
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 23 January 2023
Sec. Drugs Outcomes Research and Policies
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1023250
This article is part of the Research TopicDisease-on-a-chip: from point-of-care to personalized medicineView all 6 articles
 Olena Litvinova1*
Olena Litvinova1* Elisabeth Klager2
Elisabeth Klager2 Andy Wai Kan Yeung2,3
Andy Wai Kan Yeung2,3 Nikolay T. Tzvetkov4
Nikolay T. Tzvetkov4 Oliver Kimberger2,5
Oliver Kimberger2,5 Maria Kletecka-Pulker2,6
Maria Kletecka-Pulker2,6 Harald Willschke2,5
Harald Willschke2,5 Atanas G. Atanasov2,7*
Atanas G. Atanasov2,7*Objectives: Digital pills are new technologies that aim to improve healthcare by increasing medication adherence. The aim of the work was a bibliometric analysis of clinical studies of digital pills and an assessment of the level of evidence of their effectiveness, safety, and prospects for the future.
Materials and Methods: The studies were conducted using online databases such as ClinicalTrials.gov, Dimensions, and Web of Science for the period January 2012 to July 2022. The VOSviewer tool for building and visualizing bibliometric networks was used.
Results: Bibliometric analysis of the scientific literature revealed that over the past 10 years, the number of publications about digital pills has noticeably increased, which indicates the increasing importance of this field of knowledge. The leading positions in this area are occupied by scientists from the United States, the United Kingdom, and India. Sources of financial support for authors of publications in the field of digital pills are funds from leading developer companies, budget allocations, and funds from non-commercial organizations. Public-private partnerships are an important path to develop and implement digital pills. The four main clusters of digital pill studies were highlighted and visualized: efficacy and safety analysis for serious mental disorders; treatment and costs of tuberculosis therapy; features of the treatment of diabetes, cardiovascular diseases, and AIDS; and usage monitoring. Available publications demonstrate the efficacy potential and safety of digital pills. Nevertheless, the effects of digital pills have not yet been fully studied.
Conclusion: Priority areas for future research are further randomized controlled clinical trials and meta-analyses, which are necessary for a high level (I level) of evidence for therapeutic applications of digital pills, as well as pharmacoeconomic studies.
One of the areas of promoting health is the use of digital tools for the provision of medical care. The implementation of digital therapeutics and digital care products in health institutions is intended to speed up the exchange of information in the system of quality control of medical care and reduce risks and uncertainties. The medical industry is closely related to the accumulation and processing of significant amounts of information. The effectiveness of first aid and further treatment of the patient depend on the quality of methods of working with information.
Digital pills with ingestible sensors are an effective tool for organizing the provision of quality medical care by providing a doctor with timely and qualified information support and increasing patient adherence to treatment (Baumgartner et al., 2021). Digital pills have built-in sensors that, when used in conjunction with the software of devices like tablets and smartphones, can track the progress of pharmacotherapy. Low patient compliance (medication opt-out) is a significant barrier for all areas of medicine; hence, such monitoring is important.
Examples of well-known digital pills with ingestible sensors are the Ingestible Event Marker sensor (made by Proteus Digital Health, California, USA) and the ID-CapTM system (made by EtectRx, Florida, USA), which received the FDA’s approval (Flore, 2021).
The analysis of data from clinical trials of digital pills is the focus of many scientists and doctors of various specialties (Hafezi et al., 2015; Alipour et al., 2020; Mukherjee et al., 2022). The following types of effects from their implementation into medical practice are distinguished: increasing adherence to treatment of patients; improving the effectiveness and safety of therapy; reducing the cost of treatment and hospital stays; improving the quality of medical care; reducing medical errors; remote access to clinical data; providing mobile clinical monitoring; transparency of patient data, etc.
Currently, it can be stated that digital pills are used in various fields of medicine. An analysis of the literature shows that a number of papers focus on experimental clinical studies of them in people with mental health symptoms, monitoring the treatment regimen after transplantation, including pediatric; when taking opioids in people with acute pain; for the treatment of high-risk cardiovascular disease, uncontrolled hypertension, and type 2 diabetes; tuberculosis, hepatitis C, and AIDS (Au-Yeung et al., 2011a; Belknap et al., 2013; Eisenberger et al., 2013; DiCarlo et al., 2016; Flores et al., 2016; Noble et al., 2016; Peters-Strickland et al., 2016; Profit et al., 2016; Chai et al., 2017a; Chai et al., 2017b; Frias et al., 2017; Moorhead et al., 2017; Thompson et al., 2017; Holender et al., 2018; Triplett et al., 2019; Bonacini et al., 2020; Daar et al., 2020; Kamal et al., 2020; Sulkowski et al., 2020).
However, critical analysis of clinical trial outcomes requires attention to the challenges clinicians face, as well as questions that have yet to be answered and final decisions about the benefits and risks of using digital pills. The challenges clinicians face include the accuracy and interpretation of the data they receive, a lack of comparative randomized trials of digital pills with non-digital dosage forms, including pharmacokinetics, a small number of participants in clinical trials, and ethical questions. Following below is short discussion of selected publications discussing these problems in more detail.
Chai et al. systematized 18 pilot clinical trials of digital pills in the treatment of HIV, hepatitis C, solid organ transplantation, tuberculosis, schizophrenia, cardiovascular disease, and acute fractures (Chai et al., 2022). Studies typically included up to 50 patients, some between 120 and 288 individuals, and they lasted from 1 week to 6 months. The authors draw the conclusion that digital pills might improve medication adherence and treatment outcomes. However, randomized controlled clinical trials are necessary to compare various treatments quantitatively, which will increase the level of evidence of their effectiveness and safety.
Martani et al. analyzed the above 18 clinical trials in terms of ethical aspects (Martani et al., 2020). The authors note the importance of confidentiality in the use of digital pills, as well as the small size of the studied samples, suggesting a low level of evidence.
Other authors also address the ethical challenges of using digital pills. Thus, digital pill therapy is compared by Rosenbaum to “swallowing a spy,” with no discernible therapeutic benefit for patients (Rosenbaum, 2018). The sociologist Hatch called Abilify’s MyCite digital pills a “killer app” aimed at making a profit (Hatch, 2018).
Digital pills should not be seen as a simple change in how medications are taken, state Chevance et al. (Chevance et al., 2022). Instead, they should be seen as a complicated intervention that requires rigorous testing before being extensively employed in everyday clinical practice. The authors also stress the significance of an ethical and legal framework in order to secure the legal and moral possession and use of medicine.
Cosgrove et al. criticize the efficacy and insufficient comparative studies of digital pills in relation to non-digital dosage forms and placebo, as well as reflecting on their high financial burden (Cosgrove et al., 2019). According to the authors, there were no randomized clinical trials contrasting digital aripiprazole with a non-digital formulation, other comparators, or a placebo. There was no proof that the aripiprazole digital form had higher compliance than the analog version. The authors draw attention to the dishonest strategies used to use evergreen patents to prolong the duration of patent protection and thwart generic competition. Researchers are worried about the approval of a “new” medicine or gadget that may not be as effective or safe but will cost substantially more.
Despite the presence of a significant array of scientific research on digital pills, the problems associated with both analyzing the level of evidence of their effectiveness and safety, identifying leading developers, and assessing the costs and prospects for the future remain poorly understood. The need to solve this range of problems determines both the purpose of the study and its relevance.
At the beginning of the research, it is possible to predict the potential level of development since the dynamics of publication activity are uneven for each section of science, and thus “hot spots” can be identified. They are defined as specific thematic areas in which the amount of work exceeds the industry average. Development in this priority industry increases the chances of it being in demand, but if the work does not contain fundamentally new data, then its commercialization will not be successful. If the research is devoted to a narrow topic that goes beyond the interests of most industry and health researchers, then its commercialization becomes more complicated, regardless of the value of the results obtained. Hence, the aim of the present work was a bibliometric analysis of clinical studies of digital pills and an assessment of the level of evidence of their effectiveness, safety, and prospects for the future.
This study involved retrospective, logical, and graphic research methods and content analysis.
This study was conducted using online databases, namely ClinicalTrials.gov, Dimensions (https://app.dimensions.ai/discover/publication), and Web of Science. Searches were focused on scientific articles published between January 2012 and July 2022. The search strategy is shown in Figure 1.
