
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 03 November 2022
Sec. Respiratory Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.995051
This article is part of the Research Topic Innovative 3D models for Understanding Mechanisms underlying Lung Diseases: Powerful Tools for Translational Research View all 13 articles
 Mugdha M. Joglekar1,2*†
Mugdha M. Joglekar1,2*† Mehmet Nizamoglu1,2†
Mehmet Nizamoglu1,2† YiWen Fan1,2
YiWen Fan1,2 Sai Sneha Priya Nemani3,4
Sai Sneha Priya Nemani3,4 Markus Weckmann3,4
Markus Weckmann3,4 Simon D. Pouwels1,2,5
Simon D. Pouwels1,2,5 Irene H. Heijink1,2,5
Irene H. Heijink1,2,5 Barbro N. Melgert2,6
Barbro N. Melgert2,6 Janesh Pillay2,7
Janesh Pillay2,7 Janette K. Burgess1,2,8*
Janette K. Burgess1,2,8*Environmental insults including respiratory infections, in combination with genetic predisposition, may lead to lung diseases such as chronic obstructive pulmonary disease, lung fibrosis, asthma, and acute respiratory distress syndrome. Common characteristics of these diseases are infiltration and activation of inflammatory cells and abnormal extracellular matrix (ECM) turnover, leading to tissue damage and impairments in lung function. The ECM provides three-dimensional (3D) architectural support to the lung and crucial biochemical and biophysical cues to the cells, directing cellular processes. As immune cells travel to reach any site of injury, they encounter the composition and various mechanical features of the ECM. Emerging evidence demonstrates the crucial role played by the local environment in recruiting immune cells and their function in lung diseases. Moreover, recent developments in the field have elucidated considerable differences in responses of immune cells in two-dimensional versus 3D modeling systems. Examining the effect of individual parameters of the ECM to study their effect independently and collectively in a 3D microenvironment will help in better understanding disease pathobiology. In this article, we discuss the importance of investigating cellular migration and recent advances in this field. Moreover, we summarize changes in the ECM in lung diseases and the potential impacts on infiltrating immune cell migration in these diseases. There has been compelling progress in this field that encourages further developments, such as advanced in vitro 3D modeling using native ECM-based models, patient-derived materials, and bioprinting. We conclude with an overview of these state-of-the-art methodologies, followed by a discussion on developing novel and innovative models and the practical challenges envisaged in implementing and utilizing these systems.
Cellular migration has a fundamental role in directing development, tissue homeostasis, and disease progression (Morales et al., 2021; Yamada et al., 2022). Cells have different modes of migration–singular, amoeboid or mesenchymal, or collective fashion depending on the local tissue microenvironment and activated signaling pathways (van Helvert et al., 2018; Yamada and Sixt, 2019). The extracellular matrix (ECM) of the lung is a dynamic structural network which consists of proteins, glycosaminoglycans, and glycoproteins (Burgstaller et al., 2017). It provides structural support during important mechanical events of breathing. It is also an important bioactive component of the cellular microenvironment as it provides cues that regulate cellular processes (Theocharis et al., 2016; Yamada and Sixt, 2019). Local molecular composition (including growth factors and cytokines) and biomechanical properties (elasticity, stiffness, and compression forces) of the ECM can govern migration of (infiltrating) immune cells (van Helvert et al., 2018; Yamada and Sixt, 2019; Morales et al., 2021). Other factors that influence cellular migration include confinement of cells, ECM crosslinking and remodeling, and ECM geometry such as topology, fiber alignment, and porosity (van Helvert et al., 2018; Yamada and Sixt, 2019; Morales et al., 2021; Burgess and Harmsen, 2022). Further, ECM fragments resultant from remodeling can promote or inhibit cellular migration (Gu et al., 2018; Nissen et al., 2018; Sharma et al., 2018; de Castro Bras and Frangogiannis, 2020).
The lung is a unique organ exposed to exogenous environmental insults and infectious agents and consequently has highly regulated immune and damage repair responses. Severe or repetitive insults can cause micro-injuries leading to acute and chronic lung diseases (Labaki and Han, 2020). Chronic lung diseases are in general incurable and often have high hospitalization rates. Additionally, some patients are at risk of disease exacerbations that accelerate disease progression. Moreover, insight into the pathobiology of each of the lung diseases is still limited (Labaki and Han, 2020). Therefore, understanding the immunopathology of each of these diseases is essential for developing effective clinical management and new treatment approaches. Chronic obstructive pulmonary disease (COPD), lung fibrosis, asthma, and acute respiratory distress syndrome (ARDS) are all characterized by abnormal ECM turnover and chronic inflammatory responses in varying degrees, which lead to tissue damage (Ito et al., 2019; Burgess and Harmsen, 2022).
Investigating cell-ECM interactions as a contributing factor to the disease progression has been emerging in the last decade (McMahon et al., 2021; Burgess and Harmsen, 2022). Upon injury, the process of tissue repair is initiated, during which recruited immune cells migrate through ECM to reach the target location. Inflammation and resolution of wound healing processes are regulated by contribution of (infiltrating) immune cells (Volk et al., 2013; Manon-Jensen et al., 2016; Wang et al., 2022). It is likely that their migration, in these lung diseases, through aberrant ECM will affect the function of the infiltrating immune cells.
Conventional immune cell migration studies using standard two-dimensional (2D) cell assessment systems have provided conceptual advances (Puttur et al., 2019). Such studies have revealed a specialized mode of migration (repetitive protrusion, adhesion and contraction) that cells adopt in 2D microenvironments (Hallmann et al., 2015; Yamada and Sixt, 2019). However, it is now clear that cells implement several different modes of migration in three-dimensional (3D) environments (Hallmann et al., 2015; Yamada and Sixt, 2019). In both 2D and 3D in vitro migration assays the role of ECM in regulating these processes has been explored. Several different materials have been used as ECM-mimicking substrates. These include synthetic polymers such as poly (ethylene glycol) (Widener et al., 2021) and natural polymers such as collagen (Li et al., 2018; Surendran et al., 2021). Methodologies including precision cut lung slices (PCLS), organoids, lung-on-chip, whole decellularized lung tissues, and hydrogels have been developed over the past decades to mimic physiological environments in vitro, each with their own advantages and challenges (Gkatzis et al., 2018; Liu et al., 2019; Nizamoglu et al., 2022). However, there has been limited implementation of such models for studying immune cell infiltration, thereby providing future opportunities for exploring the dynamics between ECM and infiltrating immune cell migration in the context of lung diseases.
As the field moves ahead with innovative models, it is simultaneously important to consider new discoveries in cellular migration and how the inclusion of ECM could add to this knowledge. Cells produce and leave behind retraction fibers during migration that support the formation of vesicle-like structures called migrasomes (Tavano and Heisenberg, 2019; Fan et al., 2022). Cancer cells more frequently migrated along residual retraction fibers in microfluidic channels compared to channels without these fibers (Lee et al., 2021). Neutrophils have been shown to leave cytoplasmic trails containing chemokines for T cells upon viral infection, which may very well consist of migrasomes (Lim et al., 2015). The involvement of the ECM was highlighted through the discovery that the generation of these extracellular vesicles was being triggered by the interaction of cells with fibronectin fibers (Wu et al., 2017; Lee et al., 2021). In concert, migratory trajectories of chemotaxing neutrophils have previously been shown dependent on collagen concentration (Francois et al., 2021). Thus, migrasomes in combination with trails, could increase efficiency of directional migration. The effect of healthy and diseased ECM on the cellular source of migrasomes and trails could begin to explain the continual recruitment of immune cells. Whether a diseased ECM highway provides additional road bumps in the formation of migrasomes and migratory trails remains unexplored. Altogether, these studies highlight the role of the ECM in not just the regulation of the migratory behavior of first responders, but also the recruitment of subsequent immune cells or secondary responses such as adaptive immunity. A study using Drosophila embryos demonstrated weakened cell-ECM connections during cellular division that facilitated macrophage infiltration (Akhmanova et al., 2022). Although it may sound counter intuitive, the (increased rate of) division of cells might act as an “exit” from the ECM highway. A similar phenomenon occurring in diseases associated with hyperproliferation of stromal cells such as asthma and fibrotic lung diseases may be possible but it is unexplored to date. These new insights mentioned above on how infiltrating immune cells interact with ECM and the (resident or recruited) cells can also be further expanded in the context of the influence of ECM using in vitro models.
In this review, we highlight the importance of interactions between the “highway” ECM and infiltrating cells in the pathogenesis of various lung diseases. We review emerging technologies for in vitro modeling that better represent physiological characteristics. Some challenges that exist for implementing these models to study ECM-immune cell interactions during their migration into lung tissue will also be discussed.
Knowledge of how ECM relates to cellular migration has been the focus of recent studies illustrating that the ECM acts as a highway for the migrating/infiltrating immune cells. Biochemical and biomechanical properties of ECM influence the migratory behavior of cells, including immune cells. The importance of available adhesion ligands was established when fibroblasts were able to migrate along stiffness gradients (durotaxis) on fibronectin-coated substrates, whereas this ability was lost on substrates coated with laminin (Hartman et al., 2017). Increased fiber alignment promoted cell migration and directionality of migration (Wang et al., 2018). The inability of aged fibroblasts to produce a hyaluronan and proteoglycan cross-linking proteinresulted in the formation of a more aligned matrix that promoted metastasis while inhibiting T cell migration (Kaur et al., 2019). Accelerated ageing is a distinctive feature of some chronic lung diseases such as COPD (Brandsma et al., 2017) and idiopathic pulmonary fibrosis (IPF) (Chilosi et al., 2013; Selman and Pardo, 2021), making the above observation relevant to the field of lung research. In addition to being a reservoir for growth factors and cytokines, other factors of the ECM discussed here collectively influence the migratory behavior of infiltrating immune cells. As the composition of the ECM has been the main focus of many studies so far, most knowledge is on the influence of different ECM components on cell migration. A summary of the changes in composition of lung ECM during lung diseases can be found in Table 1. It is evident from this table that different studies have different conclusions. The diversity in these observations could be attributed to disease heterogeneity, variation in study population, and disease phenotypes. Nevertheless, the table can serve as a guide while developing in vitro models within the realms of current knowledge. A simple assumption would be that altered composition of ECM triggers changes in the mesenchymal mode of migration due to the alterations in the number of binding domains available for integrins (Yamada and Sixt, 2019). The following subsections will discuss how infiltrating immune cells participate in lung diseases and what is the role of ECM in influencing these migration patterns in the context of lung fibrosis, COPD, asthma, and ARDS.
