- 1Department of Pharmacology, Faculty of Pharmaceutical Sciences, Government College University Faisalabad, Faisalabad, Pakistan
- 2Riphah Institute of Pharmaceutical Sciences, Riphah International University, Lahore Campus, Lahore, Pakistan
- 3Department of Medical Laboratory Sciences, College of Health Sciences, Sharjah Institute for Medical Research, University of Sharjah, Sharjah, United Arab Emirates
- 4Pre-Clinical Research Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
- 5Department of Physiology, Neuroscience Unit, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
- 6Department of Pharmacy, Southeast University, Banani, Dhaka, Bangladesh
- 7Department of Medical Laboratory Sciences, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
- 8Hematology Research Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
- 9Animal House Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
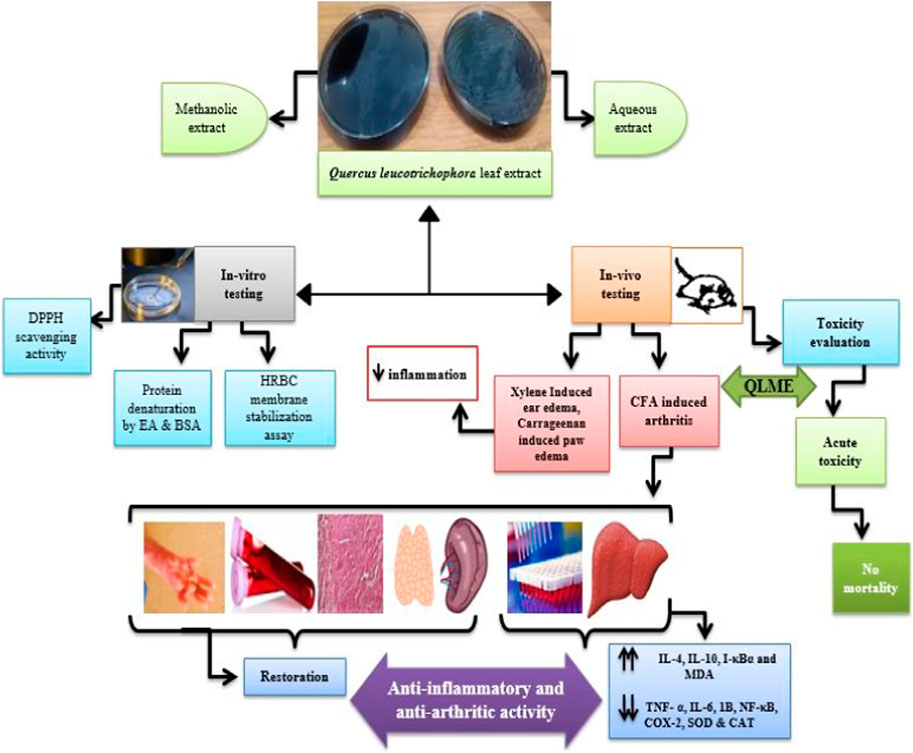
Introduction: This research was conducted to validate the folkloric use of Quercus leucotrichophora (QL) leaf extracts against inflammation and arthritis and to determine the chemical composition using HPLC.
Method: The aqueous and methanolic extracts of QL were evaluated by in vitro anti-oxidant, anti-inflammatory (inhibition of protein denaturation and membrane stabilization) assays, and in vivo anti-inflammatory (carrageenan and xylene-induced edema) and anti-arthritic models. For anti-arthritic potential, 0.1 mL Complete Freund’s Adjuvant (CFA) was inoculated into the left hind paw of a Wistar rat on day 1, and oral dosing with QL methanolic extract (QLME) at 150, 300, and 600 mg/kg was begun at day 8 till the 28th day in all groups, except disease control that was given distilled water, while methotrexate was given as standard treatment.
Results and discussion: There was a noteworthy (p < 0.05–0.0001) restoration in body weight, paw edema, arthritic index, altered blood parameters, and oxidative stress biomarkers in treated rats as compared to the diseased group. Moreover, QLME treatment significantly (p < 0.0001) downregulated TNF-α, IL-6, IL-1β, COX-2, and NF-κB, while significantly (p < 0.0001) upregulating IL-10, I-κB, and IL-4 in contrast to the diseased group. The QLME exhibited no mortality in the acute toxicity study. It was concluded that QLME possessed substantial anti-oxidant, anti-inflammatory, and anti-arthritic potential at all dosage levels prominently at 600 mg/kg might be due to the presence of quercetin, gallic, sinapic, and ferulic acids.
Introduction
Inflammation is thought to be a body’s non-specific immune response as a result of infection or injury, detecting foreign invaders and triggering an immune response or healing. The process of inflammation can be assessed by major cardinal signs at the tissue level that include tumor (swelling), calor (heat), rubor (redness), dolor (pain), and functio laesa (loss of function) (Stankov, 2012).
Rheumatoid arthritis (RA), a chronic inflammatory auto-immune syndrome of intricate etiology, is symbolized by morning stiffness, fatigue, persistent swelling of joints, erosive synovitis, and joint rigidity that begins predominantly in small diarthrodial joints of hands and feet. It affects 0.5%–1% of the adult population worldwide. Its susceptibility increases with age. The risk factors for RA include diet, smoking, hormones, caffeine, and genetic abnormalities (Firestein, 2003; Oliver and Silman, 2006).
RA is a multifaceted disease clinically identified by the existence of rheumatoid factor (RF) or anti-citrullinated protein antibody in the sera of affected persons. There is also an augmented discharge of pro-inflammatory cytokines, for instance, tumor necrosis factor (TNF)-α, interleukin (IL)-6, 1β, and metabolic enzymes like cyclooxygenase (COX)-2 and lipoxygenases (LOX) from activated immune cells. However, the level of anti-inflammatory cytokines (IL-4 and 10) is decreased in affected individuals (Harrison et al., 2021; Zhang, 2021). Oxidative stress is another trigger factor for RA in addition to pro-inflammatory cytokines.
RA management includes medications, physiotherapy, counseling of patients, nutrition, and surgery. The medications include non-steroidal anti-inflammatory drugs NSAIDs (diclofenac, ibuprofen, and piroxicam); glucocorticoids (prednisolone and dexamethasone); biologics; anti-TNF-α; gold compounds; and disease-modifying anti-rheumatic drugs (DMARDs) (Placha and Jampilek, 2021). As RA is not curable, its lifelong therapy is expensive and complicated, with various adverse effects such as ulceration, bone marrow depression, cardiovascular disorders, hypertension, hepatic damage, and nephrotoxicity. The practice of using medicinal plants such as Nigella sativa, Moringa oleifera, Polystichum braunii, and Caralluma tuberculata for treating various diseases, for instance, arthritis, asthma, diabetes, and microbial infections, provides a preferable alternative due to its low cost, ease of access, and minimum harmful implications (Shabbir et al., 2018).
Quercus leucotrichophora A. Camus (QL) belongs to the Fagaceae family. It is a deciduous evergreen tree and is commonly called “Rein”. The genus Quercus includes about 450 species. QL is widespread in Asia, Europe, North Africa, and Central and South America, among other regions with trees of this genus. QL grows in temperate and tropical climatic regions and is used as an indigenous medicine for RA in the Rawalpindi district, Pakistan. Over the centuries, the various species of the genus Quercus (Quercus dilatata and Quercus incana) have been traditionally utilized for treatment of pain, inflammation, and arthritis (Moreno-Jimenez et al., 2015); (Taib et al., 2020).
The plant (leaves, bark, and wood) is used as a folkloric remedy to treat rheumatism, asthma, dysentery, diuretic, backache, cough, fever, and joint pain (Saqib et al., 2014). The leaf, seed, and bark of QL are used for therapeutic purposes for livestock also. Its bark and leaves contained 23 and 62 constituents, respectively, with profound anti-bacterial activity (Semwal et al., 2018). It has significant antioxidant and hepato-protective activity (Singh and Bisht, 2018). The literature survey showed that the anti-arthritic potential of QL was not scientifically validated. Therefore, the current study evaluated the acute and chronic anti-inflammatory potential of QL extracts using in vitro and in vivo methods. Moreover, acute toxicity testing and chemical characterization of the plant extracts were also investigated.
Materials and methods
Sample preparation
QL leaves from a fully grown tree ( 20 m) were collected in November 2020 from Murree, Pakistan, and were identified by a taxonomist at the University of Agriculture, Faisalabad (Voucher No. 1117-20-5b), and the sample was submitted for referencing.
The leaves (5 kg) were washed to eliminate dust and foreign particles, then dried under shade, and ground to form a coarse powder. The methanol and aqueous extracts of QL (QLME and QLAQ) were prepared by maceration. The powdered plant was soaked in methanol (1:10) for 14 days at 37 °C. Filtration was carried out using a muslin cloth followed by Whatman No. 1 filter paper, and the steps were repeated twice. Finally, filtrates were gathered and condensed using a rotary evaporator at 40°C at 100 mbar. It was then air-dried at room temperature until completely dried. The same process was repeated for preparing the QLAQ extract, while QLAQ was condensed in the rotary evaporator at 90°C (Nagappan, 2012; Abubakar and Haque, 2020).
Animals for experimentation
Healthy Wistar rats of both sexes (120–170 g; age 12–15 weeks) were obtained and kept at the Animal House of the Department of Pharmacology, Government College University, Faisalabad with a continuous supply of water and a standard pellet diet. The animals were kept in 12 h of light/dark cycles at standard humidity (60%–70%) and temperature (28°C–30°C). The approval for animal testing was acquired from The Ethical Committee of Animals, GCUF (Voucher # GCUF/ERC/2220). Undue damage to the animals was evaded.
Qualitative phytochemical analysis of extracts
The QL extracts were evaluated for presence of phytochemicals like alkaloids, tannins, phenols, saponins, flavonoids, steroids, terpenoids, proteins, and glycosides by standard procedures (Saleem et al., 2020a).
For determining total phenolic contents (TPCs), the extract (0.5 mL) was mixed with 2.2 mL of distilled water (DW) and 0.15 mL of 5% NaNO2 solution. Then, after 6 min, 0.3 mL of 10% AlCl3.6H2O was mixed and left for 5 min. Then, 1 mL of 1N NaOH solution was added and vortexed; later, the absorbance was measured at 510 nm. Gallic acid was used as the standard (Saleem et al., 2020b).
