
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 03 March 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1036043
 Hui-Bo Yu1,2†
Hui-Bo Yu1,2† Jia-Qi Hu1,2†
Jia-Qi Hu1,2† Bao-Jin Han1,2†
Bao-Jin Han1,2† Hui-Juan Cao2,3
Hui-Juan Cao2,3 Shun-Tai Chen2
Shun-Tai Chen2 Xin Chen1
Xin Chen1 Hong-Tai Xiong1
Hong-Tai Xiong1 Jin Gao1,2
Jin Gao1,2 Yan-Yuan Du1
Yan-Yuan Du1 Hong-Gang Zheng1*
Hong-Gang Zheng1*Objectives: Compound Kushen injection (CKI) combined with intraperitoneal chemotherapy (IPC) is widely used in the treatment of malignant ascites (MA). However, evidence about its efficacy and safety remains limited. This review aimed to evaluate the efficacy and safety of CKI combined with IPC for the treatment of MA.
Methods: Protocol of this review was registered in PROSPERO (CRD42022304259). Randomized controlled trials (RCTs) on the efficacy and safety of IPC with CKI for the treatment of patients with MA were searched through 12 electronic databases and 2 clinical trials registration platforms from inception until 20 January 2023. The Cochrane risk-of-bias tool was used to assess the quality of the included trials through the risk of bias assessment. We included RCTs that compared IPC single used or CKI combined with IPC for patients with MA schedule to start IPC. The primary outcome was identified as an objective response rate (ORR), while the secondary outcomes were identified as the quality of life (QoL), survival time, immune functions, and adverse drug reactions (ADRs). The Revman5.4 and Stata17 software were used to calculate the risk ratio (RR) at 95% confidence intervals (CI) for binary outcomes and the mean difference (MD) at 95% CI for continuous outcomes. The certainty of the evidence was assessed according to the GRADE criteria.
Results: A total of 17 RCTs were assessed, which included 1200 patients. The risk of bias assessment of the Cochrane risk-of-bias tool revealed that one study was rated high risk and the remaining as unclear or low risk. Meta-analysis revealed that CKI combined with IPC had an advantage in increasing ORR (RR = 1.31, 95% CI 1.20 to 1.43, p < 0.00001) and QoL (RR = 1.50, 95% CI 1.23 to 1.83, p < 0.0001) when compared with IPC alone. Moreover, the combined treatment group showed a lower incidence of myelosuppression (RR = 0.51, 95%CI 0.40–0.64, p < 0.00001), liver dysfunction (RR = 0.33, 95%CI 0.16 to 0.70, p = 0.004), renal dysfunction (RR = 0.39, 95%CI 0.17 to 0.89, p = 0.02), and fever (RR = 0.51, 95%CI 0.35 to 0.75, p = 0.0007) compared to those of the control group. The quality of evidence assessment through GRADE criteria showed that ORR, myelosuppression, and fever were rated moderate, renal dysfunction and liver dysfunction were rated low, and QoL and abdominal pain were rated very low.
Conclusion: The efficacy and safety of CKI combined with IPC were superior to that with IPC alone for the treatment of MA, which indicates the potentiality of the treatment. However, more high-quality RCTs are required to validate this conclusion.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022304259], identifier [PROSPERO 2022 CRD42022304259].
Malignant ascites (MA) is a common complication of abdominal malignant tumor (Kalogeraki et al., 2012). The underlying diagnosis is associated with cancer in almost 10% of the patients with ascites, and ascites is the only manifestation in some cancer patients. (Chicago Consensus Working, 2020). MA is common in gynecological malignancies and gastrointestinal malignancies. The most common malignancy is ovarian cancer (37.7%), followed by hepatobiliary and pancreatic cancer (PC) (21%) and gastric cancer (18.3%) (Barni et al., 2011; Chao and Qin, 2019). The aggravation of MA causes distinct abdominal distension, dyspnea (Wang et al., 2017), fatigue, anorexia (Zhong and Zhao, 2015), which reduces the quality of life (QoL) of the patients, shorten the survival time, and signifies a poor prognosis. Although antitumor therapy is developing rapidly, the prevention and treatment of MA are not satisfactory. Some studies have shown that MA is closely associated with shortened survival time. The 1-year survival rate of MA is <10% (Chao and Qin, 2019). An effective control of MA is of great significance for prolonging the survival time and improving the QoL of patients with advanced cancer. Intraperitoneal chemotherapy (IPC) is widely used in the treatment of MA, as it allows increasing the local drug concentration, prolonging the contact time between drugs and cancer cells to kill cancer cells or tiny metastases, and decrease the peritoneal permeability to control MA (He et al., 2009). However, not all patients with MA can benefit from IPC because of the intolerance of adverse events, such as leukopenia, gastrointestinal reactions, renal dysfunction, fever, and pain (Armstrong et al., 2006).
Natural products have been important sources of drug discovery (Ling, 2020). Several anticancer drugs are derived from natural products and approved by the Food and Drug Administration (FDA) in the United States (Yang et al., 2021). More than 50% of new drug approvals from 1946 to 2019 are natural small molecules and their derivatives (Newman and Cragg, 2020). Compound Kushen injection (CKI) is extracted from natural botanical drugs Kushen (Sophora flavescens Aiton [Fabaceae]) and Baituling (Smilax gaudichaudiana Kunth [Smilacaceae]). CKI was made in accordance with the Ministry of Health Drug Standards (standard number: WS3-B-2752–97) and approved by China Food and Drug Administration (CFDA) (drug approval number: Z14021230) for cancer treatment in 1995. Since 1995, CKI has been widely used in anti-tumor therapy in China (Zhang L. Q, et al., 2012; Chang et al., 2021). Meanwhile a series of CKI clinical studies (NCT04204382, NCT02346318) for patients with cancer have been approved and performed in the United States. These clinical trials have exhibited that CKI combined with chemotherapy offer more advantages in decreasing MA, delaying the progression of cancer, increasing the disease control rate, prolonging the survival time, promoting the QoL, and decreasing toxic and side effects (Li et al., 2016; Huang, 2018; Xia et al., 2018; Xun et al., 2019). CKI may control MA through a series of anti-tumor activities, such as antioxidant activity (Zhang W. J, et al., 2012), promoting apoptosis (Wu et al., 2022), anti-tumor metastasis (Wang K. X et al., 2021), inhibiting angiogenesis (Wang et al., 2019; Han et al., 2020), anti-multidrug resistance of tumor cells, thereby improving immunity function (Zhang et al., 2019; Yang et al., 2020), suppressing the cell cycle, energy metabolism, and DNA repair pathways (Cui et al., 2019). Some studies have demonstrated that CKI combined with IPC has better efficacy than IPC alone, and the incidence of adverse drug reactions (ADRs) is also low. However, the scientific evidence has not been systematically reviewed. The main goal of the present systematic review was to summarize and screen the literature evidence that meets our inclusion criteria to evaluate the efficacy (including the objective response rate [ORR], QoL, survival time, and immune functions) and safety (including ADRs) of CKI combined with IPC when compared with those of IPC alone in the treatment of MA so as to obtain evidence for the treatment of MA.
The protocol of this review has been registered at PROSPERO (CRD42022304259). The report of this systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Checklist (Supplementary File S1).
CKI consists of Kushen (Sophora flavescens Aiton [Fabaceae]) and Baituling [Smilax gaudichaudiana Kunth (Smilacaceae)] (Zhao et al., 2014), which was supplied by Zhendong Pharmaceutical Co., Ltd. (Shanxi, China). CKI was prepared in accordance with the guidelines of the Ministry of Health Drug Standards (WS3-B-2752–97, Pharmacopoeia commission of the People’s Republic of China, 1997). Briefly, 1400 g of Sophora flavescens Aiton [Fabaceae] and 600 g of S.milax gaudichaudiana Kunth [Smilacaceae] were crushed with 1% acetic acid solution (solvent) and dipped for 48 h to percolate, followed by the collection of the percolate and condensation under reduced pressure (<75°C) to an appropriate amount. The residue was then decocted with water twice for 1 h each time, followed by filtration and concentration to a predefined amount. The filtrate was then combined with percolation and ethanol to add up to 65% of the volume, and the solution was kept still to allow the settlement of the particles. The solution was then filtered, ethanol was recovered, and the liquid was concentrated under pressure, with the addition of ethanol to make the ethanol content reach 90%. The resultant solution was kept still, filtered, and subjected to the recovery of all ethanol content. Then, 900 mL of the injection water was added to the filtrate along with 4 g of activated carbon, the mixture was boiled for 20 min, cooled, and then filtered. The pH value was adjusted with 20% sodium hydroxide, water was added for injection to 1000 mL, and the solution was filtered, potted, and sterilized to finally obtain the CKI. Chemical identification was performed in accordance with the procedure of thin-layer chromatography, and the alkaloid content in the matrine (C16H24N20) was ensured to be ≥ 18 mg/mL.
Patients with ascites were confirmed by imaging examination (computed tomography and/or B-ultrasound). Simultaneously, the cancer cells were detected on ascites cytology. Patients without the restrictions on age or sex were included in the trials. The baseline data of patients (such as the sex, age, type of tumor, histological type, and neoplasm staging) in the two groups were compared.
The experimental group received CKI combined with IPC. The control group received IPC only.
The primary outcome of the study was the ORR in accordance with the WHO criteria (Miller et al., 1981). The changes in the tumor condition included complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). ORR was defined as CR + PR. The secondary outcomes included the QoL as assessed by Karnofsky Performance Status (KPS); survival time assessed by overall survival (OS), progression-free survival (PFS), and disease-free survival (DFS). Immune function was assessed by the level of CD3+, CD4+, CD8+, and NK cells. Moreover, ADRs, including gastrointestinal reactions, myelosuppression, liver dysfunction, renal dysfunction, abdominal pain, and fever, were evaluated based on the incidence rate. We used ORR, QoL, survival time, and immune functions to evaluate the efficacy of the CKI in the treatment of MA as well as applied the ADRs to evaluate the safety of the CKI in the treatment of MA.
