- 1Centre for Health Management and Policy Research, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2NHC Key Laboratory of Health Economics and Policy Research (Shandong University), Jinan, China
- 3Center for Health Preference Research, Shandong University, Jinan, China
Objective: This study was aimed to investigate the cost-effectiveness of all available programmed death 1 (PD-1) inhibitors combined with chemotherapy in the first-line treatment of advanced esophageal squamous-cell carcinoma (ESCC) from the Chinese healthcare system perspective.
Methods: A partitioned survival model with a 3-week cycle and a 10-year time horizon was constructed based on a network meta-analysis. The survival data and utility values were derived from clinical trials, and the direct medical costs were collected from public drug bidding database and published literature. Total costs, quality-adjusted life-years (QALYs) and incremental cost-effectiveness ratios (ICERs) were calculated. Scenario, one-way and probabilistic sensitivity analyses were performed to assess the uncertainty around model parameters.
Results: Compared with mono-chemotherapy, toripalimab, sintilimab and camrelizumab plus chemotherapy were cost-effective treatment regimens, while serplulimab, pembrolizumab and nivolumab plus chemotherapy were not cost-effective options. Toripalimab plus chemotherapy provided the highest QALYs of 0.95 with the lower cost of $8,110.53 compared to other competing alternatives. The robustness of the base-case results was confirmed by scenario and one-way sensitivity analysis. At a willingness-to-pay threshold of three times per capita gross domestic product ($38,351.20) in 2021, the probability of toripalimab plus chemotherapy being the optimal option was 74.25% compared with other six competing alternatives.
Conclusion: Toripalimab plus chemotherapy represented the most cost-effective option as the first-line therapy for advanced ESCC patients in China.
Introduction
Esophageal cancer is the fifth most common malignancy and the fourth leading cause of cancer-related death in China (Sung et al., 2021; Zheng et al., 2022). Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma represent the predominant histological type, with the former accounting for approximately 85% of cases (Arnold et al., 2020). Many esophageal cancers are unresectable at first diagnosis (Rustgi and El-Serag, 2014). Standard fluoropyrimidine or paclitaxel plus cisplatin-based chemotherapy is recommended as first-line treatment for patients with advanced or metastatic ESCC(Muro et al., 2019). The clinical benefits, however, remain limited in patients with advanced or metastatic ESCC receiving standard of care, with a median overall survival (OS) of fewer than 1 year (Ajani et al., 2019; Shah et al., 2023). Therefore, discovering revolutionary treatment strategies to improve prognosis becomes a pressing need in these populations.
In recent years, immune checkpoint inhibitors targeting programmed death 1 (PD-1) or programmed death-ligand 1 (PD-L1) have emerged as promising antitumor regimens across multiple malignancies, including esophageal cancer (Constantinidou et al., 2019). Several prior randomized studies have demonstrated that PD-1 blockade provided significant survival benefits as second-line treatment for advanced ESCC(Kato et al., 2019; Huang et al., 2020). Further, ESCORT-first (Luo et al., 2021), CheckMate-648 (Doki et al., 2022), KEYNOTE-590 (Sun et al., 2021), ORIENT-15 (Lu et al., 2022) and JUPITER-06 (Wang et al., 2022) respectively confirmed that camrelizumab, nivolumab, pembrolizumab, sintilimab and toripalimab combined with chemotherapy produced encouraging antitumor activity compared with mono-chemotherapy. As a result, the five chemoimmunotherapies mentioned above have been in succession approved by the National Medical Products Administration and recommended by the Guidelines of Chinese Society of Clinical Oncology (CSCO, 2022). In 2021, Camrelizumab officially entered the National Reimbursement Drug List (NRDL) negotiation through an 85.2% price reduction for patients with locally advanced or metastatic ESCC, which has progressed after first-line chemotherapy (Cai et al., 2021). The other PD-1 inhibitors covered by the NRDL, such as sintilimab and toripalimab, did not yet include indications related to esophageal cancer.
A published network meta-analysis (NMA) involving five clinical trials with 3,163 patients has investigated the efficacy and safety differences between diverse chemoimmunotherapies in first-line treatment for advanced ESCC (Li et al., 2022). The results proved that toripalimab plus chemotherapy achieved the longest OS [hazard ratio (HR): 0.58, 95% confidence interval (CI): 0.43–0.78], while camrelizumab and sintilimab combined with chemotherapy engendered the longest progression-free survival (PFS) (HR: 0.56, 95% CI: 0.46–0.68) than other treatment examined (Li et al., 2022). Recently, the ASTRUM-007 trial revealed that serplulimab plus chemotherapy significantly improved PFS (HR: 0.60, 95% CI: 0.48–0.75) and OS (HR: 0.68, 95% CI: 0.53–0.87) versus mono-chemotherapy for advanced ESCC, but with a manageable safety profile (Song et al., 2023). Considering the lack of head-to-head clinical trials, clinicians confronted insurmountable quandaries in making appropriate treatment options for a given patient based on the available evidence alone, and that is before taking into account relative costs. Therefore, with the enthusiasm of health technology agencies towards life-cycle health technology assessment (Drummond et al., 2008), the selection of optimal treatment options for decision-makers essentially depended on comparative cost-effectiveness (Sanders et al., 2016; Dai et al., 2022).
Most published economic evaluations have assessed the cost-effectiveness of camrelizumab (Zhang et al., 2021), nivolumab (Liu et al., 2022), pembrolizumab (Zhu et al., 2022a) and sintilimab (Ye et al., 2022) compared to chemotherapy in the first-line setting for advanced ESCC. However, the cost-effectiveness between all available first-line chemoimmunotherapies for patients with advanced ESCC was still uncertain. As such, we aimed to evaluate the cost-effectiveness of all first-line chemoimmunotherapies for the treatment of advanced or metastatic ESCC, namely, camrelizumab, nivolumab, pembrolizumab, serplulimab, sintilimab, and toripalimab combined with chemotherapy, and mono-chemotherapy, from the perspective of Chinese healthcare system to better inform reimbursement policy and achieve optimal health resource allocation.
