
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 30 September 2022
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.998534
This article is part of the Research Topic Hepatocellular carcinoma: from personalized medicine to practical guidelines View all 10 articles
Background: Combination treatment with tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) has been widely used in patients with unresectable hepatocellular carcinoma (uHCC). As no standard guidelines exist for second-line therapy after failure of combination treatment, this study aimed to determine a better drug-switching strategy.
Methods: A total of 785 patients with uHCC who initially received a combination treatment of TKIs and ICIs between January 2017 and December 2021 at our center were screened. After applying the inclusion and exclusion criteria, a total of 102 patients were included in the study. Based on drug switching strategy, patients were divided into a single drug-switching group (A group, n = 49) and a double drug-switching group (B group, n = 53). The comparative effectiveness between groups A and B was assessed based on treatment response and survival time. Second progression-free survival (SPFS) and overall survival (OS) were compared using the Kaplan-Meier method and log-rank test.
Results: Compared to group B, group A had a higher overall response rate (16.3% vs. 3.8%; p = 0.0392) and disease control rate (61.2% vs. 49.1%; p = 0.238). The median SPFS in group A was longer than that in group B (5.47 vs. 3.8 months; HR = 1.70, p = 0.0176). In the second-line therapy, the inclusion of lenvatinib resulted in a better SPFS than other TKI treatments (5.53 vs. 2.83 months, p = 0.0038).
Conclusion: After the failure of the combination treatment of TKIs and ICIs, single-drug switching significantly prolonged median SPFS in uHCC patients, and retaining lenvatinib resulted in the survival benefit of single-drug switching.
Hepatocellular carcinoma (HCC) is one of the most frequent malignancies and the third leading cause of cancer deaths worldwide (Sung et al., 2021). Hepatectomy and liver transplantation are the most effective treatments for HCC. Because of its unobvious symptoms, many patients with HCC are diagnosed at advanced stages, and hence have a low survival rate (Yang and Heimbach, 2020). Systemic therapy, including systemic chemotherapy and local interventional therapy, is the predominant therapeutic modality for unresectable HCC (uHCC). A previous study has demonstrated that compared to the best supportive care, metronomic capecitabine was an alternative choice to sorafenib with better efficacy and safety (De Lorenzo et al., 2018). Local therapy also brings significant benefits to uHCC patients. Recently, a multi-center propensity score-matched analysis has confirmed that transarterial infusion chemotherapy with FOLFOX was an effective and safe therapy that improved the survival of advanced hepatocellular carcinoma (Li et al., 2021). Transarterial chemoembolization (TACE) is recommended as the standard therapy for HCC patients with BCLC stage B (Mei et al., 2021).
In recent years, with the progress in research and clinical application of targeted and immunotherapy drugs, the prognosis of patients with uHCC has significantly improved (Llovet et al., 2008; Kudo et al., 2018; Finn et al., 2020; Yau et al., 2022). However, less than 20% of patients with uHCC benefited from immune checkpoint inhibitors (ICIs) monotherapy (Rizzo et al., 2021). The role of ICI-based combinations warrants further evaluation, and it is exciting that better prognosis benefits were demonstrated with combination therapy of tyrosine kinase inhibitors (TKIs) and ICIs than with monotherapy (Cheng et al., 2020a). The IMbrave 150 study reported that atezolizumab plus bevacizumab could result in better overall survival (OS) and progression-free survival (PFS) in the Chinese subpopulation (Qin et al., 2021). Moreover, 90Yttrium transarterial radioembolization has an established synergism with atezolizumab plus bevacizumab treatment by enhancing antigen presentation and reducing the infiltration of immunosuppressive cells (Di Federico et al., 2022). The KEYNOTE 524 reported significant improvements with pembrolizumab plus lenvatinib, with an objective response rate (ORR) of 46% (Llovet et al., 2022). Camrelizumab combined with apatinib as the first-line therapy can significantly prolong PFS and OS in patients with advanced HCC when compared with sorafenib, and the independent data monitoring committee judged that the primary endpoint of the study met the protocol-preset superiority criteria (SHR-1210-III-310). Thus, combination treatment with TKIs and ICIs has been applied as a first-line treatment for patients in China (Zhao and Cai, 2021). Owing to tumor heterogeneity, tumor progression still occurs in patients with HCC receiving first-line treatment. Although there are some options for second-line treatment (Choi et al., 2020; Zhang et al., 2020), there is a lack of widely accepted guidelines for switching therapy.
To our knowledge, real-world outcomes of switching therapy and a comparison of its efficacy have not been reported. Based on real-world data from clinical practice, this study aimed to explore the effect of the mode of switching therapy on the prognosis of uHCC after first-line systemic therapy failure, and thus providing a reference for larger prospective clinical studies in the future to guide the complete treatment of HCC.
The Institutional Review Board of the Ethics Committee of Sun Yat-sen University Cancer Center approved this study (B2020-190-01). All procedures involving human participants were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients for anonymized information published in this article.
Patients with uHCC who received TKIs and ICIs at the Department of Liver Surgery of Sun Yat-sen University Cancer Center between January 2017 and December 2021 were included in this retrospective analysis. The inclusion criteria for patients were as follows: 1) aged 18–75 years; 2) diagnosed with uHCC according to the AASLD practice guidelines (Marrero et al., 2018); 3) Child-Pugh class A or B; 4) at least one measurable lesion based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria (Llovet and Lencioni, 2020); and 5) switched to at least one systemic therapy drug after tumor progression. The exclusion criteria of the patients were as follows: 1) presence of other malignant tumors; 2) no response evaluation after switching therapy; 3) incomplete baseline and follow-up data; and 4) clinical trials participants. A total of 102 patients with HCC were included in this study based on the inclusion and exclusion criteria. The details of the initial combination treatment and second-line treatment for the 102 uHCC patients are listed in Tables 1 and 2, respectively. All the patients were classified into two groups: group A (n = 49) and group B (n = 53), based on the mode of switching therapy. The group A included uHCC patients who switched to one systemic therapy drug after tumor progression, while the group B included patients who switched to two systemic therapy drugs after tumor progression. A flowchart of the patient disposition process is shown in Figure 1.
