- 1Department of Pharmacy, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad, Pakistan
- 2Riphah Institute of Pharmaceutical Sciences, Riphah International University, Islamabad, Pakistan
Multidrug resistance and infectious disease have enormous spread despite drug discovery and development advancements. 1, 2, 4 -triazoles have been extensively studied, playing an imperative role in many pathologic conditions. A series of Schiff base triazoles; derived from Indole -3- acetic acid with substituted Benzaldehydes (5a-5g) were designed, synthesized, and evaluated through various Spectroanalytical techniques. SwissADME was used to assess physicochemical properties and pharmacokinetic drug-likeliness behavior. (5a-5g) were evaluated for their varied biological potential through antioxidant, antimicrobial, enzyme inhibition, and cytotoxic evaluation. Schiff bases express drug-like nature as they follow Lipinski’s rule of five. 5b showed good antioxidant potential in total antioxidant capacity (TAC) and total reducing power (TRP) assays and was most active in the library in % free radical scavenging assay (%FRSA), showing 32% inhibition at 50 μg/mL concentration. Compounds showed antibacterial activity against various tested strains. 5e and 5f showed a minimum inhibitory concentration (MIC) value of 3.12 μg/mL for P.aeruginosa and K.pneumoniae, respectively. In the antifungal assay, only 5e inhibited one strain with a zone of inhibition >6 mm. These synthetic molecules possess good cytotoxic potential in the Brine Shrimp Lethality screening; 5c, 5d, and 5f exhibited LC50 =5.7 μg/mL. In the protein kinase inhibition assay, 5a, 5b, and 5g demonstrated inhibitory potential, showcasing the zone of inhibition as 7.5–10.5 mm for the bald one and 6–7.5 for the clear zone. These findings suggest that the compounds have antibacterial and cytotoxic potential, and there is a chance for further research and development in this area.
1 Introduction
Developing new strategies and advancements in combinatorial chemistry has led to substantial progress in the control and treatment of microbial infections. However, microbial resistance is still a significant concern for scientific communities and threatens public health (Wang et al., 2022). Resistant pathogens are a potential threat to increased morbidity and mortality. With increased multidrug resistance, this scenario has led to an increased urge to synthesize new drugs with improved safety profiles. To combat multidrug resistance, the dire need is to develop new classes of anti-microbial agents with unique mechanisms of action (Idhayadhulla et al., 2014). Medicinal chemists are dealing with different approaches to counter this issue, including preying on new targets, structural adaptations, and joining different pharmacophores to create hybrid molecules, the most effective strategy being linkage through covalent bonds (Alkhzem et al., 2022). An imbalance between oxidants and antioxidants in our body is called “Oxidative stress,” which, in some cases, may affect essential tissue proteins and genomic matter (Lobo et al., 2010). Reactive Oxygen or Nitrogen species may be formed in the body due to certain pathophysiological conditions, causing damage to cell structures and leading to unwanted cell growth and division (Mistry et al., 2017). As a result, free radicals are produced, which can act as oxidants; antioxidants play their role, douse these oxidants, and show their significance in opposing carcinogenesis (Kattappagari et al., 2015).
Heterocyclic compounds constitute a significant part of organic chemistry. A primary concern of modern scientists is the development of new classes of heterocycles keeping environmental and financial issues in view. Heterocycles containing three hetero atoms at symmetrical positions are more widely studied because they show various pharmacological activities (Deodware et al., 2021). Nitrogen-containing heterocycles are gaining much attention as part of many approved drugs; triazoles have shown various therapeutic activities. Triazole is a 5-membered ring structure that contains three nitrogen and two Carbon atoms, and may exist as two isomers; 1, 2, 4-triazole and 12, 3-triazole. Out of these1, 2, 4-triazoles are widely investigated and reported because of their significant binding potential and stability (Strzelecka and Swiatek, 2021). 1, 2, 4-triazole containing heterocycles and those which possess 1, 2, 4-triazoles as condensation products with another nucleus system form a very diverse class of compounds, which possess antibacterial, antifungal, anti-inflammatory, and antitumor activities. Moreover, they have been reported as CNS depressants, antiproliferative, anti-HIV, anti-tubercular, analgesic, antioxidant, and anti-inflammatory (Matin et al., 2022). Several other drugs have been reported consisting of 1, 2, 4 Triazole nucleus; these include anti-psychotic, anti-migraine, sedative and hypnotic, anti-depressant, antiviral, analgesic, and aromatase inhibitors (Aggarwal and Sumran, 2020).Schiff bases are documented with an inclusive range of chemotherapeutic doings; azomethine on Schiff bases attributes to their extended chemical and biological properties Moreover, the ability to form intermolecular hydrogen bonds and transferable protons play their part in extended bioactivity (Said et al., 2021).Schiff bases are derived from aliphatic and aromatic aldehydes, but later are more stable due to conjugation, and the former are liable to polymerization (Hasan et al., 2015).Heterocyclic Schiff bases have been reported as: antibacterial, anti-proliferative (Al-Hiyari et al., 2021), antioxidant (Kizilkaya et al., 2020) antifungal (Shafiei et al., 2021) antiviral (Alotaibi et al., 2022), anti-inflammatory (Hamid and Salih, 2022) and anti-tumoral (Iacopetta et al., 2021).
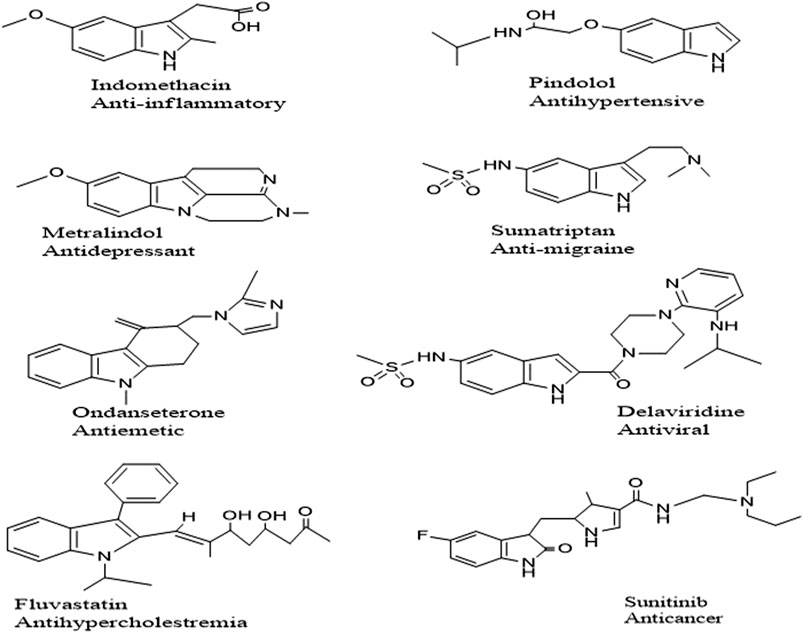
Indole is one of the most common naturally occurring Nitrogen moieties known for its diverse biological and chemical facets. Many pharmacological activities have been reported: antibacterial, antifungal, antioxidant, antidiabetic (Zhu et al., 2021), anticancer (Yousif et al., 2019) antitubercular (Ramesh et al., 2020) ulcerogenic and anti-inflammatory (Bhat et al., 2020). Indole nucleus is associated with polypharmacological activities; a few commercially available drugs are enlisted in Figure 1. Compounds incorporating the S atoms in their heterocyclic ring system have been presented as strong candidates for medicinal chemistry endeavors because of their diverse biological potential (Boraei et al., 2020). Sulfur-containing compounds are part of many natural and synthesized drug molecules.
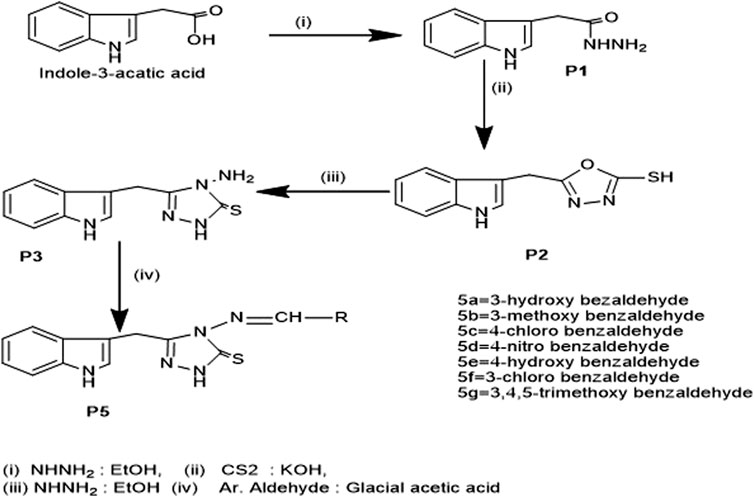
Influenced by these observations and compounds containing nitrogen, sulfur, and heterocyclic ring structures have shown promising therapeutic activities, this study intends to synthesize new compounds by combining moieties with pharmacological compatibilities and chemical veracity. Indole -3- acetic acid was used as starting material, and after its conversion into respective 1, 2, 4, triazole, Schiff bases were prepared by treatment with various benzaldehydes. The resulting compounds were recrystallized and characterized using Fourier Transform Infrared spectroscopy (FTIR), Proton and Carbon Nuclear Magnetic Resonance Spectroscopy (1H-NMR), (13C-NMR), and elemental analysis. These compounds were further screened for biological potential as antibacterial, antifungal, protein kinase inhibitors, and antioxidants, and for their cytotoxic potential.
