- 1Department of Health Sciences, University of Groningen, University Medical Center Groningen, Groningen, Netherlands
- 2Department of Pharmacy, Institut Teknologi Sumatera, Lampung Selatan, Indonesia
- 3Department of Pharmacy, Universitas Islam Indonesia, Yogyakarta, Indonesia
- 4Faculty of Pharmacy, Universitas Muhammadiyah Purwokerto, Banyumas, Indonesia
- 5Center for Health Economic Studies, Universitas Muhammadiyah Purwokerto, Banyumas, Indonesia
- 6Department of Economics, Econometrics and Finance, University of Groningen, Groningen, Netherlands
- 7Department of Pharmacology and Therapy, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
- 8Centre of Excellence in Higher Education for Pharmaceutical Care Innovation, Universitas Padjadjaran, Bandung, Indonesia
Background: Despite the benefits of trastuzumab in many trials, evidence of its impact on health-related quality of life (HRQoL) in early treatment has not been summarized. This study explored the effects of trastuzumab treatment on HRQoL, including pooled meta-analysis, in an effort to provide an integrated assessment of HRQoL for Her2-positive early breast cancer patients.
Methods: A comprehensive literature review to February 2023 using three databases, focusing on treatment using trastuzumab during the early stage, was performed. The mean changes from baseline during and after treatment were extracted from the included randomized control trials (RCTs) papers and total HRQoL scores were obtained from cross-sectional studies included. Mean difference (MD) and 95% confidence intervals were assessed by a random effect or fixed effect model based on heterogeneity (I2).
Results: A total of ten studies were identified and reviewed, consisting of seven RCTs and three cross-sectional studies. The pooled analysis of the mean change from baseline during treatment resulted in an MD of 1.92 (95% CI = 1.59 to 2.25, p < 0.05, I2 = 0%), favoring the trastuzumab group. A non-significant result of the mean change from baseline after treatment appeared in the analysis of 12-month follow-up. In the cross-sectional studies, pooled analyses of HRQoL showed that trastuzumab meaningfully demonstrated an improved HRQoL profile (MD = 9.29, 95% CI = 1.31 to 17.27, p = 0.02, I2 = 0%).
Conclusion: Trastuzumab as a targeted therapy resulted in a favorable effect on HRQoL in the early stages of Her2-positive breast cancer. The findings of significant improvements in patients’ HRQoL and less clinically meaningful deterioration in side effects of trastuzumab-containing regimen during treatment were supported by prolonged survival.
1 Introduction
In 2020, the most diagnosed form of cancer in women was breast cancer, with almost 2.4 million incidents and 684.926 mortalities worldwide (Bray et al., 2018; Sung et al., 2021). Her2-positive breast cancer constituted approximately 20% of this total number (Slamon et al., 1989; Wolff et al., 2013). This subtype is typically characterized by an overexpression of the epidermal growth factor receptor 2 (Her2 or erbB2) and rapid growth, both recurring and metastatic (Ross et al., 2009; Goddard et al., 2011; Rimawi et al., 2015). In particular, the poor prognosis of Her2-overexpressed patients is associated with lower disease-free survival and overall survival (Piccart et al., 2001). Likewise, the progression of Her2-positive breast cancer severely decreases the survivors health-related quality of life (HRQoL) due to the detrimental effects of the disease. Patients experience irreversible physical and psychosocial syndromes, mental distress, and symptoms as a tresult of long-term treatment and disease progression (Perry et al., 2007; Bouskill and Kramer, 2016; Hamer et al., 2016; Park et al., 2019). HRQoL has been taken into account in the consideration as a relevant clinical outcome of the treatment strategies for advanced disease (Goodwin et al., 2003; Lemieux et al., 2011; Meldahl et al., 2013). Yet, analyses of trastuzumab treatment in early disease are still scarce.
Trastuzumab, a targeted therapy, is remarkably effective in treating the Her2 overexpression of breast cancer (Piccart-Gebhart et al., 2005; Romond et al., 2012; Goldhirsch et al., 2013; Perez et al., 2014). Trastuzumab has proven to be effective in improving the clinical outcome of the management of the early stages of Her2-positive breast cancer, including disease-free survival, overall survival, and invasive disease-free survival (Perez et al., 2014; Cameron et al., 2017; Piccart et al., 2021). Furthermore, trastuzumab combined with chemotherapy has been used as standard of care in the adjuvant setting and is a preferable choice in high-risk node-negative Her2-positive cases (von Minckwitz et al., 2017). Many studies (Adamowicz and Baczkowska-Waliszewska, 2020; Oh et al., 2021; Dal Lago et al., 2022) evaluating the HRQoL of breast cancer patients treated with monoclonal antibody showed a beneficial effect compared to those who did not receive the treatment.
Despite the benefits of trastuzumab in many trials (Gonzalez-Angulo et al., 2006; Viani et al., 2007; Bria et al., 2008; Dahabreh et al., 2008; Madarnas et al., 2008; Ward et al., 2009; Yin et al., 2011; Mates et al., 2015; O’Sullivan et al., 2015; Long et al., 2016; Genuino et al., 2019), evidence of its impact on HRQoL in early treatment has not been summarized. In particular, this study explored the effects of trastuzumab treatment on HRQoL, including pooled meta-analysis in an effort to provide an integrated assessment of HRQoL for Her2-positive early breast cancer patients.
2 Methods
2.1 Review strategy
Three electronic databases, PubMed, Embase, and Scopus, were used to conduct a comprehensive review of articles published until February 2023. The search terms included the domains of a) breast cancer, b) trastuzumab, c) quality of life, and d) quality-adjusted life years. A snowball search for additional potential articles found in the reference list of the main articles was subsequently carried out manually to prevent unretrieved papers not being discovered. The search terms and strategies are shown in the Supplementary material.
2.2 Eligibility criteria
The articles were included according to the following criteria: 1) studies included a female population above 18 years old with Her2-positive breast cancer in early stage who received trastuzumab in any cancer treatment regimen; 2) studies reported HRQoL data, using validated instruments; 3) studies were designed as both randomized controlled trials (RCTs) and observational studies; 4) studies presented the mean change in HRQoL from baseline for RCTs; 5) studies presented the mean HRQoL for cross-sectional studies. The following articles were excluded from this review: systematic reviews, opinion pieces, protocols, commentaries, studies presenting outcomes other than HRQoL studies providing only an abstract, posters and oral presentations. Year and language restrictions were not applied in this systematic review. Duplicates or the same data from multiple publications were only used once.
2.3 Study selection
Two reviewers (SMK and FDAS) independently assessed potential articles, including titles and abstracts, based on the eligibility criteria mentioned above. Studies fitting the inclusion criteria were retrieved and assessed if the full-text was available. Full-text articles were then evaluated for final inclusion. Any disparities between the two assessors were solved through discussion until consensus was achieved. The justifications for the eligibility of each study were recorded and presented in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
2.4 Data extraction
The data from the selected articles were copied to a Microsoft Excel® spreadsheet by SMK and double-checked by FDAS. The details of the data included were: author, date of publication, study design, study period, country, age range, stage of breast cancer, type of instrument, interventions, outcome, and conclusion. Outcomes are presented as changes in HRQoL measurements from baseline to specified moments of follow-up and/or the mean of HRQoL.
2.5 Quality evaluation
Two authors (SMK and FDAS) performed the quality evaluations and solved disagreements through consensus. Methodological quality evaluation was assessed using the Cochrane Risk of Bias tool (RoB 2.0) for randomized studies and Risk of Bias in Non-randomised Studies of Interventions (ROBINS-I) for cross-sectional studies. RoB 2.0 consists of five main domains which provide extensive points of analysis, including deviations of post-randomization for intervention. The new version of this tool also enables more up-to-date explanations regarding the measurement of the trials treatment effect and explicitly concerns the particular outcome estimations. In addition, the tool makes use of a series of signaling questions, prompting a reasonable response and simple answers and providing an algorithm to summarize the outcome of each question (Sterne et al., n.d.; Hernán and Robins, 2017). ROBINS-I consists of seven bias domains and signaling questions to inform judgments of the risk of bias and provides a structure approach in evaluating non-randomized studies of interventions (Sterne et al., 2016).
