
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol. , 12 January 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1026135
Background: Lung cancer is a major public health issue and an enormous burden on society in China. Most lung cancers occur in elderly patients with non-small cell lung cancer (NSCLC), and many factors limit their treatment options. Chemotherapy-free therapy can avoid psychological fear, treatment pain, and adverse reactions caused by chemotherapy. Patients with non-small cell lung cancer with tumour protein p53 (TP53) gene mutations or Kirsten rat sarcoma viral oncogene homologue (KRAS) gene mutations tend to be more sensitive to anlotinib or programmed cell death protein 1 (PD-1) drugs. However, Kirsten rat sarcoma viral oncogene homologue is a proto-oncogene downstream of the epidermal growth factor receptor (EGFR) gene; therefore, if the Kirsten rat sarcoma viral oncogene homologue gene has an activating mutation, EGFR-targeted drug resistance may occur. Further studies are needed to explore whether patients with dual Kirsten rat sarcoma viral oncogene homologue and tumour protein p53 mutations can be treated with targeted immunotherapy without chemotherapy.
Case presentation: A 74-year-old man was referred to the Lanzhou University Second Hospital due to chest tightness, shortness of breath, and weight loss for 2 months and was diagnosed with moderately to poorly differentiated adenocarcinoma. Laboratory examinations showed increased alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), cancer antigen (CA)-125, and CA199 levels, and gene sequencing indicated mutations in Kirsten rat sarcoma viral oncogene homologue and tumour protein p53. Immunohistochemical analysis showed positive PD-L1 and PD-1 expression. Peripheral blood immune checkpoint test using flow cytometry indicated that the PD-1 + CD8 levels were positive. After multi-disciplinary treatment, therapy with a combination of anlotinib and camrelizumab was initiated. Camrelizumab 200 mg was administered intravenously once every 3 weeks. Anlotinib 12 mg was administered orally daily before breakfast for 2 weeks with a week of rest in every cycle of 21 days. A reduction in alpha-fetoprotein, carcinoembryonic antigen, CA125, CA199, and CA724 levels was observed up to the first cycle, which decreased within the normal limits up to the second cycle and continued until the eighteenth cycle. The patient’s chest tightness, shortness of breath, weight loss, and other symptoms significantly improved following treatment. Computed tomography imaging showed that the neoplastic lesion was dramatically reduced. The patient is currently being followed-up for more than 2 years to evaluate the duration of the response.
Conclusion: Chemotherapy-free immunotherapy combined with targeted therapy is an effective treatment for advanced non-small cell lung cancer in elderly patients with Kirsten rat sarcoma viral oncogene homologue and tumour protein p53 mutations. Such therapies should be supported with further clinical studies with larger sample sizes.
Lung cancer ranks second in incidence and first in mortality worldwide (Sung et al., 2021), with non-small cell lung cancer (NSCLC) comprising 85% of all lung cancer cases (Govindan et al., 2006). Surgery, chemoradiotherapy, chemotherapy, immunotherapy, and targeted drugs are common treatment strategies (Gridelli et al., 2015). Elderly patients make up a large proportion of patients with lung cancer; their age, physical condition, and possible postoperative complications can significantly limit treatment options. A comprehensive assessment of the overall situation of patients aged ≥70 years using effective tools is required. It is worth mentioning that chemotherapy-free status can eliminate psychological fear, treatment pain, and chemotherapy-related adverse reactions in patients.
In cancer progression, the PD-L1/PD-1 axis plays an important role in the immune evasion mechanisms of cancer cells by regulating T cell activity (Pardoll, 2012). Immune checkpoint inhibitors (ICI) targeting PD-1, including nivolumab (Borghaei et al., 2015), pembrolizumab (Herbst et al., 2016), and camrelizumab (Jiangsu Hengrui Pharmaceuticals Co., Ltd.) (Zhou et al., 2021), have become effective treatment options for patients with NSCLC (Borghaei et al., 2015; Herbst et al., 2016; Zhou et al., 2021). Nivolumab significantly improved overall survival (OS) in patients with advanced NSCLC in two phase III trials (median OS, nivolumab vs. docetaxel: 12.2 months vs. 9.4 months) (Borghaei et al., 2015; Brahmer et al., 2015). Pembrolizumab also prolonged OS in the aforementioned patients, with at least 1% PD-L1 expression in tumour cells (median OS, pembrolizumab vs. docetaxel: 12.7 months vs. 8.5 months) (Herbst et al., 2016). Apatinib combined with camrelizumab demonstrated potent antitumour activity and acceptable toxicity in patients with advanced NSCLC as a second-line treatment (OS, 15.5 months; 95% CI, 10.9–24.5) (Zhou et al., 2021). Moreover, studies have shown that ICI efficacy in patients with Kirsten rat sarcoma viral oncogene homologue (KRAS) mutant NSCLC is similar to that in patients with other NSCLCs. PD-L1 expression is more predictive of ICI efficacy in patients with KRAS-mutant NSCLC than in those with other NSCLC (Jeanson et al., 2019). Immunotherapy has shown promising results for patients with NSCLC. However, the population that can benefit from this treatment is small. Hence, selecting the dominant population for specific immunotherapy is a matter of utmost importance.
