- 1Department of Anesthesiology, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang, China
- 2Department of Pain Medicine, Shanghai Fourth People’s Hospital Affiliated to Tongji University School of Medicine, Shanghai, China
- 3Department of Anesthesiology, Cancer Hospital Chinese Academy of Medical Science, Shenzhen Center, Shenzhen, China
Background: Postoperative poor sleep quality and decreased gastrointestinal motility function are common clinical problems. This study investigated the effects of dexmedetomidine (DEX) combined with sufentanil for patient-controlled analgesia (PCA) on postoperative sleep quality and gastrointestinal motility function after surgery in patients with colorectal cancer.
Methods: Patients undergoing colorectal cancer surgery were randomly divided into three groups, DEX 0, 200, or 400 μg, each combined with sufentanil 150 μg for PCA immediately after surgery. The primary outcome was sleep quality in the first 7 days after surgery based on the Athens Insomnia Scale (AIS) score. The secondary outcome was postoperative gastrointestinal motility recovery evaluated by the time of first flatus, first feces and first diet. Postoperative pain intensity, side effects and the length of postoperative hospital stay were also compared among groups. The study was registered with the Chinese Clinical Trial Registry (https://www.chictr.org.cn/enIndex.aspx, ChiCTR2000032601).
Results: Ultimately, 210 cases were included. Sleep quality was better in the DEX 200 μg group and DEX 400 μg group than in the DEX 0 μg group. Overall, in the DEX 200 μg group and DEX 400 μg group, the AIS score (p < 0.05) and the incidence of sleep disturbance (7.3%, 4.5% vs. 19.6%, p < 0.001) were lower than those in the DEX 0 μg group in the first 7 days after surgery. There were no significant differences in postoperative gastrointestinal motility among the three groups in the total surgical categories (p > 0.05). In the laparoscopic surgery patients of each group, the time of postoperative first flatus (p = 0.02) and first feces (p = 0.01) was significantly longer in the DEX 400 μg group than in the DEX 0 μg group. There were no differences in postoperative pain intensity, side effects or length of postoperative hospital stay (p > 0.05).
Conclusion: The continuous infusion of DEX (200 or 400 μg) for PCA significantly improved postoperative sleep quality after colorectal cancer surgery. DEX (200 μg) was better at improving postoperative sleep quality without affecting gastrointestinal motility function than DEX (400 μg) in patients who underwent laparoscopic colorectal cancer surgery.
Clinical Trial Registration: https://www.chictr.org.cn/enIndex.aspx, identifier ChiCTR2000032601
Introduction
Sleep is essential for energy conservation, temperature regulation, immune response and brain recovery. Postoperative sleep disturbance is a common clinical problem in surgical patients and is related to surgery, anesthesia and environment (Jiang et al., 2018). Poor sleep quality increases the incidence of postoperative delirium and postoperative cognitive dysfunction, increases the incidence of cardiovascular events and delays the postoperative recovery process (Aldecoa et al., 2017; Huang et al., 2021). Sleep disturbance is the second most bothersome symptom in cancer patients, and it increases the chances of cancer recurrence (Otte et al., 2015). Many attempts have been made to alleviate severe sleep disturbance after surgery by eliminating noise and light through the use of eye masks or earplugs in surgical wards, but the effectiveness of these strategies is limited, and adjuvant medication is needed in some circumstances (Ouslander et al., 2006; Leong et al., 2021). Pharmacological interventions such as benzodiazepines, propofol or analgesics, are used to improve postoperative sleep quality. Unfortunately, these drugs may produce sleep architecture disruption and increase the incidence of postoperative delirium (Pandharipande and Ely, 2006).
Dexmedetomidine (DEX) is a potent and highly selective α2 adrenergic agonist with sedative, analgesic and sympatholytic properties (Lee, 2019). In contrast to other commonly used sedative and anesthetic agents to induce neuronal apoptosis, DEX has shown neuroprotective effects (Duan et al., 2014). The sedation induced by the administration of DEX neurophysiologically approximates natural sleep (Akeju et al., 2018). Some clinical trials have demonstrated that the intraoperative use of DEX can decrease the incidence of sleep disturbance in postoperative patients (Wu et al., 2016; Chen et al., 2017; Huyan et al., 2019; Jin et al., 2021). A recent randomized study found that DEX used during a daytime operation can better improve postoperative sleep quality in patients undergoing laparoscopic abdominal surgeries (Song et al., 2019). However, patients undergoing abdominal surgery usually suffer from reduced gastrointestinal transit and prohibition of intestinal peristalsis, which is described as a temporary impairment of gastrointestinal motility (Cho et al., 2017; Turkay et al., 2020). These patients present with a variety of clinical symptoms, including postoperative nausea and vomiting (PONV), delayed passage of flatus and feces, and the inability to tolerate solid food, resulting in an extended recovery time (Yang et al., 2016). In colorectal cancer surgery, changes in anatomical structure may promote intestinal obstruction and have a significant impact on gastrointestinal motility function (Bragg et al., 2015). It was found that α2 adrenergic agonists reduce vagally mediated gastric and small bowel motility (Maze and Tranquilli, 1991). However, the effect of DEX on gastrointestinal motility remains controversial (Iirola et al., 2011; Chen et al., 2016).
DEX has been effectively used for patient-controlled analgesia (PCA). Whether DEX has a positive impact on postoperative sleep quality and gastrointestinal motility function in patients after colorectal cancer surgery remains uninvestigated. The purpose of this study was to determine the effects of DEX combined with sufentanil for PCA on postoperative sleep quality and gastrointestinal motility function in patients after colorectal cancer surgery.
Methods
Trial design
This study was a single-center, prospective, randomized, double-blinded, controlled trial performed in Harbin Medical University Cancer Hospital from 1 July 2019 to 30 December 2019. The study was approved by the Ethics Committee of Harbin Medical University Cancer Hospital (2019-189-ⅡT) and registered with the Chinese Clinical Trial Registry (ChiCTR2000032601). Written informed consent was obtained from all subjects participating in this trial before surgery.
