
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 16 September 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.977660
This article is part of the Research Topic New Mechanistic Insights into Cancer Precision Medicine View all 5 articles
Triple-negative breast cancer (TNBC) is a highly malignant subtype of breast cancer (BC) with vicious behaviors. TNBC is usually associated with relatively poor clinical outcomes, earlier recurrence, and high propensity for visceral metastases than other BC types. TNBC has been increasingly recognized to constitute a very molecular heterogeneous subtype, which may offer additional therapeutic opportunities due to newly discovered cancer-causing drivers and targets. At present, there are multiple novel targeted therapeutic drugs in preclinical researches, clinical trial designs, and clinical practices, such as platinum drugs, poly ADP-ribose polymerase (PARP) inhibitors, immunocheckpoint inhibitors, androgen receptor inhibitors as well as PI3K/AKT/mTOR targeted inhibitors. These personalized, single, or combinational therapies based on molecular heterogeneity are currently showing positive results. The scope of this review is to highlight the latest knowledge about these potential TNBC therapeutic drugs, which will provide comprehensive insights into the personalized therapeutic strategies and options for combating TNBC.
Breast cancer (BC) is a common cancer and is one of the leading causes of cancer-related morbidity and death among women worldwide (Sung et al., 2021). Triple-negative breast cancer (TNBC) is immunohistochemically defined by the lack of expression of estrogen receptor (ER) and progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2). TNBC is a highly complex and malignant subtype of BC, representing 15–20% of BC (Perou et al., 2000). TNBC usually manifests as the form of high-grade invasive ductal carcinoma and is characterized by a high recurrence rate, often with distant metastases and shorter overall survival (OS) compared to other major BC subtypes (Walsh et al., 2020).
At present, surgery, radiotherapy, and chemotherapy alone or in intriguing combinations, are the main choice for TNBC therapy (Borri and Granaglia, 2021). However, several factors have weakened the therapeutic efficacy of TNBC, including the high propensity to other organ metastases and molecular heterogeneity of TNBC (Li et al., 2021). The FDA approved adjuvants and neoadjuvant regimens, including antimetabolites, taxanes, and Anthracycline, have shown some initial benefit in early TNBC cases, but poor prognosis in late TNBC (Slade, 2020a). Furthermore, unlike hormone therapy and HER2-guided therapy, which produce considerable positive results in hormone receptor (HR)-positive and HER2-positive BC, readily targeted drugs are absent to treat TNBCs compared to other molecular subtypes of BC.
Currently, TNBC is distinguishingly recognized as a very heterogeneous mass with potential therapeutic potential due to newly elucidated carcinogens and targets. Importantly, with the discovery and development of novel treatments, represented by the platinum, poly ADP-ribose polymerase (PARP) inhibitors, immune checkpoint inhibitors (ICIs), androgen receptor (AR) inhibitors, and phosphoinositide-3 kinase (PI3K)/AKT/mTOR targeted inhibitors, the therapeutic options for TNBC are increasing. Translational studies in TNBC have focused on subsets defined by defects in homologous recombination repair, immune cell infiltration, over-activated PI3K pathway, and expression of AR. This article highlights the current landscape of personalized clinical treatments for TNBC therapy (Figure 1). The deep understanding of these novel drugs will provide insight into the therapeutic prospects and possibly redefine the TNBC terminology for combating TNBC.
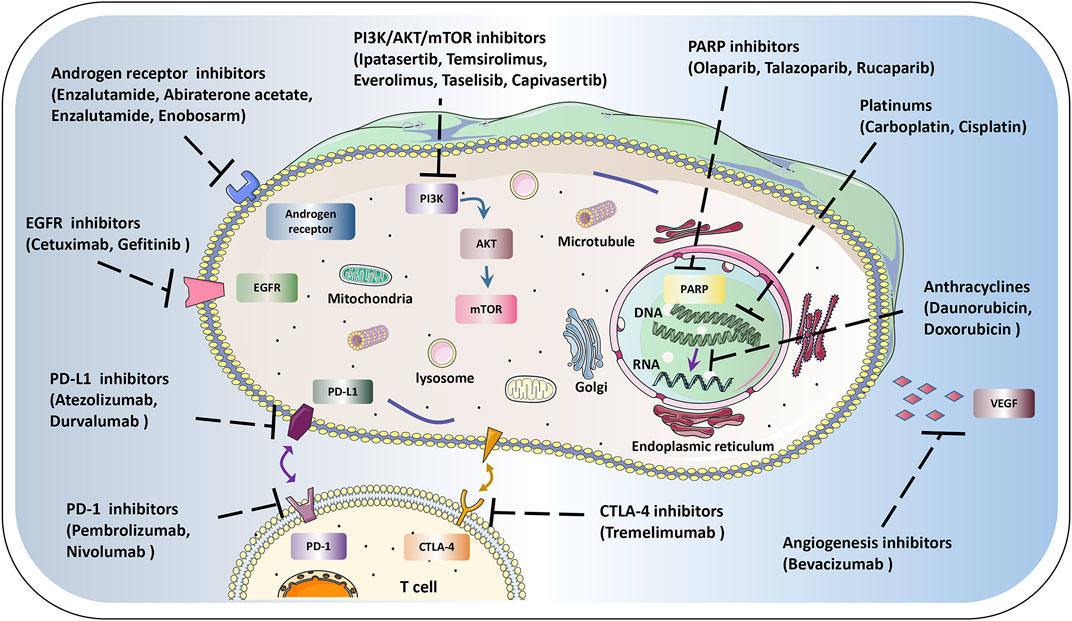
FIGURE 1. Schematic diagram of therapeutic targets in tumor cells. The current landscape of personalized clinical treatments for TNBC, mainly include anthracyclines platinums, PARP inhibitors, AR inhibitors, ICIs, PI3K/AKT/mTOR targeted inhibitors, EGFR and VEGF inhibitors, etc. Using these novel single or combination therapies will benefit the prognosis of TNBC.
TNBC is the subtype with the worst prognosis due to its very high tumor heterogeneity, drug resistance, and the long-term lack of effective treatment other than chemotherapy (Schmid et al., 2020a). In terms of histological classification, about 95% of TNBC cases are histologically defined as non-specific invasive BC or invasive ductal carcinoma accompanied by no specific histological features (Weigelt and Reis-Filho, 2009). Compared with other BC subtypes, TNBC is more often related to genetic conditions. The histopathological features of TNBC may be caused by different genes and proteins, which provides a theoretical basis for the different pathological features of TNBC at different stages and different levels (Weigelt and Reis-Filho, 2009).
In the early stage, the transcriptome analyses of BC using microarrays were performed to categorize the tumors into five intrinsic subtypes: luminal-A, luminal-B, HER2-enriched, basal-like, and a normal breast-like group (Perou et al., 2000). Although all inherent subtypes can be detected in immunohistochemically defined triple-negative disease, basal-like neoplasms show the greatest degree of overlap with TNBC. Approximately 50% and 75% of TNBC possess a basal phenotype, and approximately 80% of basal-like tumors are ER-negative/HER2-negative (Garrido-Castro et al., 2019). To better demonstrate TNBC-specific tumor heterogeneity, Lehmann et al. determined six TNBC subtypes with a distinct gene expression, including two basal-like subtypes (BL1 and BL2), an immunomodulatory subtype (IM), a mesenchymal subtype (M), a mesenchymal stem-like subtype (MSL), and an intraluminal androgen receptor (LAR) subtype (Lehmann et al., 2011). These types possess distinct gene expression signatures and distinct clinical behaviors. Specifically, there was relatively high expression of cell cycle and DNA damage reaction genes in BL1 and BL2 isoforms. The M and MSL types are characterized by enhanced expression of genes of the epithelial-mesenchymal transition (EMT) and growth-related pathways. The LAR subtype comprises patients with reduced relapse-free survival (RFS) and is characterized by AR signaling (Lehmann et al., 2011).
