- 1Department of Interventional Radiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Interventional Radiology, Jingzhou Central Hospital, The Second Clinical Medical College of Yangtze University, Jingzhou, China
Aim: Drug-eluting beads transarterial chemoembolization (DEB-TACE) has not been widely used in patients with advanced and inoperable lung cancer. We aimed to report the preliminary outcomes of DEB-TACE with gemcitabine-loaded CalliSpheres beads for patients with advanced and inoperable lung cancer.
Methods: From November 2017 to October 2021, 37 patients (29 males, mean age 64.7 ± 10.3 years) with advanced and inoperable lung cancer underwent DEB-TACE with gemcitabine-loaded CalliSpheres beads. The primary endpoint was overall response rate, and the secondary endpoints were overall survival and progression-free survival.
Results: A total of 54 sessions of DEB-TACE were performed in 37 patients, with a technique success rate of 100%. Fourteen patients received a second session of DEB-TACE. The mean follow-up time was 18.7 ± 11.9 months. After 1, 3, and 6 months, overall response rate and disease control rate were 27.8% and 91.7%, 25.8% and 74.2%, 32.1%, and 67.9%, respectively. The median progression-free survival was 8.8 months (95% CI 7.5, 12.5 months). The 3-, 6- and 12- month progression-free survival rates were 67.1%, 57.0%, and 30.1%, respectively. The median overall survival was 10.0 months (95% CI 4.5, 13.1 months). The 3-, 6-, and 12- month overall survival rates were 88.5%, 72.7%, and 40.9%, respectively. Minor complications were observed in 14 patients (37.8%), with no procedure-related deaths or severe adverse events.
Conclusion: DEB-TACE with gemcitabine-loaded CalliSpheres beads is a safe, feasible and effective treatment strategy for patients with advanced and inoperable lung cancer.
Introduction
Lung cancer is a common leading cause of deaths worldwide. Platinum-containing regimens (Schiller et al., 2002) or non-platinum-containing regimen (Pujol et al., 2005) are recommended in the treatment of advanced non-small-cell lung cancer (NSCLC). Transarterial chemoembolization (TACE) has been used for locally advanced non-small cell lung cancer after failure of concurrent chemoradiotherapy (Chen et al., 2020). As a novel palliative treatment, drug-eluting beads TACE (DEB-TACE) can not only embolize tumor supply vessels, but also slowly release the loaded chemotherapeutic drugs (Bi et al., 2021a). CalliSpheres bead is the first DEB product made in China. DEB-TACE with CalliSpheres beads has been widely used for advanced and inoperable hepatocellular carcinoma (Zhou et al., 2018; Xiang et al., 2019) and sometimes used for advanced or recurrent cervical cancer (Bi et al., 2021d) and so on (Bi et al., 2021b; a; Bi et al., 2022a; Bi et al., 2022b). It is reported that DEB-TACE using CalliSpheres beads is efficient and well tolerated in patients with hepatocellular carcinoma, which achieves better progression-free survival and treatment response while equal safety compared to conventional TACE (Zhou et al., 2018; Xiang et al., 2019).
DEB-TACE with pirarubicin-loaded CalliSpheres (Shang et al., 2020; Bi et al., 2021c; Lin et al., 2021) or cisplatin-loaded HepaSphere (Yu and Hu, 2020) has been used for advanced lung cancer. Currently, few studies reported the clinical outcomes of gemcitabine-loaded CalliSpheres beads for advanced and inoperable lung cancer. In a previous case report (Bie et al., 2019), DEB-TACE loaded with gemcitabine (800 mg) using CalliSpheres beads and transarterial infusion with nedaplatin (80–100 mg) was performed in only 6 patients. However, pirarubicin or nedaplatin are not standard regimens for advanced lung cancer (Schiller et al., 2002). In this current study, we performed DEB-TACE using gemcitabine-loaded CalliSpheres beads and transarterial infusion with cisplatin/carboplatin or docetaxel for the treatment of advanced and inoperable lung cancer, and we aimed to assess its safety and clinical efficacy.
