
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 15 February 2023
Sec. Pharmacoepidemiology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1062290
 Theresa Reinhild Haerig1†
Theresa Reinhild Haerig1† Dietmar Krause1†*
Dietmar Krause1†* Renate Klaassen-Mielke1†
Renate Klaassen-Mielke1† Henrik Rudolf2†
Henrik Rudolf2† Hans Joachim Trampisch1
Hans Joachim Trampisch1 Petra Thuermann3
Petra Thuermann3Introduction: With growing age, multiple chronic diseases may result in polypharmacy. Drugs that should be avoided in older adults are called potentially inappropriate medications (PIM). Beyond PIM, drug-drug interactions (DDI) are known to be related to adverse drug events. This analysis examines the risk of frequent falling, hospital admission, and death in older adults associated with PIM and/or DDI (PIM/DDI) prescription.
Materials and methods: This post hoc analysis used data of a subgroup of the getABI study participants, a large cohort of community-dwelling older adults. The subgroup comprised 2120 participants who provided a detailed medication report by telephone interview at the 5-year getABI follow-up. The risks of frequent falling, hospital admission, and death in the course of the following 2 years were analysed by logistic regression in uni- and multivariable models with adjustment for established risk factors.
Results: Data of all 2,120 participants was available for the analysis of the endpoint death, of 1,799 participants for hospital admission, and of 1,349 participants for frequent falling. The multivariable models showed an association of PIM/DDI prescription with frequent falling (odds ratio (OR) 1.66, 95% confidence interval (CI) 1.06–2.60, p = 0.027) as well as with hospital admission (OR 1.29, 95% CI 1.04–1.58, p = 0.018), but not with death (OR 1.00, 95% CI 0.58–1.72, p = 0.999).
Conclusion: PIM/DDI prescription was associated with the risk of hospital admission and frequent falling. No association was found with death by 2 years. This result should alert physicians to provide a closer look at PIM/DDI prescriptions.
With the improvement of living conditions and medical care, life expectancy is growing. Advanced age increases the risk of multimorbidity and polypharmacy that may cause drug-drug interactions (DDI) and adverse drug events (ADE), resulting in profound medical and safety problems for older adults and in economic effects on the healthcare system (Pohl-Dernick et al., 2016).
Drugs with an increased risk of potentially ADE for older adults are classified as potentially inappropriate medications (PIM) for this age group (Holt et al., 2010). Despite contradictory results, numerous studies have shown that PIM use is associated with an increased number of hospital admissions and – in some studies - also with higher morbidity and mortality (Reich et al., 2014; Henschel et al., 2015; Heider et al., 2017; Muhlack et al., 2017; Xing et al., 2019). In addition to PIM, DDIs are of great concern, being related to adverse drug reactions and frequent hospital stays (Johnell and Klarin, 2007; Xing et al., 2019). Almost half of preventable ADE is based on DDI, often occurring between antiplatelet drugs, oral anticoagulants, and non-steroidal anti-inflammatory drugs (NSAID). These drugs, as well as diuretics, have been identified as a frequent cause of preventable hospital admissions in older adults (Howard et al., 2007). The common combination of ACE-inhibitor or angiotensin receptor blocker plus diuretic plus NSAID was ascertained to be the unhappy triad resulting in deterioration of renal function (Lapi et al., 2013).
The first list with explicit criteria for PIMs was published 20 years ago (Beers 1991 criteria); the most recent update is reflected in the American Geriatrics Society Beers criteria (AGS Beers Criteria). The AGS Beers Criteria comprise an explicit list of PIMs that are typically best avoided by older adults in most circumstances or specific situations, for example with certain diseases or conditions (American Geriatics Society Beers Criteria® Update Expert Panel, 2019). Due to differences in the drug market, PIM lists for older adults were published in different countries between 1991 and 2017 (Motter et al., 2018). The European Union (EU) (7)-PIM list is a screening tool, developed with participation of experts from seven European countries that allows identification and comparison of PIM prescribing patterns for older adults across European countries (Renom-Guiteras et al., 2015). For the German drug market, the PRISCUS list has been established in 2010 (Holt et al., 2010). The PRISCUS list includes 18 drug groups with a total of 83 drugs and has been applied for several pharmaco-epidemiological analyses as well as for prospective studies.
Some contradictory results regarding the association of PIM use with adverse outcomes have been published (Xing et al., 2019). This may be a result of different populations studied (community-dwelling seniors versus nursing home residents), but also an effect of different methods to estimate drug exposure. Many studies used prescription and claims data to calculate exposure and to detect adverse outcomes. The present study aims to analyse the risk of frequent falling, hospital admission, and death associated with PIM/DDI prescription in a prospective cohort study observing community-dwelling older persons with regular follow-ups including patients’ interviews. PIM was defined by the PRISCUS list (Holt et al., 2010) and DDI according to the study “Reduction of potentially Inappropriate Medication in the Elderly” (RIME) (Rudolf et al., 2021). Data about medication use were available from direct patient reports.
