
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 14 February 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1083746
This article is part of the Research Topic Safety of Drugs and CAM Products in Pregnancy and Breastfeeding: Evidence From Clinical Toxicology View all 9 articles
 Hongliang Xie1,2†
Hongliang Xie1,2† Aolin Zhang1,2†
Aolin Zhang1,2† Xuan Mou1,2
Xuan Mou1,2 Tao He1,2
Tao He1,2 Junwei Li1,2
Junwei Li1,2 Chi Chiu Wang3,4
Chi Chiu Wang3,4 Xiaohui Fan1,2,5*
Xiaohui Fan1,2,5* Lu Li1,2,3,5,6,7*
Lu Li1,2,3,5,6,7*Objective: To conduct an updated systematic review and meta-analysis on the efficacy and safety of Chinese herbal medicine (CHM) for threatened miscarriage.
Data Sources: Electronic databases were searched from inception to 30 June 2022. Study Eligibility Criteria: Only randomized controlled trials (RCTs) that assessed the efficacy and safety of CHM or combined CHM and Western medicine (CHM-WM) and compared with other treatments for threatened miscarriage were included for analysis.
Methods: Three review authors independently evaluated included studies, assessed the risk of bias and extracted data for meta-analysis (continuation of pregnancy after 28 gestational weeks, continuation of pregnancy after treatment, preterm birth, adverse maternal outcomes, neonatal death, TCM syndrome severity, β-hCG levels after treatment), sensitivity analysis (β-hCG level) and subgroup analysis (TCM syndrome severity, β-hCG level). The risk ratio and 95% confidence interval were calculated by RevMan. Certainty of the evidence was assessed according to GRADE.
Results: Overall, 57 RCTs involving 5,881 patients met the inclusion criteria. Compared with WM alone, CHM alone showed significant higher incidence of continuation of pregnancy after 28 gestational weeks (Risk Ratio (RR) 1.11; 95% CI 1.02 to 1.21; n = 1; moderate quality of evidence), continuation of pregnancy after treatment (RR 1.30; 95% CI 1.21 to 1.38; n = 10; moderate quality of evidence), higher β-hCG level (Standardized Mean Difference (SMD) 6.88; 95% CI 1.74 to 12.03; n = 4) and lower Traditional Chinese medicine (TCM) syndrome severity (SMD −2.94; 95% CI −4.27 to −1.61; n = 2). Compared with WM alone, combined CHM-WM showed significant higher incidence of continuation of pregnancy after 28 gestational weeks (RR 1.21; 95% CI 1.16 to 1.27; n = 15; moderate quality of evidence), continuation of pregnancy after treatment (RR 1.19; 95% CI 1.16 to 1.23; n = 41; moderate quality of evidence), higher β-hCG level (SMD 2.27; 95% CI 1.72 to 2.83; n = 37) and lower TCM syndrome severity (SMD −1.74; 95% CI −2.21 to −1.27; n = 15). No significant differences in reducing the adverse maternal outcomes and neonatal death were found in combined CHM-WM compared with WM alone (RR 0.97; 95% CI 0.62 to 1.52; n = 8; RR 0.39; 95% CI 0.12 to 1.21; n = 2).
Conclusion: Current evidence supported CHM could be a potential treatment for threatened miscarriage. However, results should be interpreted with caution considering the low to moderate quality of the available evidence.
Systematic Review Registration: [https://inplasy.com/inplasy-2022-6-0107/], identifier [INPLASY20220107].
Threatened miscarriage is the most common pregnancy complication with an incidence of about 15%–20% and 20%–25% of the cases end up in spontaneous miscarriage. [(Wahabi et al., 2018), (Kouk et al., 2013)] Women with threatened miscarriages are 2.5 times more likely to miscarry than healthy women, which causes a huge physical and mental stress on women and their families. [(Sotiriadis et al., 2004), (Makrydimas et al., 2003)].
In clinical practice, many interventions have been used to prevent threatened miscarriage including bed rest (the most routinely prescribed intervention), anti-D immunoglobulin, endocrine regulation (e.g., the use of estrogen, progesterone, β-hCG), assisted reproductive technology, etc. [(Devall et al., 2021), (Sotiriadis et al., 2004), (Wahabi et al., 2018), (Aleman et al., 2005)] Although various interventions have been used to treat threatened miscarriage, most of them lack sufficient high-quality evidence to support their efficacy due to the unclear etiology and pathogenesis of threatened miscarriage. Thus, alternative medicines are greatly needed especially when Western medicine (WM) and other treatments are unable to provide a satisfactory therapeutic effect.
In China, Chinese herbal medicine (CHM) has been widely used in threatened miscarriage treatment for a long time. [(Giovanni, 1989)] For instance, “Shou Tai Wan (Quiet Foetus Pill)” or “An Tai Yin (Quiet Foetus Drink)” showed good protective effects against threatened miscarriage. (Chuang et al., 2007). Research showed that CHM can improve maternal-fetal immune ability, reduce the occurrence of inflammatory reactions and improve the level of endocrine in threatened miscarriage treatment. [(Ushiroyama et al., 2006), (Shen et al., 2022), (Deng et al., 2011)] Compared with WM alone, combined CHM-WM can achieve better curative effects for treating threatened miscarriage and no significant differences were found in adverse effects (Li et al., 2012a) However, more high-quality clinical evidence is needed to verify the effectiveness and safety of CHM on threatened miscarriage.
In our previous study, we made a systematic evaluation of the effectiveness of CHM for threatened miscarriage in 2012. (Li et al., 2012b). The meta-analysis showed a combination of CHM and WM was more effective than WM alone for treating threatened miscarriage, but all trials were methodologically poor and at unclear risk of bias overall. A large amount of randomized controlled trials (RCTs) was published over the past few years (Han et al., 2014; Duan et al., 2016; He, 2017; Guo et al., 2020; He, 2020; Cao and Yan, 2021; Dong and Zhang, 2021). Thus, we plan to update our review and systematically evaluate the effectiveness and safety of CHM for threatened miscarriage, ultimately providing more scientific and effective guidance for the clinical treatment of threatened miscarriage.
This systematic review and meta-analysis was conducted following a prospectively registered protocol (International Platform of Registered Systematic Review and Meta-analysis Protocols [INPLASY], INPLASY number INPLASY202260107, Supplementary Appendix A) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (PRISMA Checklist 2020; Supplementary Appendix B).
Only randomized controlled trials (RCTs) were included. Quasi-randomized, cluster-randomized trials, non-randomized trials, observational studies and cross-over trials were excluded. There were no language restrictions among all the included RCTs.
Women with threatened miscarriage at or before 28 gestational weeks regardless of underlying causes were included.
All types of CHM in either standard or combined regimens for the treatment of threatened miscarriage, regardless of the dose or duration of administration, were compared with placebo, no treatment or WM. We planned the following comparisons.
• CHM versus placebo.
• CHM versus no treatment (including bed rest).
• CHM alone versus WM alone.
• Combined CHM and WM (CHM-WM) versus WM alone.
Primary outcome
Pregnancy after 28 gestational weeks is generally considered viable, and miscarriage before 28 weeks is considered non-viable due to the extremely low birth weight and underdevelopment. In this review, only viable pregnancies and continuation of pregnancy after 28 gestational weeks were considered as the primary outcome.
The incidence of continuation of pregnancy after 28 gestational weeks = (total cases-cases of miscarriage)/total cases × 100%.
Secondary outcomes
The incidence of continuation of pregnancy after treatment = (total cases-ineffective cases)/total cases × 100%.
Ineffective cases: no significant decrease in abdominal pain, vaginal bleeding and other symptoms, but even aggravated. B ultrasound showed that the embryo size did not conform to the gestational age. (National Administation of Traditional Chinese Medidine, 2002).
The incidence of preterm birth = preterm birth cases/total cases × 100%.
Patients were reported to have nausea, vomiting, headaches, mouth dryness, constipation, insomnia, diarrhea, rash, breast swelling and pain, etc. after treatment.
Neonatal were reported to have malformation, jaundice and genital bleeding, etc. after treatment.
The incidence of neonatal death = neonatal death cases/total cases × 100%.
TCM syndrome severity was evaluated by TCM syndrome score. (National Administation of Traditional Chinese Medidine, 2002). The TCM syndrome score was recorded and graded based on the degree of individual symptoms and all indicators were determined by comparing the values obtained after treatment with the baseline values. The scores of TCM syndromes were recorded according to the standards in ‘Guiding Principles for Clinical Research of New Chinese Medicine’. (National Administation of Traditional Chinese Medidine, 2002). According to the severity of symptoms, the scores were calculated from a 0–3 scale: 0 as light, 1 as mild, 2 as moderate and 3 as severe. Higher scores indicated severer symptoms. (Zheng, 2002).
