- 1Intensive Care Unit, Lanzhou University First Affiliated Hospital, Lanzhou, China
- 2Clinical Medicine Department, First Clinical Medical Academy, Lanzhou University, Lanzhou, China
- 3Critical Care Department, Gansu Provincial Maternal and Child Health Hospital, Lanzhou, China
Background: No consensus exists on the antibiotic treatment course for patients with acute exacerbations of chronic obstructive pulmonary disease (AECOPD). Former studies indicate that shorter courses might have the same efficacy with fewer adverse events, which is inconsistent with guidelines and general practice. Existing evidence allows us to conduct a systematic review and Bayesian analysis on this topic.
Methods: Four databases were searched from their inception to January 5, 2023. All statistical estimations were performed using R. “Gemtc” was the core package of analysis. CINeMA was used to assess the grade of confidence of the results.
Results: Fourteen studies were included in the Bayesian meta-analysis. No difference in the clinical success rate of antibiotic treatment was observed from a super short course (1–3 days) to a long course (≥10 days). Considering the adverse events, the short course (4–6 days) might be the safest. The majority of results were of high or moderate confidence grade.
Conclusion: Short course might cause the fewest adverse events. The clinical efficacy of antibiotics might not depend on the course length. Undeniably, more systematic explorations are warranted to investigate the clinical application of a shorter course of antibiotic treatment.
Introduction
Chronic obstructive pulmonary disease (COPD) is the third largest cause of death on a global scale (GBD Chronic Respiratory Disease Collaborators, 2020). Acute exacerbation (AE) is the leading cause of hospitalization and mortality among the COPD population. Bacterial infection attributes to about 50% of AECOPD (Bafadhel et al., 2011). Therefore, antibiotic treatment is the fundamental element of routine therapy in AECOPD (Anthonisen et al., 1987; Saint et al., 1995; Allegra et al., 2001). However, antimicrobial resistance (AMR) is a huge challenge to global healthcare (Antimicrobial Resistance Collaborators, 2022). By leading to substantial additional death and expense, it may cause enormous burden to patients and the society (Cassini et al., 2019). Consequently, AMR and safety concerns caused by drug overuse in AECOPD have been increasingly discussed and have attracted significant attention (López-Campos et al., 2015).
Till date, the antibiotics treatment course for AECOPD has been poorly discussed and implemented. According to the guidelines, 7–10 days is the suggested treating course in most cases of bacterial infection, which has been applied widely in the treatment of AECOPD. For over 2 decades, more studies have shown that a shorter duration of antibiotics might have the same efficacy. This may draw attention to several guidelines. For instance, the Dutch guideline suggests the 7-day-course, and the global initiative for chronic obstructive pulmonary lung disease (GOLD) report 2022 suggests a 5 to 7-day-course (Geijer et al., 1997; GOLD Reports, 2022). However, the main issue with these guidelines is that these suggestions are usually based on minimal evidence. For example, the advice of “5–7-day course” from GOLD 2022 was based on only one randomized controlled trial (RCT) comparing 5–7 days of antibiotic treatment, which is not convincing (Rg and Cj, 2001; GOLD Reports, 2022). With the increasing evidence, two published systematic reviews highlight comparing shorter and longer antibiotic treatment courses in AECOPD. They suggested that a shorter course surely has similar treating efficacy as a longer one with fewer adverse events (Stolbrink et al., 2018; Llor et al., 2022). However, limitations still exist within these studies. For example, they roughly divided the studies into two groups, limiting their meaning for clinical practice. Considering that shorter treating courses generally means lower cost, higher adherence rate, and lower incidence of AMR and adverse events, it should be given further attention and explored in greater depth.
The Bayesian analysis compares direct and indirect relationships between interventions. This enables us to rank and compare interventions without directly comparing them. Noticeably, some related studies indicate that the treating course could be divided into the following four groups: 1) super short course: 1–3 days; 2) short course: 4–6 days; 3) standard course: 7–9 days; and 4) long course: 10 days or more. Thus, a result of Bayesian analysis depending on such a grouping is more clinically applicable.
This systematic review aimed to comprehensively gather RCT evidence and compare the efficacy and safety of different antibiotic treatment courses in AECOPD using Bayesian analysis. Most importantly, the objective is to provide more detailed and practical evidence for clinical practice within this field.
Materials and Methods
The current study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and was registered on PROSPERO (CRD42022343479) (Page et al., 2021; Haichuan and Ting, 2022). PRISMA checklist for network meta-analysis is provided in the Supplementary Material Table S1.
Literature search
We searched PubMed, Embase, Cochrane library, and Web of Science to gather studies published from the establishment of the databases to January 5, 2023. Major terms that were used to build the search strategy included “antibiotics,” “COPD,” and “exacerbation” (The complete search strategy of the four databases has been listed in Supplementary Material Table S2). Only English articles were included during the screening phase.
Literature screening
Two reviewers (Ting Lei and Xiaojie Su) independently conducted the screening process, and a third reviewer (Haichuan Yu) held discussions in case of disagreements. Inclusion criteria: 1) patients with COPD confirmed by spirometry or physician, 2) patients diagnosed with AE by a physician, 3) antibiotics treatment course should be the focus of the study, and 4) all the included studies were RCTs. Exclusion criteria: 1) patients with comorbid conditions and significant infection in systems other than the respiratory system, 2) the antibiotics of interest have been banned for clinical use, and 3) articles without the success rate or adverse events of antibiotic treatment. The reference lists of included studies were also scrutinized for potential studies wrongly omitted during the previous process.
