- 1Department of Traditional Chinese Medicine, Nanfang Hospital of Southern Medical University, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Medical Biomechanics and Guangdong Engineering Research Center for Translation of Medical 3D Printing Application and National Key Discipline of Human Anatomy, School of Basic Medical Sciences, Southern Medical University, Guangzhou, China
Background: Fibrosis-related diseases (FRD) include cerebral fibrosis, pulmonary fibrosis, cardiac fibrosis, liver fibrosis, renal fibrosis, peritoneal fibrosis, etc. The effects of fibrosis can be severe, resulting in organ dysfunction, functional decline, and even organ failure, which can cause serious health problems.
Aim: Currently, there is no effective modern medicine for anti-fibrosis in the clinics; however, Chinese medicine has a certain beneficial effect on treating such diseases. Astragalus Mongholicus (AM) has rich medicinal value, and its anti-fibrosis effect has been recently investigated. In recent years, more and more experimental studies have been conducted on the intervention of astragaloside IV (AS-IV), astragalus polysaccharide (APS), astragalus flavone, cycloastragalus alcohol, astragalus water extract and other pharmacological components in fibrosis-related diseases, attracting the interest of researchers. We aim to provide ideas for future research by summarizing recent research advances of AM in treating fibrosis-related diseases.
Methods: A literature search was conducted from the core collections of electronic databases such as Baidu Literature, Sciencen.com, Google Scholar, PubMed, and Science Direct using the above keywords and the pharmacological and phytochemical details of the plant.
Results: AM can be used to intervene in fibrosis-disease progression by regulating inflammation, oxidative stress, the immune system, and metabolism.
Conclusion: AS-IV, APS, and astragalus flavone were studied and discussed in detail. These components have high potential anti-fibrosis activity. Overall, this review aims to gain insight into the AM’s role in treating fibro-related diseases.
Introduction
Fibrosis is a traumatic healing reaction after acute or chronic injury. It can occur in different tissues and organs, such as the brain, lungs, heart, liver, kidneys, intestine, peritoneum, or skin. Fibrosis can develop into severe scarring, and cause abnormalities, weakening of function, and even the failure of organs, which seriously affect human health and quality of life. Studies have indicated that fibrosis-related diseases (FRD) are closely related to inflammation, oxidative stress, fibroblast proliferation, and excessive deposition of the extracellular matrix (ECM) (Wang et al., 2018a).
The researchers turned to traditional Chinese medicine (TCM) because modern medicine has been unable to treat fibrosis effectively. Literature studies have found that most of the drugs used in TCM treatment of FRD are single drugs, monomer components, TCM injections or TCM compounds with the effects of supplementing qi and generating blood, promoting blood circulation and removing blood stasis, while A. mongholicus (AM) and its pharmacodynamic components are the focus of relevant research. Astragalus Mongholicus is a perennial herb in the genus A. of the leguminous family. The dried root of A. mongholicus Bunge or/and Astragalus Amembranaceus (Fisch.) Bge. was used as medicine (Shuangcheng et al., 2012). AM is a widely used TCM formulation.
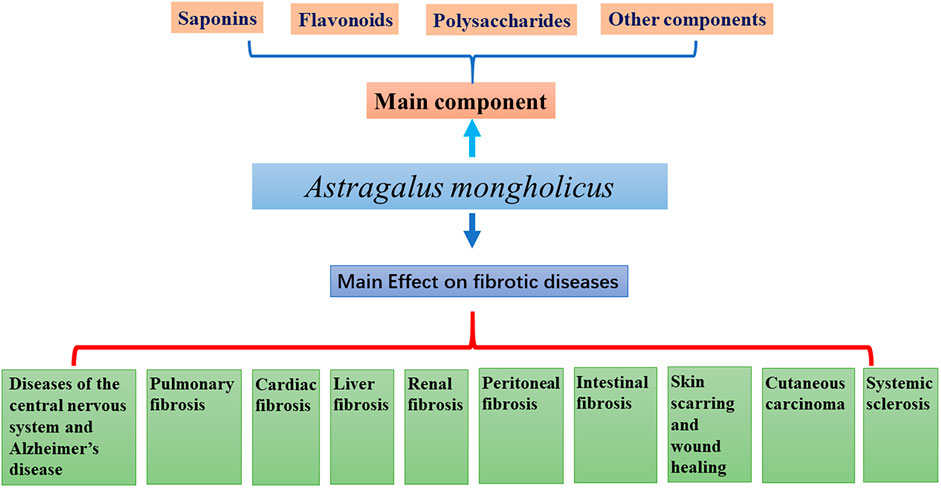
AM contains saponins, flavonoids, polysaccharides, and trace elements beneficial to the human body. Combined with modern pharmacological studies, it has been found that AM has anti-tumor properties (Gu et al., 2022) and also, it improves immune function (Liu et al., 2021), protects cardio-cerebrovascular (Li et al., 2022), lung (Qian et al., 2018), kidney (Zhou et al., 2020), liver function (Wang et al., 2022). Besides, it shows an ability to improve intestinal fucntion (Tian et al., 2021), peritoneal function (Li et al., 2014b), and anti-aging ability (Gong et al., 2021). It has also been used for the prevention and treatment of osteoporosis (Kaczmarczyk-Sedlak et al., 2013), antioxidant stress protection (Sheng et al., 2021) and anti-radiation (Wen et al., 2018). This review summarizes the relevant experimental studies on the anti-fibrosis effect of AM (Figure 1).
Main components of a. mongholicus
About 2,000–3,000 species of Astragalus are in the legume family (Li et al., 2014a; Podlech, 2008), distributed in the Northern Hemisphere, South America, and Africa. There are ∼278 species of Astragalus in China, which are distributed mainly in Tibet (Himalayas), Central Asia, and northeast Asia. AM has important medicinal value; as it is a TCM material used for replenishing qi (Shuangcheng et al., 2012).
More than 200 components have been isolated from Astragalus species. The main active components are saponins, flavonoids, and polysaccharides (Ibrahim et al., 2013), but they also contain anthraquinones, alkaloids, amino acids, β-sitosterol, and metal elements (Tan et al., 2020). About 40% of constituent studies have focused on the aboveground parts of AM. Pharmacologic studies have shown that the crude extract of AM and its isolated components have different biological activities: anti-inflammatory, antioxidant, immune stimulation, anti-cancer, anti-diabetes mellitus (DM), heart protection, liver protection, and anti-fibrosis (Wang et al., 2019; Zhao et al., 2012; Zhou et al., 2016).
Saponins
Saponins are the main chemical constituents of AM. Astragaloside components mainly include Astragaloside I-VIII, Isoastragaloside I, II, and IV, Acetylastragaloside, Cycloastragaloside E, F and G, Agroastragaloside I-IV, and soybean saponin I. Astragaloside is the main active component of saponins, and Astragaloside IV (AS-Ⅳ) is often used as a qualitative and quantitative indices of AM (Ren et al., 2013). AS-Ⅳ shows various activities, such as regulation of calcium balance (Lu et al., 2014; Xu et al., 2008a) as well as an antioxidant (Hu et al., 2009), anti-apoptotic (Liu et al., 2013; Sun et al., 2016), and anti-fibrosis (Hu et al., 2009; Yu et al., 2016) effects.
Flavonoids
Flavonoid derivatives are one of the main chemical components of AM. Flavonoids include flavonol, flavone, isoflavane, chalcone, flavanol, and thiaxanthin (Berry et al., 1992), and they have antibacterial, antioxidant, and cytotoxic activities, as well as promoting glucose consumption and inhibiting α-glucosidase. The flavonoids of AM have been shown to have an inhibitory effect on tissue fibrosis (Liu et al., 2005; Sun et al., 2007). Phytoestrogen called calycosterone imparts estrogen-like effects by attaching to estrogen receptors and binding to them. Besides being an antioxidant, it has also been reported to have anti-osteoporosis, anti-tumor, and immunomodulatory properties (Song et al., 2017).
