
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 05 October 2022
Sec. Pharmacoepidemiology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.989903
Introduction: Whether aspirin or other antiplatelet drugs can reduce mortality among patients with coronavirus disease (COVID-19) remains controversial.
Methods: We identified randomized controlled trials, prospective cohort studies, and retrospective studies on associations between aspirin or other antiplatelet drug use and all-cause mortality among patients with COVID-19 in the PubMed database between March 2019 and September 2021. Newcastle–Ottawa Scale and Cochrane Risk of Bias Assessment Tool were used to assess the risk of bias. The I2 statistic was used to assess inconsistency among trial results. The summary risk ratio (RR) and odds ratio (OR) were obtained through the meta-analysis.
Results: The 34 included studies comprised three randomized controlled trials, 27 retrospective studies, and 4 prospective cohort studies. The retrospective and prospective cohort studies showed low-to-moderate risks of bias per the Newcastle–Ottawa Scale score, while the randomized controlled trials showed low-to-high risks of bias per the Cochrane Risk of Bias Assessment Tool. The randomized controlled trials showed no significant effect of aspirin use on all-cause mortality in patients with COVID-19 {risk ratio (RR), 0.96 [95% confidence interval (CI) 0.90–1.03]}. In retrospective studies, aspirin reduced all-cause mortality in patients with COVID-19 by 20% [odds ratio (OR), 0.80 (95% CI 0.70–0.93)], while other antiplatelet drugs had no significant effects. In prospective cohort studies, aspirin decreased all-cause mortality in patients with COVID-19 by 15% [OR, 0.85 (95% CI 0.80–0.90)].
Conclusion: The administration of aspirin may reduce all-cause mortality in patients with COVID-19.
Coronavirus disease (COVID-19) is associated with a state of prothrombosis that leads to adverse clinical outcomes (Oudkerk et al., 2020; Lopes et al., 2021). Infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induces platelet activation and platelet-mediated immune inflammation, which also contributes to thrombosis in patients with COVID-19 (Mei et al., 2020). Meizlish et al. (2021) first reported that intermediate doses of anticoagulants and aspirin were associated with a lower cumulative incidence of in-hospital death in patients with COVID-19. In addition to clopidogrel, dipyridamole, and P2Y12 inhibitors, (Mega and Simon, 2015), aspirin is a powerful antiplatelet drug (Schrör, 1997) that plays an important role in anti-thrombosis, anti-inflammation, and antiviral treatments (Bianconi et al., 2020; Simon et al., 2020; Tantry et al., 2021; Connors and Ridker, 2022).
To our knowledge, published meta-analyses on this topic have only included observational studies, with controversial results (Kow and Hasan, 2021; Martha et al., 2021; Salah and Mehta, 2021; Srivastava and Kumar, 2021; Wang et al., 2021). As of July 2022, a comprehensive systematic search of ClinicalTrials.gov showed 24 registered trials and published results from four randomized controlled trials. To further explore the relationship between aspirin or other antiplatelet drugs and all-cause mortality in patients with COVID-19, we conducted a meta-analysis of randomized controlled trials, prospective cohort studies, and retrospective studies.
This meta-analysis aimed to assess the association between aspirin or other antiplatelet drugs and all-cause mortality in patients with COVID-19.
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines.
Relevant articles were searched in the PubMed database from the database inception to July 2022 using the keywords: “COVID-19” and “aspirin”, as well as “COVID-19” and “antiplatelet drugs.”
Studies were included according to the inclusion and exclusion criteria by screening the titles and abstracts of the articles to assess their eligibility. Disagreements were resolved through discussion.
The inclusion criteria were: 1) randomized controlled trials, prospective cohort studies, or retrospective studies; 2) patients with confirmed COVID-19; 3) an exposure group administered aspirin or other antiplatelet drugs and a control group receiving usual care or placebo; 4) an outcome of mortality; and 5) reported mortality among patients who received aspirin or other antiplatelet drugs or mortality could be calculated or compared to that of the control group. All other studies were excluded from the analysis. No language restrictions were imposed.
The primary outcome was all-cause mortality, as reported directly in the study or calculated from data included in the paper. The secondary outcomes were serious adverse events, including bleeding and thrombotic events.
Data were abstracted by four authors (WS, HM, ZG, and QC) from 34 studies, (Abu-Jamous et al., 2020; Alamdari et al., 2020; Giacomelli et al., 2020; Lodigiani et al., 2020; Russo et al., 2020; Sahai et al., 2020; Tremblay et al., 2020; Chow et al., 2021a; Chow et al., 2021b; Connors et al., 2021; Fröhlich et al., 2021; Haji Aghajani et al., 2021; Ho et al., 2021; Kim et al., 2021; Liu et al., 2021; Meizlish et al., 2021; Merzon et al., 2021; Mura et al., 2021; Osborne et al., 2021; Pan et al., 2021; Sisinni et al., 2021; Son et al., 2021; Xiang et al., 2021; Yuan et al., 2021; Zhao et al., 2021; Zhou et al., 2021; Al Harthi et al., 2022; Santoro et al., 2022a; Chow et al., 2022; Formiga et al., 2022; Gogtay et al., 2022; RECOVERY Collaborative GroupAbbas et al., 2022; REMAP-CAP Writing Committee for the REMAP-CAP InvestigatorsBradbury et al., 2022; Sullerot et al., 2022), including three randomized controlled trials, (Connors et al., 2021; RECOVERY Collaborative GroupAbbas et al., 2022; REMAP-CAP Writing Committee for the REMAP-CAP InvestigatorsBradbury et al., 2022), 27 retrospective studies, (Abu-Jamous et al., 2020; Alamdari et al., 2020; Lodigiani et al., 2020; Russo et al., 2020; Sahai et al., 2020; Tremblay et al., 2020; Chow et al., 2021a; Chow et al., 2021b; Fröhlich et al., 2021; Haji Aghajani et al., 2021; Ho et al., 2021; Kim et al., 2021; Liu et al., 2021; Meizlish et al., 2021; Merzon et al., 2021; Mura et al., 2021; Osborne et al., 2021; Pan et al., 2021; Sisinni et al., 2021; Son et al., 2021; Yuan et al., 2021; Zhao et al., 2021; Zhou et al., 2021; Al Harthi et al., 2022; Formiga et al., 2022; Gogtay et al., 2022; Sullerot et al., 2022), and four prospective cohort studies (Giacomelli et al., 2020; Xiang et al., 2021; Santoro et al., 2022a; Chow et al., 2022). The extracted data are shown in Supplementary Tables S1–S3.
The risks of bias in retrospective and prospective cohort studies were assessed using the Newcastle Ottawa Scale (NOS) (Wells et al., 2000), which was also used for the quality assessment of all included retrospective and prospective cohort studies. The risk of bias in the randomized controlled trials was assessed using the Cochrane Risk of Bias Assessment Tool (Sterne et al., 2019).
STATA 16.0 was used to perform the meta-analysis. The risk ratios (RRs) for randomized controlled trials and odds ratios (ORs) for retrospective and prospective cohort studies with their corresponding 95% confidence intervals (95% CIs) were calculated. Owing to the low heterogeneity (I2 < 25%), a fixed-effects model was used for the randomized controlled trials with aspirin as an intervention. The random-effects model was used for analyses of retrospective and prospective cohort studies with aspirin or other antiplatelet drugs as interventions. The heterogeneity among the studies was evaluated using the I2 statistic, with I2 > 25% indicating substantial heterogeneity. Cochran’s Q statistic was used to derive the precise p-values for heterogeneity.