For reporting the evidence quality of the review, the grading of recommendation, assessment, development and evaluation (GRADE) was employed. It offers a systematic method for formulating clinical practice recommendations and is a straightforward framework for creating and presenting evidence summaries (Guyatt et al., 2008; Meader et al., 2014).
2.6 Data synthesis and analysis
Forinclusion into the subsequent meta-analysis, the studies should provide the mean change from baseline and/or the mean of HRQoL including the standard deviation (SD) as well as explicitly comparing trastuzumab-containing regimen versus treatment regimen without trastuzumab. The quantitative analysis was done separately for the RCTs and the observational studies. The interpretation of the pooled analysis was determined by the heterogeneity, quantified by Cochran’s Q and the heterogeneity index I2. A random-effects model was used if the heterogeneity (I2) was high. A result of p < 0.05 in Cochran’s Q test was considered to indicate that the variability of the forest plot was significant (Higgins and Thompson, 2002).
3 Results
3.1 Selection criteria
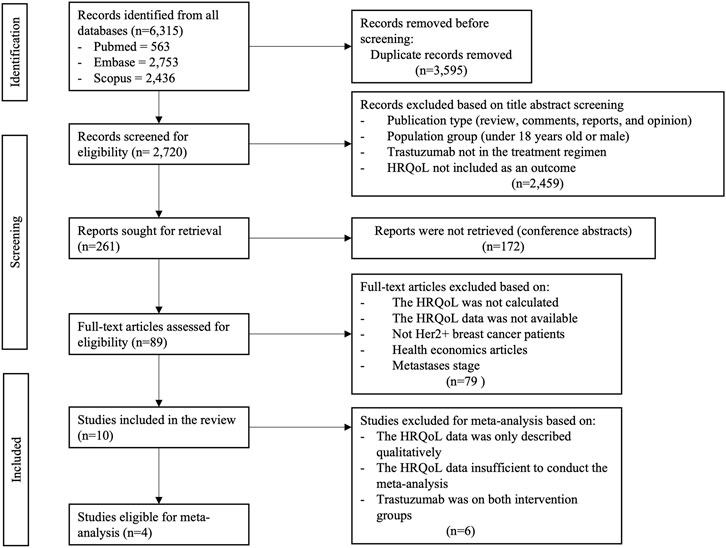
A total of 6,315 articles were extracted during the initial electronic databases search. 2,720 articles remained after removing duplicates. These articles were then filtered by title, abstract, and text, yielding 261 full-text articles. After retrieving the full texts, 172 articles appeared to be only abstract, leaving 89 articles to review for eligibility.
Eighty-one articles were eliminated according to stricter application of the inclusion criteria, leaving ten studies for our present analysis, with four of these studies being eligible for meta-analysis (Au et al., 2013; Syrios et al., 2018; Trinca et al., 2019; Conte et al., 2020). Six articles were excluded from quantitative meta-analysis due to lack of detailed data for allowing meta-analysis (Earl et al., 2020; Sawaki et al., 2020, 2022; Sella et al., 2022) or comparing trastuzumab in both arms (Bines et al., 2021; Taira et al., 2021). The details of the selection process are described in Figure 1.
3.2 Characteristics of the included studies
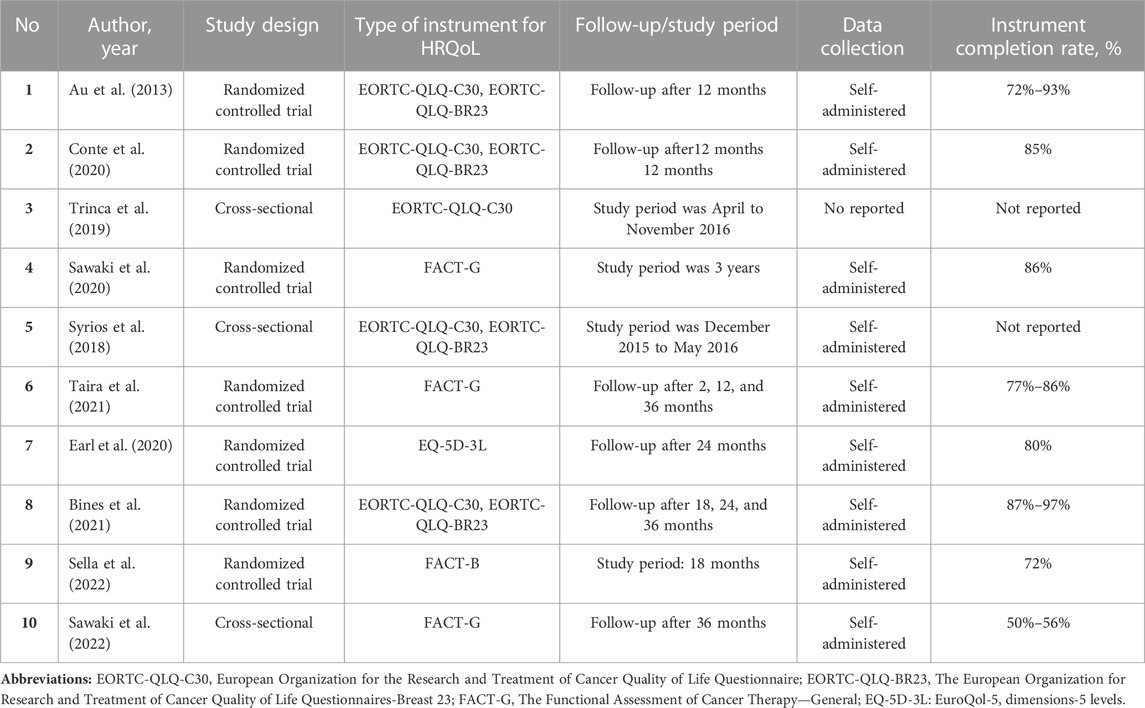
The included studies consisted of seven RCTs (Au et al., 2013; Conte et al., 2020; Earl et al., 2020; Sawaki et al., 2020; Bines et al., 2021; Taira et al., 2021; Sella et al., 2022) and three observational studies of cross-sectional design (Syrios et al., 2018; Trinca et al., 2019; Sawaki et al., 2022). Two studies (Trinca et al., 2019; Conte et al., 2020) involved the comparison of a group receiving trastuzumab treatment with a group receiving chemotherapy. Two studies (Au et al., 2013; Syrios et al., 2018) included more than three comparisons of interventions. Three studies (Sawaki et al., 2020, 2022; Taira et al., 2021) investigated the use of trastuzumab monotherapy compared to a combination of trastuzumab and chemotherapy in the treatment of elderly breast cancer patients. Two studies (Conte et al., 2020; Sella et al., 2022) compared trastuzumab regimens with trastuzumab emtansine (T-DM1) was utilized in a study. In addition, one study (Earl et al., 2020) presented a comparison of the HRQoL profile after 6 months of trastuzumab with the HRQoL profile after 12 months of trastuzumab and a study (Bines et al., 2021) evaluated the addition of pertuzumab in trastuzumab in HER-2 positive early breast cancer. Characteristics of the included studies are shown in Table 1.