Data extraction
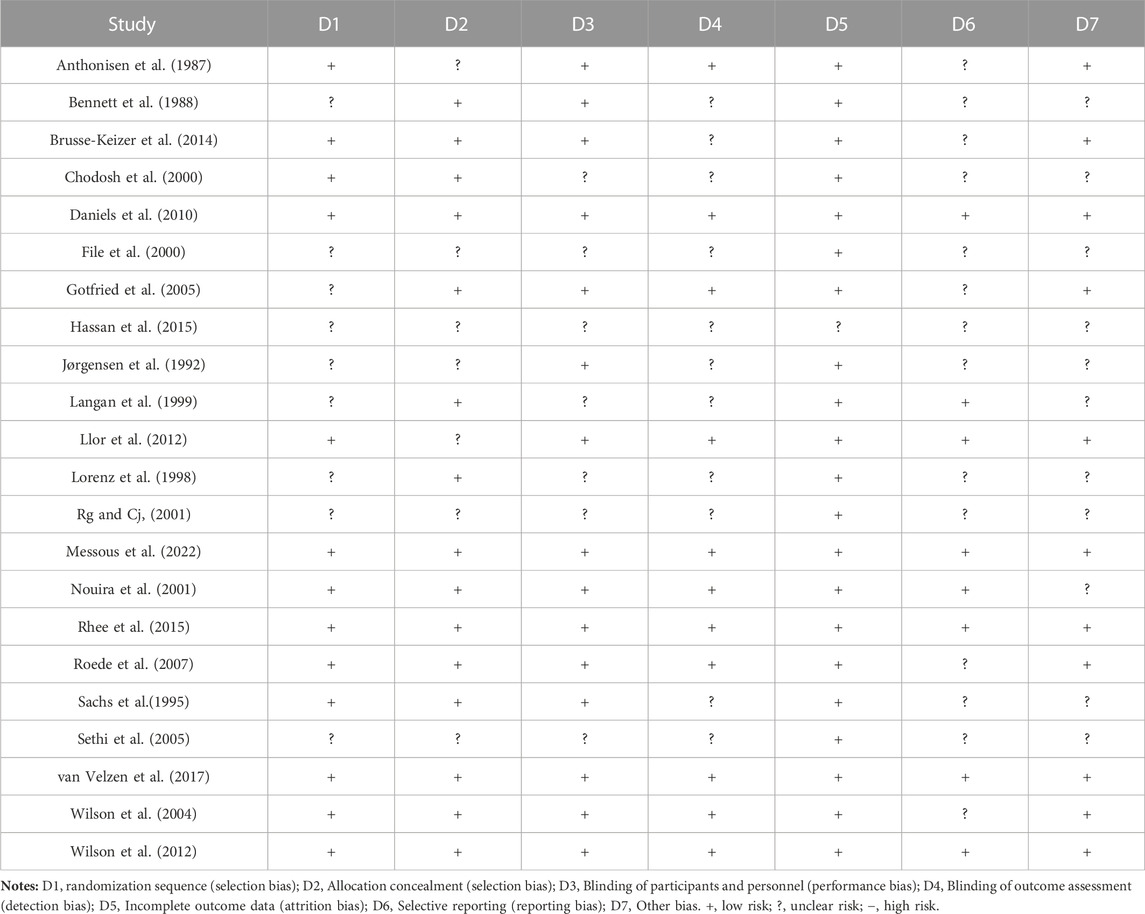
Two reviewers (Lu Zhang and Zhouzhou Feng) independently extracted the data through a pre-designed data form. The data form consisted of three parts: the essential characteristics of included studies, the risk of bias in the reports, and targeted outcomes. A third reviewer (Haichuan Yu) combined the two versions of the form into one. Cochrane Risk of Bias (ROB) tool 1.0 was utilized in this study. ROB 1.0 contains seven domains, each with a low, medium, or high rating. We aggregated an overall study evaluation based on assessing these domains.
Herein, the included treating courses were divided into four groups: 1) super short course: 1–3 days; 2) short course: 4–6 days; 3) standard course: 7–9 days; and 4) long course: 10 days or more. Moreover, placebo-controlled trials with clearly defined treating courses were also included.
Statistical analysis
R (version 4.1.3) was used to undergo the complete analysis, and “Gemtc” (version 1.0–1) was the major package utilized. First, a network was built using the function “mtc.network,” and then a model was generated using “mtc.model.” Finally, the Bayesian analysis was conducted using the “mtc.run” function. In function “mtc.model,” likelihood/link was set as “binom/log” to calculate the log risk ratio (logRR) with 95% credible interval (95%CrI) depending on the dichotomous data gathered. Models were estimated using JAGS (through the “rjags” package). Markov Chain Monte Carlo method computed a fixed effect model based on simulations of 5,000 adaptations and 20,000 iterations within the four chains. A league table was generated to present the relationships among all the treating courses. Function “exp” was used to calculate RR from LogRR when generating the league table. Furthermore, a relative effect forest plot was provided to visualize the relative effect of different treating courses than the standard one. A potential scale reduced factor (PSRF) value was calculated to quantify the convergence of the iteration. The node-split method was used to test the consistency assumption (when p < 0.05, inconsistency seemed significant). Furthermore, “mtc.anohe” was used to conduct the test of homogeneity assumption. I2> 50% indicated the significance of the heterogeneity. Sensitivity analysis was performed by comparing the random effect model pooling results with the fixed effect model.
Confidence in Network Meta-Analysis (CINeMA) was used to assess the quality of results of the network meta-analysis (Nikolakopoulou et al., 2020). CINeMA can also be used to perform the network meta-analysis under the “netmeta” package in R. Therefore, in addition to obtaining the grading results for the confidence of the analysis results, the results of the network meta-analysis received using CINeMA were also compared with the results from the “gemtc” package.