Methods
Patients and treatment
This study was guided by the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) updated reporting guidelines (Supplementary Table S1) (Husereau et al., 2022). This economic evaluation was based on modelling techniques and published literature, and did not require approval of the institutional research ethics board because no real human participants or animals were involved.
A hypothetical cohort of patients, aged at least 18 years, with histologically or cytologically confirmed unresectable locally advanced, recurrent, or metastatic ESCC with the same characteristics as those patients enrolled in ESCORT-first (Luo et al., 2021), CheckMate-648 (Doki et al., 2022), KEYNOTE-590 (Sun et al., 2021), ASTRUM-007 (Song et al., 2023), ORIENT-15 (Lu et al., 2022) and JUPITER-06 (Wang et al., 2022) clinical trials. Eligible patients received one of seven first-line interventions: (1) Chemotherapy (Cisplatin, 75 mg/m2, day 1 plus Paclitaxel, 175 mg/m2, day 1 or Fluorouracil, 800 mg/m2, days 1 through 5; 3-week); (2) Camrelizumab (200 mg; 3-week) plus chemotherapy; (3) Nivolumab (240 mg; 2-week) plus chemotherapy; (4) Pembrolizumab (200 mg; 3-week) plus chemotherapy; (5) Serplulimab (75 mg/kg; 2-week) plus chemotherapy; (6) Sintilimab (200 mg; 3-week) plus chemotherapy; (7) Toripalimab (240 mg; 3-week) plus chemotherapy (Supplementary). After disease progression, we assumed that the remaining patients would receive subsequent best supportive anti-cancer regimens to accurately capture the cost-effectiveness associated with first-line treatment.
Model construction
A partitioned survival model was constructed with three exclusive health states [PFS, progression-disease (PD), and death] to portray disease progression and treatment efficacy (Figure 1). The cycle length was 3 weeks, which was consistent with the treatment protocol in clinical trials, and half-cycle correction was implemented to calibrate the timing of events. The 10-year time horizon was adequate to guarantee that ESCC patients completely entered the terminal state. The primary endpoint of the model included overall costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs; incremental cost per additional QALY gained) for pairwise comparison between chemoimmunotherapy-related groups. According to China Guidelines for Pharmacoeconomic Evaluations, a discount of 5% was applied to health outcomes and costs beyond the first year over the time horizon (Liu et al., 2020). All costs were adjusted to 2022 prices with the local Consumer Price Index and converted into US dollars (1$ = 6.33 CNY). As recommended by the World Health Organization (Marseille et al., 2015), 3 times per capita gross domestic product (GDP) in China in 2021 ($38,351.20) was implemented as the willingness-to-pay (WTP) threshold to investigate the most cost-effective competing alternatives.
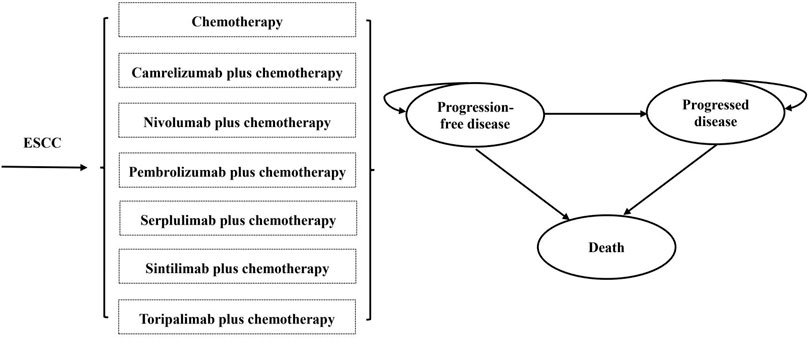
FIGURE 1. The structure of the partitioned survival model. (ESCC, esophageal squamous-cell carcinoma).
Clinical inputs
As a result of the absence of head-to-head clinical trials comparing chemotherapy and all available chemoimmunotherapies, a systematic review was conducted in February 2023 to identify randomized controlled trials (RCTs) of relevant treatment strategies in advanced ESCC. Web of Science, PubMed, Embase, and Cochrane Library databases were searched using search terms: “camrelizumab or nivolumab or pembrolizumab or serplulimab or sintilimab or toripalimab or PD-1 or PD-L1”, “chemotherapy”, “esophageal squamous cell cancer or esophageal cancer or esophageal carcinoma” and “randomized clinical trial or randomized controlled trial”. The literature search identified 157 publications (Supplementary Figure S1). After rigorous screening, a total of six relevant phase III RCTs with 3,683 patients were included in the systematic review and network meta-analysis. The basic characteristics and bias risk assessment of included studies were summarized in Supplementary Table S2, Figure S2. The results of the network meta-analysis were shown in Supplementary Table S3.