Anlotinib (Focus V® Jiangsu Chia Tai-Tianqing Pharmaceutical Co., Ltd.) is an oral small-molecule inhibitor of multiple receptor tyrosine kinases that has a broad inhibitory effect on tumour angiogenesis and growth (Sun et al., 2016). Anlotinib prolongs OS and is well-tolerated in Chinese patients with advanced NSCLC treated with third-line therapy (median OS, anlotinib vs. placebo: 9.6 vs. 6.3 months) (Han et al., 2018). Furthermore, patients with advanced NSCLC and tumour protein p53 (TP53) mutations treated with anlotinib showed longer progression-free survival (Fang et al., 2020). TP53 mutations may be a biomarker for the selection of anlotinib therapy.
KRAS mutations are found in approximately 10%–15% of cases of NSCLC in Asia (Dearden et al., 2013; Yoshizawa et al., 2013). KRAS is the most frequently activated oncogene in NSCLC, and KRAS-mutant lung cancers have generally been associated with poorer OS than KRAS wild-type tumours (Mascaux et al., 2005; Johnson et al., 2013; Marabese et al., 2015; Jordan et al., 2017). Mutations in the tumour suppressor gene TP53 are also common in lung cancer and are often accompanied by KRAS mutation (Ding et al., 2008; Pao and Girard, 2011; Gibbons et al., 2014). TP53 and KRAS genes were found to have significant effects on PD-L1 expression, infiltration of immune T cells, and enhancement of tumour immunogenicity (Dong et al., 2017). The clinical efficacy of PD-1 inhibitors is significant in patients with TP53 or KRAS mutations and TP53/KRAS mutations (Dong et al., 2017). In patients with TP53/KRAS co-mutation, the response rate of patients to anti-PD-1 is up to 57% (median progression-free survival (PFS), TP53 and KRAS vs. TP53 vs. KRAS vs. wild-type: 30 vs. 14.5 vs. 14.7 vs. 3.5 months) (Dong et al., 2017). TP53 and KRAS mutations in patients with NSCLC may be effective predictors of immunotherapy efficacy.
Here, we report an elderly patient with KRAS and TP53-mutated advanced NSCLC adenocarcinoma for whom camrelizumab and anlotinib therapy was administered.
On 28 April 2020, a 74-year-old man was referred to our hospital with complaints of chest tightness, shortness of breath, and weight loss for 2 months. Computed tomography (CT) (Figure 1A) revealed a mass in the left lung with bilateral adrenal invasion (Figure 1B) and right neck lymph node invasion (Figure 1C). AFP (8.95 ng/ml; normal value < 7.00), CEA (15.03 ng/ml; normal value < 3.40), CA125 (175.10 U/ml; normal value < 35.00), CA199 (79.27 U/ml; normal value < 27.00), and CA724 (6.03 U/ml; normal value < 6.90) levels were abnormal (Supplementary Figure S1). The patient underwent endobronchial ultrasonography-guided transbronchial needle aspiration, and the tumour was diagnosed as a moderately to poorly differentiated adenocarcinoma (90% for acinar adenocarcinoma; 10% for solid adenocarcinoma; T4N3M1 stage IV) (Figure 2A) with mutations in KRAS and TP53 (Table 1). The former mutation was a missense mutation in exon two of the gene, which is predicted to have led to the functional activation of its encoded protein, whereas the TP53 mutation was a splicing mutation in the intron of this gene, which was predicted to have caused the inactivation of protein function. Results of the immunohistochemical analysis showed that the PD-L1 expression score (SP263) in tumour cells was 0.8% and PD-1 staining (Servicebio: GB12338) of CD8 cells in tumor lesions was positive (Figure 2B). The results of peripheral blood immune checkpoint tests demonstrated that the proportions of LAG3+CD8, TIM3+CD8, PD-1+CD8, and CD3-CD19-CD14+CD16-HLA-DR levels were higher than the reference range (Supplementary Table S1).

FIGURE 1. Changes in lung tumor and metastatic tumor during camrelizumab and anlotinib treatment. (A) CT showed that the lung tumor began to shrink after first cycle of treatment until the end of treatment. (B) CT showed that the volume of bilateral adrenal invasion lesions was significantly reduced after first cycle of treatment and disappeared after that. (C) CT showed that the volume of the right neck invasion lymph node lesion was significantly reduced to normal after three cycles until the end of treatment.
