
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 10 October 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.984475
 Mingxia Xie1,2
Mingxia Xie1,2 Hanqing Wang3
Hanqing Wang3 Jun Peng4
Jun Peng4 Dongqin Qing3
Dongqin Qing3 Xi Zhang1
Xi Zhang1 Dongwei Guo1
Dongwei Guo1 Pan Meng1,2
Pan Meng1,2 Zhihong Luo1
Zhihong Luo1 Xiaoye Wang1*
Xiaoye Wang1* Qinghua Peng1,2,4*
Qinghua Peng1,2,4*Dry eye disease (DED) is a multifactorial syndrome that commonly occurs with depression. However, therapies targeting depression-related dry eye disease are rare. In the current study, we studied the beneficial effect of a natural flavone, acacetin, in depression-associated dry eye disease by utilizing the chronic unpredictable mild stress (CUMS) depression model. Our data showed that acacetin improved the depressive behaviors in sucrose preference test (SPT), tail suspension test (TST) and forced swim test (FST); relieved the dry eye symptoms including corneal epithelial impairments, tear production decrease and goblet cell loss in CUMS mice. Acacetin also inhibited NOD-like receptor protein 3 (NLRP3) inflammasome expression levels and suppressed inflammatory responses via enhancing glycoprotein 78 (gp78)/Insulin induced gene-1 (Insig-1)-controlled NLRP3 ubiquitination in CUMS mice. Furthermore, knockdown of gp78 compromised acacetin-conferred protective efficacy in depression-related dry eye disease. In summary, our findings indicated that acacetin exerts beneficial effect in depression-associated dry eye disease, which is tightly related to gp78-mediated NLRP3 ubiquitination.
Depression is one of the most prevalent and disabling mental disorder characterized by sadness, anhedonia, and a feeling of worthlessness (Hao et al., 2019). According to the World Health Organization (WHO), 350 million people worldwide suffer from depression, and it is estimated to be a major cause of disability by 2030 (Wang et al., 2019). Dry eye disease is defined as a “multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface (Messmer, 2015). Previous researches demonstrate that it frequently occurs with depression (Zhang et al., 2019; Zhou et al., 2022). However, the treatment for depression-associated dry eye disease has not been fully studied.
Inflammasomes are a group of cytosolic protein complexes that underlies a wide variety of disease (Schnappauf et al., 2019; Abbate et al., 2020). Existing evidence suggest that the NLRP3 inflammasome plays an important role in the pathological process and treatment of depression and dry eye disease (Arioz et al., 2019; Park et al., 2019). The NLRP3 inflammasome is an oligomeric complex comprised of the sensors NLRP3, the adaptor apoptosis-associated speck-like protein containing a CARD (ASC) and the effector protein-Caspase-1. In the presence of immune activators such as pathogen-associated molecular patterns (PAMPs), danger-associated molecular patterns (DAMPs), or other exogenous invaders, the pyrin domains of NLRP3 binds to that of ASC, and subsequently the caspase recruitment domain of ASC recruits and interacts with pro-caspase-1 (Shao et al., 2015). These interactions form the NLRP3 inflammasome and promote the autocatalytic cleavage of pro-caspase-1 (Luo et al., 2017). Thereafter, the activated caspase-1 converses pro–interleukin (IL)-1 β and pro-IL-18 to bioactive IL-1β and IL-18, respectively, and cleaves the gasdermin D (GSDMD) to generate the N-terminal fragment to induce pore formation, cytokine release and pyroptotic cell death (Sharif et al., 2019; Wang et al., 2021). Recently, the membrane-bound E3 ubiquitin ligase gp78 has been demonstrated to mediate the ubiquitination (a key determinator of protein fate by tagging proteins for proteasomal degradation) of NLRP3 via Insig-1, which ultimately affects the activation of NLRP3 inflammasome (Xu et al., 2022).
Acacetin (5,7-dihydroxy-4′-methoxyflavone) is a natural flavone present in a variety of plants, for instance, Turnera diffusa and Saussurea involucrate (Singh et al., 2020). A broad spectrum of pharmacological and biochemical activities, e.g., antioxidant, anti-inflammatory and neuroprotective effects, have been detected in acacetin (Xiao et al., 2019; Singh et al., 2020; Wu et al., 2021). Acacetin suppresses activation of the NLRP3 inflammasome in mice with cerebral ischemia-reperfusion injury (Bu et al., 2019). Moreover, chronic administration of acacetin exerts evident antidepressant-like efficacy in mice (Xiao et al., 2019). However, the effect of acacetin in depression-related dry eye disease remains unknown. Thus, in this study we investigated the function of acacetin in depression-associated dry eye disease and the underlying mechanisms with the usage of CUMS mice.
Male C57BL/6 mice, weighing 18–22 g, were purchased from Changzhou Cavens Laboratory Animal Co., Ltd. All animal procedures were carried out in accordance with Hunan University of Chinese Medicine Medical Ethical committee (NO. 2021.22). Animals were housed on a 12-h light/dark cycle (lights on at 6 a.m.) at room temperature of 22 ± 1°C, with access to food and water ad libitum.
Following a 1-week acclimatization period for the mice, the animals were randomly assigned to five groups: Control (non-stress + normal saline treatment); Vehicle (CUMS+normal saline treatment); Acacetin-L (CUMS+5 mg/kg acacetin treatment); Acacetin-H (CUMS+15 mg/kg acacetin treatment); Escitalopram (CUMS +10 mg/kg escitalopram treatment). Acacetin was purchased from Sigma-Aldrich (00,017) and dissolved in normal saline. The CUMS groups were singly housed and exposed to two unpredictable stressors each day for 7 weeks according to a method previously described with minor modifications (Zhang et al., 2019; Xu J et al., 2020). The stressors included: 24 h food deprivation; 24 h water deprivation; 24 h wet bedding (100 g sawdust bedding added with 200 ml water); 24 h no bedding; 24 h cage tilting; 6 h 50 ml centrifuge tube confinement; 15 min cage shaking; 5 min tail pinching; overnight illumination (twice per week). The unstressed controls were group housed under normal conditions. At 4 weeks after the starting of CUMS modeling, the sucrose preference ratio and tear production were tested (Supplementary Figure S1). Acacetin (5, 15 mg/kg/day) or escitalopram (10 mg/kg/day) was intragastrically administrated once daily for 3 consecutive weeks starting at the 5th week of CUMS modeling. Control and vehicle groups received equal volume of normal saline administration. Afterwards, behavioral assessment including sucrose preference test (SPT), tail suspension test (TST), forced swim test (FST), and open field test (OFT) was conducted, and the development of dry eye disease was evaluated. Finally, mice were sacrificed for tissue collection at the end of the experiment. For the gp78 knockdown experiment, 100 ul of gp78 siRNAs (Universal Biosystems (Anhui) Co., Ltd.; Interfering sequence: 5′-AGCTTATCCAGTGTATTGTGT-3’; Sense strand siRNA: 5′-AGCUUAUCCAGUGUAUUGUGUtt-3’; Antisense strand siRNA: 5′-ACACAAUACACUGGAUAAGCUtt-3′) was infected to mice through vein injection 4 weeks after the starting of CUMS modeling.
