
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 16 February 2023
Sec. Drugs Outcomes Research and Policies
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1089272
 Rui Hu1†
Rui Hu1† Tao Yuan1†
Tao Yuan1† Hui Wang1
Hui Wang1 Jianglin Zhao1
Jianglin Zhao1 Liya Shi1
Liya Shi1 Quankai Li1
Quankai Li1 Chunmei Zhu1,2
Chunmei Zhu1,2 Na Su3,4*
Na Su3,4* Shengzhao Zhang1*
Shengzhao Zhang1*Background: Although with the application of etanercept biosimilars in the field of rheumatoid arthritis, the evidences of their efficacy, safety, and immunogenicity are still limited. We conducted this meta-analysis to evaluate the efficacy, safety and immunogenicity of etanercept biosimilars for treating active rheumatoid arthritis compared to reference biologics (Enbrel®).
Methods: PubMed, Embase, Central, and ClinicalTrials.gov were searched for randomized controlled trials of etanercept biosimilars treated in adult patients diagnosed with rheumatoid arthritis from their earliest records to 15 August 2022. The outcomes included ACR20, ACR50, and ACR70 response rate at different time points from FAS or PPS, adverse events, and proportion of patients developed anti-drug antibodies. The risk of bias of each included study was assessed using the revised Cochrane Risk of Bias in Randomised Trials tool, and the certainty of evidence was rated according to the Grading of Recommendation Assessment, Development, and Evaluation.
Results: Six RCTs with 2432 patients were included in this meta-analysis. Etanercept biosimilars showed more benefits in ACR50 at 24 weeks from PPS [5 RCTs, OR = 1.22 (1.01, 1.47), p = 0.04, I2 = 49%, high certainty], ACR50 at 1 year from PPS [3 RCTs, OR = 1.43 (1.10, 1.86), p < 0.01, I2 = 0%, high certainty] or FAS [2 RCTs, OR = 1.36 (1.04, 1.78), p = 0.03, I2 = 0%, high certainty], and ACR70 at 1 year from PPS [3 RCTs, OR = 1.32 (1.01, 1.71), p = 0.04, I2 = 0%, high certainty]. In terms of other outcomes about efficacy, safety, and immunogenicity, the results showed that there was no significant difference between etanercept biosimilars and reference biologics, and the certainty of evidences ranged from low to moderate.
Conclusion: Etanercept biosimilars showed more benefits in ACR50 response rate at 1 year than reference biologics (Enbrel®), other outcomes for clinical efficacy, safety, and immunogenicity of etanercept biosimilars were comparable with originator in patients with rheumatoid arthritis.
Systematic Review Registration: PROSPERO, identifier CRD42022358709
Rheumatoid arthritis (RA) is a type of autoimmune inflammatory arthritis with joint pain, stiffness, swelling, as well as systemic manifestations (Smolen et al., 2016). According to relevant epidemiological data, RA showed a global prevalence of 0.22% (Abbafati et al., 2020). RA not only can lead to progressive joint damages, but also may lead to the destruction of cartilage and bone, reducing patients’ quality of life and even disability, if treatment is delayed or not controlled properly (Laugisch et al., 2016; Cush, 2021).
General management measures for RA include disease-modifying anti-rheumatic drugs (DMARDs), anti-inflammatory therapy with non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids or biologicals (Allen et al., 2018; Smolen et al., 2020). However, many of these drugs become less effective and exhibit increased toxicity over time (Kerschbaumer et al., 2020). Etanercept as a biological disease-modifying anti-rheumatic drug (bDMARD) has been shown to have salutary treatment of moderate-to-severe rheumatoid arthritis (RA) psoriatic arthritis, axial spondyloarthritis and psoriasis in adults, and juvenile idiopathic arthritis in pediatric patients (Goldenberg, 1999; Genovese et al., 2002). In addition, it has been reported that etanercept leads to less serious adverse reactions when compared with the traditional or conventional DMARDs (Emery et al., 2008).
Like other biologicals, the higher prices of etanercept increase the financial burdens, biosimilars can bring cost savings for patients and emphasizes the necessity of patients access to therapies (Abraham et al., 2014; Simoens, 2021). Meanwhile, biosimilars for patients with RA are needed, particularly in countries with high levels of co-pay such as Central and Eastern European countries, due to concerns with their funding and utilization (Putrik et al., 2014; Yoo 2014; Baumgart et al., 2019). We have seen considerable reductions in the prices of biosimilars to help with their usage (Jensen et al., 2020; Moorkens et al., 2020), lower prices can also help increase the number of potential patients eligible for treatment within universal healthcare systems (Dutta et al., 2020). Despite this still see low use of biosimilars for anti-TNFs in some countries especially where limited price differences between the biosimilars and originators and limited demand-side measures encouraging the preferential prescribing of biosimilars (Kim et al., 2020; Tubic et al., 2021). This low uptake is exacerbated by concerns regarding immunogenicity leading to a heightened “Nocebo” effect and doubts about the efficacy and safety of biosimilars in practice (Colloca et al., 2019). The Norwegian Government-sponsored NOR SWITCH study on Infliximab was a good step in addressing the effectiveness and safety concerns associated with biosimilars, thus helping to enhance their use (Jørgensen et al., 2017), however, it is good to build upon this with studies and reviews like this one! Therefore, clarifying the differences in efficacy, safety, and immunogenicity of etanercept biosimilars versus reference biologics (Enbrel®) for RA by meta-analysis is necessary.
