
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 03 October 2022
Sec. Experimental Pharmacology and Drug Discovery
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.992731
This article is part of the Research Topic Recent Advances in Molecular Pharmacology of Immune-Modulating Agents: Disease Management and Treatment of Autoimmune Disorders View all 6 articles
 Mohamed Sayed Zaazouee1*
Mohamed Sayed Zaazouee1* Asmaa Gomaa Alwarraqi2
Asmaa Gomaa Alwarraqi2 Yasmine Adel Mohammed3
Yasmine Adel Mohammed3 Mohamed A. Badheeb4
Mohamed A. Badheeb4 Abdullah Mohamed Farhat5
Abdullah Mohamed Farhat5 Mohammed Eleyan6,7
Mohammed Eleyan6,7 Afnan Morad3
Afnan Morad3 Marwa Abdel-Aziz Zeid8,9
Marwa Abdel-Aziz Zeid8,9 Aya Shaban Mohamed10
Aya Shaban Mohamed10 Hazem AbuEl-Enien11
Hazem AbuEl-Enien11 Ahmed Abdelalim12
Ahmed Abdelalim12 Ahmed Bostamy Elsnhory13
Ahmed Bostamy Elsnhory13 Yasmin S. M. Hrizat14
Yasmin S. M. Hrizat14 Nagat Taha Altahir15
Nagat Taha Altahir15 Doaa Atef16
Doaa Atef16 Alaa Ahmed Elshanbary2
Alaa Ahmed Elshanbary2 Khalaf F. Alsharif17
Khalaf F. Alsharif17 Khalid J. Alzahrani17
Khalid J. Alzahrani17 Mohammad Algahtani18
Mohammad Algahtani18 Abdulrahman Theyab18,19
Abdulrahman Theyab18,19 Yousef M. Hawsawi19,20
Yousef M. Hawsawi19,20 Ahmed A. Aldarmahi21
Ahmed A. Aldarmahi21 Mohamed M. Abdel-Daim22,23
Mohamed M. Abdel-Daim22,23Background: Dupilumab is a human monoclonal antibody directed against the alpha subunit of the interleukin-4 receptor and inhibits the signaling of IL-4 and IL-13. It is approved for treating asthma and other type-2 inflammatory diseases. There is a conflict in the literature regarding the safety and efficacy of dupilumab. Thus, we aimed to assess the safety and efficacy of dupilumab in patients with moderate to severe asthma.
Methods: Six databases (PubMed, Embase, Scopus, Web of Science, Cochrane library, and clinicaltrials.gov registry) were searched until January 2022. We included randomized controlled trials that compared dupilumab with the placebo in moderate to severe asthma patients. We extracted the data at 12 and 24 weeks and analyzed them using review manager 5.4.
Findings: Thirteen trials were included. Dupilumab significantly improved the forced expiratory volume in 1 s, asthma control questionnaire score, the fraction of exhaled nitric oxide level, and immunoglobulin E level at 12 and 24 weeks (p < 0.05). However, it was associated with increased blood eosinophils at 12 and 24 weeks. Dupilumab was generally a safe agent for asthmatic patients. It showed no significant difference compared with the placebo regarding most adverse events.
Conclusion: Dupilumab improves pulmonary function and reduces local and systemic inflammatory markers with minimal adverse events in patients with moderate to severe asthma.
Worldwide, asthma affected approximately 262 million people and caused 461,000 deaths (JL Murray, 2020). It is a major non-communicable disease that affects children and adults of both sexes, with a higher incidence in females (Wu et al., 2019). The disease prevalence has both genetic and environmental factors (Arrieta et al., 2015; Chen et al., 2017). Despite high-dose treatment, nearly more than 25% of the patients have uncontrolled asthma (JL Murray, 2020). In addition, those patients are at increased risk for respiratory function impairment, frequent asthmatic exacerbation, hospitalization, medical and societal costs, and poor quality of life (Bellin et al., 2015; Fleming et al., 2015).
Bronchial asthma is a disease of the air conducting system. It is characterized by a long-term airway inflammatory process even if the patient is in an asymptomatic period (Robinson et al., 1992). Major symptoms include cough, chest tightness, shortness of breath, and reversible episodic wheezes resulting from airway inflammation and hyperresponsiveness (Wu et al., 2019). The inflammatory process of asthma is mediated by helper T-2 cells and eosinophils in addition to the released cytokines, including interleukins (IL); IL-4, IL-5, and IL-13 (Robinson et al., 1992; Fahy, 2015). Interleukin-4 (IL-4) is one of the most important pro-inflammatory mediators in asthma. It mediates essential functions in asthma, including induction of the IgE isotype switch, expression of vascular cell adhesion molecule-1 (VCAM-1), and promotion of eosinophil transmigration across the endothelium, mucus secretion, and differentiation of T helper type-2 lymphocytes leading to cytokine release which causes asthma symptoms (Steinke and Borish, 2001). So, inhibiting the main ILs as IL-4 receptors will reduce the signaling and activity of the asthma inflammatory process, enhancing the pulmonary function and reducing the systemic and local inflammatory mediators.
Traditional pharmacological treatments are classified into controller medication and rescue medication. This comprises long-acting beta-agonists (LABA), inhaled corticosteroids, or leukotriene modifiers that interfere with the inflammatory process and prevent progression into irreversible airway remodeling (Newman, 2004; Chauhan and Ducharme, 2014; Wu et al., 2019). Dupilumab is a human monoclonal antibody directed against the alpha subunit of the interleukin-4 receptor and inhibits the signaling of IL-4 and IL-13 (Le Floc’h et al., 2020). The literature revealed significant improvement in clinical outcomes of asthmatic patients (Castro et al., 2018; Bachert et al., 2019; Castro et al., 2020; Bacharier et al., 2021). The effect of dupilumab starts early after the beginning of the treatment course. Most studies reported that it is maintained to the end of the follow-up periods of different RCTs up to 52 weeks. Moreover, it is approved for treating asthma and other type-2 inflammatory diseases in adults and adolescents. The global initiative for asthma (GINA) 2022 report (Reddel et al., 2022) suggests using anti-IL-4 receptors such as dupilumab in the management of patients with severe eosinophilic/type-2 asthma (step 5). This is suitable for patients of ≥6 years old, adolescents, and adults. However, other literature works revealed discrepancies regarding its efficacy (Wenzel et al., 2016; Weinstein et al., 2018; Laidlaw et al., 2021; Wechsler et al., 2021). This may be explained by different dosage regimens or comorbidities with asthma. Hence, in this systematic review and meta-analysis, we aimed to solve this contrast by evaluating the safety and efficacy of dupilumab in patients with moderate to severe asthma.