In the first stage of research, clinical studies of digital pills were searched in the database ClinicalTrials.gov. The following keywords were used in the search: ingestible sensor, ingestible event marker, digital health feedback system, ID-Cap System, digital pill, and their combinations. By manual screening, relevant clinical trials were identified and their trial numbers were passed to the second stage of the research.
In the second stage of the research, an analysis of publications was carried out using keywords, clinical trial numbers, and their combinations in the Dimensions Database.
In the third stage, original articles and scientific reviews were selected from Dimensions Database documents, which were then considered in more detail based on analytical tools. For bibliometric analysis, the VOSviewer tool for building and visualizing bibliometric networks was used. It can build bibliometric maps based on citation analysis, keyword co-occurrence, co-authorship, and other parameters.
It is possible to deal with a sizable number of publications when using bibliometric data analysis tools, and this makes it possible to spot trends in research activities that are challenging to find without the use of specialized digital tools. Using this strategy, it is possible to pinpoint the direction of research with the necessary level of specificity and depth as well as spot trends in the global publication activity of scientists. Bibliometric analysis is widely regarded as one of the practical techniques that allow one to gain a comprehensive understanding of the issues confronting researchers as well as become acquainted with the potential future directions of scientific inquiry.
In the fourth stage, a detailed analysis of publications related to clinical trials was carried out. The fourth stage evaluated the evidence of efficacy and safety from available publications related to clinical randomized trials and meta-analyses; the identification of gaps in this direction; and problems such as the high cost of the intervention (Cosgrove et al., 2019).
At the fifth stage of research, an analysis of organizations that finance scientists working in the field of digital pills was carried out. The scientometric base of the Web of Science makes it possible to conduct such an analysis. Studies were conducted with keywords as in the Dimensions Database, as well as identifiers of PubMed articles corresponding to clinical trial numbers. The search for organizations financing research in the field of digital pills was carried out using the commercial names of leading developer companies, non-commercial organizations, and state funds.
A search using keywords and clinical trial numbers (as indicated in Figure 1) found 977 results in the Dimensions Database for the field of digital pills.
Figure 2 shows the dynamics of publications related to clinical trials of digital pills and their review papers published between January 2012 and July 2022 in the Dimensions Database. At the same time, the largest numbers of articles were published in 2020 and 2021, covering 18% and 20% of all publications, respectively, for 2012–2022. The number of articles in 2016, 2017, and 2018 compared to 2012 increased by 1.96, 2.61, and 4.3 times, respectively.
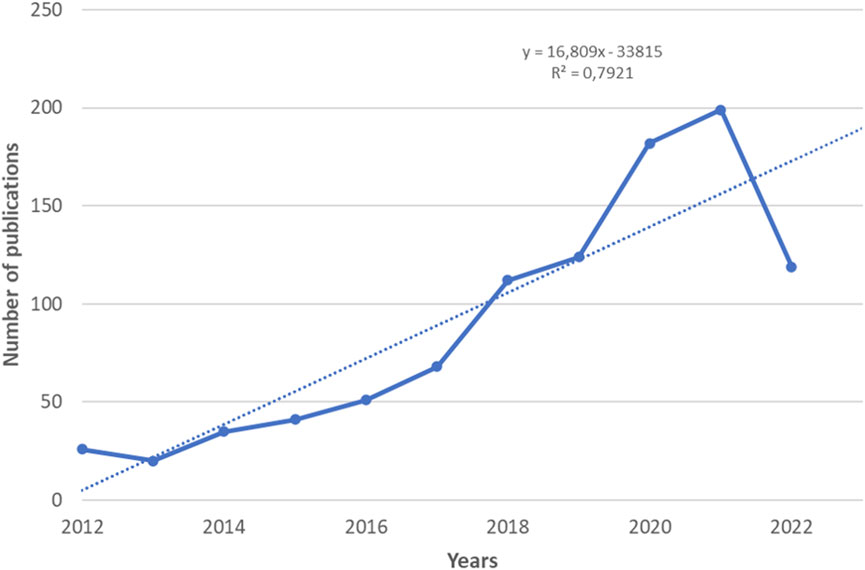
FIGURE 2. The dynamics of publications related to clinical trials of digital pills and their review papers for the period January 2012 to July 2022.
Analysis of the data obtained allows us to conclude that the dynamics of publication activity has a positive growth trend during the period under review. The trend model predicts further growth in research in this scientific area. The above confirms intensive research in the area.
Harvard University, Proteus Digital Health, Brigham and women’s hospital, University of Manchester, King’s college London are leaders in publishing and scientific activity in the field of digital pills among research organizations (Table 1).
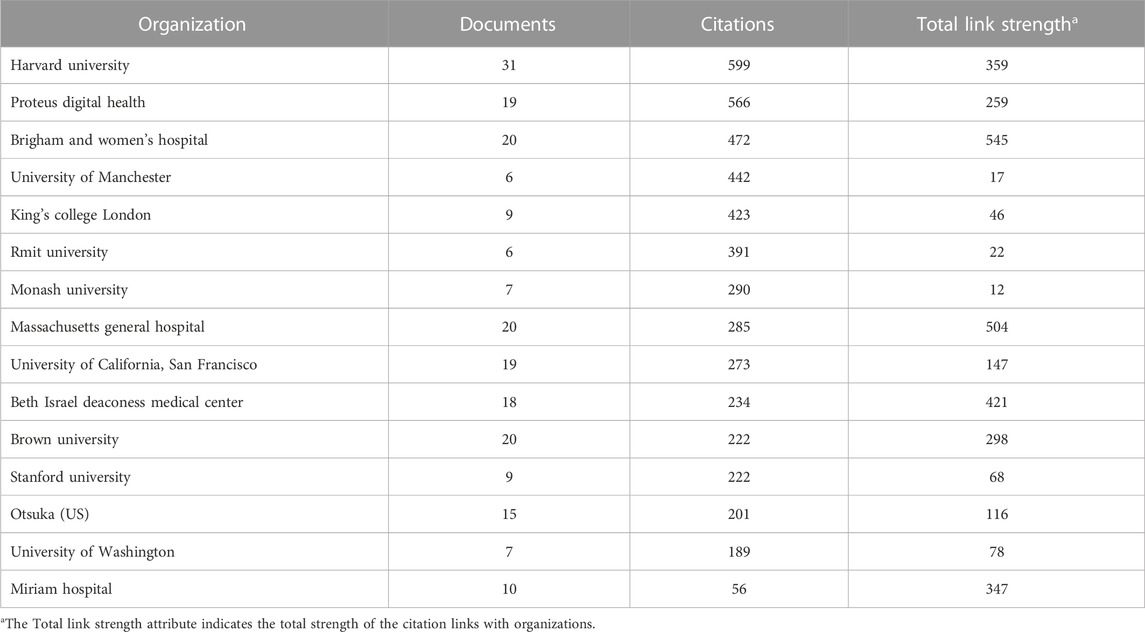
TABLE 1. The top leading organizations in publishing and scientific activity in the field of digital pills for the period January 2012 to July 2022.
Researchers from the USA contributed the most to the development of theories about digital pills, publishing 315 papers (315/977, or 32.24% of the total number of publications on the problem under study). Researchers from the United Kingdom came in second with 101 papers (101/977, or 10.33%), and researchers from India came in third with 46 papers (46/977, or 4.71%) (Table 2).
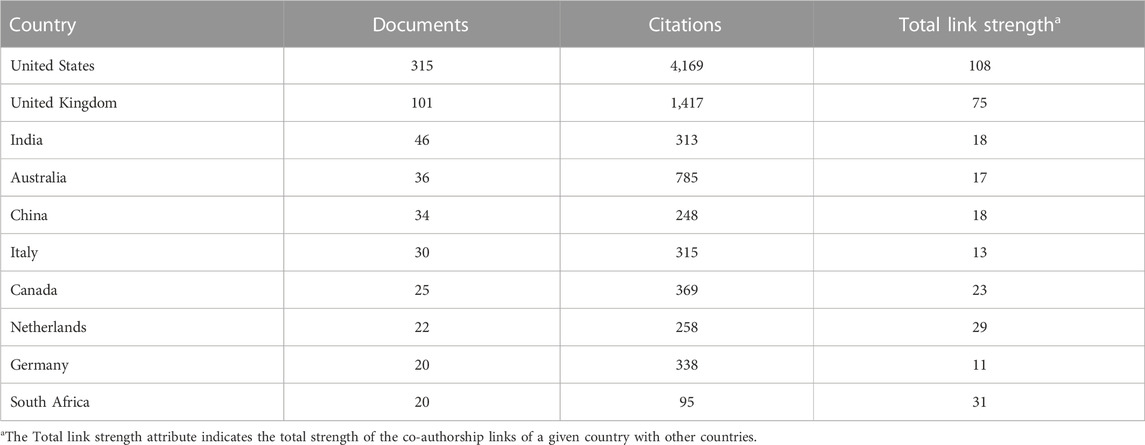
TABLE 2. The top leading countries in publishing and scientific activity in the field of digital pills for the period January 2012 to July 2022.