The basis of the present study is the getABI study that was set up to obtain reliable data on the epidemiology, comorbidities, and risk factor profile of peripheral arterial disease (PAD) in general medical practices and has been described elsewhere (Diehm et al., 2004). It started in 2001 in Germany and included unselected primary care patients at the age of 65 years or older who were observed for 7 years (getABI study group, 2002). At getABI-baseline, general and medical history were obtained, a physical examination including ankle-brachial index (ABI) measurement and blood-sampling were performed. Follow-up visits with ABI measurements and physicians’ assessments, documented in a case report form (CRF) took place after one, three, five, and 7 years. The 5-year and 7-year follow-up additionally comprised patients’ reports with a detailed listing of medications and questionnaires, e. g. concerning health-related quality of life (HRQOL), obtained by telephone interviews. A standardized assessment on information about name, pharmaceutical central number, dose, and the pharmaceutical form of medication was conducted by trained interviewers.
All patients with an available interview at the 5-year getABI follow-up were included in this analysis. For this post hoc analysis we labelled the 5-year getABI follow-up as ‘PIM/DDI baseline’, where study endpoints were taken from the 7-year follow-up.
‘Frequent falling’ defined as 2 or more falls per year was recorded retrospectively 2 years after PIM/DDI baseline for the preceding 12 months. Hospital admission was determined by the CRF 2 years after PIM/DDI baseline for the preceding 2 years. The vital status was also taken by the CRF. If there was no information in the CRF and the telephone interview was missing, the vital status was requested at the civil registration office.
As secondary endpoint, HRQOL was measured by the three-level version of the EuroQol five-dimensional questionnaire (EQ-5D-3L) (Fuller et al., 2017). We dichotomized the data of the EQ-5D-3L at the 25th percentile of the age- and sex-adjusted general population of Germany and labelled values from the lowest quarter ‘low HRQOL’ (Szende et al., 2014). The secondary endpoint was defined by low HRQOL as documented during the interview 2 years after PIM/DDI baseline.
All medication data were coded according to the German Anatomical Therapeutical Chemical (ATC)-code in the version of 2006 (Fricke et al., 2019). Combination preparations were counted according to the number of active ingredients. There were two categories of medication risk factors of interest recorded at PIM/DDI baseline:
1) PIM according to the PRISCUS list (Holt et al., 2010);
2) Four DDIs, as defined in the study ‘Reduction of potentially Inappropriate Medication in the Elderly’ (RIME) (Rudolf et al., 2021):
- antiplatelet drug/oral anticoagulation plus non-steroidal anti-inflammatory drug (NSAID) without co-prescription of a proton pump inhibitor (PPI),
- multiple antiplatelet drugs without PPI,
- antiplatelet drug plus oral anticoagulation without PPI,
- ACE-inhibitors/AT1-antagonists plus NSAIDs.
In this analysis, PIM and/or DDI prescriptions are labelled as ‘PIM/DDI’.
Other risk factors taken into account were age, male sex, low education as defined by an International Standard Classification of Education (ISCED) score of 0–2, and smoker status, taken at baseline of the getABI cohort study. Arterial hypertension was either diagnosed at getABI baseline or defined as use of antihypertensive medication at getABI baseline or at the PIM/DDI baseline. Likewise, information about diabetes was either taken from the getABI baseline (HbA1c > 47.5 mmol/mol) or defined as use of antidiabetic medication at any time point. From the baseline investigation of the getABI study information was taken about cholesterol levels. Peripheral artery disease was defined by ABI <0.9 (ABI >1.5 was labelled mediasclerosis) or in case of symptoms/events like peripheral revascularisation, necrosis, gangrene, intermittent claudication, or amputation before PIM/DDI baseline. Impaired renal function was defined as eGFR <60 ml/min/1.73 m2 measured before PIM/DDI baseline.
The characteristics of the participants at PIM/DDI baseline (including getABI baseline data as mentioned above) are presented as means and standard deviations or numbers and percentages, as appropriate. Differences between the PIM/DDI group and the non-PIM/DDI group were analysed using the t-test or the chi-square-test, respectively; p-values are displayed.
Logistic regression was applied in univariable and multivariable models. For all the endpoints we used univariable and multivariable models including the above-mentioned risk factors. Missing values of risk factor data were randomly replaced by values of the entire cohort up to a proportion of 2%.
The results were presented as odds ratios (OR) with 95% confidence interval (CI). We used two-sided p-values and labelled p-values <0.05 as significant. Analyses were performed using SAS, version 9.4 (2013, SAS Institute Inc., Cary, NC, United States).
The institutional review board of the University of Heidelberg had approved the getABI trial. Each patient had provided written informed consent. The getABI trial followed the recommendations of Good Epidemiological Practice and was supported by unrestricted grants from Sanofi-Aventis GmbH, Berlin, Germany, and the German Federal Ministry of Education and Research. The protocol of this post hoc analysis was reviewed and approved by the ethics committee of the Ruhr University Bochum, Germany (approval number: 17-6103); the data were analysed anonymously. Trial registration DRKS00014098.
The getABI cohort comprised 6,880 participants at the getABI baseline. At the 5-year getABI follow-up (PIM/DDI baseline), 6,088 of them were still alive and 2,120 gave written informed consent to a telephone interview. These patients made up the cohort of this post hoc analysis (Figure 1). Two years later, sufficient information concerning survival could be obtained from all 2,120 patients, concerning hospital admission from 1,799 (84.9%) patients, and from 1,349 (63.6%) patients for frequent falling (Figure 1).
