
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 19 September 2022
Sec. Drug Metabolism and Transport
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.991879
 Yinchen Shen1,2,3,4,5
Yinchen Shen1,2,3,4,5 Hanying Wang1,2,3,4,5
Hanying Wang1,2,3,4,5 Xiaoyin Xu1,2,3,4,5
Xiaoyin Xu1,2,3,4,5 Chong Chen1,2,3,4,5
Chong Chen1,2,3,4,5 Shaopin Zhu1,2,3,4,5
Shaopin Zhu1,2,3,4,5 Lu Cheng1,2,3,4,5
Lu Cheng1,2,3,4,5 Junwei Fang1,2,3,4,5*
Junwei Fang1,2,3,4,5* Kun Liu1,2,3,4,5*
Kun Liu1,2,3,4,5* Xun Xu1,2,3,4,5
Xun Xu1,2,3,4,5Background: Neovascular age-related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV) are major causes of blindness in aged people. 30% of the patients show unsatisfactory response to anti-vascular endothelial growth factor (anti-VEGF) drugs. This study aims to investigate the relationship between serum metabolome and treatment response to anti-VEGF therapy.
Methods: A prospective longitudinal study was conducted between March 2017 and April 2019 in 13 clinical sites in China. The discovery group were enrolled from Shanghai General Hospital. The validation group consisted of patients from the other 12 sites. Participants received at least one intravitreal injection of 0.5 mg anti-VEGF drug, conbercept, and were divided into two groups - responders and non-responders. Serum samples of both groups were processed for UHPLC-MS/MS analysis. We constructed principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) models to investigate the metabolic differences between two groups using SIMCA-P. Area under curve (AUC) was calculated to screen the biomarkers to predict treatment response. Metabolites sub-classes and enriched pathways were obtained using MetaboAnalyst5.0.
Results: 219 eyes from 219 patients (nAMD = 126; PCV = 93) were enrolled. A total of 248 metabolites were detected. PCA and PLS-DA models of the discovery group demonstrated that the metabolic profiles of responders and non-responders clearly differed. Eighty-five differential metabolites were identified, including sub-classes of diacylglycerophosphocholines, lysophosphatidylcholine (LPC), fatty acids, phosphocholine, etc. Responders and non-responders differed most significantly in metabolism of LPC (p = 7.16 × 10^-19) and diacylglycerophosphocholine (p = 6.96 × 10^-17). LPC 18:0 exhibited the highest AUC, which is 0.896 with 95% confidence internal between 0.833 and 0.949, to discriminate responders. The predictive accuracy of LPC 18:0 was 72.4% in the validation group.
Conclusions: This study suggests that differential metabolites may be useful for guiding treatment options for nAMD and PCV. Metabolism of LPC and diacylglycerophosphocholine were found to affect response to conbercept treatment. LPC 18:0 was a potential biomarker to discriminate responders from non-responders.
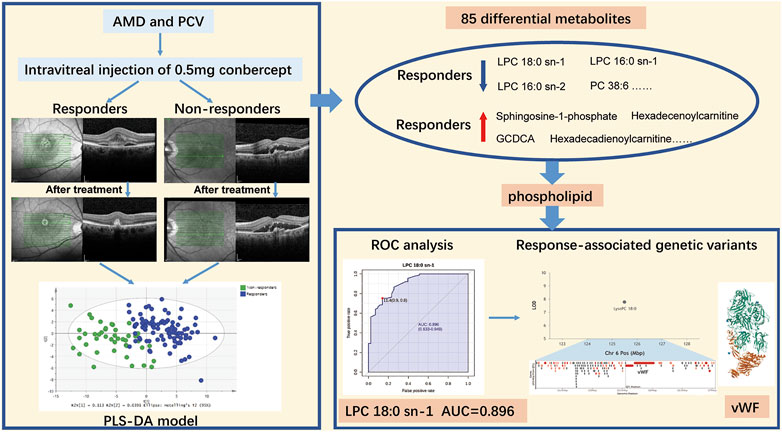
GRAPHICAL ABSTRACTAMD, age-related macular degeneration; PCV, polypoidal choroidal vasculopathy; PLS-DA, partial least squares discriminant analysis; LPC, lysophosphatidylcholine; PC, phosphocholine; ROC, operating characteristic curve; AUC, area under the receiver operating characteristic curve; vWF, von Willebrand Factor. Note: The protein structure of vWF is downloaded from PDB (https://www.rcsb.org/).
Neovascular age-related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV) are major causes of blindness in people worldwide over 50 years old. More than 20% of the ageing population may suffer from the disorders (Lim et al., 2012). The Global Burden of Disease Study 2010 reported an increase of 160% in years lived with disability related to AMD (Vos et al., 2012). nAMD, characterized by the subfoveal choroidal neovascularization (CNV), have been found to cause structural damage to the macular and lead to irreversible central vision impairment or even blindness within a few years (Rastoin et al., 2020). PCV, a subtype of nAMD, however, is thought to potentially have different pathogenic mechanisms. It is mostly found in Asian population, manifested as orange-red nodular lesions (Ho et al., 2018).
The common etiology of nAMD and PCV is due to the pathological angiogenesis mediated by the vascular endothelial growth factor (VEGF) (Rastoin et al., 2020). Therefore, intravitreal anti-VEGF therapy is recommended as the first-line treatment option for this kind of disease (Campa et al., 2011; Cheung et al., 2018; Toto et al., 2021). Despite the efficacy of anti-VEGF drugs, real-world outcomes were found to be less favorable than the results of randomized-controlled clinical trials (Schmidt-Erfurth et al., 2014). Anti-VEGF drugs only delayed the progression to blindness, and about 30% of the patients show unsatisfactory response to treatment (Rastoin et al., 2020; Mettu et al., 2021). Recent studies demonstrated that the inter-individual differences in treatment response depended on a variety of factors, such as age, lifestyles, anatomical structure of the lesions, genetic polymorphism, etc (Amoaku et al., 2015). Although intravitreal injection has been proved to be safe, intraocular infection remains a severe and destructive complication (Storey et al., 2020). In the developing countries, the economic burden of multiple anti-VEGF treatments is also an unavoidable social problem.
