
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 03 July 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1069268
This article is part of the Research TopicGinsenosides: Exploring Their Pharmacodynamic Potential and Pharmacokinetics as Adjuvant MedicinesView all 8 articles
Background: Ginseng consumption has been associated with various health outcomes. However, there are no review articles summarizing these reports.
Methods: PubMed, Embase, the Cochrane Library of Systematic Reviews, Scopus, CNKI and Wanfang databases were searched from inception to 31 July 2022. The Assessment of Multiple Systematic Reviews-2 (AMSTAR-2) and Grading of Recommendations Assessment, Development and Evaluation (GRADE) systems were used to assess the methodological quality and quality of evidence in each meta-analysis, and the results were summarized in a narrative form.
Results: Nineteen meta-analyses that met the eligibility criteria were identified from among 1,233 papers. The overall methodological quality was relatively poor, with only five studies being low-quality, and 14 critically low-quality. When compared with control treatments (mainly placebo), ginseng was beneficial for improving fatigue and physical function, sexual function, menopausal symptoms, metabolic indicators, inflammatory markers, unstable angina and respiratory diseases. Adverse events included gastrointestinal symptoms and potential bleeding; however, no serious adverse events were reported.
Conclusion: This umbrella review suggests that ginseng intake has beneficial therapeutic effects for diverse diseases. However, the methodological quality of studies needs to be improved considerably. In addition, it is imperative to establish the clinical efficacy of ginseng through high-quality randomized controlled trials.
Panax, belonging to the Acanthopanax family, is a traditional botanical drug used worldwide (Wang et al., 2020; Li et al., 2022). Ginseng plants include several species in the Panax genus, such as Panax ginseng C.A.Mey. (Korean ginseng), Panax quinquefolius L. (American ginseng), and Panax notoginseng (Burkill) F.H.Chen (Chinese ginseng) (Wang et al., 2020). It also be divided into the following categories based on the processing method: fresh ginseng (under 4 years old, freshly consumed), white ginseng (between four and 6 years old, prepared by peeling and oven- or air-dried), sun ginseng (produced by steaming white ginseng under high temperatures and pressure), and red ginseng (6 years old, steamed without peeling) (Yun, 2001; Lü et al., 2009). Ginseng contains multiple chemically active ingredients (Kim, 2012) that exert positive pharmacological effects, including anti-diabetic (Yang et al., 2022), anti-inflammation and antioxidative stress (Bak et al., 2012; Yu et al., 2016), lowering-lipid levels (Liu et al., 2021), antitumor (Huang et al., 2022), and cardioprotective effects (Sun et al., 2016),etc. In addition, it has been noted that the daily consumption of ginseng could enhance human physical performance as well as quality of life (QoL) (Coleman et al., 2003; Bahrke et al., 2009). The therapeutic potential, diverse applications, and fast advancement of ginseng products has gained increased research attention in the correlation between ginseng and health outcomes. And numerous clinical trials have been conducted to validate the impact of ginseng on human health (Shergis et al., 2013; He et al., 2018; Chen et al., 2021). However, there is still a lack of a high-quality synthesis of the existing evidence in this field.
In recent years, umbrella review has been a novel approach evaluating the methodological quality and evidence of systematic reviews and meta-analyses (Aromataris et al., 2015). This approach has been implemented across various medical fields, involving psychotherapy (Leichsenring et al., 2022; Rabasco et al., 2022), nutritional supplementation (Fong et al., 2022), as well as herbal medicine (Zhang et al., 2022). Nevertheless, to our knowledge, there are no reviews assessing the methodological quality, and summarizing findings of ginseng. Therefore, this review aimed to provide a comprehensive overview of the correlation between ginseng and health outcomes. Based on this, we provide an evidence-based approach for ginseng use that would be useful for patients and may contribute to decision-making by clinicians.
This umbrella review adhered to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2009) guidelines (Moher et al., 2009). There were no ethical requirements, as the analysis was based on published studies.
The PubMed, Embase, the Cochrane Library of Systematic Reviews, Scopus, CNKI and Wanfang databases were searched from their inception until 31 July 2022. We used the following search terms: “ginseng”, “panax”, “systematic review”, or “meta-analysis” without language restrictions. Furthermore, the references of the involved reviews were screened to identify additional articles which fulfilled the inclusion criteria. The comprehensive search strategies are elaborated in Supplementary Table S1. Two reviewers (Y.W. and Q.X.) independently screened the titles and/or abstracts and reviewed full-text of articles for eligibility. Any disagreements were resolved by consulting a third researcher (YD. W.).
The selection of studies for inclusion was based on specific criteria related to population, interventions or exposures, comparators, outcomes, and study design: (1) population: adults aged ≥18 years; (2) interventions/exposures: oral ginseng administered either alone or as a supplement; (3) comparators: placebo, no treatment, or conventional therapy; (4) outcomes: any health outcomes (e.g., inflammatory markers, blood glucose levels); (5) study design: meta-analysis of randomized controlled trials (RCTs). To ensure the isolation of ginseng effects, studies that employed multi-herbal formulas were excluded. Furthermore, studies incorporating ginseng administration via topical application or injection were excluded due to different compositions and mechanisms. When multiple meta-analyses were available for the same topic and outcome, only the most recent meta-analysis was included in our analysis.
Two reviewers (J.X.M. and X.L.) independently extracted data using a tailored data extraction form. For each eligible study, we extracted the following details: the first author’s name, year of publication, country, disease status, study design, number of primary studies, sample size, intervention and comparison, types of ginseng, dosage, frequency, treatment duration, outcomes, and safety. In cases of disagreement, a third reviewer (T.C.) was consulted for resolution. To ensure data integrity, discrepancies arising from incomplete data were resolved through communication with the authors of the original research.
The Assessment of Multiple Systematic Reviews 2 (AMSTAR-2) checklist was used to assess the methodological quality of each meta-analysis (Shea et al., 2017). The AMSTAR-2 checklist comprises 16 items that are evaluated based on three rating options, namely, “yes” (Y), “partially yes” (PY), or “no” (N). The studies were evaluated based on their methodological quality and were categorized as “high”, “moderate”, “low” or “critically low”.
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system was used to grade the quality of evidence (Guyatt et al., 2011). The risk of bias, inconsistency, indirectness, imprecision, and publication bias were assessed for each study, and the quality of evidence for each outcome was categorized as “high”, “moderate”, “low”, or “very low”.
Two researchers (J.X.Y and Y.B. T) independently evaluated the methodological and evidence quality. Any discrepancies were resolved through discussions and consultations with a third reviewer (Y.D. W.).
Health-related outcomes were extracted as descriptive summaries for every meta-analysis (Crichton et al., 2022; Zhang et al., 2022)). Estimated pooled effects—mean difference (MD), standardized mean difference (SMD), risk ratio (RR), and odds ratio (OR)—were extracted from the eligible meta-analyses, along with p-values and 95% confidence intervals (CIs) obtained using random-effects or fixed-effects models. I2 was used for evaluating heterogeneity. The evaluation of publication bias was conducted by funnel plots, as well as Egger’s and Begg’s tests. The compliance rates for items in the AMSTAR-2 checklist were measured for all meta-analyses and recorded as numbers and percentages of “Y”, “PY” or “N”. The data analysis and visualization were performed utilizing Excel 2016 (Microsoft Corporation, WA, United States).
