- 1Department of Clinical Chinese Pharmacy, School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China
- 2Institute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu, China
- 3Jiangsu Jiuxu Pharmaceutical Co. Ltd, Xuzhou, China
- 4Beijing Zest Bridge Medical Technology lnc., Beijing, China
Introduction: Systematic evaluation of the clinical efficacy and safety of Brucea javanica oil emulsion injection (BJOEI) in combination with chemotherapy in the treatment of malignant pleural effusion (MPE).
Methods: The study searched CNKI, Wanfang database, VIP database, SinoMed, PubMed, Embase, the Cochrane Library, and the Web of Science database and retrieved randomized controlled trials (RCTs) on the treatment of MPE with BJOEI in combination with chemotherapy from seven electronic databases from inception to 31 March 2022. Meta-analysis and sensitivity analysis were performed using Revman 5.4 and Stata 13.0 software.
Results: Ultimately, 30 RCTs with 2035 patients were included, including 1002 cases in the control group and 1033 cases in the treatment group. The results of the meta-analysis showed that the overall efficacy rate of BJOEI combined with chemotherapy was higher in the treatment of MPE compared with chemotherapy alone (RR = 1.45, 95%CI: 1.36–1.54, p < 0.00001). And it could improve the Karnofsky (KPS) score (RR = 1.54, 95%CI: 1.41–1.68, p < 0.00001), reduce adverse reactions such as fever (RR = 0.82, 95%CI:0.60–1.12), chest pain (RR = 0.90, 95%CI: 0.67–1.21), gastrointestinal reactions (RR = 0.70, 95%CI: 0.57–0.87, p < 0.005), and leukopenia (RR = 0.51, 95%CI: 0.43–0.61, p < 0.00001).
Conclusion: BJOEI combined with chemotherapy has better clinical efficacy than chemotherapy alone in the treatment of MPE. It can further improve KPS score, improve patients’ quality of life, and reduce the occurrence of adverse reactions. However, the conclusions of this study need to be confirmed by further randomized, double-blind, controlled trials with large sample size, reasonable design, and strict implementation.
1 Introduction
Malignant pleural effusion (MPE), also known as cancerous pleural effusion, refers to pleural effusion caused by metastasis of a primary malignant tumor of the pleura or a malignant tumor in other parts of the pleura. MPE is one of the most common complications in patients with advanced cancer, directly affecting patients’ survival, quality of life, and prognosis. If not treated actively, it can even be life-threatening. Lung and breast cancer are the most common causes, followed by malignant lymphoma and ovarian cancer, as well as metastases and other diseases. The main clinical symptoms are chest tightness, pain, dyspnea, etc. The effect of systemic treatment alone is poor. Therapeutic thoracentesis alone and intercostal thoracic drainage may temporarily relieve dyspnea, but the recurrence rate is high in the short term. The usual clinical treatment modality is to use chemotherapy drugs for local or systemic treatment after thoracic puncture and drainage to relieve symptoms. Chemotherapy controls disease progression by including apoptosis of tumor cells. Cisplatin, bleomycin, and other clinical chemotherapy drugs are also commonly used to treat MPE. However, chemotherapy not only destroys the tumor cells but also impairs normal cellular functions. The adverse reactions are more obvious and often difficult for patients to tolerate. As a result, various complications occur, and the patient’s quality of life is further reduced. Therefore, people are urgently seeking safer and more effective treatment options.
Brucea javanica oil emulsion injection (BJOEI) is a new type of anti-cancer drug consisting of the active ingredients extracted from the mature fruits of the sorrelaceae plant, mainly containing oleic acid and linoleic acid. BJOEI is a non-specific anti-cancer drug that targets the cell cycle. It can inhibit DNA synthesis by inhibiting G0, G1, S, G2 and M phases of tumor cells, and it can also enhance the immune function and hematopoietic function of the human body. When combined with chemotherapy, BJOEI can enhance efficacy and reduce toxicity. In recent years, some studies have shown that BJOEI can reverse the multidrug resistance to chemotherapeutic drugs and also induce apoptosis of tumor cells. Therefore, based on clinical data, this study compared the efficacy and safety of BJOEI in combination with chemotherapy and chemotherapy alone in the treatment of MPE to provide suggestions for doctors to improve clinical treatment plans.
2 Methods
2.1 Eligibility criteria and exclusion criteria
Studies were considered eligible for inclusion if the following criteria were met.
Study type: Randomized controlled trials (RCTs) of BJOEI combined with a chemotherapy regimen in the treatment of MPE were included. Efficacy results are clear, and data-based outcome indicators are available. RCTs that mention “random” can be included, and their general information should be complete, regardless of the language and country of the literature, regardless of whether concealed allocation and blinding were used. If the RCTs involve multiple treatment regimens simultaneously, only two groups will be selected: Chemotherapy regimens alone and BJOEI in combination with chemotherapy.
Patient: Patients with MPE confirmed to be malignant by pathology, cytology, or imaging examinations, with clinically evident symptoms of pleural effusion such as cough, shortness of breath, chest tightness, and an expected survival period of more than 3 months. There are no restrictions on patient age, gender, race, and cancer type.