We performed this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Page et al., 2021) and the Cochrane Handbook of Systematic Review and Meta-analysis of Interventions (Higgins, 2019).
Six databases (PubMed, Embase, Scopus, Web of Science, Cochrane library, and clinicaltrials.gov registry) were used for literature search from inception until January 2022. We used the following keywords (Dupilumab, SAR231893, SAR-231893, Dupixent, REGN668, REGN-668, and Asthma*).
Human-based, English-written randomized controlled trials (RCTs) were included with no restriction on age, sex, settings, or publication dates. The included RCTs compared dupilumab with the placebo in moderate to severe asthma patients. Exclusion criteria included protocols, non-English–written studies, conference abstracts, book chapters, review articles, observational studies, and non-human studies.
We used the EndNote X8 version for citation management and duplicate removal. The full text of the eligible studies in the non-open access journals were obtained through academic institution access or by contacting authors requesting full texts of their studies.
The authors selected the studies according to two steps; first, we performed the title and abstract screening, and second, full-text screening to identify studies that fulfill our inclusion criteria. We manually screened the reference list in the included studies and citations of the identified articles. Four independent authors performed each step, and a discussion with the supervisor solved any disagreements.
Four authors extracted the following data (I) summary of included studies, including study design, NCT numbers, participants’ details, intervention period, follow-up period, primary outcomes, and (II) baseline characteristics of included studies, including study arms, sample size, age, sex, forced expiratory volume in 1 s (FEV1) reversibility, history of nasal polyposis, history of smoking, and allergic conditions. Another three authors extracted the outcomes of interest.
FEV1 change per liter, Asthma Control Questionnaire (ACQ) change.
Any treatment-emergent adverse events, any treatment-emergent adverse events leading to permanent discontinuation.
Fraction of exhaled nitric oxide (FeNO) change, blood eosinophil change, and IgE changes all at 12 and 24 weeks.
Any adverse events, any adverse events leading to permanent discontinuation, serious adverse events, serious treatment-emergent adverse events, any adverse events leading to death, any treatment-emergent adverse events leading to death, nasopharyngitis, upper respiratory tract infection, viral upper respiratory tract infection, headache, erythema, injection-site reaction, cough, allergic rhinitis, bronchitis, influenza, urinary tract infection, back pain, sinusitis, and eosinophilia.
The risk of bias was assessed according to the Cochrane risk of bias tool, using the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins et al., 2011). It includes seven main domains, namely, random sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other biases.
We used Review Manager (Version 5.4) to analyze the data. We used the risk ratio (RR) with a 95% confidence interval (CI) for dichotomous data and mean difference (MD) and 95% CI for continuous data. The data were pooled under a random-effect model. Heterogeneity among the studies was examined using Cochrane’s p values and I2. We considered the data heterogeneous when chi-square p < 0.1 and I2 >50%. We used a sensitivity analysis by leaving one out method to overcome heterogeneity. According to the Cochrane Handbook, we could not assess the publication bias as all outcomes were reported in less than 10 studies. The efficacy outcomes were pooled at different time points, 12 and 24 weeks. In addition, we performed a subgroup analysis according to the treatment regimen. This includes the following groups: 100–200 mg of dupilumab every 2 weeks, 200 mg dupilumab every 2 weeks, 200 mg dupilumab every 4 weeks, 300 mg dupilumab every 2 weeks, and 300 mg dupilumab every 4 weeks.
A total of 2,268 studies were retrieved from different databases after duplicate removal. Of them, only 31 studies were eligible for full-text assessment. According to our inclusion and exclusion criteria, we included 13 RCTs (Wenzel et al., 2013; Wenzel et al., 2016; Castro et al., 2018; Rabe et al., 2018; Weinstein et al., 2018; Bachert et al., 2019; Castro et al., 2020; Corren et al., 2020; Tohda et al., 2020; Bacharier et al., 2021; Corren et al., 2021; Laidlaw et al., 2021; Wechsler et al., 2021) in our systematic review; of them, nine trials were eligible for our meta-analysis (Wenzel et al., 2013; Wenzel et al., 2016; Castro et al., 2018; Rabe et al., 2018; Weinstein et al., 2018; Bachert et al., 2019; Bacharier et al., 2021; Laidlaw et al., 2021; Wechsler et al., 2021). Figure 1 shows the PRISMA flow diagram of our meta-analysis.
We included data of 4,482 patients. Of them, 726 (16.2%) were smokers. A total of 1,092 patients (24.4%) had a history of polyposis, while 2,558 patients (57%) had a history of allergic conditions. The mean age of our population was 45 years old. Most of them were females (59.2%). The mean FEV1 reversibility at baseline was 21.2%. The follow-up periods ranged from 12 to 52 weeks. Tables 1, 2 show the summary and baseline characteristics of the included population.
All the included studies revealed a low risk of bias regarding all the assessed domains of the Cochrane risk of bias tool, except for other bias domains. It was put at a high risk as all of the included studies were funded by the drug manufacturer. In addition, in the trial by Wechesler et al. (Laidlaw et al., 2021), data about the allocation process were unclear. Figure 2 shows the summary of the quality assessment.
This outcome was reported by five trials (Wenzel et al., 2013; Wenzel et al., 2016; Castro et al., 2018; Laidlaw et al., 2021; Wechsler et al., 2021). Dupilumab significantly improved the absolute (dose-independent) FEV1 (L) at week 12 among 2,198 patients in this group compared with 1,450 patients in the placebo group; MD = 0.14, 95% CI = 0.11, 0.16, p < 0.01. This outcome was homogeneous p = 0.47, I2 = 0%. The subgroup analysis of different dupilumab doses did not reveal any significant difference between all subgroups p = 0.57 (Figure 3).