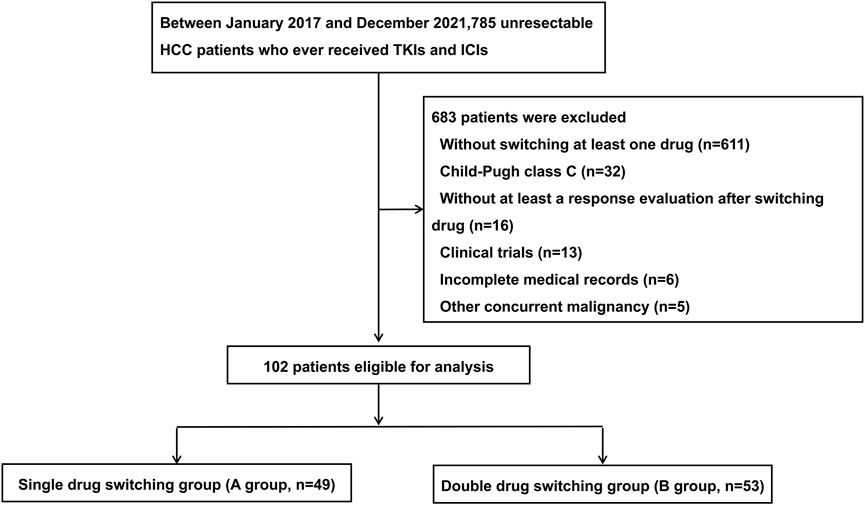
FIGURE 1. The flow chart of the disposition process of patients. HCC, hepatocellular carcinoma; TKIs, tyrosine kinase inhibitors (TKIs); ICIs, immune checkpoint inhibitors.
Patients with uHCC received a combination of ICIs and TKIs as initial treatment. TKIs including regorafenib, apatinib, sorafenib, and lenvatinib were administered orally once daily. The ICIs included PD-1 and PD-L1 inhibitors which were intravenously injected every 3 weeks. The initial doses of TKIs and ICIs used are listed in Supplementary Table S1. The interval between the initiation of ICIs and TKIs was less than 7 days. Combination treatment with ICIs and TKIs was continued until the occurrence of disease progression or intolerable toxicity. After tumor progression, the decision to switch drugs was based on resistance to TKIs and ICIs or liver function. All patients with uHCC underwent scheduled enhanced computed tomography or magnetic resonance imaging assessment every 2-3 months.
All baseline data before second-line treatment were retrieved from medical records and imaging examinations, including age, sex, Child-Pugh class, α-fetoprotein (AFP), protein induced by vitamin K absence or antagonist-II (PIVKA-II), albumin, total bilirubin (TB), etiology, Barcelona Clinic Liver Cancer stage (BCLC stage), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), progressive, macroscopic portal vein invasion, portal hypertension, and extrahepatic metastases. Tumor response to treatment was defined as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD), based on the mRECIST criteria.
Second progression-free survival (SPFS) and OS were the clinical outcomes of interest. SPFS was defined as the interval from the initiation of second-line treatment to tumor progression, while OS was measured from the initiation of second-line treatment to death. The secondary outcomes included objective response rate (ORR) and disease control rate (DCR). ORR was defined as achieving CR or PR, and DCR was defined as achieving CR, PR, or SD. Treatment-related adverse events (AEs) were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
The baseline characteristics were compared between the different modes of switching therapy. Continuous variables with normal distribution were expressed as means and standard deviations and those with abnormal distribution were expressed as medians and interquartile ranges. Continuous variables were analyzed using an unpaired Student’s t-test for parametric data and the Mann–Whitney rank sum test for non-parametric data. Categorical variables were compared using Pearson’s chi-squared test or Fisher’s exact test. The survival analysis between the different treatment groups was performed by plotting Kaplan-Meier curves and their differences were verified using the log-rank test. Univariate Cox regression analysis was used to identify survival-associated factors, which were sequentially subjected to multivariate Cox regression analysis to identify the independent prognostic factors. All statistical analyses were performed using the SPSS software (version 20.0), MedCalc (version 20.027), and R software (version 4.1.1). Two-sided p-values <0.05 were considered statistically significant.
The clinical characteristics of the patients and therapy given are summarized in Table 3. The median age of the study population was 54 years old. The majority of the patients were Child-Pugh class A (n = 91, 89.22%) and chronically infected with the hepatitis B virus (n = 92, 90.2%). Of the patients, 89.2% received other treatments, such as radiofrequency ablation, radiotherapy, hepatic artery infusion chemotherapy, transhepatic arterial chemotherapy, and embolization. Males were predominant (n = 80, 78.43%) and 2/3rd of the patients were in BCLC stage C (n = 76, 74.51%). The patients with extrahepatic metastases were approximately 60%. Almost half of the patients had macroscopic portal vein invasion (n = 41, 40.2%) and single intrahepatic progression (n = 42, 41.18%). In addition, 36.27% of patients had portal hypertension. The duration of first-line treatment and baseline characteristics were not significantly different between the groups (p >0.05).
The treatment responses are summarized in Table 4. Based on mRESIST, four patients had CR, six patients had PR, forty-six patients had SD and forty-six patients had PD. ORR and DCR were 9.8% and 54.9%, respectively. Notably, the ORR was higher in group A (16.3%) than in group B (3.8%) (p = 0.0392). A higher DCR was observed in group A than in group B (61.2% vs. 49.1%; p = 0.238). Collectively, the single drug switching strategy might provide clinical benefits to patients with uHCC.