Intervention and comparison: After pleural effusion was drained by therapeutic thoracentesis or intercostal pleural drainage, the control group received an intrapleural injection of chemotherapy drugs or systemic chemotherapy (including cisplatin, carboplatin, oxaliplatin, gemcitabine, pemetrexed, bleomycin, mitomycin, and gefitinib); the treatment group received BJOEI in combination with the control group’s pleural injection treatment regimen.
Outcome: All clinical data, including total effective rate, Karnofsky (KPS) score improvement rate, and adverse reactions, can be included. ① Total effective rate: according to the WHO standard, it is divided into complete remission (CR): the effusion disappears, symptoms are relieved and maintained for more than 4 weeks; partial remission (PR): effusion is significantly reduced (more than 50%), symptoms are relieved, and are maintained for 4 weeks or longer; stable disease (SD): less than 50% reduction in pleural effusion, or increase, but not more than 25%; progressive disease (PD): more than 25% increase in pleural effusion or patient death. Total effective rate (%) = (CR + PR)/total number of cases × 100%. In some literature, the efficacy criteria are expressed slightly differently, but they hardly affect the total amount of CR + PR data. The SD data of 11 papers [6,9,15,17–18,22,24,26–28,30] was merged with the PD data, but this did not affect the total efficiency of the calculation. ② Quality of life improvement rate: according to the KPS scoring standard for quality of life, an increase of 20 points after treatment compared with before treatment was considered markedly effective; an increase of 10 points was considered effective; an increase or decrease of <10 points were considered stable; a decrease of more than 10 points was considered a decline. The markedly effective data and effective data give the total improvement rate data. There are eight literatures [3,6–7,15,16,24,26,30] with slightly different KPS scoring standards, of which 4 literatures [6,24,26,30] combined stable data and declining data but did not affect the improvement rate data. ③ Adverse reactions: Fever, chest pain, gastrointestinal reactions, and leukopenia occur more frequently, and forest plots are made separately for analysis.
Documents that meet the following conditions are excluded: ①The article could not be obtained in full text. ②No related outcome. ③the literature with the repeated publication or unknown data.
2.2 Search strategy
Use the computer to search CNKI, Wanfang Database, VIP Database, SinoMed, PubMed, Embase, the Cochrane Library, and the Web of Science database. “Brucea javanica oil emulsion” and “Javanica oil emulsion injection” are the names of the drug. “Malignant pleural effusion”, “cancerous pleural effusion”, “cancerous pleural fluid”, etc. are the names of the diseases, and RCTs or randomized controlled trials are the search terms. Furthermore, correlative reference documents were retrieved manually.
#1 “Malignant pleural effusion” [Mesh]
#2 “MPE” [Title/Abstract] OR “Cancerous Pleural Fluid” [Title/Abstract] OR “Cancerous Pleural Effusion” [Title/Abstract]
2#3 #1 OR #
#4 “yadanziyouru zhusheye” [Title/Abstract] OR “yadanziyouru zhusheji” [Title/Abstract]) OR “yadanziyouru injection” [Title/Abstract]) OR “yadanziyouru” [Title/Abstract]) OR “Brucea javanica oil emulsion” [Title/Abstract]) OR “Javanica oil emulsion injection”
4#5 #3 AND #
2.3 Literature selection and data extraction
Two researchers independently read the titles and abstracts of the literature and then filtered out duplicates, reviews, pharmacological experiments, and irrelevant articles. The full text of the remaining RCTs was read to assess whether they met the inclusion criteria. If any disagreements occurred, they were resolved by discussion or consultation with a third researcher. After screening, the following information was extracted from the RCTs: ① Basic information of the study (including first author and publication date); ② Study characteristics (including the number of patients in the treatment and control group, sex ratio, mean age, intervention measures and course of treatment); ③ Outcomes; ④ RCTs types and quality assessment factors.
2.4 Risk of bias assessment
Two independent researchers assessed the methodological quality according to the Cochrane Risk of Bias Assessment Tool 1.0, and any disagreements were resolved by discussion or consultation with a third researcher. The assessment included the following: ① Sequence generation (selection bias); ② Allocation concealment (selection bias); ③ Blinding of patients and personnel (performance bias); ④ Implementation of blinding of outcome assessors (detection bias); ⑤ Incomplete outcome data (attrition bias); ⑥ Selective outcome reporting (reporting bias); ⑦ Bias from other sources. Each bias includes three judgments: “high risk”, “unclear” and “low risk”. “High risk” means the research used wrong blinding methods, wrong implementation methods, missing data, etc.; “Unclear” means it was not mentioned in the study and cannot be judged; “Low risk” means the implementation method was correct, or did not affect the outcome measurements etc.