As for dupilumab 200 mg q2w, two trials reported this outcome (Wenzel et al., 2016; Castro et al., 2018). They revealed a significant improvement in FEV1 (L) compared with the placebo; MD = 0.16 (95% CI = 0.11, 0.20), p < 0.01. This subgroup pooled data were homogeneous; p = 0.33, I2 = 0%.
Regarding the 300 mg dupilumab q2w, five trials reported this outcome (Wenzel et al., 2013; Wenzel et al., 2016; Castro et al., 2018; Laidlaw et al., 2021; Wechsler et al., 2021). Dupilumab significantly improved the FEV1(L) compared with the placebo; MD = 0.14, 95% CI = 0.09, 0.18, p < 0.01. This subgroup pooled data were homogeneous; p = 0.32, I2 = 14%.
This outcome was reported by five trials (Wenzel et al., 2016; Castro et al., 2018; Rabe et al., 2018; Weinstein et al., 2018; Laidlaw et al., 2021). Dupilumab significantly improved the absolute (dose-independent) FEV1 (L) at week 24 among 2,144 patients in the treatment group compared with 1,445 patients in the placebo group; MD = 0.13, (95% CI = 0.11, 0.16), p < 0.00001. This outcome was homogeneous; p = 0.66, I2 = 0%. The subgroup analysis revealed no significant difference between them, p = 0.34 (Figure 4).
In the dupilumab 200 mg q2w subgroup, two included trials (Wenzel et al., 2016; Castro et al., 2018) revealed a significant improvement in FEV1 in the dupilumab group; MD = 0.17 (95% CI = 0.12, 0.21), p < 0.00001. This subgroup pooled data were homogeneous; p = 0.84, I2 = 0%.
As for 200 mg dupilumab q4w, it significantly improved the FEV1 compared with the placebo; MD = 0.12 (95% CI = 0.03, 0.22), p = 0.01. This subgroup data were homogeneous; p = 0.6, I2 = 0%.
In four trials (Castro et al., 2018; Rabe et al., 2018; Weinstein et al., 2018; Laidlaw et al., 2021), the dupilumab 300 mg q2w regimen revealed a significant improvement in FEV1 compared with the placebo; MD = 0.11 (95% CI = 0.07, 0.15), p < 0.00001. This subgroup data were homogeneous; p = 0.54, I2 = 0%.
This outcome was reported in five trials (Wenzel et al., 2013; Wenzel et al., 2016; Bacharier et al., 2021; Laidlaw et al., 2021; Wechsler et al., 2021). They revealed a significant reduction in the absolute ACQ score at 12 weeks In the dupilumab group compared with the placebo; MD = −0.74 (95% CI = −1.20, −0.28), p = 0.001. The overall analysis revealed heterogeneity p < 0.01, I2 = 96%. The subgroup analysis revealed no significant difference between all subgroups, p = 0.11 (Figure 5).
Regarding the 300 mg q2w regimen reported by four trials (Wenzel et al., 2013; Wenzel et al., 2016; Laidlaw et al., 2021; Wechsler et al., 2021), the dupilumab revealed a significant reduction in the ACQ score compared with the placebo; MD = −1.34 (95% CI = −2.42, −0.26), p < 0.01. This subgroup data were heterogeneous; p < 0.001, I2 = 98%. The sensitivity analysis could not solve this heterogeneity.
This outcome was reported in four trials (Wenzel et al., 2013; Castro et al., 2018; Bachert et al., 2019; Bacharier et al., 2021). They revealed that dupilumab significantly reduced the absolute ACQ score at 24 weeks Compared with the placebo; MD = −0.43 (95% CI = −0.67, −0.19), p = 0.0005. The pooled analysis was heterogeneous; p <0.001, I2 = 88%. The subgroup analysis revealed no significant difference between subgroups, p = 0.68 (Figures 6A,B). As for 300 mg q2w, dupilumab revealed a significant reduction in ACQ at 24 weeks Compared with the placebo; MD = −0.53 (95% CI = −1.04, −0.02), p = 0.04. This subgroup pooled data were heterogeneous; p < 0.001, I2 = 94%. To solve this heterogeneity, we excluded Castro et al. (2018). After sensitivity analysis, there was a significant ACQ score reduction in the dupilumab group compared with the placebo; MD = −0.77 (95% CI = −1.07, −0.47), p < 0.001. The subgroup analysis was homogeneous; p = 0.19, I2 = 42%.
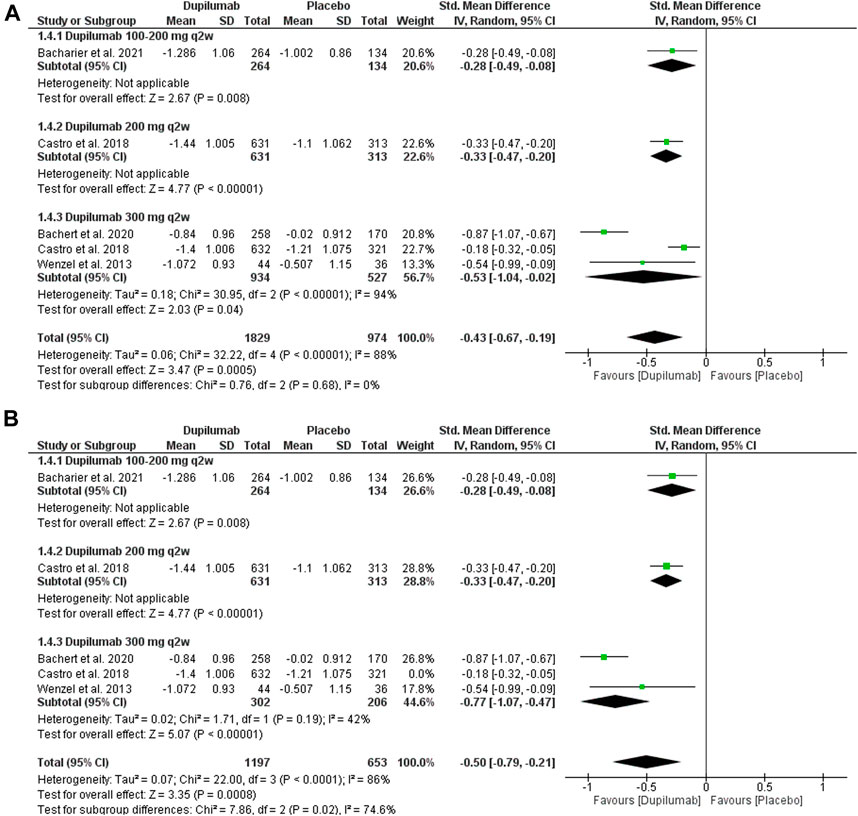
FIGURE 6. Results of the ACQ change at the 24th week. (A) Before sensitivity analysis. (B) After sensitivity analysis.
