- Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing, China
Objective: Xueshuantong (lyophilized) for injection (XST) is an effective botanical drug for treating unstable angina pectoris (UAP). However, a meta-analysis of XST combined with conventional treatment (CT) against UAP has not been conducted. Therefore, this study aimed to investigate the effectiveness and safety of XST combined with CT for UAP patients compared to CT alone.
Methods: Randomized controlled trials (RCT) of XST in UAP patients were retrieved from the Cochrane Library, PubMed, Web of Science, EMBASE, CNKI, VIP, Wanfang, and Chinese Biological Medicine Database databases. A meta-analysis was performed using Revman 5.4 and Stata 16.0, and the quality of the included literature was evaluated based on the Cochrane risk-of-bias 2.0 (RoB2.0) tool. The aggregate 95% confidence intervals (CIs), mean difference (MD), and relative risk (RR) estimates were calculated. A GRADE assessment was performed using GRADEprofiler 3.6, and trial sequent analysis was performed using TSA 0.9.
Results: Thirty-four studies involving 3,518 patients were included in the analysis. The combination of CT with XST improved the comprehensive clinical efficacy (RR = 1.22, 95% CI: 1.18–1.26, p < 0.00001) and ECG improvement (RR = 1.24, 95% CI: 1.18–1.31, p < 0.00001). The frequency of angina attacks was lower (MD = −0.73, 95% CI: −0.92 to −0.55, p < 0.00001), and the duration was shorter (MD = −1.08, 95% CI: −1.44 to −0.72, p < 0.00001) in the group that received CT combined with XST compared to the one without XST. Total cholesterol levels (MD = −1.30, 95% CI: −1.83 to −0.78, p < 0.00001) and triglyceride levels (MD = −0.76, 95% CI: −0.93 to −0.59, p < 0.00001) were lower in patients who received CT in combination with XST than those who received CT alone. CT combined with XST reduced whole blood viscosity (MD = −0.72, 95% CI = −0.99 to −0.44, p < 0.00001) and plasma viscosity (MD = −0.24, 95% CI: −0.46 to −0.03, p = 0.03). There was no statistically significant difference in the incidence of cardiovascular events or adverse events among patients treated with the combination of XST and CT compared to CT alone. The GRADE assessment indicated that the composite quality of the evidence was low. The trial sequent analysis showed an adequate sample size and stable findings for the clinical efficacy of CT combined with XST for unstable angina.
Conclusion: The present systematic review and meta-analysis conditionally indicate that XST combined with CT improved the clinical outcomes of patients with unstable angina more than CT alone with a better safety profile. However, the results need further validation due to limitations in the quality of the included studies.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022357395.
1 Introduction
Unstable angina pectoris (UAP) is integral to acute coronary syndrome (ACS). An epidemiological investigation in China reported that 122 of 100,000 people had coronary heart disease, imposing a huge burden on families and society (Summary of Chinese Cardiovascular Health and Disease report 2019, 2020). As a non-ST segment-elevated acute coronary syndrome, UAP can develop into acute myocardial infarction and is the leading cause of death in patients with coronary heart disease. The main UAP treatments in modern medicine include anti-myocardial ischemia, antiplatelet, anticoagulation and statin medications, and revascularization (Guidelines for diagnosis and treatment of non-ST segment elevation acute coronary syndrome 2016, 2017). However, the long-term use of these drugs produces side effects and drug resistance, affecting treatment efficacy. Chinese medicine has been used in clinical practice for over 3,000 years (Jia et al., 2017; Zhang et al., 2017). Chinese patent medicine (CPM) refers to drugs prepared from various botanical drugs through unique methods and specific combinations. Modern evidence-based evaluation methods and basic research evidence have confirmed that CPM is advantageous and safe for treating UAP and is widely used for treating UAP. Chinese botanical drug injections are fast-acting and have better bioavailability than general CPM (Li et al., 2022).
Xueshuantong (lyophilized) for injection (XST) is a standardized botanical drug in the Pharmacopoeia of the People’s Republic of China. It is listed in the 2018 National List of Essential Medicines as one of the most popular Chinese botanical drug injections for activating blood circulation and resolving blood stasis. Panax notoginseng saponins were isolated from the root and rhizome of Panax notoginseng (Burk.) F. H. Chen [Araliaceae; Notoginseng Radix], is the primary active component of XST (Shen et al., 2017). Panax notoginseng is a well-known botanical drug. It has the ability to invigorate blood, remove blood stasis, reduce swelling and relieve pain. Pharmaceutical manufacturer obtained 250 g of total saponins of panax notoginseng, and an appropriate amount of water was added for injection to dissolve it, followed by activated carbon. After homogenizing and filtering, water was injected into the specified volume. Then, 20% sodium citrate solution was added to adjust the pH value to 5.5–7.0, followed by filtering, filling, and freeze drying, to obtain XST. There are five main components of PNS: ginsenoside R1, ginsenoside Rg1, ginsenoside Rd, ginsenoside Re, ginsenoside Rb1. Previous studies confirmed that Panax ginseng total saponin could correct intracellular calcium ion (Ca2+) overload in cardiomyocytes by increasing the calcium pump activity on the sarcoplasmic reticulum (SR) membrane of cardiomyocytes, thereby increasing left ventricular myocardial energy (Feng and Jiang, 1998). Animal experiments established that Panax ginseng total saponin could inhibit nuclear factor (NF)-κB activation in neutrophils and reduce intercellular adhesion molecule (ICAM)-1 expression and neutrophil infiltration. Thus, it improves myocardial microcirculation, narrowing the infarct range of myocardial ischemia and effectively counteracting T-wave elevations due to acute myocardial ischemia (Tang et al., 2002; Li and Wang, 2016). The literature search yielded a previous meta-analysis of XST in treating UAP (Gao et al., 2019), but with certain limitations. The study did not consider long-term outcomes, such as the incidence of adverse cardiovascular events. The level of evidence was not evaluated, and the findings were not updated with the results of clinical trials conducted in recent years. Simultaneously, various dosage forms of XST were analyzed without any quality evaluation or sample size estimation. The current analysis used only studies of high-purity XST freeze-dried powder for the test sequential analysis to explore the stability of the study conclusions. GRADE was also used to evaluate the evidence quality, hoping to provide physicians with an accurate and objective reference on XST.
2 Methods
This meta-analysis was conducted and reported according to the PRISMA 2020 statement (Page et al., 2021) (Supplementary Material S1). The study protocol was registered with PROSPERO (registration number: CRD42022357395).
2.1 Eligibility criteria
2.1.1 Type of study
Randomized controlled trials (RCTs) were included, and the subjects met the diagnostic criteria of unstable angina pectoris (Chinese Medical Association, 2007).
2.1.2 Type of intervention
The test group was treated with XST, or XST was added to the control group. The control group was treated with routine medications (vasodilators, anticoagulants, antihypertensive drugs, and statins) (Chinese Medical Association, 2007) or other drugs.
2.1.3 Types of outcomes
The main index used to evaluate the effectiveness of this study was the comprehensive clinical efficacy, which referred to the sum of the percentage of patients whose curative effects were “significantly effective” and “effective” (FDA, 2007). Among them, a significant effect indicated that the number of angina pectoris events was reduced by more than 80%, and more than two grades improved the UAP grade. “Effective” suggested that the number of angina pectoris events decreased by 50% to more than 80%, and the UAP grade was improved by more than 1. The secondary outcomes were ECG improvement, the number of angina attacks, angina duration, blood lipid levels, and blood rheology assessments. ECG improvement was either the return of the ECG to normal, ST segment recovery >0.05 mV or the shallowing amplitude of the inverted T wave of the main lead is greater than 25% of the original or a change in the T wave from flat to upright. The primary outcome index used to evaluate safety was the incidence of adverse reactions, and the secondary outcome index was the incidence of adverse cardiovascular events.
2.2 Retrieval strategy
We searched PubMed, Embase, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP Chinese Sci-tech Periodical Database (VIP), Chinese Biological Medicine Database (CBM), and the Chinese Wan Fang Database (Wan fang) from the inception of the database to October 2022. The retrieval strategies for each database are depicted in Supplementary Material S2.
2.3 Selection of research studies
The study selection process followed the PRISMA statement guidelines (Page et al., 2021). JX and XC independently screened the literature with predefined study selection criteria. The literature retrieved by subject terms was first imported into NoteExpress software for weight-checking, followed by a preliminary screening based on the titles of the publications. Afterward, the abstracts were read to exclude literature that did not meet the inclusion criteria. Finally, the full text of the studies was used to finalize inclusion in the current study. Disagreements were resolved by discussing with a third party. The data extraction table was created using Excel. The extracted data mainly included the first author, year of publication, the baseline characteristics of the study population, interventions (including dose and duration of treatment), and outcome indicators.
2.4 Methodological quality evaluation
Two investigators independently evaluated the methodological quality of the studies from relevant specialties, and a third party was consulted to resolve any disagreement. The quality of the included literature was assessed using the Cochrane risk-of-bias 2.0 (RoB2.0) tool (Liu et al., 2019). It focuses on randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Five aspects were evaluated, and the included studies were judged as having a low or high risk of bias, or some concerns.
2.5 Statistical analysis
Data analysis was performed using Revman 5.3 statistical software provided by the International Cochrane Collaboration. Mean difference (MD) (continuous variable) and relative risk (RR) (dichotomous variable) were selected as effect sizes, and 95% confidence intervals (CI) were utilized to express interval estimates. When the heterogeneity among the studies was small (p ≥ 0.1, I2 ≤ 50%), the fixed-effects model was selected for analysis. The reason was analyzed when the heterogeneity of each study was significant. If it was caused by clinical factors or research methods, subgroup or sensitivity analysis was performed. If the heterogeneity was still significant after analysis (p < 0.1, I2 > 50%), the random-effects model was selected for analysis. When more than 10 articles were included in the outcome index, a funnel chart was made using Stata.16.0 to analyze whether the studies had publication bias. TSA0.9 was used to sequentially analyze comprehensive clinical efficacy to evaluate whether the sample size of this meta-analysis was sufficient and whether there were false-positive results.