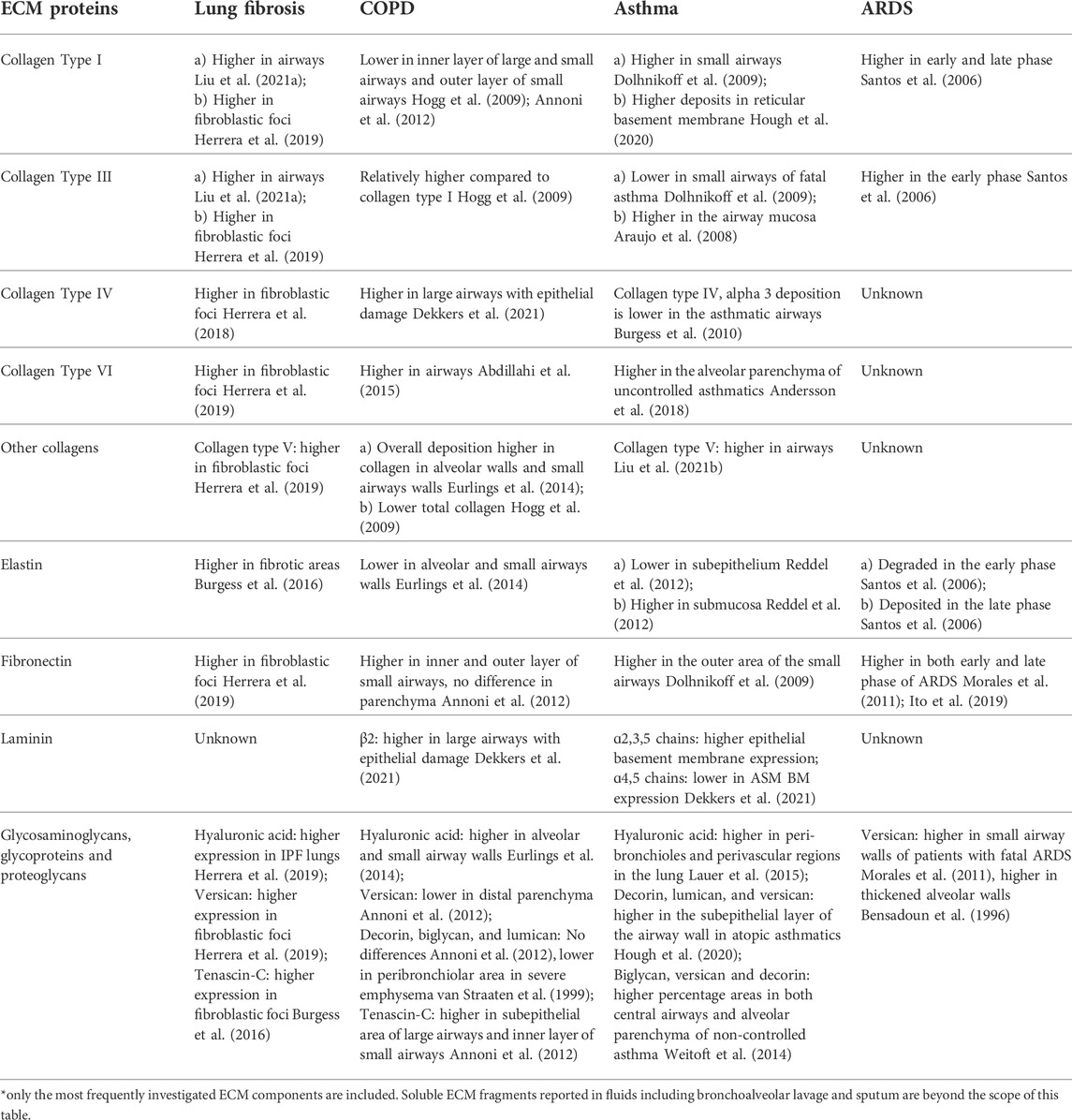
TABLE 1. Changes in the components of extracellular matrix in lung diseases compared to non-diseased controls (unless otherwise specified). *
ECM in lung parenchyma during lung fibrosis is substantially altered from healthy lungs. This has been illustrated both in terms of amounts and/or ratios of ECM components and with respect to the 3D organization of the ECM network (Burgess et al., 2016; Burgstaller et al., 2017; Burgess and Harmsen, 2022; Nizamoglu and Burgess, 2022). Along with altered biochemical composition (Table 1), altered mechanical environment with increased stiffness, decreased viscoelastic relaxation, as well as disorganized fibers and abnormal topography are well-documented changes in ECM in lung fibrosis (Booth et al., 2012; Tjin et al., 2017; de Hilster et al., 2020).
The involvement of circulating immune cells in lung fibrosis is well recognized: among these cells are monocytes and neutrophils (Ishikawa et al., 2021). While the details of recruitment and involvement of these cells are outside scope of this review, these processes can take place through both soluble mediators (Huang et al., 2020; van Geffen et al., 2021) and mechanical factors (Du et al., 2022). Higher counts of monocytes in blood were associated with faster disease progression in interstitial lung diseases (Kim et al., 2022). In mice, monocytes arriving in fibrotic lung tissue transform to macrophages to repopulate lung tissue and remain in the tissue with higher profibrotic activity compared to tissue-resident macrophages (Misharin et al., 2017). Monocytes and neutrophils were found in higher numbers in bronchoalveolar lavage (BAL) fluid of IPF patients (Kinder et al., 2008). Neutrophils were also increased during acute exacerbations of lung fibrosis (Lee et al., 2012). Due to their dynamic nature, both monocytes/macrophages and neutrophils are readily instructed by their microenvironment (Nissen et al., 2018; Vasse et al., 2018; Vasse et al., 2021).
The altered (fibrotic) microenvironment influences infiltrating immune cells in several different ways. In a study, fibroblasts cultured for different durations resulted in varying degrees of fiber organization in collagen matrices (Pakshir et al., 2019). These alterations in fiber organization, however, were unable to influence macrophage migration speed in 3D (Pakshir et al., 2019). On the other hand, neutrophil migration speed but not the directionality, was lower in denser 3D collagen networks (Francois et al., 2021). When fiber crosslinking was applied, increased crosslinking of 2D fibrin surfaces promoted macrophage migration. However, the fiber crosslinking also changed other mechanical parameters, such as stiffness. This unintended change might have also influenced the migrational behavior of the infiltrating immune cells (Hsieh et al., 2019). The influence of ECM crosslinking on migration of neutrophils has yet to be described. Similarly, the influence of altered stress relaxation, another important property of altered microenvironment in fibrotic lung ECM, on infiltrating immune cells has not been examined. New insights for lung fibrosis research can be drawn from a recent study illustrating minimal migration of cancer cells on 2D surfaces which lack stress relaxation, but robust migration of the same cells on the surfaces with high stress relaxation capacity (Adebowale et al., 2021). In addition to the changes in the ECM organization, released ECM fragments can also alter the migratory behavior of the lung resident cells (Nizamoglu and Burgess, 2022). Although there are recent studies focusing on these fragments (Burgess and Harmsen, 2022), their potential influence on migratory behavior and function of infiltrating immune cells remains unknown.
COPD is characterized by excessive ECM remodeling and ECM deposition around the small airways, while the alveolar region is characterized by ECM disruption and tissue destruction (Burgess et al., 2016; Brandsma et al., 2020). Inflammatory responses are central to COPD and understanding the immunopathology is particularly important as current treatments are ineffective in mitigating disease progression and lung tissue damage. In the context of migration in COPD, neutrophils, monocytes, and T cells to an extent, and have received most attention. These cells, and associated secreted factors, have been reported elevated in patients’ sputum, blood, and BAL, and often correlate with the progression of COPD (Hogg et al., 2004; Vargas-Rojas et al., 2011). In addition, neutrophils and macrophages from patients with COPD display impaired effector functions such as efferocytosis and phagocytosis (Taylor et al., 2010; Tan et al., 2017; Dicker et al., 2018; Belchamber et al., 2019), likely extending to a variation in normal migratory behavior of infiltrating immune cells. This has been previously demonstrated with respect to chemotactic cytokines (Sapey et al., 2011; Costa et al., 2016).
While studies exploring the influence of the ECM and ECM fragments on immune cell migration in COPD are limited, sputum has often been investigated as a chemotactic agent. CD14+ monocytes from healthy individuals not only migrated more than CD14+ monocytes from patients with COPD, but also more towards COPD sputum compared to normal sputum (Ravi et al., 2017). Similarly, neutrophils from patients with COPD migrated more towards COPD sputum compared to normal sputum, although T cells from these patients did not show the same trend (Wu et al., 2015). These studies did not identify specific sputum factors responsible for the induction of immune migratory responses. Thus, there can be multiple constituents of the sputum that can have chemotactic effects on cells including ECM fragments (Nissen et al., 2018). Indeed, alterations in sputum composition between health and disease have been demonstrated (Titz et al., 2015; Moon et al., 2018), also with respect to differential levels of ECM fragments that can alter cellular migration in patients with COPD. For example, fragments of production or degradation of collagen (Schumann et al., 2018), elastin (Ronnow et al., 2019), and fibrinogen (Manon-Jensen et al., 2019) have also been detected in sputum and serum/plasma in patients with COPD and are proposed as biomarkers of disease progression. Proline-glycine-proline (PGP), a matrikine derived from collagen, is elevated in sputum of patients with COPD and is a potent chemoattractant for neutrophils (Gaggar et al., 2008; O'Reilly et al., 2013; Patel et al., 2018). However, the role of abnormal ECM in recruitment and regulation of migratory behavior of immune cells remains unexplored.
Secreted pro-inflammatory factors such as cytokines and proteases perpetuate immune responses and remodel ECM (Ni and Dong, 2018; Brightling and Greening, 2019). In COPD, higher neutrophil elastase activity was associated with emphysematous tissue destruction (Walton et al., 2016), and lower trans-endothelial T cell migration (Rao et al., 2004). Consequently, biomechanical properties of lung tissue of patients with COPD are altered, such as loss of elasticity, increased stiffness around small airways, and decreased stiffness in the emphysematous regions (Burgess and Harmsen, 2022). These changes are bound to alter the characteristics of cellular migration.