The SPT was performed to assess anhedonia as described previously (Li et al., 2021). Mice were first habituated to 1% sucrose for 3 days. After food and water deprivation for 24 h, each mouse was provided with two bottles, one containing 1% sucrose solution, and the other containing drinking water. The test lasted for 24 h, and the positions of the bottles were switched in the middle of the test to avoid side preference. Sucrose preference ratio was calculated by using the following formula: sucrose preference (%) = sucrose intake/(sucrose intake + water intake) × 100%.
Behavioral despair of mice was evaluated by the TST and FST as reported previously (Abdelhamid et al., 2014; Li et al., 2021).
For TST, the mouse was hung on a suspension bar (30 cm above the apparatus floor) by attaching an adhesive tape to a position 1 cm from the tip of its tail. The test lasted for 6 min, and the immobility time of the last 4 min was analyzed by ANY-maze software.
For FST, each mouse was placed in a glass cylinder (20 cm height, 15 cm diameter) filled with water (23 ± 1°C). Mice were forced to swim for 6 min, and the amount of time spent immobile during the last 4 min was analyzed by ANY-maze software.
Locomotor activity was evaluated using the OFT. Each mouse was placed in the center of the open-field apparatus (40 × 40 × 25 cm) and allowed to freely explore the open area for 5 min. Distance traveled by each animal was analyzed using ANY-maze software. The apparatus was cleaned with 75% ethanol after each trial (Shen et al., 2021).
Tear production was examined using cotton phenol red threads (Jingming, Tianjin, China) as previous report (Fakih et al., 2019). In brief, the top of the thread (yellow) was placed on the lower palpebral conjunctiva for 20 s. After absorbing tears, the color of the thread would change to red. Tear production was determined by measuring the length of the wet portion of the thread.
To examine corneal epithelial defects, 1 µl of 1% sodium fluorescein was instilled onto the right eye of anaesthetized mice using a micro-pipette (Musayeva et al., 2021). Ocular surface staining was then observed using a slit lamp under a cobalt blue light. Quantification of the corneal defect area was carried out by using the following formula: Corneal defect area (%) = (fluorescein sodium positive area/the whole cornea) × 100%
The specimens were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 5-μm-thick sections. The sections were stained with periodic acid-Schiff (ab150680, Abcam) for goblet cells or Nissl staining solution (C0117, Beyotime) to identify Nissl bodies according to the manufacturer’s instructions. Subsequently, the slices were dehydrated in a gradient ethanol, cleared in xylene, and covered with neutral resins. The images were acquired by a microscope and analyzed with ImageJ.
The immunohistochemical staining was carried out according to a previous protocol (Liu et al., 2021). Briefly, tissue sections were fixed in 4% paraformaldehyde for 20 min, and permeabilized with 0.5% Triton X-100 for 10 min. Nonspecific antibody binding was blocked by 3% BSA for 1 h. The samples were then incubated with anti-NLRP3 (ab270449, Abcam) overnight at 4°C, followed by the incubation of secondary antibody for 1 h at room temperature. Positive cells were detected by 3,3′-diaminobenzidine (DAB) staining, and the nuclei was counterstained with hematoxylin. Finally, the slices were cleared with xylene, mounted by neutral resin, and observed under a microscope.
Western blotting assays were performed as previously described (Xu X et al., 2020). For protein extraction, hippocampus or cornea samples were homogenized in ice-cold RIPA lysis buffer supplemented with protease and phosphatase inhibitors. Homogenates were centrifuged at 12,000×g for 15 min at 4°C, and supernatants were collected. The concentrations of proteins were quantified by bicinchoninic acid (BCA) protein assay, and proteins were denatured by boiling for 5 min. Equal amounts of protein samples were subjected to electrophoresis on a sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gels, followed by being transferred onto polyvinylidene difluoride (PVDF) membranes. After being blocked with 3% bovine serum albumin (BSA) for 1 h, the membranes were probed with primary antibodies including anti-gp78 (ab227450, Abcam), anti-Insig-1 (55282-1-AP, Proteintech), anti-NLRP3 (ab270449, Abcam), anti-Caspase-1 (AF5418, Affinity), anti-Cleaved-Caspase-1 (AF4022, Affinity), anti-gasdermin D (GSDMD)-N (DF13758, Affinity), anti-GSDMD (AF4012, Affinity) and anti-GAPDH (10494-1-AP, Proteintech) overnight at 4°C, and then incubated with secondary antibodies for 1 h at room temperature. Afterwards, the protein bands were visualized with enhanced chemiluminescence (ECL) detection system. Protein expression levels were analyzed using ImageJ software and normalized to GAPDH.
The immunoprecipitation was conducted following a previously reported protocol (Lin et al., 2017). Hippocampus tissues were homogenized in ice-cold NP-40 lysis buffer supplemented with protease and phosphatase inhibitors. Insoluble debris was removed by centrifugation at 12,000×g for 15 min at 4°C. Protein concentrations were determined using the BCA method. Tissue lysates containing 500 μg of total protein were incubated with anti-NLRP3 overnight at 4°C with constant rotation. Next, 50 μl of protein A/G agarose beads was added to incubate for 4 h. The beads were extensively washed and boiled in SDS loading buffer for 5 min. The ubiquitination of NLRP3 was evaluated in the western blot analysis by using Ubiquitin antibody (3936, Cell Signaling Technology).
Sample preparation: Tears were collected from the lateral canthus of mice using glass microcapillary tubes. One sample consisted of tears from both eyes of one mouse that were pooled in phosphate buffered saline (PBS) + 0.1% BSA and stored at −80°C until the assay was performed (Dogru et al., 2022). Hippocampus tissues of mice were isolated, snap-frozen in liquid nitrogen, and stored at − 80°C for protein extraction. The tissues rinsed with cold PBS to remove residual blood, weighed, and added with PBS containing protease inhibitor cocktail (1:9 w/v). Samples were then homogenized, centrifuged at 3500 ×g for 20 min, and supernatants were collected (Xue et al., 2021; Dang et al., 2022).
The levels of inflammatory cytokines including tumor necrosis factor (TNF)-α, IL-1β, and IL-18 in hippocampi and tears were detected using Mouse TNF-α ELISA Kit (PT512, Beyotime), Mouse IL-1β ELISA Kit (PI301, Beyotime), and Mouse IL-18 ELISA Kit (PI553, Beyotime), respectively, as per company instructions.
Statistical analyses were completed in GraphPad Prism 7.0 software. All results are presented as the mean ± standard error of the mean (SEM) values. Differences between two groups were evaluated by Student’s t-test. One-way analysis of variance (ANOVA) was used when comparing multiple groups. P < 0.05 was considered statistically significant.