2.6 Assessment of evidence quality
JLX and RLW independently assessed the evidence quality according to previous rating standards (Balshem et al., 2011; Guyatt et al., 2011). The quality of the evidence included in the results was classified as high, medium, low, or very low. RCTs were initially classified as having high-quality evidence. The quality of each outcome was further graded according to five factors: deviation risk, inconsistency, indirectness, inaccuracy, and publication deviation. GRADEpro 3.6.1 software was utilized for data analysis and synthesis.
3 Results
3.1 Literature screening process
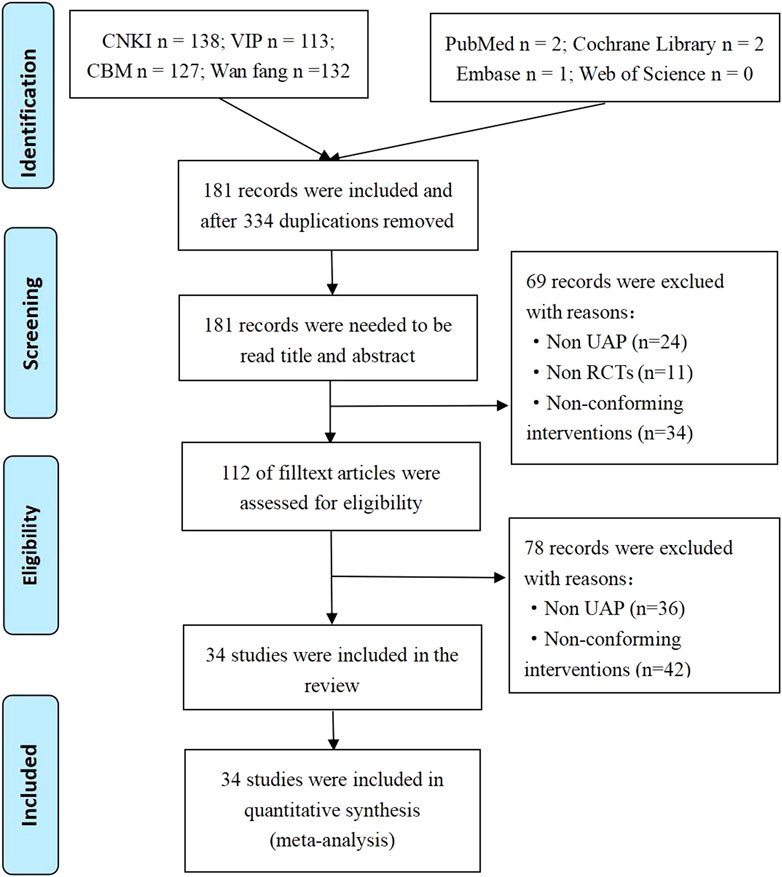
We systematically searched the databases and obtained 515 potentially related original studies. Of them, 334 duplicate studies were deleted, and the titles and abstracts of the remaining 181 articles were examined. Sixty-nine studies were deleted since they did not meet the eligibility criteria. Of these, 11 did not meet the inclusion criteria for the type of research, 34 did not meet the intervention criteria, and 24 were non-UAP studies. After further screening the full text of the studies, 78 articles were excluded since the study or intervention measures did not meet the inclusion criteria. Finally, a total of 34 original studies were included in the meta-analysis. The PRISMA flow chart study selection is represented in Figure 1.
3.2 Research and participant characteristics
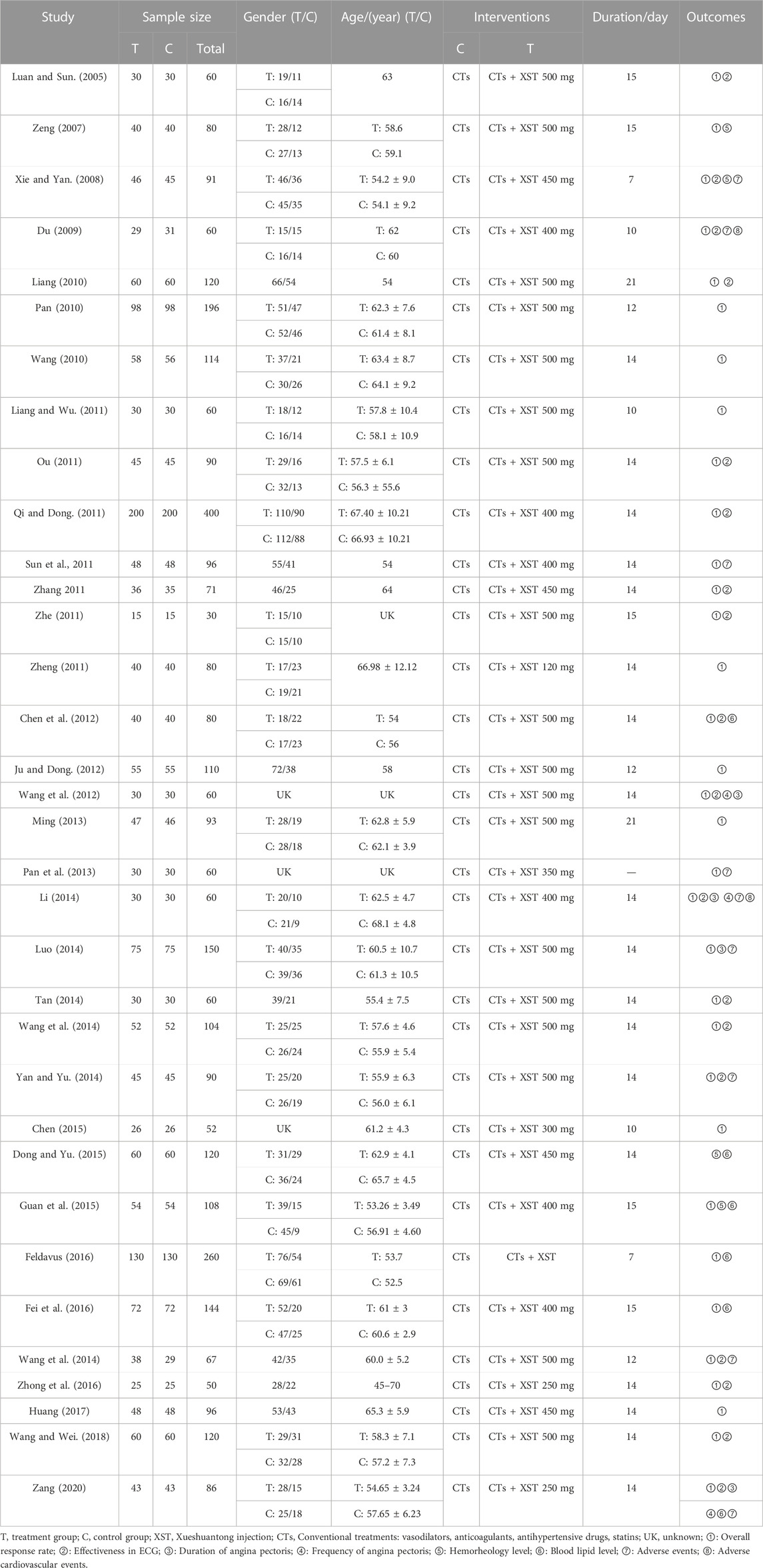
The 34 included studies were all Chinese literature (Luan and Sun, 2005; Zeng, 2007; Xie and Yan, 2008; Du, 2009; Liang, 2010; Pan, 2010; Wang, 2010; Liang and Wu, 2011; Ou, 2011; Qi and Dong, 2011; Sun et al., 2011; Zhang, 2011; Zhe, 2011; Zheng, 2011; Chen et al., 2012; Ju and Dong, 2012; Wang et al., 2012; Ming, 2013; Pan et al., 2013; Li, 2014; Luo, 2014; Tan, 2014; Wang et al., 2014; Yan and Yu, 2014; Chen, 2015; Dong and Yu, 2015; Guan et al., 2015; Fei et al., 2016; Feldavus and Sarchen, 2016; Wang, 2016; Zhong et al., 2016; Huang, 2017; Wang and Wei, 2018; Zang, 2020). The total sample size included 3,518 subjects, 1,765 in the experimental group and 1,753 in the control group. The maximum sample size of the trial group was 200, and the minimum sample size was 15. In the control group, the maximum sample size was 200, and the minimum sample size was 15. The shortest intervention period was 7 days while the longest was 21 and 14 days were the main ones (11/42, 52.94.19%). The mean age of the participants ranged from 45 to 79.1 years, as shown in Table 1.