At PIM/DDI baseline, 441 (20.8%) of these 2,120 patients had at least one PIM, 359 (16.9%) at least one DDI, and 685 patients (32.3%) made up the PIM/DDI subgroup. The mean age of all 2,120 patients was 76 years, 54% were women, and 77% had arterial hypertension, with nearly 87% in the PIM/DDI subgroup. Peripheral artery disease (PAD), myocardial infarction, stroke, kidney diseases/impaired renal function, and a significantly lower HRQOL were more often found in the PIM/DDI subgroup (Table 1).
The endpoint death was met by 63 (3.0%) participants, 22 (3.2%) in the PIM/DDI group and 41 (2.9%) in the non-PIM/DDI group (Table 2). No significant effect on mortality could be shown for the risk factor PIM/DDI (multivariable model: odds ratio (OR) 1.00, confidence interval (CI) 0.58–1.72, p = 0.999).
721 (40.1) out of 1,799 participants had hospital stays (255 in the PIM/DDI group (45.2%), 466 in the non-PIM/DDI group (37.7%)). In the univariable model, the prescription of PIM/DDI was significantly associated with hospital admission (OR 1.36, 95% CI 1.11–1.67, p = 0.003). In the multivariable model with adjustment for age, sex, and other risk factors, PIM/DDI prescription showed an OR of 1.29 (95% CI 1.04–1.58, p = 0.018) (Table 3).
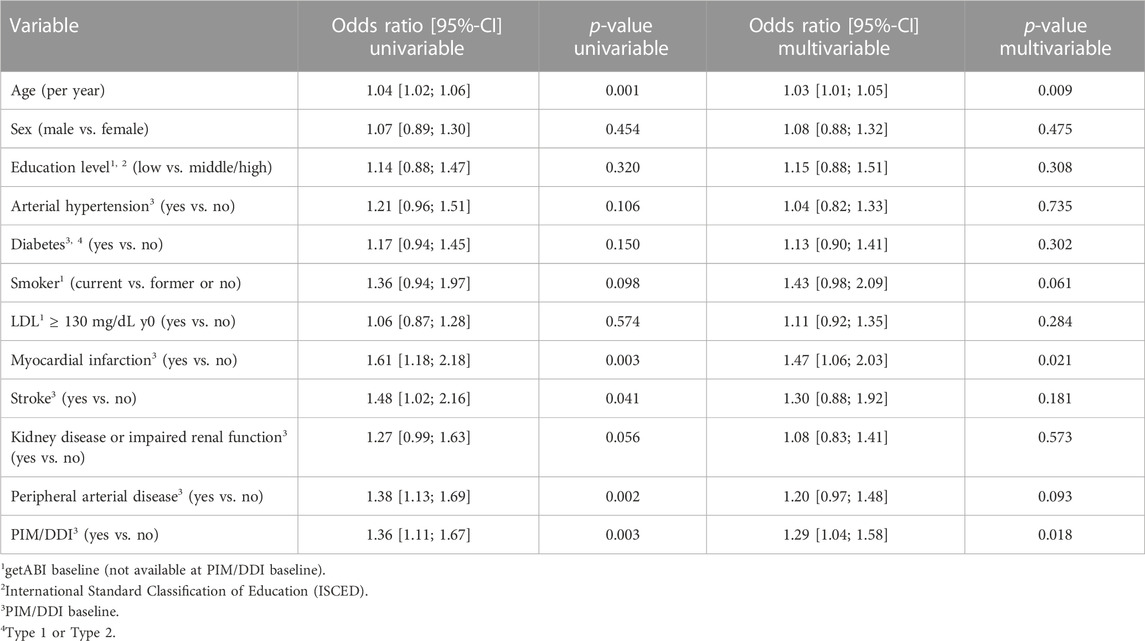
TABLE 3. Univariable models and multivariable model for the endpoint hospital admission (1,799 patients).
98 (7.3%) out of 1,349 participants were frequent fallers (43 in the PIM/DDI group (10.2%), 55 in the non-PIM/DDI group (5.9%)). In the univariable model, the prescription of PIM/DDI was significantly associated with frequent falling (OR 1.80, 95% CI 1.19–2.73, p = 0.006). In the multivariable model with adjustment for age, sex, and other risk factors, PIM/DDI prescription showed an OR of 1.66 (95% CI 1.06–2.60, p = 0.027) (Table 4).