All randomized controlled trials (RCTs) published in English or Chinese language were included in the analyses.
All patients were included in the ward, with clear dates of case collection, at least one complete course of the treatment cycle, and unlimited follow-up time. All data were collected before the beginning of each trial and after the ending of the treatment course.
(1) Articles whose full text cannot be obtained through electronic database or manual retrieval. (2) Reviews, animal experiments, and other unrelated study types; (3) paper with insufficient data that did not allow further analysis; (4) paper including duplicate data; (5) paper lacking a suitable control group; (6) study in which treatment for MA included other anti-tumor drugs, except for chemotherapy drugs and CKI.
All RCTs, both in English or Chinese languages, that were published until 20 January 2023 were searched on the following electronic databases and clinical trials registration platforms: PubMed, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), Turning Research into Practice (TRIP) medical database, Latin American and Caribbean Health Sciences Literature (LILACS), Alt HealthWatch, Web of Science, Google Scholar, China National Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP database), Wangfang Data Knowledge Service Platform, Chinese Biomedical Literature Database (CBM), clinicaltrials.gov, and Chinese Clinical Trial Registry.
The searched terms of retrieval strategy were different based on a different database. The following terms were used in the English databases: “Kushen”, “matrine”, “sophora”, “Yanshu”, “CKI”, “ascitic fluid”, “ascites”, “peritoneal fluid”, “peritoneal effusion”, and “random”. The equivalent search words were used in the Chinese databases (the detailed search strategy is illustrated in Supplementary File S2). We searched for additional studies by reviewing the reference lists of the related studies. All studies were searched by two reviewers (HBY and BJH). Any disagreement was sorted through discussion with a third reviewer (JQH).
Two reviewers (HBY and BJH) independently imported the studies into the Endnote X9 software. After the exclusion of duplicate studies, the remaining studies were independently assessed for eligibility by two reviewers (STC and XC). Any disagreement was resolved through discussion with the third reviewer (HJC). Two reviewers (HTX and JG) imported the relevant data into EpiData 3.1. The extracted information included the following: 1. General information (title, first author, year(s), etc.); 2. Methodological information (i.e., study design type, random scheme concealment, random allocation method, randomization blind method, statistical analyst blinded, the loss of follow-up, baseline comparability, and selective reporting); 3. Participants information (i.e., diagnostic criteria, inclusion criteria, source, sample size, age, gender, and the types of carcinoma); 4. Intervention information (i.e., chemotherapy regimens, the dose of drugs, and treatment duration); 5. Study outcomes.
Two reviewers (STC and YYD) evaluated the enrolled studies independently, and any disagreements arising from this process were resolved through discussion with a third reviewer (JQH). Any risk of bias was assessed with the use of the Cochrane risk-of-bias tool to ensure the quality of included studies, in which the following seven domains were assessed: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. An overall judgment for each domain included three response options (low/unclear/high risk of bias) (Julian et al., 2022).
Two reviewers (HBY and BJH) conducted a meta-analysis on the included studies using RevMan5.4 and Stata17 software. The data was summarized by using risk ratio (RR) calculations and 95% confidence intervals (CI) for binary outcomes or mean difference (MD) with 95% CI for continuous outcomes.
Statistical heterogeneity among all trials were evaluated by the I2 test. Meta-analysis was conducted in case of no significant clinical and statistical heterogeneity (I2 < 75%) among the included trials. If I2 ≤ 25%, a fixed-effect model (FEM) was used to pool the data. If 25% < I2 < 75%, we first assessed the sources of the heterogeneity. If the statistical heterogeneity was explained successfully by sensitive analysis or subgroup analysis (I2 ≤ 25%), the FEM was used to pool the data; otherwise, the random-effects model (REM) was used in the meta-analysis. Pooling analysis was not performed in the case of a significant statistical heterogeneity (I2 ≥ 75%) among the trials, as subgroup analysis could not explain the huge heterogeneity. The funnel plot and Begg’s test were performed to explore the potential publication bias in case of ≥10 trials in the meta-analysis.
Subgroup analyses were conducted to determine the effects of the chemotherapy regimen, the dose of CKI, course of treatment, cancer types, and KPS score. Sensitivity analysis was performed to challenge the robustness of the primary analysis for trials with/without a high risk of bias and for meta-analyses conducted using the FEM or the REM.
Two reviewers (HBY and JQH) independently evaluated the quality of the evidence for outcomes with meta-analysis with reference to the Grading of Recommendations Assessment Development and Evaluation criteria (GRADE), which including the following five domains: risk of bias, inconsistency of trials, indirectness evidence, imprecision of results, and publication bias. The evidence was assessed on four different levels: high, moderate, low, or very low. Any disagreements were resolved through consensus or discussion with a third reviewer (HGZ).
A total of 940 potential studies were assessed, from which only 687 articles were included after removing the duplicated articles. A total of 554 articles were excluded through the title screening, and 133 articles remained after the abstracts screening. A total of 50 articles were subjected to full-text screening. Base on the exclusion criteria, 33 studies were excluded. Finally, 17 eligible studies were included for data extraction and quantitative synthesis. The specific study process is depicted in Figure 1.
A total of 17 RCTs, comprising 1,200 patients, were presented in this review. The sample size of the studies ranged from 32 to 106. In one study (Sun and Yang, 2009), the age of the patients was not reported, and, in another (Li et al., 2016), the sex of the patients was not reported. The age of the patients in the other study was between 28 and 80 years. A total of 13 studies involved gastric cancer and nine studies involved ovarian cancer, which was the most common causes of MA. In all studies, MA was controlled by CKI combined with IPC. Among the included studies, 11 studies (Cheng et al., 2006; Sun and Yang, 2009; Huang, 2010; Chen and Chen, 2011; Zhang Y et al., 2012; Jia et al., 2015; Zhang et al., 2016; Tao et al., 2017; Jiang et al., 2018; Wu, 2018; Zhang Y et al., 2020) used cisplatin, 2 studies (Zhang D, et al., 2017; Gao L et al., 2018) used lobaplatin, 2 studies (Tang et al., 2015; Li et al., 2016) used paclitaxel liposome, 1 study used carboplatin, and 1 study (Ma, 2016) used fluorouracil. The dosage of CKI was 20–40 mL. A total of 14 studies (Cheng et al., 2006; Huang, 2010; Chen and Chen, 2011; Zhang L. Q, et al., 2012; Tang et al., 2015; Li et al., 2016; Ma, 2016; Zhang et al., 2016; Tao et al., 2017; Zhang T et al., 2017; Gao S. T et al., 2018; Wu, 2018; Zhang D et al., 2020; Zhou, 2022) reported the ORR and seven studies (Cheng et al., 2006; Huang, 2010; Chen and Chen, 2011; Jia et al., 2015; Li et al., 2016; Zhang et al., 2016; Zhou, 2022) reported the change in the QoL of the patients. Adverse events were reported in 12 studies (Sun and Yang, 2009; Huang, 2010; Chen and Chen, 2011; Jia et al., 2015; Tang et al., 2015; Li et al., 2016; Zhang et al., 2016; Tao et al., 2017; Zhang D, et al., 2017; Gao L et al., 2018; Jiang et al., 2018; Zhang Y et al., 2020), 11 studies (Sun and Yang, 2009; Huang, 2010; Chen and Chen, 2011; Tang et al., 2015; Li et al., 2016; Zhang et al., 2016; Tao et al., 2017; Zhang T et al., 2017; Gao S. T et al., 2018; Jiang et al., 2018; Zhang Y et al., 2020) reported gastrointestinal reactions, 11 studies (Sun and Yang, 2009; Huang, 2010; Chen and Chen, 2011; Tang et al., 2015; Li et al., 2016; Zhang et al., 2016; Tao et al., 2017; Zhang D, et al., 2017; Gao L et al., 2018; Jiang et al., 2018; Zhang Y et al., 2020) reported myelosuppression, 3 studies (Jia et al., 2015; Tao et al., 2017; Gao S. T et al., 2018) reported liver dysfunction, 3 studies (Jia et al., 2015; Tao et al., 2017; Gao L et al., 2018) reported renal dysfunction, 2 studies (Sun and Yang, 2009; Huang, 2010) reported abdominal pain, and 7 studies (Huang, 2010; Jia et al., 2015; Tang et al., 2015; Li et al., 2016; Zhang et al., 2016; Jiang et al., 2018; Zhang Y et al., 2020) reported fever. The basic characteristics of the included studies are shown in Table 1.
We assessed the risk of bias for 17 included studies. For “Random sequence generation,” five studies (Tang et al., 2015; Li et al., 2016; Zhang et al., 2016; Zhang T et al., 2017; Zhou, 2022) were assessed as low risk base on the random number table method. Other studies (Cheng et al., 2006; Sun and Yang, 2009; Huang, 2010; Chen and Chen, 2011; Zhang W. J, et al., 2012; Jia et al., 2015; Ma, 2016; Tao et al., 2017; Gao L et al., 2018; Jiang et al., 2018; Wu, 2018; Zhang D et al., 2020) only mentioned “random” and did not describe the specific methods, and hence they were assessed as unclear. None of studies reported allocation concealment and blind methods; therefore, the risk was assessed as unclear. For “Incomplete outcome data,” all the included studies were evaluated as low risk because of the potential completeness of the data. For “Selective reporting,” all studies were assessed as low risk because their experimental analysis methods were consistent with the preset methods. For “Other bias,” one study (Chen and Chen, 2011) was assessed as high risk because the dose of chemotherapy drugs was inconsistent between the experimental and the control groups. Due to the presence of unclear/insufficient information related to age, KPS score, sex, and CKI manufacturers in 9 trials (Cheng et al., 2006; Sun and Yang, 2009; Zhang L. Q, et al., 2012; Tang et al., 2015; Li et al., 2016; Ma, 2016; Tao et al., 2017; Wu, 2018; Zhou, 2022) were assessed as unclear. A summary of the results of risk of bias assessment for the included studies is shown in Figure 2.