For the reasons above, it is urgent to develop effective methods to predict response to anti-VEGF therapy and to personalize treatment options. Metabolomics studies are developed to uncover metabolic alteration in response to external or internal subtle perturbation. As metabolism is located at the end of life activities, metabolomics can reflect the changes that have occurred in the organism. Therefore, metabolomics data can be more easily correlated with clinical phenotypes. Dysregulation of lipid and metabolic pathways were found to play important roles in the development of AMD (Hou et al., 2020) and PCV (Li et al., 2016). Nevertheless, the serum lipid and metabolic differences between responders and non-responders remained unclear. We believe that there is a great need to better understand response to anti-VEGF therapy and that metabolomics is a potential area for addressing it. Therefore, the aim of this study is to conduct metabolic profiling to identify reliable serum biomarkers to discriminate responders to anti-VEGF treatment.
A prospective longitudinal study was conducted between March 2017 and April 2019 in 13 clinical sites in China. The discovery group were enrolled from Department of Ophthalmology, Shanghai General Hospital, School of medicine, Shanghai Jiao Tong University, Shanghai, China, from 13 March 2017 to 1 March 2019. The last patient last visit (LPLV) of the discovery group was on 1 April 2019. The validation group consisted of patients from another 12 clinical sites in mainland China, from 28 April 2017 to 20 December 2018. The LPLV of the validation group was on 21 January 2019. Figure 1 shows the procedure of the study. This study was approved by the ethics committee of Shanghai General Hospital (permit No. 2016KY115-2), in accordance with the Declaration of Helsinki, and the other clinical sites also obtained the ethical approval. This study was registered on www.ClinicalTrial.gov (NCT03128463). All subjects provided written consent forms. The study protocol has been published (Jin et al., 2018), and here we report the metabolic analysis results.
The inclusion criteria were: (i) Age ≥50 years; (ii) Diagnosed as nAMD or PCV, and the diagnosis criteria was according to the guideline of Chinese Ocular Fundus Disease Society (The Clinical Guideline and Clinical Pathway Development Committee of Age-Related Macular Degeneration, Ocular Fundus Diseases Society, Chinese Ophthalmological Society, Chinese Medical Association, 2013); (iii) Received at least one intravitreal injection of conbercept (Lumitin; Chengdu Kanghong Biotech Co., Ltd., Chengdu, China). The exclusion criteria included: (i) Intravitreal or systemic administration of anti-VEGF drugs within 3 months; (ii) Other interventional therapies within 3 months (e.g. photodynamic therapy, retinal photocoagulation, pars plana vitrectomy, etc); (iii) Other reasons that caused subretinal of intraretinal fluid/hemorrhage, such as diabetic retinopathy, retinal venous occlusion, etc.; (iv) Serious systemic diseases, such as renal failure.
At Visit 1 (V1), all participants underwent comprehensive ophthalmologic examinations, including slit-lamp biomicroscopy, best-corrected visual acuity (BCVA) using standard Early Treatment Diabetic Retinopathy Study (ETDRS) letters, intraocular pressure (IOP) measurement, color fundus photography, and spectral-domain optical coherence tomography (SD-OCT) (Spectralis; Heidelberg Engineering, Heidelberg, Germany). All OCT scans were centered on the fovea, using a centrally oriented internal fixation mark. Central retinal thickness (CRT) within 1 mm of the central fovea were calculated automatically by the instrument. Fundus fluorescein angiography and indocyanine green angiography were also performed for the differential diagnosis of nAMD and PCV, except for the participants who had a history of allergy. The retinal specialists confirmed the diagnosis based on the fundus and morphology examinations, and then they administered intravitreal injections of 0.5 mg conbercept in the operation room under topical anesthesia. One month after treatment, a routine follow-up Visit 2 (V2) was performed. The results of BCVA, IOP, and SD-OCT were collected in V2.
In the discovery group, grading of response was based on the morphology changes on SD-OCT scans from V1 to V2, such as reduction in intraretinal fluid (IRF), subretinal fluid (SRF), and retinal thickening, independently judged by two experienced graders (Yinchen Shen, Hanying Wang). The grading was confirmed by a senior retinal specialist (Xun Xu). In case of discrepancy, a final agreement was reached by all the graders. Responders were defined as patients with a significant reduction in IRF or SRF and retinal thickening. Non-responders were defined as patients with an increase or no change in IRF, SRF, and retinal thickening, from V1 to V2 (Amoaku et al., 2015). In the validation group, responders were defined as patients with a reduction of CRT ≥10% of the baseline values, while non-responders were those with a reduction of CRT <10% of the baseline values or an increase of CRT 1 month after one injection of conbercept.
10 ml patients’ peripheral blood was obtained fasting in the morning before anti-VEGF injection at V1 and at V2, respectively, then was centrifuged within 30 min (1,500 rpm, 10 min at 20°C). The serum aliquots were collected and stored at −80°C immediately for further analysis. The procedures to extract metabolites were as follows: 100 μL sample was extracted by 4-fold volume methanol extraction agent. The mixture was then vortexed and centrifuged. 180 μL lyophilized samples were used for positive and negative ion analysis, respectively. Before analysis, the lyophilized samples were re-dissolved with 50 μl 25% acetonitrile, and the system was balanced with blank samples. We carried quality control (QC) to monitor the stability and repeatability of the analysis. The QC samples were prepared the same way by pooling equal volume of all serum samples, injected after every ten samples.