This meta-analysis was mainly carried out and reported according to the guidelines of the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA 2020) statement (Page et al., 2021). We register this meta-analysis on the International Prospective Register of Systematic Review (PROSPERO, CRD42022358709).
With a combination of keywords and MeSH (Medical Subject Headings) terms related to rheumatoid arthritis, etanercept, and biosimilars, we searched PubMed, Embase (via ovid), Central (via ovid) comprehensively from inception to 15 August 2022 (Supplementary Table S2). We also searched ClinicalTrials.gov from database creation to August 15th for trials without published articles. In addition, reference lists of included literatures were screened to identify potential eligible studies.
The inclusion criteria were as follows: 1) Participants: adult patients diagnosed with rheumatoid arthritis who had an inadequate clinical response to methotrexate (MTX). 2) Interventions: an etanercept biosimilar with background MTX and folic acid. 3) Comparisons: an etanercept reference biologic (Enbrel®). 4) Outcomes: efficacy endpoints included proportion of patients achieving at least 20%, 50%, or 70% improvement in the American College of Rheumatology (ACR) response criteria (ACR20, ACR50, or ACR70) from per-protocol set (PPS) or full analysis set (FAS) at different time points; safety assessments included monitoring and recording of any adverse events, serious adverse events, withdrawal due to adverse events, and all-cause mortality; immunogenicity outcome was assessed using a bridging assay with anti-Hu-IgG detection of bound antidrug antibodies (ADAs). 5) Study design: randomized controlled trials (RCTs) in English language with follow up of at least 24 weeks.
Major exclusion criteria included 1) patients were previously treated with any other biologicals therapy for RA except etanercept within 3 months; 2) patients diagnosed with any active inflammatory or immune diseases except RA, congestive heart failure, active tuberculosis, or pregnancy.
After an initial screening of titles and abstracts, full-text articles were identified and reviewed in detail. As a quality check, two independent researchers (RH and SZ) screened separately titles and abstracts and assessed full-text articles, with a third researcher (TY) resolving any disagreements, if needed. For each selected article, the two researchers (RH and SZ) extracted the following data in an Excel spreadsheet: study characteristics (country, design, study period, and setting) and study population (case definition, eligible criteria, sample size, age, and sex).
In this study, we assessed risk of bias (RH and SZ) using the revised Cochrane Risk of Bias in Randomised Trials tool (RoB 2) (Sterne et al., 2019), which consists of five dimensions, bias arising due to: 1) the randomization process (D1); 2) deviations from intended interventions (D2); 3) missing outcome data (D3); 4) measurement of the outcome (D4); and 5) selection of the reported result (D5). After the first assessment, the tables were compared and disagreements were discussed.
The Grading of Recommendation Assessment, Development, and Evaluation (GRADE) framework was used to assess the certainty of the evidence according to risk of bias, inconsistency, imprecision, publication bias and indirectness (Guyatt et al., 2008; Zeng et al., 2021). Two authors assessed the certainty of evidence independently and rated to each comparison an overall qualitative judgment based on four levels of quality of evidence: high, moderate, low, very low; any disagreements were solved by discussions.
We performed two-stage meta-analysis pooling the Odds Ratio (OR) with their 95% Confidence Intervals (CIs) from extracted data to evaluate efficacy at different time points, safety, and immunogenicity of etanercept biosimilars versus etanercept reference biologics. Inconsistency test (I2) was used to assess heterogeneity among included studies. The meta-analysis was calculated using the random effect model when I2 was greater than 50%, which indicates a high probability of heterogeneity; as an alternative, the fixed-effects-based meta-analysis was conducted; Mantel-Haenszel method was used in our meta-analysis (Greenland and Robins, 1985; Robins et al., 1986; Borenstein et al., 2010; Bakbergenuly et al., 2020). We considered p values below 0.05 to be statistically significant.
We planned to address publication bias visually with funnel plots and statistically with the Egger regression when 10 or more studies were available. When fewer than 10 and at least five studies were included, we planned to use funnel plots and trim-and-fill analyses only (Duval and Tweedie, 2000).
We planned sensitivity analyses of studies with low risk of bias.
Our meta-analysis was conducted using the meta and metafor package in R software, version 4.2.1 (R foundation) (Viechtbauer, 2010; Polanin et al., 2017; Balduzzi et al., 2019).
A total of 366 records were identified from the databases, of which 206 unique records were screened after removing duplicate records. Out of these, the team assessed 33 full manuscripts for eligibility; 7 records from six RCTs with 2432 patients were included in our meta-analysis (Figure 1) (Bae et al., 2016; O'Dell et al., 2016; Emery et al., 2017a; Emery et al., 2017b; Matsuno et al., 2018; Matucci-Cerinic et al., 2018; Strusberg et al., 2021).