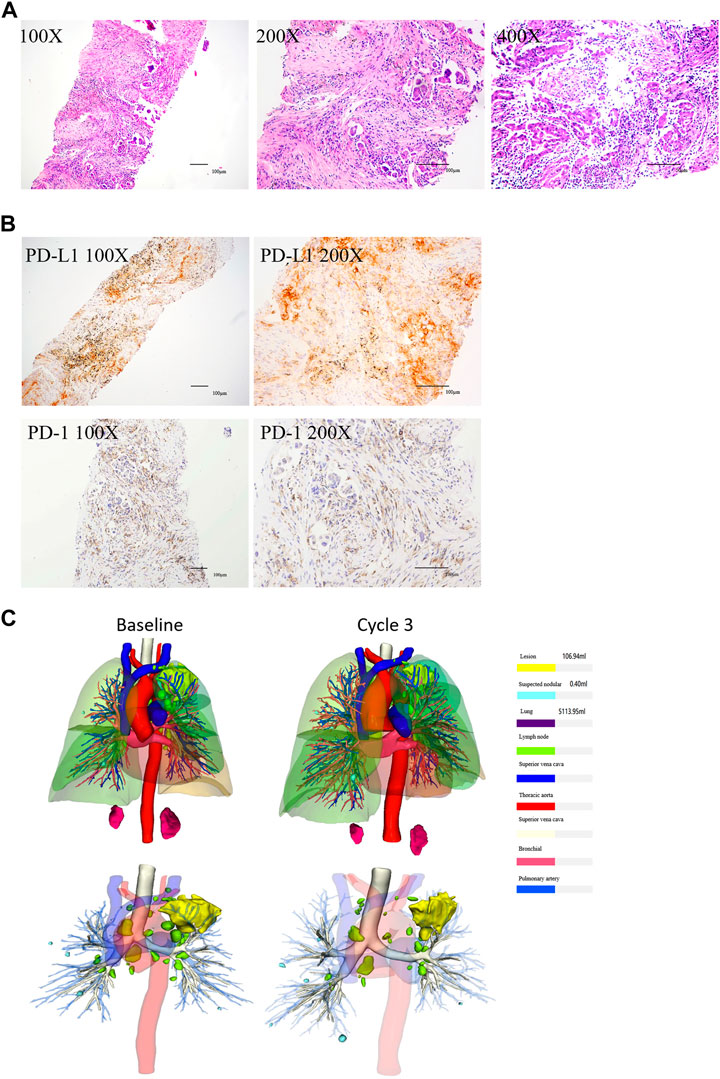
FIGURE 2. HE and IHC of NSCLC tissues, and the three-dimensional reconstruction of tumor. (A) HE showed that the tissues were moderately and poorly differentiated adenocarcinoma (From left to right: ×100 magnification, ×200 magnification, and ×400 magnification). (B) IHC showed that the expression of PD-L1 and PD-1 were positive (Left: ×100 magnification, right: ×200 magnification). (C) Three-dimensional reconstruction showed that the tumor shrinked after the third cycle.
Since the patient was elderly with poor constitution, and his family members refused chemotherapy, and given that the patient had driver gene TP53 and KRAS mutations, it was recommended through multi-disciplinary discussion that immunotherapy combined with targeted therapy should be administered. Consequently, the patient was treated with a combination of camrelizumab and anlotinib. Camrelizumab (200 mg) was administered intravenously once every 3 weeks. Anlotinib (12 mg) was administered orally every day before breakfast for 2 weeks with a week’s rest in every cycle of 21 days. The patient provided written informed consent for treatment.
After one cycle of combination therapy, AFP, CEA, CA125, CA199, and CA724 serum levels decreased to 6.22, 10.17 ng/ml, 148.20, 42.84 U/ml, and 1.26 ng/ml, respectively (Supplementary Figure S1). Three-phase multidetector CT highlighted a marked dimensional reduction of infiltrating focality in the left lung (from 168.48 mm3 to 85.14 mm3) (Figure 1A) and adrenal glands (Figure 1B). Furthermore, the patient’s chest tightness, shortness of breath, weight loss, and other symptoms significantly improved. To evaluate the efficacy more clearly, we used a three-dimensional reconstruction system (Hubei Inlook Technology Co., Ltd.) to precisely measure the volume of the lesion and found that the tumour size significantly decreased after treatment (Figure 2C). To evaluate safety, changes in blood cell counts (Supplementary Table S2; Figure 3A), liver function (Supplementary Table S3; Figure 3B), and thyroid function (Supplementary Table S4; Figure 3C) were detected and are shown. Changes in pancreatic function are shown in Figure 2.