Our initial search identified 1,233 potentially eligible records. Of these, 800 records remained after removing duplicates. Subsequently, 719 records were excluded after reviewing the titles and abstracts. The full-texts of the remaining 81 records were evaluated, and 19 studies (Jang et al., 2008; Seida et al., 2011; Bach et al., 2016; Duan et al., 2018; Ghorbani and Mirghafourvand, 2019; Mohammadi et al., 2019; Saboori et al., 2019; Antonelli et al., 2020; Ghavami et al., 2020; Miraghajani et al., 2020; Lee et al., 2021; Sha’ari et al., 2021; Zhu et al., 2021; Ikeuchi et al., 2022; Lee et al., 2022; Luo and Huang, 2022; Naseri et al., 2022; Park et al., 2022; Zhu et al., 2022) were involved in the final analysis. The flow chart for the study selection process is shown in Figure 1.
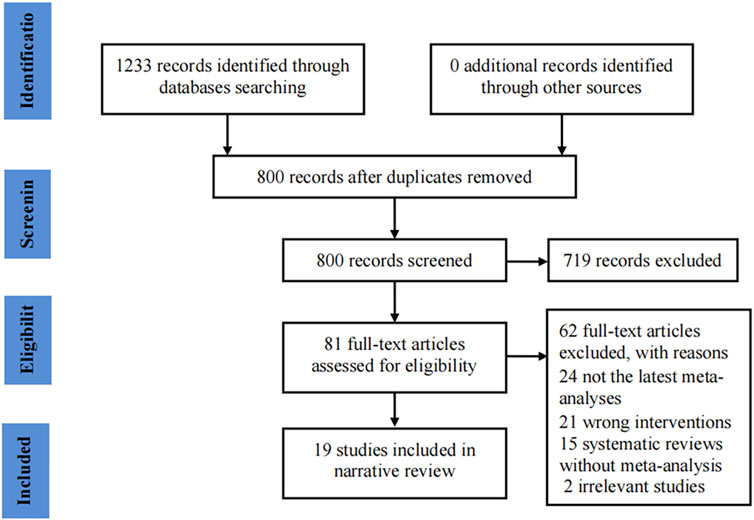
FIGURE 1. Flowchart of study selection for the umbrella review on ginseng consumption and health outcomes.
Table 1 presents the key characteristics of the included meta-analyses. All meta-analyses were published from 2008 to 2022. These studies were published in peer-reviewed journals in eight geographical regions, including five studies each from Korea (Jang et al., 2008; Bach et al., 2016; Lee et al., 2021; Lee et al., 2022; Park et al., 2022) and Iran (Ghorbani and Mirghafourvand, 2019; Mohammadi et al., 2019; Saboori et al., 2019; Ghavami et al., 2020; Miraghajani et al., 2020); four from China (Duan et al., 2018; Zhu et al., 2021; Luo and Huang, 2022; Zhu et al., 2022); and one each from Italy (Antonelli et al., 2020), Canada (Seida et al., 2011), Japan (Ikeuchi et al., 2022), Australia (An et al., 2011), and Malaysia (Sha’ari et al., 2021). Eight studies had registered their protocols prior to publication (Antonelli et al., 2020; Lee et al., 2021; Sha’ari et al., 2021; Zhu et al., 2021; Ikeuchi et al., 2022; Lee et al., 2022; Luo and Huang, 2022; Park et al., 2022). The RCTs number involved in every meta-analysis varied between two and 28, and the total number of participants ranged between 123 and 2,503. Except for two studies conducted in healthy participants (Seida et al., 2011; Ikeuchi et al., 2022) and four that included both healthy controls and patients (Bach et al., 2016; Antonelli et al., 2020; Ghavami et al., 2020; Miraghajani et al., 2020), all remaining studies were conducted in patients with indications for ginseng use (Jang et al., 2008; Duan et al., 2018; Ghorbani and Mirghafourvand, 2019; Mohammadi et al., 2019; Saboori et al., 2019; Lee et al., 2021; Sha’ari et al., 2021; Zhu et al., 2021; Lee et al., 2022; Luo and Huang, 2022; Naseri et al., 2022; Park et al., 2022; Zhu et al., 2022). Moreover, 17 meta-analyses focused on placebo-controlled trials (Jang et al., 2008; Seida et al., 2011; Bach et al., 2016; Ghorbani and Mirghafourvand, 2019; Mohammadi et al., 2019; Saboori et al., 2019; Antonelli et al., 2020; Ghavami et al., 2020; Miraghajani et al., 2020; Lee et al., 2021; Sha’ari et al., 2021; Ikeuchi et al., 2022; Lee et al., 2022; Luo and Huang, 2022; Naseri et al., 2022; Park et al., 2022; Zhu et al., 2022), whereas two focused on eligible combinations (such as with usual medication) (Duan et al., 2018; Zhu et al., 2021). Most studies reported the type of ginseng, which mainly included Panax ginseng, Panax notoginseng, Panax quinquefolius, and red ginseng. The doses ranged from 5 mg to 8,000 mg. Two studies also reported the dosing frequency, which varied from one to three times a day (Lee et al., 2021; Luo and Huang, 2022). The treatment duration range was between 2 and 32 weeks. Regarding the risk of bias tools, the Cochrane risk of bias tool was employed in 15 studies (Duan et al., 2018; Ghorbani and Mirghafourvand, 2019; Mohammadi et al., 2019; Saboori et al., 2019; Antonelli et al., 2020; Ghavami et al., 2020; Miraghajani et al., 2020; Lee et al., 2021; Zhu et al., 2021; Ikeuchi et al., 2022; Lee et al., 2022; Luo and Huang, 2022; Naseri et al., 2022; Park et al., 2022; Zhu et al., 2022), three used the Jadad scale (Jang et al., 2008; Seida et al., 2011; Bach et al., 2016), and one used the McMaster Critical Appraisal Tool (Sha’ari et al., 2021).
The overall methodological quality of each study was evaluated based on the AMSTAR-2 checklist. The results showed that five studies were of low-quality and 14 were of critically low-quality (Table 2). The methodological quality limitations were mainly related to these items: item two, which requires the registration protocol to be established before conducting the review; item three, which necessitates an explanation of the chosen study designs for inclusion in the review; item four, which mandates the use of a comprehensive literature search strategy; item seven, which obliges the provision of a list of excluded studies and a justification for their exclusions; item 10, which establishes the reporting of funding sources for individual studies; and item 15, which entails the investigation of publication bias, (Figure 2).
Of the 91 outcomes, the majority (81.32%) were associated with evidence of very low or low-quality, indicating that there was limited confidence that the estimated effects represented the true values. Evidence quality was considered moderate for the remaining 18.68% of outcomes. The GRADE levels were mostly downgraded owing to the risk of bias, inconsistency, and imprecision (Supplementary Table S2).