Participants
Patients who underwent elective colorectal cancer surgery and were expected to require postoperative PCA were enrolled in this study. The inclusion criteria were an age of over 18 years and an American Society of Anesthesiologists (ASA) physical status of I-III. The exclusion criteria included long-term use of opioids, sedatives, antidepressants, or anxiolytic drugs prior to surgery; drug addiction; a preoperative history of schizophrenia, epilepsy, Parkinsonism, or myasthenia gravis; a preoperative sleep disorder; the use of sleep-promoting medications; sleep apnea syndrome; the inability to provide informed consent (coma, profound dementia, or language barriers); sick sinus syndrome, severe sinus bradycardia (<50/min), or a second-degree or greater atrioventricular block without a pacemaker; serious hepatic dysfunction (Child–Pugh class C); serious renal dysfunction (undergoing dialysis before surgery); and an allergy to opioid analgesics or DEX.
Randomization and masking
Patients were randomized through the use of a random number table. Enrolled patients were randomly allocated into the DEX 0 μg group, DEX 200 μg group or DEX 400 μg group. To explore the effects of DEX for PCA on gastrointestinal motility function after different surgical categories, patients in the three groups were further divided into laparoscopic surgery and open surgery subgroups. Two investigators carried out this study in a blinded manner. The first investigator was responsible for enrollment and the assignment of participants to groups by randomization. The variables were recorded by the second investigator, who was blinded to each subject’s assigned group. Participants were blinded to group assignments throughout the study period. In case of an emergency (unexpected or rapid deterioration of the patient’s clinical status), physicians were allowed to request the unmasking of the treatment assignment or the adjusting or interrupting of the study, if necessary. In such a case, the patient was excluded from the final analysis.
Anesthesia and analgesia procedures
Anesthesia methods were standardized in all three groups. General anesthesia was induced with propofol 1.0–1.5 mg/kg, sufentanil 0.3–0.5 μg/kg, and cisatracurium 0.15 mg/kg. Tracheal intubation was facilitated after 3 min of cisatracurium administration and was connected to a ventilator. The ventilation rate was 12 breaths/min. The tidal volume (8–10 ml/kg) was adjusted to maintain the end-tidal CO2 (EtCO2) level at 35–45 mmHg. Propofol 4–10 mg/kg/h and remifentanil 5–20 μg/kg/h were administered and adjusted according to the hemodynamic responses to maintain a bispectral index (BIS) between 40 and 60 during anesthesia. The neuromuscular blockade was maintained by intermittent injection of 0.05 mg/kg cisatracurium as needed. All patients in the three groups received PCA after surgery. The PCA regimen consisted of sufentanil 150 μg (in 300 ml normal saline), which was set up as a continuous infusion dose of 4 ml/h with a bolus dose of 3 ml (if needed), with a lock-out time of 15 min in all groups. DEX (200 and 400 μg) was mixed with PCA in the DEX 200 μg group and DEX 400 μg group, respectively. The PCA pump was used for up to 3 days after surgery until all of the solution was exhausted. Refilling the PCA pump was not allowed for any group. The acute rescue analgesic drug flurbiprofen axetil (50 mg i.v.), was given when the visual analog scale (VAS: 0, no pain; 10, severe pain) score was more than 4 after three continuous bolus infusions of PCA.
Outcome measures
During the postoperative period, the following variables were assessed: the Athens Insomnia Scale (AIS) score; the VAS score, both at rest and with movement at 2, 24 and 48 h after surgery; the time of first flatus, first feces and first diet; the dosage of consumed sufentanil; the number of PCA attempts; the rate of rescue analgesia; side effects (PONV, bradycardia or hypotension); and the length of postoperative hospital stay.
The primary outcome of this study was sleep quality for 7 continuous days after surgery. Postoperative sleep quality was assessed with the AIS score and the incidence of postoperative sleep disturbance. The AIS is a self-reported questionnaire that quantifies sleep disturbances in accordance with the criteria set by the International Classification of Diseases (ICD-10). It consists of 8 items: sleep induction, awakenings during the night, early morning awakening, total sleep time, overall quality of sleep, sleep quality well-being, functioning capacity, and daytime sleepiness. Item scores range from 0 (no problem at all) to 3 (very serious problem), for a total score of 0–24. The AIS scores were recorded at 5:00 p.m. the following day by investigator who was blinded to each subject’s assigned group. A lower AIS score indicates better sleep quality. A total score of > 6 points reflects the diagnosis of sleep disturbance. The overall incidence of postoperative sleep disturbance was defined as the proportion of score > 6 points within 7 days after surgery.
The secondary outcome was postoperative gastrointestinal motility recovery based on the time of first flatus, first feces and first diet. Other outcomes included postoperative pain intensity at rest and with movement at 2, 24 and 48 h after surgery; the dosage of consumed sufentanil of PCA; the number of PCA attempts at 24 and 48 h after surgery and the dosage of acute rescue analgesic drugs (flurbiprofen axetil); side effects (PONV, bradycardia or hypotension) and postoperative hospital stay. Hypotension was defined as a mean arterial pressure of 30% below baseline, and bradycardia was defined as a heart rate of < 50 beats/min. The clinical characteristics of patients, such as age, sex, height, body weight, surgical site (rectal vs. colon), and surgical category (laparoscopic vs. open) were recorded.
Statistical analysis
Based on an expected incidence of the primary endpoint as a 16.6% occurrence of postoperative sleep disturbance in the DEX 0 μg group, 7.1% in the DEX 200 μg group and 4.8% in the DEX 400 μg group in our preliminary experiments, the minimum sample size was calculated. For a two-sided difference with 80% power at the 0.05 significance level, sixty-one participants in each group were required. Considering subjects who may be lost to follow-up or may otherwise drop out, seventy participants in each group were enrolled.
IBM SPSS Statistics version 26.0 software (IBM Corp., Armonk, NY, United States) was used to perform statistical analyses. The Kolmogorov–Smirnov test was applied to assess the distribution of the variables. Homogeneity of variance was compared among the three groups by Levene tests. Continuous variables are presented as the mean ± SD. Stratified continous variables are presented as the median with an interquartile range. Categorical variables are presented as a percentage. The incidence of sleep disturbance, rate of rescue analgesia, sex, ASA, surgical site, surgical category, and side effects among groups were analyzed using the chi-squared test or Fisher’s exact test. VAS score and PCA attempts were analyzed using a Kruskal–Wallis H test. The surgery time, AIS score, time to postoperative first flatus, time to postoperative first feces, time to postoperative first diet, dosage of consumed sufentanil, and general characteristics of the patients, including age, height, and weight, were analyzed using a one-way ANOVA followed by a post hoc least significant differences test. Time to postoperative first flatus, first feces, and first diet classified by surgical category were analyzed using a two-way ANOVA. p < 0.05 was considered statistically significant.