Research reported that the well-developed embryos in early pregnancy indicated β-hCG doubly increasing every other day. [(Brady et al., 2020), (Zhong et al., 2000), (Batzer et al., 1983), (Barnhart et al., 2004)] The level of β-hCG varies from individual to individual. With the prolongation of pregnancy, β-hCG will continue increasing, generally reaching a peak around the 10th week of pregnancy, and then decreasing. A significant increase in β-hCG predicts a successful pregnancy. While an abnormally low β-hCG level before 24 gestational weeks was associated with a risk of spontaneous loss. [(Braunstein et al., 1978), (Dugoff et al., 2004)]
Databases including PubMed, Cochrane Pregnancy and Childbirth’s Trials Register, VIP, Central, Embase, Medline, China National Knowledge Infrastructure (CNKI), and WanFang Database were searched for all published RCTs. The search for the previous publication ended on 31 January 2012. Therefore, we searched from 1 February 2012 until 30 June 2022. Search strategies were designed with terms related to “threatened miscarriage”, “CHM”, “WM”, “RCT”, etc. No language restrictions were used. For the complete search strategy, see Supplementary Appendix C.
Two review authors (HL. Xie and AL. Zhang) independently assessed each trial for inclusion and any disagreements were resolved through discussion. If the disagreements could not be resolved, the arbiter (L. Li) made a final decision on the selected study. Details of the study selection were shown in the PRISMA study flow diagram, see Figure 1.
Two review authors (HL. Xie and AL. Zhang) developed a checklist for data recording, and independently extracted data through a standardized eligibility form. We resolved discrepancies through discussion or consulted the arbiter (L. Li) if necessary. When the data of RCTs were insufficient or ambiguous, we contacted corresponding authors for detailed information.
Two review authors (HL. Xie and AL. Zhang) independently performed the risk of bias using the Risk of Bias 2 (RoB-2) tool. (Sterne et al., 2019). It included five domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome and bias in selection of the reported result. Each domain was assessed at low, or high risk of bias, or can be expressed as “some concerns”.
We used Review Manager software (RevMan 5.4.1, 2020) for statistical analysis. Risk ratio (RR) and 95% confidence interval (CI) were used to analyze the effect size of dichotomous data. Besides, we used the standardized mean difference (SMD) to eliminate inconsistencies in units of measurement and measurement variances. I2 quantitative tests were used to test the heterogeneity among the RCTs. When p < 0.10, I2 > 50%, it suggested that there was high heterogeneity between studies, and the random-effect model shall be selected for meta-analysis. When p > 0.10, I2 < 50%, no obvious heterogeneity is suggested, and the fixed-effect model was selected for meta-analysis. Subgroup analysis was performed on the treatment course (short-term treatment (one course only) versus long-term treatment (more than one course)); gestational weeks (≤12 versus>12). As a sensitivity analysis, estimates were performed by excluding studies and analyzing the remaining studies to test the robustness of our results. The certainty of outcomes was interpreted using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach. [(GRADEpro, 2021), (The GRADE working group Schünemann et al., 2013)].
A total of 798 clinical studies were identified by our updated search. After the removal of duplicates, 629 studies were screened by title and/or abstract, and 569 studies were excluded initially according to the inclusion and exclusion criteria. Full texts of 60 studies were further reviewed, and 8 studies were further excluded with the reasons: 1 study only had abstract without accessible data; (Chen, 2013) 3 studies had the wrong randomization that the patients were randomly grouped by researchers;[(Xie, 2016), (Liu, 2015), (Yu and Yang, 2017)] 1 study reported incomplete outcome; (Zhang and Liu, 2016); 1 study increased the number of patients after randomization, thus we didn’t know how many patients were involved in different outcome assessment; (Tian and Chen, 2016); 1 study used TCM nursing which is inappropriate for our types of intervention; (Zhou and Jiang, 2015) 1 study was an ongoing study without accessible data. (Wu, 2021) We also re-assessed the 44 RCTs included and 2 RCTs awaiting classification in the previously published review. Only 5 RCTs meet the inclusion in this update, therefore, a total of 57 RCTs including 5,881 patients were included in this updated review, see Figure 1. Amongst them, 11 RCTs (involving 1,029 women) compared CHM alone with WM alone, and 46 RCTs (involving 4,852 women) compared combined CHM-WM with WM alone. [(Cao and Yan, 2021), (Dong and Zhang, 2021), (Duan et al., 2016), (Guo et al., 2020), (Han et al., 2014), (He, 2017), (He, 2020), (Huang, 2020), (Jiang, 2019), (Ju, 2017), (Kang and Wang, 2018), (Kong et al., 2021), (Lai et al., 2020a), (Lai et al., 2020b), (Li et al., 2004), (Li et al., 2006), (Li, 2019), (Li et al., 2020), (Liu et al., 2012), (Liu and Cui, 2016), (Liu, 2018), (Liu et al., 2020), (Liu, 2020), (Liu, 2021), (Long, 2018), (Luo, 2020), (Ma, 2019), (Mi et al., 2021), (Nong, 2019), (Shang et al., 2021), (Song and Ni, 2013), (Song et al., 2018), (Su, 2016), (Teng, 2018), (Wang and Niu, 2019), (Wu et al., 2017), (Xiao, 2008), (Xie, 2014), (Xin et al., 2018), (Yang, 2006), (Yang, 2012), (Ye and Tao, 2021), (Yu, 2018), (Yu et al., 2019), (Yu et al., 2021), (Yu and Jiang, 2021), (Xiao, 2008), (Zhang and Ding, 2015), (Zhang, 2017), (Zhang et al., 2019), (Zhang, 2020), (Zhang and Hou, 2020), (Zhang, 2021a), (Zhang, 2021b), (Zheng et al., 2020), (Zhu and Wang, 2019), (Zhuang, 2016)] We did not find any comparisons of CHM with placebo or no treatment in the included studies. WM treatments included tocolytic drugs (e.g., aspirin and dydrogesterone), hormonal supplementations (e.g., HCG and progesterone) and supportive supplements (e.g., vitamin E and folic acid). CHM treatments included Chinese formulae (e.g., Jiawei Shoutai pill, Zishen Yutai pill, Shoutai Yigong powder, Guben Antai decoction, etc.), as shown in Table 1 and Table 2. These Chinese formulae were composed of different botanical drugs, of which the detailed information was listed in Supplementary Appendix D.
The included RCTs were conducted in different cities in China. Both inpatients and outpatients were included. Although all the included studies did not report the registration of a clinical trial, they all received ethical approval following Chinese Good Clinical Practice. (gongbao, 2020). No trials included in our analysis have been retracted. Three studies reported there was no conflict of interest, [(Lai et al., 2020b), (Shang et al., 2021), (Zheng et al., 2020)] while other studies did not report whether there was a conflict of interest. A summary of the characteristics of 57 RCTs including randomization, method, patients, interventions and outcomes was shown in Table 1 and Table 2.
The results of the RoB-2 evaluation were presented in Supplementary Figures S1,S2. No study was judged to be at low risk of bias for all 5 domains. The most common shortcoming was the failure to report the allocation sequence concealment. It was impossible to carry out the blinding for CHM in the clinical trials because patients in China receiving CHM insist on knowing the specific drugs to ensure drug safety and the potential harm to their pregnancies. Besides, there are obvious differences between CHM and WM in packaging, way of administration and taste. However, because most outcomes were objectively measured, the included RCTs were considered at a ‘low’ risk of bias despite a lack of outcomes assessment blinding. We judged the domain of reporting at low risk of bias even though the pre-specified plan of each included trial is inaccessible or unavailable. Because according to Good Clinical Practice of China, clinical trials must provide a trial protocol including ethics approval and must be reviewed and approved by experts before they were carried out. [92] Overall, all RCTs were rated as having an unclear risk of bias in allocation concealment and judged to be at low risk of bias for the other four domains.
Primary outcome
Only 1 study including 166 patients reported the incidence of continuation of pregnancy after 28 gestational weeks was significantly increased in CHM alone group compared with WM alone group (RR 1.11, 95% CI 1.02 to 1.21; p = 0.02, Figure 2A). (Li et al., 2020) The certainty of the evidence was judged as moderate due to imprecision caused by few patients, see Supplementary Table S1.