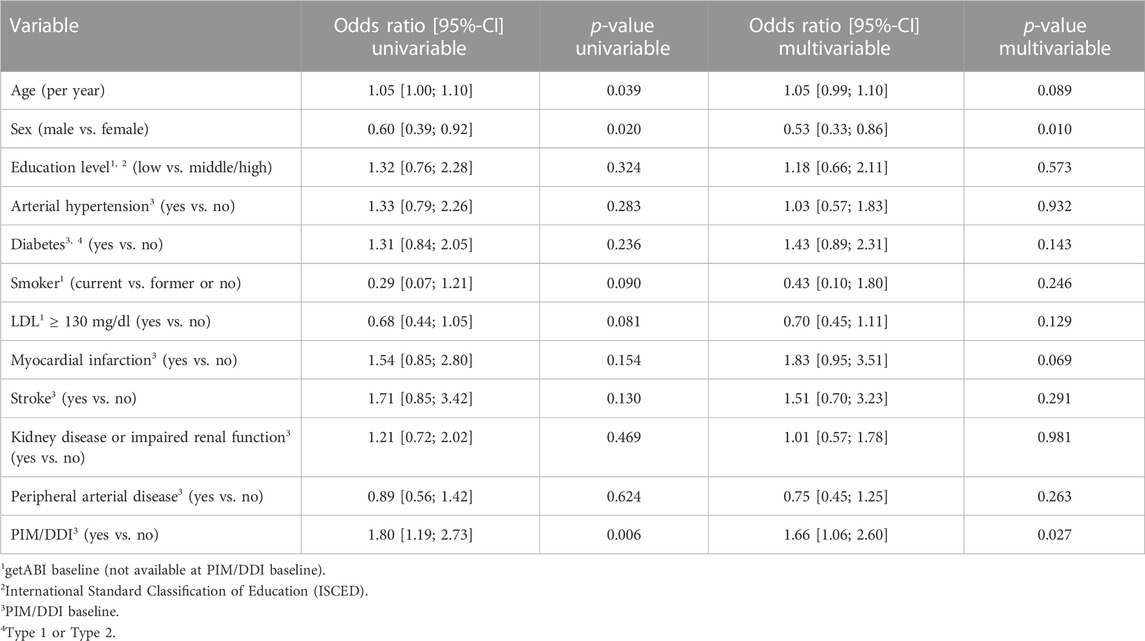
TABLE 4. Univariable models and multivariable model for the endpoint frequent falling (1,349 patients).
Considering the secondary endpoint low HRQOL, 1,322 (62.4%) participants provided sufficient data for the analysis. 336 (25.4%) out of 1,799 participants reported low HRQOL (139 in the PIM/DDI group (33.7%), 197 in the non-PIM/DDI group (21.6%)). In the univariable model, the OR of PIM/DDI for this endpoint was 1.85 (95% CI 1.43–2.39, p < 0.001). The OR remained significant in the multivariable model (1.40 (95% CI 1.04–1.88, p = 0.027). Thus, despite adjustment for the PIM/DDI baseline data (and other risk factors), PIM/DDI prescription was associated with low HRQOL after 2 years (Table 5).
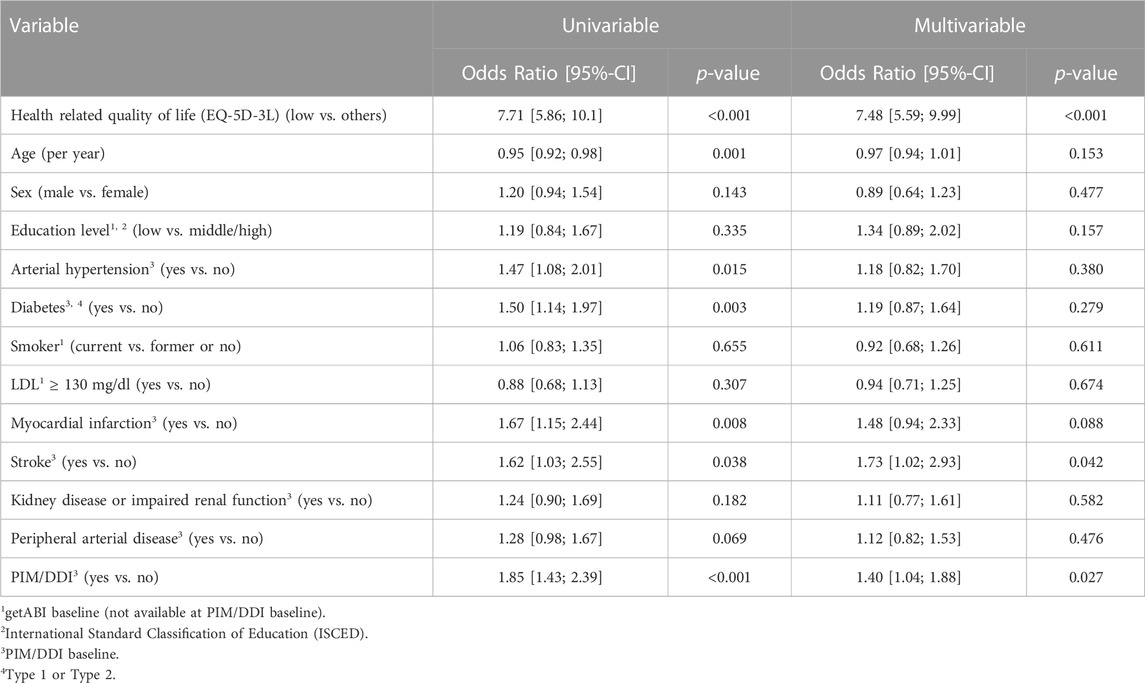
TABLE 5. Univariable models and multivariable model for the secondary endpoint lowHROQL 2 years after PIM/DDI baseline measured by the EQ-5D-3L (1,322 patients).
This post hoc analysis comprised 2,120 participants 70 years or older in a primary care setting, 685 of them (32.3%) had a PIM or a DDI. This PIM/DDI prevalence is comparable to other German and international studies. In one of them based on health insurance data of more than 800.000 persons at least 65 years old, 25.0% received at least one PIM prescription (Amann et al., 2012). A German claims data analysis with 73,665 insurants of the same age group yielded a PIM prevalence of 22% (Schubert et al., 2013). In the German health interview and examination survey (DEGS1) only 13% were PIM users (Endres et al., 2017). In contrast, in a cohort of elderly Austrian primary care patients with polypharmacy, a PIM prevalence of 37% was found (Koper et al., 2013). These data correspond well with the prevalence of PIM use observed in the US population of community dwelling seniors ranging between 11 and 51% (Nothelle et al., 2019) as well with the prevalence in European older citizens ranging calculated with 22.6% (Tommelein et al., 2015). Differences in PIM prevalence can be explained by the different populations analysed, criteria applied, the sources of information used and the methods to calculate the prevalence (Xing et al., 2019). Irrespective of these differences, the actual taking of these drugs is not proven in any study. We used patients’ interviews and thus documented patients’ statements rather than claims data. However, patient reports on medication use as well as health outcomes depend on social desirability, cognitive function and recall bias.
