- 1The Second Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China
- 2The People’s Hospital of JiangMen, Jiangmen Hospital, Southern Medical University, Jiangmen, China
- 3Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, Guangdong, China
- 4Taishan Hospital of Traditional Chinese Medicine, Jiangmen, Guangdong, China
- 5State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China
Objective: A meta-analysis is conducted to evaluate the effectiveness and safety of bevacizumab in hereditary hemorrhagic telangiectasia (HHT) epistaxis.
Method: Two researchers search PubMed, EMBASE and Web of Science databases from their inception until September 3th, 2023. The literature is read and screened, and valid data extracted, collated and analyzed. Its quality is then assessed using the Cochrane risk assessment scale. This study uses Endnote 9.3 software for literature management and RevMan 5.3.1 software for evaluation.
Results: A total of 7 documents met the requirements, including a total of 359 patients, and the literature quality evaluation was grade B. The Meta-analysis results showed that:Bevacizumab reduces the Epistaxis Severity Score (ESS) in patients with HHT epistaxis compared with the control [WMD = −0.22,95%CI (−0.38, −0.05), p = 0.01]. However, there is no significant effect on duration of epistaxis [WMD = −15.59, 95%CI (−70.41,39.23), p = 0.58] and number of epistaxes [WMD = −1.27,95%CI (−10.23,7.70), p = 0.78] in patients with HHT epistaxis. In terms of adverse effects, there is no significant difference between the bevacizumab group and control group [OR = 1.36, 95% CI (0.54, 3.44), p = 0.52].
Conclusion: Bevacizumab is superior to the control group in the treatment of HHT epistaxis, and adverse reactions are not further increased in the bevacizumab group than in the control group, suggesting that bevacizumab has clinical value in the treatment of HHT epistaxis.
1 Introduction
Hereditary hemorrhagic telangiectasia (HHT) or Osler-Weber-Rendu syndrome is an autosomal-dominant inherited vascular disease (Fuchizaki et al., 2003) which occurs due to mutations in protein-coding genes that mediate salivary secretion through the transforming growth factor-β (TGF-β) superfamily (Kritharis et al., 2018). The vast majority of HHT epistaxis patients have mutations in endorphilin (ENG) or activin receptor-like kinase 1 (ACVRL1/ALK1), resulting in dysangiogenesis, telangiectasia on the mucosal surface, local hyperfibrinolysis in telangiectasia and arteriovenous malformation in the internal organs (Kwaan and Silverman, 1973; Watanabe et al., 1985; Shovlin, 2010). It is characterized by vascular malformations in the nasal mucosa, skin, gastrointestinal tract, brain, lungs and liver, (Guttmacher et al., 1995) and causes telangiectasia of the nasal mucosa with varying degrees of recurrent epistaxis that can occur in 95% of HHT patients, wherein the mean age of first onset is 12 years and the frequency of epistaxis is approximately 18/month (GRIGG et al., 2017). Severe recurrent epistaxis may last several hours a day, causing severe iron deficiency anemia and often transfusion dependence, and even causing social isolation, as well adversely affecting the patients’ employment, travel and daily activities. Clinical treatments for HHT-related epistaxis mainly include laser, argon plasma coagulation, sclerotherapy, septoplasty of nasal lesions, bevacizumab injection and interventional embolization of epistaxis (Richmon et al., 2007; Harvey et al., 2008). At present, there is no effective treatment for HHT. The bleeding of HHT patients is mostly refractory, and the clinical treatment is still mainly supportive treatment and symptom relief. With the further deepening of research on the pathogenesis of vascular malformation in HHT, newly developed drugs such as bevacizumab (an anti-vascular endothelial growth factor monoclonal antibody) have been used in clinical practice, and bevacizumab has been proven to effectively correct the angiogenesis defects (Li et al., 2018). Studies have shown that bevacizumab is effective in the treatment of familial refractory epistaxis caused by HHT with a high safety profile (Mitchell et al., 2008; Al-Samkari et al., 2019; Vázquez et al., 2020). However, there is currently a lack of multicenter and large-sample randomized controlled trials, and there is little published evidence-based medical literature worldwide. This study aims to conduct a meta-analysis of existing clinical studies to explore the efficacy and safety of bevacizumab in the treatment of HHT epistaxis, thereby providing medical evidence for the use of bevacizumab in patients with HHT epistaxis.