Depressive behavior was assessed on all mice using SPT, TST and FST tests (Figures 1A–C). The stressed vehicles displayed higher sucrose preference ratio in SPT and longer immobility time in both TST and FST compared to unstressed controls, indicating the development of depression following CUMS. Acacetin or escitalopram, however, alleviated CUMS-induced depressive symptoms above. No differences were observed on the locomotor activity indicated by the total distance travelled in the OFT test (Figure 1D).
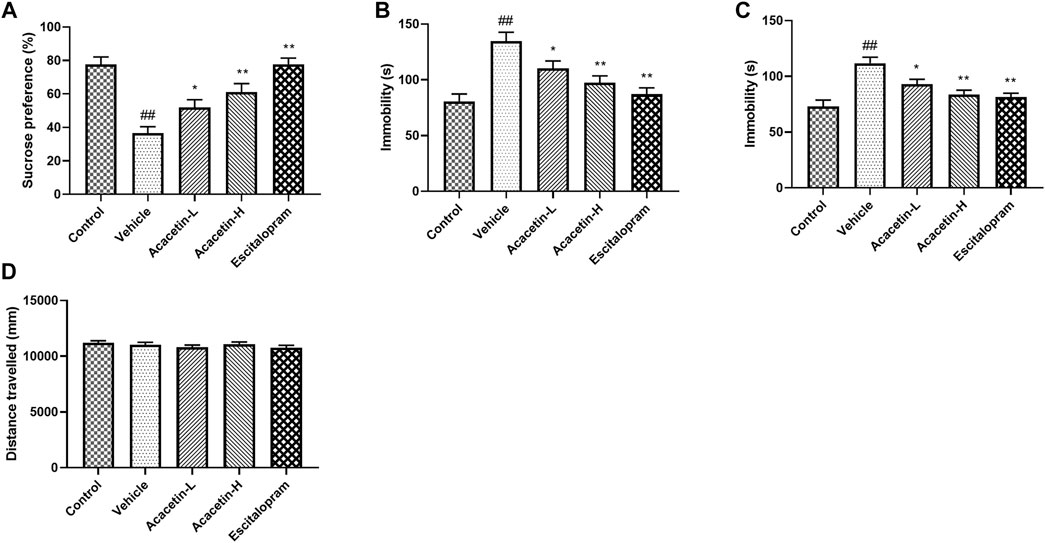
FIGURE 1. Acacetin attenuates depression-like behavior in chronic unpredictable mild stress (CUMS) mice. (A) The preference ratio of mice in the sucrose preference test. (B) Immobility duration of mice in the tail suspension test. (C) Immobility duration of mice in the forced swim test. (D) Total distance travelled in the open field test. Results are expressed as the mean ± standard error of the mean (SEM; n = 9 per group). ##p < 0.01 vs. Control; *p < 0.05, **p < 0.01 vs. Vehicle.
Next, we assessed whether acacetin would be an effective therapy for CUMS-associated dry eye disease. Corneal fluorescein staining was used to examine corneal epitheliopathy after exposure to CUMS. As shown in Figures 2A–B, almost no stained spots were found in the cornea of control mice, whereas large number of stained spots were observed in the corneal surface of vehicle-treated mice, indicating corneal damage following CUMS. Tear production and goblet cells (responsible for the release of tear-stabilizing mucin) were assessed by cotton phenol red threads and periodic acid-Schiff (PAS) staining, respectively (Puro, 2020). As shown in Figures 2C–E, CUMS resulted in reduced wetted length and goblet cells in mice. Interestingly, acacetin, but not escitalopram, rescued CUMS-induced corneal defects, tear reduction, and loss of goblet cells in mice. These results suggest that acacetin protects depressive mice from dry eye disease.
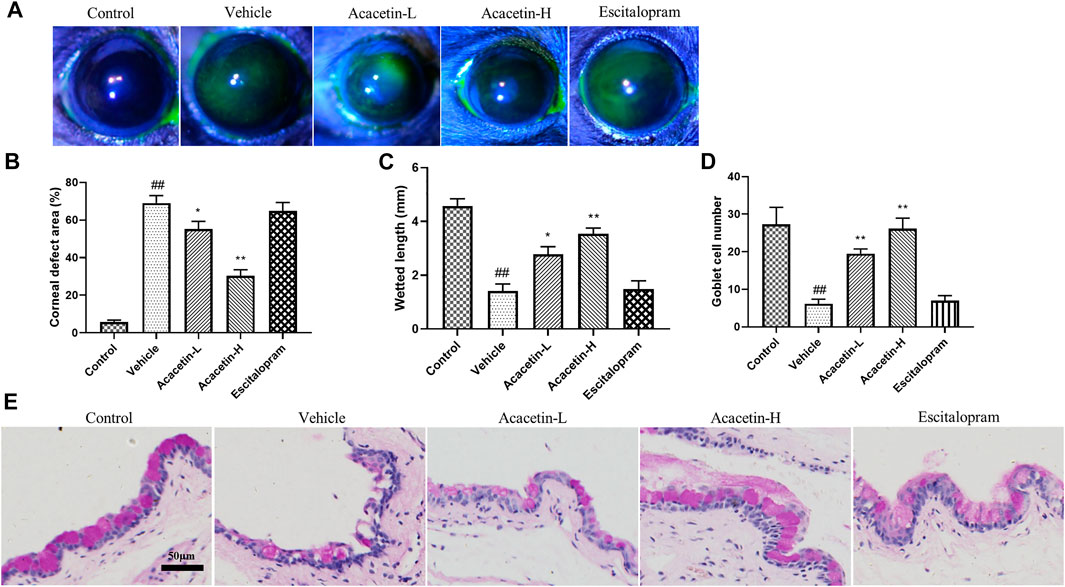
FIGURE 2. Acacetin improves dry eye symptoms in CUMS mice. (A–B) Determination of corneal epithelial defects by fluorescein staining (n = 9 per group). (C) Tear volumes of mice were measured using cotton phenol red threads (n = 9 per group). (D–E) The number of goblet cells was determined by periodic acid-Schiff (PAS) staining (n = 6 per group). Results are expressed as the mean ± SEM. ##p < 0.01 vs. Control; *p < 0.05, **p < 0.01 vs. Vehicle.
We then determined the involvement of NLRP3 ubiquitination-mediated NLRP3 inflammasome activation in the protective effect of acacetin. The co-ip results revealed that the CUMS modeling decreased ubiquitinated NLRP3 expression (though did not reach significance), and significantly increased NLRP3 protein levels in vehicle-treated mice (Figure 3). Interestingly, acacetin intervention greatly enhanced NLRP3 ubiquitination and suppressed NLRP3 protein levels in CUMS mice. Additionally, the western blotting and ELISA assays demonstrated that with the activation of gp78/Insig-1 signal, acacetin promoted hippocampal and corneal NLRP3 inflammasome (NLRP3, cleaved-Caspase1, GSDMD-N) protein expression, and boosted the production of proinflammatory factors (TNF-α, IL-1β, and IL-18) in hippocampi and cornea (Figures 4–6). Collectively, these findings demonstrated that acacetin deactivates NLRP3 inflammasome via gp78/Insig-1-mediateed NLRP3 ubiquitination in CUMS-induced dry eye disease.
