021; Park et al., 2021; Powles et al., 2021; Pujade-Lauraine et al., 2021; Reck et al., 2021; Sezer et al., 2021; Spigel et al., 2021; van der Heijden et al., 2021; Winer et al., 2021); (Fuchs et al., 2022)] with 13,206 participants were included, among which 30 studies (Hamid et al., 2017; Bang et al., 2018; Fehrenbacher et al., 2018; Cohen et al., 2019; Fradet et al., 2019; Mok et al., 2019; Robert et al., 2019; Siu et al., 2019; Theelen et al., 2019; Andre et al., 2020; Finn et al., 2020b; Powles et al., 2020b; Galsky et al., 2020; Herbst et al., 2020; Huang et al., 2020; Kojima et al., 2020; Rizvi et al., 2020; Shitara et al., 2020; Boyer et al., 2021; Gettinger et al., 2021; Hamanishi et al., 2021; Jassem et al., 2021; Kuruvilla et al., 2021; Liu et al., 2021; Park et al., 2021; Pujade-Lauraine et al., 2021; Reck et al., 2021; Sezer et al., 2021; Winer et al., 2021; Fuchs et al., 2022) involving 8,638 participants were included in the pooled analysis of the total incidence of irAEs. Incidences of any grade and grade ≥3 irAEs for PD-(L)1 inhibitors were 0.25 (95% CI 0.20–0.32) and 0.06 (95% CI 0.05–0.07), respectively. The most common any grade irAEs were skin and subcutaneous, gastrointestinal, and endocrine (>10%). Grade ≥3 hepatic irAE was more frequent than other sites (0.02, 95% CI 0.01–0.03). Regarding the concrete type of any grade irAEs, reactive cutaneous capillary endothelial proliferation (RCCEP), which was reported in one study, occurred in up to 80% of the participants though most of them were assessed as grade 1 to 2. Apart from that, hypothyroidism, pneumonitis, hyperthyroidism, and hepatitis were relatively frequent (≥2%). Concerning grade ≥3 irAEs, grade ≥3 pneumonitis was more frequent. (See Figure 2).
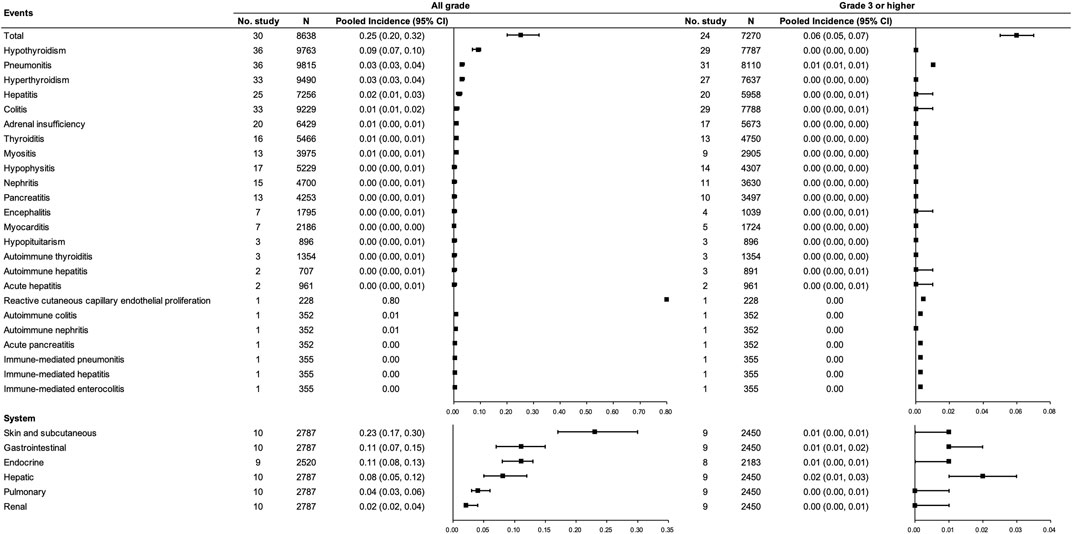
FIGURE 2. Incidence of irAEs for PD-(L)1 inhibitor monotherapy. Note: an incidence of 0.00 indicates a rate <0.005.
Regarding the combination therapy, five studies (Motzer et al., 2019; Rini et al., 2019; Powles et al., 2020a; Choueiri et al., 2021; Nayak et al., 2021) with 1,684 participants were included, of which two studies (Motzer et al., 2019; Powles et al., 2020a) with 863 participants were included in the pooled analysis of the total incidence of any grade irAEs for PD-(L)1 inhibitors and anti-VEGF(R) agents dual combination therapy. Incidences of any grade and grade ≥3 irAEs were 0.47 (95% CI 0.30–0.65) and 0.11 (95% CI 0.08–0.16), respectively. The most common any grade irAEs were hypothyroidism and hyperthyroidism (≥10%) (See Figure 3).
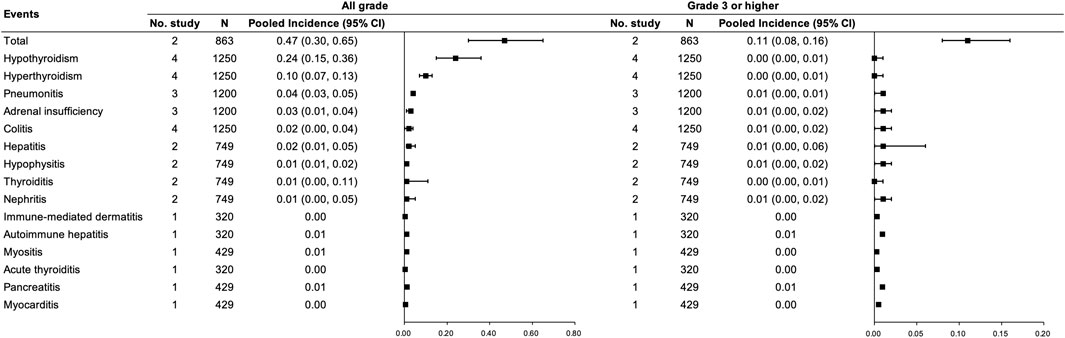
FIGURE 3. Incidence of irAEs for PD-(L)1 inhibitors combined with anti-VEGF(R) agents therapy. Note: an incidence of 0.00 indicates a rate <0.005.
As for direct comparisons for irAEs, only one study was included (Nayak et al., 2021), indicating no significant difference between the monotherapy and the dual combination regimen in terms of colitis, hyperthyroidism, and hypothyroidism for any grade and grade 3 or higher. There is a trend of higher incidence for any grade hyperthyroidism under combination therapy. (See Figure 4).