Besides, by using RNA profiling, Burstein et al. also confirmed 4 stable, clinically relevant TNBC subtypes: LAR, mesenchymal (MES), basal-like immunosuppressed (BLIS), and basal-like immune-activated (BLIA) (Burstein et al., 2015). In addition, these newly described subtypes demonstrate biological diversity, activate diverse molecular pathways, have specific DNA copy number variants (CNVs), and display varied clinical results. “Claudin-low” (CL) tumors are another intrinsic subtype (Prat et al., 2010). Clinically, CL tumors are typified by a lack of expression of luminal differentiation markers, a high degree of enrichment for EMT markers, immunoreactive genes, and cancer stem cell-like signatures. Notably, the majority of CL type is poor prognoses. According to genomic, and transcriptomic information of TNBC patients, Shao et al. divided the TNBC groups into 4 subtypes, including LAR, IM, MES, and BLIS (Jiang et al., 2019). Among them, LAR subtype presents a relatively increased frequency of ERBB2 somatic mutations, less frequency of mutational signature 3, and more CDKN2A loss. Each TNBC subtype contains specific potential therapeutic targets. Therefore, the comprehensive profile of TNBCs will valuable reference for precise tumor treatment.
Platinum-based chemotherapy (PBC) has been extensively studied in TNBC over the past decade, showing potential as an important primary treatment for metastatic triple-negative breast cancer (mTNBC) (Dent et al., 2021). Among the multiple platinum-based drug administrations, the cisplatin-based treatment program performed best (Dieci et al., 2019). These platinum-based agents can generate intra-chain and inter-chain double-stranded DNA crosslinks, which inhibit replication fork formation and mediate apoptosis of tumor cells (Zhou et al., 2020). Sensitivity to PBC might attribute to the damage and dysfunction by DNA cross-linking in tumor cells.
BRCA mutation status is a valuable and reliable diagnostic marker in TNBC (Sporikova et al., 2018). The therapeutic effect of platinum drugs (including carboplatin and cisplatin) in TNBC is significantly associated with BRCA mutations (Reis-Filho and Tutt, 2007). Advanced TNBC patients benefit from are prone to get benefit from BRCA1/2 mutation profile for providing platinum selection (Tutt et al., 2018). Single cisplatin or carboplatin has a significant objective response rate (ORR) in TNBC patients with BRCA1 mutation, but the role of platinum in non-BRCA-mutated mTNBC needs to be verified (Ghebeh et al., 2021). In the trial of TBCRC 030, Mayer et al. noted that homologous recombination defect (HRD) was not predictive of pathological response to preoperative cisplatin or paclitaxel in TNBC patients (Mayer et al., 2020). Wang et al. enrolled in a trial to compare the efficacy of PBC and non-PBC regimens in treating advanced TNBC (Wang et al., 2020). The median progression-free survival (PFS) in patients with or without deleterious mutations was 14.9 and 5.3 months, and the median OS was 26.5 and 15.5 months. In addition, because PBC was more functional in patients with harmful mutations, genetic testing in patients with advanced TNBC might yield better results.
Current ongoing trials evaluating the effectiveness of PBC versus other chemotherapy regimens will contribute to better management of TNBC. Chen et al. compared the effectiveness of PBC and non-PBC in advanced TNBC patients in four cancer centers in China. Notably, PBC doublets exhibited superior efficacy and tolerable toxicity compared with non-PBC doublets in the first-line treatment for mTNBC patients (Xu et al., 2020). This approach and conclusion were similar to the previous Canadian multicenter study of Villarreal-Garz et al. group, which confirmed that PBC-treated TNBC patients exhibited a more positive OS compared with conventionally managed TNBC patients with non-platinum chemotherapy (Sirohi et al., 2008). In the phase I/II MBC-10 trial, Rinnerthaler et al. assessed the efficacy of ixazomib, a proteasome inhibitor, combined with carboplatin, confirming that this combination was effective in treating patients with advanced TNBC (Rinnerthaler et al., 2018).
Neoadjuvant chemotherapy (NAC), which refers to the use of chemotherapeutic drugs before surgery, is a non-elimination disease modality but can improve the outcome of surgical procedures and is mainly applicable to patients with malignant tumors that have not developed distant metastases and are locally progressive (Wang and Mao, 2020). In a retrospective study, Elaine et al. demonstrated that the pathological complete response (pCR) rate was able to predicate the therapeutic ends and supported the utilization of carboplatin in NAC for TNBC (Walsh et al., 2019). Dieci et al. proposed a clinical protocol to include platinum in anthracycline/taxane-based NAC therapy for TNBC (Dieci et al., 2019). The incidence of pCR was higher in the PBC group than in the control group. Du et al. enrolled a non-inferior randomized phase 2 trial to confirm that carboplatin with taxanes was of capability in reliable adjuvant chemotherapy for TNBC patients in early-stage who did not withstand the chemotherapy with anthracycline (Du et al., 2020). Nevertheless, in the cohort of patients with basal subtype TNBC and residual invasive disease, platinum-based drugs did not improve the prognosis and exhibited a more toxic feature than capecitabine, accompanied by lower anticipated invasive disease-free survival (iDFS) (Mayer et al., 2021). This finding suggested that platinums were not appropriate or forbidden as the adjuvant agents in this population, highlighting better treatment strategies for this high-risk group. Furthermore, since incomplete pCR response in patients after NAC results in poor prognosis, effective characterization of the molecular profile to facilitate pCR prediction is critical. Ademuyiwa et al. reported a stable pCR rate of 45.7% in TNBC patients treated with neoadjuvant docetaxel and carboplatin regimen, and posed that tumor-associated mutation of epidermal growth factor receptor (EGFR), RB1, RAD51AP2, SDK2 and so on, together with immune-related gene (IRGs), might differentiate TNBC patients who would obtain pCR in this regimen (Slade, 2020b).
Platinum-based adjuvant chemotherapy is still controversial in TNBC patients regardless of whether the BRCA1 and BRCA2 (BRCA1/2) germline variants are related to treatment with platinum. Yu et al. designed a phase 3 randomized clinical trial comparing paclitaxel plus carboplatin (PCb) at 6 cycles with standard-dose regiments of cyclophosphamide, epirubicin, fluorouracil, and docetaxel at 3 cycles (Yu et al., 2020). These findings indicated that the PCb regimen was a viable selection for adjuvant chemotherapy in those cohorts with operable TNBC. Now, the subgroups sensitive to PCb need to be further studied. The addition of carboplatin to anthracycline, cyclophosphamide, and taxane regimen was associated with improved complete pathologic response (pCR), whereas patients who did not achieve pCR had a high risk of recurrence (Lee et al., 2020). Another Phase II trial examined the outcomes of the non-anthracycline plus carboplatin and nab-paclitaxel in treating early-stage TNBC (Bianchini et al., 2022). The results showed well-tolerated, highly effective effects and a pCR of 48%. Besides, they found that the GeparSixto immune profile was associated with a higher pCR, with positive implications for downgrading to NAC.
Despite these encouraging clinical data of PBC in TNBC, it is worth noting that there are some challenges in incorporating platinums into standard clinical practice. First of all, as not everyone has an exact response to treatment, predictive biomarkers are still needed to stratify patients with TNBC to avoid the adverse toxicity of these drugs. Besides, to date, there is not enough evidence have been powered to prove the long-term survival outcomes. Besides, the toxicities, dosing and regimen strategies are important clinical questions to ponder. It is very important for platinum to cooperate with other chemotherapeutic medicines to improve pCR and survival. And, Overall, platinum chemotherapy is promising but still deserves deeper and further exploration.
The inherent genomic instability of TNBC is closely connected to DNA repair defects. This is identifiable by mutational signature analysis and might be targetable with poly (ADP-ribose) polymerase (PARP) inhibitors (Slade, 2020c). BRCAness is an index with great predictive value of curative effect of platinum and PARP inhibitors, which are validated to preclinical and clinical activity (Pilié et al., 2019). The PPAR inhibitors olaparib and talazoparib, have been allowed for metastatic BC therapy, including TNBC cohort with germline mutations. It also has been demonstrated that PARP suppression reinforces the functions of ionizing radiation agents, DNA-methylating compounds, and PBC (Hastak et al., 2010).
Olaparib could enhance the anti-tumor immune by governing T-cell infiltration mediated through STING/TBK1/IRF3 pathway activation (Pantelidou et al., 2019). It elucidates another mechanism of PARP inhibitors and provides the basic principle of combining PARP inhibition with immunotherapy to treat TNBC. In phase I/II trial in Japanese patients with advanced or mTNBC, the combination treatment of olaparib with eribulin exhibited effective anti-tumor ability, with caution in the presence of febrile neutropenia (Voorwerk et al., 2019). GeparOLA (NCT02789332) studied the efficacy of olaparib in combination with paclitaxel in the treatment of BC (Fasching et al., 2021). PCR rates of olaparib and carboplatin in TNBC patients were 56.0% and 59.3%, respectively. In a phase 2 window clinical trial, RIO trial (EudraCT 2014–003319-12), exploration of 43 patients with untreated TNBC, PARP inhibitor rucaparib induced expression of interferon response genes and suppressed circulating tumor DNA (ctDNA) in homologous recombination-deficient TNBC (Chopra et al., 2020).