Results
Patient characteristics
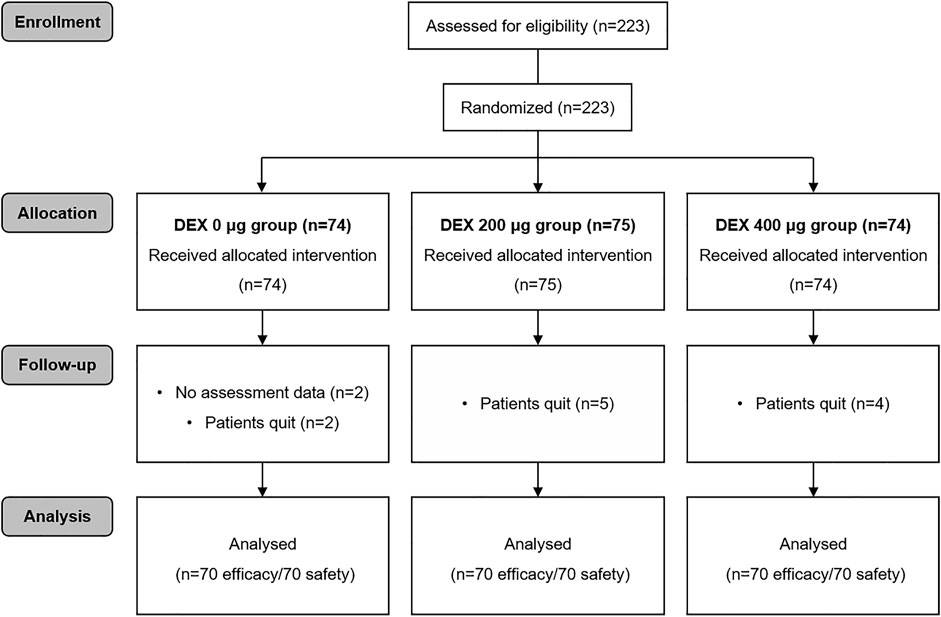
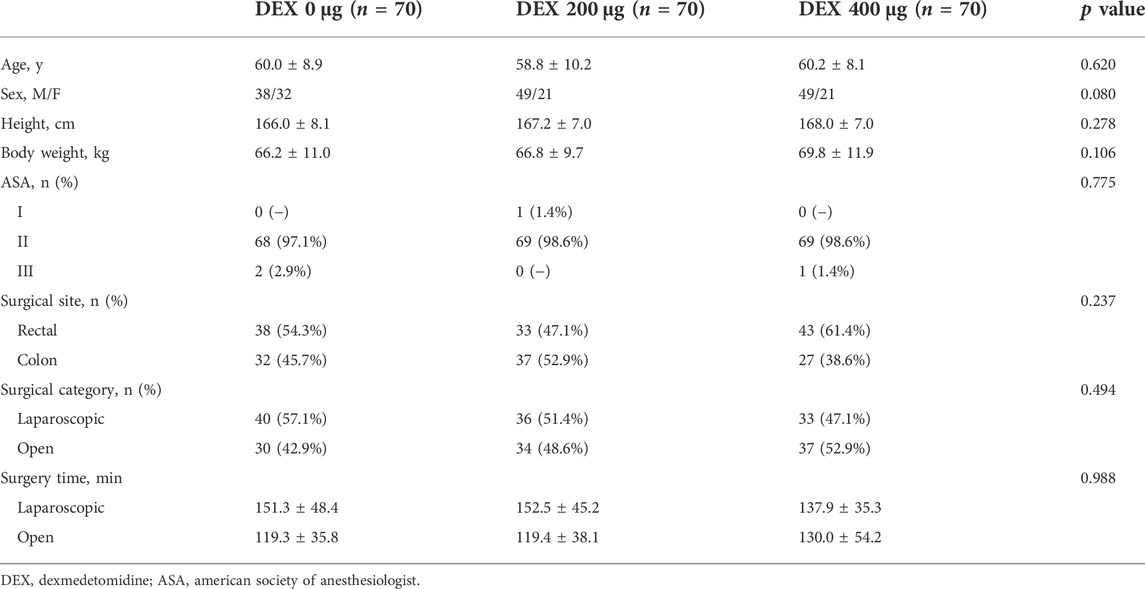
During the study period, 223 patients were involved (Figure 1). Thirteen patients were subsequently excluded from the analysis. Eleven of these patients voluntarily discontinued the trial, and the assessment data for 2 patients were unavailable. The study included 210 cases eligible for analysis, with 70 cases in each group. There were no differences in the clinical characteristics of the patients among the three groups, including age, sex, height, body weight, ASA physical status, surgical site surgical category and surgery time (Table 1).
The primary outcome
Compared with that of the DEX 0 μg group, the AIS score in the DEX 200 μg group was significantly lower on postoperative days 1, 2, 3, 4, 5, and 7 (p < 0.05, Figure 2A). The AIS score in the DEX 400 μg group was significantly lower than that in the DEX 0 μg group within all 7 days after surgery (p < 0.05). The AIS score in the DEX 400 μg group was significantly lower than that in the DEX 200 μg group on postoperative day 6 (p = 0.004). The incidence of postoperative sleep disturbance in the DEX 200 μg group was significantly lower than that in the DEX 0 μg group 1 and 3 days after surgery (p < 0.05, Figure 2B). The incidence of postoperative sleep disturbance in the DEX 400 μg group was significantly lower than that in the DEX 0 μg group 1, 3, 4, and 6 days after surgery (p < 0.05, Figure 2B). There were no differences in the incidence of postoperative sleep disturbance between the DEX 200 µg group and DEX 400 µg group. The overall incidence of postoperative sleep disturbance during the first 7 days after surgery was significantly lower in the DEX 200 µg and DEX 400 µg groups than in the DEX 0 µg group (7.3%, 4.5% vs. 19.6%, p < 0.001, Figure 2C).