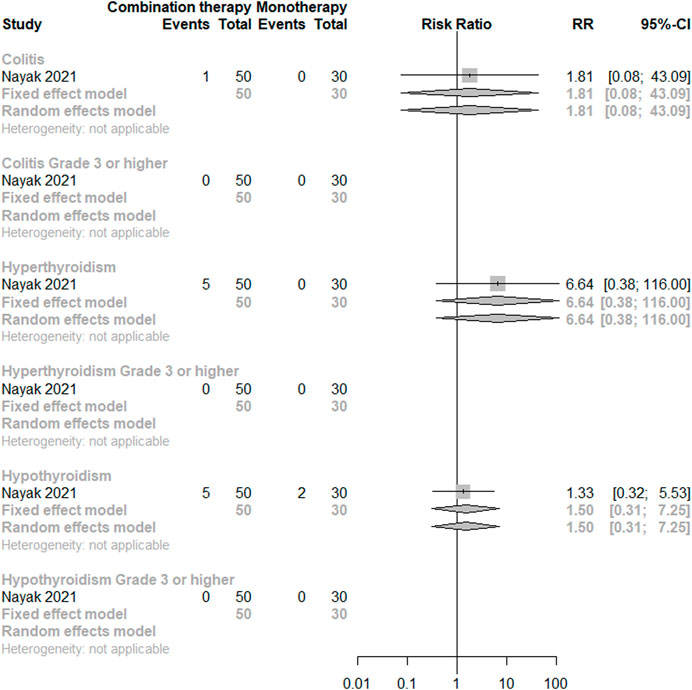
FIGURE 4. Direct comparisons of PD-(L)1 inhibitor monotherapy and in combination with anti-VEGF(R) agents therapy for irAEs.
A total of 71 studies [(Borghaei et al., 2015; Brahmer et al., 2015; Fehrenbacher et al., 2016; Carbone et al., 2017; Hamid et al., 2017; Bang et al., 2018; D'Angelo et al., 2018; Fehrenbacher et al., 2018; Ferris et al., 2018; Larkin et al., 2018; Long et al., 2018; McDermott et al., 2018; Bonomi et al., 2019; Burtness et al., 2019; Cohen et al., 2019; Eng et al., 2019; Fradet et al., 2019; Hellmann et al., 2019; Kato et al., 2019; Larkin et al., 2019; Levy et al., 2019; Long et al., 2019; Mok et al., 2019; Necchi et al., 2019; O'Reilly et al., 2019; Pujol et al., 2019); (Robert et al., 2019; Scherpereel et al., 2019; Siu et al., 2019; Theelen et al., 2019; Andre et al., 2020; Finn et al., 2020b; Motzer et al., 2020b; Powles et al., 2020b; Ferris et al., 2020; Galsky et al., 2020; Herbst et al., 2020; Huang et al., 2020; Kojima et al., 2020; Planchard et al., 2020; Popat et al., 2020; Reardon et al., 2020; Rizvi et al., 2020; Robert et al., 2020; Shitara et al., 2020; Zamarin et al., 2020; Zhang et al., 2020; Boku et al., 2021; Boyer et al., 2021; Gettinger et al., 2021; Gogas et al., 2021; Hamanishi et al., 2021; Heudobler et al., 2021; Jassem et al., 2021; Kuruvilla et al., 2021; Liu et al., 2021; Lu et al., 2021; Mahmood et al., 2021; McBride et al., 2021); (Nayak et al., 2021; Park et al., 2021; Powles et al., 2021; Pujade-Lauraine et al., 2021; Reck et al., 2021; Sezer et al., 2021; Spigel et al., 2021; van der Heijden et al., 2021; Winer et al., 2021; Chawla et al., 2022; Fuchs et al., 2022; Singh et al., 2022)] with 15,465 participants were included, among which 59 studies [(Borghaei et al., 2015; Brahmer et al., 2015; Fehrenbacher et al., 2016; Carbone et al., 2017; Hamid et al., 2017; Bang et al., 2018); (Fehrenbacher et al., 2018; Ferris et al., 2018; Larkin et al., 2018; Long et al., 2018; McDermott et al., 2018); (Burtness et al., 2019; Cohen et al., 2019; Eng et al., 2019; Fradet et al., 2019; Hellmann et al., 2019; Kato et al., 2019; Larkin et al., 2019; Levy et al., 2019; Long et al., 2019; Mok et al., 2019); (O'Reilly et al., 2019); (Robert et al., 2019; Scherpereel et al., 2019; Siu et al., 2019); (Andre et al., 2020; Finn et al., 2020b; Motzer et al., 2020b; Powles et al., 2020b; Ferris et al., 2020; Galsky et al., 2020; Herbst et al., 2020; Huang et al., 2020; Kojima et al., 2020; Planchard et al., 2020; Popat et al., 2020; Reardon et al., 2020; Rizvi et al., 2020; Robert et al., 2020; Shitara et al., 2020); (Zhang et al., 2020); (Boyer et al., 2021); (Gogas et al., 2021; Hamanishi et al., 2021; Heudobler et al., 2021; Jassem et al., 2021; Kuruvilla et al., 2021; Liu et al., 2021; Lu et al., 2021); (McBride et al., 2021); (Park et al., 2021; Powles et al., 2021; Pujade-Lauraine et al., 2021; Reck et al., 2021; Sezer et al., 2021; Spigel et al., 2021; van der Heijden et al., 2021); (Chawla et al., 2022); (Fuchs et al., 2022)] involving 14,430 participants and 63 studies [(Borghaei et al., 2015; Brahmer et al., 2015; Fehrenbacher et al., 2016; Carbone et al., 2017; Hamid et al., 2017; Bang et al., 2018; D'Angelo et al., 2018; Fehrenbacher et al., 2018; Ferris et al., 2018; Larkin et al., 2018; Long et al., 2018); (Bonomi et al., 2019; Burtness et al., 2019; Cohen et al., 2019; Eng et al., 2019; Fradet et al., 2019; Hellmann et al., 2019; Kato et al., 2019; Larkin et al., 2019; Levy et al., 2019; Long et al., 2019; Mok et al., 2019); (O'Reilly et al., 2019); (Robert et al., 2019; Scherpereel et al., 2019; Siu et al., 2019); (Andre et al., 2020; Finn et al., 2020b; Motzer et al., 2020b; Powles et al., 2020b; Ferris et al., 2020; Galsky et al., 2020; Herbst et al., 2020; Huang et al., 2020; Kojima et al., 2020; Planchard et al., 2020; Popat et al., 2020; Reardon et al., 2020; Rizvi et al., 2020; Robert et al., 2020; Shitara et al., 2020; Zamarin et al., 2020; Zhang et al., 2020); (Boyer et al., 2021; Gettinger et al., 2021; Gogas et al., 2021; Hamanishi et al., 2021; Heudobler et al., 2021; Jassem et al., 2021; Kuruvilla et al., 2021; Liu et al., 2021; Lu et al., 2021); (McBride et al., 2021); (Park et al., 2021; Powles et al., 2021; Pujade-Lauraine et al., 2021; Reck et al., 2021; Sezer et al., 2021; Spigel et al., 2021; van der Heijden et al., 2021; Winer et al., 2021; Chawla et al., 2022; Fuchs et al., 2022)] with 14,860 participants were included for the pooled analysis of the incidence of any grade trAEs and grade ≥3 trAEs for PD-(L)1 inhibitor monotherapy, respectively. Incidences of any grade and grade ≥3 trAEs were 0.70 (95% CI 0.66–0.74) and 0.17 (95% CI 0.15–0.20), respectively. The most frequent (≥10%) any grade trAEs were reactive cutaneous capillary endothelial proliferation (RCCEP), nausea/vomiting, fatigue, pruritus, and diarrhea in the pooled analysis. In particular, RCCEP had an extremely high risk of 0.80 (0.75, 0.85), though most were assessed as grades 1 to 2. (See Figure 5).