3.3 Data collection method
Health-related quality of life was calculated using valid instruments in all the selected studies. The majority of the studies (Au et al., 2013; Syrios et al., 2018; Trinca et al., 2019; Conte et al., 2020; Bines et al., 2021) made use of the EORTC-QLQ-C30 questionnaire to estimate the HRQoL of women suffering from Her2-positive breast cancer, with three (Syrios et al., 2018; Conte et al., 2020; Bines et al., 2021) of these studies also making use of EORTC-QLQ-BR23. Other types of questionnaires identified were FACT-G (Sawaki et al., 2020, 2022; Taira et al., 2021), FACT-B (Sella et al., 2022), and EQ-5D-3L (Earl et al., 2020). Most of the instruments of the studies included was self-administered with the completion rate ranged from 50% to 97% (Table 1).
3.4 Timeline of observations
One trial (Conte et al., 2020) described the mean change of HRQoL during cycles 5 and 11, at the end of chemotherapy, after 6 months of follow-up and after 1 year while another (Au et al., 2013) described the mean change during cycle 4, at the end of chemotherapy, and after 12 months of follow-up. One study (Sawaki et al., 2020) presented observations made after 2 months and 1 year and two studies (Taira et al., 2021; Sawaki et al., 2022) observed the HRQoL after 36 months. The HRQoL of early breast cancer patients filled the questionnaire at 0, 3, 6, 9, 12, 18, and 24 months of follow-up (Earl et al., 2020). Patients in a study (Bines et al., 2021) completed a questionnaire on weeks 10, 13, 25, and at the end of HER2-targeteted therapy (12 months) while another study on weeks 18 (Sella et al., 2022). Finally, an HRQoL profile was made of patients receiving treatment during the observational studies (Syrios et al., 2018; Trinca et al., 2019).
3.5 Characteristics of patients
The patients characteristics, interventions used, and qualitative summary of the HRQoL profile was presented in Tables 2, 3. Two studies (Conte et al., 2020; Earl et al., 2020) recruited patients who had previously been treated with anthracycline, taxane, and trastuzumab in the neo or adjuvant setting. One study (Trinca et al., 2019) observed female patients who had already undergone a breast cancer regimen 2 months before the study was conducted and excluded cerebral metastasis patients. Elderly patients in the early stages of breast cancer who underwent curative surgery but who had not been previously treated with chemotherapy or endocrine therapy, had Eastern Coopertive Oncology Group (ECOG) performance status score 0 or 1, and had sufficient organ function were involved in three studies (Sawaki et al., 2020, 2022; Taira et al., 2021). A study allowed patients who has been received surgery for the treatment within 90 days (Sella et al., 2022). One specific study allowed for concurrent treatment with pertuzumab (Syrios et al., 2018). Finally, one trial (Au et al., 2013) involved women aged 18 to 70 with HER2-positive early breast cancer who had undergone either mastectomy or lumpectomy and had Karnofsky performance status ≥80%.
3.6 Interventions
Three studies (Trinca et al., 2019; Sawaki et al., 2020; Taira et al., 2021) compared trastuzumab monotherapy as a frontline treatment in the early stages of Her2-positive breast cancer to trastuzumab combined with chemotherapy. One study (Earl et al., 2020) compared the results of 6 months of trastuzumab with 12 months of trastuzumab. A study (Bines et al., 2021) treated patients with 840 mg loading dose of pertuzumab followed by 420 mg every 3 weeks. All five studies mentioned previously used 8 mg/kg of intravenous trastuzumab as the loading dose, followed by a maintenance dose of 6 mg/kg every 3 weeks. Two studies compared the use of T-DM1 with the use of intravenous trastuzumab at 6 mg/kg combined with adjuvant endocrine (Conte et al., 2020) and paclitaxel 80 mg/m2 (Sella et al., 2022). One study (Syrios et al., 2018) compared the use of 600 mg of subcutaneous trastuzumab every 3 weeks combined with chemotherapy and endocrine therapy to a control group using chemotherapy without trastuzumab. Furthermore, an adjuvant trastuzumab-docetaxel-based regimen was used in one study (Au et al., 2013), with a loading dose of 4 mg/kg of trastuzumab.
3.7 Health-related quality of life profile
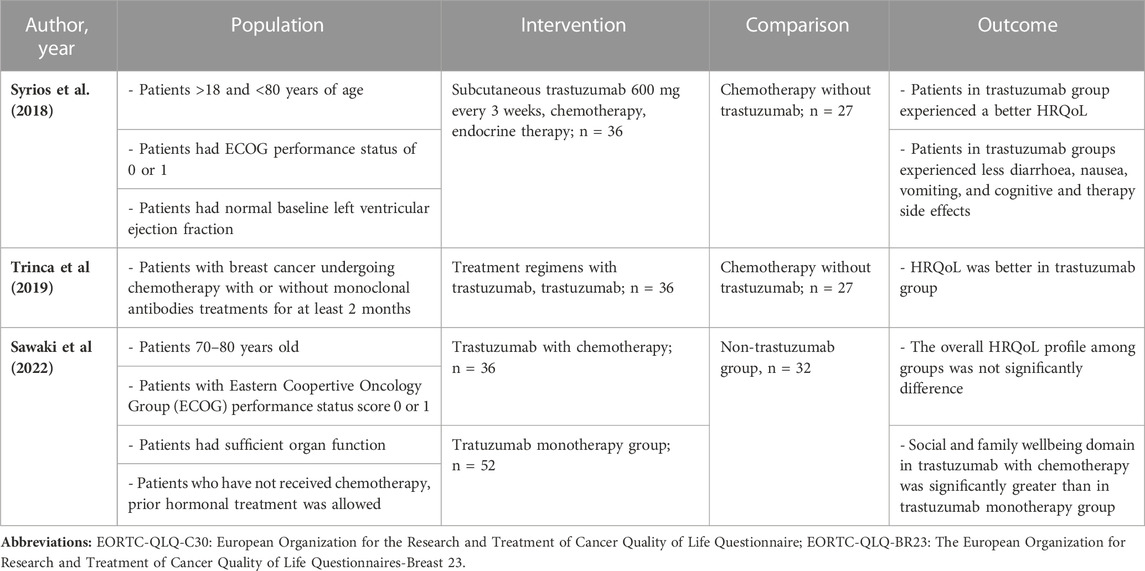
Overall, the patient’s HRQoL score during treatment measured by EORTC-QLQ-C30 favored the trastuzumab group, showing an improvement score from the baseline (Au et al., 2013; Conte et al., 2020). A similar pattern also depicted in a cross-sectional study that patients in the trastuzumab arm experienced a better HRQoL (Syrios et al., 2018; Trinca et al., 2019; Sawaki et al., 2022).
After withdrawing the treatment, the mean change of baseline of HRQoL returned to the baseline levels, showing a better score of HRQoL in the trastuzumab arm (Au et al., 2013; Conte et al., 2020). For physical functioning, the trastuzumab group exhibited a significant improvement from baseline at the mid-point of observations and demonstrated a similar profile after 12months of follow-up (Au et al., 2013; Conte et al., 2020; Bines et al., 2021).
3.8 Methodological quality
The methodological quality assessment in this systematic review and meta-analysis was conducted according to each article’s study design. The assessment results using RoB 2.0 are summarized in Figure 2. An adequate description of the randomization process was present in all studies. Deviations from the intended interventions were adequately explained in four trials (Au et al., 2013; Conte et al., 2020; Sawaki et al., 2020; Taira et al., 2021). All studies made use of sufficiently large study populations but only two studies (Au et al., 2013; Conte et al., 2020) included an intention-to-treat analysis to overcome the loss of follow-up participants. The outcomes of the trials were assessed appropriately in each of the studies. Only one study insufficiently reported the result which may lead to bias in selection of the reported result (Bines et al., 2021). However, a concern shown in the selection of the reported result for studies (Au et al., 2013; Conte et al., 2020; Earl et al., 2020; Sawaki et al., 2020; Bines et al., 2021) does not present whether the assessed result is likely to have been selected based on the result and from multiple eligible outcome measurements within the outcome domain.