2 Materials and methods
2.1 Chemistry
Commercially available chemicals were procured from Sigma Aldrich and used without refinement. M.P. was determined in sealed capillaries on a digital Gallen Kamp SAYO model MPD BM 3.5 apparatus and are uncorrected; I.R. spectra were recorded at alpha Bruker FT-IR spectrophotometer (ATR eco ZnSe, Vmax in cm -1, (Germany), 1HNMR and 13C NMR spectra were elucidated via Bruker AV_400 spectrophotometer in DMSO at 400 MHz using tetramethyl silane (TMS); an internal standard. COSTECH Elemental Combustion System CHNS-O Elementalanalyzer from PINSTECH was used for Combustion analysis. Compound purity was assessed through thin-layer chromatography
2.1.1 2- (1H-indol-3-yl) acetohydrazide
Indole -3- acetic acid (10.0 g) and 85% hydrazine hydrate (10.0 g) as a mixture was refluxed for 12 h at 80°C. Progress of reaction and completion was observed with the TLC system (Ethyl acetate: n-hexane: 1:1), the resultant precipitate was filtered, and the filtrate was washed with n-Hexane to give compound P1.
Yield 80%, MP 116°C, 1HNMR (DMSO -d6, 400 MHz) 10.1 (s, 1H, indole-NH), 8.00 (s, 1H, Indole-sec amine) 7.32–7.18 (m, 2H, Ph-H) 3.73 (s, 2H, -CH2-) 13C NMR (DMSO-d6, 400 MHz) 124.1, 135.5, 102.1, 127.6, 111.0, 120.5, 119.6, 121.7, 165.0, 35.9 IR tmax 3051, 3260, 3387, 1620, 1541, 1378 cm -1 Elemental analysis (calculated) for C10H11N3O C 63.43, H 5.87, N 22.221, O 8.46, M.S. Est m/z ratio 189.0
2.1.2 5-((1H-indol-3-yl) methyl)-1, 3, 4-oxadiazole-2-thiol
A mixture of P1 (10 g) and carbon disulfide 3.1 g in 95% ethanol was refluxed for 13 h to maintain a basic media, KOH (3 g) was added. Once a single spot was obtained on TLC (3:1, n-Hexane: Ethyl acetate), the temperature of the reaction mix. was lowered to room temperature, and upon treatment with conc. HCl pH was maintained. The contents were filtered, and washed with methanol. The result was P2.
Yield 79%, M.P. 125 C,1HNMR (DMSO-d6,400 MHz) 13.05 (s, 1H, S.H.) 10.1 (s, 1H, Indole -N.H.), 7.60–7.11 (m, 4H, Ph-H), 3.69 (s, 2H, -CH2-), 13CNMR 166.4, 124.1,135.5,102.1,127.6,111,120.5,119.6, 121.7, I.R. tmax 3395, 3198,2183,1617, 1541,1275,1217, 1092, Elemental analysis calc for C11H9N3OS C 57.12; H 3.93; N 18.14; O 6.92; S 13.89, M.S. Est m/z ratio 231.05.
2.1.3 5-((1H-indol3-yl) methyl)-4-amino-4H-1, 2, 4-triazole-3-thione
A mixture of P2 and 85% hydrazine Hydrate (1:1) in ethanol was refluxed for 12 h; reaction development was supervised through TLC using Ethyl acetate and n-Hexane. The resultant precipitates were filtered off and washed with n-Hexane.
Yield 85%, M.P. 175°C, 1HNMR (DMSO-d6,400 MHz)13.79 (s, 1H, -S.H.), 10.1 (s, 1H, indole-NH) 5.77 (s, 2H, indole amine) 7.60–7.11 (m, 4H, Ph-H), 3.89 (s, 2H, -CH2-) 13CNMR; 166.8,152.0,123.0,136.5, 108.1, 127.4, 111.1, 118.8, 121.7, 119.8, 22.1, IRtmax 3395, 3198, 3031, 1629, 1541, 1452, 1277, Elemental analysis calc for C11H11N5S, C,53.83; H,4.55; N, 28.54; S, 13.07; M.S. Est m/z ratio 245.07.
2.1.4 General synthetic procedure for Schiff Bases (5a-5g)
A solution of corresponding compound P3 and appropriate aldehyde in ethanolic media was refluxed for 8–10 h; glacial acetic acid was used as a catalyst. After cooling the mixture to room temperature and removing the solvent under reduced pressure, the crude product was recrystallized from Ethyl acetate. TLC was used to monitor the reaction progress using n-Hexane and Ethyl acetate (1:2) as mobile phase.
2.1.4.1 3-((1 H-indol-3-yl) methyl)-4-((3- hydroxybenzylidene) amin o-1 H-1, 2, 4-triazole-5 (4H)-thione (5a)
% Yield 74%, M.P. 215°C, 1HNMR (DMSO-d6,400 MHz) 3.579 (s, 1H,-CH2),9.906 (s, 1H, = C.H.), 6.99–7.88 (m, Aryl H =8H), 10.852 (s, 1H, -N.H.) 13CNMR 193.23, 173.43, 158.14, 157.78, I.R. tmax 3207 (-N.H.), 1657 (-C=N), 1276 (-C=S), Elemental analysis calc for C18H15N5OS C, 61.86; H, 4.35; N, 20.08; O,4.53; S, 9.18 Found: C, 61.89, H,4.32; N, 20.12; O; 4.57; M.S. Est m/z 49.10 (100.0%), 350.10 (22.2%), 351.11(1.8%)
2.1.4.2 3-((1 H-indol-3-yl) methyl)-4-((3- methoxybenzylidene) amino-1 H-1, 2, 4-triazole-5 (4H)-thione (5b)
% Yield 72%, M.P. 213°C, 1HNMR (DMSO-d6,400 MHz) 3.579 (s, 1H,-CH2), 9.906 (s, 1H, = C.H.),6.99–7.88 (m, Aryl H =8H), 10.852 (s, 1H, -N.H.) 13CNMR 193.23, 173.43, 158.14, 157.78, I.R. tmax 3207 (-N.H.), 657 (-C=N), 1276 (-C=S), Est m/z: 349.10 (100.0%), 350.10 (22.2%), 351.10 (5.3%), 351.11 (1.8%); Elemental Analysis for C18H15N5OS: C, 61.89; H, 4.34; N, 20.08; O, 4.56; S, 9.18
2.1.4.3 3-((1 H-indol-3-yl) methyl)-4-((4-chlorobenzylidene) amino-1 H-1, 2, 4-triazole-5 (4H)-thione (5c)
% Yield 75%, M.P. 219°C, 1HNMR (DMSO-d6,400 MHz) 3.579 (s, 1H,-CH2), 9.906 (s, 1H, = C.H.), 6.99–7.88 (m, Aryl H =8H), 10.852 (s, 1H, -N.H.) 13CNMR 193.23, 173.43, 158.14, 157.78, I.R. tmax 3207 (-N.H.), 657 (-C=N), 1276 (-C=S),m/z: 367.07 (100.0%), 369.06 (36.5%), 368.07 (20.4%), 370.07 (6.3%), 369.07 (2.3%), 368.06 (1.8%), 370.06 (1.8%), 371.06 (1.6%); Elemental Analysis for C18H15ClN5S: C, 58.72; H, 3.83; Cl, 9.62; N, 19.07; S, 8.79
2.1.4.4 3-((1 H-indol-3-yl) methyl)-4-((4-nitrorobenzylidene) amino-1 H-1, 2, 4-triazole-5 (4H)-thione (5d)
% Yield 75%, M.P. 220°C, 1HNMR (DMSO-d6,400 MHz) 3.579 (s, 1H,-CH2), 9.906 (s, 1H, = C.H.), 6.99–7.88 (m, Aryl H =8H), 10.852 (s, 1H, -N.H.) 13CNMR 193.23, 173.43, 158.14, 157.78, I.R. tmax 3207 (-NH) 657 (-C=N), 1276 (-C=S), Elemental analysis for C18H14N6O2S; C 57.18; H, 3.77; N, 22.29; O, 8.46; S,8.47; M.S. Est m/z: 378.09(100.0%), 379.09(22.6%), 380.11(5.5%), 380.11(1.8%).