During the phase II I-SPY2 trial, the united therapy of durvalumab and olaparib was given to the standard NAC therapy with paclitaxel, denoted as DOP (Pusztai et al., 2021). The pCR rates with 27%–47% were markedly improved in TNBC patients with DOP, than those cohorts with paclitaxel standard. Eikesdal et al. enrolled phase II PETREMAC trial that TNBC patients with solid tumors larger than 2 cm received olaparib for 10 weeks prior to chemotherapy (Oliveira et al., 2019). Olaparib achieved an objective response in 18 of 32 patients (56.3%) with mild side effects and did not affect the tolerability of subsequent chemotherapy. Furthermore, HRD could predict the olaparib efficacy and response to olaparib over germinal BRCA1/2 and gPALB2 mutations. Therefore, the germline BRCA1/2 mutations, HRD, as well as homozygous BRCA1 promoter methylation are potentially useful indicators for evaluating the stratification of TNBC patients who would reap the benefits of PARP inhibitor olaparib/eribulin co-administration (Kawachi et al., 2020).
AR is another area of great interest and is emerging as an important factor in the pathogenesis of BC. AR is expressed in BC tissues and normal tissues to varying degrees, and AR expression of BC tissues shows significant differences in different stages, pathological types, and malignant degrees. (Finn et al., 2020). About 10–15% distinct subgroups of TNBC with AR present more benign processes, which may be responsive to AR blockade. Besides, the genome of these tumors is featured by an enriched rate of PIK3CA-activated mutations. The first proof-of-concept trial confirmed the efficacy of the AR antagonist bicalutamide in patients with advanced AR-positive TNBC (Gucalp et al., 2013). Since then, evidence of clinical efficacy further supports the therapies of other next-generation AR-targeted agents such as enzalutamide, abiraterone acetate, and enzalutamide. Abiraterone acetate plus prednisone treatment was beneficial for some patients with AR-positive locally advanced or mTNBC (Bonnefoi et al., 2016). Biological insight into AR-positive TNBC has positive significance for signaling pathway cross-talk, TNBC subtype classification, early tumor detection, and synergistic treatment.
Preclinical data have demonstrated that AR-positive TNBC cells respond effectively to AR antagonists. Enobosarm, also known as GTx-024 or ostarine, is a first-in-class, nonsteroidal selective androgen receptor modulator (SARM) being developed for diverse indications in medical oncology, including AR-positive BC (Dalton et al., 2011). Yuan et al. explored the potency and biosafety of enobosarm and pembrolizumab combination in AR + mTNBC patients (Yuan et al., 2021). The enobosarm plus pembrolizumab was well tolerated, in heavily pre-treated AR TNBC without pre-selected PD-L1. Therefore, it is necessary to further conduct clinical trials on the combination of AR-antagonistic treatment with immunosuppressant for AR + TNBC. A phase II study in 2018 was performed to figure out the benefit of enzalutamide in patients with locally advanced or metastatic AR-positive TNBC (Traina et al., 2018). The clinical benefit rate (CBR) was 25% and 33%, median PFS was 2.9 months and 3.3 months, respectively in the intention to treat (ITT) population and the evaluable subgroup. Thus, enzalutamide exhibited a positive profile in clinical activity and tolerance in advanced AR-positive TNBC patients. In the multi-institutional phase Ib/II study TBCRC032, patients with stage II TNBC were randomized to be treated with enzalutamide alone or plus taselisib (Lehmann et al., 2020). In this study, Lehmann et al. validated the treatment effectiveness of enzalutamide combined plus taselisib in AR + TNBC with a better CBR (35.7%) and PFS (3.4 months). Moreover, genomic analyses revealed that only AR protein expression was not sufficient for identifying patients, confirming the necessity of identifying tumor LAR subtypes or AR splice variants in AR antagonist therapy.
However, the prognostic characteristics of AR expression in TNBC patients continue to be debated. A meta-analysis study explored the relationship between AR expression and survival outcomes and found that there was no correlation (Xu et al., 2020). It seems difficult to identify and stratify patients who will benefit from AR-targeted therapy. AR-based prognostic and predictive value and the related biomarker identification improve response rates would be encouraging.
Abnormal PI3K/AKT/mTOR is frequently activated in TNBC and is linked with oncogenesis, progress, and chemoresistance. Activation of the PI3K pathway is mainly triggered upon the protein level, commonly accompanied by less dependent on PIK3CA mutations and loss of the negative regulators PTEN (Oliveira et al., 2019). High-frequency alterations in PI3K pathway gene provide a theoretical basis for designing inhibitors targeting PI3K/AKT. In BRCA-proficient TNBC, PI3K inhibition impairs BRCA1/2 expression and DNA homologous recombination, and sensitizes TNBC to PARP inhibition (Ibrahim et al., 2012). Recently, PI3K blockers have displayed several desirable therapeutic effects in patients with stage II-III TNBC with PIK3CA mutations (Gupta et al., 2020).
Ipatasertib is a highly selective AKT kinase small molecule inhibitor currently for treating BC and prostate cancer (Sweeney et al., 2021). In a phase 2 trial (LOTUS), the results supported the AKT-targeted therapy for TNBC. Median PFS in the intention to treat (ITT) population was 6.2 months with ipatasertib versus 4.9 months with placebo. In 48 patients with tumors with low PTEN levels, median PFS was 6.2 months for ipatasertib compared with 3.7 months for placebo (Sweeney et al., 2021). The final result of this program in 2021 revealed that patients with advanced TNBC could benefit from the ipatasertib-paclitaxel combination (Dent et al., 2021). In the PAKT trial of AKT inhibitor capivasertib mTNBC, Schmid et al. verified that adding the capivasertib to the first-line paclitaxel treatment in TNBC could observably prolong PFS and OS for benefiting TNBC patients (Schmid et al., 2020b). The FAIRLANE trial showed a consistent result from LOTUS and PAKT in metastatic TNBC (Oliveira et al., 2019). In FAIRLANE, the addition of ipatasertib to paclitaxel NAT in early TNBC did not pose a clinically significant enhancement in pCR rates, but ipatasertib had a more pronounced antitumor effect in patients with biomarker selection.
To ascertain whether the prognosis is affected by tumor subtype, Basho et al. evaluated the results of therapy in patients with mesenchymal breast cancer (MpBC) versus non-MpBC, undergoing mTOR inhibition (temsirolimus or everolimus) with liposomal doxorubicin and bevacizumab (Basho et al., 2018). The results demonstrated that patients with advanced MpBC showed better long-term outcomes, which indicated that metaplasia histology might predict the efficacy of agent targeting the PI3K/Akt/mTOR pathway. In multi-institutional phase Ib/II study TBCRC032, the combination of AR-antagonist enzalutamide and PI3K inhibitor taselisib could promote CBR (Lehmann et al., 2020). In the SAFIR02 trial (NCT02299999), Mosele et al. selected a total of 649 patients with metastatic BC with available mutational profiles for outcome analysis, interestingly showing that TNBC recipients with PIK3CA mutation exhibited superior OS. This might be due to the accumulation of PIK3CA mutations in luminal BC and the reduction of HR performance during metastasis (Mosele et al., 2020). Anand et al. evaluated the efficacy of targeted therapy following standard anthracycline and taxane NAC, which showed that everolimus combined with cisplatin was effective for TNBC patients with residual lesions following standard NAC (Anand et al., 2021).
However, in a randomized phase II neoadjuvant study, the adjuvant treatment with mTOR inhibitor everolimus did not increase response rates and was associated with more adverse events, in stage II/III patients with TNBC who received weekly preoperative weekly cisplatin, paclitaxel, and daily everolimus or placebo before surgery (Anand et al., 2021). Therefore, how to reconcile the dose of the three drugs, synergistically exert the function of inhibitors, and more effectively “attack” TNBC is a challenge. In addition, drugs targeting distinct PI3K/AKT/mTOR components and homologous molecules (e.g., MAPK) are in urgent development. In this evolving field, in addition to the effects and application range of these drugs, it is also necessary to consider synergistic treatment with the other targeted TNBC drugs.