As shown in Figures 2A,B, the median SPFS was significantly longer in group A (5.47 months) than in group B (3.8 months) (HR = 1.70, 95%CI: 1.089–2.641, p = 0.0176), while there was no significant difference in OS between group A and group B (HR = 1.12, 95%CI: 0.55–2.26, p = 0.7556). The median OS in groups A and B were 20.7 and 21.6 months, respectively.
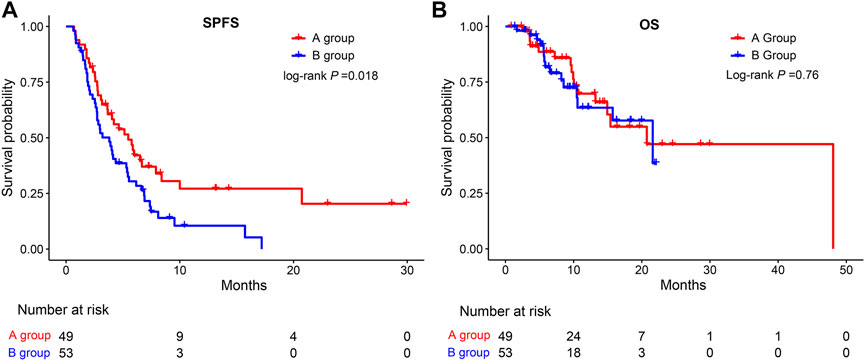
FIGURE 2. Kaplan-Meier survival curves for SPFS (A) and OS (B) of patients in the A group and the B group. SPFS, second progression-free survival; OS, overall survival.
A subgroup analysis was performed to identify the subset of patients who could benefit from a single drug-switching strategy. Interestingly, single drug switching strategy extended the SPFS of HCC patients with AFP<400 ng/ml (HR = 1.89, 95%CI: 1.01–3.55, p = 0.0365), Child-Pugh class A (HR = 2.12, 95%CI: 1.32–3.41, p = 0.0018), absence of macroscopic portal vein invasion (HR = 1.88, 95%CI: 1.05–3.35, p = 0.0275), BCLC stage A or B (HR = 2.78, 95%CI: 1.04–7.45, p = 0.0414), absence of extrahepatic metastasis (HR = 2.48, 95%CI: 1.20–5.14, p = 0.0166), and single progression pattern (HR = 2.45, 95%CI:1.40–4.27, p = 0.0019). However, SPFS was not extended in patients with macroscopic portal vein invasion (Figure 3). No significant difference in OS was observed among the different subgroups (Figure 4). Collectively, the mode of single drug switching could extend SPFS in patients, especially in those without BCLC stage A or B.
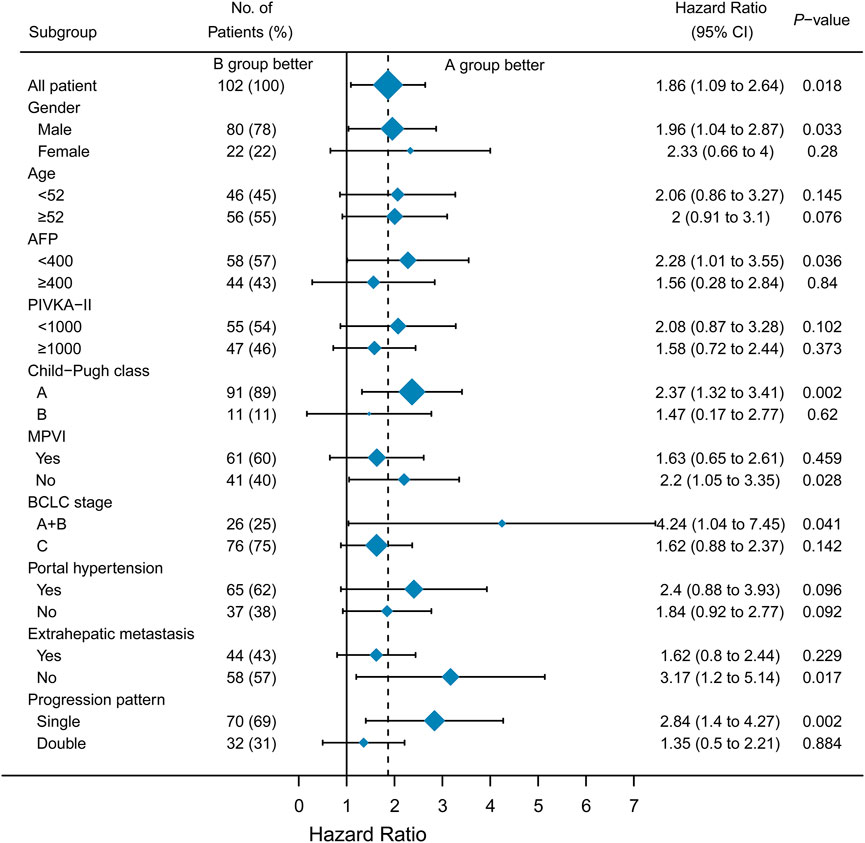
FIGURE 3. Subgroup analysis of second progression-free survival. MPVI, macroscopic portal vein invasion.
We further divided group A into TKIs switching and ICIs switching groups. No significant difference was observed between the TKIs switching and B groups (HR = 0.63, 95%CI; 0.35–1.13, p >0.05) (Figure 5A). However, compared to group B, the ICIs switching sub-group could significantly extend the SPFS (HR = 0.58, 95%CI: 0.36–0.95, p = 0.029) (Figure 5B). The majority of uHCC patients in the ICIs switching group retained lenvatinib. Based on these results, we hypothesized that lenvatinib could be an important factor affecting the treatment efficacy. Thus, the effectiveness of lenvatinib treatment with other TKI treatments as second-line therapies was compared. As shown in Figure 6, lenvatinib treatment accounted for better SPFS than other TKI treatments (5.53 vs. 2.83 months, p = 0.0038).