Leading scientists involved in clinical research of digital pills and the systematization of the obtained results are shown in Table 3. The high citation of these authors is revealed. The most productive authors in this context were Giovanni Kenneth Hugh Mayer (US), Peter Ray Chai (US), Edward W Boyer (US), Rochelle K Rosen (US), Conall Michael O’Cleirigh (US), Yoona A Kim (US), Timothy S Peters-Strickland (US), and Lorenzo Angelo Dicarlo (US).
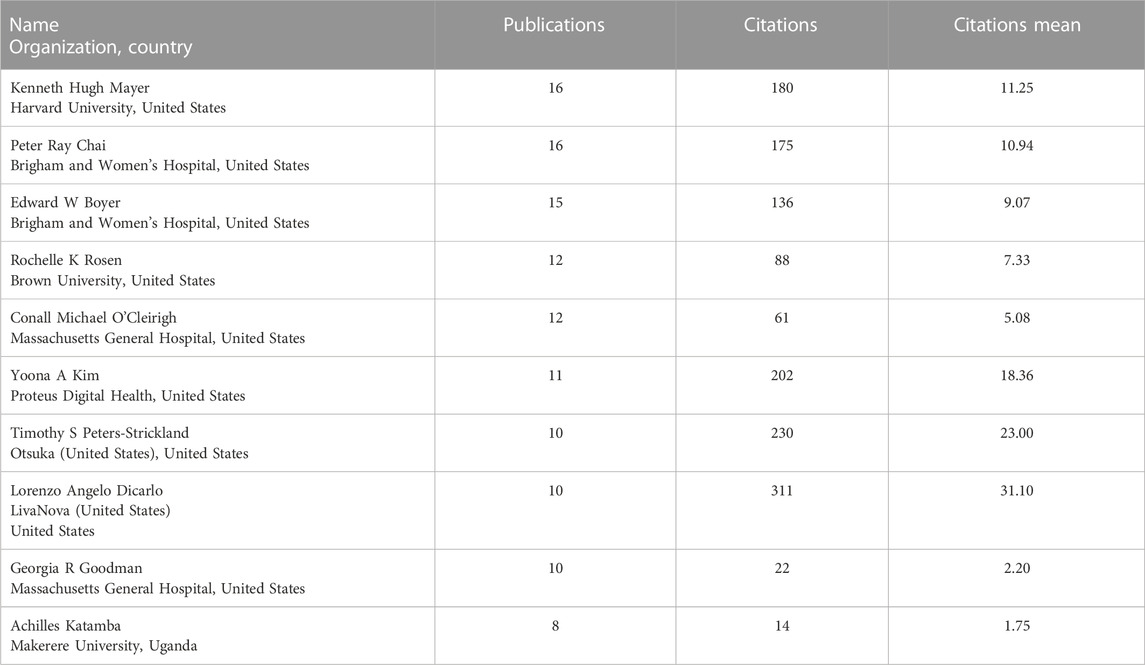
TABLE 3. Leading scientists in publishing and scientific activity in the field of digital pills for the period January 2012 to July 2022.
An analysis of the sources of funding for scientific research in the field of systematization, development, and implementation of digital pills in the Web of Science Database was carried out. For the period 2012–2022, 784 publications were identified. A correlation analysis of the number of publications associated with digital pills by year in the Web of Science and Dimensions Databases showed that there is a high correlation in the indexed studies. The correlation coefficient was found to be 0.95.
An examination of publications in the Web of Science Database pointed that Proteus Digital Health (California, USA), the leading developer of digital pills, is an important player in funding research. In 2% (16) of publications in the field of digital pills, funding by Proteus Digital Health for the period 2012–2022 is indicated as the source. It is noted that Proteus Digital Health, which funded the study, is also the employer of some authors (Belknap et al., 2013).
A digital ingestion tracking system—a sensor known as an “Ingestible Event Marker”—made by Proteus Digital Health was used to create the medicine Abilify MyCite by Otsuka Pharmaceutical Company (Japan). Otsuka Pharmaceutical Company was also involved in financing digital pill developments. Authors of 3.3% (26) of publications noted the receipt of funding by Otsuka Pharmaceutical Company in the field of digital pills.
It should be noted that the company EtectRx (Florida, USA) also funded research in the field of digital pills. EtectRx funded the authors of 1.4% (11) of the publications identified.
Another company funding digital pill research is Gilead Sciences, with 1.8% (14) of publications related to the financing of digital pill development.
It should also be noted the active patenting of technologies related to digital pills by Proteus Digital Health, EtectRx, and Otsuka Pharmaceutical Company, which indicates their commercial interest (Litvinova et al., 2022).
Information on funding by companies for developments in the field of digital tablets is provided for illustration only and not as a limitation. However, the analysis of the data obtained allows us to conclude that financing by development companies is not the main one.
It has been established that the predominant type of financial resources used by researchers in the development of digital pills are proceeds from budget funds, and non-profit organizations. For example, it was revealed that in the field of digital pills, 14% (110) of publications received funding from the National Institutes of Health (NIH, USA), 14.6% (115) from the United States Department of Health and Human Services, 1.4% (11) from the Alliance Healthcare Foundation, and 1.4% (11) from Specialists in Global Health. The budgetary financing of scientific developments is primarily associated with the conduct of fundamental scientific research, which provides technological breakthroughs.
Healthcare and pharmacy are areas where public-private partnerships have become quite widespread (Davis et al., 2021). Thus, the experience of joint financing of the creation of digital pills, both by developer companies and government funds, should be noted (Browne et al., 2018). The development of public-private partnerships to intensify the processes of creating digital pills for the treatment of socially threatening diseases (hepatitis, HIV, oncopathology, tuberculosis, etc.) contributes to the pooling of unique innovative resources and the distribution of risks associated with a high degree of uncertainty in the results of innovative activities. Public-private partnerships build the innovative potential of the pharmaceutical industry through the joint activities of organizations, increase the financial resources needed to reform the healthcare system, contribute to the optimization of budgetary expenditures, and increase the efficiency of investments in healthcare, which ultimately contribute to improving the quality of medical and pharmaceutical services.
Hingle M. et al. note the benefits of academic and industrial partnerships in digital medicine (Hingle et al., 2019). There are also publications in the field of creating digital tablets, the authors of which are representatives of the university and the developer company (Belknap et al., 2013).
Leading journals and preprint servers presenting publications in the field of digital pills are given in Table 4. They are connected with scientific directions such as Medical and Health Sciences; Public Health and Health Services; Clinical Sciences; etc.
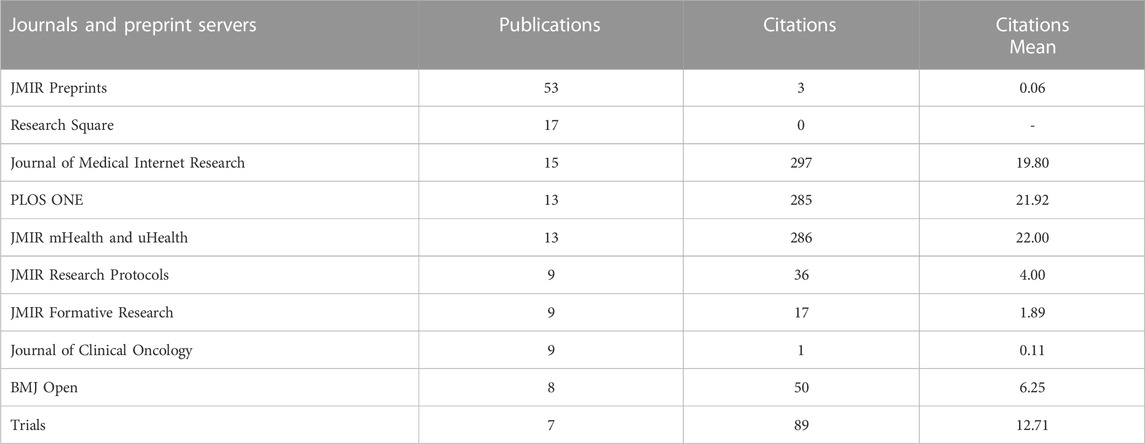
TABLE 4. Leading journals and preprint servers presenting publications in the field of digital pills for the period January 2012 to July 2022.