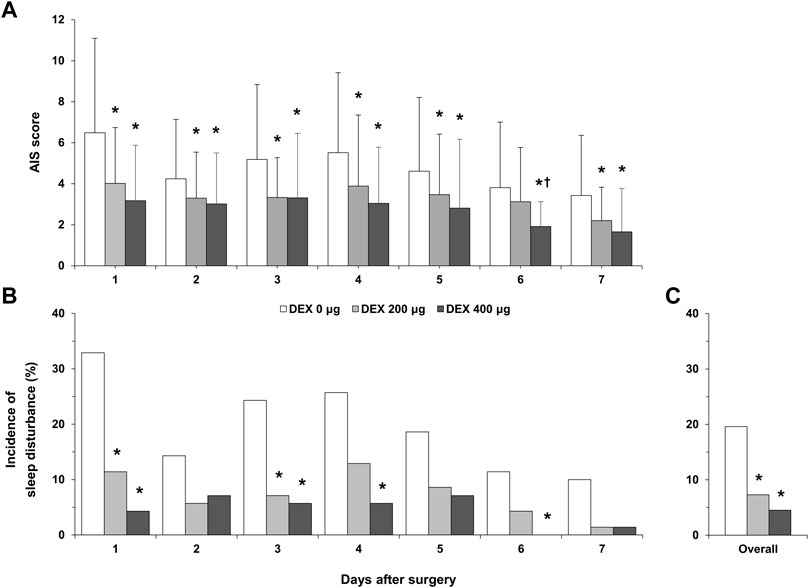
FIGURE 2. Postoperative sleep quality. (A), AIS score over 7 postoperative days; (B), incidence of postoperative sleep disturbance over 7 postoperative days; (C), overall incidence of postoperative sleep disturbance during the first 7 days after surgery. DEX, dexmedetomidine; AIS, Athens Insomnia Scale; *p < 0.05, vs. DEX 0 μg; †p < 0.05, vs. DEX 200 μg.
The secondary outcomes
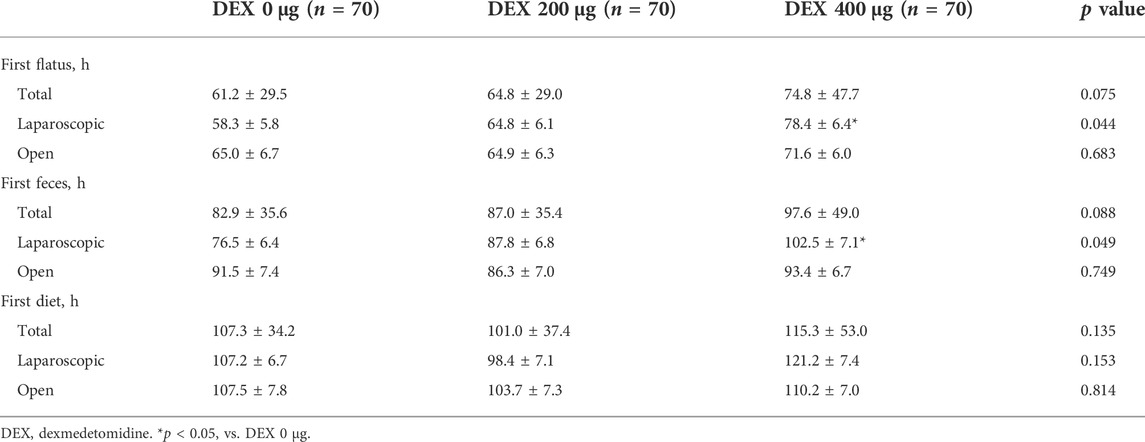
There were no significant differences in postoperative gastrointestinal motility among the three groups in the total surgical category (Table 2). In the laparoscopic surgery group, the time of postoperative first flatus and first feces was significantly longer in the DEX 400 μg group than in the DEX 0 μg group. There was no significant difference in the time of the postoperative first diet among all groups.
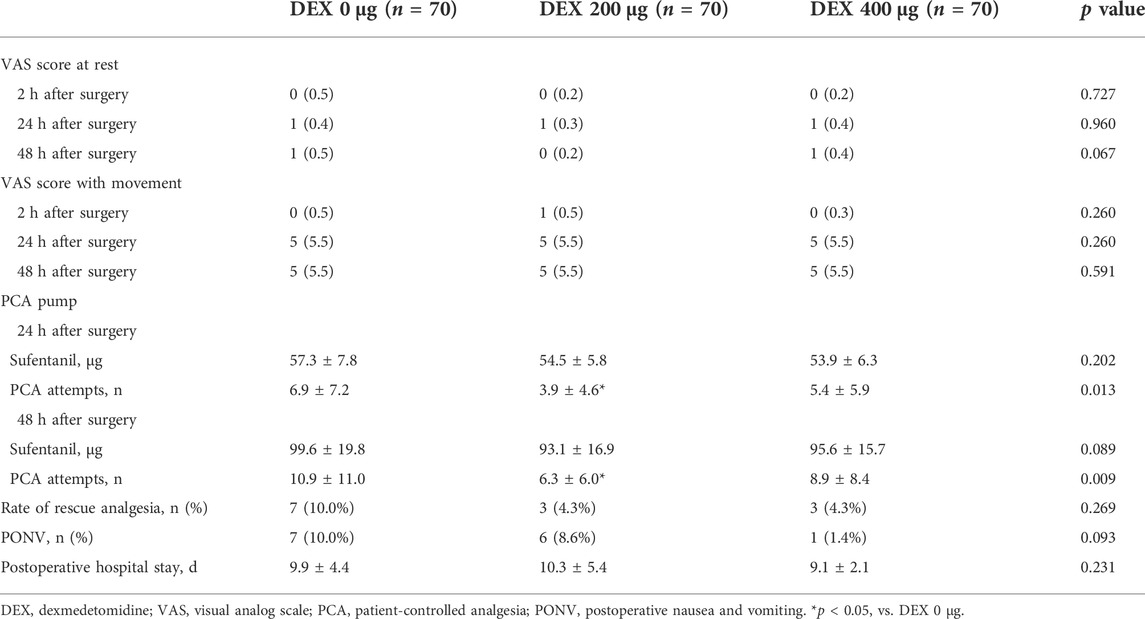
The VAS score at rest or with movement at 2, 24 and 48 h after surgery showed no significant differences among the three groups (Table 3). There was no difference in the dosage of consumed sufentanil among all groups 24 and 48 h after surgery. The number of PCA attempts in the DEX 200 μg group was significantly lower than that in the DEX 0 μg and DEX 400 μg groups at 24 h (p = 0.013) and 48 h (p = 0.009) after surgery. There were no significant differences in the rate of rescue analgesia or postoperative hospital stay among the groups. No episodes of PONV, bradycardia or hypotension were recorded.