The host immune system is an important orchestrator in reshaping the response to TNBC treatment and prognosis. High levels of intratumoral tumor-infiltrating lymphocytes (TILs) in TNBC are a potential biomarker for indicating a more favorable survival outcome and response to immunotherapy in TNBC (Lotfinejad et al., 2020). In addition, immunosuppressive programmed cell death ligand 1 (PD-L1) is expressed in 20% of TNBC (Dieci et al., 2015). TNBC contains high levels of TILs and CD8+ lymphocytes, as well as expressing PD-L1, which seems to have greater clinical benefits (Ghebeh et al., 2021). Immunomodulatory antibody-based strategies are emerging as promising approaches to bring breakthroughs to TNBC. Several ICIs, including PD-L1-targeted (atezolizumab, and durvalumab), PD-1-targeted (pembrolizumab, nivolumab, and camrelizumab), CTLA-4-targeted (tremelimumab) antibodies, are currently being investigated either as monotherapy or co-therapy for TNBC in many immunotherapy trials.
ICIs have the capabilities to dramatically enlarge the treatment efficacy in TNBC. Besides, other hopeful immunotherapy methods of TNBC, like anti-protease cathepsin D, or EGFR therapy, personalized vaccination, adoptive cell therapy, Immunotherapy combination, are also under comprehensive and deep investigation (Ashraf et al., 2019) (Jia et al., 2017). Other ICIs for single agent treatment, or in association with pharmacotherapy for advanced TNBC, and for early stage disease, are emerging and ongoing areas of research. This will be a key direction for future clinical trials to characterize highly predictable biomarkers of response, as well as strategies for personalized immunotherapy in the treatment of TNBC.
Atezolizumab is a humanized, PD-L1-targeted monoclonal antibody that is well tolerated and clinically improves the anti-tumor activity in multiple cancer types (Finn et al., 2020). Atezolizumab coupled with nab-paclitaxel is representative of a promising novel first-line care standard for those with PD-L1-positive mTNBC. In a phase I study, the women with mTNBC were treated with single-agent atezolizumab every 3 weeks (Emens et al., 2019). This study preliminarily verified the safety and clinical activity of atezolizumab with a 10% ORR and an OS of 17.6 months in the entire cohort. To extend the optimal anti-tumor activity, combinations with standard chemotherapy strategies are being investigated to synergistically elicit the death of immune tumor cells. Adams et al. further conducted a phase Ib trial of atezolizumab in the context of nab-paclitaxel the cohort of mTNBC (Adams et al., 2019a). The ORR was 39.4% and the median PFS and OS were 5.5 months and 14.7 months, respectively. It demonstrated that this combination mTNBC was reliable and offered a manageable safety profile. Next, Schmid et al. reported a second interim OS analysis of the phase 3 IMpassion130 study, which indicated a valuable OS benefit of this regimen in patients with PD-L1 immune cell-positive BC by receiving atzolizumab plus nab-paclitaxel in cohort with unresectable, localized advanced, or mTNBC (Schmid et al., 2020a). It was also intriguing that early TNBC patients receiving neoadjuvant therapy combining azolizumab with sequential nab-paclitaxel and anthracycline-based treatment, presented markedly higher pCR and excellent biosafety characteristics (Mittendorf et al., 2020).
In GeparNuevo trial, durvalumab was added to standard NAC therapy in early TNBC patients (Loibl et al., 2019). The results deciphered that the joining of durvalumab to anthracycline/paclitaxel-based NAC strategy might improve pCR rates to some extent, particularly in patients treated with durvalumab alone prior to chemotherapy initiation. Meanwhile, the cohort of this randomized phase II placebo-controlled study was too small to give very definitive evidence. The usage of durvalumab has some positive implications in patients with higher tumor load. Ghebeh’s study investigated the efficacy of durvalumab and paclitaxel in combination for TNBC treatment (Ghebeh et al., 2021). The results showed that patients who underwent at minimum one period of combined therapy demonstrated a positive safety profile, indicating the promise and window for further use of the therapy. In addition, Ahmed et al. suggested that higher levels of PD-L1 expression in tumor cells, stromal immune cells, and co-localization of CD68-positive cells were strongly linked to superior pCR of NAC durvalumab in TNBC group (Ahmed et al., 2020).
Notably, the combination of ICI antibodies and other tumor-targeting drugs can produce significant synergistic effects. To explore the optimal setting and potential mechanisms of anti-angiogenesis inhibitors and anti-PD-1 therapy, Liu et al. preliminarily revealed dose-dependent synergistic effects of this combination at animal and TNBC patient levels and showed that increased osteopontin (OPN) and TGF-β expressions were positively correlated with favorable therapeutic effect (Li et al., 2019). In their subsequent clinical trial (NCT03394287), the ORR of camrelizumab and apatinib combination therapy was significantly higher than that of camrelizumab or apatinib alone (Liu et al., 2020). Therefore, camrelizumab in combination with apatinib displayed good therapeutic function and controllable results in the advanced TNBC cohort. Within this therapy, several responsive biomarkers could predict better ORR, including higher baseline TIL and tumor-infiltrating CD8+ T cells, and enhanced plasma TIM-3/CD152, as well as lower baseline plasma HGF/IL-8 and decreased plasma IL-8 level (Liu et al., 2021).
Pembrolizumab is a PD-1 inhibitor currently being used in clinical trials that possesses positive efficacy in solid tumors, represented by recurrent/metastatic cervical cancer and advanced melanoma (De Felice et al., 2021) (Slade, 2020a). The phase Ib KEYNOTE-173 aimed to evaluate the security and preliminary efficacy of NAC in combination with pembrolizumab utilized in the cohort of high-risk, early-stage non-mTNBC (Dent et al., 2021). The pCR rates for all cohorts ranged from 49% to 71%, indicating that this treatment, NAC plus pembrolizumab, had definite antitumor activity and was associated with low side effect symptoms.
The efficacy of therapeutic strategies of PD-1 blockade alone is insufficient in metastatic TNBC. This is because many patients with metastatic tumors exhibit resistance to therapy. Moreover, although some patients can benefit from PD-1/PD-L1 blockade therapy in the setting of PD-L1-positive TME, overall the majority of patients benefit less (Adams et al., 2019b). Therefore, how to effectively improve or amplify the therapeutic effect of PD-1/PD-L1 blockade is an important obstacle to be solved urgently. In the TONIC trial, Voorwerk et al. pointed out that short-term doxorubicin and cisplatin might trigger a more improved TME, manifested by up-regulation of PD-1, PD-L1, and IRG expression in T cell cytotoxicity signal pathway (Voorwerk et al., 2019). Therefore, the combination of chemotherapy and PD-1 blockade makes TNBC tumor cells more sensitive to PD-1 blockade, and most patients in the treatment group showed effective ORR (Table 1).
Antibody-drug conjugates (ADCs) are novel engineered therapeutic agents consisting of a humanized monoclonal antibody targeting a tumor-specific antigen and a loaded cytotoxic drug. Many ADCs have demonstrated impressive performance of high efficacy and safety in a variety of cancers including breast, lung, and hematological malignancies. Some ADCs, including glembatumumab vedotin (CDX-011, GV), sacituzumab govitecan, PF-06647263, and mirvetuximab soravtansine, have becoming increasingly important options in TNBC trials.
GV belongs to a ADC with a glycoprotein NMB-targeting monoclonal antibody conjugated with monomethyl auristatin E (MMAE) (Bardia, 2017). Vahdat et al. conducted 2 breast cancer clinical trials based on Glembatumumab vedotin. They firstly enrolled 42 patients with advanced/metastatic breast cancer, who were treated with standard 3 + 3 dose escalation followed by a phase II extension, and were confirmed by immunohistochemical gpNMB staining of tumor tissue (Bendell et al., 2014). Median PFS was 17.9 weeks for TNBC patients, and 18 weeks for patients with gpNMB + tumors, preliminarily confirming the tolerability of this ADC. Subsequent EMERGE clinical trial results showed that glembatumumab vedotin had a good and controlled safety feature, and that its activity was possibly intensified in patients with TNBC and/or gpNMB tumor expression (Yardley et al., 2015).