We used the VOSviewer program’s method of keyword clustering (terms from “Title and Abstract”). The diameter of the circle indicates how frequently the term is used. The separation between the circles acts as a barometer for the degree of association between terms, which is interpreted as the frequency of terms occurring together frequently. Relationships get stronger the closer they get. Thus, keywords form specific thematic clusters.
On the terminological map in Figure 3, clusters are indicated with various colors; each keyword’s size is determined by the “total link strength” indicator, which measures how strongly a specific keyword is associated with all other phrases; and the lines demonstrate associations between two different keywords.
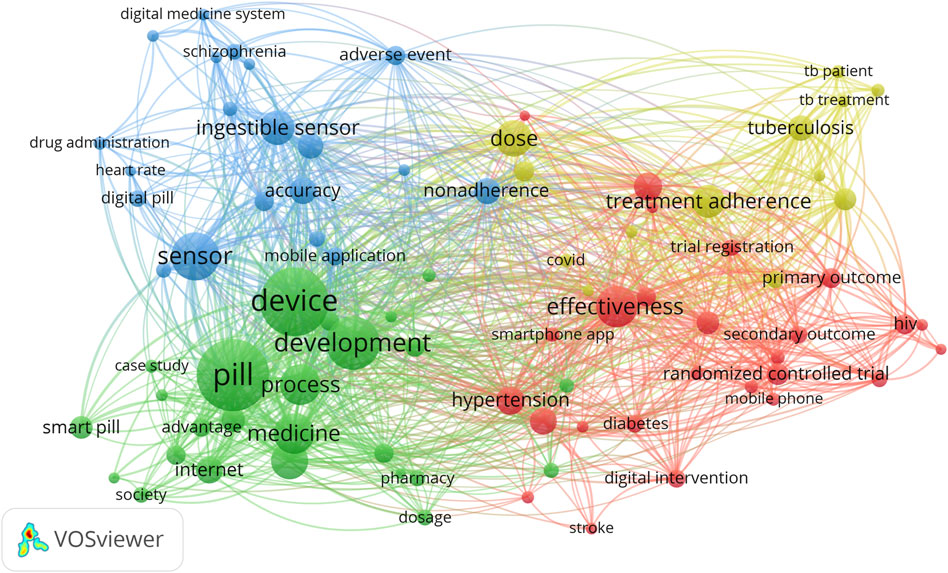
FIGURE 3. The terminological map of publications in the field of development and research of digital pills for the period January 2012 to July 2022.
The degree to which one phrase is used frequently in conjunction with another was evaluated. A specific thesaurus (446 terms) was created to integrate related terms and get rid of keyword typos. Keywords that appear at least 15 times in the sample were chosen in order to construct a terminological map. There are 84 terms in the final set of keywords.
Based on the analysis’s findings, 4 clusters were identified to be related to digital pills and comprised of the subjects of the most referenced authors’ scientific studies.
The first cluster (marked in blue) is devoted to the analysis of the effectiveness and safety of digital pills in patients with severe mental disorders, such as schizophrenia. “Accuracy”, “adverse events”, “aripiprazole”, “caregiver”, “digital health”, “digital medicine system”, “nonadherence”, “safety”, “schizophrenia”, and “serious mental illness” are the main terms of this cluster.
The second cluster (marked in yellow) is related to the treatment and cost-effectiveness issues of using digital pills in patients with tuberculosis. This cluster is represented by the following terms: “cost effectiveness”, “digital adherence technology”, “dose”, “tuberculosis patient”, “tuberculosis treatment”, “treatment adherence”, etc.
The third cluster (in red) relates to the features of the treatment of diabetes, cardiovascular diseases, and antiretroviral therapy using digital pills. The most common terms are “antiretroviral therapy”, “HIV”, “cardiovascular diseases”, “blood pressure”, “effectiveness”, “feedback”, “clinical outcome”, “digital intervention”, “hypertension”, “stroke”, “mobile health”, etc.
The fourth cluster (marked in green) is connected to the usage monitoring of digital pills. This cluster includes such terms as “advantage”, “adverse effect”, “artificial intelligence”, “elderly person”, “dosage”, “healthcare”, “health outcome”, “innovation”, “society”, etc.
These clusters show the links between scientific topics and potential key areas of further research.
The resulting network has a high density, which is expressed by the multiplicity of links between the terms and indicates their high compatibility and occurrence in various studies. The terms given in Table 5 have the greatest weight (according to the occurrence numbers in the analysed literature).
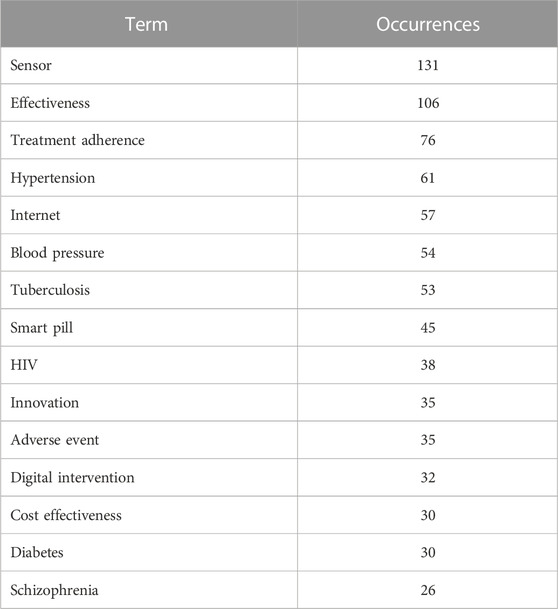
TABLE 5. The top keywords in publishing and scientific activity in the field of digital pills for the period January 2012 to July 2022.
The complex nature of the topic of digital pills allows us to conclude that promising areas of research may be at the intersection of two or more clusters.
Of greatest interest in terms of identifying the most significant and discussed problems in the area of digital pills are the most cited research publications. The works that caused most resonance in academic science are given in Table 6.
A study by Haddad et al. (Haddad et al., 2014), aimed at investigating the problems of nonadherence with treatment regimens in schizophrenia and management strategies, takes first place in the ranking. According to VOSviewer, this study was cited the most (329 times) from 2014 to the present. The authors note that nonadherence with the treatment regimen is observed in all chronic diseases but is especially dangerous in schizophrenia. Assessing plasma neuroleptic levels for adherence monitoring is challenging. The individual variability of the drug in plasma is high. In addition, metabolism, smoking, and drug interactions affect the concentrations of antipsychotics. The benefits of an ingestible event marker developed by Proteus are noted. The need for further research on electronic monitoring of the use of neuroleptics is emphasized.
The next most cited study is by Schueler et al. (Schueler et al., 2012), dedicated to the emergence of “smart sensors” with computing capabilities connected to each other wirelessly. Sensors form networks and identify and monitor various processes, health conditions, etc.
The third most cited work by Kalantar-Zade et al. (Kalantar-Zadeh et al., 2018) was aimed at directly analysing pilot trials of electronic capsules, providing information on the chemical composition of the intestine. The authors proposed an accurate and safe tool to monitor the effects of diet on people’s digestion, which can be used as a diagnostic tool for the gut. Subject gas profiles were obtained by varying dietary fiber intake and modulating the enzymatic activity of gut microbes. Ultrasound imaging confirmed that the oxygen equivalent concentration profile could be used as an accurate marker of the capsule location.
A significant contribution to the systematization of digital pill representations is made by the study by Alipanah et al. (Alipanah et al., 2018). Unlike previously published papers, there has been a systematic review and meta-analysis of 129 studies to ensure adherence to tuberculosis treatment, including directly observed therapy (DOT), training and counselling, financial reward, reminders, psychological support, digital tools, and combinations thereof. Tuberculosis outcomes were found to improve with the use of combined adherence measures. The addition of other compliance interventions to the DOT correlates with reduced mortality rates and higher rates of treatment and recovery. The conducted meta-analysis has limitations. First, contemporary, more integrated approaches to HIV/TB care were not assessed in this review. Second, the studies under review were heterogeneous in their methodology.