Polysaccharides
There is a type of water-soluble polysaccharide called Astragalus polysaccharide (APS), obtained from dried roots or stems of AM. Studies have shown that the monosaccharide components of APS include arabinose, fructose, glucose, and mannose. Xu et al. (Xu et al., 2008b) isolated and purified APS-I and APS-II from the water extract of AM. Kiyohara et al. (Kiyohara et al., 2010) isolated 13 polysaccharides from A mongolicus, all showing immunomodulatory activity.
Other components
AM also contains amino acids, microelements (Li et al., 2014a), sterols, chlorogenic acid (Guzhva, 2010), emodin, and vitamins (Gong et al., 2018a).
Effect of a. mongholicus on fibrotic diseases
Effect of a. mongholicus on diseases of the central nervous system and Alzheimer’s disease
AD is the most common form of senile dementia. The main pathologic features of AD are extensive extracellular amyloid plaques and neurofibrillary tangles (Ramezani et al., 2016; Sperling et al., 2014). Misfolding and aggregation of proteins are linked directly to several neurodegenerative diseases, such as AD, Parkinson’s disease, and Huntington’s disease (Haiyan et al., 2016; Soto and Pritzkow, 2018; Wang et al., 2017). Different types of proteins and peptides misfold and accumulate in the form of filamentous structures that further induce the formation of amyloid fibers. The latter can be found in the extracellular environment around the brain’s arteries and within neurons (Owen et al., 2019; Tahaei Gilan et al., 2019). They cause inflammation and oxidative-stress responses, resulting in decreased neuronal function in the cerebral cortex and hippocampus (Nam et al., 2018).
Song et al. (Song et al., 2017) found that the effects of calycosin on spatial learning and memory were significant and dose-dependent in APP/PS1 transgenic mice (Alzheimer’s disease model). Also, activation of the protein kinase C pathway normalized hippocampal levels of β-amyloid, tau inflammation, oxidative stress, and that neuroprotection depend on it. Wang and Zhao (Wang and Zhao, 2016) reported that Calycosin had a beneficial effect on the improvement, prevention, and treatment of DM-related cognitive deficits by affecting oxidative stress, synaptic function, and the phosphatidylinositol 3-hydroxy kinase/protein kinase B/glycogen synthase kinase-3β (PI3K/AKT/GSK-3β) pathway, and contributed to the improvement of the pathologic process of AD.
Effect of the a. mongholicus on subarachnoid hemorrhage
The mortality associated with SAH is up to 40%–60%, and SAH is the most dangerous subtype of stroke subtypes (Dong et al., 2018). Chronic hydrocephalus after SAH is closely related to subarachnoid fibrosis. Transforming growth factor β1 (TGF-β1) can promote subarachnoid fibrosis and chronic hydrocephalus by activating the TGF-β1-mothers against decapentaplegic (Smad)-connective tissue growth factor axis to produce different endogenous factors and the ECM (Kuo and Huang, 2021; Yan et al., 2016). SAH can lead to increased malondialdehyde, neuronal apoptosis, caspase-3 rupture, cerebral edema, and decreased superoxide dismutase and glutathione peroxidase activities. AS-IV has been shown to reverse those changes and improve neurobehavioral outcomes in rats with SAH. Those results suggest that AS-IV may alleviate early brain injury after SAH through antioxidant and anti-apoptotic effects (Shao et al., 2014). AS-IV can downregulate the expression of apoptosis-related proteins (forkhead box O1 (Foxo1), Bim, Bax) and inhibit cleavage of caspase-3 through the PI3K/AKT signaling pathway, and alleviate the brain injury caused by SAH (Yang et al., 2020).
Effect of a.s mongholicus on ischemic stroke
In ischemic stroke, the blood supply is blocked due to the narrowing or blockage of lumina, which results in hypoxia, ischemic necrosis, and loss of nerve function (Lai et al., 2019). Neuroinflammation is involved in almost every step of ischemic brain repair, including neurogenesis (Sun et al., 2020b).
AM, through its antioxidant, anti-inflammatory, and anti-apoptotic properties, has beneficial effects on cognitive impairment after stroke (Xue et al., 2019; L. Zhang et al., 2019b. Zhang et al., 2019a). AS-IV has been found to enhance hippocampal neurogenesis in adults (Huang et al., 2018; Yang et al., 2017) and promote the proliferation of neural stem cells in the brain with transient ischemia (Chen et al., 2019a). Therefore, AS-IV may be a promising strategy for the treatment of ischemic stroke because it promotes neurogenesis (Sun et al., 2020a).
In mice who had suffered a stroke, AS-IV helped inhibit neuronal apoptosis, promote neurogenesis and alleviate cognitive deficits. In vitro and in vivo, AS-IV can downregulate protein expression of interleukin (IL)-17, antagonize neurogenesis by regulating the AKT/GSK-3β pathway, and significantly regulate apoptosis (Sun et al., 2020b). Calycosin-7-O-β-D-glucoside (CG) was shown to significantly reduce the volume of infarct, the extent of histopathology damage, and the permeability of the blood-brain barrier in a rat model of occluded middle cerebral arteries. CG treatment significantly inhibited the expression and activity of matrix metalloproteinases (MMPs) in the cortical microvessels of ischemic rats and ensured the expression of alveolar protein-1 and tight-junction proteins. CG can also clear nitric oxide (NO), inhibit the activity of MMP-2 and MMP-9, and reduce the death of cultured microvascular endothelial cells of the brain under oxygen-glucose deprivation (OGD) conditions (Fu et al., 2014).
AS-Ⅳ stimulates hippocampal neurogenesis after stroke and downregulates IL-17 expression through the wingless-type (Wnt) pathway, which can promote remodeling and repair of the brain (Sun et al., 2020a). It has been reported that compensatory angiogenesis can occur in the ischemic area after ischemic injury, which somewhat promotes the repair of nerve function in the ischemic area. Studies have shown that AS-IV has a brain-protective effect and is important in promoting angiogenesis. AS-IV has been shown to significantly reduce infarct size, promote cell proliferation and duct formation, and inhibit expression of the ephrinA3 target gene by increasing miRNA-210 expression and inducing activation of hypoxic inducible factor/vascular endothelial growth factor Notch (HIF/VEGF/Notch) signaling pathway (Liang et al., 2020).
AS-IV has a potential neuroprotective in experimental models of Parkinson’s disease, AD, and cerebral ischemia by reducing inflammation and oxidative stress through the antioxidant system (Costa et al., 2019). AS-IV and Calycosin isoflavones may have more important roles in treating cerebral fibrosis.
Effect of a. mongholicus on pulmonary fibrosis
Pulmonary fibrosis is a proliferative disease mainly involving the lung’s interstitium, alveoli, and bronchioles. Gradual loss of alveolar-capillary functional units eventually developed into diffuse pulmonary fibrosis and a “honeycomb” lung. Several types of interstitial lung disease can lead to extensive fibrosis and respiratory failure. A significant proportion of the fibroblasts in pulmonary fibrosis are epithelial cells (Degryse et al., 2011; Tanjore et al., 2009). It has been shown that when epithelial-mesenchymal transformation (EMT) occurs in the lung, epithelial cells show lower levels of E-cadherin while mesenchymal cells have a higher level of a-smooth muscle actin (a-SMA) (Sakuma, 2017). In the development of pulmonary fibrosis, the EMT plays a significant role. The cytokine TGF-β is an important mediator of fibrogenesis. TGF-β1 induces fibroblasts to undergo a phenotypic transition to myofibroblasts, which are the effectors of the fibrotic state (Hinz et al., 2007; Lekkerkerker et al., 2012). Among the potential pro-fibrotic factors known, TGF-α1 is an important one that induces EMT in pulmonary fibrosis (Willis et al., 2005). Therefore, a novel approach employed to treat pulmonary fibrosis could include using a novel anti-EMT pathway or a method that inhibits TGF-β1 signaling to offer a potential target.
