- 1Institute of Biomedical and Allied Health Sciences, University of Health Sciences, Lahore, Pakistan
- 2Department of Microbiology, University of Health Sciences, Lahore, Pakistan
- 3Department of Pharmacology, University of Health Sciences, Lahore, Pakistan
- 4Department of Molecular Biology and Umeå Centre for Microbial Research (UCMR), Umeå University, Umeå, Sweden
Carbapenem resistant Acinetobacter baumannii has emerged as one of the most difficult to treat nosocomial bacterial infections in recent years. It was one of the major causes of secondary infections in Covid-19 patients in developing countries. The polycationic polypeptide antibiotic colistin is used as a last resort drug to treat carbapenem resistant A. baumannii infections. Therefore, resistance to colistin is considered as a serious medical threat. The purpose of this study was to assess the current status of colistin resistance in Pakistan, a country where carbapenem resistant A. bumannii infections are endemic, to understand the impact of colistin resistance on virulence in mice and to assess alternative strategies to treat such infections. Out of 150 isolates collected from five hospitals in Pakistan during 2019–20, 84% were carbapenem resistant and 7.3% were additionally resistant to colistin. There were two isolates resistant to all tested antibiotics and 83% of colistin resistant isolates were susceptible to only tetracycline family drugs doxycycline and minocycline. Doxycycline exhibited a synergetic bactericidal effect with colistin even in colistin resistant isolates. Exposure of A. baumannii 17978 to sub inhibitory concentrations of colistin identified novel point mutations associated with colistin resistance. Colistin tolerance acquired independent of mutations in lpxA, lpxB, lpxC, lpxD, and pmrAB supressed the proinflammatory immune response in epithelial cells and the virulence in a mouse infection model. Moreover, the oral administration of water extract of Saussuria lappa, although not showing antimicrobial activity against A. baumannii in vitro, lowered the number of colonizing bacteria in liver, spleen and lung of the mouse model and also lowered the levels of neutrophils and interleukin 8 in mice. Our findings suggest that the S. lappa extract exhibits an immunomodulatory effect with potential to reduce and cure systemic infections by both opaque and translucent colony variants of A. baumannii.
Introduction
Acinetobacter baumannii is an emerging nosocomial pathogen responsible for a diverse range of infections, including blood stream infections, meningitis, urinary tract infections and ventilator-associated pneumonia (Rice, 2008). Bacteremia and acute respiratory distress syndrome associated with A. baumannii infections are often fatal (Mcconnell et al., 2013). Over the last 2 decades, a tremendous rise in antimicrobial resistance of A. baumannii has been reported throughout the world (Antunes et al., 2014; Ayoub Moubareck and Hammoudi Halat, 2020; Karah et al., 2020; Khalid et al., 2020; Ibrahim et al., 2021). In contrast, new additions to the arsenal of useful antimicrobial drugs are remarkably low. Consequently, the spectrum of available drugs for treatment is being narrowed down (Kempf and Rolain, 2012). The carbapenems have been in frequent use to treat infections caused by Gram-negative rods, but resistance to this class of drugs has been rapidly observed in Acinetobacter species (Paterson, 2006).
Pakistan stands among countries where carbapenem resistant A. baumannii are reported at very high frequency (Begum et al., 2013; Evans et al., 2011; Hasan et al., 2014; Hsu et al., 2017; Karah et al., 2020; Khalid et al., 2020). Carbapenem resistant A. baumannii infections are challenging to treat due to limited available options. Colistin is considered as an antibiotic of last option for the treatment of carbapenem resistant A. baumannii (Isler et al., 2019).
Colistin is a cationic polypeptide antibiotic that consists of a cyclic decapeptide joined by an α-amide linkage to a fatty acyl chain. With a single amino acid difference to polymyxins B, both antibiotics exhibit similar anti-microbial activities against Gram-negative bacteria (Gallardo-Godoy et al., 2016; Poirel et al., 2017). Polymyxins directly target bacterial outer membranes and exert the antibacterial effect by a two-step mechanism. In the first step, the positively charged antibiotic binds to negatively charged lipopolysaccharide. The LPS-polymyxin interactions cause an electrostatic effect leading to the permeabilization of the outer membrane followed by its destabilization (Poirel et al., 2017). Any alteration in lipid A bilayer that nullifies its charge can prevent its binding to colistin. Thus, the distinct mechanism of resistance usually involves alterations to lipid A that reduces or nullifies the charge-based interaction with polymyxins. In a number of Gram-negative bacteria, the modification of lipid A by addition of 4-amino-4-deoxy-l-arabinose (l-Ara4N) and/or phospho ethanolamine (PEtn) reduces the net LPS negative charge to decrease the LPS-polymyxin interactions. In A. baumannii, modifications of LPS, in order to achieve colistin resistance is mainly achieved by point mutations in two component system pmrAB that cause an addition of phospho ethanolamine to LPS (Moffatt et al., 2010a). Alternatively, loss of LPS due to mutations in LPS biogenesis machinery involving loci lpxA, lpxC, and lpxD also lead to colistin resistance (Moffatt et al., 2010a).
Here, we focus on the status of colistin usage in Pakistan, a country where carbapenem resistant A. baumannii infections are endemic, by analysing the impact of colistin resistance on virulence and inflammation in a mouse model. We evaluate the potency of second line drug doxycycline against colistin resistance isolates and assess the efficacy of Saussurea lappa extracts against inflammation caused by A. baumannii.
Results
High frequency of colistin resistance in extreme drug resistant isolates of Acinetobacter baumannii in Pakistan
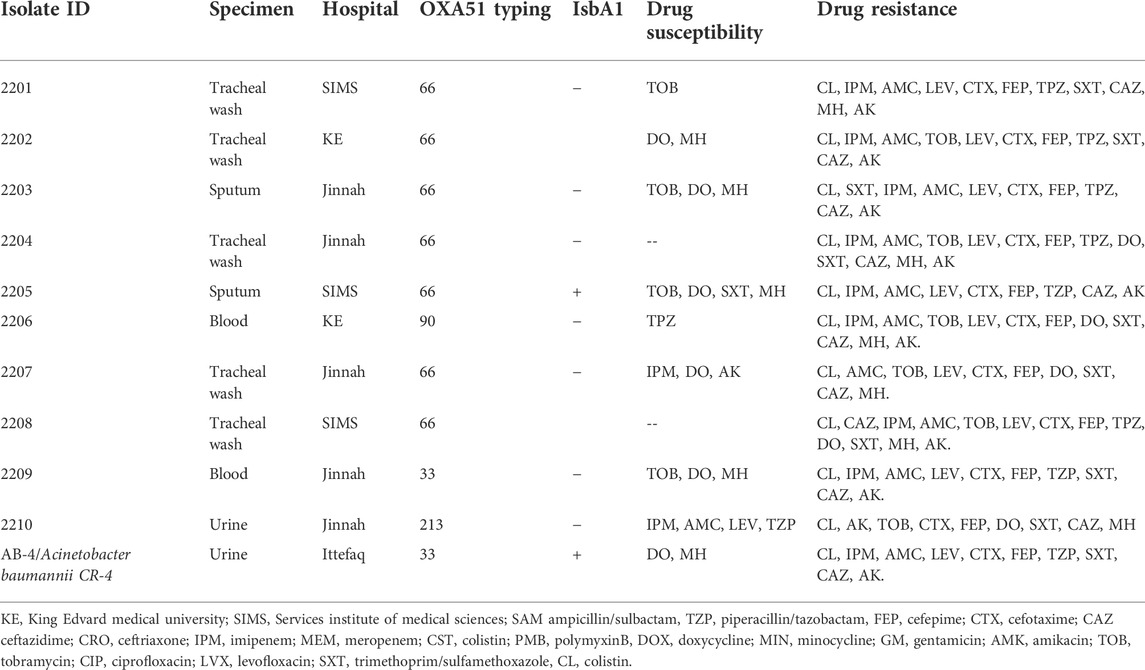
Previously, we reported on the molecular epidemiology and drug resistant features of A. baumannii isolates collected from hospitals in Pakistan during 2013–2015. No colistin resistance was found among those isolates (Karah et al., 2020). In the follow up, here we report the current status of colistin resistance in isolates collected from five hospitals in Pakistan during 2019–2020. For this study, a collection of 150 clinical isolates was obtained. Out of the 150 clinical isolates, 28 (19.4%) were from the respiratory tract infections, 56 (38.8%) from the blood or central venous catheter, 23 (16.3%) from the wound infections and 36 (25%) from the urinary tract infections. The isolates showed resistance rates of 100% (150/150) to ampicillin/sulbactam, piperacillin/tazobactam, cefepime, cefotaxime, ceftazidime, ceftriaxone, gentamicin, tobramycin, ciprofloxacin, levofloxacin, and trimethoprim/sulfamethoxazole. In addition, 84% (126/150) of the isolates were resistant to imipenem, 9,3% (14/150) to minocycline and doxycycline, and 7,3% (11/150) to colistin and polymyxin B (Table 1). Except for the findings on colistin resistance, data were in line with previous studies describing extensive occurrence of multidrug-resistant strains of A. baumannii in Pakistan (Begum et al., 2013; Khalid et al., 2020). More alarmingly, there were two isolates resistant to all tested 2nd line and 3rd line antibiotics including colistin, moxifloxacin and doxycycline. Both of these isolates (2204 and 2208) were obtained from tracheal secretion of persons admitted to two different hospitals in 2020 and belong to OXA-66 type of strains with OXA-51 beta lactamase (Table 2). The MIC of colistin varied from strain to strain in colistin resistant isolate with a range between 8 ug/ml to 32 ug/ml. Nine out of eleven colistin resistant isolates were hetero resistant to all tested antibiotics except tetracycline drugs doxycycline and minocycline whereas the remaining two isolates were resistant to all tested antibiotics.