A study by Choudhry et al. (Choudhry et al., 2017) examines the comparative effects of diverse reminder devices on treatment adherence in 53,480 patients. It was found that reminder devices did not improve patient adherence to treatment. The authors attribute the obtained results to patients’ initial suboptimal adherence and device use difficulties. Further research is needed to implement effective tools to increase adherence to treatment.
The obtained Dimensions Database results were analysed and systematized regarding comparative clinical trials, pharmacokinetic parameters, physicians’ opinions, and the socio-economic aspect of digital tablets with a swallowed sensor. Discussion related to the respective findings is given below.
The data presented indicate that companies and government funding agencies are interested in conducting research on the possibility of using digital pills.
The conducted investigation reveals uses of digital pills in the following fields: cancer, tuberculosis, transplantology, HIV/AIDS, cardiovascular disorders, diabetes mellitus, gastroenterology (including hepatitis C), and pain relief. According to the studies mentioned above, the use of digital pills would boost patient safety, enhance treatment outcomes, promote compliance, shorten hospital stays, and provide mobile clinical monitoring.
It should be noted that the incorporation of information technologies into medicine is undeniably important because missed diagnoses, delayed treatment, and poor adherence to treatment result in disease progression, disability, and, in some cases, death.
The available publications also confirm the safety of Proteus digital pills. The ingestible sensor is made of copper chloride, magnesium, and silicon and signals the patch when it collides with gastric acid. The amount of minerals released into the body is much less than the levels commonly found in a typical diet (Flore, 2021).
Plowman R. S., et al., note that under Section 21 of the Code of Federal Regulations (CFR), Proteus digital pills have been deemed safe and effective for general use by the public in multiple domains, including: performance testing, human factors, electrical safety, electromagnetic compatibility, toxicology, clinical testing, and biocompatibility testing, which meet the special controls of the FDA (Plowman et al., 2018).
However, there is evidence of the side effects of the Proteus digital pills. Peter Chai notes that of the 16 studies that used the Proteus digital pills, 10 studies reported local skin irritation, described as inflammation, at the patch site in 13.2% of participants (Chai et al., 2022).
Studies in the Dimensions Database have revealed that recently, data from pharmacokinetic parameter studies have been obtained for digital pills. This can serve as evidence of bioequivalence relative to non-digital dosage forms or reveal possible benefits.
Thus, comparative pharmacokinetic studies of the traditional form of Truvada (enofovir disoproxil fumarate/emtricitabine) and its encapsulated form (n = 24) were carried out in a digital system with an oral sensor (Proteus Digital Health) (Ibrahim et al., 2018). The authors conclude that the investigational formulations are bioequivalent.
Another study (n = 49) examined the pharmacokinetics of combined antiretroviral (ARV) medicines encapsulated in an ingestible sensor system (Proteus Digital Health Feedback) (Liu et al., 2020). The following combinations were used as ARVs: emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF); FTC/tenofovir alafenamide (TAF); efavirenz (EFV)/FTC/TDF; abacavir (ABC)/lamivudine (3 TC); dolutegravir (DTG)/ABC/3TC; rilpivirine (RPV)/TAF/FTC; elvitegravir (EVG)/cobicistat (COBI)/FTC/TAF; and bictegravir (BIC)/FTC/TAF. The obtained experimental results were compared with the literature data. The Cmax and AUC values were not statistically significantly different from the literature data for all formulations except for the Cmax of FTC/TDF, the Cmax of BIC, and the Cmax of RPV. The authors attribute the observed deviation in FTC/TDF (Truvada) to participants’ characteristics and fasting/feeding conditions. There is no doubt that further pharmacokinetic studies of digital pills with encapsulated ARVs and traditional forms of ARVs are necessary in order to identify bioequivalence and possible benefits.
The presented data indicates that in pharmacokinetic parameters, encapsulated ARVs with digital pills are not inferior to traditional forms. That is, they are bioequivalent but have advantages in adherence to treatment.
While the increase in adherence when using digital pills was not in doubt, the comparative effectiveness of digital pills and traditional forms has not been sufficiently studied, and it is also important to identify side effects.
In this regard, it is of interest to acknowledge the work of Browne et al. on the comparative study of therapy with wirelessly observed therapy using digital pills (WOT) and directly observed therapy (DOT) during the treatment of tuberculosis (n = 77) with a combination of isoniazid 150 mg/rifampin 300 mg. 100% of the participants chose to use WOT (Browne et al., 2019). WOT-related adverse events were less than 10% and consisted of minor patches of skin rash and itching. The authors conclude that in terms of accuracy parameters, WOT was equivalent to DOT, and the vast majority of participants preferred digital therapy. This study has a number of limitations, including collecting data only during the continuation phase of tuberculosis therapy. Data are needed on the efficacy of WOT during the intensive phase of tuberculosis treatment. The authors note that DOT is resource-intensive, especially over geographical distances, time-consuming, and represents the single largest cost of tuberculosis treatment.
The use of digital pills for the treatment of tuberculosis is being investigated by other scientists. A quantitative survey was conducted among stakeholders directly involved in tuberculosis management, revealing that digital pills are seen as an acceptable, practicable, and beneficial technology for tracking antitubercular medication adherence (Vaz et al., 2022).
Of special note is the 8-week multicenter study of the efficacy and safety of antipsychotics (aripiprazole, quetiapine, olanzapine, or risperidone) encapsulated in the Proteus digital medical system in patients with schizophrenia, schizoaffective disorder, or the first episode of psychosis (n = 55), which had sufficient efficacy and tolerability (Fowler et al., 2021). There is no data on the difference in pharmacological effects of the antipsychotics studied. The study’s potential weakness was its small sample size, particularly in the schizoaffective disorder group (n = 10). It might have contributed to the group’s high degree of variability in the percentage of time with satisfactory patch coverage and adherence score. The authors concluded that it required further clinical trials.
Of particular interest also are works that are related to the study of the opinions of doctors regarding digital pills in medical practice.
A survey of 56 tuberculosis control programs in the United States revealed the promise of using directly monitored electronic therapies, including digital pills (Macaraig et al., 2018).
Ruetsch et al., performed a survey of physicians (n = 131) prescribing digital pills (Ruetsch et al., 2021). It has been established that doctors are ready to use digital technologies to improve the accuracy of the assessment of compliance with the treatment regimen.
In a pilot study (n = 49), an integrated call center is reported to have contributed to the implementation of a digital medical system with aripiprazole in patients with bipolar I disorder, major depressive disorder, and schizophrenia (Kopelowicz et al., 2017).
The issue of using digital pills has not only a medical but also a socio-economic aspect. On the one hand, timely and effective therapy with digital pills combined with a high degree of adherence to treatment significantly reduces the number of patients with disability and severe degrees of disability. Along with this, it should be noted that treatment with digital pills has a high cost. This jeopardizes the possibility of prolonged access for patients to therapy.
In this regard, the study of Forma et al. (Forma et al., 2022) involved an online survey on willingness to pay for adherence monitoring tools among individuals (n = 184) caring for patients with serious mental disorders. A high preference for the use of digital pills, which monitor not only the use of medicines but also the physical activity of patients, the quality of rest, and mood, has been identified. In addition, respondents reported a willingness to pay $255 more for digital pills versus a pill organizer.
According to the literature, there is currently insufficient information on pharmacoeconomic studies of digital pills. The emergence of such information will expand the data on the benefits of digital medicine.