A total of 1002 patients in 14 studies (Chen and Chen, 2011; Cheng et al., 2006; Gao S. T et al., 2018; Huang, 2010; Li et al., 2016; Ma, 2016; Tang et al., 2015; Tao et al., 2017; Wu, 2018; Zhang et al., 2016; Zhang W. J, et al., 2012; Zhang D, et al., 2017; Zhang D et al., 2020+; Zhou, 2022) reported ORR. Meta-analysis showed that, when compared with IPC alone, CKI combined with IPC had an advantage in increasing ORR (REM, RR = 1.31, 95%CI 1.20 to 1.43, 1002 participants, I2 = 28%, p < 0.00001) (Shown in Figure 3).
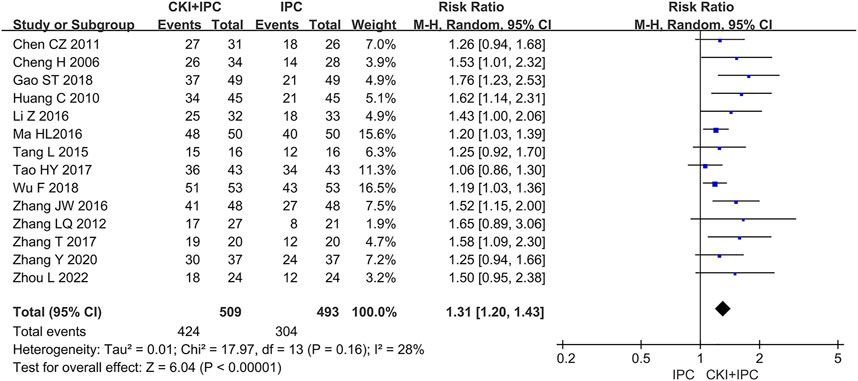
FIGURE 3. Forest plot and pooled risk ratios for the association of objective response rate (ORR) with CKI + IPC and IPC. CKI, Compound Kushen Injection; IPC, intraperitoneal chemotherapy.
Subgroup analysis of ORR was performed base on the chemotherapy regimen, dose of CKI, the course of treatment, cancer types, and KPS score (Shown in Table 2). The dose of CKI was 30 mL and 40 mL. Subgroup analysis revealed that higher dosage of CKI might have a better effect on ORR (Shown in Figure 4). The chemotherapy regimen included cisplatin, lobaplatin, carboplatin, paclitaxel liposome, and 5-Fu. Subgroup analysis revealed that CKI may have more advantage when combined with lobaplatin (Shown in Figure 5). Subgroup analysis classification by the course of treatment, cancer types, and KPS score did not explain the heterogeneity (Shown in Supplementary Figure S1–S3). Furthermore, subgroup analysis on the cancer types showed that the ovarian cancer subgroup had the highest effect value (REM, RR = 1.58, 95%CI 1.09 to 2.30, 31 participants, p = 0.02), followed by gastric cancer (REM, RR = 1.49, 95%CI 1.19 to 1.85, 111 participants, p = 0.0004), mixed cancer (REM, RR = 1.34, 95%CI 1.17 to 1.53, 404 participants, p < 0.0001), and liver cancer (REM, RR = 1.19, 95%CI 1.08 to 1.32, 182 participants, p = 0.0007). However, the number of studies reported by a single cancer subgroup was very small to draw convincing conclusions. Further, we used meta -regression to determine the degree of correlation between subgroups and intervention effects. The result indicated that only CKI dose had statistically significant (p = 0.042) (shown in Table 2).
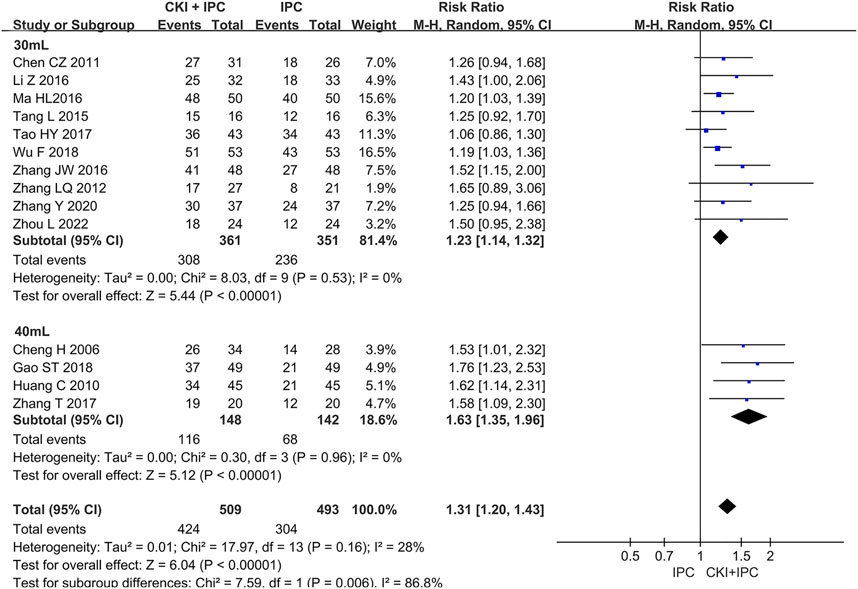
FIGURE 4. Forest plot and pooled risk ratios for the association of objective response rate (ORR) with CKI + IPC and IPC. Subgroup analysis of different CKI dosage.
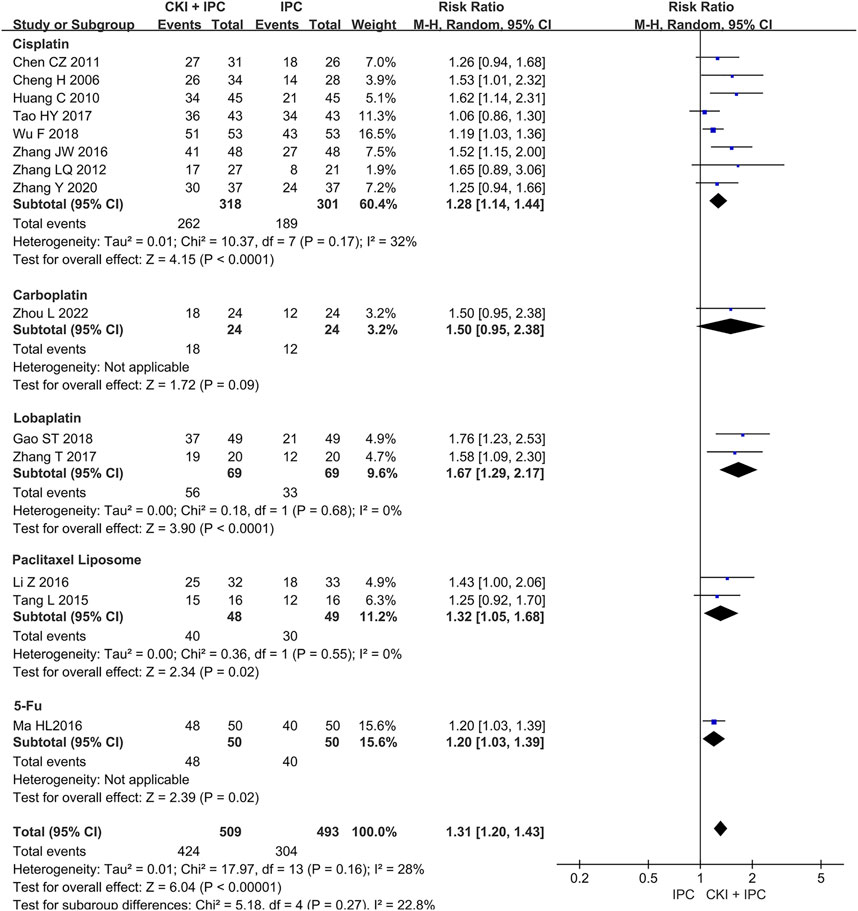
FIGURE 5. Forest plot and pooled risk ratios for the association of objective response rate (ORR) with CKI + IPC and IPC. Subgroup analysis of different chemotherapy regimen.
No study reported this outcome.
A total of 234 patients in 3 studies (Huang, 2010; Zhang et al., 2016; Zhou, 2022) reported the QoL by dichotomous data. Meta-analysis revealed that CKI combined with IPC could increase number of patients whose added KPS scores >10 when compared with IPC alone based on the baseline level (FEM, RR = 1.50, 95%CI 1.23 to 1.83, 234 participants, I2 = 0%, p < 0.0001). (Shown in Figure 6).
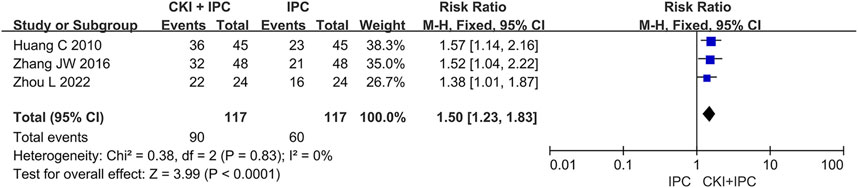
FIGURE 6. Forest plot and pooled risk ratios for the association of QoL (continuous data) with CKI + IPC and IPC.