Asthma is characterized by hallmark features such as airway inflammation and remodelling. Airway remodeling, a feature of asthma—but also seen in COPD, refers to the structural and ECM changes in both small and large airways (Hough et al., 2020). The profile of ECM is altered in the asthmatic airways with less deposition of collagen type IV, elastin, and more deposition of collagen type I, fibronectin, laminin, periostin, versican, decorin, and lumican (Burgess et al., 2010; Hough et al., 2020; Dekkers et al., 2021). Recently, also fibrillar collagen was shown to be fragmented and disorganized in the lamina propria of large and small airways from patients with asthma (Mostaco-Guidolin et al., 2019). Several factors have been identified in asthma that led to abnormal turnover of ECM components such as epigenetic modifications, recurrent viral infections and excess fibrolysis (Pech et al., 2018; Nemani et al., 2021; Weckmann et al., 2021; Ronnow et al., 2022).
Several immune cells, including neutrophils, eosinophils, monocytes, macrophages, and mast cells, among others, are considered to play an important role in airway remodeling in asthma (Holgate et al., 2015; Helfrich et al., 2019). Alveolar macrophages, mast cells, eosinophils, and neutrophils were shown to degrade ECM by releasing matrix metalloproteinase (MMP)−9 (Hough et al., 2020). MMP-driven degradation of collagen released biologically active fragments in asthma such as the pro-neutrophilic matrikine PGP (Patel and Snelgrove, 2018). Another significant matrikine in asthma is tumstatin, a non-collagenous domain of collagen type IV α3 which was shown to be significantly reduced in airways from patients with asthma (Burgess et al., 2010). Interestingly, when mice were treated with tumstatin the inflammatory cell counts in the lungs were reduced (Burgess et al., 2010).
It was recently suggested that migration of tissue eosinophils in ECM - likely occurs via periostin interactions which were particularly higher in T2-high asthmaand correlated with recruitment of eosinophils to the airway (Johansson, 2017; Burgess et al., 2020). Another study reported the chemotaxis of neutrophils was reduced on tumstatin-induced asthmatic airway smooth muscle cell-derived ECM (Harkness et al., 2017). Using a human airway-on-chip, transmigration of immune cells to the epithelial lumen from the vascular microchannel during a viral infection was analyzed. Greatest neutrophil adhesion at the surface of the microvascular endothelium was observed in presence of IL-13 stimulation (to mimic T-helper cell type 2 asthmatic phenotype) rapidly followed by neutrophil trans-endothelial migration through a combination of migratory events (Nawroth et al., 2020).
In ARDS, changes in pulmonary ECM are a direct consequence of the inflammatory injury and subsequent repair responses (Tomashefski, 2000; Ito et al., 2019). The changes in ECM can be divided in distinct ARDS phases, with ECM destruction and alveolar and capillary damage predominating in the early phase, which transitions to a fibroproliferative repair phase later. However, the phases are not strictly separated as early fibroblast activation and matrix deposition are also present (Meduri et al., 1998; Boyd et al., 2020).
Early recruitment of neutrophils and monocytes, following lung injury results in ECM degradation, predominantly through the production of MMPs (Torii et al., 1997; Davey et al., 2011). Fragments generated from degradation of ECM play a role in amplifying recruitment of inflammatory cells. The matrikines PGP and its acetylated form induce neutrophil chemotactic activity and migration (Biesalski, 2007; Pierpaoli et al., 2011; Sava et al., 2015; Misiura and Miltyk, 2019; Palmieri et al., 2019). This effect was dose dependent (van Houwelingen et al., 2008) and occurred through C-X-C motif chemokine receptor two interaction on leukocytes (Weathington et al., 2006; Braber et al., 2011; Kim et al., 2011; Hahn et al., 2015; Sharma et al., 2018; Robison et al., 2021). Additionally, the matricellular protein cellular communication network factor 1 (CCN1) was found in high concentrations in BAL fluid of patients with ARDS, while mice overexpressing CCN1 spontaneously developed ARDS coinciding with neutrophil influx (Grazioli et al., 2015; Morrell et al., 2020). The direct effect of CCN1 on cell migration is not straightforward, as it appeared to increase chemokinesis by interaction with αMβ2 integrins. However, prolonged presence of CCN1 inhibited cell migration and played a role in neutrophil clearance through efferocytosis (Lobel et al., 2012; Jun et al., 2015). Fibronectin deposition in the acute phase of ARDS facilitated neutrophil migration partly by higher expression and redistribution of intracellular adhesion molecule-1 (ICAM-1) in endothelial cells (Sava et al., 2015). In a model of S. aureus induced skin infection, hyaluronic acid deposition was increased in ARDS and the failure to digest this ECM increased neutrophil influx (Hällgren et al., 1989; Dokoshi et al., 2020).
Less is known about the exact ECM changes in the fibroproliferative phase of ARDS. Synthesis of collagen types I and III was present, but unlike lung fibrosis; at this stage no information on the crosslinking states of these collagens exists. Interestingly, commonalities including similar ECM composition and distribution between IPF and ARDS have been recognized (Raghu et al., 1985). In patients with ARDS, epithelial lining fluid levels of C-terminal propeptide (marker of collagen type I synthesis) were increased, while degradation products of collagen type I/II were reduced compared to individuals at risk of ARDS/ALI (Armstrong et al., 1999). BAL measurement of N-terminal peptide of alveolar procollagen type III, a precursor of collagen type III, has been validated as a diagnostic tool to indicate fibroproliferation in ARDS patients as well as to identify patients who can benefit from corticosteroid treatment (Forel et al., 2015; Hamon et al., 2019). Serological and BAL levels of hyaluronic acid were found associated with ARDS severity and organ failure (Esposito et al., 2017). Recently, lung tissue obtained from patients with coronavirus disease (COVID-19) induced ARDS, stained positively for hyaluronic acid which was associated with the degree of alveolar damage (Hellman et al., 2020). An in vitro chemotaxis model recently showed that collagen type III had an inhibitory effect on neutrophil migration regarding track length, direction, and targeting (Kraus et al., 2021). However, it is still unknown whether these mechanisms are active in ARDS.
To further strengthen understanding of interactions between infiltrating immune cells and ECM in the context of migration, development of innovative in vitro models is key. Some of the ideal properties for in vitro modeling of immune cell migration in different types of lung diseases are illustrated in Figure 1.
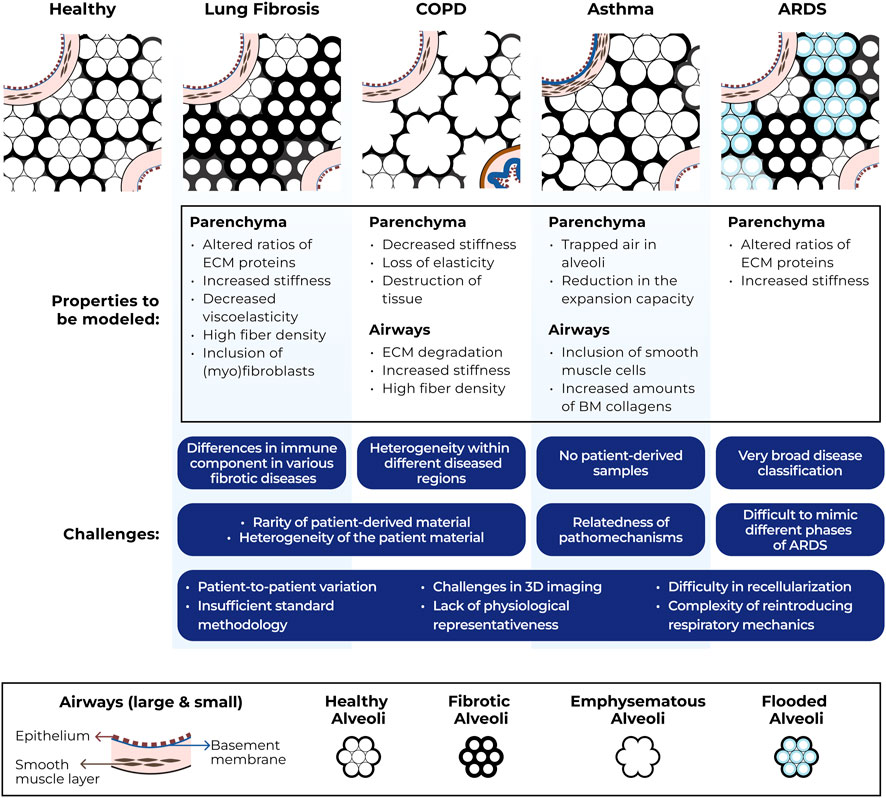
FIGURE 1. Schematic representation of structural ECM changes in lung diseases, ideal properties for modeling immune cell migration through ECM in these disease conditions and challenges associated with generating such models. COPD = chronic obstructive pulmonary disease, ARDS = acute respiratory distress syndrome, ECM = extracellular matrix, BM = basement membrane, 3D = three-dimensional.
Moving towards in vitro models for studying ECM influences on immune cell migration, ECM-derived in vitro models are emerging as a novel methodology. ECM-derived systems have been established using single proteins or by decellularization of native lung tissue. These models recapitulate the biochemical and mechanical properties of native ECM more closely than 2D models in which cells are cultured on plastic with unrepresentative polarity. To facilitate the investigation of altered biomechanics separately or in combination with altered composition of lung ECM, development of novel methodologies and ECM-mimicking biomaterials is warranted. This includes but is not limited to: changing pore size or fiber density without changing ECM-composition or altering mechanical properties without changing fiber density. In a recent study from our group, we demonstrated the possibility of modulating stromal mechanical properties without altering composition (Nizamoglu et al., 2022). Another study implemented macromolecular crowding to induce changes in the collagen fibril networks, without significantly changing the bulk stiffness (Ranamukhaarachchi et al., 2019).
In addition to native ECM-based models, patient-derived materials are an important source of cells that are essential for establishing in vivo representative models. Involving multiple cell types (such as epithelial cells or fibroblasts) adds to the physiological relevance of a model and these cell-cell interactions can provide invaluable information about disease-driving mechanisms. Effects of cell-cell interactions on immune cell migration has been demonstrated for many types of immune cells, such as between peripheral blood-derived monocytes and leukocytes (Costa et al., 2016), between fibroblasts and macrophages (Ford et al., 2019), and between epithelial spheroids and neutrophils (Surendran et al., 2021). Investigating the influence of these cell-cell interactions on (infiltrating) cell migration within the context of diseased ECM could bring new perspectives to our current understanding of lung disease pathobiology.