In our analysis, the endpoint death was not significantly associated with the use of PIMs or occurrence of the three selected DDIs. PIM use as defined by the Beers criteria or the HEDIS-DAE list was associated with 1.6-fold increased mortality in older adults (Muhlack et al., 2017). Heider et al. applied the PRISCUS list to a German claims database where approximately 500.000 PIM users were matched to almost four million non-users. PIM use was associated with a 1.8-fold higher risk of mortality compared to non-users (Heider et al., 2017). However, most studies also failed to demonstrate a higher odds ratio for death under PIM use (Mekkonnen et al., 2021). Interestingly, in one meta-analysis, the association between PIM use and mortality became significant when considering only studies with a new user design indicating an increased risk particularly at the initiation of PIM therapy (Xing et al., 2019). Moreover, the significance of the association between PIM use and adverse outcomes may also be influenced by the overall prescribing prevalence of PIM (Xing et al., 2019). Furthermore, the negative results for the endpoint mortality may also be explained by the fact, that the complexity of the telephone interview and the medication questionnaire might have resulted in a selection of healthier participants in this post hoc study.
Inappropriate medication use in older multimorbid people is associated with a range of negative healthcare consequences including adverse drug events and unplanned hospitalizations (Xing et al., 2019; Mekkonnen et al., 2021). In our study, PIM/DDI use was associated with a significant higher rate of hospitalisations (OR = 1.29; 95% CI 1.04-1.58). Another study, also using the PRISCUS list, showed that PIM use compared to PIM alternatives was associated with an increased risk of all-cause hospitalization in the 180 days following individual index date (OR = 1.38; 95% CI: 1.35–1.41) (Endres et al., 2016). In a 5-year prospective cohort study with 196 patients using modified Beers Criteria, PIMs were correlated with first hospitalization (HR 1.91, 95% CI 1.17-3.09) (Huang et al., 2019). 647,073 patients aged ≥65 years with PIM were compared to matched patients without PIM. The OR for hospitalization was 1.54 [95% CI 1.23–1.93] for PIM patients compared to non-PIM patients (Henschel et al., 2015). In an elderly managed care population in Switzerland, multiple cox regression analysis revealed a significant association with adverse outcomes in terms of hospitalizations (Reich et al., 2014).
98 (7.3%) out of 1,349 participants in our study were frequent fallers (43 in the PIM/DDI group (10.2%), 55 in the non-PIM/DDI group (5.9%)), yielding an OR of 1.66 in the multivariable model. Bauer et al. used the PRISCUS list to analyse the risk of fall-related injuries in a sample of frail patients above the age of 65 years. In their cohort, use of psychotropics and/or drugs from the PRISCUS list was significantly associated with fall-related injuries derived from administrative data (Bauer et al., 2012). A correlation between falls and PIMs, as well as the class of medication, was seen in community-dwelling older adults aged 55 and older (Lawson et al., 2018). Also, in a retrospective study with 667 patients, PIM use as defined by the Beers criteria was associated with the risk of falls (OR 2.24, 95% CI 1.51-3.32) (Kucugdagli et al., 2020). In their meta-analysis, Mekkonen et al. reported a significant association between PIM use and falls (Mekkonnen et al., 2021). However, as emphasized by Bauer et al. (Bauer et al., 2012), not all drugs from the PRISCUS list are specific for fall risk and so-called fall-risk increasing drugs (FRIDS) (Seppala et al., 2018), some of which are on the PRISCUS list (e.g. long-acting benzodiazepines) and are closer correlated with falls (Leipzig et al., 1999; Dhalwani et al., 2017). Unfortunately, we did not differentiate between PIM subclasses with a special subgroup of PIM-FRIDS.
Furthermore, our study shows that PIM/DDI prescription may be associated with a reduced HRQOL. An American study investigated the relationship between two widely used generic HRQOL measures (Short Form-12 (SF-12) and EQ-5D-3L) and potentially inappropriate drug use in a cohort of higher aged people (Franic and Jiang, 2006). PIM use was not a significant predictor of HRQOL in any of the models tested, but the number of prescriptions was a significant predictor of HRQOL, as measured by using the SF-12 and the EQ-5D. The same was found in hospitalized older patients (Akkawi et al., 2019). The contradictory results reported in the literature (Mekkonnen et al., 2018) may rely on different methodological approaches, but also on the fact that many drugs on the PIM lists are psychotropics and analgesics and such results might be confounded by indication.