Subgroup analysis was performed to assess the associations between aspirin and all-cause mortality at different time points of administration (before or after admission). The associations in the subgroups were compared by calculating the ORs. ORs further from 1 indicated a greater difference between the estimated associations in the two subgroups.
The initial search of PubMed from its inception through July 2022 revealed a total of 722 studies. A review of the titles and abstracts removed 113 duplicate articles and excluded an additional 564 articles. Finally, the full texts of 45 articles were downloaded and an additional 11 articles were excluded. Four studies (a randomized controlled trial, a prospective cohort study, and two retrospective studies) were excluded as the studies mainly aimed at assessing the effect of anticoagulants, not antiplatelet drugs, on the prognosis of patients with COVID-19 (Viecca et al., 2020; Matli et al., 2021; Berger et al., 2022; Santoro et al., 2022b). One study was excluded as the interventions did not meet the inclusion criteria (Kevorkian et al., 2021). Six studies were excluded as the outcomes were not of interest for our analysis (Argenziano et al., 2020; Dreher et al., 2020; Middeldorp et al., 2020; Regina et al., 2020; Sivaloganathan et al., 2020; Abdelwahab et al., 2021). Finally, the quantitative synthesis comprised 34 articles, including three randomized controlled trials and 17,225 patients with COVID-19, 27 retrospective studies and 96,552 patients, and four prospective cohort studies and 120,019 patients (Figure 1). Among the randomized controlled trials, the sample size of the RECOVERY study was large with a weight of 86.47% (RECOVERY Collaborative GroupAbbas et al., 2022). One retrospective study with enough patients was only applicable to a specific group because it included a homogenous sample from the Veterans Health Administration (Osborne et al., 2021). The study characteristics are summarized in Supplementary Tables 1–3.
Among randomized controlled trials exploring the association between aspirin and all-cause mortality, 1,387 (17.2%) and 1,470 (17.9%) deaths were reported in the aspirin and control groups, respectively. With a summary fixed-effects RR of 0.96 (95% CI 0.90–1.03; p = 0.222), no between-study heterogeneity was detected (I2 = 0.0%; p = 0.696) (Figure 2). Thus, aspirin use showed no significant effect on all-cause mortality in patients with COVID-19.

FIGURE 2. Summary hazard ratio of all-cause mortality for aspirin use in randomized controlled trials.
The randomized controlled trial on the association between P2Y12 inhibitors and all-cause mortality reported 127 deaths among 448 patients taking P2Y12 inhibitors and 167 deaths among 521 patients receiving usual care, with an RR of 0.88 (95% CI 0.73–1.07). No significant effect was observed between P2Y12 inhibitor use and all-cause mortality among patients with COVID-19.
One prospective cohort study on the association between aspirin use and all-cause mortality reported 1,410 deaths among 13,795 patients taking aspirin and 11,577 deaths among 98,275 patients receiving usual care, with an OR of 0.85 (95% CI 0.80–0.90). A 15% reduction in all-cause mortality was observed after aspirin use among patients with COVID-19.
Prospective cohort studies showed no significant effect between the use of other antiplatelet drugs and all-cause mortality among patients with COVID-19 [OR, 1.49 (95% CI 0.62–3.60); p = 0.375] (Figure 3).
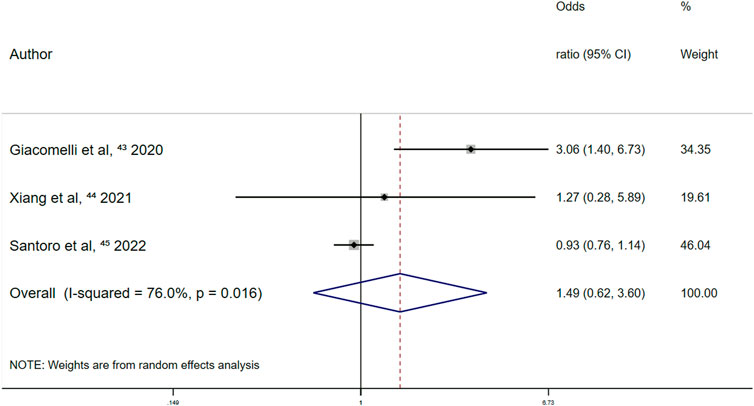
FIGURE 3. Summary odds ratio of all-cause mortality for other antiplatelet drugs use in prospective cohort studies.
Retrospective studies on the association between aspirin and all-cause mortality reported 2,152 deaths among 12,668 patients administered aspirin and 3,227 deaths among 27,645 patients in the control group. The summary random-effects OR was 0.80 (95% CI 0.70–0.93; p = 0.003) and high between-study heterogeneity was observed (I2 = 64.8%; p = 0.000) (Figure 4). A 20% reduction in all-cause mortality was observed after aspirin use among patients with COVID-19.
Retrospective studies reported no significant effect between the use of other antiplatelet drugs and all-cause mortality among patients with COVID-19 [OR, 1.23 (95% CI 0.85–1.78; p = 0.283)] (Figure 5).

FIGURE 5. Summary odds ratio of all-cause mortality for other antiplatelet drugs use in retrospective studies.
In studies in which aspirin was administered after admission, the overall random-effects OR was 0.65 (95% CI 0.46–0.93; p = 0.018) (Figure 6). A 35% reduction in all-cause mortality among COVID-19 patients was observed for aspirin use after admission. In studies where aspirin was administered before admission, the overall random-effects OR was 0.81 (95% CI 0.66–1.00; p = 0.046) (Figure 6). Therefore, aspirin use before admission was independently associated with a 19% reduction in all-cause mortality in patients with COVID-19. The ratio of ORs was 1.25.
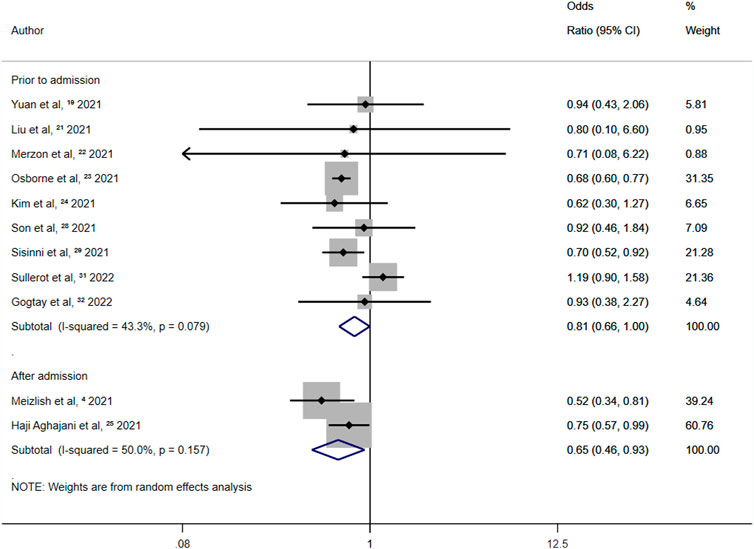
FIGURE 6. Summary odds ratio of all-cause mortality for aspirin use after admission and prior to admission.