Results
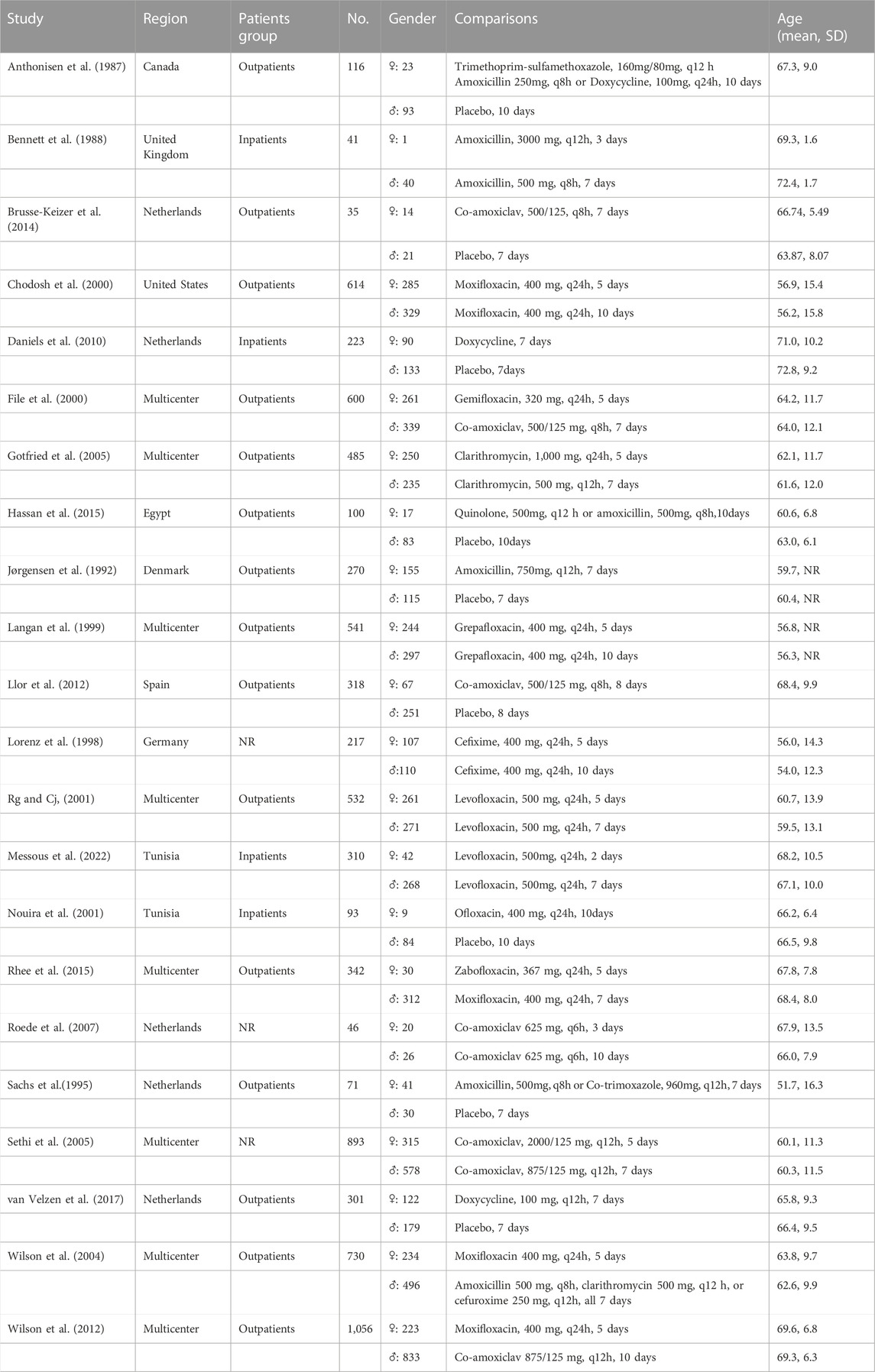
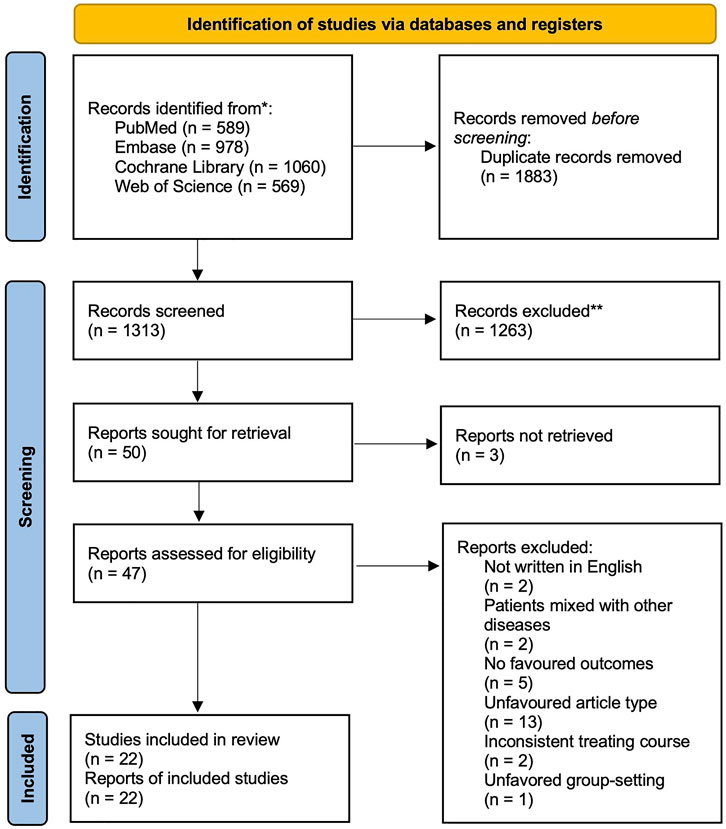
After the search, 3196 articles were included in the screening (search strategy and results are listed in the Supplementary Material Table S2). After discarding the duplicates and excluding the studies that did not match PICOS based on their title and abstract, 50 full articles were retrieved. The full articles were carefully read, and finally, 22 studies with a population of 7934 were included in the Bayesian analysis (essential characteristics of the included studies are presented in Table 1; the PRISMA flow diagram is shown in Figure 1). Most patients were outpatients of similar age. All the articles reported the clinical success rate and adverse effect incidence. 10 studies focused on “chronic bronchitis”, however included patients with airflow limitation. Considering their design would have predated the global use of the term “COPD” so that the population they focused might be consistent with ours, we still included these studies.