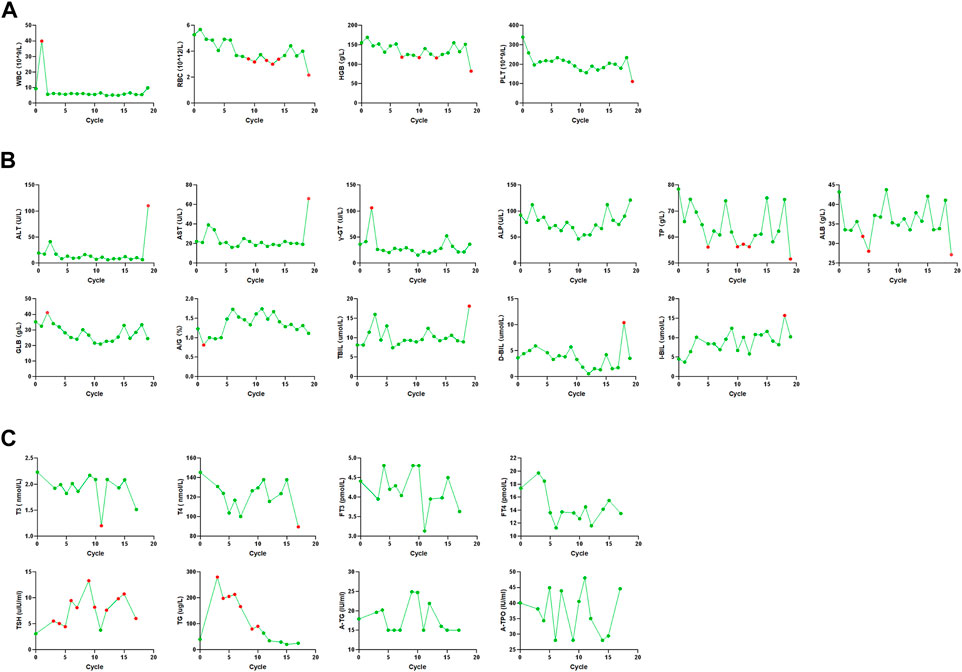
FIGURE 3. Changes in blood cell counts, liver function, and thyroid function. (A) Blood cell counts was almost within normal limits until the end of treatment. (B) Liver function was almost within normal limits until the end of treatment. (C) Thyroid function was almost within normal limits until the end of treatment.
Treatment continued up to 18 cycles (till 26 November 2021). CT demonstrated a good response with the reduction of mass in the lung (Figure 1A), adrenal (Figure 1B), and right neck lymph node invasion (Figure 1C). The tumour marker values remained within the normal range (Supplementary Figure S1). Three-dimensional reconstruction revealed a marked reduction in infiltrating focality in the left lung (Figure 2C). Blood cell counts (Supplementary Table S2; Figure 3A), liver function (Supplementary Table S3; Figure 3B), thyroid function (Supplementary Table S4; Figure 3C), and pancreatic function (Supplementary Figure S1) were nearly within normal limits.
After cycle 19 (2021.12.30), the patient did not visit the hospital on time due to personal reasons. Furthermore, AFP and CA724 levels were elevated. Blood cell counts (Supplementary Table S2; Figure 3A), liver function (Supplementary Table S3; Figure 3B), and thyroid function (Supplementary Table S4; Figure 3C) were not within normal limits. The patient developed elevated blood pressure, fluid and electrolyte disturbances, systemic infections, and liver, thyroid, and pancreatic dysfunctions. This condition is mainly due to the side effects of drugs. Treatment ended owing to subjective and objective reasons. The patient is still being followed-up. The therapeutic process is shown in Figure 4.
During the treatment, the patient had good compliance, no serious adverse reactions, and the minor adverse reactions were acceptable, manageable, and improved after symptomatic treatment. The study was reviewed and approved by the Institutional Ethics Committee of the Lanzhou University Second Hospital.
The main first-line treatment for advanced NSCLC is chemotherapy, which mainly includes paclitaxel and gemcitabine. This case achieved a good treatment outcome without chemotherapy, indicating that chemotherapy is not necessary for some patients. The latest research showed that the objective response rate, disease control rate, median PFS, and 12-month OS rate of sindilizumab combined with anlotinib as a first-line therapy were 72.7%, 100%, 15 months, and 95.5% (Chu et al., 2021), respectively, effectively improving the survival time and life quality of first-line lung cancer patients and truly achieving a “chemotherapy-free” status. The results of this study are consistent with those of ours. However, individual cases may not be appropriate for the general patient.
Generally, the toxicity associated with treatment increases with age (Movsas et al., 1999; Atagi et al., 2012; Dawe et al., 2016). However, age is not the only limitation that excludes patients from the most effective treatment; other factors such as comorbidities, social and personal circumstances, and performance need to be collectively assessed (Ng et al., 2005). Simultaneously, clinical trials are being conducted to evaluate older patients (≥75 years of age) with stage III NSCLC, including frail, vulnerable, and healthy older patients, with quality-adjusted survival as the primary endpoint (Driessen et al., 2018). Considering the complex physical condition of elderly patients, multi-disciplinary evaluation is strongly recommended for all elderly NSCLCs patients. Chemotherapy-free treatment can reduce the physical burden of treatment, prolong survival, and the improve quality of life in elderly patients; thus, it is recommended for elderly patients with poor tolerance.