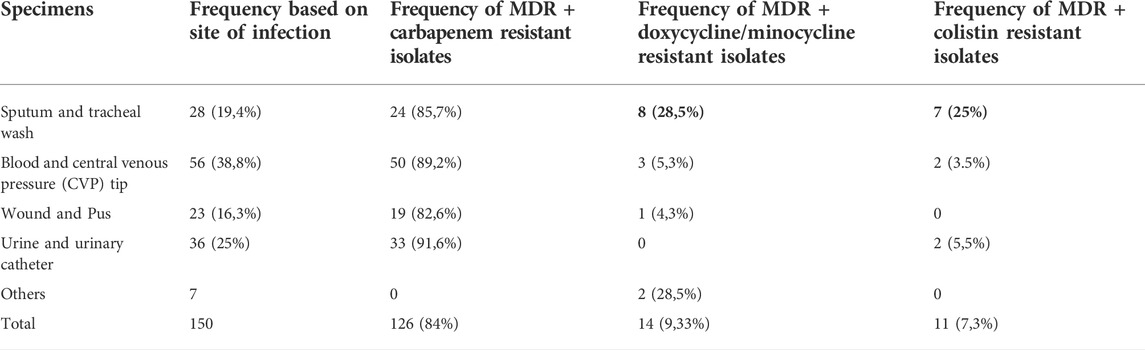
TABLE 1. Frequency of carbapenem, colistin and doxycycline resistant A. baumannii during 2019–2020 in public hospitals of Lahore, Pakistan.
Identification of novel mutations associated with colistin resistance
In order to monitor the potential effect of colistin resistance on the fitness and virulence of A. baumannii, a colistin resistant derivative of the well characterized colistin sensitive strain A. baumannii17978 was obtained by challenging it with sub optimal concentration of 0,25 ug/ml colistin by passing the bacteria through three consecutive overnight subcultures. The resulting colistin resistant variant of A. baumannii 17978, hereafter referred to as CR-1, exhibited a MIC value of 16 ug/ml for colistin. Whole genome sequencing of the isolate CR-1 was performed to assess adaptive response to colistin at genetic level. Mutations in one of the genes involved in encoding LPS biosynthetic machinery lpxA, lpxC or lpxD or mutations in pmrAB operon involved in the addition of phospho ethanolamine on LPS have been known to cause colistin resistance. However, no mutation was detected in these genes in the CR-1 isolate (Whole genome sequence Accession no. JANIHK000000000). Hence, the colistin resistance acquired by CR-1 appeared independent of mutations in these genes. In depth analysis of the whole genome sequence of CR-1 identified four single base pair mutations in loci AIS_1585, AIS_2445, AIS_3424 and AIS_2454 in the genome of CR-1 (Table 3). None of these point mutations have been shown to be associated with colistin resistance previously. Interestingly, the transposons mutants of these four genes along with several other mutants were shown to be associated with a salt induced colistin tolerance phenotype (Hood et al., 2013) suggesting the relevance of these single nucleotide mutations in colistin resistance acquired by CR-1. These mutations appear to be associated with colistin resistance phenotype. However to find role of these mutations in colistin resistance further investigations are required.

TABLE 3. Mutations associated with colistin tolerance in A. baumannii 17978 as revealed from whole genome sequencing of CR-1.
The reduced virulence associated with colistin resistance can be independent of mutations in pmrAB, lpxA, lpxB, or lpxD
The CR-1 isolate was then tested for LPS biogenesis. Silver staining of LPS preparations resolved in acrylamide gel identified that CR-1 lacks a distinct band of lipopolysaccharide rather than an overall decrease in LPS content (Figure 1A). It is known that A. baumannii acquires colistin resistance at the cost of virulence loss due to mutations in lpxA, lpxC, lpxD or pmrAB (Da Silva and Domingues, 2017). However, these genes were intact in CR-1. Therefore, the CR-1 isolate was then tested for virulence phenotypes and capability to induce a pre-inflammatory immune response in epithelial cells. Lung epithelial cell line A549 and rectal carcinoma epithelial cells SW480 were infected with CR-1 and wild type A. baumannii 17978. The adherence capability of CR-1 with both cell lines was significantly decreased (Figures 1B,C). Consistently, the pro-inflammatory response induced by CR-1 in SW480 cells, measured as IL-6 and IL-8 levels, was significantly decreased in case of the CR-1 isolate as compare to wild type (Figures 1D,E). Since colistin resistance in CR-1 was acquired under laboratory conditions, to validate these findings, naturally colistin resistant clinical isolate AB-4 was also tested for interaction with epithelial cells in comparison to colistin sensitive hyper virulent strain A. baumannii AB5075. The cell adherence and cytokine induction capabilities of AB-4 were significantly decreased as compare to A. baumannii AB5075 (Figures 1B–D). The identity and molecular typing of AB-4 was determined by whole genome sequencing. The multi locus sequence (MLS) typing shows that the isolate belongs to international clone II and MLS type 2 (Supplementary Table S2). AB-4 is found to be equipped with beta lactamases OXA-66 and blaADC25 of A. baumannii. The broad range beta lactamase encoding genes bla-PER-1 and blaOXA-23 were also detected in the genome of AB-4. In addition, streptogramin B and macrolide resistance genes msr(E) and mph (E) were detected. The isolate was found to harbour aminoglycoside resistance genes aph (3”)-Ib, aph (3”)-VIb, aph (3”)-Id, aph (3”)-Via, aph (3”)-Ia and armA and sulfonamide resistance gene sul-2 (Supplementary Table S3).