Six trials (Wenzel et al., 2013; Wenzel et al., 2016; Castro et al., 2018; Rabe et al., 2018; Bacharier et al., 2021; Wechsler et al., 2021) reported this outcome. Dupilumab significantly reduced the FeNO (ppb) compared with the placebo; MD = −17.58 (95% CI = −21.87, −13.29), p < 0.001. The pooled analysis was heterogeneous p = 0.005, I2 = 62%. The subgroup analysis revealed no significant difference between those regimens regarding the dupilumab efficacy, p = 0.84 (Supplementary Figure S1).
Regarding the 200 mg q2w regimens, the pooled two trials (Wenzel et al., 2016; Castro et al., 2018) revealed no significant reduction in the FeNO (ppb) compared with the placebo; MD = −22.25 (95% CI = −44.73, 0.23), p = 0.05. The pooled analysis was heterogeneous, p = 0.01, I2 = 84%.
Five trials (Wenzel et al., 2013; Wenzel et al., 2016; Castro et al., 2018; Rabe et al., 2018; Wechsler et al., 2021) evaluated the 300 mg q2w regimen. Dupilumab significantly reduced the FeNO (ppb) compared with the placebo; MD = −19.56 (95% CI = −27.21, −11.90), p < 0.001. The pooled analysis was heterogeneous, p = 0.004, I2 = 74%. We could not resolve this heterogeneity by sensitivity analysis.
Four trials reported this outcome (Wenzel et al., 2016; Castro et al., 2018; Rabe et al., 2018; Bacharier et al., 2021). At the week 24, dupilumab significantly reduced the FeNO (ppb) compared with the placebo; MD = −19.50 (95% CI = −24.74, −14.25), p < 0.001. The pooled analysis was heterogeneous, p = 0.001, I2 = 71%. The subgroup analysis revealed no significant difference between them, p = 0.88 (Supplementary Figures S2A,B).
Two trials (Wenzel et al., 2016; Castro et al., 2018) reported the 200 mg q2w regimen. They reported a significant reduction in the FeNO (ppb) in the dupilumab group compared with the placebo group; MD = −21.61 (95% CI = −40.37, −2.85), p = 0.02. The pooled analysis was heterogeneous, p = 0.01, I2 = 83%.
In addition, three trials (Wenzel et al., 2016; Castro et al., 2018; Rabe et al., 2018) reported the 300 mg q2w revealing that the dupilumab significantly reduced the FeNO compared with the placebo; MD = −21.18 (95% CI = −33.97, −8.38], p = 0.001. The pooled analysis was heterogeneous, p = 0.0009, I2 = 86%. We solved this heterogeneity by exclusion of Wenzel et al. (2016); p = 0.21, I2 = 36%. The pooled analysis of subgroup remained significant; MD = −13.34 (95% CI = −18.67, −8.00), p < 0.001.
This outcome was reported in four trials (Wenzel et al., 2013; Castro et al., 2018; Bacharier et al., 2021; Wechsler et al., 2021). The placebo group showed significantly lower serum eosinophil levels than the dupilumab group, which showed an increase in their levels; MD = 133.05 (95% CI = 97.46, 168.64), p < 0.001. The pooled analysis was homogeneous; p = 0.41, I2 = 0%. The subgroup analysis showed no significant difference between each group; p = 0.51, I2 = 0% (Supplementary Figure S3).
In three trials (Wenzel et al., 2013; Castro et al., 2018; Wechsler et al., 2021), the dupilumab 300 mg q2w showed a significant increase in eosinophil levels in the dupilumab group; MD = 168.27, (95% CI = 76.12, 260.41), p = 0.0003. The pooled data were homogeneous; p = 0.23, I2 = 33%.
This outcome was reported in two trials (Castro et al., 2018; Bacharier et al., 2021). Similarly, in the 24th week, the changes in the placebo group were significantly lower than those in the dupilumab group; MD = 94.66 (95% CI = 54.92, 134.40), p < 0.001. The pooled analysis was homogeneous; p = 0.87, I2 = 0%. The subgroups of different dupilumab regimens did not show a significant difference; p = 0.87 (Supplementary Figure S4).
This outcome was reported in three trials (Wenzel et al., 2013; Castro et al., 2018; Wechsler et al., 2021). The dupilumab significantly reduced the IgE levels compared with the placebo; MD = −149.27 (95% CI = −176.39, −122.16), p < 0.001. The pooled data were homogeneous; p = 0.34, I2 = 11%. The subgroups did not show a significant difference between both regimens; p = 0.18 (Supplementary Figure S5).
This outcome was reported by three trials (Castro et al., 2018; Bachert et al., 2019; Bacharier et al., 2021). Dupilumab significantly reduced the IgE levels at the 24th week compared with the placebo; MD = −210.28, (95% CI = −365.02, −55.55), p = 0.008. The pooled analysis was heterogeneous, p < 0.001, I2 = 98%. The subgroup analysis revealed no significant difference between dupilumab regimens, p = 0.55 (Supplementary Figure S6).
The adverse events of dupilumab were reported by most of the included trials. Compared to the placebo, dupilumab revealed a significantly higher incidence of upper respiratory tract infections (URTI), injection-site reaction, and eosinophilia, p < 0.05 (Table 3; Supplementary Figures S7, S14).
On the other hand, there was no significant difference between dupilumab and placebo groups (p ≥ 0.05), regarding the following outcomes; any adverse events, any treatment-emergent adverse events, any adverse events leading to permanent discontinuation, any treatment-emergent adverse events leading to permanent discontinuation, serious adverse events, serious treatment-emergent adverse events, any adverse events leading to death, any treatment-emergent adverse events leading to death, viral upper respiratory tract infection, influenza, bronchitis, nasopharyngitis, sinusitis, headache, allergic rhinitis, cough, urinary tract infection, back pain, and erythema.