Sacituzumab govitecan is a FDA-approved ADC for pretreated mTNBC patients. It is consisted of two components, the active metabolite of irinotecan SN-38 and humanized RS7 antibody targeting glycoprotein Trop-2 (Marks and Kalinsky, 2017). Vahdat et al. also conducted a single-arm, multicenter trial to assess the effect of sacituzumab govitecan in patients with advanced/refractory mTNBC, administered 10 mg/kg starting dose on days 1 and 8 of 21-days repeated cycles (Bardia et al., 2017). Trop-2 was moderate to strongly positive in tumor tissue from most enrolled patients. In this cohort of heavily treated m TNBC patients, sacituzumab govitecan was shown to be very well tolerated and to produce early and long-lasting therapeutic effects.
PF-06647263, an ADC consisting of an anti-EFNA4 antibody linked to a calicheamicin payload, was shown to exhibit preliminary effects in some xenograft models (Fraguas-Sánchez et al., 2022). Ignacio et al. implemented a first-in-human study of PF-06647263 using every 3 weeks and every week regimens in patients with TNBC, ovarian cancer, and other advanced solid tumors (Garrido-Laguna et al., 2019). But as adequate exposure of PF-06647263 did not elicit enough anti-tumor activity in patients with TNBC and ovarian cancer, this study was definitively discontinued.
Mirvetuximab soravtansine is denoted as a novel ADC that has posed potential efficacy for targeting FRα-positive solid tumors (O’Malley et al., 2020). Mirvetuximab soravtansine was investigated in a prospective phase II trial for the benefit of metastatic TNBC (Yam et al., 2021). The study was terminated early due to a low rate of FRα positivity in the selected patient population and no patients had a partial or complete response. This suggested that mirvetuximab soravtansine might be an optional therapeutic ADC only when there are sufficient clinical indications or characteristic expressions.
Trastuzumab deruxtecan (T-DXD) is a conjugate of a HER2 antibody and a DNA topoisomerase I inhibitor. Trastuzumab deruxtecan has shown positive therapeutic value in a variety of tumors, such as breast cancer, gastric cancer, and non-small cell lung cancer. Several studies by Modi et al. have documented that trastuzumab deruxtecan exhibited persistent antitumor activity in a pretreated HER2-positive metastatic BC patient cohort (Tamura et al., 2019; Modi et al., 2020a). In a phase 3 trial, the percentage of patients surviving at 12 months in the trastuzumab deruxtecan group was 94.1%, among patients with previously treated HER2-positive metastatic breast cancer (Cortés et al., 2022). In another trial involving patients with HER2 low metastatic BC, Modi et al. also showed that trastuzumab deruxtecan significantly prolonged PFS and OS compared to the other chemotherapy regimens (Modi et al., 2020b; Modi et al., 2022).
In conclusion, ADC drugs can have synergistic therapeutic effects on a variety of tumors due to both the targeting and therapeutic properties of antibodies and the tumor cytotoxicity of coupled small molecule drugs. Several ADC-based therapies have been approved or are undergoing clinical trials in recent years. Although the efficacy, tolerability, and safety of ADC are the tradeoff factors affecting the efficacy of ADC, it shows a certain therapeutic prospect for TNBC tumors.
In addition to the above, targeting EGFR in TNBC also represents a hopeful treatment option. Studies have shown that EGFR is frequently over-represented in TNBC, which suggests the potential of EGFR serving as a therapeutic target for TNBC (Carey et al., 2012). EGFR-targeted monoclonal antibodies and/or tyrosine kinase (TK) inhibitors, such as cetuximab, gefitinib, erlotinib, lapatinib, and panitumumab, allow efficient therapy for TNBC. Besides, bevacizumab is known as a world-famous monoclonal antibody targeting vascular endothelial growth factor A (VEGF-A), which is crucial for tumor growth and TNBC metastasis. For TNBC patients with BRCA1/2 mutations, adding bevacizumab after standard NAC might increase pCR (Fasching et al., 2018).
At present, the above novel targeted therapeutic drugs comprise the landscape of personalized clinical treatments for TNBC, such as platinum drugs, PARP inhibitors, ICIs, AR inhibitors as well as PI3K/AKT/mTOR targeted inhibitors (Table 2). While standard anthracycline- and taxane-based chemotherapy regimens remain the classic approach to early systemic therapy, emerging information on cancer genomes has prompted molecular characterization-driven therapeutic strategies. These personalized, single, or combinational therapies based on molecular heterogeneity are currently showing positive results.
Although the current studies have all made some research progress, they are still challenging at this time. Firstly, it must be emphasized that TNBC is a highly heterogeneous tumor disease, including many different entities with different biological and clinical behaviors. The molecular characteristics of TNBC are not only important indicators for tumor evaluation, but also important references for the “tailored” targeted therapy and diagnosis of TNBC. Increasingly updated multiomics studies, including genomic, transcriptome, metabolome and epigenetic, provide detailed information on the deeper characterization of TNBC. There is a need to establish specific molecular profiles or metrics to precisely evaluate treatment benefits and risks and to determine better trial design options. Integrating multiple strategies such as tumor proliferation/immune-related markers and PAM50 subtypes allows adjustment for further step-down chemotherapy and/or other therapies in treating TNBC. Secondly, the cohorts included in some studies contain small numbers of patients and these patients are from a single institution. In addition, for some combination therapies, the optimal dose ratios or sequential dosing regimens need to be further explored to determine the best combination patterns, for optimal effects. Thus, at present, the main focus of existing studies is on the ongoing and completed early trials, the efficacy outcomes and common adverse events of each type of treatment, and to guide the adjustment and protocol involved in subsequent drug therapy Multicenter, long-follow, large-cohort clinical treatment investigation are still necessary to determine exact efficacy of these drugs.
Precision oncology, by analyzing the transcriptome, proteome and metabolome of the tumor genome in order to identify targets that can be sought to attack the tumor and develop treatment strategies accordingly. Classification of specific molecular profiles of TNBC to provide diagnosis, prognosis, and treatment information has been applied in clinical trails for choosing targeted therapies such as small molecule inhibitors or monoclonal antibodies and for predicting therapeutic resistance. Since tumors have a huge number of molecular alterations, it may be arduous to achieve clinical efficacy by targeting just one or several of the mutation. There is no denying that precision oncology does bring a ray of hope to the treatment of TNBC. Collectively, based on TNBC molecular classification, it is currently necessary to mine therapeutic targets within each subtype and formulate corresponding therapeutic strategies. Future research still needs to develop highly effective targeted drugs and identify relevant biomarkers to evaluate therapeutic benefits.
Conceptualization, JZ, YX, and XZ; writing-original draft preparation, JZ and YX; writing-review, XZ; investigation, HY and YT; data curation, YX and YT; supervision, YW, YD and QZ. All authors have reviewed and approved the final version.
This research was funded by China Guanghua Science and Technology Foundation (2019JZXM001), Wuhan Science and Technology Bureau (2020020601012241), and National Natural Science Foundation of China (NSFC, 81902933).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adams, S., Diamond, J. R., Hamilton, E., Pohlmann, P. R., Tolaney, S. M., Chang, C-W., et al. (2019). Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up: A phase 1b clinical trial. JAMA Oncol. 5 (3), 334–342. doi:10.1001/jamaoncol.2018.5152
Adams, S., Loi, S., Toppmeyer, D., Cescon, D. W., De Laurentiis, M., Nanda, R., et al. (2019). Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 30 (3), 405–411. doi:10.1093/annonc/mdy518
Ahmed, F. S., Gaule, P., McGuire, J., Patel, K., Blenman, K., Pusztai, L., et al. (2020). PD-L1 protein expression on both tumor cells and macrophages are associated with response to neoadjuvant durvalumab with chemotherapy in triple-negative breast cancer. Clin. Cancer Res. 26 (20), 5456–5461. doi:10.1158/1078-0432.CCR-20-1303
Anand, K., Patel, T., Niravath, P., Rodriguez, A., Darcourt, J., Belcheva, A., et al. (2021). Targeting mTOR and DNA repair pathways in residual triple negative breast cancer post neoadjuvant chemotherapy. Sci. Rep. 11 (1), 82. doi:10.1038/s41598-020-80081-y
Ashraf, Y., Mansouri, H., Laurent-Matha, V., Alcaraz, L. B., Roger, P., Guiu, S., et al. (2019). Immunotherapy of triple-negative breast cancer with cathepsin D-targeting antibodies. J. Immunother. Cancer 7 (1), 29. doi:10.1186/s40425-019-0498-z
Bardia, A. (2017). Antibody drug conjugates for triple-negative breast cancer: Targeting positive in the negative. Oncologist 22, S3.