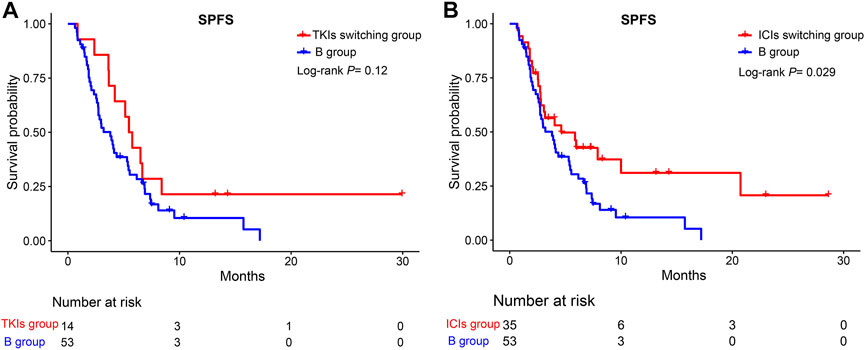
FIGURE 5. Kaplan-Meier curves for SPFS of patients in the TKIs switching group, ICIs switching group, and the B group. (A) TKIs switching group vs. B group; (B) ICIs switching group vs. B group. SPFS, second progression-free survival; TKIs, tyrosine kinase inhibitors; ICIs, immune checkpoint inhibitors.
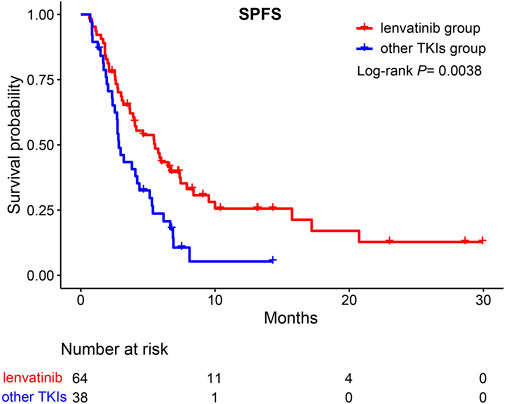
FIGURE 6. Kaplan-Meier curves for SPFS of patients moving to second-line therapy. SPFS, second progression-free survival; TKIs, tyrosine kinase inhibitors.
In addition, the efficacy of lenvatinib as a first-line sequential treatment was investigated. As shown in Figure 7, patients who received lenvatinib as first-line therapy, compared to other TKIs treatments, could still benefit from retaining lenvatinib as the second-line treatment (5.97 vs. 2.73 months, p = 0.0033). However, for those patients receiving other TKIs treatment as a first-line treatment, no survival benefit was reported between lenvatinib and other TKIs treatments in the second-line treatment (5.43 vs. 4.36 months, p >0.05).
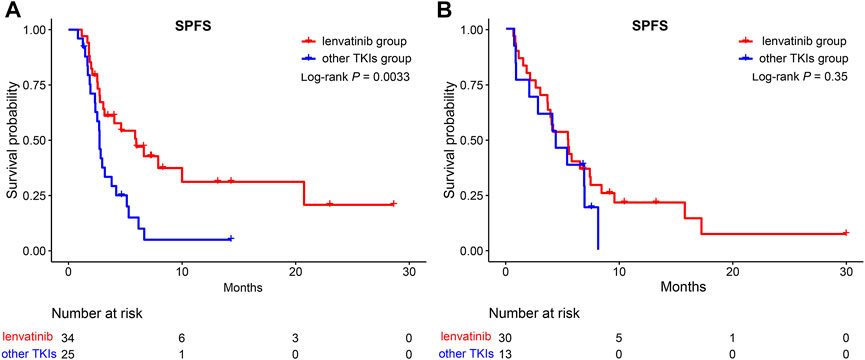
FIGURE 7. Kaplan-Meier curves for SPFS of patients in the lenvatinib group and the other TKIs group. (A) A subgroup of patients after lenvatinib as first-line therapy; (B) A subgroup of patients after other TKIs as first-line therapy. SPFS, second progression-free survival; TKIs, tyrosine kinase inhibitors.
As shown in Table 5, no AE-associated deaths were observed during the follow-up. The most common AEs were increased AST, followed by increased ALT, and pain in both groups. Seven (14.3%) and eleven (20.8%) patients in groups A and B experienced at least one grade 3/4 AE. Grade 3 AEs are severe, serious, or medically significant but not immediately life-threatening, requiring hospitalization or prolonged hospitalization and partial loss of self-care. Grade 4 AEs are life-threatening, which may lead to fatal consequences, and urgent intervention is required. The AEs in Groups A and B were manageable.
The results of univariate Cox regression analysis indicated that AFP≥400 (HR = 1.797, p = 0.0116), BCLC stage C (HR = 1.959, p = 0.0173), Child-Pugh class B (HR = 2.649, p = 0.0049), extrahepatic metastasis (HR = 1.769, p = 0.0165), PIVKA-II≥1,000 (HR = 1.874, p = 0.0036), progression pattern (HR = 1.735, p = 0.007), and switching to two systemic therapy drugs after tumor progression (HR = 1.722, p = 0.0192) were potential prognostic biomarkers of SPFS. The potentially predictive biomarkers were introduced into multivariate Cox regression analysis which confirmed that Child-Pugh class B (HR = 4.060, p <0.001) and switching to two systemic therapy drugs after tumor progression (HR = 4.060, p = 0.0123) were independent prognostic factors for SPFS (Table 6). In addition, extrahepatic metastasis (HR = 2.212, p = 0.055), PIVKA-II≥1,000 (HR = 2.603, P= 0.0119), and progression pattern (HR = 2.684, p <0.001) were potential prognostic biomarkers for OS. Further analysis indicated that PIVKA-II≥1,000 (HR = 2.651, P= 0.0118) was an adverse prognostic factor for OS (Table 7).
