- 1State Key Laboratory of Component-Based Chinese Medicine, Institute of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, Hong Kong, Hong Kong SAR, China
- 3School of Integrative Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China
It is widely acknowledged that the climacteric syndrome negatively affects women’s quality of life and leads to cerebral ischemic injury, osteoporosis and cardiovascular disease. One of the main active ingredients in Radix Scutellariae, Baicalin, has been established to possess a wide range of pharmacological effects and is beneficial in enhancing osteogenic differentiation and cardiovascular disease. Baicalin’s profound metabolic impact on various stem cell populations and their fate specification could improve the efficiency of stem cell therapy for climacteric syndrome. However, Baicalin-mediated processes are complex and many of the underlying mechanisms are not fully fathomed yet. This review aims to shed light on the regulatory role of Baicalin on the diverse behaviors of distinct stem cell populations and provide a good cell source for stem cell therapy to broaden the therapeutic landscape for climacteric syndrome patients.
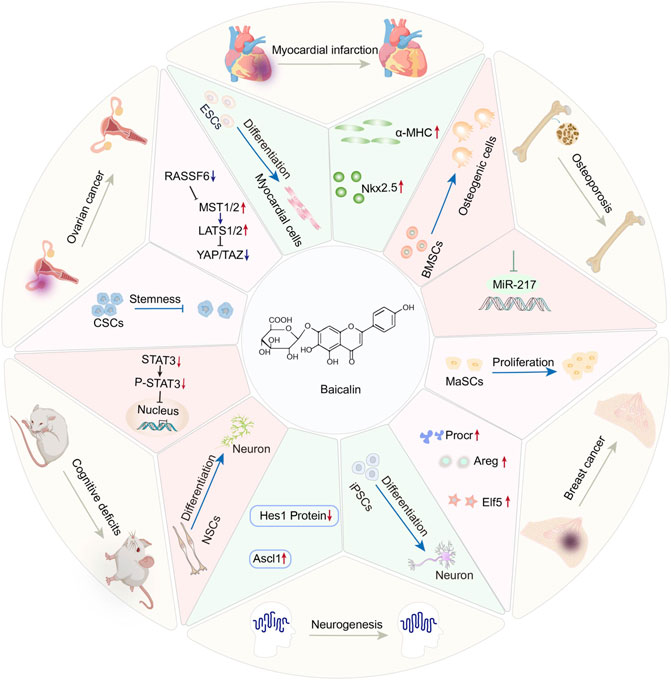
GRAPHICAL ABSTRACT. This review summarizes the regulatory role of Baicalin on the diverse behaviors of distinct stem cell populations and emphasizes the potential applications of Baicalin and stem cell therapy in climacteric syndrome.
Introduction
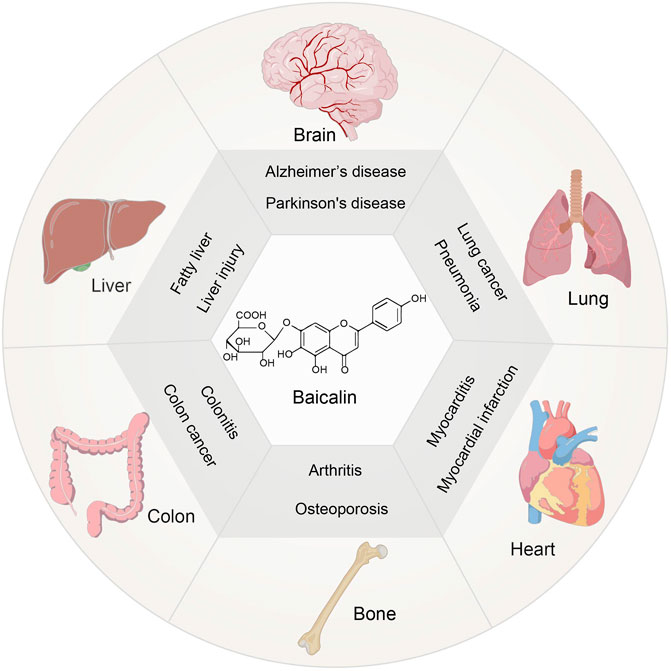
Scutellaria baicalensis Georgi has a long history of therapeutic and commercial value in traditional Chinese medicine (Zhao et al., 2019). One of the key constituents is Baicalin, which belongs to the flavonoid family (Zhao Q. et al., 2018). It has been established that Baicalin exerts anti-inflammatory, antioxidant, and anti-apoptotic properties (Guo et al., 2019). Currently, the focus is on studying its pharmacological action before clinical trials for the treatment of various diseases, including liver injury (Shi et al., 2020) and fatty liver(Liu et al., 2020), neurological dysfunction(Jin et al., 2019),lung injury(Zhang H. et al., 2021),osteoporosis(Zhao et al., 2020), inflammation of the colon(Xu et al., 2021) and cardiovascular disease(Xiping et al., 2007) (Figure 1). Moreover, menopause leads to an increase in risk for degenerative diseases and cardiovascular diseases, due to the fluctuation of hormones in women (Brooks et al., 2016), which indicates the possibility of Baicalin in the treatment of climacteric syndrome. Clinical investigations on the treatment of injuries, gum damage, and influenza demonstrate the broad-spectrum pharmacological benefits of baicalin (Table 1). Thus, the therapeutic roles of Baicalin in these severe illnesses emphasizes its potential capacity for management of climacteric syndrome.
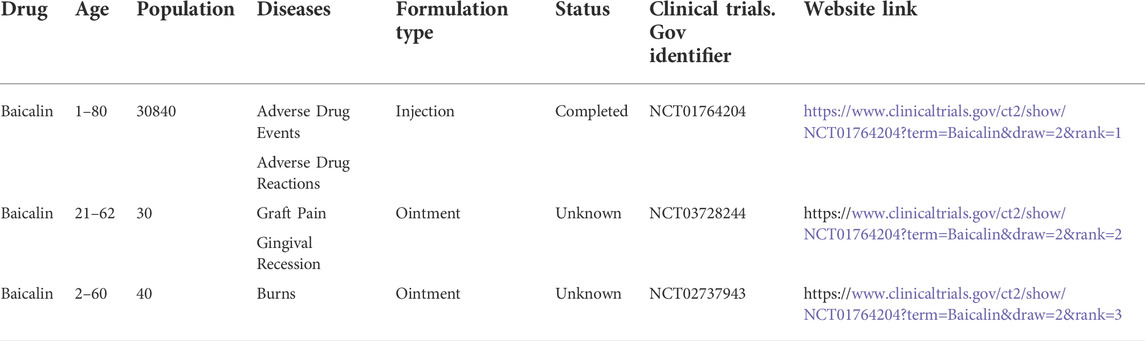
TABLE 1. Clinical trials of Baicalin in various diseases (www.clinicaltrials.gov).
Menopause is a physiological condition that naturally develops in women as they age. Climacteric syndrome is a set of symptoms that often occur throughout the perimenopausal and postmenopausal periods (Wong et al., 2015). Accompanied by mood swings and anxiety and diseases such as osteoporosis(Wang B. et al., 2020), breast cancer (Kabat et al., 2017) and cardiovascular disease (Jaballah et al., 2021) caused by metabolic abnormalities. Cognitive impairment disorders such as Alzheimer’s disease arise because hormone levels have a large impact on the brain (Song et al., 2020). These can negatively burden the quality of life and work efficiency of women. Hormone therapy remains the mainstay of treatment, but it has been associated with risks and side effects (Yang et al., 2022). These findings highlight the need for a new therapeutic approach to the broad spectrum of menopausal symptoms without causing severe side effects. The advent of stem cell technology has offered optimism in treating various diseases in recent decades (Yam et al., 2022). The various mechanisms of action of Baicalin on cells and its ability to regulate various signaling pathways can be used to develop strategies to treat menopause.
Stem cells are self-renewing cells with multi-lineage differentiation potential (McKenzie et al., 2006), which is critical for their involvement in tissue repair and homeostasis (Mi et al., 2022). An increasing body of evidence from recently published studies has demonstrated the efficacy of stem cell therapy in the prevention and treatment of a variety of disorders, including cancer (Steenbruggen et al., 2020), Alzheimer’s disease (Zhu et al., 2020), psoriasis disease (Ali et al., 2020) and dominant optic atrophy (Weiss and Levy, 2019), suggesting that stem cell therapy has great therapeutic potential. Various natural substances are currently available to support stem cell therapy in the treatment of obesity (Hong et al., 2018) and the cure of ischemic cardiomyopathy including myocardial infarction (Han et al., 2019), as well as to increase the treatment effect of osteoporosis and nervous system disorders. As a result, stem cell treatments and natural substances show great promise for tissue regeneration.
Although it is well-established that Baicalin has several positive physiological functions, this paper is focused on how Baicalin regulates physiological mechanisms in embryonic stem cells, neural stem cells and other stem cell populations (Table 2) by demonstrating the physiological mechanisms influencing stem cells for the treatment of different diseases.