Effect of a. mongholicus on bronchial pulmonary hypoplasia
BPD is a group of heterogeneous pulmonary diseases beginning in the neonatal period. Premature infants suffer from chronic lung injury most commonly caused by barotrauma, volvulus, and oxygen deprivation (Zhang et al., 2022). BPD is characterized by stagnation of lung growth, fewer alveoli, and vascular malformation (Wang and Tsao, 2020). Wang et al. (Wang and Huang, 2014) showed that APS upregulated expression of epidermal growth factor-like domain 7 (EGFL7) and B-cell lymphoma 2, downregulated Bax expression, significantly reduced alveolar injury, and had a protective effect on the lung tissues of BPD patients, which was closely related to inhibition of apoptosis of endothelial cells. Studies conducted on rats with hyperoxia-induced brain damage found that APS has both antioxidant and anti-inflammatory effects.
Effect of a. mongholicus on idiopathic pulmonary fibrosis
Idiopathic pulmonary fibrosis is the most common type of pulmonary fibrosis. It is characterized by excessive proliferation of fibroblasts/myofibroblasts, excessive deposition of the ECM, alveolar re-epithelialization, and abnormal repair and remodeling of blood vessels (Li et al., 2017). TGF-β1 mediates fibroblast differentiation and plays an important part in pulmonary fibrosis by activating monocytes and fibroblasts through recruitment and increasing ECM production (Wolters et al., 2014). Qian et al. (Qian et al., 2018) found, in vivo and in vitro, that AS-iV inhibited the FOXO3a hyperphosphorylation induced by TGF-β1/PI3K/AKT, which reversed EMT in the fibrosis process.
Effect of a. mongholicus on cardiac fibrosis
Cardiac fibrosis leads to cardiac dysfunction and arrhythmia in which normal cardiac fibroblasts and circulating fibroblasts proliferate and activate myofibroblasts (Du et al., 2021; Ghosh et al., 2012). Studies have shown that AS-IV has a protective effect on the cardiovascular system. AS-IV can eliminate hypoxia-induced changes, including increased proliferation, decreased apoptosis, and cell death (Lu et al., 2017). Zhang et al. (Zhang et al., 2012) reported that AS-IVcould alleviate hypoxia/reoxygenation-induced cardiomyocyte injury in neonatal rats and that AS-IV may inhibit expression of the long non-coding-RNA growth arrest-specific 5 (GAS5) by activating the PI3K/mammalian target of rapamycin (mTOR) pathway and protect hypoxia-stimulated H9C2 cells (Du et al., 2019). AS-IV may protect cardiomyocytes from hypoxia-induced injury by downregulating the expression of miRNA-23a and miRNA-92a and activating the PI3K/AKT and mitogen-activated protein kinase/extracellular regulated protein kinase (MAPK/ERK) signaling pathways (Gong et al., 2018b). Li et al. (Liang et al., 2017) demonstrated that AS-IV could promote the proliferation of mouse cardiomyocytes and act as cardiac-regenerative cells.
The attenuating effect of AS-IV on myocardial hypertrophy and fibrosis in rats is also closely related to pro-inflammatory signaling pathways. Studies have shown that AS-IV promotes the expression of the suppressor of IKKε (SIKE, an important negative regulator of myocardial hypertrophy) by inhibiting the TANK-binding kinase 1 (TBK1)/PI3K pathway, thereby ablating inflammation and apoptosis (Liu et al., 2018). Wan et al. (2018) found that AS-IV and its active saponin cycloastragalus alcohol could prevent myocardial fibrosis in mice by inhibiting the NLR family pyrin domain containing-3 inflammasome (Wan et al., 2018). AS-Ⅳ protects isoproterenol-induced cardiac hypertrophy by inhibiting the toll-like receptor 4/nuclear factor-kappa B (TLR4/NF-κB) signaling pathway and reducing inflammation (Yang et al., 2013). AS-Ⅳ can prevent (at least in part) isoproterenol-induced hypertrophy and alleviate energy-metabolism disorders through the NF-κB/Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α (PGC-1α) pathway (Zhang et al., 2015b). AS-IV inhibits (at least in part) cardiac hypertrophy by activating the nuclear factor-erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway (Nie et al., 2019). In one study, Liu and colleagues demonstrated that AS-IV might work as a potential therapeutic for cardiac hypertrophy by inhibiting apoptosis and inflammation and enhancing the action of IKK inhibitors by inhibiting TBK1/PI3K/AKT active pathways (Liu et al., 2018).
The preventive effect of AS-Ⅳ on fibrosis may be related to the TGF-β1-Smad signaling pathway (Chen et al., 2011). Downregulation of the expression of TGF-β1 and its downstream phosphorylated (p)Smad2/3 and p-Smad4 is carried out by AS-Ⅳ, which ultimately decreases the level of type-I collagen (Chen et al., 2021; Wei et al., 2020). In addition, In vivo and in vitro, AS-IV protects against hypoxia-induced cardiac fibrosis by inhibiting transient receptor potential cationic channel M subfamily member 7 expression (Lu et al., 2017; Wei et al., 2020).
The mechanism by which AS-Ⅳ inhibits isoproterenol-induced myocardial fibrosis may be related to reactive oxygen species (ROS)-mediated responses. Studies have shown that AS-IV can reduce myocardial ROS content and inhibit cardiotrophin-1 expression, ROS-mediated MAPK activation, myocardial-fibroblast proliferation, and collagen production, thereby inhibiting myocardial fibrosis (Dai et al., 2017; Jia et al., 2017; Xu et al., 2016).
In the development of dilated cardiomyopathy, toxic myocardial lipids arising from abnormal lipid metabolism play a significant role. (e.g., myocardial inflammation and fibrosis) which, ultimately, leads to cardiac dysfunction and myocardial remodeling (Kim et al., 2018; Kocabaş et al., 2019). AS-Ⅳ has been shown to improve systolic and diastolic function and histopathologic changes in rats suffering from type-2 diabetes mellitus (T2DM). In addition, Myocardial fibrosis in T2DM rats was inhibited significantly by AS-IV, providing evidence that AS-IV may improve myocardial lipid metabolism in the context of protection from myocardial injury (Wang et al., 2020).
The studies mentioned above suggest that AS-Ⅳ may be an effective therapeutic strategy for preventing myocardial fibrosis.
Effect of a. mongholicus on liver fibrosis
Excessive ECM protein deposition is a hallmark of hepatic fibrosis, which can develop into liver cirrhosis and hepatocellular carcinoma (Friedman, 2008). Most chronic liver diseases are characterized by fibrosis, which shows different developmental patterns depending on the cause of liver damage. As liver fibrosis progresses to cirrhosis, it often leads to the end-stage disease of the organ, so it is vital to limit the progression of chronic liver disease to cirrhosis. Hepatic stellate cell (HSC) activation plays an important part in liver fibrosis (Chen et al., 2019b), and can increase ECM generation by activating the TGF-β1/Smad signaling pathway in HSCs (Yu et al., 2018). Flavonoids have an inhibitory effect on liver fibrosis (An et al., 2021; Cheng et al., 2017; Tan et al., 2006). Network pharmacological analyses have suggested that the mechanism of action of flavonoids against liver fibrosis may be related to inhibition of the NF-κB pathway by inhibition of IKKβ expression (An et al., 2021).
Using porcine serum-induced rats, Liu et al. (Liu et al., 2009) found AS-IV inhibited collagen synthesis in activated HSCs by inhibiting the P38 MAPK pathway mediated by oxidative stress. (Li et al., 2013). Studies have shown that flavonoids can improve dimethylnitrosamine-induced fibrosis through antioxidant activity. The anti-fibrotic activity of flavonoids in chronic liver injury may be due to decreased synthesis of lipid peroxidation (LPO) and collagen and increased collagen degradation (Liu et al., 2005).