The samples were processed in the same batch for UHPLC-MS/MS analysis on a hybrid quadrupole-Orbitrap mass spectrometer (Vanquish UPLC-Q Exactive, Thermo Fisher Scientific, Rockford, IL, USA). For positive ion mode, we used Waters BEH C8 column (50 mm × 2.1 mm, 1.7 μm) (Waters, Milford, MA) to separate mixture at 60°C. Phase A: water with 0.1% formic acid; phase B: acetonitrile with 0.1% formic acid. Flow rate was set at 0.4 ml/min with the following gradient: 0–0.5 min, 5%B; 0.5–2 min, linearly increased to 40%B; 2–8 min, linearly increased to 100% B; maintained for 2 min; 10.1 min, decreased back to 5% B; balanced for 2 min. For negative ion mode, we used ACQUITY UPLC HSS T3 (50 mm × 2.1 mm, 1.8 μm) (Waters, Milford, MA) to separate mixture at 60°C. Phase A: 6.5 mm NH4HCO3 added to water; phase B: 95% methanol and 6.5 mM NH4HCO3 aqueous solution. Flow rate was set at 0.4 ml/min with the following gradient: 0–0.5 min, 2%B; 0.5–2 min, linearly increased to 40%B; 2–8 min, linearly increased to 100% B; maintained for 2 min; 10.1 min, decreased back to 2% B; balanced for 1.9 min. The acquisition setting for metabolomics data: full scan mode; capillary temperature, 300°C; sheath gas, 45; auxiliary gas, 10; mass resolution, 7e4. For positive ions: spray voltage, 3.50 kV; m/z range, 80–1,200. For negative ions: spray voltage, 3.00 kV; m/z range, 80–1,200.
The descriptive statistics were performed with SPSS software version 22.0 (SPSS Inc. Chicago, IL, United States). Continuous variables were expressed as mean (95% confidence internal). Comparisons of patients’ age, BCVA, CRT, IOP were performed using independent-samples t-tests or Mann-Whitney U. Categorical variables were expressed as number (percentage). Comparisons of patients’ gender, ethnicity, study eye, lesion type, systemic diseases, and medicine treatments were analyzed by Chi-square tests. Meanwhile, the weighted kappa for inter-observer imaging grading was calculated to verify the reliability and accuracy of the judgement. A p value <0.05 was considered statistically significant.
For metabolomics analysis, we constructed principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) models to investigate the differences of metabolic profiles between responders and non-responders using SIMCA-P version 14.0 (Umetrics AB, Umea, Sweden). Fold change (FC) and false discovery rate (FDR) were calculated to reveal the metabolites with significant differences. The metabolites with FC > 1.2 and FDR <0.01 were defined as differential metabolites. Area under the receiver operating characteristic curve (AUC) was calculated to screen the biomarkers to discriminate responders. In order to verify the potential biomarker, its optimal cut-off value of the discovery group was employed with the validation group to test the prediction accuracy. Volcano plot, venn plot, interactive pie chart of metabolites sub-classes, and bar plot of enriched pathways were drawn using MetaboAnalyst5.0 (http://www.metaboanalyst.ca/). We created a Github page and upload all related scripts and supported data. The link of the Github page is https://github.com/epang2022/Metabolomics-Study-of-Treatment-Response-to-Conbercept-of-Patients-with-Neovascular-Age-related-Macu.
In order to reveal possible genetic variants related to identified metabolites in this study, a public platform Lipid Genie’s Lipid Viewer (http://www.lipidgenie.com/qtlViewer.html) was used to explore genome-metabolite connections. After selecting a specific lipid species, the Logarithm-of-Odds (LOD) and the allele effect plots for mapped Quantitative Trait Loci (QTLs) were visualized in parallel to genes located within a 3 megabase pair window of QTL’s apex LOD score.
In total, 219 eyes from 219 patients (nAMD, n = 126; PCV, n = 93) were enrolled. The discovery group included 132 eyes from 132 patients (nAMD, n = 62; PCV, n = 70). Another 87 independent subjects (nAMD, n = 64; PCV, n = 23) were recruited as the validation group. A total of 248 metabolites were detected by untargeted LC-MS metabolomics analysis, and the reproducibility of analysis was confirmed by QC samples. The relative standard deviations of 226 (91.1%) metabolites were less than 30%.
The clinical characteristics are in Table 1. There were no statistical differences in age, gender, ethnicity, study eye, baseline BCVA, and baseline CRT between responders and non-responders for both populations. The weighted kappa for inter-observer imaging grading of the discovery set was 0.862 (p < 0.001). In the discovery group, the mean CRT of responders reduced significantly from 502.8 (458.8–546.8) μm to 342.9 (315.0–370.8) μm, p < 0.001. However, CRT slightly increased from 524.5 (451.2–597.8) μm to 530.3 (455.0–605.7) μm for non-responders, p = 0.348. There were also more gains in BCVA for responders. For the validation group, CRT of responders decreased from 397.5 (351.8–443.3) μm to 260.7 (233.3–288.2) μm, p < 0.001. Nevertheless, CRT increased slightly for non-responders, from 348.9 (304.0–393.7) μm to 364.7 (319.1–410.4) μm, p = 0.076. BCVA gains for responders did not reach statistical significance.
Both PCA and PLS-DA models were constructed for serum metabolic profiles of patients with nAMD before and after treatment. Neither model showed significant differences. The permutation test indicated that PLS-DA model of nAMD overfitted. Meanwhile, PCA model was constructed for patients with PCV before and after treatment, indicating no difference. PLS-DA model of PCV could not be established because of insignificant difference (Figure 2).