Four trials (Castro et al., 2020; Corren et al., 2020; Tohda et al., 2020; Corren et al., 2021) were included in our qualitative synthesis. In their 2020 trial, Corren et al. (2020) assessed the efficacy of the dupilumab during a treatment period of 52 weeks in 1,902 patients (allergic and eosinophilic asthma). They found dupilumab reduced asthma exacerbation and the inflammatory biomarkers and FEV1 improvement in both types of asthma. Moreover, in a 2021 trial by Corren et al. (2021), they assessed the same outcomes in patients with more than one, two, or three exacerbations in the year before the trial. In addition, they classified patients in different subgroups according to the baseline blood eosinophils, FeNO, and inhaled corticosteroid doses. Corren et al. (2021) in a 2021 post hoc analysis reported similar results to their previous 2020 trial. Another post hoc analysis by Castro et al. (2020) investigated how dupilumab affects lung function in total participants and according to inflammatory biomarker levels. They concluded that dupilumab enhances lung function results, especially in patients with increased type-2 inflammatory biomarkers. Tohda et al. (2020), in their 2020 trial, evaluated the efficacy of dupilumab in the Japanese subpopulation of the QUEST trial (114). They found dupilumab reduced asthma exacerbation and the inflammatory and improved FEV1 in the Japanese population of QUEST, indicating the significance of dupilumab in different ethnic groups.
Our pooled data of 13 RCTs found that dupilumab significantly improved the FEV1 at the 12th and 24th weeks. In addition, it reduced FeNO levels, IgE levels, and ACQ scores of asthmatic patients at the 12th and 24th weeks. However, it was associated with an increase in blood eosinophils at the 12th and 24th weeks. Dupilumab was generally a safe agent for asthmatic patients. It showed no significant difference compared with the placebo regarding all adverse effects, except for upper respiratory tract infection, injection-site reaction, and eosinophilia, which had a significantly higher incidence in the dupilumab group. Furthermore, those findings seem to be dose-independent as there was no significant difference between different subgroups.
Those results were consistent with most of the results of these trials (Wenzel et al., 2013; Wenzel et al., 2016; Castro et al., 2018; Rabe et al., 2018; Weinstein et al., 2018; Bachert et al., 2019; Castro et al., 2020; Corren et al., 2020; Tohda et al., 2020; Bacharier et al., 2021; Corren et al., 2021; Laidlaw et al., 2021; Wechsler et al., 2021) and with previous meta-analyses (Edris et al., 2019; Xiong et al., 2019; Zayed et al., 2019). However, Weinstein et al. (2018) trial reported that the 200 mg q2w dupilumab regimen was associated with a statistically insignificant improvement in FEV1 compared with the placebo in patients with perennial allergic rhinitis (PAR). But this was different in non-PAR patients, whereas dupilumab 200 mg/2 weeks regimen significantly increased FEV1 by 0.15 L compared to the placebo. In contrast, the 300 mg q2w dupilumab regimen showed no significant difference regarding FEV1 compared with the placebo. This indicates the importance of classifying asthmatic patients according to their medical conditions or comorbidities and the importance of the choice of treatment regimens. Moreover, similar results were found regarding the annualized rate of severe exacerbations.
The effect of dupilumab starts early after the beginning of the treatment course and is maintained to the end of the follow-up periods of different RCTs up to 52 weeks, as reported by most of our included studies. In addition, it is reported that dupilumab reduced the annualized rate of severe asthma exacerbations by 47%, especially when added to inhaled corticosteroids and other controllers compared with the placebo (Castro et al., 2018).
Asthmatic children require special attention as uncontrolled asthma affects pulmonary functions and limits the airflow, leading to COPD in adulthood (Tagiyeva et al., 2016; McGeachie, 2017). However, the protective role of dupilumab against COPD development or restoring normal pulmonary growth and function is still unclear. Similarly, the role of the different treatment doses and duration is still a query. Although there was no significant difference between different regimens of dupilumab in our trial, we think that the actual effect may be detected in the long-term course of treatment beyond our follow-up periods.
Furthermore, Bacharier et al. (2021) reported that 78% of children using dupilumab as an add-on therapy experienced an exacerbation-free period during the 52 weeks of treatment, compared with 60% of children in the placebo group. Those patients required less use of systemic corticosteroids. This is a critical indicator of efficacy and safety, especially among pediatric asthmatic patients, as they avoided the long-term use of corticosteroids with their subsequent complications.
IL-13 promotes the activity of NO-synthase with increased NO levels. This indicates NO’s role as a biomarker of asthmatic activity and could be correlated to the levels of IL in the airway mucosa (Chibana et al., 2008; Barranco et al., 2017). Our results were consistent with these mechanisms. We observed that dupilumab significantly reduced the local and systemic inflammatory biomarkers such as FeNO and IgE. This confirms the role of dupilumab in the inflammatory process signaling and activity. In addition, those biomarkers may be used as a screening test for the response to dupilumab and other agents targeting the type-2 inflammatory pathway.
On the other hand, our pooled analysis revealed a relatively higher serum eosinophilic count in the dupilumab group compared with the placebo. This increase seems to be transient at the beginning of dupilumab treatment in adults (Castro et al., 2018) and children (Bacharier et al., 2021). During the inflammatory process, the IL-4 and IL-13 produce eotaxin and vascular cell adhesion molecule, which stimulates eosinophils’ migration to targeted tissues. Dupilumab blocks this sequence of events retaining eosinophils in the circulation (Barthel et al., 2008; Tozawa et al., 2011). Moreover, Rabe et al. (2018) explained the increase in blood eosinophil levels due to different corticosteroid dosages between both study arms as glucocorticoids reduce the levels of blood eosinophils. In the dupilumab group, the dose of the glucocorticoids was reduced compared with that in the placebo group. This seems to be responsible for eosinophilia. In addition, the elevation of blood eosinophil levels was not associated with clinical consequences; it was only a laboratory finding, as reported by Castro et al. (2018). Nevertheless, we think dupilumab is still effective in treating asthma, as it significantly reduces the key inflammatory mediators. However, it causes eosinophilia but of no significant role in the efficacy of the dupilumab against inflammation.