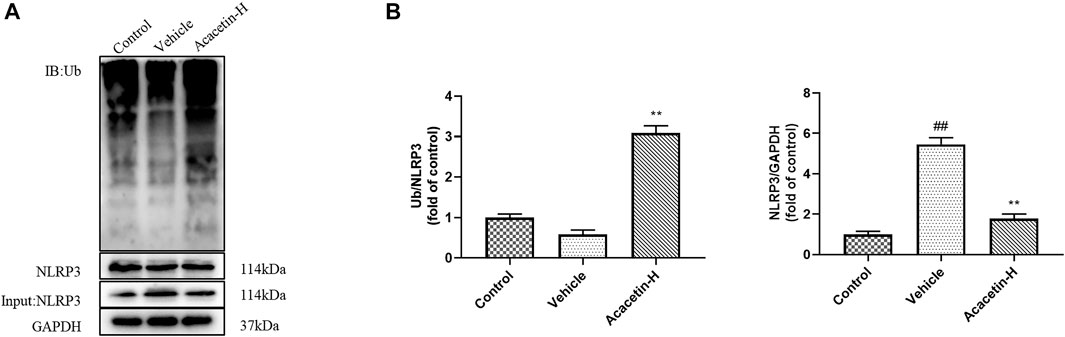
FIGURE 3. Effect of acacetin on NLRP3 ubiquitination in the hippocampus. (A) The ubiquitination of NLRP3 in the hippocampus of mice was detected by co-immunoprecipitation (co-ip). (B) Quantification of ubiquitinated NLRP3 expression levels and NLRP3 protein expression in the hippocampus of mice. Results are expressed as the mean ± SEM (n = 3 per group). ##p < 0.01 vs. Control; **p < 0.01 vs. Vehicle.
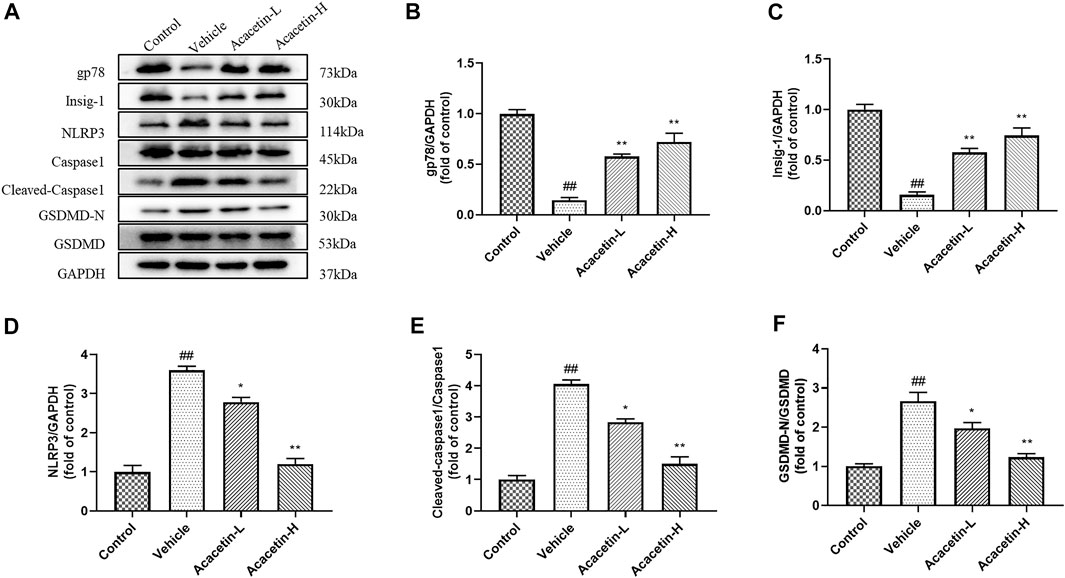
FIGURE 4. Effect of acacetin on gp78-mediated NLRP3 inflammasome in the hippocampus. (A) Representative western blots of gp78, Insig-1, NLRP3, Caspase1, cleaved Caspase1, GSDMD-N, and GSDMD. (B–F) Quantitative analysis of western blot results. Results are expressed as the mean ± SEM (n = 3 per group). ##p < 0.01 vs. Control; *p < 0.05, **p < 0.01 vs. Vehicle.
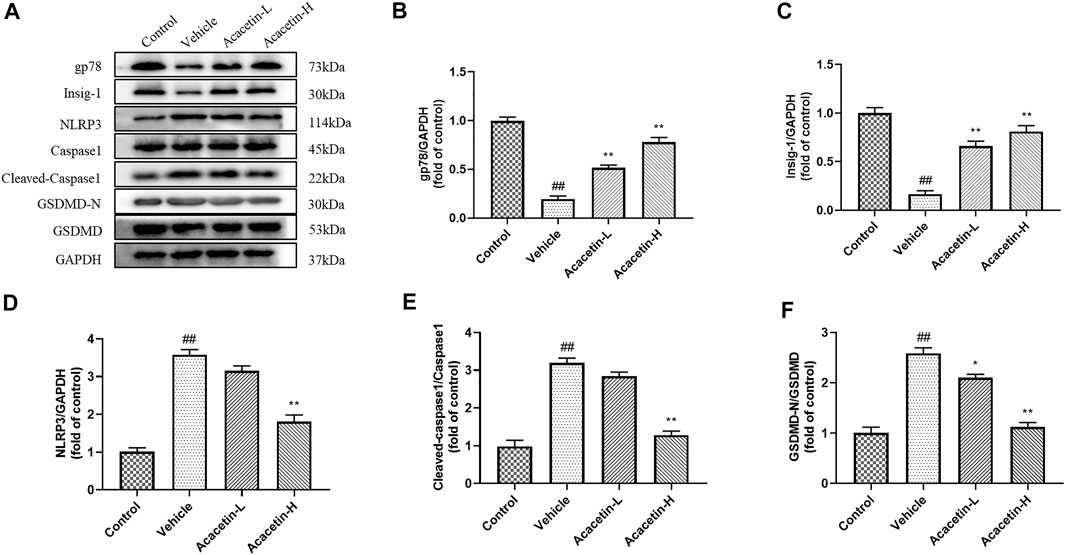
FIGURE 5. Effect of acacetin on gp78-mediated NLRP3 inflammasome in the cornea. (A) Representative western blots of gp78, Insig-1, NLRP3, Caspase1, cleaved Caspase1, GSDMD-N, and GSDMD. (B–F) Quantitative analysis of western blot results. Results are expressed as the mean ± SEM (n = 3 per group). ##p < 0.01 vs. Control; *p < 0.05, **p < 0.01 vs. Vehicle.