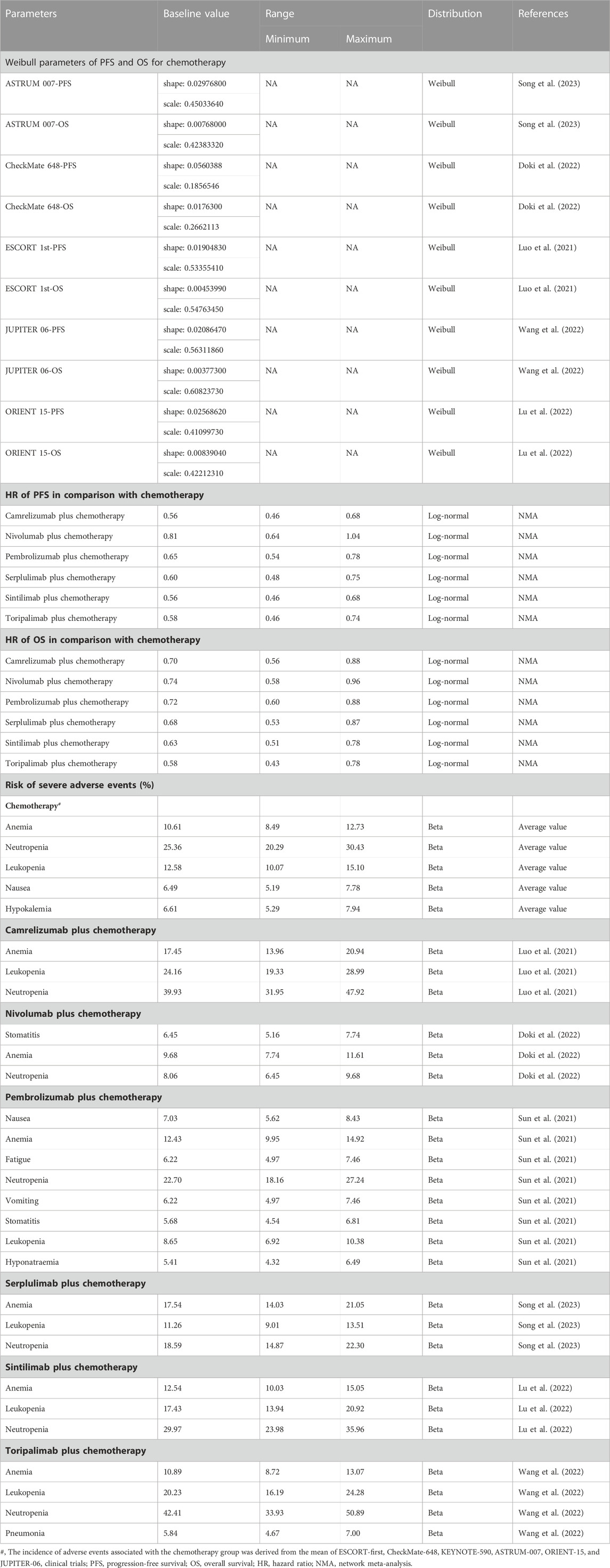
GetData Graph Digitizer 2.26 (http://www.getdata-graph-digitizer.com/) was applied to extract PFS and OS data points from the Kaplan-Meier curves reported in the six RCTs (Supplementary Table S4, S5). To optimally extrapolate the lifetime survival outcome, Guyot’s parametric survival models were considered for each endpoint of chemotherapy (Guyot et al., 2012), including Exponential, Weibull, Log-logistic, Log-normal, and Gompertz distributions (Supplementary Table S6, Figures S3, S4). Weibull distribution provided eligible survival function based on clinical plausibility, statistical goodness-of-fit (Akaike Information Criterion and Bayesian Information Criterion), and visual examination (Latimer, 2013). The estimated shape parameters (γ) and scale parameters (λ) were shown in Table 1.
The baseline hazards for chemotherapy were estimated by averaging the patient survival data fitted by Weibull distribution (Supplementary Figure S5). We then derived the expected survival curves for chemoimmunotherapies by applying the HRs to the reference arm of chemotherapy. The Weibull parameter γ for chemoimmunotherapies was equal to the reference arm, and the Weibull parameter λ for chemoimmunotherapies was calculated as λ for reference arm multiplied by the HRs between alternative treatments and mono-chemotherapy (Hoyle et al., 2010).
Cost inputs
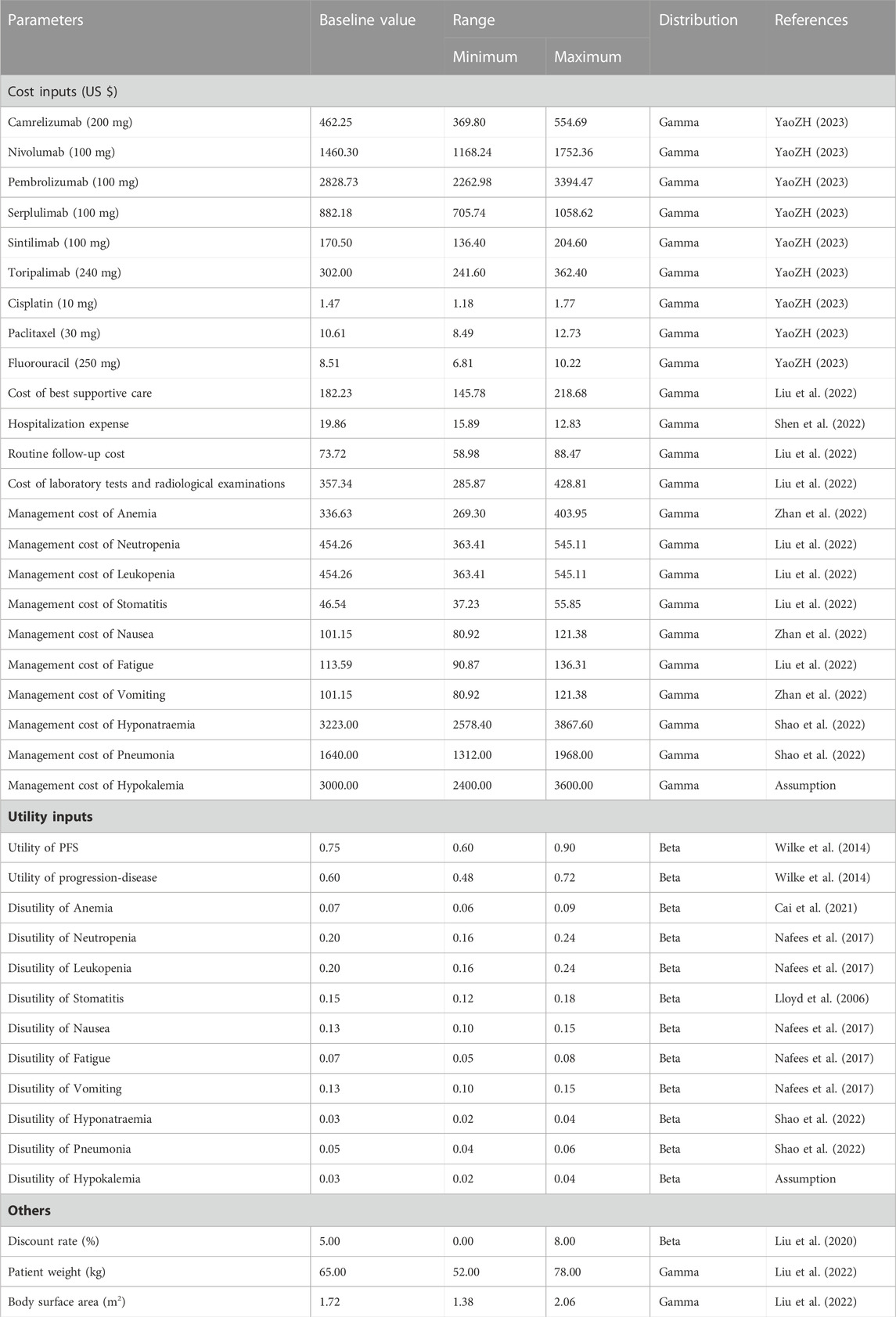
Our model considered only direct medical costs, which included drug costs, subsequent treatment, hospitalization expense, routine follow-up and radiological examinations, and administration costs associated with adverse events (AEs) (Table 2). To estimate drug costs, we calculated the average winning bids in 2023 from YAOZHI database (https://data.yaozh.com/), which aggregated the latest price data around the country. The default height of 165 cm and body weight of 65 kg, with an average body surface area (BSA) of 1.72 m2 were assumed for the Chinese ESCC patients to determine the dosage and expenditure of chemotherapies (Liu et al., 2022). Other healthcare-related costs were retrieved from recently published literature (Liu et al., 2022; Shen et al., 2022). Grade 3 or above AEs with an incidence of greater than 5% reported in the clinical trial were included as they exerted a considerable effect on the course of survival and treatment, including anemia, neutropenia, leukopenia, stomatitis, nausea, fatigue, vomiting, hyponatraemia, hypokalemia and pneumonia (Liu et al., 2022; Shao et al., 2022; Zhan et al., 2022). For each treatment regimen, the management cost of serious AEs were determined by multiplying the unite cost (per event) by the corresponding incidence rate.
Health state utility
Health state utilities were estimated based on the EuroQoL five-dimension, three-level questionnaire reported from a double-blind, randomised phase 3 trial, which recruited participants with metastatic or locally advanced gastric or gastro-oesophageal junction adenocarcinoma (Wilke et al., 2014). The baseline utility values for PFS and PD states were 0.75 and 0.60, respectively, which were in compliance with previously published cost-effectiveness analyses (Yang et al., 2021; Liu et al., 2022). The disutility values caused by grade 3 or above treatment-related AEs were considered by multiplying the duration-adjusted disutilities by the prevalence rates of specific AEs (Lloyd et al., 2006; Nafees et al., 2017; Cai et al., 2021; Shao et al., 2022) (Table 2).