Baicalin and embryonic stem cells
Embryonic stem cells (ESCs) have a remarkable capacity for maintaining an undifferentiated condition before differentiating for a long period (Kehat et al., 2001). Since ESCs can proliferate indefinitely and differentiate into any cell type (Xu et al., 2020), they have huge prospects for clinical application (Li et al., 2021). It has been reported that post-menopausal women have a higher risk of cardiovascular disease than when they were younger (Gabriel et al., 2005; Welten et al., 2021). Post-menopausal women also exhibit a greater increase in systolic blood pressure, and total cholesterol and triglyceride levels, as well as low density lipoprotein associated with development of cardiovascular disease, including coronary artery disease. The incidence of myocardial infarction (MI) increases gradually in the post-menopausal women, which is likely to the incidence of men at the age of 80. The result suggest that the onset of myocardial infarction has a sex-specific pathogenesis and seriously affect the quality of life in women (Savonitto et al., 2018). However, the low efficiency of ESCs limits their widespread use. Interestingly, it has been discovered that Baicalin can influence ESC differentiation into cardiomyocytes (Tang et al., 2013) and inhibit cell proliferation (Wang J. et al., 2015). They provide an excellent cell source for myocardial infarction through the differentiation of ESCs, as a novel approach to treating myocardial infarction brought on by female menopause.
Myocardial infarction is a common cardiovascular disease of rapid onset (Zhao et al., 2021). Current evidence suggests that although reperfusion can reduce cardiac tissue injury, it increases perfusion injury (Zhang G. et al., 2021). Accordingly, a safer approach is warranted to minimize reperfusion injury in myocardial infarction. Nkx2.5 is an early cardiovascular transcription factor (Thiele et al., 2019), and its specific deletion can lead to cardiac abnormalities(He et al., 2022), suggesting the importance of Nkx2.5 for heart development and growth. Continuous Baicalin treatment can induce functional myocardium formation of embryonic stem cell line D3 by up-regulating the transcription of Nkx2.5 at the intermediate and late stages of differentiation (Tang et al., 2013) (Figure 2A). In addition, the α -myosin heavy chain (α-MHC) is a heart-specific gene (Zhu and Lou, 2006) that Baicalin can upregulate. Baicalin stimulates cardiomyocyte differentiation via induction of ESCs to restore function after myocardial infarction via cell transplantation, providing a new cell source for the treatment of ischemic heart disease, emphasizing the role of Baicalin in the treatment of heart disease. Notably, the Src-Yap1 signaling axis is highly activated in ESCs, and ESC differentiated cells and regulates embryonic stem cell differentiation (Luo et al., 2021). Therefore, it is essential to investigate whether the level of Src-Yap1 fluctuates during treatment with Baicalin on ESC differentiation.
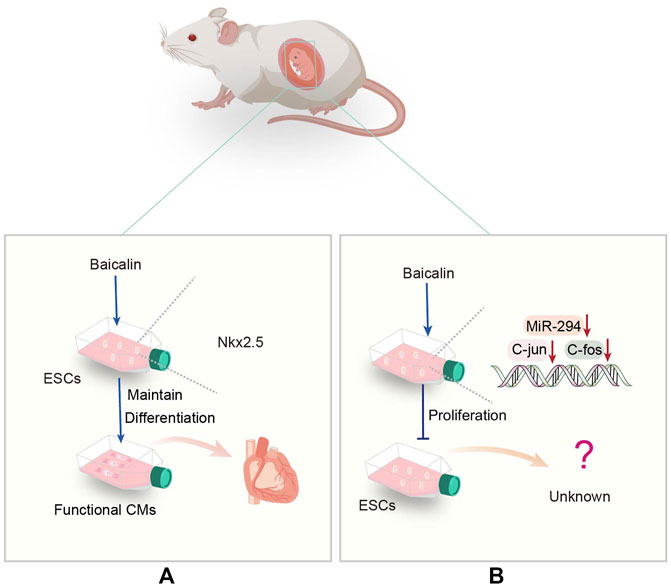
FIGURE 2. (A) Representative scheme illustrating the potential mechanisms underlying the Baicalin regulating the differentiation of ESCs. (B) Representative scheme illustrating the potential mechanisms underlying the Baicalin regulation in the proliferation of ESCs.
Moreover, it has been demonstrated that the miR-290 family promotes ESC self-renewal by influencing the cell cycle and processes in ESCs (Marson et al., 2008). As a member of this family, miR-294 can promote cell cycle progression and be used in the treatment of myocardial infarction (Borden et al., 2019). Inhibition of miR-294 expression by Baicalin down-regulates the expression of c-Jun and c-Fos genes, leading to an increase in G1 phase, but a decrease in S or G2/M phase of mouse embryonic stem cell line D3 (D3-mESCs), associated with cell proliferation phenotype (Wang J. et al., 2015) (Figure 2B). More importantly, it has been discovered that Baicalin inhibits ESC proliferation. Consequently, more emphasis should be placed on intracellular molecular regulation to improve the current diagnosis and treatment approaches.
In response to hypoxia, Baicalin may activate the HIF1/BNIP3 pathway and produce the upregulated hypoxia-inducible factor 1α (HIF1α), which reduces apoptosis and viability produced by this state, thereby increasing cardiac protection (Yu et al., 2019). Baicalin’s regulatory effect on ESCs highlights that it has huge prospects for myocardial cell differentiation and functional recovery after myocardial infarction. It can inhibit proliferation while promoting differentiation, allowing for better differentiation of ESCs into required cells for clinical application, and offers more therapeutic options for myocardial infarction.
Baicalin and cancer stem cells
Cancer is characterized by aberrant cell development and the potential for metastatic spread and is associated with a high global mortality rate. Reports suggest 19.3 million new cancer diagnoses and 10 million cancer deaths globally in 2020 (Sung et al., 2021). Cancer cells are formed partly from the differentiation of cancer stem cells (CSCs), which has become a major research hotspot for treatment in recent decades (Bie et al., 2021). Baicalin has been used to treat a wide variety of cancers since it exhibits anticancer properties in ovarian cancer (Gao et al., 2017). Given the numerous methods of action of Baicalin, the following sections concentrate on the therapeutic benefits of Baicalin on ovarian cancer via lowering the stemness of CSCs.
It has been established that CSCs is closely related to many signaling pathways, such as YAP (Gao et al., 2020) and Wnt (Tang et al., 2020), which affect the growth and proliferation of cancer cells. The hippocampal/YAP signaling pathway (Hippo/YAP) is a conservative kinase cascade pathway found in Drosophila melanogaster (Cordenonsi et al., 2011), containing ste20-like kinase 1/2 (MST1/2) and a large tumor suppressor (Lats1/2), which can be phosphorylated and activated by MST1/2 (Zhou et al., 2020). YAP and transcriptional co-activator (TAZ) are the main downstream effectors of the Hippo pathway, and Lats1/2 can inhibit YAP by direct phosphorylation of S127 (Yu et al., 2012), which play a crucial role in cell fate and maintaining cell stemness (Huang et al., 2020; Quinn et al., 2021). Moreover, Baicalin reduces YAP activity by inhibiting the transcription of RASSF6, a negative regulator of MST1/2 (Li et al., 2020), leading to further inhibition of the stemness of ovarian CSCs (Figure 3), indicating that Baicalin could be utilized to block the YAP signaling pathway in vitro. This finding suggests that Baicalin may be utilized to inhibit the YAP signaling pathway. Accordingly, it has huge potential as an anticancer medication to suppress ovarian CSCs.
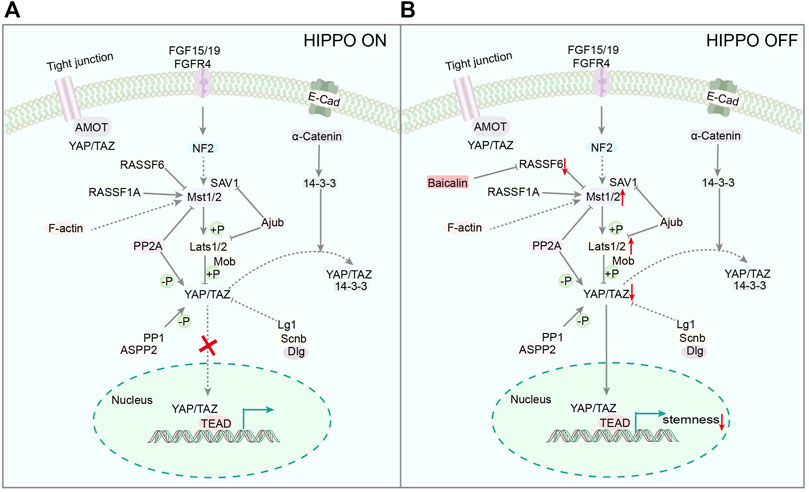
FIGURE 3. (A) Representative scheme illustrating the Hippo Signaling pathway. (B) Representative scheme illustrating Baicalin’s role in the proliferation of CSCs by regulating the Hippo Signaling pathway.