2 Data and methods
2.1 Literature search
PubMed, Embase and Web of science databases were searched for the following terms: “hereditary hemorrhagic telangiectasia”, “Osler-Weber-Rendu disease”, “epistaxis” and “bevacizumab”. Literature references were traced, the search language was limited to English and the search time was limited to September 3th, 2023.
2.2 Inclusion criteria
① Study type: case-control study; ② study subjects: patients with HHT epistaxis; ③ intervention: bevacizumab treatment; ④ outcome indicators: effectiveness indicators (e.g., duration of epistaxis, Epistaxis Severity Score (ESS) and number of epistaxes) and adverse reactions(all treatment-related adverse reactions, such as headache, nausea and vomiting, musculoskeletal pain, edema, dizziness, etc.); ⑤ language: English.
2.3 Exclusion criteria
① Abstract, review, or case report; ② duplicate data; ③ does not include test indicators to be evaluated; ④ treatment that affects the efficacy of bevacizumab has been carried out; ⑤ grade C quality standard; ⑥ animal experiments.
2.4 Literature screening and data extraction
The titles, abstracts and main texts were independently examined by two researchers to select eligible studies. Selected articles were then reviewed at the full-text level by the same two researchers. Both researchers then independently extracted data from eligible original articles. The main data extracted was author, year, country, study type, sample size, duration of epistaxis, number of epistaxes, ESS, etc. Disagreements regarding literature selection and data extraction were resolved by discussion with a third author.
2.5 Literature quality evaluation
The two researchers rated on the Cochrane risk assessment scale as follows: ① random sequence generation; ② allocation concealment; ③ blindness (patients, staff); ④ outcome assessors blinded; ⑤ outcome data completeness; ⑥ selective report outcomes; ⑦ other bias. A “low” (low risk of bias), “high” (high risk of bias) or “unclear” (uncertain) judgment was required for each item. When the evaluation results were inconsistent, a discussion was held with a third author. According to the library RCT article evaluation system, the articles were ultimately graded as A, B or C.
2.6 Statistical analysis
Data was analyzed with RevMan 5.3.1 software. The heterogeneity test was conducted first. If the heterogeneity among the studies was not statistically significant (I2 < 50% and p > 0.1), a fixed effects model was used, and in case of significant heterogeneity (I2 > 50% and p < 0.1), a random effects model was used. The continuous variables were analyzed by weighted mean difference (WMD) and its 95% confidence interval (CI). The odds ratio (OR) or risk ratio (RR) and its 95% CI were used to analyze the binary variables.
3 Results
3.1 Literature search results
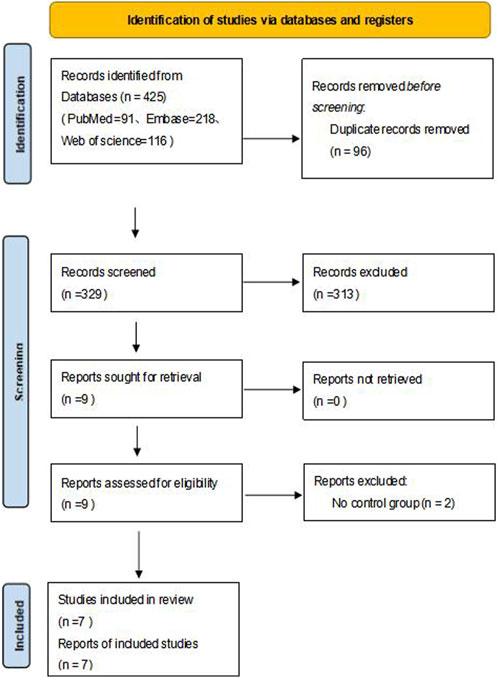
A total of 425 relevant articles were obtained after a preliminary search, and 96 remained after duplicate articles were excluded. After the preliminary screening of titles and abstracts, a total of nine articles met the requirements after excluding case reports, reviews, letters, abstracts, guidelines and articles not belonging to the research field. After reading the full texts, seven articles met the inclusion and exclusion criteria (Simonds et al., 2009; Dupuis-Girod et al., 2014; Riss et al., 2015; Dupuis-Girod et al., 2016; Whitehead et al., 2016; Khanwalkar et al., 2022; Dupuis-Girod et al., 2023). The literature selection flow chart is shown in Figure 1.