Patients and methods
Study design
Ethical approval was waived by the Institutional Review Board of University due to its retrospective characteristics. Written informed consent was obtained from all patients. This current retrospective study enrolled 37 patients with advanced and inoperable lung cancer who received DEB-TACE with gemcitabine-loaded CalliSpheres beads from November 2017 to October 2021. Indications for DEB-TACE: age range from 18 to 85 years; pathological diagnosis of lung cancer, local progression after standard treatments; ineligible or refused to receive standard treatments due to emphysema, heart disease, or severe pulmonary fibrosis; without other life-threatening diseases. Exclusion criteria: combined with other malignant tumor but with no curative treatment; lower white blood cell count (< 3.0 × 109/L) or lower platelets (< 40.0 × 109/L); severe infection; pregnant or breastfeeding women. DEB-TACE treatment for lung cancer patients is discussed and individually tailored. The final choice of DEB-TACE strategy needs to consider the factors such as the treatment willingness and economic ability of the patients and their families.
Data collection
Patients’ data were retrospectively collected and analyzed, such as clinical/demographic features, Eastern Cooperative Oncology Group (ECOG) performance status, the tumor-node-metastasis (TNM) classification, illness history, location and size of tumor, DEB-TACE procedure, tumor markers, adverse events, data of computed tomography, and so on (Figures 2A–C; Figures 3A,B; Figures 4A,B). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines a standard for pulmonary severity levels based on the predicted ratio of forced expiratory volume in one second (FEV1%) values (Rabe et al., 2007).
CalliSpheres beads
A CalliSpheres bead (Jiangsu Hengrui Medicine Co. Ltd. Jiangsu, China) is a type of ion-exchange bead with some negatively charged functional groups, which are responsible for the loading of some positively charged drugs (Zhang et al., 2017). It is a blue polyvinyl alcohol particles beads with a smooth surface, uniform size, and usually 100–300 μm or 300–500 μm in diameter. About 7 ml of physiological sodium chloride solution and 2 ml beads were kept in bottles with no pharmacological agent (Figure 1). The pharmacological agent are loaded extemporaneously about 30 min before DEB-TACE.

FIGURE 1. Photograph of CalliSpheres beads. It is a blue polyvinyl alcohol particles beads with a smooth surface, uniform size, and usually100–300 μm or 300–500 μm in diameter. About 7 ml of physiological sodium chloride solution and 2 ml beads were kept in bottles with no pharmacological agent.
DEB-TACE procedures
Under local anesthesia, the right femoral artery was accessed and a 5F-Cobra catheter (Terumo, Japan) was introduced. Tumor-feeding vessels were catheterized and embolized, including bronchial arteries, internal thoracic arteries, phrenic arteries. A 2.7-F microcatheter (Progreat, Terumo, Japan) was used for super-selection of all tumor-feeding arteries. Cisplatin or carboplatin was initially infused for patients with no previous platinum-based chemotherapy. Docetaxel was infused for patients receiving non-platinum-containing regimen. Gemcitabine (0.4–1.0 g) was pre-loaded with CalliSpheres beads for 30 min, and then mixed with iodixanol (ratio 1:1) as contrast developer before embolization. CalliSpheres beads were slowly injected into the tumor-feeding vessels under fluoroscopic guidance. One vial of CalliSpheres beads embolization was used for each patient, additional embolization was performed by using polyvinyl alcohol particles (Merit, American) or gelatin sponge particles if embolization is insufficient (Figure 2D; Figures 3C,D; Figures 4C,E).