Discussion
This randomized clinical trial investigated the effect of 0, 200, and 400 μg of DEX combined with sufentanil for PCA on postoperative sleep quality and the recovery of gastrointestinal motility function in patients after colorectal cancer surgery. Our results demonstrated that the continuous infusion of 200 and 400 μg of DEX for PCA after surgery improves sleep quality in the first 7 days. The recovery of postoperative gastrointestinal motility function was not significantly different across the whole sample (both laparoscopic and open surgeries included) among the three comparison groups. However, in patients who underwent laparoscopic surgery, the DEX 400 μg group inhibited postoperative recovery of gastrointestinal motility more than the DEX 0 μg group. Postoperative pain intensity, side effects and postoperative hospital stay did not differ among the three groups. According to this study, DEX 200 μg with sufentanil for PCA is preferred in patients with colorectal cancer surgery for improving postoperative sleep quality and provides effective analgesia without affecting gastrointestinal motility function.
Studies have shown that DEX used for PCA at a rate of 0.03 μg/kg/h improves postoperative sleep quality (Chen et al., 2017; Hong et al., 2021). The total dosage of DEX used in this study was 0.03–0.05 and 0.07–0.09 μg/kg/h according to the body weight of enrolled patients in the DEX 200 μg group and DEX 400 μg group, respectively, which was higher than that used in the above report. The higher dosage of DEX used for PCA in this study was due to the results of our previous study, where 200 and 400 μg of DEX for PCA reduced the incidence of postoperative delirium in elderly patients without increasing side effects, and 100 μg of DEX (0.02–0.03 μg/kg/h) had no effect on the improvement of postoperative delirium (Zhao et al., 2020).
Sleep is an active, complex process that is necessary for mental and physical restoration. Sleep disturbances are known to result in poor healing, reduced cognitive function, and an increased chance of cancer recurrence (Weinhouse et al., 2009; Otte et al., 2015). One study demonstrated that postoperative sleep disturbance is most serious within the first 3 days after surgery, which manifests with decreased sleep time, increased awakenings, and disturbance of sleep rhythm (Knill et al., 1990). Patients frequently report postoperative sleep disturbance in response to surgical stress; 42% of patients complained of unsatisfactory sleep after orthopedic, vascular, and general surgery (vs. 28% the night before surgery), and their sleep remained unsatisfactory after 4 days in 23% of cases (Chouchou et al., 2014). In our study, the overall incidence of postoperative sleep disturbance within 7 days after surgery was 19.6%, and both 200 and 400 μg of DEX used for PCA reduced the incidence of sleep disturbances (7.3% vs. 4.5%), improving the sleep quality of patients. Postoperative sleep disturbances may be the result of a variety of factors, including pain and hospital environment-related factors, noise and light exposure from procedures or intensive monitoring during the night (Dolan et al., 2016). Postoperative pain is one of the most common factors affecting postoperative sleep quality (Miller et al., 2015; Dolan et al., 2016; Chen et al., 2017). However, in the present study, postoperative analgesia was similar among the groups. Thus, the improvement of postoperative sleep quality by DEX was not due to analgesia. The locus coeruleus nucleus is the site that receives external stimuli and sleep arousal (Song et al., 2017). DEX exerts its hypnotic action through selective activation of central pre- and postsynaptic adrenergic receptors in the locus coeruleus (Bamgbade, 2006). DEX inhibits locus coeruleus-derived noradrenergic neurotransmission to the ventrolateral preoptic nucleus, thus disinhibiting the ventrolateral preoptic nucleus and provoking an inhibition of cortical arousal nuclei (Guldenmund et al., 2017).
Previous studies have shown that the intraoperative use of DEX was associated with a shorter gastrointestinal motility recovery time than the use of a placebo (Chen et al., 2016; Mah et al., 2021). It was found that the intraoperative use of DEX was associated with reductions in time of first flatus, first feces, and the return to a regular solid diet in laparoscopic resection of colorectal cancer (Chen et al., 2016). A recent multicenter, placebo-controlled randomized clinical trial also suggested that the administration of intraoperative DEX reduced the time of first flatus and first feces in patients undergoing open and laparoscopic abdominal surgeries (Mah et al., 2021). However, the reported effects of DEX on gastrointestinal motility function are inconsistent. In patients undergoing abdominal hysterectomy, no difference in time to first bowel sounds and flatus was observed after receipt of intraoperative DEX (Xu et al., 2017). Two preclinical studies and one study involving healthy participants found that DEX inhibits gastrointestinal motility function (Asai et al., 1998; Herbert et al., 2002; Iirola et al., 2011). DEX markedly inhibited gastric emptying and gastrointestinal transit when healthy participants received 1 μg/kg of DEX over 20 min followed by a continuous infusion of 0.7 μg/kg/h for 190 min (Iirola et al., 2011). The current study found that, compared with the DEX 0 μg group, the use of 400 μg of DEX combined with sufentanil for PCA prolonged the time to the first flatus (74.8 ± 47.7 vs. 61.2 ± 29.5), first feces (97.6 ± 49.0 vs. 82.9 ± 35.6) and first diet (115.3 ± 53.0 vs. 107.3 ± 34.2), although there were no statistically significant differences in the recovery time of gastrointestinal motility function in either the laparoscopic or open surgical categories. However, based on the subgroup analysis, the effect of DEX on gastrointestinal motility function was dependent on the surgical category. In the laparoscopic groups, the time of postoperative first flatus and feces of the DEX 400 μg group was longer than that in the DEX 0 μg group, and there was no difference between the DEX 200 μg and DEX 0 μg groups, indicating that 400 μg of DEX, but not 200 μg of DEX, inhibited the recovery of postoperative gastrointestinal function. In the open surgery groups, the recovery of gastrointestinal motility function was not different between DEX groups.