Patient-derived material has the highest physiological relevance when used for modeling in vitro systems, however, such samples pose various challenges associated with their nature. The availability of human lung tissue for scientific research is rare, except in some specialized clinical centers. Moreover, large volumes of tissue cannot be obtained for every disease; for instance, for asthma and ARDS usually only small bronchial biopsies are available. Furthermore, obtaining true healthy control tissue is an added obstacle. Control “healthy” material is often obtained from lung tissue resected during lobectomies, tumors, or transplantation. The resected tissue is assessed for morphological and anatomical normalcy and although the cells and tissues may appear to be healthy, their microenvironment is possibly altered as a consequence of disease compared to a healthy individual. Patient-to-patient variability creates additional challenges while working with the small(er) sample sizes that are inherent to such models. Modeling chronic diseases should also be accompanied by modeling with appropriate controls, which include important considerations such as matching for age, sex, and smoking history. However, the limitations in the availability of precious patient material also constrains the inclusion of proper controls to perform appropriate comparisons. This challenge of limited availability of donor material also extends to models that utilize human-derived ECM. Another important consideration for ECM-based models is heterogeneity of mechanical properties in different compartments of the available human material. For example, small airways in COPD become stiffer but the parenchyma on the whole appears softer due to enlarged emphysematous regions while the remaining alveolar walls are measured as having stiffness similar to control alveolar walls (Burgess and Harmsen, 2022). There are several well-established protocols to decellularize the lung to obtain either intact scaffolds or solubilized ECM, that is, reconstituted to form hydrogels (Wagner et al., 2013; Gilpin and Wagner, 2018; de Hilster et al., 2020). An unmet challenge for these models, however, is recellularization (Wagner et al., 2013). Current efforts at recellularization are unable to ensure appropriate 3D distribution of cells. Advances in 3D bioprinting technology such as ECM based bioinks reinforced with cells can bolster the development of models with correct spatial distribution of cells (De Santis et al., 2021; Falcones et al., 2021).
Conducting experiments in 3D provides a plethora of information in addition to the physiological relevance. An extra dimension goes hand-in-hand with added challenges for retrieving readouts to generate these data. Visualizing the network of ECM with varying degrees of resolution, is possible using histological staining (Masson Trichrome, Picrosirius Red), immunohistochemistry and/or immunofluorescence staining, scanning electron microscopy, and atomic force microscopy. However, sample processing techniques may limit the extent of visualization and/or introduce artefacts. For example, sectioning the sample for staining procedures limits the information provided to only one plane, the harsh treatments necessary for scanning electron microscopy sample preparation can alter ECM structure. Similarly, fluorescence imaging approaches might be hindered by auto-fluorescence of native ECM. Second harmonics generation and multiphoton microscopy are emerging as prominent high-resolution imaging techniques for visualizing the matrix and overcoming these limitations (Mayorca-Guiliani et al., 2017; Tjin et al., 2017). Fluorescent-labeling of cells or matrix has also allowed deciphering matrix changes and cellular movement in different studies (Fischer et al., 2022). Digital holographic microscopy has been utilized to visualize cell migration in 3D Matrigel matrices (Hellesvik et al., 2020). While each visualization method has advantages and disadvantages (Martinez-Garcia et al., 2022), combination of different techniques for the visualization of migrating cells and the ECM network might be the key for advancing knowledge.
Another important feature of the lung is the presence of oxygen gradient. The alveolar-arterial (A-a) oxygen gradient has been previously used as an indicator of disease severity and outcome in pneumonia and recently in COVID-19 (Singh et al., 2022). Often, acute and chronic lung diseases are also characterized by hypoxemia and hypoxia. Thus, modeling gradients in vitro systems, although challenging, is crucial as cells modulate their responses depending on the oxygen levels in their microenvironment (Zenewicz, 2017). Most 3D migration systems are modeled under static conditions, missing the dynamic state of the lung. Inclusion of respiratory mechanics associated with breathing and blood flow also poses a challenge while modeling these systems. One such event is cyclic deformations which have been mimicked in lung-on-chip models recently (Kumar et al., 2022; Zhu et al., 2022). Including cyclic deformations in the state-of-the-art ECM-based migration models would increase the translational capacity of the models and bring them one step closer to in vivo. Similarly, the lack of (interstitial) flow is an important aspect that can add another dimension to these migration models. The effect of interstitial flow was elucidated when tumor-associated interstitial flow promoted tumor-like characteristics in healthy macrophages (Li et al., 2018). Similarly, neutrophils were shown to infiltrate cancer-derived spheroids deeper when a flow was present in the in vitro system (Surendran et al., 2021).
Altogether, using innovative 3D in vitro models to mimic migration of infiltrating immune cells in lung diseases has been emerging as a new possibility. Developing new systems to represent altered ECM composition, structure, organization and mechanics in each of these lung diseases will help us advance our understanding how the ECM-immune cell interplay influences the migration of these cells.
Interactions with the microenvironment critically direct cell behavior, including cell migration. Therefore, it is highly likely that disrupted ECM homeostasis in lung diseases such as lung fibrosis, COPD, asthma and/or ARDS alters the behavior of infiltrating inflammatory cells, similar to how a hazardous highway would hinder the smooth flow of traffic. Advances in methodologies for 3D culture systems and advances in the biomaterials field in the last decade have greatly improved our understanding of how migrating cells interact with their microenvironment with respect to the biochemical and biomechanical properties. Emerging data suggest that the contributions of different ECM properties may differ when assessed individually as compared to when in combinations. Targeting isolated parameters within an altered ECM is one of the important questions upon which future research should focus. Another important aspect that remains unknown is the influence that lung-resident cells, such as epithelial cells, endothelial cells and fibroblasts, have on migration of immune cells. Multicellular in vitro models are necessary to investigate whether resident lung cells modulate immune cell migration through abnormal ECM in lung diseases. Developing 3D ECM in vitro models helps to further our understanding of the pathobiology of a disease (Tabdanov et al., 2021). Recently, modulating cancer ECM has been shown to have potential for therapeutic targeting as weakening cell-matrix adhesion and reducing fiber rigidity reduced cancer cell invasiveness (Pal et al., 2021). Therefore, it is not unlikely that similar approaches targeting the contribution of altered ECM to immune cell recruitment could be employed as therapeutic strategies against lung diseases.
The lack of techniques to obtain information from these novel models poses a future challenge. Nevertheless, steady progress has led to advances in new qualitative and quantitative methodologies for studying disease mechanisms using 3D models. Newer approaches for better imaging, improved compositional analyses, recellularization, and modeling dynamic conditions are paving the way for improved and innovative models. Incorporation of patient-derived material such as native ECM and cells in research will play an important role in our understanding of disease origin and progression.
In summary, understanding the recruitment of immune cells from peripheral blood during lung diseases and how the diseased ECM alters their behavior is a key factor to deepen our knowledge of these diseases and to start generating hypotheses revolving around targeting these interactions for the development of new treatment strategies.
MMJ, MN, and JKB conceptualized and designed the manuscript. The content was the manuscript was written by MMJ, MN, YF, SSPN, and JP. The figure was prepared by MN. All authors contributed towards editing and reviewing the manuscript. All authors also approved the final version of the manuscript.
MMJ is funded by the Graduate School of Medical Sciences of the University of Groningen. MN, IHH, BNM, and JKB receive unrestricted research funds from Boehringer Ingelheim. YF acknowledges support from the State Scholarship Fund by the China Scholarship Council (202006230092). SSPN and MW were funded by grants from the German Federal Ministry of Education and Research (BMBF, 82DZL001A6) and MW by a Junior Research cluster grant from the University of Lübeck (JC01-2016). JKB also acknowledges support from the NWO (Aspasia 015.013.010).