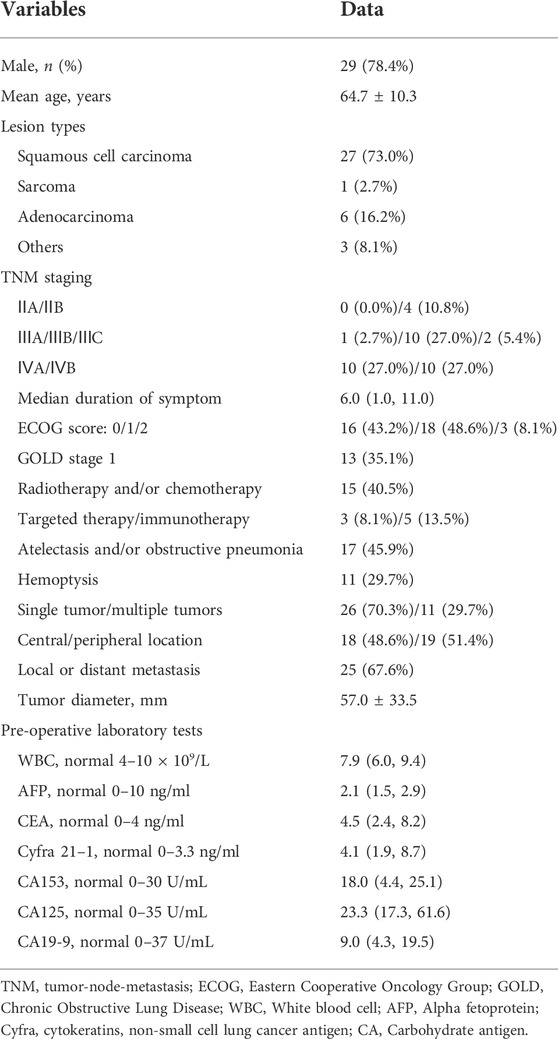
FIGURE 2. A 65-years male treated by CalliSpheres beads for advanced squamous cell carcinoma. (A) Computed tomography examination on admission revealed malignant tumor, severe bronchial stenosis and complete atelectasis of right lung. (B) Fibrobronchoscopy revealed a right main bronchial occlusion. (C) Pathological diagnosis of squamous cell carcinoma by biopsy of the lesion. (D) The right bronchial artery was the blood supply artery of the tumor, and was embolized by drug-loaded beads. (E) The right lung tumor was found to shrink after 1.5 months’ follow-up. (F) The airway stent was in good position and the tumor was almost disappeared after 1 year.
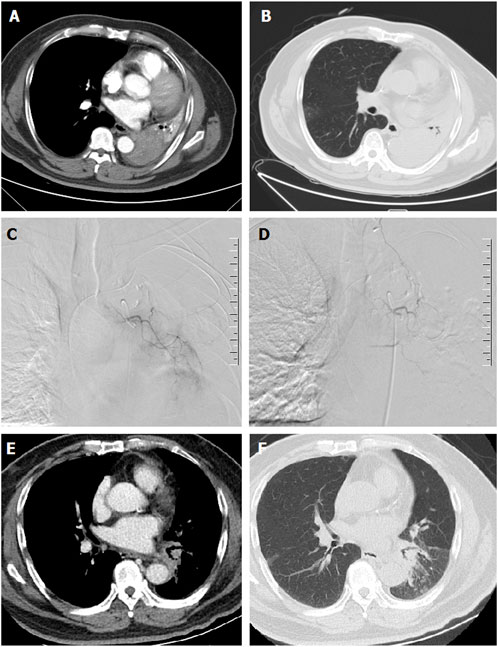
FIGURE 3. A 67-years male treated by CalliSpheres beads for advanced squamous cell carcinoma. (A,B) Computed tomography preoperative examination revealed a large tumor of the left lung with complete atelectasis. (C,D) The left bronchial artery was super-selectively incubated and embolized by drug-loaded beads. (E,F) The left lung tumor was found to shrink and the left lung atelectasis disappeared after 2 weeks’ follow-up.