Furthermore, Baicalin can reportedly increase the chemical sensitivity of cancer cells, reducing drug resistance (Zeng et al., 2020), providing a good auxiliary effect against drug resistance during the cancer treatment process. These discoveries highlight Baicalin’s potential to regulate tumor stem cell growth and how it can benefit humanity by providing the foothold for developing new cancer treatments. As our present understanding of this issue is primarily based on in vitro studies, further in vivo studies will be required to fully define Baicalin’s ability and promise in the treatment of ovarian cancer.
Baicalin and neural stem cells
Neural stem cells (NSCs) are undifferentiated cells that can proliferate, self-renew indefinitely, and differentiate into all types of neurons and glial cells (Andreotti et al., 2019). Adult NSCs generate new neurons with active functions throughout their lives, which are integrated into the original neural network to facilitate the development of learning and memory functions (Goncalves et al., 2016). NSCs are crucial in brain development, maturation, and neurogenesis(Di Bernardini et al., 2014). It has been shown that postmenopausal women are more susceptible to neurodegenerative conditions such as ischemic injury and cognitive decline (Ma et al., 2020). Early menopause is associated with an increased risk of stroke (Welten et al., 2021). Moreover, menopause can lead to cognitive issues like Alzheimer’s disease (AD) (Mosconi et al., 2021). These findings highlight the need for new therapeutic approaches to alleviate the symptoms of menopause, thereby reducing cognitive decline and ischemic damage.
Alzheimer’s disease is a degenerative disorder characterized by brain atrophy, loss of neurons, associated with behavioral changes and cognitive decline (Ma et al., 2022). As a common form of dementia, AD affects about 50 million people worldwide and is expected to diagnose a new case every 3 s. However, there is currently no effective treatment available (Liu et al., 2022a). Interestingly, Baicalin has the potential to treat AD through inhibiting Ras-ERK signaling pathway and altering the cell cycle composition ratio, thereby preventing apoptosis caused by Aβ accumulation (Song et al., 2022). In addition, Baicalin can improve synaptic plasticity, mitochondrial fragmentation and dysfunction by inhibiting PDE4 activation in a mouse model of AD (Yu et al., 2022). In addition, Baicalin also affects the differentiation and proliferation of NSCs in AD mouse model (Zhao J. et al., 2018). Accordingly, investigating the effect of Baicalin on NSCs is important to provide novel therapeutic concepts for this patient population. In recent years, research has primarily focused on the role of Baicalin in disease treatment by regulating NSC differentiation. Many signaling pathways, including STAT and ERK, can impact neurodevelopment and NSC fate determination (Boku et al., 2013).
Jak-STAT is an intracellular signal transduction pathway, including Janus Kinases (JAK) and the signal and activator of transcription (STAT). The JAK-STAT pathway is a highly regulated and efficient system that regulates gene expression (Wang T. et al., 2015). STAT3 is involved in NSC differentiation (Boku et al., 2013) and synaptic plasticity (Long et al., 2021). Baicalin can promote neuronal differentiation of embryonic neural stem cells by down-regulating STAT3 phosphorylation (Li Y. et al., 2012) (Figure 4). Baicalin can reportedly ameliorate the usual features of degenerative disorders, such as decreased memory and cognitive function, by promoting differentiation of NSCs. It is widely thought that Baicalin may direct various cells to perform different functions in the central nervous system (CNS). Thus, Baicalin can facilitate the rehabilitation of cognitive impairment caused by aberrant inflammation and age.
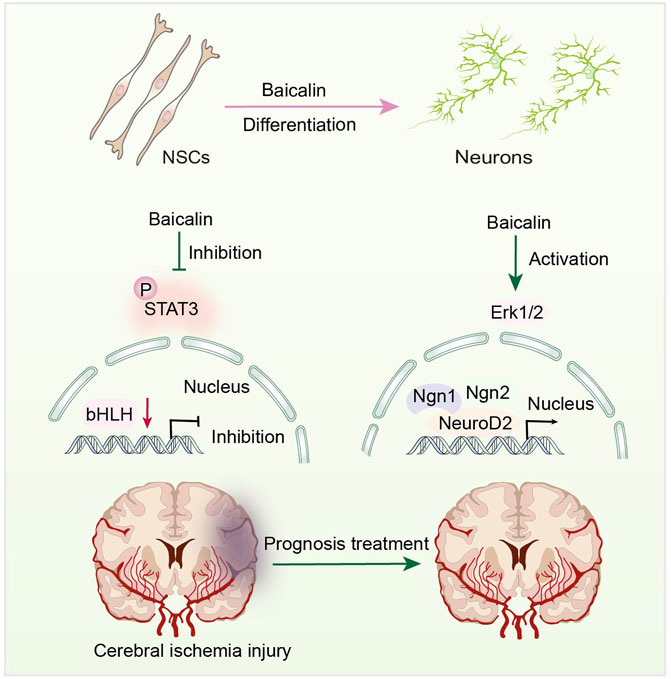
FIGURE 4. Representative scheme illustrating Baicalin’s role in the proliferation and differentiation of NSCs by regulating the JAK/STAT3 Signaling pathway.
Overwhelming evidence substantiates that activating Erk1/2 can increase neurogenesis in C17.2 NSCs (Song et al., 2011; Liu D. et al., 2018), which is employed as a model for assessing the neural differentiation-inducing features of many compounds. Baicalin can stimulate differentiation of C17.2 NSCs by activating Erk1/2 (Li et al., 2011), which controls neural differentiation-related gene expression. Under the effects of Baicalin, the expression levels of NeuroD2, Ngn1, and Ngn2 mRNA are upregulated (Li M. et al., 2012) (Figure 4). Neurogenic proteins (Ngns) and neurogenic differentiation factors (NeuroDs) are pro-transcriptional factors that govern neurogenesis and play a vital role in the development of NSCs into neuronal lineages (Chen W. C. et al., 2019). This finding suggests that Baicalin positively affects NSC differentiation. Moreover, Baicalin has been shown to have no influence on the mRNA expression of split one enhancer (Hes1), Hes5, and DNA binding inhibitor 2 (Id2) (Li M. et al., 2012). These factors inhibit the formation of glial cells while inhibiting neurogenesis (Zhang et al., 2010).
Furthermore, Baicalin has been shown to induce hippocampus regeneration and improve cognitive function following cerebral ischemia injury (Zhuang et al., 2013), providing new insights into the prognosis and treatment of cerebral ischemic injury. These findings account for the ability of Baicalin to increase neurogenesis in clinical trials and provide a novel perspective on NSCs. It is widely thought that Baicalin has the potential to become a small molecule medicine for the regeneration therapy of nervous system illnesses, utilized to alleviate the cognitive impairment and stroke induced by menopause, based on the evaluation of stem cell proliferation and differentiation.
Baicalin and induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) are pluripotent, which refer to the cells produced after gene reprogram in somatic cells (Ramotowski et al., 2019). IPSCs have a wide range of sources and are often used to study developmental processes, medical regeneration, and so on (Yamamoto et al., 2021). This phenomenon is notably evident in the nervous system, particularly in light of recent findings on the neuronal differentiation capacity of iPSCs and the benefits of such models for the in vitro modeling of AD (Mungenast et al., 2016).
The basic-helix-loop-helix (bHLH) family of transcription factors, such as Hes1, Ascl1 and Oligo2, play a crucial role in the neural development and fate determination of iPSCs (Wang et al., 2018). Hes1 can induce the expression of glial fibrillary acidic protein (GFAP), which in turn encodes an intermediate filament protein, and inhibit neuronal differentiation by suppressing Ascl1 expression (Ramotowski et al., 2019). Interestingly, Baicalin has been shown to promote neuronal differentiation, but inhibit astroglial differentiation of iPSCs by up-regulating gene expression of Ascl1 and reducing Hes1 protein expression, respectively. The results verify the regulatory effect of Baicalin in bHLH protein family, which is essential for neuronal differentiation of iPSCs (Morita et al., 2015) (Figure 5). Therefore, the ability of iPSCs to differentiate into neurons has made possible therapeutic strategy for neurodegenerative diseases. However, current achievements are only focused on in vitro studies, and future research needs on in vivo studies in order to obtain more exploratory findings.
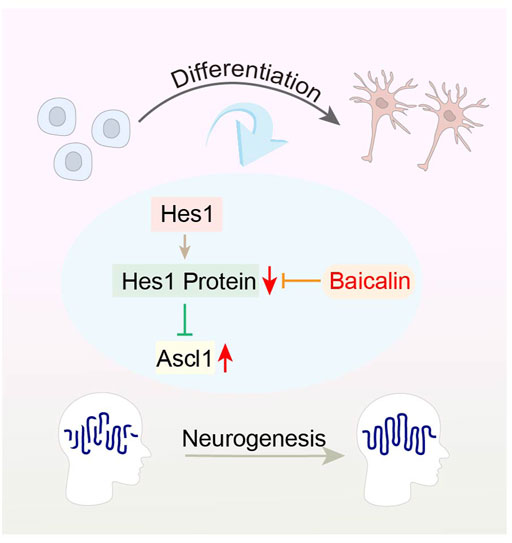
FIGURE 5. Representative schemes illustrating the potential mechanism of Baicalin regulation in the differentiation of iPSCs.