Quantitative phytochemical analysis of extracts
For total flavonoid contents (TFCs), 100 µL of the stock sample (10 mg/mL) was added to 2 mL (2% Na2CO3) and left for 2 min at room temperature. Afterward, 100 µL of 50% Folin–Ciocalteu’s reagent was added. Catechin was used as the reference. After 30 min of incubation at room temperature, absorbance was determined at 765 nm (Cheruth et al., 2016).
The HPLC analysis of QLME and QLAQ was performed by following the previously mentioned procedure (Saleem et al., 2020a). The sample preparation was performed by adding 10 mg of the extract to 5 mL of DW, and then, 12 mL of ethanol was mixed. After 5 min, 6 mL DW was added and held for 5 min, along with the addition of 10 mL of 15 M HCl, and then placed in the oven for 2 h at 90°C. A syringe filter was used for injecting the sample into HPLC. The separation of compounds was conducted using the Shim–Pack Column (Shimadzu, Japan) CLC-ODS. The mobile phase included methanol and acetonitrile in a ratio of 30:70 as solvent A and double-DW with glacial acetic acid (0.5%) as solvent B. The UV–Visible detector (SPD-10AV) was used at a wavelength of 280 nm. The retention time of standards was used to identify and quantify detected compounds.
In vitro evaluation
i. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging assay
For determining antioxidant potential, 2 mL of DPPH solution (0.04/100 mg methanol) was mixed with 1 mL solution of plant extract and 1 mL of methanol. The two-fold dilution method was used to prepare the sample solution of extracts in methanol. Absorbance was measured after 30 min at 517 nm using ascorbic acid as a reference (Saleem et al., 2020a). The % DPPH radical scavenging of the mean was calculated.
ii. Inhibition of protein denaturation assays
In the egg albumin (EA) denaturation assay, a 5 mL reaction mixture containing 0.2 mL of the EA (from a fresh egg of a hen); phosphate-buffered saline 2.8 mL (PBS) of pH 6.4; and 2 mL of plant extracts (50, 100, 200, 400, 800, and 1,600 μg/mL) was used, while the same volumes of piroxicam solution and DW were used as standard and control solutions, respectively, in place of extract solutions. Afterward, these mixtures were incubated for 15 min at 37 ± 2°C. Later, they were warmed for 5 min at 70°C. The absorbance of the mixture was measured at 660 nm (Akhtar, 2020).
For the BSA denaturation assay, a previously implemented procedure was followed (Saleem et al., 2019). Briefly, the test control (0.5 mL) contained 0.45 mL of BSA (5% w/v) and 0.05 mL of extract dilutions. The product control solution contained DW (0.45 mL) in place of the BSA solution. The standard solution contained piroxicam instead of extract solution. The pH was adjusted to 6.3, incubated for 20 min at 37°֯C, and afterward, heated at 57°C for 3 min. The absorbance was measured at 660 nm.
iii. Human red blood cell (HRBC) membrane stabilization assay
This assay was performed according to a previous procedure (Saleem et al., 2019). In brief, 3 mL of blood from a healthy volunteer was mixed with Alsever’s solution, and RBC suspension (10% v/v) was prepared using an isosaline solution after centrifugation at 3,000 rpm for 15 min.
The test solution contained phosphate buffer (1 mL), hypotonic saline (2 mL), 0.5 mL of extract, and 10% human red blood cells (0.5 mL). The test control solution contained DW, while the standard solution contained piroxicam instead of the extract. The solutions were incubated for 30 min at 37°C and centrifuged at 3,000 rpm for 15 min. The sample absorbance was measured at 560 nm, and % protection was calculated.
Acute toxicity study
It was performed by following OECD guidelines 425 up-and-down procedure. A total of 10 female rats (120–170 g) were distributed into two groups (n = 5). Briefly, l00 mg/kg dose of QLME was given and change in behavior, gait, movements, respiration, and mortality was observed at 0.5, 1, 2, and 4 h till the 48th hour. While in NC, only 1 mL of DW was given. The rats were observed for clinical signs of toxicity like mortality, body temperature, respiratory rate, and motor movements during this period. The body weight was also measured on 1, 7, and 14th days (Saleem et al., 2020b).
Acute anti-inflammatory potential in rats
i- Carrageenan-induced paw edema
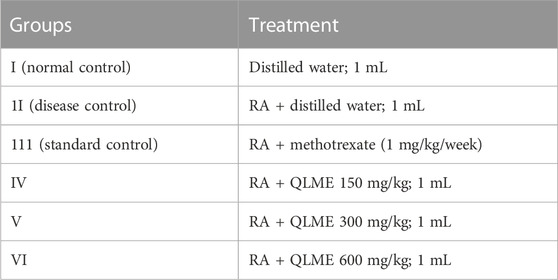
The 54 Wistar rats (120–170 g) were randomly allocated into six groups (six rats in each group): Group I served as normal control (NC) provided with DW; Group II as disease control (DC) provided with DW; Group III as standard control (SC) receiving piroxicam (10 mg/kg); and Groups IV, V and VI were given QLME at 150, 300, and 600 mg/kg and QLAQ at 150, 300, and 600 mg/kg orally, respectively.
The rats in all groups were treated as aforementioned. One hour later, 0.1 mL of carrageenan (1% w/v) solution was administered via subcutaneous injection in the left hind paw (sub-plantar region) of all rats, except NC. Paw diameter (mm) was measured at 0, 1, 2, 3, and 4 h till the 8th hour using a digital Vernier caliper (Shabbir et al., 2018).
ii- Xylene-induced ear edema
A previous procedure was adopted for xylene-induced ear edema (Shabbir et al., 2018). Briefly, a drop of xylene was applied to the inner surface of the right ear of each animal except NC, 60 min post-administration of the previously mentioned treatments. After that, the anesthetized rats were euthanized after 15 min to remove and weigh both ears. The percentage inhibition was calculated.
C: disease control; T: treatment.
Anti-arthritic potential in rats
Complete Freund’s Adjuvant- induced arthritis
The 36 Wistar rats (120–170 g) were indiscriminately allocated into six groups (n = 6). On day 1, 0.1 mL CFA (Sigma Aldrich®, United Kingdom) was injected in the left hind paw in all, excluding normal control rats (Han et al., 2016).The treatment was started from day 8 after inoculation till 28th day, and the treatment procedure is as follows. Group I served as normal control (NC) and was provided with DW. Group II was the disease control (DC) and was provided with DW. Group III was standard control (SC) and was given methotrexate (MTX) intraperitoneally. Groups IV, V, and VI were treated with QLME at 150, 300, and 600 mg/kg via orally, respectively, for 21 days.
Treatment plan
Evaluation of arthritis
The body weight and diameter of the left hind paw were determined before the first immunization and then on days 7, 12, 16, 20, 24, and 28th. The percentage inhibition of paw edema was measured. The severity of arthritis was assessed by an arthritic scoring system that ranged from 0 to 4 scales. The arthritic scoring included 0: no swelling; 1: swelling of toe joints; 2: swelling of both toes and toe joints; 3: ankle joint swelling; and 4: entire paw swelling leading to immobility (Han et al., 2016).
Effect on blood parameters
After the 28th day, the blood was collected via cardiac puncture in collecting tubes from anesthetized rats. The commercially available kit (Antec Diagnostic Products®, United Kingdom) was utilized to determine RF, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP), while an automated chemistry analyzer (Microlab 300®, Germany) was used to determine urea and creatinine. The automated hemocytometer (Sysmex, Roche®, Germany) was used to count complete blood count (CBC).
Effect on immune organs and histopathology of ankle joint
The blood collected by cardiac puncture in the EDTA tube was processed for RNA extraction. The abdomen was dissected to remove the spleen and thymus, which were then washed with DW and weighed (Saleem et al., 2020c). For histopathological evaluation, left hind limb ankle joints were removed, rinsed with DW, and placed for 24 h in 10% v/v neutral-buffered formalin. The decalcification of joints was performed by decalcifying solution (10% w/v EDTA). After mounting on slides, the tissues were stained, and the slides were observed for histopathological changes under a light microscope at 40X (Shabbir et al., 2018).
Quantification of inflammatory biomarkers by real-time (RT)-PCR
RT-PCR was used for estimating the levels of IL-4, IL-10, IL-6, IL-1β, NF-κB, TNF-α, I- ΚB, and COX-2 in the blood of rats. The RNA was removed from the collected blood by the TRIzol method as per the manufacturer’s instructions (Invitrogen®Pure Link RNA). The complementary DNA synthesis was performed using the kit manufacturer’s protocol (K1622: Thermo Scientific®, Germany). For quantification and amplification, the SYBR Select Master Mix kit (Applied Biosystems Thermo Scientific®, Germany) was used on qRT-PCR (Applied Biosystems Thermo Scientific®). The primers were selected from a previous study using GAPDH as the housekeeping gene (Saleem et al., 2020d).
Assessment of oxidative stress biomarkers
After the 28th day, rats were euthanized, and the liver was taken out for estimating superoxide dismutase (SOD), catalase (CAT) , and malondialdehyde (MDA) levels. The 10% liver homogenate (LH) was prepared (Akhtar et al., 2016). Lowry’s method was used for estimating protein content in LH (Saleem et al., 2021). The SOD, CAT, and MDA in the LH were estimated by xanthine oxidase, hydrogen peroxide, and thiobarbituric acid (TBA) assays, respectively (Bhangale and Acharya, 2016).
Statistical analysis
The data were expressed as mean ± standard deviation (S.D). In vitro assays, xylene-induced ear edema, and blood results were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test and others by two-way ANOVA followed by Tukey’ s test using GraphPad Prism® software version 7.0. p < 0.05 was considered statistically significant.
Results
The percentage yields of QLME and QLAQ were 6.1% and 4.05%, respectively.
Phytochemical analysis
It was observed that tannins, phenols, glycosides, terpenoids, and flavonoids were present in QLME and QLAQ, while alkaloids and carbohydrates were present only in QLAQ. Saponins, proteins, and steroids were absent in both extracts. QLME contained a higher amount of phenolic acid and flavonoids (TPC: 201.2 ± 0.72 mg GAE/g and TFC: 30.30 ± 0.66 mg CE/g) than QLAQ (TPC:188.07 ± 0.50 mg GAE/g and TFC: 23.37 ± 0.85 mg CE/g).