The baseline characteristics of the included studies were summarized in Table 1. The included trials registered were two-arm, conducted in various countries or regions (Korea, Europe, United States, Mexico, Argentina, Japan, South Africa), all of which were published in English. The mean age of participants ranged from 46 to 55 years old, the proportion of females ranged from 79.4% to 88.8%, the disease duration of patients ranged from 6 to 10 years, and the length of follow up of ranged from 24 to 52 weeks.
The assessment for risk of bias about included RCTs are presented in Figure 2. Specifically, NCT03332719 was at some concern for risk of bias because of evaluator-blinded and early termination; three trials (NCT02638259, NCT02357069, and NCT02115750) were at some concern for risk of bias because of early termination; other two trials were evaluated at low risk of bias in all domain.
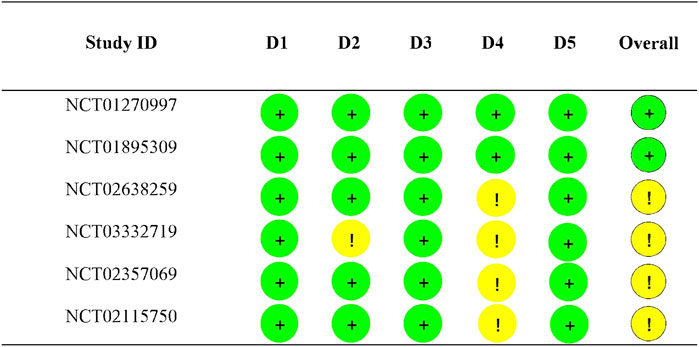
FIGURE 2. Risk of bias assessment results. D1: Randomization process; D2: Deviations from the intended interventions; D3: Missing outcome data; D4: Measurement of the outcome; D5: Selection of the reported results; + represents low risk; ! represents some concerns.
ACR20, ACR50, or ACR70 from PPS at different time points: the pooled results showed no significant difference in ACR20 at 24 weeks [5 RCTs, OR = 0.92 (0.54, 1.58), p = 0.79, I2 = 73%, low certainty] or 1 year [3 RCTs, OR = 1.08 (0.76, 1.55), p = 0.66, I2 = 0%, moderate certainty], and ACR70 at 24 weeks [5 RCTs, OR = 1.06 (0.87, 1.28), p = 0.58, I2 = 45%, moderate certainty] between biosimilars and reference biologics (Enbrel®); biosimilars showed more benefits in ACR50 at 24 weeks [5 RCTs, OR = 1.22 (1.01, 1.47), p = 0.04, I2 = 49%, high certainty] or 1 year [3 RCTs, OR = 1.43 (1.10, 1.86), p < 0.01, I2 = 0%, high certainty], and ACR70 at 1 year [3 RCTs, OR = 1.32 (1.01, 1.71), p = 0.04, I2 = 0%, high certainty] than reference biologics.
ACR20, ACR50, or ACR70 from FAS at different time points: the pooled results showed no significant difference in ACR20 at 24 weeks [2 RCTs, OR = 1.14 (0.84, 1.55), p = 0.39, I2 = 0%, moderate certainty] or 32 weeks [2 RCTs, OR = 1.02 (0.72, 1.45), p = 0.92, I2 = 0%, moderate certainty] or 1 year [2 RCTs, OR = 1.22 (0.91, 1.65), p = 0.19, I2 = 0%, moderate certainty], ACR50 at 24 weeks [2RCTs, OR = 1.31 (1.00, 1.71), p = 0.053, I2 = 21%, moderate certainty], and ACR70 at 24 weeks [2 RCTs, OR = 1.14 (0.83, 1.56), p = 0.42, I2 = 0%, moderate certainty] or 1 year [2 RCTs, OR = 1.25 (0.93, 1.68), p = 0.13, I2 = 0%, moderate certainty] between biosimilars and reference biologics; biosimilars showed more benefits in ACR50 at 1 year [2 RCTs, OR = 1.36 (1.04, 1.78), p = 0.03, I2 = 0%, high certainty] than reference biologics.
The forest plots of efficacy assessment are presented in Figure 3.