Among the included studies, four compared two or more different antibiotic regimens, nine compared different treating courses with placebo, and the other nine compared various treating courses of the same drugs. Notably, the standard course is the most frequently studied treating course (13 trials), followed by short (10 trials), long (7 trials), and super short (3 trials). Eight trials are multicentered, and studies were mostly conducted in developed areas such as Europe or North America.
17 out of 22 (77.27%) trials included more than 100 patients per group. Inclusion in the report was split 40/60 between the low and medium risk of bias based on the Cochrane ROB (1.0) tool. Based on the comparative analysis of study design, outcome measures, patients involved, and inclusion and exclusion criteria, it was observed that a quantitative synthesis of the evidence using network-based meta-analysis was appropriate. The homogeneity and consistency assumptions were statistically confirmed (see the results of analysis of heterogeneity and inconsistency in Supplementary Data S1).
Before undergoing meta-analysis, it could be roughly judged that in all the included studies, no superiority or inferiority existed between the groups. The overall risk of bias was graded “medium to low.” The primary reason that increased the risk was that some of the included studies could not report the methods for avoiding publication bias (see ROB results in Table 2).
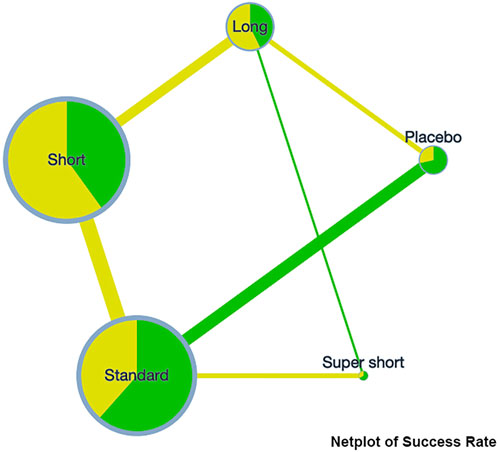
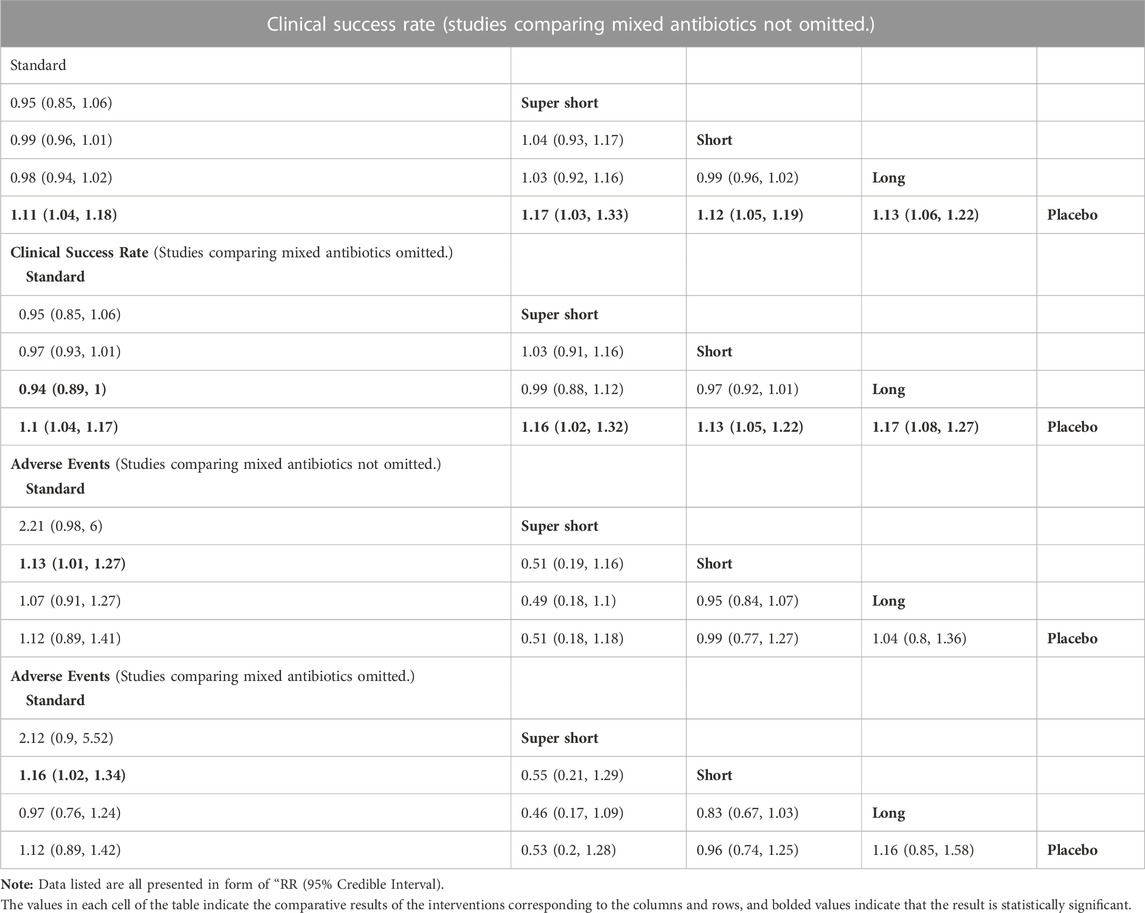
A network plot was presented on the clinical success rate to show the relationship among the different treatment courses (see Figure 2). Compared to placebo, the RR [95%CrI] of each course are standard, 1.11 [1.04, 1.18], super short, 1.17 [1.03, 1.33]; short, 1.12 [1.05, 1.19]; and long, 1.13 [1.06, 1.22]. This indicates that any course of antibiotics might cause benefit to COPD exacerbation. Detailed comparative analysis has been presented in the league table, and no significant difference could be observed among the different treating courses (see Table 3).