3.2 Basic characteristics of included studies
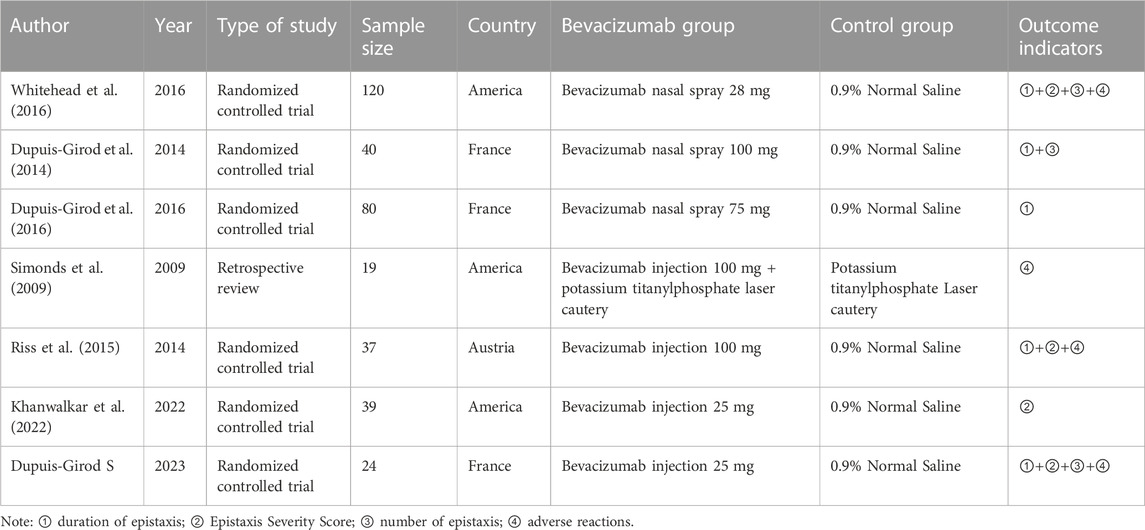
Of the seven included studies, the total number of cases was 359. The basic characteristics of all included literature is shown in Table 1.
3.3 Literature quality evaluation results
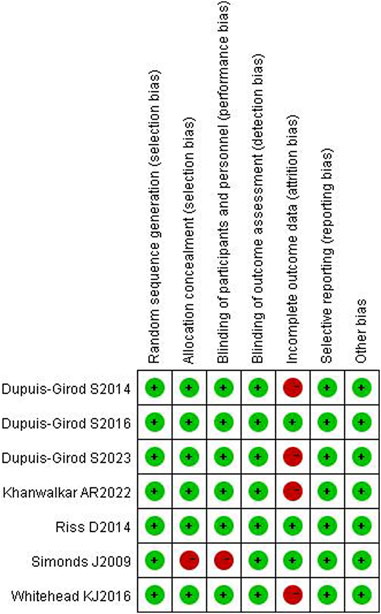
Of all included studies, allocation concealment and blindness were not mentioned in the Simonds J (Simonds et al., 2009) study, which was assessed as high risk; in the Whitehead K J (Whitehead et al., 2016) and Khanwalkar AR (Khanwalkar et al., 2022; Dupuis-Girod et al., 2023) studies, case shedding was mentioned, and the completeness of the outcome data was at high risk; the rest were assessed as low risk. All six articles were grade B or above. The results are detailed in Figures 2, 3.
3.4 Meta-analysis results
3.4.1 Duration of the epistaxis episode
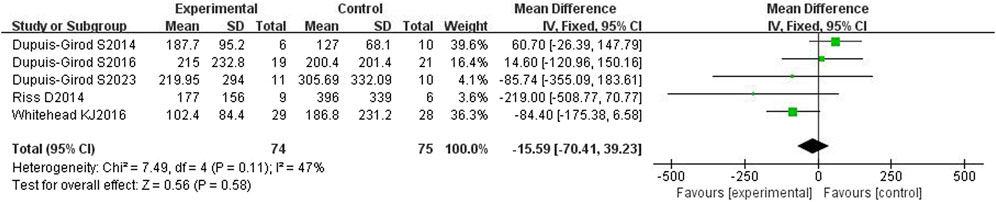
All five included articles (Dupuis-Girod et al., 2014; Riss et al., 2015; Dupuis-Girod et al., 2016; Whitehead et al., 2016; Dupuis-Girod et al., 2023) reported the duration of epistaxis and had a total of 149 patients. The heterogeneity test showed moderate heterogeneity (p = 0.11, I2 = 47%), so a fixed effects model was used. The results showed that the duration of epistaxis was not significantly different between the bevacizumab group and control group [WMD = −15.59, 95%CI (−70.41, 39.23), p = 0.58], as shown in Figure 4. After excluding each study one by one for sensitivity analysis, it was found that the duration of nasal hemorrhage in the bevacizumab group was still not significantly different from that in the control group, and the results were stable.