Our analysis revealed that some of the funding for digital pills research is provided by private companies. Since such companies are a stakeholders with financial interests linked to research outcomes, proper disclosure of competing interests in the resulting academic works is of a great importance. From the perspective of the World Health Organisation Commission on Intellectual Property Rights, Innovation, and Public Health, public-private partnerships in general are an effective way to benefit from public and private sector opportunities to address health problems that, alone, neither party can properly address (Commission on Intellectual Property Rights and Innovation and Public Health, 2006). A bright example of a public-private research and development partnership in the European Union is the implementation of projects under the Innovative Health Initiative. The goals of this initiative are to translate health research and innovation into tangible benefits for patients and society and ensure that Europe remains at the cutting edge of interdisciplinary, sustainable, patient-centric health research. In the area of accelerating the development and admission of medicines to the market in the United States, a public-private partnership is being implemented that combines the National Institutes of Health, the US Food and Drug Administration (FDA), pharmaceutical companies, and non-profit organizations. As world experience shows, a clear organizational structure of management and appropriate regulatory support are important conditions for successful scientific research. It should be noted that research transparency and protection against unfair competition are required. These practices are meant to ensure impartiality and prevent conflicts of interest. Consequently, it should be noted the key importance of adhering to established intellectual property policy, ensuring the protection of authors’ and copyright holders’ rights, and using intellectual property while taking into account the interests of all interested parties in conjunction with public interests.
Of particular note is the comparative cost study for direct confirmation of tuberculosis treatment with wirelessly observed therapy using digital pills (WOT) and directly observed therapy (DOT) (Au-Yeung et al., 2011b). Utilizing information from public treatment sources, staff expenses, patient costs, and interview responses, the costs for the two methods of follow-up were compared. In terms of the cost of treatment in a public health facility, the cost of WOT was projected to be 36 percent of the 7-day DOT and 71 percent of the 3-day DOT. WOT was estimated to cost 4% of the 7-day DOT and 8% of the 3-day DOT to treat a patient. The authors come to the conclusion that using WOT to treat M. tuberculosis looks to be much less expensive than using DOT.
A number of clinical trials of digital pills are currently underway. A number of clinical trials are presented in Table 7. It should be noted that most clinical studies are single-arm cohort studies (uncontrolled). It is interesting to observe that none of the studies in Table 7 deals with mental.
Thus, the possibilities of using digital pills seem very promising for their further testing within the framework of various pathologies.
Nevertheless, the effects of digital pills have not yet been fully studied. Additional randomized controlled clinical trials and meta-analyses are necessary for a high level of evidence for therapeutic investigations (I level) (Burns et al., 2011).
There are a number of restrictions on both the pharmacological and clinical plans connected with their practical application.
These restrictions are defined as follows.
a) scarcity of large-scale comparative randomized clinical trials comparing the efficacy and safety of digital and non-digital forms; data from meta-analyses;
b) there is an insufficient number of pharmacokinetic studies of digital pills confirming their bioequivalence with non-digital dosage forms or identifying benefits;
c) issues related to the high cost of pharmacotherapy;
d) privacy, informed consent, data handling, and ethics issues.
The emergence of such information will expand the therapeutic arsenal of digital medicine.
The growing cost, complexity, and riskiness of innovative projects necessitate the search for new forms of interaction and cooperation between participants in the scientific and private business sphere. The pharmaceutical industry is special, given the depth of its dependence on the knowledge, intensity, and effectiveness of basic research (The 10 biggest science stories of 2022, 2022). In recent decades, the global costs of pharmaceutical companies for innovative medicine development have increased significantly (Ten years on Measuring the return from pharmaceutical innovation 2019, 2022). The listed challenges pose new tasks and problems regarding innovation policy, forcing the most powerful participants in the pharmaceutical industry and the state to look for new mechanisms to support innovative activities.
According to studies, mechanisms of public-private partnership are implemented in the field of digital medicine creation with the participation of many interested parties (states, companies, universities, non-profit organizations). The possibility of combining resources and sharing the financial burden and risks makes such projects more attractive and viable.
In conclusion, it should be noted that the importance of the problem of adherence to treatment will steadily increase in the coming years, which is associated both with the further actualization of cardiovascular disease, diabetes, HIV/AIDS, and tuberculosis in the world and with an increase in life expectancy. Further study, clinical testing, and the implementation into the practice of new effective and safe digital pills for pharmacotherapy will continue to be high priority areas of medicine.
Bibliometric analysis of the scientific literature revealed that over the past 10 years, the number of publications about digital pills has noticeably increased, which indicates the increasing importance of this field of knowledge. The leading positions in this area are occupied by scientists from the USA, the United Kingdom, and India, and the leaders in publishing and scientific activities among research organizations are Harvard University, Proteus digital health, Brigham and women’s hospital, University of Manchester, King’s college London. Sources of financial resources for authors of publications in the field of digital pills are funds from leading developer companies, budget allocations, and funds from non-commercial organizations. Public-private partnerships are an effective way to develop and implement digital pills. Clearly there is a high need for research on cheaper, non-invasive alternatives to enhance medication adherence, whereby non-profit institutions including public universities should play a more active role in such endeavors.
Using the VOSviewer program, terminology maps were built according to the selected concepts of the respective scientific literature, and their main intersection points were found. The four main clusters of digital pill studies were highlighted and visualized: efficacy and safety analysis for serious mental disorders; treatment and costs of tuberculosis therapy; features of the treatment of diabetes, cardiovascular diseases, and AIDS; and usage monitoring.
An analysis of the five most cited publications on the problems of digital tablets from 2012 to 2022 showed that the most resonant and debatable topics are: strategies for increasing adherence to treatment; types of sensors for digital therapy; and results of clinical studies and meta-analyzes.
Promising directions for further clinical studies of digital pills are: the conduct of randomized controlled trials and meta-analyses to increase the level of evidence of their effectiveness and safety; studying their pharmacokinetic parameters to confirm bioequivalence or to identify the benefit; and pharmacoeconomic analysis.
This study had several limitations. First, we conducted a review with stringent inclusion criteria; this review may have missed non-indexed articles that consider digital pills. Second, because we only included English-language papers, it is possible that we overlooked advancements in ingestible sensor technologies for digital pills that were written about in other languages.
OL and AGA designed the study. OL collected and analyzed data and wrote the first draft. EK, AWKY, NTT, OK, MK-P, and HW critically revised the draft. All authors agreed to the final version.
This study was supported by a grant from the Austrian Academy of Sciences through the academic mobility program “Joint Excellence in Science and Humanities” (JESH) for applications from Ukrainian researchers (OL is a fellowship recipient) grant number 80611.
The Ukrainian researcher acknowledge support from the Austrian Academy of Sciences and the Ludwig Boltzmann Institute for Digital Health and Patient Safety.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alipanah, N., Jarlsberg, L., Miller, C., Linh, N. N., Falzon, D., Jaramillo, E., et al. (2018). Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. PLoS Med. 15 (7), e1002595. doi:10.1371/journal.pmed.1002595
Alipour, A., Gabrielson, S., and Patel, P. B. (2020). Ingestible sensors and medication adherence: Focus on use in serious mental illness. Pharmacy 8 (2), 103. doi:10.3390/pharmacy8020103
Au-Yeung, K. Y., Moon, G. D., Robertson, T. L., Dicarlo, L. A., Epstein, M. S., Weis, S. E., et al. (2011). Early clinical experience with networked system for promoting patient self-management. Am. J. Manag. Care 17 (7), e277–e287.