2.1.4.5 3-((1 H-indol-3-yl) methyl)-4-((4- hydroxybenzylidene) amino-1 H-1, 2, 4-triazole-5 (4H)-thione (5e)
% Yield 77%, M.P. 215°C, 1HNMR (DMSO-d6,400 MHz) 3.693 (s, 1H, -CH2), 9.881 (s, 1H, = C.H.), 7.1–7.81 (m, Aryl H =8H), 10.863 (s, 1H, -NH) 13 CNMR 193.77, 173.80 158.30, 138.03, I.R. tmax 3207 (-N.H.), 1657 (-C=N), 1276 (-C=S), Elemental analysis calc for C18H15N5OS C, 61.85; H, 4.36; N, 20.09; O,4.53; S, 9.15; M.S. Est m/z 349.10 (100.0%), 350.10 (22.2%), 351.11(1.8%)
2.1.4.6 3-((1 H-indol-3-yl) methyl)-4-((3-chlorobenzylidene) amino-1 H-1, 2, 4-triazole-5 (4H)-thione (5f)
% Yield 75%, M. P 219°C, 1HNMR (DMSO-d6,400 MHz) 3.693 (s, 1H, -CH2), 9.881 (s, 1H, = C.H.), 7.1–7.81 (m, Aryl H =8H), 10.863 (s, 1H, -N.H.),13CNMR 193.77, 173.80 158.30, 138.03, I.R. tmax3207 (-N.H.), 1657 (-C=N), 1276 (-C=S), M.S. Est m/z: 349.10 (100.0%), 350.10 (22.2%), 351.11(1.8%)
2.1.4.7 3-((1 H-indol-3-yl) methyl)-4-((3,4,5-trimethoxybenzylidene) amino-1 H-1, 2, 4-triazole-5 (4H)-thione (5g)
% Yield 71%, M.P. 220°C, 1HNMR (DMSO-d6,400 MHz) 3.693 (s, 1H, -CH2), 9.881 (s, 1H, = C.H.), 7.1–7.81 (m, Aryl H =8H), 10.863 (s, 1H, -N.H.) 13CNMR 193.77, 173.80 158.30, 138.03, I.R. tmax 3207 (-N.H.), 1657 (-C=N), 1276 (-C=S), Est m/z: 409.12 (100.0%), 410.12 (24.3%), 411.12 (5.1%), 411.13 (2.9%), 412.12 (1.1%; Elemental Analysis for C20H19N5O3S: C, 58.63; H, 4.64; N, 17.13; O, 11.77; S, 7.87
2.2 In silico studies
Using an available software (SWISS ADME), pharmacokinetic parameters were determined. Employing a computer-aided program, we assessed the newly synthesized compounds for five different parameters: Molecular Weight (Mol.wt.). Moriguchi log of the Partition coefficient (MLOGP), octanal-water partition coefficient. (AlogP), hydrogen bond acceptor (H-BA) and donor (H-BD) and drug likeliness according to Lipinski’s rule of five (RO5).
2.3 Pharmacological evaluation
2.3.1 Antioxidant estimation
2.3.1.1 DPPH assay
The DPPH assay was executed via the technique stated by (Bibi et al., 2011). DPPH(Diphenyl-1-picrylhydrazyl), a free radical, is bagged to inquire about the antioxidant ability of the synthesized compounds. 10 μL from the synthesized compound (1 mg/mL DMSO) is added to 190 μL DPPH solution., making up the final conc. of 50 μg/mL. Absorbance was observed at 517 nm using a microplate reader after incubating at 37 C for 30 min approx. the given formula was computed
DPPH assay was repeated in triplicate using DMSO as -ve and ascorbic acid as + ve control; findings are expressed as means standard deviation.
2.3.1.2 Assessment of total antioxidant capacity (TAC assay)
This evaluation was based on the protocol by (Bibi et al., 2011). This mechanism’s key point is reducing Mo (VI) to Mo (V). A solution consisting of 4 mM Ammonium molybdate, 28 mM SodiumPhosphate, and 0.6 M Sulphuric acid is utilized, which forms Phosphate Molybdenum Complex which is green in color and gives absorption = 630 nm. 20 mL of the sample is added to 180 mL of reagent, which is incubated at 95°C for one and a half hours and cooled to 25°C.
2.3.1.3 Approximation of total reducing power (TRP assay)
The process used for TRP was illustrated by (Bibi et al., 2011). The potassium ferrocyanide calorimetric method is used for 100 μL of each solution, 200 μL Phosphate buffer (0.2 M, pH 6.6), and 250 μL of potassium ferricyanide was mixed and incubated at 50°C for 20 min, to this solution, 10 percent (200 μL) trichloroacetic acid was added, centrifugation was done at 3,000 rpm for 10 min, the supernatant was collected, added with FeCl3 (50 μL), and shifted to the microplate. Absorbance was recorded at 700 nm. Ascorbic acid (100 μg/mL DMSO) is the +ve control for the test, and dimethyl sulfoxide (DMSO) is the blank.
2.3.2.Antimicrobial assay
2.3.2.1 Antibacterial assay
The micro broth dilution method was used to establish the antibacterial potential of test samples against Staphylococcus aureus (ATCC-6538), Bacillus subtilis (ATCC-6633), Escherichia coli (ATCC-25922), Klebsiella pneumoniae (ATCC-1705), and Pseudomonas aeruginosa (ATCC-15442) and for the resistant strains of MRSA Staphylococcus aureus, P. aeruginosa and Escherichia coli. The procedure used is described by (Sarker et al., 2007). Bacterial inoculum was formed under aseptic conditions keeping the density near 5 × 104 CFU/mL in pre-autoclaved nutrient broth. Test samples of an aliquot of 5 µL were shifted to labeled wells of 96 well plates, and after that addition of 195 µL of nutrient broth; was accomplished. After that, a 2-fold serial dilution of every sample was arranged to get final concentrations of 12.5 μg/mL. Then, with the help of a micropipette, 195 µL of bacterial culture of respective strains was poured into each well; further advancement was incubating of plates for 30 min at the temperature of 37°C. Zero-time readings at 630 nm were observed with the help of a microplate reader. Incubation of plates was again carried out at 37°C for 1 day, absorbance was noted, and net change in turbidity was the difference between two values of absorbance.
2.3.2.2 Antifungal examination
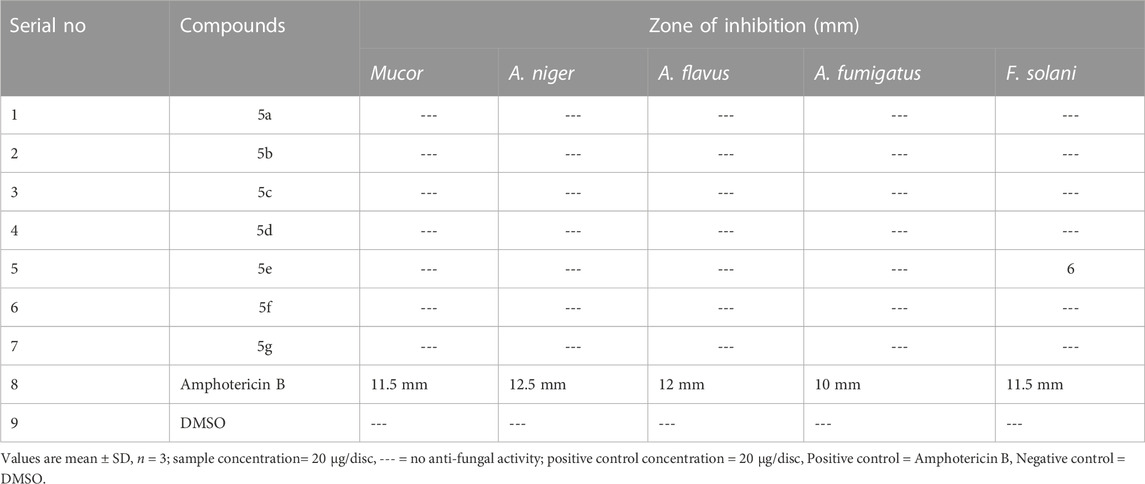
The antifungal response to these chemical moieties was evaluated through a procedure designated by (Zahra et al., 2017). Amphotericin B (4 mg/mL DMSO) is used as + ve control and DMSO as-ve one (5 μL/disc). Sterile SDA-coated plates were used, on which 100 µL of suspension of spores for each strain (previously reaped-in-Tween-20 solution) was spread. Discs of sterile filter paper containing 5 µL of sample solution (4 mg/mL DMSO) were kept on plates and incubated for 2 days at 28°C. Zones of inhibition were recorded.