Quantitative analysis
The HPLC analysis revealed that QLME contained the highest amount of quercetin (265.53 ppm) followed by gallic acid (190.0389 ppm). The QLAQ had the highest amount of p-coumaric acid (255.34 ppm) followed by catechin (236.9 ppm). The phenolic acid and flavonoids detected in the plant extracts are listed in Table 1.
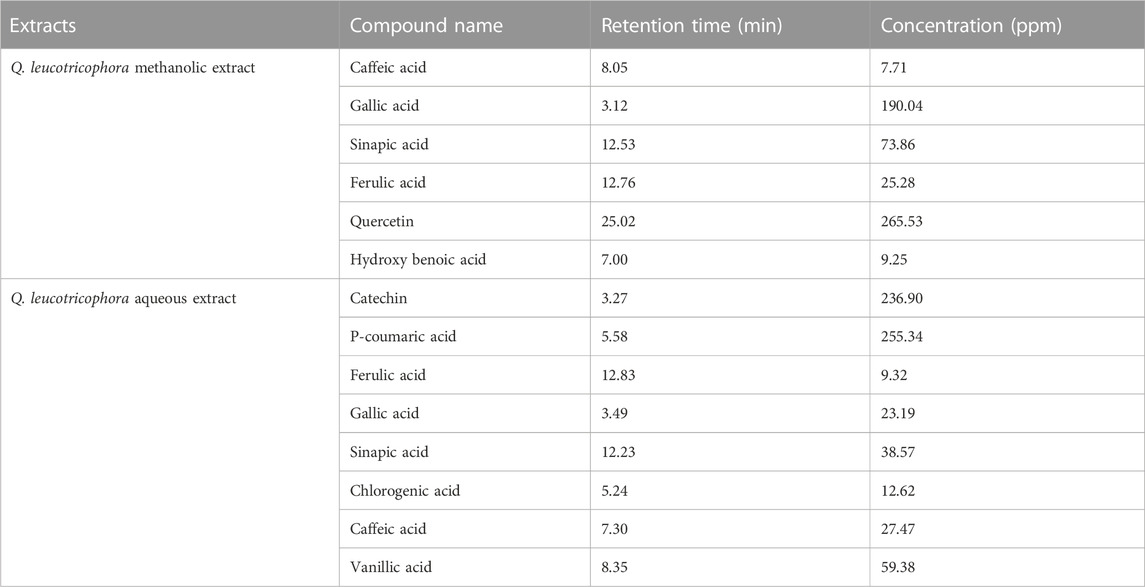
TABLE 1. HPLC profile of phytochemicals detected in methanolic and aqueous extracts of Quercus leucotrichophora.
In vitro antioxidant activity
The DPPH assay revealed that both extracts exhibited profound dose-dependent antioxidant activity. The highest % scavenging was presented by the QLME 1600 µg/mL solution than the QLAQ 1600 µg/mL solution. The antioxidant activity of QLME (67.64% ± 0.43%) and QLAQ (63.09% ± 0.65%) at 1,600 µg/mL was significantly (p < 0.0001) different as compared to that of ascorbic acid (72.75% ± 0.21%), as shown in Figure 1. The QLME exhibited significant (p < 0.05) antioxidant activity at all tested concentrations (50, 100, 200, 400, 800, and 1,600 µg/mL) in contrast to QLAQ at the respective concentration.
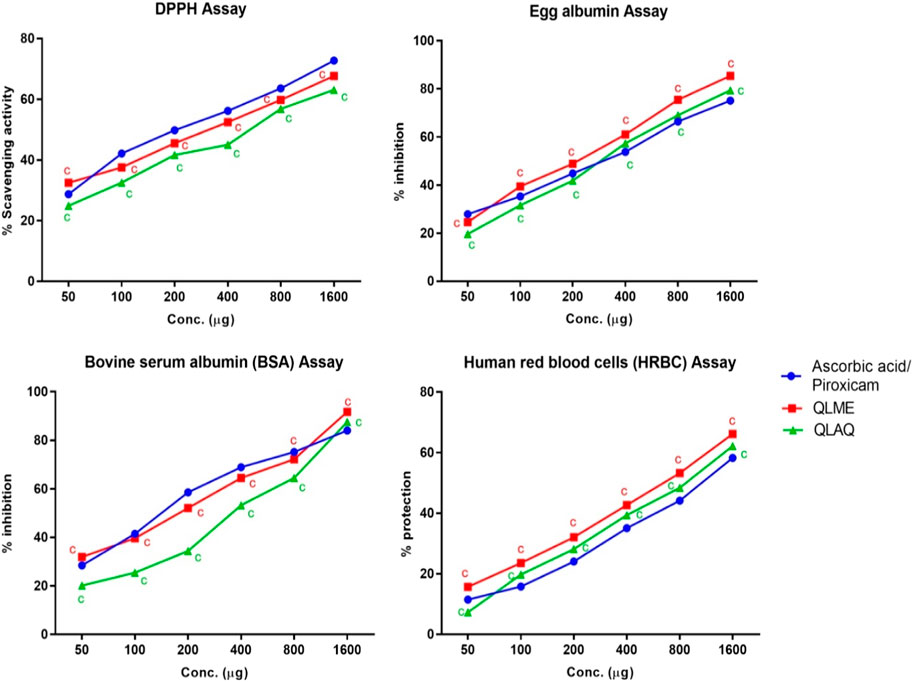
FIGURE 1. In vitro antioxidant (DPPH assay) and in vitro anti-inflammatory (protein denaturation and HRBC membrane stabilization assays) activities of Quercus leucotrichophora extracts. Results presented as mean ± S.D (n = 3). ‘c’ (p < 0.0001) in contrast to standard control. QLME: Q. leucotrichophora methanolic extracts; QLAQ: Q. leucotrichophora aqueous extracts.
Inhibition of protein denaturation
The EA and BSA denaturation inhibition assays revealed that both extracts inhibited protein denaturation at all concentrations. In the egg albumin assay, the maximum % inhibition was shown by QLME (85.42% ± 0.42%) than QLAQ (79.44% ± 0.52%) at 1,600 µg/mL, and both were significantly different as compared to piroxicam (75.13% ± 0.81%). The QLME profoundly (p < 0.0001) inhibited EA denaturation at all concentrations in contrast to QLAQ at respective concentrations. In the BSA assay, the maximum % per inhibition exerted by QLME (87.54% ± 0.73%) and QLAQ (91.74% ± 0.44%) at 1,600 µg/mL was considerably different from that of piroxicam (84.05% ± 0.54%), as displayed in Figure 1. The QLME notably inhibited (p < 0.0001) BSA denaturation in contrast to QLAQ at respective concentrations.
HRBC membrane stabilization assay
It was revealed that both extracts dose-dependently inhibited membrane lysis in RBCs (Figure 1). The increased % protection of RBC’s membrane lysis by QLME (66.16% ± 0.83%) and QLAQ (62.17% ± 0.32%) at 1,600 µg/mL was pointedly different (p < 0.0001) in comparison to that of piroxicam (58.23% ± 0.22%). The membrane stabilization activity of QLME was notably (p < 0.0001) higher than that of QLAQ.
Acute toxicity study
After administering 2000 mg/kg QLME, no marked changes in behavioral and physiological parameters were observed. The QLME LD50 was more than 2000 mg/kg as mortality was not noticed within 14 days of extract administration. There were non-significant (p > 0.05) changes in body weight of the treated group (140.3 ± 1.53 g) as compared to the NC group (139.3 ± 2.52 g) on the 14th day.
Effect on carrageenan-induced paw edema
The carrageenan administration produced a considerable (p < 0.0001) increase in paw diameter in DC rats as compared to NC and standard control from 2nd hr till 8hr hr. All treated groups showed significant (p < 0.05–0.0001) reduction in paw edema in comparison with DC and NC at respective time intervals (Table 2). The maximum reduction in paw edema exhibited by QLME (3.59 ± 0.08 mm) and QLAQ (3.77 ± 0.09) at 600 mg/kg was notably (p < 0.0001) different as compared to that of piroxicam (3.72 ± 0.49 mm). The paw diameter in all treated groups was insignificantly different from the standard control on 3–8 h except QLME 150 mg/kg. The maximum % inhibition was exhibited by QLME (29.03%) at 600 mg/kg, which was more than that of piroxicam and other groups as given in Table 2. The effect of QLME (150, 300, and 600 mg/kg) and QLAQ (150, 300, and 600 mg/kg) on reduction of paw edema was non-significant (p > 0.05) at each hour.
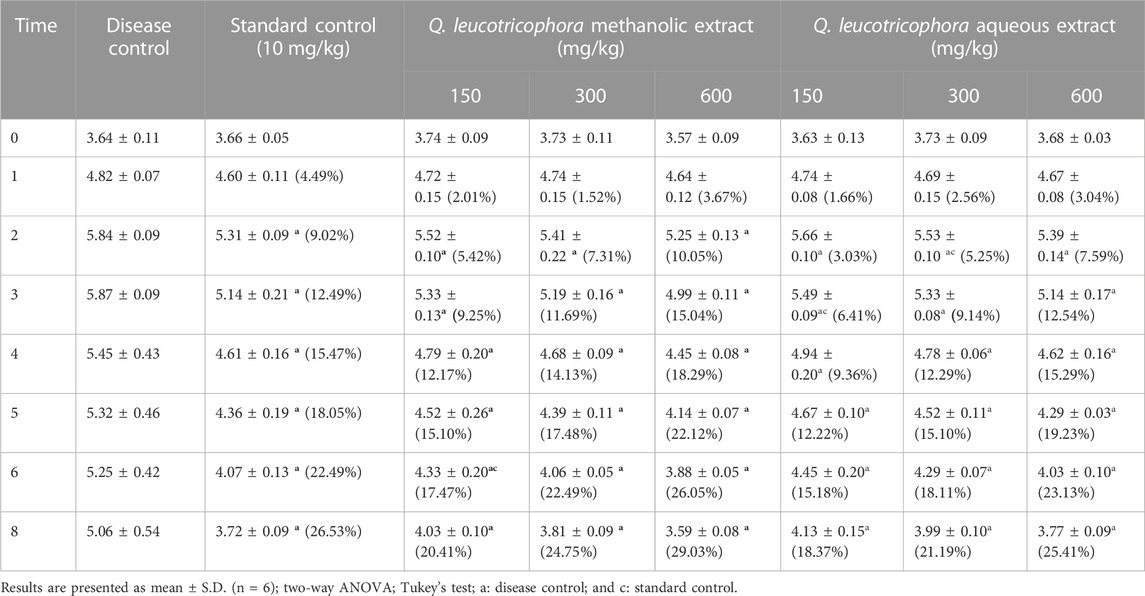
TABLE 2. Effect of Quercus leucotrichophora extracts in carrageenan-induced paw edema in rats as a function of time and administered doses.