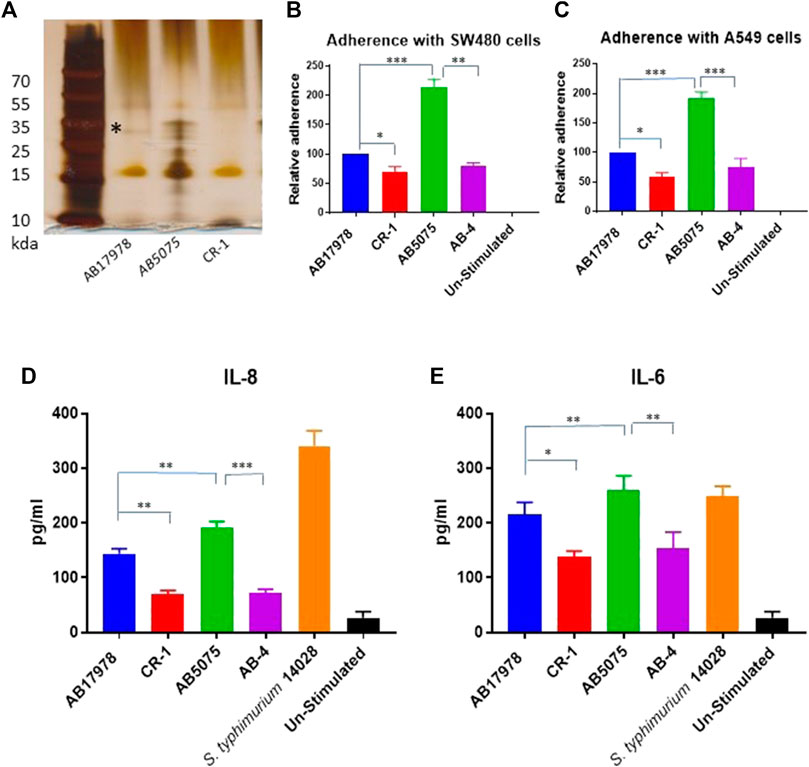
FIGURE 1. Consequences of colistin resistance in A. baumannii for capabilities to adhere with epithelial cells and to pro-inflammatory immune response induction. (A) Silver stained polyacrylamide gel elucidating a total LPS content of CR-1 as compared to the parental strain A. baumannii 17978 (AB 17978) and the hyper virulent strain AB5075. The band indicated by asterisk is corresponding to 35 kda with respect to protein ladder is diminished in CR-1 isolate (B) Relative adherence of A. baumannii strains with respect to A. baumannii 17978 wild type with SW480 cell lines and (C) A549 cell lines upon 4 h of co-infection with MOI of 1:100. Absolute values of cytokines IL-8 (D) and IL -6 (E) induced by bacterial strains from SW480 cells upon 4 h of co-infection with MOI of 1:100. S. typhimurium 14028 was used as a positive control. Un-stimulated refers to cells without any infection. Bars show mean ± standard deviation from at least five independent biological experiments performed in three technical replicates. Statistical significance is indicated by *p < 0.05, **p < 0.01 and ***p < 0.001 using non-parametric t test.
These in vitro findings triggered us to test CR-1 for virulence potential and inflammatory response associated with colistin resistance in a mouse infection model. For that, BALB/c mice were intraperitoneally infected with wild type A. baumannii 17978 and its colistin resistant mutant strain CR-1. The hyper virulent strain AB5075 was used as a positive control to establish virulence phenotypes. The mice infected with A. baumannii AB5075 and A. baumannii 17978 exhibited a strong inflammatory response with respectively 4-fold and 2-fold increase in neutrophil count in the blood after 24 h of the post infection (Figure 2A). The colistin resistant mutant strain CR-1 also induced an IL-8 and neutrophil increase upon intraperitoneal infection. However, there was a significant decrease in the number of colonized bacteria into lungs and liver of the mice infected with colistin resistant isolates as compare to colistin sensitive isolates (Figures 2B,C). There was no significant difference detected in the colonization of CR-1 bacteria into spleen (Figure 2D). Altogether these findings suggest that the colistin resistant strain CR-1, although it harbours intact lpxA+, lpxC+, lpxD+ and pmrA+B+ loci although it exhibited a significant reduced virulence in the mouse model.
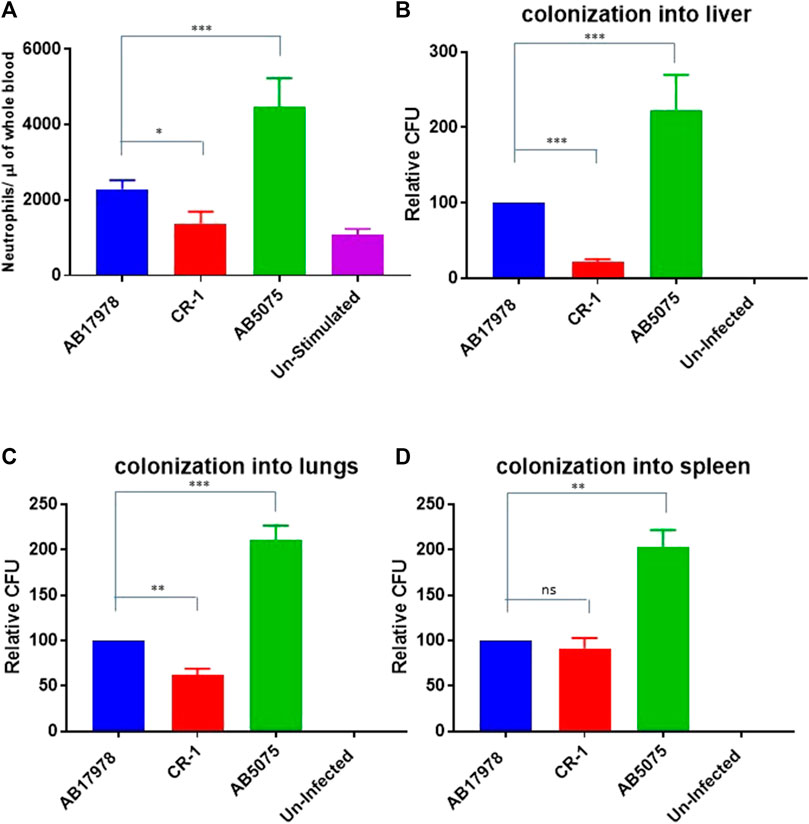
FIGURE 2. Consequences of colistin resistance in CR-1 for virulence in BALB/c mice. (A) Total neutrophil counts in the whole blood of mice withdrawn through heart puncture after 20 h of infection. Number of viable bacterial counts shown as colony forming unit (CFU) count into liver (B), lungs (C) and spleen (D) of the mice sacrificed after 20 h of intraperitoneal innocula consisting of 107 cells suspended in phosphate buffer saline. Bars show mean ± standard deviation of the individual values obtained from six mice in each group. Statistical significance is indicated by *p < 0.05, **p < 0.01and ***p < 0.001 using non-parametric t test.
Synergism of doxycycline and colistin in colistin resistant isolates
Nine out of 11 colistin resistant isolates were detected susceptible to tetracycline drugs, doxycycline and minocycline (Table 2). In order to determine potency of doxycycline against colistin resistant isolates, time dependent bactericidal and bacteriostatic effects of doxycycline in the presence and absence of colistin were investigated in a representative isolate. The colistin resistant but doxycycline susceptible strain AB-4 was selected as a representative strain for this purpose.
In time-kill curve studies, doxycycline showed a 4-log10 reduction in CFU/ml at 4xMIC concentration in comparison to growth control after 2 h of incubation and 3-log10 reduction at 2xMIC after 18 h of incubation against AB-04 and AB17978 (Figures 3A,B). There were no viable bacteria detected with the treatment of 4xMIC after 8 h of incubation. However, there was 4-log10 reduction of CFU with 2xMIC treatment and 2-log10 reduction with MIC with a ½ MIC treatment of doxycycline.
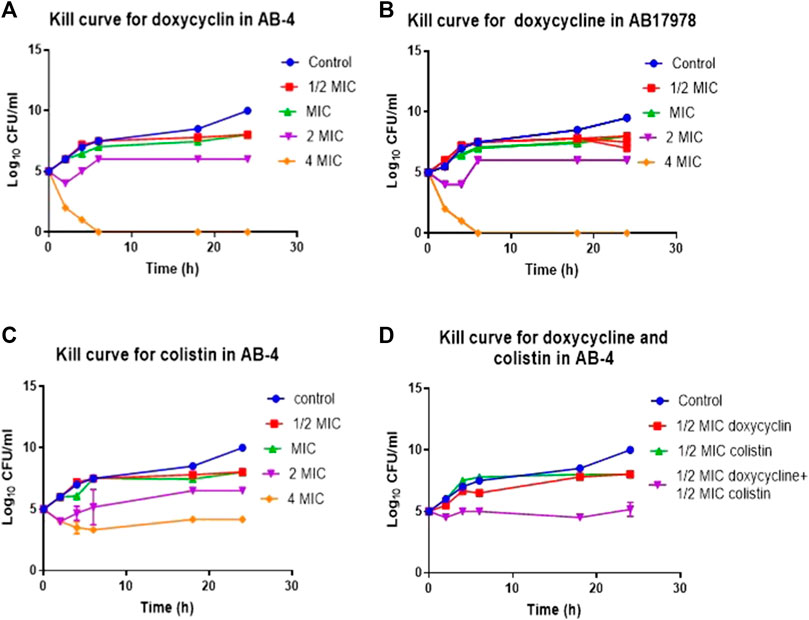
FIGURE 3. Time kill curve demonstrating in vitro efficacy of doxycycline in colistin resistant clinical isolate AB-4 (A) Kill curve of AB-4 upon the treatment with different concentrations of doxycycline. The MIC value for doxycycline in AB-4 is 0.5 ug/ml (B) Kill curve of A. baumannii 17978 (AB17978 upon the treatment with different concentrations of doxycycline. The MIC value for doxycycline in AB17978 is 0.125 ug/ml (C) Kill curve of AB-4 upon the treatment with different concentrations of colistin. The MIC value for colistin in AB-4 is 16 ug/ml (D) Kill curve of AB-4 upon the treatment with colistin and doxycycline. Bars show mean ± standard deviation from at least five independent biological experiments.