3.3 Methodological quality
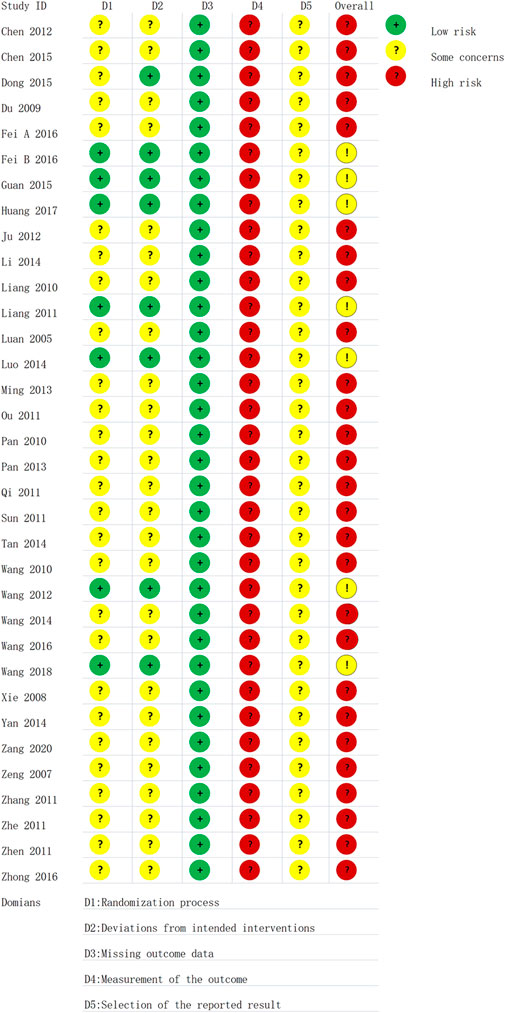
All included studies were RCTs, seven of which reported specific randomization methods, particularly the random number table method. The others only mentioned randomization. One study said that patients provided written informed consent. Sample size estimates and protocol concealment for all studies were not adequately reported. The presence of other biases was unclear in all of the studies. The risk of bias assessment is shown in Figure 2.
3.4 Meta-analysis
3.4.1 Primary efficacy outcome
3.4.1.1 Comprehensive clinical efficacy
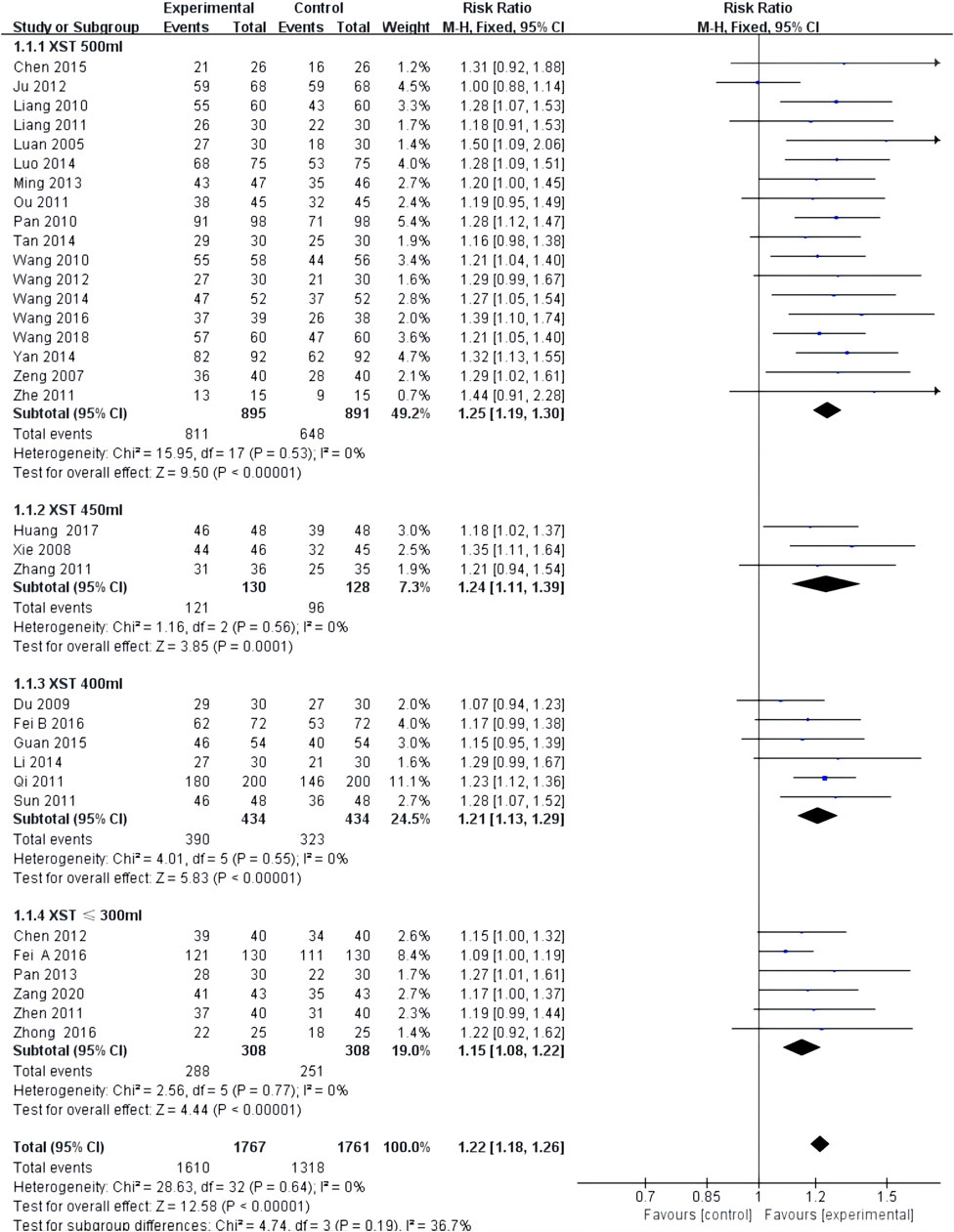
Comprehensive clinical efficacy was reported in 33 studies with low heterogeneity among the studies (I2 = 0.0%, p = 0.64) and was merged with a fixed-effects model. In a subgroup analysis based on XST dosage, the comprehensive clinical efficacy of the combination of CT and 500 mL of XST (RR = 1.25, 95% CI: 1.19–1.30, p < 0.00001; Figure 3), 450 ml of XST (RR = 1.24, 95% CI: 1.11–1.39, p = 0.0001; Figure 3), 400 ml of XST (RR = 1.21, 95% CI: 1.13–1.29, p < 0.00001; Figure 3), and less than 300 ml (RR = 1.15, 95% CI: 1.08–1.22, p < 0.00001; Figure 3) was significantly different from the control group.
3.4.2 Secondary efficacy outcomes
3.4.2.1 ECG improvement
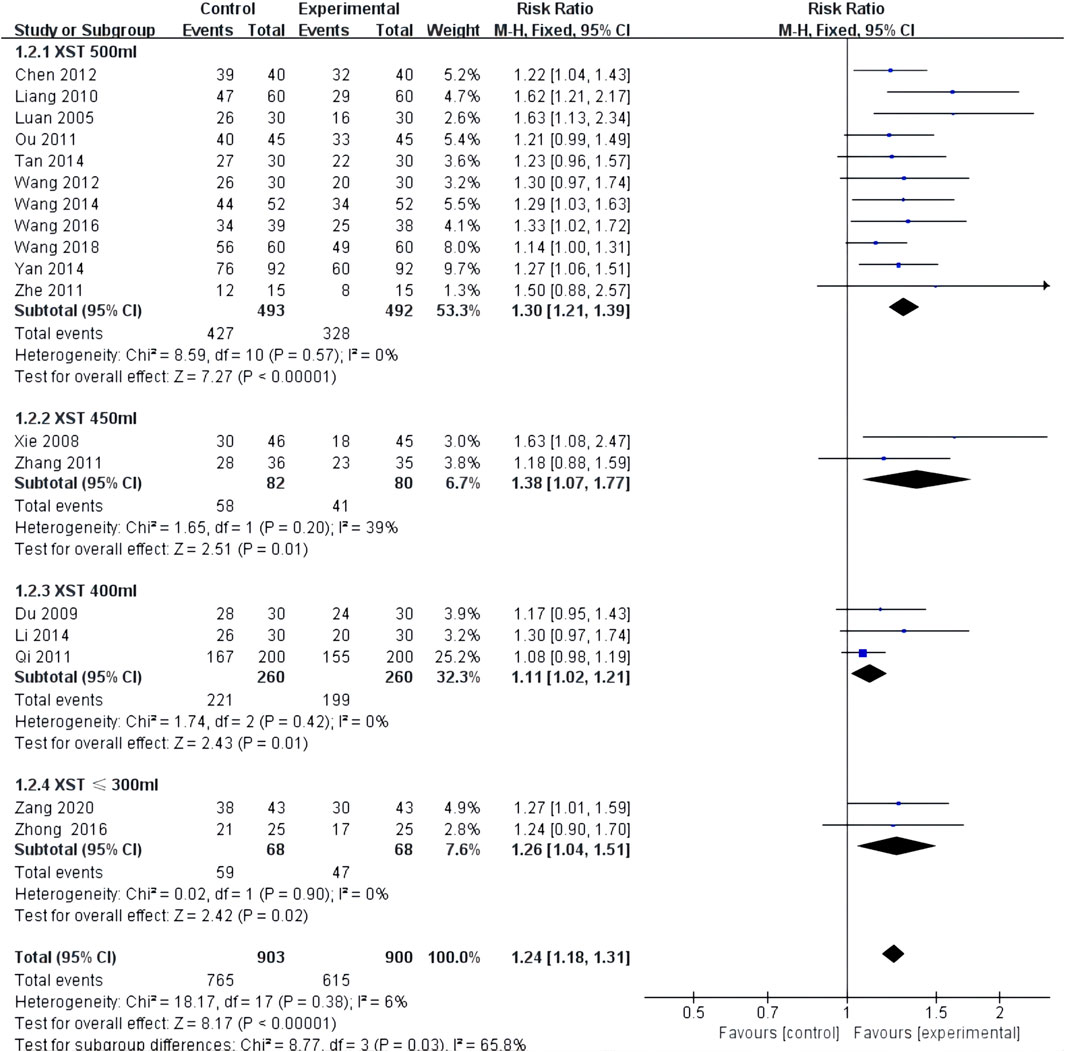
ECG improvement was reported in 18 studies with low heterogeneity among the studies (I2 = 0.0%, p = 0.54) and was merged with a fixed-effects model. In a subgroup analysis based on the dose of XST, the ECG improvement by the combination of CT with 500 mL of XST (RR = 1.30, 95% CI: 1.21–1.39, p < 0.00001; Figure 4), 450 mL of XST (RR = 1.38, 95% CI: 1.07–1.77, p = 0.01; Figure 4), 400 ml of XST (RR = 1.11, 95% CI: 1.02–1.21, p = 0.01; Figure 4), or less than 300 ml (RR = 1.26, 95% CI: 1.04–1.51, p = 0.02; Figure 4) was significantly different from the control group.