Effect on xylene-induced ear edema
There was a considerable increase in the ear weight of DC in comparison to NC. The QLME (91.37% ± 3.24%) and QLAQ (86.42% ± 2.88%) significantly (p < 0.001) reduced ear edema at 600 mg/kg as equated to the standard control (54.94% ± 2.31%), as presented in Figure 2. The % age inhibitions in QLME and QLAQ 600 mg/kg were notably different from those in QLME and QLAQ 150 and 300 mg/kg treated groups. The QLME at 300 and 600 mg/kg insignificantly (p > 0.05) inhibited ear edema in contrast to the corresponding dosage of QLAQ, while QLME 150 mg/kg showed a significant (p < 0.05) effect than QLAQ 150 mg/kg in reducing ear edema.
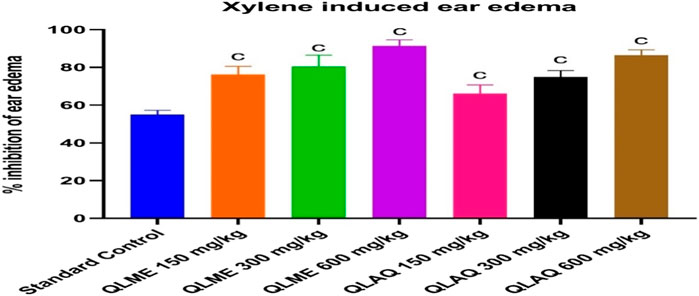
FIGURE 2. Effect of Quercus leucotrichophora extracts in xylene-induced ear edema in rats. Results presented as mean ± S.D. (n = 6); c. p < 0.001 as compared to standard control (10 mg/kg). QLME: Q. leucotricophora methanolic extract; QLAE: Q. leucotricophora aqueous extract. In the previously mentioned in vitro testing, QLME was more effective than QLAQ; therefore, QLME was selected for further study to reveal its therapeutic value in treating arthritis.
Effect on paw diameter of arthritic rats
The anti-arthritic effect of QLME was evaluated against CFA-induced arthritis in rats because it exhibited notable in vitro antioxidant and anti-inflammatory activities. On day 8, there was a considerable increase in paw diameter in all arthritic rats in contrast to NC. A substantial increase in paw diameter in DC rats was noted until the 28th day as compared to NC and standard control groups. The paw diameter in treated groups was notably different from that in NC and DC groups, while QLME-treated groups were insignificantly different from standard control groups on the 12th day (Figure 3). The dosage of 600 mg/kg QLME (4.05 ± 0.07 mm) considerably (p < 0.05) reduced paw edema along with MTX-treated rats (3.92 ± 0.16 mm) from 16 to 28th day in contrast to the NC and DC groups. The reduction in paw diameter in standard control rats insignificantly varied from the NC group on the 28th day. The maximum % inhibition was exhibited by MTX (50.02%) followed by QLME 600 mg/kg/day-treated rats (48.34%) on the 28th day, as shown in Table 3. The inhibitory effect of QLME 600 mg/kg on paw edema was insignificantly different from that of MTX on 16 to 28th day (Figure 3).
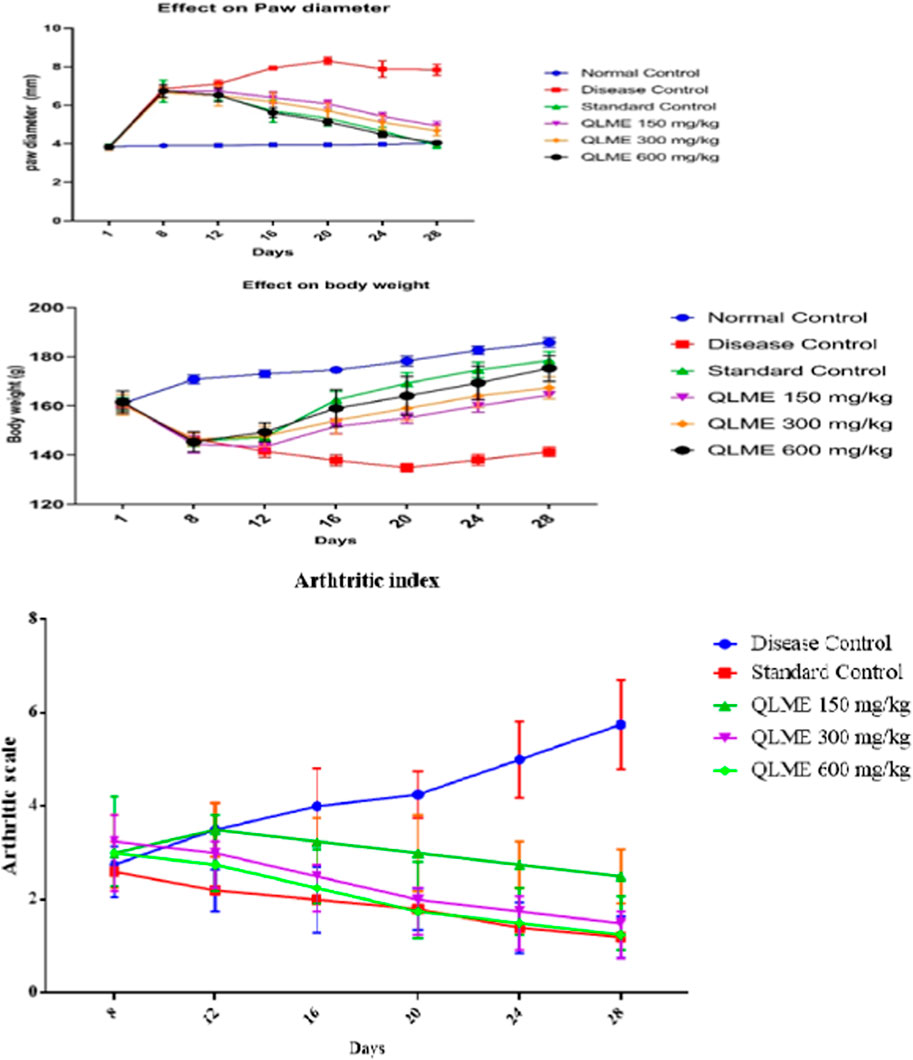
FIGURE 3. Effect of Quercus leucotrichophora extracts on paw diameter, body weight (g), and arthritic index in CFA-induced arthritic rats. Results as mean ± S.D. (n = 6). Analyzed by two-way ANOVA followed by Tukey’s test. QLME: Quercus leucotricophora methanolic extract.
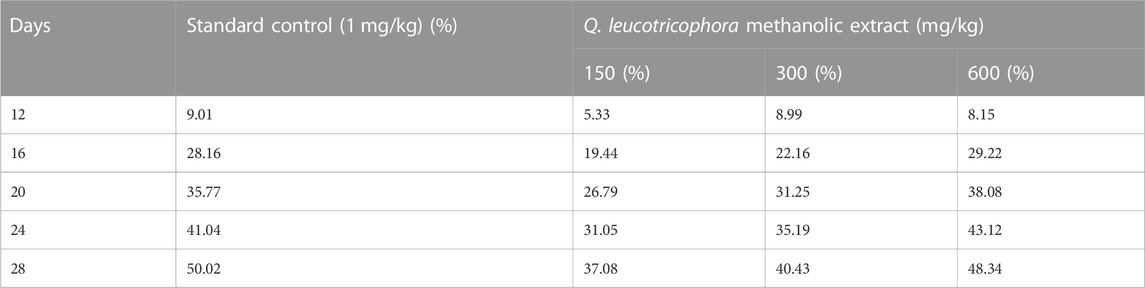
TABLE 3. Effect of Quercus leucotrichophora extract on % reduction of paw edema in CFA-induced arthritic rats.
Effect on body weight of arthritic rats
There was a significant decrease in body weight after CFA inoculation in all groups compared to NC on the eighth day. The decrease in weight was continuous in DC from the eighth day as compared to NC till the end of the study. Body weight in all treatment groups was insignificantly different from DC and standard control groups, while significantly (p < 0.0001) varying from NC on the 12th day. Body weight in all treatment groups was notably different from NC and DC groups on 16th and 20th days of the study.
However, treatment with QLME (150–600 mg/kg) and MTX caused a significant (p < 0.05) restoration of body weight from the 16 to 28th day as compared to DC and NC. Body weight in all treatment groups was notably different from NC and DC groups on 16th and 20th days of the study. Body weight in QLME 600 mg/kg-treated rats was insignificantly different from that in the standard control group on the 20th day. On the 24th day, body weight in all treated groups was significantly different from that of DC and NC, while standard control insignificantly varied from NC and QLME 600 mg/kg. On the 28th day, body weight in all treated groups was notably varied from DC, NC, and standard control groups (Figure 3).
The MTX (178.4 ± 3.65 g)- and QLME 600 mg/kg/day (175.3 ± 5.19 g)-treated rats exhibited considerable (p < 0.05) restoration in body weight on the 28th day, in contrast to DC, while the effect of QLME 600 mg/kg/day on body weight was insignificantly varied from MTX on the respective day, as given in Figure 3.
Effect on arthritic index
During the whole study, the DC group showed continuous increase in arthritic index. The treatment groups exhibited substantial (p < 0.05) restoration of the arthritic index from day 16 till the end of the study in contrast to the DC group. The maximum arthritic index was observed in the DC group on the 28th day (4.25 ± 0.96). The plant extract exerted the most pronounced effect on the 28th day in contrast to DC and standard control groups (Figure 3).