MN, IHH, BNM, and JKB receive unrestricted research funds from Boehringer Ingelheim.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdillahi, S. M., Bober, M., Nordin, S., Hallgren, O., Baumgarten, M., Erjefalt, J., et al. (2015). Collagen VI is upregulated in COPD and serves both as an adhesive target and a bactericidal barrier for Moraxella catarrhalis. J. Innate Immun. 7 (5), 506–517. doi:10.1159/000381213
Adebowale, K., Gong, Z., Hou, J. C., Wisdom, K. M., Garbett, D., Lee, H. P., et al. (2021). Enhanced substrate stress relaxation promotes filopodia-mediated cell migration. Nat. Mat. 20 (9), 1290–1299. doi:10.1038/s41563-021-00981-w
Akhmanova, M., Emtenani, S., Krueger, D., Gyoergy, A., Guarda, M., Vlasov, M., et al. (2022). Cell division in tissues enables macrophage infiltration. Science 376 (6591), 394–396. doi:10.1126/science.abj0425
Andersson, C. K., Weitoft, M., Rydell-Tormanen, K., Bjermer, L., Westergren-Thorsson, G., and Erjefalt, J. S. (2018). Uncontrolled asthmatics have increased FceRI(+) and TGF-beta-positive MCTC mast cells and collagen VI in the alveolar parenchyma. Clin. Exp. Allergy 48 (3), 266–277. doi:10.1111/cea.13092
Annoni, R., Lancas, T., Yukimatsu Tanigawa, R., de Medeiros Matsushita, M., de Morais Fernezlian, S., Bruno, A., et al. (2012). Extracellular matrix composition in COPD. Eur. Respir. J. 40 (6), 1362–1373. doi:10.1183/09031936.00192611
Araujo, B. B., Dolhnikoff, M., Silva, L. F., Elliot, J., Lindeman, J. H., Ferreira, D. S., et al. (2008). Extracellular matrix components and regulators in the airway smooth muscle in asthma. Eur. Respir. J. 32 (1), 61–69. doi:10.1183/09031936.00147807
Armstrong, L., Thickett, D. R., Mansell, J. P., Ionescu, M., Hoyle, E., Billinghurst, R. C., et al. (1999). Changes in collagen turnover in early acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 160 (6), 1910–1915. doi:10.1164/ajrccm.160.6.9811084
Belchamber, K. B. R., Singh, R., Batista, C. M., Whyte, M. K., Dockrell, D. H., Kilty, I., et al. (2019). Defective bacterial phagocytosis is associated with dysfunctional mitochondria in COPD macrophages. Eur. Respir. J. 54 (4), 1802244. doi:10.1183/13993003.02244-2018
Bensadoun, E. S., Burke, A. K., Hogg, J. C., and Roberts, C. R. (1996). Proteoglycan deposition in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 154 (6), 1819–1828. doi:10.1164/ajrccm.154.6.8970376
Biesalski, H. K. (2007). Polyphenols and inflammation: Basic interactions. Curr. Opin. Clin. Nutr. Metab. Care 10 (6), 724–728. doi:10.1097/MCO.0b013e3282f0cef2
Booth, A. J., Hadley, R., Cornett, A. M., Dreffs, A. A., Matthes, S. A., Tsui, J. L., et al. (2012). Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 186, 866–876. doi:10.1164/rccm.201204-0754OC
Boyd, D. F., Allen, E. K., Randolph, A. G., Guo, X. J., Weng, Y., Sanders, C. J., et al. (2020). Exuberant fibroblast activity compromises lung function via ADAMTS4. Nature 587 (7834), 466–471. doi:10.1038/s41586-020-2877-5
Braber, S., Overbeek, S. A., Koelink, P. J., Henricks, P. A., Zaman, G. J., Garssen, J., et al. (2011). CXCR2 antagonists block the N-Ac-PGP-induced neutrophil influx in the airways of mice, but not the production of the chemokine CXCL1. Eur. J. Pharmacol. 668 (3), 443–449. doi:10.1016/j.ejphar.2011.03.025
Brandsma, C.-A., de Vries, M., Costa, R., Woldhuis, R. R., Königshoff, M., and Timens, W. (2017). Lung ageing and COPD: Is there a role for ageing in abnormal tissue repair? Eur. Respir. Rev. 26, 170073. doi:10.1183/16000617.0073-2017
Brandsma, C. A., Van den Berge, M., Hackett, T. L., Brusselle, G., and Timens, W. (2020). Recent advances in chronic obstructive pulmonary disease pathogenesis: From disease mechanisms to precision medicine. J. Pathol. 250 (5), 624–635. doi:10.1002/path.5364
Brightling, C., and Greening, N. (2019). Airway inflammation in COPD: Progress to precision medicine. Eur. Respir. J. 54 (2), 1900651. doi:10.1183/13993003.00651-2019
Burgess, J. K., Boustany, S., Moir, L. M., Weckmann, M., Lau, J. Y., Grafton, K., et al. (2010). Reduction of tumstatin in asthmatic airways contributes to angiogenesis, inflammation, and hyperresponsiveness. Am. J. Respir. Crit. Care Med. 181 (2), 106–115. doi:10.1164/rccm.200904-0631OC
Burgess, J. K., and Harmsen, M. C. (2022). Chronic lung diseases: Entangled in extracellular matrix. Eur. Respir. Rev. 31 (163), 210202. doi:10.1183/16000617.0202-2021
Burgess, J. K., Jonker, M. R., Berg, M., ten Hacken, N. T. H., Meyer, K. B., van den Berge, M., et al. (2020). Periostin: Contributor to abnormal airway epithelial function in asthma? Eur. Respir. J. 57, 2001286. doi:10.1183/13993003.01286-2020
Burgess, J. K., Mauad, T., Tjin, G., Karlsson, J. C., and Westergren-Thorsson, G. (2016). The extracellular matrix - the under-recognized element in lung disease? J. Pathol. 240 (4), 397–409. doi:10.1002/path.4808
Burgstaller, G., Oehrle, B., Gerckens, M., White, E. S., Schiller, H. B., and Eickelberg, O. (2017). The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 50 (1), 1601805. doi:10.1183/13993003.01805-2016
Chilosi, M., Carloni, A., Rossi, A., and Poletti, V. (2013). Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl. Res. 162 (3), 156–173. doi:10.1016/j.trsl.2013.06.004
Costa, C., Traves, S. L., Tudhope, S. J., Fenwick, P. S., Belchamber, K. B. R., Russell, R. E. K., et al. (2016). Enhanced monocyte migration to CXCR3 and CCR5 chemokines in COPD. Eur. Respir. J. 47 (4), 1093–1102. doi:10.1183/13993003.01642-2015
Davey, A., McAuley, D. F., and O'Kane, C. M. (2011). Matrix metalloproteinases in acute lung injury: Mediators of injury and drivers of repair. Eur. Respir. J. 38 (4), 959–970. doi:10.1183/09031936.00032111
de Castro Bras, L. E., and Frangogiannis, N. G. (2020). Extracellular matrix-derived peptides in tissue remodeling and fibrosis. Matrix Biol. 91-92, 176–187. doi:10.1016/j.matbio.2020.04.006
de Hilster, R. H. J., Sharma, P. K., Jonker, M. R., White, E. S., Gercama, E. A., Roobeek, M., et al. (2020). Human lung extracellular matrix hydrogels resemble the stiffness and viscoelasticity of native lung tissue. Am. J. Physiol. Lung Cell. Mol. Physiol. 318 (4), L698-L704–L704. doi:10.1152/ajplung.00451.2019
De Santis, M. M., Alsafadi, H. N., Tas, S., Bolukbas, D. A., Prithiviraj, S., Da Silva, I. A. N., et al. (2021). Extracellular-matrix-reinforced bioinks for 3D bioprinting human tissue. Adv. Mat. 33 (3), e2005476. doi:10.1002/adma.202005476
Dekkers, B. G. J., Saad, S. I., van Spelde, L. J., and Burgess, J. K. (2021). Basement membranes in obstructive pulmonary diseases. Matrix Biol. Plus 12, 100092. doi:10.1016/j.mbplus.2021.100092
Dicker, A. J., Crichton, M. L., Pumphrey, E. G., Cassidy, A. J., Suarez-Cuartin, G., Sibila, O., et al. (2018). Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 141 (1), 117–127. doi:10.1016/j.jaci.2017.04.022
Dokoshi, T., Zhang, L. J., Li, F., Nakatsuji, T., Butcher, A., Yoshida, H., et al. (2020). Hyaluronan degradation by cemip regulates host defense against Staphylococcus aureus skin infection. Cell. Rep. 30 (1), 61–68. e64. doi:10.1016/j.celrep.2019.12.001
Dolhnikoff, M., da Silva, L. F., de Araujo, B. B., Gomes, H. A., Fernezlian, S., Mulder, A., et al. (2009). The outer wall of small airways is a major site of remodeling in fatal asthma. J. Allergy Clin. Immunol. 123 (5), 1090–1097. doi:10.1016/j.jaci.2009.02.032
Du, H., Bartleson, J. M., Butenko, S., Alonso, V., Liu, W. F., Winer, D. A., et al. (2022). Tuning immunity through tissue mechanotransduction. Nat. Rev. Immunol. doi:10.1038/s41577-022-00761-w
Esposito, A. J., Bhatraju, P. K., Stapleton, R. D., Wurfel, M. M., and Mikacenic, C. (2017). Hyaluronic acid is associated with organ dysfunction in acute respiratory distress syndrome. Crit. Care 21 (1), 304. doi:10.1186/s13054-017-1895-7
Eurlings, I. M., Dentener, M. A., Cleutjens, J. P., Peutz, C. J., Rohde, G. G., Wouters, E. F., et al. (2014). Similar matrix alterations in alveolar and small airway walls of COPD patients. BMC Pulm. Med. 14 (1), 90. doi:10.1186/1471-2466-14-90
Falcones, B., Sanz-Fraile, H., Marhuenda, E., Mendizabal, I., Cabrera-Aguilera, I., Malandain, N., et al. (2021). Bioprintable lung extracellular matrix hydrogel scaffolds for 3D culture of mesenchymal stromal cells. Polymers 13 (14), 2350. doi:10.3390/polym13142350
Fan, C., Shi, X., Zhao, K., Wang, L., Shi, K., Liu, Y. J., et al. (2022). Cell migration orchestrates migrasome formation by shaping retraction fibers. J. Cell. Biol. 221 (4), e202109168. doi:10.1083/jcb.202109168
Fischer, A., Wannemacher, J., Christ, S., Koopmans, T., Kadri, S., Zhao, J., et al. (2022). Neutrophils direct preexisting matrix to initiate repair in damaged tissues. Nat. Immunol. 23 (4), 518–531. doi:10.1038/s41590-022-01166-6
Ford, A. J., Orbach, S. M., and Rajagopalan, P. (2019). Fibroblasts stimulate macrophage migration in interconnected extracellular matrices through tunnel formation and fiber alignment. Biomaterials 209, 88–102. doi:10.1016/j.biomaterials.2019.03.044