2.5 Statistical analysis
Revman 5.4 and Stata 13.0 software were used to synthesize and analyze the data. The relative risk (RR) was used as the statistic for the analysis of dichotomous variables of the index effect. 95% confidence intervals (95%CIs) were calculated to indicate the range of results. Chi-square analysis was used to study heterogeneity, and I2 assessed the magnitude of heterogeneity. For the meta-analysis, the fixed-effects model was used when p > 0.1, I2 < 50%; otherwise, a random-effects model was used. After the results were obtained, p ≤ 0.05 indicates that the results are significant; otherwise, the risk difference (RD) is used instead of RR as the indicator effect analysis statistic of the dichotomous variable. If there is no qualitative change in the Meta analysis results, this indicates that the actual results of the RR value are reliable. For publication bias analysis, a funnel plot was drawn, and a sensitivity analysis was performed by Stata 13.0 to assess the stability of the results.
3 Results
3.1 Literature retrieval and screening result
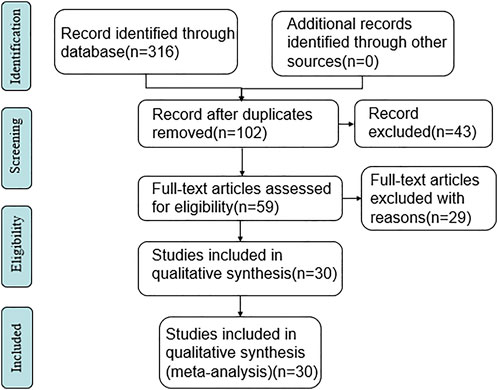
A total of 316 relevant literatures were detected, and all of them were in Chinese. After deduplication, reading titles, and abstracts to exclude irrelevant literature, there were 60 articles left. Further, read the full text of the literature that may meet the criteria after the initial screening and excluded 29 literature, such as non-RCT studies and data that could not be extracted, 30 RCTs were finally included. The specific search situation is shown in Figure 1.
3.2 Characteristics of the included studies
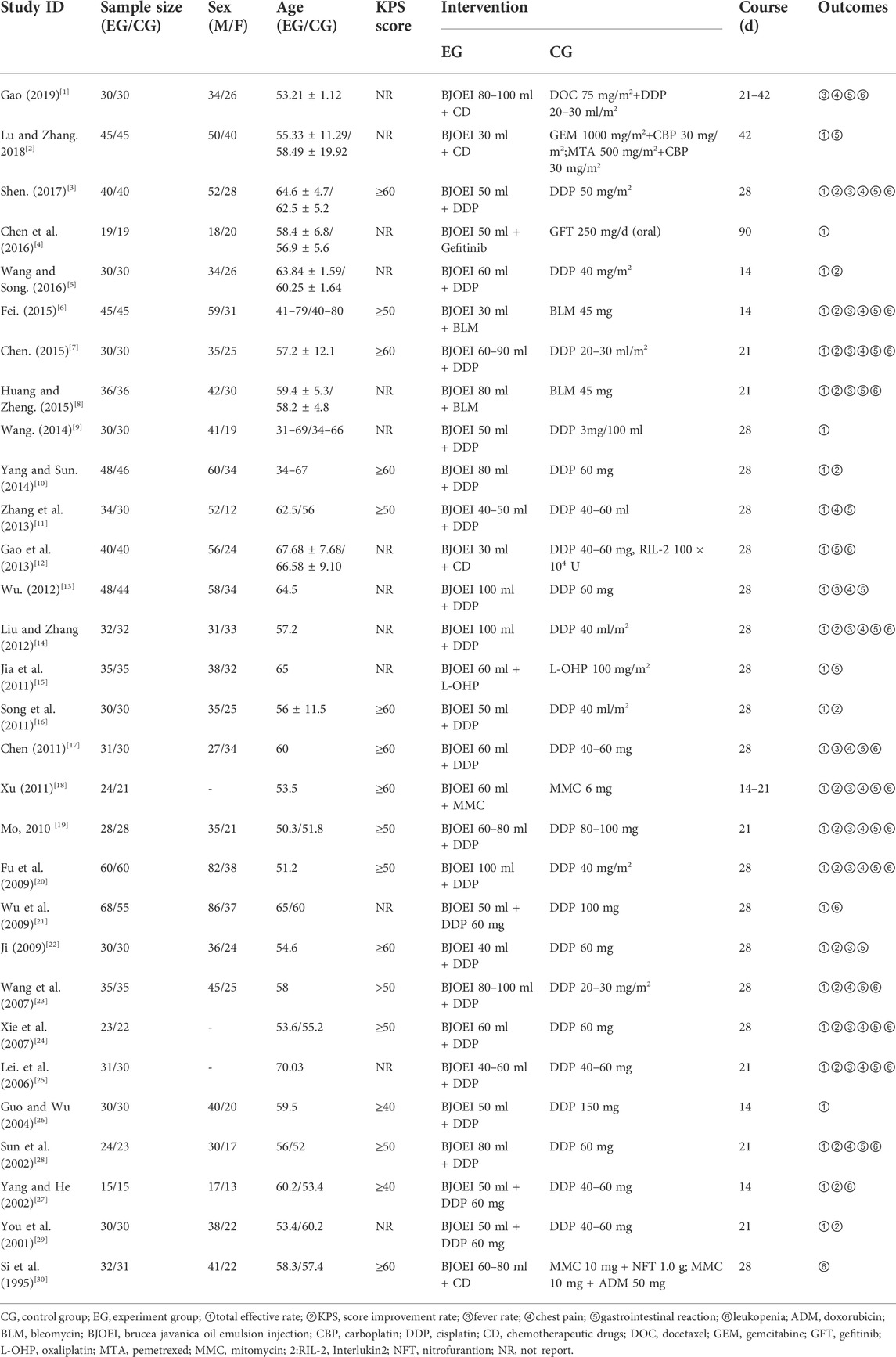
A total of 30 RCTs with 2035 patients were included in this study, 1002 in the control group and 1033 in the treatment group. The details are shown in Table 1.