An AM extract inhibits fibrosis and adhesion in rats with chronic liver disease through antioxidant activity (Liu et al., 2005). Dang et al. (Dang et al., 2008) found that APS could reduce hepatomegaly and liver fibrosis by decreasing serum levels of alanine aminotransferase and total bilirubin and increasing serum albumin levels. The alleviating effect of AM on chronic liver injury may be related to its ability to enhance antioxidant enzymes. Cheng et al. (Cheng et al., 2017) found that total flavonoids could reduce collagen deposition and regulate the interaction between the peroxisome proliferation-activated receptor-γ signaling pathway and farnesoid X receptors to achieve an anti-fibrosis effect. AS-Ⅳ had an anti-fibrosis effect on porcine-serum-induced rats, possibly inhibiting collagen synthesis and proliferation of hematopoietic stem cells (Liu et al., 2009).
Compound Astragalus and Salvia miltiorrhiza extract (CASE) inhibits diethylnitrosamine-induced hepatocellular carcinoma by reducing the expression of pre-tumor markers (gamma-glutamyl transferase and glutathione S-transferase placental type (GST-P) and reducing fibrosis severity. CASE may ameliorate liver fibrosis by reducing plasminogen activator inhibitor-1 (PAI-1) mRNA transcription in hepatocellular carcinoma (Rui et al., 2014). Paeonia lactiflora and A. Mongholicus extract (PAE) has an anti-fibrosis effect on PS-induced rats, and the mechanism of action may be related to PAE scavenging free radicals, reducing platelet-derived growth factor receptor beta (PDGFR-β) expression, as well as inhibiting HSC proliferation and MAPK activation (Sun et al., 2012).
Effect of a. mongholicus on renal fibrosis
Renal fibrosis is common pathogenesis of chronic kidney disease that ultimately leads to end-stage renal failure. Ureteral obstruction and DM can induce interstitial infiltration of inflammatory cells into the kidney, apoptosis of renal tubular epithelial cells, myofibroblast accumulation, promote the production of pro-fibrotic factors, increase ECM production, and reduce ECM degradation. Those actions lead to the development of renal interstitial fibrosis and damage to renal function (Che et al., 2015; Tan et al., 2006).
Effect of a. mongholicus on renal interstitial fibrosis
An important factor contributing to renal interstitial fibrosis is the deposition of excessive amounts of ECM through the infiltration of interstitial inflammatory cells, the release of pro-inflammatory mediators, and the activation and proliferation of interstitial cells. (Zhou et al., 2017). After the renal injury, the number of interstitial lymphocytes, macrophages, and interstitial collagen fibers increases. A tubular interstitial injury is a key event in the progression of chronic kidney disease because it leads to fibrosis, tubular atrophy, and interstitial peritubular capillary occlusion, thereby resulting in persistent disorders of renal hemodynamics. Renal tubular epithelial cells are the targets of injury and repair of the kidney (Zhou et al., 2017). These cells secrete different pro-inflammatory cytokines and the ECM after inflammatory stimulation. AS-Ⅳ has been shown to prevent renal fibrosis caused by unilateral ureteral obstruction by reducing the inflammatory response via the TLR4/NF-κB pathway. EMT induces the progression of renal tubular interstitial fibrosis. TGF-β1 is a well-characterized pro-fibrotic cytokine associated with renal disease and plays a key part in EMT (Hills and Squires, 2011; Matsuda et al., 2011; Qi et al., 2008; Shan et al., 2016; Suzuki et al., 2004).
AM antagonizes the EMT and accumulation of ECM in renal tubules by the TGF-β/Smad2/3 pathway, thereby improving renal fibrosis. Che et al. (2015) found that AS-Ⅳ regulated the activity of MAPK and NF-κB signaling pathways in a dose-dependent manner, and inhibited TGF-β1-induced proliferation, trans-differentiation and ECM formation of fibroblasts. AM can inhibit renal interstitial fibrosis in vivo, which may be related to inhibiting myofibroblast activation, inducing hepatocyte growth factor (HGF) expression, and inhibiting TGF-β1 expression (Zuo et al., 2009). AS-IV alleviates the progression of renal fibrosis by inhibiting the MAPK and TGF-β/Smad signaling pathways (Wang et al., 2014).
AS-Ⅳ and Ferulic acid (the main component of Astragalus and Angelica) synergistic inhibition of obstructive nephropathy renal tubular interstitial fibrosis in rats, and this inhibition of mesenchymal into renal tubular epithelial - EMT and fibroblast activation, and increase the production of NO in the kidney (Meng et al., 2011). A decoction combined with AM and Angelica sinensis had an anti-fibrosis effect on rats with chronic kidney disease caused by ureteral obstruction and could improve renal blood flow in rats with acute ischemic injury to the kidney. The mechanism of action may be related to the activation of endothelial nitric oxide synthase and ROS clearance, promoting NO production (Meng et al., 2007; Wojcikowski et al., 2010).
Effect of a. mongholicus on diabetic nephropathy
DN is one of the most common causes of end-stage renal disease, and its pathogenesis involves mesangial dilation, thickening of the basement membrane, glomerular hypertrophy, and renal fibrosis, among which progressive renal fibrosis is an important pathologic feature of DN (Chen et al., 2019b). DN’s development depends on oxidative stress and inflammation (Kandhare et al., 2017; Zheng et al., 2016).
Zhang et al. (2020) used a rat model of DN to explore the transcriptome characteristics of the kidney after AS-IV treatment: the latter could significantly reduce the level of advanced glycation end-products, IL-1β expression, and IL-18 expression in the serum and kidney of rats, and the Renal Fibrosis Index. Studies have shown that AS-IV reduces high glucose-stimulated renal tubular EMT through the mammalian target of rapamycin complex 1 (mTORC1)/ribosomal protein S6 kinaseβ-1 (p70S6K) signaling pathway and downregulates the expression of the transcription factors SNAIL and TWIST in HK-2 cells (Chen et al., 2019b). AS-Ⅳ treatment has been shown to improve endoplasmic reticulum stress in renal tubular epithelial cells and renal function and fibrosis in animal models of DN (Ju et al., 2019; Liu et al., 2017; Wang et al., 2018b; Wang et al., 2018c). Wu et al.(2018) pointed out the anti-fibrosis effect of total flavonoids in vivo and in vitro, and discussed the inhibitory effect of total flavonoids on renal fibrosis through the miRNA-21/Smad7 signaling pathway.
Effect of a. mongholicus on peritoneal fibrosis
Peritoneal dialysis (PD) and hemodialysis are treatment options for patients with end-stage renal disease. However, PD predisposes to encapsulated peritoneal sclerosis, a severe disease with a high mortality rate that can lead to failure of ultrafiltration and eventual discontinuation of treatment (Jagirdar et al., 2019). AM can inhibit the recruitment and activation of monocytes/macrophages, thereby reducing TGF-β1 production in the peritoneum of people undergoing dialysis. Peritoneal fibrosis has been shown to be reduced significantly after AM treatment. Expression of Monocyte Chemoattractant Protein (MCP)-1 was positively correlated with TGF-β1 sensitivity, suggesting that the anti-fibrosis effect of AM is related to McP-1 and TGF-β1 pathways (Li et al., 2014b). AM might be an efficacious agent against PD-induced peritoneal fibrosis. In a study by Zhang et al. (Zhang et al., 2015a), AS-IV was demonstrated to have the therapeutic ability to regulate peritoneal fibrosis by promoting upregulation of Smad7, a signaling component involved in TGF- β1/Smad signaling.
Effect of a. mongholicus on intestinal fibrosis
Intestinal fibrosis is a major complication of Crohn’s disease. Celiac disease is a difficult disease to treat. Liu et al. (Liu et al., 2019) found that Calycoflavones inhibited the expression of p-Smad2, p-Smad3, Smad4, and TGF-β1, inhibited the TGF-β/Smad pathway, and increased Smad7 expression. Therefore, Calycoflavones may inhibit intestinal fibrosis by inhibiting the TGF-β/Smad pathway.
Effect of a.s mongholicus on skin scarring and wound healing
A keloid is a benign skin tumor caused by the proliferation of fibroblasts and capillary endothelial cells after skin injury (Ogawa, 2022). Chen et al. (Chen et al., 2012,2013) showed that Astragalus armour glycosides of scar have shown significant dose-dependent inhibition; its mechanism is to reduce fibroblasts' secrete collagen type I/III and TGF-β1 levels.