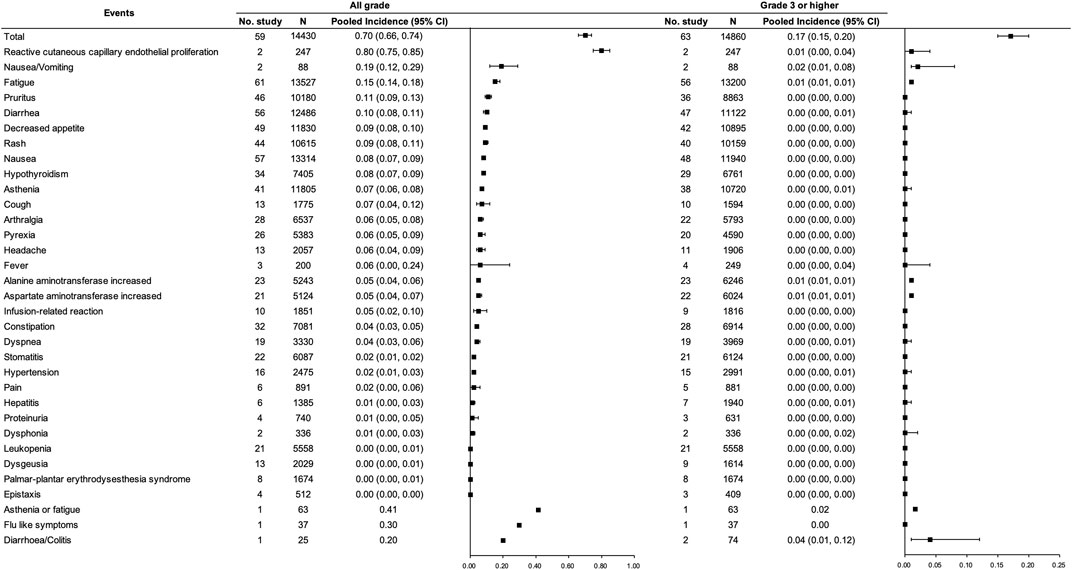
FIGURE 5. Incidence of trAEs for PD-(L)1 inhibitor monotherapy. Note: an incidence of 0.00 indicates a rate <0.005.
With respect to the combination therapy, eight studies (McDermott et al., 2018; Motzer et al., 2019; Rini et al., 2019; Finn et al., 2020a; Powles et al., 2020a; Choueiri et al., 2021; Motzer et al., 2021; Nayak et al., 2021) with 2,466 participants were included, among which six studies (McDermott et al., 2018; Motzer et al., 2019; Rini et al., 2019; Powles et al., 2020a; Choueiri et al., 2021; Motzer et al., 2021) involving 2087 participants were included for the pooled analysis of the incidence of trAEs for PD-(L)1 inhibitors and anti-VEGF(R) agents dual combination therapy. Incidences of any grade and grade ≥3 trAEs were 0.97 (95% CI 0.94–0.98) and 0.60 (95% CI 0.49–0.70), respectively. Any grade diarrhea, hypertension, hypothyroidism, fatigue, proteinuria, and nausea were frequent (≥20%). In addition, constipation and pyrexia reported in only one study were with an incidence of more than 0.20 (McDermott et al., 2018). Regarding grade ≥3 trAEs, hypertension was the most frequent (>10%). (See Figure 6).
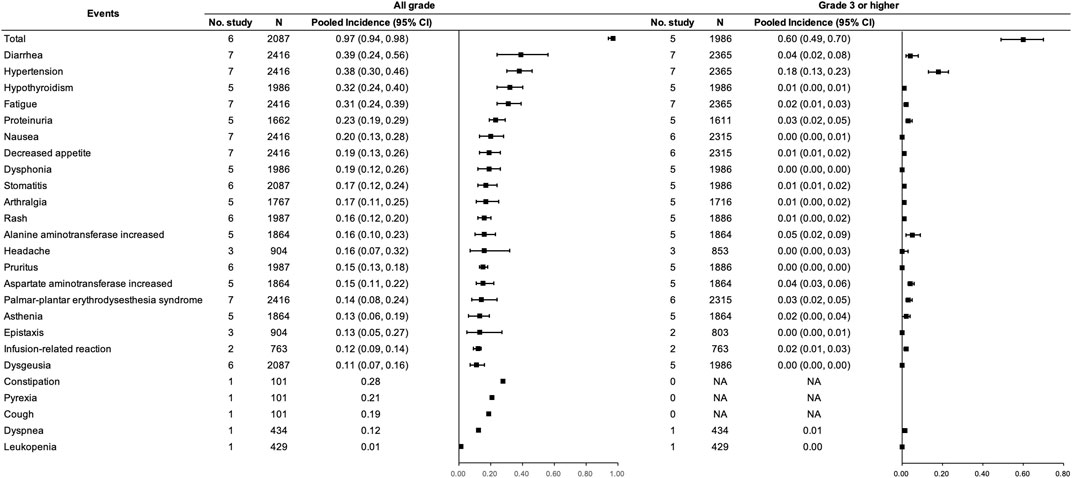
FIGURE 6. Incidence of trAEs for PD-(L)1 inhibitors combined with anti-VEGF(R) agents therapy. Note: An incidence of 0.00 indicates a rate <0.005.
Concerning direct comparisons for trAEs, two studies (McDermott et al., 2018; Nayak et al., 2021) were included and no significant difference was observed between the two regimens. However, risks of gastrointestinal disorders (constipation, nausea, diarrhea, and stomatitis), metabolism and nutrition disorders (decreased appetite), musculoskeletal and connective tissue disorders (arthralgia), nervous system disorders (dysgeusia and headache), respiratory, thoracic, and mediastinal disorders (epistaxis), vascular disorders (hypertension), and renal and urinary disorders (proteinuria) were significantly higher in the combination treatment group than in the monotherapy group (p < 0.05). (See Figure 7).