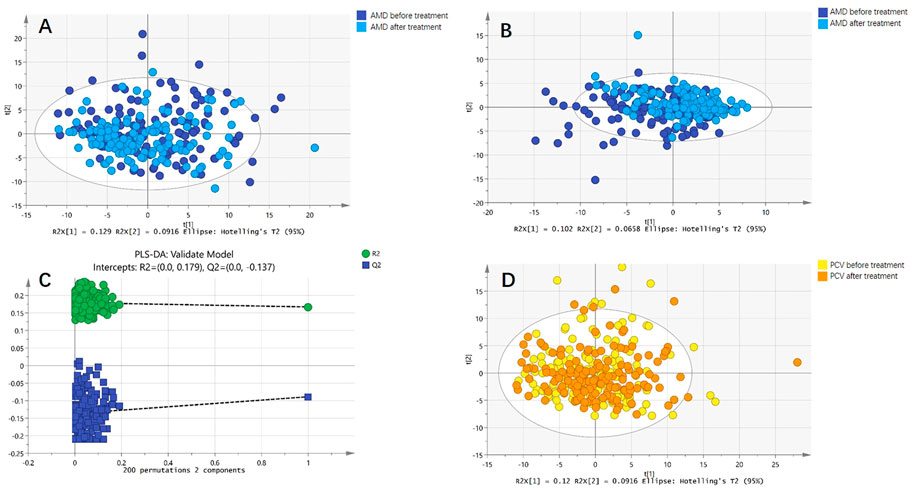
FIGURE 2. Comparison of metabolic profiles before and after anti-VEGF treatment. (A) Principal component analysis (PCA) model of patients with nAMD before and after anti-VEGF treatment. The PCA model showed no difference. (B) Partial least squares discriminant analysis (PLS-DA) model of patients with nAMD before and after anti-VEGF treatment. The PLS-DA model also showed no difference. (C) The permutation test indicated that PLS-DA model of nAMD overfitted. (D) PCA model of patients with PCV before and after anti-VEGF treatment. The PCA model showed no difference.
For the discovery group, PCA and PLS-DA models both demonstrated that the baseline metabolic profile of responders and that of non-responders showed significant differences combining nAMD and PCV (Figure 3). In all, eighty-five differential metabolites were identified (see Supplementary Table S1). Table 2 lists the top 20 metabolites ranked by AUC. In addition, PCA and PLS-DA models were established for patients with nAMD and PCV, respectively. We also identified a clear separation between responders and non-responders in these models (Figure 4).
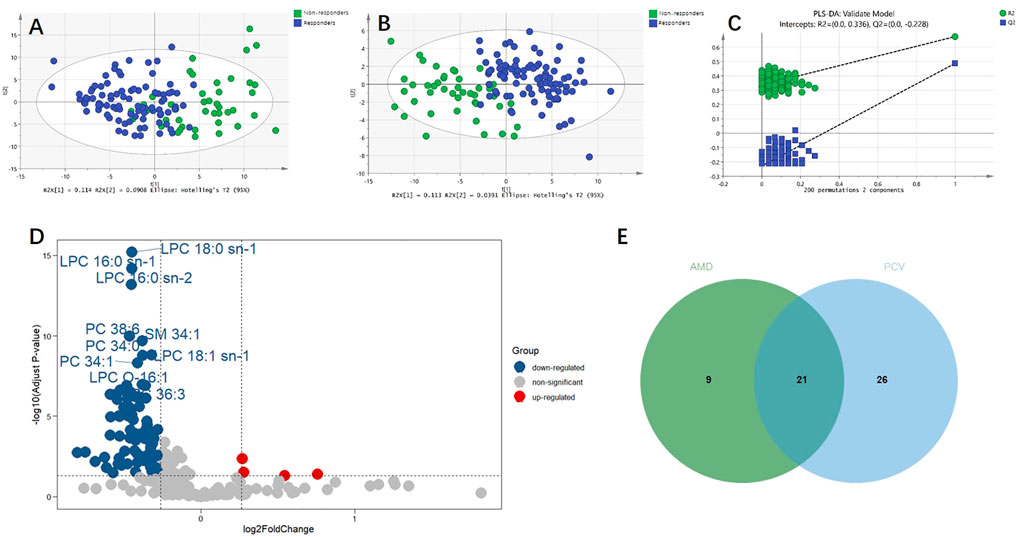
FIGURE 3. Metabolic differences between responders and non-responders to anti-VEGF treatment combining nAMD and PCV. (A) Principal component analysis (PCA) model showed significant difference. (B) Partial least squares discriminant analysis (PLS-DA) model showed significant difference. (C) The permutation test demonstrated that PLS-DA model did not overfit. (D) Volcano plot of the differential metabolites between responders and non-responders. Blue and red dots indicated down-regulated and up-regulated metabolites, respectively. (E) Venn plot of the differential metabolites between responders and non-responders in AMD (30 metabolites) and the differential metabolites between responders and non-responders in PCV (47 metabolites).
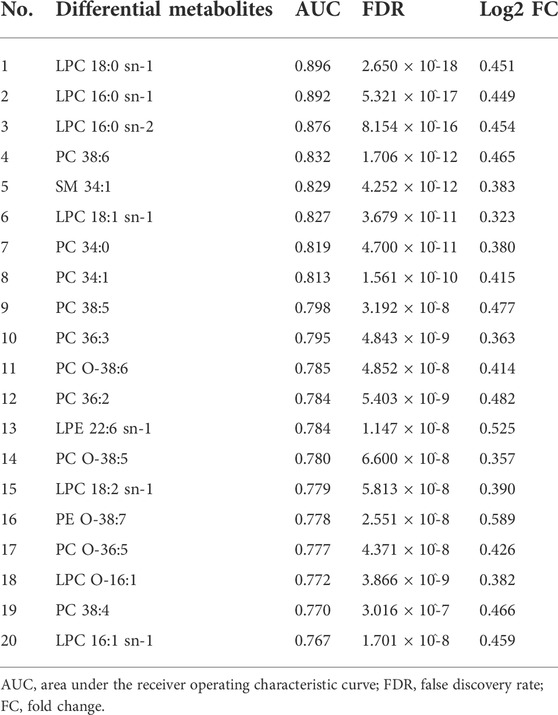
TABLE 2. Top 20 differential metabolites between responders and non-responders ranked by the area under the receiver operating characteristic curve (AUC).