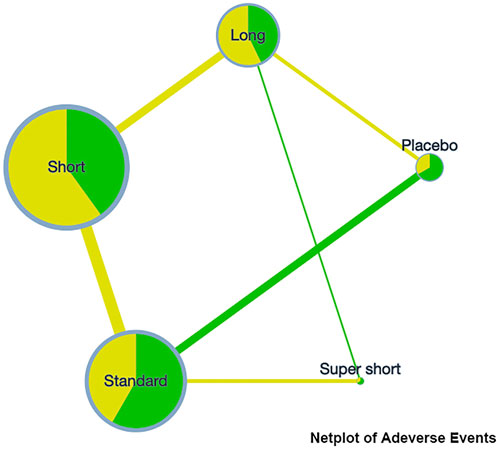
On the incidence rate of adverse events, the network plot was similar to the one with a clinical success rate (see Figure 3). Based on the league table, a short course has a significantly lower risk (RR [95%CrI], 0.88 [0.79, 0.99]) of adverse events compared with the standard course. However, no further difference was observed among the other courses (see Table 3).
Notably, the difference is absent in most cases. Therefore, rank plots and SUCRA were not provided since they could be meaningless under this circumstance.
PSRF value was 1.00 for clinical success rate and 1.01 for adverse events, indicating that the convergence of iteration was satisfying. The node-split analysis results showed no significant inconsistency (all p > 0.05). The heterogeneity of the analysis was acceptable (all I2 < 50%). Results of sensitivity analysis indicated that the results were robust to the different pooling methods (see the results of analysis of heterogeneity and inconsistency in Supplementary Data S1).
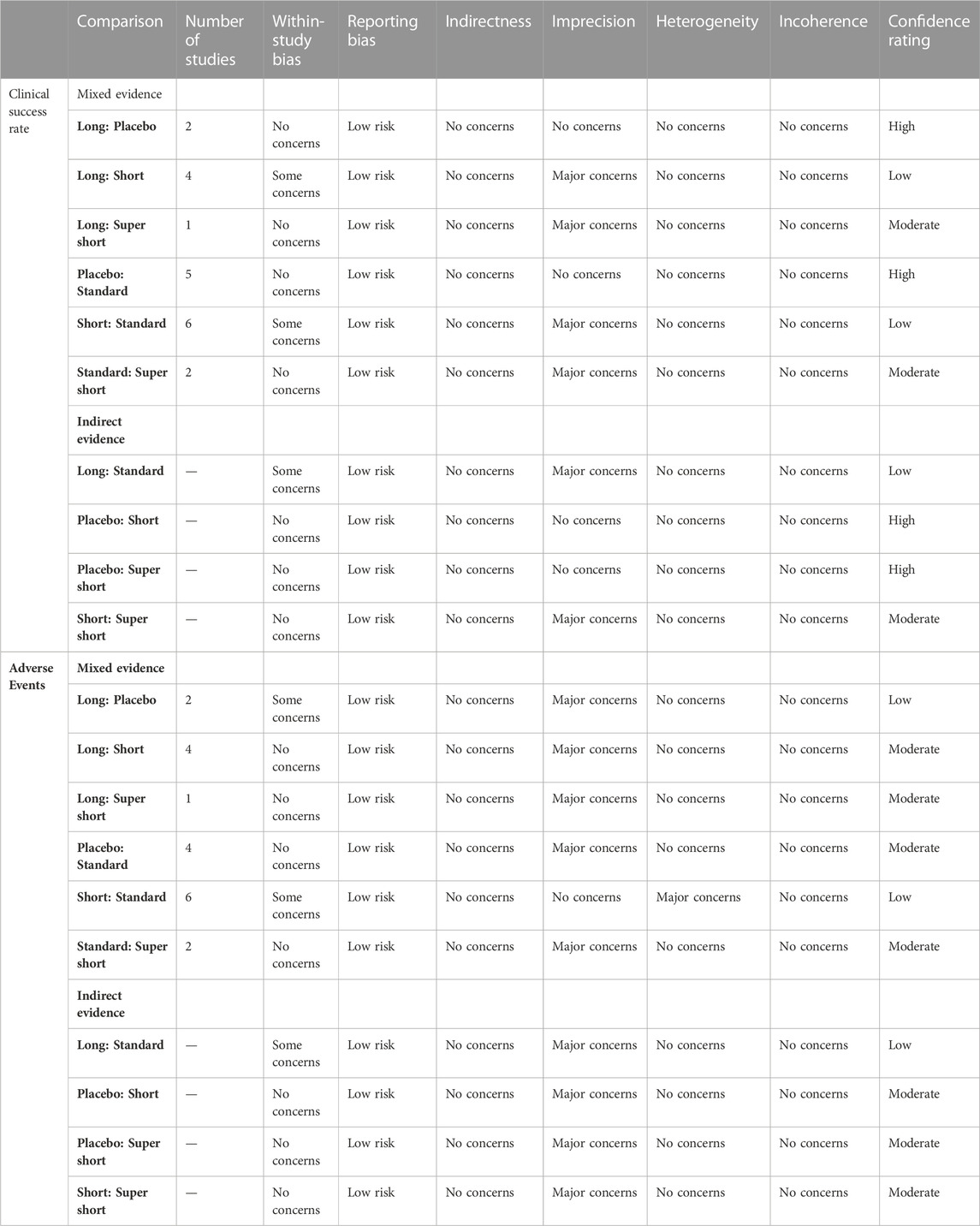
Furthermore, the confidence grade of our results was assessed using CINeMA online application. A full assessment was presented in the Supplementary Material S1. All the comparison results were of high confidence. Imprecision was the main reason for downgrading, mainly because the estimated 95%CrI of RR included “1". Reporting this bias raised some concerns since there was no comparison including more than 10 trials, so publication bias could not be tested (Egger et al., 1997). The full assessment is presented in Table 4. Moreover, CINeMA provided a result of the network analysis based on the “network” package in R, which was highly similar to what we obtained from the “gemtc” package (see the results of analysis of heterogeneity and inconsistency in Supplementary Data S1).
Furthermore, considering four studies with two or more different antibiotic regimens, the Bayesian analysis was rerun after omitting the studies. No significant difference between the two results was observed (see Table 3).