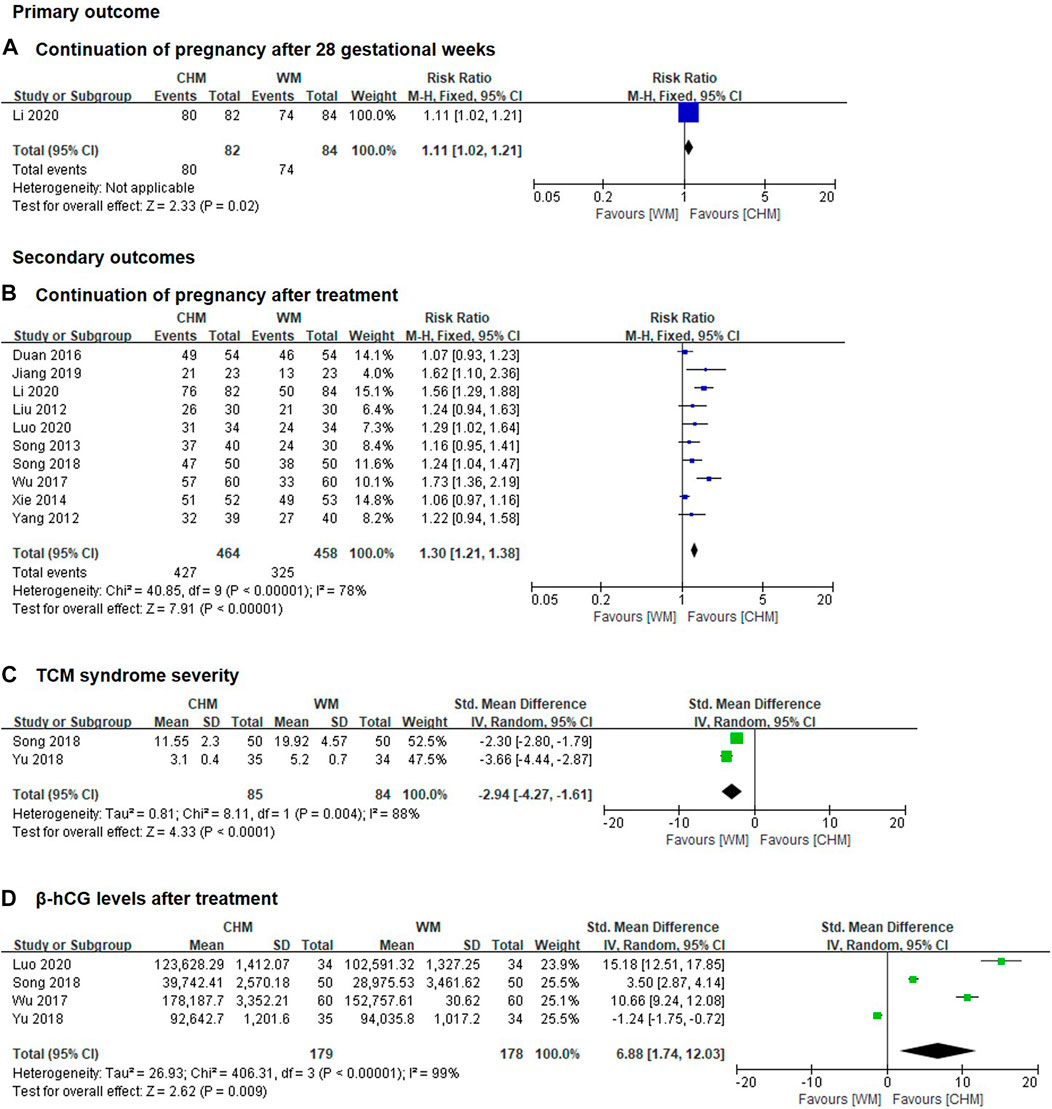
FIGURE 2. Forest plot of CHM alone group vs. WM alone group (A) continuation of pregnancy after 28 gestational weeks; (B) continuation of pregnancy after treatment; (C) TCM syndrome severity; (D) β-hcG levels after treatment.
Secondary outcomes
Ten trials including 922 patients reported the incidence of continuation of pregnancy after treatment was significantly increased in CHM alone group compared with WM alone group (RR 1.30, 95% CI 1.21 to 1.38, I2 = 78%, p < 0.00001, Figure 2B). (Liu et al., 2012; Yang, 2012; Song and Ni, 2013; Xie, 2014; Duan et al., 2016; Wu et al., 2017; Song et al., 2018; Jiang, 2019; Li et al., 2020; Luo, 2020). The certainty of the evidence was judged as moderate due to imprecision caused by few patients, see Supplementary Table S2.
No data is available for preterm birth in this comparison group.
No data is available for adverse maternal outcomes in this comparison group.
No data is available for adverse neonatal outcomes in this comparison group.
No data is available for neonatal death in this comparison group.
SMD with 95% CI was used as a measurement to eliminate the effect of dimension because different definitions of TCM syndrome severity were reported in different studies. Two trials included 169 patients reported the TCM syndrome severity was significantly reduced in CHM alone group compared with WM alone group (SMD -2.94, 95% CI -4.27 to −1.61, I2 = 88%, p < 0.0001, Figure 2C). (Song et al., 2018; Yu, 2018). The certainty of the evidence for this outcome was assessed as low due to imprecision caused by a wide 95% CI and a small number of trials and patients, see Supplementary Table S1.
SMD with 95% CI was used as a measurement to eliminate the effect of dimension because the data of β-hCG extracted from different studies were reported using different units. Research has shown that a higher level of β-hCG was associated with a better pregnancy success rate. Four clinical trials including 357 patients reported that the level of β-hCG was significantly increased in CHM alone group compared with WM alone group after treatment (SMD 6.88, 95% CI 1.74 to 12.03, I2 = 99%, p = 0.009, Figure 2D). (Wu et al., 2017; Song et al., 2018; Yu, 2018; Luo, 2020). The certainty of the evidence for this outcome was assessed as low due to imprecision caused by a wide 95% CI and a small number of trials and patients, see Supplementary Table S1.
Primary outcome
In total, 15 clinical trials with 1,519 patients suggested that the incidence of continuation of pregnancy after 28 gestational weeks was significantly increased in combined CHM-WM group compared with WM alone group (RR 1.21, 95% CI 1.16 to 1.27, I2 = 0%, p < 0.00001, Figure 3A). [(Cao and Yan, 2021), (He, 2020), (Huang, 2020), (Kong et al., 2021), (Lai et al., 2020b), (Liu et al., 2020), (Liu, 2020), (Liu, 2021), (Long, 2018), (Ma, 2019), (Nong, 2019), (Shang et al., 2021), (Xin et al., 2018), (Ye and Tao, 2021), (Zhu and Wang, 2019)] The certainty of the evidence was assessed as moderate due to imprecision caused by small number of patients, see Supplementary Table S2.
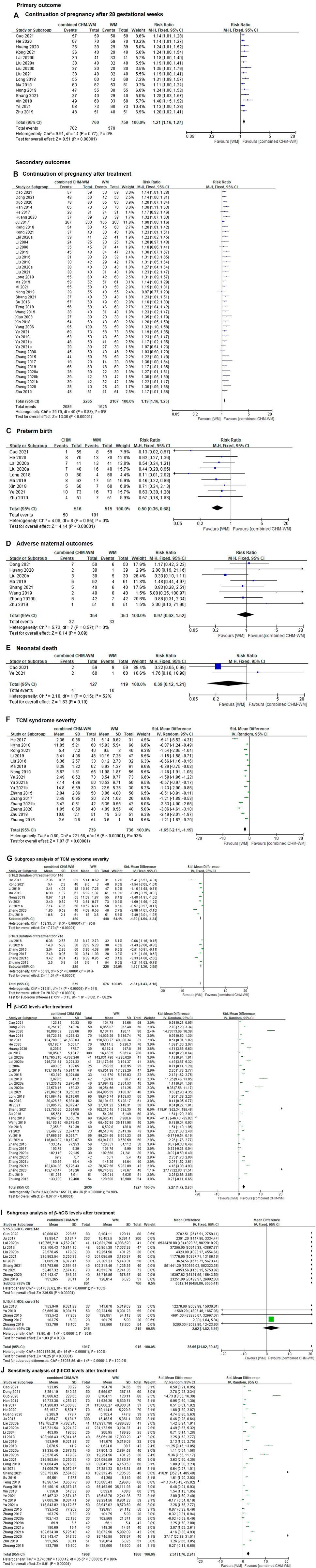
FIGURE 3. Forest plot of combined CHM-WM group versus WM alone group. (A) Continuation of pregnancy after 28 gestational weeks; (B) Continuation of pregnancy after treatment; Preterm birth; (D) Adverse maternal outcomes; Neonatal death; TCM syndrome severity; (G) Subgroup analysis of TCM syndrome severity; β-hCG levels after treatment; Subgroup analysis of β-hCG levels after treatment; Sensitivity analysis of β-hCG levels after treatment. (C) preterm birth; (E) neonatal death; (F) TCM syndrome severity; (H) β-hCG levels after treatment; (I) subgroup analysis of β-hCG levels after treatment; (J) sensitivity analysis of β-hCG levels after treatment.