Three studies examined ginseng’s effects on serum lipids (Duan et al., 2018; Naseri et al., 2022; Park et al., 2022). In patients with metabolic syndrome (Mets), ginseng was found to reduce the total cholesterol (TC) (GRADE level: moderate), triglycerides (TG) (GRADE level: moderate), and low-density lipoprotein cholesterol (LDL-C) (GRADE level: moderate) when compared with placebo (Park et al., 2022). Although ginseng intake elevated high-density lipoprotein cholesterol (HDL-C) (GRADE level: moderate), the differences was not statistically significant (Park et al., 2022). In patients with prediabetes and type 2 diabetes mellitus (T2DM) patients, ginseng intake decreased TC (GRADE level: very low) but had no significant effect on TG (GRADE level: very low), LDL-C (GRADE level: very low), or HDL-C (GRADE level: very low) when compared to placebo (Naseri et al., 2022). When compared with lipid-lowering drugs alone, Panax notoginseng combined with lipid-lowering drugs significantly reduced TC, TG, and LDL-C levels and increased HDL-C levels, although the level of evidence was considered low (Duan et al., 2018).
In terms of glucose metabolism, ginseng significantly reduced the serum concentrations of fasting plasma glucose (FPG) (GRADE level: very low) when compared with the placebo in patients with prediabetes and T2DM (Naseri et al., 2022). However, ginseng failed to reduce the oral glucose tolerance test results (OGTT) (GRADE level: very low) and hemoglobin A1c (HbA1c) levels (GRADE level: very low) (Naseri et al., 2022). In terms of insulin resistance and secretion, ginseng significantly reduced the Homeostatic Model Assessment of Insulin Resistance scores (GRADE level: low), but did not affect fasting insulin levels (GRADE level: very low) (Naseri et al., 2022).
In terms of weight management, ginseng supplementation had no significant effect on body weight (BW) (GRADE level: very low), body mass index (BMI) (GRADE level: low), or waist circumference (WC) (GRADE level: very low to low) when compared with the control treatment (mainly placebo) in adults/prediabetes and T2DM (Miraghajani et al., 2020; Naseri et al., 2022). However, there were contradictory results in body fat percentage (BF%). One study reported that ginseng had no effect on BF% in adults (GRADE level: very low) when compared to placebo (Miraghajani et al., 2020), whereas another study reported that ginseng significantly reduced BF% by 2.11% in patients with Mets (GRADE level: low) (Park et al., 2022).
In terms of blood pressure, one study found that ginseng significantly reduced systolic blood pressure (SBP) (GRADE level: low), but failed to control diastolic blood pressure (DBP) (GRADE level: very low) in patients with metabolic diseases patients (Park et al., 2022). Another study showed that ginseng did not significantly affect SBP (GRADE level: very low) or DBP (GRADE level: very low) in patients with prediabetes and T2DM, but could increase the heart rate (GRADE level: low) (Naseri et al., 2022).
Three studies evaluated the effects of ginseng on serum inflammatory parameters and adipocytokines (Mohammadi et al., 2019; Saboori et al., 2019; Naseri et al., 2022). One study indicated that ginseng reduced the serum interleukin 6 (IL-6) levels (GRADE level: very low) and tumor necrosis factor alpha (TNF-α) (GRADE level: very low) but had no effect on the high-sensitivity C-reactive protein (hs-CRP) levels (GRADE level: very low) in adults (Mohammadi et al., 2019). Similarly, in adults with any underlying health problem, ginseng did not reduce the serum levels of C-reactive protein (CRP) (GRADE level: very low) when compared to placebo (Saboori et al., 2019). Nevertheless, ginseng was associated with lower IL-6 levels (GRADE level: very low) and higher TNF-α levels (GRADE level: very low) than placebo, but had no effect on CRP (GRADE level: very low), lipocalin (GRADE level: very low) or leptin levels (GRADE level: very low) in patients with prediabetes and T2DM patients (Naseri et al., 2022).
Four studies evaluated the effects of ginseng on physical functioning (Bach et al., 2016; Ikeuchi et al., 2022; Luo and Huang, 2022; Zhu et al., 2022). When compared with the placebo, ginseng was beneficial for reducing fatigue (GRADE level: moderate) (Bach et al., 2016), disease-related fatigue (GRADE level: moderate) (Zhu et al., 2022), and cancer-related fatigue (GRADE level: low) (Luo and Huang, 2022) in healthy people, patients with underlying diseases, or patients with cancer, respectively. Moreover, ginseng consumption was found to improve exercise endurance (GRADE level: low) in adults (Ikeuchi et al., 2022). However, there was no association between ginseng intake and physical performance (GRADE level: very low) (Bach et al., 2016).
Five studies evaluated the effects of ginseng on sexual function (Jang et al., 2008; Ghorbani and Mirghafourvand, 2019; Lee et al., 2021; Sha’ari et al., 2021; Lee et al., 2022). According to the Erectile Function Domain of the International Index of Erectile Function-15, ginseng had a trivial effect on erectile function (GRADE level: very low) (Lee et al., 2021). In addition, ginseng improved men’s self-reported ability to perform sexual intercourse (GRADE level: low) but had little effect on sexual satisfaction (GRADE level: low) (Lee et al., 2021). Another study showed that red ginseng was more effective in improving the erectile function than the placebo (GRADE level: low) (Jang et al., 2008). Among female patients with sexual dysfunction, ginseng failed to improve overall sexual function (GRADE level: very low), but had a positive effect in treating sexual arousal (GRADE level: very low) and sexual desire (GRADE level: very low) (Sha’ari et al., 2021). Similarly, ginseng failed to improve sexual function in menopausal women (GRADE level: very low) when compared with the placebo (Ghorbani and Mirghafourvand, 2019; Lee et al., 2022). However, ginseng could significantly reduce menopausal symptoms (GRADE level: low), hot flashes (GRADE level: low), and improve the QoL (GRADE level: moderate) in menopausal women (Lee et al., 2022).
Three studies evaluated the effects of ginseng supplements on respiratory disease (Seida et al., 2011; Antonelli et al., 2020; Zhu et al., 2021). Compared with the placebo, ginseng reduced the incidence of seasonal acute upper respiratory infections (SAURIs) during the intervention period (GRADE level: very low), but had no effect on their duration (GRADE level: very low) (Antonelli et al., 2020). One study reported a trend of ginseng intake reducing the incidence of common cold infections (GRADE level: low), although there was no statistically significant difference (Seida et al., 2011). In patients with non-small cell lung cancer (NSCLC), ginseng combined with chemotherapy was associated with improvements in the overall response rate (GRADE level: moderate), disease control rate (GRADE level: low), and QoL (GRADE level: low) (Zhu et al., 2021). In addition, ginseng supplementation had beneficial effects on chemotherapy-related complications; in particular, ginseng reduced the incidence of leukopenia (GRADE level: moderate), thrombocytopenia (GRADE level: moderate), myelosuppression (GRADE level: moderate), low hemoglobin levels (GRADE level: low), hepatotoxicity (GRADE level: moderate), nausea/vomiting (GRADE level: moderate), and diarrhea (GRADE level: moderate) (Zhu et al., 2021). Ginseng supplementation also significantly improved immune parameters, including the levels of CD3+ (GRADE level: low), CD4+ (GRADE level: very low), CD8+ (GRADE level: very low), and CD4+/CD8+ (GRADE level: very low) (Zhu et al., 2021). Importantly, ginseng supplementation also had positive effects on the 1-year survival rate (GRADE level: moderate) and 2-year survival rates (GRADE level: moderate) of patients with NSCLC (Zhu et al., 2021).