Discussion
This systematic review and Bayesian meta-analysis found that it is not “the longer, the better” when treating AECOPD with antibiotics. In particular, super short or short courses were not worse than standard or long courses in clinical success rate. Moreover, a short course (4–6 days) might be the safest choice when considering the incidence of adverse events. These results were based on 22 RCTs rated “medium to low risk” using ROB Tools. Four studies included groups with different antibiotics, but after we omitted them, the results did not change significantly.
Shorter courses of antibiotic treatment in AECOPD are not worse in terms of clinical success rate. In this study, the clinical success rate among different courses did not differ. This phenomenon could be attributed to several reasons. Non-etheless, about half of AECOPD is caused by infection, but the other half is for non-infective reasons (Costelloe et al., 2010). Moreover, there is still a lack of cost-effective, reliable, and instant methods to recognize viral infection in the infected population (GOLD Reports, 2022). These two factors lead to uncertainty and controversy about the need to administrate antibiotics in a large population and might contribute to the uncompetitive course.
A shorter course of antibiotic treatment in AECOPD might cause equal or fewer adverse events. A recent systematic review reported that excess antibiotic treatment is commonly used in the respiratory department, which causes significantly more adverse events (Vaughn et al., 2019). In this study, it was found that a short course (4–6 days) led to significantly fewer adverse events, which is coherent with former systematic reviews.
A shorter course of antibiotic treatment in AECOPD might have some other benefits. On the one hand, it has been well known that “more antibiotics, more resistance”; as a result, shorter courses could be an effective way to slow down the development of antibiotic-resistant bacterial strains (Goossens et al., 2005). On the other hand, minimal use of antibiotics is associated with lower treatment costs. The outcomes might be attributed to decreased individual costs and fewer population-based antibiotic resistance (Zhen et al., 2019).
WHO introduced a classification system called “AWaRe” (A, access; Wa, watch; Re, reserve) to guide the use of 180 antibiotics (Organization, 2019). Of the 22 trials included in this study, 11 used “Access” drugs and the remaining 11 used “Watch” drugs. This widespread use of antibiotic class that are not recommended as first-line in exacerbations not necessarily caused by bacterial infections is also a cause for concern. It is reassuring to note that there exists clinical trial examining the application of antibiotics based on evidence of biomarkers reflecting bacterial infection now (Mohamed Amine et al., 2021).
This study has some advantages. For instance, this is the first study using methods of the systematic review and Bayesian meta-analysis to compare the efficacy and safety of different antibiotic treatment courses. By dividing treatment courses into four groups, our study provides more guiding significance for clinical practice than former systematic reviews.
Non-etheless, there remain some limitations in this study: 1) There are only 22 studies included in the analysis, and no comparison had more than 10 trials. Moreover, no direct comparisons existed between long and standard courses. All these issues impaired the certainty of our estimation. 2) Half of the included reports were graded as medium risk of bias, which could impair the strength of evidence. 3) Because of the different mechanisms of action and bactericidal efficacy, the results of comparing different duration of antibiotic courses of different antibiotics remains debatable. 4) Within the study, publication bias could not be tested for included trials of each comparison of less than 10. 5) Many of the studies included in this study are multicentre studies and those that are not also widely spread across Asia, Europe, Africa, and North America. Coupled with the limited number of original studies included, it may not have been possible to conduct subgroup analyses on different regions, especially for low- and middle-income countries (LMICs) which suffered more from AMR.
Based on the discussions mentioned above, particular suggestions have been made for clinical practice and future study: 1) The results of this study should be cautiously interpreted due to its limitations. 2) Shorter courses should be considered more in clinical practice; short courses (4–6 days) would be optimal based on the existing evidence. 3) Notably, 2 days is the shortest course we observed. Many systematic explorations are required to determine whether it is the shortest effective course, which will be carried out in future studies. 3) Possibly, more flexible antibiotic regimens should be developed and tested, for instance, the instant use of antibiotics, symptom (sputum purulence, fever, etc.) based treatment, biomarker (e.g., C-reactive protein) guided medication, among others. 4) As aforementioned, only 50% of AECOPD is caused by bacterial infection, so caution needs to be exercised when administering antibiotics to this population, and the WHO ‘Access’ classification should probably be considered first when using antibiotics empirically. 5) Although it was found in this study that a short course of antibiotic treatment may have similar efficacy and fewer adverse events to a standard course of treatment, this does not mean “the shorter, the better,” as inadequate treatment duration is also an important cause of AMR. Caution should be exercised when using shorter than conventional antibiotic courses, and population-based follow-up should be done to monitor the occurrence of AMR.
Conclusion
By systematic review and Bayesian meta-analysis, it was found that in terms of the antibiotic treatment course of AECOPD, super short (1–3 days), short (4–6 days), and long course (≥10 days) could have similar treating efficacy with the standard course (7–9 days). Short course possesses statistically significant superiority in adverse events compared to standard ones. The limitations of this study are the need for further studies and keeping cautious when interpreting our results.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. Conception (HY), study design (HY and XS), literature obtaining (LZ, XS and XC), data extraction (TL, ZF and HY), analysis and interpretation (HY) and revising or critically reviewing the article (HG and JL).