Meanwhile, 4 studies (Cheng et al., 2006; Chen and Chen, 2011; Jia et al., 2015; Li et al., 2016) with 226 patients were reported by continuous data based on the KPS scale. Owing to the huge heterogeneity, we could not conduct a meta-analysis. However, subgroup analysis showed that the dosage of CKI could be a reason for heterogeneity; CKI (30 mL) plus IPC might have a better effect on improving QoL when compared to IPC alone. (REM, RR = 11.05, 95%CI 9.54 to 12.56, 164 participants, I2 = 0%, p < 0.00001) (Shown in Supplementary Figure S4).
A total of 4 studies (Zhang L. Q, et al., 2012; Zhang et al., 2016; Jiang et al., 2018; Zhang D et al., 2020) reported immune functions including the level of CD3+, CD4+, CD8+, CD4+/CD8+. Owing to the huge heterogeneity, we could not conduct a meta-analysis. All studies showed that the combined group could increase the level of CD3+, CD4+, CD4+/CD8+. Furthermore, three studies reported that the combined group could decrease the level of CD8+, and another study reported the opposite results. Only one study (Zhang et al., 2016) reported the level of NK cells, which suggested that the combined group could increase the level of NK cells. The specific data is depicted in Supplementary Table S1.
A total of 12 studies (Sun and Yang, 2009; Huang, 2010; Chen and Chen, 2011; Jia et al., 2015; Tang et al., 2015; Li et al., 2016; Zhang et al., 2016; Tao et al., 2017; Zhang T et al., 2017; Gao L et al., 2018; Jiang et al., 2018; Zhang Y et al., 2020) reported ADRs in total. A total of 11 studies (Sun and Yang, 2009; Huang, 2010; Chen and Chen, 2011; Tang et al., 2015; Li et al., 2016; Zhang et al., 2016; Tao et al., 2017; Zhang D, et al., 2017; Gao S. T et al., 2018; Jiang et al., 2018; Zhang D et al., 2020) with 794 patients reported gastrointestinal reactions (nausea or/and vomiting or/and diarrhea), 11 studies (Sun and Yang, 2009; Huang, 2010; Chen and Chen, 2011; Tang et al., 2015; Li et al., 2016; Zhang et al., 2016; Tao et al., 2017; Zhang T et al., 2017; Gao L et al., 2018; Jiang et al., 2018; Zhang Y et al., 2020) with 794 patients reported myelosuppression, three studies (Jia et al., 2015; Tao et al., 2017; Gao S. T et al., 2018) with 226 patients reported liver dysfunction, three studies (Jia et al., 2015; Tao et al., 2017; Gao L et al., 2018) with 226 patients reported renal dysfunction, two studies (Sun and Yang, 2009; Huang, 2010) with 146 patients reported abdominal pain, and seven studies (Huang, 2010; Jia et al., 2015; Tang et al., 2015; Li et al., 2016; Zhang et al., 2016; Jiang et al., 2018; Zhang D et al., 2020) with 499 patients reported fever (Shown in Table 1).
Meta-analysis showed that the incidence of myelosuppression was lower than that in the control group (FEM, RR = 0.51, 95%CI 0.40 to 0.64, 794 participants, I2 = 0, p < 0.00001). The treatment group had an advantage in decreasing the incidence of liver dysfunction (FEM, RR = 0.33, 95%CI 0.16 to 0.70, 226 participants, I2 = 0, p = 0.004), renal dysfunction (FEM, RR = 0.39, 95%CI 0.17 to 0.89, 226 participants, I2 = 0, p = 0.02) and fever (FEM, RR = 0.51, 95%CI 0.35 to 0.75, 499 participants, I2 = 0, p = 0.0007). However, no significant difference was found in the incidence of abdominal pain between groups (FEM, RR = 0.29, 95%CI 0.08 to 1.01, 146 participants, I2 = 0, p = 0.05) (Shown in Figure 7; Table 3).
Moreover, 794 patients in 11 studies (Sun and Yang, 2009; Huang, 2010; Chen and Chen, 2011; Tang et al., 2015; Li et al., 2016; Zhang et al., 2016; Tao et al., 2017; Zhang D, et al., 2017; Gao S. T et al., 2018; Jiang et al., 2018; Zhang Y et al., 2020) reported gastrointestinal reactions. Owing to the large heterogeneity, we could not conduct a meta-analysis. However, subgroup analysis showed that, when compared with cisplatin alone, CKI combined with cisplatin offered an advantage in decreasing the incidence of gastrointestinal reactions (REM, RR = 0.52, 95%CI 0.39 to 0.69, 559 participants, I2 = 0%, p < 0.00001) (Shown in Figure 8).
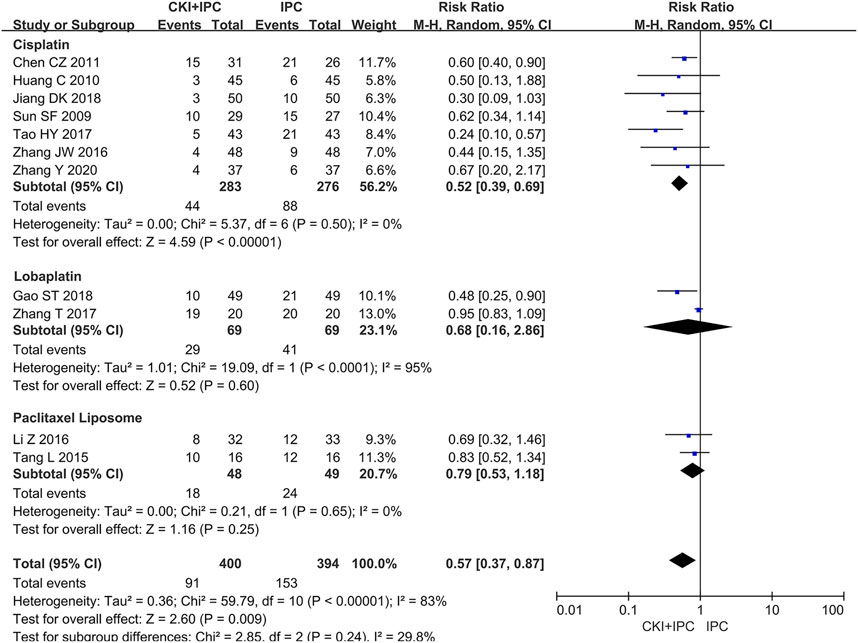
FIGURE 8. Forest plot and pooled risk ratios for the association of gastrointestinal reactions with CKI + IPC and IPC. Subgroup analysis of different chemotherapy regimen.
When compared with the funnel plot of IPC alone, the ORR and myelosuppression in the studies with CKI combined with IPC were distributed on both the sides symmetrically (Shown in Figure 9). Begg’s test indicated no significant publication bias in the meta-analysis of ORR (p = 0.1005) and myelosuppression (p = 0.2129).
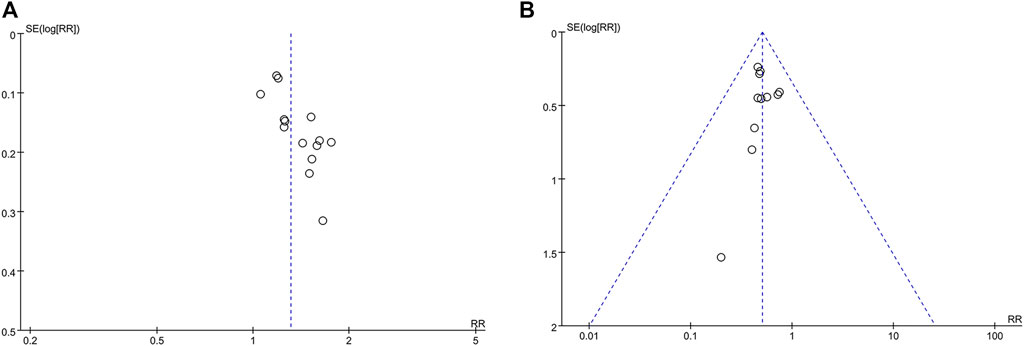
FIGURE 9. Funnel plot (A) CKI + IPC compared to IPC with the ORR reported in 14 trails; (B) CKI + IPC compared to IPC with the myelosuppression reported in 11 trails.
Base on the protocol, sensitivity analysis was performed to determine the effect of removing the high-risk study and switching REM/FEM on the results. Only one high risk trial (Chen and Chen, 2011) was included in our review, and the results showed that there was no difference after removing the high-risk trials from the meta-analysis (the result from original Figure 3 to RR = 1.32, 95%CI 1.20 to 1.46, p < 0.00001). After switching the REM/FEM, we found that our results were stable.
As shown in Table 4, based on the GRADE criteria, the quality of evidence was found to be moderate for ORR, myelosuppression, fever, and low for renal dysfunction, liver dysfunction, and very low for QoL and abdominal pain.
CKI combined with IPC has been widely used for treating patients with MA. However, because of the small sample size and insufficient quality of the single study, the efficacy and safety of this treatment plan should be investigated. Therefore, we conducted this systematic review and meta-analysis after systematic searching and screening and included 17 RCTs. The outcomes included ORR, survival time, immune function, QoL, and ADRs.