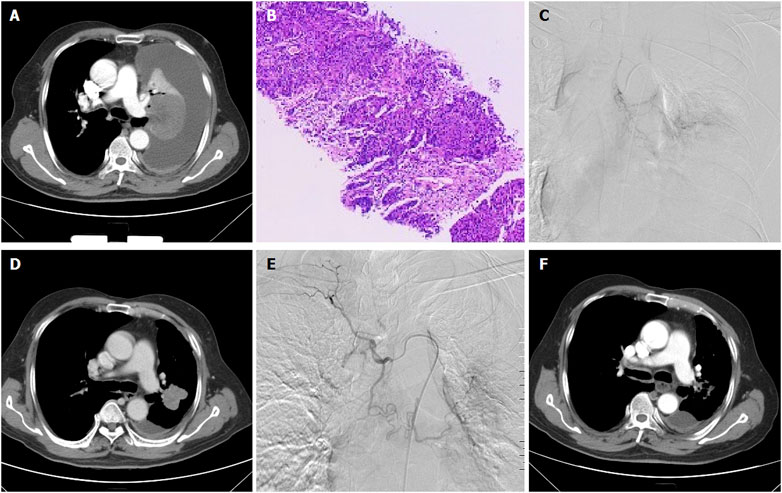
FIGURE 4. A 66-years male treated by CalliSpheres beads for advanced squamous cell carcinoma in left lung. (A) Computed tomography examination before procedure revealed malignant central tumor of left lung, with complete atelectasis and massive pleural effusion. (B) Pathological diagnosis of squamous cell carcinoma by biopsy of the lesion. (C) The left lung tumor was treated by CalliSpheres beads. (D) The left lung tumor shrunk and atelectasis disappeared 1 month after the first session of DEB-TACE. (E) The left tumor was treated again by drug-loaded beads 1 month later. (F) The tumor was almost disappeared 3 months after first DEB-TACE.
Endpoint
The primary endpoint was overall response rate, which was assessed by chest computed tomography before procedure and during follow up. The overall response rate was defined as the sum of complete response and partial response. Disease control rate was calculated as the sum of complete response, partial response and stable disease. The secondary endpoints were overall survival and progression-free survival.
Safety assessment
According to the Common Terminology Criteria for Adverse Events (CTCAE) (version 4.0) (National Cancer Institute NIH, US Department of Health and Human Services, 2009), serious adverse events and adverse events were recorded perioperatively and during the follow up.
Follow-up
Patients were followed up by chest computed tomography about 1, 3 and 6 months after DEB-TACE (Figures 2E,F; Figures 3E,F; Figures 4D,F). Patients were followed up by phone calls, and the last follow-up date was 17 November 2021.
Results
Patient characteristics
A total of 37 patients were enrolled in this study, including 29 males and 8 females (mean age 64.7 ± 10.3 years, range 38–83 years). As shown in Table 1, 27 patients (73.0%) were diagnosed with squamous cell carcinoma and 6 patients (16.2%) were adenocarcinoma. Local or distant metastases were present in 25 patients (67.6%). Atelectasis and/or obstructive pneumonia were found in 17 patients (45.9%). Five patients showed symptomatic relief in atelectasis and/or obstructive pneumonia about 1–2 months after DEB-TACE. Twenty-eight patients complained of cough or expectoration, and median duration of symptom was 6.0 months. Sixteen patients showed symptomatic relief in cough or expectoration about 1–2 months after DEB-TACE. A total of 16 patients (43.2%) received other synchronous treatments, such as radiotherapy and/or chemotherapy (n = 15), targeted therapy (n = 3) or immunotherapy (n = 5). There were 13 GOLD one patients, and the remained 24 patients showed normal pulmonary function.
DEB-TACE treatments
A total of 54 sessions of DEB-TACE were performed in 37 patients, and 14 patients received a second session of DEB-TACE, with a mean of 1.5 ± 0.7 sessions per patient. All DEB-TACE procedures were successfully performed, with a technique success rate of 100%. Fourteen Patients were all treated with gemcitabine-loaded DEB-TACE. The median dose of gemcitabine was 0.5 ± 0.2 g. A total of 98 tumor supply vessels were embolized, including 62 bronchial arteries (double bronchial arteries in 11 sessions), 27 internal thoracic arteries, three phrenic arteries and 6 other arteries. Except for one session of DEB-TACE with CalliSpheres beads of 100–300 μm, the remaining sessions of DEB-TACE was performed by using CalliSpheres beads of 300–500 μm in diameter. All patients received a bottle of beads, 16 of whom (43.2%) received additional embolization. Polyvinyl alcohol or gelatin sponge particles of 350–560 μm were used in 10 and 5 patients after DEB-TACE. Thirteen patients (35.1%) received other treatments, of which, 6 patients underwent airway stent placement due to malignant airway stricture. Eight patients underwent 125I seeds implantation and two patients underwent upper vena cava stenting. The median inpatient duration was 13.0 days and the mean cost of hospitalizations was 6.8 × 104 Renminbi (Table 2).