Secondary outcomes
Forty-one clinical trials including 4,372 patients suggested that the incidence of continuation of pregnancy after treatment was significantly increased in combined CHM-WM compared with WM alone group (RR 1.19, 95% CI 1.16 to 1.23, I2 = 0%, p < 0.00001, Figure 3B). [(Cao and Yan, 2021), (Dong and Zhang, 2021), (Guo et al., 2020), (Han et al., 2014), (He, 2017), (Huang, 2020), (Ju, 2017), (Kang and Wang, 2018), (Kong et al., 2021), (Lai et al., 2020a), (Li et al., 2004), (Li et al., 2006), (Li, 2019), (Liu and Cui, 2016), (Liu, 2018), (Liu et al., 2020), (Liu, 2021), (Long, 2018), (Ma, 2019), (Mi et al., 2021), (Nong, 2019), (Shang et al., 2021), (Su, 2016), (Teng, 2018), (Wang and Niu, 2019), (Xiao, 2008), (Xin et al., 2018), (Yang, 2006), (Ye and Tao, 2021), (Yu et al., 2019), (Yu et al., 2021), (Yu and Jiang, 2021), (Xiao, 2008), (Zhang and Ding, 2015), (Zhang, 2017), (Zhang et al., 2019), (Zhang, 2020), (Zhang and Hou, 2020), (Zhang, 2021b), (Zheng et al., 2020), (Zhu and Wang, 2019)] The certainty of the evidence was assessed as moderate due to imprecision caused by small number of patients, see Supplementary Table S2.
Preterm birth rates were reported in nine trials including 1,031 patients, which showed the preterm birth rate was significantly reduced in combined CHM-WM group compared with WM alone group (RR 0.50, 95% CI 0.36 to 0.68, I2 = 0%, p < 0.00001, Figure 3C). [(Cao and Yan, 2021), (He, 2020), (Lai et al., 2020b), (Liu et al., 2020), (Long, 2018), (Ma, 2019), (Xin et al., 2018), (Ye and Tao, 2021), (Zhu and Wang, 2019)] The certainty of the evidence for this outcome was assessed as low due to imprecision caused by a wide 95% CI and a small number of trials and patients, see Supplementary Table S2.
In total, eight trials including 707 patients reported no significant difference in reducing the incidence of adverse in combined CHM-WM group compared with WM alone group (RR 0.97, 95% CI 0.62 to 1.52, I2 = 0%; p = 0.89, Figure 3D). [(Dong and Zhang, 2021), (Huang, 2020), (Liu, 2020), (Ma, 2019), (Shang et al., 2021), (Wang and Niu, 2019), (Zhang and Hou, 2020), (Zhu and Wang, 2019)] The certainty of the evidence for this outcome was assessed as low due to imprecision caused by a wide 95% CI and a small number of trials and patients, see Supplementary Table S2. Detailed adverse outcomes of each study were shown in the summary of adverse events in the included studies, see Supplementary Table S3.
Only 1 trial reported neonatal malformation in combined CHM-WM group. (Ye and Tao, 2021).
Two trials including 246 patients reported neonatal death, of these, four neonatal death cases were reported in patients receiving combined CHM-WM and 10 neonatal death cases were reported in patients who received WM alone. No significant difference in reducing the incidence of neonatal death was found in combined CHM-WM group compared with WM alone group. (RR 0.39; 95% CI 0.12 to 1.21, I2 = 52%, p = 0.15, Figure 3E). (Cao and Yan, 2021; Ye and Tao, 2021) The certainty of the evidence for this outcome was assessed as low due to imprecision caused by a wide 95% CI and a small number of trials and patients, see Supplementary Table S2.
Sixteen trials included 1,475 patients suggested that the TCM syndrome severity was significantly reduced in the combined CHM-WM group compared with WM alone group (SMD -1.65, 95% CI –2.11 to −1.19, I2 = 93%, p < 0.00001, Figure 3F). [(He, 2017), (Kang and Wang, 2018), (Kong et al., 2021), (Li, 2019), (Liu and Cui, 2016), (Ma, 2019), (Nong, 2019), (Ye and Tao, 2021), (Yu et al., 2021), (Yu and Jiang, 2021), (Zhang and Ding, 2015), (Zhang, 2017), (Zhang, 2021b), (Zheng et al., 2020), (Zhu and Wang, 2019), (Zhuang, 2016)] The reason for using continuous outcome was the same as we have discussed in the CHM alone versus WM alone section. The certainty of the evidence for this outcome was assessed as low due to imprecision caused by a wide 95% CI and a small number of trials and patients, see Supplementary Table S2.
Subgroup analysis
We decided to conduct a subgroup analysis to investigate the source of the heterogeneity in the TCM syndrome score. Although the duration of treatment showed a lower TCM syndrome score in combined CHM-WM group compared with WM alone group, the heterogeneity was still high (I2 = 94%, Figure 3G). Short-term treatment (one course only) and long-term treatment (more than one course) did not show any difference in reducing the TCM syndrome score (p = 0.08, Figure 3G). Subgroup analysis for other planned comparisons was not available.
Thirty-seven RCTs including 3,957 patients suggested that the level of β-hCG was significantly reduced in combined CHM-WM group compared with WM alone group after treatment (SMD 2.27, 95% CI 1.72 to 2.83, I2 = 98%, p < 0.00001, Figure 3H). [(Cao and Yan, 2021), (Dong and Zhang, 2021), (Guo et al., 2020), (Han et al., 2014), (He, 2017), (He, 2020), (Huang, 2020), (Ju, 2017), (Lai et al., 2020a), (Lai et al., 2020b), (Li et al., 2004), (Li, 2019), (Liu and Cui, 2016), (Liu, 2018), (Liu et al., 2020), (Liu, 2020), (Liu, 2021), (Long, 2018), (Ma, 2019), (Mi et al., 2021), (Shang et al., 2021), (Su, 2016), (Teng, 2018), (Wang and Niu, 2019), (Xin et al., 2018), (Ye and Tao, 2021), (Yu et al., 2019), (Yu et al., 2021), (Zhang and Ding, 2015), (Zhang, 2017), (Zhang, 2020), (Zhang and Hou, 2020), (Zhang, 2021a), (Zhang, 2021b), (Zheng et al., 2020), (Zhu and Wang, 2019), (Zhuang, 2016)] The reason for using continuous outcome was the same as we have discussed in the CHM alone versus WM alone section. The certainty of the evidence for this outcome was assessed as low due to imprecision caused by a wide 95% CI and a small number of trials and patients, see Supplementary Table S2.
Subgroup analysis
Duration of treatment showed a higher level of β-hCG in combined CHM-WM when compared to WM alone after treatment, but heterogeneity was still high (I2 = 100%, Figure 3I). Short-term treatment (one course only) had a higher level of β-hCG when compared to long-term treatment (more than one course) (p < 0.00001, Figure 3I). Subgroup analysis for other planned comparisons was not available.
Sensitivity analysis
Sensitivity analysis was carried out by removing one trial with a gestation week range from 7 to 28. However, the heterogeneity was still high (SMD 2.34, 95% CI 1.76 to 2.91, I2 = 98%, Figure 3J). (Ma, 2019).
The certainty of the evidence for continuation of pregnancy after 28 gestational weeks and continuation of pregnancy after treatment were judged to be moderate. And the certainty of the evidence for adverse maternal outcomes, preterm birth, neonatal death, TCM syndrome severity and β-hCG levels were judged to be low. Downgrading of evidence was based on different serious limitations of the imprecision due to the wide 95% CI and a small number of trials and patients. Although high heterogeneity in TCM syndrome severity and β-hCG levels were reported in this meta-analysis, heterogeneity was inevitable. Because the TCM syndrome severity was recorded by TCM syndrome score, and the judgments were made by TCM practitioners. Besides, the level of β-hCG changed a lot during the first trimester of pregnancy and β-hCG itself varies greatly among different participants. As the trials have provided the reason for the inconsistency in TCM syndrome severity and β-hCG levels and it is not related to the nature of the results. Thus, we decided not to downgrade the certainty of inconsistency. See Supplementary Tables S1,S2 for the details of the GRADE assessment.