PD-1/PD-L1 antibodies significantly improved the rate of durable response. It also prolongs long-term survival with advanced NSCLC (Xia et al., 2019), with limited adverse effects. However, the overall objective response rate of second-line therapy was less than 20%, and the progression-free survival (PFS) was similar or worse than that of conventional second-line chemotherapy (Borghaei et al., 2015; Herbst et al., 2016; Rittmeyer et al., 2017). Patients who are not expected to benefit from monotherapy with PD-1/PD-L1 antibodies, such as patients with PD-L1 negative tumors or those refractory to first-line therapy, should be treated in combination. Recent studies have shown that anlotinib can change the tumor immune microenvironment by down-regulating the expression of PD-L1 on vascular endothelial cells (Liu et al., 2020). Antiangiogenic drugs can enhance tumor immune response, whereas immune checkpoint inhibitors can normalize blood vessels in the tumor microenvironment (Vanneman and Dranoff, 2012; Ciciola et al., 2020). Furthermore, preclinical studies have shown that the combination of immunotherapy and anti-angiogenic drugs can reprogram the tumour microenvironment and enhance anti-tumour efficacy by inhibiting tumour angiogenesis, promoting vascular normalisation and increasing tumour T lymphocytes infiltration (Manegold et al., 2017). Recent studies have shown that the results of sintilimab in combination with anlotinib as a first-line treatment for advanced NSCLC showed that the objective response rate was 72.7%, and the disease control rate was 100% (Chu et al., 2021). Anti-angiogenic drugs combined with immunotherapy have a synergistic effect in the treatment of various cancers, including advanced NSCLC (Manegold et al., 2017). In this case, we analyzed the efficacy and safety of anlotinib combined with camrelizumab (a PD-1 inhibitor) in the treatment of advanced NSCLC patients, and explored the synergistic effect of antiangiogenic agents and immunotherapy, both of which showed favorable results.
However, some patients may develop adaptive resistance to current immunotherapy regimen and many patients do not respond to this treatment. Combined with relevant references and Supplementary Table S1, the anti-LAG-3 antibody or an antibody cocktail (anti-LAG-3, anti-TIM-3 and anti-PD-1) may improve clinical outcomes to overcome immune resistance (Baumeister et al., 2016). Studies on patients with NSCLC have found that Treg cells expressing LAG-3 produce high levels of immunoregulatory cytokines IL-10 and TGF-β, and inhibit the activation of TIL (Camisaschi et al., 2010). LAG-3 binds to its ligand to form an immune checkpoint pathway independent of PD-1/PD-L1, leading to T cell dysfunction and tumor cell evasion of immune surveillance (Camisaschi et al., 2010). Preclinical cancer treatment models using LAG-3 blocking show that antigen-specific T cells at the tumor site are activated, tumor growth is inhibited, and tumor parenchyma is destroyed (Grosso et al., 2007). However, some studies have shown that although mice lacking LAG-3 alone will not produce spontaneous autoimmunity, mice lacking both LAG-3 and PD-1 will produce fatal systemic autoimmunity (Okazaki et al., 2011; Woo et al., 2012), which highlights the synergy of these two approaches in controlling T cell tolerance. T cells in tumor and chronic viral infection usually coexpress PD-1 and LAG-3, and the therapeutic effect of blocking PD-1 and LAG-3 jointly in tumor models is greater than that of blocking alone (Blackburn et al., 2009; Matsuzaki et al., 2010; Woo et al., 2012). Currently, clinical trials related to the treatment of NSCLC with LAG-3 antibody are ongoing [IBI110, IMP321]. TIM-3 is a promising inhibitory receptor among many emerging immune checkpoints. TIM-3 is expressed on CD4+ TH1 helper T cells and CD8+ Tc1 cytotoxic T cells, and is associated with the prognosis of various cancers, including NSCLC (Das et al., 2017). It has been reported that up-regulation of TIM-3 in NSCLC patients is one of the mechanisms of adaptive resistance to PD-1 inhibitor (Koyama et al., 2016). Preclinical studies in mouse tumor models have shown that anti-TIM-3 monotherapy can shrink tumors, and its combination with anti-PD-1 or anti-PD-L1 significantly reduces tumor load and improves anti-tumor immune response (Zhu et al., 2005; Ngiow et al., 2011). Several NSCLC drugs targeting TIM-3 (Cobolimab, INCAGN-02390, RO7121661, BGB-A425) are currently in clinical trials as monotherapy or in combination with anti-PD-1/PD-L1 drugs.
Why do only a few people benefit from targeted therapy? Among the factors used for assessment, biological markers may be the most important. The present case report describes a patient with advanced NSCLC involving a KRAS and TP53 mutant, treated with camrelizumab and anlotinib. Gene detection and evaluation have extensively supported the efficacy of immunotherapy and targeted therapy in the absence of chemotherapy. Activation of KRAS mutations is reported to be considered as a driver of tumour progression. KRAS has been reported to be associated with increases in tumour-infiltrating lymphocytes, PD-L1 expression, and tumor mutational burden (Lee et al., 2018). There is evidence that KRAS mutation is a genetic marker of the benefit of emerging direct inhibitors and is combined with immunotherapy during clinical development (Adderley et al., 2019). TP53 mutations are associated with genomic stability and defects in DNA damage repair (Soussi and Beroud, 2001). One study illustrated a significant response to anlotinib in patients with advanced NSCLC with TP53 mutations (Fang et al., 2020). Dong et al. (2017) found that the TP53/KRAS co-mutation led to an increased PD-L1+/CD8A + ratio and longer PFS with PD-1 inhibitors (NCT01295827) (Cortez MA et al., 2015; Ji et al., 2016). In our case, PD-L1 expression was also positive and the proportions of LAG3+CD8, TIM3+CD8, PD-1+CD8, and CD3-CD19-CD14+CD16-HLA-DR levels were higher than the reference range. The patient in our case achieved encouraging efficacy with mutations in TP53 and KRAS, further proving that these two genes can be used as indicators for immunotherapy and targeted therapy.