Endpoint
Only one patient was lost to follow-up, the median follow-up time was 18.7 ± 11.9 months. As shown in Table 3, overall response rate and disease control rate were 27.8%, 91.7%, 25.8%, 74.2%, 32.1%, and 67.9% respectively at 1, 3, and 6 months after DEB-TACE. The median progression-free survival was 8.8 months (95%CI 7.5, 12.5 months). The 3-, 6- and 12 months progression-free survival rates were 67.1%, 57.0%, and 30.1%, respectively. The median overall survival was 10.0 months (95%CI 4.5, 13.1 months). The 3-, 6 and 12 months overall survival rates were 88.5%, 72.7%, and 40.9%, respectively (Figure 5).
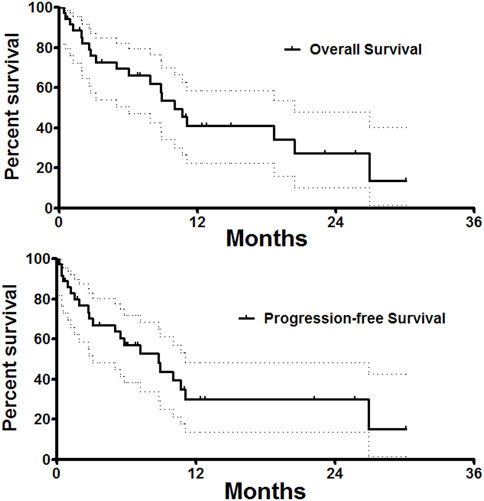
FIGURE 5. Follow up. Median overall survival and progression-free survival was 10.0 and 8.8 months. The 3-, 6 and 12 months overall survival rates were 88.5%, 72.7%, and 40.9%, respectively.
Safety
Minor complications were observed in 14 patients (37.8%), with no severe adverse events, such as procedure-related deaths, nontarget embolization, catheter-related adverse events, or massive bleeding. Minor complications were observed in 14 patients (37.8%). Only one patient (2.7%) had angina pectoris attacks about half an hour after the second session of DEB-TACE procedure. This patient had a history of hypertension for many years, a history of coronary heart disease bypass surgery, and had acute myocardial infarction more than 1 month ago. It showed decreased oxygen saturation, increased heart rate and increased blood pressure, and symptoms relieved the symptoms after antihypertensive, diuretic and dexamethasone. Five patients showed nausea and/or vomiting and seven patients (18.9%) had mild chest pain, those patients were well controlled within 2–3 days.
Discussion
Platinum-containing regimens (cisplatin + paclitaxel, cisplatin + gemcitabine, cisplatin + docetaxel, or carboplatin + paclitaxel) (Schiller et al., 2002) or non-platinum-containing regimen (gemcitabine–docetaxel combination) (Pujol et al., 2005) are recommended in the treatment of advanced NSCLC. According to the National Comprehensive Cancer Network (NCCN) Guidelines Insights (Ettinger et al., 2021), several new targeted therapies or new indications for therapies, including capmatinib, lorlatinib, pralsetinib, selpercatinib, and fam-trastuzumab deruxtecan, are now recommended for eligible patients with metastatic NSCLC who have certain actionable biomarkers. Lorlatinib is recommended as another preferred first-line therapy option for patients with ALK rearrangement–positive metastatic NSCLC. Entrectinib is recommended as a subsequent therapy option for patients with ROS1-positive disease who have central nervous system progression after crizotinib.