CHM alone versus WM alone for threatened miscarriage
Eleven RCTs with 1,029 women were included in this comparison of this updated review. In the treatment of threatened miscarriage, CHM alone group showed significant benefits in improving continuation of pregnancy after 28 weeks of gestation, continuation of pregnancy after treatment, TCM syndrome severity and β-hCG levels compared with WM alone group. No trials in this comparison reported adverse maternal outcomes, adverse neonatal outcomes, preterm birth and neonatal death. Due to a small number of trials and lack of detailed information, it was impossible to carry out the planned subgroup analyses.
Combined CHM-WM versus WM alone for threatened miscarriage
Forty-six RCTs with 4,852 women were included in this comparison of this updated review. In the treatment of threatened miscarriage, combined CHM-WM group showed significant improvement in the incidence of continuation of pregnancy after 28 weeks of gestation, continuation of pregnancy after treatment, preterm birth, TCM syndrome severity and β-hCG levels compared with WM alone group. While no significant differences in reducing the incidence of adverse maternal outcomes and neonatal death were found in this comparison group. Only one trial reported adverse neonatal outcomes in combined CHM-WM group. As more RCTs were included in this comparison and showed combined CHM-WM was more effective, CHM therapy could be a potential treatment for threatened miscarriage.
Meta-analysis of β-hCG and TCM syndrome severity showed high heterogeneity, thus we carried out subgroup analysis and sensitivity analysis to identify the potential source of the heterogeneity. However, the heterogeneity did not decrease significantly. After analysis of the article, the reason for high heterogeneity might be: 1) The level of β-hCG varies greatly among different patients during the first trimester of pregnancy (gestational week <12 weeks). Studies published by other teams also showed high heterogeneity of β-hCG but without any further investigation for the cause of high heterogeneity; [(Li et al., 2021), (Salari et al., 2020)] 2) Different studies used different CHM prescription ingredients, doses and treatment duration. Therefore, high heterogeneity is inevitable among the included studies. The certainty of the evidence for the main outcomes was from moderate to low due to different serious limitations of the imprecision caused by the range of 95% CI and the number of trials and patients. As most of the included trials had different CHM formulations, dosage, and duration of therapy, making a uniform protocol for standard treatment may be difficult and it also violates the principle of TCM syndrome differentiation and treatment.
Compared with other published meta-analyses by different teams, the same conclusion (CHM alone or combined CHM-WM was more effective than WM alone for threatened miscarriage treatment) on CHM for threatened miscarriage was reported. [(Gao et al., 2016), (He et al., 2019)] However, they are all limited by poor methodologies, such as, only a small number of RCTs were included, and most of them were assessed at high risk of bias and published years ago. Besides, they mainly focus on the efficacy evaluation of CHM treatment and barely consider the safety assessment due to the limited data.
Comparisons between this updated review and our previously published review in 2012 are: 1) In our previous review, 44 RCTs were included for meta-analysis but only 11 trials reported the details of randomization methods, so the sample size and quality of the included studies are limited. Based on the limitation, in this updated review we only included studies with details of randomization methods, and in total 57 RCTs were included for meta-analysis, more rigorous methodology and qualified RCTs. Besides, all the included RCTs were reported at low risk of bias at intervention deviation, result selection and outcome measurement. Hence, the results of the updated review provided a higher quality of evidence and a credible conclusion to the clinical practice; 2) In our previous review, there was no laboratory data available for meta-analysis. In this updated review, we collected and compared the values of laboratory investigations, for example, improvement in β-hCG levels after treatment as a surrogate indicator of efficacy. In addition, we also included other outcome measures, including TCM syndrome severity as an important indicator to evaluate the efficacy of TCM based on Chinese medicine theory. Our updated review provided more comprehensive information and both laboratory data and TCM syndrome severity supported the efficacy of CHM in the treatment of threatened miscarriage; 3) In our previous review, no safety outcome was reported, thus we were unable to draw any conclusion about the safety of CHM for mothers and fetuses. In this updated review, we have included and compared the adverse outcomes, including adverse maternal outcomes and neonatal death. The results showed no significant difference between the combined CHM-WM group and WM alone group, suggesting that CHM is safe for pregnancy.
Therefore, based on the above advantages in this updated review, we have a larger sample size, more high-quality studies and stronger evidence to confirm both the efficacy and safety of CHM for threatened miscarriage to recommend CHM to be a potential alternative therapy for physicians and patients in the treatment of threatened miscarriage.
The main strengths of our study included: 1) a rigorous methodology was conducted for performing the updated systematic review and meta-analysis; 2) the inclusion of a relatively large number of RCTs and most of which were recently published; 3) strict risk of bias assessment were conducted in all RCTs, which made the finding more persuasive and reliable; 4) A broad possible range of reported outcomes were included, which provide a comprehensive summary of relevant information to support the efficacy of CHM for threatened miscarriage; 5) Potential sources of heterogeneity were determined and discussed in details through several subgroup analyses and sensitivity analysis.
Several limitations need to be considered in the interpretation of our results. 1) High heterogeneity of TCM syndrome severity and β-hCG were reported, which have been discussed in the ‘GRADE certainty of the evidence’ section and ‘Principal findings’ section; 2) Chinese medicine practitioners would slightly modify the classical formula by adding or removing some CHM for better treatment effects according to the TCM syndrome differentiation and treatment principle. Therefore, outcome data were not reported according to the CHM formula, making relevant subgroup analysis impracticable. 3) Very few RCTs reported adverse events, due to the limited available data, planned sensitivity analysis could not be performed. Therefore, more high-quality RCTs are needed to provide sufficient information for the long-term study of CHM’s safety in treating threatened miscarriage.
Worldwide, there is currently no effective treatment for threatened miscarriage. Our meta-analysis of 57 RCTs suggested that CHM could be a potential treatment for threatened miscarriage, given that it showed a significant immediate effect on continuation of pregnancy after treatment and improvement in a longer gestational stage after 28 weeks. Besides, no increased risk of adverse events was found in either the mother or the newborn in combined CHM-WM group compared with WM alone group, but this finding was partial and requires further evaluation. Furthermore, according to the reported studies and clinical evidence, it showed that CHM could be a potential intervention when given for the prevention of miscarriage [(Li et al., 2016), (Zhou et al., 2022), Therefore, our updated review recommended CHM therapy as an alternative for threatened miscarriage, as it can prevent miscarriage better, without increasing maternal and neonatal risk. However, long-term safety information is still lacking which requires further investigation. Therefore, larger sample size and RCTs with a low risk of bias are needed to facilitate the accumulation of high-certainty evidence and the translation of clinical practice.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
LL, XF, and CCW contributed to the study conception and design; HX, AZ, XM, TH, and JL collected the data; HX and AZ analyzed the data; HX, AZ, XM, and LL interpreted the work; HX and LL drafted the manuscript; LL, CCW, and XF critically revised the manuscript. All authors commented on the drafts and approved the final draft; LL, XF, and CCW are the guarantors.
This work was supported by the Source of Funding: Zhejiang Provincial Natural Science Foundation (LY20H180004), Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-D-202002), Hong Kong Research Grant Council General Research Fund 14122021, Hong Kong Food and Health Bureau Health and Medical Research Fund 15160971, and National Public Welfare Projects for Chinese Medicine (201507001). Fundamental Research Funds for the Central Universities (226-2022-00226).