2.3.3 Investigation of the compounds for cytotoxic attributes
2.3.3.1 Brine Shrimp Lethality assessment
The cytotoxic evaluation was accomplished using the decorum designed by (Khan et al., 2011). To a plate of 96 wells, artificial seawater and nauplii were added. Test samples were taken in wells (40, 20, 10, 5 μg/mL) after making up the final volume with seawater, the plates were left uncovered under the lamp, and after 24 h, the number of surviving larvae was calculated. For samples having ≥50% mortality, the median lethal conc was calculated using the table curve software, Doxorubicin was used as + ve control, and DMSO was the -ve. The percentage of killed organisms was found using the formula.
2.3.3.2 Protein kinase inhibition assay
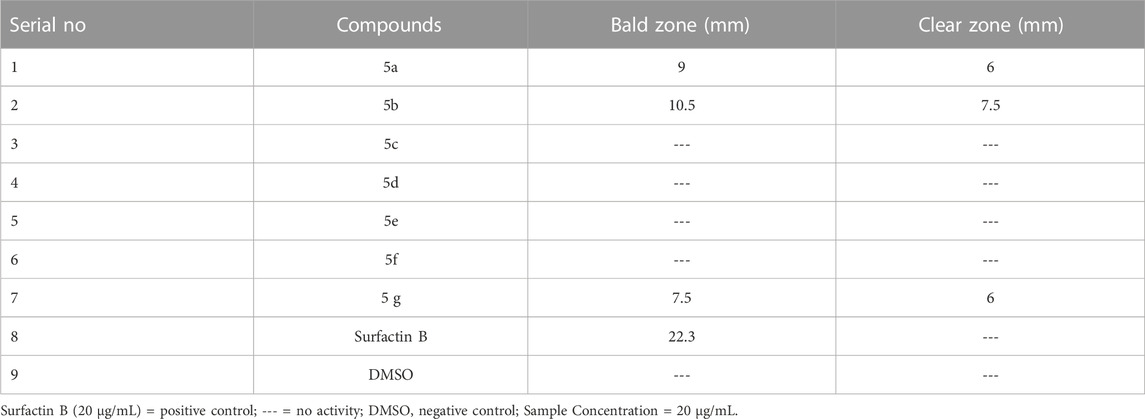
The protocol followed for this is mentioned (Fatima et al., 2015). To assess the test compound’s inhibitory potential, Streptomyces 85 E strains were used, which were refreshed in tryptone soya broth at 37 for 1 day. Spores from refreshed cultures were utilized to prepare the bacterial culture. 100 μg compound was loaded on 5 mm discs of filter paper. These were kept on sowed plates. DMSO-permeated discs were the -ve control. The incubation period was 7 days, and outcomes were stated as bald and clear zones of inhibition.
2.4 Statistical analysis
The results of all the experiments were articulated as mean ± SD. Each test was performed in triplicate. TAC assay was analyzed by one-way analysis of variance (ANOVA) following DUNCAN’S test using the statistical package for social sciences 18 and p < 0.05 was considered as significant where appropriate. For samples having ≥50% mortality, the median lethal concentration (LC50) was calculated using the table curve software.
3 Result and discussion
3.1 Chemistry
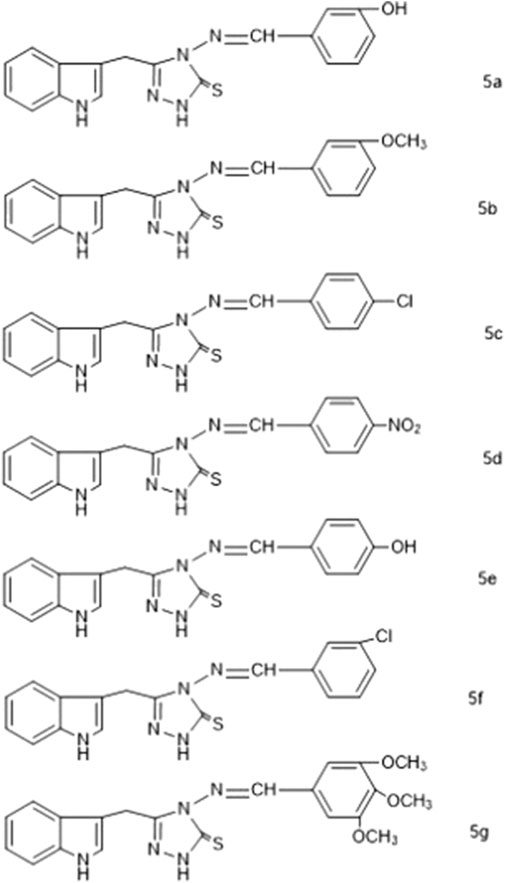
We synthesized the desired compounds according to scheme 1, following the method described by (Bayrak et al., 2009). The whole scheme is mentioned in Figure 2. Condensation of 1 with hydrazine in ethanol as reaction media yielded 2-(1H-indol-3-yl) acetohydrazide P1, In I.R., the C=O for acetohydrazide gives the impression at 1,620 cm-1, and for -NHNH2 around 3,260, 3,387 confirms the formation of P1. P2 was obtained from the reaction between P1 and CS2 in basic media; the final product was 1, 3, 4 -oxadiazole which was obtained by treatment of filtrate with conc. HCl and in FTIR were confirmed through characteristic peaks at 1,275 (-C=S) and 1,223 (-C-O-C) cm-1. P3; the key intermediate was the product of P2 and hydrazine hydrate reaction, confirmed via FTIR 1,277 (C=S), 1,629 (C=N) cm-1. P3 on treatment with aromatic_aldehydes in the ethanolic medium using Glacial acetic acid as catalyst yielded compounds 5a-5g. These Schiff base triazoles were confirmed through various spectroscopic techniques, i.e., FTIR, 1HNMR, and 13CNMR. The appearance of a singlet at 9.88–9.90 indicates -C=N for Schiff bases in 1HNMR, and at 158.30ppm for the same in 13CNMR, and at 1657cm-1 in FTIR, confirming it. During the synthesis of 5a-5g, it was observed that substituted aromatic aldehydes possessing electron-withdrawing groups, e.g., -Cl, tend to end up reaction process early with good yield as compared to compounds affording electron-releasing groups, e.g., -OCH3. Figure 3 enlists the structures of synthesized compounds (5a-5g).
3.2 In silico studies
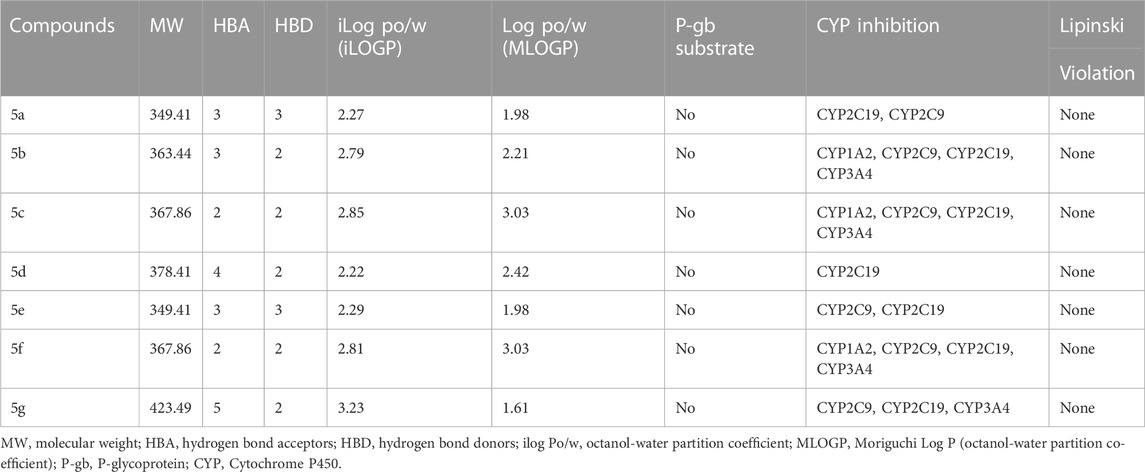
Prediction of compounds for their drug-like traits is critical to inquire about their pharmaceutical, pharmacokinetic and pharmacological behavior. Many drugs cannot go through clinical trials as per poor ADME (Adsorption, Distribution, Metabolism and Excretion) characteristics; in silico studies are acquiring an outstanding reputation in the drug development phase. (Arjun et al., 2019). Online tools were used to estimate the drug likeliness of newly synthesized compounds. SWISS ADME software was used to predict ADME properties; the data obtained are given in Table 1. All of these compounds are drug-like following RO5. In complete accordance with the Lipinski rule, any tested compound should fulfill the following mentioned conditions.