Unlike side effects such as rash, elevated blood pressure, liver dysfunction, and diarrhoea caused by targeted therapy, immunotherapy can produce immune-related side effects, such as fluid and electrolyte disturbances, immune pneumonia, immune hepatitis, systemic infections, and liver, thyroid, and pancreas dysfunction (Borghaei et al., 2015; Brahmer et al., 2015; Herbst et al., 2016; Rittmeyer et al., 2017). Furthermore, targeted therapy may cause tumours to develop drug resistance, and long-term application may reduce its efficacy. During treatment, the adverse reactions caused by these two drugs were tolerable. After 19 cycles, the patient did not attend the hospital on time for personal reasons and developed a series of adverse reactions, and the treatment was, therefore, ended. Generally, combining immunisation and targeted therapy significantly prolongs patient survival with a manageable safety profile.
To the best of our knowledge, there are few studies on PD-1 antibodies combined with targeted agents in patients with first-line NSCLC who did not receive chemotherapy. In view of its encouraging efficacy, durability, and safety profile, camrelizumab plus anlotinib represents a novel chemotherapy-free regimen for elderly patients with TP53 and KRAS mutations.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of Lanzhou University Second Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HC developed the idea and revised the paper; WQ wrote the manuscript; all authors read, revised and approved the final manuscript.
This study was supported by National Natural Science Foundation of China (No. 82160129); Key Talents Project of Gansu Province (No. 2019RCXM020); Key Project of Science and Technology in Gansu province (19ZD2WA001); Key Project of Science and Technology in Gansu province (22ZD6FA054); Science and Technology Project of Chengguan District of Lanzhou City (2020SHFZ0039); Science and Technology Project of Chengguan District of Lanzhou City (2020JSCX0073); Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (No. CY2017-ZD01); Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2020-BJ06); Medical Innovation and Development Project of Lanzhou University (lzuyxcx-2022-160); Medical Innovation and Development Project of Lanzhou University (lzuyxcx-2022-45); Medical Innovation and Development Project of Lanzhou University (lzuyxcx-2022-88).
We would like to express our gratitude to Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1026135/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | A change of tumor markers and amylase level. The levels of AFP, CEA, CA125, CA199 and CA724 decreased to normal levels after the second treatment cycle and continued to the 19th cycle; The levels of AMY and AMY-U decreased to normal levels after the second treatment cycle until cycle 15.
Adderley, H., Blackhall, F. H., and Lindsay, C. R. (2019). KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. EBioMedicine 41, 711–716. doi:10.1016/j.ebiom.2019.02.049
Atagi, S., Kawahara, M., Yokoyama, A., Okamoto, H., Yamamoto, N., Ohe, Y., et al. (2012). Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: A randomised, controlled, phase 3 trial by the Japan clinical oncology group (JCOG0301). Lancet Oncol. 13, 671–678. doi:10.1016/S1470-2045(12)70139-0
Baumeister, S. H., Freeman, G. J., Dranoff, G., and Sharpe, A. H. (2016). Coinhibitory pathways in immunotherapy for cancer. Annu. Rev. Immunol. 34, 539–573. doi:10.1146/annurev-immunol-032414-112049
Blackburn, S. D., Shin, H., Haining, W. N., Zou, T., Workman, C. J., Polley, A., et al. (2009). Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10, 29–37. doi:10.1038/ni.1679
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., et al. (2015). Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639. doi:10.1056/NEJMoa1507643
Brahmer, J., Reckamp, K. L., Baas, P., Crinò, L., Eberhardt, W. E., Poddubskaya, E., et al. (2015). Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373, 123–135. doi:10.1056/NEJMoa1504627
Camisaschi, C., Casati, C., Rini, F., Perego, M., De Filippo, A., Triebel, F., et al. (2010). LAG-3 expression defines a subset of CD4(+) CD25(high)Foxp3(+) regulatory T cells that are expanded at tumor sites. J. Immunol. 184, 6545–6551. doi:10.4049/jimmunol.0903879
Chu, T. Q., Zhong, R. B., Zhong, H., Zhang, B., Zhang, W., Shi, C. L., et al. (2021). Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J. Thorac. Oncol. 16, 643–652. doi:10.1016/j.jtho.2020.11.026
Ciciola, P., Cascetta, P., Bianco, C., Formisano, L., and Bianco, R. (2020). Combining immune checkpoint inhibitors with anti-angiogenic agents. J. Clin. Med. 9, 675–740. doi:10.3390/jcm9030675