The MIC for colistin of AB-4 was 16 μg/ml. In the time-kill curve studies, colistin showed a 2-log10 reduction in CFU/ml at 4xMIC concentration in comparison to growth control after 2 h of incubation and a 1-log10 reduction at 2xMIC after 18 h of incubation (Figure 3C). Colistin showed bactericidal effect towards strain AB-4 at a concentration twice of MIC at second hour of incubation. A bactericidal effect was also noted at the second hour of incubation at a concentration of four times of their MICs whereas no bactericidal effect was found at their MIC during 24 h of incubation.
Subsequently, the time kill assay was performed to assess the effect of colistin in the presence of doxycycline with strain AB-4 at ½ MICs of colistin and doxycycline (Figure 3D). No bactericidal effect was observed with ½ MICs of doxycycline or colistin alone in first hour of incubation. However, treatment with both doxycycline and colistin at ½ MICs each significantly reduced the CFU, 3-5 log10 at all tested time points. This finding suggests that a synergetic effect of antimicrobial activity of colistin and doxycycline exists in colistin resistant isolate AB-4.
Efficacy of an extract from the herb Saussurea lappa against colistin resistant A. baumannii isolates
Considering very limited, treatment options against colistin resistant isolates (Table 1), we were interested to explore other alternative treatment strategies. In this regard, extracts of the traditional medicinal plant Saussurea lappa have been shown to possess antimicrobial activity against Gram-negative and Gram-positive and anti-inflammatory activity in tracheal inflammation (Hasson et al., 2013; Rao Vadaparthi et al., 2015; Pyun et al., 2018). In vitro antimicrobial activity of various fractions of the herb Saussurea lappa was tested against colistin resistant A. baumannii isolates. However, a remarkable antimicrobial activity of S. lappa against these isolates was not observed in vitro at the concentration of less than up to the concentration of 10 mg/ml. In order to assess its anti-inflammatory role, a water extract of S. lappa was tested in the mouse model of systemic infection against the extreme drug resistant isolate A. bauamnnii AB-4 upon intraperitoneal infection and with the hyper virulent strain A. baumannii AB5075. The mice were treated with the water-soluble fraction from S. lappa. For that, a dose consisting of 10 mg of S. lappa powder was orally administrated to each mouse after 3 h of intraperitoneal infection. The inflammation induced by A. baumannii strains was measured as IL-8 levels and total neutrophil count in the blood of infected mice after 20 h of infection. There was a statistically significant reduction in IL-8 levels and total neutrophil count in the blood of the infected mice treated with S. lappa or tetracycline as compare to uninfected group (Figure 4A). This finding suggests that the compound from S. lappa modulates the inflammatory response induced by A. baumannii infection. Also, there was a significant decrease in numbers of viable bacteria colonized into liver and lungs of infected mice treated with S. lappa extract or tetracycline as compare to the untreated group (Figures 4B–D). Altogether, these findings suggest that the water-soluble fraction of S. lappa possess some immunomodulatory compound that may be used to treat A. baumannii infection in mice.
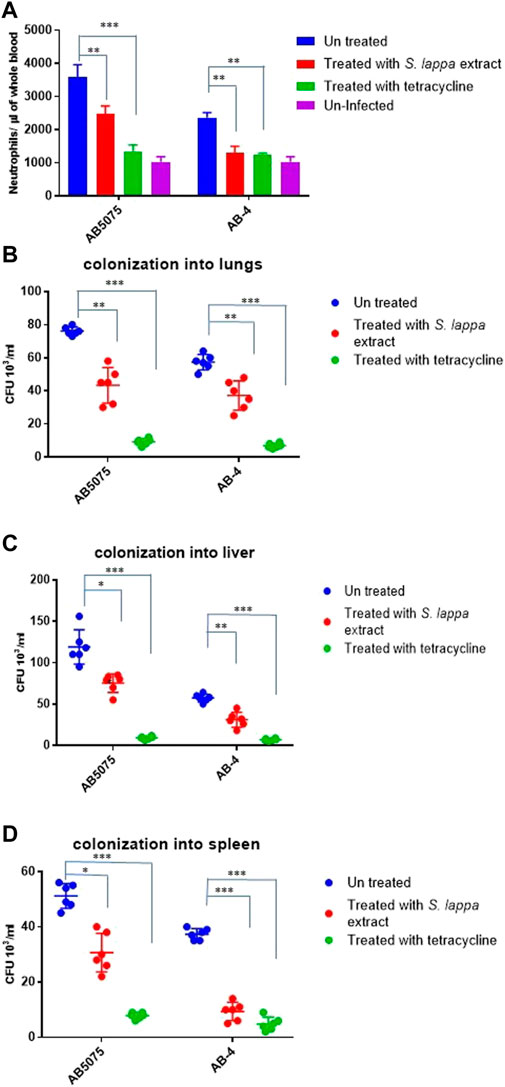
FIGURE 4. Effect of the oral administration of the extract from S. lappa in mice infected with the AB5075 or AB-4 strains of A. baumannii (A) Total neutrophil counts in the whole blood of mice withdrawn through heart puncture after 20 h of infection. Number of viable bacterial counts shown as colony forming unit (CFU) count in liver (B), lungs (C) and spleen (D) of the mice sacrificed after 20 h of intraperitoneal infection consisting of 107 cells suspended in phosphate buffer saline. Bars show mean ± standard deviation of the individual values obtained from six mice in each group. Statistical significance is indicated by *p < 0.05 and **p < 0.01 and ***p < 0.001 using non-parametric t test.
Immunomodulatory effect of Saussura lappa with respect to colony phase variation in Acinetobacter baumannii AB5075
A. baumannii AB5075 displays a recognized capability of switching into an avirulent subpopulation of bacterial cells that appears as translucent colonies on agar plates (Figure 5A) (Tipton et al., 2015; Chin et al., 2018; Ahmad et al., 2019). The findings of the immunomodulatory role of Saussurea lappa extracts towards A. baumanii infection inspired us to investigate whether such effects would be limited to a certain subpopulation of A. baumannii AB5075. The effect of the S. lappa extract was therefore tested upon infecting mice with opaque and translucent colony variants of A. baumannii AB5075. As reported previously, the opaque variant was more virulent as compare to translucent counterpart in the mouse. The administration of S. lappa extract to mice groups infected with opaque and translucent variants exerted similar effect in lowering cytokine levels, white blood cell count neutrophils and colonization of bacteria into organs (Figures 5B–E).