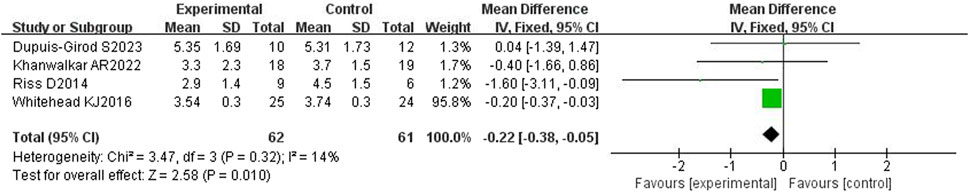
3.4.2 ESS score
All four included articles (Riss et al., 2015; Whitehead et al., 2016; Khanwalkar et al., 2022; Dupuis-Girod et al., 2023) reported the ESS and had a total of 131 patients. The heterogeneity test showed mild heterogeneity (p = 0.32, I2 = 14%), so a fixed effects model was used. The results showed that the ESS in the bevacizumab group was statistically significant compared with the control group [WMD = −0.22,95%CI (−0.38, −0.05), p = 0.01], as shown in Figure 5. However, there was no statistical difference between the duration of epistaxis in the bevacizumab group and the ESS score in the control group, which requires further confirmation by large sample and multi-center clinical studies.
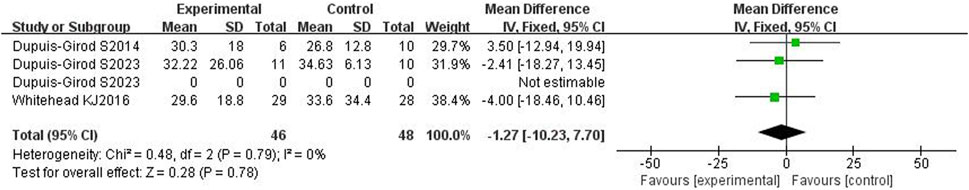
3.4.3 Number of epistaxes
All three included articles (Dupuis-Girod et al., 2014; Whitehead et al., 2016; Dupuis-Girod et al., 2023) reported the number of epistaxes and had a total of 94 patients. The heterogeneity test showed no heterogeneity (p = 0.79, I2 = 0%), so a fixed effects model was used. The results showed that the number of epistaxes in bevacizumab group was not statistically significant compared with the control group [WMD = −1.27,95%CI(−10.23, 7.70), p = 0.78], as shown in Figure 6. After removing each study one by one for sensitivity analysis, it was found that there was no significant difference in the number of epistaxis between the bevacizumab group and the control group, and the results were stable.
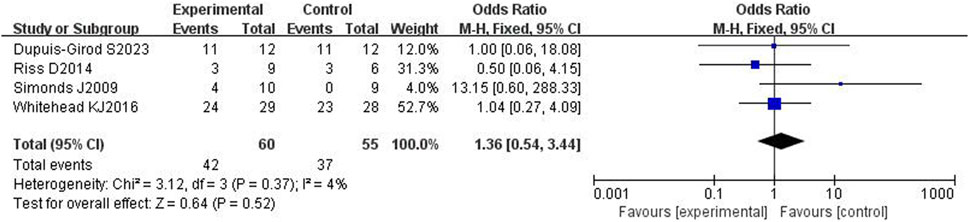
3.4.4 Adverse reactions
All four included articles[14, 17–18,20] reported adverse effects in a total of 115 patients. The heterogeneity test showed mild heterogeneity (p = 0.37, I2 = 4%), so a fixed effects model was used. The results showed no significant difference in adverse effects between the bevacizumab group and control group [OR = 1.36, 95% CI (0.54,3.44), p = 0.52], as shown in Figure 7. After removing each study one by one for sensitivity analysis, it was found that there was no significant difference in adverse reactions between the bevacizumab group and the control group, and the results were stable.
3.5 Publication bias evaluation
The publication bias of the included studies was analyzed by mapping the funnel plots. The left and right distributions of each study site were largely symmetrical without severe publication bias, as shown in Figure 8.