Two studies evaluated the effects of ginseng on liver function markers (Ghavami et al., 2020; Naseri et al., 2022). When compared with the control group, orally administered ginseng had no effect on alanine aminotransferase (ALT) (GRADE level: low), aspartate aminotransferase (AST) (GRADE level: very low), gamma-glutamyl transferase (GGT) (GRADE level: low), alkaline phosphatase (ALP) (GRADE level: low), or albumin (ALB) (GRADE level: very low) in adults, although it was associated with a slight increase in bilirubin (BIL) levels (GRADE level: moderate) (Ghavami et al., 2020). Similarly, when compared with the placebo, ginseng exerted no effect on AST (GRADE level: very low), ALT (GRADE level: very low), or GGT (GRADE level: very low) in patients with prediabetes and T2DM patients (Naseri et al., 2022). Compared with conventional therapy, P.notoginseng saponin combined with traditional therapy effectively improved the values of electrocardiogram parameters (GRADE level: low) and reduced the occurrence of primary endpoint events (GRADE level: very low), frequency and duration of angina attacks (GRADE level: low to moderate), and dosage of nitroglycerin (GRADE level: low) in patients with unstable angina (Duan et al., 2018).
Ten studies reported the safety of ginseng (Jang et al., 2008; Seida et al., 2011; Duan et al., 2018; Ghorbani and Mirghafourvand, 2019; Antonelli et al., 2020; Lee et al., 2021; Sha’ari et al., 2021; Ikeuchi et al., 2022; Lee et al., 2022; Luo and Huang, 2022). The most common adverse events were gastrointestinal symptoms (Jang et al., 2008; Seida et al., 2011; Duan et al., 2018; Ghorbani and Mirghafourvand, 2019; Sha’ari et al., 2021), including abdominal discomfort (Jang et al., 2008), constipation (Jang et al., 2008), dyspepsia (Ghorbani and Mirghafourvand, 2019; Sha’ari et al., 2021), and fecal occult blood (Duan et al., 2018). Some hematological adverse events were also reported, including neutropenia (Luo and Huang, 2022), vaginal bleeding (Ghorbani and Mirghafourvand, 2019; Sha’ari et al., 2021), subcutaneous hemorrhage, and rash (Duan et al., 2018). Other adverse reactions included headache (Jang et al., 2008), insomnia (Jang et al., 2008; Lee et al., 2022), palpitations (Lee et al., 2022), and flushing (Lee et al., 2022). In male patients with erectile dysfunction, ginseng had few side effects relative to placebo (RR: 1.45, 95% CI: 0.69 to 3.03; I2 = 0%; GRADE level: low) (Lee et al., 2021). The remaining studies used a descriptive approach and concluded that ginseng consumption appeared to be safe and well-tolerated (Antonelli et al., 2020; Lee et al., 2021; Ikeuchi et al., 2022).
To our knowledge, this is the first review to assess the methodological quality and evidence of readily available meta-analyses on ginseng. Our review included 19 meta-analyses that provided a range of evidence related to the therapeutic effects of ginseng. The current findings suggest that ginseng is associated with improvements in physical strength, respiratory disease, sexual dysfunction and female menopausal symptoms, glucolipid metabolism, inflammatory markers, blood pressure, body weight management, and unstable angina. In addition, ginseng has a good safety profile.
Traditionally, ginseng has been used as a folk remedy for preventing and treating metabolic diseases, including diabetes, obesity, dyslipidemia, and cardiovascular disease (Yin et al., 2008). Several studies have showed the regulatory mechanisms underlying these effects of ginseng. For example, ginsenosides have been shown to improve insulin resistance and glucolipid metabolism through triggering IRS-1/PI3K/AKT as well as AMPK signaling pathways (Wang et al., 2022). It may also exert anti-obesity effects by regulating thermogenesis, lipogenesis, and lipolysis (Chen et al., 2017; Yoon et al., 2021). Ginseng may also exert beneficial pharmacological effects on blood pressure by mediating the inhibition of vascular myogenic activity (Qin et al., 2008). Furthermore, ginseng has been shown to exert cardioprotective effects via its antioxidant activity, by increasing coronary perfusion flow (Chen, 1996) and by enhancing contractile function during ischemic and reperfusion events (Deng and Zhang, 1991; Xie et al., 2006; Park et al., 2021). Ginseng also can negatively regulate pro-inflammatory cytokines expression and accelerate inflammation regression (Zhou et al., 2017; Kang et al., 2018). Ginseng consumption may aid in alleviating fatigue and help improving physical performance and exercise endurance by increasing SIRT1 deacetylase activity (Yang et al., 2018), improving energy metabolism, and inhibiting oxidative stress in skeletal muscles (Tan et al., 2013; Tan et al., 2014).
Ginseng may also have a positive impact on sexual function by promoting the release of endothelial nitric oxide, which improves penile hemodynamics and attenuates impairments in endothelial L-arginine-NO activity(Castela and Costa, 2016). This can directly influence the cavernous tissues and trigger erection via corporal smooth muscle relaxation (Castela and Costa, 2016; Ying et al., 2018). In addition, ginseng may have beneficial effects on preventing respiratory diseases. Indeed, ginseng can significantly amplify the serum antibody response to relevant vaccines (Song et al., 2010), inhibite lung inflammation, and reduce the infiltration of inflammatory cells into the lung tissue (Lee et al., 2018). Several studies on ginseng used in NSCLC patients have shown that ginseng extract inhibits tumor growth by altering the proliferation and morphology of tumor cells (Duan et al., 2017; Li et al., 2018; Lev-Ari et al., 2021), which highlights the potential for its widespread usage in clinical practice.
The active ingredients of ginseng have not been well reported in previous reviews. The quality and composition of its active ingredients may vary depending on numerous factors, including the plant species, cultivation methods, age at harvest, and the part of the plant used for extraction (Leung and Wong, 2010). Furthermore, there are studies reporting that the oral bioavailability of ginsenosides is very low. It may subject to poor permeability, low membrane permeability and biotransformation (Murugesan et al., 2022). Therefore, it is not possible to make appropriate recommendations on the dosage and dosing frequency of ginseng use based on current evidence. Although ginseng was not related to any significant side effects, it should not be used as a substitute for medication. Moreover, ginseng may not be appropriate for all populations, and it should only be administered under the care of physicians and/or nutritionists. The adverse events reported in most studies mainly including “gastrointestinal symptoms” and potential bleeding. However, patients and clinicians should remain aware of potential interactions between ginseng and conventional medicines, and further studies are required to identify any dangerous interactions (Izzo and Ernst, 2009; Dong et al., 2017). Because there is limited information on the safety of ginseng from clinical trials, it is difficult to draw definitive conclusions regarding its safety profile. Therefore, we hope that toxicological trials on ginseng, especially related to its long-term and frequent use, will be conducted in future. These studies will also help to provide more comprehensive safety data that can be used to standardize herbal medicine regimens (Qu et al., 2018).