Randomized controlled trials on the association between aspirin use and major bleeding events reported a summary fixed-effects RR of 1.63 (95% CI 1.23–2.15; p = 0.001). Slight between-study heterogeneity was observed (I2 = 15.4%; p = 0.307) (Figure 7). Thus, aspirin use was independently associated with a 63% increased risk of major bleeding events in patients with COVID-19.
The randomized controlled trial on the association between P2Y12 inhibitors and major bleeding events reported an RR of 5.81 (95% CI 1.28–26.4). Thus, the use of P2Y12 inhibitors increased the risk of major bleeding events among patients with COVID-19.
Randomized controlled trials on the association between aspirin use and overt thrombosis events reported a summary fixed-effects RR of 0.90 (95% CI 0.79–1.02; p = 0.097). No between-study heterogeneity was observed (I2 = 0.0%; p = 0.541) (Figure 8). This indicated no significant effect of aspirin use on overt thrombosis events in patients with COVID-19.
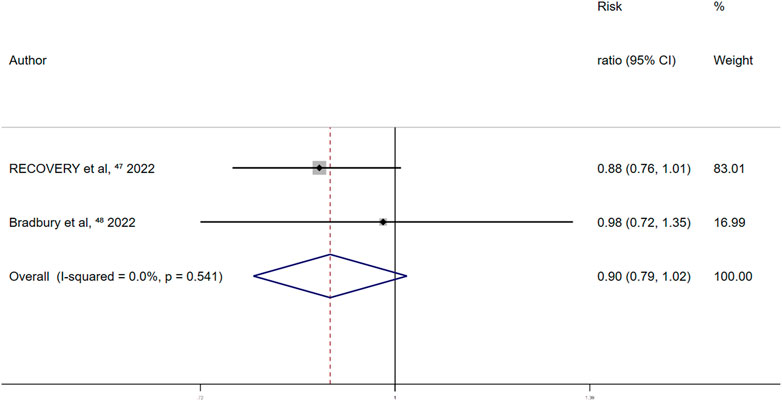
FIGURE 8. Summary hazard ratio of thrombotic events for aspirin use in randomized controlled trials.
The randomized controlled trial on the association between P2Y12 inhibitors and overt thrombosis events reported an RR of 0.77 (95% CI 0.53–1.11). This indicated no significant effect between the use of P2Y12 inhibitors and overt thrombosis events in patients with COVID-19.
Retrospective studies on the association between the use of other antiplatelet drugs and major bleeding events reported a summary random-effects OR of 1.72 (95% CI 0.50–5.90; p = 0.387) (Figure 9). This indicated that the use of other antiplatelet drugs was not significantly associated with major bleeding events in patients with COVID-19.
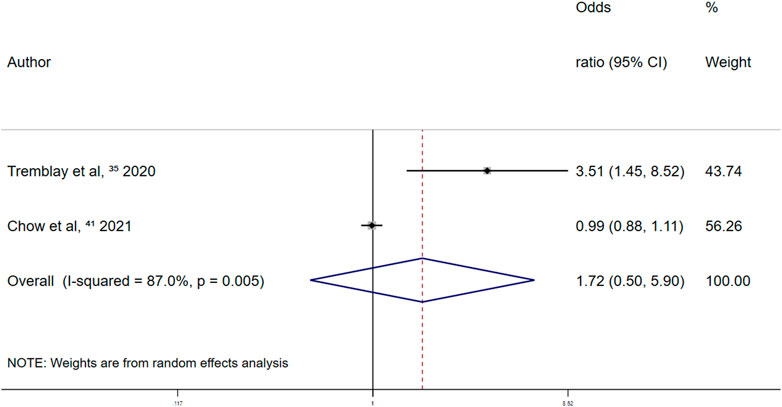
FIGURE 9. Summary odds ratio of bleeding events for other antiplatelet drugs use in retrospective studies.
One retrospective study on the association between aspirin and major bleeding events reported an OR of 0.79 (95% CI 0.31–1.99). This indicated that the use of aspirin was not significantly associated with major bleeding events in patients with COVID-19.
One prospective cohort study on the association between aspirin and major bleeding events reported an OR of 1.14 (95% CI 1.07–1.22). Aspirin use resulted in a 14% increase in major bleeding events among patients with COVID-19.
One prospective cohort study that explored the association between other antiplatelet drugs and major bleeding events, reported an OR of 0.85 (95% CI 0.5–1.45). This indicated that the use of other antiplatelet drugs was not significantly associated with major bleeding events in patients with COVID-19.
In the retrospective studies on the association between aspirin and overt thrombosis events, the summary fixed-effects OR was 1.05 (95% CI 0.76–1.43; p = 0.779) (Figure 10). Aspirin use and overt thrombosis events were not significantly associated in patients with COVID-19.
In the retrospective studies on the association between other antiplatelet drugs and overt thrombosis events, the summary random-effects OR was 1.29 (95% CI 0.58–2.88; p = 0.530) (Figure 11). Thus, the use of other antiplatelet drugs was not significantly associated with overt thrombosis events in patients with COVID-19.
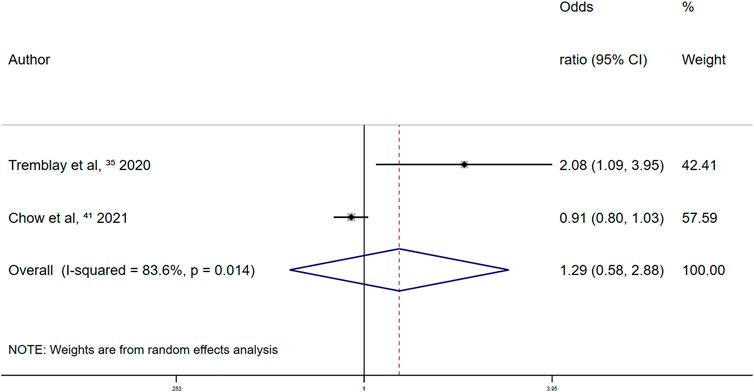
FIGURE 11. Summary odds ratio of thrombotic events for other antiplatelet drugs use in retrospective studies.
One prospective cohort study on the association between aspirin and overt thrombosis events reported an OR of 0.55 (95% CI 0.47–0.64), corresponding to 45% fewer overt thrombosis events in patients with COVID-19 after aspirin use.
One prospective cohort study on the association between other antiplatelet drug use and overt thrombosis events reported an OR of 1.15 (95% CI 0.73–1.82). Hence, the use of other antiplatelet drugs was not significantly associated with overt thrombosis events in patients with COVID-19.
The retrospective and prospective cohort studies showed low-to-moderate risks of bias based on the NOS. Table 1 shows the results of these quality assessments, indicating that of the retrospective and prospective cohort studies, 29 were good, while the remaining two were of fair quality. Regarding the three randomized controlled trials assessed by the Cochrane Risk of Bias Assessment Tool, the risk of bias was low in one trial in terms of mortality results and high in the other two trials, as these trials followed an open-label design (Table 2).

TABLE 1. The qualities of retrospective and prospective cohort studies assessed by the Newcastle-Ottawa scale.