CKI is a botanical drug with broad-spectrum and multi-target anti-tumor effect. The main compounds of CKI are matrine (MT) and oxymatrine, which exhibit diverse anti-tumor pharmacological activities (Chen et al., 2022). Basic research showed that CKI could induce tumor apoptosis. Wu, X (Wu et al., 2022). reported that CKI combined with cisplatin exerted a synergistic anti-tumor effect against the p53-R273H/P309S mutant (SW480 cell) in colorectal cancer cells by inducing apoptosis. Yang, Y (Yang et al., 2020). indicated that CKI relieved the immunosuppression of tumor-associated macrophages and promoted the proliferation and cytotoxic ability of CD8+ T cells, thus resulting in the apoptosis of hepatocellular carcinoma cells. In addition, CKI could inhibit metastasis and angiogenesis in tumor. Through live cell imaging, Nourmohammadi (Nourmohammadi et al., 2019) confirmed that CKI strongly reduced the migration of HT-29 and MDA-MB-231 cells, moderately slowed brain cancer cells, and had a small effect on HEK-293 cells (Wang Q. G et al., 2021). indicated that CKI intervened in the metabolic reprogramming and epithelial-mesenchymal transition of HCC by regulating β-catenin/c-Myc signaling pathway and reducing their migration. CKI can also reduce the angiogenesis in the tumor tissues and play a role in inhibiting tumor growth (Wang et al., 2019). With the increasing CKI dose, the microvessel density of transplanted tumors decreased significantly, whereas the vascular maturity index increased significantly. CKI also regulated the immune functions. By performing an enzyme-linked immunosorbent assay, Shen (Shen et al., 2019) found that CKI could upregulate IL-1β gene, thereby increasing the IL-1β levels. The pathophysiology of MA is a combination of obstructed lymphatic drainage and altered vascular permeability. [12] Base on the present experimental evidence, CKI could inhibit tumor cells to relieve lymphatic obstruction and inhibit neoangiogenesis in the tumor tissues, which could be the mechanism of CKI for controlling MA. Animal experiments have demonstrated the positive effect of CKI on ascites in mice (Zhang L. Q, et al., 2012; Wang K. X et al., 2021). Several clinical trials have also shown that CKI combined with chemotherapy could increase the anti-tumor efficacy, improve QoL, and decrease ADRs (Pu et al., 2019; Zhang D et al., 2020; Zhang et al., 2021), which are consistent with our study results.
Moderate evidence obtained in the meta-analysis showed that CKI combined with IPC was more effective than IPC alone regarding ORR (19.1% more than IPC). The subgroup analysis showed that CKI may exert a better effect when combined with lobaplatin regimen, CKI 40 mL. It also showed that CKI played a better role in treating MA, which may be closely related to the multi-target anti-tumor activities of CKI, as confirmed by some network pharmacology studies. The anti-gastric cancer effect of CKI and its key targets were verified through in vivo and in vitro experiments. CKI could regulate phosphoinositide-3-kinase (PI3K)/Akt and toll-like receptor signaling pathways by interfering with hub genes such as AKR1B1, MMP2 and PTGERR3 (Zhou et al., 2021). CKI could exert anti-HCC effects through key targets such as MMP2, MYC, CASP3, REG1A, and the key pathways of glycometabolism and amino acid metabolism (Gao L et al., 2018). Wu, C (Wu et al., 2021). reported that, in PC treatment, the mechanism was related to cell cycle, Janus kinase/signal transducers, and the activators of transcription, ErbB, PI3K/Akt and mammalian target of the rapamycin signaling pathways. Furthermore, CDK1, JAK1, EGFR, MAPK1 and MAPK3 served as the core genes regulated by CKI in PC treatment.
In this review, we included “survival time” as an outcome, which is different from the protocol, for the following reasons: First, in a later study, we found that most patients with MA had a shorter survival time (approximately 5.7 months). (Hodge and Badgwell, 2019). We propose the inclusion of “survival time” as one of the outcomes to obtain evidence on the effect of CKI combined with IPC on this outcome in patients with MA. Second, “survival time” is one of the recommended evaluation measures for the clinical trials of cancer-related drugs according to the U.S. Food and Drug Administration (Services et al., 2018) and other studies (Wilson, et al., 2015a; Wilson, et al., 2015). “Survival time” is an important outcome for evaluating drug efficacy in many cancers. After comprehensive consideration, we decided to include this outcome in the scope of the review and truthfully reported the results.
None of the studies included in this review reported survival times. Most of them consisted of treatments that lasted between 1 and 2 months. However, the median survival of patients with MA was 5.7 months (Hodge and Badgwell, 2019). Several researchers have not yet observed this outcome and have neglected to follow up these patients. Nevertheless, several studies have demonstrated the positive effect of CKI on prolonging the survival of patients with multiple cancers. The meta-analysis revealed that CKI combined with platinum-based chemotherapy could improve the 1-year survival rate of patients with advanced non-small cell lung cancer (RR = 1.51, 95% CI 1.18 to 1.94, p = 0.001) (Chen et al., 2020). Some clinical studies have shown that CKI combined with chemotherapy could prolong the survival of patients with MA (Chen et al., 2011; Zhang et al., 2015). Furthermore, past studies have shown that CKI combined with chemotherapy could prolong the survival of patients with non-small cell lung cancer (Xiao et al., 2012), cervical cancer (Yang et al., 2018), and bladder cancer (Liu, 2018). Zhang Y found that CKI may improve the survival of Ehrlich ascites carcinoma in mice by exerting an antioxidant effect (Zhang W. J, et al., 2012). These clinical and elementary studies indicate that CKI might potentially prolong the survival of patients with MA, albeit this warrants further exploration.
With the popularization of bio-psycho-social medicine model, doctors, and patients are paying attention to the QoL, which is considered an additional outcome to evaluate cancer treatment by several researchers (Anota et al., 2015). MA is a poor prognostic indicator and detrimental to the QoL (Hodge and Badgwell, 2019). In this meta-analysis, very low evidence indicated that CKI combined with IPC might improve the QoL of patients with MA (25.6% more than IPC). The subgroup analysis showed that the effect of the 30 mL subgroup of CKI was stronger than that of the 40 mL subgroup of CKI. We found only one study in the 40 mL dose subgroup, and the present results are based on the differences between the two groups after treatment. However, as the baseline KPS score of the 40 mL subgroup was lower than that of the 30 mL subgroup in the original study, the general condition of the patients was worse, and the degree of improvement was poor, which may explain the lower effect of the 40 mL subgroup. We found that the dose of CKI may be a factor affecting the QoL of patients with MA; however, we could not conclude the specific extent of the effect based on the present data. The results regarding the effect of CKI dose on QoL improvement warrants further investigation.
Due to the limitations of the original study, although some clinical (Zhao et al., 2016) and animal studies (Zhou et al., 2012) have suggested that CKI may enhance immune function, we cannot conclude the effect of CKI combined with IPC on immune functions. In this study, one study showed different results from the other three in terms of the characteristics studied based on the level of CD8+, implying the uncertainty of the effect of CKI combined with IPC on immune function. Thus, the effect of CKI on immune functions warrants further exploration.
The symptoms of MA are generally caused by abdominal distention, visceral compression, and the loss of proteins and electrolytes. These symptoms include abdominal pain, nausea, vomiting, dyspnea, anorexia, dyskinesia, and fatigue, which severely affects the QoL of patients with MA (Cavazzoni et al., 2013). The decrease in the QoL of patients with MA can be alleviated by treating ascites itself (Hodge and Badgwell, 2019). Interestingly, the effect of CKI on QoL may also be related to energy metabolism. Previous studies have demonstrated that CKI could affect energy synthesis by regulating glucose metabolism, which may be the mechanism for improving the QoL. Cui, J (Cui et al., 2019). found reduced energy metabolism in cancer cells based on reduced glucose consumption and cellular energy charges. CKI might improve the QoL by increasing the immune function of patients with cancer. In an experiment, Shen (Zhou et al., 2012) indicated that CKI could blocks gastric carcinogenesis, thereby protecting against carcinogen-induced oxidative damage and improving immunity.
IPC is a widely used and effective treatment strategy for MA. However, not all patients can benefit from it because of intolerant ADRs to chemotherapy drugs, including gastrointestinal reactions, myelosuppression, liver and renal dysfunction, abdominal pain, and fever. Moreover, the expected treatment cycle could not be achieved because of severe ADRs, which may confuse physicians. In this study, moderate evidence supports that the combination group exhibited a lower incidence of myelosuppression (16.3% lower than the control group) and fever (11.6% lower than the control group) in patients with MA. Due to the huge heterogeneity, we could not conduct a meta-analysis for gastrointestinal reactions. However, the subgroup analysis showed that CKI combined with cisplatin could decrease the incidence of gastrointestinal reactions when compared with cisplatin alone (RR = 0.52, p < 0.00001). In addition, less evidence indicated that the combination group showed a lower incidence of liver dysfunction (14.4% lower than the control group) and renal dysfunction (9.8% lower than the control group), but it did not show an increased burden of abdominal pain (RR = 0.29, p = 0.05). Saeed Nourmohammadi (Nourmohammadi et al., 2019) reported that CKI uniformly blocked invasiveness via the extracellular matrix. CKI increased apoptosis in breast cancer cells, but not in the non-cancerous cell lines. CKI did not affect the viability of all cell lines, which may explain why CKI did not increase the burden of ADRs. To summarize, our cumulative results indicate that CKI combined with IPC is safe and can reduce the incidence of some ADRs in MA treatment.
Although several systematic reviews published previously are similar to our research topics, they are all different from this review. Methodologically, we used a recognized authoritative tool (GRADE criteria) and performed subgroup analysis and meta-regression to further explore the sources of heterogeneity. The present study conforms to the PRISMA guideline (McInnes et al., 2018). Considering the evidence obtained, our study is newer and more standardized than related past studies. Moreover, unlike previous studies, we included additional outcomes such as the survival time and immune functions in the evaluation. For evaluating the safety of CKI, we included more types of adverse events than the previous studies did. Moreover, Wang Peipei (Wang, 2020) focused on the differences between the efficacies of different botanical injections, whereas we focused on the efficacy and safety of CKI combined with IPC. Tang Ziwei (Tang, 2020) only evaluated CKI combined with cisplatin for treating MA. Xu Zhong (Xu et al., 2014) included some studies in which intravenous CKIs were used. Some studies reported that the different routes of drug administration may affect drug efficacy and safety outcomes (Dai et al., 2020). Therefore, to reduce bias, our inclusion criteria were the intraperitoneal infusion of both CKI and chemotherapy drugs. Moreover, previous studies have indicated the exact efficacy of CKI combined with chemotherapy regimen for treating liver cancer (Ma et al., 2016), digestive tract tumors (Zhang et al., 2021), breast cancer (Liu et al., 2020), lung cancer (Li et al., 2022), gastric cancer (Zhang T et al., 2017), and cancer pain (Yanju et al., 2014), which indirectly supports the results. We hope to provide the best and latest available evidence by adopting evidence-based medicine methods and providing clinicians with a clear and effective treatment plan.