Forel, J.-M., Guervilly, C., Hraiech, S., Voillet, F., Thomas, G., Somma, C., et al. (2015). Type III procollagen is a reliable marker of ARDS-associated lung fibroproliferation. Intensive Care Med. 41 (1), 1–11. doi:10.1007/s00134-014-3524-0
Francois, J., Kandasamy, A., Yeh, Y. T., Schwartz, A., Ayala, C., Meili, R., et al. (2021). The interplay between matrix deformation and the coordination of turning events governs directed neutrophil migration in 3D matrices. Sci. Adv. 7 (29), eabf3882. doi:10.1126/sciadv.abf3882
Gaggar, A., Jackson, P. L., Noerager, B. D., O’Reilly, P. J., Mcquaid, D. B., Rowe, S. M., et al. (2008). A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J. Immunol. 180 (8), 5662–5669. doi:10.4049/jimmunol.180.8.5662
Gilpin, S. E., and Wagner, D. E. (2018). Acellular human lung scaffolds to model lung disease and tissue regeneration. Eur. Respir. Rev. 27, 180021. doi:10.1183/16000617.0021-2018
Gkatzis, K., Taghizadeh, S., Huh, D., Stainier, D. Y. R., and Bellusci, S. (2018). Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease. Eur. Respir. J. 52 (5), 1800876. doi:10.1183/13993003.00876-2018
Grazioli, S., Gil, S., An, D., Kajikawa, O., Farnand, A. W., Hanson, J. F., et al. (2015). CYR61 (CCN1) overexpression induces lung injury in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 308 (8), L759–L765. doi:10.1152/ajplung.00190.2014
Gu, B. H., Madison, M. C., Corry, D., and Kheradmand, F. (2018). Matrix remodeling in chronic lung diseases. Matrix Biol. 73, 52–63. doi:10.1016/j.matbio.2018.03.012
Hahn, C. S., Scott, D. W., Xu, X., Roda, M. A., Payne, G. A., Wells, J. M., et al. (2015). The matrikine N-alpha-PGP couples extracellular matrix fragmentation to endothelial permeability. Sci. Adv. 1 (3), e1500175. doi:10.1126/sciadv.1500175
Hällgren, R., Samuelsson, T., Laurent, T. C., and Modig, J. (1989). Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am. Rev. Respir. Dis. 139 (3), 682–687. doi:10.1164/ajrccm/139.3.682
Hallmann, R., Zhang, X., Di Russo, J., Li, L., Song, J., Hannocks, M. J., et al. (2015). The regulation of immune cell trafficking by the extracellular matrix. Curr. Opin. Cell. Biol. 36, 54–61. doi:10.1016/j.ceb.2015.06.006
Hamon, A., Scemama, U., Bourenne, J., Daviet, F., Coiffard, B., Persico, N., et al. (2019). Chest CT scan and alveolar procollagen III to predict lung fibroproliferation in acute respiratory distress syndrome. Ann. Intensive Care 9 (1), 42. doi:10.1186/s13613-019-0516-9
Harkness, L. M., Weckmann, M., Kopp, M., Becker, T., Ashton, A. W., and Burgess, J. K. (2017). Tumstatin regulates the angiogenic and inflammatory potential of airway smooth muscle extracellular matrix. J. Cell. Mol. Med. 21 (12), 3288–3297. doi:10.1111/jcmm.13232
Hartman, C. D., Isenberg, B. C., Chua, S. G., and Wong, J. Y. (2017). Extracellular matrix type modulates cell migration on mechanical gradients. Exp. Cell. Res. 359 (2), 361–366. doi:10.1016/j.yexcr.2017.08.018
Helfrich, S., Mindt, B. C., Fritz, J. H., and Duerr, C. U. (2019). Group 2 innate lymphoid cells in respiratory allergic inflammation. Front. Immunol. 10, 930. doi:10.3389/fimmu.2019.00930
Hellesvik, M., Oye, H., and Aksnes, H. (2020). Exploiting the potential of commercial digital holographic microscopy by combining it with 3D matrix cell culture assays. Sci. Rep. 10 (1), 14680. doi:10.1038/s41598-020-71538-1
Hellman, U., Karlsson, M. G., Engström-Laurent, A., Cajander, S., Dorofte, L., Ahlm, C., et al. (2020). Presence of hyaluronan in lung alveoli in severe Covid-19: An opening for new treatment options? J. Biol. Chem. 295 (45), 15418–15422. doi:10.1074/jbc.ac120.015967
Herrera, J., Forster, C., Pengo, T., Montero, A., Swift, J., Schwartz, M. A., et al. (2019). Registration of the extracellular matrix components constituting the fibroblastic focus in idiopathic pulmonary fibrosis. JCI Insight 4 (1), 125185. doi:10.1172/jci.insight.125185
Herrera, J., Henke, C. A., and Bitterman, P. B. (2018). Extracellular matrix as a driver of progressive fibrosis. J. Clin. Investig. 128 (1), 45–53. doi:10.1172/JCI93557
Hogg, J. C., Chu, F., Utokaparch, S., Woods, R., Elliott, W. M., Buzatu, L., et al. (2004). The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 350 (26), 2645–2653. doi:10.1056/NEJMoa032158
Hogg, J. C., McDonough, J. E., Gosselink, J. V., and Hayashi, S. (2009). What drives the peripheral lung-remodeling process in chronic obstructive pulmonary disease? Proc. Am. Thorac. Soc. 6 (8), 668–672. doi:10.1513/pats.200907-079DP
Holgate, S. T., Wenzel, S., Postma, D. S., Weiss, S. T., Renz, H., and Sly, P. D. (2015). Asthma. Nat. Rev. Dis. Prim. 1 (1), 15025. doi:10.1038/nrdp.2015.25
Hough, K. P., Curtiss, M. L., Blain, T. J., Liu, R. M., Trevor, J., Deshane, J. S., et al. (2020). Airway remodeling in asthma. Front. Med. 7, 191. doi:10.3389/fmed.2020.00191
Hsieh, J. Y., Keating, M. T., Smith, T. D., Meli, V. S., Botvinick, E. L., and Liu, W. F. (2019). Matrix crosslinking enhances macrophage adhesion, migration, and inflammatory activation. Apl. Bioeng. 3 (1), 016103. doi:10.1063/1.5067301
Huang, E., Peng, N., Xiao, F., Hu, D., Wang, X., and Lu, L. (2020). The roles of immune cells in the pathogenesis of fibrosis. Int. J. Mol. Sci. 21 (15), 5203. doi:10.3390/ijms21155203
Ishikawa, G., Liu, A., and Herzog, E. L. (2021). Evolving perspectives on innate immune mechanisms of IPF. Front. Mol. Biosci. 8, 676569. doi:10.3389/fmolb.2021.676569
Ito, J. T., Lourenco, J. D., Righetti, R. F., Tiberio, I., Prado, C. M., and Lopes, F. (2019). Extracellular matrix component remodeling in respiratory diseases: What has been found in clinical and experimental studies? Cells 8 (4), 342. doi:10.3390/cells8040342
Johansson, M. W. (2017). Eosinophil activation status in separate compartments and association with asthma. Front. Med. 4, 75. doi:10.3389/fmed.2017.00075
Jun, J. I., Kim, K. H., and Lau, L. F. (2015). The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat. Commun. 6 (1), 7386. doi:10.1038/ncomms8386
Kaur, A., Ecker, B. L., Douglass, S. M., Kugel, C. H., Webster, M. R., Almeida, F. V., et al. (2019). Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 9 (1), 64–81. doi:10.1158/2159-8290.CD-18-0193
Kim, J. S., Axelsson, G. T., Moll, M., Anderson, M. R., Bernstein, E. J., Putman, R. K., et al. (2022). Associations of monocyte count and other immune cell types with interstitial lung abnormalities. Am. J. Respir. Crit. Care Med. 205 (7), 795–805. doi:10.1164/rccm.202108-1967OC
Kim, S. D., Lee, H. Y., Shim, J. W., Kim, H. J., Yoo, Y. H., Park, J. S., et al. (2011). Activation of CXCR2 by extracellular matrix degradation product acetylated Pro-Gly-Pro has therapeutic effects against sepsis. Am. J. Respir. Crit. Care Med. 184 (2), 243–251. doi:10.1164/rccm.201101-0004OC
Kinder, B. W., Brown, K. K., Schwarz, M. I., Ix, J. H., Kervitsky, A., and King, T. E. (2008). Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest 133 (1), 226–232. doi:10.1378/chest.07-1948
Kraus, R. F., Gruber, M. A., and Kieninger, M. (2021). The influence of extracellular tissue on neutrophil function and its possible linkage to inflammatory diseases. Immun. Inflamm. Dis. 9 (4), 1237–1251. doi:10.1002/iid3.472
Kumar, V., Madhurakkat Perikamana, S. K., Tata, A., Hoque, J., Gilpin, A., Tata, P. R., et al. (2022). An in vitro microfluidic alveolus model to study lung biomechanics. Front. Bioeng. Biotechnol. 10, 848699. doi:10.3389/fbioe.2022.848699
Labaki, W. W., and Han, M. K. (2020). Chronic respiratory diseases: A global view. Lancet. Respir. Med. 8 (6), 531–533. doi:10.1016/S2213-2600(20)30157-0
Lauer, M. E., Majors, A. K., Comhair, S., Ruple, L. M., Matuska, B., Subramanian, A., et al. (2015). Hyaluronan and its heavy chain modification in asthma severity and experimental asthma exacerbation. J. Biol. Chem. 290 (38), 23124–23134. doi:10.1074/jbc.M115.663823
Lee, J. S., Song, J. W., Wolters, P. J., Elicker, B. M., King, T. E., Kim, D. S., et al. (2012). Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur. Respir. J. 39 (2), 352–358. doi:10.1183/09031936.00050911
Lee, S. Y., Choi, S. H., Lee, M. S., Kurmashev, A., Lee, H. N., Ko, Y. G., et al. (2021). Retraction fibers produced by fibronectin-integrin α5β1 interaction promote motility of brain tumor cells. FASEB J. 35 (10), e21906. doi:10.1096/fj.202100452RR
Li, R., Serrano, J. C., Xing, H., Lee, T. A., Azizgolshani, H., Zaman, M., et al. (2018). Interstitial flow promotes macrophage polarization toward an M2 phenotype. Mol. Biol. Cell. 29 (16), 1927–1940. doi:10.1091/mbc.E18-03-0164
Lim, K., Hyun, Y. M., Lambert-Emo, K., Capece, T., Bae, S., Miller, R., et al. (2015). Neutrophil trails guide influenza-specific CD8⁺ T cells in the airways. Science 349 (6252), aaa4352. doi:10.1126/science.aaa4352
Liu, G., Betts, C., Cunoosamy, D. M., Åberg, P. M., Hornberg, J. J., Sivars, K. B., et al. (2019). Use of precision cut lung slices as a translational model for the study of lung biology. Respir. Res. 20 (1), 162. doi:10.1186/s12931-019-1131-x
Liu, G., Philp, A. M., Corte, T., Travis, M. A., Schilter, H., Hansbro, N. G., et al. (2021a). Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol. Ther. 225, 107839. doi:10.1016/j.pharmthera.2021.107839
Liu, L., Stephens, B., Bergman, M., May, A., and Chiang, T. (2021b). Role of collagen in airway mechanics. Bioengineering 8 (1), 13. doi:10.3390/bioengineering8010013