3.4.2.2 Frequency of angina attacks
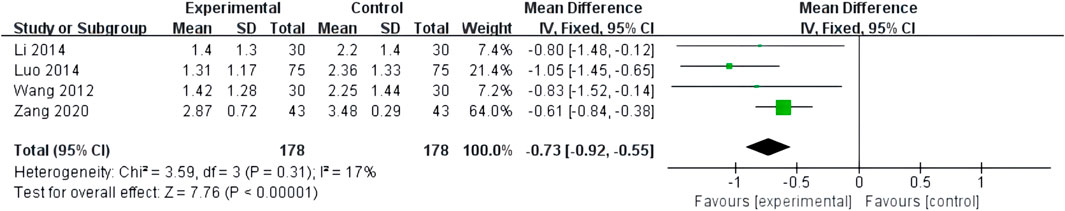
The frequency of angina attacks was reported in four studies with low heterogeneity (I2 = 17%, p = 0.31) and was analyzed using a fixed-effects model. The combination of XST and CT decreased the frequency of angina attacks (MD = −0.73, 95% CI: −0.92 to −0.55, p < 0.00001; Figure 5).
3.4.2.3 Duration of angina pectoris
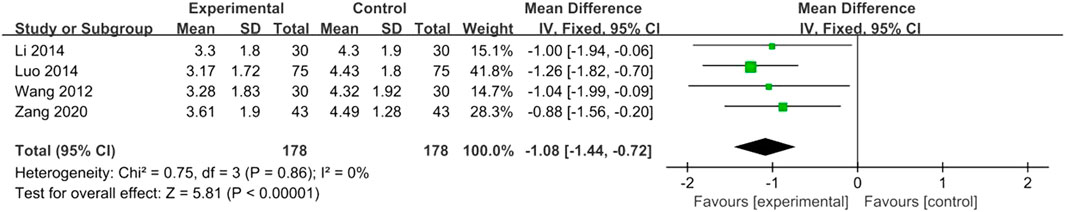
The duration of angina was reported in four studies with low heterogeneity (I2 = 0%, p = 0.86) and was analyzed using a fixed-effects model. The combined use of XST and CT reduced the duration of angina pectoris (MD = −1.08, 95% CI: −1.44 to −0.72, p < 0.00001; Figure 6).
3.4.2.4 Hemorheology levels
Five studies reported total cholesterol and triglyceride levels, and four reported high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels. There was high heterogeneity among the studies (I2 = 99%, p < 0.00001; I2 = 100%, p < 0.00001; I2 = 79%, p = 0.003; I2 = 100%, p < 0.00001), and the random-effects model was utilized for analysis. The results indicated that XST combined with CT decreased total cholesterol (MD = −1.30, 95% CI:−1.83 to −0.78, p < 0.00001; Figure 7A) and triglyceride levels (MD = −0.76, 95% CI: −0.93 to −0.59, p < 0.00001; Figure 7B) and increased HDL levels (MD = 0.17, 95% CI = 0.00 to 0.33, p = 0.05; Figure 7C). However, there was no significant difference in LDL levels in patients treated with XST combined with CT compared to CT alone (MD = −0.68, 95% CI: −1.87 to 0.51, p = 0.26; Figure 7D). The greater heterogeneity in the analyses could be due to significant differences in basal lipid levels in the patients included in the studies and differences in XST doses and CT used in the included studies. In addition, the forest plot showed that in terms of total cholesterol and triglycerides, Fei A and Zang studies are more homogenous, and Fei B et al. and Guan et al. studies are more homogenous. This may be because CTs in the studies by Fei A et al. and Zang et al. were very similar, with a similar average age of the patients. In contrast, the CTs used in the studies by Fei B et al. and Guan et al. were similar.
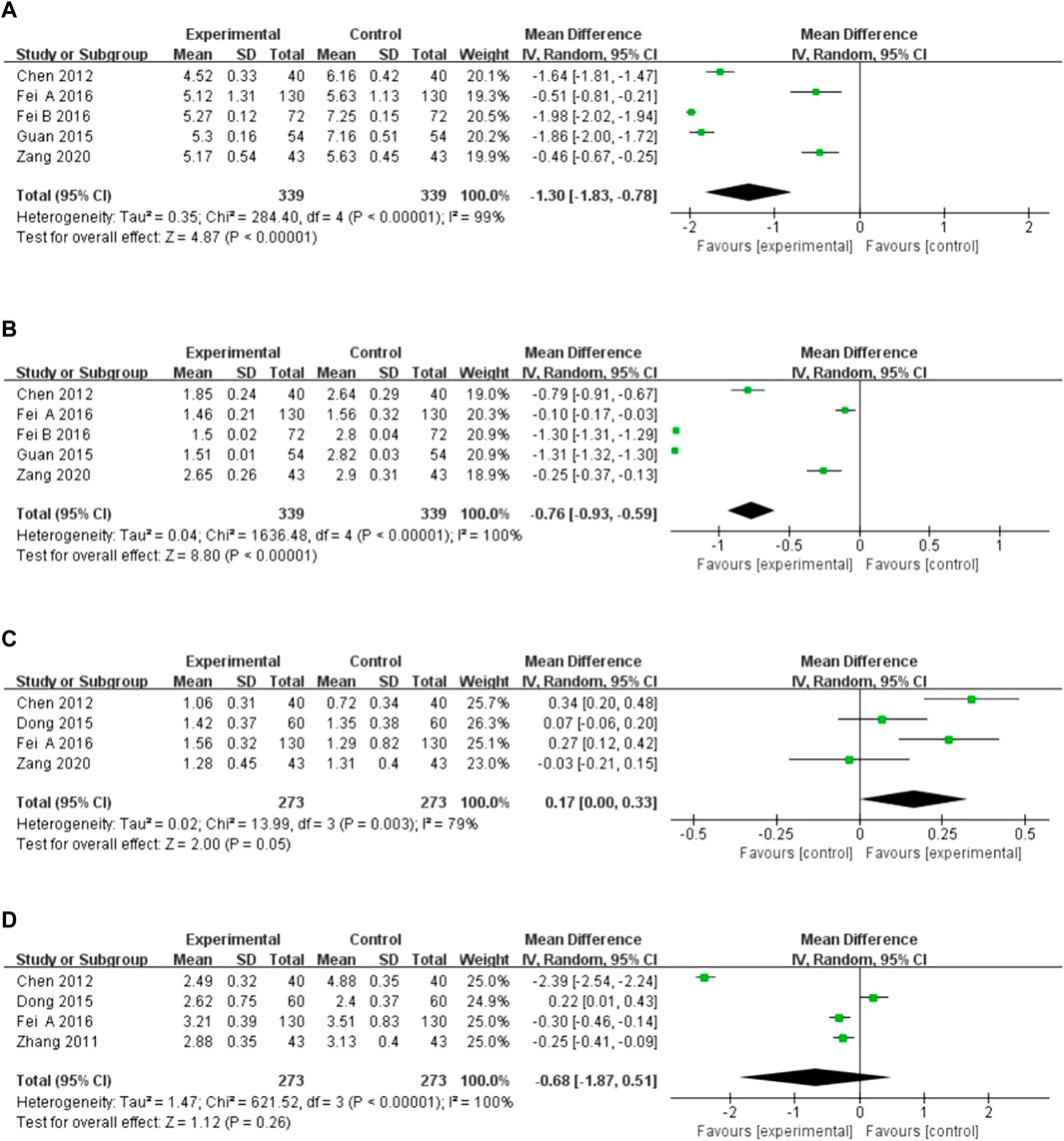
FIGURE 7. Forest plot of hemorheology leve. (A) Total cholesterol. (B) Triglyceride levels. (C) High-density lipoprotein. (D) Low-density lipoprotein.
3.4.2.5 Blood lipid levels
Four studies reported the level of blood lipid levels assessed by different indicators. There was high heterogeneity among the studies, and the random-effects model was used for the analysis (I2 = 72%, p = 0.03; I2 = 80%, p = 0.006; I2 = 87%, p = 0.0006; I2 = 99%, p < 0.00001). The results showed that XST combined with CT decreased whole blood viscosity at a high shear rate (MD = −0.60, 95% CI: −0.83 to −0.37, p < 0.00001; Figure 8A), whole blood viscosity at a low shear rate (MD = −0.94, 95% CI: −1.69 to −0.19, p = 0.01; Figure 8A), and plasma viscosity (MD = −0.24, 95% CI: −0.46 to −0.03, p = 0.03; Figure 8B). However, no significant difference in the decreases in fibrinogen levels was observed between the patients who received CT combined with XST and those who received CT alone (MD = −1.07, 95% CI: −2.27 to 0.12, p = 0.08; Figure 8C). Sensitivity analysis was conducted to explore the sources of heterogeneity, but the sources could not be identified. Differences in dosage, duration, and CT regimens among the four included studies could have been responsible for this heterogeneity. Therefore, these results were limited by substantial heterogeneity. More trials with good homogeneity are required to validate the results.