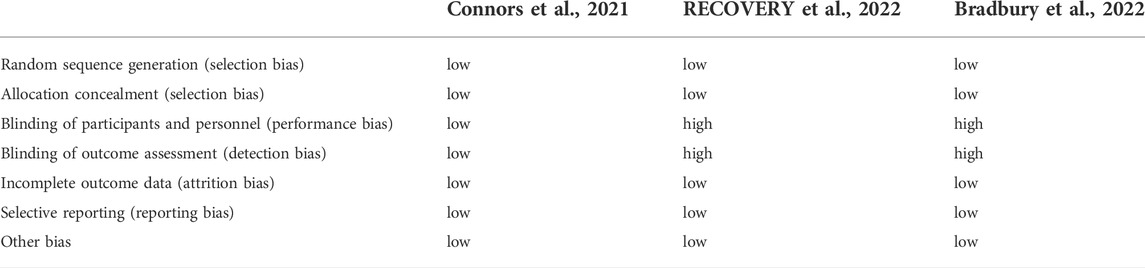
TABLE 2. The qualities of randomized controlled trials assessed by Cochrane Risk of Bias Assessment Tool.
This meta-analysis included three randomized controlled trials comprising 17,225 patients, 27 retrospective studies including 96,552 patients, and four prospective cohort studies including 120,019 patients. The retrospective and prospective cohort studies showed that the administration of aspirin was associated with lower mortality among patients with COVID-19. Therefore, aspirin may be an effective treatment for COVID-19.
In one randomized controlled trial, other drugs may have affected the antithrombotic effect of aspirin. In the RECOVERY trial (RECOVERY Collaborative GroupAbbas et al., 2022), 94% and 93% of patients were taking corticosteroids and heparin, respectively, compared to 98% and 93% of patients in the study by Bradbury et al. (REMAP-CAP Writing Committee for the REMAP-CAP InvestigatorsBradbury et al., 2022) Both corticosteroids and heparin have antithrombotic effects, (Steinlin et al., 2017; Qiu et al., 2021), which may have prevented the exposed group from gaining significant benefits from aspirin.
The OR of the association between aspirin and all-cause mortality was lower when aspirin was administered after admission compared to that before admission. Thus, aspirin may be more beneficial in reducing all-cause mortality in patients with COVID-19 when administered after admission.
Traditionally, antithrombotic effects are achieved at low doses (75 mg/day–81 mg/day), analgesic and antipyretic effects are achieved at intermediate doses (650 mg–4 g/day), and anti-inflammatory effects are observed at high doses (4 g/day–8 g/day) of aspirin (Pillinger et al., 1998). Among the studies on aspirin dose included in the present meta-analysis, the aspirin dose was generally low to achieve an antithrombotic effect.
Evidence regarding aspirin reducing mortality in patients with COVID-19 is unclear. Previous studies mainly recognized the antiplatelet, antithrombotic, and anti-inflammatory effects of aspirin (Simon et al., 2020; Connors and Ridker, 2022). Aspirin is part of an effective antiplatelet regimen, (Antithrombotic Trialists' Collaboration, 2002), and exerts antithrombotic effects due to its inhibition of platelet function by the acetylation of platelet cyclooxygenase (COX)-1 at the functionally important amino acid residue serine 529 (Schrör, 1997). Our results showed that aspirin reduced mortality among patients with COVID-19, whereas other antiplatelet drugs did not, suggesting that the antiplatelet effects of aspirin may not reduce mortality among patients with COVID-19. However, the antiplatelet effects of aspirin may play a role in cases with bleeding, which is a serious adverse reaction.
The relationship between SARS-CoV-2 infection and the development of acute respiratory distress syndrome (ARDS) cannot be ignored (Asselah et al., 2021). SARS-CoV-2 infection can elevate plasma proinflammatory cytokine levels and cause an inflammatory “cytokine storm.” (Mehta et al., 2020; Ramasamy and Subbian, 2021) In sepsis and ARDS, multiple pathogenic mechanisms implicated in the development of multiple organ dysfunction can be modulated by aspirin (Toner et al., 2015; Yu et al., 2018). Regarding the pathogenesis and complications of COVID-19, inflammatory mediators are critical, among which aspirin has demonstrated a potential survival benefit through its anti-inflammatory actions in COVID-19 (Hussain et al., 2012; Kelleni, 2022). However, it is not clear whether antiplatelet therapy is associated with reduced morbidity in high-risk patients with ARDS (Wang et al., 2018).
The enzyme COX-2, which leads to the formation of prostaglandins, causes inflammation, swelling, pain, and fever (Schrör, 1997; Vane and Botting, 2003). However, aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) inhibit the activity of this enzyme to exert anti-inflammatory, analgesic, and antipyretic actions. NSAIDs do not significantly reduce the risk of SARS-COV-2 infection, (Moore et al., 2021), which may mean that aspirin does not reduce the mortality of patients with COVID-19 through its anti-inflammatory, analgesic, and antipyretic actions.
Aspirin has specific antiviral activities against influenza A and human rhinovirus (Glatthaar-Saalmüller et al., 2017). Published articles indicate the significant antiviral activities of aspirin against DNA and RNA viruses, including different human coronaviruses (Bianconi et al., 2020). Aspirin targeting of intracellular signaling pathways critical for viral replication could serve as a reliable adjunctive treatment option in patients with COVID-19 (Tantry et al., 2021).
Among deaths due to COVID-19, 11.8% of patients without underlying cardiovascular disease had severe heart damage, according to the National Health Commission (NHC) report (Zheng et al., 2020). Previous studies also reported that COVID-19 can cause myocardial damage and increase the incidence of cardiovascular diseases, resulting in increased mortality (Bianconi et al., 2020). The prognosis of patients with underlying cardiovascular disease but without myocardial injury is relatively favorable, (Clerkin et al., 2020; Guo et al., 2020), which highlights the correlation between COVID-19 and myocardial damage. However, various theories have been proposed regarding the mechanism by which COVID-19 causes an increased incidence of cardiovascular diseases. COVID-19 may cause the production of a variety of cytokines, resulting in coronary microvascular endothelial injury, plaque destruction, thrombosis generation and cardiomyocyte inflammation, apoptosis, and necrosis (Guo et al., 2020; Zheng et al., 2020). SARS-CoV-2 hydrolyzes the S protein through serine protease, binds with transmembrane angiotensin-converting enzyme 2 (ACE2), and enters cardiomyocytes, which may lead to viral myocarditis (Bonow et al., 2020; Zheng et al., 2020). The reduction of COVID-19 mortality due to aspirin use may be attributed to the reduction of myocardial injury by aspirin’s anti-inflammatory and antithrombotic effects, as well as its protective effects in the coronary artery. However, the outcome of the 34 studies included in this analysis was all-cause rather than cardiovascular disease mortality. Therefore, whether aspirin reduces the mortality of patients with COVID-19 owing to its protective effect on the coronary artery and myocardium requires further research.
Prospective cohort studies showed that aspirin increases the risk of bleeding events and decreases the risk of thrombotic events. The three randomized controlled trials assessed bleeding events and concluded that aspirin was associated with higher risks of bleeding events. In the randomized controlled trials, the random-effects RR for the trials that did and did not use heparin were 2.21 (95% CI 0.76–6.44) (Figure 12) and 1.89 (95% CI 0.48–7.4), respectively, suggesting an increased risk of bleeding with heparin use. Two randomized controlled trials mentioned thrombotic events, in which aspirin did not reduce the risk of thrombotic events in patients with COVID-19.