Scenario and sensitivity analyses
We performed four scenarios to examine how our model was impacted by time horizon, utility values, BSA and subsequent treatment strategies: first, health utility values from published economic evaluations associated with ESCC were employed to further validate the base-case results (Zhang et al., 2020; Marguet et al., 2021; Zhang et al., 2021); second, shorter time horizon (2, 5, and 8 years) was conducted in this scenario; third, the reasonably lower or higher weight and BSA (58 kg, 1.60 m2 and 80 kg, 1.98 m2) were investigated; fourth, according to guidelines and clinical trials (CSCO, 2022), after disease progression, we assumed that the proportion of patients receiving immunotherapy, targeted therapy, chemotherapy and BSC in the chemotherapy and chemoimmunotherapy groups were 10% and 20%, 10% and 10%, 20% and 25%, and 60% and 45%, respectively.
One-way and probabilistic sensitivity analyses (PSA) were conducted for input parameters to explore the robustness of our results. In the one-way sensitivity analyses, the estimated range of variables were either based on reported 95% confidence intervals or determined by assuming a 20% deviation from the base-case values to appraise their degree of impact on ICERs. On the basis of China Guidelines for Pharmacoeconomic Evaluations, the range of discount rate was set as 0%–8% (Liu et al., 2020). The results were represented by Tornado diagrams. For the PSA, 10,000 Monte Carlo simulations was generated by simultaneously sampling all crucial variables from the pre-specified statistical distributions. Gamma distribution was selected for costs, log-normal distribution for HRs between the competing alternatives, and beta distribution for utility values and proportions (Briggs et al., 2012). The results of PSA were presented in cost-effectiveness acceptability curves (CEAC), which illustrated the probabilities of each competing strategy being cost-effective at various WTP thresholds.
Results
Base-case results
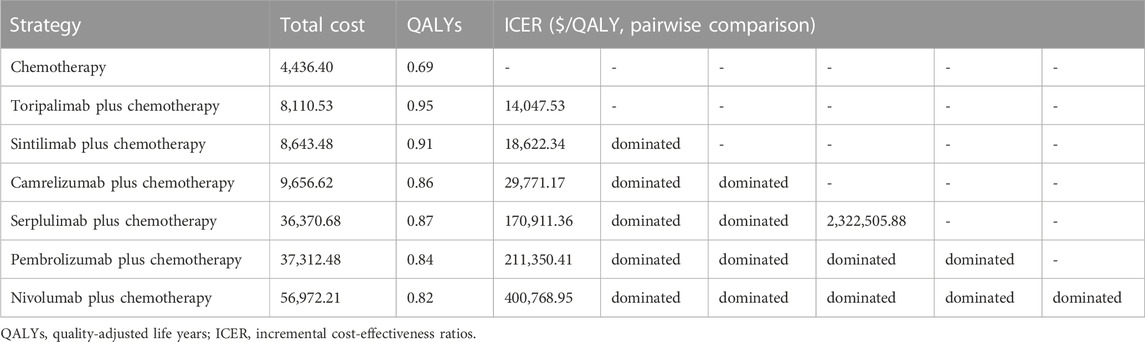
The base-case results were shown in Table 3. Compared with mono-chemotherapy, the ICERs of toripalimab, sintilimab, and camrelizumab combined with chemotherapy were $14,047.53/QALY, $18,622.34/QALY, and $29,771.17/QALY, respectively, all were lower than WTP threshold. The ICERs of serplulimab, pembrolizumab, and nivolumab plus chemotherapy versus mono-chemotherapy were $170,911.36/QALY, $211,350.41/QALY, and $400,768.95/QALY, respectively, all were more than WTP threshold. In the pairwise comparison between all competing treatments, toripalimab plus chemotherapy yielded the highest QALYs (0.95) with lower cost ($8,110.53) and represented high-value option for advanced ESCC patients at the current price and WTP threshold.