Lobel, M., Bauer, S., Meisel, C., Eisenreich, A., Kudernatsch, R., Tank, J., et al. (2012). CCN1: A novel inflammation-regulated biphasic immune cell migration modulator. Cell. Mol. Life Sci. 69 (18), 3101–3113. doi:10.1007/s00018-012-0981-x
Manon-Jensen, T., Kjeld, N. G., and Karsdal, M. A. (2016). Collagen-mediated hemostasis. J. Thromb. Haemost. 14 (3), 438–448. doi:10.1111/jth.13249
Manon-Jensen, T., Langholm, L. L., Ronnow, S. R., Karsdal, M. A., Tal-Singer, R., Vestbo, J., et al. (2019). End-product of fibrinogen is elevated in emphysematous chronic obstructive pulmonary disease and is predictive of mortality in the ECLIPSE cohort. Respir. Med. 160, 105814. doi:10.1016/j.rmed.2019.105814
Martinez-Garcia, F. D., Fischer, T., Hayn, A., Mierke, C. T., Burgess, J. K., and Harmsen, M. C. (2022). A beginner's guide to the characterization of hydrogel microarchitecture for cellular applications. Gels 8 (9), 535. doi:10.3390/gels8090535
Mayorca-Guiliani, A. E., Madsen, C. D., Cox, T. R., Horton, E. R., Venning, F. A., and Erler, J. T. (2017). ISDoT: In situ decellularization of tissues for high-resolution imaging and proteomic analysis of native extracellular matrix. Nat. Med. 23 (7), 890–898. doi:10.1038/nm.4352
McMahon, M., Ye, S., Pedrina, J., Dlugolenski, D., and Stambas, J. (2021). Extracellular matrix enzymes and immune cell biology. Front. Mol. Biosci. 8, 703868. doi:10.3389/fmolb.2021.703868
Meduri, G. U., Tolley, E. A., Chinn, A., Stentz, F., Postlethwaite, A., and PostlethwAite, A. (1998). Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am. J. Respir. Crit. Care Med. 158 (1), 1432–1441. doi:10.1164/ajrccm.158.5.9801107
Misharin, A. V., Morales-Nebreda, L., Reyfman, P. A., Cuda, C. M., Walter, J. M., McQuattie-Pimentel, A. C., et al. (2017). Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 214 (8), 2387–2404. doi:10.1084/jem.20162152
Misiura, M., and Miltyk, W. (2019). Proline-containing peptides-new insight and implications: A review. Biofactors 45 (6), 857–866. doi:10.1002/biof.1554
Moon, J. Y., Leitao Filho, F. S., Shahangian, K., Takiguchi, H., and Sin, D. D. (2018). Blood and sputum protein biomarkers for chronic obstructive pulmonary disease (COPD). Expert Rev. Proteomics 15 (11), 923–935. doi:10.1080/14789450.2018.1539670
Morales, M. M., Pires-Neto, R. C., Inforsato, N., Lancas, T., da Silva, L. F., Saldiva, P. H., et al. (2011). Small airway remodeling in acute respiratory distress syndrome: A study in autopsy lung tissue. Crit. Care 15 (1), R4. doi:10.1186/cc9401
Morales, X., Cortes-Dominguez, I., and Ortiz-de-Solorzano, C. (2021). Modeling the mechanobiology of cancer cell migration using 3D biomimetic hydrogels. Gels 7 (1), 17. doi:10.3390/gels7010017
Morrell, E. D., Grazioli, S., Hung, C., Kajikawa, O., Kosamo, S., Stapleton, R. D., et al. (2020). Alveolar CCN1 is associated with mechanical stretch and acute respiratory distress syndrome severity. Am. J. Physiol. Lung Cell. Mol. Physiol. 319 (5), L825-L832–L832. doi:10.1152/ajplung.00073.2020
Mostaco-Guidolin, L. B., Osei, E. T., Ullah, J., Hajimohammadi, S., Fouadi, M., Li, X., et al. (2019). Defective fibrillar collagen organization by fibroblasts contributes to airway remodeling in asthma. Am. J. Respir. Crit. Care Med. 200 (4), 431–443. doi:10.1164/rccm.201810-1855OC
Nawroth, J. C., Lucchesi, C., Cheng, D., Shukla, A., Ngyuen, J., Shroff, T., et al. (2020). A microengineered airway lung chip models key features of viral-induced exacerbation of asthma. Am. J. Respir. Cell. Mol. Biol. 63 (5), 591–600. doi:10.1165/rcmb.2020-0010MA
Nemani, S. S. P., Vermeulen, C. J., Pech, M., Faiz, A., Oliver, B. G. G., van den Berge, M., et al. (2021). COL4A3 expression in asthmatic epithelium depends on intronic methylation and ZNF263 binding. ERJ Open Res. 7 (2), 00802-2020–02020. doi:10.1183/23120541.00802-2020
Ni, L., and Dong, C. (2018). Roles of myeloid and lymphoid cells in the pathogenesis of chronic obstructive pulmonary disease. Front. Immunol. 9, 1431. doi:10.3389/fimmu.2018.01431
Nissen, G., Hollaender, H., Tang, F. S. M., Wegmann, M., Lunding, L., Vock, C., et al. (2018). Tumstatin fragment selectively inhibits neutrophil infiltration in experimental asthma exacerbation. Clin. Exp. Allergy 48 (11), 1483–1493. doi:10.1111/cea.13236
Nizamoglu, M., and Burgess, J. K. (2022). The multi-faceted extracellular matrix: Unlocking its secrets for understanding the perpetuation of lung fibrosis. Curr. Tissue Microenviron. Rep. 2 (4), 53–71. doi:10.1007/s43152-021-00031-2
Nizamoglu, M., de Hilster, R. H. J., Zhao, F., Sharma, P. K., Borghuis, T., Harmsen, M. C., et al. (2022). An in vitro model of fibrosis using crosslinked native extracellular matrix-derived hydrogels to modulate biomechanics without changing composition. Acta Biomater. 147, 50–62. doi:10.1016/j.actbio.2022.05.031
O'Reilly, P. J., Jackson, P. L., Wells, J. M., Dransfield, M. T., Scanlon, P. D., and Blalock, J. E. (2013). Sputum PGP is reduced by azithromycin treatment in patients with COPD and correlates with exacerbations. Bmj Open 3 (12), e004140. doi:10.1136/bmjopen-2013-004140
Pakshir, P., Alizadehgiashi, M., Wong, B., Coelho, N. M., Chen, X., Gong, Z., et al. (2019). Dynamic fibroblast contractions attract remote macrophages in fibrillar collagen matrix. Nat. Commun. 10 (1), 1850. doi:10.1038/s41467-019-09709-6
Pal, A., Haliti, P., Dharmadhikari, B., Qi, W., and Patra, P. (2021). Manipulating extracellular matrix organizations and parameters to control local cancer invasion. IEEE/ACM Trans. Comput. Biol. Bioinform. 18 (6), 2566–2576. doi:10.1109/TCBB.2020.2989223
Palmieri, B., Vadala, M., and Laurino, C. (2019). Nutrition in wound healing: Investigation of the molecular mechanisms, a narrative review. J. Wound Care 28 (10), 683–693. doi:10.12968/jowc.2019.28.10.683
Patel, D. F., Peiro, T., Shoemark, A., Akthar, S., Walker, S. A., Grabiec, A. M., et al. (2018). An extracellular matrix fragment drives epithelial remodeling and airway hyperresponsiveness. Sci. Transl. Med. 10 (455), eaaq0693. doi:10.1126/scitranslmed.aaq0693
Patel, D. F., and Snelgrove, R. J. (2018). The multifaceted roles of the matrikine Pro-Gly-Pro in pulmonary health and disease. Eur. Respir. Rev. 27 (148), 180017. doi:10.1183/16000617.0017-2018
Pech, M., Weckmann, M., Konig, I. R., Franke, A., Heinsen, F. A., Oliver, B., et al. (2018). Rhinovirus infections change DNA methylation and mRNA expression in children with asthma. PLoS One 13 (11), e0205275. doi:10.1371/journal.pone.0205275
Pierpaoli, E., Cirioni, O., Barucca, A., Orlando, F., Silvestri, C., Giacometti, A., et al. (2011). Vitamin E supplementation in old mice induces antimicrobial activity and improves the efficacy of daptomycin in an animal model of wounds infected with methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 66 (9), 2184–2185. doi:10.1093/jac/dkr254
Puttur, F., Denney, L., Gregory, L. G., Vuononvirta, J., Oliver, R., Entwistle, L. J., et al. (2019). Pulmonary environmental cues drive group 2 innate lymphoid cell dynamics in mice and humans. Sci. Immunol. 4 (36), eaav7638. doi:10.1126/sciimmunol.aav7638
Raghu, G., Striker, L. J., Hudson, L. D., and Striker, G. E. (1985). Extracellular matrix in normal and fibrotic human lungs. Am. Rev. Respir. Dis. 131 (2), 281–289. doi:10.1164/arrd.1985.131.2.281
Ranamukhaarachchi, S. K., Modi, R. N., Han, A., Velez, D. O., Kumar, A., Engler, A. J., et al. (2019). Macromolecular crowding tunes 3D collagen architecture and cell morphogenesis. Biomater. Sci. 7 (2), 618–633. doi:10.1039/c8bm01188e
Rao, R. M., Betz, T. V., Lamont, D. J., Kim, M. B., Shaw, S. K., Froio, R. M., et al. (2004). Elastase release by transmigrating neutrophils deactivates endothelial-bound SDF-1alpha and attenuates subsequent T lymphocyte transendothelial migration. J. Exp. Med. 200 (6), 713–724. doi:10.1084/jem.20040499
Ravi, A. K., Plumb, J., Gaskell, R., Mason, S., Broome, C. S., Booth, G., et al. (2017). COPD monocytes demonstrate impaired migratory ability. Respir. Res. 18 (1), 90. doi:10.1186/s12931-017-0569-y
Reddel, C. J., Weiss, A. S., and Burgess, J. K. (2012). Elastin in asthma. Pulm. Pharmacol. Ther. 25 (2), 144–153. doi:10.1016/j.pupt.2012.02.001
Robison, S. W., Li, J., Viera, L., Blackburn, J. P., Patel, R. P., Blalock, J. E., et al. (2021). A mechanism for matrikine regulation in acute inflammatory lung injury. JCI Insight 6 (7), 140750. doi:10.1172/jci.insight.140750
Ronnow, S. R., Langholm, L. L., Sand, J. M. B., Thorlacius-Ussing, J., Leeming, D. J., Manon-Jensen, T., et al. (2019). Specific elastin degradation products are associated with poor outcome in the ECLIPSE COPD cohort. Sci. Rep. 9 (1), 4064. doi:10.1038/s41598-019-40785-2
Ronnow, S. R., Sand, J. M. B., Staunstrup, L. M., Bahmer, T., Wegmann, M., Lunding, L., et al. (2022). A serological biomarker of type I collagen degradation is related to a more severe, high neutrophilic, obese asthma subtype. Asthma Res. Pract. 8 (1), 2. doi:10.1186/s40733-022-00084-6
Santos, F. B., Nagato, L. K., Boechem, N. M., Negri, E. M., Guimaraes, A., Capelozzi, V. L., et al. (2006). Time course of lung parenchyma remodeling in pulmonary and extrapulmonary acute lung injury. J. Appl. Physiol. 100 (1), 98–106. doi:10.1152/japplphysiol.00395.2005
Sapey, E., Stockley, J. A., Greenwood, H., Ahmad, A., Bayley, D., Lord, J. M., et al. (2011). Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 183 (9), 1176–1186. doi:10.1164/rccm.201008-1285OC