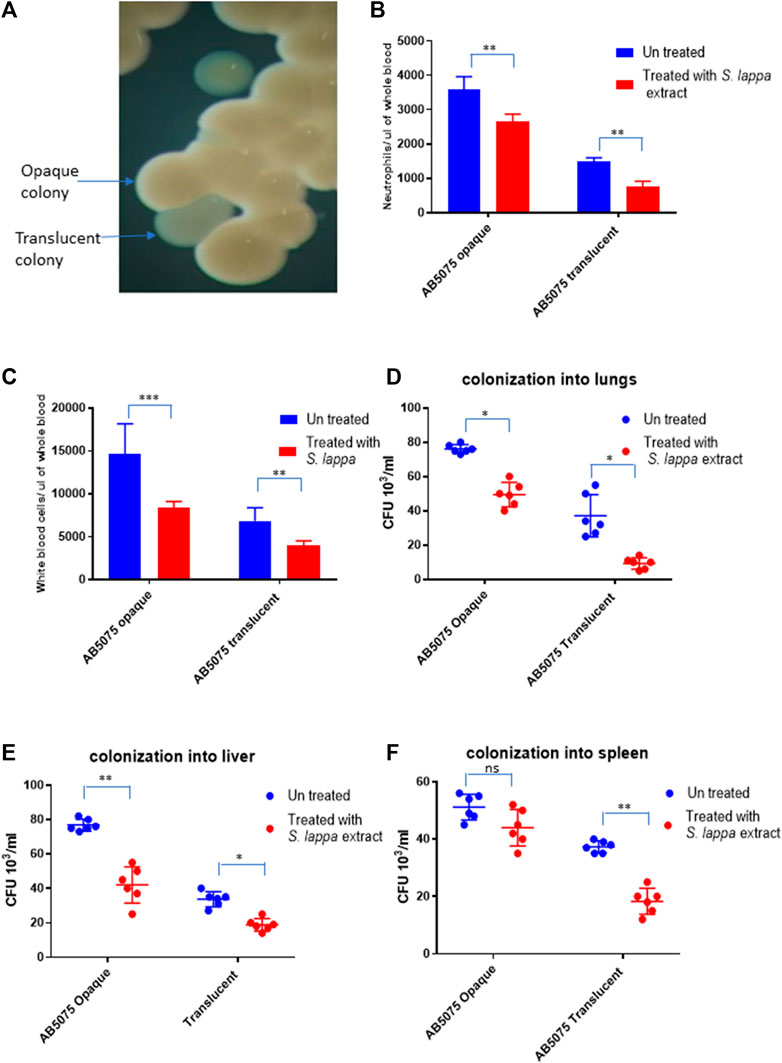
FIGURE 5. Effect of the extract from S. lappa in mice infected with opaque and translucent colony variants of A. baumannii AB 5075 (A) Stereomicroscopic image of opaque and translucent colonies in a typical LB agar culture of A. baumannii AB 5075. (B) Total neutrophils count in the whole blood of mice withdrawn through heart puncture after 20 h of infection. (C) Total white blood cells counts in the whole blood of mice withdrawn through heart puncture after 20 h of infection (D) Number of viable bacterial counts shown as colony forming unit (CFU) count in the lungs, liver (E) and spleen (F) of the mice sacrificed after 20 h of intraperitoneal innocula consisting of 107 cells suspended in phosphate buffer saline. Bars show mean ± standard deviation of the individual values obtained from six mice in each group. Statistical significance is indicated by *p < 0.05, **p < 0.01 and ***p < 0.001 non-parametric t test.
Discussion
Colistin is a last resort drug for the treatment of carbapenem resistant A. baumannii infections. The finding on the emergence of colistin resistance among several clonal types of carbapenem resistant A. baumannii isolates in Pakistani hospitals is an alarming situation. Particularly, there were two isolates detected with resistance to all tested antibiotics from two different hospitals. Also, the emergence of colistin resistance among carbapenem resistant isolates have been reported in other parts of the world (Abdulzahra et al., 2018; Al-Kadmy et al., 2019; Thadtapong et al., 2021). If the situation is not tackled vigilantly, the frequent outbreaks of pan-drug resistant strains of A. baumannii could be expected in the near future.
Colistin resistant clinical isolate AB-4 as well as colistin resistant derivative of reference strain AB17978CR-1 appeared deficient in their capabilities to induce a proinflammatory response and virulence in mice. It is a well evident fact that A. baumannii acquires colistin resistance at the cost of virulence potential (Da Silva and Domingues, 2017). A. baumannii can acquire resistance to colistin via complete loss of lipopolysaccharide (LPS) biosynthesis due to mutations in the lpxA, lpxC and lpxD genes (Moffatt et al., 2010a). Consistently, a LPS-deficient derivative of the ATCC 19606 strain was not only defective in causing virulence in mice but also acquired growth defects (Carretero-Ledesma et al., 2018). LPS loss altered several other bacterial traits such as biofilm formation, motility and growth rate in the presence of disinfectants. The mutations in genes encoding the LPS biosynthesis machinery were found to be associated with higher fitness costs than in case of mutations in the pmrAB operon (Mu et al., 2016). Similarly, colistin resistance associated with mutations in pmrB upon the long-term exposure of colistin with extreme drug resistant isolates did not cause any virulence defect (Durante-Mangoni et al., 2015). In contrast, LPS loss mediated due to mutations in the lpx operon and phospho ethanolamine addition mediated by mutations in pmrAB, both caused a defect in fitness of A. baumannii 19606 while pmrAB mutants acquired a higher viability loss as compared to lpx mutants upon interaction with A549 epithelial cells. Also, host dependent effects of virulence loss was observed in lpx and pmrB mutants in C. elegans and mice models (Beceiro et al., 2014).
The findings suggest that the cause of colistin resistance determines the virulence potential of A. baumannii. The treatment strategy could also have differential impact on colistin resistance with or without alteration in LPS biosynthesis. Therefore, a detailed investigation is required to determine the impact of second line drugs such as doxycycline, minocycline and doxycycline on treatment of colistin resistant A. baumannii. Doxycycline is found to be an effective choice to treat colistin resistant A. baumannii. Particularly, in the presence of colistin ½ MIC (8 ug/ml), the bactericidal effect of doxycycline is found to increase at least 3-log. A systematic evaluation of alternative antibiotics against colistin resistant A. baumannii will be required.
Attempts to find alternative treatment options for colistin resistant A. baumannii lead us to identify the root extract of Saussurea lappa as an effective immunomodulatory agent to treat such infections. However, in vitro antimicrobial activity was not detected against colistin resistant A. baumannii. In contrast, the root extract of S. lappa has been shown to possess in vitro antibacterial activity against several types of Gram-positive and Gram-negative bacteria such as Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and carbapenem resistant A. baumannii (Hasson et al., 2013).
The idea to use plant extracts as anti-bacterial agent is not new but have been tested in several plants successfully. For example, the extracts of Lythrum salicaria was shown to possess antimicrobial activity against A. baumannii and P. aeruginosa (Ertugrul Guclu et al., 2013). Similarly, herbs like Syzygium aromaticum and Cinnamomum zeylanicum, have been shown to have promising results against multidrug-resistant A. baumannii (Burt, 2004). The use of epigallocatechin gallate along with curcumin harbours a synergetic effect to inhibit the growth of A. baumannii. The combinational use of both herbs at the concentration of 4 µg/ml inhibits the growth of A. baumannii without any antagonistic effect (Betts and Wareham, 2014). The extracts of plant Magnolia dealbata have also been shown to exhibit anti-bacterial activity against many bacteria such as P. aeruginosa, Clavibacter michiganensis, A. baumannii and A. lwoffii. Honokiol and magnolol are important active ingredients in this plant that possess antimicrobial activity (Jacobo-Salcedo Mdel et al., 2011).
Plant extracts are not only found to exhibit antimicrobial activity but there are multiple plant derived molecules found to enhance antimicrobial potential of existing antibiotics. For example, plant derived compounds tannic acid and ellagic acid have been shown to enhance antimicrobial activity of many drugs such as novobiocin, chlorobiocin, coumermycin rifampicin and fusidic acid (Chusri et al., 2009). An alkaloid compound, berberine, found in different kinds of plants such as Rhizoma coptidis, Berberis fremontii and Hydrastis canadensis possess antibacterial activities (Lewis and Ausubel, 2006). Plant derived antibacterial molecules are generally weak but work better in synergy with antibiotics (Lewis and Ausubel, 2006). Picatechin, a tea polyphenol having no antibacterial properties can potentiate theaflavin in vivo and thus increases its activity against A. baumannii and Stenotrophomonas maltophilia isolates. The probable mechanism may be that epicatechin inhibits theaflavin oxidation thus enhancing its antibacterial effect, but the exact mechanism of synergy is not yet understood and needs further study (Betts and Wareham, 2014).
Water extracts of the roots of S. lappa, although it lacks an antimicrobial activity in vitro against A. baumannii, showed a significant potential of suppressing virulence and inflammation induced upon the infection by A. baumannii in the mouse model. The key ingredients of this herb include sesquiterpene lactones and dehydrocostus lactone (Rao Vadaparthi et al., 2015). The immune modulatory role of sesquiterpens in the inhibition of proinflammatory cytokine production and lymphocyte proliferation is well evident (Koch et al., 2001). Also, Dehydrocostus lactone has been shown to suppresses allergic airway inflammation by binding to dimerized translationally controlled tumor protein in a mouse model and thus possesses important role in immunomodulation (Pyun et al., 2018). Further studies are required to reveal the active ingredient of S. lappa involved in the presently observed modulation of immune response that suppress the systemic infection of A. baumannii in mouse.
In summary, we have elucidated the status of colistin resistance in carbapenem resistant A. baumannii isolates from Pakistan, the synergetic effect of colistin and doxycycline against colistin resistant isolates and the inhibitory potential of an extract from S. lappa on the infection and inflammation induced by A. baumannii in mice. However, further detailed analysis is required to identify the active ingredient(s) and mechanisms of this inhibitory role.