1. M.W. should be ≤500 Da.
2. HBA ≤10
3. HBD ≤5
4. iLOGP ≤5
5. MLOG ≤5
These given ADME parameters of compounds; help in assessing the potential of compounds for various factors, which may include distribution to the site of action, access to the bloodstream, and metabolization as non-toxic water-soluble excretes. SwissADME provides an insight into some basic parameters; solubility in water, ability to cross BBB, intestinal absorption, lipophilicity, attributes to bind with plasma proteins, and the ability to interfere with Cytochrome P 450 enzymes (Waseem et al., 2017). These compounds are prophesied for their CYP450 enzyme inhibition capacity, which accounts for CYP1A2, CYP2C19, CYP2C9, and CYP3A4. 5b, 5c, and 5f were able to inhibit all of these; 5g inhibited 2C19, 2C9, and 3A4, 5a and 5e inhibited 2C19 and 2C9, 5d inhibited 2C19 only. These findings state that these synthesized molecules may potentially affect the other drugs during metabolism, which are supposed to be metabolized by these enzymes. These compounds have a middling profile, can be investigated in vivo, and are selected for further inquiry. The BOILED-EGG method gives insight into the compound’s gut absorption and BBB permeability. In our synthetic library, all the compounds exhibited high gastrointestinal absorption. The appearance of a red dot indicates; being a non-substrate for globulin protein. The position of dots is linked with BBB permeability and gut absorption. The BOILED-Egg depiction of compounds is shown in Figure 4.
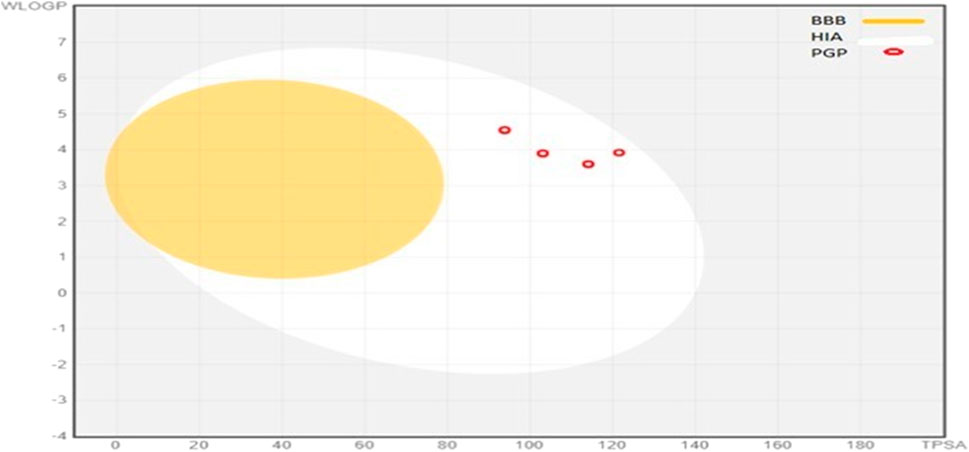
FIGURE 4. BOILED-Egg representations of absorption and distribution properties of (5a-5g) compounds. Egg white represen uman intestinal absorption (HIA), whereas egg yolk (yellow) shows blood-brain barrier permeability (BBB).
3.3 Pharmacologic evaluation
3.3.1.Antioxidant assays
3.3.1.1 DPPH assay
It is one of the very commonly utilized methods for the assessment of the antioxidant ability of compounds. DPPH turns from purple to yellow, which indicates the synthetic compound’s scavenging potential (Rahman et al., 2017). DMSO and A.A. were used as -ve and +ve control. The antioxidant potential of compounds was assessed at 50 μg/mL, 25 μg/mL, 12.5 μg/mL, and 6.25 μg/mL. All the compounds showed very slight antioxidant potential, with 5b (32%) being the most active in the series, followed by 5a (22.55%), then 5c and 5d (11.1%), and the least active was 5e (8.3%). The results are summarized in Figure 5. Production of free radicals is an outcome of regular oxygen consumption in the body; whenever something is missing with the natural antioxidant mechanism, the accumulation of these free radicals may lead to cytotoxic interaction with the body systems and may cause damage to genomic content of various cells, and proteins in the body (Barbuceanu et al., 2014). Free radical accumulation is also associated with different diseases, e.g., coronary heart disease, neuronic disorders, diabetes, necrosis, and various tumors (Ismaili.et al., 2008). The antioxidant potential of the compound depends upon its reducing power; the introduction of hydroxy and methoxy groups on the Benzene ring led to better reducing power or promising antioxidant activity (Rakesh et al., 2015). In this study, compounds 5b and 5a exhibited good antioxidant potential as having -OCH3 and groups at position three of the benzene ring. However, a change in position from three to four for Hydroxy benzaldehyde showed a significant loss in reducing potential. The basicity of the nitrogen atom also plays a significant role, i.e., the more basic is the nitrogen atom, the more active the compound will be in terms of its reducing potential (Hayun et al., 2018).
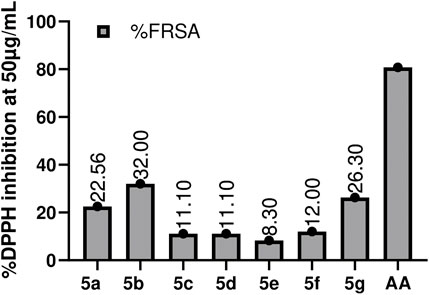
FIGURE 5. Graphical representation of DPPH Assay estimation of the synthesized compounds (5a-5g). Values given are expressed as the mean of triplicate ±standard deviation.
3.3.1.2 Investigation of total antioxidant capacity (TAC) and total reducing power (TRP)
The antioxidant potential of test compounds was estimated by TAC &TRP assays. The spectrophotometric method was used to analyze the formation of the green-colored phosphomolibdenum compound. The newly synthesized compounds have shown noteworthy results in the TAC assay; 5b showed the maximum activity among the synthetic compounds, whereas 5c showed the least. The findings are depicted in Figure 6.
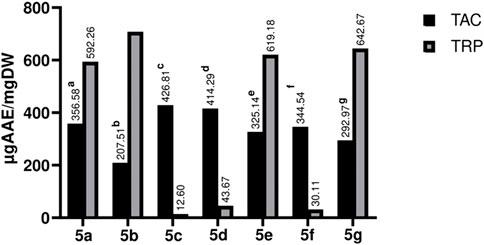
FIGURE 6. Graphical representation of TAC and TRP estimation of Library compounds (5a-5g). Values given are expressed as the mean of triplicate +standard deviation.
For estimation of reducing potential TRP assay was carried out, compound 5b (706.42 ± 0.004 µg AAE/mg) showed the highest activity, followed by 5g (642.67 ± 0.001 µg AAE/mg), 5e (619.18 ± 0.010 µg AAE/mg), and 5a (592.26 ± 0.007 µg AAE/mg). It is apparent from the results that compounds having hydroxy and methoxy in their structure showed promising activities as compared to those having nitro and chloro moieties at various positions. The primary mechanism of TRP assay is based on the compound’s capacity to reduce free radicals, which are generated during the procedure. i.e., Fe3+ is reduced to Fe2+ (Lee et al., 2015). Various factors determine the antioxidant potential of the compound; a few to explain include the chemical attributes, nature of antioxidant moieties, conditions and mechanism of the reaction, which accounts for the reason for a compound’s activity in one assay and none-to-less response in the other (Francenia Santos-Sanchez et al., 2019). However, compounds with hydroxyl and methoxy groups form the bases for potential candidates as an antioxidant (Rakesh et al., 2015).
3.3.2 Anti-microbial screening
3.3.2.1 Antibacterial assessment
Schiff base tri-azo compounds were investigated for antibacterial potential. The antibacterial assays were performed for nine different bacterial strains, which include; Gram-positive (, S. aureus, B. subtilis), Gram-negative (Pseudomonas aeruginosa, K. pneumoniae, E. coli), and resistant bacterial strains (S. aureus, MRSA, Pseudomonas aeruginosa, E. coli. Compounds that exhibited significant activity, producing inhibitory growth zone ≥10 mm, their MIC values were determined (through broth microdilution method), ranging from 25 to 3.12 μg/mL. The Antibacterial activity of compounds and their minimum inhibitory concentration values are mentioned in Tables 2, 3. These results indicate that all the compounds showed in-vitro anti-microbial activity to some extent. None of the compounds in the given series was able to show inhibitory activity for B. subtilis, and only 5g and 5f were effective against S. aureus with MIC 25 μg/mL (5a-5g) showed activity against K. pneumoniae, and exhibited different values for MIC, ranging from 12.5 μg/mL for 5a, 5c, 5d, and 5e. 6.25 μg/mL for 5b and 5g, and the least, Table 4 3.12 μg/mL was for 5f. 5a, 5b (MIC =12.5 μg/mL), 5e (MIC = 3.12 μg/mL, same as Ciprofloxacin) were active against Pseudomonas aeruginosa, and 5c, 5d, 5e (MIC = 25 μg/mL) were active against E. coli. These compounds were effective against only one resistant bacterial strain, i.e., MRSA, and showed a MIC value of 12.5 μg/mL. Substituents with electron-withdrawing attributes, e.g., -NO2, -Cl, -OH on the aromatic ring, assert a great impact on the antibacterial activity of synthetic compounds. It is also observed that an increase in the number of -OH groups tend to enhance the activity (Hosamani and Shingalapur, 2011). Due to the difference in cell-wall composition of Gram + ve and -ve bacteria, different compounds show different features towards the same micro-organism (Goszczyn’ska et al., 2015).