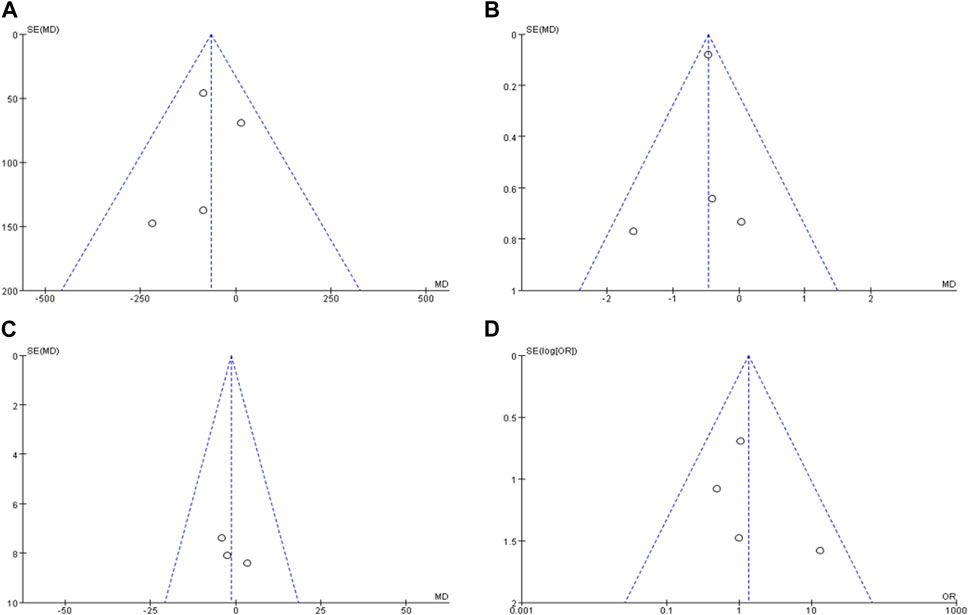
FIGURE 8. Publication bias funnel plots of included literature. Note: (A) is the funnel plot of the duration of the epistaxis episode, (B) is the funnel plot of ESS score, (C) is the funnel plot number of epistaxes, (D) is the funnel plot of adverse reactions.
4 Discussion
Spontaneous, repeated and massive epistaxis is the most obvious feature of HHT, which often leads to chronic and severe anemia, and requires iron supplements and even frequent blood transfusions to keep patients alive. Although electrocoagulation or arterial embolization can immediately relieve epistaxis, their effects are short-lived. HHT can be divided into six types according to different pathogenic genes: HHT1 (ENG gene), HHT2 (ACVRL1 gene), HHT3 and HHT4 (on chromosomes 5 and 7 respectively, with pathogenic gene mapping but not yet cloned), juvenile polyposis and HHT syndrome (SMAD4 gene), and HHT5 (GDF2 gene). Pathologically, the relevant proteins encoded by these genes play a role in the TGF-β and vascular endothelial growth factor (VEGF) signaling cascade, with TGF-β and VEGF concentrations elevated in patient serum and mucosa. Currently, the inhibition of VEGF is a rational way to treat HHT and a pharmacological basis for the application of bevacizumab, a known VEGF inhibitor, in the treatment of the disease (ten Dijke and Arthur, 2007; Sadick et al., 2005; Iyer et al., 2018). The ability of bevacizumab to bind to and inhibit the biological activity of VEGF prevents endothelial cell proliferation and angiogenesis, and its efficacy in treating HHT-associated epistaxis has been demonstrated (Dupuis-Girod, 2020). Bevacizumab is a recombinant humanized antibody directed against vascular endothelial growth factor, and is approved by the US Food and Drug Administration for use in patients with colorectal or other cancers. After initial case reports that intravenous bevacizumab with oncology dosing improves bleeding in patients with HHT, (Flieger et al., 2006; Bose et al., 2009) subsequent reports suggest efficacy at lower doses (Suppressa et al., 2011; Thompson et al., 2014) or with submucosal24or topical treatment (Davidson et al., 2010).
The efficacy of HHT related to epistaxis in each current study was mainly assessed by duration of epistaxis, number of epistaxes and ESS. The ESS was created as a standardized measure for assessing the extent of epistaxis (Hoag et al., 2010). The ESS is based on six questions, four of which document the frequency, duration, intensity and treatment need of epistaxis, while the other two detail the presence of anemia and whether the patient requires a blood transfusion. The severity weights vary on a scale of 0–10, with 0 as disease-free and 10 for severe disease. To date, ESS has been used as an excellent measure of epistaxis severity.