Effect on blood parameters in arthritic rats
There was a remarkable (p < 0.0001) decrease in the level of hemoglobin (Hb) and RBCs, while increase (p < 0.05) in platelets, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), RF, and total leukocyte count (TLC) in DC as compared to NC and standard control groups was observed (Figure 4). Treatment with plant extract (150–600 mg/kg) notably restored Hb and RBCs in treated groups in contrast to NC and DC groups. TLC and Hb in standard control were insignificantly varied from NC. The ESR, CRP, and RF in QLME 600 mg/kg-treated group was insignificantly varied from those of standard control. Moreover, all the treatment groups at 150–600 mg/kg restored the hematological parameters in arthritic rats, as shown in Figure 4.
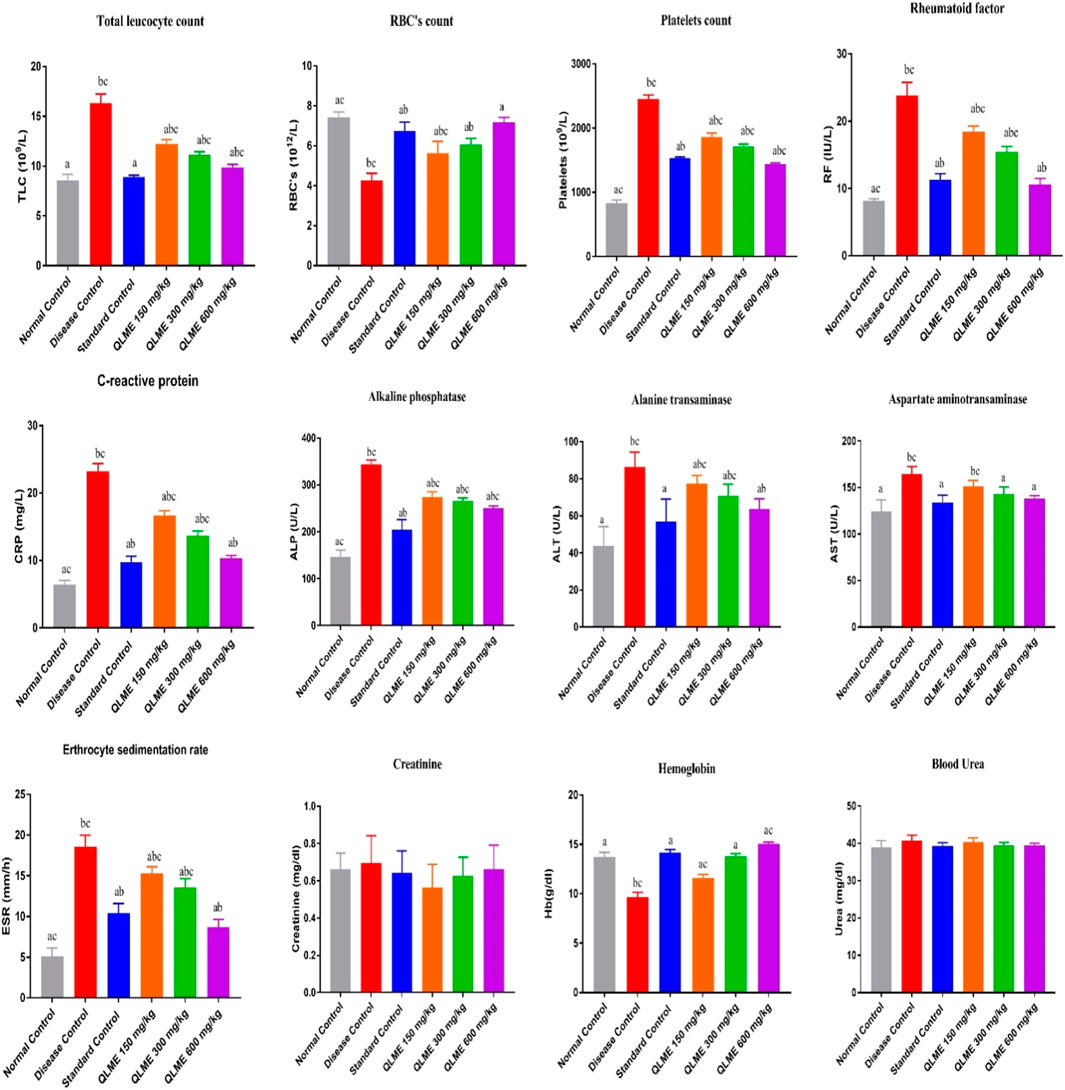
FIGURE 4. Effects of Quercus leucotrichophora extracts on blood parameters in CFA-induced arthritic rats. Results are expressed as mean ± S.D (n = 6) and one-way ANOVA followed by Tukey’s test. a: disease control; b: normal control; and c: standard control. QLME: Quercus leucotricophora methanolic extract.
There were non-significant changes on urea and creatinine levels in all arthritic rats in the 28-day study of CFA induced arthritis. The levels of ALT, ALP and AST were considerably (p < 0.05) altered in DC as compared to those of NC and standard control, and all the treatment groups significantly restored their level in arthritic rats profoundly varied from DC and NC, except AST level in QLME 150 mg/kg, which was non-significantly varied from DC (Figure 4). The level of AST in QLME 300- and 600 mg/kg-treated groups was insignificantly varied from standard control. ALP in QLME-treated groups was significantly varied from that of standard control. The ALT in the QLME 600 mg/kg-treated group was insignificantly varied from that of standard control.
Effect on weight of spleen and thymus in arthritic rats
The weight of the spleen and thymus increased in DC than in NC. The weight of the spleen and thymus was significantly (p < 0.05) restored by QLME (spleen: 0.45 ± 0.03 g; thymus: 0.23 ± 0.02 g) at 600 mg/kg- and MTX (spleen: 0.42 ± 0.01 g; thymus: 0.20 ± 0.02 g)-treated groups in contrast to DC and NC, but the weight of immune organs in the QLME 600 mg/kg-treated group was insignificantly varied from NC and standard control, as shown in Figure 5.
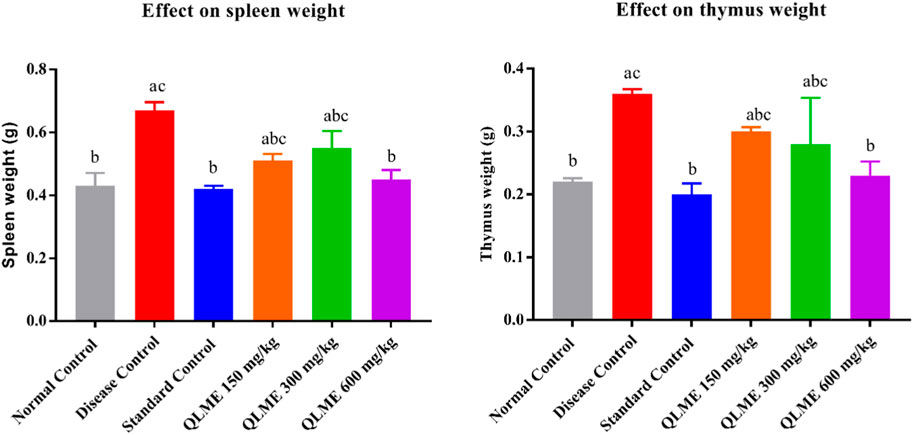
FIGURE 5. Effects of Quercus leucotrichophora extracts on immune organ weight in CFA-induced arthritic rats. Results are expressed as mean ± S.D (n = 6) and one-way ANOVA followed by Tukey’s test. a: normal control; b: disease control; and c: standard control.
Inflammatory biomarkers
The expressions of multiple inflammatory biomarkers were quantified by qRT-PCR in all rats after 28 days of CFA inoculation. It was observed that there was a remarkable (p < 0.05) downregulation of IL-10 (36.87% ± 2.23%), IL-4 (45.03% ± 1.30%), and I-kBα (46.17% ± 1.86%) along with upregulation of NF-kB (4.67 ± 0.40); COX-2 (6.23 ± 0.30); IL-6 (6.03 ± 0.42); TNF-α (4.97 ± 0.31); and IL-1β (4.17 ± 0.31-fold change) in DC as compared to NC and standard control groups. The level of these biomarkers was restored in all treatment groups in contrast to DC, NC, and standard control groups (Figure 6). However, treatment with MTX (IL-10: 95.67 ± 2.52; IL-4: 94.67 ± 3.01; I-kBα: 93.33% ± 2.52%) and QLME (IL-10: 90.67 ± 2.52; IL-4: 85.33 ± 1.53; I-kBα: 93.33% ± 2.52%) at 600 mg/kg/day had significantly (p < 0.0001) increased the expression of these genes in comparison to the DC and NC groups. Moreover, the expressions of IL-6, IL-1β, NF-κB, TNF-α, and COX-2 were significantly reduced by the QLME (NF-kB: 1.71 ± 0.20; COX-2: 1.72 ± 0.19; IL-6: 1.66 ± 0.09; TNF-α: 1.62 ± 0.29; and IL-1β: 1.67 ± 0.20-fold change) 600 mg/kg and MTX (NF-kB: 1.48 ± 0.15; COX-2: 1.45 ± 0.06; IL-6: 1.52 ± 0.09; TNF-α: 1.34 ± 0.14; and IL-1β: 1.47 ± 0.12-fold change)-treated rats in contrast to DC and NC, as given in Figure 6. TNF-α, IL-1β, IL-6, NF-κB, and COX-2 levels in QLME 600 mg/kg were insignificantly varied from those of the standard, while standard control was insignificantly different from NC. IL-4, IL-10, and I-kBα in all the QLME-treated groups were notably (p < 0.05–0.0001) varied from NC and standard control.