3.3 Risk of bias assessment
The 30 included studies had a consistent baseline, and the interventions were parallel. All 30 studies were randomized controlled trials, and all mentioned random assignment. Among the 30 RCTs, one RCT (Lu and Zhang, 2018) used the random sampling method, and three RCTs (Gao et al., 2013; Chen, 2015;, 2019) used the random number table method. The above four RCTs (Gao et al., 2013; Chen, 2015; Lu and Zhang, 2018;, 2019) were rated as “low risk” for selection bias because they generated an adequate random sequence. All studies reported complete outcome data and were rated as “low risk” for attrition bias due to incomplete data. The remaining studies were rated as “unclear” due to insufficient information. The overall situation of quality assessment was as shown in Figure 2.
3.4 Outcomes
3.4.1 Clinical total effective rate
A total of 29 RCTs [2–30] reported the clinical total effective rate. Since the heterogeneity results showed p = 0.84 and I2 = 0%, the fixed effects model was selected for analysis in this study. The results showed that the total effective rate of the treatment of MPE with BJOEI in combination with chemotherapy drugs was significantly improved compared with using chemotherapy drugs alone, and the difference between the two groups was statistically significant (RR = 1.45, 95%CI:1.36–1.54, p < 0.00001), as shown in Figure 3.
3.4.2 KPS score improvement rate
A total of 18 RCTs [3,5,6–8,10,14,16,18,20–21,23–26,28–30] reported the KPS score improvement rate. Since the heterogeneity results showed p = 0.95 and I2 = 0%, the fixed effects model was selected for analysis in this study. The results showed that the treatment of MPE with BJOEI in combination with chemotherapeutic drugs could further improve the KPS score and improve the patient’s quality of life compared with the use of chemotherapeutic drugs alone. The difference between the two groups was statistically significant(RR = 1.54, 95%CI: 1.41–1.68, p < 0.00001), as shown in Figure 4.
According to the KPS score of the included cases, patients were divided into three groups: KPS score ≥60 points, KPS score ≥50 points, KPS score ≥40 points, and subgroup analysis was performed. There was little difference in the results between the groups, so the results were pooled.
3.4.3 Fever rate
A total of 14 RCTs [1,3,6–8,13–14,17–18,20–21,23,25–26] reported on fever rate. Since the results of heterogeneity showed p = 0.87 and I2 = 0%, the fixed effects model was selected for analysis in this study. The results showed that the treatment group could reduce the probability of fever to a certain extent compared with the control group. However, it was not statistically significant(RR = 0.82, 95%CI: 0.60–1.12), as shown in Figure 5.
3.4.4 Chest pain
A total of 15 RCTs [1,3,6–7,11,13–14,17–20,23–25,27] reported the chest pain. Since the results of heterogeneity showed p = 0.37 and I2 = 8%, the fixed effects model was selected for analysis in this study. The results showed that the treatment group could reduce the occurrence of chest pain to a certain extent compared with the control group, but it was not statistically significant(RR = 0.90, 95%CI: 0.67–1.21), as shown in Figure 6.
3.4.5 Gastrointestinal reactions
A total of 20 RCTs [1–3,6–8,11–15,17–20,21–25,27] reported on gastrointestinal reaction. Since the results of heterogeneity showed p = 0.46 and I2 = 0%, the fixed effects model was selected for analysis in this study. The results showed that treatment of MPE with BJOEI in combination with chemotherapeutic drugs compared with the use of chemotherapeutic drugs alone, can reduce the probability of gastrointestinal reactions to some extent. The results were statistically significant (RR = 0.70, 95%CI: 0.57–0.87, p < 0.005), as shown in Figure 7.
3.4.6 Leukopenia
A total of 18 RCTs [1,3,6–8,12,14,17–21,23–25,27–28,30] reported leukopenia. Since the results of heterogeneity showed p = 0.53 and I2 = 0%, the fixed effects model was selected for analysis in this study. The results showed that treatment of MPE with BJOEI in combination with chemotherapeutic drugs can reduce the incidence of leukopenia compared with the use of chemotherapeutic drugs alone, and the results were statistically significant (RR = 0.51, 95%CI: 0.43–0.61, p < 0.00001), as shown in Figure 8.