We have some instructions for future research. First, the present study has some limitations regarding the methodological quality of the trials for the risk of bias assessment. Most studies possessed an unclear risk regarding factors including random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of the outcome assessment. Future studies should focus on applying random methods, implementing blinding methods, and properly managing missing data (e.g., intention to treat). Reporting baseline data (e.g., sex, age, type of tumor, histological type, neoplasm staging of patients) and drug manufacturer information are also essential. Registration of clinical trials is also necessary so that more information about the study design can be made public. Furthermore, researchers should conduct well-designed and high-quality clinical studies per the CONSORT guidelines (Schulz et al., 2010) to verify the efficacy and safety of CKI combined with IPC for treating MA. Second, MA is the manifestation of tumor deterioration, and the survival time of such patients is usually short. Thus, the survival data of these patients should be observed and reported. Third, most of the studies included in this review only reported ADR incidence. We recommend reporting ADRs using standardized scales and/or criteria such as the National Cancer Institute-Common Toxicity Criteria for Adverse Events to better reflect the extent of ADRs. Fourth, we recommend that patients with the same type of cancer should be considered as a study population and those with different types of cancers should be reported by subgroups to decrease the effect of cancer types on outcomes including the survival time and ORR. Finally, as we found that the dose of CKI may affect the QoL of patients, we suggest that subsequent analyses should further clarify the relationship between the dose of CKI and QoL.
The study has the following limitations. First, we have only searched English and Chinese databases. All included studies are from China, which may have incurred regional and ethnic differences. Second, most studies included in this review may not strictly adhere to the CONSORT reporting standards; hence, some results are rated as low or very low at the time of GRADE rating. Third, this study was not a reticular meta-analysis; hence, we could not evaluate the difference between the efficacies of CKI and other botanical injections. However, the cluster analysis of 29 RCTs (Zhang D et al., 2020) of 8 botanical injections showed that CKI combined with chemotherapy was the optimal choice for improving the clinical efficacy rate and ADRs in patients with esophageal cancer. Finally, most studies included MA caused by multiple types of tumors as a study population, which may have increased the confounding bias. Hence, subsequent studies should address these limitations.
This study result suggests that CKI combined with IPC can increase ORR and improve QoL of patients with MA. In addition, this combination treatment can partially reduce toxicity caused by chemotherapy drugs. However, the efficacy and safety of CKI combined with IPC for patients with MA needs to be verified in future by conducting well-designed and high-quality clinical trials that adhere to the CONSORT guidelines.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
H-GZ designed the research. H-BY, B-JH, and J-QH performed literature search. H-BY, B-JH, H-JC, S-TC, XC, H-TX, Y-YD, and JG performed article selection and data extraction. S-TC, XC, and J-QH assessed methodological bias risk. H-BY, B-JH, and J-QH conducted a meta-analysis. H-BY, J-QH, and H-GZ assessed study quality. H-BY finished the manuscript draft. H-GZ and H-JC reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (No. CI 2021A01804), Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-C-202205).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer NL declared a shared parent affiliation with the authors HY, JH, BH, XC, HX, JG, YD, and HZ to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1036043/full#supplementary-material
CKI, Compound Kushen Injection; IPC, intraperitoneal chemotherapy; MA, malignant ascites; RCTs, randomized controlled trials; ORR, objective response rate; QoL, quality of life; ADRs, adverse drug reactions; GRADE, Grading of Recommendations Assessment Development and Evaluation criteria; CI, confidence intervals; RR, risk ratio; FDA, Food and Drug Administration; CFDA, China Food and Drug Administration; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; MD, mean difference; KPS, Karnofsky Performance Status; FEM, fixed-effects model; REM, random-effects model; MT, matrine; OMT, oxymatrine; CRC, colorectal cancer cells; MVD, micro vessel density; PC, pancreatic cancer; OS, overall survival; PFS, progression-free survival; DFS, Disease-free survival; HCC, Hepatocellular Carcinoma; SM, statistical method.
Anota, A., Hamidou, Z., Paget-Bailly, S., Chibaudel, B., Bascoul-Mollevi, C., Auquier, P., et al. (2015). Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: Do we need RECIST for quality of life to achieve standardization? Qual. Life Res. 24 (1), 5–18. doi:10.1007/s11136-013-0583-6
Armstrong, D. K., Bundy, B., Wenzel, L., Huang, H. Q., Baergen, R., Lele, S., et al. (2006). Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 354 (1), 34–43. doi:10.1056/NEJMoa052985
Barni, S., Cabiddu, M., Ghilardi, M., and Petrelli, F. (2011). A novel perspective for an orphan problem: Old and new drugs for the medical management of malignant ascites. Crit. Rev. Oncol. Hematol. 79 (2), 144–153. doi:10.1016/j.critrevonc.2010.07.016
Cavazzoni, E., Bugiantella, W., Graziosi, L., Franceschini, M. S., and Donini, A. (2013). Malignant ascites: Pathophysiology and treatment. Int. J. Clin. Oncol. 18 (1), 1–9. doi:10.1007/s10147-012-0396-6
Chang, Z. G., Ma, L., Chen, X. B., Guo, Y. W., Zhang, X. D., Yan, W. W., et al. (2021). Clinical study of compound kushen injection combined with oxaliplatin and tigio in the treatment of stage Ⅳ gastric cancer. Chin. J. Cancer Prev. Treat. 28 (16), 1242–1246. doi:10.16073/j.cnki.cjcpt.2021.16.09
Chao, J., and Qin, S. K. (2019). Progression of recombinant human endostatin (endostar) intraperitoneal injection for the treatment of malignant ascites. Chin. Clin. Oncol. 24 (09), 856–861. doi:10.3969/j.issn.1009-0460.2019.09.018
Chen, C. Z., and Chen, Y. H. (2011). Matrine injection and intraperitoneal injection of cisplatin efficacy in treatment of cancer ascites. Chin. FOREIGN Med. Res. 9 (17), 16–17. doi:10.3969/j.issn.1674-6805.2011.17.009
Chen, F., Pan, Y., Xu, J., Liu, B., and Song, H. (2022). Research progress of matrine's anticancer activity and its molecular mechanism. J. Ethnopharmacol. 286, 114914. doi:10.1016/j.jep.2021.114914
Chen, H., Yao, X., Li, T., Lam, C. W., Zhang, R., Zhang, H., et al. (2020). Compound kushen injection combined with platinum-based chemotherapy for stage III/IV non-small cell lung cancer: A meta-analysis of 37 RCTs following the PRISMA guidelines. J. Cancer 11 (7), 1883–1898. doi:10.7150/jca.40267
Chen, Y. F., Li, Q., Xiang, S., and Yang, G. H. (2011). Compound kusheng injection combined with cisplatin for malignant pleural effusion: A clinical study. Eval. Analysis Drug-Use Hosp. China 11 (04), 366–367. doi:10.14009/j.issn.1672-2124.2011.04.016
Cheng, H., Zhang, H. Y., Wang, S. X., and Ni, M. (2006). Treatment of malignant ascites with intraperitoneal injection of compound Kushen injection combined cisplatin in 34 patients. Chin. J. Integr. Tradit. West Med. Dig. 14 (6), 407–408. doi:10.3969/j.issn.1671-038X.2006.06.022
Chicago Consensus Working, G. (2020). The Chicago Consensus on peritoneal surface malignancies: Palliative care considerations. Cancer 126 (11), 2571–2576. doi:10.1002/cncr.32826
Cui, J., Qu, Z., Harata-Lee, Y., Nwe Aung, T., Shen, H., Wang, W., et al. (2019). Cell cycle, energy metabolism and DNA repair pathways in cancer cells are suppressed by Compound Kushen Injection. BMC Cancer 19 (1), 103. doi:10.1186/s12885-018-5230-8
Dai, B., Zhan, M., and Xu, T. (2020). Meta-analysis of the efficacy and safety of different administration routes of Bevacizumab in the treatment of malignant pleural effusion. China Pharm. 31 (06), 734–739. doi:10.6039/j.issn.1001-0408.2020.06.19
Gao, L, L., Wang, K. X., Zhou, Y. Z., Fang, J. S., Qin, X. M., and Du, G. H. (2018). Uncovering the anticancer mechanism of Compound Kushen Injection against HCC by integrating quantitative analysis, network analysis and experimental validation. Sci. Rep. 8 (1), 624. doi:10.1038/s41598-017-18325-7
Gao, S. T, S. T., Shan, G. Y., and Wang, L. (2018). Therapeutic effect of compound Kushen injection and Lobaplatin in treating malignant ascites. J. Mod. Oncol. 26 (15), 2392–2395. doi:10.3969/j.issn.1672-4992.2018.15.020
Han, L., Zhang, W., Li, X., He, Q., Han, J., Zhang, Y., et al. (2020). Investigating the anti-angiogenic effects of Fufang Kushen Injection in combination with cisplatin using a zebrafish model. Pak J. Pharm. Sci. 33 (5), 1955–1960.