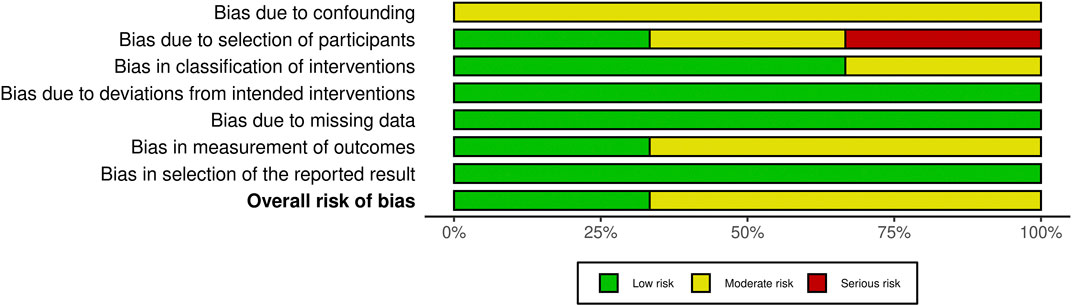
The risk of bias using the ROBINS-I tool is reported in Figure 3. According to the evaluation, the results suggest that the studies quality of reporting was adequate by presenting a low and risk of bias. All confounders in all the studies were measured and controlled, and the reliability and validity of measurement of important domains were sufficient. However, in one study (Sawaki et al., 2022), the selection into the study may have been related to intervention and outcome and there was no information regarding the adjustment techniques used to correct the presence of selection biases. The study failed to explain how to calculate the sample size. In terms of statistical analysis, the study also failed to adequately report on missing data. The quality assessment of the studies also discovered a flaw in the ROBINS-I tool, which lacked a way to address the missing participants in the research and the calculation of the sensitivity analysis. Moreover, the number of outcomes was only shown in the study using a single moment of observation.
3.9 Quality of the evidence
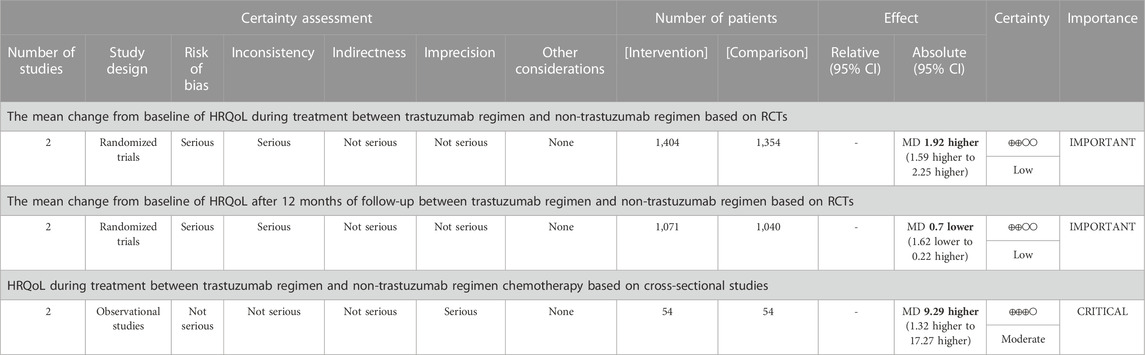
There is low-quality of evidence that in RCTs, patients in trastuzumab arm had a better profile of HRQoL than those in chemotherapy group during receiving treatment (MD = 1.92, 95% CI = 1.59 to 2.25, two studies with 2,758 participants). After 12 months of treatment, there is also the low quality of evidence that the HRQoL profile in the trastuzumab arm was comparable with chemotherapy arm (MD = −0.70, 95% CI = −1.62 to 0.22, two studies with 2,111 participants). For observational studies, moderate quality of evidence was presented in showing the higher HRQoL profile in trastuzumab patients compared to the chemotherapy group (MD = 9.29, 95% CI = 1.31 to 17.27, p = 0.02, two studies with 108 participants). The result of the quality of the evidence was depicted in Table 4.
3.10 Meta-analysis
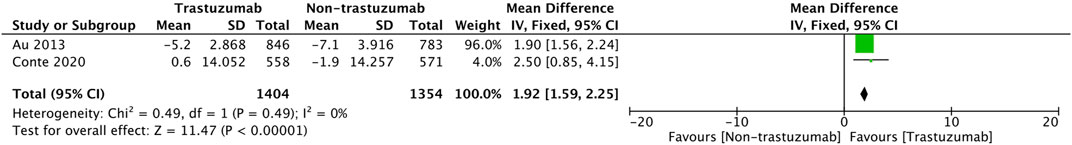
For RCT studies, a pooled analysis was performed to calculate the pool’s difference in mean change from baseline during treatment in 5 cycles of treatment. A total of 2,758 patients (1,404 patients in the trastuzumab group and 1,354 in the control group) were included in the trials. In Figure 4, it is shown that the mean change from baseline significantly favors the trastuzumab group (MD = 1.92, 95% CI = 1.59 to 2.25, p < 0.05) with low heterogeneity (I2 = 0%).
Another pooled analysis calculated the pool’s difference in mean change from the baseline of HRQOL after 12 months, which is presented in Figure 5. The result shows no significant difference between the two groups of comparison (MD = −0.70, 95% CI = −1.62 to 0.22, p = 0.13) with low heterogeneity (I2 = 29%).
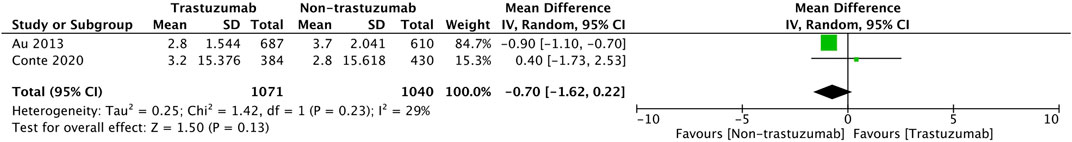
FIGURE 5. Pooled analysis of the difference in mean change from baseline of HRQoL after 12 months of follow-up based on RCTs.
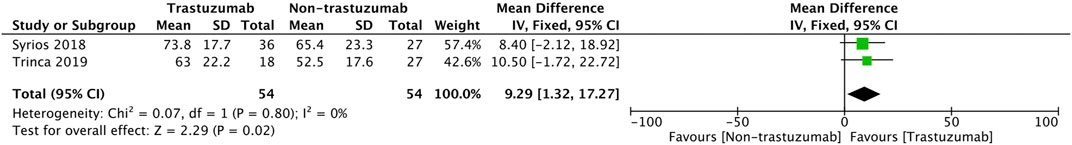
For observational studies (Figure 6), the pool’s mean difference in HRQoL between the two comparison groups tended significantly in favor of the trastuzumab regimen (MD = 9.29, 95% CI = 1.31 to 17.27, p = 0.02) and a homogenous observation was shown (I2 = 0%).