3.5 Safety
Among the 30 RCTs, 23 RCTs recorded adverse reactions, including fever, chest pain, gastrointestinal reactions, leukopenia, liver and kidney damage, hair loss, etc. None of the remaining seven RCTs [4–5,9–10,16,26,29] mentioned adverse reactions.
3.6 Sensitivity analysis
Sensitivity analysis was performed using the one-by-one exclusion method for the clinical total effective rate, i.e., one study was excluded each time and the remaining studies were re-analyzed to obtain the RR value and 95%CI and to assess the stability of the results. The results showed that there was no qualitative change in the RR value and 95% CI, indicating that the results of this study were stable, as shown in Figure 9.
3.7 Publication bias
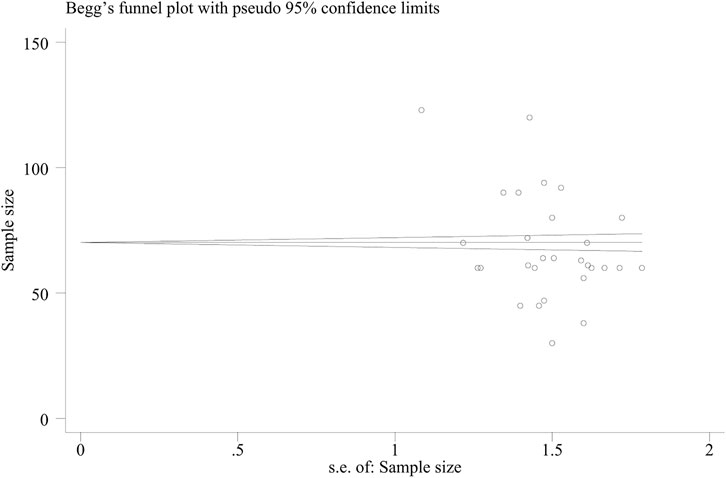
A funnel plot (Figure 10) was used to evaluate the publication bias in the literature for the total effective rate, with the RR value on the horizontal axis and the standard error of the logRR value on the vertical axis. The results showed that the funnel plot was skewed to some degree, the distribution of included studies on the left and right sides of the midline was not completely symmetrical, and one study was outside the auxiliary line, indicating some publication bias.
The Beggar test yielded a value of p = 0.019, indicating that there was no significant publication bias (Figure 11).
4 Discussion
MPE is a common complication in advanced cancer patients whose effusion is exudate. After the tumor invades the pleura of tumor patients, the permeability increases, a large amount of protein invades the pleural cavity, and the colloid osmotic pressure in the pleural cavity continues to increase. Therefore, when a malignant pleural effusion occurs, the amount of effusion is often large. A large amount of pleural effusion compresses the lungs and affects cardiopulmonary function. Without timely and effective treatment, the pleural effusion will further inhibit the patient’s respiratory and circulatory function, eventually leading to respiratory failure or death.
In the theory of Traditional Chinese medicine, MPE belongs to xuanyin, which is caused by the accumulation of phlegm, turbidity, blood stasis and toxins, the blockage of the body’s water channels, and the stagnation of water in the chest and abdomen, which damages the human’s zhengqi. BJOEI can protect the zhengqi, warm and disperse the knots, and promote the flow of qi and blood circulation to treat MPE.
As a new pure anti-cancer drug of Traditional Chinese medicine, BJOEI is known to treat MPE through the following main mechanisms: ① By improving the body’s non-specific immunity, enhancing its ability to generate antibodies, and strengthening the function of phagocytes to achieve the purpose of anti-tumor treatment. ② BJOEI has a specific affinity for tumor cells and can selectively destroy the membrane system of tumor cells. The unsaturated fat in it can directly act on the membrane of the tumor cells, change their surface activity, destroy their biological structure, and finally cause the degeneration and necrosis of tumor cells without affecting the normal cells. ③ Blocks the proliferation of tumor cells by inhibiting their growth and the cell cycle of DNA synthesis. ④ It can specifically inhibit the activity of intracellular POPO II (topoisomerase II), interfere with the normal function of the enzyme, and then cause cell death. ⑤ By stimulating mesothelial cells in the patient’s visceral pleura and parietal pleura to induce chemical pleurisy, the cells proliferate and become fibrotic, resulting in pleural adhesions and pleural atresia, blocking channels for the fluid formation and helping chemotherapeutic drugs to exert their effects. In addition, BJOEI can also reverse tumor resistance to chemotherapy drugs and prolong the application cycle of chemotherapy drugs.
In this study, the average effective rate of the treatment group using BJOEI in combination with chemotherapy in the treatment of MPE was 83.06%, which was a significant improvement over the average effective rate of chemotherapy drugs alone in the control group (57.13%) (RR = 1.45, 95%CI: 1.36–1.54, p < 0.00001), and the difference between the two groups was statistically significant. In terms of improving the quality of life, according to the change in KPS score, the average improvement rate in the treatment group was 80.54%, which was also better than the average improvement rate in the control group (52.32%) (RR = 1.54, 95%CI: 1.41–1.68, p < 0.00001), the difference was statistically significant.