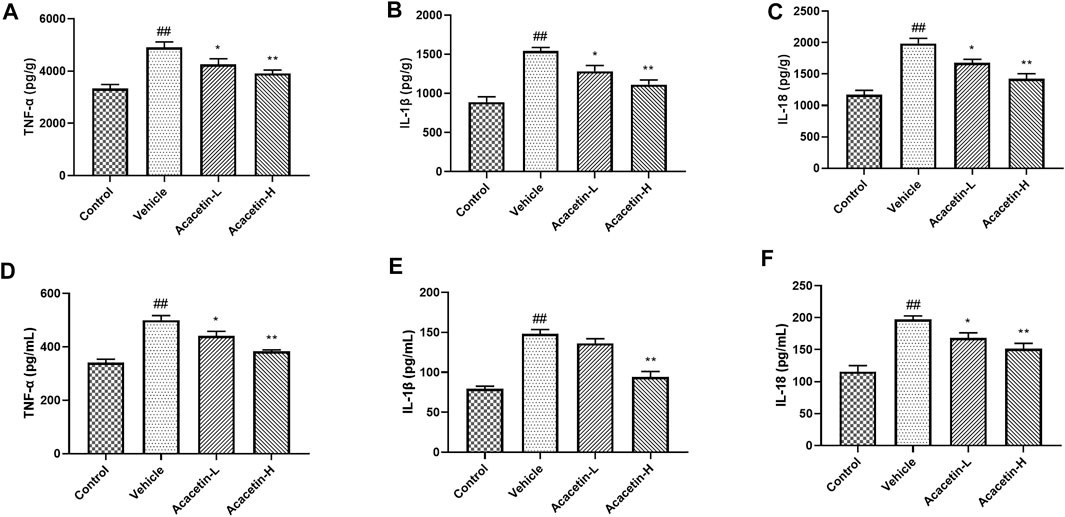
FIGURE 6. ELISA analyses of inflammatory factors in the hippocampus and tears. (A–C) Production of TNF-α, IL-1β, and IL-18 in the hippocampus of mice. (D–F) Concentrations of TNF-α, IL-1β, and IL-18 in the tears of mice. Results are expressed as the mean ± SEM (n = 6 per group). ##p < 0.01 vs. Control; *p < 0.05, **p < 0.01 vs. Vehicle.
To elucidate the role of gp78 in acacetin-conferred beneficial effect, we injected CUMS mice with gp78 siRNAs (Supplementary Figure S2). As shown in Figures 7,8, gp78 siRNA infection greatly inhibited the increase of NLRP3 ubiquitination in CUMS mice resulted from acacetin treatment, leading to the enhancement of NLRP3 and Cleaved-Caspase1 expression as well as the reduction of neurons and goblet cells. Moreover, although acacetin significantly ameliorated the depressive behavior (measured by SPT, TST, and FST) and dry eye symptoms (determined by wetted length and corneal defects) triggered by CUMS, this effect was greatly blocked by gp78 siRNA intervention (Figure 9). Taken together, these results suggest that gp78 activation is required for acacetin to prevent depression-related dry eye disease.

FIGURE 7. Influence of gp78 siRNAs on hippocampal NLRP3 inflammasome in CUMS mice. (A–B) NLRP3 ubiquitination levels in the hippocampus of mice were determined by co-ip. (C–D) Hippocampal NLRP3 expression levels of the experimental groups were detected with the immunohistochemistry. (E) Protein expression of NLRP3 and Cleaved-Caspase1 in the western blotting. Results are expressed as the mean ± SEM (n = 3 per group).
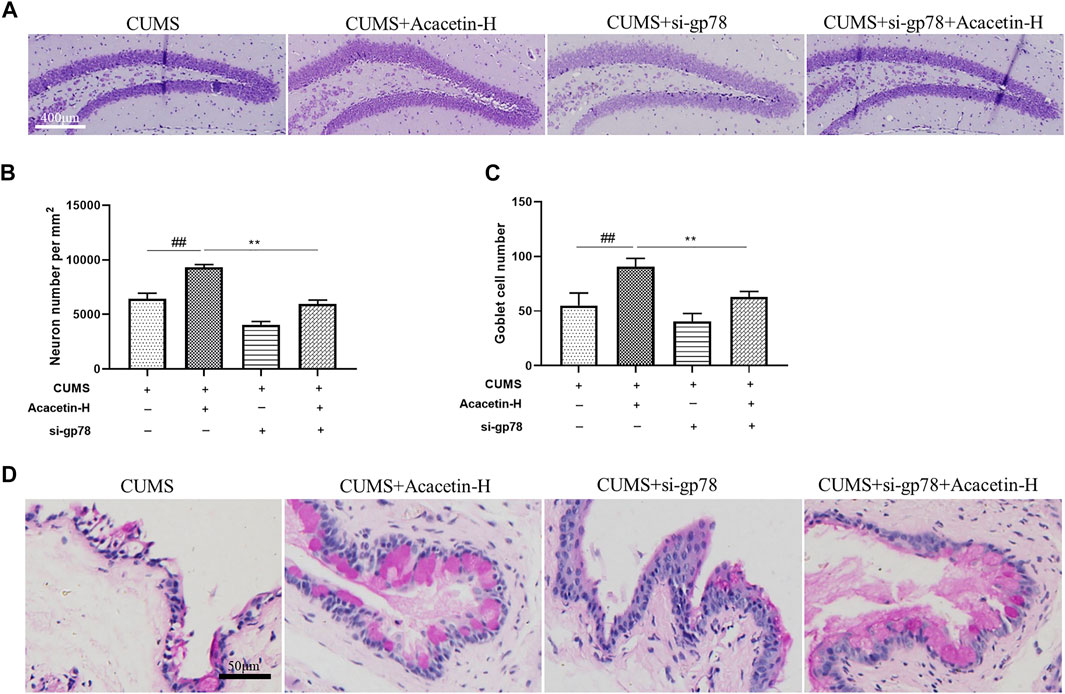
FIGURE 8. Influence of gp78 siRNAs on hippocampus Nissl bodies and goblet cells in CUMS mice. (A–B) Representative images of Nissl staining and quantitative analysis of Nissl bodies in the hippocampus. (C–D) Representative images of PAS staining and quantitative analysis of goblet cells in the cornea. Results are expressed as the mean ± SEM (n = 3 per group).

FIGURE 9. Impact of gp78 siRNAs on depressive behaviors and dry eye symptoms in CUMS mice. (A) Sucrose preference percentage of mice in the sucrose preference test. (B) Immobility time of mice in the tail suspension test. (C) Tear volumes of mice were measured using cotton phenol red threads. (D) Determination of corneal epithelial defects by fluorescein staining in CUMS mice. Results are expressed as the mean ± SEM (n = 9 per group).