In this review, we used the AMSTAR-2 checklist to evaluate the methodological quality of the included meta-analyses, and the findings indicated the existence of several possible constraints. Our findings indicate a need for significant improvements related to item two, three, four, seven, 10 and 15. As we know, registration and publication of protocols can promote the transparency and reproducibility of the meta-analyses (Page et al., 2018). It also reduces redundant efforts among diverse research teams (Sideri et al., 2018; Rombey et al., 2020). It is recommended that authors register their protocols in publicly available and open databases, such as the PROSPERO platform and Cochrane Library, to prevent possible bias in the study (Chien et al., 2012). The AMSTAR-2 checklist necessitates that review authors provide a rationale for their selection of a specific study design for meta-analysis (Shea et al., 2017), as study designs serve various purposes. In addition, a comprehensive literature search strategy is the foundation for conducting meta-analysis and guarantee the reliability of the findings. This also helps authors include all relevant studies and obtain accurate conclusions without the risk of selection bias (Qiu and Wang, 2016). Furthermore, review authors are required to provide a complete list, along with the rationale for their exclusion. This practice can enhance the transparency of the selection procedure and facilitate evaluating the integrity of the results. Authors should clearly disclose their sources of funding. Most studies indicate the absence of independent financial support, which is typically associated with financial conflicts of interest; for example, authors may present favorable outcomes and/or amplifying the effects of pharmaceuticals or devices supplied by industry sponsors (Lundh et al., 2018). Finally, publication bias may influence the pooled estimates by exaggerating the efficacy of a drug or downplaying the safety outcomes (Herrmann et al., 2017; Furuya-Kanamori et al., 2020). In this regard, funnel plots, Egger’s test, Begg’s test, and Macaskill’s test are all effective methods for assessing publication bias (Hayashino et al., 2005).
The GRADE system indicated that the evidence quality was very low to low. This emphasized the need for considerable improvements in future studies, which would help ensure that any clinical recommendations are based on high-quality data. The review revealed that the most frequent downgrade factor was the risk of bias. The primary cause was that RCTs lacked transparent or entire information on randomization, blinding, and allocation concealment. Hence, it is recommended that researchers directing upcoming investigations allocate more significant consideration toward the design framework and execution processes. Additionally, researchers should comply with basic guidelines for reporting clinical trials, such as—CONSORT statement (Schulz et al., 2010)—so as to provide better evidence to support their healthcare recommendations. Another downgrade factor was the significant heterogeneity across studies, which may be related to differences in the type of ginseng used and the dosage, dosing frequency, and treatment duration. Future RCTs should standardize the bioactive components of ginseng and provide detailed data related to the above parameters, as well as treatment adherence. Additional evidence is also required to verify the value of ginseng for clinical use in patients with high blood pressure and liver diseases. Other priority research areas include the anti-aging, immunomodulatory, and neuroprotective effects of ginseng; although there is plenty of evidence from animal and mechanistic studies supporting the effects of ginseng, its impact on humans has not been thoroughly investigated in clinical trials.
This review had several strengths and limitations. One strength of this review is that it synthesized evidence-based data from clinical practice, and the findings improved our understanding of the effects of ginseng use in the clinical setting. Notably, we adopted a rigorous study design that included assessments of methodological quality and quality of evidence employing AMSTAR-2 (Shea et al., 2017) and GRADE system (Guyatt et al., 2011) latest versions. The findings can guide future research and aid in making clinical decisions. However, although two trained researchers independently evaluated the quality of methodology and evidence in this review, any subjective biases could not be eliminated. Moreover, as most studies did not mention the side effects, which made it difficult to precisely evaluate ginseng safety in clinical practice.
Our umbrella review suggests that ginseng has beneficial effects on health outcomes, including metabolic indicators (e.g., TC, TG, LDL-C, HDL-C, FBG, HOMA-IR, BW,WC, BMI, SBP, DBP), inflammatory markers (e.g., IL-6 and TNF-α), fatigue and exercise endurance, seasonal upper respiratory infections, colds, sexual function, female menopausal symptoms, unstable angina as well as NSCLC and related complications. Adverse events included gastrointestinal symptoms and potential bleeding, but no serious adverse events were reported. However, there are several limitations in methodological quality across studies. Therefore, researchers should pay attention to the design and implementation of RCTs. Moreover, researchers need to comply with basic guidelines for reporting clinical trials so as to provide better evidence to support the healthcare needs of patients.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
All authors participated in the design and implementation of this study and were responsible for the data’s integrity and precision. ZL and TC designed the study. YW, QX, JM, XL, and YT conducted literature retrieval and data extraction. ZL and TC examined and comprehended the data. YW evaluated the article. All authors contributed to the article and approved the submitted version.
This paper was supported by the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (CI2021A01005).
We would like to thank Editage [www.editage.cn] for English language editing. We would also like to acknowledge Jiaxing Yan for accessing quality.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer LL declared a shared parent affiliation with all of the author(s) to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1069268/full#supplementary-material
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; ALB, albumin; BIL, bilirubin; BW, body weight; BMI, body mass index; BF%, body fat percentage; DBP, diastolic blood pressure; FPG, fasting plasma glucose; GGT, gamma-glutamyl transferase; HDL-C, high-density lipoprotein cholesterol; HbA1c, hemoglobin A1c; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance scores; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; LDL-C, low-density lipoprotein cholesterol; MD, mean difference; Mets, metabolic syndrome; NSCLC, non-small cell lung cancer; OR, odds ratio; Qol, quality of life; RR, risk ratio; SBP, systolic blood pressure; SMD, standardized mean difference; SAURIs, seasonal acute upper respiratory infections; TC, total cholesterol; TG, triglycerides; T2DM, type 2 diabetes mellitus; TNF-α, tumor necrosis factor alpha; OGTT, oral glucose tolerance test results; WC, waist circumference.
An, X., Zhang, A. L., Yang, A. W., Lin, L., Wu, D., Guo, X., et al. (2011). Oral ginseng formulae for stable chronic obstructive pulmonary disease: A systematic review. Respir. Med. 105 (2), 165–176. doi:10.1016/j.rmed.2010.11.007
Antonelli, M., Donelli, D., and Firenzuoli, F. (2020). Ginseng integrative supplementation for seasonal acute upper respiratory infections: A systematic review and meta-analysis. Complementary Ther. Med. 52, 102457. doi:10.1016/j.ctim.2020.102457
Aromataris, E., Fernandez, R., Godfrey, C. M., Holly, C., Khalil, H., and Tungpunkom, P. (2015). Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evidence-based Healthc. 13 (3), 132–140. doi:10.1097/XEB.0000000000000055
Bach, H. V., Kim, J., Myung, S. K., and Cho, Y. A. (2016). Efficacy of ginseng supplements on fatigue and physical performance: A meta-analysis. J. Korean Med. Sci. 31 (12), 1879–1886. doi:10.3346/jkms.2016.31.12.1879
Bahrke, M. S., Morgan, W. P., and Stegner, A. (2009). Is ginseng an ergogenic aid? Int. J. Sport Nutr. Exerc. Metabolism 19 (3), 298–322. doi:10.1123/ijsnem.19.3.298
Bak, M. J., Jun, M., and Jeong, W. S. (2012). Antioxidant and hepatoprotective effects of the red ginseng essential oil in H(2)O(2)-treated hepG2 cells and CCl(4)-treated mice. Int. J. Mol. Sci. 13 (2), 2314–2330. doi:10.3390/ijms13022314
Castela, Â., and Costa, C. (2016). Molecular mechanisms associated with diabetic endothelial-erectile dysfunction. Nat. Rev. Urol. 13 (5), 266–274. doi:10.1038/nrurol.2016.23
Chen, G., Li, H., Zhao, Y., Zhu, H., Cai, E., Gao, Y., et al. (2017). Saponins from stems and leaves of Panax ginseng prevent obesity via regulating thermogenesis, lipogenesis and lipolysis in high-fat diet-induced obese C57BL/6 mice. Food Chem. Toxicol. Int. J. Publ. Br. Industrial Biol. Res. Assoc. 106, 393–403. doi:10.1016/j.fct.2017.06.012
Chen, W., Yao, P., Vong, C. T., Li, X., Chen, Z., Xiao, J., et al. (2021). Ginseng: A bibliometric analysis of 40-year journey of global clinical trials. J. Adv. Res. 34, 187–197. doi:10.1016/j.jare.2020.07.016
Chen, X. (1996). Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin. Exp. Pharmacol. Physiology 23 (8), 728–732. doi:10.1111/j.1440-1681.1996.tb01767.x
Chien, P. F. W., Khan, K. S., and Siassakos, D. (2012). Registration of systematic reviews: Prospero. BJOG Int. J. Obstetrics Gynaecol. 119 (8), 903–905. doi:10.1111/j.1471-0528.2011.03242.x
Coleman, C. I., Hebert, J. H., and Reddy, P. (2003). The effects of Panax ginseng on quality of life. J. Clin. Pharm. Ther. 28 (1), 5–15. doi:10.1046/j.1365-2710.2003.00467.x
Crichton, M., Davidson, A. R., Innerarity, C., Marx, W., Lohning, A., Isenring, E., et al. (2022). Orally consumed ginger and human health: An umbrella review. Am. J. Clin. Nutr. 115 (6), 1511–1527. doi:10.1093/ajcn/nqac035
Deng, H. L., and Zhang, J. T. (1991). Anti-lipid peroxilative effect of ginsenoside Rb1 and Rg1. Chin. Med. J. 104 (5), 395–398.