Effect of a. mongholicus on cutaneous carcinoma
Exposure to ultraviolet-B radiation initiates and progresses the generation of squamous-cell carcinoma (Bowden, 2004). More than 70% of all skin-cancer cases in older people are nonmelanoma skin cancer, which is thought to be caused by excess exposure to ultraviolet light accumulated over time (Leiter et al., 2014). From the perspective of preventing photoaging and/or potential skin cancer, AM extract significantly reduces ultraviolet A-induced DNA damage in cultured lung and skin fibroblasts from humans (Curnow et al., 2016).
Effect of a. mongholicus on systemic sclerosis
SSc is a group of diseases of a complex multi-organ system of unknown etiology. Internal organs and skin fibrosis result from excessive fibroblast proliferation and ECM production in this disease. (Qi et al., 2014; Yasuoka et al., 2014). Fibrosis is a major cause of morbidity and mortality in SSc (Rimar et al., 2014). Studies have suggested that targeted fibrosis therapy may be an option for SSc (Gerber et al., 2013). The application of AS-IV consistently suppressed collagen and fibronectin production in the skin lesions of SSc mice with skin lesions and reduced collagen formation and structure. The reduction of AS-IV-induced fibrosis may be due to the deregulation of Smad 3/Fli-1, the major mediators of the fibrotic response and key molecules in TGF-β signaling. AS-IV can also reduce the p-SMAD3 level and completely block its relocation into nuclei. AS-IV attenuates fibrosis by inhibiting the TGF-β–Smads3 axis in SSc(Qi et al., 2014).
Clinical study
It is difficult for clinicians to identify the differences in clinical efficacy and safety of TCM. AM, a commonly used traditional Chinese medicine, has important medicinal value. The research community has done a lot of research on the mechanism of astragalus membranaceus against fibrosis-related diseases and achieved considerable experimental results, but the clinical application of AM drug preparations is relatively insufficient.
A. mongholicus preparation
At moderate doses, AM granules were sufficient to show the best effect in improving cardiac contraction. In improving the quality of life of patients with chronic heart failure, the effectiveness of AM granules showed a dose-dependent trend (Yang et al., 2011). A prospective randomized controlled study showed that conventional drugs plus AM capsules improved left ventricular diastolic function in hypertensive women with postmenopausal metabolic syndrome (Li et al., 2018). Tian et al.(2016) demonstrated that AM injection or AM water decoction can be used as adjuvant therapy for T2DM based on a preliminary meta-analysis. Astragalus is a traditional Chinese medicine used to treat strokes. PG2 is an insoluble polysaccharide extracted from Astragalus membranaceus. Li et al. (2018) studied the effect of PG2 on patients with spontaneous acute cerebral hemorrhage. The results showed that PG2 administration for 2 weeks did not increase the percentage of Glasgow Outcome Scale (4-5 scores) and Good Mrs Scores (0–2 scores), nor did it produce any anti-inflammatory properties.
Traditional chinese medicine compound
Wang et al. (2021) found that various effective components of AM decoction jointly act on liver fibrosis with a comprehensive strategy based on pharmacokinetics. Twenty-four prototype components and 17 metabolites in AM decoction were identified in vivo, and the pharmacokinetic characteristics of 14 components were elucidated. Among these components, AS-IV, CAG, glycyrrhizic acid, glycyrrhetinic acid, liquiritigenin, and isoliquiritigenin decreased the expression of α-SMA mRNA. CAG, CG, formononetin, glycyrrhetinic acid, liquiritin, and isoliquiritin restrained the expression of type I collagen. Calycosin, liquiritigenin, isoliquiritigenin, CAG, and glycyrrhetinic increased the apoptosis of human hepatic stellate. The multicomponent combination of AM decoction reduced serum transaminase activity and liver collagen fiber deposition in CCL4-induced liver fibrosis mice. Cheng et al.(2019) found that CDC42 and GLI1 might be the treatment target of AM decoction granules in patients with hepatitis B cirrhosis. In addition, SMAD2, EGFR, AKT1, Rho A, and GAS5 may be related to the efficacy of AM decoction granules in patients with hepatitis B cirrhosis.
A pilot study showed that CD25 expression on T cells was significantly increased after 24 h of echinacea combined with AM and licorice examined CD25 expression on T cells after the ingestion of three common herbs (echinacea, AM, and licorice) alone and in combination (Zwickey et al., 2007). Using AM, angelica Sinensis, rhubarb, and salvia miltiorrhiza, the risk of end-stage renal disease and death in patients associated with advanced chronic kidney disease was decreased. And his benefit did not increase the risk of hyperkalemia.
Summary and prospects
AM contains flavonoids, saponins, polysaccharides, and other active ingredients. These ingredients have pharmacologic effects: regulating immunity, protecting the cardiovascular and nervous systems; anti-tumor effects; liver protection; multi-target and multi-pathway anti-fibrosis actions; slowing down the progress of fibrosis and alleviating related symptoms. The main action mechanisms of AM are antioxidant, anti-inflammatory, and immunoregulatory, and their combination.
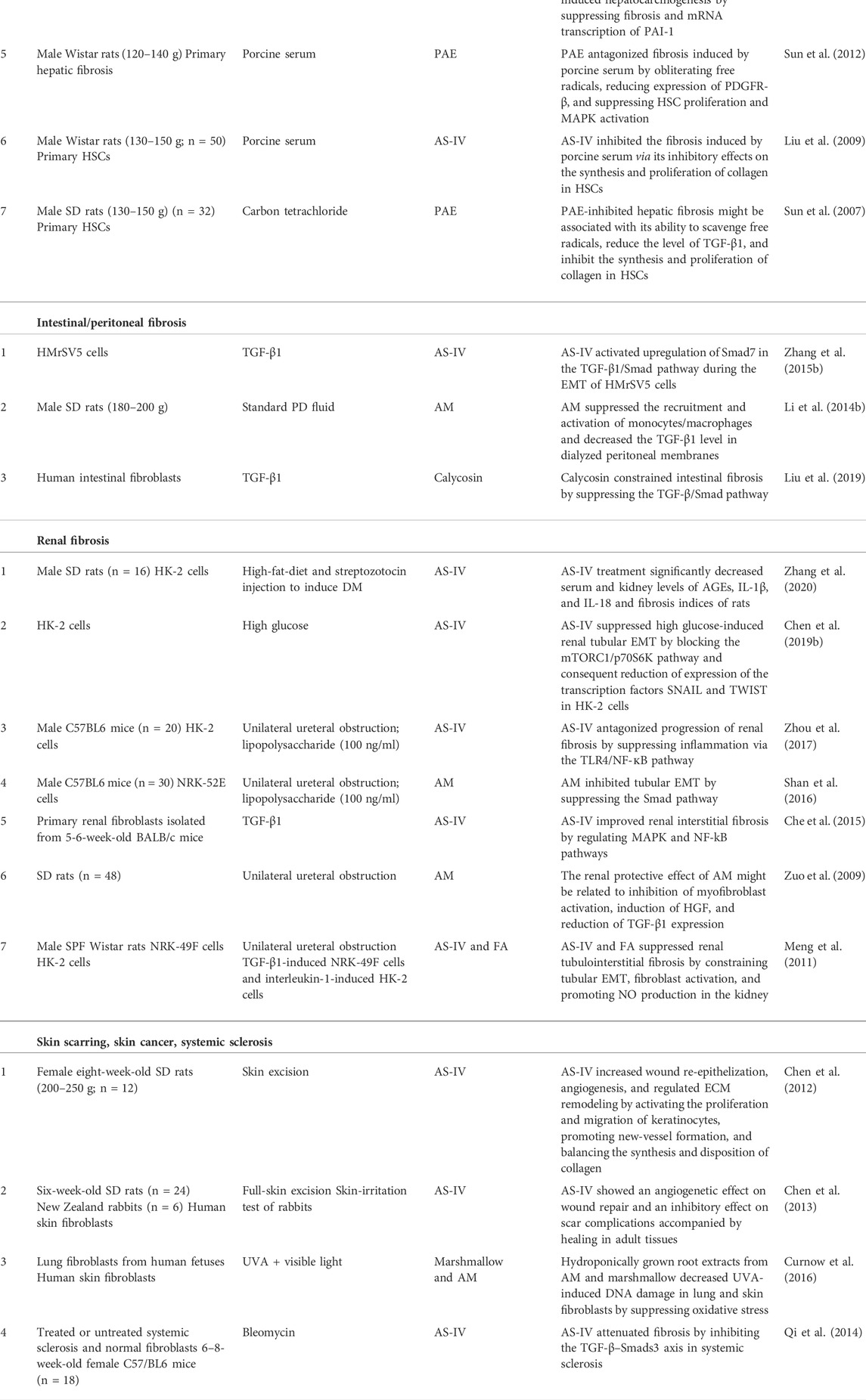
Scholars have conducted a wide range of pharmacologic studies on AM and its active components and explained its mechanism of action at whole-cell, molecular, and genetic levels. However, most of those studies have been experimental and focused on a single ingredient (Table 1). Few clinical studies have been done, so AM has not been applied clinically.