Regarding safety, Figure 5 shows that the fever rates of the two groups were very close in four studies, and the results of three studies tended to the control group. Figure 6 shows that the chest pain rates of the two groups are very close in four studies, and the results of two studies in the control group are better than those in the treatment group. Figure 7 shows that the incidence of gastrointestinal reactions is very similar between the two groups in the six included studies. Moreover, in one RCT, the results of the control group are better than those of the treatment group. As shown in Figure 8, there are three studies in which the incidence of gastrointestinal reactions is very similar between the two groups. Moreover, the results of the control group are better than those of the treatment group in the two studies. However, specific data analysis cannot prove that the control group intervention is superior to the treatment group. RD was used to re-merge and analyze the data in Figures 5–8, and the same qualitative results as the original effect index RR were obtained, which can be considered more reliable. It was concluded that the incidence of adverse reactions in the treatment group was generally lower than that in the control group. In reducing the incidence of gastrointestinal reactions, the treatment group had a more significant advantage than the control group (RR = 0.70, 95%CI: 0.57–0.87, p < 0.05). Similarly, the treatment group also had a significant advantage over the control group in reducing the incidence of leukopenia (RR = 0.51, 95%CI: 0.43–0.61, p < 0.00001). In terms of reducing the fever rate and chest pain rate, the treatment group was the same as the control group, and the treatment group had a better effect to a certain extent. However, the meta-analysis results did not show that the difference between the two groups was statistically significant.
At present, there are two meta-analysis articles on the treatment of MPE with BJOEI in combination with chemotherapy, both published in 2014. Zhengbo Hu et al. conducted a meta-analysis on the treatment of MPE with BJOEI in combination with the intrapleural injection of chemotherapeutic drugs. Kongping Wei et al. conducted a meta-analysis on the treatment of MPE with BJOEI in combination with cisplatin alone. In this study, a meta-analysis was performed on the treatment of MPE with BJOEI in combination with chemotherapeutic drugs. These studies did not limit the types of chemotherapeutic drugs, nor were they limited to local injection or systemic chemotherapy. However, the drawback is that some of the included studies did not report the randomization methods and most of the literatures did not mention blinding or allocation concealment.
5 Conclusion
In summary, this study verified that BJOEI in combination with chemotherapy drugs could enhance the therapeutic effect of MPE, effectively improve patients’ quality of life, and reduce the toxic and side effects of chemotherapy drugs, which is worth further clinical promotion.
Abbreviations
95% CI, 95% Credible intervals; BJOEI, Brucea javanica oil emulsion injection; CR, complete remission; KPS, Karnofsky performance status; MPE, malignant pleural effusion; PD, progressive disease; PR, partial remission; PRISMA, the preferred reporting items for systematic reviews and meta-analyses; RCTs, Randomized controlled trials; RD, risk difference; RR, relative risk; SD, stable disease.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Author contributions
RS: conceptualization, methodology, formal analysis, original draft writing, and visualization. ZW and HW: methodology, formal analysis, review editing. JZ and FZ: software and review editing. AS: editing and Language polishing. JW and CW: conceptualization, funding acquisition and project administration. MC, YZ and JH: formal analysis and supervision. QZ, BS and PL: formal analysis, review editing.
Funding
This work was supported by Natural Science Foundation of China (Grant NO.82074284), China National Traditional Chinese Medicine Inheritance and Innovation Team Sub-project (Grant No. ZYYCXTD-C-202005-10), China Medical Association of Minorities Research Project (Grant No. 2020MZ298-110101), Open Fund Project, Associated Key Laboratory of Traditional Mongolia Medicine Research and Development, National Ethnic Affairs Commission and Ministry of Education of China (Grant No. MDK2020013).
Conflict of interest
Authors QZ and BS were employed by the company Jiangsu Jiuxu Pharmaceutical Co. Ltd. Author CW was employed by the company Beijing Zest Bridge Medical Technology lnc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.998218/full#supplementary-material
References
Chen, S. L. (2015). Observation on the curative effect of brucei oil emulsion injection combined with cisplatin pleural perfusion in the treatment of malignant pleural effusion caused by lung cancer. Zhengzhou, Henan: Henan University of Traditional Chinese Medicine.
Chen, Y., Fan, W. C., Tseng, P. C., Tsai, C. M., Chou, T. Y., Wu, C. H., et al. (2011). Plasma epidermal growth factor receptor mutation analysis and possible clinical applications in pulmonary adenocarcinoma patients treated with erlotinib. Oncol. Lett. 21 (10), 713–717. doi:10.3892/ol.2011.534
Chen, Z. H., Huang, L. Z., Gong, H., Zhou, S. L., Xu, L., Ding, P., et al. (2016). Clinical study on the treatment of lung adenocarcinoma malignant pleural effusion by intrapleural injection of brucei oil emulsion combined with gefitinib. Traditional Chin. Med. Pharmacol. Clin. 32 (04), 131–133.