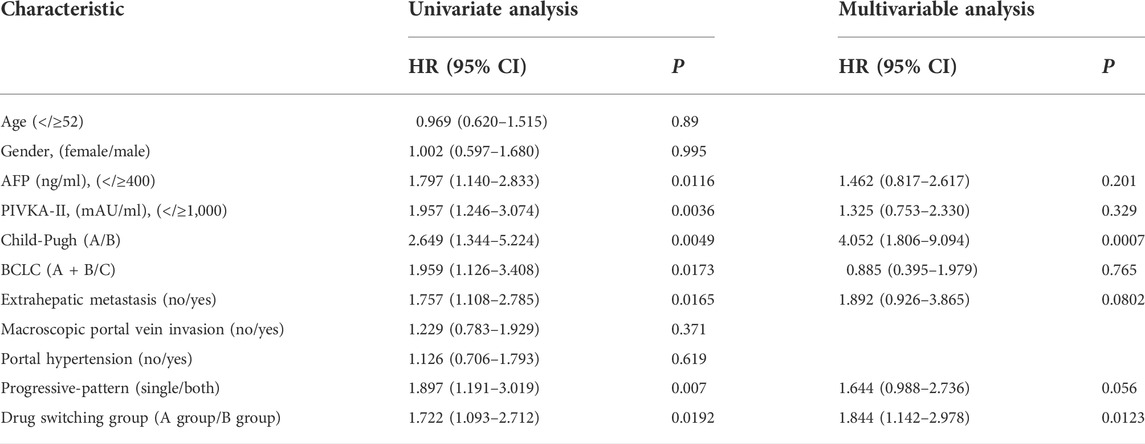
TABLE 6. Univariable and multivariable Cox regression analyses for second progression-free survival.
The treatment of uHCC is primarily based on systemic therapy. The age of patients undergoing combination treatment with TKIs and ICIs has decreased. There is abundant evidence to support that uHCC patient can benefit from a combination treatment of TKIs and ICIs (Cheng et al., 2020b). However, for HCC patients who progress on first-line combination treatment, many treatment options are available for subsequent therapies. Moreover, there is still a lack of generally accepted guidelines to guide second-line therapy after the progression of first-line combination treatment. There are two strategies for switching drugs in clinical practice: single drug switching (group A) and double drug switching (group B). This retrospective study aimed to evaluate and compare the effectiveness of two strategies of drug switching for patients with HCC who had failed combination treatment with TKIs and ICIs based on real-world data from clinical practice.
In our study, 102 patients with HCC were divided into groups A (n = 49) and B (n = 53). We observed a higher ORR (16.3%) and DCR (61.2%) in the group A. Further survival analysis indicated a significant difference in SPFS between groups A and B. Surprisingly, the median SPFS of group A was longer than that of group B (5.47 vs. 3.8 months, p = 0.0176). These data suggest that the median SPFS in our study was significantly extended compared to that of a previous study where the sequence ramucirumab for uHCC after TKI treatment (Amioka et al., 2021).
However, we observed no differences in the OS between groups A and B. The reason for this may be as follows. First, the follow-up time for SPFS was shorter and the sample size for SPFS was smaller than for OS. Our study’s sample size and follow-up time might not be sufficient for OS calculation. Second, OS might be affected by subsequent treatment and does not directly reflect the true efficacy of switching therapy. After switching therapy, patients with uHCC may receive other subsequent treatments, such as interventional therapy and radiotherapy. We did not observe a significant difference in OS between groups A and B.
We further analyzed which subgroup of patients could benefit from a single drug switch and double drug switch. In the subgroup analysis, we found that HCC patients with AFP<400, Child-Pugh class A, without macroscopic portal vein invasion, BCLC stage A or B, without extrahepatic metastasis, and a single progression pattern could benefit from the single drug switching strategy. In our study, Child-Pugh class A was associated with a better prognosis. A previous study demonstrated that uHCC patients with Child-Pugh class A could receive a sufficient relative dose intensity of lenvatinib, which sequentially affected the objective response (Sasaki et al., 2019). AFP level is used for the diagnosis of HCC. Previous studies have shown that there is a close relationship between AFP levels and response to comprehensive treatment (Chau et al., 2018). Consistent with a previous study, AFP < 400 ng/ml could predict the response to a single drug-switching strategy (Regmi et al., 2021). BCLC staging is a generally acknowledged system for the treatment of HCC (Reig et al., 2022). As for the single progression pattern, the reason it could benefit from single drug switching may be associated with the microenvironment. The sole progression pattern indicates that one of the tumor sites may be curbed or eradicated. However, this hypothesis requires further investigation. Macroscopic portal vein invasion and extrahepatic metastasis are the parameters of BCLC stage C. Mei et al. (2021) demonstrated that HCC patients with main portal vein tumor thrombus or extrahepatic metastasis could not benefit most from hepatic artery infusion chemotherapy plus lenvatinib combination therapy. In our study, these results indicate that the single-drug switching strategy might be suitable for patients with BCLC stage A or B. BCLC stage C indicates a more malignant tumor. As a result, compared with uHCC patients with stage A or B disease, patients with macroscopic portal vein invasion or extrahepatic metastasis seemed to be more inclined to progress, leading to a worse survival prognosis. Collectively, the mode of single drug switching could extend SPFS in patients, especially in those with BCLC stage A or B.