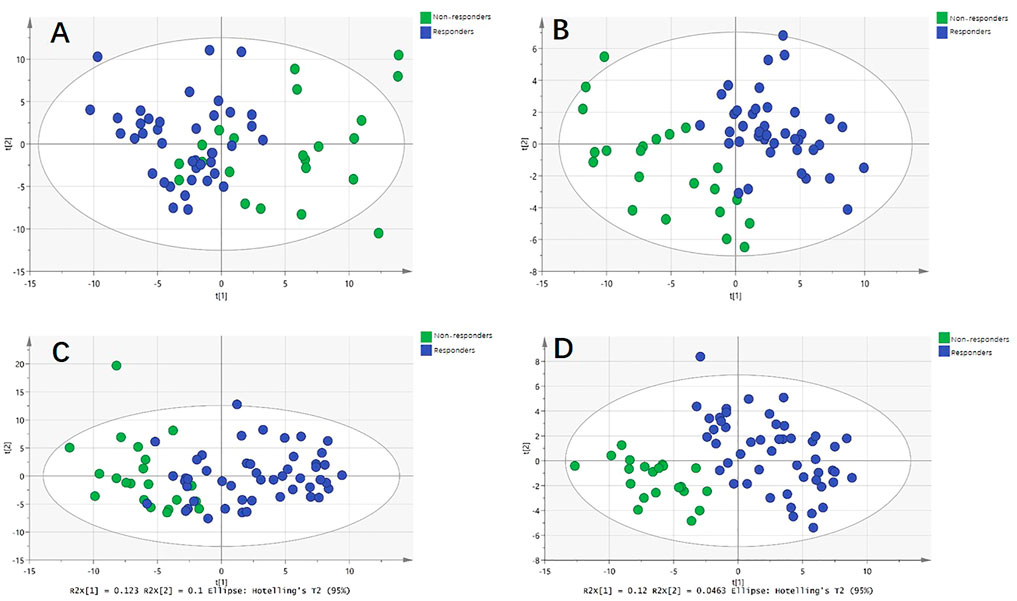
FIGURE 4. Metabolic differences between responders and non-responders to anti-VEGF treatment in nAMD and PCV, respectively. (A) Principal component analysis (PCA) model showed significant difference in nAMD. (B) Partial least squares discriminant analysis (PLS-DA) model showed significant difference in nAMD. (C) PCA model showed significant difference in PCV. (D) PLS-DA model showed significant difference in PCV.
The sub-classes of differential metabolites associated with treatment response included diacylglycerophosphocholines, lysophosphatidylcholine (LPC), branched fatty acids, unsaturated fatty acids, phosphocholine (PC), etc (Figure 5A). Responders and non-responders differed most significantly in metabolism of LPC (p = 7.16 x 10^-19) and diacylglycerophosphocholine (p = 6.96 x 10^-17). The enriched metabolites pathways are listed in Figure 5B, and the detailed information of the pathways is summarized in Supplementary Table S2. Among the differential metabolites, LPC 18:0 exhibited the highest AUC, which is 0.896 with 95% confidence internal between 0.833 and 0.949, to discriminate responders from non-responders (Figure 5C). Prediction accuracies by LPC 18:0 in the discovery and validation groups are illustrated in Figure 5D. The optimal cut-off value of LPC 18:0 in the discovery group was 11.4. This cut-off value was then used to predict treatment response in the validation group, and the predictive value was 72.4%. According to our genome-metabolite connections analysis on the platform Lipid Genie’s Lipid Viewer, the single nucleotide polymorphism (SNP) of von Willebrand Factor (vWF) was highly relevant with the level of LPC 18:0 (Figure 5E).
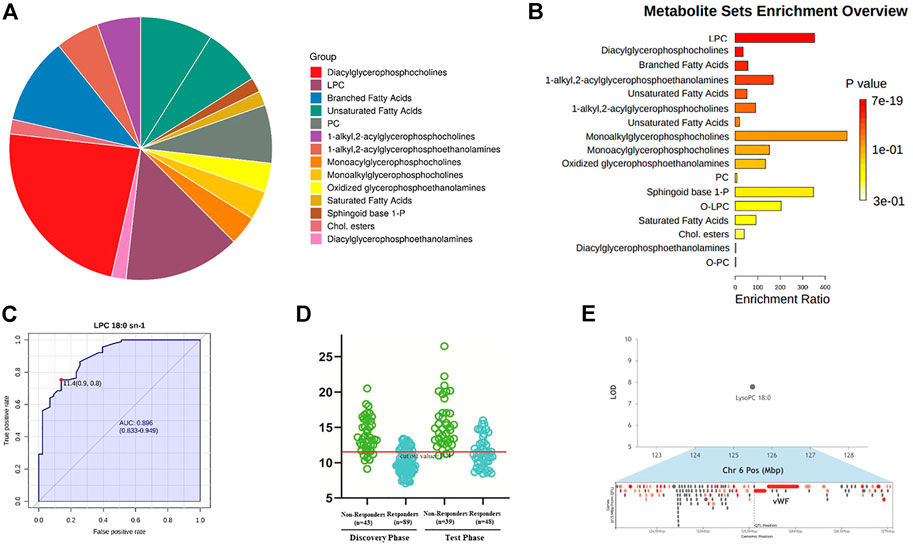
FIGURE 5. Enriched metabolite pathways and potential biomarker associated with different response to anti-VEGF treatment. (A) Interactive pie chart of sub-classes of differential metabolites. (B) Bar plot of enriched metabolite pathways. Responders and non-responders differed most significantly in metabolism of LPC (p = 7.16 x 10^-19) and diacylglycerophosphocholine (p = 6.96 x 10^-17). (C) Diagnostic outcome of LPC 18:0 in the discovery group. The area under curve is 0.896 with 95% confidence internal between 0.833 and 0.949, to discriminate responders from non-responders. The optimal cut-off value of LPC 18:0 was 11.4. (D) Prediction accuracies by LPC 18:0 in the discovery and validation group. This cut-off value 11.4 was used to predict treatment response in the validation group. The predictive value was 72.4%. (E) Hotspot mapping to chromosome 6: 125.554 Mbp indicated the gene associated with LPC 18:0 (highlighted in red). The single nucleotide polymorphism of von Willebrand Factor was highly relevant with the level of LPC 18:0.