Seven studies were included in this meta-analysis, dating from 2009 to 2023, from France, USA and Austria, and no significant publication bias was found by funnel mapping. The meta-analysis showed that bevacizumab reduced the ESS in HHT epistaxis patients compared with the control, but had no significant effect on the duration and number of epistaxes. In terms of adverse effects, there was no significant difference between the bevacizumab and control groups. Through sensitivity analysis, it can be seen that deleting any of the three studies on the duration, frequency, and adverse reactions of nasal bleeding between the bevacizumab group and the control group did not have a significant impact on the combined effect values of the remaining literature, confirming the stability of the results. However, in the ESS study, after deleting some of the literature, there was no statistical difference in the duration of nasal bleeding between the bevacizumab group and the control group’s ESS score, and further large-scale sampling is needed Further confirmation from multicenter clinical studies. Due to the limited number of literature included in this meta-analysis, subgroup analyses such as national and bevacizumab dosage and dosage forms cannot be conducted. The ESS score includes six influencing factors, namely: frequency, duration, intensity, need for medical care, anemia, and need for blood transfusion. From the above results, there is no significant difference in the duration and number of epistaxes, but the ESS is significantly decreased. Thus, in addition to the duration and number of epistaxes, greater attention should be paid to the two important indicators of intensity and need for treatment(such as the need for medical care, anemia, and the need for blood transfusion). However, the current literature has paid relatively little attention to these indicators, so a combined meta-analysis is not yet possible. It is hoped that in the future, a multicenter and large-sample randomized controlled trial will observe intensity and need for treatment indicators.
This study has some limitations. First, the combined sample size of the included articles was small and may have had some impact on the results. Second, the included articles were from the United States, France and Austria, which may have generated some regional bias. Third, the heterogeneity in some articles may have had a certain influence on the results of the pooled analysis. In the future, multicenter studies should be designed in order to further explore the effectiveness and safety of bevacizumab in the treatment of HHT epistaxis, thereby providing a more reliable decision basis for clinical diagnosis and treatment.
5 Conclusion
Bevacizumab was superior to the control group in the treatment of HHT epistaxis, and adverse reactions were not further increased in the bevacizumab group than in the control group, suggesting that bevacizumab has clinical value in the treatment of HHT epistaxis.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
RC, HC, JC, WY, CW, ZZ, and XC are the guarantor of the manuscript and take responsibility for the content of this manuscript. HC, YY, and RC contributed to the design of the study. HC, JZ, MC, and HL were involved in the data analysis. HC and RC contributed to the acquisition of primary data. HC, JC, CW, and RC wrote the initial draft of the manuscript. WY, YY, XY, and ZZ contributed significantly to the revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Natural Science Foundation of Guangdong Province (2021A1515011373).
Acknowledgments
We would also like to thank Professor Nanshan Zhong from State Key Laboratory of Respiratory Disease for the constructive advice he gave.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Samkari, H., Albitar, H. A., Olitsky, S. E., Clancy, M. S., and Iyer, V. N. (2019). Systemic bevacizumab for high-output cardiac failure in hereditary hemorrhagic telangiectasia: an international survey of HHT centers. Orphanet J. Rare Dis. 14 (1), 256. doi:10.1186/s13023-019-1239-6
Bose, P., Holter, J. L., and Selby, G. B. (2009). Bevacizumab in hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 360 (20), 2143–2144. doi:10.1056/NEJMc0901421
Davidson, T. M., Olitsky, S. E., and Wei, J. L. (2010). Hereditary hemorrhagic telangiectasia/avastin. Laryngoscope 120 (2), 432–435. doi:10.1002/lary.20757
Dupuis-Girod, S. (2020). Intravenous bevacizumab in hereditary hemorrhagic telangiectasia: a role that is still to be defined. Mayo Clin. Proc. 95 (8), 1565–1566. doi:10.1016/j.mayocp.2020.06.038