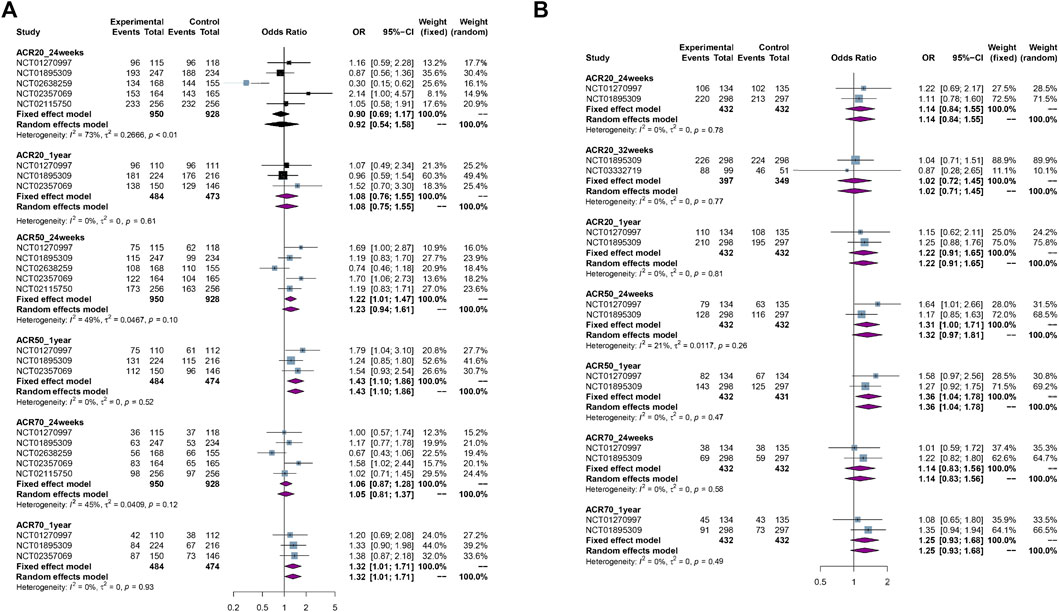
FIGURE 3. The forest plots of efficacy assessment. (A) ACR20, ACR50, ACR70 response rate from per-protocol set (PPS) at different time points. (B) ACR20, ACR50, ACR70 response rate from per-protocol set (PPS) at different time points.
The results of meta-analysis showed there was no significant difference in incidence of any adverse events [4 RCTs, OR = 0.94 (0.76, 1.18), p = 0.61, I2 = 0%, moderate certainty], incidence of serious adverse events [5 RCTs, OR = 1.17 (0.82, 1.68), p = 0.39, I2 = 11%, moderate certainty], incidence of withdrawal due to adverse events [4 RCTs, OR = 0.75 (0.49, 1.15), p = 0.19, I2 = 0%, moderate certainty], and all-cause mortality [4 RCTs, OR = 1.18 (0.38, 3.70), p = 0.77, I2 = 13%, low certainty] between biosimilars and reference biologics. The forest plots of safety assessment are presented in Figure 4.
The results of meta-analysis showed there was no significant difference in immunogenicity between biosimilars and reference biologics [5 RCTs, OR = 0.26 (0.06, 1.09), p = 0.07, I2 = 84%, low certainty]. The forest plot of immunogenicity assessment are presented in Figure 5.
The results of sensitivity analyses are presented in supplementary materials (Supplementary Section S3), and the robustness of our meta-analysis was confirmed by all sensitivity analyses.
Due to the limited number of included studies, we did not assess the publication bias by Egger regression. Funnel plots were performed to qualitatively evaluate the potential publication bias for outcomes involving at least five studies and trim-and-fill analyses were conducted to quantitatively assess the robustness. The assessment of publication bias is presented in supplementary materials (Supplementary Section S4). The results confirmed the robustness of primary findings.
Our meta-analysis provides a summary of evidence regarding the efficacy, safety, and immunogenicity between an etanercept biosimilar and a reference biologic (Enbrel®) in the treatment of patients with RA. Our study suggests etanercept biosimilars are beneficial for RA patients with an inadequate response to MTX, with highly strong evidences showing ACR50 improvements at 24 weeks from PPS and at 1 year from both PPS and FAS, as well as ACR70 improvement at 1 year from PPS; and moderate and low evidence presented in the supplementary materials. In combination with the results of the evaluation of efficacy from PPS or FAS above, we hold the opinion that etanercept biosimilars may show more benefits in ACR50 at 1 year than reference biologics, and are similar to the reference biologic in terms of other outcomes including efficacy, safety and immunogenicity.
Although all six of the RCTs included had etanercept as a reference biologic in their control groups, biosimilars in each study was different, which may result in heterogeneity among the included studies. However, the results of sensitivity analyses and assessments of publication bias confirmed the robustness of our main results, so the heterogeneity in our meta-analysis was acceptable to us.
Before our study, there was a network meta-analysis which only included three etanercept biosimilars from three RCTs, indicated that no significant differences was found between etanercept biosimilars and etanercept originators in patients with RA despite treatment with MTX in terms of ACR20 response rate and incidence of serious adverse events, which was consistent with our findings (Lee and Song, 2021). Furthermore, some systematic literature reviews or network meta-analyses evaluated the efficacy and safety of tumor necrosis factor (TNF) inhibitors biosimilars versus TNF inhibitors originators, demonstrated that these biosimilars had an overall comparable efficacy and safety profile compared with their originators in RA patients, which was also similar to what we found (Komaki et al., 2017; Moots et al., 2018; Graudal et al., 2019; Ho Lee and Gyu Song, 2021). Nevertheless, theses previous related studies have some weakness, such as limited outcome indicators, failure to consider different time points and a lack of evidence assessment.