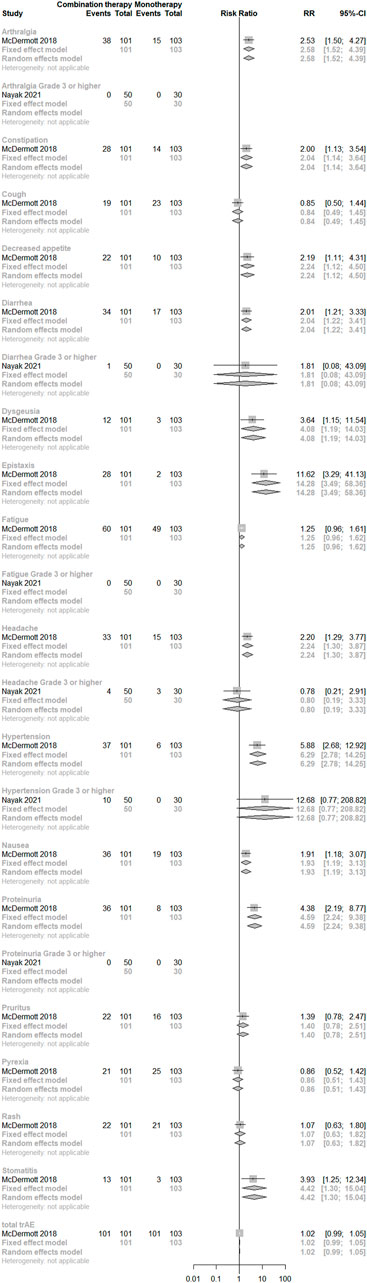
FIGURE 7. Direct comparisons of PD-(L)1 inhibitor monotherapy and in combination with anti-VEGF(R) agents therapy for trAEs.
We completed a systematic review to comprehensively summarize the incidence of irAEs and trAEs with PD-(L)1 inhibitor alone and in combination with VEGF(R) blockades. This study was the first systematic review comparing the safety of the two regimens directly. Our meta-analysis demonstrated that the incidence of irAEs was 0.25 (95% CI 0.20–0.32) for PD-(L)1 inhibitor monotherapy and 0.47 (95% CI 0.30–0.65) for PD-(L)1 and VEGF(R) inhibitor combination therapy, which were usually underestimated. Sensitivity analyses did not reveal essential changes in pooled results.
A preclinical study illustrated that dual blockades of PD-1 and VEGFR-2 could promote vascular normalization and enhance antitumor immune responses (Shigeta et al., 2020). Simultaneously, some irAEs tended to increase. For example, the risk of thyroid disorders, although no significant difference was observed in the head-to-head comparison (N = 80), appeared to be higher under combination therapy in the indirect comparison (N > 1,000), which may require the clinician’s attention. Moreover, several VEGF(R) blockades also have an influence on the thyroid (Ivy et al., 2009), which may affect the judgment on the relevance between irAE and PD-(L)1 inhibitors. However, according to the guideline of the Center for Drug Evaluation in China, it is also judged as an irAE conservatively, which may lead to an increase in the proportion of such toxicities. Therefore, thyroid hormone levels should be regularly monitored. Additionally, our study displayed that pneumonitis and myocarditis, which were serious and were deteriorating patients’ prognosis and quality of life were of similar risks between the two groups. What’s more, the incidence of grade ≥3 hepatic irAE was higher for PD-1 inhibitor monotherapy than combination therapy in our study (0.02 vs. 0.01). It is likely that the incidence of grade ≥3 hepatic irAE (0.02, 95% CI 0.01–0.03) referred to the hepatic system including several subtypes of adverse events, while the combination therapy group only reported hepatitis and autoimmune hepatitis with hepatic system risk not mentioned.
IrAEs can affect a broad spectrum of organs (Kennedy and Salama, 2020), and the skin and subcutaneous system were most common under ICIs therapy. It is noteworthy that camrelizumab, a PD-1 antibody approved for the treatment of Hodgkin’s lymphoma, hepatocellular carcinoma, non-small cell lung cancer, esophageal cancer, and nasopharyngeal carcinoma by the National Medical Products Administration in China and was designated as an orphan drug by the United States. Food and Drug Administration, has a specific irAE—RCCEP with quite a high incidence ranging from 67% to 79.8% when used alone though the majority of which were grade 1 to 2 (Huang et al., 2020; Qin et al., 2020). Nonetheless, it is reported in phase II trials that camrelizumab combined with apatinib appreciably decreased the risk of RCCEP to 29.5% in advanced hepatocellular carcinoma (Xu et al., 2021) and to 8.9% in advanced cervical cancer patients (Lan et al., 2020). Overall, the toxicity of camrelizumab was acceptable. The mechanism of RCCEP remains unclarified and the VEGFR-2 signaling pathway possibly plays a crucial role in the formation of RCCEP.
To our knowledge, this is the first systematic review and meta-analysis that reported the incidence of various types of irAEs of anti-VEGF(R) agents combined with PD-(L)1 inhibitors, which could provide a reference for clinical decision-making. Additionally, we provided evidence of direct and indirect comparisons between PD-(L)1 blockade monotherapy and combined with VEGF(R) blockade therapy, which was more persuasive.
There are some limitations in our study to be improved. Predominantly, scarce direct comparisons were included in the meta-analysis. Additionally, publication bias and small-study effects based on funnel plot and Egger’s test were observed in the meta-analyses of some adverse events. What’s more. cancer types, lines of treatment, and duration of follow-up, which may bring in high heterogeneity across several studies, should be taken into consideration. Further, versions of CTCAE were not consistent between the earlier and later conducted studies. Moreover, the incidence of all-cause or treatment-emergent AEs was regarded as the incidence of trAEs when trAEs were not reported or were not reported in detail in the included studies (McDermott et al., 2018; Bonomi et al., 2019; Eng et al., 2019; Levy et al., 2019; Necchi et al., 2019; Pujol et al., 2019; Theelen et al., 2019; Gogas et al., 2021; Jassem et al., 2021; McBride et al., 2021; van der Heijden et al., 2021). There were no trials that either compared different kinds of PD-1 blockade monotherapy (except for drug compared with its biosimilar) or compared different combination regimens, so that we were unable to conduct a network meta-analysis. Risks of irAEs and trAEs differ across different types and dosages of PD-(L)1 antibodies and their combinations with various anti-VEGF(R) drugs, which could be further explored through a network meta-analysis when there are more head-to-head comparisons performed in the future.
Total incidences of any grade and grade ≥3 irAEs were higher in the combination treatment group. Direct comparisons indicated that no significant difference was observed between the two regimens for any grade and grade ≥3 specific irAEs. RCCEP and thyroid disorders need to be paid attention to clinically. Moreover, there is a need for more trials conducted to directly compare PD-(L)1 inhibitor monotherapy and its combination therapy with anti-VEGF(R) agents. The safety profiles of the two regimens should be further explored. Exploration of the mechanism of action and regulatory management of adverse events should be enhanced.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
NL: conceptualization, writing–reviewing and editing, and funding acquisition. DW: conceptualization, investigation, supervision, and writing–reviewing and editing. QT: methodology, software, formal analysis, investigation, writing–original draft, and visualization. HH: methodology and validation. HF: writing–reviewing and editing. YW: data curation. FL: data curation. All authors contributed to the article and approved the submitted version.