Dupuis-Girod, S., Ambrun, A., Decullier, E., Fargeton, A. E., Roux, A., Bréant, V., et al. (2016). Effect of bevacizumab nasal spray on epistaxis duration in hereditary hemorrhagic telangectasia: a randomized clinical trial. JAMA 316 (9), 934–942. doi:10.1001/jama.2016.11387
Dupuis-Girod, S., Ambrun, A., Decullier, E., Samson, G., Roux, A., Fargeton, A. E., et al. (2014). ELLIPSE Study: a Phase 1 study evaluating the tolerance of bevacizumab nasal spray in the treatment of epistaxis in hereditary hemorrhagic telangiectasia. MAbs 6 (3), 794–799. doi:10.4161/mabs.28025
Dupuis-Girod, S., Rivière, S., Lavigne, C., Fargeton, A. E., Gilbert-Dussardier, B., Grobost, V., et al. (2023). Efficacy and safety of intravenous bevacizumab on severe bleeding associated with hemorrhagic hereditary telangiectasia: a national,randomized multicenter trial. J. Intern Med. 00, 1–14. doi:10.1111/joim.13714
Flieger, D., Hainke, S., and Fischbach, W. (2006). Dramatic improvement in hereditary hemorrhagic telangiectasia after treatment with the vascular endothelial growth factor (VEGF) antagonist bevacizumab. Ann. Hematol. 85 (9), 631–632. doi:10.1007/s00277-006-0147-8
Fuchizaki, U., Miyamori, H., Kitagawa, S., Kaneko, S., and Kobayashi, K. (2003). Hereditary haemorrhagic telangiectasia (Rendu-Osler-Weber disease). Lancet 362 (9394), 1490–1494. doi:10.1016/S0140-6736(03)14696-X
Grigg, C., Anderson, D., and Earnshaw, J. (2017). Diagnosis and treatment of hereditary hemorrhagic telangiectasia. Ochsner 17 (2), 157–161.
Guttmacher, A. E., Marchuk, D. A., and White, R. I. (1995). Hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 333, 918–924. doi:10.1056/NEJM199510053331407
Harvey, R. J., Kanagalingam, M. A., and Lund, V. J. (2008). The impact of septo dermoplasty and potassium-titanyl-phosphate (KTP) laser therapy in the treatment of hereditary hemorrhagic telangiectasia-related epistaxis. Am. J. Rhinol. 22, 182–187. doi:10.2500/ajr.2008.22.3145
Hoag, J. B., Terry, P., Mitchell, S., Reh, D., and Merlo, C. A. (2010). An epistaxis severity score for hereditary hemorrhagic telangiectasia. Laryngoscope 120, 838–843. doi:10.1002/lary.20818
Iyer, V. N., Apala, D. R., Pannu, B. S., Kotecha, A., Brinjikji, W., Leise, M. D., et al. (2018). Intravenous bevacizumab for refractory hereditary hemorrhagic telangiectasia-related epistaxis and gastrointestinal bleeding. Mayo Clin. Proc. 93 (2), 155–166. doi:10.1016/j.mayocp.2017.11.013
Khanwalkar, A. R., Rathor, A., Read, A. K., Paknezhad, H., Ma, Y., and Hwang, P. H. (2022). Randomized, controlled, double-blinded clinical trial of effect of bevacizumab injection in management of epistaxis in hereditary hemorrhagic telangiectasia patients undergoing surgical cauterization. Int. Forum Allergy Rhinol. 12 (8), 1034–1042. doi:10.1002/alr.22961
Kritharis, A., Al-Samkari, H., and Kuter, D. J. (2018). Hereditary hemorrhagic telangiectasia: diagnosis and management from the hematologist's perspective. Haematologica 103 (9), 1433–1443. doi:10.3324/haematol.2018.193003
Kwaan, H. C., and Silverman, S. (1973). Fibrinolytic activity in lesions of hereditary hemorrhagic telangiectasia. Arch. Dermatol 107 (4), 571–573. doi:10.1001/archderm.107.4.571
Li, S., Wang, S., and Zhao, Y. (2018). Clinical features and treatment of hereditary hemorrhagic telangiectasia. Medicine 97 (31), e11687. doi:10.1097/MD.0000000000011687
Mitchell, A., Adams, L. A., MacQuillan, G., Tibballs, J., vanden Driesen, R., and Delriviere, L. (2008). Bevacizumab reverses need for liver transplantation in hereditary hemorrhagic telangiectasia. Liver Transpl. 14 (2), 210–213. doi:10.1002/lt.21417
Richmon, J. D., Tian, Y., Husseman, J., and Davidson, T. M. (2007). Use of a sprayed fibrin hemostatic sealant after laser therapy for hereditary hemorrhagic telangiectasia epistaxis. Am. J. Rhinol. 21, 187–191. doi:10.2500/ajr.2007.21.2969
Riss, D., Burian, M., Wolf, A., Kranebitter, V., Kaider, A., and Arnoldner, C. (2015). Intranasal submucosal bevacizumab for epistaxis in hereditary hemorrhagic telangiectasia: a double-blind, randomized, placebo-controlled trial. Head. Neck 37 (6), 783–787. doi:10.1002/hed.23655
Sadick, H., Riedel, F., Naim, R., Goessler, U., Hörmann, K., Hafner, M., et al. (2005). Patients with hereditary hemorrhagic telangiectasia have increased plasma levels of vascular endothelial growth factor and transforming growth factor-beta1 as well as high ALK1 tissue expression. Haematologica 90 (6), 818–828.