To the best of our knowledge, our meta-analysis is the first meta-analysis with the focus on efficacy, safety, and immunogenicity including as many outcome indicators in different time points as possible for RA patients. In addition, we used sensitivity analyses to confirm the robustness of our main results and trim-and-fill analyses to address the publication bias. Our meta-analysis is not only an update of the data of relevant meta-analysis or network meta-analysis before, but also provides an overview of the evidence for comparisons between etanercept biosimilars and reference biologics.
There are several limitations to our study. First, we only included phase III RCTs which had strict eligible criteria and may not truly reflect real-world conditions due to the differences between large randomized controlled trials and real-world practice (Zhou et al., 2021). At present there are some studies evaluated etanercept biosimilars versus etanercept originators in RA patients based on real-world data. And all these studies demonstrated the biosimilars were as effective, safe, and acceptable as the original (Codreanu et al., 2019; Atzeni et al., 2021; Gharibdoost et al., 2021; Rojas-Giménez et al., 2021; Selmi et al., 2021; Pinto et al., 2022). The conclusions of other real-world studies are similar to ours; we considered the efficiency outcomes at different time points and found that biosimilars had relative benefits in some efficacy outcomes at those times. Therefore, our study provides a reference for clinical practice and decision making, and offers preliminary evidence to support future observational studies. We would not discuss any superiority of the biosimilar compared to the originator; rather, we would just state that they are as good. This may be difficult for key personnel to appreciate, especially if they initially harbored doubts about biosimilars. Differences in efficacy may be attributed to the fact that originator manufacturers often change their manufacturing processes, which could have an effect on effectiveness. However, it is difficult to definitively state this since originator manufacturers do not need to conduct new studies following changes in manufacturing processes (Vezér et al., 2016; Jiménez-Pichardo et al., 2017; Godman et al., 2020). Now the published studies address the lack of problems for patients switching between biosimilars, which is the next big hurdle to address in order to further accelerate biosimilar use (Allocati et al., 2022).
The conclusions of these real-world studies are somewhat similar to ours, but we considered the efficiency outcomes at different time points and we found biosimilars showed relative benefits in some efficacy outcomes at different times. Thus, our study still provides reference for clinical practice and decision making, and preliminary evidence to support future observational studies. Second, as RA is a chronic disease, it would be preferable to conduct time-to-event outcome analysis, but relevant data are not available, so we can only evaluate outcomes at different time points by two-stage meta-analysis. Third, we did not conduct Egger regression due to the limited number of included studies, the risk of publication bias could not be excluded by funnel plots, but the trim-and-fill analysis confirmed the robustness of our results.
Etanercept biosimilars showed more benefits in ACR50 response rate at 1 year than reference biologics, other outcomes for clinical efficacy, safety, and immunogenicity of etanercept biosimilars were comparable with originator in RA patients with background MTX and folic acid. The main results of our study further support the utilization of etanercept biosimilars in clinical practice and decision making. And more future studies based on real-world data are needed to validate the findings of our study.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
RH, TY, and SZ were in charge of study design, RH and SZ completed data collection and interpretation, quality assessment of evidence, RH and TY wrote this manuscript together, SZ and NS critically reviewed the manuscript and provided revisions, SZ was the primary monitor of this study, HW, QL, LS, CZ, and JZ mainly checked the final data of the study. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
This study sponsored by Karamay Central Hospital and Bethune Charitable Foundation (grant number YSP-2014). SZ was supported under grants from Xinjiang Natural Science Foundation (grant number 2022D01B194).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1089272/full#supplementary-material.
Abbafati, C., Abbas, K. M., Abbasi, M., Abbasifard, M., Abbasi-Kangevari, M., Abbastabar, H., et al. (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet 396, 1204–1222. doi:10.1016/S0140-6736(20)30925-9
Abraham, I., Han, L., Sun, D., MacDonald, K., and Aapro, M. (2014). Cost savings from anemia management with biosimilar epoetin alfa and increased access to targeted antineoplastic treatment: A simulation for the EU G5 countries. Future Oncol. Lond. Engl. 10, 1599–1609. doi:10.2217/fon.14.43
Allen, A., Carville, S., and McKenna, F.Guideline Development Group (2018). Diagnosis and management of rheumatoid arthritis in adults: Summary of updated NICE guidance. BMJ Clin. Res. ed.) 362, k3015. doi:10.1136/bmj.k3015
Allocati, E., Godman, B., Gobbi, M., Garattini, S., and Banzi, R. (2022). Switching among biosimilars: A review of clinical evidence. Front. Pharmacol. 13, 917814. doi:10.3389/fphar.2022.917814
Atzeni, F., Gerratana, E., Bongiovanni, S., Talotta, R., Miceli, G., Salaffi, F., et al. (2021). Efficacy and safety of biosimilar and originator etanercept in rheumatoid arthritis patients: Real-life data. Isr. Med. Assoc. J. 23, 344–349.