Baicalin and mammary stem cells
Mammary stem cells (MaSCs) have the potential to self-renew and change, developing into luminal progenitors and basal cells, which then differentiate into ducts or alveolar cells (Patwardhan et al., 2014). As a result, MaSCs can sustain mammary gland epithelial and internal environment growth (Jiang et al., 2010) and stimulate mammary gland development and regeneration (Wang et al., 2021). Besides, abnormal regulatory pathways of MaSCs can affect cell activities, leading to breast cancer (Liu R. et al., 2022). Interestingly, it has been reported that hormone levels are associated with the etiology of breast cancer (Ranjan et al., 2021). By 2020, more than 2.3 million women were diagnosed with breast cancer, and 685000 people were killed, exceeding lung cancer-related death for the first time. Although curative rates are dismal, hormone treatment remains the mainstay of therapy (Chen X. et al., 2019). As a result, it is critical to address the process and mechanism of MaSCs in treating breast cancer and mammary gland development, allowing patients to receive better care.
Many natural monomer compounds exhibit hormone-like properties and may be used for medical therapy (Yang et al., 2017). Research has demonstrated that Baicalin can facilitate MaSCs amplification and directly facilitate Protein C receptor (Procr) gene transcription (Chen W. et al., 2021). The Procr gene is essential for the proliferation of MaSCs and the mammary environment (Liu C. et al., 2022). Current evidence suggests that Procr can be used as a surface marker of MaSCs and a therapeutic target for breast cancer (Wang et al., 2019). Several important genes related to basal cell mammary gland development, such as Procr, Areg, Elf5, Socs2, and Bax, are upregulated at the same time, implying that Baicalin promotes mammary gland growth (Chen W. et al., 2021). Baicalin can regulate MaSCs via hormone-like activities, providing a good supply of stem cells for stem cell therapy as well as a novel treatment for breast cancer.
Baicalin and bone marrow mesenchymal stem cells
As pluripotent stem cells, bone marrow mesenchymal stem cells (BMSCs) can differentiate into chondrocytes, osteoblast and fat cells (Khoshsirat et al., 2019). Over the years, BMSCs have been used to treat neurodegenerative diseases (Xue et al., 2019; Liu et al., 2022b) and osteoporosis (Chen M. et al., 2021). As a result, BMSCs represent an important source for the effective treatment of osteoporosis induced by endocrine abnormalities in postmenopausal women. It has been reported that oxidative stress could reduce the osteogenic development of BMSCs during the osteoporosis era (Yang et al., 2021), and even cause BMSCs senescence and apoptosis (Liu Z. et al., 2018). These factors restrict the use of BMSCs in treating neurological illnesses, blood-brain barrier disorders, and bone diseases in the clinic. An efficient antioxidant is accordingly required to minimize oxidative stress and enhance the survival rate of BMSCs.
Interestingly, it is widely thought that Scutellaria baicalensis Georgi has an antioxidant effect (Liau et al., 2019). It has been discovered that BMSCs transplantation might be employed to increase BMSCs transplantation survival rates and bone mechanical strength. Baicalin has been shown to promote BMSC differentiation into osteoblasts and the emergence of mineralized nodules in vitro (Zhang et al., 2017), implying that it has an anti-osteoporosis effect (Figure 6). This finding provides compelling evidence for BMSCs transplantation and higher-quality BMSCs for stem cell therapy. Additionally, Baicalin might increase microRNA 217, activate the Wnt/β-catenin and MEK/ERK pathways, and quicken the process of enhancing cell viability and osteogenic differentiation (Wang Q. et al., 2020). Baicalin has huge prospects for application in the treatment of osteoporosis. A study reports that the osteogenic-specific molecules runt-related protein 2 (Runx2) and osteocalcin (Ocn) are expressed more frequently when Baicalin is present (Wang Q. et al., 2020). However, another study demonstrates that miR-217 can bind to Runx2 to prevent rat BMSCs from differentiating into osteoblasts (Zhu et al., 2017). The discrepancy in results may be attributed to different cell lines used in the experiment, and further investigating the effect of miR-217 on osteogenic differentiation is worthwhile. Importantly, Baicalin can increase osteogenic activity and promote bone repair and remodeling, which could have many applications in bone transplantation or osteoporosis.
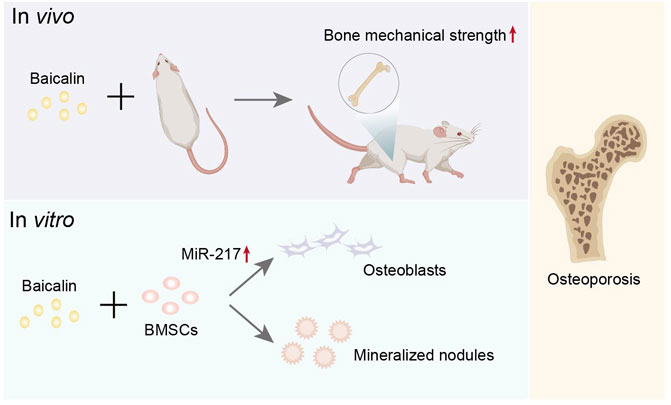
FIGURE 6. Representative schemes illustrating the potential mechanism of Baicalin regulation in the differentiation of BMSCs.
Conclusion and future perspectives
This review sought to highlight the effect of Baicalin on stem cells in alleviating several disorders associated with menopause, providing novel insights into the treatment and prevention of climacteric syndrome (Graphical abstract). Current evidence substantiates that Baicalin can be utilized to treat climacteric sickness and control stem cell proliferation, differentiation and self-renewal.
It is widely acknowledged that natural substances like Baicalin have various pharmacological effects, with low toxicity and can cross the blood-brain barrier (Zhao et al., 2022), which offers a basic platform for the treatment of diseases. More study is required to establish the fate of stem cells after treatment with Baicalin, although it has been established that stem cells provide novel insights and therapeutic alternatives for many disorders.
Stem cell therapy has been widely used in recent years and has been clinically used for treating arthritis (Cai et al., 2023), brain injury (Jiao et al., 2022) and other diseases. However, its disadvantages cannot be ignored, such as immunologic rejection and poor cell viability (Chen et al., 2022). As it turns out, Baicalin can enhance stem cell efficacy, serve as a good source of stem cells for stem cell therapy, and augment the regulatory function of stem cells. Baicalin and stem cell therapy can work synergistically to extend the drug’s scope of use and therapeutic potential. In order to unlock the therapeutic potential of Baicalin at a deeper level, an interdisciplinary approach is required to uncover the cellular and molecular mechanisms of Baicalin in these processes and the associated pathological pathways.
Indeed, various shortcomings of Baicalin should be addressed before its implementation for the treatment of climacteric illness. Baicalin, for example, has been demonstrated in vivo to limit the proliferation of hematopoietic stem cells (HSCs) in a concentration-dependent way (Abbasi et al., 2015) After hydrolysis into baicalein in vivo (Wang et al., 2018), baicalein activates ERK by reducing MKP3 aquaporin and triggering the Nrf-2 pathway, causing upregulation of cytokines (Patwardhan et al., 2014) and boosting the number of HSCs even further. Further research is warranted on the in vivo transformation of the two and the combined regulation of stem cells. Furthermore, Baicalin has poor water solubility and a limited bioavailability (Mi et al., 2021), limiting its future clinical applicability. To increase medication usage, dosage formulations such as cyclodextrin inclusion and hydrogel are currently being explored (Li et al., 2018; Wang et al., 2022). Therefore, further dosage optimization represents a major future challenge, and the therapeutic effects of different doses still need to be explored. Prior studies on the molecular pharmacology of Baicalin on stem cells showed that both could be utilized to treat diseases that are stem cell-related. Treatment for illnesses connected to climacteric syndrome has benefited from all these investigations. We anticipate our assessment will generate fresh perspectives for further debate on Baicalin and stem cell research.
Author contributions
YL and SW contributed to the conception, designed the paper, supervised the work, administered the project and its final editing. QW drafted the work and revised it critically for important intellectual content. All authors participated in the revision of the paper and approved the final manuscript.