4 Discussion
Evaluating patients health status during the early stages of breast cancer is essential in defining the patients perceptions of long-term treatment, treatment benefits and potential adverse impacts. This review focuses on the HRQoL of patients, specifically the changes in HRQoL during and after treatment, to create a comprehensive picture of patients HRQoL when following a regimen including trastuzumab in early disease. The result from this review revealed that in the middle of treatment, the favorable HRQoL profile of patients using EORTC-QLQ C30 in RCT studies (Au et al., 2013; Conte et al., 2020; Earl et al., 2020; Sawaki et al., 2020; Taira et al., 2021) was shown in the trastuzumab arm by demonstrating a better score in the HRQoL, physical, and systemic side effect domains. Patients in trastuzumab arms showed a more tolerable profile of systemic effects domain compared to those in the chemotherapy group and the scores were significantly improved until the end of treatment. A chemotherapy regimen arm showed more deterioration of the mean HRQoL score from baseline until cycle 5 of the treatment, including the cognitive, physical, and fatigue domains. A significant worsening of the nausea/vomiting domain was also observed in the chemotherapy group compared to the trastuzumab group, which included worse nausea, decreased platelet count, and increased liver enzyme. After 12 months of treatment, the HRQoL status had increased in both trastuzumab and chemotherapy groups, presenting a similar pattern but was slightly higher in the trastuzumab group. The deterioration caused by the adverse effects of the treatment seemed resolved, leading to a pattern of increased HRQoL in both groups over time (Conte et al., 2020).
Trastuzumab also demonstrated a beneficial effect for the elderly by providing a clinically meaningful improvement in HRQoL score. For the elderly, HRQoL as an outcome is an essential factor besides a greater chance of survival (Wedding et al., 2007; Muss et al., 2009; Kornblith et al., 2011). Trastuzumab monotherapy exhibited a better score of HRQoL and the combination of trastuzumab with chemotherapy indicated a significant deterioration of patients HRQoL at 2 months and 1 year of treatment.
Furthermore, fewer adverse effects appeared during the first 36 months of observation, and it performed impressively in all functional domains. After 36 months, diminishing adverse effects were observed and HRQoL was unaffected (Taira et al., 2021). Another published study presented a deterioration of the HRQoL during the initial stages of treatment of elderly patients receiving good adjuvant therapy (Cheng et al., 2018). Based on these findings, adding chemotherapy to a trastuzumab regimen leads to adverse effects in older women and a meaningful deterioration of HRQoL (Sawaki et al., 2020). Trastuzumab combined with chemotherapy was related to worse outcome despite favorable cardiotoxicity (Goldvaser et al., 2019).
The above results were supported by the pooled analysis that calculated the mean change from baseline during treatment (Figure 3) and in the follow-up observation (Figure 4) based on two studies (Au et al., 2013; Conte et al., 2020). The pooled result showed that patients in the trastuzumab group experienced a meaningfully better HRQoL during treatment, and deterioration was more prevalent in the chemotherapy group. The lower mean improvement from baseline in HRQoL in the chemotherapy group was associated with the adverse events caused by chemotherapy. Breast cancer patients undergoing chemotherapy demonstrate less favorable score of HRQoL due to increasing symptoms attributed to systemic treatment (Leinert et al., 2017; Binotto et al., 2020). A cohort study also demonstrated a worsening score of HRQoL and functional scales while there was a meaningful increase in the domain of fatigue, nausea and vomiting, insomnia, appetite loss and diarrhea (Binotto et al., 2020). These findings are consistent with the APHINITY trial (Bines et al., 2021) investigating the HRQoL of patients in the early stages of breast cancer, which, using EORTC-QLQ-BR30, revealed that patients experienced a deterioration of HRQoL and functioning domain while receiving taxane-based chemotherapy. The patients using anthracycline-cyclophosphamide-based chemotherapy and docetaxel experienced a lower score in general health, physical functioning, and systemic side effects during treatment (Au et al., 2013) due to cardiotoxicity, fatigue, and nausea (Mills et al., 2005; Cohen et al., 2007; Cardinale et al., 2020).
For cross-sectional studies (Syrios et al., 2018; Trinca et al., 2019; Sawaki et al., 2022), patients had higher scores of HRQoL in the trastuzumab group compared to the chemotherapy group. Patients receiving trastuzumab had a better HRQoL score than the chemotherapy group, and more patients experienced decreased nausea/vomiting, cognitive side effects, and systemic therapy side effects (Syrios et al., 2018). However, patients who followed a chemotherapy regimen combined with intravenous trastuzumab had more diarrhea (Trinca et al., 2019). Another meta-analysis (J, 2019) also revealed a higher risk of diarrhea during trastuzumab therapy. That said, another study assessing subcutaneous trastuzumab demonstrated less diarrhea, as well as a significantly lower score in nausea/vomiting (Syrios et al., 2018). Generally, patients undergoing subcutaneous trastuzumab had a similar HRQoL profile to those receiving intravenous administration (Syrios et al., 2018). Subcutaneous trastuzumab has comparable efficacy, safety, and profile and requires a shorter period of administration than intravenous trastuzumab (G et al., 2012; Shpilberg and Jackisch, 2013; Schmidt et al., 2021). The PrefHer trial (Pivot et al., 2014) mentioned that most patients (88.9%) preferred SC administration due to shorter administration, less pain, and fewer side effects. The patients receiving subcutaneous trastuzumab during the early stages of breast cancer experienced less severe symptoms and a general improvement in functioning. The pooled analysis (Figure 5) also supports this result, as the pooled difference of the HRQoL between the two studies trended meaningfully towards the regimen including trastuzumab (Syrios et al., 2018; Trinca et al., 2019).
When evaluating treatment benefits, it is essential to consider the effect on HRQoL of adding a new drug to a treatment regimen (Johansson et al., 2008; Osoba et al., 2016). Ideally, the addition should improve treatment efficacy without causing deterioration in the HRQoL. This review shows that adding trastuzumab to chemotherapy supports efficacy and doesn’t lead to increased deterioration of the patient’s HRQoL. The improvement in HRQoL is likely associated with a decrease in tumor mass (Osoba et al., 2002). According to an efficacy study (Slamon et al., 2001), a high proportion of patients treated with trastuzumab and chemotherapy showed a reduction in tumors that contributed to a longer time of disease progression and the survival was longer compared to those undergoing chemotherapy alone. Several RCTs (Gianni et al., 2011; Perez et al., 2011; Slamon et al., 2011) and a large cohort study (Bonifazi et al., 2014) evaluating the efficacy of trastuzumab in the Her2-positive early breast cancer management exhibited that the addition of trastuzumab in the chemotherapy regimen was associated in the longer survival. Despite a comparable HRQoL profile between trastuzumab and chemotherapy 1 year after treatment, the benefit of the trastuzumab in the HRQoL of patients potentially supports the overall survival due to the favorable score of the mean change of baseline during treatment in trastuzumab patients. A deterioration of HRQoL leads to significantly related to shorter survival (van Nieuwenhuizen et al., 2021). In addition, the improved chances of survival as a result of trastuzumab should balance out the impact on patients HRQoL. Another published article found that a better score of HRQoL was meaningfully associated with an improvement of 5-year survival (van Nieuwenhuizen et al., 2021). Additionally, this result of this review may answer the limited used of trastuzumab in low- and middle-income countries (LMIC) due to its high cost.
To the best of our knowledge, this is the first systematic review and meta-analysis of the effect of trastuzumab in the early stages of breast cancer management on HRQoL. Three large electronic databases without year and language limitations were used to conduct a systematic search and we applied strict inclusion criteria to the articles we found. The RoB 2.0. ROBINS-I, and GRADE were used to comprehensively assess the risk of bias and the level of evidence of the studied included in this review. In addition, we deemed the studies of sufficient quality despite some of the cross-sectional studies lacking certain information. This review may provide a comprehensive assessment of the available evidence on this topic, including studies that have been published up until the present day, that can potentially inform and enhance use in settings where trastuzumab is not yet used large scale in all countries, such as a low-middle income country like Indonesia with numerous remote areas. However, there were several limitations to this review. The variety of treatment regimens in the trastuzumab and control groups may affect the different HRQoL profiles and result in high risk of bias which reduce the quality of the evidence. However, in the synthesis process, the heterogeneity was still possible to reduce from another viewpoint as the studies have the same HRQoL instrument, further enhancing the relevance of this analysis. In this case, we suggest that future studies in this area should consider using consistent comparator arms. Of the overall results of the four articles presented in the meta-analysis in this study, two lent themselves less clearly to interpretation, despite the high level of homogeneity in the calculation. The meta-analysis result was supported by the GRADE, in which shows low to moderate quality of evidence.