Although our study, as many others (Mekkonnen et al., 2018) show significant associations between PIM use, certain DDIs and adverse outcomes, it is still unclear whether reduction of PIMs cleary results in improvements of outcomes. Recent systematic reviews stated that even any effect of interventions to improve the appropriate use of polypharmacy for older people is rather small and there is no evidence for reduction of unwanted outcomes such as hospital admissions, morbidity, and mortality as well as improvement of QoL (Rankin et al., 2018; Anderson et al., 2020). However, several analyses of claims data as well as cohort studies from Germany suggest a substantial decrease of PIM prevalence during the last 10 years (Zimmermann et al., 2013; Selke-Krulichova et al., 2021). It is unclear, if this is the result of increased awareness after the publication of the PRISCUS list in 2010, or a generally higher recognition of problematic prescribing in older patients supported by an increase in seminars and lectures. In addition, the PRISCUS list has been adopted in most major electronic prescribing tools in Germany. As interdisciplinary teams involving clinical pharmacists, some of them specialised in geriatric pharmacy, are getting more and more into practice, this may also contribute to safer prescribing (Moecker et al., 2022).
This study has some limitations. We did not capture the changes in medication and PIM/DDI status during the 2-year follow-up period. In addition, we limited our DDI definition to 4 major interactions and disregarded other possible DDIs (de Oliveira et al., 2021). No differentiation was made between PIM subclasses, nor was any differentiation made with respect to causes of hospitalization or death. Data on hospitalizations were missing for 282 patients and on frequent falls for 771 patients (of whom 63 died). Restricting 6,088 getABI patients who were still alive to the 2,120 participants in this study with written informed consent for telephone interview could introduce substantial selection bias.
A strength of this study is that data about medication use was available from direct patient reports increasing the probability of actual intake and that there was a direct assessment of the quality of life by telephone interviews. A further strength is the joint recording of PIM and DDI according to the RIME study.
In summary, our results show that PIM/DDI prescription may have an impact on hospital admission and frequent falling and may be associated with a reduction of health-related quality of life. As an aging society bears the burden of an increasing rate of chronic diseases and polypharmacy, the development of effective tools to improve medication management is urgently needed.
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics committee of the Ruhr University Bochum, Germany (approval number: 17-6103). The patients/participants provided their written informed consent to participate in this study.
DK, RK-M, HR, and HT contributed to conception and design of the study. TH, RK-M, and HR organized the database. TH, RK-M, and HR performed the statistical analysis. TH, PT, and DK wrote the first draft of the manuscript. PT, DK and RK-M wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version. All authors agree to be accountable for the content of the work.
The authors highly appreciate the participation of the patients as well as the cooperation of the primary care and the specialized physicians of the getABI study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akkawi, M. E., Nik Mohamed, M. H., and Md Aris, M. A. (2019). Does inappropriate prescribing affect elderly patients' quality of life? A study from a Malaysian tertiary hospital. Qual. Life Res. 28, 1913–1920. Epub 2019 Mar 4. PMID: 30830646. doi:10.1007/s11136-019-02153-5
Amann, U., Schmedt, N., and Garbe, E. (2012). Prescribing of potentially inappropriate medications for the elderly: An analysis based on the PRISCUS list. Dtsch. Arztebl. Int. 109, 69–75. doi:10.3238/arztebl.2012.0069
American Geriatics Society Beers Criteria® Update Expert Panel (2019). American geriatrics society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 67, 674–694. doi:10.1111/jgs.15767
Anderson, L. J., Nuckols, T. K., Coles, C., Le, M. M., Schnipper, J. L., and Shane, R. Members of the PHARM-DC Group (2020). A systematic overview of systematic reviews evaluating medication adherence interventions. Am. J. health-system Pharm. AJHP 77, 138–147. doi:10.1093/ajhp/zxz284
Bauer, T. K., Lindenbaum, K., Stroka, M. A., Engel, S., Linder, R., and Verheyen, F. (2012). Fall risk increasing drugs and injuries of the frail elderly - evidence from administrative data. Pharmacoepidemiol. Drug Saf. 21, 1321–1327. Epub 2012 Oct 25. PMID: 23097414. doi:10.1002/pds.3357
de Oliveira, L. M., Diel, J. D. A. C., Nunes, A., and da Silva Dal Pizzol, T. (2021). Prevalence of drug interactions in hospitalised elderly patients: A systematic review. Eur. J. Hosp. Pharm. 28, 4–9. doi:10.1136/ejhpharm-2019-002111
Dhalwani, N. N., Fahami, R., Sathanapally, H., Seidu, S., Davies, M. J., and Khunti, K. (2017). Association between polypharmacy and falls in older adults: A longitudinal study from england. BMJ Open 16 (7), e016358. PMID: 29042378; PMCID: PMC5652576. doi:10.1136/bmjopen-2017-016358
Diehm, C., Schuster, A., Allenberg, J. R., Darius, H., Haberl, R., Lange, S., et al. (2004). High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: Cross-sectional study. Atherosclerosis 172, 95–105. PMID: 14709362. doi:10.1016/s0021-9150(03)00204-1
Endres, H. G., Kaufmann-Kolle, P., Knopf, H., and Thürmann, P. A. (2017). Welche Faktoren begünstigen die Anwendung potenziell ungeeigneter Medikamente bei älteren Menschen? Berlin: Robert Koch-Institut.