Funding
This work was supported by Tianjin Municipal Education Commission Scientific Research Project (Natural Science, Grant No. 2019ZD11 to YL, China).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.986436/full#supplementary-material
Abbreviations
AD, Alzheimer’s disease; BMSCs, bone marrow mesenchymal stem cells; CSC, cancer stem cells; CNS, central nervous system; Id2, DNA binding inhibitor two; ESCs, embryonic stem cells; HSC, hematopoietic stem cell; Hippo/YAP, hippocampal/YAP signaling pathway; iPSCs, human-induced pluripotent stem cells; HIF1α, hypoxia-inducible factor 1α; JAK, Janus Kinases; Lats1/2, large tumor suppressor; MaSCs, Mammary stem cells; NSCs, Neural stem cells; NeuroDs, neurogenic differentiation factors; Ngns, Neurogenic proteins; Ocn, osteocalcin; Procr, Protein C receptor; Runx2, runt-related protein two; STAT, signal and activator of transcription; Hes1, split one enhancer; MST1/2, ste20-like kinase 1/2; TAZ, transcriptional co-activator; α-MHC, α-myosin heavy chain.
References
Abbasi, P., Shamsasenjan, K., Movassaghpour Akbari, A. A., Akbarzadehlaleh, P., Dehdilani, N., and Ejtehadifar, M. (2015). The effect of baicalin as A PPAR activator on erythroid differentiation of CD133(+)Hematopoietic stem cells in umbilical cord blood. Cell J. 17 (1), 15–26. doi:10.22074/cellj.2015.508
Ali, G., Elsayed, A. K., Nandakumar, M., Bashir, M., Younis, I., Abu Aqel, Y., et al. (2020). Keratinocytes derived from patient-specific induced pluripotent stem cells recapitulate the genetic signature of psoriasis disease. Stem Cells Dev. 29 (7), 383–400. doi:10.1089/scd.2019.0150
Andreotti, J. P., Silva, W. N., Costa, A. C., Picoli, C. C., Bitencourt, F. C. O., Coimbra-Campos, L. M. C., et al. (2019). Neural stem cell niche heterogeneity. Semin. Cell Dev. Biol. 95, 42–53. doi:10.1016/j.semcdb.2019.01.005
Bie, Q., Song, H., Chen, X., Yang, X., Shi, S., Zhang, L., et al. (2021). IL-17B/IL-17RB signaling cascade contributes to self-renewal and tumorigenesis of cancer stem cells by regulating Beclin-1 ubiquitination. Oncogene 40, 2200–2216. doi:10.1038/s41388-021-01699-4
Boku, S., Nakagawa, S., Takamura, N., Kato, A., Takebayashi, M., Hisaoka-Nakashima, K., et al. (2013). GDNF facilitates differentiation of the adult dentate gyrus-derived neural precursor cells into astrocytes via STAT3. Biochem. Biophys. Res. Commun. 434 (4), 779–784. doi:10.1016/j.bbrc.2013.04.011
Borden, A., Kurian, J., Nickoloff, E., Yang, Y., Troupes, C. D., Ibetti, J., et al. (2019). Transient introduction of miR-294 in the heart promotes cardiomyocyte cell cycle reentry after injury. Circ. Res. 125 (1), 14–25. doi:10.1161/CIRCRESAHA.118.314223
Brooks, H. L., Pollow, D. P., and Hoyer, P. B. (2016). The VCD mouse model of menopause and perimenopause for the study of sex differences in cardiovascular disease and the metabolic syndrome. Physiology 31 (4), 250–257. doi:10.1152/physiol.00057.2014
Cai, Y., Wu, C., Ou, Q., Zeng, M., Xue, S., Chen, J., et al. (2023). Enhanced osteoarthritis therapy by nanoengineered mesenchymal stem cells using biomimetic CuS nanoparticles loaded with plasmid DNA encoding TGF-β1. Bioact. Mat. 19, 444–457. doi:10.1016/j.bioactmat.2022.04.021
Chen, M., Han, H., Zhou, S., Wen, Y., and Chen, L. (2021). Morusin induces osteogenic differentiation of bone marrow mesenchymal stem cells by canonical Wnt/β-catenin pathway and prevents bone loss in an ovariectomized rat model. Stem Cell Res. Ther. 12 (1), 173. doi:10.1186/s13287-021-02239-3
Chen, P., Ning, X., Li, W., Pan, Y., Wang, L., Li, H., et al. (2022). Fabrication of Tβ4-Exosome-releasing artificial stem cells for myocardial infarction therapy by improving coronary collateralization. Bioact. Mat. 14, 416–429. doi:10.1016/j.bioactmat.2022.01.029
Chen, W. C., Chang, L. H., Huang, S. S., Huang, Y. J., Chih, C. L., Kuo, H. C., et al. (2019). Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J. Neuroinflammation 16 (1), 187. doi:10.1186/s12974-019-1572-7
Chen, W., Wei, W., Yu, L., Zhang, X., Huang, F., Zheng, Q., et al. (2021). Baicalin promotes mammary gland development via steroid-like activities. Front. Cell Dev. Biol. 9, 682469. doi:10.3389/fcell.2021.682469
Chen, X., Xu, D., Li, X., Zhang, J., Xu, W., Hou, J., et al. (2019). Latest overview of the cyclin-dependent kinases 4/6 inhibitors in breast cancer: The past, the present and the future. J. Cancer 10 (26), 6608–6617. doi:10.7150/jca.33079
Cordenonsi, M., Zanconato, F., Azzolin, L., Forcato, M., Rosato, A., Frasson, C., et al. (2011). The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147 (4), 759–772. doi:10.1016/j.cell.2011.09.048
Di Bernardini, E., Campagnolo, P., Margariti, A., Zampetaki, A., Karamariti, E., Hu, Y., et al. (2014). Endothelial lineage differentiation from induced pluripotent stem cells is regulated by microRNA-21 and transforming growth factor β2 (TGF-β2) pathways. J. Biol. Chem. 289 (6), 3383–3393. doi:10.1074/jbc.M113.495531
Gabriel, S. R., Carmona, L., Roque, M., Sánchez, G. L. M., and Bonfill, X. (2005). Hormone replacement therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst. Rev. 2, CD002229. doi:10.1002/14651858.CD002229.pub2
Gao, C., Zhou, Y., Li, H., Cong, X., Jiang, Z., Wang, X., et al. (2017). Antitumor effects of baicalin on ovarian cancer cells through induction of cell apoptosis and inhibition of cell migration in vitro. Mol. Med. Rep. 16 (6), 8729–8734. doi:10.3892/mmr.2017.7757
Gao, Y., Li, J., Xi, H., Cui, J., Zhang, K., Zhang, J., et al. (2020). Stearoyl-CoA-desaturase-1 regulates gastric cancer stem-like properties and promotes tumour metastasis via Hippo/YAP pathway. Br. J. Cancer 122 (12), 1837–1847. doi:10.1038/s41416-020-0827-5
Goncalves, J. T., Schafer, S. T., and Gage, F. H. (2016). Adult neurogenesis in the Hippocampus: From stem cells to behavior. Cell 167 (4), 897–914. doi:10.1016/j.cell.2016.10.021
Guo, L. T., Wang, S. Q., Su, J., Xu, L. X., Ji, Z. Y., Zhang, R. Y., et al. (2019). Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflammation 16 (1), 95. doi:10.1186/s12974-019-1474-8
Han, X. J., Li, H., Liu, C. B., Luo, Z. R., Wang, Q. L., Mou, F. F., et al. (2019). Guanxin Danshen Formulation improved the effect of mesenchymal stem cells transplantation for the treatment of myocardial infarction probably via enhancing the engraftment. Life Sci. 233, 116740. doi:10.1016/j.lfs.2019.116740
He, L., Zhang, Q., Jiang, D., Zhang, Y., Wei, Y., Yang, Y., et al. (2022). Zebrafish Foxc1a controls ventricular chamber maturation by directly regulating wwtr1 and nkx2.5 expression. J. Genet. Genomics 49 (6), 559–568. doi:10.1016/j.jgg.2021.12.002
Hong, W., Park, J., Yun, W., Kang, P. J., Son, D., Jang, J., et al. (2018). Inhibitory effect of celastrol on adipogenic differentiation of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 507 (1-4), 236–241. doi:10.1016/j.bbrc.2018.11.014
Huang, C., Yuan, W., Lai, C., Zhong, S., Yang, C., Wang, R., et al. (2020). EphA2-to-YAP pathway drives gastric cancer growth and therapy resistance. Int. J. Cancer 146 (7), 1937–1949. doi:10.1002/ijc.32609
Jaballah, A., Soltani, I., Bahia, W., Dandana, A., Hasni, Y., Miled, A., et al. (2021). The relationship between menopause and metabolic syndrome: Experimental and bioinformatics analysis. Biochem. Genet. 59 (6), 1558–1581. doi:10.1007/s10528-021-10066-7