In our study, patients could benefit from single-drug switching rather than double-drug switching. To explain the reasons for this result, we further divided group A into TKIs switching and ICIs switching groups. Surprisingly, compared with group B, the ICIs switching group could significantly extend the SPFS. However, no significant difference was reported between the TKIs switching and the B group. Both uHCC patients in the ICIs switching group and B group switched ICIs after tumor progression. Why could the former group extend the SPFS? We found that the majority of uHCC patients in the ICIs switching group retained lenvatinib. Moreover, for second-line therapy, lenvatinib treatment accounted for a better SPFS than other TKI treatments (5.53 vs. 2.83 months, p = 0.0038). This result further confirms our hypothesis. The REFLECT clinical trial indicated that the overall survival time of the lenvatinib group was not inferior to the sorafenib group (Kudo et al., 2018). Further studies indicated that, compared with sorafenib, lenvatinib exhibited stronger inhibitory activity targeting the fibroblast growth factor receptor (Tohyama et al., 2014). Shi et al. (2021) found that lenvatinib may be a suitable second-line treatment for uHCC patients who progressed on sorafenib by regulating FGFR4-ERK signaling. Apatinib is a small-molecule tyrosine kinase inhibitor that selectively inhibits the activity of VEGFR-2 (Tian et al., 2011). Moreover, a previous study indicated that lenvatinib had immunomodulatory activity, which contributed to the antitumor effect of lenvatinib and enhanced the synergistic effect with the anti-PD-1 antibody (Kimura et al., 2018). Moreover, Chen et al. demonstrated that lenvatinib could reduce the expression of PD-L1 in HCC and regulate T-cell differentiation by blocking FGFR4 to improve anti-PD-1 efficacy (Yi et al., 2021). Collectively, retaining lenvatinib accounted for the survival benefits of single-drug switching, especially in SPFS. However, lenvatinib led to better SPFS, but did not translate into OS benefits. The use of longer SPFS with lenvatinib to enable patients to obtain longer OS benefits still needs to be explored by oncologists.
Further analysis indicated that for those patients who selected lenvatinib as the first-line treatment, compared to other TKIs treatment, they could still benefit from retaining lenvatinib as the second-line treatment (5.97 vs. 2.73 months, p = 0.0033). However, for patients who selected other TKIs as the first-line treatment, no survival benefit was reported between lenvatinib and other TKIs treatments. Chen et al. retrospectively analyzed 26 cases of advanced uHCC from October 2018 to October 2019 in the real world in China and found that lenvatinib combined with the PD-1 antibody was expected to further improve the prognosis of patients who progressed on lenvatinib (Chen et al., 2020). Thus, lenvatinib should be used for the comprehensive treatment of uHCC. However, high-quality randomized controlled studies are required to validate this conclusion.
In the prognostic factor analysis, the Child-Pugh class and drug-switching strategy were identified as independent prognostic factors for SPFS. The Child-Pugh class is an evaluation system for liver reserve function, including five parameters (Kok and Abraldes, 2019). In our study, Child-Pugh class A could predict better SPFS, and HCC patients with Child-Pugh class A could obtain a longer SPFS benefit from the single drug switching strategy. The reason for this might be that HCC patients with Child-Pugh class A could better tolerate the combination therapy’s toxicity. Moreover, a PIVKA-II>1,000 was regarded as an adverse prognostic factor for OS. Another prognostic factor is the drug-switching strategy. Based on the results of the comparison of the two drug-switching strategies in clinical practice, we found that single-drug switching could extend the SPFS. PIVKA-II is produced because of the incomplete carboxylation of amino acid residues (Liebman et al., 1984). What is clear is that PIVKA-II is not only a diagnostic predictor but also a prognostic predictor of liver cancer (Yang et al., 2021). PIVKA-II exhibited stronger mitogenic capacity and migratory activity during angiogenesis in HCC patients (Bertino et al., 2010).
As for safety, consistent with a previous study, toxicities were manageable with no unexpected safety signals (Mo et al., 2021). No AE-associated death was observed during follow-up, and the most common AEs were damage to liver function. Dose adjustments of TKIs and ICIs accounted for safety in the present study. In our study, the percentages of interruption and dose reduction in groups A and B were 30% and 35%, respectively. Half of the routine dosage or weekends-off administration of lenvatinib (Iwamoto et al., 2020) was the primary method of dose adjustment.
We acknowledge the potential limitations of this study. First, a selection bias was unavoidable because this was a retrospective study. Liver function was worse in group A than in group B, and it was positively correlated with survival rate. However, the survival analysis indicated that the treatment response and SPFS of group A were better than those of group B. The potential selection bias worked unfavorably against the single-drug switching strategy, leading to an opposite result. Secondly, one hundred and two patients with uHCC were included in our study. The sample size was small, and the observation period was short. All included patients were Asian, and their data were obtained from a single Chinese institute. A single drug-switching strategy might be beneficial only to the Asian population. A great amount of evidence has demonstrated that the carcinogenic factors of patients with HCC in Asia and the West are different, which limits the ability to draw general conclusions from the results (Marengo et al., 2016). Collectively, our conclusion requires further confirmation by a large international multicenter clinical study in the future. Third, confounding factors are one of the limitations. We defined drug-switching strategies for second-line therapy after combination treatment with TKIs and ICIs, but the optional treatment for HCC patients lacks clear guidelines. Subsequent treatments after first-line treatment were not chosen in a randomized manner. Thus, the therapeutic molecules used in the second line might vary between groups A and B, which influenced the uniformity of the treatment procedure. Such division of patients into different groups may bring about a certain degree of heterogeneity; thus, this was a preliminary study on a drug-switching strategy for second-line therapy after combination treatment with tyrosine kinase inhibitors and immune checkpoint inhibitors for unresectable hepatocellular carcinoma. The findings of this study should be further validated using higher-level randomized controlled trials. Finally, lenvatinib was the main TKIs used in combination with ICIs in our study. Thus, the value of other TKIs, such as sorafenib and regorafenib, in combination treatment should be further investigated.
After combination treatment with TKIs and ICIs failure, single-drug switching significantly prolonged the median SPFS in uHCC patients, and retaining lenvatinib accounted for the survival benefit brought by single-drug switching.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by The Institutional Review Board of the Ethics Committee of Sun Yat-Sen University Cancer Center approved this study (B2020-190-01). The patients/participants provided their written informed consent to participate in this study.
RG: data curation; formal analysis; software; methodology; writing-original draft; writing-review and editing. CY: formal analysis; investigation; software; writing-original draft. JM: project administration; visualization; writing-review and editing. SL: investigation; supervision; visualization; writing-review and editing. WW: investigation; supervision; visualization; writing-review and editing. RoG: conceptualization; funding acquisition; project administration; writing-review and editing.