The effects of DEX on postoperative gastrointestinal function may be related to the dosage and the time of administration. A preclinical study revealed that DEX concentration dependently inhibited peristalsis of the guinea pig ileum in vitro, and that the inhibition was caused by the interaction with α2-adrenoceptors (Herbert et al., 2002). Two clinical studies with opposite results demonstrated the dosage-dependent effect of DEX. Patients who received DEX at a total dosage of 3 μg/kg showed inhibited gastric emptying and gastrointestinal transit (Iirola et al., 2011). However, gastric emptying was not delayed when the total dosage was 1 μg/kg (Memiş et al., 2006). Low-dose DEX may improve gastrointestinal transit by acting on central α2-adrenoceptor agonists to reduce sympathetic tone (Cho et al., 2015). High-dose DEX may inhibit peristalsis by activating inhibitory α1-adrenoceptors located postsynaptically on the smooth muscle or by activating inhibitory α2-adrenoceptors on excitatory cholinergic pathways in the enteric nervous system, such as opioid, purinergic, and nitrergic neurons (De Ponti et al., 1996). The effects of DEX on postoperative gastrointestinal function were different depending on the time of administration. Low perfusion of intestinal smooth muscle disturbed intestinal motility during surgery. DEX improved postoperative gastrointestinal function by its global hemodynamic stabilizing effect, preventing the violent alteration of gastrointestinal microcirculation, attenuating intestinal ischemia–reperfusion injury, and improving stress response (Kiliç et al., 2012; Zhang et al., 2012). However, during the nonsurgical period, DEX inhibits gastrointestinal function by affecting the α2-adrenoceptor of enteric neurons (Herbert et al., 2002; Iirola et al., 2011). Furthermore, the effects of DEX may vary depending on the degree of physiological impairment in the participant. Abdominal open surgery causes severe hemodynamic changes and extensive trauma, which is a representative of long-term major surgery. Evidence suggests that compared to laparoscopic surgery, open approaches quickly induce an inflammatory cascade and stress responses by causing greater tissue injury (Raygor et al., 2015). Under physiological conditions, DEX inhibited gastrointestinal motility in the study of healthy participants (Iirola et al., 2011). In a study involving critically ill patients, no difference in gastric emptying time was observed after receipt of DEX vs. propofol (Memiş et al., 2006). In the present study, high-dose DEX did inhibit the recovery time of postoperative first flatus and first feces in laparoscopic surgery rather than open surgery, suggesting the dose and the degree of physiological impairment are relevant in determining the benefits of DEX associated with return of gastrointestinal function.
DEX at a rate of 0.02–0.05 μg/kg/h with sufentanil at a rate of 0.015–0.02 μg/kg/h used for PCA potentiated the analgesic effect of sufentanil and reduced the sufentanil consumption in abdominal surgery (Feng et al., 2019). In the present study, the VAS scores and sufentanil consumption was not statistically different among three groups, which is consistent with our previous study (Zhao et al., 2020). The dosage of sufentanil used in this study was 0.03 μg/kg/h (total dosage up to 150 μg) according to the body weight of enrolled patients, which was higher than that used in the above report (Feng et al., 2019). This indicated that dosage of sufentanil in our study for PCA was sufficient for postoperative analgesia, the combination of DEX did not further potentiate the analgesia effect of sufentanil. A study with a higher dosage of sufentanil (a rate of 0.04 μg/kg/h) supports the result that DEX did not potentiate sufentanil in PCA (Chen et al., 2016). The PCA was set up at a 3 ml bolus if needed with a background infusion dosage of 4 ml/h for up to 3 days. Although the PCA attempts varied among groups, the relatively smaller proportion of bolus consumption compared with the consistent background infusion of the three groups did not result in a statistically difference in sufentanil consumption among three groups. The number of additional rescue analgesia requirement in the DEX 0 μg group was higher than the DEX 200 μg group and the DEX 400 μg group, but it was no statistics difference. The number of PCA attempts in the DEX 200 μg group were significantly lower than in the 0 μg group. There were no statistics difference between the DEX 200 μg group and DEX 400 μg group or between the DEX 0 μg group and the 400 μg group. The DEX 200 μg group had less number of the PCA attempts while with large standard deviation.
This present study had a couple of limitations. First, there was no long-term (>7 days postoperatively) evaluation of postoperative sleep quality. A study showed that 25% of patients had not returned to normal sleep quality 2 weeks after discharge (Chouchou et al., 2014). Considering the short hospital stays of the patients, this analysis was limited to evaluating sleep quality within 7 days after surgery. Second, the sample size estimation in our study was based on postoperative sleep disturbance, which was powered solely as the primary endpoint in our present study. It is worth noting that the 400 μg group prolonged the time to the first flatus, first feces and first diet for almost 10 h in total surgical categories, although the differences were only numerically different. It cannot be excluded that statistically significant differences in secondary outcomes may have become apparent after the inclusion of a larger sample size.
Conclusion
Our study indicated that, compared with the use of sufentanil alone for PCA, a continuous infusion of DEX (200 or 400 μg) with sufentanil for PCA for up to 3 days significantly improved sleep quality in the first 7 days for patients after colorectal cancer surgery without increasing any side effects or prolonging their hospital stay. Compared with 400 μg of DEX, 200 μg of DEX was better at improving postoperative sleep quality without affecting gastrointestinal motility function in patients who underwent laparoscopic surgery. According to this study, 200 μg of DEX with sufentanil for PCA is preferred in patients with colorectal cancer surgery for improving postoperative sleep quality and providing effective analgesia without affecting gastrointestinal motility function.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Harbin Medical University Cancer Hospital (2019-189-ⅡT) and registered with the Chinese Clinical Trial Registry (ChiCTR2000032601). The patients/participants provided their written informed consent to participate in this study.