Jiang, S., Lee, B. C., Fu, Y., Avraham, S., Lim, B., and Avraham, H. K. (2010). Reconstitution of mammary epithelial morphogenesis by murine embryonic stem cells undergoing hematopoietic stem cell differentiation. PLoS One 5 (3), e9707. doi:10.1371/journal.pone.0009707
Jiao, Y., Sun, Y. T., Chen, N. F., Zhou, L. N., Guan, X., Wang, J. Y., et al. (2022). Human umbilical cord-derived mesenchymal stem cells promote repair of neonatal brain injury caused by hypoxia/ischemia in rats. Neural Regen. Res. 17 (11), 2518–2525. doi:10.4103/1673-5374.339002
Jin, X., Liu, M.-Y., Zhang, D.-F., Zhong, X., Du, K., Qian, P., et al. (2019). Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci. Ther. 25 (5), 575–590. doi:10.1111/cns.13086
Kabat, G. C., Kim, M. Y., Lee, J. S., Ho, G. Y., Going, S. B., Beebe-Dimmer, J., et al. (2017). Metabolic obesity phenotypes and risk of breast cancer in postmenopausal women. Cancer Epidemiol. Biomarkers Prev. 26 (12), 1730–1735. doi:10.1158/1055-9965.EPI-17-0495
Kehat, I., Kenyagin-Karsenti, D., Snir, M., Segev, H., Amit, M., Gepstein, A., et al. (2001). Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest.. 108 (3), 407–414. doi:10.1172/JCI12131
Khoshsirat, S., Abbaszadeh, H. A., Khoramgah, M. S., Darabi, S., Mansouri, V., Ahmady-Roozbahany, N., et al. (2019). Protective effect of photobiomodulation therapy and bone marrow stromal stem cells conditioned media on pheochromocytoma cell line 12 against oxidative stress induced by hydrogen peroxide. J. Lasers Med. Sci. 10 (3), 163–170. doi:10.15171/jlms.2019.26
Li, M, M., Choi, S. T., Tsang, K. S., Shaw, P. C., and Lau, K. F. (2012). DNA microarray expression analysis of baicalin-induced differentiation of C17.2 neural stem cells. Chembiochem 13 (9), 1286–1290. doi:10.1002/cbic.201200145
Li, M., Tsang, K. S., Choi, S. T., Li, K., Shaw, P. C., and Lau, K. F. (2011). Neuronal differentiation of C17.2 neural stem cells induced by a natural flavonoid, baicalin. Chembiochem 12 (3), 449–456. doi:10.1002/cbic.201000570
Li, S. Y., Liu, Y., Wang, L., Wang, F., Zhao, T. T., Li, Q. Y., et al. (2021). A phase I clinical trial of human embryonic stem cell-derived retinal pigment epithelial cells for early-stage stargardt macular degeneration: 5-years' follow-up. Cell Prolif. 54 (9), e13100. doi:10.1111/cpr.13100
Li, Y., He, Z. D., Zheng, Q. E., Hu, C., and Lai, W. F. (2018). Hydroxypropyl-beta-cyclodextrin for delivery of baicalin via inclusion complexation by supercritical fluid encapsulation. Molecules 23 (5), E1169. doi:10.3390/molecules23051169
Li, Y., Wang, D., Liu, J., Li, Y., Chen, D., Zhou, L., et al. (2020). Baicalin attenuates YAP activity to suppress ovarian cancer stemness. Onco. Targets. Ther. 13, 7151–7163. doi:10.2147/OTT.S254607
Li, Y., Zhuang, P., Shen, B., Zhang, Y., and Shen, J. (2012). Baicalin promotes neuronal differentiation of neural stem/progenitor cells through modulating p-stat3 and bHLH family protein expression. Brain Res. 1429, 36–42. doi:10.1016/j.brainres.2011.10.030
Liau, P. R., Wu, M. S., and Lee, C. K. (2019). Inhibitory effects of Scutellaria baicalensis root extract on linoleic acid hydroperoxide-induced lung mitochondrial lipid peroxidation and antioxidant activities. Molecules 24 (11), E2143. doi:10.3390/molecules24112143
Liu, C., Lin, C., Wang, D., Wang, J., Tao, Y., Li, Y., et al. (2022). Procr functions as a signaling receptor and is essential for the maintenance and self-renewal of mammary stem cells. Cell Rep. 38 (12), 110548. doi:10.1016/j.celrep.2022.110548
Liu, D., Ye, Y., Xu, L., Yuan, W., and Zhang, Q. (2018). Icariin and mesenchymal stem cells synergistically promote angiogenesis and neurogenesis after cerebral ischemia via PI3K and ERK1/2 pathways. Biomed. Pharmacother. 108, 663–669. doi:10.1016/j.biopha.2018.09.071
Liu, J., Yuan, Y., Gong, X., Zhang, L., Zhou, Q., Wu, S., et al. (2020). Baicalin and its nanoliposomes ameliorates nonalcoholic fatty liver disease via suppression of TLR4 signaling cascade in mice. Int. Immunopharmacol. 80, 106208. doi:10.1016/j.intimp.2020.106208
Liu, R., Hu, H., McNeil, M., Xu, J., Bi, X., Lou, P., et al. (2022). Dormant Nfatc1 reporter-marked basal stem/progenitor cells contribute to mammary lobuloalveoli formation. iScience 25 (3), 103982. doi:10.1016/j.isci.2022.103982
Liu, S., Fan, M., Xu, J. X., Yang, L. J., Qi, C. C., Xia, Q. R., et al. (2022a). Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J. Neuroinflammation 19 (1), 35. doi:10.1186/s12974-022-02393-2
Liu, S., Fan, M., Zheng, Q., Hao, S., Yang, L., Xia, Q., et al. (2022b). MicroRNAs in Alzheimer's disease: Potential diagnostic markers and therapeutic targets. Biomed. Pharmacother. 148, 112681. doi:10.1016/j.biopha.2022.112681
Liu, Z., Li, T., Deng, S., Fu, S., Zhou, X., and He, Y. (2018). Radiation induces apoptosis and osteogenic impairment through miR-22-mediated intracellular oxidative stress in bone marrow mesenchymal stem cells. Stem Cells Int. 2018, 5845402. doi:10.1155/2018/5845402
Long, Q. H., Wu, Y. G., He, L. L., Ding, L., Tan, A. H., Shi, H. Y., et al. (2021). Suan-Zao-Ren Decoction ameliorates synaptic plasticity through inhibition of the Aβ deposition and JAK2/STAT3 signaling pathway in AD model of APP/PS1 transgenic mice. Chin. Med. 16 (1), 14. doi:10.1186/s13020-021-00425-2
Luo, J., Zou, H., and Li, P. (2021). Src-Yap1 signaling axis controls the trophectoderm and epiblast lineage differentiation in mouse embryonic stem cells. Stem Cell Res. 54, 102413. doi:10.1016/j.scr.2021.102413
Ma, C., Hong, F., and Yang, S. (2022). Amyloidosis in Alzheimer's disease: Pathogeny, etiology, and related therapeutic directions. Molecules 27 (4), 1210. doi:10.3390/molecules27041210
Ma, Y., Liu, M., Yang, L., Zhang, L., Guo, H., Hou, W., et al. (2020). Loss of estrogen efficacy against Hippocampus damage in long-term OVX mice is related to the reduction of Hippocampus local estrogen production and estrogen receptor degradation. Mol. Neurobiol. 57 (8), 3540–3551. doi:10.1007/s12035-020-01960-z
Marson, A., Levine, S. S., Cole, M. F., Frampton, G. M., Brambrink, T., Johnstone, S., et al. (2008). Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 134 (3), 521–533. doi:10.1016/j.cell.2008.07.020
McKenzie, J. L., Gan, O. I., Doedens, M., Wang, J. C., and Dick, J. E. (2006). Individual stem cells with highly variable proliferation and self-renewal properties comprise the human hematopoietic stem cell compartment. Nat. Immunol. 7 (11), 1225–1233. doi:10.1038/ni1393
Mi, L., Hu, J., Li, N., Gao, J., Huo, R., Peng, X., et al. (2022). The mechanism of stem cell aging. Stem Cell Rev. Rep. 18 (4), 1281–1293. doi:10.1007/s12015-021-10317-5
Mi, X., Hu, M., Dong, M., Yang, Z., Zhan, X., Chang, X., et al. (2021). Folic acid decorated zeolitic imidazolate framework (ZIF-8) loaded with baicalin as a nano-drug delivery system for breast cancer therapy. Int. J. Nanomedicine 16, 8337–8352. doi:10.2147/IJN.S340764
Morita, A., Soga, K., Nakayama, H., Ishida, T., Kawanishi, S., and Sato, E. F. (2015). Neuronal differentiation of human iPS cells induced by baicalin via regulation of bHLH gene expression. Biochem. Biophys. Res. Commun. 465 (3), 458–463. doi:10.1016/j.bbrc.2015.08.039