5 Conclusion
Trastuzumab as a targeted therapy may result in an improvement of HRQoL in the early stages of Her2-positive breast cancer. The measurement from RCTs revealed a more favorable score of HRQoL and a tolerable profile in the systemic side effects domain. A meaningfully better score of HRQoL in cross-sectional studies also provided a beneficial effect of trastuzumab in the management of Her-2 positive early breast cancer. These findings of significant improvements in patients HRQoL and less clinically significant deterioration in side effects of trastuzumab-containing regimen during treatment were supported by prolonged survival.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
SMK designed the study protocol, screened the included studies, analysed the data, and drafted the manuscript. FDAS contributed by reviewing the included studies, analysing the data and drafting the manuscript. DS and MP supported the manuscript by reviewing the data and the draft of the manuscript and approving the final version to be published. All authors read and approved the final manuscript.
Funding
This study was supported by Indonesia Endowment Funds for Education (LPDP) and the University of Groningen/University Medical Center Groningen.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1090326/full#supplementary-material
References
Adamowicz, K., and Baczkowska-Waliszewska, Z. (2020). Quality of life during chemotherapy, hormonotherapy or antiHER2 therapy of patients with advanced, metastatic breast cancer in clinical practice. Health Qual. Life Outcomes 18, 134–139. doi:10.1186/s12955-020-01389-x
Au, H.-J., Eiermann, W., Robert, N. J., Pienkowski, T., Crown, J., Martin, M., et al. (2013). Health-related quality of life with adjuvant docetaxel- and trastuzumab-based regimens in patients with node-positive and high-risk node-negative, HER2-positive early breast cancer: Results from the BCIRG 006 study. Oncologist 18, 812–818. doi:10.1634/theoncologist.2013-0091
Bines, J., Clark, E., Barton, C., Restuccia, E., Procter, M., Sonnenblick, A., et al. (2021). Patient-reported function, health-related quality of life, and symptoms in APHINITY: Pertuzumab plus trastuzumab and chemotherapy in HER2-positive early breast cancer. Br. J. Cancer 125, 38–47. doi:10.1038/s41416-021-01323-y
Binotto, M., Reinert, T., Werutsky, G., Zaffaroni, F., and Schwartsmann, G. (2020). Health-related quality of life before and during chemotherapy in patients with early-stage breast cancer. Ecancermedicalscience 14, 1007. doi:10.3332/ECANCER.2020.1007
Bonifazi, M., Franchi, M., Rossi, M., Zambelli, A., Moja, L., Zambon, A., et al. (2014). Long term survival of HER2-positive early breast cancer treated with trastuzumab-based adjuvant regimen: A large cohort study from clinical practice. Breast 23, 573–578. doi:10.1016/J.BREAST.2014.05.022
Bouskill, K., and Kramer, M. (2016). The impact of cancer and quality of life among long-term survivors of breast cancer in Austria. Support Care Cancer 24, 4705–4712. doi:10.1007/S00520-016-3319-7
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi:10.3322/caac.21492
Bria, E., Cuppone, F., Fornier, M., Nisticò, C., Carlini, P., Milella, M., et al. (2008). Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: The dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res. Treat. 109, 231–239. doi:10.1007/s10549-007-9663-z
Cameron, D., Piccart-Gebhart, M. J., Gelber, R. D., Procter, M., Goldhirsch, A., de Azambuja, E., et al. (2017). 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin adjuvant (HERA) trial. Lancet 389, 1195–1205. doi:10.1016/S0140-6736(16)32616-2
Cardinale, D., Iacopo, F., and Cipolla, C. M. (2020). Cardiotoxicity of anthracyclines. Front. Cardiovasc Med. 7, 26. doi:10.3389/fcvm.2020.00026
Cheng, K. K. F., Lim, E. Y. T., and Kanesvaran, R. (2018). Quality of life of elderly patients with solid tumours undergoing adjuvant cancer therapy: A systematic review. BMJ Open 8, e018101. doi:10.1136/BMJOPEN-2017-018101
Cohen, L., De Moor, C. A., Eisenberg, P., Ming, E. E., and Hu, H. (2007). Chemotherapy-induced nausea and vomiting: Incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 15, 497–503. doi:10.1007/S00520-006-0173-Z
Conte, P., Schneeweiss, A., Loibl, S., Mamounas, E. P., von Minckwitz, G., Mano, M. S., et al. (2020). Patient-reported outcomes from katherine: A phase 3 study of adjuvant trastuzumab emtansine versus trastuzumab in patients with residual invasive disease after neoadjuvant therapy for human epidermal growth factor receptor 2-positive breast cancer. Cancer 126, 3132–3139. doi:10.1002/cncr.32873
Dahabreh, I. J., Linardou, H., Siannis, F., Fountzilas, G., and Murray, S. (2008). Trastuzumab in the adjuvant treatment of early-stage breast cancer: A systematic review and meta-analysis of randomized controlled trials. Oncologist 13, 620–630. doi:10.1634/theoncologist.2008-0001
Dal Lago, L., Uwimana, A. L., Coens, C., Vuylsteke, P., Curigliano, G., Brouwers, B., et al. (2022). Health-related quality of life in older patients with HER2+ metastatic breast cancer: Comparing pertuzumab plus trastuzumab with or without metronomic chemotherapy in a randomised open-label phase II clinical trial. J. Geriatr. Oncol. 13, 582–593. doi:10.1016/J.JGO.2022.01.009
Earl, H., Hiller, L., Vallier, A.-L., Loi, S., McAdam, K., Hughes-Davies, L., et al. (2020). Six versus 12 months’ adjuvant trastuzumab in patients with HER2-positive early breast cancer: The PERSEPHONE non-inferiority RCT. Health Technol. Assess. 24, 1–190. doi:10.3310/hta24400
Genuino, A. J., Chaikledkaew, U., The, D. O., Reungwetwattana, T., and Thakkinstian, A. (2019). Adjuvant trastuzumab regimen for HER2-positive early-stage breast cancer: A systematic review and meta-analysis. Expert Rev. Clin. Pharmacol. 12, 815–824. doi:10.1080/17512433.2019.1637252
Gianni, L., Dafni, U., Gelber, R. D., Azambuja, E., Muehlbauer, S., Goldhirsch, A., et al. (2011). Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: A 4-year follow-up of a randomised controlled trial. Lancet Oncol. 12, 236–244. doi:10.1016/S1470-2045(11)70033-X
Goddard, K. A. B., Weinmann, S., Richert-Boe, K., Chen, C., Bulkley, J., and Wax, C. (2011). HER2 evaluation and its impact on breast cancer treatment decisions. Public Health Genomics 15, 1–10. doi:10.1159/000325746
Goldhirsch, A., Gelber, R. D., Piccart-Gebhart, M. J., de Azambuja, E., Procter, M., Suter, T. M., et al. (2013). 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): An open-label, randomised controlled trial. Available at: www.thelancet.com, 382. doi:10.1016/S0140-6736(13)61094-6
Goldvaser, H., Korzets, Y., Shepshelovich, D., Yerushalmi, R., Sarfaty, M., Ribnikar, D., et al. (2019). Deescalating adjuvant trastuzumab in HER2-positive early-stage breast cancer: A systemic review and meta-analysis. JNCI Cancer Spectr. 3, pkz033. doi:10.1093/JNCICS/PKZ033