As a novel delivery and embolization system, DEB-TACE can not only embolize tumor-feeding vessels to block the blood supply of lung cancer, but also increase the retention time and drug concentration of antitumor drugs by mean of slow release. Thus, DEB-TACE can theoretically both increase effectiveness of tumor necrosis and reduce systemic drug concentration and adverse events (Kunisada et al., 2013; Choi et al., 2014; Mikhail et al., 2018). Yu and Hu (2020) performed DEB-TACE with cisplatin-loaded HepaSphere and concurrent transarterial infusion with docetaxel (120 mg) for a 69-year-old male with locally advanced squamous non-small cell lung cancer. DEB-TACE with pirarubicin-loaded CalliSpheres beads have been used for advanced lung cancer (Shang et al., 2020; Bi et al., 2021c; Lin et al., 2021); however, pirarubicin is not standard regimens (Schiller et al., 2002). Nowadays, few studies reported the clinical outcomes of gemcitabine-loaded CalliSpheres beads for advanced and inoperable lung cancer. In a previous case report of Bie et al. (2019), DEB-TACE was performed with gemcitabine (800 mg) loading CalliSpheres beads and transarterial infusion with nedaplatin (80–100 mg). However, only 6 patients received DEB-TACE and nedaplatin is not standard regimens for advanced and inoperable lung cancer (Schiller et al., 2002).
In this current study, we performed DEB-TACE using gemcitabine-loaded CalliSpheres beads and transarterial infusion with cisplatin/carboplatin or docetaxel for the treatment of advanced and inoperable lung cancer. After 1, 3, and 6 months, overall response rate and disease control rate were 27.8%, 91.7%, 25.8%, 74.2%, 32.1%, and 67.9%, respectively. The median progression-free survival and the 6-months progression-free survival rate after DEB-TACE were 8.8 and 57.0%, respectively. Our data was similar to that of DEB-TACE with gemcitabine-loaded CalliSpheres beads in a previous case report (Bie et al., 2019). The median progression-free survival was 8.0 months and the 6-months progression-free survival rate was 66.7%. Our preliminary data indicated that DEB-TACE with gemcitabine-loaded CalliSpheres beads was associated with good disease control during a short-term follow-up period.
A larger tumor volume and failure previous systematic treatments may predict a poorer therapeutic response to DEB-TACE, and a combination of other therapies can improve the disease prognosis (Bie et al., 2019). For example, a combined radioactive iodine-125 implantation was associated with a better prognosis (Yang et al., 2016; Chen et al., 2017). In this study, 8 patients received 125I seeds implantation after progressive disease. Besides, it is also important to treat severe complications caused by tumor progression, such as severe airway stenosis (Bi et al., 2020). In this study, three patients underwent airway stenting and two patients underwent upper vena cava stenting.
Regarding the safety, no serious adverse events or procedure-related death were observed in this study. Minor complications were observed in 14 patients (37.8%). Only one patient (2.7%) had angina pectoris attacks after procedure. The symptoms relieved after antihypertensive, diuretic and dexamethasone. DEB-TACE using gemcitabine loaded CalliSpheres beads appear to be safe for patients with advanced and inoperable lung cancer.
There are some limitations. This is a retrospective observational study performed in a single center. There is more than one medication regimen, which is not conducive to drawing a clear conclusion. The limited sample size does not allow for statistically powered subgroup analysis. More studies are wanted to verify the safety and efficacy. In conclusion, DEB-TACE with gemcitabine-loaded CalliSpheres beads is safe, feasible and well-tolerated for patients with advanced and inoperable lung cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer [WZ] declared a shared affiliation with the authors [YB, JR, and XH] to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bi, Y., Shi, X., Ren, J., Yi, M., Han, X., and Song, M. (2021a). Clinical outcomes of doxorubicin-eluting CalliSpheres® beads-transarterial chemoembolization for unresectable or recurrent esophageal carcinoma. BMC Gastroenterol. 21 (1), 231. doi:10.1186/s12876-021-01816-3