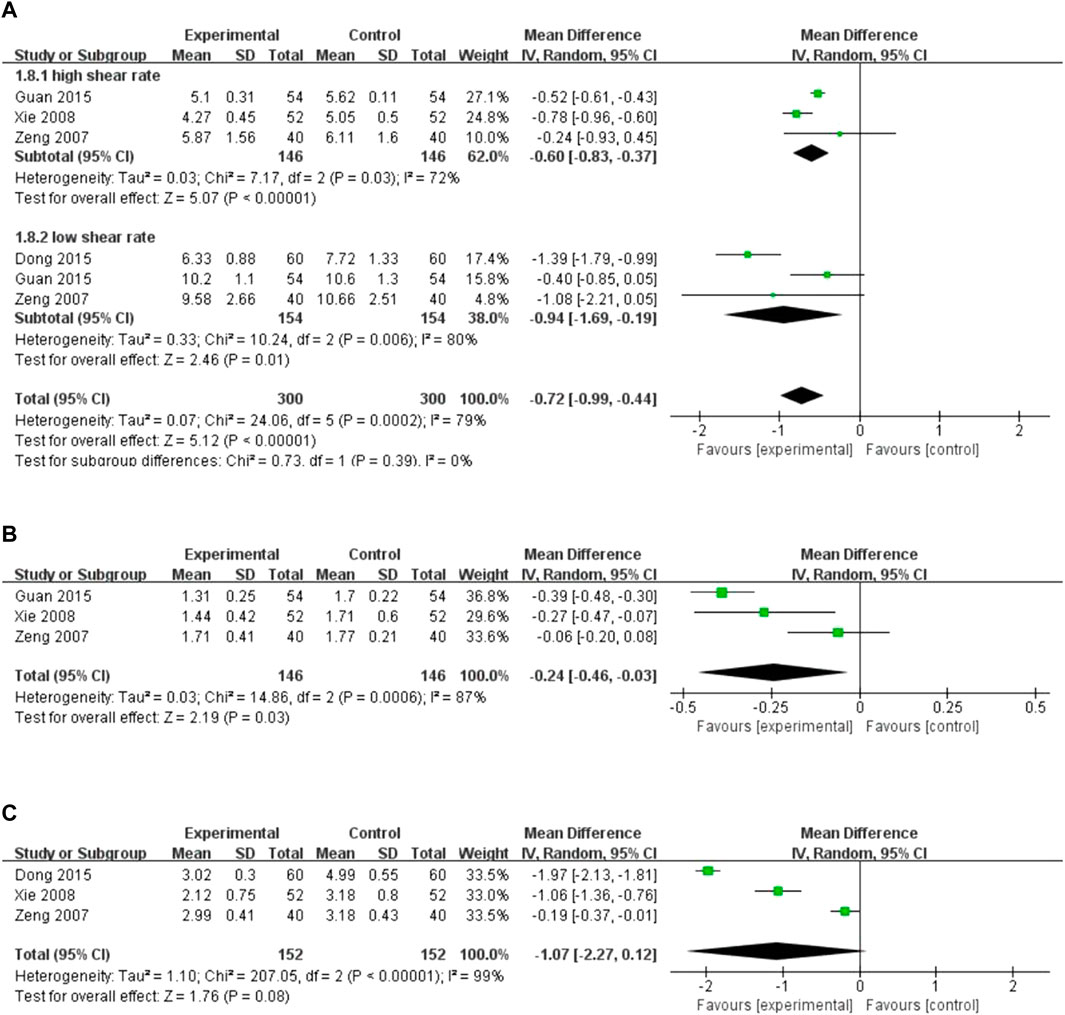
FIGURE 8. Forest plot of blood lipids level. (A) Whole blood viscosity. (B) Plasma viscosity. (C) Fibrinogen levels.
3.4.3 Primary safety outcome
3.4.3.1 Adverse cardiovascular events
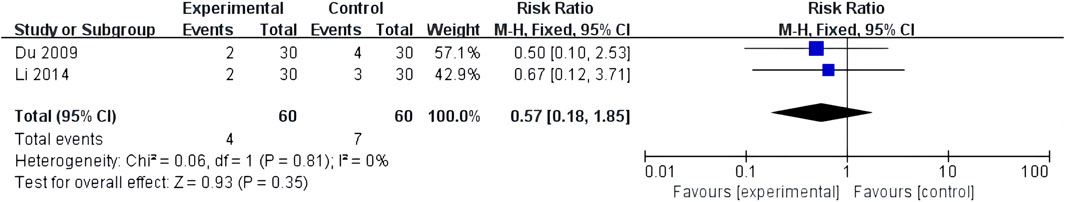
Two studies reported adverse cardiovascular events, with low heterogeneity between the two studies (I2 = 0%, p = 0.81). The fixed-effects model analyzed the results. The results revealed no significant difference in cardiovascular events between patients treated with XST combined with CT and those treated with CT alone (RR = 0.57, 95% CI = 0.18–1.85, p = 0.35; Figure 9). The details are shown in Supplementary Material S3.
3.4.4 Secondary safety outcomes
3.4.4.1 Adverse events
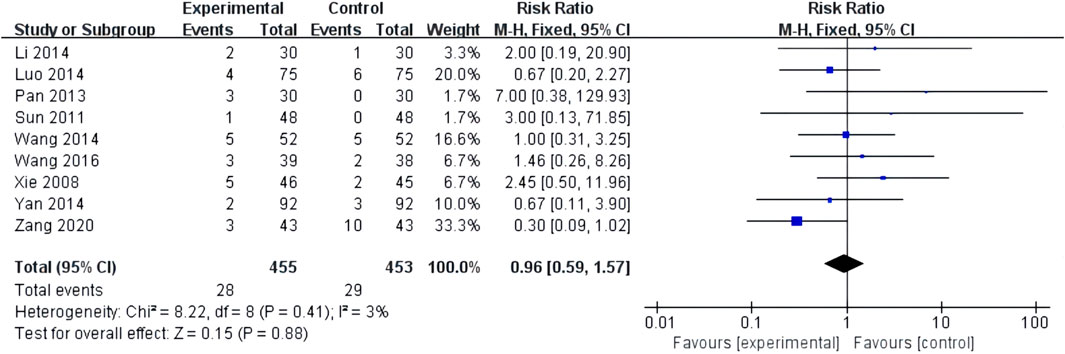
Nine studies reported adverse events with low heterogeneity among the studies (I2 = 3%, p = 0.41). The random-effects model helped analyze the adverse reactions, with no significant difference between patients treated with the combination of XST and CT and those treated with CT alone (RR = 0.96, 95% CI = 0.59–1.57, p = 0.88; Figure 10). Our results revealed that gastrointestinal discomfort, tetter, allergy, and subcutaneous ecchymosis constitute the most frequently occurring AEs. These adverse reactions disappeared with symptomatic management. No participants discontinued the study drug due to adverse events. The details are shown in Supplementary Material S4.
3.5 Publication bias
We analyzed the outcome indicators for more than 10 studies for publication bias. Since the heterogeneity was low, the Harbord test was adopted. The results showed publication bias for comprehensive clinical efficacy (p = 0.003; Figure 11A) and ECG improvement (p = 0.000; Figure 11B). There were multiple reasons for the biases, including the ease of publication, the retrieval of positive results, the small sample size of the included studies, and a small sample effect. All the included studies in the analysis were in Chinese, so there was language publication bias.
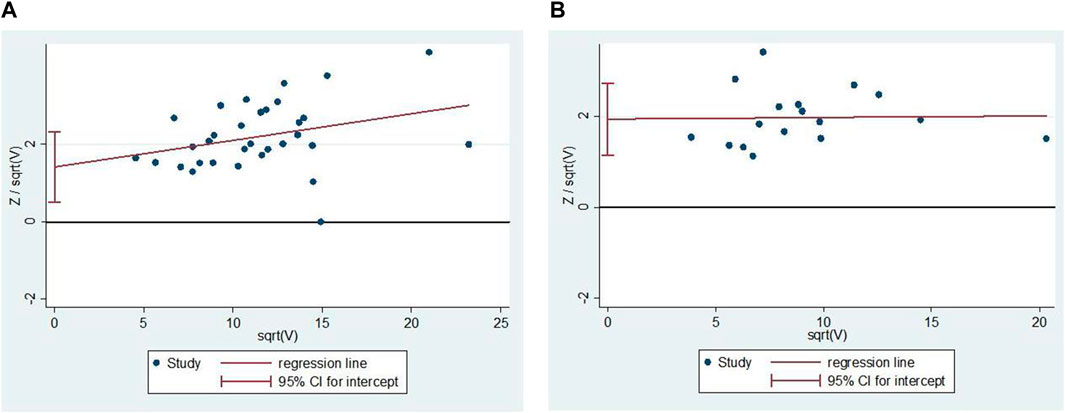
FIGURE 11. Publication bias. (A) The Harbord’s test on comprehensive clinical efficacy. (B) The Harbord’s test on ECG improvement.