Gonzalez-Angulo, A. M., Hortobágyi, G. N., and Esteva, F. J. (2006). Adjuvant therapy with trastuzumab for HER-2/neu-positive breast cancer. Oncologist 11, 857–867. doi:10.1634/theoncologist.11-8-857
Goodwin, P. J., Black, J. T., Bordeleau, L. J., and Ganz, P. A. (2003). Health-related quality-of-life measurement in randomized clinical trials in breast cancer-taking stock. J. Natl. Cancer Inst. 95, 263–281. doi:10.1093/JNCI/95.4.263
Gustavo, I., Roberto, H., Susanne, M., Dominik, H., Bert, L., Sung-Bae, K., et al. (2012). Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-iii breast cancer (HannaH study): A phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 13, 869–878. doi:10.1016/S1470-2045(12)70329-7
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). Grade: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926. doi:10.1136/BMJ.39489.470347.AD
Hamer, J., McDonald, R., Zhang, L., Verma, S., Leahey, A., Ecclestone, C., et al. (2016). Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Support Care Cancer 25, 409–419. doi:10.1007/S00520-016-3417-6
Hernán, M. A., and Robins, J. M. (2017). Per-protocol analyses of pragmatic trials. 1391–1398. doi:10.1056/NEJMSM1605385
Higgins, J. P. T., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. STATISTICS Med. Statist. Med 21, 1539–1558. doi:10.1002/sim.1186
Jing, L. (2019). Diarrhea with HER2-targeted agents in cancer patients: A systematic review and meta-analysis. J. Clin. Pharmacol. 59, 935–946. doi:10.1002/JCPH.1382
Johansson, B., Brandberg, Y., Hellbom, M., Persson, C., Petersson, L. M., Berglund, G., et al. (2008). Health-related quality of life and distress in cancer patients: Results from a large randomised study. Br. J. Cancer 99, 1975–1983. doi:10.1038/SJ.BJC.6604789
Kornblith, A. B., Lan, L., Archer, L., Partridge, A., Kimmick, G., Hudis, C., et al. (2011). Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: A companion study to cancer and leukemia group B 49907. J. Clin. Oncol. 29, 1022–1028. doi:10.1200/JCO.2010.29.9859
Leinert, E., Singer, S., Janni, W., Harbeck, N., Weissenbacher, T., Rack, B., et al. (2017). The impact of age on quality of life in breast cancer patients receiving adjuvant chemotherapy: A comparative analysis from the prospective multicenter randomized adebar trial. Clin. Breast Cancer 17, 100–106. doi:10.1016/J.CLBC.2016.10.008
Lemieux, J., Goodwin, P. J., Bordeleau, L. J., Lauzier, S., and Théberge, V. (2011). Quality-of-life measurement in randomized clinical trials in breast cancer: An updated systematic review (2001-2009). J. Natl. Cancer Inst. 103, 178–231. doi:10.1093/jnci/djq508
Long, H., Lin, Y., Zhang, J., Zhong, W., and Zheng, R. (2016). Risk of congestive heart failure in early breast cancer patients undergoing adjuvant treatment with trastuzumab: A meta-analysis. Oncologist 21, 547–554. doi:10.1634/THEONCOLOGIST.2015-0424
Madarnas, Y., Trudeau, M., Franek, J. A., McCready, D., Pritchard, K. I., and Messersmith, H. (2008). Adjuvant/neoadjuvant trastuzumab therapy in women with HER-2/neu-overexpressing breast cancer: A systematic review. Cancer Treat. Rev. 34, 539–557. doi:10.1016/j.ctrv.2008.03.013
Mates, M., Fletcher, G. G., Freedman, O. C., Eisen, A., Gandhi, S., Trudeau, M. E., et al. (2015). Systemic targeted therapy for her2-positive early female breast cancer: A systematic review of the evidence for the 2014 cancer care ontario systemic therapy guideline. Curr. Oncol. 22, S114–S122. doi:10.3747/CO.22.2322
Meader, N., King, K., Llewellyn, A., Norman, G., Brown, J., Rodgers, M., et al. (2014). A checklist designed to aid consistency and reproducibility of GRADE assessments: Development and pilot validation. Syst. Rev. 3, 82. doi:10.1186/2046-4053-3-82
Meldahl, M. L., Acaster, S., and Hayes, R. P. (2013). Exploration of oncologists’ attitudes toward and perceived value of patient-reported outcomes. Qual. Life Res. 22, 725–731. doi:10.1007/S11136-012-0209-4
Mills, P. J., Parker, B., Dimsdale, J. E., Sadler, G. R., and Ancoli-Israel, S. (2005). The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol. Psychol. 69, 85–96. doi:10.1016/J.BIOPSYCHO.2004.11.007
Muss, H. B., Berry, D. A., Cirrincione, C. T., Theodoulou, M., Mauer, A. M., Kornblith, A. B., et al. (2009). Adjuvant chemotherapy in older women with early-stage breast cancer. N. Engl. J. Med., 2055–2065. doi:10.1056/NEJMOA0810266
Oh, H. J. S., Menéndez, Á. F., Santos, V. S., Martínez, Á. R., Ribeiro, F. F., Vilanova-Trillo, L., et al. (2021). Evaluating health related quality of life in outpatients receiving anti-cancer treatment: Results from an observational, cross-sectional study. Health Qual. Life Outcomes 19, 245. doi:10.1186/S12955-021-01876-9
Osoba, D., Rodrigues, G., Myles, J., Zee, B., and Pater, J. (2016). Interpreting the significance of changes in health-related quality-of-life scores. 139–144. doi:10.1200/JCO.1998.16.1.139
Osoba, D., Slamon, D. J., Burchmore, M., and Murphy, M. (2002). Effects on quality of life of combined trastuzumab and chemotherapy in women with metastatic breast cancer. J. Clin. Oncol. 20, 3106–3113. doi:10.1200/JCO.2002.03.090
O’Sullivan, C. C., Bradbury, I., Campbell, C., Spielmann, M., Perez, E. A., Joensuu, H., et al. (2015). Efficacy of adjuvant trastuzumab for patients with human epidermal growth factor receptor 2-positive early breast cancer and tumors ≤ 2 cm: A meta-analysis of the randomized trastuzumab trials. J. Clin. Oncol. 33, 2600–2608. doi:10.1200/JCO.2015.60.8620
Park, J. H., Jung, Y. S., Jung, Y. M., and Bae, S. H. (2019). The role of depression in the relationship between cognitive decline and quality of life among breast cancer patients. Support Care Cancer 27, 2707–2714. doi:10.1007/S00520-018-4546-X
Perez, E. A., Romond, E. H., Suman, V. J., Jeong, J. H., Davidson, N. E., Geyer, C. E., et al. (2011). Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2–positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J. Clin. Oncol. 29, 3366–3373. doi:10.1200/JCO.2011.35.0868
Perez, E. A., Romond, E. H., Suman, V. J., Jeong, J. H., Sledge, G., Geyer, C. E., et al. (2014). Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2 - positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 32, 3744–3752. doi:10.1200/JCO.2014.55.5730
Perry, S., Kowalski, T. L., and Chang, C. H. (2007). Quality of life assessment in women with breast cancer: Benefits, acceptability and utilization. Health Qual. Life Outcomes 5, 24. doi:10.1186/1477-7525-5-24
Piccart, M., Lohrisch, C., Di Leo, A., and Larsimont, D. (2001). The predictive value of HER2 in breast cancer. Oncology 61 (2), 73–82. doi:10.1159/000055405
Piccart, M., Procter, M., Fumagalli, D., de Azambuja, E., Clark, E., Ewer, M. S., et al. (2021). Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 Years’ follow-up. J. Clin. Oncol. 39, 1448–1457. doi:10.1200/JCO.20.01204