Author contributions
FG, YL, and JD: organization of content and structure, writing and reviewing. RQ and YiL: writing and reviewing. All authors agree to be accountable for the content of this work.
Funding
This study was supported by Scientific Research Project of Traditional Chinese Medicine Bureau of Guangdong Province (20201232) and the President’s Fund of Nanfang Hospital (2019C033).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
An, L., Lin, Y., Li, L., Kong, M., Lou, Y., Wu, J., et al. (2021). Integrating network pharmacology and experimental validation to investigate the effects and mechanism of Astragalus Flavonoids against hepatic fibrosis. Front. Pharmacol. 11, 618262. doi:10.3389/fphar.2020.618262
Berry, M. N., Halls, H. J., and Grivell, M. B. (1992). Techniques for pharmacological and toxicological studies with isolated hepatocyte suspensions. Life Sci. 51 (1), 1–16. doi:10.1016/0024-3205(92)90212-8
Bowden, G. T. (2004). Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat. Rev. Cancer 4 (1), 23–35. doi:10.1038/nrc1253
Che, X., Wang, Q., Xie, Y., Xu, W., Shao, X., Mou, S., et al. (2015). Astragaloside IV suppresses transforming growth factor-β1 induced fibrosis of cultured mouse renal fibroblasts via inhibition of the MAPK and NF-κB signaling pathways. Biochem. Biophys. Res. Commun. 464 (4), 1260–1266. doi:10.1016/j.bbrc.2015.07.116
Chen, F., Yang, D., Cheng, X., Yang, H., Yang, X., Liu, H., et al. (2021). Astragaloside IV ameliorates cognitive impairment and neuroinflammation in an oligomeric Aβ induced Alzheimer's disease mouse model via inhibition of microglial activation and NADPH oxidase expression. Biol. Pharm. Bull. 44 (11), 1688–1696. doi:10.1248/bpb.b21-00381
Chen, P., Xie, Y., Shen, E., Li, G. G., Yu, Y., Zhang, C. B., et al. (2011). Astragaloside IV attenuates myocardial fibrosis by inhibiting TGF-β1 signaling in coxsackievirus B3-induced cardiomyopathy. Eur. J. Pharmacol. 658 (2-3), 168–174. doi:10.1016/j.ejphar.2011.02.040
Chen, X., Peng, L., Li, N., Li, Q., Li, P., Fung, K., et al. (2012). The healing and anti-scar effects of astragaloside IV on the wound repair in vitro and in vivo. J. Ethnopharmacol. 139 (3), 721–727. doi:10.1016/j.jep.2011.11.035
Chen, X., Peng, L., Shan, Y., Li, N., Wei, W., Yu, L., et al. (2013). Astragaloside IV-loaded nanoparticle-enriched hydrogel induces wound healing and anti-scar activity through topical delivery. Int. J. Pharm. 447 (1-2), 171–181. doi:10.1016/j.ijpharm.2013.02.054
Chen, X., Wu, H., Chen, H., Wang, Q., Xie, X., and Shen, J. (2019a). Astragaloside VI promotes neural stem cell proliferation and enhances neurological function recovery in transient cerebral ischemic injury via activating EGFR/MAPK signaling cascades. Mol. Neurobiol. 56 (4), 3053–3067. doi:10.1007/s12035-018-1294-3
Chen, X., Yang, Y., Liu, C., Chen, Z., and Wang, D. (2019b). Astragaloside IV ameliorates high glucose-induced renal tubular epithelial-mesenchymal transition by blocking mTORC1/p70S6K signaling in HK-2 cells. Int. J. Mol. Med. 43 (2), 709–716. doi:10.3892/ijmm.2018.3999
Chen, Z., Yao, L., Liu, Y., Pan, Z., Peng, S., Wan, G., et al. (2019c). Astragaloside IV regulates NF-κB-mediated cellular senescence and apoptosis of hepatic stellate cells to suppress PDGF-BB-induced activation. Exp. Ther. Med. 18 (5), 3741–3750. doi:10.3892/etm.2019.8047
Cheng, Y., Liu, P., Hou, T., Maimaitisidike, M., Ababaikeli, R., and Abudureyimu, A. (2019c). Mechanisms of huangqi decoction granules on hepatitis B cirrhosis patients based on RNA-sequencing. Chin. J. Integr. Med. 25 (7), 507–514. doi:10.1007/s11655-018-3013-3
Cheng, Y., Mai, J., Wang, M., Chen, G., and Ping, J. (2017). Antifibrotic effect of total flavonoids of Astmgali Radix on dimethylnitrosamine-induced liver cirrhosis in rats. Chin. J. Integr. Med. 23 (1), 48–54. doi:10.1007/s11655-016-2627-6
Costa, I. M., Lima, F., Fernandes, L., Norrara, B., Neta, F. I., Alves, R. D., et al. (2019). Astragaloside IV supplementation promotes a neuroprotective effect in experimental models of neurological disorders: A systematic review. Curr. Neuropharmacol. 17 (7), 648–665. doi:10.2174/1570159X16666180911123341
Curnow, A., Owen, S. J., and El Enshasy, H. A. (2016). An evaluation of root phytochemicals derived from Althea officinalis (Marshmallow) and Astragalus membranaceus as potential natural components of UV protecting dermatological formulations. Oxid. Med. Cell. Longev. 2016, 7053897. doi:10.1155/2016/7053897
Dai, H., Jia, G., Lu, M., Liang, C., Wang, Y., and Wang, H. (2017). Astragaloside IV inhibits isoprenaline-induced cardiac fibrosis by targeting the reactive oxygen species/mitogen-activated protein kinase signaling axis. Mol. Med. Rep. 15 (4), 1765–1770. doi:10.3892/mmr.2017.6220
Dang, S., Zhang, X., Jia, X., Cheng, Y., Song, P., Liu, E., et al. (2008). Protective effects of emodin and astragalus polysaccharides on chronic hepatic injury in rats. Chin. Med. J. 121 (11), 1010–1014. doi:10.1097/00029330-200806010-00009
Degryse, A. L., Tanjore, H., Xu, X. C., Polosukhin, V. V., Jones, B. R., Boomershine, C. S., et al. (2011). TGFβ signaling in lung epithelium regulates bleomycin-induced alveolar injury and fibroblast recruitment. Am. J. Physiol. Lung Cell. Mol. Physiol. 300 (6), L887–L897. doi:10.1152/ajplung.00397.2010
Dong, C., Ming, X., Ye, Z., Wang, P., Wang, L., Li, Z., et al. (2018). Icariside II attenuates chronic hydrocephalus in an experimental subarachnoid hemorrhage rat model. J. Pharm. Pharm. Sci. 21 (1), 318–325. doi:10.18433/jpps29811
Du, J., Liu, J., Zhen, J., Yang, S., Zheng, E., and Leng, J. (2019). Astragaloside IV protects cardiomyocytes from hypoxia-induced injury by down-regulation of lncRNA GAS5. Biomed. Pharmacother. 116, 109028. doi:10.1016/j.biopha.2019.109028
Du, J., Yu, Q., Liu, Y., Du, S., Huang, L., Xu, D., et al. (2021). A novel role of kallikrein-related peptidase 8 in the pathogenesis of diabetic cardiac fibrosis. Theranostics 11 (9), 4207–4231. doi:10.7150/thno.48530