Materials and methods
Bacterial strains
Clinical isolates of A. baumannii (n = 150) were collected from clinical samples of patients visited or hospitalized in Jinnah hospital, Ittefaq Hospital Lahore, Services Institute of Medical Sciences, King Edward Medical University Laboratory and Mayo Hospital Lahore during 2019–2020. The strains were identified by their morphological and biochemical characteristics using API 20-NE (Bio Merieux, France) following manufacturer`s instructions. A. baumanniis 17978 was used for isolation of a colistin resistant derivative CR-1. The hyper virulent strain AB5075 and its opaque and translucent colony variants were used for infection tests (Ahmad et al., 2019). Escherichia coli ATCC 25922 was used as a control strain in MIC value tests. Salmonella typhimurium 14028 was used as positive control to induce proinflammatory response from epithelial cells. The strains were stored at minus 80°C in 10% glycerol prepared in LB broth.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed by the standard Kirby-Bauer disk diffusion method using cation adjusted Mueller-Hinton agar (MHA) (Oxoid, United Kingdom), according to Clinical Laboratory Standards Institute (CLSI) guidelines (Clinical and Laboratory Standards Institute (CLSI), 2019) Antibiotic discs (Oxoid, United Kingdom) used were piperacillin (100 µg), ampicillin-sulbactam (10 µg/10 µg), piperacillin-tazobactam (100 µg/10 µg), ticarcillin-clavulanic acid (75µ/10 µg), ceftazidime (30 µg), ceftriaxone (30 µg), cefepime (30 µg), imipenem (10 µg), gentamicin (10 µg), doxycycline (30 µg) and ciprofloxacin (5 µg).
MIC determination of colistin and tetracycline
The minimal inhibitory concentration of colistin was determined by micro broth dilution method. The base material colistin (Glaxosmith Kline pharmaceuticals) was prepared in water and tetracycline in 70% ethanol and stored at minus 20°C. MICs of all strains for each antibiotic were determined by standard microbroth dilution method. Bacterial inoculum equivalent to 0.5 McFarland (5 × 108) was prepared and diluted 1:10 to achieve the final inoculum of 5 × 107 CFU/ml. Concentration range of the antibiotics to be tested was; 0.125–256 µg in MHA using 96 well plates. The plates were incubated for 24 h at 37°C and the lowest concentration at which bacterial growth was completely inhibited was noted and declared as MIC. Escherichia coli ATCC 25922 was used as a control strain. MIC results were read and interpreted according to the CLSI 2019 breakpoints for A. baumannii.
Strain typing
The colistin resistant isolates were typed using single-locus molecular schemes based on the allelic identity of the A. baumannii-intrinsic blaOXA-51-like gene (Pournaras et al., 2014). PCR amplification of blaOXA-51-like was performed using in-house designed primers (Supplementary Table S1) and followed by Sanger sequencing of the amplicons. The thermal cycling program used for PCR consisted of: initial denaturation; 94°C for 5 min: 30 cycles of amplification: denaturation at 95°C for 20 s; annealing at 56°C for 20 s, and extension at 72°C for 1 min and 30 s: Final extension; 72°C for 8 min. This approach was able to detect the occurrence of insertion sequence (IS) elements, such as ISAba1, in the bordering regions of blaOXA-51-like.
Whole-genome sequence analysis
The whole genome sequencing of colistin resistant clinical isolate AB-4 and colistin resistant variant of A. baumannii 17978 CR-1 was performed using the MiSeq Desktop Sequencer and MiSeq Reagent Kit v3 (Illumina, San Diego, CA, United States). DNA preparation, library construction, and genome sequencing were done according to the manufacturer’s instructions. Sequence data were assembled and analyzed using the CLC genomics workbench (v7.0.4; CLC bio, Aarhus, Denmark). The whole genome sequence of A. baumannii ATCC 17978 (DDBJ/EMBL/GenBank database accession number CP000521) was used as a reference sequence.
The MLST web-based search engine, hosted by the Center for Genomic Epidemiology in Denmark (http://www.genomicepidemiology.org/), was used to assign the isolate into STs according to the Institute Pasteur’s MLST scheme (http://www.pasteur.fr/mlst) (Larsen et al., 2012). The occurrence of acquired antimicrobial resistance genes was detected using the Res Finder service, also hosted by the Center for Genomic Epidemiology in Denmark [24]. The occurrence of resistance genes was verified, and genetic surroundings were annotated based on the yields of nucleotide similarities obtained using the Basic Local Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi) against the “Nucleotide collection (nr/nt)” and/or “Whole-genome shotgun contigs (wgs)” databases (Zhang et al., 2000).
Nucleotide sequence accession numbers
Draft genome sequences of the isolates were deposited in the DDBJ/EMBL/GenBank database under the BioProject accession number: PRJNA862891. The isolates described in this paper are AB-4 with accession number JANIEU000000000 and CR-1 with accession number JANIHK000000000.
Time-kill assay
The colistin resistant strain A. baumannii AB-4 and colistin sensitive strain A. baumannii 17978 were selected for time kill assay. Bacterial suspensions equivalent to 1.0 MF (3 × 108 CFU/ml) was prepared for each isolate to be tested by inoculating four to 5 colonies into 5 ml of CAMHB and incubating for 6 h. One ml of 1.0 MF suspension was diluted in 4 ml of sterile saline to achieve 6 × 107 CFU/ml (1:5 dilution). The same was further diluted (1:100) by dispensing 0.1 ml of 1:5 diluted suspension in 10 ml of saline. The colony count was 6 × 105 CFU/ml. This was used as a starting inoculum in this method.
MICs were determined by micro broth dilution method found to be 0,125 ug/ml in AB17978 and 0,5 ug/ml in AB-4. The following concentrations were tested along with growth and sterility control tubes; MIC, two times the MIC, four times the MIC, and one half the MIC.
The antibiotic agent was dispensed in a sterile screw capped glass tube containing 9.9 ml of CAMHB as 0.1 ml of 100-fold concentrated antibiotic agent. The tubes were incubated at 37°C, and test samples were taken at 0, 2, 4, 6, 18, 24 h after inoculation, for colony count. Serial dilution of contents were prepared and 0.1 ml aliquot of each dilution was plated on Tryptic Soya agar and incubated overnight at 37°C. Antibiotic concentration was determined that resulted in 3-log10 reduction in CFU/ml as compared to the growth control curve. The time was determined that was required to achieve 3-log10 reduction.
Cell adherence assays
Lung epithelial cell line A549 and human rectal carcinoma epithelial cell line SW480 were tested for interaction with A. baumannii. Cells were cultured to confluence in 24-well cell culture treated plates in RPMI 1640 cell culture medium supplemented with fetal bovine serum at 37°C with a continuous supply of 5% CO2. After a change of the medium, confluent layers cells grown in wells of 24 well plates were infected with respective bacterial strains at MOI of 100. For cell infection experiments, bacterial strains were grown to the logarithmic growth phase in LB broth at 37°C. Upon infection, the plate was incubated at 37°C with 5% CO2 for 4 h. The eukaryotic cell monolayer was washed three times with PBS and subsequently to be treated with 1 ml of 5% trypsin. After dissociation of cells attached to the surface, harvested sample was diluted in PBS. The 2nd dilution of every well was processed for enumeration of CFU by point inoculation following overnight incubation on LB agar plates. The CFU numbers of each strain were recorded. Bacterial adherence was determined as the percentage of bacterial cells associated with eukaryotic cells relative to the original inoculums. Each experiment was performed as five biological replicates and three technical replicates.
Levels of IL-6 and IL-8 measurement
A-549 and SW-480 cells were cultured in 24-well plates in RPMI-1640 medium supplemented with 5% fetal bovine serum (FBS) at 37°C with a continuous supply of 5% CO2. After a change of the medium, confluent layers cells were infected with respective bacterial strains at MOI of 100. For cell infection experiments, bacterial strains were grown into the logarithmic growth phase in LB broth at 37°C. Then the bacteria were harvested by centrifugation at 5000 rpm for 10 min and resuspended in RPMI. After infection, cells were incubated at 37°C with 5% CO2 for 4 h. The suspension was, centrifuged after 4 h. The supernatant was analysed for production of IL-8 and IL-6 using ELISA according to manufacturer’s instructions (Bio Assay Technology Laboratory). Salmonella typhimurium 14028 was used a positive control to induce IL-8 from epithelial cells.