This study was supported by the National Natural Science Foundation of China (No. 82172579, No. 81871985), Natural Science Foundation of Guangdong Province (No. 2018A0303130098, No. 2017A030310203), Science and Technology Planning Project of Guangdong Province (No. 2017A020215112), and Medical Scientific Research Foundation of Guangdong Province (No. A2017477); Science and Technology Planning Project of Guangzhou (No. 201903010017, No. 201904010479); and Clinical Trials Project (5010 Project) of Sun Yat-Sen University (No. 5010-2017009).
The authors thank all the patients who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.998534/full#supplementary-material
AEs, Adverse events; AFP, α-fetoprotein; ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer stage; CR, Complete response; DCR, Disease control rate; HCC, Hepatocellular carcinoma; HR, Hazards ratio; ICIs, Immune checkpoint inhibitors; OS, Overall survival; ORR, Objective response rate; PD, Progressive disease; PFS, Progression-free survival; PIVKA-II, Protein induced by vitamin K absence or antagonist-II; PR, Partial response; SD, Stable disease; SPFS, Second progression-free survival; TB, Total bilirubin; TKIs, Tyrosine kinase inhibitors; uHCC, Unresectable hepatocellular carcinoma.
Amioka, K., Kawaoka, T., Ogawa, Y., Kikukawa, C., Naruto, K., Yoshikawa, Y., et al. (2021). Comparison of the clinical outcome of ramucirumab for unresectable hepatocellular carcinoma with that of prior tyrosine kinase inhibitor therapy. Oncology 99, 327–335. doi:10.1159/000514315
Bertino, G., Ardiri, A. M., Calvagno, G. S., Bertino, N., and Boemi, P. M. (2010). Prognostic and diagnostic value of des-gamma-carboxy prothrombin in liver cancer. Drug News Perspect. 23, 498–508. doi:10.1358/dnp.2010.23.8.1444236
Chau, I., Park, J. O., Ryoo, B. Y., Yen, C. J., Poon, R., Pastorelli, D., et al. (2018). Alpha-fetoprotein kinetics in patients with hepatocellular carcinoma receiving ramucirumab or placebo: An analysis of the phase 3 REACH study. Br. J. Cancer 119, 19–26. doi:10.1038/s41416-018-0103-0
Chen, Y., Zhang, L., Ge, N., Wang, Y., and Ren, Z. (2020). Clinical effect of sequential therapy of LEN combination with anti-PD1 antibody in uHCC patients who progressed on LEN treatment: A real-world data in China. J. Clin. Oncol. 38, e16654–e16654. doi:10.1200/JCO.2020.38.15_suppl.e16654
Cheng, A. L., Hsu, C., Chan, S. L., Choo, S. P., and Kudo, M. (2020). Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J. Hepatol. 72, 307–319. doi:10.1016/j.jhep.2019.09.025
Cheng, S., Chen, M., Cai, J., Sun, J., Guo, R., Bi, X., et al. (2020). Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus (2018 edition). Liver Cancer 9, 28–40. doi:10.1159/000503685
Choi, W. M., Choi, J., Lee, D., Shim, J. H., Lim, Y. S., Lee, H. C., et al. (2020). Regorafenib versus nivolumab after sorafenib failure: Real-world data in patients with hepatocellular carcinoma. Hepatol. Commun. 4, 1073–1086. doi:10.1002/hep4.1523
De Lorenzo, S., Tovoli, F., Barbera, M. A., Garuti, F., Palloni, A., Frega, G., et al. (2018). Metronomic capecitabine vs. best supportive care in child-pugh B hepatocellular carcinoma: A proof of concept. Sci. Rep. 8, 9997. doi:10.1038/s41598-018-28337-6
Di Federico, A., Rizzo, A., Carloni, R., De Giglio, A., Bruno, R., Ricci, D., et al. (2022). Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: Preclinical rationale and ongoing clinical trials. Expert Opin. Investig. Drugs 31, 361–369. doi:10.1080/13543784.2022.2009455
Finn, R. S., Ryoo, B. Y., Merle, P., Kudo, M., Bouattour, M., Lim, H. Y., et al. (2020). Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J. Clin. Oncol. 38, 193–202. doi:10.1200/JCO.19.01307
Iwamoto, H., Suzuki, H., Shimose, S., Niizeki, T., Nakano, M., Shirono, T., et al. (2020). Weekends-off lenvatinib for unresectable hepatocellular carcinoma improves therapeutic response and tolerability toward adverse events. Cancers (Basel) 12, E1010. doi:10.3390/cancers12041010
Kimura, T., Kato, Y., Ozawa, Y., Kodama, K., Ito, J., Ichikawa, K., et al. (2018). Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 109, 3993–4002. doi:10.1111/cas.13806
Kok, B., and Abraldes, J. G. (2019). Child-pugh classification: Time to abandon? Semin. Liver Dis. 39, 96–103. doi:10.1055/s-0038-1676805
Kudo, M., Finn, R. S., Qin, S., Han, K. H., Ikeda, K., Piscaglia, F., et al. (2018). Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173. doi:10.1016/S0140-6736(18)30207-1
Li, S., Mei, J., Wang, Q., Shi, F., Liu, H., Zhao, M., et al. (2021). Transarterial infusion chemotherapy with FOLFOX for advanced hepatocellular carcinoma: A multi-center propensity score matched analysis of real-world practice. Hepatobiliary Surg. Nutr. 10, 631–645. doi:10.21037/hbsn.2020.03.14
Liebman, H. A., Furie, B. C., Tong, M. J., Blanchard, R. A., Lo, K. J., Lee, S. D., et al. (1984). Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N. Engl. J. Med. 310, 1427–1431. doi:10.1056/NEJM198405313102204
Llovet, J. M., Castet, F., Heikenwalder, M., Maini, M. K., Mazzaferro, V., Pinato, D. J., et al. (2022). Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 19, 151–172. doi:10.1038/s41571-021-00573-2