The literature lacks the exact mechanism of action of dupilumab either in vivo or in vitro studies (Harb and Chatila, 2020). Dupilumab is a human monoclonal antibody directed against the alpha subunit of the interleukin-4 receptor and inhibits the signaling of IL-4 and IL-13 (Le Floc’h et al., 2020). Dupilumab acts on the alpha subunit of IL-4 receptor and prevents the binding of the IL-4 to type 1 receptor. In addition, it may inhibit the protein assembly of the type-2 receptor complex. This process may be explained by the inhibition of binding of IL-13 to IL-13 receptor, which is needed for the mobilization of the IL-4 alpha receptor. Moreover, the binding of IL-4 and 13 to their targeted receptors conducts a series of events leading to the recruitment of the other receptors subunits (Harb and Chatila, 2020). Dupilumab has different sites of action, which are fundamental for the Th2 inflammatory process of various diseases. Apart from inflammatory cells, it can also act on endothelium, reducing the cellular recruitment and vascular permeability for those cells (Harb and Chatila, 2020) (Figure 7).
Viral infections have been shown to exacerbate asthma symptoms. Few data exist on COVID-19 immunological responses in biologic-treated asthmatics, and using biological agents during the COVID-19 pandemic is still debatable. A multicenter study by Eger et al. (2020) revealed that biologic-treated asthmatics were highly vulnerable to COVID-19 infection and had a higher severity than the general population. In contrast, other studies (Klimek et al., 2020; Patruno et al., 2020; Bhalla et al., 2021; Grieco et al., 2021; Tanabe et al., 2021; Ungar et al., 2022a; Ungar et al., 2022b) reported that dupilumab is a safe agent to use during the COVID-19 pandemic and may reduce the severity of COVID-19 symptoms. However, the role of biological agents on the COVID-19 response is still unclear, so each patient should be carefully assessed, and the patient should be involved in considering the therapy’s benefits and hazards.
Regarding the other adverse events, all the included studies reported similar incidence of adverse events in both dupilumab and placebo regarding most adverse events. The injection-site reaction was increased with dupilumab treatment in addition to upper respiratory tract infection. This points to the importance of reaching an explanation of those inflammatory conditions in a drug that is thought to reduce inflammation. This may be due to different mechanisms related to the treatment regimen, duration, or the associated add-ons; however, it is not reported. Reaching an explanation of such adverse events is essential to avoid them and to reach more efficient results while manufacturing those agents.
The heterogeneity of some outcomes is the main limitation of this meta-analysis. This heterogeneity might be due to some of the included studies including asthmatic patients associated with other type-2 inflammatory diseases. The dupilumab regimens were not reported in multiple studies, so we could not have a definite conclusion about all regimens. Also, some of the included studies were post hoc analyses; thus, we could not pool these data when the results were reported in the original one. In addition, we could not conduct an age-dependent analysis of the efficacy of the dupilumab on different outcomes to determine the best age group to benefit from the targeted medication. There was no data beyond 52 weeks of treatment, and we could not determine the least period for the most effective results. Also, the pediatric population needs special care to detect the least effective dose to avoid the toxic doses. Other trials comparing dupilumab with the placebo and other drugs are needed. We suggest further RCTs that assess the safety and efficacy of dupilumab according to the biomarker level of type-2 inflammation, types of asthma, and age groups. Furthermore, a network meta-analysis to compare dupilumab with other standard treatments is recommended to show the best option for asthmatic patients.
Dupilumab improves pulmonary function and reduces local and systemic inflammatory markers with minimal adverse events in patients with moderate to severe asthma. Those effects seem to be dose-independent as there was no significant difference between different regimen subgroups.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
MSZ, AGE, YAM, and MAB: Conceptualization. MSZ, AAE, and MAB: Methodology. AM, MAZ, DA, NTA, KFA, and KJA: Data collection. ABE, NTA, YSH, AA, HA, MA, AT, and YMH: Screening. ABE, YAM, MAB, ME, ASM, HA, and AAA: Data extraction. AGE, MAZ, ASM, and ME: Risk of bias. MSZ, ME, and AMF: Analysis. AMF, AGE, YAM, AM, DA, KFA, KJA, and MA: Writing - Original Draft Preparation. YSH, AA, AAE, AT, YMH, MMA, and AAA: Writing - Review & Editing. MSZ, AAE, and MMA: Supervision. All authors reviewed the manuscript and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.992731/full#supplementary-material
Arrieta, M-C., Stiemsma, L. T., Dimitriu, P. A., Thorson, L., Russell, S., Yurist-Doutsch, S., et al. (2015). Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 7 (307), 307ra152. doi:10.1126/scitranslmed.aab2271
Bacharier, L. B., Maspero, J. F., Katelaris, C. H., Fiocchi, A. G., Gagnon, R., de Mir, I., et al. (2021). Dupilumab in children with uncontrolled moderate-to-severe asthma. N. Engl. J. Med. 385 (24), 2230–2240. doi:10.1056/NEJMoa2106567
Bachert, C., Han, J. K., Desrosiers, M., Hellings, P. W., Amin, N., Lee, S. E., et al. (2019). Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 394 (10209), 1638–1650. doi:10.1016/S0140-6736(19)31881-1