Funding
This work was supported by the Science and Technology Projects of Gansu Province (Grant Number 18JR3RA344), and Special Funding for Big Data Research of Analgesic Sedation for Severe Infections. (Grant number Z-2019-1-002). Funders had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1024807/full#supplementary-material
References
Allegra, L., Blasi, F., de Bernardi, B., Cosentini, R., and Tarsia, P. (2001). Antibiotic treatment and baseline severity of disease in acute exacerbations of chronic bronchitis: A re-evaluation of previously published data of a placebo-controlled randomized study. Pulm. Pharmacol. Ther. 14 (2), 149–155. doi:10.1006/pupt.2001.0289
Anthonisen, N. R., Manfreda, J., Warren, C. P., Hershfield, E. S., Harding, G. K., and Nelson, N. A. (1987). Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann. Intern Med. 106 (2), 196–204. doi:10.7326/0003-4819-106-2-196
Antimicrobial Resistance Collaborators (2022). Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 399 (10325), 629–655. doi:10.1016/S0140-6736(21)02724-0
Bafadhel, M., McKenna, S., Terry, S., Mistry, V., Reid, C., Haldar, P., et al. (2011). Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 184 (6), 662–671. doi:10.1164/rccm.201104-0597OC
Bennett, J. B., Crook, S. J., Shaw, E. J., and Davies, R. J. (1988). A randomized double blind controlled trial comparing two amoxycillin regimens in the treatment of acute exacerbations of chronic bronchitis. J. Antimicrob. Chemother. 21 (2), 225–232. doi:10.1093/jac/21.2.225
Brusse-Keizer, M., VanderValk, P., Hendrix, R., Kerstjens, H., and van der Palen, J. (2014). Necessity of amoxicillin clavulanic acid in addition to prednisolone in mild-to-moderate COPD exacerbations. BMJ Open Respir. Res. 1 (1), e000052. doi:10.1136/bmjresp-2014-000052
Cassini, A., Högberg, L. D., Plachouras, D., Quattrocchi, A., Hoxha, A., Simonsen, G. S., et al. (2019). Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 19 (1), 56–66. doi:10.1016/S1473-3099(18)30605-4
Chodosh, S., DeAbate, C. A., Haverstock, D., Aneiro, L., and Church, D. (2000). Short-course moxifloxacin therapy for treatment of acute bacterial exacerbations of chronic bronchitis. The Bronchitis Study Group. Respir. Med. 94 (1), 18–27. doi:10.1053/rmed.1999.0708
Costelloe, C., Metcalfe, C., Lovering, A., Mant, D., and Hay, A. D. (2010). Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 340, c2096. doi:10.1136/bmj.c2096
Daniels, J. M. A., Schoorl, M., Snijders, D., Knol, D. L., Lutter, R., Jansen, H. M., et al. (2010). Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest 138 (5), 1108–1115. doi:10.1378/chest.09-2927
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
File, T., Schlemmer, B., Garau, J., Lode, H., Lynch, S., and Young, C. (2000). Gemifloxacin versus amoxicillin/clavulanate in the treatment of acute exacerbations of chronic bronchitis. The 070 Clinical Study group. J. Chemother. 12 (4), 314–325. doi:10.1179/joc.2000.12.4.314
GBD Chronic Respiratory Disease Collaborators (2020). Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet Respir. Med. 8 (6), 585–596. doi:10.1016/S2213-2600(20)30105-3
Geijer, R. M. M., Schayck, C. P. V., Weel, C. V., Sachs, A. P. E., and Rosmalen, C. F. H. (1997). NHG-Standaard COPD: Behandeling. Huisarts Wet., 430–442.
GOLD Reports (2022). GOLD reports - global initiative for chronic obstructive lung disease - GOLD. (Accessed July 8, 2022).
Goossens, H., Ferech, M., Vander Stichele, R., and Elseviers, M.ESAC Project Group (2005). Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 365 (9459), 579–587. doi:10.1016/S0140-6736(05)17907-0
Gotfried, M., Notario, G., Spiller, J., Palmer, R., and Busman, T. (2005). Comparative efficacy of once daily, 5-day short-course therapy with clarithromycin extended-release versus twice daily, 7-day therapy with clarithromycin immediate-release in acute bacterial exacerbation of chronic bronchitis. Curr. Med. Res. Opin. 21 (2), 245–254. doi:10.1185/030079905X26243
Haichuan, Y., and Ting, L. (2022). Does the administrating term of antibiotics matter in the treatment of the acute bacterial exacerbation of chronic respiratory disease: A systematic review and meta-analysis. Available at: (Accessed July 11, 2022).
Hassan, W. A., Shalan, I., and Elsobhy, M. (2015). Impact of antibiotics on acute exacerbations of COPD. Egypt. J. Chest Dis. Tuberc. 64 (3), 579–585. doi:10.1016/j.ejcdt.2015.03.007
Jørgensen, A. F., Coolidge, J., Pedersen, P. A., Petersen, K. P., Waldorff, S., and Widding, E. (1992). Amoxicillin in treatment of acute uncomplicated exacerbations of chronic bronchitis. A double-blind, placebo-controlled multicentre study in general practice. Scand. J. Prim. Health Care 10 (1), 7–11. doi:10.3109/02813439209014027
Langan, C. E., Zuck, P., Vogel, F., McIvor, A., Peirzchala, W., Smakal, M., et al. (1999). Randomized, double-blind study of short-course (5 day) grepafloxacin versus 10 day clarithromycin in patients with acute bacterial exacerbations of chronic bronchitis. J. Antimicrob. Chemother. 44 (4), 515–523. doi:10.1093/jac/44.4.515
Llor, C., Moragas, A., Hernández, S., Bayona, C., and Miravitlles, M. (2012). Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 186 (8), 716–723. doi:10.1164/rccm.201206-0996OC
Llor, C., Moragas, A., Miravitlles, M., Mesquita, P., and Cordoba, G. (2022). Are short courses of antibiotic therapy as effective as standard courses for COPD exacerbations? A systematic review and meta-analysis. Pulm. Pharmacol. Ther. 72, 102111. doi:10.1016/j.pupt.2022.102111
López-Campos, J. L., Hartl, S., Pozo-Rodriguez, F., and Roberts, C. M.European COPD Audit team (2015). Antibiotic prescription for COPD exacerbations admitted to hospital: European COPD audit. PLOS ONE 10 (4), e0124374. doi:10.1371/journal.pone.0124374
Lorenz, J., Steinfeld, P., Drath, L., Keienburg, T., and Troester, K.Cefixime Short-Course versus Standard-Course Study Group (1998). Efficacy and tolerability of 5- vs 10-day cefixime therapy in acute exacerbations of chronic bronchitis. Clin. Drug Investig. 15 (1), 13–20. doi:10.2165/00044011-199815010-00002
Messous, S., Trabelsi, I., Bel Haj Ali, K., Abdelghani, A., Ben Daya, Y., Razgallah, R., et al. (2022). Two-day versus seven-day course of levofloxacin in acute COPD exacerbation: A randomized controlled trial. Ther. Adv. Respir. Dis. 16, 17534666221099729. doi:10.1177/17534666221099729
Mohamed Amine, M., Selma, M., Adel, S., Khaoula, B. H. A., Mohamed Hassene, K., Imen, T., et al. (2021). 2-Day versus C-reactive protein guided antibiotherapy with levofloxacin in acute COPD exacerbation: A randomized controlled trial. PLoS One 16 (5), e0251716. doi:10.1371/journal.pone.0251716
Nikolakopoulou, A., Higgins, J. P. T., Papakonstantinou, T., Chaimani, A., Del Giovane, C., Egger, M., et al. (2020). CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLOS Med. 17 (4), e1003082. doi:10.1371/journal.pmed.1003082
Nouira, S., Marghli, S., Belghith, M., Besbes, L., Elatrous, S., and Abroug, F. (2001). Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: A randomised placebo-controlled trial. Lancet 358 (9298), 2020–2025. doi:10.1016/S0140-6736(01)07097-0
Organization, W. H. (2019). The 2019 WHO AWaRe classification of antibiotics for evaluation and monitoring of use. 2019 WHO AWaRe Classif. antibiotics Eval. Monit. use. (Accessed January 9, 2023).