Author contributions
MJ, KL, GJ, JZ, and FH designed this work. XS and FH wrote the paper. AS, CY, and LW performed the experiments. XS, ZH, and FH analyzed the data. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81871515, 82171859).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akeju, O., Hobbs, L. E., Gao, L., Burns, S. M., Pavone, K. J., Plummer, G. S., et al. (2018). Dexmedetomidine promotes biomimetic non-rapid eye movement stage 3 sleep in humans: A pilot study. Clin. Neurophysiol. 129, 69–78. doi:10.1016/j.clinph.2017.10.005
Aldecoa, C., Bettelli, G., Bilotta, F., Sanders, R. D., Audisio, R., Borozdina, A., et al. (2017). European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur. J. Anaesthesiol. 34, 192–214. doi:10.1097/EJA.0000000000000594
Asai, T., Mapleson, W. W., and Power, I. (1998). Interactive effect of morphine and dexmedetomidine on gastric emptying and gastrointestinal transit in the rat. Br. J. Anaesth. 80, 63–67. doi:10.1093/bja/80.1.63
Bamgbade, O. A. (2006). Dexmedetomidine for peri-operative sedation and analgesia in alcohol addiction. Anaesthesia 61, 299–300. doi:10.1111/j.1365-2044.2006.04559.x
Bragg, D., El-Sharkawy, A. M., Psaltis, E., Maxwell-Armstrong, C. A., and Lobo, D. N. (2015). Postoperative ileus: Recent developments in pathophysiology and management. Clin. Nutr. 34, 367–376. doi:10.1016/j.clnu.2015.01.016
Chen, C., Huang, P., Lai, L., Luo, C., Ge, M., Hei, Z., et al. (2016). Dexmedetomidine improves gastrointestinal motility after laparoscopic resection of colorectal cancer: A randomized clinical trial. Med. Baltim. 95, e4295. doi:10.1097/MD.0000000000004295
Chen, Z., Tang, R., Zhang, R., Jiang, Y., and Liu, Y. (2017). Effects of dexmedetomidine administered for postoperative analgesia on sleep quality in patients undergoing abdominal hysterectomy. J. Clin. Anesth. 36, 118–122. doi:10.1016/j.jclinane.2016.10.022
Cho, J. S., Kim, H. I., Lee, K. Y., An, J. Y., Bai, S. J., Cho, J. Y., et al. (2015). Effect of intraoperative dexmedetomidine infusion on postoperative bowel movements in patients undergoing laparoscopic gastrectomy: A prospective, randomized, placebo-controlled study. Med. Baltim. 94, e959. doi:10.1097/MD.0000000000000959
Cho, J. S., Kim, H. I., Lee, K. Y., Son, T., Bai, S. J., Choi, H., et al. (2017). Comparison of the effects of patient-controlled epidural and intravenous analgesia on postoperative bowel function after laparoscopic gastrectomy: A prospective randomized study. Surg. Endosc. 31, 4688–4696. doi:10.1007/s00464-017-5537-6
Chouchou, F., Khoury, S., Chauny, J. M., Denis, R., and Lavigne, G. J. (2014). Postoperative sleep disruptions: A potential catalyst of acute pain? Sleep. Med. Rev. 18, 273–282. doi:10.1016/j.smrv.2013.07.002
De Ponti, F., Giaroni, C., Cosentino, M., Lecchini, S., and Frigo, G. (1996). Adrenergic mechanisms in the control of gastrointestinal motility: From basic science to clinical applications. Pharmacol. Ther. 69, 59–78. doi:10.1016/0163-7258(95)02031-4
Dolan, R., Huh, J., Tiwari, N., Sproat, T., and Camilleri-Brennan, J. (2016). A prospective analysis of sleep deprivation and disturbance in surgical patients. Ann. Med. Surg. 6, 1–5. doi:10.1016/j.amsu.2015.12.046
Duan, X., Li, Y., Zhou, C., Huang, L., and Dong, Z. (2014). Dexmedetomidine provides neuroprotection: Impact on ketamine-induced neuroapoptosis in the developing rat brain. Acta Anaesthesiol. Scand. 58, 1121–1126. doi:10.1111/aas.12356
Feng, M., Chen, X., Liu, T., Zhang, C., Wan, L., and Yao, W. (2019). Dexmedetomidine and sufentanil combination versus sufentanil alone for postoperative intravenous patient-controlled analgesia: A systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. 19, 81. doi:10.1186/s12871-019-0756-0
Guldenmund, P., Vanhaudenhuyse, A., Sanders, R. D., Sleigh, J., Bruno, M. A., Demertzi, A., et al. (2017). Brain functional connectivity differentiates dexmedetomidine from propofol and natural sleep. Br. J. Anaesth. 119, 674–684. doi:10.1093/bja/aex257
Herbert, M. K., Roth-Goldbrunner, S., Holzer, P., and Roewer, N. (2002). Clonidine and dexmedetomidine potently inhibit peristalsis in the Guinea pig ileum in vitro. Anesthesiology 97, 1491–1499. doi:10.1097/00000542-200212000-00022
Hong, H., Zhang, D. Z., Li, M., Wang, G., Zhu, S. N., Zhang, Y., et al. (2021). Impact of dexmedetomidine supplemented analgesia on delirium in patients recovering from orthopedic surgery: A randomized controlled trial. BMC Anesthesiol. 21, 223. doi:10.1186/s12871-021-01441-3
Huang, B. H., Duncan, M. J., Cistulli, P. A., Nassar, N., Hamer, M., and Stamatakis, E. (2021). Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Br. J. Sports Med. 56 (13), 718–724. doi:10.1136/bjsports-2021-104046
Huyan, T., Hu, X., Peng, H., Zhu, Z., Li, Q., and Zhang, W. (2019). Perioperative dexmedetomidine reduces delirium in elderly patients after lung cancer surgery. Psychiatr. Danub. 31, 95–101. doi:10.24869/psyd.2019.95
Iirola, T., Vilo, S., Aantaa, R., Wendelin-Saarenhovi, M., Neuvonen, P. J., Scheinin, M., et al. (2011). Dexmedetomidine inhibits gastric emptying and oro-caecal transit in healthy volunteers. Br. J. Anaesth. 106, 522–527. doi:10.1093/bja/aer004
Jiang, Z., Zhou, G., Song, Q., Bao, C., Wang, H., and Chen, Z. (2018). Effect of intravenous oxycodone in combination with different doses of dexmedetomdine on sleep quality and visceral pain in patients after abdominal surgery: A randomized study. Clin. J. Pain 34, 1126–1132. doi:10.1097/AJP.0000000000000645
Jin, X. B., Xiao, R., Zhou, W., Liu, C., Luo, Y. R., Liu, R. H., et al. (2021). Effect of different modes of administration of dexmedetomidine combined with nerve block on postoperative analgesia in total knee arthroplasty. Pain Ther. 10, 1649–1662. doi:10.1007/s40122-021-00320-6
Kiliç, K., Hanci, V., Selek, S., SöZMEN, M., Kiliç, N., Citil, M., et al. (2012). The effects of dexmedetomidine on mesenteric arterial occlusion-associated gut ischemia and reperfusion-induced gut and kidney injury in rabbits. J. Surg. Res. 178, 223–232. doi:10.1016/j.jss.2012.03.073