This systematic review and meta-analysis were supported by Construction of Exemplary Clinical Research Ward in Beijing (Grant number BCRW202003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XZ declared a shared affiliation with the authors QT, DW, HH, HF, and NL to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1093194/full#supplementary-material
Andre, T., Shiu, K. K., Kim, T. W., Jensen, B. V., Jensen, L. H., Punt, C., et al. (2020). Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 383 (23), 2207–2218. doi:10.1056/NEJMoa2017699
Bang, Y. J., Ruiz, E. Y., Van Cutsem, E., Lee, K. W., Wyrwicz, L., Schenker, M., et al. (2018). Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: Primary analysis of JAVELIN gastric 300. Ann. Oncol. 29 (10), 2052–2060. doi:10.1093/annonc/mdy264
Baxi, S., Yang, A., Gennarelli, R. L., Khan, N., Wang, Z., Boyce, L., et al. (2018). Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ 360, k793. doi:10.1136/bmj.k793
Boku, N., Satoh, T., Ryu, M. H., Chao, Y., Kato, K., Chung, H. C., et al. (2021). Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer 24 (4), 946–958. doi:10.1007/s10120-021-01173-w
Bonomi, M., Ahmed, T., Addo, S., Kooshki, M., Palmieri, D., Levine, B. J., et al. (2019). Circulating immune biomarkers as predictors of the response to pembrolizumab and weekly low dose carboplatin and paclitaxel in NSCLC and poor PS: An interim analysis. Oncol. Lett. 17 (1), 1349–1356. doi:10.3892/ol.2018.9724
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., et al. (2015). Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373 (17), 1627–1639. doi:10.1056/NEJMoa1507643
Boyer, M., Sendur, M. A. N., Rodriguez-Abreu, D., Park, K., Lee, D. H., Cicin, I., et al. (2021). Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%: Randomized, double-blind phase III KEYNOTE-598 study. J. Clin. Oncol. 39 (21), 2327–2338. doi:10.1200/JCO.20.03579
Brahmer, J., Reckamp, K. L., Baas, P., Crino, L., Eberhardt, W. E., Poddubskaya, E., et al. (2015). Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373 (2), 123–135. doi:10.1056/NEJMoa1504627
Brahmer, J. R., Abu-Sbeih, H., Ascierto, P. A., Brufsky, J., Cappelli, L. C., Cortazar, F. B., et al. (2021). Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 9 (6), e002435. doi:10.1136/jitc-2021-002435
Burtness, B., Harrington, K. J., Greil, R., Soulieres, D., Tahara, M., de Castro, G., et al. (2019). Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 394 (10212), 1915–1928. doi:10.1016/S0140-6736(19)32591-7
Carbone, D. P., Reck, M., Paz-Ares, L., Creelan, B., Horn, L., Steins, M., et al. (2017). First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N. Engl. J. Med. 376 (25), 2415–2426. doi:10.1056/NEJMoa1613493
Chawla, S. P., Van Tine, B. A., Pollack, S. M., Ganjoo, K. N., Elias, A. D., Riedel, R. F., et al. (2022). Phase II randomized study of CMB305 and atezolizumab compared with atezolizumab alone in soft-tissue sarcomas expressing NY-ESO-1. J. Clin. Oncol. 40 (12), 1291–1300. doi:10.1200/JCO.20.03452
Chen, S. C., Huang, Y. H., Chen, M. H., Hung, Y. P., Lee, R. C., Shao, Y. Y., et al. (2022). Anti-PD-1 combined sorafenib versus anti-PD-1 alone in the treatment of advanced hepatocellular cell carcinoma: A propensity score-matching study. BMC Cancer 22 (1), 55. doi:10.1186/s12885-022-09173-4
Choueiri, T. K., Powles, T., Burotto, M., Escudier, B., Bourlon, M. T., Zurawski, B., et al. (2021). Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 384 (9), 829–841. doi:10.1056/NEJMoa2026982
Cohen, E. E. W., Soulieres, D., Le Tourneau, C., Dinis, J., Licitra, L., Ahn, M. J., et al. (2019). Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 393 (10167), 156–167. doi:10.1016/S0140-6736(18)31999-8
CTCAE (2017). Common terminology criteria for adverse events (CTCAE) V5. [Online]. Available: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm ([Accessed].
D'Angelo, S. P., Mahoney, M. R., Van Tine, B. A., Atkins, J., Milhem, M. M., Jahagirdar, B. N., et al. (2018). Nivolumab with or without ipilimumab treatment for metastatic sarcoma (alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 19 (3), 416–426. doi:10.1016/S1470-2045(18)30006-8
Eng, C., Kim, T. W., Bendell, J., Argiles, G., Tebbutt, N. C., Di Bartolomeo, M., et al. (2019). Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 20 (6), 849–861. doi:10.1016/S1470-2045(19)30027-0
Fehrenbacher, L., Spira, A., Ballinger, M., Kowanetz, M., Vansteenkiste, J., Mazieres, J., et al. (2016). Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 387 (10030), 1837–1846. doi:10.1016/S0140-6736(16)00587-0
Fehrenbacher, L., von Pawel, J., Park, K., Rittmeyer, A., Gandara, D. R., Ponce Aix, S., et al. (2018). Updated efficacy analysis including secondary population results for oak: A randomized phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J. Thorac. Oncol. 13 (8), 1156–1170. doi:10.1016/j.jtho.2018.04.039
Ferris, R. L., Blumenschein, G., Fayette, J., Guigay, J., Colevas, A. D., Licitra, L., et al. (2018). Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 81, 45–51. doi:10.1016/j.oraloncology.2018.04.008
Ferris, R. L., Haddad, R., Even, C., Tahara, M., Dvorkin, M., Ciuleanu, T. E., et al. (2020). Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann. Oncol. 31 (7), 942–950. doi:10.1016/j.annonc.2020.04.001
Finn, R. S., Qin, S., Ikeda, M., Galle, P. R., Ducreux, M., Kim, T. Y., et al. (2020). Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382 (20), 1894–1905. doi:10.1056/NEJMoa1915745
Finn, R. S., Ryoo, B. Y., Merle, P., Kudo, M., Bouattour, M., Lim, H. Y., et al. (2020). Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J. Clin. Oncol. 38 (3), 193–202. doi:10.1200/JCO.19.01307
Fradet, Y., Bellmunt, J., Vaughn, D. J., Lee, J. L., Fong, L., Vogelzang, N. J., et al. (2019). Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann. Oncol. 30 (6), 970–976. doi:10.1093/annonc/mdz127
Fuchs, C. S., Ozguroglu, M., Bang, Y. J., Di Bartolomeo, M., Mandala, M., Ryu, M. H., et al. (2022). Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer 25 (1), 197–206. doi:10.1007/s10120-021-01227-z