This study had several limitations. First, retrospective and prospective cohort studies accounted for a large proportion (92.6%) of the studies included in the meta-analyses, which may be prone to bias. Second, owing to the observational nature of the present study, selection bias was possible, as patients requiring aspirin may be at high risk for potential comorbidities or thrombosis. Third, the data were only searched in PubMed, which may have missed relevant articles. Fourth, our study only included an assessment of mortality over a relatively short period and did not include reports of long-term mortality, including post-discharge mortality. Fifth, the trials recruited only adults; thus, the effect of aspirin on children remains unclear. Sixth, the endpoint difference in mortality may have led to inconsistencies among trial results. Seventh, some ongoing randomized controlled trials were not included in our study. Eighth, in the retrospective studies, the dosage of aspirin was mainly the conventional dose, and high doses were ignored, which may have led to incomplete results. Ninth, among randomized controlled trials, the sample size of the RECOVERY study was large, with a weight of 86.47%, (RECOVERY Collaborative GroupAbbas et al., 2022), which may have caused bias. Tenth, studies on the effect of anticoagulants were excluded, (Viecca et al., 2020; Matli et al., 2021; Rivera-Caravaca et al., 2021; Berger et al., 2022; Santoro et al., 2022b; Rivera-Caravaca et al., 2022), which could lead to bias. For example, although approximately 10% of the patients in these two studies were taking antiplatelet drugs, the main purpose aimed at determining the effect of oral anticoagulants on the prognosis of patients with COVID-19; thus, some studies were excluded (Rivera-Caravaca et al., 2021; Rivera-Caravaca et al., 2022). More recently, Santoro et al. demonstrated the association of aspirin combined with prophylactic anticoagulation therapy with lower mortality risk among patients with COVID-19 (Santoro et al., 2022b). Eleventh, the prognosis of patients with COVID-19 is influenced by many factors, such as cancer, (Pérez-Segura et al., 2021), arterial hypertension, (El-Battrawy et al., 2021), and kidney disease, (Uribarri et al., 2020), which may bias the results of this study, as not all 34 studies were well matched with these factors.
The results of this meta-analysis of patients with COVID-19 showed that the administration of aspirin may reduce all-cause mortality; however, these findings require confirmation by randomized controlled trials.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
WS, RD and TH had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. WS is first author. RD and TH designed the research. All authors contributed to the acquisition, analysis, or interpretation of data. WS drafted the manuscript. RD contributed to the critical revision of the manuscript for important intellectual content. WS, HM, ZG and QC contributed to the statistical analysis. RD and TH obtained funding. All authors read and approved the final manuscript.
The study was supported by grants from the National Key R&D Program of China (2019YFC2003401), the National Natural Science Foundation of China (82173499), LiaoNing Revitalization Talents Program (XLYC2007001), Changjiang Scholars Program of Ministry of Education of China (TG2019081) and High-performance Computing Platform of Peking University. The funders had no role in the study design, data collection, data analysis and interpretation, writing of the report, or the decision to submit the article for publication.
We thank the study authors who provided data and extra information for this meta-analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.989903/full#supplementary-material.
Abdelwahab, H. W., Shaltout, S. W., Sayed Ahmed, H. A., Fouad, A. M., Merrell, E., Riley, J. B., et al. (2021). Acetylsalicylic acid compared with enoxaparin for the prevention of thrombosis and mechanical ventilation in COVID-19 patients: A retrospective cohort study. Clin. Drug Investig. 41, 723–732. Epub 2021 Jul 30. doi:10.1007/s40261-021-01061-2
Abu-Jamous, B., Anisimovich, A., Baxter, J., Mackillop, L., Vizcaychipi, M. P., McCarthy, P., et al. (2020). Associations of comorbidities and medications with COVID-19 outcome: A retrospective analysis of real-world evidence data. Prepr. Medrxiv. 2020. doi:10.1101/2020.08.20.20174169
Al Harthi, A. F., Aljuhani, O., Korayem, G. B., Altebainawi, A. F., Alenezi, R. S., Al Harbi, S., et al. (2022). Evaluation of low-dose aspirin use among critically Ill patients with COVID-19: A multicenter propensity score matched study. J. Intensive Care Med. 37, 1238–1249. Epub ahead of print. doi:10.1177/08850666221093229
Alamdari, N. M., Afaghi, S., Rahimi, F. S., Tarki, F. E., Tavana, S., Zali, A., et al. (2020). Mortality risk factors among hospitalized COVID-19 patients in a major referral center in Iran. Tohoku J. Exp. Med. 252, 73–84. doi:10.1620/tjem.252.73
Antithrombotic Trialists' Collaboration (2002). Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324, 71–86. doi:10.1136/bmj.324.7329.71
Argenziano, M. G., Bruce, S. L., Slater, C. L., Tiao, J. R., Baldwin, M. R., Barr, R. G., et al. (2020). Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ 369, m1996. doi:10.1136/bmj.m1996
Asselah, T., Durantel, D., Pasmant, E., Lau, G., and Schinazi, R. F. (2021). COVID-19: Discovery, diagnostics and drug development. J. Hepatol. 74, 168–184. Epub 2020 Oct 8. doi:10.1016/j.jhep.2020.09.031
Berger, J. S., Kornblith, L. Z., Gong, M. N., Reynolds, H. R., Cushman, M., Cheng, Y., et al. (2022). Effect of P2Y12 inhibitors on survival free of organ Support among non-critically Ill hospitalized patients with COVID-19: A randomized clinical trial. JAMA 327, 227–236. doi:10.1001/jama.2021.23605
Bianconi, V., Violi, F., Fallarino, F., Pignatelli, P., Sahebkar, A., and Pirro, M. (2020). Is acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID-19? Drugs 80, 1383–1396. doi:10.1007/s40265-020-01365-1
Bonow, R. O., Fonarow, G. C., O'Gara, P. T., and Yancy, C. W. (2020). Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 5, 751–753. doi:10.1001/jamacardio.2020.1105