Scenario and sensitivity analyses results
Across all scenario analyses, the general conclusions of the primary analyses were robust and reliable, namely, toripalimab plus chemotherapy was the most cost-effective option against competing regimens (Supplementary Tables S7, S8, S9, S10). One-way sensitivity analyses demonstrated that HR-related parameters, drug costs, utility values and BSA played a considerable role in the base-case results, but alterations in these variables did not significantly alter the conclusion (Supplementary Figure S6). At the WTP thresholds of 3 times per capita GDP in China, the CEAC revealed that approximately 74.25%, 23.38%, and 2.37% probabilities of toripalimab, sintilimab, and camrelizumabplus chemotherapy being cost-effective options in simultaneous comparisons of competing strategies (Figure 2).
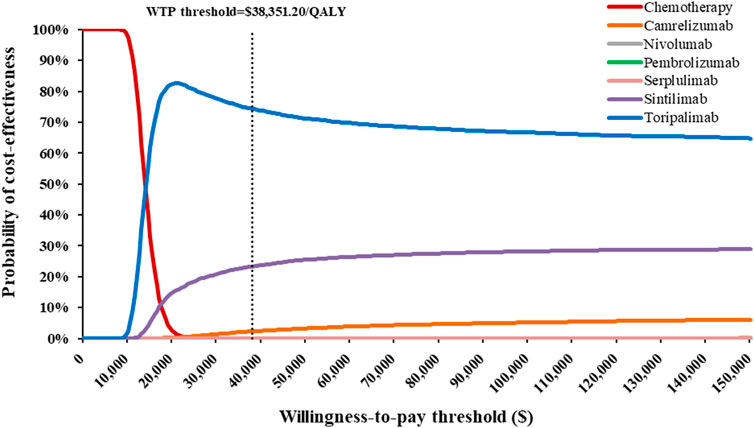
FIGURE 2. Cost-effectiveness acceptability curves indicating the probability of each treatment regimen to be cost-effectiveness in the treatment of advanced esophageal squamous-cell carcinoma at various willingness-to-pay thresholds in China.
Discussion
To our knowledge, this is the first study to comprehensively appraise the cost-effectiveness of currently available first-line chemoimmunotherapies for patients with advanced ESCC from the Chinese healthcare system perspective. Our findings indicated that toripalimab, sintilimab, and camrelizumab combined with chemotherapy were cost-effectiveness compared to chemotherapy. Toripalimab plus chemotherapy was the most cost-effective treatment paradigm under the current WTP threshold by virtue of the highest QALYs and lower cost. The base-case results were upheld by the scenario and sensitivity analyses.
Toripalimab was the first approved PD-1 inhibitor developed independently by Chinese pharmaceutical companies, which not only greatly reduced transportation costs compared to imported immunotherapeutic agents, but also provided more substantial price reductions than comparable inhibitors (Tian et al., 2022). Therefore, toripalimab could be more accessible and widely applied for Chinese patients. The NMA demonstrated that sintilimab and camrelizumab plus chemotherapy provided more significant improvements in PFS and OS than nivolumab and pembrolizumab plus chemotherapy. Due to the considerable price advantage and accessibility, sintilimab and camrelizumab plus chemotherapy may be appropriate alternatives for advanced ESCC patients. Serplulimab, a novel domestic PD-1 inhibitor, plus chemotherapy for first-line treatment has not shown an economic advantage, although it may be cost-effective in patients with extensive-stage small cell lung cancer (Zhu et al., 2022b). Therefore, a substantial price reduction for serplulimab was essential to improve patient affordability. Moreover, PD-1 inhibitors plus chemotherapy improved clinical benefits as first-line therapy for advanced ESCC patients, at the cost of greater but controllable toxicity including increased frequency of serious AEs (Li et al., 2022). However, one-way sensitivity analyses showed that these tolerable toxicity-related costs and disutilities exerted a minimal impact on cost-effectiveness and, hence, would not substantially alter the results.
In recent years, the Chinese self-developed innovative PD-1 inhibitors have gradually provided better survival benefits, clinical tolerability and cost-effective treatment options for various cancer patients. This situation is mainly driven by the centralized price-negotiated mechanisms to improve the accessibility and afordability of patients (Zhang et al., 2022a; Zhang et al., 2022b). The National Medical Products Administration, previously called the China Food and Drug Administration, has strengthened regulatory capacity and launched a series of priority procedures to expedite the development, review and approval of innovative anti-cancer medicines (Zhou et al., 2017; Zhang et al., 2022a). Furthermore, to temper rapidly increasing costs, value-based pricing and national medical insurance negotiations became critical criterion for innovative drugs to be covered by national medical insurance (Si et al., 2020; Tang et al., 2020). These mechanisms have reduced drug prices by half, safeguarding both patient affordability and the sustainability of medical insurance (Zhang et al., 2022b).