Piccart-Gebhart, M. J., Procter, M., Leyland-Jones, B., Goldhirsch, A., Untch, M., Smith, I., et al. (2005). Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353, 1659–1672. doi:10.1056/NEJMOA052306
Pivot, X., Gligorov, J., Müller, V., Curigliano, G., Knoop, A., Verma, S., et al. (2014). Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: Final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann. Oncol. 25, 1979–1987. doi:10.1093/ANNONC/MDU364
Rimawi, M. F., Schiff, R., and Osborne, C. K. (2015). Targeting HER2 for the treatment of breast cancer. Annu. Rev. Med. 66, 111–128. doi:10.1146/ANNUREV-MED-042513-015127
Romond, E. H., Jeong, J.-H. H., Rastogi, P., Swain, S. M., Geyer, C. E. J., Ewer, M. S., et al. (2012). Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 30, 3792–3799. doi:10.1200/JCO.2011.40.0010
Ross, J. S., Slodkowska, E. A., Symmans, W. F., Pusztai, L., Ravdin, P. M., and Hortobagyi, G. N. (2009). The HER-2 receptor and breast cancer: Ten years of targeted anti–HER-2 therapy and personalized medicine. Oncologist 14, 320–368. doi:10.1634/THEONCOLOGIST.2008-0230
Sawaki, M., Taira, N., Uemura, Y., Saito, T., Baba, S., Kobayashi, K., et al. (2022). Adjuvant trastuzumab without chemotherapy for treating early HER2-positive breast cancer in older patients: A propensity score-adjusted analysis of a prospective cohort study. Breast Official J. Eur. Soc. Mastology 66, 245–254. doi:10.1016/J.BREAST.2022.10.017
Sawaki, M., Taira, N., Uemura, Y., Saito, T., Baba, S., Kobayashi, K., et al. (2020). Randomized controlled trial of trastuzumab with or without chemotherapy for HER2-positive early breast cancer in older patients. J. Clin. Oncol. 38, 3743–3752. doi:10.1200/JCO.20.00184
Schmidt, M., Kümmel, S., Ruf-Doerdelmann, A., Distelrath, A., Wacker, Jü., Schmatloch, S., et al. (2021). Neo-adjuvant and/or adjuvant subcutaneous trastuzumab (Herceptin(®)) in patients with early HER2-positive breast cancer: Real world data from a German observational study - (NIS HerSCin). Anticancer Res. 41, 485–496. doi:10.21873/anticanres.14799
Sella, T., Zheng, Y., Tayob, N., Ruddy, K. J., Freedman, R. A., Dang, C., et al. (2022). Treatment discontinuation, patient-reported toxicities and quality-of-life by age following trastuzumab emtansine or paclitaxel/trastuzumab (ATEMPT). NPJ Breast Cancer 8, 127. doi:10.1038/s41523-022-00495-x
Shpilberg, O., and Jackisch, C. (2013). Subcutaneous administration of rituximab (MabThera) and trastuzumab (Herceptin) using hyaluronidase. Br. J. Cancer 109, 1556–1561. doi:10.1038/BJC.2013.371
Slamon, D., Eiermann, W., Robert, N., Pienkowski, T., Martin, M., Press, M., et al. (2011). Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 365, 1273–1283. doi:10.1056/NEJMOA0910383
Slamon, D. J., Godolphin, W., Jones, L. A., Holt, J. A., Wong, S. G., Keith, D. E., et al. (1989). Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244, 707–712. doi:10.1126/SCIENCE.2470152
Slamon, D. J., Leyland-Jones, B., Shak, S., Fuchs, H., Paton, V., Bajamonde, A., et al. (2001). Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792. doi:10.1056/NEJM200103153441101
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. Res. Methods. doi:10.1136/bmj.l4898
Sterne, J. A., Hernán, M. A., Reeves, B. C., Savović, J., Berkman, N. D., Viswanathan, M., et al. (2016). ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919. doi:10.1136/BMJ.I4919
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Syrios, J., Pappa, E., Volakakis, N., Grivas, A., Alafis, J., Manioudaki, S., et al. (2018). Real-world data on health-related quality of life assessment in patients with breast cancer receiving subcutaneous trastuzumab. Breast Cancer (Auckl) 12, 1178223418758031. doi:10.1177/1178223418758031
Taira, N., Sawaki, M., Uemura, Y., Saito, T., Baba, S., Kobayashi, K., et al. (2021). Health-related quality of life with trastuzumab monotherapy versus trastuzumab plus standard chemotherapy as adjuvant therapy in older patients with HER2-positive breast cancer. J. Clin. Oncol. 39, 2452–2462. doi:10.1200/JCO.20.02751
Trinca, F., Infante, P., Dinis, R., Inácio, M., Bravo, E., Caravana, J., et al. (2019). Depression and quality of life in patients with breast cancer undergoing chemotherapy and monoclonal antibodies. Ecancermedicalscience 13, 937. doi:10.3332/ECANCER.2019.937
van Nieuwenhuizen, A. J., Buffart, L. M., Langendijk, J. A., Vergeer, M. R., Voortman, J., Leemans, C. R., et al. (2021). Health-related quality of life and overall survival: A prospective study in patients with head and neck cancer treated with radiotherapy. Qual. Life Res. 30, 1145–1153. doi:10.1007/S11136-020-02716-X
Viani, G. A., Afonso, S. L., Stefano, E. J., De Fendi, L. I., and Soares, F. V. (2007). Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: A meta-analysis of published randomized trials. BMC Cancer 7, 153. doi:10.1186/1471-2407-7-153
von Minckwitz, G., Procter, M., de Azambuja, E., Zardavas, D., Benyunes, M., Viale, G., et al. (2017). Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 377, 122–131. doi:10.1056/NEJMOA1703643
Ward, S., Pilgrim, H., and Hind, D. (2009). Trastuzumab for the treatment of primary breast cancer in HER2-positive women: A single technology appraisal. Health Technol. Assess. 13 (1), 1–6. doi:10.3310/hta13suppl1/01
Wedding, U., Pientka, L., and Höffken, K. (2007). Quality-of-life in elderly patients with cancer: A short review. Eur. J. Cancer 43, 2203–2210. doi:10.1016/J.EJCA.2007.06.001
Wolff, A. C., Hammond, M. E. H., Hicks, D. G., Dowsett, M., McShane, L. M., Allison, K. H., et al. (2013). Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. J. Clin. Oncol. 31, 3997–4013. doi:10.1200/JCO.2013.50.9984
Keywords: trastuzumab, breast cancer, meta-analysis, health-related quality of life, Her2-positive breast cancer
Citation: Khoirunnisa SM, Suryanegara FDA, Setiawan D and Postma MJ (2023) Health-related quality of life in Her2-positive early breast cancer woman using trastuzumab: A systematic review and meta-analysis. Front. Pharmacol. 14:1090326. doi: 10.3389/fphar.2023.1090326
Received: 05 November 2022; Accepted: 31 March 2023;
Published: 14 April 2023.
Edited by:
Domenico Criscuolo, Italian Society of Pharmaceutical Medicine, ItalyReviewed by:
Daniele Mengato, University Hospital of Padua, ItalyCoy D. Heldermon, University of Florida, United States
Copyright © 2023 Khoirunnisa, Suryanegara, Setiawan and Postma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sudewi Mukaromah Khoirunnisa, cy5tLmtob2lydW5uaXNhQHJ1Zy5ubA==, c3VkZXdpLm11a2Fyb21haEBmYS5pdGVyYS5hYy5pZA==
 Sudewi Mukaromah Khoirunnisa
Sudewi Mukaromah Khoirunnisa Fithria Dyah Ayu Suryanegara
Fithria Dyah Ayu Suryanegara Didik Setiawan4,5
Didik Setiawan4,5 Maarten Jacobus Postma
Maarten Jacobus Postma