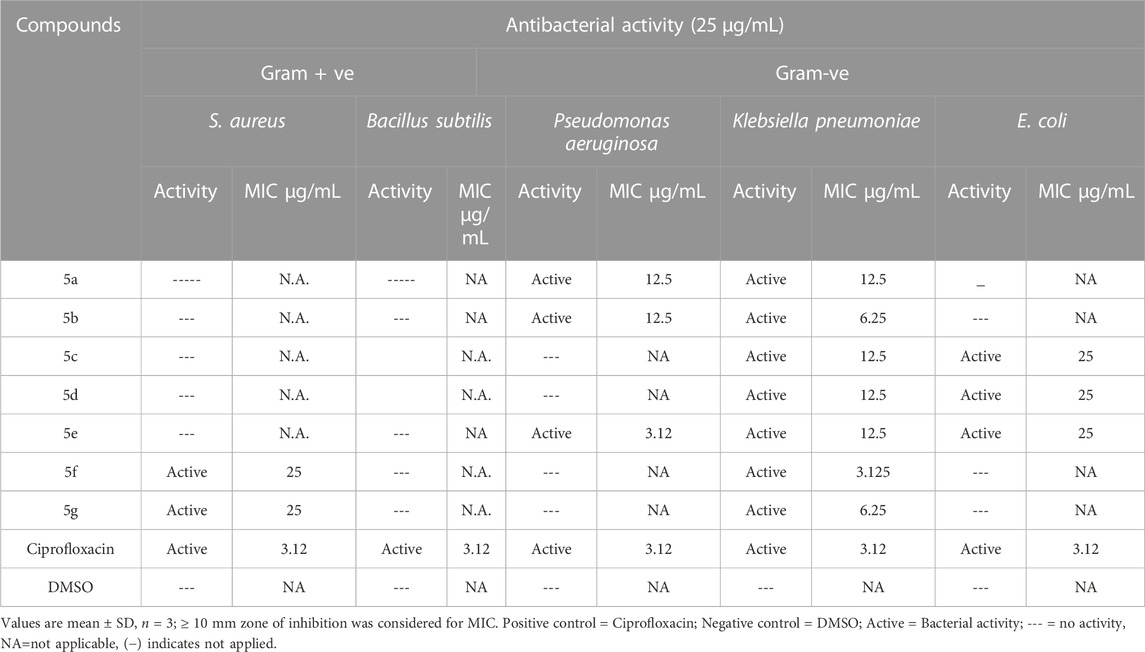
TABLE 2. Antibacterial evaluation of the synthetic compounds of Library compounds (5a-5g) against gram-positive and gram-negative bacteria and their MIC values.
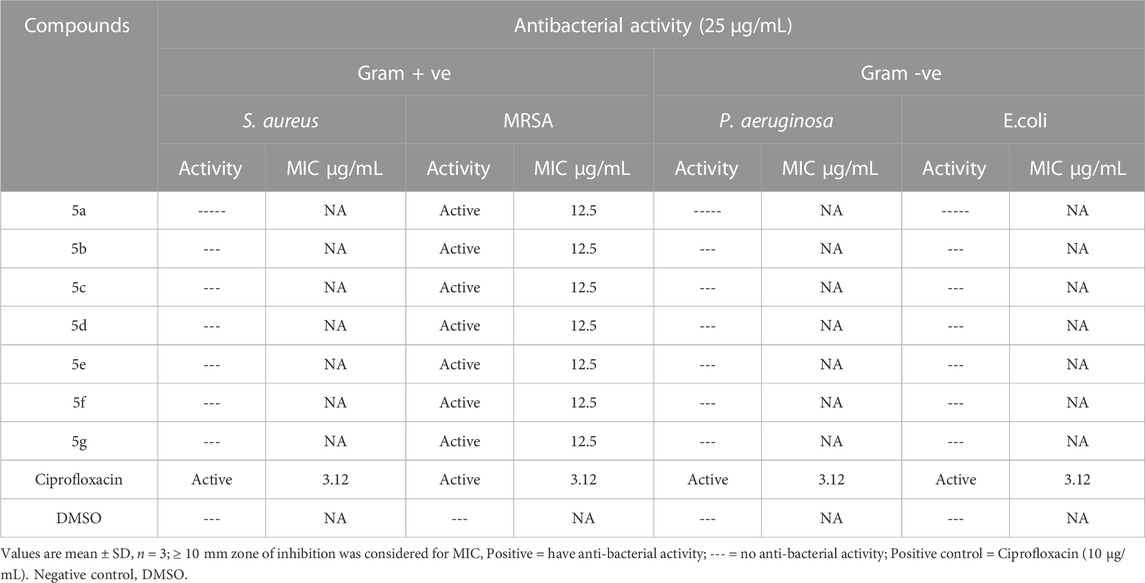
TABLE 3. Antibacterial evaluation of the synthetic compounds of Library compounds (5a-5g) against resistant gram-positive and gram-negative bacteria and their MIC values.
3.3.2.2 Antifungal estimation
The Synthetic compound library was subjected to an antifungal assay using five different strains of Fungai, i.e., Fusarium solani (FCBP-0291), Aspergillus fumigatus (FCBP-66), Aspergillus flavus (FCBP-0064), Aspergillus niger (FCBP-0198) and Mucor species (FCBP-0300). Amphotericin B was the standard drug used. None of the compounds showed antifungal activity, except 5e, which was active against only one strain of Fusarium solani. The outcomes are concise in Table 5.
3.3.3.Cytotoxic evaluation
3.3.3.1.Brine shrimp lethality assay
This evaluation test forms the basis for the bioactive scanning of compounds for their potential as anti-cancer moieties, to be investigated on a large scale (Amanullah et al., 2012). Through research, it has been found that the compound’s anti-cancer potential and B.S. lethality has direct relation (El-Gohary and Shaaban, 2014). Similarities between Artemia salina and mammalian cells have been reported due to DNA-dependent RNA polymerases (Birndorf et al., 1975). These Schiff bases were investigated for their cytotoxic potential. The values for the toxicity test of all 1, 2.4-Triazole compounds against brine shrimp assay and LC50 values are described in Figure 7. Doxorubicin was used as the positive control. All of the compounds showed cytotoxic potential, and a few expressed activities similar to standard; doxorubicin, some have less value of LC50 than Doxorubicin 5c, 5d, and 5f showed max. Activity with LC50 values of 5.7 μg/mL, followed by 5b, 5e 14.14 μg/mL, and the maximum value was observed for 5g, which is 18.18 μg/mL. Compounds that possess -Cl, -OH, at 2, 3, and -NO2 at position four at the side chain of the main nucleus show good cytotoxic potential (Hosamani and Shingalapur, 2011). The substantial LC50 values of Schiff bases indicate that these possess cytotoxic capacity, which allows further studies to be carried -out. From a pharmacologic point of view, it is pertinent that the compounds with promising activity in B.S. assay are well-thought-out as better antitumoral agents (Carballo et al., 2002).
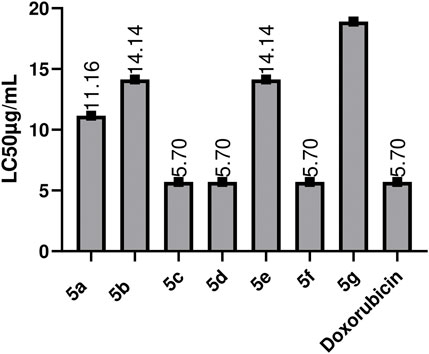
FIGURE 7. Toxicity evaluation of the synthetic compounds by using Brine shoxorubicin Values are expressed as Mean ± SEM(standard error of the mean, n = 3) lethality assay.