We appreciate the help given by the Deputy Managing Editor and other colleagues in the editorial office of the Cochrane Pregnancy and Childbirth Group, Department of Women’s and Children’s Health, Institute of Translational Medicine, University of Liverpool in UK.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1083746/full#supplementary-material
Aleman, A., Althabe, F., Belizán, J., and Bergel, E. (2005). Bed rest during pregnancy for preventing miscarriage. Cochrane Database Syst. Rev. 2005, CD003576. doi:10.1002/14651858.CD003576.pub2
Barnhart, K. T., Sammel, M. D., Rinaudo, P. F., Zhou, L., Hummel, A. C., and Guo, W. (2004). Symptomatic patients with an early viable intrauterine pregnancy: HCG curves redefined. Obstet. Gynecol. 104, 50–55. doi:10.1097/01.AOG.0000128174.48843.12
Batzer, F. R., Weiner, S., Corson, S. L., Schlaff, S., and Otis, C. (1983). Landmarks during the first forty-two days of gestation demonstrated by the beta-subunit of human chorionic gonadotropin and ultrasound. Am. J. Obstet. Gynecol. 146, 973–979. doi:10.1016/0002-9378(83)90977-8
Brady, P. C., Farland, L. V., Racowsky, C., and Ginsburg, E. S. (2020). Hyperglycosylated human chorionic gonadotropin as a predictor of ongoing pregnancy. Am. J. Obstet. Gynecol. 222, 68.e1–68. doi:10.1016/j.ajog.2019.08.004
Braunstein, G. D., Karow, W. G., Gentry, W. C., Rasor, J., and Wade, M. E. (1978). First-trimester chorionic gonadotropin measurements as an aid in the diagnosis of early pregnancy disorders. Am. J. Obstet. Gynecol. 131, 25–32. doi:10.1016/0002-9378(78)90469-6
Cao, K., and Yan, T. Q. (2021). Effect of integrated traditional Chinese and Western medicine on early threatened abortion and pregnancy outcome. China Med. Her. [Zhong Guo Yi Yao Dao. Bao 18, 107–110.
Chen, Y. (2013). Clinical analysis of combined Traditional Chinese and Western medicine for threatened abortion. Road Health Mag. [Jian Kang Zhi Lu 12, 268–269.
Chuang, C. H., Hsieh, W. S., Guo, Y. L., Tsai, Y. J., Chang, P. J., Lin, S. J., et al. (2007). Chinese herbal medicines used in pregnancy: A population-based survey in taiwan. Pharmacoepidemiol Drug Saf. 16, 464–468. doi:10.1002/pds.1332
Deng, J. S., Chi, C. S., Huang, S. S., Shie, P. H., Lin, T. H., and Huang, G. J. (2011). Antioxidant, analgesic, and anti-inflammatory activities of the ethanolic extracts of Taxillus liquidambaricola. J. Ethnopharmacol. 137, 1161–1171. doi:10.1016/j.jep.2011.07.041
Devall, A. J., Papadopoulou, A., Podesek, M., Haas, D. M., Price, M. J., Coomarasamy, A., et al. (2021). Progestogens for preventing miscarriage: A network meta-analysis. Cochrane Database Syst. Rev. 4, CD013792. doi:10.1002/14651858.CD013792.pub2
Dong, R., and Zhang, Y. (2021). Clinical study on Zishen Yutai Pills combined with progesterone in treatment of early threatened abortion. China Med. Her. [Xian Dai Yao Wu Yu Lin. Chuang] 36, 1194–1198.
Duan, Z. Z., Pan, H. Y., Chen, L., and Zhong, H. Z. (2016). 54 cases of early threatened abortion treated with Bushen Jianpi Antai Decoction and emotional therapy. Jiangxi J. Traditional Chin. Med. [Jiang Xi Zhong Yi Yao] 47, 49–51.
Dugoff, L., Hobbins, J. C., Malone, F. D., Porter, T. F., Luthy, D., Comstock, C. H., et al. (2004). First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: A population-based screening study (the FASTER trial). Am. J. Obstet. Gynecol. 191, 1446–1451. doi:10.1016/j.ajog.2004.06.052
Gao, F. X., Wu, H. W., Gao, J., and Luo, S. P. (2016). Shoutai pill for threatened miscarriage: Meta-analysis of randomized controlled trials. Chin. Archives Traditional Chin. [Zhong Hua Zhong Yi Yao Xue Kan] 34, 2637–2642.
Giovanni, M. (1989). The foundations of Chinese medicine: A comprehensive text for acupuncturists and herbalists. Edinburgh, UK: Churchill Livingstone, 219–268.
gongbao (2020). Announcement of the national Food and drug administration and the national Health commission on the issuance of quality management standards for drug clinical trials. Beijing, China: National Medical Products Administration. Availabe at: http://www.gov.cn/gongbao/content/2020/content_5525106.htm (accessed April 23, 2020).
Gradepro, G. D. T. (2021). GRADEpro guideline development tool. Hamilton, ON: McMaster Univ Evid Prime. Available at: https://www.gradepro.org/ (accessed November 30, 2021).
Guo, Y., Zhang, S. R., and Wang, H. W. (2020). Treatment of 80 cases of early threatened abortion with Xiongguijiaoai Decoction and progesterone capsule. Henan Tradit. Chin. Med. [Hen Nan Zhong Yi] 40, 363–366.
Han, C. Y., Sun, Z. X., Han, J. J., and Zhang, J. D. (2014). Clinical observation on treating 70 cases of threatened abortion in the integrative medicine. Clin. J. Chin. Med. [Zhong Yi Lin Chuang Yan Jiu] 6, 83–84.
He, D. M., Huang, J. M., Zhuang, J. L., and Li, J. B. (2019). Meta analysis of the clinical effect of Shoutai pill on threatened abortion. Traditional Chin. Med. Res. [Zhong Yi Yan Jiu] 32, 60–63.
He, J. L. (2017). Observation on the curative effect of Shoutai Yigong San combined with progesterone in the treatment of early threatened abortion of Kidney Deficiency and blood stasis type. Mod. J. Integr. Traditional Chin. West. Med. [Xian Dai Zhong Xi Yi Jie He Za Zhi] 26, 1568–1570.
He, P. (2020). Clinical effect of Guben Antai Decoction on pregnant women with early threatened abortion. Maternal Child Health Care China [Zhong Guo Fu You Bao Jian 35, 4546–4548.
Huang, L. (2020). Effect of Gushen Antai Pills and dydrogesterone on serum endocrine hormone levels and pregnancy outcomes in patients with early threatened abortion. Guangming J. Chin. Med. [Guang Ming Zhong Yi] 35, 3969–3971.
Jiang, G. X. (2019). Clinical application study of Chinese medicine dialectical treatment of threatened abortion with insufficient corpus luteum function. China Health Care and Nutr. [Zhongguo Bao Jian Yang 29, 93.
Ju, J. (2017). Curative effect of TCM syndrome differentiation on early threatened abortion. Biped Health [Shuang Zu Yu Bao Jian 26, 191–195.
Kang, Y. F., and Wang, C. H. (2018). Observation on therapeutic effect of integrated Traditional Chinese and Western medicine on threatened abortion. Shanxi J. Traditional Chin. Med. [Shan Xi Zhong Yi] 34, 29–32.
Kong, D. J., Guo, Q. G., and Huang, F. X. (2021). Effect of Bushenhuoxue Recipe combined with Dydrogesterone in treating early threatened abortion of Kidney Deficiency and blood stasis type. Chin. J. Rural Med. Pharm. [Zhong Guo Xiang Cun Yi Yao] 28 (02), 23–24.
Kouk, L. J., Neo, G. H., Malhotra, R., Allen, J. C., Beh, S. T., Tan, T. C., et al. (2013). A prospective study of risk factors for first trimester miscarriage in Asian women with threatened miscarriage. Singap. Med. J. 54, 425–431. doi:10.11622/smedj.2013148
Lai, J., Liu, F., Qian, L. F., Ke, Z. M., Deng, J., and Ma, R. F. (2020). Clinical effect of addition and subtraction of traditional Chinese medicine adjuvant therapy on threatened abortion and its influence on related factors. Chin. J. General Pract. [Zhong Hua Quan Ke Yi Xue] 18 (5), 783–786.
Lai, L., Wang, C., and Chen, D. J. (2020). Effect of modified prescription of Shoutai Pills on pregnancy outcomes of patients with Spleen-Kidney Deficiency type of early threatened abortion. J. Sichuan Traditional Chin. Med. [Si Chuan Zhong Yi] 38 (9), 158–161.
Li, H. (2019). Clinical effects of CHU’s Antai Decoction combined with progesterone on patients with early threatened abortion due to Kidney Deficiency. Chin. Tradit. Pat. Med. [Zhong Chen Yao] 41, 2646–2650.