Bae, S. C., Kim, J., Choe, J. Y., Park, W., Lee, S. H., Park, Y. B., et al. (2016). A phase III, multicentre, randomised, double-blind, active-controlled, parallel-group trial comparing safety and efficacy of HD203, with innovator etanercept, in combination with methotrexate, in patients with rheumatoid arthritis: The HERA study. Ann. Rheum. Dis. 76, 65–71. doi:10.1136/annrheumdis-2015-207613
Bakbergenuly, I., Hoaglin, D. C., and Kulinskaya, E. (2020). Methods for estimating between-study variance and overall effect in meta-analysis of odds ratios. Res. Synth. Methods 11, 426–442. doi:10.1002/jrsm.1404
Balduzzi, S., Ruecker, G., and Schwarzer, G. (2019). How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 22, 153–160. doi:10.1136/ebmental-2019-300117
Baumgart, D. C., Misery, L., Naeyaert, S., and Taylor, P. C. J. F. i. P. (2019). Biological therapies in immune-mediated inflammatory diseases: Can biosimilars reduce access inequities? Front. Pharmacol. 10, 279. doi:10.3389/fphar.2019.00279
Borenstein, M., Hedges, L. V., Higgins, J. P., and Rothstein, H. R. (2010). A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 1, 97–111. doi:10.1002/jrsm.12
Codreanu, C., Popescu, C. C., Mogoșan, C., Enache, L., Daia, S., Ionescu, R., et al. (2019). Efficacy and safety of original and biosimilar etanercept (SB4) in active rheumatoid arthritis - a comparison in a real-world national cohort. Biologicals 62, 27–32. doi:10.1016/j.biologicals.2019.10.009
Colloca, L., Panaccione, R., and Murphy, T. K. J. F. i. P. (2019). The clinical implications of nocebo effects for biosimilar therapy. Front. Pharmacol. 10, 1372. doi:10.3389/fphar.2019.01372
Cush, J. J. (2021). Rheumatoid arthritis: Early diagnosis and treatment. Med. Clin. North Am. 105, 355–365. doi:10.1016/j.mcna.2020.10.006
Dutta, B., Huys, I., Vulto, A. G., and Simoens, S. (2020). Identifying key benefits in European off-patent biologics and biosimilar markets: It is not only about price. Bio Drugs 34, 159–170. doi:10.1007/s40259-019-00395-w
Duval, S., and Tweedie, R. (2000). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463. doi:10.1111/j.0006-341x.2000.00455.x
Emery, P., Breedveld, F. C., Hall, S., Durez, P., Chang, D. J., Robertson, D., et al. (2008). Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): A randomised, double-blind, parallel treatment trial. Lancet 372, 375–382. doi:10.1016/S0140-6736(08)61000-4
Emery, P., Vencovský, J., Sylwestrzak, A., Leszczy&nacuteski, P., Porawska, W., Baranauskaite, A., et al. (2017a). A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann. Rheum. Dis. 76, 51–57. doi:10.1136/annrheumdis-2015-207588
Emery, P., Vencovský, J., Sylwestrzak, A., Leszczynski, P., Porawska, W., Baranauskaite, A., et al. (2017b). 52-week results of the phase 3 randomized study comparing SB4 with reference etanercept in patients with active rheumatoid arthritis. Rheumatol. Oxf. Engl. 56, 2093–2101. doi:10.1093/rheumatology/kex269
Genovese, M. C., Bathon, J. M., Martin, R. W., Fleischmann, R. M., Tesser, J. R., Schiff, M. H., et al. (2002). Etanercept versus methotrexate in patients with early rheumatoid arthritis: Two-year radiographic and clinical outcomes. Arthritis Rheum. 46, 1443–1450. doi:10.1002/art.10308
Gharibdoost, F., Salari, A. H., Salesi, M., Ebrahimi Chaharom, F., Mottaghi, P., Hosseini, M., et al. (2021). Assessment of treatment safety and quality of life in patients receiving etanercept biosimilar for autoimmune arthritis (asqa): A multicenter post-marketing surveillance study. Adv. Ther. 38, 1290–1300. doi:10.1007/s12325-020-01611-8
Godman, B., Allocati, E., Moorkens, E., Kwon, H. Y. J. G., and Journal, B. I. (2020). Can local policies on biosimilars optimize the use of freed resources – experiences from Italy. Generics Biosimilars Initiative J. 9, 183–187. doi:10.5639/gabij.2020.0904.029
Goldenberg, M. M. (1999). Etanercept, a novel drug for the treatment of patients with severe, active rheumatoid arthritis. Clin. Ther. 21, 75–87. doi:10.1016/S0149-2918(00)88269-7
Graudal, N., Kaas-Hansen, B. S., Guski, L., Hubeck-Graudal, T., Welton, N. J., and Jürgens, G. (2019). Different original and biosimilar TNF inhibitors similarly reduce joint destruction in rheumatoid arthritis-A network meta-analysis of 36 randomized controlled trials. Int. J. Mol. Sci. 20, 4350. doi:10.3390/ijms20184350
Greenland, S., and Robins, J. M. (1985). Estimation of a common effect parameter from sparse follow-up data. Biometrics 41, 55–68. doi:10.2307/2530643
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). Grade: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ Clin. Res. ed.) 336, 924–926. doi:10.1136/bmj.39489.470347.AD