Friedman, S. L. (2008). Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 88 (1), 125–172. doi:10.1152/physrev.00013.2007
Fu, S., Gu, Y., Jiang, J. Q., Chen, X., Xu, M., Chen, X., et al. (2014). Calycosin-7-O-β-D-glucoside regulates nitric oxide/caveolin-1/matrix metalloproteinases pathway and protects blood-brain barrier integrity in experimental cerebral ischemia-reperfusion injury. J. Ethnopharmacol. 155 (1), 692–701. doi:10.1016/j.jep.2014.06.015
Gerber, E. E., Gallo, E. M., Fontana, S. C., Davis, E. C., Wigley, F. M., Huso, D. L., et al. (2013). Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature 503 (7474), 126–130. doi:10.1038/nature12614
Ghosh, A. K., Nagpal, V., Covington, J. W., Michaels, M. A., and Vaughan, D. E. (2012). Molecular basis of cardiac endothelial-to-mesenchymal transition (EndMT): Differential expression of microRNAs during EndMT. Cell. Signal. 24 (5), 1031–1036. doi:10.1016/j.cellsig.2011.12.024
Gong, A., Duan, R., Wang, H., Kong, X., Dong, T., Tsim, K., et al. (2018a). Evaluation of the pharmaceutical properties and value of astragali radix. Medicines 5 (2), 46. doi:10.3390/medicines5020046
Gong, L., Chang, H., Zhang, J., Guo, G., Shi, J., and Xu, H. (2018b). Astragaloside IV protects rat cardiomyocytes from hypoxia-induced Injury by down-regulation of miR-23a and miR-92a. Cell. Physiol. biochem. 49 (6), 2240–2253. doi:10.1159/000493827
Gong, P., Wang, D., Cui, D., Yang, Q., Wang, P., Yang, W., et al. (2021). Anti-aging function and molecular mechanism of Radix Astragali and Radix Astragali preparata via network pharmacology and PI3K/Akt signaling pathway. Phytomedicine. 84, 153509. doi:10.1016/j.phymed.2021.153509
Gu, J., Sun, R., Tang, D., Liu, F., Chang, X., and Wang, Q. (2022). Astragalus mongholicus Bunge-Curcuma aromatica Salisb. Suppresses growth and metastasis of colorectal cancer cells by inhibiting M2 macrophage polarization via a Sp1/ZFAS1/miR-153-3p/CCR5 regulatory axis. Cell Biol. Toxicol. 38, 679–697. doi:10.1007/s10565-021-09679-w
Guzhva, N. N. (2010). Flavonoids and hydroxycinnamic acids from Astragalus asper. Chem. Nat. Compd. 46 (2), 303–304. doi:10.1007/s10600-010-9597-2
Haiyan, H., Rensong, Y., Guoqin, J., Xueli, Z., Huaying, X., and Yanwu, X. (2016). Effect of Astragaloside IV on neural stem cell transplantation in Alzheimer's disease rat models. Evid. Based. Complement. Altern. Med. 2016, 3106980–3106988. doi:10.1155/2016/3106980
Hills, C. E., and Squires, P. E. (2011). The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. 22, 131–139. doi:10.1016/j.cytogfr.2011.06.002
Hinz, B., Phan, S. H., Thannickal, V. J., Galli, A., Bochaton-Piallat, M., and Gabbiani, G. (2007). The myofibroblast: One function, multiple origins. Am. J. Pathol. 170 (6), 1807–1816. doi:10.2353/ajpath.2007.070112
Hu, J. Y., Han, J., Chu, Z. G., Song, H. P., Zhang, D. X., Zhang, Q., et al. (2009). Astragaloside IV attenuates hypoxia-induced cardiomyocyte damage in rats by upregulating superoxide dismutase-1 levels. Clin. Exp. Pharmacol. Physiol. 36 (4), 351–357. doi:10.1111/j.1440-1681.2008.05059.x
Huang, F., Lan, Y., Qin, L., Dong, H., Shi, H., Wu, H., et al. (2018). Astragaloside IV promotes adult neurogenesis in hippocampal dentate gyrus of mouse through CXCL1/CXCR2 signaling. Molecules 23 (9), 2178. doi:10.3390/molecules23092178
Ibrahim, L. F., Marzouk, M. M., Hussein, S. R., Kawashty, S. A., Mahmoud, K., and Saleh, N. A. (2013). Flavonoid constituents and biological screening of Astragalus bombycinus Boiss. Nat. Prod. Res. 27 (4-5), 386–393. doi:10.1080/14786419.2012.701213
Jagirdar, R. M., Bozikas, A., Zarogiannis, S. G., Bartosova, M., Schmitt, C. P., and Liakopoulos, V. (2019). Encapsulating peritoneal sclerosis: Pathophysiology and current treatment options. Int. J. Mol. Sci. 20 (22), 5765. doi:10.3390/ijms20225765
Jia, G., Leng, B., Wang, H., and Dai, H. (2017). Inhibition of cardiotrophin-1 overexpression is involved in the antifibrotic effect of Astrogaloside IV. Mol. Med. Rep. 16 (6), 8365–8370. doi:10.3892/mmr.2017.7676
Ju, Y., Su, Y., Chen, Q., Ma, K., Ji, T., Wang, Z., et al. (2019). Protective effects of Astragaloside IV on endoplasmic reticulum stress-induced renal tubular epithelial cells apoptosis in type 2 diabetic nephropathy rats. Biomed. Pharmacother. 109, 84–92. doi:10.1016/j.biopha.2018.10.041
Kaczmarczyk-Sedlak, I., Wojnar, W., Zych, M., Ozimina-Kamińska, E., Taranowicz, J., and Siwek, A. (2013). Effect of formononetin on mechanical properties and chemical composition of bones in rats with Ovariectomy-Induced osteoporosis. Evid. Based. Complement. Altern. Med. 2013, 457052. doi:10.1155/2013/457052
Kandhare, A. D., Mukherjee, A., and Bodhankar, S. L. (2017). Antioxidant for treatment of diabetic nephropathy: A systematic review and meta-analysis. Chem. Biol. Interact. 278, 212–221. doi:10.1016/j.cbi.2017.10.031
Kim, J., Joo, S., Eom, G. H., Lee, S. H., Lee, M., Lee, M., et al. (2018). CCN5 knockout mice exhibit lipotoxic cardiomyopathy with mild obesity and diabetes. PLoS One 13 (11), e0207228. doi:10.1371/journal.pone.0207228
Kiyohara, H., Uchida, T., Takakiwa, M., Matsuzaki, T., Hada, N., Takeda, T., et al. (2010). Different contributions of side-chains in β-d-(1→3, 6)-galactans on intestinal Peyer's patch-immunomodulation by polysaccharides from Astragalus mongholics Bunge. Phytochemistry 71 (2-3), 280–293. doi:10.1016/j.phytochem.2009.10.001
Kocabaş, U., Yılmaz, Ö., and Kurtoğlu, V. (2019). Diabetic cardiomyopathy: Acute and reversible left ventricular systolic dysfunction due to cardiotoxicity of hyperglycaemic hyperosmolar state-a case report. Eur. Heart J. Case Rep. 3 (2), ytz049. doi:10.1093/ehjcr/ytz049
Kuo, L., and Huang, A. P. (2021). The pathogenesis of hydrocephalus following aneurysmal subarachnoid hemorrhage. Int. J. Mol. Sci. 22 (9), 5050. doi:10.3390/ijms22095050
Lai, Y., Hanneman, S. K., Casarez, R. L., Wang, J., and McCullough, L. D. (2019). Blood biomarkers for physical recovery in ischemic stroke: A systematic review. Am. J. Transl. Res. 11 (8), 4603–4613.