Llovet, J. M., and Lencioni, R. (2020). mRECIST for HCC: Performance and novel refinements. J. Hepatol. 72, 288–306. doi:10.1016/j.jhep.2019.09.026
Llovet, J. M., Ricci, S., Mazzaferro, V., Hilgard, P., Gane, E., Blanc, J. F., et al. (2008). Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390. doi:10.1056/NEJMoa0708857
Marengo, A., Rosso, C., and Bugianesi, E. (2016). Liver cancer: Connections with obesity, fatty liver, and cirrhosis. Annu. Rev. Med. 67, 103–117. doi:10.1146/annurev-med-090514-013832
Marrero, J. A., Kulik, L. M., Sirlin, C. B., Zhu, A. X., Finn, R. S., Abecassis, M. M., et al. (2018). Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 68, 723–750. doi:10.1002/hep.29913
Mei, J., Tang, Y. H., Wei, W., Shi, M., Zheng, L., Li, S. H., et al. (2021). Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front. Oncol. 11, 618206. doi:10.3389/fonc.2021.618206
Mo, D. C., Luo, P. H., Huang, S. X., Wang, H. L., and Huang, J. F. (2021). Safety and efficacy of pembrolizumab plus lenvatinib versus pembrolizumab and lenvatinib monotherapies in cancers: A systematic review. Int. Immunopharmacol. 91, 107281. doi:10.1016/j.intimp.2020.107281
Qin, S., Ren, Z., Feng, Y. H., Yau, T., Wang, B., Zhao, H., et al. (2021). Atezolizumab plus bevacizumab versus sorafenib in the Chinese subpopulation with unresectable hepatocellular carcinoma: Phase 3 randomized, open-label IMbrave150 study. Liver Cancer 10, 296–308. doi:10.1159/000513486
Regmi, P., Hu, H. J., Lv, T. R., Paudyal, A., Sah, R. B., Ma, W. J., et al. (2021). Efficacy and safety of sorafenib plus hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Surg. Oncol. 39, 101663. doi:10.1016/j.suronc.2021.101663
Reig, M., Forner, A., Rimola, J., Ferrer-Fabrega, J., Burrel, M., Garcia-Criado, A., et al. (2022). BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 76, 681–693. doi:10.1016/j.jhep.2021.11.018
Rizzo, A., Ricci, A. D., Gadaleta-Caldarola, G., and Brandi, G. (2021). First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: Current management and future challenges. Expert Rev. Gastroenterol. Hepatol. 15, 1245–1251. doi:10.1080/17474124.2021.1973431
Sasaki, R., Fukushima, M., Haraguchi, M., Miuma, S., Miyaaki, H., Hidaka, M., et al. (2019). Response to lenvatinib is associated with optimal RelativeDose intensity in hepatocellular carcinoma: Experience in clinical settings. Cancers (Basel) 11, E1769. doi:10.3390/cancers11111769
Shi, T., Iwama, H., Fujita, K., Kobara, H., Nishiyama, N., Fujihara, S., et al. (2021). Evaluating the effect of lenvatinib on sorafenib-resistant hepatocellular carcinoma cells. Int. J. Mol. Sci. 22, 13071. doi:10.3390/ijms222313071
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Tian, S., Quan, H., Xie, C., Guo, H., Lu, F., Xu, Y., et al. (2011). YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 102, 1374–1380. doi:10.1111/j.1349-7006.2011.01939.x
Tohyama, O., Matsui, J., Kodama, K., Hata-Sugi, N., Kimura, T., Okamoto, K., et al. (2014). Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J. Thyroid. Res. 2014, 638747. doi:10.1155/2014/638747
Yang, J. D., and Heimbach, J. K. (2020). New advances in the diagnosis and management of hepatocellular carcinoma. BMJ 371, m3544. doi:10.1136/bmj.m3544
Yang, Y., Li, G., Lu, Z., Liu, Y., Kong, J., and Liu, J. (2021). Progression of prothrombin induced by vitamin K absence-II in hepatocellular carcinoma. Front. Oncol. 11, 726213. doi:10.3389/fonc.2021.726213
Yau, T., Park, J. W., Finn, R. S., Cheng, A. L., Mathurin, P., Edeline, J., et al. (2022). Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet. Oncol. 23, 77–90. doi:10.1016/S1470-2045(21)00604-5
Yi, C., Chen, L., Lin, Z., Liu, L., Shao, W., Zhang, R., et al. (2021). Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology 74, 2544–2560. doi:10.1002/hep.31921
Zhang, X. H., Cao, M. Q., Li, X. X., and Zhang, T. (2020). Apatinib as an alternative therapy for advanced hepatocellular carcinoma. World J. Hepatol. 12, 766–774. doi:10.4254/wjh.v12.i10.766
Keywords: hepatocellular carcinoma, combination treatment, tyrosine kinase inhibitor, immune checkpoint inhibitor, drug switching, second-line therapy
Citation: Guan R, Yu C, Li S, Mei J, Wei W and Guo R (2022) A preliminary study on drug switching strategy for second-line therapy after combination treatment of tyrosine kinase inhibitors and immune checkpoint inhibitors for unresectable hepatocellular carcinoma. Front. Pharmacol. 13:998534. doi: 10.3389/fphar.2022.998534
Received: 29 July 2022; Accepted: 31 August 2022;
Published: 30 September 2022.
Edited by:
Sarah El-Nakeep, Ain Shams University, EgyptReviewed by:
Giovanni Brandi, University of Bologna, ItalyCopyright © 2022 Guan, Yu, Li, Mei, Wei and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renguo Guan, Z3VhbnJnMUBzeXN1Y2Mub3JnLmNu; Wei Wei, d2Vpd2VpQHN5c3VjYy5vcmcuY24=; Rongping Guo, Z3VvcnBAc3lzdWNjLm9yZy5jbg==
†These authors share the first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.