Shovlin, C. L. (2010). Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Rev. 24 (6), 203–219. doi:10.1016/j.blre.2010.07.001
Simonds, J., Miller, F., Mandel, J., and Davidson, T. M. (2009). The effect of bevacizumab (Avastin) treatment on epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope 119 (5), 988–992. doi:10.1002/lary.20159
Suppressa, P., Liso, A., Sabbà, C., Arcangelo, L., Sabbà, C., and Carlo, S. (2011). Low dose intravenous bevacizumab for the treatment of anaemia in hereditary haemorrhagic telangiectasia. Br. J. Haematol. 152 (4), 365. doi:10.1111/j.1365-2141.2010.08481.x
ten Dijke, P., and Arthur, H. M. (2007). Extracellular control of TGFbeta signalling in vascular development and disease. Nat. Rev. Mol. Cell Biol. 8 (11), 857–869. doi:10.1038/nrm2262
Thompson, A. B., Ross, D. A., Berard, P., Figueroa-Bodine, J., Livada, N., and Richer, S. L. (2014). Very low dose bevacizumab for the treatment of epistaxis in patients with hereditary hemorrhagic telangiectasia. Allergy Rhinol. Provid. 5 (2), 91–95. doi:10.2500/ar.2014.5.0091
Vázquez, C., Gonzalez, M. L., Ferraris, A., Bandi, J. C., and Serra, M. M. (2020). Bevacizumab for treating hereditary hemorrhagic telangiectasia patients with severe hepatic involvement or refractory anemia. PLoS One 15 (2), e0228486. doi:10.1371/journal.pone.0228486
Watanabe, M., Hanawa, S., and Morishima, T. (1985). Fibrinolytic activity in cutaneous lesions of hereditary hemorrhagic telangiectasia. Nihon Hifuka Gakkai Zasshi 95 (1), 11–16.
Whitehead, K. J., Sautter, N. B., McWilliams, J. P., Chakinala, M. M., Merlo, C. A., Johnson, M. H., et al. (2016). Effect of topical intranasal therapy on epistaxis frequency in patients with hereditary hemorrhagic telangiectasia: a randomized clinical trial. JAMA 316 (9), 943–951. doi:10.1001/jama.2016.11724
Keywords: bevacizumab, hereditary hemorrhagic telangiectasia epistaxis, treatment, efficacy, meta-analysis
Citation: Chen H, Zhang Z, Chen X, Wang C, Chen M, Liao H, Zhu J, Zheng Z and Chen R (2023) Meta-analysis of efficacy and safety of bevacizumab in the treatment of hereditary hemorrhagic telangiectasia epistaxis. Front. Pharmacol. 14:1089847. doi: 10.3389/fphar.2023.1089847
Received: 11 November 2022; Accepted: 06 November 2023;
Published: 12 December 2023.
Edited by:
Jean-Marie Boeynaems, Université libre de Bruxelles, BelgiumReviewed by:
Yi Liao, Affiliated Hospital of Southwest Medical University, ChinaAndong He, First Affiliated Hospital of Jinan University, China
Daibing Zhou, Fudan University, China
Copyright © 2023 Chen, Zhang, Chen, Wang, Chen, Liao, Zhu, Zheng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenzhen Zheng, emhlbmd6aGVuemhlbjIwMThAMTYzLmNvbQ==; Riken Chen, Y2hlbnJpa2VuQDEyNi5jb20=
†These authors have contributed equally to this work
 Huimin Chen
Huimin Chen Zhiping Zhang2†
Zhiping Zhang2† Zhenzhen Zheng
Zhenzhen Zheng Riken Chen
Riken Chen