Mosconi, L., Berti, V., Dyke, J., Schelbaum, E., Jett, S., Loughlin, L., et al. (2021). Menopause impacts human brain structure, connectivity, energy metabolism, and amyloid-beta deposition. Sci. Rep. 11 (1), 10867. doi:10.1038/s41598-021-90084-y
Mungenast, A. E., Siegert, S., and Tsai, L. H. (2016). Modeling Alzheimer's disease with human induced pluripotent stem (iPS) cells. Mol. Cell. Neurosci. 73, 13–31. doi:10.1016/j.mcn.2015.11.010
Patwardhan, R. S., Sharma, D., Checker, R., and Sandur, S. K. (2014). Mitigation of radiation-induced hematopoietic injury via regulation of cellular MAPK/phosphatase levels and increasing hematopoietic stem cells. Free Radic. Biol. Med. 68, 52–64. doi:10.1016/j.freeradbiomed.2013.11.004
Quinn, H. M., Vogel, R., Popp, O., Mertins, P., Lan, L., Messerschmidt, C., et al. (2021). YAP and beta-catenin cooperate to drive oncogenesis in basal breast cancer. Cancer Res. 81 (8), 2116–2127. doi:10.1158/0008-5472.CAN-20-2801
Ramotowski, C., Qu, X., and Villa-Diaz, L. G. (2019). Progress in the use of induced pluripotent stem cell-derived neural cells for traumatic spinal cord injuries in animal populations: Meta-analysis and review. Stem Cells Transl. Med. 8 (7), 681–693. doi:10.1002/sctm.18-0225
Ranjan, M., Lee, O., Cottone, G., Mirzaei Mehrabad, E., Spike, B. T., Zeng, Z., et al. (2021). Progesterone receptor antagonists reverse stem cell expansion and the paracrine effectors of progesterone action in the mouse mammary gland. Breast Cancer Res. 23 (1), 78. doi:10.1186/s13058-021-01455-2
Savonitto, S., Ferri, L. A., and Colombo, D. (2018). Perimenopause vasomotor symptoms, coronary atherosclerosis and risk of myocardial infarction during menopause: The cardiologist's perspective. Prz. Menopauzalny 17 (2), 53–56. doi:10.5114/pm.2018.77301
Shi, L., Zhang, S., Huang, Z., Hu, F., Zhang, T., Wei, M., et al. (2020). Baicalin promotes liver regeneration after acetaminophen-induced liver injury by inducing NLRP3 inflammasome activation. Free Radic. Biol. Med. 160, 163–177. doi:10.1016/j.freeradbiomed.2020.05.012
Song, H. W., Kumar, B. K., Kim, S. H., Jeon, Y. H., Lee, Y. A., Lee, W. T., et al. (2011). Agmatine enhances neurogenesis by increasing ERK1/2 expression, and suppresses astrogenesis by decreasing BMP 2, 4 and SMAD 1, 5, 8 expression in subventricular zone neural stem cells. Life Sci. 89 (13-14), 439–449. doi:10.1016/j.lfs.2011.07.003
Song, Y. J., Li, S. R., Li, X. W., Chen, X., Wei, Z. X., Liu, Q. S., et al. (2020). The effect of estrogen replacement therapy on Alzheimer's disease and Parkinson's disease in postmenopausal women: A meta-analysis. Front. Neurosci. 14, 157. doi:10.3389/fnins.2020.00157
Song, Z., He, C., Yu, W., Yang, M., Li, Z., Li, P., et al. (2022). Baicalin attenuated aβ1-42-induced apoptosis in SH-SY5Y cells by inhibiting the ras-ERK signaling pathway. Biomed. Res. Int. 2022, 9491755. doi:10.1155/2022/9491755
Steenbruggen, T. G., Steggink, L. C., Seynaeve, C. M., van der Hoeven, J. J. M., Hooning, M. J., Jager, A., et al. (2020). High-dose chemotherapy with hematopoietic stem cell transplant in patients with high-risk breast cancer and 4 or more involved axillary lymph nodes: 20-Year follow-up of a phase 3 randomized clinical trial. JAMA Oncol. 6 (4), 528–534. doi:10.1001/jamaoncol.2019.6276
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tang, M., Yin, M., Tang, M., Liang, H., Yu, C., Hu, X., et al. (2013). Baicalin maintains late-stage functional cardiomyocytes in embryoid bodies derived from murine embryonic stem cells. Cell. Physiol. biochem. 32 (1), 86–99. doi:10.1159/000350127
Tang, Q., Chen, J., Di, Z., Yuan, W., Zhou, Z., Liu, Z., et al. (2020). TM4SF1 promotes EMT and cancer stemness via the Wnt/β-catenin/SOX2 pathway in colorectal cancer. J. Exp. Clin. Cancer Res. 39 (1), 232. doi:10.1186/s13046-020-01690-z
Thiele, F., Voelkner, C., Krebs, V., Muller, P., Jung, J. J., Rimmbach, C., et al. (2019). Nkx2.5 based ventricular programming of murine ESC-derived cardiomyocytes. Cell. Physiol. biochem. 53 (2), 337–354. doi:10.33594/000000142
Wang, B., Huang, T., Fang, Q., Zhang, X., Yuan, J., Li, M., et al. (2020). Bone-protective and anti-tumor effect of baicalin in osteotropic breast cancer via induction of apoptosis. Breast Cancer Res. Treat. 184 (3), 711–721. doi:10.1007/s10549-020-05904-y
Wang, D., Hu, X., Liu, C., Jia, Y., Bai, Y., Cai, C., et al. (2019). Protein C receptor is a therapeutic stem cell target in a distinct group of breast cancers. Cell Res. 29 (10), 832–845. doi:10.1038/s41422-019-0225-9
Wang, J., Masika, J., Zhou, J., Wang, J., Zhu, M., Luo, H., et al. (2015). Traditional Chinese medicine baicalin suppresses mESCs proliferation through inhibition of miR-294 expression. Cell. Physiol. biochem. 35 (5), 1868–1876. doi:10.1159/000373997
Wang, Q., Shi, D., Geng, Y., Huang, Q., and Xiang, L. (2020). Baicalin augments the differentiation of osteoblasts via enhancement of microRNA-217. Mol. Cell. Biochem. 463 (1-2), 91–100. doi:10.1007/s11010-019-03632-6
Wang, R., Huang, F., Wei, W., Zhou, Y., Ye, Z., Yu, L., et al. (2021). Programmed cell death ligand 1 is enriched in mammary stem cells and promotes mammary development and regeneration. Front. Cell Dev. Biol. 9, 772669. doi:10.3389/fcell.2021.772669
Wang, T., Yuan, W., Liu, Y., Zhang, Y., Wang, Z., Zhou, X., et al. (2015). The role of the JAK-STAT pathway in neural stem cells, neural progenitor cells and reactive astrocytes after spinal cord injury. Biomed. Rep. 3 (2), 141–146. doi:10.3892/br.2014.401
Wang, Y. S., Cho, J. G., Hwang, E. S., Yang, J. E., Gao, W., Fang, M. Z., et al. (2018). Enhancement of protective effects of Radix Scutellariae on UVB-induced photo damage in human HaCaT keratinocytes. Appl. Biochem. Biotechnol. 184 (4), 1073–1093. doi:10.1007/s12010-017-2611-4
Wang, Z. Z., Jia, Y., Wang, G., He, H., Cao, L., Shi, Y., et al. (2022). Dynamic covalent hydrogel of natural product baicalin with antibacterial activities. RSC Adv. 12 (14), 8737–8742. doi:10.1039/d1ra07553e
Weiss, J. N., and Levy, S. (2019). Stem cell ophthalmology treatment study (Scots): Bone marrow derived stem cells in the treatment of dominant optic atrophy. Stem Cell Investig. 6, 41. doi:10.21037/sci.2019.11.01
Welten, S., Onland-Moret, N. C., Boer, J. M. A., Verschuren, W. M. M., and van der Schouw, Y. T. (2021). Age at menopause and risk of ischemic and hemorrhagic stroke. Stroke 52 (8), 2583–2591. doi:10.1161/STROKEAHA.120.030558
Wong, K. L., Lai, Y. M., Li, K. W., Lee, K. F., Ng, T. B., Cheung, H. P., et al. (2015). A novel, stable, estradiol-stimulating, osteogenic yam protein with potential for the treatment of menopausal syndrome. Sci. Rep. 5, 10179. doi:10.1038/srep10179
Xiping, Z., Hua, T., Hanqing, C., Li, C., Zhiwei, W., Keyi, W., et al. (2007). The protecting effects and mechanisms of Baicalin and Octreotide on heart injury in rats with SAP. Mediat. Inflamm. 2007, 19469. doi:10.1155/2007/19469
Xu, B., Huang, S., Chen, Y., Wang, Q., Luo, S., Li, Y., et al. (2021). Synergistic effect of combined treatment with baicalin and emodin on DSS-induced colitis in mouse. Phytother. Res. 35 (10), 5708–5719. doi:10.1002/ptr.7230