Endres, H. G., Kaufmann-Kolle, P., Steeb, V., Bauer, E., Böttner, C., and Thürmann, P. (2016). Association between potentially inappropriate medication (PIM) use and risk of hospitalization in older adults: An observational study based on routine data comparing PIM use with use of PIM alternatives. PLoS One 11, e0146811. doi:10.1371/journal.pone.0146811
Franic, D. M., and Jiang, J. Z. (2006). Potentially inappropriate drug use and health-related quality of life in the elderly. Pharmacotherapy 26, 768–778. doi:10.1592/phco.26.6.768
Fricke, U., Günther, J., Niepraschk-von Dollen, K., and Zawinell, A. (2019). Anatomisch-therapeutisch-chemische klassifikation mit tagesdosen für den deutschen arzneimittelmarkt: ATC-index mit DDD-angaben 2019. 18th ed. Berlin: Wissenschaftliches Institute der AOK.
Fuller, J. C., Levesque, P. A., and Lindsay, R. W. (2017). Assessment of the EuroQol 5-dimension questionnaire for detection of clinically significant global health-related quality-of-life improvement following functional septorhinoplasty. JAMA Facial. Plast. Surg. 19, 95–100. doi:10.1001/jamafacial.2016.1410
getABI study group (2002). getABI: German epidemiological trial on ankle brachial index for elderly patients in family practice to dedect peripheral arterial disease, significant marker for high mortality. Vasa 31, 241–248. doi:10.1024/0301-1526.31.4.241
Heider, D., Matschinger, H., Meid, A. D., Quinzler, R., Adler, J.-B., Günster, C., et al. (2017). Health service use, costs, and adverse events associated with potentially inappropriate medication in old age in Germany: Retrospective matched cohort study. Drugs Aging 34, 289–301. doi:10.1007/s40266-017-0441-2
Henschel, F., Redaelli, M., Siegel, M., and Stock, S. (2015). Correlation of incident potentially inappropriate medication prescriptions and hospitalization: An analysis based on the PRISCUS list. Drugs Real World Outcomes 2, 249–259. doi:10.1007/s40801-015-0035-4
Holt, S., Schmiedl, S., and Thürmann, P. A. (2010). Potentially inappropriate medications in the elderly: The PRISCUS list. Dtsch. Arztebl. Int. 107, 543–551. doi:10.3238/arztebl.2010.0543
Howard, R. L., Avery, A. J., Slavenburg, S., Royal, S., Pipe, G., Lucassen, P., et al. (2007). Which drugs cause preventable admissions to hospital? A systematic review. Br. J. Clin. Pharmacol. 63, 136–147. doi:10.1111/j.1365-2125.2006.02698.x
Huang, C.-H., Umegaki, H., Watanabe, Y., Kamitani, H., Asai, A., Kanda, S., et al. (2019). Potentially inappropriate medications according to STOPP-J criteria and risks of hospitalization and mortality in elderly patients receiving home-based medical services. PLoS One 14, e0211947. doi:10.1371/journal.pone.0211947
Johnell, K., and Klarin, I. (2007). The relationship between number of drugs and potential drug-drug interactions in the elderly: A study of over 600,000 elderly patients from the Swedish prescribed drug register. Drug Saf. 30, 911–918. doi:10.2165/00002018-200730100-00009
Koper, D., Kamenski, G., Flamm, M., Böhmdorfer, B., and Sönnichsen, A. (2013). Frequency of medication errors in primary care patients with polypharmacy. Fam. Pract. 30, 313–319. doi:10.1093/fampra/cms070
Kucukdagli, P., Bahat, G., Bay, I., Kilic, C., Oren, M. M., Turkmen, B. O., et al. (2020). The relationship between common geriatric syndromes and potentially inappropriate medication use among older adults. Aging Clin. Exp. Res. 32, 681–687. Epub 2019 Jun 12. PMID: 31190200. doi:10.1007/s40520-019-01239-x
Lapi, F., Azoulay, L., Yin, H., Nessim, S. J., and Suissa, S. (2013). Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: Nested case-control study. BMJ 346, e8525. doi:10.1136/bmj.e8525
Lawson, K., Vinluan, C. M., Oganesyan, A., Gonzalez, E. C., Loya, A., and Strate, J. J. (2018). A retrospective analysis of prescription medications as it correlates to falls for older adults. Pharm. Pract. 16, 1283. Epub 2018 Nov 13. PMID: 30637029; PMCID: PMC6322985. doi:10.18549/PharmPract.2018.04.1283
Leipzig, R. M., Cumming, R. G., and Tinetti, M. E. (1999). Drugs and falls in older people: A systematic review and meta-analysis: I. Psychotropic drugs. J. Am. Geriatr. Soc. 47, 30–39. doi:10.1111/j.1532-5415.1999.tb01898.x
Mekonnen, A. B., Redley, B., de Courten, B., and Manias, E. (2021). Potentially inappropriate prescribing and its associations with health-related and system-related outcomes in hospitalised older adults: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 87, 4150–4172. Epub 2021 May 18. PMID: 34008195; PMCID: PMC8597090. doi:10.1111/bcp.14870
Moecker, R., Weissenborn, M., Klingenberg, A., Wirbka, L., Fuchs, A., Eickhoff, C., et al. (2022). ARMIN Study GroupTask sharing in an interprofessional medication management program - a survey of general practitioners and community pharmacists. BMC Health Serv. Res. 22, 1005. doi:10.1186/s12913-022-08378-4