Au-Yeung, K. Y., Moon, G. D., Robertson, T. L., Dicarlo, L. A., Epstein, M. S., Weis, S. E., et al. (2011). Early clinical experience with networked system for promoting patient self-management. Am. J. Manag. Care 17 (7), e277–e287. doi:10.5588/ijtld.11.0868
Baumgartner, S. L., Buffkin, D. E., Rukavina, E., Jones, J., Weiler, E., and Carnes, T. C. (2021). A novel digital pill system for medication adherence measurement and reporting: Usability validation study. JMIR Hum. Factors 8 (4), e30786. doi:10.2196/30786
Belknap, R., Weis, S., Brookens, A., Au-Yeung, K. Y., Moon, G., DiCarlo, L., et al. (2013). Feasibility of an ingestible sensor-based system for monitoring adherence to tuberculosis therapy. PLoS ONE 8 (1), e53373. doi:10.1371/journal.pone.0053373
Bonacini, M., Kim, Y., Pitney, C., McKoin, L., Tran, M., and Landis, C. (2020). Wirelessly observed therapy to optimize adherence and target interventions for oral hepatitis C treatment: Observational pilot study. J. Med. Internet Res. 22 (2), e15532. doi:10.2196/15532
Browne, S. H., Umlauf, A., Tucker, A. J., Low, J., Moser, K., Gonzalez Garcia, J., et al. (2019). Wirelessly observed therapy compared to directly observed therapy to confirm and support tuberculosis treatment adherence: A randomized controlled trial. PLoS Med. 16 (10), e1002891. doi:10.1371/journal.pmed.1002891
Browne, S., Peloquin, C., Santillo, F., Haubrich, R., Muttera, L., Moser, K., et al. (2018). Digitizing medicines for remote capture of oral medication adherence using Co-encapsulation. Clin. Pharmacol. Ther. 103 (3), 502–510. doi:10.1002/cpt.760
Burns, P. B., Rohrich, R. J., and Chung, K. C. (2011). The levels of evidence and their role in evidence-based medicine. Plastic Reconstr. Surg. 128 (1), 305–310. doi:10.1097/PRS.0b013e318219c171
Chai, P. R., Carreiro, S., Innes, B. J., Chapman, B., Schreiber, K. L., Edwards, R. R., et al. (2017). Oxycodone ingestion patterns in acute fracture pain with digital pills. Anesth. Analgesia 125 (6), 2105–2112. doi:10.1213/ANE.0000000000002574
Chai, P. R., Carreiro, S., Innes, B. J., Rosen, R. K., O’Cleirigh, C., Mayer, K. H., et al. (2017). Digital pills to measure opioid ingestion patterns in emergency department patients with acute fracture pain: A pilot study. J. Med. Internet Res. 19 (1), e19. doi:10.2196/jmir.7050
Chai, P. R., Vaz, C., Goodman, G. R., Albrechta, H., Huang, H., Rosen, R. K., et al. (2022). Ingestible electronic sensors to measure instantaneous medication adherence: A narrative review. Digit. Health 8, 20552076221083119. doi:10.1177/20552076221083119
Chevance, A., Fortel, A., Jouannin, A., Denis, F., Mamzer, M. F., Ravaud, P., et al. (2022). Acceptability of and willingness to take digital pills by patients, the public, and health care professionals: Qualitative content analysis of a large online survey. J. Med. Internet Res. 24 (2), e25597. doi:10.2196/25597
Choudhry, N. K., Krumme, A. A., Ercole, P. M., Girdish, C., Tong, A. Y., Khan, N. F., et al. (2017). Effect of reminder devices on medication adherence: The REMIND randomized clinical trial. JAMA Intern Med. 177 (5), 624–631. doi:10.1001/jamainternmed.2016.9627
Commission on Intellectual Property Rights, Innovation and Public Health (2006). Public health, innovation and intellectual property rights: Report of the commission on intellectual property rights, innovation and public health. Geneva, Switzerland: World Health Organization. Avaliable at: https://apps.who.int/iris/handle/10665/43460.
Cosgrove, L., Cristea, I. A., Shaughnessy, A. F., Mintzes, B., and Naudet, F. (2019). Digital aripiprazole or digital evergreening? A systematic review of the evidence and its dissemination in the scientific literature and in the media. BMJ EBM 24 (6), 231–238. doi:10.1136/bmjebm-2019-111204
Daar, E. S., Rosen, M. I., Wang, Y., Siqueiros, L., Shen, J., Guerrero, M., et al. (2020). Real-time and wireless assessment of adherence to antiretroviral therapy with Co-encapsulated ingestion sensor in HIV -infected patients: A pilot study. Clin. Transl. Sci. 13 (1), 189–194. doi:10.1111/cts.12701
Davis, A. M., Engkvist, O., Fairclough, R. J., Feierberg, I., Freeman, A., and Iyer, P. (2021). Public-private partnerships: Compound and data sharing in drug discovery and development. SLAS Discov. Adv. Life Sci. R D 26 (5), 604–619. doi:10.1177/2472555220982268
DHFS for Medication Adherence Support During Hospital Admissions for Person Living With HIV (2022). ClinicalTrials.gov identifier: NCT04418037. Avaliable at: https://clinicaltrials.gov/ct2/show/NCT04418037?term=NCT04418037&draw=2&rank=1 (Accessed July 25, 2022).
DiCarlo, L. A., Weinstein, R. L., Morimoto, C. B., Savage, G. M., Moon, G. L., Au-Yeung, K., et al. (2016). Patient-centered home care using digital medicine and telemetric data for hypertension: Feasibility and acceptability of objective ambulatory assessment. J. Clin. Hypertens. 18 (9), 901–906. doi:10.1111/jch.12787
Digital Health Feedback System (DHFS) for Longitudinal Monitoring of ARVs Used in HIV Pre-exposure Prophylaxis (PrEP) (2022). ClinicalTrials.gov identifier: NCT03693040. Avaliable at: https://clinicaltrials.gov/ct2/show/NCT03693040?term=NCT03693040&draw=2&rank=1 (Accessed July 27, 2022).
Digital Health Feedback System for Longitudinal Measurement of Medication Adherence During Anti-Retroviral (ARV)Therapy (2022). ClinicalTrials.gov identifier: NCT02800655. Avaliable at: https://clinicaltrials.gov/ct2/show/NCT02800655?term=NCT02800655&draw=2&rank=1 (Accessed July 27, 2022).
Eisenberger, U., Wüthrich, R. P., Bock, A., Ambühl, P., Steiger, J., Intondi, A., et al. (2013). Medication adherence assessment: High accuracy of the new ingestible sensor system in kidney transplants. Transplantation 96 (3), 245–250. doi:10.1097/TP.0b013e31829b7571
Flore, J. (2021). Ingestible sensors, data, and pharmaceuticals: Subjectivity in the era of digital mental health. New Media and Soc. 23 (7), 2034–2051. doi:10.1177/1461444820931024
Flores, G. P., Peace, B., Carnes, T. C., Baumgartner, S. L., Buffkin, D. E., Euliano, N. R., et al. (2016). Performance, reliability, usability, and safety of the ID-cap system for ingestion event monitoring in healthy volunteers: A pilot study. Innov. Clin. Neurosci. 13 (9–10), 12–19.
Forma, F., Chiu, K., Shafrin, J., Boskovic, D. H., and Veeranki, S. P. (2022). Are caregivers ready for digital? Caregiver preferences for health technology tools to monitor medication adherence among patients with serious mental illness. Digit. Health 8, 20552076221084472. doi:10.1177/20552076221084472
Fowler, J. C., Cope, N., Knights, J., Fang, H., Skubiak, T., Shergill, S. S., et al. (2021). Hummingbird study: Results from an exploratory trial assessing the performance and acceptance of a digital medicine system in adults with schizophrenia, schizoaffective disorder, or first-episode psychosis. NDT 17, 483–492. doi:10.2147/NDT.S290793
Frias, J., Virdi, N., Raja, P., Kim, Y., Savage, G., and Osterberg, L. (2017). Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension and type 2 diabetes: Prospective, open-label, cluster-randomized pilot clinical trial. J. Med. Internet Res. 19 (7), e246. doi:10.2196/jmir.7833
Haddad, P., Brain, C., and Scott, J. (2014). Nonadherence with antipsychotic medication in schizophrenia: Challenges and management strategies. PROM 5, 43–62. doi:10.2147/PROM.S42735
Hafezi, H., Robertson, T. L., Moon, G. D., Au-Yeung, K. Y., Zdeblick, M. J., and Savage, G. M. (2015). An ingestible sensor for measuring medication adherence. IEEE Trans. Biomed. Eng. 62 (1), 99–109. doi:10.1109/TBME.2014.2341272
Hatch, A. R. (2018). Digital mental health drug raises troubling questions. Avaliable at: https://theconversation.com/digital-mental-health-drug-raises-troubling-questions-97510 (Accessed July 12, 2022).