3.3.3.2 Protein kinase inhibition assay
These synthetic products were evaluated to estimate the inhibitory potential for protein kinase of Streptomycin 85 E. Protein kinase is involved with the mycelium production of this species, so the growth patterns were observed as the bald zone. The clear zone specifies no cytotoxic bacterial growth; as far as the bald zone is concerned, it shows inhibition of protein kinase. The results for this assay for compounds (5a-5g) are given in Table 5. One of the propitious targets in cancer treatment is the inhibition of protein kinases. Protein phosphorylation by protein kinases at various residues regulates diverse biological processes, which may include apoptosis, proliferation and differentiation of cells. Deregulation at any step in early tumorigenesis may lead to cancer (Yao et al., 2011). Aerial hyphae formation in Streptomyces depends upon protein kinase activity; this scenario is exploited in this test; to inquire about these compounds’ kinase inhibition potential and to look at their anti-cancer potential. The advantage of using Streptomyces for kinase inhibition assay is its predisposition to a wide range of eukaryotic cells. Whole-cell assay of Streptomyces not only identifies the compound’s cytotoxic potential but also identifies signal transduction inhibitors for various activities, e.g., antitumor, anti-mycobacterial, and and anti-infective (Waters et al., 2002). Among the tested compounds, only 5a, 5b, and 5g showed inhibitory activity. 5b showed the max. Activity with a bald zone of 10.5 mm and a clear zone of 7.5 mm, following is 5a with a bald zone of 9 mm and a clear zone of 6 mm, and for 5g, values are 7.5 and 6 mm, respectively. It means that these compounds have the potential for protein kinase inhibition as well as the toxicity of the compound.
4 Conclusion
The synthesized Schiff bases showed encouraging results in these assays. The whole series was found to follow Lipinski’s rule of five. Among these synthesized compounds, no one was able to show promising activity in DPPH assay; 5b showed max. Antioxidant potential in TAC and TRP assays. In antibacterial evaluation, 5e was effective against max. the number of the strains, which include P. aeruginosa, K. pneumoniae, E. coli, MRSA, 5g, and 5f, were active for Gram + ve, Gram -ve, and resistant strains, with MIC values comparable to reference drug Ciprofloxacin. 5b was most active in series as a Protein kinase inhibitor, and 5c, 5d, and 5f showed max. Cytotoxic potential in Brine shrimp lethality assay with LC50 5.7 μg/mL, equal to Doxorubicin. SAR studies reveal that the -OH group plays a vital role. Compounds with -Cl, -NO2, and -OH are good candidates for cytotoxic potential. In short, this study has revealed the scope for further research in these areas to develop novel bioactive compounds and launch rational QSAR studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MJ: Conceptualization, investigation, data curation and is responsible for the whole study. I-UH, HN: HF: Methodology, resources. Visualization. NI: HN: Contributed in write -up and supervised the study. A-UK: Reviewed the manuscript, validation, and formal analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal, R., and Sumran, G. (2020). An insight on medicinal attributes of 1,2,4-triazoles. Eur. J. Med. Chem. 205, 112652. doi:10.1016/j.ejmech.2020.112652
Al-Hiyari, B. A., Shakya, A. K., Naik, R. R., and Bardaweel, S. (2021). Microwave-assisted synthesis of schiff bases of isoniazid and evaluation of their anti-proliferative and antibacterial activities. Molbank 2021, M1189. doi:10.3390/M1189
Alkhzem, A. H., Woodman, T. J., and Blagbrough, I. S. (2022). Design and synthesis of hybrid compounds as novel drugs and medicines. RSC Adv. 12, 19470–19484. doi:10.1039/D2RA03281C
Alotaibi, S. H., Amer, H. H., Touil, N., Abdel-Moneim, A. S., Soliman, M. M., and Zaki, Y. H. (2022). Synthesis, characterization and molecular docking of new nucleosides and schiff bases derived from ampyrone as antiviral agents to contain the COVID-19 virus. Polycycl. Aromat. Compd. 1, 1–12. doi:10.1080/10406638.2022.2045329
Amanullah, M., Rehman, W., Khan, A., Akram, M., and Naseer, F. (2012). Syntheses, spectral characterization and biological elucidation of some mannich bases. Asian J. Chem. 24, 5179–5182.
Arjun, H. A., Elancheran, R., Manikandan, N., Lakshmithendral, K., Ramanathan, M., Bhattacharjee, A., et al. (2019). Design, synthesis, and biological evaluation of (E)-N’-((1-Chloro-3,4-Dihydronaphthalen-2-yl)Methylene)Benzohydrazide derivatives as anti-prostate cancer agents. Front. Chem. 7, 474. doi:10.3389/fchem.2019.00474
Barbuceanu, S. F., Ilies, D. C., Saramet, G., Uivarosi, V., Draghici, C., and Radulescu, V. (2014). Synthesis and antioxidant activity evaluation of new compounds from hydrazinecarbothioamide and 1,2,4-triazole class containing diarylsulfone and 2,4-difluorophenyl moieties. Int. J. Mol. Sci. 15, 10908–10925. doi:10.3390/ijms150610908
Bayrak, H., Demirbas, A., Karaoglu, S. A., and Demirbas, N. (2009). Synthesis of some new 1,2,4-triazoles, their Mannich and Schiff bases and evaluation of their antimicrobial activities. Eur. J. Med. Chem. 44, 1057–1066. doi:10.1016/j.ejmech.2008.06.019
Bhat, M. A., Al-Omar, M. A., Alsaif, N. A., Almehizia, A. A., Naglah, A. M., Razak, S., et al. (2020). Novel sulindac derivatives: Synthesis, characterisation, evaluation of antioxidant, analgesic, anti-inflammatory, ulcerogenic and COX-2 inhibition activity. J. Enzyme Inhib. Med. Chem. 35, 921–934. doi:10.1080/14756366.2020.1746783
Bibi, G., Ullah, N., Mannan, A., and Mirza, B. (2011). Antitumor, cytotoxic and antioxidant potential of Aster thomsonii extracts. Afr. J. Pharm. Pharmacol. 5, 252–258. doi:10.5897/AJPP10.417
Birndorf, H. C., D’Alessio, J., and Bagshaw, J. C. (1975). DNA-dependent RNA-polymerases from Artemia embryos. Characterization of polymerases I and II from nauplius larvae. Dev. Biol. 45, 34–43. doi:10.1016/0012-1606(75)90238-9
Boraei, A. T. A., Sarhan, A. A. M., Yousuf, S., and Barakat, A. (2020). Synthesis of a new series of nitrogen/sulfur heterocycles by linking four rings: Indole; 1,2,4-triazole; pyridazine; and quinoxaline. Molecules 25, 450. doi:10.3390/molecules25030450
Carballo, J., Hernández-Inda, Z. L., Pérez, P., and García-Grávalos, M. D. (2002). A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol. 2, 17. doi:10.1186/1472-6750-2-17
Deodware, S. A., Barache, U. B., Chanshetti, U. B., Sathe, D. J., Panchsheela Ashok, U., Gaikwad, S. H., et al. (2021). Newly synthesized triazole-based Schiff base ligands and their Co(II) complexes as antimicrobial and anticancer agents: Chemical synthesis, structure and biological investigations. Results Chem. 3, 100162. doi:10.1016/j.rechem.2021.100162
El-Gohary, N., and Shaaban, M. (2014). Antimicrobial and antiquorum-sensing studies. Part 2: Synthesis, antimicrobial, antiquorum-sensing and cytotoxic activities of new series of fused[1,3,4]thiadiazole and [1,3]benzothiazole derivatives. Med. Chem. Res. 23, 287–299. doi:10.1007/s00044-013-0637-x
Fatima, H., Khan, K., Zia, M., Ur-Rehman, T., Mirza, B., and Haq, I. U. (2015). Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: An in vitro biological and phytochemical investigation. BMC Complement. Altern. Med. 15, 376. doi:10.1186/s12906-015-0891-1
Francenia Santos-Sánchez, N., Salas-Coronado, R., Villanueva-Cañongo, C., and Hernández-Carlos, B. (2019). Antioxidant compounds and their antioxidant mechanism. Antioxidants. doi:10.5772/intechopen.85270