Bardia, A., Mayer, I. A., Diamond, J. R., Moroose, R. L., Isakoff, S. J., Starodub, A. N., et al. (2017). Efficacy and safety of anti-trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J. Clin. Oncol. 35 (19), 2141–2148. doi:10.1200/JCO.2016.70.8297
Basho, R. K., Yam, C., Gilcrease, M., Murthy, R. K., Helgason, T., Karp, D. D., et al. (2018). Comparative effectiveness of an mTOR-based systemic therapy regimen in advanced, metaplastic and nonmetaplastic triple-negative breast cancer. Oncologist 23 (11), 1300–1309. doi:10.1634/theoncologist.2017-0498
Bendell, J., Saleh, M., Rose, A. A. N., Siegel, P. M., Hart, L., Sirpal, S., et al. (2014). Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer. J. Clin. Oncol. 32 (32), 3619–3625. doi:10.1200/JCO.2013.52.5683
Bianchini, G., De Angelis, C., Licata, L., and Gianni, L. (2022). Treatment landscape of triple-negative breast cancer-expanded options, evolving needs. Nat. Rev. Clin. Oncol. 19, 91–113. doi:10.1038/s41571-021-00565-2
Bonnefoi, H., Grellety, T., Tredan, O., Saghatchian, M., Dalenc, F., Mailliez, A., et al. (2016). A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1). Ann. Oncol. 27 (5), 812–818. doi:10.1093/annonc/mdw067
Borri, F., and Granaglia, A. (2021). Pathology of triple negative breast cancer. Semin. Cancer Biol. 72, 136–145. doi:10.1016/j.semcancer.2020.06.005
Burstein, M. D., Tsimelzon, A., Poage, G. M., Covington, K. R., Contreras, A., Fuqua, S. A. W., et al. (2015). Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 21 (7), 1688–1698.
Carey, L. A., Rugo, H. S., Marcom, P. K., Mayer, E. L., Esteva, F. J., Ma, C. X., et al. (2012). Tbcrc 001: Randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J. Clin. Oncol. 30 (21), 2615–2623. doi:10.1200/JCO.2010.34.5579
Chopra, N., Tovey, H., Pearson, A., Cutts, R., Toms, C., Proszek, P., et al. (2020). Homologous recombination DNA repair deficiency and PARP inhibition activity in primary triple negative breast cancer. Nat. Commun. 11 (1), 2662. doi:10.1038/s41467-020-16142-7
Cortés, J., Kim, S-B., Chung, W-P., Im, S-A., Park, Y. H., Hegg, R., et al. (2022). Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 386 (12), 1143–1154. doi:10.1056/NEJMoa2115022
Dalton, J. T., Barnette, K. G., Bohl, C. E., Hancock, M. L., Rodriguez, D., Dodson, S. T., et al. (2011). The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: Results of a double-blind, placebo-controlled phase II trial. J. Cachexia Sarcopenia Muscle 2 (3), 153–161. doi:10.1007/s13539-011-0034-6
De Felice, F., Giudice, E., Bolomini, G., Distefano, M. G., Scambia, G., Fagotti, A., et al. (2021). Pembrolizumab for advanced cervical cancer: Safety and efficacy. Expert Rev. Anticancer Ther. 21, 1–8. doi:10.1080/14737140.2021.1850279
Dent, R., Oliveira, M., Isakoff, S. J., Im, S-A., Espié, M., Blau, S., et al. (2021). Final results of the double-blind placebo-controlled randomized phase 2 LOTUS trial of first-line ipatasertib plus paclitaxel for inoperable locally advanced/metastatic triple-negative breast cancer. Breast Cancer Res. Treat. 189, 377–386. doi:10.1007/s10549-021-06143-5
Dieci, M. V., Del Mastro, L., Cinquini, M., Montemurro, F., Biganzoli, L., Cortesi, L., et al. (2019). Inclusion of platinum agents in neoadjuvant chemotherapy regimens for triple-negative breast cancer patients: Development of GRADE (grades of recommendation, assessment, development and evaluation) recommendation by the Italian association of medical oncology (AIOM). Cancers (Basel) 11 (8), 1137. doi:10.3390/cancers11081137
Dieci, M. V., Mathieu, M. C., Guarneri, V., Conte, P., Delaloge, S., Andre, F., et al. (2015). Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann. Oncol. 26 (8), 1698–1704. doi:10.1093/annonc/mdv239
Du, F., Wang, W., Wang, Y., Li, M., Zhu, A., Wang, J., et al. (2020). Carboplatin plus taxanes are non-inferior to epirubicin plus cyclophosphamide followed by taxanes as adjuvant chemotherapy for early triple-negative breast cancer. Breast Cancer Res. Treat. 182 (1), 67–77. doi:10.1007/s10549-020-05648-9
Emens, L. A., Cruz, C., Eder, J. P., Braiteh, F., Chung, C., Tolaney, S. M., et al. (2019). Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: A phase 1 study. JAMA Oncol. 5 (1), 74–82. doi:10.1001/jamaoncol.2018.4224
Fasching, P. A., Link, T., Hauke, J., Seither, F., Jackisch, C., Klare, P., et al. (2021). Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study). Ann. Oncol. 32 (1), 49–57. doi:10.1016/j.annonc.2020.10.471
Fasching, P. A., Loibl, S., Hu, C., Hart, S. N., Shimelis, H., Moore, R., et al. (2018). BRCA1/2 mutations and bevacizumab in the neoadjuvant treatment of breast cancer: Response and prognosis results in patients with triple-negative breast cancer from the GeparQuinto study. J. Clin. Oncol. 36 (22), 2281–2287. doi:10.1200/JCO.2017.77.2285
Finn, R. S., Qin, S., Ikeda, M., Galle, P. R., Ducreux, M., Kim, T-Y., et al. (2020). Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905. doi:10.1056/NEJMoa1915745
Fraguas-Sánchez, A. I., Lozza, I., and Torres-Suárez, A. I. (2022). Actively targeted nanomedicines in breast cancer: From pre-clinal investigation to clinic. Cancers (Basel) 14 (5), 1198. doi:10.3390/cancers14051198
Garrido-Castro, A. C., Lin, N. U., and Polyak, K. (2019). Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discov. 9 (2), 176–198. doi:10.1158/2159-8290.CD-18-1177
Garrido-Laguna, I., Krop, I., Burris, H. A., Hamilton, E., Braiteh, F., Weise, A. M., et al. (2019). First-in-human, phase I study of PF-06647263, an anti-EFNA4 calicheamicin antibody–drug conjugate, in patients with advanced solid tumors. Int. J. Cancer 145 (7), 1798–1808. doi:10.1002/ijc.32154
Ghebeh, H., Al-Sayed, A., Eiada, R., Cabangon, L., Ajarim, D., Suleman, K., et al. (2021). Weekly Paclitaxel given concurrently with Durvalumab has a favorable safety profile in triple-negative metastatic breast cancer. Sci. Rep. 11 (1), 19154. doi:10.1038/s41598-021-98113-6
Gucalp, A., Tolaney, S., Isakoff, S. J., Ingle, J. N., Liu, M. C., Carey, L. A., et al. (2013). Phase II trial of bicalutamide in patients with androgen receptor–positive, estrogen receptor–negative metastatic breast cancer. Clin. Cancer Res. 19 (19), 5505–5512. doi:10.1158/1078-0432.CCR-12-3327
Gupta, G. K., Collier, A. L., Lee, D., Hoefer, R. A., Zheleva, V., Siewertsz van Reesema, L. L., et al. (2020). Perspectives on triple-negative breast cancer: Current treatment strategies, unmet needs, and potential targets for future therapies. Cancers (Basel) 12 (9), 2392. doi:10.3390/cancers12092392
Hastak, K., Alli, E., and Ford, J. M. (2010). Synergistic chemosensitivity of triple-negative breast cancer cell lines to poly(ADP-ribose) polymerase inhibition, gemcitabine, and cisplatin. Cancer Res. 70 (20), 7970–7980. doi:10.1158/0008-5472.CAN-09-4521
Ibrahim, Y. H., García-García, C., Serra, V., He, L., Torres-Lockhart, K., Prat, A., et al. (2012). PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2 (11), 1036–1047. doi:10.1158/2159-8290.CD-11-0348