Li, J. J., Li, H. Y., Jiang, J., Zhang, X. L., Shan, S. Z., Zhao, X., et al. (2021). Dilatation and curettage versus lesion resection in the treatment of cesarean-scar-pregnancy: A systematic review and meta-analysis. Taiwan J. Obstet. Gynecol. 60, 412–421. doi:10.1016/j.tjog.2021.03.006
Li, L., Dou, L., Leung, P. C., Chung, T. K., and Wang, C. C. (2016). Chinese herbal medicines for unexplained recurrent miscarriage. Cochrane Database Syst. Rev. 2016 (1), CD010568. doi:10.1002/14651858.CD010568.pub2
Li, L., Dou, L., Leung, P. C., and Wang, C. C. (2012). Chinese herbal medicines for threatened miscarriage. Cochrane Database Syst. Rev., CD008510. doi:10.1002/14651858.CD008510.pub2
Li, L., Dou, L. X., Neilson, J. P., Leung, P. C., and Wang, C. C. (2012). Adverse outcomes of Chinese medicines used for threatened miscarriage: A systematic review and meta-analysis. Hum. Reprod. Update 18, 504–524. doi:10.1093/humupd/dms025
Li, L., Zhou, Z. X., and Liu, J. Y. (2006). Guangxi journal of traditional Chinese medicine. Guangxi, China: Guang Xi Zhong Yi Yao, 17–18.Clinical observation of self-made Bushen Gutei decoction combined with hormone in the treatment of threatened abortion
Li, W. L., Li, D. J., Lu, Y., Zhou, J., and Liu, C. L. (2004). Clinical journal of traditional Chinese medicine. Anhui, China: Zhong Yi Yao Lin Chuang Za Zhi, 232–233.Therapeutic effect of bushen antaiyin on early threatened abortion
Li, Y. Q., Zhao, F., Ji, M. M., Li, K., and Wang, Y. H. (2020). Efficacy of Jiawei Shoutai Wan in the treatment of threatened abortion and its influence on reproductive immune-endocrine function. Lishizhen Med. Materia Medica Res. [Shi Zhen Guo Yi Guo Yao] 31, 2971–2973.
Liu, C. D. (2021). Efficacy of Zishen Yangtai recipe on pregnant women with threatened abortion and effects on prognosis. Liaoning J. traditional Chin. Med. [Liao Ning Zhong Yi Za Zhi] 48, 124–127.
Liu, F. H. (2018). Clinical observation on 42 cases of early threatened abortion treated by integrated Traditional Chinese and Western medicine. Inn. Mong. J. Traditional Chin. Med. [Nei Meng Gu Zhong Yi Yao] 37, 54–55.
Liu, F. P., Ou, Y. W., and Tang, L. A. (2020). Analysis of the effect of guben antai decoction combined with western medicine in the treatment of threatened abortion with spleen-kidney deficiency. Clin. J. Chin. Med. [Zhong Yi Lin Chuang Yan Jiu 12, 84–87.
Liu, H. X. (2020). Effect of self-made Antai Pill on serum progesterone, β-HCG levels and fetal protection in patients with early threatened abortion with Kidney Qi Deficiency. Guangming J. Chin. Med. [Guang Ming Zhong Yi] 35, 2857–2859.
Liu, X. F., and Cui, X. D. (2016). With Chinese Medicine kidney and spleen side of acupoint sticking therapy clinical research threatened abortion. Clin. J. Traditional Chin. Med. [Zhong Yi Yao Lin Chuang Za Zhi] 28, 550–553.
Liu, Z. H., Liu, X. Q., and Ding, L. L. (2012). Treating 30 cases of threatened abortion with Yangtai Decoction. China's Naturop. [Zhong Guo Min. Jian Liao Fa 20, 30–31.
Liu, Z. Q. (2015). The effect of the modified treatment of the birth of the fetus on the serum sex hormone in the treatment of threatened abortion. China and Foreign Med. Treat. [Zhong Wai Yi Liao] 34, 168–170.
Long, X. P. (2018). Clinical research of Bushenhuoxuefang in treating threatened abortion of kidney deficiency type. J. Sichuan Traditional Chin. Med. [Si Chuan Zhong Yi 36, 174–177.
Luo, Y. L. (2020). Treatment of threatened abortion in early pregnancy with Traditional Chinese Medicine. Shanxi, China: Chinese Baby [Mu Ying Shi Jie, 69–70.
Ma, W. Z. (2019). Clinical outcomes of Guben Antai Decoction combined with Western medication in the treatment of threatened abortion. Inf. Traditional Chin. Med. [Zhong Yi Yao Xin Xi 36, 73–77.
Makrydimas, G., Sebire, N. J., Lolis, D., Vlassis, N., and Nicolaides, K. H. (2003). Fetal loss following ultrasound diagnosis of a live fetus at 6-10 weeks of gestation. Ultrasound Obstet. Gynecol. 22, 368–372. doi:10.1002/uog.204
Mi, Y. R., Li, X. Q., You, J. P., and Zhe, N. (2021). Effects of Guyuan Wentai Decoction combined with progestin on hemorheological indexes, serum levels of Mcp-1 and Il-1β in patients with threatened abortion. Traditional Chin. Drug Res. Clin. Pharmacol. [Zhong Yao Xin Yao Yu Lin Chuang Yao Li] 32, 123–127.
National Administation of Traditional Chinese Medidine (2002). Guiding principles for clinical research of New Chinese medicine. Beijing: China Medical Science Press, 253–260.
Nong, X. X. (2019). Effect of comprehensive intervention program of traditional Chinese medicine on pregnancy outcome of patients with early threatened abortion. Hebei J. Traditional Chin. Med. [ He Bei Zhong Yi ] 41, 1810–1814.
Salari, N., Kazeminia, M., Shohaimi, S., Nankali, A. A., and Mohammadi, M. (2020). Evaluation of treatment of previous cesarean scar pregnancy with methotrexate: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 18, 108. doi:10.1186/s12958-020-00666-0
The GRADE working group (2013). in GRADE handbook for grading quality of evidence and strength of recommendations. Editors H. Schünemann, J. Bro_ zek, G. Guyatt, and A. Oxman, Available at: guidelinedevelopment.org/handbook (accessed November 30, 2021).
Shang, X. T., Huang, N., and Qiu, Y. F. (2021). Effect of Chinese Herbal Decoction combined with dydrogesterone in the treatment of early threatened abortion. Chin. J. General Pract. [Zhong Hua Quan Ke Yi Xue] 19, 1206–1209.
Shen, H. S., Chang, W. C., Chen, Y. L., Wu, D. L., Wen, S. H., and Wu, H. C. (2022). Chinese herbal medicines have potentially beneficial effects on the perinatal outcomes of pregnant women. Front. Pharmacol. 13, 831690. doi:10.3389/fphar.2022.831690
Song, Y. L., and Ni, T. T. (2013). Clin. study Treat. Threat. Abort. Shoutai Pill Shaoyao Liquor. Decoction Acta Chin. Med. [Zhong Yi Xue Bao] 28, 556–557.
Song, Y. W., Lu, Z. P., Fu, D. Y., Li, R. F., Liu, Y. H., and Chen, Y. (2018). Clinical effects of Yuetai decoction on blood heat type fetal leakage and fetal irritability and its effect on blood beta human chorionic gonadotropin, progesterone, estradiol and gestational sac in patients. Hebei J. Traditional Chin. Med. [Hei Bei Zhong Yi] 40, 497–500+79.
Sotiriadis, A., Papatheodorou, S., and Makrydimas, G. (2004). Threatened miscarriage: Evaluation and management. BMJ 329, 152–155. doi:10.1136/bmj.329.7458.152
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Su, Y. X. (2016). Cases of early threatened abortion of kidney deficiency treated by Bushen Gutai Decoction and Antai powder. J. Extern. Ther. Traditional Chin. Med. [Zhong Yi Wai Zhi Za Zhi] 25, 23–24.
Teng, L. D. (2018). Clinical efficacy of modified Xuanyu Tongjing Decoction in adjuvant treatment of threatened abortion. Chin. Pract. J. Rural Dr. [Zhongguo Shi Yong Xiang Cun Yi Sheng Za Zhi] 25, 5762–5769.
Tian, C. M., and Chen, B. (2016). Effects of Gushen Antai pills combined with progestin on serum β-HCG, P, E2 and CA125 in patients with threatened abortion. China J. Chin. Materia Medica [Zhongguo Zhong Yao Za Zhi] 4, 321–325. doi:10.4268/cjcmm20160225
Ushiroyama, T., Araki, R., Sakuma, K., Nosaka, S., Yamashita, Y., and Kamegai, H. (2006). Efficacy of the Kampo medicine Xiong-gui-jiao-ai-tang, a traditional herbal medicine, in the treatment of threatened abortion in early pregnancy. Am. J. Chin. Med. 34, 731–740. doi:10.1142/S0192415X06004247
Wahabi, H. A., Fayed, A. A., Esmaeil, S. A., and Bahkali, K. H. (2018). Progestogen for treating threatened miscarriage. Cochrane Database Syst. Rev. 8, CD005943. doi:10.1002/14651858.CD005943.pub5
Wang, Y. M., and Niu, C. F. (2019). Clinical study on tonifying kidney for preventing miscarriage decoction combined with progesterone in treating early threatened abortion of Kidney Deficiency type. World J. Complex Med. [Shi Jie Fu He Yi Xue 5, 124–126.