Dry eye disease represents a heterogeneous group of conditions with tear film insufficiency and symptoms of ocular surface defects, and affects tens of millions of people (Thulasi and Djalilian, 2017; Tsubota et al., 2020). Depression is an important factor involved in the development of dry eye disease (Rakofsky et al., 2021). Severity of dry eye disease is associated with symptoms of depression (Weatherby et al., 2019). Patients with depression may suffer from central sensitization which affects pain perception and pain related behavior (Wan et al., 2016). While effective management of depression can help attenuate symptoms of dry eye disease, the anti-cholinergic effect of antidepressants may contribute to dry eye symptoms due to their potential side effects on the tear film status (Wan et al., 2016; Weatherby et al., 2019). Treatment of depression with the currently used antidepressants such as selective inhibitors of serotonin receptors promotes inflammatory cytokine secretion with subsequent inflammation and apoptosis of cells on the ocular surface (Weatherby et al., 2019). Therefore, drugs targeting depression-associated dry eye disease is urgently needed (Zhang et al., 2019). Acacetin is a plant flavone with diverse therapeutic potential. Acacetin alleviates diabetes-accelerated atherosclerosis by preserving mitochondrial function via activating Sirtuin1 (Sirt1)/Sirtuin3 (Sirt3)/AMP-activated protein kinase (AMPK) pathway (Han et al., 2020). Acacetin also protects mice from dextran sulfate sodium-induced acute colitis through suppressing macrophage inflammation and regulating the composition of gut microbiota (Ren et al., 2020). Doxorubicin cardiomyopathy is antagonized by acacetin treatment through Sirt1-mediated activation of AMPK/nuclear factor E2-related factor 2 (Nrf2) signaling. Here, we found that acacetin rescued corneal defects and the decrease of tear production and goblet cells in CUMS mice, indicating the protective effect of acacetin in depression-associated dry eye disease.
Inflammation is an important immune response regulating the interaction between organisms and the environment, and contributes to numerous diseases, including dry eye disease (Bordoni et al., 2017). Fakih et al. (2019) observed enhanced neuro-inflammatory responses in the trigeminal ganglion, as well as increased proinflammatory markers and activated astrocytes and microglia in the trigeminal brainstem sensory complex of mice with dry eye disease. Chen et al. (2020) found NOD-like receptor 12 (NLRP12) collaborates with NLR family CARD domain-containing protein 4 (NLRC4) inflammasomes to trigger GSDMD-dependent pyroptosis and exacerbate dry eye disease. One dose of carbonized nanogels with anti-inflammatory property profoundly relieved the symptoms of dry eye disease in vivo (Lin et al., 2022).
The NLRP3 inflammasome is a multimeric cytosolic protein complex that controls inflammatory response and influences the process of dry eye disease (Sharma and Kanneganti, 2021). MiR-223 decreases hyperosmolarity-induced inflammation through downregulating NLRP3 activation in human corneal epithelial cells and dry eye patients (Ren et al., 2022). Calcitriol inhibits the NLRP3 inflammasome-GSDMD pyroptosis pathway leading to the alleviation of hyperosmotic stress-induced corneal epithelial cell damage in dry eye disease (Zhang et al., 2021). The upregulation of NLRP3 inflammasome is noticed in the tears and ocular surface of dry eye patients (Niu et al., 2015). The artemisinin analog β-aminoarteether maleate (SM934) mitigates dry eye disease in rodent models by regulating Toll-like Receptor 4 (TLR4)/nuclear factor (NF)-κB/NLRP3 signaling (Yang et al., 2021). Consistently, our results showed that acacetin blocked NLRP3 inflammasome/GSDMD signaling pathway and reduced the concentrations of proinflammatory cytokines TNF-α, IL-1β, and IL-18, supporting the essential role of NLRP3 inflammasome-mediated inflammation in the development and treatment of dry eye disease.
Ubiquitination is a type of posttranslational modification of intracellular proteins, in which the ubiquitin moiety is covalently attached to a target protein to influence protein stability, interaction partner and biological function (Gu and Jan 2020). Glycoprotein 78 (gp78) is an E3 ubiquitin ligase within the endoplasmic reticulum-associated degradation pathway, which plays an essential role in multiple physiological and pathological processes (Joshi et al., 2017). Deacetylation of heat shock protein A5 (HSPA5) by histone deacetylase6 (HDAC6) results in gp78-mediated HSPA5 ubiquitination at K447 and suppresses metastasis of breast cancer (Chang et al., 2016). High expression levels of gp78 are related to poor outcomes in both endoplasmic reticulum (ER)-positive and ER-negative tumors (Singhal et al., 2022). Cyclin-dependent kinase5 (CDK5)-mediated phosphorylation-dependent ubiquitination and degradation of E3 ubiquitin ligases gp78 accelerates neuronal death in Parkinson’s disease (Wang et al., 2018). Xu et al. (2022) reported that gp78 mediates the ubiquitination of NLRP3, which suppresses NLRP3 inflammasome activation by inhibiting the oligomerization and subcellular translocation of NLRP3. In our study, the upregulation of gp78/Insig-1 elicited by acacetin promoted NLRP3 ubiquitination and attenuated CUMS-induced inflammatory response and dry eye disease in mice; however, interference of gp78 blunted acacetin-conferred beneficial effects in CUMS-related dry eye disease, confirming the crucial role of gp78 in regulating NLRP3 ubiquitination-regulated inflammation and dry eye disease in depressive rodents.
To conclude, the present study demonstrated that acacetin prevents depression-associated dry eye disease through suppression of NLRP3 ubiquitination-mediated inflammatory response via gp78 signaling. Our results suggest that acacetin may be a powerful therapeutic candidate for depression-related dry eye disease.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by Hunan University of Chinese Medicine Medical Ethical committee.
This study was conceived and designed by MX, Methodology and project management MX, JP, DQ, and HW. Data curation and analysis, XZ, PM, ZL, and DG, Interpretation of data, statistical analysis, article drafting, and revising were performed by XW and QP. All authors approved the submission of the manuscript.