Anti-VEGF drugs have revolutionized the treatment of retinal neovascular diseases. Conbercept, an anti-VEGF agent developed recently in China, reduced CRT in patients with nAMD in previous clinical studies (Zhang et al., 2011; Li et al., 2014; Liu et al., 2019). Nevertheless, there was still a lack of reliable and accurate methods to predict the efficacy of anti-VEGF treatment. The authors have conducted a prospective longitudinal study (NCT03128463) to investigate metabolic biomarkers related to treatment response to conbercept. The main findings of this study included three aspects.
Firstly, there were no significant differences in serum metabolic profiles before and after intravitreal injection of conbercept. Previous studies revealed that plasma level of VEGF was reduced after injection of aflibercept, but no change was found after ranibizumab treatment in patients with AMD (Wang et al., 2014; Yoshida et al., 2014; Zehetner et al., 2015). However, the influence of anti-VEGF treatment on serum metabolomics has not been reported previously. To our knowledge, our study was the first to disclose that intraocular anti-VEGF administration had little influence on serum metabolism. The diversity of treatment response was not due to the metabolic changes caused by the drug.
Secondly, the authors identified a clear difference of baseline metabolic profiles between responders and non-responders, and inferred that metabolism was one of the factors determining treatment response. Although some metabolic pathways related to the onset and progression of AMD and PCV have been studied these years, there is still no consensus over the effect of metabolomics on treatment response. The well-recognized pathways associated with AMD progression included dysregulation of lipid metabolism, nucleotide metabolism, carbohydrate metabolism, amino acid metabolism, etc (Hou et al., 2020). Interestingly, the differential metabolites we identified were mostly enriched to lipids, including lipids from subclasses of LPC, PC, carnitine, fatty acids, etc. Research on the relationship between lipids and angiogenesis was just beginning. Kananen et al. reported that high level of serum total cholesterol led to the development of AMD (Kananen et al., 2021). Samson et al. utilized chorioallantoic membrane assay of chick embryo and gas chromatography-mass spectrometry analysis to uncover the specific lipids related to angiogenesis. High levels of LPC, lysophosphatidylethanolamine and cholesterol were detected in the vessel area (Samson et al., 2021). We speculated that specific lipids might regulate the retino-choroidal angiogenesis microenvironment, resolve the activity of CNV, and therefore affect anti-VEGF response. Gao et al. implicated that the serum levels of glycerophosphocholine, LysoPC (18:2) and PS (18:0/20:4) were increased in non-responders (Gao et al., 2020), which was consistent with ours.
The most important disorder pathway we identified was LPC metabolism. In a study of patients at different stages of AMD, patients with nAMD demonstrated increased serum levels of total LPC and LPC 18:0, indicating that LPC participated in angiogenesis (Semba et al., 2019). Fundamental experiment showed that LPC was related to endothelial dysfunction via activation of PKC signaling pathway (Zhao et al., 2021). We performed ROC curve analysis to screen potential biomarkers to discriminate responders. LPC18:0 showed the highest diagnostic efficiency to predict treatment response to conbercept at the beginning of the primary phase, but the mechanism needs further research.
Apart from LPC, pathway enrichment analysis also suggested alterations of diacylglycerophosphocholine metabolism, and the principle metabolites of this pathway were PCs. Increased circulating oxidized phospholipids were related to the pathogenesis of various diseases (Karki et al., 2020). The function of PCs in eye diseases was deemed to reflect oxidative stress of lipids (Cabrerizo et al., 2017a; Cabrerizo et al., 2017b). PCs were identified as discriminating metabolites of AMD (Kersten et al., 2019) and PCV (Li et al., 2016), and our results suggested that PCs could be biomarkers to predict treatment response. Another important metabolite identified was carnitine C 14:3, and the accumulation of carnitine might have resulted from disorders of carnitine metabolism (Knottnerus et al., 2018). The major function of carnitine was to shuttle long-chain fatty acids into mitochondrial matrix (Almannai et al., 2019). Recent research revealed that the carnitine shuttle pathway was more significantly changed in nAMD than intermediate AMD (Mitchell et al., 2021), implying its role in CNV generation.
Thirdly, the authors identified genetic variants associated with differential metabolite through public data analysis, with the purpose of clarifying genome-metabolite connections. SNP of vWF was closely associated with LPC 18:0. Despite the main function of regulating bleeding disorder, vWF was one of the specific surface markers of endothelial cells (Lagarkova et al., 2008). Immunohistochemistry of CNV membranes and polypoidal vessels of human eyes verified that vWF was expressed in the vascular endothelial cells (Wakusawa et al., 2008). Yamashita et al. reported elevated plasma levels of vWF:Antigen in patients with AMD, elucidating the participation of vWF in AMD (Yamashita et al., 2018). Nevertheless, how vWF regulates LPC 18:0 needs further investigation.
Honestly, there are some limitations to this study. (i) The relative small sample size of the study population, which could influence repeatability of the results. Hence, the authors recruited 87 independent subjects from other 12 clinical sites as the validation group to verify the main findings. (ii) According to the protocol of the study, only patients receiving conbercept treatment were enrolled, which could lead to bias. Therefore, the results should be objectively interpreted, and further research is required to elucidate whether our results can be expanded to other anti-VEGF drugs. (iii) The time point to define treatment response was 1 month after the first injection. Although ‘3 + PRN’ regimen was recommended for nAMD based on the results of randomized controlled clinical trials, many patients could not afford three loading doses. Thus, it is critical to develop metabolic biomarkers to predict response at the beginning of the treatment. More longitudinal studies are needed to clarify the relationship between metabolites and response to anti-VEGF treatment in the primary phase as well as in the maintained phase.