To date, several economic evaluations were relevant to ours and warrant discussion. Zhang et al. (Zhang et al., 2021) estimated the cost-effectiveness of camrelizumab plus chemotherapy in the first-line treatment of advanced or metastatic ESCC based on ESCORT-first clinical trial, and suggested that camrelizumab plus chemotherapy might not be cost-effective compared with standard chemotherapy in China. Nevertheless, this previous assessment used non-negotiated prices for camrelizumab, which are no longer relevant at present, as the medical insurance negotiation mechanism has dramatically improved accessibility for patients. Zhu et al. (Zhu et al., 2022a) and Liu et al. (Liu et al., 2022) evaluated the cost-effectiveness of pembrolizumab and nivolumab combined with chemotherapy from the Chinese healthcare system perspective, respectively, and the conclusions aligned well with those of this analysis. Nivolumab and pembrolizumab combined with chemotherapy was extremely unlikely to be economical compared to chemotherapy (Malmberg et al., 2022), and substantial price reductions or generous patient assistance programs were required to improve affordability (Howard, 2014). The latest economic evidence suggested that sintilimab and toripalimab plus chemotherapy were cost-effective compared with chemotherapy regimens in the first-line treatment of patients with advanced ESCC(Shao et al., 2022; Fang et al., 2023). Our results were consistent with available studies. Camrelizumab, sintilimab, and toripalimab plus chemotherapy were high-value innovative options for advanced ESCC patients in China.
Our study had some limitations that merited discussion, many of which were governed by data availability and model assumptions. Foremost, because the head-to-head clinical trial was unavailable, an indirect comparison was performed based on NMA to evaluate all available chemoimmunotherapies as first-line treatment for advanced ESCC, although there was moderate heterogeneity in the pairwise comparison. Second, we assumed best supportive care as the primary treatment after disease progression, which might be different from the actual clinical situations. Scenario analysis demonstrated that the alternative of subsequent treatment options would not substantially alter the outcome of the base-case analysis. Third, since the utility values of specific health states were limited in China, the utilities and disutilities were determined based on published clinical trial, which might cause some deviations in the cumulative QALYs. Fourth, due to the absence of data, the costs and disutilities associated with grade 1/2 treatment-related AEs were excluded from this model, although one-way sensitivity analyses implied that only minimal impact on the base-case results. Fifth, PD-L1 expression was enriched in ESCC patients. Prior economic evidence indicated that PD-1 inhibitors were potentially more sensitive to PD-L1-positive ESCC patients against overall population (Zhu et al., 2022a; Liu et al., 2022; Shao et al., 2022). Because PD-L1-positive was inconsistently defined across clinical trials, subgroup analyses were not feasible in this study. Consequently, subgroup analyses based on head-to-head trials or real-world data warranted further studies to support healthcare decision-making and precision medicine.
Conclusion
In summary, our findings showed that toripalimab, sintilimab, and camrelizumab combined with chemotherapy were cost-effective treatment options over chemotherapy, and toripalimab plus chemotherapy was the most cost-effective regimen compared with other competing alternatives as the first-line treatment for advanced ESCC patients in China.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
SXL and SPL were responsible for study design, model building and statistical analysis. SXL prepared the manuscript. SXL and LD searched literatures and collected data. All authors critically reviewed the model structure, verified results and revised the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1055727/full#supplementary-material
References
Ajani, J. A., D'Amico, T. A., Bentrem, D. J., Chao, J., Corvera, C., Das, P., et al. (2019). Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in Oncology. J. Natl. Compr. Canc Netw. 17 (7), 855–883. doi:10.6004/jnccn.2019.0033
Arnold, M., Ferlay, J., van Berge Henegouwen, M. I., and Soerjomataram, I. (2020). Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 69 (9), 1564–1571. doi:10.1136/gutjnl-2020-321600
Briggs, A. H., Weinstein, M. C., Fenwick, E. A., Karnon, J., Sculpher, M. J., Paltiel, A. D., et al. (2012). Model parameter estimation and uncertainty analysis: A report of the ISPOR-SMDM modeling good research practices task force working group-6. Med. Decis. Mak. 32 (5), 722–732. doi:10.1177/0272989x12458348
Cai, H., Xu, B., Li, N., Zheng, B., Zheng, Z., and Liu, M. (2021). Cost-effectiveness analysis of camrelizumab versus chemotherapy as second-line treatment of advanced or metastatic esophageal squamous cell carcinoma. Front. Pharmacol. 12 (1), 732912. doi:10.3389/fphar.2021.732912
Constantinidou, A., Alifieris, C., and Trafalis, D. T. (2019). Targeting programmed cell death -1 (PD-1) and ligand (PD-L1): A new era in cancer active immunotherapy. Pharmacol. Ther. 194 (2), 84–106. doi:10.1016/j.pharmthera.2018.09.008
CSCO (2022). Guidelines of Chinese society of clinical oncologhy(CSCO) esophageal cancer. Beijing: People's Medical Publishing House.