Sava, P., Cook, I. O., Mahal, R. S., and Gonzalez, A. L. (2015). Human microvascular pericyte basement membrane remodeling regulates neutrophil recruitment. Microcirculation 22 (1), 54–67. doi:10.1111/micc.12173
Schumann, D. M., Leeming, D., Papakonstantinou, E., Blasi, F., Kostikas, K., Boersma, W., et al. (2018). Collagen degradation and formation are elevated in exacerbated COPD compared with stable disease. Chest 154 (4), 798–807. doi:10.1016/j.chest.2018.06.028
Selman, M., and Pardo, A. (2021). Fibroageing: An ageing pathological feature driven by dysregulated extracellular matrix-cell mechanobiology. Ageing Res. Rev. 70, 101393. doi:10.1016/j.arr.2021.101393
Sharma, N. S., Lal, C. V., Li, J. D., Lou, X. Y., Viera, L., Abdallah, T., et al. (2018). The neutrophil chemoattractant peptide proline-glycine-proline is associated with acute respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 315 (5), L653-L661–L661. doi:10.1152/ajplung.00308.2017
Singh, A., Soni, K. D., Singh, Y., Aggarwal, R., Venkateswaran, V., Ashar, M. S., et al. (2022). Alveolar arterial gradient and respiratory index in predicting the outcome of COVID-19 patients; a retrospective cross-sectional study. Arch. Acad. Emerg. Med. 10 (1), e28. doi:10.22037/aaem.v10i1.1543
Surendran, V., Rutledge, D., Colmon, R., and Chandrasekaran, A. (2021). A novel tumor-immune microenvironment (TIME)-on-Chip mimics three dimensional neutrophil-tumor dynamics and neutrophil extracellular traps (NETs)-mediated collective tumor invasion. Biofabrication 13 (3), 035029. doi:10.1088/1758-5090/abe1cf
Tabdanov, E. A.-O. X., Rodríguez-Merced, N. A.-O., Cartagena-Rivera, A. A.-O., Puram, V. A.-O., Callaway, M. A.-O., Ensminger, E. A.-O., et al. (2021). Engineering T cells to enhance 3D migration through structurally and mechanically complex tumor microenvironments. Nat. Commun. 12, 2815–1723. doi:10.1038/s41467-021-22985-5
Tan, D. B. A., Teo, T. H., Setiawan, A. M., Ong, N. E., Zimmermann, M., Price, P., et al. (2017). Increased CTLA-4(+) T cells may contribute to impaired T helper type 1 immune responses in patients with chronic obstructive pulmonary disease. Immunology 151 (2), 219–226. doi:10.1111/imm.12725
Tavano, S., and Heisenberg, C. P. (2019). Migrasomes take center stage. Nat. Cell. Biol. 21 (8), 918–920. doi:10.1038/s41556-019-0369-3
Taylor, A. E., Finney-Hayward, T. K., Quint, J. K., Thomas, C. M., Tudhope, S. J., Wedzicha, J. A., et al. (2010). Defective macrophage phagocytosis of bacteria in COPD. Eur. Respir. J. 35 (5), 1039–1047. doi:10.1183/09031936.00036709
Theocharis, A. D., Skandalis, S. S., Gialeli, C., and Karamanos, N. K. (2016). Extracellular matrix structure. Adv. Drug Deliv. Rev. 97, 4–27. doi:10.1016/j.addr.2015.11.001
Titz, B., Sewer, A., Schneider, T., Elamin, A., Martin, F., Dijon, S., et al. (2015). Alterations in the sputum proteome and transcriptome in smokers and early-stage COPD subjects. J. Proteomics 128 (1876-7737), 306–320. (Electronic)). doi:10.1016/j.jprot.2015.08.009
Tjin, G., White, E. S., Faiz, A., Sicard, D., Tschumperlin, D. J., Mahar, A., et al. (2017). Lysyl oxidases regulate fibrillar collagen remodelling in idiopathic pulmonary fibrosis. Dis. Model. Mech. 10 (11), 1301–1312. doi:10.1242/dmm.030114
Tomashefski, J. F. (2000). Pulmonary pathology of acute respiratory distress syndrome. Clin. Chest Med. 21 (3), 435–466. doi:10.1016/s0272-5231(05)70158-1
Torii, K., Iida, K., Miyazaki, Y., Saga, S., Kondoh, Y., Taniguchi, H., et al. (1997). Higher concentrations of matrix metalloproteinases in bronchoalveolar lavage fluid of patients with adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 155 (1), 43–46. doi:10.1164/ajrccm.155.1.9001287
van Geffen, C., Deißler, A., Quante, M., Renz, H., Hartl, D., and Kolahian, S. (2021). Regulatory immune cells in idiopathic pulmonary fibrosis: Friends or foes? Front. Immunol. 12, 663203. doi:10.3389/fimmu.2021.663203
van Helvert, S., Storm, C., and Friedl, P. (2018). Mechanoreciprocity in cell migration. Nat. Cell. Biol. 20 (1), 8–20. doi:10.1038/s41556-017-0012-0
van Houwelingen, A. H., Weathington, N. M., Verweij, V., Blalock, J. E., Nijkamp, F. P., and Folkerts, G. (2008). Induction of lung emphysema is prevented by L-arginine-threonine-arginine. FASEB J. 22 (9), 3403–3408. doi:10.1096/fj.07-096230
van Straaten, J. F., Coers, W., Noordhoek, J. A., Huitema, S., Flipsen, J. T., Kauffman, H. F., et al. (1999). Proteoglycan changes in the extracellular matrix of lung tissue from patients with pulmonary emphysema. Mod. Pathol. 12 (7), 697–705.
Vargas-Rojas, M. I., Ramirez-Venegas, A., Limon-Camacho, L., Ochoa, L., Hernandez-Zenteno, R., and Sansores, R. H. (2011). Increase of Th17 cells in peripheral blood of patients with chronic obstructive pulmonary disease. Respir. Med. 105 (11), 1648–1654. doi:10.1016/j.rmed.2011.05.017
Vasse, G. F., Kühn, P. T., Zhou, Q., Bhusari, S. A., Reker-Smit, C., Melgert, B. N., et al. (2018). Collagen morphology influences macrophage shape and marker expression in vitro. J. Immunol. Regen. Med. 1, 13–20. doi:10.1016/j.regen.2018.01.002
Vasse, G. F., Nizamoglu, M., Heijink, I. H., Schleputz, M., van Rijn, P., Thomas, M. J., et al. (2021). Macrophage-stroma interactions in fibrosis: Biochemical, biophysical, and cellular perspectives. J. Pathol. 254 (4), 344–357. doi:10.1002/path.5632
Volk, S. W., Iqbal, S. A., and Bayat, A. (2013). Interactions of the extracellular matrix and progenitor cells in cutaneous wound healing. Adv. Wound Care 2 (6), 261–272. doi:10.1089/wound.2012.0417
Wagner, D. E., Bonvillain, R. W., Jensen, T., Girard, E. D., Bunnell, B. A., Finck, C. M., et al. (2013). Can stem cells be used to generate new lungs? ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology 18 (6), 895–911. doi:10.1111/resp.12102
Walton, G. M., Stockley, J. A., Griffiths, D., Sadhra, C. S., Purvis, T., and Sapey, E. (2016). Repurposing treatments to enhance innate immunity. Can statins improve neutrophil functions and clinical outcomes in COPD? J. Clin. Med. 5 (10), 89. doi:10.3390/jcm5100089
Wang, W. Y., Pearson, A. T., Kutys, M. L., Choi, C. K., Wozniak, M. A., Baker, B. M., et al. (2018). Extracellular matrix alignment dictates the organization of focal adhesions and directs uniaxial cell migration. Apl. Bioeng. 2 (4), 046107. doi:10.1063/1.5052239
Wang, Z., Qi, F., Luo, H., Xu, G., and Wang, D. (2022). Inflammatory microenvironment of skin wounds. Front. Immunol. 13, 789274. doi:10.3389/fimmu.2022.789274
Weathington, N. M., van Houwelingen, A. H., Noerager, B. D., Jackson, P. L., Kraneveld, A. D., Galin, F. S., et al. (2006). A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 12 (3), 317–323. doi:10.1038/nm1361
Weckmann, M., Bahmer, T., Sand, J. M., Ronnow, S. R., Pech, M., Vermeulen, C., et al. (2021). COL4A3 is degraded in allergic asthma and degradation predicts response to anti-IgE therapy. Eur. Respir. J. 58 (6), 2003969. doi:10.1183/13993003.03969-2020
Weitoft, M., Andersson, C., Andersson-Sjoland, A., Tufvesson, E., Bjermer, L., Erjefalt, J., et al. (2014). Controlled and uncontrolled asthma display distinct alveolar tissue matrix compositions. Respir. Res. 15 (1), 67. doi:10.1186/1465-9921-15-67
Widener, A. E., Bhatta, M., Angelini, T. E., and Phelps, E. A. (2021). Guest-host interlinked PEG-MAL granular hydrogels as an engineered cellular microenvironment. Biomater. Sci. 9 (7), 2480–2493. doi:10.1039/d0bm01499k
Wu, D., Xu, Y., Ding, T., Zu, Y., Yang, C., and Yu, L. (2017). Pairing of integrins with ECM proteins determines migrasome formation. Cell. Res. 27 (11), 1397–1400. doi:10.1038/cr.2017.108
Wu, J., Hillier, C., Komenda, P., Lobato de Faria, R., Levin, D., Zhang, M., et al. (2015). A microfluidic platform for evaluating neutrophil chemotaxis induced by sputum from COPD patients. PLoS One 10 (5), e0126523. doi:10.1371/journal.pone.0126523
Yamada, K. M., Doyle, A. D., and Lu, J. (2022). Cell-3D matrix interactions: Recent advances and opportunities. Trends Cell. Biol. 32, 883–895. doi:10.1016/j.tcb.2022.03.002
Yamada, K. M., and Sixt, M. (2019). Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell. Biol. 20 (12), 738–752. doi:10.1038/s41580-019-0172-9
Zenewicz, L. A. (2017). Oxygen levels and immunological studies. Front. Immunol. 8, 324. doi:10.3389/fimmu.2017.00324
Keywords: extracellular matrix, migration, infiltrating immune cells, in vitro models, lung diseases, three-dimensional
Citation: Joglekar MM, Nizamoglu M, Fan Y, Nemani SSP, Weckmann M, Pouwels SD, Heijink IH, Melgert BN, Pillay J and Burgess JK (2022) Highway to heal: Influence of altered extracellular matrix on infiltrating immune cells during acute and chronic lung diseases. Front. Pharmacol. 13:995051. doi: 10.3389/fphar.2022.995051
Received: 15 July 2022; Accepted: 19 October 2022;
Published: 03 November 2022.
Edited by:
Isaac Kirubakaran Sundar, University of Kansas Medical Center, United StatesReviewed by:
Ranu Surolia, University of Alabama at Birmingham, United StatesCopyright © 2022 Joglekar, Nizamoglu, Fan, Nemani, Weckmann, Pouwels, Heijink, Melgert, Pillay and Burgess. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mugdha M. Joglekar, bS5tLmpvZ2xla2FyQHVtY2cubmw=; Janette K. Burgess, ai5rLmJ1cmdlc3NAdW1jZy5ubA==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.