Responders and non-responders treated with conbercept differed most significantly in metabolism of LPC and diacylglycerophosphocholine. LPC 18:0 could be a potential biomarker to discriminate responders. Further research is needed to confirm the relationship between lipids and angiogenesis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the ethics committee of Shanghai General Hospital, School of medicine, Shanghai Jiao Tong University, Shanghai, China (permit No. 2016KY115-2). The patients/participants provided their written informed consent to participate in this study.
YS, JF, and KL conceived and designed the study and drafted the manuscript. HW, XiX, CC, SZ, LC, and XuX provided analytical technical support. All authors have read and approved the final manuscript.
This work was funded by the National Natural Science Foundation of China (No. 8217040425, 81870667, 81800831, 82101141), National Key R&D Program of China (No. 2016YFC0904800, 2019YFC0840607), Program of Shanghai Academic Research Leader (21XD1402700), Bethune•Lumitin Young and Middle-aged Ophthalmic Research Fund (No. BJ-LM2021010J), and Science and Technology Research Project of Songjiang District (No. 2020SJ307). The sponsor or funding organization had no role in the design or conduct of this research.
The authors thank the doctors from Xijing Hospital, Air Force Medical University, Xi’an, China; Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China; Eye and ENT Hospital of Fudan University, Shanghai, China; Eye Hospital and School of Ophthalmology and Optometry, Wenzhou Medical University, Wenzhou, China; Shanghai 10th People’s Hospital Affiliated to Tongji University School of Medicine, Shanghai, China; Zhongshan Hospital, Fudan University, Shanghai, China; The First Affiliated Hospital of Guangxi Medical University, Nanning, China; Wuhan General Hospital of Guangzhou Military Region, Wuhan, China; The Affiliated Eye Hospital of Nanjing Medical University, Nanjing, China; Ningbo Eye Hospital, Ningbo, China; Inner Mongolia Chaoju Eye Hospital, Hohhot, China; Ningbo First Hospital, Ningbo, China for their help during the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.991879/full#supplementary-material
Almannai, M., Alfadhel, M., and El-Hattab, A. W. (2019). Carnitine inborn errors of metabolism. Molecules 24, 3251. doi:10.3390/molecules24183251
Amoaku, W. M., Chakravarthy, U., Gale, R., Gavin, M., Ghanchi, F., Gibson, J., et al. (2015). Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond.) 29, 721–731. doi:10.1038/eye.2015.48
Cabrerizo, J., Urcola, J. A., and Vecino, E. (2017b). Changes in the lipidomic profile of aqueous humor in open-angle glaucoma. J. Glaucoma 26, 349–355. doi:10.1097/IJG.0000000000000603
Cabrerizo, J., Urcola, J. A., Vecino, E., and Melles, G. (2017a). Changes in lipidomic profile of aqueous humour in Fuchs endothelial dystrophy. Acta Ophthalmol. 95, 727–732. doi:10.1111/aos.13374
Campa, C., and Harding, S. P. (2011). Anti-VEGF compounds in the treatment of neovascular age related macular degeneration. Curr. Drug Targets 12, 173–181. doi:10.2174/138945011794182674
Cheung, C., Lai, T., Ruamviboonsuk, P., Chen, S. J., Chen, Y., Freund, K. B., et al. (2018). Polypoidal choroidal vasculopathy: Definition, pathogenesis, diagnosis, and management. Ophthalmology 125, 708–724. doi:10.1016/j.ophtha.2017.11.019
Gao, Y., Teo, Y., Beuerman, R. W., Wong, T. Y., Zhou, L., and Cheung, C. (2020). A serum metabolomics study of patients with nAMD in response to anti-VEGF therapy. Sci. Rep. 10, 1341. doi:10.1038/s41598-020-58346-3
Ho, C., and Lai, T. (2018). Current management strategy of polypoidal choroidal vasculopathy. Indian J. Ophthalmol. 66, 1727–1735. doi:10.4103/ijo.IJO_975_18
Hou, X. W., Wang, Y., and Pan, C. W. (2020). Metabolomics in age-related macular degeneration: A systematic review. Invest. Ophthalmol. Vis. Sci. 61, 13. doi:10.1167/iovs.61.14.13
Jin, J., Shen, Y., Chen, X., Wang, J., Chen, C., Xu, X., et al. (2018). Pharmacogenomic study on anti-VEGF medicine in treatment of macular neovascular diseases: A study protocol for a prospective observational study. BMC Ophthalmol. 18, 181. doi:10.1186/s12886-018-0812-4
Kananen, F., Strandberg, T., Loukovaara, S., and Immonen, I. (2021). Early middle age cholesterol levels and the association with age-related macular degeneration. Acta Ophthalmol. 99, e1063–e1069. doi:10.1111/aos.14774
Karki, P., and Birukov, K. G. (2020). Oxidized phospholipids in healthy and diseased lung endothelium. Cells 9, 981. doi:10.3390/cells9040981
Kersten, E., Dammeier, S., Ajana, S., Groenewoud, J., Codrea, M., Klose, F., et al. (2019). Metabolomics in serum of patients with non-advanced age-related macular degeneration reveals aberrations in the glutamine pathway. PloS one 14, edoi:0218457doi:10.1371/journal.pone.0218457
Knottnerus, S., Bleeker, J. C., Wüst, R., Ferdinandusse, S., IJlst, L., Wijburg, F. A., et al. (2018). Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev. Endocr. Metab. Disord. 19, 93–106. doi:10.1007/s11154-018-9448-1
Lagarkova, M. A., Volchkov, P. Y., Philonenko, E. S., and Kiselev, S. L. (2008). Efficient differentiation of hESCs into endothelial cells in vitro is secured by epigenetic changes. Cell cycle 7, 2929–2935. doi:10.4161/cc.7.18.6700