Jia, H., Truica, C. I., Wang, B., Wang, Y., Ren, X., Harvey, H. A., et al. (2017). Immunotherapy for triple-negative breast cancer: Existing challenges and exciting prospects. Drug resist. updat. 32, 1–15. doi:10.1016/j.drup.2017.07.002
Jiang, Y-Z., Ma, D., Suo, C., Shi, J., Xue, M., Hu, X., et al. (2019). Genomic and transcriptomic landscape of triple-negative breast cancers: Subtypes and treatment strategies. Cancer Cell. 35 (3), 428–440. doi:10.1016/j.ccell.2019.02.001
Kawachi, A., Yamashita, S., Okochi-Takada, E., Hirakawa, A., Tsuda, H., Shimomura, A., et al. (2020). BRCA1 promoter methylation in breast cancer patients is associated with response to olaparib/eribulin combination therapy. Breast Cancer Res. Treat. 181 (2), 323–329. doi:10.1007/s10549-020-05647-w
Lee, J. S., Yost, S. E., and Yuan, Y. (2020). Neoadjuvant treatment for triple negative breast cancer: Recent progresses and challenges. Cancers (Basel) 12 (6), 1404. doi:10.3390/cancers12061404
Lehmann, B. D., Abramson, V. G., Sanders, M. E., Mayer, E. L., Haddad, T. C., Nanda, R., et al. (2020). TBCRC 032 IB/II multicenter study: Molecular insights to AR antagonist and PI3K inhibitor efficacy in patients with AR + metastatic triple-negative breast cancer. Clin. Cancer Res. 26 (9), 2111–2123. doi:10.1158/1078-0432.CCR-19-2170
Lehmann, B. D., Bauer, J. A., Chen, X., Sanders, M. E., Chakravarthy, A. B., Shyr, Y., et al. (2011). Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 121 (7), 2750–2767. doi:10.1172/JCI45014
Li, C-J., Tzeng, Y-D. T., Chiu, Y-H., Lin, H-Y., Hou, M-F., and Chu, P-Y. (2021). Pathogenesis and potential therapeutic targets for triple-negative breast cancer. Cancers (Basel) 13 (12), 2978. doi:10.3390/cancers13122978
Li, Q., Wang, Y., Jia, W., Deng, H., Li, G., Deng, W., et al. (2019). Low-dose anti-angiogenic therapy sensitizes breast cancer to PD-1 blockade. Clin. Cancer Res. 26 (7), 1712–1724. doi:10.1158/1078-0432.CCR-19-2179
Liu, J., Li, Y., Li, Q., Liang, D., Wang, Q., and Liu, Q. (2021). Biomarkers of response to camrelizumab combined with apatinib: An analysis from a phase II trial in advanced triple-negative breast cancer patients. Breast Cancer Res. Treat. 186, 687–697. doi:10.1007/s10549-021-06128-4
Liu, J., Liu, Q., Li, Y., Li, Q., Su, F., Yao, H., et al. (2020). Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: An open-label phase II trial. J. Immunother. Cancer 8 (1), e000696. doi:10.1136/jitc-2020-000696
Loibl, S., Untch, M., Burchardi, N., Huober, J., Sinn, B. V., Ju, Blohmer, et al. (2019). A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 30 (8), 1279–1288. doi:10.1093/annonc/mdz158
Lotfinejad, P., Asghari Jafarabadi, M., Abdoli Shadbad, M., Kazemi, T., Pashazadeh, F., Sandoghchian Shotorbani, S., et al. (2020). Prognostic role and clinical significance of tumor-infiltrating lymphocyte (TIL) and programmed death ligand 1 (PD-L1) expression in triple-negative breast cancer (TNBC): A systematic review and meta-analysis study. Diagnostics 10 (9), 704. doi:10.3390/diagnostics10090704
Marks, D. K., and Kalinsky, K. (2017). Sacituzumab govitecan. Trop-2-targeted antibody-drug conjugate, Treatment of epithelial cancers. Drugs Future 42 (2), 87. doi:10.1358/dof.2017.042.02.2560076
Mayer, E. L., Abramson, V., Jankowitz, R., Falkson, C., Marcom, P. K., Traina, T., et al. (2020). Tbcrc 030: A phase II study of preoperative cisplatin versus paclitaxel in triple-negative breast cancer: Evaluating the homologous recombination deficiency (HRD) biomarker. Ann. Oncol. 31 (11), 1518–1525. doi:10.1016/j.annonc.2020.08.2064
Mayer, I. A., Zhao, F., Arteaga, C. L., Symmans, W. F., Park, B. H., Burnette, B. L., et al. (2021). Randomized phase III postoperative trial of platinum-based chemotherapy versus capecitabine in patients with residual triple-negative breast cancer following neoadjuvant chemotherapy: ECOG-ACRIN EA1131. J. Clin. Oncol. [Internet 39 (23), 2539–2551. doi:10.1200/JCO.21.00976
Mittendorf, E. A., Zhang, H., Barrios, C. H., Saji, S., Jung, K. H., Hegg, R., et al. (2020). Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 tria. Lancet 396, 10257.
Modi, S., Jacot, W., Yamashita, T., Sohn, J., Vidal, M., Tokunaga, E., et al. (2022). Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 1–12, 9–20. doi:10.1056/NEJMoa2203690
Modi, S., Park, H., Murthy, R. K., Iwata, H., Tamura, K., Tsurutani, J., et al. (2020). Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low–expressing advanced breast cancer: Results from a phase Ib study. J. Clin. Oncol. 38 (17), 1887–1896. doi:10.1200/JCO.19.02318
Modi, S., Saura, C., Yamashita, T., Park, Y. H., Kim, S-B., Tamura, K., et al. (2020). Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 382 (7), 610–621. doi:10.1056/NEJMoa1914510
Mosele, F., Stefanovska, B., Lusque, A., Tran Dien, A., Garberis, I., Droin, N., et al. (2020). Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann. Oncol. 31 (3), 377–386. doi:10.1016/j.annonc.2019.11.006
Oliveira, M., Saura, C., Nuciforo, P., Calvo, I., Andersen, J., Passos-Coelho, J. L., et al. (2019). FAIRLANE, a double-blind placebo-controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple-negative breast cancer. Ann. Oncol. 30 (8), 1289–1297. doi:10.1093/annonc/mdz177
O’Malley, D. M., Matulonis, U. A., Birrer, M. J., Castro, C. M., Gilbert, L., Vergote, I., et al. (2020). Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 157 (2), 379–385. doi:10.1016/j.ygyno.2020.01.037
Pantelidou, C., Sonzogni, O., De Oliveria Taveira, M., Mehta, A. K., Kothari, A., Wang, D., et al. (2019). PARP inhibitor efficacy depends on CD8 + T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 9 (6), 722–737. doi:10.1158/2159-8290.CD-18-1218
Perou, C. M., Sørlie, T., Eisen, M. B., van de Rijn, M., Jeffrey, S. S., Rees, C. A., et al. (2000). Molecular portraits of human breast tumours. Nature 406 (6797), 747–752. doi:10.1038/35021093
Pilié, P. G., Gay, C. M., Byers, L. A., O’Connor, M. J., and Yap, T. A. (2019). PARP inhibitors: Extending benefit beyond BRCA -mutant cancers. Clin. Cancer Res. 25 (13), 3759–3771. doi:10.1158/1078-0432.CCR-18-0968
Prat, A., Parker, J. S., Karginova, O., Fan, C., Livasy, C., Herschkowitz, J. I., et al. (2010). Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 12 (5), R68. doi:10.1186/bcr2635
Pusztai, L., Yau, C., Wolf, D. M., Han, H. S., Du, L., Wallace, A. M., et al. (2021). Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: Results from the adaptively randomized I-SPY2 trial. Cancer Cell. 39 (7), 989–998.e5. doi:10.1016/j.ccell.2021.05.009
Reis-Filho, J. S., and Tutt, A. N. J. (2007). Triple negative tumours: A critical review. Histopathology 52 (1), 108–118. doi:10.1111/j.1365-2559.2007.02889.x
Rinnerthaler, G., Gampenrieder, S. P., Petzer, A., Burgstaller, S., Fuchs, D., Rossmann, D., et al. (2018). Ixazomib in combination with carboplatin in pretreated women with advanced triple-negative breast cancer, a phase I/II trial of the AGMT (AGMT MBC-10 trial). BMC Cancer 18 (1), 1074. doi:10.1186/s12885-018-4979-0
Schmid, P., Abraham, J., Chan, S., Wheatley, D., Brunt, A. M., Nemsadze, G., et al. (2020). Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: The PAKT trial. J. Clin. Oncol. 38 (5), 423–433.