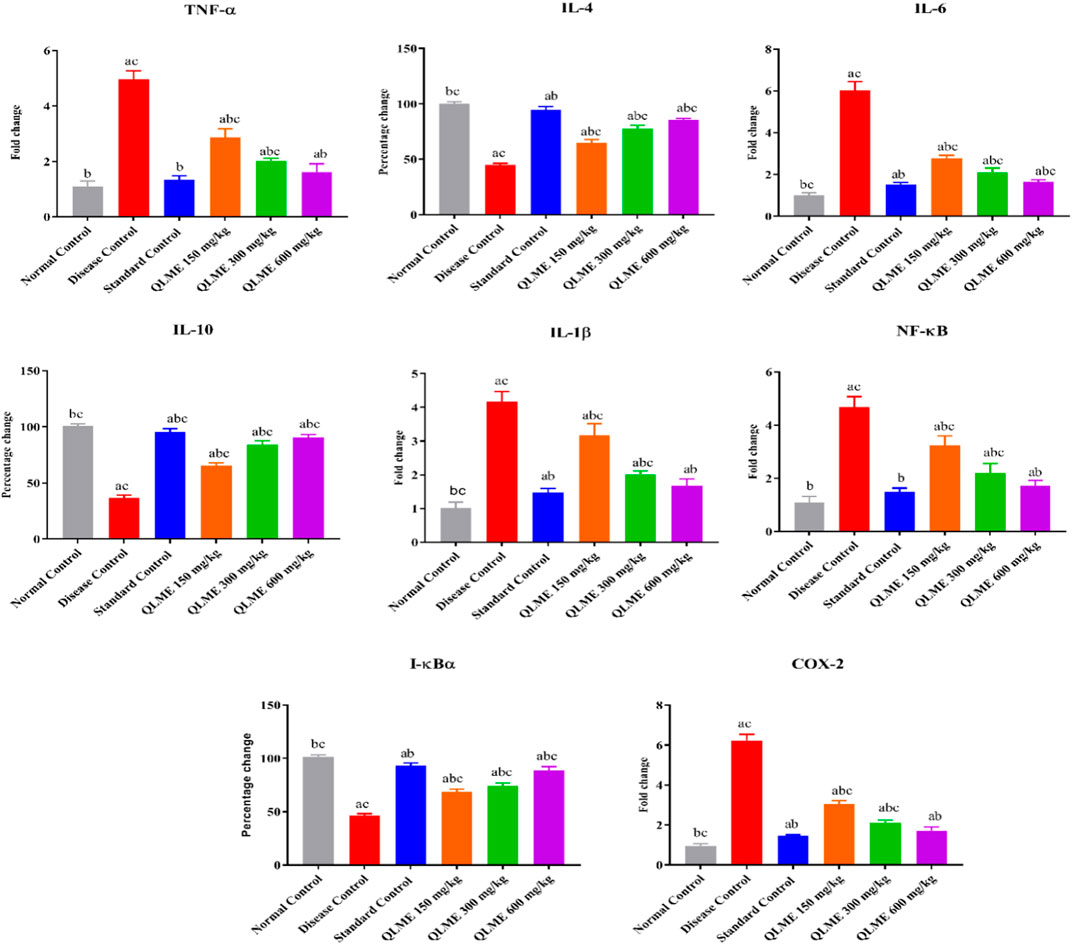
FIGURE 6. Effects of Quercus leucotrichophora extract on mRNA expression of inflammatory biomarkers in arthritic rats. Results are expressed as mean ± S.D (n = 6) and one-way ANOVA followed by Tukey’s test. a: normal control; b: disease control; and c: standard control. IL: interleukin; TNF-α: tumor necrosis factor; COX-2: cyclooxygenase-2. p < 0.05 in contrast to DC, NC, and standard control groups.
Effect on oxidative stress biomarkers
Oxidative stress was developed after CFA immunization in all rats, which was restored with the extract at all tested dosages and MTX-treated rats (Figure 7). CAT and SOD activities were significantly (p < 0.05) lowered in DC in contrast to NC and standard control. There was also an increased level of MDA in LH of DC as compared to NC and standard control. All the treatment groups (MTX and QLME) significantly (p < 0.05) improved the activities of CAT and SOD and reduced the level of MDA in arthritic rats as compared to DC and NC. The MDA level and CAT activity in standard control were insignificantly varied from those of NC. The QLME 600 mg/kg (CAT: 22.64 ± 0.35; SOD: 15.08 ± 0.34 U/mg of protein and MDA: 7.26 ± 0.12 µM/mg protein)- and MTX (CAT: 24.89 ± 0.25; SOD: 16.17 ± 0.11 U/mg of protein and MDA: 7.61 ± 0.40 µM/mg protein)-treated groups significantly (p < 0.0001) restored the levels of oxidative stress biomarkers in contrast to DC and NC, as shown in Figure 7.
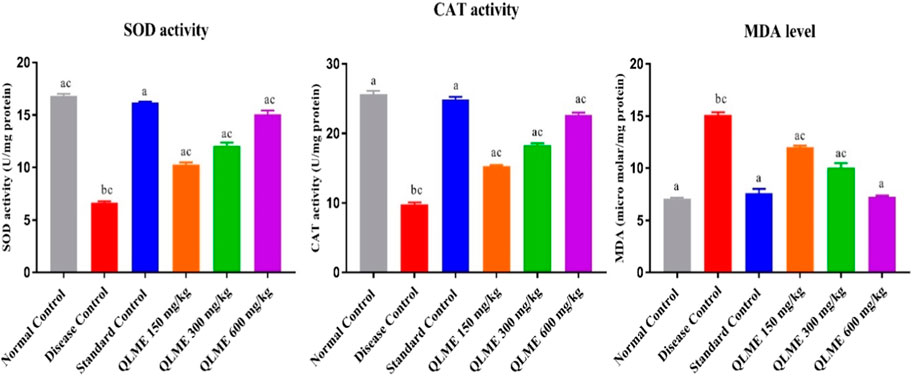
FIGURE 7. Effects of Quercus leucotrichophora extract on oxidative stress biomarkers in CFA-induced arthritic rats. Results are expressed as mean ± S.D (n = 6) and one-way ANOVA followed by Tukey’s test. a: disease control; b: normal control; and c: standard control. p < 0.05 in contrast to DC, NC, and standard control.
Effect on joint histopathology
At the end of the 28th day, the histopathology of ankle joints showed the formation of pannus, inflammation, bone erosion, and mononuclear cell infiltration in DC (Figure 8). Furthermore, NC rats were devoid of bone erosion and inflammation (Figure 8). The treatment with QLME (150, 300, and 600 mg/kg) and MTX profoundly reduced inflammation, pannus formation, bone erosion, and cellular infiltration in arthritic groups in comparison to DC, as exhibited in Figures 8C–F.
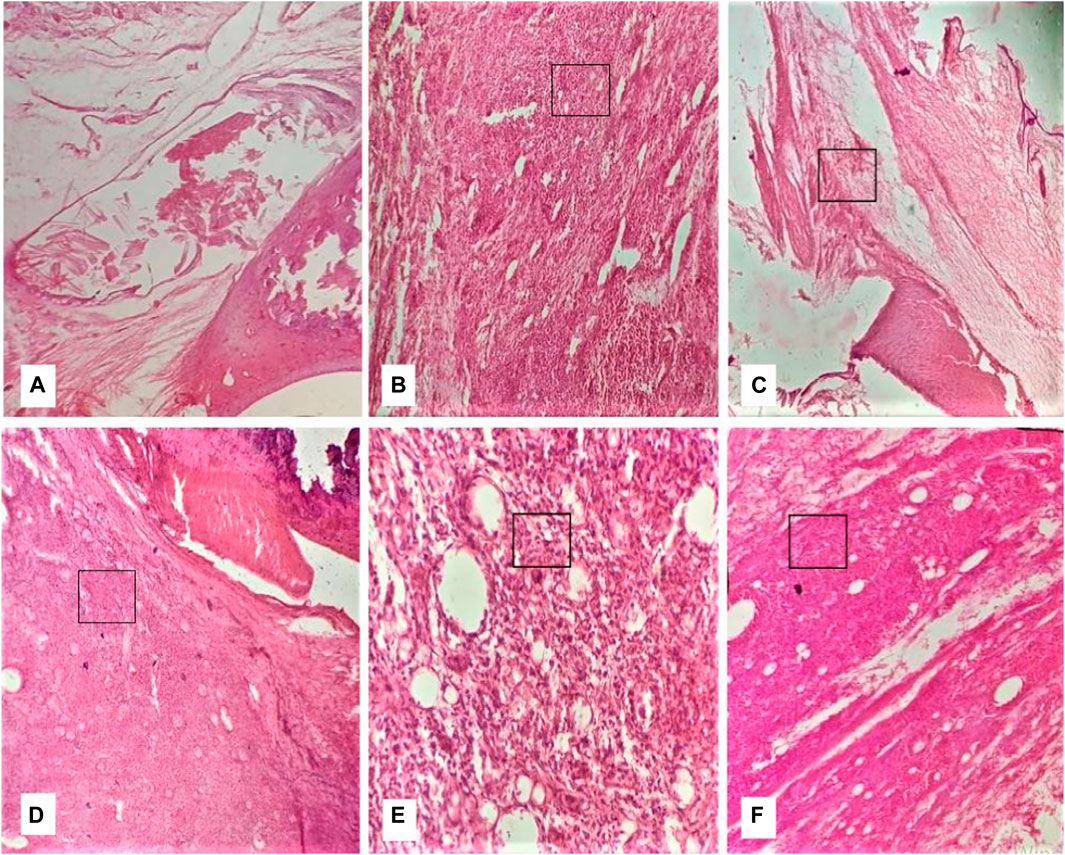
FIGURE 8. Effect of Quercus leucotrichophora on histopathology of CFA-induced arthritic rats. (A) normal group, (B) diseased group, (C) standard group, (D–F) QLME at 150, 300, and 600 mg/kg, respectively.

Discussion
The current study assessed the anti-inflammatory and anti-arthritic potential of QL. The QLME extract had shown notable anti-oxidant and in vitro anti-arthritic activities than the QLAQ extract; therefore, it was further evaluated in animals using acute anti-inflammatory tests and CFA-induced arthritis.
Excessive production of free radicals causes oxidative stress, a hallmark of numerous diseases like arthritis, cancer, and diabetes mellitus. Medicinal plants containing anti-oxidants reduce the intensity of disease associated with oxidative stress in humans. The QLME exhibited prominent anti-oxidant activity than QLAQ, probably due to the occurrence of high amounts of TFC and TPC. The flavonoids and phenols having high amounts of hydroxyl groups in plants reduced the free radicals and suppressed oxidative stress as previously described (Arulselvan et al., 2016). The QLME was safe in the acute toxicity study as it did not cause mortality till the 14th day, and LD50 was more than 2000 mg/kg.