Preparation of Saussurea lappa fractions for in vitro and in vivo testing against A. baumannii
The root of S. lappa weighing 200 g was washed with water and kept in incubator at 37°C until get dried. Upon drying, roots were crushed into powder form and dissolved in autoclaved distilled water. The mixture was incubated overnight at room temperature followed by gentle centrifugation. The supernatant was collected in sterile tubes. The contents were dried in rotary evaporator. Left over powder was dissolved in water at the concentration of 100 mg/ml. The MIC of this water extract was tested against colistin resistant isolates described in Table 2 using the method described above for colistin.
Infection in mouse model
BALB/c mice were used in the study. All animals were maintained and treated in accordance with the recommended rules of the WMA Helsinki declaration and the permit granted by University of Health Sciences, Lahore, Pakistan, ethical and research committee wide letter number UHS/REG-19/ERC/1236.
For infection experiments, bacterial strains were grown on LB agar plats overnight at 37°C. From the overnight culture, the isolates were sub-cultured into LB broth until the logarithmic phase. An inoculum consisting of 107 cells of the fresh culture in log phase were used to infect animals. Adult mice were inoculated with A. baumannii isolates by intraperitoneal injection. When required, animals were feed with 10 mg of S. lappa extract or 100 ug of tetracycline drug. Animals were maintained under standard laboratory conditions with free access to food and water throughout the experimental period. Mice were sacrificed 20 h post inoculation (hpi) after anesthesia. For neutrophil count, 500 micro liters of the blood were withdrawn through a heart puncture and stored in vials containing 50 ul of 0.5 M EDTA. The relevant organs (such as lungs, liver and spleens were aseptically removed and used for quantitative bacteriology. Organs were homogenized in sterile saline using aerosol-proof homogenizers. Aliquots (100 μl) of 10-fold serial dilutions of the homogenates were cultured on LB agar plates to quantify the number of viable A. baumannii organisms in the respective organs. Total white blood cells and neutrophil count was measured by hematology analzer (Beckman Coulter DxH 900) and cytokine levels were measured by ELISA according to manufacturer’s instructions (Bio Assay Technology Laboratory).
Statistical analysis
Graph Pad Prism seven was used to construct graphs and perform statistical analysis of results. The type of statistical analysis preformed on each set of data is indicated in the respective figure legend.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The animal study was reviewed and approved by Ethical review committee University of Health Sciences, Lahore vide letter number UHS/REG-19/ERC/1236.
Author contributions
IA, conceived the study, planned experiments, analysed data and wrote the manuscript; BU contributed in funding and materials acquisition, data analysis and manuscript editing; UA, FM, SS, AM, and HS performed experiments and analysed data and MS contributed in experimental design and data analysis.
Funding
The study was supported by grants from the Higher Education Commission Pakistan (Project grant No: 8666/Punjab/NRPU/R&D/HEC/2017) and from Swedish Research Council (Research Network grant No: 2020-06136) to IA, as well as, in part by a grant from the Kempe Foundations (Project grant No: SMK21-0076) and from the Swedish Research Council (Project grant No: 2019-01720) to BU.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.986802/full#supplementary-material
References
Abdulzahra, A. T., Khalil, M. A. F., and Elkhatib, W. F. (2018). First report of colistin resistance among carbapenem-resistant Acinetobacter baumannii isolates recovered from hospitalized patients in Egypt. New Microbes New Infect. 26, 53–58. doi:10.1016/j.nmni.2018.08.007 | |
Ahmad, I., Karah, N., Nadeem, A., Wai, S. N., and Uhlin, B. E. (2019). Analysis of colony phase variation switch in Acinetobacter baumannii clinical isolates. PLoS One 14, e0210082. doi:10.1371/journal.pone.0210082 | |
Al-Kadmy, I. M. S., Ibrahim, S. A., Al-Saryi, N., Aziz, S. N., Besinis, A., and Hetta, H. F. (2019). Prevalence of genes involved in colistin resistance in acinetobacter baumannii: First report from Iraq. Microb. Drug Resist. 26, 616–622. doi:10.1089/mdr.2019.0243 | |
Antunes, L. C., Visca, P., and Towner, K. J. (2014). Acinetobacter baumannii: evolution of a global pathogen. Pathog. Dis. 71, 292–301. doi:10.1111/2049-632X.12125 | |
Ayoub Moubareck, C., and Hammoudi Halat, D. (2020)., 9. Antibiotics (Basel), E119. doi:10.3390/antibiotics9030119Insights into acinetobacter baumannii: A review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogenAntibiotics | |
Beceiro, A., Moreno, A., Fernández, N., Vallejo, J. A., Aranda, J., Adler, B., et al. (2014). Biological cost of different mechanisms of colistin resistance and their impact on virulence in acinetobacter baumannii. Antimicrob. Agents Chemother. 58, 518–526. doi:10.1128/AAC.01597-13 | |
Begum, S., Hasan, F., Hussain, S., and Ali Shah, A. (2013). Prevalence of multi drug resistant Acinetobacter baumannii in the clinical samples from Tertiary Care Hospital in Islamabad, Pakistan. Pak. J. Med. Sci. 29, 1253–1258. doi:10.12669/pjms.295.3695 | |
Betts, J. W., and Wareham, D. W. (2014). In vitro activity of curcumin in combination with epigallocatechin gallate (EGCG) versus multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 14, 172. doi:10.1186/1471-2180-14-172 | |
Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in foods--a review. Int. J. Food Microbiol. 94, 223–253. doi:10.1016/j.ijfoodmicro.2004.03.022 | |
Carretero-Ledesma, M., García-Quintanilla, M., Martín-Peña, R., Pulido, M. R., Pachón, J., and Mcconnell, M. J. (2018). Phenotypic changes associated with Colistin resistance due to Lipopolysaccharide loss in Acinetobacter baumannii. Virulence 9, 930–942. doi:10.1080/21505594.2018.1460187 | |
Chin, C. Y., Tipton, K. A., Farokhyfar, M., Burd, E. M., Weiss, D. S., and Rather, P. N. (2018). A high-frequency phenotypic switch links bacterial virulence and environmental survival in Acinetobacter baumannii. Nat. Microbiol. 3, 563–569. doi:10.1038/s41564-018-0151-5 | |
Chusri, S., Villanueva, I., Voravuthikunchai, S. P., and Davies, J. (2009). Enhancing antibiotic activity: a strategy to control acinetobacter infections. J. Antimicrob. Chemother. 64, 1203–1211. doi:10.1093/jac/dkp381 | |
Coetzee, J., Corcoran, C., Prentice, E., Moodley, M., Mendelson, M., Poirel, L., et al. (2016). Clinical alert-emergence of plasmid-mediated colistin resistance (MCR-1) among Escherichia coli isolated from South African patients: in practice. S. Afr. Med. J. 106, 449–450. doi:10.7196/samj.2016.v106i5.10710 | |
Clinical and Laboratory Standards Institute (CLSI) (2019). Performance standards for antimicrobial susceptibility testing 29th edn. Wayne: Clinical and Laboratory Standards Institute (CLSI).