Barranco, P., Phillips-Angles, E., Dominguez-Ortega, J., and Quirce, S. (2017). Dupilumab in the management of moderate-to-severe asthma: The data so far. Ther. Clin. Risk Manag. 13, 1139–1149. doi:10.2147/TCRM.S125964
Barthel, S. R., Johansson, M. W., McNamee, D. M., and Mosher, D. F. (2008). Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J. Leukoc. Biol. 83 (1), 1–12. doi:10.1189/jlb.0607344
Bellin, M. H., Osteen, P., Kub, J., Bollinger, M. E., Tsoukleris, M., Chaikind, L., et al. (2015). Stress and quality of life in urban caregivers of children with poorly controlled asthma: A longitudinal analysis. J. Pediatr. Health Care. 29 (6), 536–546. doi:10.1016/j.pedhc.2015.04.018
Bhalla, A., Mukherjee, M., Radford, K., Nazy, I., Kjarsgaard, M., Bowdish, D. M. E., et al. (2021). Dupilumab, severe asthma airway responses, and SARS-CoV-2 serology. Allergy 76 (3), 957–958. doi:10.1111/all.14534
Castro, M., Corren, J., Pavord, I. D., Maspero, J., Wenzel, S., Rabe, K. F., et al. (2018). Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N. Engl. J. Med. 378 (26), 2486–2496. doi:10.1056/NEJMoa1804092
Castro, M., Rabe, K. F., Corren, J., Pavord, I. D., Katelaris, C. H., Tohda, Y., et al. (2020). Dupilumab improves lung function in patients with uncontrolled, moderate-to-severe asthma. ERJ Open Res. 6 (1), 00204–02019. doi:10.1183/23120541.00204-2019
Chauhan, B. F., and Ducharme, F. M. (2014). Addition to inhaled corticosteroids of long acting beta 2 agonists versus anti leukotrienes for chronic asthma. Cochrane Database Syst. Rev. (1), CD003137. doi:10.1002/14651858.CD003137.pub5
Chen, Z., Salam, M. T., Alderete, T. L., Habre, R., Bastain, T. M., Berhane, K., et al. (2017). Effects of childhood asthma on the development of obesity among school-aged children. Am. J. Respir. Crit. Care Med. 195 (9), 1181–1188. doi:10.1164/rccm.201608-1691OC
Chibana, K., Trudeau, J. B., Mustovich, A. T., Hu, H., Zhao, J., Balzar, S., et al. (2008). IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin. Exp. Allergy 38 (6), 936–946. doi:10.1111/j.1365-2222.2008.02969.x
Corren, J., Castro, M., O'Riordan, T., Hanania, N. A., Pavord, I. D., Quirce, S., et al. (2020). Dupilumab efficacy in patients with uncontrolled, moderate-to-severe allergic asthma. J. Allergy Clin. Immunol. Pract. 8 (2), 516–526. doi:10.1016/j.jaip.2019.08.050
Corren, J., Katelaris, C. H., Castro, M., Maspero, J. F., Ford, L. B., Halpin, D. M. G., et al. (2021). Effect of exacerbation history on clinical response to dupilumab in moderate-to-severe uncontrolled asthma. Eur. Respir. J. 58 (4), 2004498. doi:10.1183/13993003.04498-2020
Edris, A., De Feyter, S., Maes, T., Joos, G., and Lahousse, L. (2019). Monoclonal antibodies in type 2 asthma: A systematic review and network meta-analysis. Respir. Res. 20 (1), 179. doi:10.1186/s12931-019-1138-3
Eger, K., Hashimoto, S., Braunstahl, G. J., Brinke, A. T., Patberg, K. W., Beukert, A., et al. (2020). Poor outcome of SARS-CoV-2 infection in patients with severe asthma on biologic therapy. Respir. Med. 177, 106287. doi:10.1016/j.rmed.2020.106287
Fahy, J. V. (2015). Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 15 (1), 57–65. doi:10.1038/nri3786
Fleming, L., Murray, C., Bansal, A. T., Hashimoto, S., Bisgaard, H., Bush, A., et al. (2015). The burden of severe asthma in childhood and adolescence: Results from the paediatric U-biopred cohorts. Eur. Respir. J. 46 (5), 1322–1333. doi:10.1183/13993003.00780-2015
Grieco, T., Chello, C., Sernicola, A., Muharremi, R., Michelini, S., Paolino, G., et al. (2021). Impact of COVID-19 on patients with atopic dermatitis. Clin. Dermatol. 39 (6), 1083–1087. doi:10.1016/j.clindermatol.2021.07.008
Harb, H., and Chatila, T. A. (2020). Mechanisms of dupilumab. Clin. Exp. Allergy 50 (1), 5–14. doi:10.1111/cea.13491
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P. T. (2019). Cochrane handbook for systematic reviews of interventions. Second edition. Hoboken, NJ: Wiley-Blackwell.
Jl Murray, C. (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet 396 (10258), 1204–1222. doi:10.1016/S0140-6736(20)30925-9
Klimek, L., Pfaar, O., Worm, M., Eiwegger, T., Hagemann, J., Ollert, M., et al. (2020). Use of biologicals in allergic and type-2 inflammatory diseases during the current COVID-19 pandemic: Position paper of Ärzteverband Deutscher Allergologen (AeDA)(A), Deutsche Gesellschaft für Allergologie und Klinische Immunologie (DGAKI)(B), Gesellschaft für Pädiatrische Allergologie und Umweltmedizin (GPA)(C), Österreichische Gesellschaft für Allergologie und Immunologie (ÖGAI)(D), Luxemburgische Gesellschaft für Allergologie und Immunologie (LGAI)(E), Österreichische Gesellschaft für Pneumologie (ÖGP)(F) in co-operation with the German, Austrian, and Swiss ARIA groups(G), and the European Academy of Allergy and Clinical Immunology (EAACI)(H). Allergol. Sel. 4, 53–68. doi:10.5414/ALX02166E
Laidlaw, T. M., Bachert, C., Amin, N., Desrosiers, M., Hellings, P. W., Mullol, J., et al. (2021). Dupilumab improves upper and lower airway disease control in chronic rhinosinusitis with nasal polyps and asthma. Ann. Allergy Asthma Immunol. 126 (5), 584–592.e1. doi:10.1016/j.anai.2021.01.012
Le Floc’h, A., Allinne, J., Nagashima, K., Scott, G., Birchard, D., Asrat, S., et al. (2020). Dual blockade of IL‐4 and IL‐13 with dupilumab, an IL‐4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy 75 (5), 1188–1204. doi:10.1111/all.14151