Knill, R. L., Moote, C. A., Skinner, M. I., and Rose, E. A. (1990). Anesthesia with abdominal surgery leads to intense REM sleep during the first postoperative week. Anesthesiology 73, 52–61. doi:10.1097/00000542-199007000-00009
Lee, S. (2019). Dexmedetomidine: Present and future directions. Korean J. Anesthesiol. 72, 323–330. doi:10.4097/kja.19259
Leong, R. W., Davies, L. J., Fook-Chong, S., Ng, S. Y., and Lee, Y. L. (2021). Effect of the use of earplugs and eye masks on the quality of sleep after major abdominal surgery: A randomised controlled trial. Anaesthesia 76, 1482–1491. doi:10.1111/anae.15468
Mah, K. E., Alten, J. A., Cornell, T. T., Selewski, D. T., Askenazi, D., Fitzgerald, J. C., et al. (2021). Acute kidney injury after in-hospital cardiac arrest. Resuscitation 160, 49–58. doi:10.1016/j.resuscitation.2020.12.023
Maze, M., and Tranquilli, W. (1991). Alpha-2 adrenoceptor agonists: Defining the role in clinical anesthesia. Anesthesiology 74, 581–605. doi:10.1097/00000542-199103000-00029
Memiş, D., DöKMECI, D., Karamanlioğlu, B., Turan, A., and TüRE, M. (2006). A comparison of the effect on gastric emptying of propofol or dexmedetomidine in critically ill patients: Preliminary study. Eur. J. Anaesthesiol. 23, 700–704. doi:10.1017/S0265021506000512
Miller, A., Roth, T., Roehrs, T., and Yaremchuk, K. (2015). Correlation between sleep disruption on postoperative pain. Otolaryngol. Head. Neck Surg. 152, 964–968. doi:10.1177/0194599815572127
Otte, J. L., Carpenter, J. S., Manchanda, S., Rand, K. L., Skaar, T. C., Weaver, M., et al. (2015). Systematic review of sleep disorders in cancer patients: Can the prevalence of sleep disorders be ascertained? Cancer Med. 4, 183–200. doi:10.1002/cam4.356
Ouslander, J. G., Connell, B. R., Bliwise, D. L., Endeshaw, Y., Griffiths, P., and Schnelle, J. F. (2006). A nonpharmacological intervention to improve sleep in nursing home patients: Results of a controlled clinical trial. J. Am. Geriatr. Soc. 54, 38–47. doi:10.1111/j.1532-5415.2005.00562.x
Pandharipande, P., and Ely, E. W. (2006). Sedative and analgesic medications: Risk factors for delirium and sleep disturbances in the critically ill. Crit. Care Clin. 22, 313–327. doi:10.1016/j.ccc.2006.02.010
Raygor, K. P., Than, K. D., Chou, D., and Mummaneni, P. V. (2015). Comparison of minimally invasive transspinous and open approaches for thoracolumbar intradural-extramedullary spinal tumors. Neurosurg. Focus 39, E12. doi:10.3171/2015.5.FOCUS15187
Song, A. H., Kucyi, A., Napadow, V., Brown, E. N., Loggia, M. L., and Akeju, O. (2017). Pharmacological modulation of noradrenergic arousal circuitry disrupts functional connectivity of the locus ceruleus in humans. J. Neurosci. 37, 6938–6945. doi:10.1523/JNEUROSCI.0446-17.2017
Song, B., Li, Y., Teng, X., Li, X., Yang, Y., and Zhu, J. (2019). The effect of intraoperative use of dexmedetomidine during the daytime operation vs the nighttime operation on postoperative sleep quality and pain under general anesthesia. Nat. Sci. Sleep. 11, 207–215. doi:10.2147/NSS.S225041
Turkay, Ü., Yavuz, A., Hortu, İ., Terzi, H., and Kale, A. (2020). The impact of chewing gum on postoperative bowel activity and postoperative pain after total laparoscopic hysterectomy. J. Obstet. Gynaecol. 40, 705–709. doi:10.1080/01443615.2019.1652891
Weinhouse, G. L., Schwab, R. J., Watson, P. L., Patil, N., Vaccaro, B., Pandharipande, P., et al. (2009). Bench-to-bedside review: Delirium in ICU patients - importance of sleep deprivation. Crit. Care 13, 234. doi:10.1186/cc8131
Wu, X. H., Cui, F., Zhang, C., Meng, Z. T., Wang, D. X., Ma, J., et al. (2016). Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: A pilot randomized controlled trial. Anesthesiology 125, 979–991. doi:10.1097/ALN.0000000000001325
Xu, S. Q., Li, Y. H., Wang, S. B., Hu, S. H., Ju, X., and Xiao, J. B. (2017). Effects of intravenous lidocaine, dexmedetomidine and their combination on postoperative pain and bowel function recovery after abdominal hysterectomy. Minerva Anestesiol. 83, 685–694. doi:10.23736/S0375-9393.16.11472-5
Yang, R., Tao, W., Chen, Y. Y., Zhang, B. H., Tang, J. M., Zhong, S., et al. (2016). Enhanced recovery after surgery programs versus traditional perioperative care in laparoscopic hepatectomy: A meta-analysis. Int. J. Surg. 36, 274–282. doi:10.1016/j.ijsu.2016.11.017
Zhang, X. Y., Liu, Z. M., Wen, S. H., Li, Y. S., Li, Y., Yao, X., et al. (2012). Dexmedetomidine administration before, but not after, ischemia attenuates intestinal injury induced by intestinal ischemia-reperfusion in rats. Anesthesiology 116, 1035–1046. doi:10.1097/ALN.0b013e3182503964
Zhao, W., Hu, Y., Chen, H., Wang, X., Wang, L., Wang, Y., et al. (2020). The effect and optimal dosage of dexmedetomidine plus sufentanil for postoperative analgesia in elderly patients with postoperative delirium and early postoperative cognitive dysfunction: A single-center, prospective, randomized, double-blind, controlled trial. Front. Neurosci. 14, 549516. doi:10.3389/fnins.2020.549516
Keywords: dexmedetomidine, patient-controlled analgesia, postoperative sleep quality, postoperative gastrointestinal motility function, colorectal cancer surgery
Citation: Sui X, Wang Y, Jin M, Li K, Jiang G, Song A, He Z, Yin C, Zhao J, Wang L and Han F (2022) The effects of dexmedetomidine for patient-controlled analgesia on postoperative sleep quality and gastrointestinal motility function after surgery: A prospective, randomized, double-blind, and controlled trial. Front. Pharmacol. 13:990358. doi: 10.3389/fphar.2022.990358
Received: 09 July 2022; Accepted: 26 September 2022;
Published: 10 October 2022.
Edited by:
Tingting Wang, Huazhong University of Science and Technology, ChinaReviewed by:
Yu Wu, Bethune International Peace Hospital, ChinaSang-Hwan Do, Seoul National University Bundang Hospital, South Korea
Copyright © 2022 Sui, Wang, Jin, Li, Jiang, Song, He, Yin, Zhao, Wang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Han, ZmguZmVpaEB5YWhvby5jb20=
†These authors have contributed equally to this work
 Xin Sui1†
Xin Sui1† Fei Han
Fei Han