Chow, J. H., Khanna, A. K., Kethireddy, S., Yamane, D., Levine, A., Jackson, A. M., et al. (2021). Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth. Analg. 132, 930–941. doi:10.1213/ANE.0000000000005292
Chow, J. H., Rahnavard, A., Gomberg-Maitland, M., Chatterjee, R., Patodi, P., Yamane, D. P., et al. (2022). Association of early aspirin use with in-hospital mortality in patients with moderate COVID-19. JAMA Netw. Open 5, e223890. doi:10.1001/jamanetworkopen.2022.3890
Chow, J. H., Yin, Y., Yamane, D. P., Davison, D., Keneally, R. J., Hawkins, K., et al. (2021). Association of prehospital antiplatelet therapy with survival in patients hospitalized with COVID-19: A propensity score-matched analysis. J. Thromb. Haemost. 19, 2814–2824. Epub. doi:10.1111/jth.15517
Clerkin, K. J., Fried, J. A., Raikhelkar, J., Sayer, G., Griffin, J. M., Masoumi, A., et al. (2020). COVID-19 and cardiovascular disease. Circulation 141, 1648–1655. Epub 2020 Mar 21. doi:10.1161/CIRCULATIONAHA.120.046941
Connors, J. M., Brooks, M. M., Sciurba, F. C., Krishnan, J. A., Bledsoe, J. R., Kindzelski, A., et al. (2021). Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: The ACTIV-4B randomized clinical trial. JAMA 326, 1703–1712. doi:10.1001/jama.2021.17272
Connors, J. M., and Ridker, P. M. (2022). Thromboinflammation and antithrombotics in COVID-19: Accumulating evidence and current status. JAMA 327, 1234–1235. doi:10.1001/jama.2022.2361
Dreher, M., Kersten, A., Bickenbach, J., Balfanz, P., Hartmann, B., Cornelissen, C., et al. (2020). The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch. Arztebl. Int. 117, 271–278. doi:10.3238/arztebl.2020.0271
El-Battrawy, I., Nuñez-Gil, I. J., Abumayyaleh, M., Estrada, V., Manuel Becerra-Muñoz, V., Uribarri, A., et al. (2021). COVID-19 and the impact of arterial hypertension-An analysis of the international HOPE COVID-19 Registry (Italy-Spain-Germany). Eur. J. Clin. Invest. 51, e13582. Epub 2021 Aug 19. doi:10.1111/eci.13582
Formiga, F., Rubio-Rivas, M., Mora-Luján, J. M., Escudero, S. C., Martinez, R. F. M., Mendez-Bailón, M., et al. (2022). Does admission acetylsalicylic acid uptake in hospitalized COVID-19 patients have a protective role? Data from the Spanish SEMI-COVID-19 registry. Intern. Emerg. Med. 17, 761–775. Epub 2021 Nov 29. doi:10.1007/s11739-021-02870-1
Fröhlich, G. M., Jeschke, E., Eichler, U., Thiele, H., Alhariri, L., Reinthaler, M., et al. (2021). Impact of oral anticoagulation on clinical outcomes of COVID-19: A nationwide cohort study of hospitalized patients in Germany. Clin. Res. Cardiol. 110, 1041–1050. Epub 2021 Jan 8. doi:10.1007/s00392-020-01783-x
Giacomelli, A., Ridolfo, A. L., Milazzo, L., Oreni, L., Bernacchia, D., Siano, M., et al. (2020). 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: A prospective cohort study. Pharmacol. Res. 158, 104931. doi:10.1016/j.phrs.2020.104931
Glatthaar-Saalmüller, B., Mair, K. H., and Saalmüller, A. (2017). Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study. Influenza Other Respir. Viruses 11, 85–92. Epub 2016 Sep 22. doi:10.1111/irv.12421
Gogtay, M., Singh, Y., Bullappa, A., and Scott, J. (2022). Retrospective analysis of aspirin's role in the severity of COVID-19 pneumonia. World J. Crit. Care Med. 11, 92–101. doi:10.5492/wjccm.v11.i2.92
Guo, T., Fan, Y., Chen, M., Wu, X., Zhang, L., He, T., et al. (2020). Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5, 811–818. doi:10.1001/jamacardio.2020.1017
Haji Aghajani, M., Moradi, O., Amini, H., Azhdari Tehrani, H., Pourheidar, E., Rabiei, M. M., et al. (2021). Decreased in-hospital mortality associated with aspirin administration in hospitalized patients due to severe COVID-19. J. Med. Virol. 93, 5390–5395. doi:10.1002/jmv.27053
Ho, G., Dusendang, J. R., Schmittdiel, J., Kavecansky, J., Tavakoli, J., and Pai, A. (2021). Association of chronic anticoagulant and antiplatelet use on disease severity in SARS-COV-2 infected patients. J. Thromb. Thrombolysis 52, 476–481. Epub 2021 Feb 1. doi:10.1007/s11239-021-02383-w
Hussain, M., Javeed, A., Ashraf, M., Zhao, Y., Mukhtar, M. M., and Rehman, M. U. (2012). Aspirin and immune system. Int. Immunopharmacol. 12, 10–20. Epub 2011 Dec 13. doi:10.1016/j.intimp.2011.11.021
Kelleni, M. T. (2022). NSAIDs/nitazoxanide/azithromycin repurposed for COVID-19: Potential mitigation of the cytokine storm interleukin-6 amplifier via immunomodulatory effects. Expert Rev. anti. Infect. Ther. 20, 17–21. Epub 2021 Jun 15. doi:10.1080/14787210.2021.1939683
Kevorkian, J. P., Lopes, A., Sène, D., Riveline, J. P., Vandiedonck, C., Féron, F., et al. (2021). Oral corticoid, aspirin, anticoagulant, colchicine, and furosemide to improve the outcome of hospitalized COVID-19 patients - the COCAA-COLA cohort study. J. Infect. 82, 276–316. Epub 2021 Feb 9. doi:10.1016/j.jinf.2021.02.008
Kim, I., Yoon, S., Kim, M., Lee, H., Park, S., Kim, W., et al. (2021). Aspirin is related to worse clinical outcomes of COVID-19. Med. Kaunas. 57, 931. doi:10.3390/medicina57090931
Kow, C. S., and Hasan, S. S. (2021). Use of antiplatelet drugs and the risk of mortality in patients with COVID-19: A meta-analysis. J. Thromb. Thrombolysis 52, 124–129. Epub 2021 Apr 4. doi:10.1007/s11239-021-02436-0
Liu, Q., Huang, N., Li, A., Zhou, Y., Liang, L., Song, X., et al. (2021). Effect of low-dose aspirin on mortality and viral duration of the hospitalized adults with COVID-19. Med. Baltim. 100, e24544. doi:10.1097/MD.0000000000024544
Lodigiani, C., Iapichino, G., Carenzo, L., Cecconi, M., Ferrazzi, P., Sebastian, T., et al. (2020). Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 191, 9–14. Epub 2020 Apr 23. doi:10.1016/j.thromres.2020.04.024
Lopes, R. D., de Barros E Silva, P. G. M., Furtado, R. H. M., Macedo, A. V. S., Bronhara, B., Damiani, L. P., et al. (2021). Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): An open-label, multicentre, randomised, controlled trial. Lancet 397, 2253–2263. Epub 2021 Jun 4. doi:10.1016/S0140-6736(21)01203-4
Martha, J. W., Pranata, R., Lim, M. A., Wibowo, A., and Akbar, M. R. (2021). Active prescription of low-dose aspirin during or prior to hospitalization and mortality in COVID-19: A systematic review and meta-analysis of adjusted effect estimates. Int. J. Infect. Dis. 108, 6–12. Epub 2021 May 15. doi:10.1016/j.ijid.2021.05.016