Galsky, M. D., Arija, J. A. A., Bamias, A., Davis, I. D., De Santis, M., Kikuchi, E., et al. (2020). Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 395 (10236), 1547–1557. doi:10.1016/S0140-6736(20)30230-0
Gettinger, S. N., Redman, M. W., Bazhenova, L., Hirsch, F. R., Mack, P. C., Schwartz, L. H., et al. (2021). Nivolumab plus ipilimumab vs nivolumab for previously treated patients with stage IV squamous cell lung cancer: The lung-MAP S1400I phase 3 randomized clinical trial. JAMA Oncol. 7 (9), 1368–1377. doi:10.1001/jamaoncol.2021.2209
Gogas, H., Dreno, B., Larkin, J., Demidov, L., Stroyakovskiy, D., Eroglu, Z., et al. (2021). Cobimetinib plus atezolizumab in BRAF(V600) wild-type melanoma: Primary results from the randomized phase III IMspire170 study. Ann. Oncol. 32 (3), 384–394. doi:10.1016/j.annonc.2020.12.004
Hamanishi, J., Takeshima, N., Katsumata, N., Ushijima, K., Kimura, T., Takeuchi, S., et al. (2021). Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer: Open-label, randomized trial in Japan (NINJA). J. Clin. Oncol. 39 (33), 3671–3681. doi:10.1200/JCO.21.00334
Hamid, O., Puzanov, I., Dummer, R., Schachter, J., Daud, A., Schadendorf, D., et al. (2017). Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur. J. Cancer 86, 37–45. doi:10.1016/j.ejca.2017.07.022
Hellmann, M. D., Paz-Ares, L., Bernabe Caro, R., Zurawski, B., Kim, S. W., Carcereny Costa, E., et al. (2019). Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 381 (21), 2020–2031. doi:10.1056/NEJMoa1910231
Herbst, R. S., Garon, E. B., Kim, D. W., Cho, B. C., Perez-Gracia, J. L., Han, J. Y., et al. (2020). Long-Term outcomes and retreatment among patients with previously treated, programmed death-ligand 1Positive, advanced NonSmall-cell lung cancer in the KEYNOTE-010 study. J. Clin. Oncol. 38 (14), 1580–1590. doi:10.1200/JCO.19.02446
Heudobler, D., Schulz, C., Fischer, J. R., Staib, P., Wehler, T., Sudhoff, T., et al. (2021). A randomized phase II trial comparing the efficacy and safety of pioglitazone, clarithromycin and metronomic low-dose chemotherapy with single-agent nivolumab therapy in patients with advanced non-small cell lung cancer treated in second or further line (ModuLung). Front. Pharmacol. 12, 599598. doi:10.3389/fphar.2021.599598
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Huang, J., Xu, J., Chen, Y., Zhuang, W., Zhang, Y., Chen, Z., et al. (2020). Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): A multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 21 (6), 832–842. doi:10.1016/S1470-2045(20)30110-8
Huang, D., Cui, P., Huang, Z., Wu, Z., Tao, H., Zhang, S., et al. (2021). Anti-PD-1/L1 plus anti-angiogenesis therapy as second-line or later treatment in advanced lung adenocarcinoma. J. Cancer Res. Clin. Oncol. 147 (3), 881–891. doi:10.1007/s00432-020-03380-x
Ivy, S. P., Wick, J. Y., and Kaufman, B. M. (2009). An overview of small-molecule inhibitors of VEGFR signaling. Nat. Rev. Clin. Oncol. 6 (10), 569–579. doi:10.1038/nrclinonc.2009.130
Jassem, J., de Marinis, F., Giaccone, G., Vergnenegre, A., Barrios, C. H., Morise, M., et al. (2021). Updated Overall survival analysis from IMpower110: Atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J. Thorac. Oncol. 16 (11), 1872–1882. doi:10.1016/j.jtho.2021.06.019
Kato, K., Cho, B. C., Takahashi, M., Okada, M., Lin, C. Y., Chin, K., et al. (2019). Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 20 (11), 1506–1517. doi:10.1016/S1470-2045(19)30626-6
Kennedy, L. B., and Salama, A. K. S. (2020). A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 70 (2), 86–104. doi:10.3322/caac.21596
Kim, C. G., Jang, M., Kim, Y., Leem, G., Kim, K. H., Lee, H., et al. (2019). VEGF-A drives TOX-dependent T cell exhaustion in anti-PD-1-resistant microsatellite stable colorectal cancers. Sci. Immunol. 4 (41), eaay0555. doi:10.1126/sciimmunol.aay0555
Kojima, T., Shah, M. A., Muro, K., Francois, E., Adenis, A., Hsu, C. H., et al. (2020). Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J. Clin. Oncol. 38 (35), 4138–4148. doi:10.1200/JCO.20.01888
Kuruvilla, J., Ramchandren, R., Santoro, A., Paszkiewicz-Kozik, E., Gasiorowski, R., Johnson, N. A., et al. (2021). Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): An interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 22 (4), 512–524. doi:10.1016/S1470-2045(21)00005-X
Lan, C., Shen, J., Wang, Y., Li, J., Liu, Z., He, M., et al. (2020). Camrelizumab plus apatinib in patients with advanced cervical cancer (clap): A multicenter, open-label, single-arm, phase II trial. J. Clin. Oncol. 38 (34), 4095–4106. doi:10.1200/JCO.20.01920
Larkin, J., Minor, D., D'Angelo, S., Neyns, B., Smylie, M., Miller, W. H., et al. (2018). Overall survival in patients with advanced melanoma who received nivolumab versus investigator's choice chemotherapy in CheckMate 037: A randomized, controlled, open-label phase III trial. J. Clin. Oncol. 36 (4), 383–390. doi:10.1200/JCO.2016.71.8023
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Rutkowski, P., Lao, C. D., et al. (2019). Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381 (16), 1535–1546. doi:10.1056/NEJMoa1910836
Levy, B. P., Giaccone, G., Besse, B., Felip, E., Garassino, M. C., Domine Gomez, M., et al. (2019). Randomised phase 2 study of pembrolizumab plus CC-486 versus pembrolizumab plus placebo in patients with previously treated advanced non-small cell lung cancer. Eur. J. Cancer 108, 120–128. doi:10.1016/j.ejca.2018.11.028
Liu, Y., Wang, C., Li, X., Dong, L., Yang, Q., Chen, M., et al. (2021). Improved clinical outcome in a randomized phase II study of anti-PD-1 camrelizumab plus decitabine in relapsed/refractory Hodgkin lymphoma. J. Immunother. Cancer 9 (4), e002347. doi:10.1136/jitc-2021-002347
Long, G. V., Atkinson, V., Lo, S., Sandhu, S., Guminski, A. D., Brown, M. P., et al. (2018). Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 19 (5), 672–681. doi:10.1016/S1470-2045(18)30139-6
Long, G. V., Dummer, R., Hamid, O., Gajewski, T. F., Caglevic, C., Dalle, S., et al. (2019). Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol. 20 (8), 1083–1097. doi:10.1016/S1470-2045(19)30274-8