Wu, X., Li, Y. O., and Hu, J. P. (2017). Clinical research of Yunbao Decoction on the endocrine and PIBF of patients with threatened abortion. China J. Traditional Chin. Med. Pharm. [Zhong Hua Zhong Yi Yao Za Zhi] 32, 5678–5681.
Wu, Xiaoke (2021). Chinese herbal medicine and micronized progesterone for live births in threatened miscarriage. Available at: https://clinicaltrials.gov/ct2/show/study/NCT02633878 (accessed September 23, 2021).
Xiao, Z. X. (2008). Guiding journal of traditional Chinese medicine and pharmacy, 2008. Hunan, China: Zhong Yi Yao Dao Bao, 59–60.Clinical observation on 30 cases of threatened abortion treated with integrated traditional Chinese and western medicine
Xie, L. J. (2016). Observation on the effect of syndrome differentiation nursing and treatment of threatened abortion. Chin. Med. Mod. Distance Educ. China [Zhong Guo Zhong Yi Yao Xian Dai Yuan Cheng Jiao Yu ] 14, 128–129.
Xie, P. (2014). Clinical effect of Traditional Chinese Medicine Anzi mixture in treatment of threatened abortion. Clin. Misdiagnosis Mistherapy [Lin Chuang Wu Zhen Wu Zhi] 27, 101–103.
Xin, N., Gao, J. Q., Yang, L. J., and Wu, Y. J. (2018). The clinical effect of Zishen Yutai Pills, human chorionic gonadotropin combined with vitamin E capsules in the treatment of early threatened abortion. Guangxi, China: Home Medicine [Jia Ting Yi Yao, 177.
Yang, J. X. (2012). Clinical research on treating threatened abortion by Antai recipe. Clin. J. Chin. Med. [Zhong Yi Lin Chuan Yan Jiu] 4, 82–83.
Yang, M. Q. (2006). 100 cases of threatened abortion in early pregnancy treated with integrated traditional Chinese and Western Medicine, 2006. Guangxi, China: Guangxi Medical Journal [Guang Xi Yi Xue, 1984–1985.
Ye, S., and Tao, H. J. (2021). Clinical study on Yangxue Guchong Tang combined with Dydrogesterone for early threatened abortion. J. New Chin. Med. [Xin Zhong Yi] 53, 84–87.
Yu, J., Ding, C. F., and Zhang, W. (2019). Clinical observation of TCM formula granule in treating threatened abortion with kidney deficiency and blood stasis type. Chin. Archives Traditional Chin. Med. [Zhong Hua Zhong Yi Yao Xue Kan] 37, 2522–2525.
Yu, L., Li, Q., and Huang, J. Y. (2021). Clinical effect of Zishen Yutai Pills in the treatment of threatened abortion due to low estradiol. Guangdong Med. J. [Guang Dong Yi Xue 42, 832–836.
Yu, L. M. (2018). Evaluation of the effect of Antai Fangleu Decoction in the treatment of threatened abortion in early pregnancy. Contemp. Med. Symp. [Dang Dai Yi Yao Lun. Cong] 16, 98–99.
Yu, M., and Yang, X. (2017). Observation on the effect of treating threatened abortion with kidney deficiency in early pregnancy with integrated Traditional Chinese and Western medicine. Contemp. Med. Symp. [Dang Dai Yi Yao Lun. Cong] 15, 50–52.
Yu, X. H., and Jiang, P. (2021). Treatment of 30 cases of early threatened abortion with kidney deficiency with bushen antai granules and dydrogesterone. J. Anhui Univ. Chin. Med. [An Hui Zhong Yi Yao Da Xue Xue Bao] 40, 19–23.
Zhang, D. M. (2017). 20 cases of early threatened abortion treated by Gushan Yangshi Baotai Decoction combined with progesterone. Journal of Anhui University of Chinese Medicine [An Hui Zhong Yi Yao Da Xue Xue Bao] 36, 29–32.
Zhang, H. Z. (2020). The effects of the Bushen Baotai Zhuyun decoction on fetal leakage of the Shenxu type and fetal motion. Clinical Journal of Chinese Medicine [Zhong Yi Lin Chuang Yan Jiu] 12, 83–86.
Zhang, L. L., and Hou, L. L. (2020). The clinical effect of Baoyinjian combined with dydrogesterone tablets in the treatment of early threatened abortion. Journal of China Prescription Drug [Zhong Guo Chu Fang Yao] 18, 145–146.
Zhang, S. F. (2021). Analysis of the effect of Yuantu Gutai Decoction combined with progesterone in the treatment of threatened abortion in early pregnancy. Clinical Research [Lin Chuang Yan Jiu] 29, 126–127.
Zhang, W., and Ding, C. F. (2015). Clinical observation on the treatment of 100 cases of threatened abortion with Yishenangong Decoction and progesterone injection. Journal of Traditional Chinese Medicine [Zhong Yi Za Zhi] 56, 773–777.
Zhang, X., and Liu, J. J. (2016). Effect of Yishen antai Prescription combined with Didrogesterone tablets on early threatened abortion with kidney deficiency. Yunnan Journal of Traditional Chinese Medicine and Materia Medica [Yun Nan Zhong Yi Zhong Yao Za Zhi] 37, 50–51.
Zhang, X. (2021). Treatment of 42 cases of early threatened abortion of kidney deficiency type with modified Shoutai pill combined with western medicine. Capital Food Medicine [Shou Du Shi Pin Yu Yao] 28, 161–162.
Zhang, Z. R., Luo, S. X., and Zhou, X. Y. (2019). To observe the clinical effect of the method of invigorating the kidney and spleen combined with progesterone in the treatment of early threatened abortion. Inner Mongolia Journal of Traditional Chinese Medicine [Nei Meng Gu Zhong Yi Yao] 38, 36–37.
Zheng, H. J., Sun, Y., and Ma, D. Z. (2020). Analysis of effect of acupoint sticking combined with Bushen Yiqi and Guchong Antai devotion in the treatment of threatened abortion with kidney deficiency. Chinese Journal of Primary Medicine and Pharmacy Zhong Guo Ji Ceng Yi Yao 27, 1698–1702.
Zheng, X. Y. (2002). Guiding principles for clinical study of New Chinese medicine. Beijing: China Pharmaceutical Science and Technology Press, 219.
Zhong, H., Fu, C., and Xue, K. (2000). The. first edition. Beijing: People’s Medical Publishing House, 1315–1318.
Zhou, H., Yang, Y., Deng, L., Yao, Y., and Liao, X. (2022). A potential mechanism of kidney-tonifying herbs treating unexplained recurrent spontaneous abortion: Clinical evidence from the homogeneity of embryo implantation and tumor invasion. Front Pharmacol 12, 775245. doi:10.3389/fphar.2021.775245
Zhou, Y. H., and Jiang, J. (2015). Eighty cases of threatened early abortion treated with TCM nursing according to syndrome. Henan Traditional Chinese Medicine [He Nan Zhong Yi] 35, 2577–2578.
Zhu, G. X., and Wang, J. (2019). Effect of Bushen baotai zhuyun decoction combined with dydrogesterone for treating pregnant women with threatened abortion and fetal irritability because of Shenxu. Chinese Journal of Family Planning [Zhong Guo Ji Hua Sheng Yu Za Zhi] 27, 593–598.
Keywords: Chinese herbal medicine, threatened miscarriage, systematic review, meta-analysis, efficacy, safety
Citation: Xie H, Zhang A, Mou X, He T, Li J, Wang CC, Fan X and Li L (2023) Chinese herbal medicine for threatened miscarriage: An updated systematic review and meta-analysis. Front. Pharmacol. 14:1083746. doi: 10.3389/fphar.2023.1083746
Received: 29 October 2022; Accepted: 25 January 2023;
Published: 14 February 2023.
Edited by:
Giada Crescioli, University of Florence, ItalyReviewed by:
Dazhi Fan, Foshan Women and Children Hospital, ChinaCopyright © 2023 Xie, Zhang, Mou, He, Li, Wang, Fan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Fan, ZmFueGhAemp1LmVkdS5jbg==; Lu Li, bHVjaWFsaUB6anUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.