Dai, W. F., Beca, J. M., Nagamuthu, C., Liu, N., de Oliveira, C., Earle, C. C., et al. (2022). Cost-effectiveness analysis of pertuzumab with trastuzumab in patients with metastatic breast cancer. JAMA Oncol. 8 (4), 597–606. doi:10.1001/jamaoncol.2021.8049
Doki, Y., Ajani, J. A., Kato, K., Xu, J., Wyrwicz, L., Motoyama, S., et al. (2022). Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N. Engl. J. Med. 386 (5), 449–462. doi:10.1056/NEJMoa2111380
Drummond, M. F., Schwartz, J. S., Jönsson, B., Luce, B. R., Neumann, P. J., Siebert, U., et al. (2008). Key principles for the improved conduct of health technology assessments for resource allocation decisions. Int. J. Technol. Assess. Health Care 24 (3), 244–258. doi:10.1017/s0266462308080343
Fang, R., Wang, S., Liu, Y., and Xu, J. (2023). Cost-effectiveness analysis of toripalimab plus paclitaxel and cisplatin as first-line treatment for advanced or metastatic esophageal squamous cell carcinoma. Adv. Ther. doi:10.1007/s12325-022-02402-z
Guyot, P., Ades, A. E., Ouwens, M. J., and Welton, N. J. (2012). Enhanced secondary analysis of survival data: Reconstructing the data from published kaplan-meier survival curves. BMC Med. Res. Methodol. 12 (1), 9. doi:10.1186/1471-2288-12-9
Howard, D. H. (2014). Drug companies' patient-assistance programs--helping patients or profits? N. Engl. J. Med. 371 (2), 97–99. doi:10.1056/NEJMp1401658
Hoyle, M., Green, C., Thompson-Coon, J., Liu, Z., Welch, K., Moxham, T., et al. (2010). Cost-effectiveness of temsirolimus for first line treatment of advanced renal cell carcinoma. Value Health 13 (1), 61–68. doi:10.1111/j.1524-4733.2009.00617.x
Huang, J., Xu, J., Chen, Y., Zhuang, W., Zhang, Y., Chen, Z., et al. (2020). Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): A multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 21 (6), 832–842. doi:10.1016/s1470-2045(20)30110-8
Husereau, D., Drummond, M., Augustovski, F., de Bekker-Grob, E., Briggs, A. H., Carswell, C., et al. (2022). Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. Value Health 25 (1), 3–9. doi:10.1016/j.jval.2021.11.1351
Kato, K., Cho, B. C., Takahashi, M., Okada, M., Lin, C. Y., Chin, K., et al. (2019). Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 20 (11), 1506–1517. doi:10.1016/s1470-2045(19)30626-6
Latimer, N. R. (2013). Survival analysis for economic evaluations alongside clinical trials--extrapolation with patient-level data: Inconsistencies, limitations, and a practical guide. Med. Decis. Mak. 33 (6), 743–754. doi:10.1177/0272989x12472398
Li, Z. C., Sun, Y. T., Lai, M. Y., Zhou, Y. X., and Qiu, M. Z. (2022). Efficacy and safety of PD-1 inhibitors combined with chemotherapy as first-line therapy for advanced esophageal cancer: A systematic review and network meta-analysis. Int. Immunopharmacol. 109 (8), 108790. doi:10.1016/j.intimp.2022.108790
Liu, G., Hu, S., Wu, J., and Wu, J. (2020). China guidelines for pharmacoeconomic evaluations (2020). Beijing: China Market Press.
Liu, S., Dou, L., Wang, K., Shi, Z., Wang, R., Zhu, X., et al. (2022). Cost-effectiveness analysis of nivolumab combination therapy in the first-line treatment for advanced esophageal squamous-cell carcinoma. Front. Oncol. 12 (1), 899966. doi:10.3389/fonc.2022.899966
Lloyd, A., Nafees, B., Narewska, J., Dewilde, S., and Watkins, J. (2006). Health state utilities for metastatic breast cancer. Br. J. Cancer 95 (6), 683–690. doi:10.1038/sj.bjc.6603326
Lu, Z., Wang, J., Shu, Y., Liu, L., Kong, L., Yang, L., et al. (2022). Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): Multicentre, randomised, double blind, phase 3 trial. BMJ 377 (17), e068714. doi:10.1136/bmj-2021-068714
Luo, H., Lu, J., Bai, Y., Mao, T., Wang, J., Fan, Q., et al. (2021). Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: The ESCORT-1st randomized clinical trial. JAMA 326 (10), 916–925. doi:10.1001/jama.2021.12836
Malmberg, R., Zietse, M., Dumoulin, D. W., Hendrikx, J., Aerts, J., van der Veldt, A. A. M., et al. (2022). Alternative dosing strategies for immune checkpoint inhibitors to improve cost-effectiveness: A special focus on nivolumab and pembrolizumab. Lancet Oncol. 23 (12), e552–e561. doi:10.1016/s1470-2045(22)00554-x
Marguet, S., Adenis, A., Delaine-Clisant, S., Penel, N., and Bonastre, J. (2021). Cost-utility analysis of continuation versus discontinuation of first-line chemotherapy in patients with metastatic squamous-cell esophageal cancer: Economic evaluation alongside the E-DIS trial. Value Health 24 (5), 676–682. doi:10.1016/j.jval.2020.11.017
Marseille, E., Larson, B., Kazi, D. S., Kahn, J. G., and Rosen, S. (2015). Thresholds for the cost-effectiveness of interventions: Alternative approaches. Bull. World Health Organ 93 (2), 118–124. doi:10.2471/blt.14.138206
Muro, K., Lordick, F., Tsushima, T., Pentheroudakis, G., Baba, E., Lu, Z., et al. (2019). Pan-asian adapted ESMO clinical practice guidelines for the management of patients with metastatic oesophageal cancer: A JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann. Oncol. 30 (1), 34–43. doi:10.1093/annonc/mdy498
Nafees, B., Lloyd, A. J., Dewilde, S., Rajan, N., and Lorenzo, M. (2017). Health state utilities in non-small cell lung cancer: An international study. Asia Pac J. Clin. Oncol. 13 (5), e195–e203. doi:10.1111/ajco.12477
Rustgi, A. K., and El-Serag, H. B. (2014). Esophageal carcinoma. N. Engl. J. Med. 371 (26), 2499–2509. doi:10.1056/NEJMra1314530
Sanders, G. D., Neumann, P. J., Basu, A., Brock, D. W., Feeny, D., Krahn, M., et al. (2016). Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA 316 (10), 1093–1103. doi:10.1001/jama.2016.12195