Matli, K., Chamoun, N., Fares, A., Zibara, V., Al-Osta, S., Nasrallah, R., et al. (2021). Combined anticoagulant and antiplatelet therapy is associated with an improved outcome in hospitalised patients with COVID-19: A propensity matched cohort study. Open Heart 8, e001785. doi:10.1136/openhrt-2021-001785
Mega, J. L., and Simon, T. (2015). Pharmacology of antithrombotic drugs: An assessment of oral antiplatelet and anticoagulant treatments. Lancet 386, 281–291. Epub 2015 Mar 14. doi:10.1016/S0140-6736(15)60243-4
Mehta, P., McAuley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., and Manson, J. J.HLH Across Speciality Collaboration, UK (2020). COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034. doi:10.1016/S0140-6736(20)30628-0
Mei, H., Luo, L., and Hu, Y. (2020). Thrombocytopenia and thrombosis in hospitalized patients with COVID-19. J. Hematol. Oncol. 13, 161. doi:10.1186/s13045-020-01003-z
Meizlish, M. L., Goshua, G., Liu, Y., Fine, R., Amin, K., Chang, E., et al. (2021). Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: A propensity score-matched analysis. Am. J. Hematol. 96, 471–479. Epub 2021 Feb 22. doi:10.1002/ajh.26102
Merzon, E., Green, I., Vinker, S., Golan-Cohen, A., Gorohovski, A., Avramovich, E., et al. (2021). The use of aspirin for primary prevention of cardiovascular disease is associated with a lower likelihood of COVID-19 infection. FEBS J. 288, 5179–5189. Epub 2021 Apr 19. doi:10.1111/febs.15784
Middeldorp, S., Coppens, M., van Haaps, T. F., Foppen, M., Vlaar, A. P., Müller, M. C. A., et al. (2020). Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 18, 1995–2002. Epub 2020 Jul 27. doi:10.1111/jth.14888
Moore, N., Bosco-Levy, P., Thurin, N., Blin, P., and Droz-Perroteau, C. (2021). NSAIDs and COVID-19: A systematic review and meta-analysis. Drug Saf. 44, 929–938. Epub 2021 Aug 2. doi:10.1007/s40264-021-01089-5
Mura, C., Preissner, S., Nahles, S., Heiland, M., Bourne, P. E., and Preissner, R. (2021). Real-world evidence for improved outcomes with histamine antagonists and aspirin in 22, 560 COVID-19 patients. Signal Transduct. Target. Ther. 6, 267. doi:10.1038/s41392-021-00689-y
Osborne, T. F., Veigulis, Z. P., Arreola, D. M., Mahajan, S. M., Röösli, E., and Curtin, C. M. (2021). Association of mortality and aspirin prescription for COVID-19 patients at the Veterans Health Administration. PLoS One 16, e0246825. doi:10.1371/journal.pone.0246825
Oudkerk, M., Büller, H. R., Kuijpers, D., van Es, N., Oudkerk, S. F., McLoud, T., et al. (2020). Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the national institute for public Health of The Netherlands. Radiology 297, E216–E222. Epub 2020 Apr 23. doi:10.1148/radiol.2020201629
Pan, D., Ip, A., Zhan, S., Wasserman, I., Snyder, D. J., Agathis, A. Z., et al. (2021). Pre-hospital antiplatelet medication use on COVID-19 disease severity. Heart Lung. 50, 618–621. doi:10.1016/j.hrtlng.2021.04.010
Pérez-Segura, P., Paz-Cabezas, M., Núñez-Gil, I. J., Arroyo-Espliguero, R., Maroun Eid, C., Romero, R., et al. (2021). Prognostic factors at admission on patients with cancer and COVID-19: Analysis of HOPE registry data. Med. Clin. 157, 318–324. doi:10.1016/j.medcli.2021.02.021
Pillinger, M. H., Capodici, C., Rosenthal, P., Kheterpal, N., Hanft, S., Philips, M. R., et al. (1998). Modes of action of aspirin-like drugs: Salicylates inhibit erk activation and integrin-dependent neutrophil adhesion. Proc. Natl. Acad. Sci. U. S. A. 95, 14540–14545. doi:10.1073/pnas.95.24.14540
Qiu, M., Huang, S., Luo, C., Wu, Z., Liang, B., Huang, H., et al. (2021). Pharmacological and clinical application of heparin progress: An essential drug for modern medicine. Biomed. Pharmacother. 139, 111561. doi:10.1016/j.biopha.2021.111561
Ramasamy, S., and Subbian, S. (2021). Critical determinants of cytokine storm and type I interferon response in COVID-19 pathogenesis. Clin. Microbiol. Rev. 34, 002999–e320. doi:10.1128/CMR.00299-20
Recovery Collaborative Group, , Abbas, A., Abbas, F., Abbas, M., Abbasi, S., Abbass, H., et al. (2022). Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 399, 143–151. Epub 2021 Nov 17. doi:10.1016/S0140-6736(21)01825-0
Regina, J., Papadimitriou-Olivgeris, M., Burger, R., Le Pogam, M. A., Niemi, T., Filippidis, P., et al. (2020). Epidemiology, risk factors and clinical course of SARS-CoV-2 infected patients in a Swiss University hospital: An observational retrospective study. PLoS One 15, e0240781. doi:10.1371/journal.pone.0240781
Remap-Cap Writing Committee for the Remap-Cap Investigators Bradbury, C. A., Lawler, P. R., Stanworth, S. J., McVerry, B. J., McQuilten, Z., et al. (2022). Effect of antiplatelet therapy on survival and organ support-free days in critically Ill patients with COVID-19: A randomized clinical trial. JAMA 327, 1247–1259. doi:10.1001/jama.2022.2910
Rivera-Caravaca, J. M., Núñez-Gil, I. J., Lip, G. Y. H., Uribarri, A., Viana-Llamas, M. C., Gonzalez, A., et al. (2022). Chronic oral anticoagulation therapy and prognosis of patients admitted to hospital for COVID-19: Insights from the HOPE COVID-19 registry. Int. J. Clin. Pract. 2022, 7325060. doi:10.1155/2022/7325060
Rivera-Caravaca, J. M., Núñez-Gil, I. J., Vivas, D., Viana-Llamas, M. C., Uribarri, A., Becerra-Muñoz, V. M., et al. (2021). Clinical profile and prognosis in patients on oral anticoagulation before admission for COVID-19. Eur. J. Clin. Invest. 51, e13436. Epub 2020 Nov 7. doi:10.1111/eci.13436
Russo, V., Di Maio, M., Attena, E., Silverio, A., Scudiero, F., Celentani, D., et al. (2020). Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: A multicenter observational study. Pharmacol. Res. 159, 104965. doi:10.1016/j.phrs.2020.104965
Sahai, A., Bhandari, R., Koupenova, M., Freedman, J., Godwin, M., McIntyre, T., et al. (2020). SARS-CoV-2 receptors are expressed on human platelets and the effect of aspirin on clinical outcomes in COVID-19 patients. Res. Sq. rs.3, rs-119031. doi:10.21203/rs.3.rs-119031/v1):
Salah, H. M., and Mehta, J. L. (2021). Meta-analysis of the effect of aspirin on mortality in COVID-19. Am. J. Cardiol. 142, 158–159. Epub 2021 Jan 6. doi:10.1016/j.amjcard.2020.12.073
Santoro, F., Nuñez-Gil, I. J., Vitale, E., Viana-Llamas, M. C., Reche-Martinez, B., Romero-Pareja, R., et al. (2022). Antiplatelet therapy and outcome in COVID-19: The Health outcome predictive evaluation registry. Heart 108, 130–136. Epub 2021 Oct 5. doi:10.1136/heartjnl-2021-319552