Schmid, P., Rugo, H. S., Adams, S., Schneeweiss, A., Barrios, C. H., Iwata, H., et al. (2020). Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Oncol. 21 (1), 44–59. doi:10.1016/S1470-2045(19)30689-8
Sirohi, B., Arnedos, M., Popat, S., Ashley, S., Nerurkar, A., Walsh, G., et al. (2008). Platinum-based chemotherapy in triple-negative breast cancer. Ann. Oncol. 19 (11), 1847–1852. doi:10.1093/annonc/mdn395
Slade, D. (2020). PARP and PARG inhibitors in cancer treatment. Genes. Dev. 34, 360–394. doi:10.1101/gad.334516.119
Sporikova, Z., Koudelakova, V., Trojanec, R., and Hajduch, M. (2018). Genetic markers in triple-negative breast cancer. Clin. Breast Cancer 18 (5), e841–e850. doi:10.1016/j.clbc.2018.07.023
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Sweeney, C., Bracarda, S., Sternberg, C. N., Chi, K. N., Olmos, D., Sandhu, S., et al. (2021). Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): A multicentre, randomised, double-blind, phase 3 trial. Lancet 398, 131–142. doi:10.1016/S0140-6736(21)00580-8
Tamura, K., Tsurutani, J., Takahashi, S., Iwata, H., Krop, I. E., Redfern, C., et al. (2019). Trastuzumab deruxtecan (DS-8201a)in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: A dose-expansion, phase 1 study. Lancet. Oncol. 20 (6), 816–826. doi:10.1016/S1470-2045(19)30097-X
Traina, T. A., Miller, K., Yardley, D. A., Eakle, J., Schwartzberg, L. S., O’Shaughnessy, J., et al. (2018). Enzalutamide for the treatment of androgen receptor–expressing triple-negative breast cancer. J. Clin. Oncol. 36 (9), 884–890. doi:10.1200/JCO.2016.71.3495
Tutt, A., Tovey, H., Cheang, M. C. U., Kernaghan, S., Kilburn, L., Gazinska, P., et al. (2018). Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT trial. Nat. Med. 24 (5), 628–637. doi:10.1038/s41591-018-0009-7
Voorwerk, L., Slagter, M., Horlings, H. M., Sikorska, K., van de Vijver, K. K., de Maaker, M., et al. (2019). Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 25 (6), 920–928. doi:10.1038/s41591-019-0432-4
Walsh, E. M., Shalaby, A., O’Loughlin, M., Keane, N., Webber, M. J., Kerin, M. J., et al. (2019). Outcome for triple negative breast cancer in a retrospective cohort with an emphasis on response to platinum-based neoadjuvant therapy. Breast Cancer Res. Treat. 174 (1), 1–13. doi:10.1007/s10549-018-5066-6
Walsh, R. J., Ngoi, N., Ong, R. J. M., Ow, S. G. W., Wong, A., Eng, L. S., et al. (2020). Molecular profiling of metastatic breast cancer (MBC) and target-based therapeutic matching in an Asian tertiary phase I oncology unit. J. Clin. Oncol. [Internet] 38, 3561. doi:10.1200/JCO.2020.38.15_suppl.3561
Wang, H., and Mao, X. (2020). Evaluation of the efficacy of neoadjuvant chemotherapy for breast cancer. Drug Des. Devel Ther. 4, 2423–2433.
Wang, N., Li, K., Huang, W., Kong, W., Liu, X., Shi, W., et al. (2020). Efficacy of platinum in advanced triple-negative breast cancer with germline BRCA mutation determined by next generation sequencing. Chin. J. Cancer Res. [Internet] 32 (2), 149–162.
Weigelt, B., and Reis-Filho, J. S. (2009). Histological and molecular types of breast cancer: Is there a unifying taxonomy? Nat. Rev. Clin. Oncol. 6 (12), 718–730. doi:10.1038/nrclinonc.2009.166
Xu, M., Yuan, Y., Yan, P., Jiang, J., Ma, P., Niu, X., et al. (2020). Prognostic significance of androgen receptor expression in triple negative breast cancer: A systematic review and meta-analysis. Clin. Breast Cancer 20 (4), e385–e396. doi:10.1016/j.clbc.2020.01.002
Yam, C., Rauch, G. M., Rahman, T., Karuturi, M., Ravenberg, E., White, J., et al. (2021). A phase II study of Mirvetuximab Soravtansine in triple-negative breast cancer. Invest. New Drugs 39 (2), 509–515. doi:10.1007/s10637-020-00995-2
Yardley, D. A., Weaver, R., Melisko, M. E., Saleh, M. N., Arena, F. P., Forero, A., et al. (2015). Emerge: A randomized phase II study of the antibody-drug conjugate glembatumumab vedotin in advanced glycoprotein nmb - expressing breast cancer. J. Clin. Oncol. 33 (14), 1609–1619. doi:10.1200/JCO.2014.56.2959
Yu, K-D., Ye, F-G., He, M., Fan, L., Ma, D., Mo, M., et al. (2020). Effect of adjuvant paclitaxel and carboplatin on survival in women with triple-negative breast cancer: A phase 3 randomized clinical trial. JAMA Oncol. 6 (9), 1390–1396. doi:10.1001/jamaoncol.2020.2965
Yuan, Y., Lee, J. S., Yost, S. E., Frankel, P. H., Ruel, C., Egelston, C. A., et al. (2021). A phase II clinical trial of pembrolizumab and enobosarm in patients with androgen receptor-positive metastatic triple-negative breast cancer. Oncologist 26 (2), 99–e217. doi:10.1002/onco.13583
Zhou, J., Kang, Y., Chen, L., Wang, H., Liu, J., Zeng, S., et al. (2020). The drug-resistance mechanisms of five platinum-based antitumor agents. Front. Pharmacol. 11, 343. doi:10.3389/fphar.2020.00343
ADCs antibody-drug conjugates
AR androgen receptor
LAR luminal androgen receptor luminal androgen receptor
BLIA basal-like immune-activated
BLIS basal-like immunosuppressed
BL basal-like subtypes
BC Breast cancer
CNVs copy number variants
ctDNA circulating tumor DNA
CL claudin-low
EGFR epidermal growth factor receptor
EMT epithelial-to-mesenchymal transition
ER estrogen receptor
CDX-011, GV glembatumumab vedotin
HR hormone receptor
HRD homologous recombination defect
HER2 human epidermal growth factor receptor 2
ICIs immune checkpoint inhibitors
IRGs immune-related gene
IM immunomodulatory subtypes
ITT intention to treat
iDFS invasive disease-free survival
LAR luminal androgen receptor luminal androgen receptor
MES mesenchymal
MMAE monomethyl auristatin E
MSL mesenchymal stem-like subtypes
MpBC metaplastic breast cancers
mTNBC metastatic triple-negative breast cancer
ORR objective response rate
OPN osteopontin
OS overall survival
PCb paclitaxel plus carboplatin
pCR pathological complete response
PI3K phosphoinositide-3 kinase
PBC platinum-based chemotherapy
PARP poly ADP-ribose polymerase
PR progesterone receptor
PD-L1 programmed cell death ligand 1
PFS progression-free survival
RFS recurrence-free survival
SARM selective androgen receptor modulator
T-DXD Trastuzumab deruxtecan
TNBC triple-negative breast cancer
TILs tumor-infiltrating lymphocytes
TK tyrosine kinase
Keywords: triple-negative breast cancer, metastasis, biomarkers, targeted therapy, immune therapy
Citation: Zhang J, Xia Y, Zhou X, Yu H, Tan Y, Du Y, Zhang Q and Wu Y (2022) Current landscape of personalized clinical treatments for triple-negative breast cancer. Front. Pharmacol. 13:977660. doi: 10.3389/fphar.2022.977660
Received: 24 June 2022; Accepted: 05 September 2022;
Published: 16 September 2022.
Edited by:
Lei Wang, Queen’s University Belfast, United KingdomReviewed by:
Eva Valentina Klocker, University Hospital Graz, AustriaCopyright © 2022 Zhang, Xia, Zhou, Yu, Tan, Du, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaying Du, eWF5aW5nZHVAaHVzdC5lZHUuY24=; Qi Zhang, emhhbmdxaTA2MTcyQDE2My5jb20=; Yiping Wu, dG9uZ2ppcGxhc3RpY0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.