3.6 Trial sequential analysis
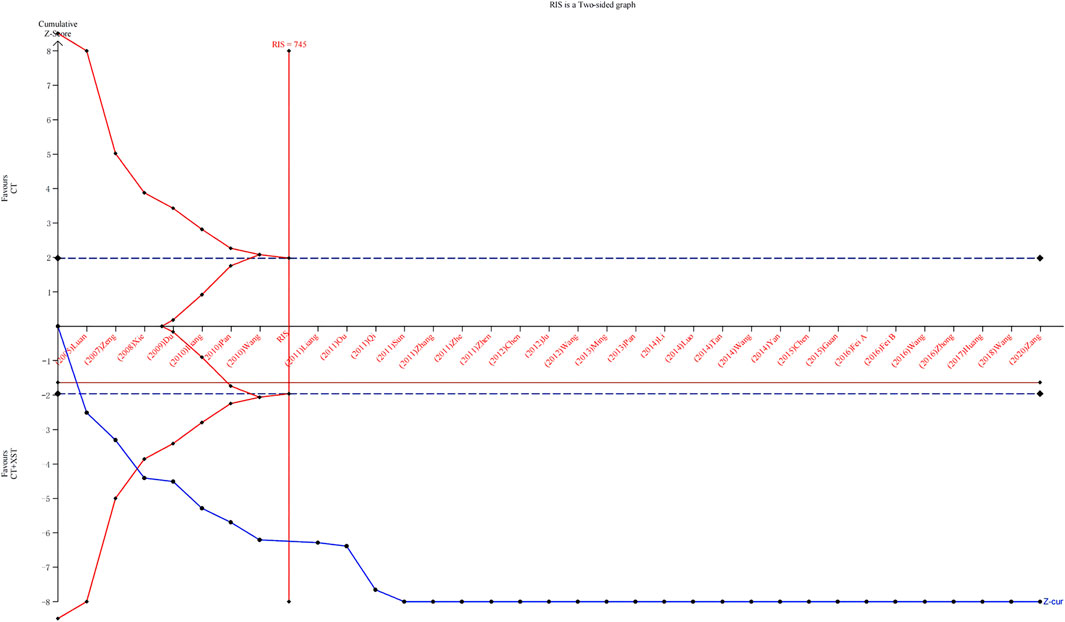
Thirty-three studies reporting comprehensive clinical efficacy were estimated by trial sequential analysis (TSA). Based on the results of related research and this meta-analysis, the class I error definition in this study was set to 0.05, and the statistical efficiency was 0.8. The relative risk reduction (RRR) was 15%, the relative incident rate in the control group was 76.8% (based on the meta-analysis results), and the information axis was set as the cumulative sample size. The TSA analysis showed that the cumulative Z value crossed the traditional boundary value curve (Z = 1.96) and the required information size (RIS) curve. Thus, the cumulative sample size achieved the expected value, the result was stable, and additional tests could not change the conclusion (Figure 12).
3.7 GRADE assessment
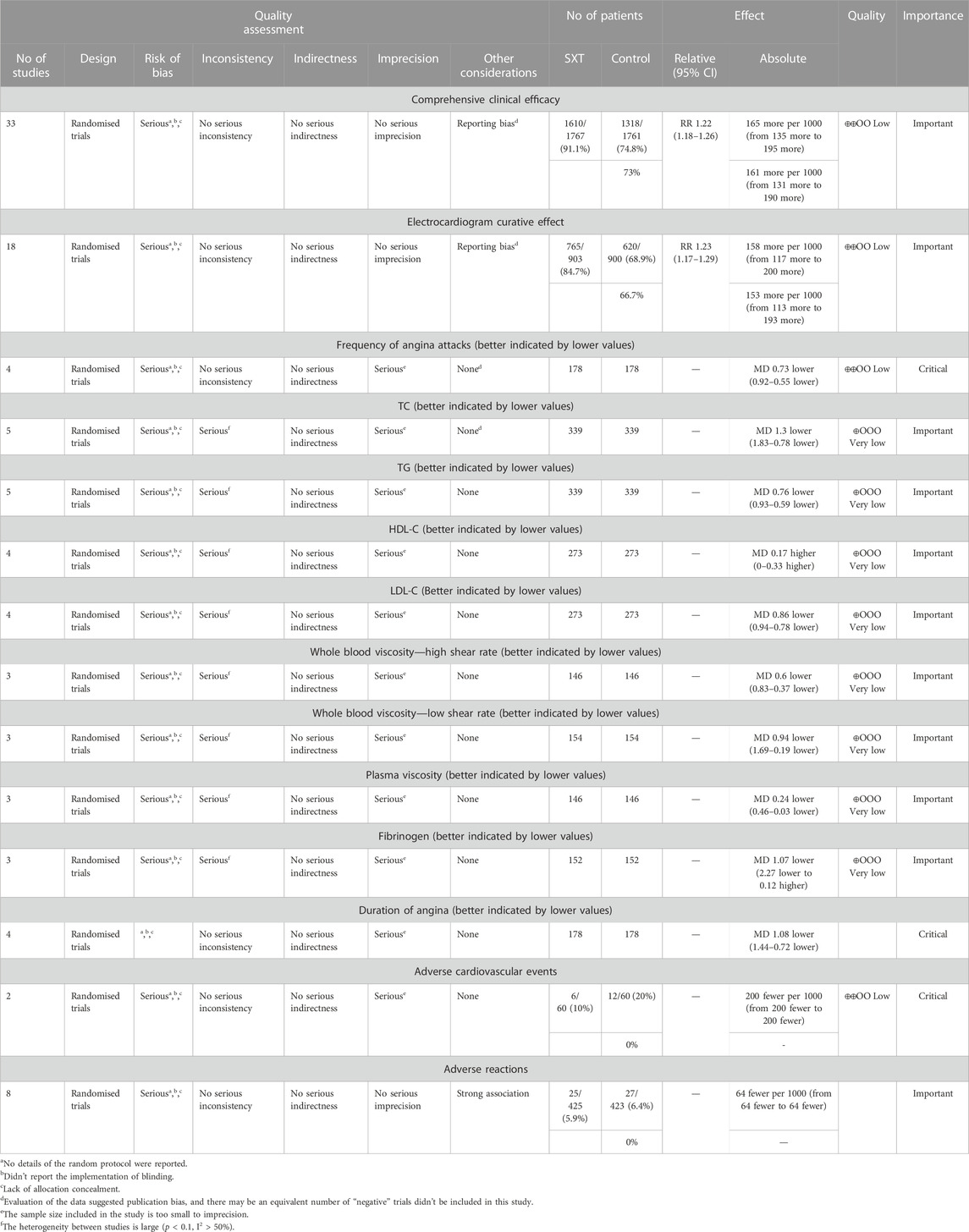
All outcome indicators were evaluated using the GRADE system. Due to the low risk of bias, accuracy, and heterogeneity, the certainty quality of the evidence was low or very low. An overview of the GRADE evidence is shown in Table 2. The following six reasons cause the serious risks of deviations and reported deviations: 1) No details on the randomization protocol were reported. 2) The implementation of blinding was not reported. 3) There was a lack of allocation concealment. 4) The heterogeneity between studies was high (p < 0.1, I2 > 50%). 5) Evaluation of the data suggested publication bias, and an equivalent number of “negative” trials may not be included in this study. 6) The sample size included in the study is too small for imprecision. The results of the evidence quality evaluation of the above outcomes provide a reference basis for guiding clinical practice. More largesamples and high-quality RCTs are needed to improve the evidence of the effectiveness of XST in treating UAP.
4 Discussion
Through systematic searching and screening, 34 RCTs were finally included in this meta-analysis to compare the efficacy and safety of XST combined with CT compared to CT alone for treating patients with UAP. We found that the combined use of XST and CT showed improved clinical efficacy in treating UAP by reducing the frequency and duration of angina pectoris. Moreover, it improves blood lipid levels and hemorheology parameter values, with no increases in adverse cardiovascular events or reactions.
A noticeable increase in blood viscosity can cause dyslipidemia, a significant risk factor for coronary atherosclerosis. Atherosclerosis causes coronary artery lumen stenosis or blockage, which leads to temporary myocardial blood supply insufficiency and the production of excess pyruvate, lactic acid, and polypeptides. These can stimulate cardiac autonomic ganglions and eventually cause angina pectoralis symptoms, including precordial pain, chest tightness, and palpitations (Qian et al., 2018; Sincer I et al., 2018; Chen and Wang, 2019). The results of a Chinese cohort study (n = 20,954, age 35–64 years) with a 20-year follow-up revealed (Zhang et al., 2018) that low-density lipoprotein cholesterol (LDL-C) levels (from <1.0 mmol/L to ≥4.1 mmol/L) were significantly and positively associated with the risk of coronary heart disease. The lower LDL-C levels were associated with a lower risk of coronary heart disease over the next 20 years and vice versa. Based on the results of this meta-analysis, combined treatment with XST and CT could reduce total cholesterol, triglyceride levels, and whole blood viscosity and increase HDL levels compared to CT alone. Qin et al., (2013) found that XST reduced total cholesterol, triglyceride, and LDL-C levels; increased HDL-C levels, and decreased the degree of aortic intimal lesions in atherosclerotic rabbits. The core drug in XST is Panax notoginseng, a typical traditional Chinese medicine used to promote blood circulation and remove blood stasis. Some studies (Han et al., 2017) found that Panax notoginseng saponins could inhibit platelet adhesion to the injured endothelial cell surface by inhibiting the expression of vascular cell adhesion molecule-1 (VCAM-1) and the secretion of thromboxane A2 (TXA2) by vascular endothelial cells. Panax notoginseng saponins also inhibited the expression of VCAM-1 and the release of thromboxane B2 to inhibit platelet activation and play a role in the prevention and treatment of thrombosis. Another study (Wang, 2020) found that Panax notoginseng inhibited thromboxane A2 production by increasing cyclic adenosine monophosphate (cAMP) levels in platelets. Additionally, it inhibited calcium ion and 5-hydroxytryptamine (5-HT) release to inhibit thrombus formation. Acute myocardial ischemia is a direct cause of UAP. Wu et al. (Wu et al., 2015) found that XST could reduce the extent of myocardial infarction, lower serum lactate dehydrogenase, creatine kinase, and creatine kinase isoenzyme levels, promote thrombus lysis, inhibit platelet aggregation, and thus, alleviate myocardial ischemic injury in myocardial ischemia model rats.