Ho Lee, Y., and Gyu Song, G. (2021). Comparative efficacy and safety of tumor necrosis factor inhibitors and their biosimilars in patients with rheumatoid arthritis having an insufficient response to methotrexate. Z Rheumatol. doi:10.1007/s00393-021-01041-z
Jensen, T. B., Kim, S. C., Jimenez-Solem, E., Bartels, D., Christensen, H. R., and Andersen, J. T. (2020). Shift from adalimumab originator to biosimilars in Denmark. JAMA Intern. Med. 180, 902–903. doi:10.1001/jamainternmed.2020.0338
Jiménez-Pichardo, L., Gázquez-Pérez, R., and Sierra-Sánchez, J. F. (2017). Degree of prescriber's knowledge about variability in biological drugs "innovators" in manufacturing process. Eur. J. Clin. Pharmacol. 74, 505–511. doi:10.1007/s00228-017-2397-x
Jørgensen, K. K., Olsen, I. C., Goll, G. L., Lorentzen, M., Bolstad, N., Haavardsholm, E. A., et al. (2017). Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): A 52-week, randomised, double-blind, non-inferiority trial. Lancet 389, 2304–2316. doi:10.1016/S0140-6736(17)30068-5
Kerschbaumer, A., Sepriano, A., Smolen, J. S., van der Heijde, D., Dougados, M., van Vollenhoven, R., et al. (2020). Efficacy of pharmacological treatment in rheumatoid arthritis: A systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 79, 744–759. doi:10.1136/annrheumdis-2019-216656
Kim, Y., Kwon, H. Y., Godman, B., Moorkens, E., Simoens, S., and Bae, S. (2020). Uptake of biosimilar infliximab in the UK, France, Japan, and korea: Budget savings or market expansion across countries? Front. Pharmacol. 11, 970. doi:10.3389/fphar.2020.00970
Komaki, Y., Yamada, A., Komaki, F., Kudaravalli, P., Micic, D., Ido, A., et al. (2017). Efficacy, safety and pharmacokinetics of biosimilars of anti-tumor necrosis factor-α agents in rheumatic diseases; A systematic review and meta-analysis. J. Autoimmun. 79, 4–16. doi:10.1016/j.jaut.2017.02.003
Laugisch, O., Wong, A., Sroka, A., Kantyka, T., Koziel, J., Neuhaus, K., et al. (2016). Citrullination in the periodontium--a possible link between periodontitis and rheumatoid arthritis. Clin. Oral Investig. 20, 675–683. doi:10.1007/s00784-015-1556-7
Lee, Y. H., and Song, G. G. (2021). Comparative efficacy and safety of etanercept biosimilars in comparison with etanercept in patients with rheumatoid arthritis who have insufficient response to methotrexate: A network meta-analysis. Int. J. Clin. Pharmacol. Ther. 59, 760–767. doi:10.5414/CP204049
Matsuno, H., Tomomitsu, M., Hagino, A., Shin, S., Lee, J., and Song, Y. W. (2018). Phase III, multicentre, double-blind, randomised, parallel-group study to evaluate the similarities between LBEC0101 and etanercept reference product in terms of efficacy and safety in patients with active rheumatoid arthritis inadequately responding to methotrexate. Ann. Rheum. Dis. 77, 488–494. doi:10.1136/annrheumdis-2017-212172
Matucci-Cerinic, M., Allanore, Y., Kavanaugh, A., Buch, M. H., Schulze-Koops, H., Kucharz, E. J., et al. (2018). Efficacy, safety and immunogenicity of GP2015, an etanercept biosimilar, compared with the reference etanercept in patients with moderate-to-severe rheumatoid arthritis: 24-week results from the comparative phase III, randomised, double-blind EQUIRA study. RMD Open 4, e000757. doi:10.1136/rmdopen-2018-000757
Moorkens, E., Godman, B., Huys, I., Hoxha, I., Malaj, A., Keuerleber, S., et al. (2020). The expiry of Humira(®) market exclusivity and the entry of adalimumab biosimilars in Europe: An overview of pricing and national policy measures. Front. Pharmacol. 11, 591134. doi:10.3389/fphar.2020.591134
Moots, R. J., Curiale, C., Petersel, D., Rolland, C., Jones, H., and Mysler, E. (2018). Efficacy and safety outcomes for originator TNF inhibitors and biosimilars in rheumatoid arthritis and psoriasis trials: A systematic literature review. BioDrugs 32, 193–199. doi:10.1007/s40259-018-0283-4
O'Dell, J., Takeuchi, T., Tanaka, Y., Louw, I., Tiabut, T., Kai, M., et al. (2016). OP0226 Randomized, double-blind study comparing chs-0214 with etanercept in patients with active rheumatoid arthritis (RA) despite methotrexate (MTX) therapy. Ann. Rheum. Dis. 75, 143.1–143. doi:10.1136/annrheumdis-2016-eular.1800
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ Clin. Res. ed.) 372, n71. doi:10.1136/bmj.n71
Pinto, A. S., Cunha, M. M., Pinheiro, F., Bernardes, M., Assunção, H., Martins-Martinho, J., et al. (2022). Effectiveness and safety of original and biosimilar etanercept (Enbrel® vs Benepali®) in bDMARD-naïve patients in a real-world cohort of Portugal. ARP Rheumatol. 1, 109–116.