Xu, Y., Zhang, Y., Garcia-Canaveras, J. C., Guo, L., Kan, M., Yu, S., et al. (2020). Chaperone-mediated autophagy regulates the pluripotency of embryonic stem cells. Science 369 (6502), 397–403. doi:10.1126/science.abb4467
Xue, J., Liu, Y., Darabi, M. A., Tu, G., Huang, L., Ying, L., et al. (2019). An injectable conductive Gelatin-PANI hydrogel system serves as a promising carrier to deliver BMSCs for Parkinson's disease treatment. Mat. Sci. Eng. C Mat. Biol. Appl. 100, 584–597. doi:10.1016/j.msec.2019.03.024
Yam, G. H., Yang, T., Geary, M. L., Santra, M., Funderburgh, M., Rubin, E., et al. (2022). Human corneal stromal stem cells express anti-fibrotic microRNA-29a and 381-5p - a robust cell selection tool for stem cell therapy of corneal scarring. J. Adv. Res. S2090-1232 (22), 00120–00125. doi:10.1016/j.jare.2022.05.008
Yamamoto, N., Hiramatsu, N., Ohkuma, M., Hatsusaka, N., Takeda, S., Nagai, N., et al. (2021). Novel technique for retinal nerve cell regeneration with electrophysiological functions using human iris-derived iPS cells. Cells 10 (4), 743. doi:10.3390/cells10040743
Yang, I. H., Lin, I. E., Liang, Y. J., Lin, J. N., Chen, T. C., Chen, Z. Y., et al. (2022). Development of di(2-ethylhexyl) phthalate-containing thioglycolic acid immobilized chitosan mucoadhesive gel as an alternative hormone therapy for menopausal syndrome. Bioeng. Transl. Med. 7 (2), e10267. doi:10.1002/btm2.10267
Yang, R., Yuan, B. C., Ma, Y. S., Zhou, S., and Liu, Y. (2017). The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 55 (1), 5–18. doi:10.1080/13880209.2016.1225775
Yang, Y., Sun, Y., Mao, W. W., Zhang, H., Ni, B., and Jiang, L. (2021). Oxidative stress induces downregulation of TP53INP2 and suppresses osteogenic differentiation of BMSCs during osteoporosis through the autophagy degradation pathway. Free Radic. Biol. Med. 166, 226–237. doi:10.1016/j.freeradbiomed.2021.02.025
Yu, F. X., Zhao, B., Panupinthu, N., Jewell, J. L., Lian, I., Wang, L. H., et al. (2012). Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150 (4), 780–791. doi:10.1016/j.cell.2012.06.037
Yu, H., Chen, B., and Ren, Q. (2019). Baicalin relieves hypoxia-aroused H9c2 cell apoptosis by activating Nrf2/HO-1-mediated HIF1α/BNIP3 pathway. Artif. Cells Nanomed. Biotechnol. 47 (1), 3657–3663. doi:10.1080/21691401.2019.1657879
Yu, H. Y., Zhu, Y., Zhang, X. L., Wang, L., Zhou, Y. M., Zhang, F. F., et al. (2022). Baicalin attenuates amyloid beta oligomers induced memory deficits and mitochondria fragmentation through regulation of PDE-PKA-Drp1 signalling. Psychopharmacol. Berl. 239 (3), 851–865. doi:10.1007/s00213-022-06076-x
Zeng, A., Liang, X., Zhu, S., Liu, C., Luo, X., Zhang, Q., et al. (2020). Baicalin, a potent inhibitor of NF-κB signaling pathway, enhances chemosensitivity of breast cancer cells to docetaxel and inhibits tumor growth and metastasis both in vitro and in vivo. Front. Pharmacol. 11, 879. doi:10.3389/fphar.2020.00879
Zhang, C., Zhang, Z., Shu, H., Liu, S., Song, Y., Qiu, K., et al. (2010). The modulatory effects of bHLH transcription factors with the Wnt/beta-catenin pathway on differentiation of neural progenitor cells derived from neonatal mouse anterior subventricular zone. Brain Res. 1315, 1–10. doi:10.1016/j.brainres.2009.12.013
Zhang, G., Li, C., Niu, Y., Yu, Q., Chen, Y., and Liu, E. (2017). Osteoprotective effect of Radix Scutellariae in female hindlimb-suspended sprague-dawley rats and the osteogenic differentiation effect of its major constituent. Molecules 22 (7), E1044. doi:10.3390/molecules22071044
Zhang, G., Wang, X., Li, C., Li, Q., An, Y. A., Luo, X., et al. (2021). Integrated stress response couples mitochondrial protein translation with oxidative stress control. Circulation 144 (18), 1500–1515. doi:10.1161/CIRCULATIONAHA.120.053125
Zhang, H., Li, X., Wang, J., Cheng, Q., Shang, Y., and Wang, G. (2021). Baicalin relieves Mycoplasma pneumoniae infection‑induced lung injury through regulating microRNA‑221 to inhibit the TLR4/NF‑κB signaling pathway. Mol. Med. Rep. 24 (2), 571. doi:10.3892/mmr.2021.12210
Zhao, J., Lu, S., Yu, H., Duan, S., and Zhao, J. (2018). Baicalin and ginsenoside Rb1 promote the proliferation and differentiation of neural stem cells in Alzheimer's disease model rats. Brain Res. 1678, 187–194. doi:10.1016/j.brainres.2017.10.003
Zhao, Q., Cui, M. Y., Levsh, O., Yang, D., Liu, J., Li, J., et al. (2018). Two CYP82D enzymes function as flavone hydroxylases in the biosynthesis of root-specific 4'-deoxyflavones in Scutellaria baicalensis. Mol. Plant 11 (1), 135–148. doi:10.1016/j.molp.2017.08.009
Zhao, R., Wu, X., Bi, X. Y., Yang, H., and Zhang, Q. (2022). Baicalin attenuates blood-spinal cord barrier disruption and apoptosis through PI3K/Akt signaling pathway after spinal cord injury. Neural Regen. Res. 17 (5), 1080–1087. doi:10.4103/1673-5374.324857
Zhao, T., Tang, H., Xie, L., Zheng, Y., Ma, Z., Sun, Q., et al. (2019). Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 71 (9), 1353–1369. doi:10.1111/jphp.13129
Zhao, W., Zhang, X., and Rong, J. (2021). SUMOylation as a therapeutic target for myocardial infarction. Front. Cardiovasc. Med. 8, 701583. doi:10.3389/fcvm.2021.701583
Zhao, Y., Wang, H.-L., Li, T.-T., Yang, F., and Tzeng, C.-M. (2020). Baicalin ameliorates dexamethasone-induced osteoporosis by regulation of the RANK/RANKL/OPG signaling pathway. Drug Des. devel. Ther. 14, 195–206. doi:10.2147/DDDT.S225516
Zhou, X., Li, Y., Wang, W., Wang, S., Hou, J., Zhang, A., et al. (2020). Regulation of Hippo/YAP signaling and esophageal squamous carcinoma progression by an E3 ubiquitin ligase PARK2. Theranostics 10 (21), 9443–9457. doi:10.7150/thno.46078
Zhu, D. Y., and Lou, Y. J. (2006). Icariin-mediated expression of cardiac genes and modulation of nitric oxide signaling pathway during differentiation of mouse embryonic stem cells into cardiomyocytes in vitro. Acta Pharmacol. Sin. 27 (3), 311–320. doi:10.1111/j.1745-7254.2006.00275.x
Zhu, Q., Zhang, N., Hu, N., Jiang, R., Lu, H., Xuan, A., et al. (2020). Neural stem cell transplantation improves learning and memory by protecting cholinergic neurons and restoring synaptic impairment in an amyloid precursor protein/presenilin 1 transgenic mouse model of Alzheimer's disease. Mol. Med. Rep. 21 (3), 1172–1180. doi:10.3892/mmr.2020.10918
Zhu, Y. L., Wang, S., Ding, D. G., Xu, L., and Zhu, H. T. (2017). miR217 inhibits osteogenic differentiation of rat bone marrowderived mesenchymal stem cells by binding to Runx2. Mol. Med. Rep. 15 (5), 3271–3277. doi:10.3892/mmr.2017.6349
Keywords: climacteric syndrome, baicalin, stem cell, proliferation, differentiation
Citation: Wei Q, Hao X, Lau BW-M, Wang S and Li Y (2022) Baicalin regulates stem cells as a creative point in the treatment of climacteric syndrome. Front. Pharmacol. 13:986436. doi: 10.3389/fphar.2022.986436
Received: 05 July 2022; Accepted: 17 October 2022;
Published: 02 November 2022.
Edited by:
Wei Cui, Ningbo University, ChinaReviewed by:
Dongxu Wang, Jilin University, ChinaJia Zhao, The University of Hong Kong, Hong Kong SAR, China
Copyright © 2022 Wei, Hao, Lau, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoxia Wang, d2FuZ3NoYW94aWExOTc4QGhvdG1haWwuY29t; Yue Li, bHl1ZTIwMDZAMTYzLmNvbQ==
 Qian Wei
Qian Wei Xia Hao1
Xia Hao1 Benson Wui-Man Lau
Benson Wui-Man Lau Yue Li
Yue Li