The safety indicators for this study were the incidence of adverse cardiovascular events and the incidence of adverse reactions. The incidence of adverse cardiovascular events was studied in 120 patients and experienced by 10% (6/60) of the patients treated with XST plus CT and 20% (12/60) of those treated with CT only. The results showed no statistical difference in the safety of XST combined with CT versus CT alone, indicating that XST combined with CT is safe. The incidence of adverse reactions was studied in 848 patients and was observed in 5.8% (25/425) of the patients treated with XST plus CT. Moreover, the incidence of adverse reactions in patients treated with CT alone was 6.3% (27/423). The main adverse reactions were skin pruritus, rash, subcutaneous ecchymosis, and gastrointestinal reactions. The allergic reactions resolved after taking antihistamines, and the gastrointestinal reactions resolved after slowing the infusion speed. There was no statistical difference in the incidence of adverse reactions between the test and control groups, which indicated that adding XST to CT would not increase the incidence of adverse reactions.
However, this study had some limitations. Most articles did not report the severity of angina pectoris, leading to potential heterogeneity. Several included studies did not report specific randomization methods or allocation blinding. It was also unclear whether randomization or blinding methods were implemented, which may have led to implementation and measurement biases. Blinding can objectively evaluate therapeutic effects, and the quality of the blinding method will directly affect the accuracy of the research results. Therefore, future RCT test designs should refer to the items in the Cochrane Collaboration Network’s risk of bias tool. Moreover, attention should be paid to implementing randomization and blinding methods in designing trials and their execution. The sample size in the included studies was generally small. Only two studies had a sample size of more than 200 cases, and 22 had fewer than 100 cases. The therapeutic index of a study with a smaller sample size may be unstable. The asymmetrical distribution of the funnel chart indicated publication bias, and the subjective biases of the researchers may have exaggerated the therapeutic effect of UAP in the trial group. XST is a drug in China’s National Catalog of Essential Medicines, and most of the subjects were Chinese, which may have led to ethnic and geographical biases.
Therefore, the following recommendations were made: 1) The relevant RCT should classify UAP into specific types, including initial angina pectoris, worsening exertional angina pectoris, resting angina pectoris, etc., to ensure targeted conclusions. Simultaneously, the classification of angina pectoris and its course should also be fully and comprehensively reported based on the CONSORT statement (David Moher et al., 2011). This can help identify the source of heterogeneity among studies, carry out subgroup analysis, and analyze the best timing and application of XST. 2) The specific medication category, usage, and dosage of CT must be reported in the primary studies for repeating the procedure. 3) Long-term follow-up after the trials are also necessary, especially for end-point events and safety outcomes. 4) Comprehensive clinical efficacy and ECG Improvement have been reported across most of the original literature. These two indicators are widely used, but the evaluation is difficult to quantify. However, there are few reports on quantifiable indicators, such as the Frequency of Angina Attacks and Duration of Angina Pectoris. Therefore, future research should focus on measurable indicators.
5 Conclusion
The available literature data and systematic evaluation methods demonstrated that XST combined with CT improved the therapeutic effect of UPA, relieved angina symptoms, and improved hemorheology parameter values compared to CT alone. No serious adverse effects were observed in this study, and the addition of XST to CT is conditionally recommended. However, a more rigorous RCT should be designed and completed before the widespread use of XST combined with CT is recommended.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JX and YX conceived the idea of this study. JX and XC extracted the data. RW and YL performed the quality evaluation and sample size estimation of the data. JX wrote the manuscript. RW and XC revised the manuscript, and YL and YX interpreted the data based on their expertise. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key Research and Development Program of China (2018YFC1707400).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1074400/full#supplementary-material
Abbreviations
ACS, Acute Coronary Syndrome; AE, Adverse Event; cAMP, cyclic Adenosine Monophosphate; CBM, Chinese Biological Medicine Database; CI, Confidence Intervals; CNKI, China National Knowledge Infrastructure; CT, Conventional Treatment; CPM, Chinese Patent Medicine; ECG, Electrocardiograph; HDL, High-density Lipoprotein; ICAM, Intercellular Adhesion Molecule; LDL, Low-density Lipoprotein; MD, Mean Difference; RCT, Randomized Controlled Trials; RR, Relative Risk; RRR, Relative Risk Reduction; RIS, Required Information Size; SR, Sarcoplasmic Reticulum; TSA, Trial Sequential Analysis; TXA2, Thromboxane A2; UAP, Unstable Angina Pectoris; VCAM-1, Vascular Cell Adhesion Molecule-1; VIP, VIP Chinese Sci-tech Periodical Database; XST, Xueshuantong (lyophilized) for injection; 5-HT, 5-hydroxytryptamine.
References
Balshem, H., Helfand, M., Schünemann, H. J., Oxman, A. D., Kunz, R., Brozek, J., et al. (2011). GRADE guidelines: 3. Rating the quality of evidence. J. Clin.Epidemiol. 64, 401–406. doi:10.1016/j.jclinepi.2010.07.015
Chen, J. H., and Wang, J. L. (2019). Effect of ginseng and Taohong Siwu decoction on cardiac function and blood lipids in patients with angina pectoris of coronary heart disease. China Med. Her. 16 (24), 135–138.
Chen, T. N., Ge, C. J., Yang, J., and Sun, G. (2012). Clinical observation of Xueshuantong injection in the treatment of unstable angina pectoris. Mod. Med. Health 28 (19), 2909–2910.
Chen, Z. (2015). Clinical analysis of Xueshuantong in the treatment of coronary heart disease and angina pectoris in the elderly. World's latest Med. Inf. Abstr. 15 (55), 94.
Chinese Medical Association Cardiology Branch, editorial Board of Chinese Journal of Cardiovascular Diseases (2007). Guidelines for diagnosis and treatment of unstable angina pectoris and non-St segment elevation myocardial infarction. Chin. J. Cardiovasc. Dis. 35(4), 295–304.
Dong, Y., and Yu, Rui. (2015). Effect of Xueshuantong injection on blood biochemical indexes in patients with unstable angina pectoris. J. Cardiovasc. Cerebrovasc. Dis. Integr. traditional Chin. West. Med. 13 (01), 93–94.
Du, Z. A. (2009). Analysis of the efficacy of low molecular weight heparin combined with Xueshuantong in the treatment of 60 cases of unstable angina pectoris. Clin. Med. 29 (05), 99–100.
FDA (2007). Guidance for industry clinical trial endpoints for the approval of cancer drugs and biologics. May: Food Drug Admin. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071590.pdf.
Fei, H. J., Liu, P., and Xiao, Y. l. (2016). The clinical efficacy of Xueshuantong injection in the treatment of patients with unstable angina. China Pharmacoeconomics 11 (09), 58–60.
Feldavus Sarchen (2016). Clinical observation of Atto vastatin combined with Xueshuantong in the treatment of unstable angina pectoris. China Mod. Med. 10 (05), 123–124. doi:10.14164/j.cnki.cn11-5581/r.2016.05.098
Feng, P. F., and Jiang, H. F. (1998). The effect of panax notoginseng saponins on Ca∼(2+) in SHR myocardial cells and the activity of calcium pump on sarcoplasmic reticulum. China J. Traditional Chin. Med. (03), 45–47+65.
Gao, Y., Lv, J., Xie, Y. M., and Sun, M. H. (2019). Systematic evaluation on the efficacy and safety of Xueshuantong (freeze-dried powder) injection in the randomized controlled trial of unstable angina pectoris of coronary heart disease/Meta analysis. Chin. J. Traditional Chin. Med. 44 (20), 4366–4378. doi:10.19540/j.cnki.cjcmm.20190724.501
Guan, H. W., Fang, J., and Wang, C. Y. (2015 ). Effect of Xueshuantong injection on blood pressure, HR and cardiac function in patients with angina pectoris. Electron. J. Transl. Med. 2 (09), 75–78.
Guidelines for diagnosis and treatment of non-ST segment elevation acute coronary syndrome 2016 (2017). Chronicles Cardiovasc. Dis. 45 (05), 359–376.
Guyatt, G. H., Oxman, A. D., Sultan, S., Glasziou, P., Akl, E. A., Alonso-Coello, P., et al. (2011). GRADE guidelines: 9.Rating up the quality of evidence. J. Clin. Epidemiol. 64, 1311–1316. doi:10.1016/j.jclinepi.2011.06.004
Han, S. X., Chen, Y., Zhang, Q., Han, B., Ge, Y. M., Xiang, Y. H., et al. (2017). Study on the molecular pharmacological mechanism of Xueshuantong capsule against platelet adhesion under flow condition. Chin. J. traditional Chin. Med. 42 (02), 341–346. doi:10.19540/j.cnki.cjcmm.20161222.014
Huang, T. H. (2017). Clinical observation of Xueshuantong injection in the treatment of unstable angina pectoris. World's latest Med. Inf. Abstr. 17 (91), 94. doi:10.19613/j.cnki.1671-3141.2017.91.074
Jia, M. Q., Xiong, Y. J., Xue, Y., Wang, Y., and Yan, C. (2017). Using UPLC-MS/MS for characterization of active components in extracts of yupingfeng and application to a comparative pharmacokinetic study in rat plasma after oral administration. Molecules 22, 810. doi:10.3390/molecules22050810
Ju, M. Y., and Dong, J. (2012). Clinical observation on 110 cases of unstable angina pectoris treated with Xueshuantong (freeze-dried powder). Chin. community physician (medical Major. 14 (27), 36.
Li, H., Ma, S. H., Wang, Lan., Tian, B., and Sheng, J. F. (2022). Current situation and consideration of safety and aseptic guarantee system of traditional Chinese medicine injection. Propr. Chin. Med. 44 (09), 2939–2943.
Li, N. N. (2014). Clinical efficacy of Xueshuantong in the treatment of unstable angina pectoris. J. Pract. Cardiac, Cerebrovasc. Pulm. Vasc. Dis. 22 (12), 15–18.