Fei, X. F. (2015). Clinical study on the treatment of malignant pleural effusion of lung cancer with Brucea javanica oil emulsion combined with intrapleural injection of bleomycin. Hangzhou, Zhejiang: Zhejiang University of Traditional Chinese Medicine.
Fu, X. J., Fu, Y. Z., Yang, G. H., Xue, Y. H., Chen, L. S., Yu, S. Z., et al. (2009). Observation on the curative effect of continuous catheter drainage combined with Brucea javanica oil emulsion and cisplatin in the treatment of malignant pleural effusion. J. Clin. Mil. Med. 37 (05), 818–820.
Gao, C. L. (2019). Efficacy of intrathoracic injection of Brucea javanica oil emulsion combined with nursing in the treatment of lung cancer with malignant pleural effusion. Chin. Folk. Ther. 2019 (06), 53–54+72.
Gao, Z. W., Lou, J. S., Ding, T. L., Song, D. S., and Xu, Y. (2013). Clinical observation of duck gall seed oil emulsion combined with cisplatin and Interleukin-Ⅱ in treating lung cancer with malignant pleural effusion. Chin. Oncol. Clin. Rehabilitation 20 (02), 153–154.
Guo, Y. W., Wu, J. Y., Yang, H. C., and Cao, C. W. (2004). The treatment of malignant pleural effusion by intracavitary injection of Brucea javanica oil emulsion combined with cisplatin. J. Oncol. 2004 (02), 129–130.
Huang, G. P., and Zheng, Z. (2015). Clinical efficacy of brucei oil emulsion injection combined with bleomycin in the treatment of primary lung cancer with cancerous pleural effusion. Liaoning J. Traditional Chin. Med. 42 (03), 533–535.
Ji, Y. H. (2009). Cisplatin combined with Brucea javanica oil for the treatment of 30 cases of malignant pleural effusion. J. Oncol. 15 (05), 474–475.
Jia, L., Wang, Y. L., and Geng, L. M. (2011). The treatment of 35 cases of malignant pleural effusion with brucella oil emulsion combined with oxaliplatin in pleural injection. J. Traditional Chin. Med. 52 (22), 1956–1957.
Lei, H., Zhao, Y. X., and Su, L. H. (2006). Treatment of 111 elderly patients with malignant body cavity effusion by intracavitary injection of Brucea javanica oil emulsion combined with chemotherapeutic drugs. Ningxia Med. J. 2006 (05), 372–373.
Lin, J. G., Lyu, J., Sun, M. H., and Liao, X. (2020). Systematic review and meta-analysis of shenfu injection on treating acute exacerbation of chronic obstructive pulmonary disease. World J. Tradit. Chin. Med. 6, 276–283. doi:10.4103/wjtcm.wjtcm_30_20
Liu, B., Zhang, L. M., Zhu, F., Guo, C. h., and Yang, W. l. (2012). Comparison between anterior and posterior decompression for cervical spondylotic myelopathy: Subjective evaluation and cost analysis. Orthop. Surg. 19 (07), 47–54. doi:10.1111/j.1757-7861.2011.00169.x
Lu, G. J., and Zhang, Y. (2018). Observation of curative effect of Brucea javanica oil emulsion combined with systemic chemotherapy in the treatment of malignant pleural effusion. J. Med. Res. 47 (03), 97–99.
Mo, S. X. (2010). The effect of brucei oil emulsion combined with chemotherapy on the immune function of patients with non-small cell lung cancer after surgery. Mod. J. Integr. Traditional Chin. West. Med. 19 (09), 1098–1099.
Pei, D. M., Ji, Y., and Li, Z. (2021). Effects of Brucea javanica oil emulsion combined with capecitabine and oxaliplatin on immune function and tumor metastasis and infiltration in elderly patients with advanced colorectal cancer. World Clin. Med. 42 (05), 369–374.
Shen, S. L. (2017). Clinical efficacy of cisplatin combined with Brucea javanica oil emulsion in the treatment of malignant pleural effusion caused by lung cancer. J. Clin. Ration. Drugs 10 (30), 10–11.
Si, P. X., Guo, G. S., Huang, J. L., and Liu, H. L. (1995). Analysis of the efficacy of adjuvant treatment of 32 cases of malignant pleural effusion with crow milk. Clin. Metadata 1995 (12), 546.
Song, G. F., Chen, L. N., Xu, Y. F., and Liu, P. (2017). Tumor inhibitory effect and apoptosis mechanism of Brucea javanica oil emulsion on A549 lung cancer cells and A549 lung cancer nude mice. Chin. Med. Her. 23 (24), 26–29.
Song, Y. J., Wang, L. X., Hong, Y. Q., and Huang, M. (2011). Curative effect of Brucea javanica oil emulsion combined with cisplatin in the treatment of malignant pleural effusion. Jiangsu Med. 37 (21), 2527–2529.