Phytochemicals like quercetin, chlorogenic, vanillic, gallic, sinapic, and p-coumaric acids protected lysosomal degradation via free radical scavenging activity (Oyeleke et al., 2018). Protein denaturation leads to RA by the production of autoantibodies at the target site. It occurred in the presence of strong stimulus like heat, resulting in loss of the protein structure (Akhtar, 2020). Therefore, it can be assumed that the QLME showed the maximum inhibition of protein denaturation and HRBC membrane stabilization than QLAQ due to the presence of higher amounts of flavonoids and phenols as detected by HPLC analysis. It has been reported in previous studies that plants containing phenolic compounds like ferulic, gallic, vanillic, p-coumaric, and sinapic acid and quercetin and catechin have anti-oxidant, anti-inflammatory, and in vitro anti-arthritic activities (Roychoudhury et al., 2021). Quercetin inhibited neutrophil infiltration in in vitro and in vivo studies (Wang et al., 2021).
Xylene and carrageenan are considered significant inducers of inflammation. Xylene is partially involved in substance-P release, causing neurogenic inflammation, leading to release of kinins and histamine with concomitant swelling. The QLME reduced ear inflammation more than QLAQ, and its activity was comparable to that of piroxicam. A similar finding was reported previously by other Quercus species (Shabbir et al., 2018). Carrageenan induced inflammation by its actions on the complement system or via mediators of inflammation like histamine, prostaglandins (PGs), and 5-HT. In our findings, QLME at 600 mg/kg significantly reduced paw edema by inhibiting the release of inflammatory mediators like PGs, serotonin, and histamine from the activated immune cells (Peerzada et al., 2020).
The CFA was used promisingly to induce polyarthritis in rodents. The CFA suspension contains Mycobacterium tuberculosis killed by heat in un-metabolized oils that are favorably used for induction of polyarthritis in rodents that is similar in pathogenesis and symptoms to RA in humans (Saleem et al., 2020c). CFA caused arthritis in two phases. In the primary phase, inflammation occurred with the release of PGs, whereas the secondary immunological phase resulted in the production of auto-antibodies. It leads to the accumulation of mononuclear and polynuclear cells in joints, which results in structural changes in joint and cartilage via changes in cytokines (TNF-α, IL-6, and IL-1β), chemokines, tissue destructive enzymes (proteases and lysosomes), and anti-oxidant enzymes. In the current study, CFA immunization caused notable paw edema and weight loss in DC, which were restored with plant extract dose-dependently as evidenced from arthritic indices and histological findings. Weight loss is the prime feature of RA directly related to joint inflammation and might be due to malabsorption of nutrients from the intestine, muscle proteolysis, allodynia, hyperalgesia, and disease distress (Yin et al., 2019; Saleem et al., 2020c). In the current study, CFA-induced weight loss was notably restored with treatment, as evidenced by reduction in paw edema.
TNF-α causes joint destruction and synovial infiltration in RA and causes the formation of other pro-inflammatory cytokines. IL-1β causes morning stiffness and bone erosion in RA. The increased levels of CRP, anemia, and reduced levels of albumin are also due to increased level of IL-6, which leads to myocardial infarction and an extra-articular manifestation of RA (Hashizume and Mihara, 2011). The expressions of pro-inflammatory cytokines (IL-6, IL-1β, NF-κB, COX-2, and TNF-α) were reduced with treatment at all dosage levels, as evidenced by the improvement in blood parameters of arthritic rats also.
IL-4 is involved in Th2 cell production. IL-4 and IL-10 halt pro-inflammatory cytokine release from monocytes and synovial fibroblasts, and their levels were reduced in RA patients, as also evident in DC animals in the current study (Mateen et al., 2016). The levels of anti-inflammatory cytokines were increased in treated rats, as co-evidenced by the reduction of paw inflammation.
As reported in previous studies, quercetin caused inhibition of late-phase inflammation by the inhibition of TNF-α, lipoxygenase, COX-2, and phospholipase A2 (Yin et al., 2019). The QLME at 600 mg/kg/day showed profound anti-arthritic activity comparable to that of the MTX-treated group. The presence of quercetin, sinapic acid, ferulic acid, and other phenolic compounds and flavonoids in QLME might be responsible for its anti-arthritic potential by downregulating pro-inflammatory and upregulating anti-inflammatory cytokines in treated rats (Foyet et al., 2015).
Anemia is the characteristic feature of RA, as reported in diseased rats, which was noticeably restored by QLME at all dosage levels (Saleem et al., 2020c). The systemic biomarkers of arthritis like RF, CRP, ESR, WBCs, and platelets were notably altered in arthritic rats that were restored by treatment (Shabbir et al., 2018). Hyper-functioning of the immune system is recognized from splenomegaly and lymphadenopathy. The QLME notably suppresses the overactive immune system by reducing the weight of immune organ (spleen and thymus), that might be due to the presence of phytochemicals (Saleem et al., 2020c). Enhanced ROS generation occurred because of increased discharge of inflammatory cytokines from activated immune cells like neutrophils and macrophages, ultimately leading to synovium cellular infiltration (Ishibashi, 2013). In the current study, oxidative stress was reduced in treated arthritic rats in contrast to the DC group. Numerous other species of the Quercus genus such as Q. dilatata, Q. incana, Quercus sideroxyla, Quercus durifolia, Quercus eduardii, and Quercus macrocarpa have exhibited anti-inflammatory, anti-oxidant, and anti-bacterial activities (Moreno-Jimenez et al., 2015; Taib et al., 2020); (Burlacu et al., 2020).
Sinapic acid retains anti-oxidant, anti-inflammatory, anxiolytic, and anti-cancer activities (Bin Jardan et al., 2020). Catechin, a polyphenol, exhibited anti-inflammatory, antioxidant, and anti-hypertensive activities along with various other health benefits (Lee et al., 2020). Sinapic, gallic, vanillic, and ferulic acids, and quercetin and catechin detected in QLME might be responsible for in vitro and in vivo anti-inflammatory and anti-arthritic potential, as reported in previous studies (Shabbir et al., 2018). Moreover, due to the presence of these phytochemicals in QLME, the cardinal signs of inflammation have also improved in treated animals. Analysis of inflammatory and other molecular biomarkers should be performed by using other techniques, including Western blot.
Conclusion
It was concluded that QL exhibited in vitro anti-oxidant and anti-arthritic activity along with in vivo anti-inflammatory and anti-arthritic activity in Wistar rats, which might be due to the presence of ferulic acid, sinapic acid, gallic acid, and catechin and quercetin as detected with HPLC. QLME exhibited anti-arthritic potential by restoring altered blood parameters, pro-, anti-inflammatory, and oxidative stress biomarkers in treated rats. Activity-guided fractionation of Quercus leucotricophora should be performed in order to find the most active fraction. There is an immense need for activity-guided fractionation of QLME to isolate anti-arthritic components as well. HPLTC fingerprinting of plant extracts should be performed. Moreover, there should be a detailed toxicity study of QLME to assure its safety for human use.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by the Ethical Committee of Animals, Government College University Faisalabad, Faisalabad. Voucher # GCUF/ERC/2220.
Author contributions
AS, MuA: conceptualization, visualization, review, supervision, experimentation, analysis, and writing the original draft. AS and IH: experimentation and analysis, and performed literature review. AS and MuA, GA, BA, MR, and MaA: performed literature searches and editing manuscript.
Funding
This research work was funded by the Institutional Fund Projects under grant no. IFPDP-123-22. Therefore, authors gratefully acknowledge technical and financial support from Ministry of Education and Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia.