Shah, M. A., Kennedy, E. B., Alarcon-Rozas, A. E., Alcindor, T., Bartley, A. N., Malowany, A. B., et al. (2023). Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO guideline. J. Clin. Oncol., JCO2202331. Jco2202331. doi:10.1200/jco.22.02331
Shao, T., Zhao, M., and Tang, W. (2022). Cost-effectiveness analysis of sintilimab vs. placebo in combination with chemotherapy as first-line therapy for local advanced or metastatic oesophageal squamous cell carcinoma. Front. Oncol. 12 (1), 953671. doi:10.3389/fonc.2022.953671
Shen, J., Du, Y., Shao, R., and Jiang, R. (2022). First-line sintilimab plus chemotherapy in locally advanced or metastatic esophageal squamous cell carcinoma: A cost-effectiveness analysis from China. Front. Pharmacol. 13 (1), 967182. doi:10.3389/fphar.2022.967182
Si, L., Xu, L., Chen, M., and Jan, S. (2020). Using strategic price negotiations to contain costs and expand access to medicines in China. BMJ Glob. Health 5 (1), e002256. doi:10.1136/bmjgh-2019-002256
Song, Y., Zhang, B., Xin, D., Kou, X., Tan, Z., Zhang, S., et al. (2023). First-line serplulimab or placebo plus chemotherapy in PD-L1-positive esophageal squamous cell carcinoma: A randomized, double-blind phase 3 trial. Nat. Med. doi:10.1038/s41591-022-02179-2
Sun, J. M., Shen, L., Shah, M. A., Enzinger, P., Adenis, A., Doi, T., et al. (2021). Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 398 (10302), 759–771. doi:10.1016/s0140-6736(21)01234-4
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tang, M., Song, P., and He, J. (2020). Progress on drug pricing negotiations in China. Biosci. Trends 13 (6), 464–468. doi:10.5582/bst.2019.01339
Tian, K., Han, J., Wang, Z., and Chen, J. (2022). Immune checkpoint inhibition in first-line treatment for recurrent or metastatic nasopharyngeal carcinoma: A CAPTAIN-1st and JUPITER-02 trial-based cost-effectiveness analysis. Oral Oncol. 128 (5), 105842. doi:10.1016/j.oraloncology.2022.105842
Wang, Z. X., Cui, C., Yao, J., Zhang, Y., Li, M., Feng, J., et al. (2022). Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell 40 (3), 277–288.e3. doi:10.1016/j.ccell.2022.02.007
Wilke, H., Muro, K., Van Cutsem, E., Oh, S. C., Bodoky, G., Shimada, Y., et al. (2014). Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 15 (11), 1224–1235. doi:10.1016/s1470-2045(14)70420-6
Yang, F., Fu, Y., Kumar, A., Chen, M., Si, L., and Rojanasarot, S. (2021). Cost-effectiveness analysis of camrelizumab in the second-line treatment for advanced or metastatic esophageal squamous cell carcinoma in China. Ann. Transl. Med. 9 (15), 1226. doi:10.21037/atm-21-1803
YaoZH, (2023). The big data service platform for China’s health industry: Information Query of Drug Bid Winning. [Online]. Available: https://data.yaozh.com/ (Accessed February 2, 2023).
Ye, Z. M., Xu, Z., Zeng, F. Y., Tang, Z. Q., and Zhou, Q. (2022). Cost-effectiveness analysis of sintilimab combined with chemotherapy versus chemotherapy alone as the first-line treatment for advanced esophageal cancer. Front. Pharmacol. 13 (1), 934275. doi:10.3389/fphar.2022.934275
Zhan, M., Xu, T., Zheng, H., and He, Z. (2022). Cost-effectiveness analysis of pembrolizumab in patients with advanced esophageal cancer based on the KEYNOTE-181 study. Front. Public Health 10 (1), 790225. doi:10.3389/fpubh.2022.790225
Zhang, P. F., Xie, D., and Li, Q. (2020). Cost-effectiveness analysis of nivolumab in the second-line treatment for advanced esophageal squamous cell carcinoma. Future Oncol. 16 (17), 1189–1198. doi:10.2217/fon-2019-0821
Zhang, Q., Wu, P., He, X., Ding, Y., and Shu, Y. (2021). Cost-effectiveness analysis of camrelizumab vs. Placebo added to chemotherapy as first-line therapy for advanced or metastatic esophageal squamous cell carcinoma in China. Front. Oncol. 11 (1), 790373. doi:10.3389/fonc.2021.790373
Zhang, Y., Naci, H., Wagner, A. K., Xu, Z., Yang, Y., Zhu, J., et al. (2022a). Overall survival benefits of cancer drugs approved in China from 2005 to 2020. JAMA Netw. Open 5 (8), e2225973. doi:10.1001/jamanetworkopen.2022.25973
Zhang, Y., Wei, Y., Li, H., Chen, Y., Guo, Y., Han, S., et al. (2022b). Prices and clinical benefit of national price-negotiated anticancer medicines in China. Pharmacoeconomics 40 (7), 715–724. doi:10.1007/s40273-022-01161-7
Zheng, R., Zhang, S., Zeng, H., Wang, S., Sun, K., Chen, R., et al. (2022). Cancer incidence and mortality in China, 2016. J. Natl. Canc Cent. 2 (1), 1–9. doi:10.1016/j.jncc.2022.02.002
Zhou, Q., Chen, X. Y., Yang, Z. M., and Wu, Y. L. (2017). The changing landscape of clinical trial and approval processes in China. Nat. Rev. Clin. Oncol. 14 (9), 577–583. doi:10.1038/nrclinonc.2017.10
Zhu, Y., Liu, K., Ding, D., Zhou, Y., and Peng, L. (2022a). Pembrolizumab plus chemotherapy as first-line treatment for advanced esophageal cancer: A cost-effectiveness analysis. Adv. Ther. 39 (6), 2614–2629. doi:10.1007/s12325-022-02101-9
Keywords: cost-effectiveness, esophageal squamous-cell carcinoma, PD-1 inhibitors, first-line therapy, chemoimmunotherapy
Citation: Liu S, Dou L and Li S (2023) Cost-effectiveness analysis of PD-1 inhibitors combined with chemotherapy as first-line therapy for advanced esophageal squamous-cell carcinoma in China. Front. Pharmacol. 14:1055727. doi: 10.3389/fphar.2023.1055727
Received: 28 September 2022; Accepted: 20 February 2023;
Published: 02 March 2023.
Edited by:
Ileana Mardare, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Wenxi Tang, China Pharmaceutical University, ChinaBogdan Vasile Ileanu, Independent researcher, Bucharest, Romania
Copyright © 2023 Liu, Dou and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shunping Li, bGlzaHVucGluZ0BzZHUuZWR1LmNu
 Shixian Liu
Shixian Liu Lei Dou
Lei Dou Shunping Li
Shunping Li