Dong, H., Ma, J., Li, T., Xiao, Y., Zheng, N., Liu, J., et al. (2017). Global deregulation of ginseng products may be a safety hazard to warfarin takers: Solid evidence of ginseng-warfarin interaction. Sci. Rep. 7 (1), 5813. doi:10.1038/s41598-017-05825-9
Duan, L., Xiong, X., Hu, J., Liu, Y., and Wang, J. (2018). Efficacy and safety of oral panax notoginseng saponins for unstable angina patients: A meta-analysis and systematic review. Phytomedicine Int. J. Phytotherapy Phytopharm. 47, 23–33. doi:10.1016/j.phymed.2018.04.044
Duan, Z., Deng, J., Dong, Y., Zhu, C., Li, W., and Fan, D. (2017). Anticancer effects of ginsenoside Rk3 on non-small cell lung cancer cells: In vitro and in vivo. Food & Funct. 8 (10), 3723–3736. doi:10.1039/c7fo00385d
Fong, C., Alesi, S., Mousa, A., Moran, L. J., Deed, G., Grant, S., et al. (2022). Efficacy and safety of nutrient supplements for glycaemic control and insulin resistance in type 2 diabetes: An umbrella review and hierarchical evidence synthesis. Nutrients 14 (11), 2295. doi:10.3390/nu14112295
Furuya-Kanamori, L., Xu, C., Lin, L., Doan, T., Chu, H., Thalib, L., et al. (2020). P value-driven methods were underpowered to detect publication bias: Analysis of Cochrane review meta-analyses. J. Clin. Epidemiol. 118, 86–92. doi:10.1016/j.jclinepi.2019.11.011
Ghavami, A., Ziaei, R., Foshati, S., Hojati Kermani, M. A., Zare, M., and Amani, R. (2020). Benefits and harms of ginseng supplementation on liver function? A systematic review and meta-analysis. Complementary Ther. Clin. Pract. 39, 101173. doi:10.1016/j.ctcp.2020.101173
Ghorbani, Z., and Mirghafourvand, M. (2019). A meta-analysis of the efficacy of panax ginseng on menopausal women’s sexual function. Int. J. Women's Health Reproduction Sci. 7 (1), 124–133. doi:10.15296/ijwhr.2019.20
Guyatt, G., Oxman, A. D., Akl, E. A., Kunz, R., Vist, G., Brozek, J., et al. (2011). GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 64 (4), 383–394. doi:10.1016/j.jclinepi.2010.04.026
Hayashino, Y., Noguchi, Y., and Fukui, T. (2005). Systematic evaluation and comparison of statistical tests for publication bias. J. Epidemiol. 15 (6), 235–243. doi:10.2188/jea.15.235
He, Y., Yang, J., Lv, Y., Chen, J., Yin, F., Huang, J., et al. (2018). A review of ginseng clinical trials registered in the WHO international clinical trials registry platform. BioMed Res. Int. 2018, 1843142. doi:10.1155/2018/1843142
Herrmann, D., Sinnett, P., Holmes, J., Khan, S., Koller, C., and Vassar, M. (2017). Statistical controversies in clinical research: Publication bias evaluations are not routinely conducted in clinical oncology systematic reviews. Ann. Oncol. Official J. Eur. Soc. Med. Oncol. 28 (5), 931–937. doi:10.1093/annonc/mdw691
Huang, J., Liu, D., Wang, Y., Liu, L., Li, J., Yuan, J., et al. (2022). Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 71 (4), 734–745. doi:10.1136/gutjnl-2020-321031
Ikeuchi, S., Minamida, M., Nakamura, T., Konishi, M., and Kamioka, H. (2022). Exploratory systematic review and meta-analysis of panax genus plant ingestion evaluation in exercise endurance. Nutrients 14 (6), 1185. doi:10.3390/nu14061185
Izzo, A. A., and Ernst, E. (2009). Interactions between herbal medicines and prescribed drugs: An updated systematic review. Drugs 69 (13), 1777–1798. doi:10.2165/11317010-000000000-00000
Jang, D. J., Lee, M. S., Shin, B. C., Lee, Y. C., and Ernst, E. (2008). Red ginseng for treating erectile dysfunction: A systematic review. Br. J. Clin. Pharmacol. 66 (4), 444–450. doi:10.1111/j.1365-2125.2008.03236.x
Kang, S., Park, S. J., Lee, A. Y., Huang, J., Chung, H. Y., and Im, D. S. (2018). Ginsenoside Rg3 promotes inflammation resolution through M2 macrophage polarization. J. Ginseng Res. 42 (1), 68–74. doi:10.1016/j.jgr.2016.12.012
Kim, D. H. (2012). Chemical diversity of panax ginseng, panax quinquifolium, and panax notoginseng. J. Ginseng Res. 36 (1), 1–15. doi:10.5142/jgr.2012.36.1.1
Lee, H. W., Ang, L., and Lee, M. S. (2022). Using ginseng for menopausal women's health care: A systematic review of randomized placebo-controlled trials. Complementary Ther. Clin. Pract. 48, 101615. doi:10.1016/j.ctcp.2022.101615
Lee, H. W., Lee, M. S., Kim, T. H., Alraek, T., Zaslawski, C., Kim, J. W., et al. (2021). Ginseng for erectile dysfunction. Cochrane Database Syst. Rev. 4, 12654. doi:10.1002/14651858.CD012654.pub2
Lee, J. H., Min, D. S., Lee, C. W., Song, K. H., Kim, Y. S., and Kim, H. P. (2018). Ginsenosides from Korean red ginseng ameliorate lung inflammatory responses: Inhibition of the MAPKs/NF-κB/c-Fos pathways. J. Ginseng Res. 42 (4), 476–484. doi:10.1016/j.jgr.2017.05.005
Leichsenring, F., Steinert, C., Rabung, S., and Ioannidis, J. P. A. (2022). The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: An umbrella review and meta-analytic evaluation of recent meta-analyses. World Psychiatry Official J. World Psychiatric Assoc. (WPA) 21 (1), 133–145. doi:10.1002/wps.20941
Leung, K. W., and Wong, A. S. T. (2010). Pharmacology of ginsenosides: A literature review. Chin. Med. 5, 20. doi:10.1186/1749-8546-5-20
Lev-Ari, S., Starr, A. N., Vexler, A., Kalich-Philosoph, L., Yoo, H. S., Kwon, K. R., et al. (2021). Rh2-enriched Korean ginseng (Ginseng Rh2+) inhibits tumor growth and development of metastasis of non-small cell lung cancer. Food & Funct. 12 (17), 8068–8077. doi:10.1039/d1fo00643f
Li, H., Huang, N., Zhu, W., Wu, J., Yang, X., Teng, W., et al. (2018). Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer 18 (1), 579. doi:10.1186/s12885-018-4299-4