Goszczyńska, A., Kwiecień, H., and Fijałkowski, K. (2015). Synthesis and antibacterial activity of Schiff bases and amines derived from alkyl 2-(2-formyl-4-nitrophenoxy)alkanoates. Med. Chem. Res. 24, 3561–3577. doi:10.1007/s00044-015-1397-6
Hamid, S. J., and Salih, T. (2022). Design, synthesis, and anti-inflammatory activity of some coumarin schiff base derivatives: In silico and in vitro study. Drug Des. Devel Ther. 16, 2275–2288. doi:10.2147/DDDT.S364746
Hayun, H., Arrahman, A., Purwati, E. M., Yanuar, A., Fortunata, F., Suhargo, F., et al. (2018). Synthesis, anti-inflammatory and antioxidant activity of mannich bases of dehydrozingerone derivatives. J. Young Pharm. 10, S6–S10. doi:10.5530/jyp.2018.2s.2
Hosamani, K. M., and Shingalapur, R. v. (2011). Synthesis of 2-mercaptobenzimidazole derivatives as potential anti-microbial and cytotoxic agents. Arch. Pharm. Weinh. 344, 311–319. doi:10.1002/ardp.200900291
Iacopetta, D., Ceramella, J., Catalano, A., Saturnino, C., Bonomo, M. G., Franchini, C., et al. (2021). Schiff bases: Interesting scaffolds with promising antitumoral properties. Appl. Sci. 11, 1877. doi:10.3390/app11041877
Idhayadhulla, A., Surendra Kumar, R., Abdul Nasser, A. J., Selvin, J., and Manilal, A. (2014). Synthesis of some Mannich base derivatives and their antimicrobial activity study. Arab. J. Chem. 7, 994–999. doi:10.1016/J.ARABJC.2010.12.025
Ismaili, L., Nadaradjane, A., Nicod, L., Guyon, C., Xicluna, A., Robert, J. F., et al. (2008). Synthesis and antioxidant activity evaluation of new hexahydropyrimido[5,4-c]quinoline-2,5-diones and 2-thioxohexahydropyrimido[5,4-c]quinoline-5-ones obtained by Biginelli reaction in two steps. Eur. J. Med. Chem. 43, 1270–1275. doi:10.1016/j.ejmech.2007.07.012
Kattappagari, K., Ravi Teja, C., Kommalapati, R., Poosarla, C., Gontu, S., and Reddy, B. R. (2015). Role of antioxidants in facilitating the body functions: A review. J. Orofac. Sci. 7, 71. doi:10.4103/0975-8844.169745
Khan, S., Shin, E. M., Choi, R. J., Jung, Y. H., Kim, J., Tosun, A., et al. (2011). Suppression of LPS-induced inflammatory and NF-κB responses by anomalin in RAW 264.7 macrophages. J. Cell. Biochem. 112, 2179–2188. doi:10.1002/jcb.23137
Kizilkaya, H., Dag, B., Aral, T., Genc, N., and Erenler, R. (2020). Synthesis, characterization, and antioxidant activity of heterocyclic Schiff bases. J. Chin. Chem. 67, 1696–1701. doi:10.1002/jccs.202000161
Lee, Y. H., Choo, C., and Waisundara, V. Y. (2015). Determination of the total antioxidant capacity and quantification of phenolic compounds of different solvent extracts of black mustard seeds (Brassica nigra). Int. J. Food Prop. 18, 2500–2507. doi:10.1080/10942912.2014.986331
Lobo, V., Patil, A., Phatak, A., and Chandra, N. (2010). Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 4, 118–126. doi:10.4103/0973-7847.70902
Matin, M. M., Matin, P., Rahman, M. R., ben Hadda, T., Almalki, F. A., Mahmud, S., et al. (2022). Triazoles and their derivatives: Chemistry, synthesis, and therapeutic applications. Front. Mol. Biosci. 9, 864286. doi:10.3389/fmolb.2022.864286
Mistry, B., Patel, R. v., Keum, Y. S., and Kim, D. H. (2017). Synthesis of N-Mannich bases of berberine linking piperazine moieties revealing anticancer and antioxidant effects. Saudi J. Biol. Sci. 24, 36–44. doi:10.1016/j.sjbs.2015.09.005
Rahman, L., Shinwari, Z. K., Iqrar, I., Rahman, L., and Tanveer, F. (2017). An assessment on the role of endophytic microbes in the therapeutic potential of Fagonia indica. Ann. Clin. Microbiol. Antimicrob. 16, 53. doi:10.1186/s12941-017-0228-7
Rakesh, K. P., Manukumar, H. M., and Gowda, D. C. (2015). Schiff’s bases of quinazolinone derivatives: Synthesis and SAR studies of a novel series of potential anti-inflammatory and antioxidants. Bioorg Med. Chem. Lett. 25, 1072–1077. doi:10.1016/j.bmcl.2015.01.010
Ramesh, D., Joji, A., Vijayakumar, B. G., Sethumadhavan, A., Mani, M., and Kannan, T. (2020). Indole chalcones: Design, synthesis, in vitro and in silico evaluation against Mycobacterium tuberculosis. Eur. J. Med. Chem. 198, 112358. doi:10.1016/j.ejmech.2020.112358
Said, M. A., Khan, D. J. O., Al-Blewi, F. F., Al-Kaff, N. S., Ali, A. A., Rezki, N., et al. (2021). New 1,2,3-triazole scaffold schiff bases as potential anti-Covid-19: Design, synthesis, dft-molecular docking, and cytotoxicity aspects. Vaccines (Basel) 9, 1012. doi:10.3390/vaccines9091012
Sarker, S. D., Nahar, L., and Kumarasamy, Y. (2007). Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42, 321–324. doi:10.1016/j.ymeth.2007.01.006
Shafiei, M., Toreyhi, H., Firoozpour, L., Akbarzadeh, T., Amini, M., Hosseinzadeh, E., et al. (2021). Design, synthesis, and in vitro and in vivo evaluation of novel fluconazole-based compounds with promising antifungal activities. ACS Omega 6, 24981–25001. doi:10.1021/acsomega.1c04016
Strzelecka, M., and Świątek, P. (2021). 1,2,4-Triazoles as important antibacterial agents. Pharmaceuticals 14, 224. doi:10.3390/ph14030224
Wang, X., Fu, H. Y., He, W., Xiang, Y. T., Yang, Z. C., Kuang, Y., et al. (2022). Synthesis and antibacterial activity evaluation of biphenyl and dibenzofuran derivatives as potential antimicrobial agents against antibiotic-resistant bacteria. Curr. Issues Mol. Biol. 44, 4087–4099. doi:10.3390/cimb44090280
Waseem, D., Butt, A. F., Haq, I. ul, Bhatti, M. H., and Khan, G. M. (2017). Carboxylate derivatives of tributyltin (IV) complexes as anticancer and antileishmanial agents. DARU, J. Pharm. Sci. 25, 8. doi:10.1186/s40199-017-0174-0
Waters, B., Saxena, G., Waggui, Y., Kau, D., Wrigley, S., Stokes, R., et al. (2002). Identifying protein kinase inhibitors using an assay based on inhibition of aerial hyphae formation in Streptomyces. J. Antibiot. 55, 407–416. doi:10.7164/antibiotics.55.407
Yao, G., Sebisubi, F. M., Voo, L. Y. C., Ho, C. C., Tan, G. T., and Chang, L. C. (2011). Citrinin derivatives from the soil filamentous fungus Penicillium sp. H9318. J. Braz Chem. Soc. 22, 1125–1129. doi:10.1590/S0103-50532011000600018
Yousif, M. N. M., Hussein, H. A. R., Yousif, N. M., El-Manawaty, M. A., and El-Sayed, W. A. (2019). Synthesis and anticancer activity of novel 2-phenylindole linked imidazolothiazole, thiazolo-s-triazine and imidazolyl-sugar systems. J. Appl. Pharm. Sci. 9, 6–14. doi:10.7324/JAPS.2019.90102
Zahra, S. S., Ahmed, M., Qasim, M., Gul, B., Zia, M., Mirza, B., et al. (2017). Polarity based characterization of biologically active extracts of Ajuga bracteosa Wall. ex Benth. and RP-HPLC analysis. BMC Complement. Altern. Med. 17, 443. doi:10.1186/s12906-017-1951-5
Keywords: 1, 2, 4-triazoles, Schiff base, antioxidant, antibacterial, cytotoxic. protein kinase inhibition
Citation: Javaid M, Haq I-U, Nadeem H, Fatima H, Khan A-U and Irshad N (2023) Design, synthesis and screening of indole acetic acid-based tri-azo moieties as antioxidants, anti-microbial and cytotoxic agents. Front. Pharmacol. 14:1084181. doi: 10.3389/fphar.2023.1084181
Received: 30 October 2022; Accepted: 17 February 2023;
Published: 27 February 2023.
Edited by:
Ayesha Ihsan, National Institute for Biotechnology and Genetic Engineering, PakistanReviewed by:
Ashok K. Shakya, Al-Ahliyya Amman University, JordanWaqas Ahmad, University of Science Malaysia (USM), Malaysia
Syed Nasir Abbas Bukhari, Al Jouf University, Saudi Arabia
Copyright © 2023 Javaid, Haq, Nadeem, Fatima, Khan and Irshad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nadeem Irshad, bmlyc2hhZEBxYXUuZWR1LnBr
 Maryam Javaid
Maryam Javaid Ihsan-Ul Haq1
Ihsan-Ul Haq1 Nadeem Irshad
Nadeem Irshad