Da Silva, G. J., and Domingues, S. (2017). Interplay between colistin resistance, virulence and fitness in acinetobacter baumannii. Antibiot. (Basel) 6, 28. doi:10.3390/antibiotics6040028 |
Durante-Mangoni, E., Del Franco, M., Andini, R., Bernardo, M., Giannouli, M., and Zarrilli, R. (2015). Emergence of colistin resistance without loss of fitness and virulence after prolonged colistin administration in a patient with extensively drug-resistant Acinetobacter baumannii. Diagn. Microbiol. Infect. Dis. 82, 222–226. doi:10.1016/j.diagmicrobio.2015.03.013 | |
Ertugrul Guclu, H. G., Mustafa, Zengin, and Oguz, Karabay (2013). Antibacterial activity of Lythrum salicaria against multidrug-resistant acinetobacter baumannii and Pseudomonas aeruginosa. Annu. Res. Rev. Biol. 4, 1099–1105. doi:10.9734/arrb/2014/7357 |
Evans, B. A., Hamouda, A., Abbasi, S. A., Khan, F. A., and Amyes, S. G. (2011). High prevalence of unrelated multidrug-resistant Acinetobacter baumannii isolates in Pakistani military hospitals. Int. J. Antimicrob. Agents 37 (6), 580–581. doi:10.1016/j.ijantimicag.2011.01.023 | |
Gallardo-Godoy, A., Muldoon, C., Becker, B., Elliott, A. G., Lash, L. H., Huang, J. X., et al. (2016). Activity and predicted nephrotoxicity of synthetic antibiotics based on polymyxin B. J. Med. Chem. 59, 1068–1077. doi:10.1021/acs.jmedchem.5b01593 | |
Hasan, B., Perveen, K., Olsen, B., and Zahra, R. (2014). Emergence of carbapenem-resistant Acinetobacter baumannii in hospitals in Pakistan. J. Med. Microbiol. 63, 50–55. doi:10.1099/jmm.0.063925-0 | |
Hasson, S. S., Al-Balushi, M. S., Alharthy, K., Al-Busaidi, J. Z., Aldaihani, M. S., Othman, M. S., et al. (2013). Evaluation of anti-resistant activity of Auklandia (Saussurea lappa) root against some human pathogens. Asian pac. J. Trop. Biomed. 3, 557–562. doi:10.1016/S2221-1691(13)60113-6 | |
Hood, M. I., Becker, K. W., Roux, C. M., Dunman, P. M., and Skaar, E. P. (2013). genetic determinants of intrinsic colistin tolerance in Acinetobacter baumannii. Infect. Immun. 81, 542–551. doi:10.1128/IAI.00704-12 | |
Hsu, L. Y., Apisarnthanarak, A., Khan, E., Suwantarat, N., Ghafur, A., and Tambyah, P. A. (2017). Carbapenem-resistant acinetobacter baumannii and enterobacteriaceae in South and southeast asia. Clin. Microbiol. Rev. 30, 1–22. doi:10.1128/CMR.00042-16 | |
Ibrahim, S., Al-Saryi, N., Al-Kadmy, I. M. S., and Aziz, S. N. (2021). Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol. Biol. Rep. 48, 6987–6998. doi:10.1007/s11033-021-06690-6 | |
Isler, B., Doi, Y., Bonomo, R. A., and Paterson, D. L. (2019). New treatment options against Carbapenem-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 63 (1). doi:10.1128/aac.01110-18 | |
Jacobo-Salcedo Mdel, R., Gonzalez-Espindola, L. A., Alonso-Castro, A. J., Gonzalez-Martinez Mdel, R., Domínguez, F., and Garcia-Carranca, A. (2011). Antimicrobial activity and cytotoxic effects of Magnolia dealbata and its active compounds. Nat. Prod. Commun. 6, 1934578X1100600–4. doi:10.1177/1934578x1100600818 | |
Karah, N., Khalid, F., Wai, S. N., Uhlin, B. E., and Ahmad, I. (2020). Molecular epidemiology and antimicrobial resistance features of Acinetobacter baumannii clinical isolates from Pakistan. Ann. Clin. Microbiol. Antimicrob. 19, 2. doi:10.1186/s12941-019-0344-7 | |
Kempf, M., and Rolain, J.-M. (2012). Emergence of resistance to carbapenems in acinetobacter baumannii in europe: clinical impact and therapeutic options. Int. J. Antimicrob. Agents 39, 105–114. doi:10.1016/j.ijantimicag.2011.10.004 | |
Khalid, F., Saleem, S., and Ahmad, I. (2020). High prevalence of carbapenem-resistant Acinetobacter baumannii associated respiratory tract infections in Pakistani hospitals. J. Pak. Med. Assoc. 70, 1630–1632. doi:10.5455/JPMA.35384 | |
Koch, E., Klaas, C. A., Rüngeler, P., Castro, V., Mora, G., Vichnewski, W., et al. (2001). Inhibition of inflammatory cytokine production and lymphocyte proliferation by structurally different sesquiterpene lactones correlates with their effect on activation of NF-kappaB. Biochem. Pharmacol. 62, 795–801. doi:10.1016/s0006-2952(01)00714-6 | |
Larsen, M. V., Cosentino, S., Rasmussen, S., Friis, C., Hasman, H., Marvig, R. L., et al. (2012). Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 50, 1355–1361. doi:10.1128/JCM.06094-11 | |
Lewis, K., and Ausubel, F. M. (2006). Prospects for plant-derived antibacterials. Nat. Biotechnol. 24, 1504–1507. doi:10.1038/nbt1206-1504 | |
Mcconnell, M. J., Actis, L., and Pachón, J. (2013). Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol. Rev. 37, 130–155. doi:10.1111/j.1574-6976.2012.00344.x | |
Moffatt, J. H., Harper, M., Harrison, P., Hale, J. D., Vinogradov, E., Seemann, T., et al. (2010a). Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 54, 4971–4977. doi:10.1128/AAC.00834-10 | |
Mu, X., Wang, N., Li, X., Shi, K., Zhou, Z., Yu, Y., et al. (2016). The effect of colistin resistance-associated mutations on the fitness of acinetobacter baumannii. Front. Microbiol. 7, 1715. doi:10.3389/fmicb.2016.01715 | |
Paterson, D. L. (2006). Resistance in gram-negative bacteria: Enterobacteriaceae. Am. J. Infect. Control 34, S20–S28. doi:10.1016/j.ajic.2006.05.238 | |
Poirel, L., Jayol, A., and Nordmann, P. (2017). Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin. Microbiol. Rev. 30, 557–596. doi:10.1128/CMR.00064-16 | |
Pournaras, S., Gogou, V., Giannouli, M., Dimitroulia, E., Dafopoulou, K., Tsakris, A., et al. (2014). Single-locus-sequence-based typing of blaOXA-51-like genes for rapid assignment of Acinetobacter baumannii clinical isolates to international clonal lineages. J. Clin. Microbiol. 52, 1653–1657. doi:10.1128/JCM.03565-13 | |
Pyun, H., Kang, U., Seo, E. K., and Lee, K. (2018). Dehydrocostus lactone, a sesquiterpene from Saussurea lappa Clarke, suppresses allergic airway inflammation by binding to dimerized translationally controlled tumor protein. Phytomedicine 43, 46–54. doi:10.1016/j.phymed.2018.03.045 | |
Rao Vadaparthi, P. R., Kumar, K., Sarma, V. U., Hussain, Q. A., and Babu, K. S. (2015). Estimation of costunolide and dehydrocostus lactone in Saussurea lappa and its polyherbal formulations followed by their stability studies using HPLC-DAD. Pharmacogn. Mag. 11, 180–190. doi:10.4103/0973-1296.149736 | |
Rice, L. B. (2008). Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 197, 1079. doi:10.1086/533452 | |
Thadtapong, N., Chaturongakul, S., Soodvilai, S., and Dubbs, P. (2021)., Colistin and carbapenem-resistant acinetobacter baumannii Aci46 in Thailand: Genome analysis and antibiotic resistance profiling Antibiotics 10 (9). 1054. doi:10.3390/antibiotics10091054 |
Tipton, K. A., Dimitrova, D., and Rather, P. N. (2015). Phase-variable control of multiple phenotypes in acinetobacter baumannii strain AB5075. J. Bacteriol. 197, 2593–2599. doi:10.1128/JB.00188-15 | |
Keywords: colistin, multiple drug resistence, Acinetobacter baumannii, doxycycline, saussurea lappa
Citation: Ahsan U, Mushtaq F, Saleem S, Malik A, Sarfaraz H, Shahzad M, Uhlin BE and Ahmad I (2022) Emergence of high colistin resistance in carbapenem resistant Acinetobacter baumannii in Pakistan and its potential management through immunomodulatory effect of an extract from Saussurea lappa. Front. Pharmacol. 13:986802. doi: 10.3389/fphar.2022.986802
Received: 05 July 2022; Accepted: 17 August 2022;
Published: 16 September 2022.
Edited by:
Manoj Puthia, Lund University, SwedenReviewed by:
Dafne Bongiorno, University of Catania, ItalyChanghan Lee, Ajou University, South Korea
Srisuda Pannanusorn, Thammasat University, Thailand
Copyright © 2022 Ahsan, Mushtaq, Saleem, Malik, Sarfaraz, Shahzad, Uhlin and Ahmad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Irfan Ahmad, SXJmYW4uYWhtYWRAdW11LnNl, aXJmYW4uYWhtYWRAdWhzLmVkdS5waw==
†These authors have contributed equally to this work
 Umaira Ahsan1,2†
Umaira Ahsan1,2† Bernt Eric Uhlin
Bernt Eric Uhlin Irfan Ahmad
Irfan Ahmad