He, Y. F., Sun, Y. B., Chen, J., Xu, T. Y., Wang, G., Wang, Y., et al. (2009). Intraperitoneal injection of recombinant human endostatin combined with fluorouracil on advanced cancers with malignant ascites. Chin. Clin. Oncol. 14 (03), 252–255.
Hodge, C., and Badgwell, B. D. (2019). Palliation of malignant ascites. J. Surg. Oncol. 120 (1), 67–73. doi:10.1002/jso.25453
Huang, C. (2010). Treatment of malignant ascites with intraperitoneal injection of compound Kushen injection combined cisplatin in 90 patients. Chin. J. New Drugs 19 (17), 1593–1595.
Huang, Z. C. (2018). Effect of compound Kushen injection combined with oxaliplatin chemotherapy on clinical efficacy of patients with primary hepatocellular cancer. Chin. J. Integr. Tradit. West Med. Intensive Crit. Care 25 (5). doi:10.3969/j.issn.1008-9691.2018.05.023
Julian, H., James, T., JacQueline, C., Miranda, C., Tianjing, L., Matthew, P., et al. (2022). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022), Available at: www.training.cochrane.org/handbook
Jia, G., Zhang, H. Z., and Jiang, H. J. (2015). Clinical efficacy of intraperitoneal perfusion of compound matrine injection and cisplatin on malignant ascites. J. Mod. Oncol. 23 (10), 1438–1440. doi:10.3969/j.issn.1672-4992.2015.10.34
Jiang, D. K., Chen, Z. Y., Ma, D. M., and Xu, L. (2018). Effect of compound kushen injection on abdominal perfusion of malignant ascites and influence on CEA, CA125 and AFP. Mod. J. Integr. Tradit. Chin. West Med. 27 (33), 3714–3717. doi:10.3969/j.issn.1008-8849.2018.33
Kalogeraki, A., Karvela-Kalogeraki, I., Tamiolakis, D., Petraki, P., Papathanasiou, A., Saridaki, Z., et al. (2012). Cytopathologic interpretation of ascites due to malignancy. J. BUON 17 (3), 446–451.
Li, J., Zhu, G. H., Liu, T. T., Xu, B. W., and Li, J. (2022). Comparative efficacy of 10 Chinese herbal injections combined with GP regimen chemotherapy for patients with advanced NSCLC a systematic review and network meta-analysis. J. Cancer 13 (2), 465–480. doi:10.7150/jca.66410
Li, Z., Li, K., Wang, W., and Li, Q. H. (2016). Observation of paclitaxel liposome fufang kushen injection and yiqi yangwei decoction combined with in the treatment of advanced gastric cancer with malignant ascites. J. Liaoning Univ. Tradit. Chin. Med. 18 (11), 138–140. doi:10.13194/j.issn.1673-842x.2016.11.042
Ling, C. Q. (2020). Traditional Chinese medicine is a resource for drug discovery against 2019 novel coronavirus (SARS-CoV-2). J. Integr. Med. 18 (2), 87–88. doi:10.1016/j.joim.2020.02.004
Liu, S., Wang, H., Wang, M., Hu, X., Yang, W., Jin, R., et al. (2020). Comparative efficacy and safety of Chinese herbal injections combined with cyclophosphamide and 5-fluorouracil chemotherapies in treatment of breast cancer: A bayesian network meta-analysis. Front. Pharmacol. 11, 572396. doi:10.3389/fphar.2020.572396
Liu, T. (2018). Effects of matrine injection combined with GC chemotherapy on chemo tolerance and median survival time in patients with advanced bladder cancer. Drug Eval. 15 (09), 35–38. doi:10.3969/j.issn.1672-2809.2018.09.011
Ma, H. L. (2016). Analysis of nursing effect of compound matrine injection combined with chemotherapeutic drugs intraperitoneal injection in the treatment of liver cancer with malignant ascites. J. Liaoning Univ. Tradit. Chin. Med. 18 (10), 214–216. doi:10.13194/j.issn.1673-842x.2016.10.068
Ma, X., Li, R. S., Wang, J., Huang, Y. Q., Li, P. Y., Wang, J., et al. (2016). The therapeutic efficacy and safety of compound kushen injection combined with transarterial chemoembolization in unresectable hepatocellular carcinoma: An update systematic review and meta-analysis. Front. Pharmacol. 7, 70. doi:10.3389/fphar.2016.00070
McInnes, M. D. F., Moher, D., Thombs, B. D., McGrath, T. A., Bossuyt, P. M., the, P.-D. T. A. G., et al. (2018). Preferred reporting Items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA 319 (4), 388–396. doi:10.1001/jama.2017.19163
Miller, A. B., Hoogstraten, B., Staquet, M., and Winkler, A. (1981). Reporting results of cancer treatment. Cancer 47 (1), 207–214. doi:10.1002/1097-0142(19810101)47:1<207:aid-cncr2820470134>3.0.co;2-6
Newman, D. J., and Cragg, G. M. (2020). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83 (3), 770–803. doi:10.1021/acs.jnatprod.9b01285
Nourmohammadi, S., Aung, T. N., Cui, J., Pei, J. V., De Ieso, M. L., Harata-Lee, Y., et al. (2019). Effect of compound kushen injection, a natural compound mixture, and its identified chemical components on migration and invasion of colon, brain, and breast cancer cell lines. Front. Oncol. 9, 314. doi:10.3389/fonc.2019.00314
Pu, L., Chen, W. H., Cao, L. X., Wu, K. J., Chen, S. L., Lin, J. H., et al. (2019). Compound kushen injection as an adjunctive therapy for the treatment of non-small-cell lung cancer: A meta-analysis of randomized controlled trials. Evid. Based Complement. Altern. Med. 2019, 7241927. doi:10.1155/2019/7241927
Schulz, K. F., Altman, D. G., Moher, D., and Group, C. (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 340, c332. doi:10.1136/bmj.c332
Services, U., Administration, F., Excellence, O., Cder), C., and Cber, C. (2018). Clinical trial endpoints for the approval of cancer drugs and biologics guidance for industry. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trial-endpoints-approval-cancer-drugs-and-biologics.
Shen, H., Qu, Z., Harata-Lee, Y., Aung, T. N., Cui, J., Wang, W., et al. (2019). Understanding the mechanistic contribution of herbal extracts in compound kushen injection with transcriptome analysis. Front. Oncol. 9, 632. doi:10.3389/fonc.2019.00632
Sun, S. F., and Yang, Y. X. (2009). Effect of compound kushen combined with intraperitoneal chemotherapy on ovarian cancer ascites. ZHEJIANG J. Trauma. Surg. 14 (2), 128–129. doi:10.3969/j.issn.1009-7147.2009.02.024
Tang, L., Piao, Y., Ji, F. H., and Tan, L. S. (2015). United Fufangkushen paclitaxel liposome injection in the treatment of malignant ascites efficacy and safety analysis. Clin. J. Med. Off. 43 (12), 1257–1260. doi:10.16680/j.1671-3826.2015.12.14
Tang, Z. W. (2020). Compound kushen injection combined with cisplatin in the treatment of malignant intraperitoneal perfusion:A Meta-analysis. Chengdu University of Traditional Chinese Medicine. Chengdu, China, Unpublished master.
Tao, H. Y., Qu, Z. Y., Wan, L. X., Wei, G. M., Wang, W. L., and Wang, Q. C. (2017). Effect of compound kushen injection combined with intraperitoneal chemotherapy in the treatment of malignant ascites and its effect on CEA, CA125 and AFP levels. Inf. Traditional Chin. Med. 34 (3), 83–86. doi:10.3969/j.issn.1002-2406.2017.03.023
Wang, H. B., Jiang, F. L., Lai, Z. L., Song, N., Sun, M. L., and Wang, Y. H. (2017). Treatment status and progress of malignant ascites. J. Emerg. Traditional Chin. Med. 26 (12), 2162–2164. doi:10.3969/j.issn.1004-745X.2017.12.028
Wang, H., Hu, H., Rong, H., and Zhao, X. (2019). Effects of compound Kushen injection on pathology and angiogenesis of tumor tissues. Oncol. Lett. 17 (2), 2278–2282. doi:10.3892/ol.2018.9861
Wang, P. P. (2020). Traditional Chinese medicine injections combined with cisplatin intraperitoneal perfusion in the treatment of cancerous ascites: A systematic review and meta-analysis. Beijing University of Chinese Medicine. Unpublished master.