Lu, S., Wang, J., Cheng, Y., Mok, T., Chang, J., Zhang, L., et al. (2021). Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer: 2-year follow-up from a randomized, open-label, phase 3 study (CheckMate 078). Lung Cancer 152, 7–14. doi:10.1016/j.lungcan.2020.11.013
Mahmood, U., Bang, A., Chen, Y. H., Mak, R. H., Lorch, J. H., Hanna, G. J., et al. (2021). A randomized phase 2 study of pembrolizumab with or without radiation in patients with recurrent or metastatic adenoid cystic carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 109 (1), 134–144. doi:10.1016/j.ijrobp.2020.08.018
Makker, V., Rasco, D., Vogelzang, N. J., Brose, M. S., Cohn, A. L., Mier, J., et al. (2019). Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 20 (5), 711–718. doi:10.1016/S1470-2045(19)30020-8
Martins, F., Sofiya, L., Sykiotis, G. P., Lamine, F., Maillard, M., Fraga, M., et al. (2019). Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16 (9), 563–580. doi:10.1038/s41571-019-0218-0
McBride, S., Sherman, E., Tsai, C. J., Baxi, S., Aghalar, J., Eng, J., et al. (2021). Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J. Clin. Oncol. 39 (1), 30–37. doi:10.1200/JCO.20.00290
McDermott, D. F., Huseni, M. A., Atkins, M. B., Motzer, R. J., Rini, B. I., Escudier, B., et al. (2018). Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 24 (6), 749–757. doi:10.1038/s41591-018-0053-3
Meder, L., Schuldt, P., Thelen, M., Schmitt, A., Dietlein, F., Klein, S., et al. (2018). Combined VEGF and PD-L1 blockade displays synergistic treatment effects in an autochthonous mouse model of small cell lung cancer. Cancer Res. 78 (15), 4270–4281. doi:10.1158/0008-5472.CAN-17-2176
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6 (7), e1000097. doi:10.1371/journal.pmed.1000097
Mok, T. S. K., Wu, Y. L., Kudaba, I., Kowalski, D. M., Cho, B. C., Turna, H. Z., et al. (2019). Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 393 (10183), 1819–1830. doi:10.1016/S0140-6736(18)32409-7
Motzer, R. J., Penkov, K., Haanen, J., Rini, B., Albiges, L., Campbell, M. T., et al. (2019). Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380 (12), 1103–1115. doi:10.1056/NEJMoa1816047
Motzer, R. J., Robbins, P. B., Powles, T., Albiges, L., Haanen, J. B., Larkin, J., et al. (2020). Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: Biomarker analysis of the phase 3 JAVELIN renal 101 trial. Nat. Med. 26 (11), 1733–1741. doi:10.1038/s41591-020-1044-8
Motzer, R. J., Escudier, B., George, S., Hammers, H. J., Srinivas, S., Tykodi, S. S., et al. (2020). Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 126 (18), 4156–4167. doi:10.1002/cncr.33033
Motzer, R., Alekseev, B., Rha, S. Y., Porta, C., Eto, M., Powles, T., et al. (2021). Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 384 (14), 1289–1300. doi:10.1056/NEJMoa2035716
Nayak, L., Molinaro, A. M., Peters, K., Clarke, J. L., Jordan, J. T., de Groot, J., et al. (2021). Randomized phase II and biomarker study of pembrolizumab plus bevacizumab versus pembrolizumab alone for patients with recurrent glioblastoma. Clin. Cancer Res. 27 (4), 1048–1057. doi:10.1158/1078-0432.CCR-20-2500
Necchi, A., Giannatempo, P., Raggi, D., Mariani, L., Colecchia, M., Fare, E., et al. (2019). An open-label randomized phase 2 study of durvalumab alone or in combination with tremelimumab in patients with advanced germ cell tumors (apache): Results from the first planned interim analysis. Eur. Urol. 75 (1), 201–203. doi:10.1016/j.eururo.2018.09.010
O'Reilly, E. M., Oh, D. Y., Dhani, N., Renouf, D. J., Lee, M. A., Sun, W., et al. (2019). Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: A phase 2 randomized clinical trial. JAMA Oncol. 5 (10), 1431–1438. doi:10.1001/jamaoncol.2019.1588
Park, K., Ozguroglu, M., Vansteenkiste, J., Spigel, D., Yang, J. C. H., Ishii, H., et al. (2021). Avelumab versus docetaxel in patients with platinum-treated advanced NSCLC: 2-Year follow-up from the JAVELIN lung 200 phase 3 trial. J. Thorac. Oncol. 16 (8), 1369–1378. doi:10.1016/j.jtho.2021.03.009
Planchard, D., Reinmuth, N., Orlov, S., Fischer, J. R., Sugawara, S., Mandziuk, S., et al. (2020). Arctic: Durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann. Oncol. 31 (5), 609–618. doi:10.1016/j.annonc.2020.02.006
Popat, S., Curioni-Fontecedro, A., Dafni, U., Shah, R., O'Brien, M., Pope, A., et al. (2020). A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: The European thoracic oncology platform (ETOP 9-15) PROMISE-meso trial. Ann. Oncol. 31 (12), 1734–1745. doi:10.1016/j.annonc.2020.09.009
Postow, M. A., Sidlow, R., and Hellmann, M. D. (2018). Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378 (2), 158–168. doi:10.1056/NEJMra1703481
Powles, T., Plimack, E. R., Soulieres, D., Waddell, T., Stus, V., Gafanov, R., et al. (2020). Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 21 (12), 1563–1573. doi:10.1016/S1470-2045(20)30436-8
Powles, T., van der Heijden, M. S., Castellano, D., Galsky, M. D., Loriot, Y., Petrylak, D. P., et al. (2020). Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 21 (12), 1574–1588. doi:10.1016/S1470-2045(20)30541-6
Powles, T., Csoszi, T., Ozguroglu, M., Matsubara, N., Geczi, L., Cheng, S. Y., et al. (2021). Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 22 (7), 931–945. doi:10.1016/S1470-2045(21)00152-2
Pujade-Lauraine, E., Fujiwara, K., Ledermann, J. A., Oza, A. M., Kristeleit, R., Ray-Coquard, I. L., et al. (2021). Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN ovarian 200): An open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 22 (7), 1034–1046. doi:10.1016/S1470-2045(21)00216-3
Pujol, J. L., Greillier, L., Audigier-Valette, C., Moro-Sibilot, D., Uwer, L., Hureaux, J., et al. (2019). A randomized non-comparative phase II study of anti-programmed cell death-ligand 1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: Results from the IFCT-1603 trial. J. Thorac. Oncol. 14 (5), 903–913. doi:10.1016/j.jtho.2019.01.008