McGeachie, M. J. (2017). Childhood asthma is a risk factor for the development of chronic obstructive pulmonary disease. Curr. Opin. Allergy Clin. Immunol. 17 (2), 104–109. doi:10.1097/ACI.0000000000000348
Newman, S. P. (2004). Spacer devices for metered dose inhalers. Clin. Pharmacokinet. 43 (6), 349–360. doi:10.2165/00003088-200443060-00001
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Patruno, C., Stingeni, L., Fabbrocini, G., Hansel, K., and Napolitano, M. (2020). Dupilumab and COVID-19: What should we expect? Dermatol. Ther. 33 (4), e13502. doi:10.1111/dth.13502
Rabe, K. F., Nair, P., Brusselle, G., Maspero, J. F., Castro, M., Sher, L., et al. (2018). Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N. Engl. J. Med. 378 (26), 2475–2485. doi:10.1056/NEJMoa1804093
Reddel, H. K., Bacharier, L. B., Bateman, E. D., Brightling, C. E., Brusselle, G. G., Buhl, R., et al. (2022). Global initiative for asthma strategy 2021: Executive summary and rationale for key changes. Eur. Respir. J. 59 (1), 2102730. doi:10.1183/13993003.02730-2021
Robinson, D. S., Hamid, Q., Ying, S., Tsicopoulos, A., Barkans, J., Bentley, A. M., et al. (1992). Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 326 (5), 298–304. doi:10.1056/NEJM199201303260504
Steinke, J. W. B. L., and Borish, L. (2001). Th2 cytokines and asthma. Interleukin-4: Its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir. Res. 2 (2), 66–70. doi:10.1186/rr40
Tagiyeva, N. D. G., Fielding, S., Turner, S., and Douglas, G. (2016). Outcomes of childhood asthma and wheezy bronchitis. A 50-year cohort study. Am. J. Respir. Crit. Care Med. 193, 23–30. doi:10.1164/rccm.201505-0870OC
Tanabe, N., Matsumoto, H., Hamada, S., Ito, I., and Hirai, T. (2021). Dupilumab maintenance therapy in an asthmatic patient with coronavirus disease 2019 pneumonia. Allergol. Int. 70 (2), 274–276. doi:10.1016/j.alit.2020.10.005
Tohda, Y., Nakamura, Y., Fujisawa, T., Ebisawa, M., Arima, K., Miyata, M., et al. (2020). Dupilumab efficacy and safety in Japanese patients with uncontrolled, moderate-to-severe asthma in the phase 3 LIBERTY ASTHMA QUEST study. Allergol. Int. 69 (4), 578–587. doi:10.1016/j.alit.2020.04.002
Tozawa, H., Kanki, Y., Suehiro, J., Tsutsumi, S., Kohro, T., Wada, Y., et al. (2011). Genome-wide approaches reveal functional interleukin-4-inducible STAT6 binding to the vascular cell adhesion molecule 1 promoter. Mol. Cell. Biol. 31 (11), 2196–2209. doi:10.1128/MCB.01430-10
Ungar, B., Lavin, L., Golant, A. K., Gontzes, A., David, E., Estrada, Y. D., et al. (2022). The impact of dupilumab treatment on severe acute respiratory syndrome coronavirus 2-coronavirus disease 2019 antibody responses in patients with atopic dermatitis. Ann. Allergy Asthma Immunol. 128 (6), 734–736. doi:10.1016/j.anai.2022.03.019
Ungar, B., Glickman, J. W., Golant, A. K., Dubin, C., Marushchak, O., Gontzes, A., et al. (2022). COVID-19 symptoms are attenuated in moderate-to-severe atopic dermatitis patients treated with dupilumab. J. Allergy Clin. Immunol. Pract. 10 (1), 134–142. doi:10.1016/j.jaip.2021.10.050
Wechsler, M. E., Ruddy, M. K., Pavord, I. D., Israel, E., Rabe, K. F., Ford, L. B., et al. (2021). Efficacy and safety of itepekimab in patients with moderate-to-severe asthma. N. Engl. J. Med. 385 (18), 1656–1668. doi:10.1056/NEJMoa2024257
Weinstein, S. F., Katial, R., Jayawardena, S., Pirozzi, G., Staudinger, H., Eckert, L., et al. (2018). Efficacy and safety of dupilumab in perennial allergic rhinitis and comorbid asthma. J. Allergy Clin. Immunol. 142 (1), 171–177.e1. doi:10.1016/j.jaci.2017.11.051
Wenzel, S., Ford, L., Pearlman, D., Spector, S., Sher, L., Skobieranda, F., et al. (2013). Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 368 (26), 2455–2466. doi:10.1056/NEJMoa1304048
Wenzel, S., Castro, M., Corren, J., Maspero, J., Wang, L., Zhang, B., et al. (2016). Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 388 (10039), 31–44. doi:10.1016/S0140-6736(16)30307-5
Wu, T. D., Brigham, E. P., and McCormack, M. C. (2019). Asthma in the primary care setting. Med. Clin. North Am. 103 (3), 435–452. doi:10.1016/j.mcna.2018.12.004
Xiong, X. F., Zhu, M., Wu, H. X., Fan, L. L., and Cheng, D. Y. (2019). Efficacy and safety of dupilumab for the treatment of uncontrolled asthma: A meta-analysis of randomized clinical trials. Respir. Res. 20 (1), 108. doi:10.1186/s12931-019-1065-3
Keywords: asthma, dupilumab, monoclonal antibody, systematic review, meta-analysis
Citation: Zaazouee MS, Alwarraqi AG, Mohammed YA, Badheeb MA, Farhat AM, Eleyan M, Morad A, Zeid MA-A, Mohamed AS, AbuEl-Enien H, Abdelalim A, Elsnhory AB, Hrizat YSM, Altahir NT, Atef D, Elshanbary AA, Alsharif KF, Alzahrani KJ, Algahtani M, Theyab A, Hawsawi YM, Aldarmahi AA and Abdel-Daim MM (2022) Dupilumab efficacy and safety in patients with moderate to severe asthma: A systematic review and meta-analysis. Front. Pharmacol. 13:992731. doi: 10.3389/fphar.2022.992731
Received: 12 July 2022; Accepted: 11 August 2022;
Published: 03 October 2022.
Edited by:
Ahmed Esmat Abdel Moneim, Helwan University, EgyptReviewed by:
Ashraf Abdel-Naim, Ain Shams University, EgyptCopyright © 2022 Zaazouee, Alwarraqi, Mohammed, Badheeb, Farhat, Eleyan, Morad, Zeid, Mohamed, AbuEl-Enien, Abdelalim, Elsnhory, Hrizat, Altahir, Atef, Elshanbary, Alsharif, Alzahrani, Algahtani, Theyab, Hawsawi, Aldarmahi and Abdel-Daim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed Sayed Zaazouee, TW9oYW1lZFphYXpvdWVlLnN0dS42LjQ0QGF6aGFyLmVkdS5lZw==, b3JjaWQub3JnLzAwMDAtMDAwMy0wOTA0LTkxNTM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.