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Rg, M., and Cj, B. (2001). Randomized, double-blind study comparing 5- and 7-day regimens of oral levofloxacin in patients with acute exacerbation of chronic bronchitis. Int. J. Antimicrob. agents 18 (6), 503–512. doi:10.1016/s0924-8579(01)00435-6
Rhee, C. K., Chang, J. H., Choi, E. G., Kim, H. K., Kwon, Y. S., Kyung, S. Y., et al. (2015). Zabofloxacin versus moxifloxacin in patients with COPD exacerbation: A multicenter, double-blind, double-dummy, randomized, controlled, phase III, non-inferiority trial. Int. J. Chron. Obstruct Pulmon Dis. 10, 2265–2275. doi:10.2147/COPD.S90948
Roede, B. M., Bresser, P., El Moussaoui, R., Krouwels, F. H., van den Berg, B. T. J., Hooghiemstra, P. M., et al. (2007). Three vs. 10 days of amoxycillin-clavulanic acid for type 1 acute exacerbations of chronic obstructive pulmonary disease: A randomised, double-blind study. Clin. Microbiol. Infect. 13 (3), 284–290. doi:10.1111/j.1469-0691.2006.01638.x
Sachs, A. P., Koëter, G. H., Groenier, K. H., van der Waaij, D., Schiphuis, J., and Meyboom-de Jong, B. (1995). Changes in symptoms, peak expiratory flow, and sputum flora during treatment with antibiotics of exacerbations in patients with chronic obstructive pulmonary disease in general practice. Thorax 50 (7), 758–763. doi:10.1136/thx.50.7.758
Saint, S., Bent, S., Vittinghoff, E., and Grady, D. (1995). Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta-analysis. JAMA 273 (12), 957–960. doi:10.1001/jama.1995.03520360071042
Sethi, S., Breton, J., and Wynne, B. (2005). Efficacy and safety of pharmacokinetically enhanced amoxicillin-clavulanate at 2,000/125 milligrams twice daily for 5 days versus amoxicillin-clavulanate at 875/125 milligrams twice daily for 7 days in the treatment of acute exacerbations of chronic bronchitis. Antimicrob. Agents Chemother. 49 (1), 153–160. doi:10.1128/AAC.49.1.153-160.2005
Stolbrink, M., Amiry, J., and Blakey, J. D. (2018). Does antibiotic treatment duration affect the outcomes of exacerbations of asthma and COPD? A systematic review. Chron. Respir. Dis. 15 (3), 225–240. doi:10.1177/1479972317745734
van Velzen, P., Ter Riet, G., Bresser, P., Baars, J. J., van den Berg, B. T. J., van den Berg, J. W. K., et al. (2017). Doxycycline for outpatient-treated acute exacerbations of COPD: A randomised double-blind placebo-controlled trial. Lancet Respir. Med. 5 (6), 492–499. doi:10.1016/S2213-2600(17)30165-0
Vaughn, V. M., Flanders, S. A., Snyder, A., Conlon, A., Rogers, M. A. M., Malani, A. N., et al. (2019). Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann. Intern Med. 171 (3), 153–163. doi:10.7326/M18-3640
Wilson, R., Allegra, L., Huchon, G., Izquierdo, J. L., Jones, P., Schaberg, T., et al. (2004). Short-term and long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis. Chest 125 (3), 953–964. doi:10.1378/chest.125.3.953
Wilson, R., Anzueto, A., Miravitlles, M., Arvis, P., Alder, J., Haverstock, D., et al. (2012). Moxifloxacin versus amoxicillin/clavulanic acid in outpatient acute exacerbations of COPD: MAESTRAL results. Eur. Respir. J. 40 (1), 17–27. doi:10.1183/09031936.00090311
Keywords: chronic obstructive pulmonary disease, acute exacerbation, antibiotics, treatment course, Bayesian meta-analysis
Citation: Yu H, Lei T, Su X, Zhang L, Feng Z, Chen X and Liu J (2023) A systematic review and Bayesian meta-analysis of the antibiotic treatment courses in AECOPD. Front. Pharmacol. 14:1024807. doi: 10.3389/fphar.2023.1024807
Received: 23 August 2022; Accepted: 10 January 2023;
Published: 20 January 2023.
Edited by:
Jean-Marie Boeynaems, Université libre de Bruxelles, BelgiumReviewed by:
Zikria Saleem, Bahauddin Zakariya University, PakistanBrian Godman, University of Strathclyde, United Kingdom
Copyright © 2023 Yu, Lei, Su, Zhang, Feng, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Liu, bWVkZWNpbmxpdUBzaW5hLmNvbQ==
 Haichuan Yu
Haichuan Yu Ting Lei
Ting Lei Xiaojie Su
Xiaojie Su Lu Zhang1,2
Lu Zhang1,2 Xinlong Chen
Xinlong Chen Jian Liu
Jian Liu