Cortez, M. A., Ivan, C., Valdecanas, D., Wang, X., Peltier, H. J., Ye, Y., et al. (2015). PD-L1 Regulation by p53 via miR-34. J. Natl. Cancer Inst. 108, djv303–544. doi:10.1093/jnci/djv303
Das, M., Zhu, C., and Kuchroo, V. K. (2017). Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 276, 97–111. doi:10.1111/imr.12520
Dawe, D. E., Christiansen, D., Swaminath, A., Ellis, P. M., Rothney, J., Rabbani, R., et al. (2016). Chemoradiotherapy versus radiotherapy alone in elderly patients with stage III non-small cell lung cancer: A systematic review and meta-analysis. Lung Cancer 99, 180–185. doi:10.1016/j.lungcan.2016.07.016
Dearden, S., Stevens, J., Wu, Y. L., and Blowers, D. (2013). Mutation incidence and coincidence in non-small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann. Oncol. 24, 2371–2376. doi:10.1093/annonc/mdt205
Ding, L., Getz, G., Wheeler, D. A., Mardis, E. R., McLellan, M. D., Cibulskis, K., et al. (2008). Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455, 1069–1075. doi:10.1038/nature07423
Dong, Z. Y., Zhong, W. Z., Zhang, X. C., Su, J., Xie, Z., Liu, S. Y., et al. (2017). Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin. Cancer Res. 23, 3012–3024. doi:10.1158/1078-0432.CCR-16-2554
Driessen, E. J. M., Janssen-Heijnen, M. L. G., Maas, H. A., Dingemans, A. C., and van Loon, J. G. M. (2018). Study protocol of the NVALT25-ELDAPT trial: Selecting the optimal treatment for older patients with stage III non-small-cell lung cancer. Clin. Lung Cancer 19, e849–e852. doi:10.1016/j.cllc.2018.07.003
Fang, S., Cheng, W., Zhang, M., and Yang, R. (2020). Association of TP53 mutations with response to anlotinib treatment in advanced non-small cell lung cancer. Onco Targets Ther. 13, 6645–6650. doi:10.2147/OTT.S257052
Gibbons, D. L., Byers, L. A., and Kurie, J. M. (2014). Smoking, p53 mutation, and lung cancer. Mol. Cancer Res. 12, 3–13. doi:10.1158/1541-7786.MCR-13-0539
Govindan, R., Page, N., Morgensztern, D., Read, W., Tierney, R., Vlahiotis, A., et al. (2006). Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol. 24, 4539–4544. doi:10.1200/JCO.2005.04.4859
Gridelli, C., Rossi, A., Carbone, D. P., Guarize, J., Karachaliou, N., Mok, T., et al. (2015). Non-small-cell lung cancer. Nat. Rev. Dis. Prim. 1, 15009–15024. doi:10.1038/nrdp.2015.9
Grosso, J. F., Kelleher, C. C., Harris, T. J., Maris, C. H., Hipkiss, E. L., De Marzo, A., et al. (2007). LAG-3 regulates CD8+ T cell accumulation and effector function in murine selfandtumor-tolerance systems. J. Clin. Investigation 117, 3383–3392. doi:10.1172/JCI31184
Han, B. H., Li, K., Wang, Q. M., Zhang, L., Shi, J., Wang, Z., et al. (2018). Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: The ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 4, 1569–1575. doi:10.1001/jamaoncol.2018.3039
Herbst, R. S., Baas, P., Kim, D. W., Felip, E., Pérez-Gracia, J. L., Han, J. Y., et al. (2016). Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387, 1540–1550. doi:10.1016/S0140-6736(15)01281-7
Jeanson, A., Tomasini, P., Souquet-Bressand, M., Brandone, N., Boucekine, M., Grangeon, M., et al. (2019). Efficacy of immune checkpoint inhibitors in KRAS-mutant non-small cell lung cancer (NSCLC). J. Thorac. Oncol. 14, 1095–1101. doi:10.1016/j.jtho.2019.01.011
Ji, M., Liu, Y., Li, Q., Li, X., Ning, Z., Zhao, W., et al. (2016). PD-1/PD-L1 expression in non-small-cell lung cancer and its correlation with EGFR/KRAS mutations. Cancer Biol. Ther. 17, 407–413. doi:10.1080/15384047.2016.1156256
Johnson, M. L., Sima, C. S., Chaft, J., Paik, P. K., Pao, W., Kris, M. G., et al. (2013). Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinomas. Cancer 119, 356–362. doi:10.1002/cncr.27730
Jordan, E. J., Kim, H. R., Arcila, M. E., Barron, D., Chakravarty, D., Gao, J. J., et al. (2017). Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov. 7, 596–609. doi:10.1158/2159-8290.CD-16-1337
Koyama, S., Akbay, E. A., Li, Y. Y., Herter-Sprie, G. S., Buczkowski, K. A., Richards, W. G., et al. (2016). Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 17, 10501–10509. doi:10.1038/ncomms10501
Lee, C. K., Man, J., Lord, S., Cooper, W., Links, M., Gebski, V., et al. (2018). Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non small cell lung carcinoma. A systematic review and meta-analysis. JAMA Oncol. 4, 210–216. doi:10.1001/jamaoncol.2017.4427
Liu, S., Qin, T., Liu, Z., Wang, J., Jia, Y., Feng, Y., et al. (2020). Anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis. 11, 309–337. doi:10.1038/s41419-020-2511-3