Li, M., Zhang, X., Liao, N., Ye, B., Peng, Y., Ji, Y., et al. (2016). Analysis of the serum lipid profile in polypoidal choroidal vasculopathy. Sci. Rep. 6, doi:38342doi:10.1038/srep38342
Li, X., Xu, G., Wang, Y., Xu, X., Liu, X., Tang, S., et al. (2014). Safety and efficacy of conbercept in neovascular age-related macular degeneration: Results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology 121, 1740–1747. doi:10.1016/j.ophtha.2014.03.026
Lim, L. S., Mitchell, P., Seddon, J. M., Holz, F. G., and Wong, T. Y. (2012). Age-related macular degeneration. Lancet 379, 1728–1738. doi:10.1016/S0140-6736(12)60282-7
Liu, K., Song, Y., Xu, G., Ye, J., Wu, Z., Liu, X., et al. (2019). Conbercept for treatment of neovascular age-related macular degeneration: Results of the randomized phase 3 PHOENIX study. Am. J. Ophthalmol. 197, 156–167. doi:10.1016/j.ajo.2018.08.026
Mettu, P. S., Allingham, M. J., and Cousins, S. W. (2021). Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities. Prog. Retin. Eye Res. 82, doi:100906doi:10.1016/j.preteyeres.2020.100906
Mitchell, S. L., Ma, C., Scott, W. K., Agarwal, A., Pericak-Vance, M. A., Haines, J. L., et al. (2021). Plasma metabolomics of intermediate and neovascular age-related macular degeneration patients. Cells 10, 3141. doi:10.3390/cells10113141
Rastoin, O., Pagès, G., and Dufies, M. (2020). Experimental models in neovascular age related macular degeneration. Int. J. Mol. Sci. 21, 4627. doi:10.3390/ijms21134627
Samson, F. P., Fabunmi, T. E., Patrick, A. T., Jee, D., Gutsaeva, D. R., and Jahng, W. J. (2021). Fatty acid composition and stoichiometry determine the angiogenesis microenvironment. ACS omega 6, 5953–5961. doi:10.1021/acsomega.1c00196
Schmidt-Erfurth, U., Chong, V., Loewenstein, A., Larsen, M., Souied, E., Schlingemann, R., et al. (2014). Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 98, 1144–1167. doi:10.1136/bjophthalmol-2014-305702
Semba, R. D., Moaddel, R., Cotch, M. F., Jonasson, F., Eiriksdottir, G., Harris, T. B., et al. (2019). Serum lipids in adults with late age-related macular degeneration: A case-control study. Lipids Health Dis. 18, 7. doi:10.1186/s12944-018-0954-7
Storey, P. P., Patel, D., and Garg, S. (2020). Endophthalmitis following intravitreal injection of anti-vascular endothelial growth factor agents. Can. J. Ophthalmol. 55, 286–292. doi:10.1016/j.jcjo.2020.01.015
The Clinical Guideline and Clinical Pathway Development Committee of Age-Related Macular Degeneration, (2013). Ocular fundus diseases society, Chinese ophthalmological society, Chinese medical AssociationClinical pathway of age-related macular degeneration in China. Chin. J. Ocul. Fundus Dis. 29, 343–355. doi:10.3760/cma.j.issn.1005-1015.2013.04.002
Toto, L., Di Antonio, L., Costantino, O., and Mastropasqua, R. (2021). Anti-VEGF therapy in myopic CNV. Curr. Drug Targets 22, 1054–1063. doi:10.2174/1389450122999210128180725
Vos, T., Flaxman, A. D., Naghavi, M., Lozano, R., Michaud, C., Ezzati, M., et al. (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet 380, 2163–2196. doi:10.1016/S0140-6736(12)61729-2
Wakusawa, R., Abe, T., Sato, H., Yoshida, M., Kunikata, H., Sato, Y., et al. (2008). Expression of vasohibin, an antiangiogenic factor, in human choroidal neovascular membranes. Am. J. Ophthalmol. 146, 235–243. doi:10.1016/j.ajo.2008.03.019
Wang, X., Sawada, T., Sawada, O., Saishin, Y., Liu, P., and Ohji, M. (2014). Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am. J. Ophthalmol. 158, 738–744. doi:10.1016/j.ajo.2014.06.009
Yamashita, M., Matsumoto, M., Hayakawa, M., Sakai, K., Fujimura, Y., and Ogata, N. (2018). Intravitreal injection of aflibercept, an anti-VEGF antagonist, down-regulates plasma von Willebrand factor in patients with age-related macular degeneration. Sci. Rep. 8, 1491. doi:10.1038/s41598-018-19473-0
Yoshida, I., Shiba, T., Taniguchi, H., Takahashi, M., Murano, T., Hiruta, N., et al. (2014). Evaluation of plasma vascular endothelial growth factor levels after intravitreal injection of ranibizumab and aflibercept for exudative age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 252, 1483–1489. doi:10.1007/s00417-014-2717-0
Zehetner, C., Kralinger, M. T., Modi, Y. S., Waltl, I., Ulmer, H., Kirchmair, R., et al. (2015). Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: A randomised, prospective trial. Acta Ophthalmol. 93, e154–e159. doi:10.1111/aos.12604
Zhang, M., Zhang, J., Yan, M., Luo, D., Zhu, W., Kaiser, P. K., et al. (2011). A phase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology 118, 672–678. doi:10.1016/j.ophtha.2010.08.008
Keywords: anti-vegf, neovascular age-related macular degeneration, polypoidal choroidal vasculopathy, metabolomics, treatment response
Citation: Shen Y, Wang H, Xu X, Chen C, Zhu S, Cheng L, Fang J, Liu K and Xu X (2022) Metabolomics study of treatment response to conbercept of patients with neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Front. Pharmacol. 13:991879. doi: 10.3389/fphar.2022.991879
Received: 12 July 2022; Accepted: 31 August 2022;
Published: 19 September 2022.
Edited by:
Lvzhen Huang, Peking University People’s Hospital, ChinaReviewed by:
Weiye Li, Drexel University, US Minor Outlying IslandsCopyright © 2022 Shen, Wang, Xu, Chen, Zhu, Cheng, Fang, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kun Liu, ZHJsaXVrdW5Ac2p0dS5lZHUuY24=; Junwei Fang, ZmFnbmp1bndlaUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.