Hingle, M., Patrick, H., Sacher, P. M., and Sweet, C. C. (2019). The intersection of behavioral science and digital health: The case for academic-industry partnerships. Health Educ. Behav. 46 (1), 5–9. doi:10.1177/1090198118788600
Holender, A., Sutton, S., and De Simoni, A. (2018). Opinions on the use of technology to improve tablet taking in >65-year-old patients on cardiovascular medications. J. Int. Med. Res. 46 (7), 2754–2768. doi:10.1177/0300060518770578
Ibrahim, M. E., Brooks, K. M., Castillo-Mancilla, J. R., McHugh, C., Morrow, M., Brothers, J., et al. (2018). Short communication: Bioequivalence of tenofovir and emtricitabine after coencapsulation with the Proteus ingestible sensor. AIDS Res. Hum. Retroviruses 34 (10), 835–837. doi:10.1089/AID.2018.0081
Kalantar-Zadeh, K., Berean, K. J., Ha, N., Chrimes, A. F., Xu, K., Grando, D., et al. (2018). A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat. Electron 1 (1), 79–87. doi:10.1038/s41928-017-0004-x
Kamal, S., Rosen, M. I., Lazar, C., Siqueiros, L., Wang, Y., Daar, E. S., et al. (2020). Perceptions of people living with HIV and HIV healthcare providers on real-time measuring and monitoring of antiretroviral adherence using ingestible sensors: A qualitative study. AIDS Res. Treat. 2020, 10981091–10981110. doi:10.1155/2020/1098109
Kopelowicz, A., Baker, R. A., Zhao, C., Brewer, C., Lawson, E., and Peters-Strickland, T. (2017). A multicenter, open-label, pilot study evaluating the functionality of an integrated call center for a digital medicine system to optimize monitoring of adherence to oral aripiprazole in adult patients with serious mental illness. NDT 13, 2641–2651. doi:10.2147/NDT.S143091
Litvinova, O., Klager, E., Tzvetkov, N. T., Kimberger, O., Kletecka-Pulker, M., Willschke, H., et al. (2022). Digital pills with ingestible sensors: Patent landscape analysis. Pharm. (Basel, Switz. 15 (8), 1025. doi:10.3390/ph15081025
Liu, H., Daar, E., Wang, Y., Siqueiros, L., Campbell, K., Shen, J., et al. (2020). Pharmacokinetics of coencapsulated antiretrovirals with ingestible sensors. AIDS Res. Hum. Retroviruses 36 (1), 65–74. doi:10.1089/AID.2019.0202
Macaraig, M., Lobato, M. N., McGinnis Pilote, K., and Wegener, D. (2018). A national survey on the use of electronic directly observed therapy for treatment of tuberculosis. J. Public Health Manag. Pract. 24 (6), 567–570. doi:10.1097/PHH.0000000000000627
Martani, A., Geneviève, L. D., Poppe, C., Casonato, C., and Wangmo, T. (2020). Digital pills: A scoping review of the empirical literature and analysis of the ethical aspects. BMC Med. Ethics 21 (1), 3. doi:10.1186/s12910-019-0443-1
Monitoring Vital Signs With a Wireless Ingestible Device in Subjects Undergoing Polysomnography (2022). ClinicalTrials.gov identifier: NCT05183529. Avaliable at: https://clinicaltrials.gov/ct2/show/NCT05183529?term=NCT05183529&draw=2&rank=1 (Accessed July 27, 2022).
Moorhead, P., Zavala, A., Kim, Y., and Virdi, N. S. (2017). Efficacy and safety of a medication dose reminder feature in a digital health offering with the use of sensor-enabled medicines. J. Am. Pharm. Assoc. 57 (2), 155–161. doi:10.1016/j.japh.2016.12.067
Mukherjee, S., Suleman, S., Pilloton, R., Narang, J., and Rani, K. (2022). State of the art in smart portable, wearable, ingestible and implantable devices for health status monitoring and disease management. Sensors 22 (11), 4228. doi:10.3390/s22114228
Noble, K., Brown, K., Medina, M., Alvarez, F., Young, J., Leadley, S., et al. (2016). Medication adherence and activity patterns underlying uncontrolled hypertension: Assessment and recommendations by practicing pharmacists using digital health care. J. Am. Pharm. Assoc. 56 (3), 310–315. doi:10.1016/j.japh.2016.01.005
Peters-Strickland, T., Pestreich, L., Hatch, A., Rohatagi, S., Baker, R. A., Docherty, J., et al. (2016). Usability of a novel digital medicine system in adults with schizophrenia treated with sensor-embedded tablets of aripiprazole. NDT 12, 2587–2594. doi:10.2147/NDT.S116029
Plowman, R. S., Peters-Strickland, T., and Savage, G. M. (2018). Digital medicines: Clinical review on the safety of tablets with sensors. Expert Opin. Drug Saf. 17 (9), 849–852. doi:10.1080/14740338.2018.1508447
Profit, D., Rohatagi, S., Zhao, C., Hatch, A., Docherty, J. P., and Peters-Strickland, T. S. (2016). Developing a digital medicine system in psychiatry: Ingestion detection rate and latency period. J. Clin. Psychiatry 77 (09), e1095–e1100. doi:10.4088/JCP.16m10643
Quantification of Tenofovir Alafenamide Adherence (QUANTI-TAF) (2022). ClinicalTrials.gov identifier: NCT04065347. Avaliable at: https://clinicaltrials.gov/ct2/show/NCT04065347?term=NCT04065347&draw=2&rank=1 (Accessed April 27, 2022).
Remote Methadone Ingestion Surveillance Trial (RMIST) (2022). ClinicalTrials.gov identifier: NCT05259501. Avaliable at: https://clinicaltrials.gov/ct2/show/NCT05259501?term=NCT05259501&draw=2&rank=1 (Accessed July 27, 2022).
Rosenbaum, L. (2018). Swallowing a spy — the potential uses of digital adherence monitoring. N. Engl. J. Med. 378 (2), 101–103. doi:10.1056/NEJMp1716206
Ruetsch, C., Davis, T., Liberman, J. N., Velligan, D. I., Robinson, D., Jaeger, C., et al. (2021). Prescriber attitudes, experiences, and proclivities toward digital medicine and how they influence adoption of digital medicine platforms. NDT 17, 3715–3726. doi:10.2147/NDT.S318344
Schueler, M., Mandel, C., Puentes, M., and Jakoby, R. (2012). Metamaterial inspired microwave sensors. IEEE Microw. 13 (2), 57–68. doi:10.1109/MMM.2011.2181448
Sulkowski, M., Luetkemeyer, A. F., Wyles, D. L., Martorell, C., Muir, A., Weisberg, I., et al. (2020). Impact of a digital medicine programme on hepatitis C treatment adherence and efficacy in adults at high risk for non-adherence. Aliment. Pharmacol. Ther. 51 (12), 1384–1396. doi:10.1111/apt.15707
Ten years on Measuring the return from pharmaceutical innovation 2019 (2022). Report from the deloitte centre for health solutions. Avaliable at: https://www2.deloitte.com/content/dam/Deloitte/uk/Documents/life-sciences-health-care/deloitte-uk-ten-years-on-measuring-return-on-pharma-innovation-report-2019.pdf (Accessed December 26, 2022).
The 10 biggest science stories of 2022 (2022). Chosen by scientists. Avaliable at: https://www.theguardian.com/science/2022/dec/18/the-10-biggest-science-stories-of-2022-chosen-by-scientists (Accessed December 26, 2022).
Thompson, D., Mackay, T., Matthews, M., Edwards, J., Peters, N. S., and Connolly, S. B. (2017). Direct adherence measurement using an ingestible sensor compared with self-reporting in high-risk cardiovascular disease patients who knew they were being measured: A prospective intervention. JMIR Mhealth Uhealth 5 (6), e76. doi:10.2196/mhealth.6998
Triplett, K. N., El-Behadli, A. F., Masood, S. S., Sullivan, S., and Desai, D. M. (2019). Digital medicine program with pediatric solid organ transplant patients: Perceived benefits and challenges. Pediatr. Transpl. 23 (7), e13555. doi:10.1111/petr.13555
Keywords: digital pill, ingestible sensor, efficiency, safety, medication adherence, clinical trials
Citation: Litvinova O, Klager E, Yeung AWK, Tzvetkov NT, Kimberger O, Kletecka-Pulker M, Willschke H and Atanasov AG (2023) Bibliometric analysis and evidence of clinical efficacy and safety of digital pills. Front. Pharmacol. 14:1023250. doi: 10.3389/fphar.2023.1023250
Received: 21 September 2022; Accepted: 12 January 2023;
Published: 23 January 2023.
Edited by:
Diana Pinho, University of Minho, PortugalReviewed by:
Larissa J. Maier, University of California, San Francisco, United StatesCopyright © 2023 Litvinova, Klager, Yeung, Tzvetkov, Kimberger, Kletecka-Pulker, Willschke and Atanasov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olena Litvinova, aGxpdHZpbm92YUBnbWFpbC5jb20=; Atanas G. Atanasov, QXRhbmFzLkF0YW5hc292QGRocHMubGJnLmFjLmF0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.