Li, X., Liu, J., Zuo, T. T., Hu, Y., Li, Z., Wang, H. D., et al. (2022). Advances and challenges in ginseng research from 2011 to 2020: The phytochemistry, quality control, metabolism, and biosynthesis. Nat. Product. Rep. 39 (4), 875–909. doi:10.1039/d1np00071c
Liu, Z., Qu, C. Y., Li, J. X., Wang, Y. F., Li, W., Wang, C. Z., et al. (2021). Hypoglycemic and hypolipidemic effects of malonyl ginsenosides from American ginseng (Panax quinquefolius L) on type 2 diabetic mice. ACS Omega 6 (49), 33652–33664. doi:10.1021/acsomega.1c04656
Lü, J. M., Yao, Q., and Chen, C. (2009). Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr. Vasc. Pharmacol. 7 (3), 293–302. doi:10.2174/157016109788340767
Lundh, A., Lexchin, J., Mintzes, B., Schroll, J. B., and Bero, L. (2018). Industry sponsorship and research outcome: Systematic review with meta-analysis. Intensive Care Med. 44 (10), 1603–1612. doi:10.1007/s00134-018-5293-7
Luo, W. T., and Huang, T. W. (2022). Effects of ginseng on cancer-related fatigue: A systematic review and meta-analysis of randomized controlled trials. Cancer Nurs. 46, 120–127. doi:10.1097/NCC.0000000000001068
Miraghajani, M., Hadi, A., Hajishafiee, M., Arab, A., Ghaedi, E., and Moody, V. (2020). The effects of ginseng supplementation on anthropometric indices and body composition: A systematic review and meta-analysis. J. Herb. Med. 23, 100379. doi:10.1016/j.hermed.2020.100379
Mohammadi, H., Hadi, A., Kord-Varkaneh, H., Arab, A., Afshari, M., Ferguson, A. J. R., et al. (2019). Effects of ginseng supplementation on selected markers of inflammation: A systematic review and meta-analysis. Phytotherapy Res. PTR 33 (8), 1991–2001. doi:10.1002/ptr.6399
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ Clin. Res. ed.) 339, b2535. doi:10.1136/bmj.b2535
Murugesan, M., Mathiyalagan, R., Boopathi, V., Kong, B. M., Choi, S. K., Lee, C. S., et al. (2022). Production of minor ginsenoside CK from major ginsenosides by biotransformation and its advances in targeted delivery to tumor tissues using nanoformulations. Nanomater. (Basel, Switz. 12 (19), 3427. doi:10.3390/nano12193427
Naseri, K., Saadati, S., Sadeghi, A., Asbaghi, O., Ghaemi, F., Zafarani, F., et al. (2022). The efficacy of ginseng (panax) on human prediabetes and type 2 diabetes mellitus: A systematic review and meta-analysis. Nutrients 14 (12), 2401. doi:10.3390/nu14122401
Page, M. J., Shamseer, L., and Tricco, A. C. (2018). Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst. Rev. 7 (1), 32. doi:10.1186/s13643-018-0699-4
Park, S. H., Chung, S., Chung, M. Y., Choi, H. K., Hwang, J. T., and Park, J. H. (2022). Effects of Panax ginseng on hyperglycemia, hypertension, and hyperlipidemia: A systematic review and meta-analysis. J. Ginseng Res. 46 (2), 188–205. doi:10.1016/j.jgr.2021.10.002
Park, S. K., Hyun, S. H., Park, C. K., Kwak, Y. S., and Jang, Y. J. (2021). The antioxidant activities of Korean red ginseng (Panax ginseng) and ginsenosides: A systemic review through in vivo and clinical trials. J. Ginseng Res. 45 (1), 41–47. doi:10.1016/j.jgr.2020.09.006
Qin, N., Gong, Q. H., Wei, L. W., Wu, Q., and Huang, X. N. (2008). Total ginsenosides inhibit the right ventricular hypertrophy induced by monocrotaline in rats. Biol. Pharm. Bull. 31 (8), 1530–1535. doi:10.1248/bpb.31.1530
Qiu, X., and Wang, C. (2016). Literature searches in the conduct of systematic reviews and evaluations. Shanghai Archives Psychiatry 28 (3), 154–159. doi:10.11919/j.issn.1002-0829.215008
Qu, L., Zou, W., Wang, Y., and Wang, M. (2018). European regulation model for herbal medicine: The assessment of the EU monograph and the safety and efficacy evaluation in marketing authorization or registration in Member States. Phytomedicine Int. J. Phytotherapy Phytopharm. 42, 219–225. doi:10.1016/j.phymed.2018.03.048
Rabasco, A., McKay, D., Smits, J. A., Powers, M. B., Meuret, A. E., and McGrath, P. B. (2022). Psychosocial treatment for panic disorder: An umbrella review of systematic reviews and meta-analyses. J. Anxiety Disord. 86, 102528. doi:10.1016/j.janxdis.2022.102528
Rombey, T., Puljak, L., Allers, K., Ruano, J., and Pieper, D. (2020). Inconsistent views among systematic review authors toward publishing protocols as peer-reviewed articles: An international survey. J. Clin. Epidemiol. 123, 9–17. doi:10.1016/j.jclinepi.2020.03.010
Saboori, S., Falahi, E., Yousefi Rad, E., Asbaghi, O., and Khosroshahi, M. Z. (2019). Effects of ginseng on C-reactive protein level: A systematic review and meta-analysis of clinical trials. Complementary Ther. Med. 45, 98–103. doi:10.1016/j.ctim.2019.05.021
Schulz, K. F., Altman, D. G., and Moher, D.CONSORT Group (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 152 (11), 726–732. doi:10.7326/0003-4819-152-11-201006010-00232
Seida, J. K., Durec, T., and Kuhle, S. (2011). North American (panax quinquefolius) and asian ginseng (panax ginseng) preparations for prevention of the common cold in healthy adults: A systematic review. Evidence-based Complementary Altern. Med. ECAM 2011, 282151. doi:10.1093/ecam/nep068
Sha'ari, N., Woon, L. S. C., Sidi, H., Das, S., Bousman, C. A., and Mohamed Saini, S. (2021). Beneficial effects of natural products on female sexual dysfunction: A systematic review and meta-analysis. Phytomedicine Int. J. Phytotherapy Phytopharm. 93, 153760. doi:10.1016/j.phymed.2021.153760