Sun, D. X., Shen, H. C., Ji, C. L., and Wei, Q. L. (2002). Observation of curative effect of Brucea javanica oil emulsion combined with cisplatin in the treatment of malignant pleural effusion. Chin. Pat. Med. 2002 (08), 36–38.
Wang, C. S. (2014). Observation and nursing of 30 cases of malignant pleural effusion treated by Brucea javanica oil emulsion combined with cisplatin. Natl. Med. Forum 29 (05), 28–29.
Wang, C. Y., and Song, C. Y. (2016). Treatment of 30 cases of lung cancer with pleural effusion by Brucea javanica oil emulsion combined with cisplatin. Henan Tradit. Chin. Med. 36 (04), 665–666.
Wang, H. M., Liao, G. Q., Liu, P. H., Qu, Y. M., and Xie, G. Q. (2007). Treatment of 70 cases of lung cancer with pleural effusion with Brucea javanica oil emulsion combined with cisplatin. China Oncol. 12, 1035–1036.
Wu, G. G. (2012). Curative effect observation on 92 cases of cancerous pleural effusion treated by intrathoracic infusion of Brucea javanica oil and cisplatin. Front. Med. 2 (19), 211.
Wu, S. H., Rui, L., and Hong, Y. C. (2009). Curative effect observation of 68 cases of cancerous pleural effusion treated with Brucea javanica oil emulsion combined with cisplatin intrapleural injection. Hainan Med. 20 (09), 14–15.
Xie, Y. P., Nie, L., Wang, F., and Wu, J. (2007). Clinical observation on the treatment of malignant pleural effusion with brucella oil emulsion combined with cisplatin in the treatment of malignant pleural effusion. J. Clin. Pulmonol. 8, 809–810.
Xu, C. H. (2011). Study on the treatment of malignant pleural effusion with Brucea javanica oil emulsion combined with mitomycin. J. Clin. Pulmonol. 16 (01), 70–71.
Yang, G. Y., and Sun, C. H. (2014). Clinical observation of Brucea javanica oil emulsion combined with cisplatin in the treatment of malignant pleural effusion. Chin. Foreign Med. Care 33 (13), 142–143+145.
Yang, J., and He, L. (2002). Brucea javanica oil emulsion combined with intracavitary injection of cisplatin in the treatment of cancerous pleural effusion. Henan J. Oncol. 4, 281–282.
You, J. L., Xue, Q., and Zhao, Y. C. (2001). Treatment of 30 cases of cancerous pleural effusion by intrapleural injection of Brassica chinensis oil emulsion combined with cisplatin. J. Traditional Chin. Med. 9, 569–570.
Zhang, C., Lyu, J., Xie, Y. M., and Sun, M. H. (2020). Clinical evaluation on xiyanping injection in the treatment of bronchopneumonia in children based on meta-analysis. World J. Tradit. Chin. Med. 6, 307–323. doi:10.4103/wjtcm.wjtcm_29_20
Zhang, H., Jin, D. Y., and Zhao, Y. (2013). Treatment of 34 cases of malignant pleural effusion with Brucea javanica oil emulsion combined with cisplatin. Mod. Distance Educ. Chin. Med. 11 (05), 41–42.
Zhang, X. J., Wang, X. L., Zhao, F., An, C. L., and Wang, X. N. (2011). Inhibition of the proliferation of lung cancer NCI-H460 cells by Brucea javanica oil emulsion and its mechanism. Chin. J. Oncol. Biotherapy 18 (05), 519–523.
Keywords: Brucei javanica oil emulsion injection, chemotherapy, malignant pleural effusion, meta-analysis., randomzied controlled trials
Citation: Shi R, Wu Z, Wang H, Zhang J, Zhang F, Stalin A, Chen M, Huang J, Zhai Y, Zhang Q, Liu P, Wu J, Sun B and Wu C (2022) Investigation on the efficiency of Brucea javanica oil emulsion injection with chemotherapy for treating malignant pleural effusion: A meta-analysis of randomized controlled trials. Front. Pharmacol. 13:998218. doi: 10.3389/fphar.2022.998218
Received: 19 July 2022; Accepted: 22 August 2022;
Published: 16 September 2022.
Edited by:
Rong-Rong He, Jinan University, ChinaReviewed by:
Weijian Bei, Guangdong Metabolic Disease Research Center of Integrated Chinese and Western Medicine, ChinaRuixin Liu, First Affiliated Hospital of Henan University of Traditional Chinese Medicine, China
Haibo Cheng, Nanjing University of Chinese Medicine, China
Copyright © 2022 Shi, Wu, Wang, Zhang, Zhang, Stalin, Chen, Huang, Zhai, Zhang, Liu, Wu, Sun and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiarui Wu, ZXhvZ2FteUAxNjMuY29t; Bin Sun, MzY0MjY3MDU3QHFxLmNvbQ==; Chunfang Wu, d3VjaHVuZmFuZ0B6ZXFpYW8uY29t
 Rui Shi1
Rui Shi1 Antony Stalin
Antony Stalin Yiyan Zhai
Yiyan Zhai Jiarui Wu
Jiarui Wu