Santoro, F., Núñez-Gil, I. J., Vitale, E., Viana-Llamas, M. C., Romero, R., Maroun Eid, C., et al. (2022). Aspirin therapy on prophylactic anticoagulation for patients hospitalized with COVID-19: A propensity score-matched cohort analysis of the hope-COVID-19 registry. J. Am. Heart Assoc. 11, e024530. Epub ahead of print. doi:10.1161/JAHA.121.024530
Schrör, K. (1997). Aspirin and platelets: The antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin. Thromb. Hemost. 23, 349–356. doi:10.1055/s-2007-996108
Simon, T. G., Duberg, A. S., Aleman, S., Chung, R. T., Chan, A. T., and Ludvigsson, J. F. (2020). Association of aspirin with hepatocellular carcinoma and liver-related mortality. N. Engl. J. Med. 382, 1018–1028. doi:10.1056/NEJMoa1912035
Sisinni, A., Rossi, L., Battista, A., Poletti, E., Battista, F., Battista, R. A., et al. (2021). Pre-admission acetylsalicylic acid therapy and impact on in-hospital outcome in COVID-19 patients: The ASA-CARE study. Int. J. Cardiol. 344, 240–245. Epub 2021 Oct 4. doi:10.1016/j.ijcard.2021.09.058
Sivaloganathan, H., Ladikou, E. E., and Chevassut, T. (2020). COVID-19 mortality in patients on anticoagulants and antiplatelet agents. Br. J. Haematol. 190, e192–e195. Epub 2020 Jul 19. doi:10.1111/bjh.16968
Son, M., Noh, M. G., Lee, J. H., Seo, J., Park, H., and Yang, S. (2021). Effect of aspirin on coronavirus disease 2019: A nationwide case-control study in South Korea. Med. Baltim. 100, e26670. doi:10.1097/MD.0000000000026670
Srivastava, R., and Kumar, A. (2021). Use of aspirin in reduction of mortality of COVID-19 patients: A meta-analysis. Int. J. Clin. Pract. 75 (11), e14515. Epub 2021 Jun 28. doi:10.1111/ijcp.14515
Steinlin, M., Bigi, S., Stojanovski, B., Gajera, J., Regényi, M., El-Koussy, M., et al. (2017). Focal cerebral arteriopathy: Do steroids improve outcome? Stroke 48, 2375–2382. Epub 2017 Jul 21. doi:10.1161/STROKEAHA.117.016818
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Sullerot, C., Bouiller, K., Laborde, C., Gilis, M., Fèvre, A., Hacquin, A., et al. (2022). Premorbid aspirin use is not associated with lower mortality in older inpatients with SARS-CoV-2 pneumonia. Geroscience 44, 573–583. Epub 2022 Jan 7. doi:10.1007/s11357-021-00499-8
Tantry, U. S., Schror, K., Navarese, E. P., Jeong, Y. H., Kubica, J., Bliden, K. P., et al. (2021). Aspirin as an adjunctive pharmacologic therapy option for COVID-19: Anti-inflammatory, antithrombotic, and antiviral effects all in one agent. J. Exp. Pharmacol. 13, 957–970. doi:10.2147/JEP.S330776
Toner, P., McAuley, D. F., and Shyamsundar, M. (2015). Aspirin as a potential treatment in sepsis or acute respiratory distress syndrome. Crit. Care 19, 374. doi:10.1186/s13054-015-1091-6
Tremblay, D., van Gerwen, M., Alsen, M., Thibaud, S., Kessler, A., Venugopal, S., et al. (2020). Impact of anticoagulation prior to COVID-19 infection: A propensity score-matched cohort study. Blood 136, 144–147. doi:10.1182/blood.2020006941
Uribarri, A., Núñez-Gil, I. J., Aparisi, A., Becerra-Muñoz, V. M., Feltes, G., Trabattoni, D., et al. (2020). Impact of renal function on admission in COVID-19 patients: An analysis of the international HOPE COVID-19 (Health outcome predictive evaluation for COVID 19) registry. J. Nephrol. 33, 737–745. Epub 2020 Jun 29. doi:10.1007/s40620-020-00790-5
Vane, J. R., and Botting, R. M. (2003). The mechanism of action of aspirin. Thromb. Res. 110, 255–258. doi:10.1016/s0049-3848(03)00379-7
Viecca, M., Radovanovic, D., Forleo, G. B., and Santus, P. (2020). Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study. Pharmacol. Res. 158, 104950. doi:10.1016/j.phrs.2020.104950
Wang, Y., Ao, G., Nasr, B., and Qi, X. (2021). Effect of antiplatelet treatments on patients with COVID-19 infection: A systematic review and meta-analysis. Am. J. Emerg. Med. 43, 27–30. Epub 2021 Jan 13. doi:10.1016/j.ajem.2021.01.016
Wang, Y., Zhong, M., Wang, Z., Song, J., Wu, W., and Zhu, D. (2018). The preventive effect of antiplatelet therapy in acute respiratory distress syndrome: A meta-analysis. Crit. Care 22, 60. doi:10.1186/s13054-018-1988-y
Wells, G., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M., et al. (2000). The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute.
Xiang, Y., Wong, K. C., and So, H. C. (2021). Exploring drugs and vaccines associated with altered risks and severity of COVID-19: A UK biobank cohort study of all atc level-4 drug categories reveals repositioning opportunities. Pharmaceutics 13, 1514. doi:10.3390/pharmaceutics13091514
Yu, H., Ni, Y. N., Liang, Z. A., Liang, B. M., and Wang, Y. (2018). The effect of aspirin in preventing the acute respiratory distress syndrome/acute lung injury: A meta-analysis. Am. J. Emerg. Med. 36, 1486–1491. Epub 2018 May 21. doi:10.1016/j.ajem.2018.05.017
Yuan, S., Chen, P., Li, H., Chen, C., Wang, F., and Wang, D. W. (2021). Mortality and pre-hospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease. J. Cell. Mol. Med. 25, 1263–1273. Epub 2020 Dec 18. doi:10.1111/jcmm.16198
Zhao, X., Gao, C., Dai, F., Treggiari, M. M., Deshpande, R., and Meng, L. (2021). Treatments associated with lower mortality among critically Ill COVID-19 patients: A retrospective cohort study. Anesthesiology 135, 1076–1090. doi:10.1097/ALN.0000000000003999
Zheng, Y. Y., Ma, Y. T., Zhang, J. Y., and Xie, X. (2020). COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 17, 259–260. doi:10.1038/s41569-020-0360-5
Keywords: aspirin, antiplatelet drug, mortality, COVID-19, meta-analysis
Citation: Su W, Miao H, Guo Z, Chen Q, Huang T and Ding R (2022) Associations between the use of aspirin or other antiplatelet drugs and all-cause mortality among patients with COVID-19: A meta-analysis. Front. Pharmacol. 13:989903. doi: 10.3389/fphar.2022.989903
Received: 11 July 2022; Accepted: 21 September 2022;
Published: 05 October 2022.
Edited by:
Christos Kontogiorgis, Democritus University of Thrace, GreeceReviewed by:
Francesco Santoro, University of Foggia, ItalyCopyright © 2022 Su, Miao, Guo, Chen, Huang and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Huang, aHVhbmcudGFvQHBrdS5lZHUuY24=; Renyu Ding, cmVueXVkaW5nQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.