Manegold, C., Dingemans, A. C., Gray, J. E., Nakagawa, K., Nicolson, M., Peters, S., et al. (2017). The potential of combined immunotherapy and Antiangiogenesis for the synergistic treatment of advanced NSCLC. J. Thorac. Oncol. 12, 194–207. doi:10.1016/j.jtho.2016.10.003
Marabese, M., Ganzinelli, M., Garassino, M. C., Shepherd, F. A., Piva, S., Caiola, E., et al. (2015). KRAS mutations affect prognosis of non-small-cell lung cancer patients treated with first-line platinum containing chemotherapy. Oncotarget 6, 34014–34022. doi:10.18632/oncotarget.5607
Mascaux, C., Iannino, N., Martin, B., Paesmans, M., Berghmans, T., Dusart, M., et al. (2005). The role of RAS oncogene in survival of patients with lung cancer: A systematic review of the literature with meta-analysis. Br. J. Cancer 92, 131–139. doi:10.1038/sj.bjc.6602258
Matsuzaki, J., Gnjatic, S., Mhawech-Fauceglia, P., Beck, A., Miller, A., Tsuji, T., et al. (2010). Tumor-infiltrating NYESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. PNAS 107, 7875–7880. doi:10.1073/pnas.1003345107
Movsas, B., Scott, C., Sause, W., Byhardt, R., Komaki, R., Cox, J., et al. (1999). The benefit of treatment intensification is age and histology dependent in patients with locally advanced non-small cell lung cancer (NSCLC): A quality-adjusted survival analysis of radiation therapy oncology group (RTOG) chemoradiation studies. Int. J. Radiat. Oncol. Biol. Phys. 45, 1143–1149. doi:10.1016/s0360-3016(99)00325-9
Ng, R., Boer, R. D., and Green, M. D. (2005). Undertreatment of elderly patients with non small-cell lung cancer. Clin. Lung Cancer 7, 168–174. doi:10.3816/CLC.2005.n.031
Ngiow, S. F., von Scheidt, B., Akiba, H., Yagita, H., Teng, M. W., Smyth, M. J., et al. (2011). Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 71, 3540–3551. doi:10.1158/0008-5472.CAN-11-0096
Okazaki, T., Okazaki, I. M., Wang, J., Sugiura, D., Nakaki, F., Yoshida, T., et al. (2011). PD-1 and LAG-3 inhibitory coreceptors act synergistically to prevent autoimmunity in mice. J. Exp. Med. 208, 395–407. doi:10.1084/jem.20100466
Pao, W., and Girard, N. (2011). New driver mutations in non-small-cell lung cancer. Lancet Oncol. 12, 175–180. doi:10.1016/S1470-2045(10)70087-5
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264. doi:10.1038/nrc3239
Rittmeyer, A., Barlesi, F., Waterkamp, D., Park, K., Ciardiello, F., von Pawel, J., et al. (2017). Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 389, 255–265. doi:10.1016/S0140-6736(16)32517-X
Soussi, T., and Beroud, C. (2001). Assessing TP53 status in human tumours to evaluate clinical outcome. Nat. Rev. Cancer 535, 233–240. doi:10.1038/35106009
Sun, Y., Niu, W., Du, F., Du, C. X., Li, S., Wang, J. W., et al. (2016). Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J. Hematol. Oncol. 9, 105–110. doi:10.1186/s13045-016-0332-8
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Vanneman, M., and Dranoff, G. (2012). Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12, 237–251. doi:10.1038/nrc3237
Woo, S. R., Turnis, M. E., Goldberg, M. V., Bankoti, J., Selby, M., Nirschl, C. J., et al. (2012). Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72, 917–927. doi:10.1158/0008-5472.CAN-11-1620
Xia, L., Liu, Y., and Wang, Y. (2019). PD-1/PD-L1 blockade therapy in advanced non-small-cell lung cancer: Current status and future directions. Oncologist 24, S31–S41. doi:10.1634/theoncologist.2019-IO-S1-s05
Yoshizawa, A., Sumiyoshi, S., Sonobe, M., Kobayashi, M., Fujimoto, M., Kawakami, F., et al. (2013). Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: Analysis of 440 Japanese patients. J. Thorac. Oncol. 8, 52–61. doi:10.1097/JTO.0b013e3182769aa8
Zhou, C., Wang, Y., Zhao, J., Chen, G., Liu, Z., Gu, K., et al. (2021). Efficacy and biomarker analysis of camrelizumab in combination with apatinib in patients with advanced nonsquamous NSCLC previously treated with chemotherapy. Clin. Cancer Res. 27, 1296–1304. doi:10.1158/1078-0432.CCR-20-3136
Keywords: NSCLC, camrelizumab, anlotinib, KRAS, TP53, chemotherapy-free, case report
Citation: Qi W, Xi D, Bai Y, Liu L, Ma Y, Yin Z and Chen H (2023) Case Report: Chemotherapy-free treatment with camrelizumab and anlotinib for elderly patients with KRAS and TP53 mutated advanced lung cancer. Front. Pharmacol. 14:1026135. doi: 10.3389/fphar.2023.1026135
Received: 23 August 2022; Accepted: 05 January 2023;
Published: 12 January 2023.
Edited by:
Jianqiang Xu, Dalian University of Technology, ChinaReviewed by:
Ning Su, Guangzhou Chest Hospital, ChinaCopyright © 2023 Qi, Xi, Bai, Liu, Ma, Yin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Chen, ZXJ5X2NoZW5oQGx6dS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.