Li, Y. H., and ang Wang, X. M. (2016). On the pharmacological effects of Panax notoginseng on blood system. Chinese Modern Drug use. Jing 10 (08), 253–254. doi:10.14164/j.cnki.cn11-5581/r.2016.08.201
Liang, Y. C., and Wu, Rong. (2011). Clinical observation on 30 cases of unstable angina pectoris treated with Xueshuantong injection. Chin. J. Pract. Chin. West. Med. (7).
Liang, Z. L. (2010). Clinical observation of Xueshuantong combined with nitroglycerin in the treatment of unstable angina pectoris. Chin. Pract. Med. 5 (11), 154–155. doi:10.14163/j.cnki
Liu, K., Sun, D. Q., Liao, X., and Zhang, L. (2019). Interpretation of the risk of bias assessment tool for randomized controlled trials, rev. 2.0. 2016:8565907. Chin. J. Evid. Based Cardiovasc Med. 11 (03), 284–291. doi:10.3969/j.issn.1674-4055.2019.03.05
Luan, Y. D., and Sun, L. (2005). Clinical observation on 60 cases of unstable angina pectoris treated with Xueshuantong. Heilongjiang Med. Sci. 29 (6), 438.
Luo, J. E. (2014). Efficacy and safety of Xueshuantong injection in the treatment of unstable angina pectoris. J. Pract. Cardiac, Cerebrovasc. Pulm. Vasc. Dis. 22 (09), 11–12.
Ming, L. (2013). Clinical observation of nitroglycerin combined with Xueshuantong in the treatment of 93 cases of unstable angina pectoris. Chin. Foreign Health Abstr. 10 (8), 64–65. doi:10.3969/j.issn.1672-5085.2013.08.055
Moher, David, Hopewell, S., Schulz, K. F., Montori, V., Gotzsche, P. C., Devereaux, P. J., et al. (2011). CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 10 (1), 28–55. doi:10.1016/j.ijsu.2011.10.001
Ou, E. X. (2011). Effect of Xueshuantong injection on platelet activation in patients with angina pectoris of coronary atherosclerotic heart disease. Hebei Tradit. Chin. Med. 33(06), 902–906.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 88, 105906. doi:10.1016/jijsu/2021/105906
Pan, G. Z. (2010). Clinical observation of Xueshuantong combined with nitroglycerin in the treatment of 98 cases of unstable angina pectoris. Chin. Med. refers South 8 (23), 55–56. doi:10.15912/j.cnki.gocm.2010.23.056
Pan, X. L., Wang, J., and Tang, Dan. (2013). Clinical observation of Xueshuantong in treating 30 cases of unstable angina pectoris of coronary heart disease. Chin. Minkang Med. 25 (01), 81–82.
Qi, H., and Dong, Y. W. (2011). Observation on the efficacy of Xueshuantong powder acupuncture in the treatment of unstable angina pectoris. Chin. J. Clin. Electron. Ed. 5 (19), 5801–5803.
Qian, H., Chen, J., Xu, H., Luo, Z., and Xiao, C. (2018). Red cell distribution width in coronary heart disease: Prediction of restenosis and its relationship with inflammatory markers and lipids: Prediction of restenosis and itsrelationship with inflammatory markers and lipids. Postgrad. Med. J. 94 (1115), 489–494. doi:10.1136/postgradmedj-2018-135806
Qin, Y. N., Liu, H. G., Lu, S. H., Zhang, H. L., Wen, L., Chen, M., et al. (2013). Comparison of preventive effects of Panax notoginseng saponins enteric-coated pellets and Panax notoginseng saponins on atherosclerosis in rabbits. Chin. J. Exp. Pharm. J. Pharm. 19 (12), 261–264.
Sincer, I., Aktas, G., Gunes, Y., Mansiroglu, A. K., and Cosgun, M. (2018). Association of mean platelet volume and red blood cell distribution width with coronary collateral development in stable coronary artery disease. Postepy Kardiol. Interwencyjnej 14 (4), 263–269. doi:10.5114/aic.2018.78329
Summary of Chinese Cardiovascular Health and Disease report 2019 (2020). China J. Circulation 35 (09), 833–854.
Sun, Z. H., Dong, P. F., Sun, D. W., Zhao, L. Y., and Zhang, C. Y. (2011). 48 cases of unstable angina pectoris were treated with Xueshuantong injection. Harbin Med. 31 (05), 345.
Tan, J. (2014). Effect of Xueshuantong injection on angina pectoris of coronary heart disease and its effect on platelet activation function. J. Clin. Ration. Drug use 7 (16), 42–43. doi:10.15887/j.cnki.13-1389/r.2014.16.007
Tang, X. D., Jiang, J. Q., Jiang, D. C., Yan, C. W., Liu, B. Y., and Gu, D. Y. (2002). Effects of Panax notoginseng saponins on activation and adhesion of neutrophil nuclear factor-κ B during myocardial ischemia-reperfusion. China Pharmacol. Bull. 18 (05), 556–560.
Wang, F. B. (2010). Clinical observation of Xueshuantong combined with nitroglycerin in the treatment of 58 cases of unstable angina pectoris. Chin. Med. refers South 8 (32), 79–80. doi:10.15912/j.cnki.gocm.2010.32.120
Wang, H. J. (2016). Clinical observation of Xueshuantong freeze-dried powder in the treatment of unstable angina pectoris. Electron. J. Cardiovasc. Dis. Integr. traditional Chin. West. Med. 4 (15), 67+70. doi:10.16282/j.cnki.cn11-9336/r.2016.15.046
Wang, L., Gao, Y., and Yuan, Q. C. (2014). Clinical observation of Xueshuantong (lyophilized) in the treatment of unstable angina pectoris. Jilin Med. 35 (04), 689–690.
Wang, T. R., and Wei, M. (2018). Effect of Xueshuantong injection on serum IL-6 and MMP-2 in patients with unstable angina pectoris. China Med. Her. 15 (15), 127–130.
Wang, Wei, Zhang, Y. D., and He, Xin. (2012). Clinical observation of Xueshuantong in the treatment of unstable angina pectoris. Chin. Med. Eng. Program 20 (09), 14–15.
Wang, X. Q. (2020). Research progress on pharmacological effects of panax notoginseng on blood system. Shanxi Med. J. 49 (10), 1231–1233.
Wu, T., Zhang, S. F., Dong, S. F., Wu, J. Y., Jia, Z. L., Zhang, S. W., et al. (2015). Effect of Xueshuantong capsule on acute myocardial ischemia and antithrombosis. Chin. J. Comp. Med. 25(12), 10–25.
Xie, P. D., and Yan, X. X. (2008). Observation on the efficacy of two methods in the treatment of unstable angina pectoris. Chin. Trop. Med. 8 (12), 2174–2175.
Yan, H., and Yu, Z. J. (2014). Efficacy of Xueshuantong in the treatment of unstable angina pectoris. Contemp. Med. 20 (14), 124–125.
Zang, C. X. (2020). Clinical study of Xueshuantong injection combined with rosuvastatin in the treatment of unstable angina pectoris. Mod. Med. Clin. 35(03), 487–491.
Zeng, L. F. (2007). Clinical observation on 40 cases of unstable angina pectoris treated with Xueshuantong. World J. Integr. traditional Chin. West. Med. 2(10), 594,595.
Zhang, Q. G. (2011). Clinical observation of Xueshuantong in the treatment of unstable angina pectoris of coronary heart disease. Mod. Med. Health 27 (19), 2960–2961.
Zhang, R. Z., Yu, S. J., Bai, H., and Ning, K. (2017). TCM-Mesh: The database and analytical system for network pharmacology analysis for TCM preparations. Sci. Rep. 7, 2821. doi:10.1038/s41598-017-03039-7
Zhang, X., Liu, J., Wang, M., Qi, Y., Sun, J., Liu, J., et al. (2018). Twenty-year epidemiologic study on LDL-C levels in relation to the risks of atherosclerotic event, hemorrhagic stroke, and cancer death among young and middle-aged population in China. J. Clin. Lipidol. 12 (5), 1179–1189 e4. doi:10.1016/j.jacl.2018.06.011
Zhe, Y. (2011). Observation on the efficacy of Xueshuantong injection in the treatment of angina pectoris coronary heart disease. Misc. Rec. Clin. Ration. Drug use 4 (23), 18–19. doi:10.15887/j.cnki.13-1389/r.2011.23.078
Zheng, D. W. (2011). Clinical observation of Xueshuantong in the treatment of unstable angina pectoris. Clin. Res. traditional Chin. Med. 3 (09), 28–29.
Keywords: Xueshuantong for injection, unstable angina pectoris, meta-analysis, trial sequential analysis, GRADE
Citation: Xi J, Wei R, Cui X, Liu Y and Xie Y (2023) The efficacy and safety of Xueshuantong (lyophilized) for injection in the treatment of unstable angina pectoris: A systematic review and meta-analysis. Front. Pharmacol. 14:1074400. doi: 10.3389/fphar.2023.1074400
Received: 19 October 2022; Accepted: 24 March 2023;
Published: 07 April 2023.
Edited by:
Kuo Gao, Beijing University of Chinese Medicine, ChinaReviewed by:
Shihua Shi, The First Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, ChinaFengwen Yang, Tianjin University of Traditional Chinese Medicine, China
Wentai Pang, Tianjin University of Traditional Chinese Medicine, China
Xiao Ma, Chengdu University of Traditional Chinese Medicine, China
Copyright © 2023 Xi, Wei, Cui, Liu and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanming Xie, a3R6dTIwMThAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Junyu Xi
Junyu Xi Ruili Wei†
Ruili Wei† Xin Cui
Xin Cui Yi Liu
Yi Liu