Qin, S., Ren, Z., Meng, Z., Chen, Z., Chai, X., Xiong, J., et al. (2020). Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 21 (4), 571–580. doi:10.1016/S1470-2045(20)30011-5
Reardon, D. A., Brandes, A. A., Omuro, A., Mulholland, P., Lim, M., Wick, A., et al. (2020). Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: The CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 6 (7), 1003–1010. doi:10.1001/jamaoncol.2020.1024
Reck, M., Rodriguez-Abreu, D., Robinson, A. G., Hui, R., Csoszi, T., Fulop, A., et al. (2021). Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J. Clin. Oncol. 39 (21), 2339–2349. doi:10.1200/JCO.21.00174
Rini, B. I., Powles, T., Atkins, M. B., Escudier, B., McDermott, D. F., Suarez, C., et al. (2019). Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 393 (10189), 2404–2415. doi:10.1016/S0140-6736(19)30723-8
Rizvi, N. A., Cho, B. C., Reinmuth, N., Lee, K. H., Luft, A., Ahn, M. J., et al. (2020). Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: The MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 6 (5), 661–674. doi:10.1001/jamaoncol.2020.0237
Robert, C., Ribas, A., Schachter, J., Arance, A., Grob, J. J., Mortier, L., et al. (2019). Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 20 (9), 1239–1251. doi:10.1016/S1470-2045(19)30388-2
Robert, C., Long, G. V., Brady, B., Dutriaux, C., Di Giacomo, A. M., Mortier, L., et al. (2020). Five-year outcomes with nivolumab in patients with wild-type BRAF advanced melanoma. J. Clin. Oncol. 38 (33), 3937–3946. doi:10.1200/JCO.20.00995
Saeed, A., Park, R., and Sun, W. (2021). The integration of immune checkpoint inhibitors with VEGF targeted agents in advanced gastric and gastroesophageal adenocarcinoma: A review on the rationale and results of early phase trials. J. Hematol. Oncol. 14 (1), 13. doi:10.1186/s13045-021-01034-0
Scherpereel, A., Mazieres, J., Greillier, L., Lantuejoul, S., Do, P., Bylicki, O., et al. (2019). Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): A multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 20 (2), 239–253. doi:10.1016/S1470-2045(18)30765-4
Sezer, A., Kilickap, S., Gumus, M., Bondarenko, I., Ozguroglu, M., Gogishvili, M., et al. (2021). Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 397 (10274), 592–604. doi:10.1016/S0140-6736(21)00228-2
Shigeta, K., Datta, M., Hato, T., Kitahara, S., Chen, I. X., Matsui, A., et al. (2020). Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology 71 (4), 1247–1261. doi:10.1002/hep.30889
Shitara, K., Van Cutsem, E., Bang, Y. J., Fuchs, C., Wyrwicz, L., Lee, K. W., et al. (2020). Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 6 (10), 1571–1580. doi:10.1001/jamaoncol.2020.3370
Singh, A. S., Hecht, J. R., Rosen, L., Wainberg, Z. A., Wang, X., Douek, M., et al. (2022). A randomized phase II study of nivolumab monotherapy or nivolumab combined with ipilimumab in patients with advanced gastrointestinal stromal tumors. Clin. Cancer Res. 28 (1), 84–94. doi:10.1158/1078-0432.CCR-21-0878
Siu, L. L., Even, C., Mesia, R., Remenar, E., Daste, A., Delord, J. P., et al. (2019). Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: The phase 2 CONDOR randomized clinical trial. JAMA Oncol. 5 (2), 195–203. doi:10.1001/jamaoncol.2018.4628
Spigel, D. R., Vicente, D., Ciuleanu, T. E., Gettinger, S., Peters, S., Horn, L., et al. (2021). Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331. Ann. Oncol. 32 (5), 631–641. doi:10.1016/j.annonc.2021.01.071
Theelen, W., Peulen, H. M. U., Lalezari, F., van der Noort, V., de Vries, J. F., Aerts, J., et al. (2019). Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 5 (9), 1276–1282. doi:10.1001/jamaoncol.2019.1478
van der Heijden, M. S., Loriot, Y., Duran, I., Ravaud, A., Retz, M., Vogelzang, N. J., et al. (2021). Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma: A long-term Overall survival and safety update from the phase 3 IMvigor211 clinical trial. Eur. Urol. 80 (1), 7–11. doi:10.1016/j.eururo.2021.03.024
Wang, Y., Zhou, S., Yang, F., Qi, X., Wang, X., Guan, X., et al. (2019). Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol. 5 (7), 1008–1019. doi:10.1001/jamaoncol.2019.0393
Winer, E. P., Lipatov, O., Im, S. A., Goncalves, A., Munoz-Couselo, E., Lee, K. S., et al. (2021). Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 22 (4), 499–511. doi:10.1016/S1470-2045(20)30754-3
Xu, J., Shen, J., Gu, S., Zhang, Y., Wu, L., Wu, J., et al. (2021). Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (rescue): A nonrandomized, open-label, phase II trial. Clin. Cancer Res. 27 (4), 1003–1011. doi:10.1158/1078-0432.CCR-20-2571
Yi, M., Jiao, D., Qin, S., Chu, Q., Wu, K., and Li, A. (2019). Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol. Cancer 18 (1), 60. doi:10.1186/s12943-019-0974-6
Zamarin, D., Burger, R. A., Sill, M. W., Powell, D. J., Lankes, H. A., Feldman, M. D., et al. (2020). Randomized phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: An NRG oncology study. J. Clin. Oncol. 38 (16), 1814–1823. doi:10.1200/JCO.19.02059
Zhang, T., Harrison, M. R., O'Donnell, P. H., Alva, A. S., Hahn, N. M., Appleman, L. J., et al. (2020). A randomized phase 2 trial of pembrolizumab versus pembrolizumab and acalabrutinib in patients with platinum-resistant metastatic urothelial cancer. Cancer 126 (20), 4485–4497. doi:10.1002/cncr.33067
Keywords: programmed cell death (ligand) 1, vascular endothelial growth factor (receptor), immune-related adverse events, treatment-related adverse events, meta-analysis
Citation: Tang Q, Wu D, Huang H, Fang H, Wu Y, Liu F and Li N (2023) Adverse events of PD-(L)1 inhibitors plus anti-VEGF(R) agents compared with PD-(L)1 inhibitors alone for cancer patients: a systematic review and meta-analysis. Front. Pharmacol. 14:1093194. doi: 10.3389/fphar.2023.1093194
Received: 08 November 2022; Accepted: 14 April 2023;
Published: 25 April 2023.
Edited by:
Jian Yu, Beihang University, ChinaReviewed by:
Hao Ping, Capital Medical University, ChinaCopyright © 2023 Tang, Wu, Huang, Fang, Wu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Li, bGluaW5nQGNpY2Ftcy5hYy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.