Bi, Y., Shi, X., Ren, J., Yi, M., Han, X., and Song, M. (2021b). Transarterial chemoembolization with doxorubicin-loaded beads for inoperable or recurrent colorectal cancer. Abdom. Radiol. 46, 2833–2838. doi:10.1007/s00261-020-02877-w
Bi, Y., Shi, X., Ren, J., Yi, M., and Han, X. (2022a). Transarterial chemoembolization of unresectable renal cell carcinoma with doxorubicin-loaded CalliSpheres drug-eluting beads. Sci. Rep. 12 (1), 8136. doi:10.1038/s41598-022-12334-x
Bi, Y., Shi, X., Yi, M., Han, X., and Ren, J. (2021c). Pirarubicin-loaded CalliSpheres® drug-eluting beads for the treatment of patients with stage III–IV lung cancer. Acta Radiol. 63, 311–318. doi:10.1177/0284185121994298
Bi, Y., Shi, X., Zhang, W., Lu, H., Han, X., and Ren, J. (2022b). Drug-eluting embolics chemoembolization for the management of recurrent or advanced head and neck cancer. J. Vasc. Interv. Radiol. 33, 949–955. doi:10.1016/j.jvir.2022.05.002
Bi, Y., Wang, Y., Zhang, J., Shi, X., Wang, Y., Xu, M., et al. (2021d). Clinical outcomes of uterine arterial chemoembolization with drug-eluting beads for advanced-stage or recurrent cervical cancer. Abdom. Radiol. 46, 5715–5722. doi:10.1007/s00261-021-03267-6
Bi, Y., Zhu, X., Yu, Z., Yi, M., Han, X., and Ren, J. (2020). Clinical outcomes of self-expandable metallic stents for malignant obstructive atelectasis. Sci. Rep. 10 (1), 3600. doi:10.1038/s41598-020-60566-6
Bie, Z., Li, Y., Li, B., Wang, D., Li, L., and Li, X. (2019). The efficacy of drug-eluting beads bronchial arterial chemoembolization loaded with gemcitabine for treatment of non-small cell lung cancer. Thorac. Cancer 10 (9), 1770–1778. doi:10.1111/1759-7714.13139
Chen, C., Wang, W., Yu, Z., Tian, S., Li, Y., and Wang, Y. (2020). Combination of computed tomography-guided iodine-125 brachytherapy and bronchial arterial chemoembolization for locally advanced stage III non-small cell lung cancer after failure of concurrent chemoradiotherapy. Lung Cancer 146, 290–296. doi:10.1016/j.lungcan.2020.06.010
Chen, Y., Li, Y., Jia, Y., Lei, K., Zhang, X., Cao, Y., et al. (2017). Bronchial artery chemoembolization combined with radioactive iodine-125 seed implantation in the treatment of advanced nonsmall cell lung cancer. J. Cancer Res. Ther. 13 (4), 636–641. doi:10.4103/jcrt.JCRT_93_17
Choi, J. W., Cho, H. J., Park, J. H., Baek, S. Y., Chung, J. W., Kim, D. D., et al. (2014). Comparison of drug release and pharmacokinetics after transarterial chemoembolization using diverse lipiodol emulsions and drug-eluting beads. PLoS One 9 (12), e115898. doi:10.1371/journal.pone.0115898
Ettinger, D. S., Wood, D. E., Aisner, D. L., Akerley, W., Bauman, J. R., Bharat, A., et al. (2021). NCCN Guidelines Insights: Non-small cell lung cancer, version 2.2021. J. Natl. Compr. Canc. Netw. 19 (3), 254–266. doi:10.6004/jnccn.2021.0013
Kunisada, K., Ishikawa, H., Takafuji, J., and Komuta, K. (2013). A case of a 91-year-old patient with advanced squamous cell lung cancer complicated with renal dysfunction successfully treated with trans-arterial chemo-embolization. Gan Kagaku Ryoho. 40 (7), 917–919.
Lin, H., Wang, Q., Tian, F., Zhang, R., Mu, M., Zhao, W., et al. (2021). Drug-eluting beads bronchial arterial chemoembolization in treating relapsed/refractory small cell lung cancer patients: Results from a pilot study. Cancer Manag. Res. 13, 6239–6248. doi:10.2147/CMAR.S310115
Mikhail, A. S., Pritchard, W. F., Negussie, A. H., Krishnasamy, V. P., Amchin, D. B., Thompson, J. G., et al. (2018). Mapping drug dose distribution on CT images following transarterial chemoembolization with radiopaque drug-eluting beads in a rabbit tumor model. Radiology 289 (2), 396–404. doi:10.1148/radiol.2018172571
National Cancer Institute NIH, US Department of Health and Human Services (2009). Common terminology criteria for adverse events version 4.0. Available at: http://ctep.cancer.gov/protocol Development/electronic_applications/ctc.htm (Accessed May 28, 2009).