This work was supported by China Postdoctoral Science Foundation (2021M690990); The 2021 “Academician Liu Liang Workstation” guidance project (21YS002); The 2022 “Disciplinary Reveal System” project (22JBZ024); Scientific Research Project of Hunan Administration of traditional Chinese Medicine (D2022053); Scientific Research Project of Hunan Provincial Health Commission (202107021634); Hunan Provincial Department of Science and Technology, Hunan Clinical Medical Research Center (2021SK4022); Key Projects of Hunan Provincial Department of Education (21A0238).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.984475/full#supplementary-material
Abbate, A., Toldo, S., Marchetti, C., Kron, J., Van Tassell, B. W., and Dinarello, C. A. (2020). Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ. Res. 126 (9), 1260–1280. doi:10.1161/CIRCRESAHA.120.315937
Abdelhamid, R. E., Kovacs, K. J., Nunez, M. G., and Larson, A. A. (2014). Depressive behavior in the forced swim test can Be induced by TRPV1 receptor activity and is dependent on NMDA receptors. Pharmacol. Res. 79, 21–27. doi:10.1016/j.phrs.2013.10.006
Arioz, B. I., Tastan, B., Tarakcioglu, E., Tufekci, K. U., Olcum, M., Ersoy, N., et al. (2019). Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/nrf2 pathway. Front. Immunol. 10, 1511. doi:10.3389/fimmu.2019.01511
Bordoni, A., Danesi, F., Dardevet, D., Dupont, D., Fernandez, A. S., Gille, D., et al. (2017). Dairy products and inflammation: A review of the clinical evidence. Crit. Rev. Food Sci. Nutr. 57 (12), 2497–2525. doi:10.1080/10408398.2014.967385
Bu, J., Shi, S., Wang, H. Q., Niu, X. S., Zhao, Z. F., Wu, W. D., et al. (2019). Acacetin protects against cerebral ischemia-reperfusion injury via the NLRP3 signaling pathway. Neural Regen. Res. 14 (4), 605–612. doi:10.4103/1673-5374.247465
Chang, Y. W., Tseng, C. F., Wang, M. Y., Chang, W. C., Lee, C. C., Chen, L. T., et al. (2016). Deacetylation of HSPA5 by HDAC6 leads to GP78-mediated HSPA5 ubiquitination at K447 and suppresses metastasis of breast cancer. Oncogene 35 (12), 1517–1528. doi:10.1038/onc.2015.214
Chen, H., Gan, X., Li, Y., Gu, J., Liu, Y., Deng, Y., et al. (2020). NLRP12- and NLRC4-mediated corneal epithelial pyroptosis is driven by GSDMD cleavage accompanied by IL-33 processing in dry eye. Ocul. Surf. 18 (4), 783–794. doi:10.1016/j.jtos.2020.07.001
Dang, R., Wang, M., Li, X., Wang, H., Liu, L., Wu, Q., et al. (2022). Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflammation 19 (1), 41. doi:10.1186/s12974-022-02400-6
Dogru, M., Kojima, T., Simsek, C., Nagata, T., and Tsubota, K. (2022). Salivary and lacrimal gland alterations of the epidermal fatty acid-binding protein (E-FABP) in non-obese diabetic mice. Int. J. Mol. Sci. 23 (7), 3491. doi:10.3390/ijms23073491
Fakih, D., Zhao, Z., Nicolle, P., Reboussin, E., Joubert, F., Luzu, J., et al. (2019). Chronic dry eye induced corneal hypersensitivity, neuroinflammatory responses, and synaptic plasticity in the mouse trigeminal brainstem. J. Neuroinflammation 16 (1), 268. doi:10.1186/s12974-019-1656-4
Gu, H., and Jan, F. B. (2020). Specificity in ubiquitination triggered by virus infection. Int. J. Mol. Sci. 21 (11), E4088. doi:10.3390/ijms21114088
Han, W. M., Chen, X. C., Li, G. R., and Wang, Y. (2020). Acacetin protects against high glucose-induced endothelial cells injury by preserving mitochondrial function via activating sirt1/sirt3/AMPK signals. Front. Pharmacol. 11, 607796. doi:10.3389/fphar.2020.607796
Hao, Y., Ge, H., Sun, M., and Gao, Y. (2019). Selecting an appropriate animal model of depression. Int. J. Mol. Sci. 20 (19), E4827. doi:10.3390/ijms20194827
Joshi, V., Upadhyay, A., Kumar, A., and Mishra, A. (2017). Gp78 E3 ubiquitin ligase: Essential functions and contributions in proteostasis. Front. Cell. Neurosci. 11, 259. doi:10.3389/fncel.2017.00259
Li, W., Ali, T., Zheng, C., Liu, Z., He, K., Shah, F. A., et al. (2021). Fluoxetine regulates eEF2 activity (phosphorylation) via HDAC1 inhibitory mechanism in an LPS-induced mouse model of depression. J. Neuroinflammation 18 (1), 38. doi:10.1186/s12974-021-02091-5
Lin, M. C., Lin, J. J., Hsu, C. L., Juan, H. F., Lou, P. J., and Huang, M. C. (2017). GATA3 interacts with and stabilizes HIF-1α to enhance cancer cell invasiveness. Oncogene 36 (30), 4243–4252. doi:10.1038/onc.2017.8
Lin, P. H., Jian, H. J., Li, Y. J., Huang, Y. F., Anand, A., Huang, C. C., et al. (2022). Alleviation of dry eye syndrome with one dose of antioxidant, anti-inflammatory, and mucoadhesive lysine-carbonized nanogels. Acta Biomater. 141, 140–150. doi:10.1016/j.actbio.2022.01.044
Liu, Y., Miao, L., Guo, Y., and Tian, H. (2021). Preclinical evaluation of safety, pharmacokinetics, efficacy, and mechanism of radioprotective agent HL-003. Oxid. Med. Cell. Longev. 2021, 6683836. doi:10.1155/2021/6683836
Luo, B., Huang, F., Liu, Y., Liang, Y., Wei, Z., Ke, H., et al. (2017). NLRP3 inflammasome as a molecular marker in diabetic cardiomyopathy. Front. Physiol. 8, 519. doi:10.3389/fphys.2017.00519
Messmer, E. M. (2015). The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Arztebl. Int. 112 (5), 71–81. doi:10.3238/arztebl.2015.0071
Musayeva, A., Jiang, S., Ruan, Y., Zadeh, J. K., Chronopoulos, P., Pfeiffer, N., et al. (2021). Aged mice devoid of the M3 muscarinic acetylcholine receptor develop mild dry eye disease. Int. J. Mol. Sci. 22 (11), 6133. doi:10.3390/ijms22116133
Niu, L., Zhang, S., Wu, J., Chen, L., and Wang, Y. (2015). Upregulation of NLRP3 inflammasome in the tears and ocular surface of dry eye patients. PLoS One 10 (5), e0126277. doi:10.1371/journal.pone.0126277
Park, B., Jo, K., Lee, T. G., Hyun, S. W., Kim, J. S., and Kim, C. S. (2019). Polydatin inhibits NLRP3 inflammasome in dry eye disease by attenuating oxidative stress and inhibiting the NF-κB pathway. Nutrients 11 (11), E2792. doi:10.3390/nu11112792
Puro, D. G. (2020). Bioelectric responses of conjunctival goblet cells to dry eye: Impact of ion channels on exocytotic function and viability. Int. J. Mol. Sci. 21 (24), E9415. doi:10.3390/ijms21249415
Rakofsky, J. J., Rakofsky, S. I., and Dunlop, B. W. (2021). Dry those crying eyes: The role of depression and antidepressants in dry eye disease. J. Clin. Psychopharmacol. 41 (3), 295–303. doi:10.1097/JCP.0000000000001382