Acknowledgments
The preprint of this manuscript has been presented in “research square” and accessible with link: https://www.researchsquare.com/article/rs-1214323/v1 [Saleem et al., 2020c].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abubakar, A. R., and Haque, M. (2020). Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. bioallied Sci. 12, 1–10. doi:10.4103/jpbs.JPBS_175_19
Akhtar, M. F., Ashraf, M., Anjum, A. A., Javeed, A., Sharif, A., Saleem, A., et al. (2016). Textile industrial effluent induces mutagenicity and oxidative DNA damage and exploits oxidative stress biomarkers in rats. Environ. Toxicol. Pharmacol. 41, 180–186. doi:10.1016/j.etap.2015.11.022
Akhtar, M. F. (2020). HPLC analysis and pharmacological study of Quercus dilatata Lindle. ex Royle aqueous methanolic extract in Sprague Dawley rats. Pak. J. Pharm. Sci. doi:10.36721/PJPS.2020.33.3.SUP.1327-1332.1
Arulselvan, P., Tan, W. S., Gothai, S., Muniandy, K., Fakurazi, S., Esa, N. M., et al. (2016). Anti-inflammatory potential of ethyl acetate fraction of Moringa oleifera in downregulating the NF-κB signaling pathway in lipopolysaccharide-stimulated macrophages. Molecules 21, 1452. doi:10.3390/molecules21111452
Bhangale, J. O., and Acharya, S. R. (2016). Anti-Parkinson activity of petroleum ether extract of Ficus religiosa (L.) leaves. Adv. Pharmacol. Sci. 2016, 9436106. doi:10.1155/2016/9436106
Bin Jardan, Y. A., Ansari, M. A., Raish, M., Alkharfy, K. M., Ahad, A., Al-Jenoobi, F. I., et al. (2020). Sinapic acid ameliorates oxidative stress, inflammation, and apoptosis in acute doxorubicin-induced cardiotoxicity via the NF-κB-Mediated pathway. BioMed Res. Int. 2020, 3921796. doi:10.1155/2020/3921796
Burlacu, E., Nisca, A., and Tanase, C. (2020). A comprehensive review of phytochemistry and biological activities of Quercus species. Forests 11, 904. doi:10.3390/f11090904
Cheruth, A. J., Al Naqbi, K. M., El-Kaabi, A. A. A., Odeh, O. W., Kandhan, K., Maqsood, S., et al. (2016). In vitro antioxidant activities and screening of phytochemicals from methanolic and ethyl acetate extracts of Calligonum comosum L’Her. Orient. Pharm. Exp. Med. 16, 209–215. doi:10.1007/s13596-016-0232-z
Firestein, G. S. (2003). Evolving concepts of rheumatoid arthritis. Nature 423, 356–361. doi:10.1038/nature01661
Foyet, H. S., Tsala, D. E., Bodo, J. Z. E., Carine, A. N., Heroyne, L. T., and Oben, E. K. (2015). Anti-inflammatory and anti-arthritic activity of a methanol extract from Vitellaria paradoxa stem bark. Pharmacogn. Res. 7, 367–377. doi:10.4103/0974-8490.159569
Han, X., Su, D., Xian, X., Zhou, M., Li, X., Huang, J., et al. (2016). Inhibitory effects of Saussurea involucrata (Kar. et Kir.) Sch.-Bip. on adjuvant arthritis in rats. J. Ethnopharmacol. 194, 228–235. doi:10.1016/j.jep.2016.09.008
Harrison, M. A. A., Wise, R. M., Benjamin, B. P., Hochreiner, E. M., Mohiuddin, O. A., and Bunnell, B. A. (2021). Adipose-derived stem cells from obese donors polarize macrophages and microglia toward a pro-inflammatory phenotype. Cells 10, 26. doi:10.3390/cells10010026
Hashizume, M., and Mihara, M. (2011). The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis 2011, 765624, doi:10.1155/2011/765624
Ishibashi, T. (2013). Molecular hydrogen: New antioxidant and anti-inflammatory therapy for rheumatoid arthritis and related diseases. Curr. Pharm. Des. 19, 6375–6381. doi:10.2174/13816128113199990507
Lee, H. A., Song, Y. R., Park, M. H., Chung, H. Y., Na, H. S., and Chung, J. (2020). Catechin ameliorates Porphyromonas gingivalis-induced inflammation via the regulation of TLR2/4 and inflammasome signaling. J. periodontology 91, 661–670. doi:10.1002/JPER.18-0004
Mateen, S., Zafar, A., Moin, S., Khan, A. Q., and Zubair, S. (2016). Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. acta 455, 161–171. doi:10.1016/j.cca.2016.02.010
Moreno-Jimenez, M. R., Trujillo-Esquivel, F., Gallegos-Corona, M. A., Reynoso-Camacho, R., González-Laredo, R. F., Gallegos-Infante, J. A., et al. (2015). Antioxidant, anti-inflammatory and anticarcinogenic activities of edible red oak (Quercus spp.) infusions in rat colon carcinogenesis induced by 1, 2-dimethylhydrazine. Food Chem. Toxicol. 80, 144–153. doi:10.1016/j.fct.2015.03.011
Nagappan, R. (2012). Evaluation of aqueous and ethanol extract of bioactive medicinal plant, Cassia didymobotrya (fresenius) irwin & barneby against immature stages of filarial vector, Culex quinquefasciatus say (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2, 707–711. doi:10.1016/S2221-1691(12)60214-7
Oliver, J. E., and Silman, A. J. (2006). Risk factors for the development of rheumatoid arthritis. Scand. J. Rheumatology 35, 169–174. doi:10.1080/03009740600718080
Oyeleke, S. A., Ajayi, A. M., Umukoro, S., Aderibigbe, A., and Ademowo, O. G. (2018). Anti-inflammatory activity of Theobroma cacao L. stem bark ethanol extract and its fractions in experimental models. J. Ethnopharmacol. 222, 239–248. doi:10.1016/j.jep.2018.04.050
Peerzada, S., Khan, M. T., Akhtar, M. F., Saleem, A., Hamid, I., Akhtar, B., et al. (2020). Phytochemical, anti-inflammatory, anti-nociceptive and cytotoxic basis for the use of Haloxylon stocksii. Pak. J. Pharm. Sci. 33, 887–894.
Placha, D., and Jampilek, J. (2021). Chronic inflammatory diseases, anti-inflammatory agents and their delivery nanosystems. Pharmaceutics 13, 64. doi:10.3390/pharmaceutics13010064
Roychoudhury, S., Sinha, B., Choudhury, B. P., Jha, N. K., Palit, P., Kundu, S., et al. (2021). Scavenging properties of plant-derived natural biomolecule para-coumaric acid in the prevention of oxidative stress-induced diseases. Antioxidants 10, 1205. doi:10.3390/antiox10081205
Saleem, A., Saleem, M., and Akhtar, M. F. (2020a). Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: An ethnomedicinal plant of Moringaceae family. South Afr. J. Bot. 128, 246–256. doi:10.1016/j.sajb.2019.11.023
Saleem, A., Saleem, M., Akhtar, M. F., Ashraf Baig, M. M. F., and Rasul, A. (2020b). HPLC analysis, cytotoxicity, and safety study of Moringa oleifera Lam.(wild type) leaf extract. J. Food Biochem. 44, e13400. doi:10.1111/jfbc.13400
Saleem, A., Saleem, M., Akhtar, M. F., Shahzad, M., and Jahan, S. (2020c). Moringa rivae leaf extracts attenuate Complete Freund's adjuvant-induced arthritis in Wistar rats via modulation of inflammatory and oxidative stress biomarkers. Inflammopharmacology 28, 139–151. doi:10.1007/s10787-019-00596-3
Saleem, A., Saleem, M., Akhtar, M. F., Shahzad, M., and Jahan, S. (2020d). Polystichum braunii extracts inhibit Complete Freund’s adjuvant-induced arthritis via upregulation of I-κB, IL-4, and IL-10, downregulation of COX-2, PGE2, IL-1β, IL-6, NF-κB, and TNF-α, and subsiding oxidative stress. Inflammopharmacology 28, 1633–1648. doi:10.1007/s10787-020-00688-5
Saleem, A., Saleem, M., Akhtar, M. F., Sharif, A., Javaid, Z., and Sohail, K. (2019). In vitro and in vivo anti-arthritic evaluation of Polystichum braunii to validate its folkloric claim. Pak. J. Pharm. Sci. 32, 1167–1173.
Saleem, U., Akhtar, R., Anwar, F., Shah, M. A., Chaudary, Z., Ayaz, M., et al. (2021). Neuroprotective potential of Malva neglecta is mediated via down-regulation of cholinesterase and modulation of oxidative stress markers. Metab. Brain Dis. 36, 889–900. doi:10.1007/s11011-021-00683-x
Saqib, Z., Mahmood, A., Malik, R. N., Mahmood, A., Syed, J. H., and Ahmad, T. (2014). Indigenous knowledge of medicinal plants in Kotli Sattian, Rawalpindi district, Pakistan. J. Ethnopharmacol. 151, 820–828. doi:10.1016/j.jep.2013.11.034
Semwal, P., Painuli, S., Badoni, H., and Bacheti, R. K. (2018). Screening of phytoconstituents and antibacterial activity of leaves and bark of Quercus leucotrichophora A. Camus from Uttarakhand Himalaya. Clin. Phytoscience 4, 30–36. doi:10.1186/s40816-018-0090-y
Shabbir, A., Batool, S. A., Basheer, M. I., Shahzad, M., Sultana, K., Tareen, R. B., et al. (2018). Ziziphora clinopodioides ameliorated rheumatoid arthritis and inflammatory paw edema in different models of acute and chronic inflammation. Biomed. Pharmacother. 97, 1710–1721. doi:10.1016/j.biopha.2017.11.118
Singh, A., and Bisht, M. (2018). Evaluation of in-vitro antioxidant potential and in-vivo hepatoprotective activity of root extract of Quercus oblongata D. DON. J. Drug Deliv. Ther. 8, 152–161. doi:10.22270/jddt.v8i5-s.1925
Stankov, S. V. (2012). Definition of inflammation, causes of inflammation and possible anti-inflammatory strategies. open Inflamm. J. 5, 1–9. doi:10.2174/1875041901205010001
Taib, M., Rezzak, Y., Bouyazza, L., and Lyoussi, B. (2020). Medicinal uses, phytochemistry, and pharmacological activities of Quercus Species. Evidence-Based Complementary Altern. Med. 2020, 1920683. doi:10.1155/2020/1920683
Wang, Y., Chen, S., Du, K., Liang, C., Wang, S., Owusu Boadi, E., et al. (2021). Traditional herbal medicine: Therapeutic potential in rheumatoid arthritis. J. Ethnopharmacol. 279, 114368. doi:10.1016/j.jep.2021.114368
Yin, J., Kim, H. H., Hwang, I. H., Kim, D. H., and Lee, M. W. (2019). Anti-inflammatory effects of phenolic compounds isolated from Quercus mongolica Fisch. ex Ledeb. on UVB-irradiated human skin cells. Molecules 24, 3094. doi:10.3390/molecules24173094
Keywords: Quercus leucotrichophora, oxidative stress biomarkers, complete Freund’s, HPLC, TNF-α, IL-6
Citation: Saleem A, Hameed I, Akhtar MF, Ashraf GM, Alghamdi BS, Rahman MH and Almashjary MN (2023) Exploration of acute and chronic anti-inflammatory potential of Quercus leucotrichophora A. Camus extracts in Wistar rats: A mechanistic insight. Front. Pharmacol. 14:1002999. doi: 10.3389/fphar.2023.1002999
Received: 25 July 2022; Accepted: 20 March 2023;
Published: 11 April 2023.
Edited by:
Enrico Sangiovanni, University of Milan, ItalyReviewed by:
Rishi Pal, King George’s Medical University, IndiaVivekananda Mandal, Guru Ghasidas Vishwavidyalaya, India
Newman Osafo, Kwame Nkrumah University of Science and Technology, Ghana
Copyright © 2023 Saleem, Hameed, Akhtar, Ashraf, Alghamdi, Rahman and Almashjary. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ammara Saleem, YW1tYXJhc2FsZWVtQGdjdWYuZWR1LnBr, Muhammad Furqan Akhtar, ZnVycWFuLnBoYXJtYWNpc3RAZ21haWwuY29t
†ORCID: Ammara Saleem, orcid.org/0000-0002-2478-7800Muhammad Furqan Akhtar, orcid.org/0000-0003-2270-6242
 Ammara Saleem
Ammara Saleem Izza Hameed
Izza Hameed Muhammad Furqan Akhtar
Muhammad Furqan Akhtar Ghulam Md Ashraf
Ghulam Md Ashraf Badrah S. Alghamdi
Badrah S. Alghamdi Md. Habibur Rahman
Md. Habibur Rahman Majed N. Almashjary
Majed N. Almashjary