Wang, K. X, K. X., Du, G. H., Qin, X. M., and Gao, L. (2021). Compound Kushen Injection intervenes metabolic reprogramming and epithelial-mesenchymal transition of HCC via regulating beta-catenin/c-Myc signaling. Phytomedicine 93, 153781. doi:10.1016/j.phymed.2021.153781
Wang, Q. G, Q. G., Wang, Y. H., and Kong, F. Z. (2021). Anti-tumor effect of marine adjuvant chemotherapy in hepatoma ascites tumor-bearing mice. Chin. J. Clin. Pharmacol. 37 (17), 2320–2323. doi:10.13699/j.cnki.1001-6821.2021.17.018
Wilson, M. K., Collyar, D., Chingos, D. T., Friedlander, M., Ho, T. W., Karakasis, K., et al. (2015a). Outcomes and endpoints in cancer trials: Bridging the divide. Lancet Oncol. 16 (1), e43–e52. doi:10.1016/S1470-2045(14)70380-8
Wilson, M. K., Karakasis, K., and Oza, A. M. (2015b). Outcomes and endpoints in trials of cancer treatment: The past, present, and future. Lancet Oncol. 16 (1), e32–e42. doi:10.1016/S1470-2045(14)70375-4
Wu, C., Huang, Z. H., Meng, Z. Q., Fan, X. T., Lu, S., Tan, Y. Y., et al. (2021). A network pharmacology approach to reveal the pharmacological targets and biological mechanism of compound kushen injection for treating pancreatic cancer based on WGCNA and in vitro experiment validation. Chin. Med. 16 (1), 121. doi:10.1186/s13020-021-00534-y
Wu, F. (2018). Clinical trial of compound kushen injection combined with cisplatin and single cisplatin in the treatment of hepatocellular carcinoma with malignant ascites. China Pract. Med. 13 (30), 118–119. doi:10.14163/j.cnki.11-5547/r.2018.30.064
Wu, X., Lu, Y., and Qin, X. (2022). Combination of Compound Kushen Injection and cisplatin shows synergistic antitumor activity in p53-R273H/P309S mutant colorectal cancer cells through inducing apoptosis. J. Ethnopharmacol. 283, 114690. doi:10.1016/j.jep.2021.114690
Xia, N. X., Qiu, B. A., Liu, P., Zhu, J. Y., Zhao, W. C., and An, Y. (2018). Observation on the curative effect of oxycontin combined with compound sophorae injection an cancer pain in patients with adcanced liver cancer. Clin. J. Med. Off. 46 (07), 735–736+740. doi:10.16680/j.1671-3826.2018.07.01
Xiao, P., Meng, J. Q., Meng, X. R., Bai, H., Li, M., Lin, H. W., et al. (2012). Efficacy and toxicity of compound matrine injection combined with gemcitabine plus cisplatin for advanced non-small-cell lung cancer: Analysis of 56 cases. Eval. Analysis Drug-Use Hosp. China 12 (02), 155–157. doi:10.14009/j.issn.1672-2124.2012.02.014
Xu, Z., Cao, H., Huang, G. M., and Jiang, Y. (2014). Compound kushen injection plus intraperitoneal chemotherapy in the treatment of malignant ascites:a meta-analysis. Anti-Tumor Pharm. 4 (05), 383–388. doi:10.3969/j.issn.2095-1264.2014.079
Xun, G. Z., Wang, L. P., Wang, M., and Wei, Y. C. (2019). Efficacy and side effect of compound Sophora flavescens injection in adjuvant folfox chemotherapy for gastric cancer: A systematic review. Chin. J. Cancer Prev. Treat. 26 (12), 881–887. doi:10.16073/j.cnki.cjcpt.2019.12.012
Yang, X. Y., Hu, B. Y., and Jiang, Y. T. (2018). Effect of compound kushen injection and neoadjuvant chemotherapy on cervical cancer and survival. Chin. Arch. Tradit. Chin. Med. 36 (10), 2520–2524. doi:10.13193/j.issn.1673-7717.2018.10.056
Yang, Y., Li, N., Wang, T. M., and Di, L. (2021). Natural products with activity against lung cancer: A review focusing on the tumor microenvironment. Int. J. Mol. Sci. 22 (19), 10827. doi:10.3390/ijms221910827
Yang, Y., Sun, M., Yao, W., Wang, F., Li, X., Wang, W., et al. (2020). Compound kushen injection relieves tumor-associated macrophage-mediated immunosuppression through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib. J. Immunother. Cancer 8 (1), e000317. doi:10.1136/jitc-2019-000317
Yanju, B., Yang, L., Hua, B., Hou, W., Shi, Z., Li, W., et al. (2014). A systematic review and meta-analysis on the use of traditional Chinese medicine compound kushen injection for bone cancer pain. Support Care Cancer 22 (3), 825–836. doi:10.1007/s00520-013-2063-5
Zhang, D., Wu, J., Wang, H., Zhou, W., Ni, M., Liu, X., et al. (2020). Systematic review and network meta-analysis comparing Chinese herbal injections with chemotherapy for treating patients with esophageal cancer. J. Int. Med. Res. 48 (1), 300060519898336. doi:10.1177/0300060519898336
Zhang, J., Qu, Z., Yao, H., Sun, L., Harata-Lee, Y., Cui, J., et al. (2019). An effective drug sensitizing agent increases gefitinib treatment by down regulating PI3K/Akt/mTOR pathway and up regulating autophagy in non-small cell lung cancer. Biomed. Pharmacother. 118, 109169. doi:10.1016/j.biopha.2019.109169
Zhang, J. W., Duan, D. M., and Ren, Z. H. (2016). Combination of compound kushen injection and cisplatin intraperitoneal chemotherapy in patients with gastric cancer and malignant ascites. Chin. J. Exp. Tradit. Med. Formulae 22 (11), 179–183. doi:10.13422/j.cnki.syfjx.2016110179
Zhang, L. Q., Jiang, S. M., and Tian, S. Q. (2012). Clinical observation of compound matrine injection in treatment of malignant ascites by intraperitoneal perfusion. Res. Integr. Traditional Chin. West. Med. 4 (3), 128–130. doi:10.3870/j.issn.1674-4616.2012.03.005
Zhang, S. F., Chen, X. P., Chen, Y. S., Bao, L., and Zhang, T. (2015). Effiacy and safety of compound kushen injection plus nedaplatin for malignant pleural effusion in patients with lung cancer. Eval. Analysis Drug-Use Hosp. China 15 (05), 601–603. doi:10.14009/j.issn.1672-2124.2015.05.015
Zhang, W., Gong, W., He, X., Wu, C., and Tu, X. (2021). A systematic review and meta-analysis on the efficacy of Compound Kushen Injection in 3 kinds of digestive tract tumor. J. Gastrointest. Oncol. 12 (6), 2919–2929. doi:10.21037/jgo-21-774
Zhang, Y., Wang, Z. X., and Li, Z. G. (2020). Effect of compound kushen injection and cisplatin on immune function and vascular endothelial function in patients with malignant ascites. Guangming J. Chin. Med. 35 (15), 2371–2373. doi:10.3969/j.issn.1003-8914.2020.15.038
Zhang, D, D., Zheng, J., Ni, M., Wu, J., Wang, K., Duan, X., et al. (2017). Comparative efficacy and safety of Chinese herbal injections combined with the FOLFOX regimen for treating gastric cancer in China: A network meta-analysis. Oncotarget 8 (40), 68873–68889. doi:10.18632/oncotarget.20320
Zhang, T, T., Xu, H. T., Hui, H., Geng, C., Ding, M. H., and Xu, J. (2017). Therapeutic effect of losecplatin combined with compound matrine in the intraperitoneal perfusion treatment of ovarian cancer with malignant ascites and its effect on TNF. Chin. J. Prim. Med. Pharm. 24 (15), 2281–2284. doi:10.3760/cma.j.issn.1008-6706.2017.15.010
Zhang, W. J, W. J., Hai, L. N., and Lian, Z. L. (2012). Progress in the experimental study of antitumor effect and mechanism of compound kushen injection. Chin. J. Inf. Tradit. Chin. Med. 19 (08), 101–103. doi:10.3969/j.issn.1005-5304.2012.08.049
Zhang, Y, Y., Jia, Y., and Jin, S. (2012). Effect of compound kushen injection in the treatment on Ehrlich ascites carcinoma in mice. Drug Eval. Res. 35 (05), 343–344.
Zhao, Z., Fan, H., Higgins, T., Qi, J., Haines, D., Trivett, A., et al. (2014). Fufang Kushen injection inhibits sarcoma growth and tumor-induced hyperalgesia via TRPV1 signaling pathways. Cancer Lett. 355 (2), 232–241. doi:10.1016/j.canlet.2014.08.037
Zhao, Z., Liao, H., and Ju, Y. (2016). Effect of compound Kushen injection on T-cell subgroups and natural killer cells in patients with locally advanced non-small-cell lung cancer treated with concomitant radiochemotherapy. J. Tradit. Chin. Med. 36 (1), 14–18. doi:10.1016/s0254-6272(16)30002-4
Zhong, L., and Zhao, Z. (2015). Effect of anti-VEGF in the treatment of malignant ascites. J. Dig. Oncol. Version) 7 (03), 165–170.
Zhou, L. (2022). Clinical study of compound kushen injection combined with carboplatin intraperitoneal perfusion in the treatment of malignant peritoneal effusion. Liaoning J. Tradit. Chin. Med. 49 (02), 131–132. doi:10.13192/j.issn.1000-1719.2022.02.037
Zhou, S. K., Zhang, R. L., Xu, Y. F., and Bi, T. N. (2012). Antioxidant and immunity activities of fufang kushen injection liquid. Molecules 17 (6), 6481–6490. doi:10.3390/molecules17066481
Zhou, W., Wu, C., Zhao, C., Huang, Z., Lu, S., Fan, X., et al. (2021). An advanced systems pharmacology strategy reveals AKR1B1, MMP2, PTGER3 as key genes in the competing endogenous RNA network of compound kushen injection treating gastric carcinoma by integrated bioinformatics and experimental verification. Front. Cell Dev. Biol. 9, 742421. doi:10.3389/fcell.2021.742421
Keywords: compound kushen injection, malignant ascites, intraperitoneal chemotherapy, meta-analysis, botanical drug
Citation: Yu H-B, Hu J-Q, Han B-J, Cao H-J, Chen S-T, Chen X, Xiong H-T, Gao J, Du Y-Y and Zheng H-G (2023) Evaluation of efficacy and safety for compound kushen injection combined with intraperitoneal chemotherapy for patients with malignant ascites: A systematic review and meta-analysis. Front. Pharmacol. 14:1036043. doi: 10.3389/fphar.2023.1036043
Received: 03 September 2022; Accepted: 23 February 2023;
Published: 03 March 2023.
Edited by:
George Qian Li, Australian Eureka Bee Products, AustraliaReviewed by:
Ning Liang, China Academy of Chinese Medical Sciences, ChinaCopyright © 2023 Yu, Hu, Han, Cao, Chen, Chen, Xiong, Gao, Du and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Gang Zheng, aG9uZ2dhbmd6aGVuZ0AxMjYuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.