Ren, J., Yue, B., Wang, H., Zhang, B., Luo, X., Yu, Z., et al. (2020). Acacetin ameliorates experimental colitis in mice via inhibiting macrophage inflammatory response and regulating the composition of gut microbiota. Front. Physiol. 11, 577237. doi:10.3389/fphys.2020.577237
Ren, Y., Feng, J., Lin, Y., Reinach, P. S., Liu, Y., Xia, X., et al. (2022). MiR-223 inhibits hyperosmolarity-induced inflammation through downregulating NLRP3 activation in human corneal epithelial cells and dry eye patients. Exp. Eye Res. 220, 109096. doi:10.1016/j.exer.2022.109096
Schnappauf, O., Chae, J. J., Kastner, D. L., and Aksentijevich, I. (2019). The pyrin inflammasome in Health and disease. Front. Immunol. 10, 1745. doi:10.3389/fimmu.2019.01745
Shao, B. Z., Xu, Z. Q., Han, B. Z., Su, D. F., and Liu, C. (2015). NLRP3 inflammasome and its inhibitors: a review. Front. Pharmacol. 6, 262. doi:10.3389/fphar.2015.00262
Sharif, H., Wang, L., Wang, W. L., Magupalli, V. G., Andreeva, L., Qiao, Q., et al. (2019). Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570 (7761), 338–343. doi:10.1038/s41586-019-1295-z
Sharma, B. R., and Kanneganti, T. D. (2021). NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 22 (5), 550–559. doi:10.1038/s41590-021-00886-5
Shen, Z., Xu, H., Song, W., Hu, C., Guo, M., Li, J., et al. (2021). Galectin-1 ameliorates perioperative neurocognitive disorders in aged mice. CNS Neurosci. Ther. 27 (7), 842–856. doi:10.1111/cns.13645
Singh, S., Gupta, P., Meena, A., and Luqman, S. (2020). Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 145, 111708. doi:10.1016/j.fct.2020.111708
Singhal, S. K., Byun, J. S., Yan, T., Yancey, R., Caban, A., Gil, H. S., et al. (2022). Protein Expression of The Gp78 e3-Ligase Predicts Poor Breast Cancer Outcome Based on Race. JCI Insight 7, e157465. doi:10.1172/jci.insight.157465
Thulasi, P., and Djalilian, A. R. (2017). Update in current diagnostics and therapeutics of dry eye disease. Ophthalmology 124 (11S), S27–S33. doi:10.1016/j.ophtha.2017.07.022
Tsubota, K., Pflugfelder, S. C., Liu, Z., Baudouin, C., Kim, H. M., Messmer, E. M., et al. (2020). Defining dry eye from a clinical perspective. Int. J. Mol. Sci. 21 (23), E9271. doi:10.3390/ijms21239271
Wan, K. H., Chen, L. J., and Young, A. L. (2016). Depression and anxiety in dry eye disease: a systematic review and meta-analysis. Eye 30 (12), 1558–1567. doi:10.1038/eye.2016.186
Wang, Q., Jiao, F., Zhang, P., Yan, J., Zhang, Z., He, F., et al. (2018). CDK5-Mediated phosphorylation-dependent ubiquitination and degradation of E3 ubiquitin ligases GP78 accelerates neuronal death in Parkinson's disease. Mol. Neurobiol. 55 (5), 3709–3717. doi:10.1007/s12035-017-0579-2
Wang, X., Cheng, S., and Xu, H. (2019). Systematic review and meta-analysis of the relationship between sleep disorders and suicidal behaviour in patients with depression. BMC Psychiatry 19 (1), 303. doi:10.1186/s12888-019-2302-5
Wang, C., Yang, T., Xiao, J., Xu, C., Alippe, Y., Sun, K., et al. (2021). NLRP3 inflammasome activation triggers gasdermin D-independent inflammation. Sci. Immunol. 6 (64), eabj3859. doi:10.1126/sciimmunol.abj3859
Weatherby, T., Raman, V., and Agius, M. (2019). Depression and dry eye disease: a need for an interdisciplinary approach? Psychiatr. Danub. 31, 619–621.
Wu, Y., Song, F., Li, Y., Li, J., Cui, Y., Hong, Y., et al. (2021). Acacetin exerts antioxidant potential against atherosclerosis through Nrf2 pathway in apoE(-/-) mice. J. Cell. Mol. Med. 25 (1), 521–534. doi:10.1111/jcmm.16106
Xiao, W. Z., Zhou, W. H., Ma, Q., Cui, W. G., Mei, Q. Y., and Zhao, X. (2019). Serotonergically dependent antidepressant-like activity on behavior and stress Axis responsivity of acacetin. Pharmacol. Res. 146, 104310. doi:10.1016/j.phrs.2019.104310
Xu, T., Yu, W., Fang, H., Wang, Z., Chi, Z., Guo, X., et al. (2022). Ubiquitination of NLRP3 by gp78/insig-1 restrains NLRP3 inflammasome activation. Cell Death Differ. 29, 1582–1595. doi:10.1038/s41418-022-00947-8
Xu, J., Deng, Y., Wang, Y., Sun, X., Chen, S., and Fu, G. (2020). SPAG5-AS1 inhibited autophagy and aggravated apoptosis of podocytes via SPAG5/AKT/mTOR pathway. Cell Prolif. 53 (2), e12738. doi:10.1111/cpr.12738
Xu, X., Piao, H. N., Aosai, F., Zeng, X. Y., Cheng, J. H., Cui, Y. X., et al. (2020). Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br. J. Pharmacol. 177 (22), 5224–5245. doi:10.1111/bph.15261
Xue, J., Zhang, Y., Zhang, J., Zhu, Z., Lv, Q., and Su, J. (2021). Astrocyte-derived Ccl7 promotes microglia-mediated inflammation following traumatic brain injury. Int. Immunopharmacol. 99, 107975. doi:10.1016/j.intimp.2021.107975
Yang, F. M., Fan, D., Yang, X. Q., Zhu, F. H., Shao, M. J., Li, Q., et al. (2021). The artemisinin analog SM934 alleviates dry eye disease in rodent models by regulating TLR4/NF-κB/NLRP3 signaling. Acta Pharmacol. Sin. 42 (4), 593–603. doi:10.1038/s41401-020-0484-5
Zhang, X., Yin, Y., Yue, L., and Gong, L. (2019). Selective serotonin reuptake inhibitors aggravate depression-associated dry eye via activating the NF-κB pathway. Invest. Ophthalmol. Vis. Sci. 60 (1), 407–419. doi:10.1167/iovs.18-25572
Zhang, J., Dai, Y., Yang, Y., and Xu, J. (2021). Calcitriol alleviates hyperosmotic stress-induced corneal epithelial cell damage via inhibiting the NLRP3-ASC-caspase-1-GSDMD pyroptosis pathway in dry eye disease. J. Inflamm. Res. 14, 2955–2962. doi:10.2147/JIR.S310116
Keywords: dry eye disease, depression, NLRP3 ubiquitination, gp78, acacetin
Citation: Xie M, Wang H, Peng J, Qing D, Zhang X, Guo D, Meng P, Luo Z, Wang X and Peng Q (2022) Acacetin protects against depression-associated dry eye disease by regulating ubiquitination of NLRP3 through gp78 signal. Front. Pharmacol. 13:984475. doi: 10.3389/fphar.2022.984475
Received: 02 July 2022; Accepted: 23 September 2022;
Published: 10 October 2022.
Edited by:
Yue Liu, Xiyuan Hospital, ChinaReviewed by:
Wei Wei Tao, Nanjing University of Chinese Medicine, ChinaCopyright © 2022 Xie, Wang, Peng, Qing, Zhang, Guo, Meng, Luo, Wang and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoye Wang, MzMwMjQzQGhudWNtLmVkdS5jbg==; Qinghua Peng, cHFoNDEwMDA3QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.