Polanin, J. R., Hennessy, E. A., and Tanner-Smith, E. E. (2017). A review of meta-analysis packages in R. J. Educ. Behav. Statistics 42, 206–242. doi:10.3102/1076998616674315
Putrik, P., Ramiro, S., Kvien, T. K., Sokka, T., Pavlova, M., Uhlig, T., et al. (2014). Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann. Rheum. Dis. 73, 198–206. doi:10.1136/annrheumdis-2012-202603
Robins, J., Breslow, N., and Greenland, S. (1986). Estimators of the Mantel-Haenszel variance consistent in both sparse data and large-strata limiting models. Biometrics 42, 311–323. doi:10.2307/2531052
Rojas-Giménez, M., Mena-Vázquez, N., Romero-Barco, C. M., Manrique-Arija, S., Ureña-Garnica, I., Diaz-Cordovés, G., et al. (2021). Effectiveness, safety and economic analysis of Benepali in clinical practice. Reumatol. Clin. 17, 588–594. doi:10.1016/j.reumae.2020.06.010
Selmi, C., Krüger, K., Cantagrel, A., Abad Hernández, M. A., Freudensprung, U., Farouk Rezk, M., et al. (2021). Benefit: Real-world effectiveness of SB4 after transition from reference etanercept in rheumatoid arthritis and axial spondyloarthritis. Clin. Exp. Rheumatol. 39, 365–371. doi:10.55563/clinexprheumatol/usrd9z
Simoens, S. (2021). How do biosimilars sustain value, affordability, and access to oncology care. Expert Rev. Pharmacoecon Outcomes Res. 21, 327–329. doi:10.1080/14737167.2020.1813570
Smolen, J. S., Aletaha, D., and McInnes, I. B. (2016). Rheumatoid arthritis. Lancet 388, 2023–2038. doi:10.1016/S0140-6736(16)30173-8
Smolen, J. S., Landewé, R., Bijlsma, J., Burmester, G. R., Dougados, M., Kerschbaumer, A., et al. (2020). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 79, 685–699. doi:10.1136/annrheumdis-2019-216655
Sterne, J., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ Clin. Res. ed.) 366, l4898. doi:10.1136/bmj.l4898
Strusberg, I., Mysler, E., Citera, G., Siri, D., de Los Ángeles Correa, M., Lazaro, M. A., et al. (2021). Efficacy, safety, and immunogenicity of biosimilar etanercept (enerceptan) versus its original form in combination with methotrexate in patients with rheumatoid arthritis: A randomized, multicenter, evaluator-blinded, noninferiority study. J. Clin. Rheumatol. 27, S173–S179. doi:10.1097/RHU.0000000000001616
Tubic, B., Markovi-Pekovi, V., Jungi, S., Allocati, E., and Godman, B. J. (2021). Availability and accessibility of monoclonal antibodies in Bosnia and Herzegovina: Findings and implications. Med. Access Point Care 5, 23992026211027692. doi:10.1177/23992026211027692
Vezér, B., Buzás, Z., Sebeszta, M., and Zrubka, Z. (2016). Authorized manufacturing changes for therapeutic monoclonal antibodies (mAbs) in European Public Assessment Report (EPAR) documents. Curr. Med. Res. Opin. 32, 829–834. doi:10.1185/03007995.2016.1145579
Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. doi:10.18637/jss.v036.i03
Yoo, D. H. (2014). The rise of biosimilars: Potential benefits and drawbacks in rheumatoid arthritis. Expert Rev Clin Immunol 10, 981–983. doi:10.1586/1744666X.2014.932690
Zeng, L., Brignardello-Petersen, R., Hultcrantz, M., Siemieniuk, R., Santesso, N., Traversy, G., et al. (2021). GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J. Clin. Epidemiol. 137, 163–175. doi:10.1016/j.jclinepi.2021.03.026
Keywords: rheumatoid arthritis, etanercept, biosimilars, meta-analysis, reference biologic
Citation: Hu R, Yuan T, Wang H, Zhao J, Shi L, Li Q, Zhu C, Su N and Zhang S (2023) Efficacy, safety and immunogenicity of etanercept biosimilars versus reference biologics in patients with rheumatoid arthritis: A meta-analysis. Front. Pharmacol. 14:1089272. doi: 10.3389/fphar.2023.1089272
Received: 04 November 2022; Accepted: 20 January 2023;
Published: 16 February 2023.
Edited by:
Jean-Marie Boeynaems, Université libre de Bruxelles, BelgiumReviewed by:
Vanda Marković-Peković, University of Banja Luka, Bosnia and HerzegovinaCopyright © 2023 Hu, Yuan, Wang, Zhao, Shi, Li, Zhu, Su and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Su, em95YTE1OUAxNjMuY29t; Shengzhao Zhang, enN6OTA4NzdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.