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). Amstar 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ Clin. Res. ed.) 358, j4008. doi:10.1136/bmj.j4008
Shergis, J. L., Zhang, A. L., Zhou, W., and Xue, C. C. (2013). Panax ginseng in randomised controlled trials: A systematic review. Phytotherapy Res. PTR 27 (7), 949–965. doi:10.1002/ptr.4832
Sideri, S., Papageorgiou, S. N., and Eliades, T. (2018). Registration in the international prospective register of systematic reviews (PROSPERO) of systematic review protocols was associated with increased review quality. J. Clin. Epidemiol. 100, 103–110. doi:10.1016/j.jclinepi.2018.01.003
Song, X., Chen, J., Sakwiwatkul, K., Li, R., and Hu, S. (2010). Enhancement of immune responses to influenza vaccine (H3N2) by ginsenoside Re. Int. Immunopharmacol. 10 (3), 351–356. doi:10.1016/j.intimp.2009.12.009
Sun, Y., Liu, Y., and Chen, K. (2016). Roles and mechanisms of ginsenoside in cardiovascular diseases: Progress and perspectives. Sci. China Life Sci. 59 (3), 292–298. doi:10.1007/s11427-016-5007-8
Tan, S. J., Li, N., Zhou, F., Dong, Q. T., Zhang, X. D., Chen, B. C., et al. (2014). Ginsenoside Rb1 improves energy metabolism in the skeletal muscle of an animal model of postoperative fatigue syndrome. J. Surg. Res. 191 (2), 344–349. doi:10.1016/j.jss.2014.04.042
Tan, S., Zhou, F., Li, N., Dong, Q., Zhang, X., Ye, X., et al. (2013). Anti-fatigue effect of ginsenoside Rb1 on postoperative fatigue syndrome induced by major small intestinal resection in rat. Biol. Pharm. Bull. 36 (10), 1634–1639. doi:10.1248/bpb.b13-00522
Wang, D. S., Wang, J. M., Zhang, F. R., Lei, F. J., Wen, X., Song, J., et al. (2022). Ameliorative effects of malonyl ginsenoside from Panax ginseng on glucose-lipid metabolism and insulin resistance via IRS1/PI3K/akt and AMPK signaling pathways in type 2 diabetic mice. Am. J. Chin. Med. 50 (3), 863–882. doi:10.1142/S0192415X22500367
Wang, L., Huang, Y., Yin, G., Wang, J., Wang, P., Chen, Z. Y., et al. (2020). Antimicrobial activities of Asian ginseng, American ginseng, and notoginseng. Phytotherapy Res. PTR 34 (6), 1226–1236. doi:10.1002/ptr.6605
Xie, J. T., Shao, Z. H., Vanden Hoek, T. L., Chang, W. T., Li, J., Mehendale, S., et al. (2006). Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur. J. Pharmacol. 532 (3), 201–207. doi:10.1016/j.ejphar.2006.01.001
Yang, Q. Y., Lai, X. D., Ouyang, J., and Yang, J. D. (2018). Effects of Ginsenoside Rg3 on fatigue resistance and SIRT1 in aged rats. Toxicology 409, 144–151. doi:10.1016/j.tox.2018.08.010
Yang, Z., Dan, W., Li, Y., Zhou, X., Liu, T., Shi, C., et al. (2022). Untargeted metabolomics analysis of the anti-diabetic effect of Red ginseng extract in Type 2 diabetes Mellitus rats based on UHPLC-MS/MS. Biomed. Pharmacother. = Biomedecine Pharmacother. 146, 112495. doi:10.1016/j.biopha.2021.112495
Yin, J., Zhang, H., and Ye, J. (2008). Traditional Chinese medicine in treatment of metabolic syndrome. Endocr. Metabolic Immune Disord. Drug Targets 8 (2), 99–111. doi:10.2174/187153008784534330
Ying, A., Yu, Q. T., Guo, L., Zhang, W. S., Liu, J. F., Li, Y., et al. (2018). Structural-activity relationship of ginsenosides from steamed ginseng in the treatment of erectile dysfunction. Am. J. Chin. Med. 46 (1), 137–155. doi:10.1142/S0192415X18500088
Yoon, S. J., Kim, S. K., Lee, N. Y., Choi, Y. R., Kim, H. S., Gupta, H., et al. (2021). Effect of Korean red ginseng on metabolic syndrome. J. Ginseng Res. 45 (3), 380–389. doi:10.1016/j.jgr.2020.11.002
Yu, T., Rhee, M. H., Lee, J., Kim, S. H., Yang, Y., Kim, H. G., et al. (2016). Ginsenoside rc from Korean red ginseng (panax ginseng C.A. Meyer) attenuates inflammatory symptoms of gastritis, hepatitis and arthritis. Am. J. Chin. Med. 44 (3), 595–615. doi:10.1142/S0192415X16500336
Yun, T. K. (2001). Brief introduction of Panax ginseng C.A. Meyer. J. Korean Med. Sci. 16, S3–S5. doi:10.3346/jkms.2001.16.S.S3
Zhang, X., Deng, J., Tang, Y., Guan, X., Chen, X., and Fan, J. (2022). Zingiberaceae plants/curcumin consumption and multiple health outcomes: An umbrella review of systematic reviews and meta-analyses of randomized controlled trials in humans. Phytotherapy Res. PTR 36, 3080–3101. doi:10.1002/ptr.7500
Zhou, P., Lu, S., Luo, Y., Wang, S., Yang, K., Zhai, Y., et al. (2017). Attenuation of TNF-α-Induced inflammatory injury in endothelial cells by ginsenoside Rb1 via inhibiting NF-κB, JNK and p38 signaling pathways. Front. Pharmacol. 8, 464. doi:10.3389/fphar.2017.00464
Zhu, H., Liu, H., Zhu, J. H., Wang, S. Y., Zhou, S. S., Kong, M., et al. (2021). Efficacy of ginseng and its ingredients as adjuvants to chemotherapy in non-small cell lung cancer. Food & Funct. 12 (5), 2225–2241. doi:10.1039/d0fo03341c
Keywords: ginseng, health outcomes, meta-analysis, AMSTAR-2, umbrella review
Citation: Li Z, Wang Y, Xu Q, Ma J, Li X, Tian Y, Wen Y and Chen T (2023) Ginseng and health outcomes: an umbrella review. Front. Pharmacol. 14:1069268. doi: 10.3389/fphar.2023.1069268
Received: 13 October 2022; Accepted: 09 June 2023;
Published: 03 July 2023.
Edited by:
Wei Li, Jilin Agricultural University, ChinaReviewed by:
Xiaojun Gou, Baoshan District Hospital of Integrated Traditional Chinese and Western Medicine of Shanghai, ChinaCopyright © 2023 Li, Wang, Xu, Ma, Li, Tian, Wen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yandong Wen, MzE4MDIwMDE4MkBjYWEuZWR1LmNu; Ting Chen, eGluZ2xpbmNoZW50aW5nQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.