Pujol, J. L., Breton, J. L., Gervais, R., Rebattu, P., Depierre, A., Morere, J. F., et al. (2005). Gemcitabine-docetaxel versus cisplatin-vinorelbine in advanced or metastatic non-small-cell lung cancer: A phase III study addressing the case for cisplatin. Ann. Oncol. 16 (4), 602–610. doi:10.1093/annonc/mdi126
Rabe, K. F., Hurd, S., Anzueto, A., Barnes, P. J., Buist, S. A., Calverley, P., et al. (2007). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 176 (6), 532–555. doi:10.1164/rccm.200703-456SO
Schiller, J. H., Harrington, D., Belani, C. P., Langer, C., Sandler, A., Krook, J., et al. (2002). Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl. J. Med. 346 (2), 92–98. doi:10.1056/NEJMoa011954
Shang, B., Li, J., Wang, X., Li, D., Liang, B., Wang, Y., et al. (2020). Clinical effect of bronchial arterial infusion chemotherapy and CalliSpheres drug-eluting beads in patients with stage II-IV lung cancer: A prospective cohort study. Thorac. Cancer 11 (8), 2155–2162. doi:10.1111/1759-7714.13522
Xiang, H., Long, L., Yao, Y., Fang, Z., Zhang, Z., and Zhang, Y. (2019). CalliSpheres drug-eluting bead transcatheter arterial chemoembolization presents with better efficacy and equal safety compared to conventional TACE in treating patients with hepatocellular carcinoma. Technol. Cancer Res. Treat. 18, 1533033819830751. doi:10.1177/1533033819830751
Yang, X. G., Wu, G., Li, Z. W., Wu, H., Sun, Y. Y., Wen, H. H., et al. (2016). Efficacy for artery chemoembolization combined with radiofrequency ablation in the treatment of advanced non-small cell lung cancer. Zhonghua Yi Xue Za Zhi 96 (7), 539–543. doi:10.3760/cma.j.issn.0376-2491.2016.07.010
Yu, G., and Hu, J. (2020). Drug-eluting beads bronchial arterial chemoembolization as a neoadjuvant treatment for squamous non-small cell lung cancer. Postgrad. Med. 132 (6), 568–571. doi:10.1080/00325481.2020.1761711
Zhang, S., Huang, C., Li, Z., Yang, Y., Bao, T., Chen, H., et al. (2017). Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads in rabbit livers. Drug Deliv. 24 (1), 1011–1017. doi:10.1080/10717544.2017.1344336
Keywords: lung cancer, drug-eluting beads transarterial chemoembolization (DEB-TACE), callispheres beads, gemcitabine, transarterial chemoembolization (TACE)
Citation: Bi Y, Zhang B, Ren J, Han X and Wu W (2022) Clinical outcomes of gemcitabine-loaded callispheres drug-eluting beads for patients with advanced and inoperable lung cancer: A case series study. Front. Pharmacol. 13:992526. doi: 10.3389/fphar.2022.992526
Received: 12 July 2022; Accepted: 13 September 2022;
Published: 29 September 2022.
Edited by:
Andrea Messori, Regione Toscana, ItalyReviewed by:
Guanhui Zhou, Zhejiang University, ChinaWenguang Zhang, First Affiliated Hospital of Zhengzhou University, China
Weichieh Lee, Chi Mei Medical Center, Taiwan
Copyright © 2022 Bi, Zhang, Ren, Han and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinwei Han, ZHJlYW13ZWF2ZXIwOEAxMjYuY29t; Wenze Wu, MTAyOTE5MzE5MUBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
 Yonghua Bi
Yonghua Bi Bo Zhang2†
Bo Zhang2† Xinwei Han
Xinwei Han