Motter, F. R., Fritzen, J. S., Hilmer, S. N., Paniz, É. V., and Paniz, V. M. V. (2018). Potentially inappropriate medication in the elderly: A systematic review of validated explicit criteria. Eur. J. Clin. Pharmacol. 74, 679–700. doi:10.1007/s00228-018-2446-0
Muhlack, D. C., Hoppe, L. K., Weberpals, J., Brenner, H., and Schöttker, B. (2017). The association of potentially inappropriate medication at older age with cardiovascular events and overall mortality: A systematic review and meta-analysis of cohort studies. J. Am. Med. Dir. Assoc. 18, 211–220. doi:10.1016/j.jamda.2016.11.025
Nothelle, S. K., Sharma, R., Oakes, A., Jackson, M., and Segal, J. B. (2019). Factors associated with potentially inappropriate medication use in community-dwelling older adults in the United States: A systematic review. Int. J. Pharm. Pract. 27, 408–423. doi:10.1111/ijpp.12541
Pohl-Dernick, K., Meier, F., Maas, R., Schöffski, O., and Emmert, M. (2016). Potentially inappropriate medication in the elderly in Germany: An economic appraisal of the PRISCUS list. BMC Health Serv. Res. 16, 109–112. doi:10.1186/s12913-016-1366-x
Rankin, A., Cadogan, C. A., Patterson, S. M., Kerse, N., Cardwell, C. R., Bradley, M. C., et al. (2018). Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst. Rev. 9, CD008165. doi:10.1002/14651858.CD008165.pub4
Reich, O., Rosemann, T., Rapold, R., Blozik, E., and Senn, O. (2014). Potentially inappropriate medication use in older patients in Swiss managed care plans: Prevalence, determinants and association with hospitalization. PLoS One 9, 105425. doi:10.1371/journal.pone.0105425
Renom-Guiteras, A., Meyer, G., and Thürmann, P. A. (2015). The EU(7)-PIM list: A list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur. J. 8Clin. Pharmacol. 71, 861–875. doi:10.1007/s00228-015-1860-9
Rudolf, H., Thiem, U., Aust, K., Krause, D., Klaaßen-Mielke, R., Greiner, W., et al. (2021). Reduction of potentially inappropriate medication in the elderly. Dtsch. Arztebl. Int. 118, 875–882. doi:10.3238/arztebl.m2021.0372
Schubert, I., Küpper-Nybelen, J., Ihle, P., and Thürmann, P. (2013). Prescribing potentially inappropriate medication (PIM) in Germany's elderly as indicated by the PRISCUS list. An analysis based on regional claims data. Pharmacoepidemiol. Drug Saf. 22, 719–727. doi:10.1002/pds.3429
Selke Krulichová, I., Selke, G. W., and Thürmann, P. A. (2021). Trends and patterns in EU(7)-PIM prescribing to elderly patients in Germany. Eur. J. Clin. Pharmacol. 77, 1553–1561. doi:10.1007/s00228-021-03148-3
Seppala, L. J., Wermelink, A. M. A. T., de Vries, M., Ploegmakers, K. J., van de Glind, E. M. M., Daams, J. G., et al. (2018). Fall-risk-increasing drugs: A systematic review and meta-analysis: II. Psychotropics. J. Am. Med. Dir. Assoc. 19, 371.e11–371.e17. PMID: 29402652. doi:10.1016/j.jamda.2017.12.098
Szende, A., Janssen, B., and Cabases, J. (2014). Self-reported population health: An international perspective based on based on EQ-5D [internet]. Dordrecht (NL): Springer. PMID: 29787044.
Tommelein, E., Mehuys, E., Petrovic, M., Somers, A., Colin, P., and Boussery, K. (2015). Potentially inappropriate prescribing in community-dwelling older people across europe: A systematic literature review. Eur. J. Clin. Pharmacol. 71, 1415–1427. doi:10.1007/s00228-015-1954-4
Xing, X. X., Zhu, C., Liang, H. Y., Wang, K., Chu, Y. Q., Zhao, L. B., et al. (2019). Associations between potentially inappropriate medications and adverse health outcomes in the elderly: A systematic review and meta-analysis. Ann. Pharmacother. 53, 1005–1019. doi:10.1177/1060028019853069
Zimmermann, T., Kaduszkiewicz, H., van den Bussche, H., Schön, G., Brettschneider, C., König, H. H., et al. (2013). Potentially inappropriate medication in elderly primary care patients: A retrospective, longitudinal analysis. Bundesgesundheitsblatt Gesundheitsforsch. Gesundheitsschutz 56, 941–949. doi:10.1007/s00103-013-1767-5
Keywords: potentially inappropriate medication, drug-drug interaction, risk of frequent falling, hospital admission, mortality
Citation: Reinhild Haerig T, Krause D, Klaassen-Mielke R, Rudolf H, Trampisch HJ and Thuermann P (2023) Potentially inappropriate medication including drug-drug interaction and the risk of frequent falling, hospital admission, and death in older adults - results of a large cohort study (getABI). Front. Pharmacol. 14:1062290. doi: 10.3389/fphar.2023.1062290
Received: 05 October 2022; Accepted: 02 February 2023;
Published: 15 February 2023.
Edited by:
Carlos Alves, University of Coimbra, PortugalReviewed by:
Hsuei-Chen Lee, National Yang Ming Chiao Tung University, TaiwanCopyright © 2023 Reinhild Haerig, Krause, Klaassen-Mielke, Rudolf, Trampisch and Thuermann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dietmar Krause, Z3VuZGkua3JhdXNlQHQtb25saW5lLmRl
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.