Leiter, U., Eigentler, T., and Garbe, C. (2014). Epidemiology of skin cancer. Adv. Exp. Med. Biol. 810, 120–140. doi:10.1007/978-1-4939-0437-2_7
Lekkerkerker, A. N., Aarbiou, J., van Es, T., and Janssen, R. A. J. (2012). Cellular players in lung fibrosis. Curr. Pharm. Des. 18 (27), 4093–4102. doi:10.2174/138161212802430396
Li, L., Xu, L., Hu, Y., Cui, W., Cui, W., Zhou, W., et al. (2017). Astragaloside IV improves Bleomycin-Induced pulmonary fibrosis in rats by attenuating extracellular matrix deposition. Front. Pharmacol. 8, 513. doi:10.3389/fphar.2017.00513
Li, M., Han, B., Zhao, H., Xu, C., Xu, D., Sieniawska, E., et al. (2022). Biological active ingredients of Astragali Radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine. 98, 153918. doi:10.1016/j.phymed.2021.153918
Li, N. Y., Yu, H., Li, X. L., Wang, Q. Y., Zhang, X. W., Ma, R. X., et al. (2018). Astragalus membranaceus improving asymptomatic left ventricular diastolic dysfunction in postmenopausal hypertensive women with metabolic syndrome: A prospective, open-labeled, randomized controlled trial. Chin. Med. J. 131 (5), 516–526. doi:10.4103/0366-6999.226077
Li, X., Qu, L., Dong, Y., Han, L., Liu, E., Fang, S., et al. (2014a). A review of recent research progress on the Astragalus Genus. Molecules 19 (11), 18850–18880. doi:10.3390/molecules191118850
Li, X., Wang, X., Han, C., Wang, X., Xing, G., Zhou, L., et al. (2013). Astragaloside IV suppresses collagen production of activated hepatic stellate cells via oxidative stress-mediated p38 MAPK pathway. Free Radic. Biol. Med. 60, 168–176. doi:10.1016/j.freeradbiomed.2013.02.027
Li, Z., Zhang, L., He, W., Zhu, C., Yang, J., and Sheng, M. (2014b). Astragalus membranaceus inhibits peritoneal fibrosis via monocyte chemoattractant protein (MCP)-1 and the transforming growth factor-β1 (TGF-β1) pathway in rats submitted to peritoneal dialysis. Int. J. Mol. Sci. 15 (7), 12959–12971. doi:10.3390/ijms150712959
Liang, C., Ni, G., Shi, X., Jia, L., and Wang, Y. (2020). Astragaloside IV regulates the HIF/VEGF/Notch signaling pathway through miRNA-210 to promote angiogenesis after ischemic stroke. Restor. Neurol. Neurosci. 38 (3), 271–282. doi:10.3233/RNN-201001
Liang, Y., Ahmed, M., Guo, H., Soares, F., Hua, J. T., Gao, S., et al. (2017). LSD1-mediated epigenetic reprogramming drives CENPE expression and prostate cancer progression. Cancer Res. 77 (20), 5479–5490. doi:10.1158/0008-5472.CAN-17-0496
Liu, C. Y., Gu, Z. L., Zhou, W. X., and Guo, C. Y. (2005). Effect of Astragalus complanatus flavonoid on anti-liver fibrosis in rats. World J. Gastroenterol. 11 (37), 5782–5786. doi:10.3748/wjg.v11.i37.5782
Liu, G., Song, J., Guo, Y., Wang, T., and Zhou, Z. (2013). Astragalus injection protects cerebral ischemic injury by inhibiting neuronal apoptosis and the expression of JNK3 after cerebral ischemia reperfusion in rats. Behav. Brain Funct. 9, 36. doi:10.1186/1744-9081-9-36
Liu, H., Wei, W., Sun, W., and Li, X. (2009). Protective effects of Astragaloside IV on porcine-serum-induced hepatic fibrosis in rats and in vitro effects on hepatic stellate cells. J. Ethnopharmacol. 122 (3), 502–508. doi:10.1016/j.jep.2009.01.035
Liu, J., Deng, T., Wang, Y., Zhang, M., Zhu, G., Fang, H., et al. (2019). Calycosin inhibits intestinal fibrosis on CCD-18Co cells via modulating transforming growth factor-β/Smad signaling pathway. Pharmacology 104 (1-2), 81–89. doi:10.1159/000500186
Liu, X., Ma, J., Ding, G., Gong, Q., Wang, Y., Yu, H., et al. (2021). Microglia polarization from m1 toward m2 phenotype is promoted by astragalus polysaccharides mediated through inhibition of miR-155 in experimental autoimmune encephalomyelitis. Oxid. Med. Cell. Longev. 2021, 5753452. doi:10.1155/2021/5753452
Liu, X., Wang, W., Song, G., Wei, X., Zeng, Y., Han, P., et al. (2017). Astragaloside IV ameliorates diabetic nephropathy by modulating the mitochondrial quality control network. PLoS One 12 (8), e0182558. doi:10.1371/journal.pone.0182558
Liu, Z., Liu, H., and Wang, J. (2018). Astragaloside IV protects against the pathological cardiac hypertrophy in mice. Biomed. Pharmacother. 97, 1468–1478. doi:10.1016/j.biopha.2017.09.092
Lu, J., Wang, Q. Y., Zhou, Y., Lu, X. C., Liu, Y. H., Wu, Y., et al. (2017). Astragaloside against cardiac fibrosis by inhibiting TRPM7 channel. Phytomedicine 30, 10–17. doi:10.1016/j.phymed.2017.04.002
Lu, M., Wang, H., Wang, J., Zhang, J., Yang, J., Liang, L., et al. (2014). Astragaloside IV protects against cardiac hypertrophy via inhibiting the Ca2+/CaN signaling pathway. Planta Med. 80 (1), 63–69. doi:10.1055/s-0033-1360129
Matsuda, H., Fukuda, N., Ueno, T., Katakawa, M., Wang, X., Watanabe, T., et al. (2011). Transcriptional inhibition of progressive renal disease by gene silencing pyrrole–imidazole polyamide targeting of the transforming growth factor-β1 promoter. Kidney Int. 79 (1), 46–56. doi:10.1038/ki.2010.330
Meng, L. Q., Tang, J. W., Wang, Y., Zhao, J. R., Shang, M. Y., Zhang, M., et al. (2011). Astragaloside IV synergizes with ferulic acid to inhibit renal tubulointerstitial fibrosis in rats with obstructive nephropathy. Br. J. Pharmacol. 162 (8), 1805–1818. doi:10.1111/j.1476-5381.2011.01206.x
Meng, L., Qu, L., Tang, J., Cai, S., Wang, H., and Li, X. (2007). A combination of Chinese herbs, Astragalus membranaceus var. Mongholicus and Angelica sinensis, enhanced nitric oxide production in obstructed rat kidney. Vasc. Pharmacol. 47 (2-3), 174–183. doi:10.1016/j.vph.2007.06.002
Nam, E., Derrick, J. S., Lee, S., Kang, J., Han, J., Lee, S. J. C., et al. (2018). Regulatory activities of dopamine and its derivatives toward metal-free and metal-induced amyloid-β aggregation, oxidative stress, and inflammation in Alzheimer's disease. ACS Chem. Neurosci. 9 (11), 2655–2666. doi:10.1021/acschemneuro.8b00122
Nie, P., Meng, F., Zhang, J., Wei, X., and Shen, C. (2019). Astragaloside IV exerts a myocardial protective effect against cardiac hypertrophy in rats, partially via activating the Nrf2/HO-1 signaling pathway. Oxid. Med. Cell. Longev. 2019, 4625912–4625916. doi:10.1155/2019/4625912
Ogawa, R. (2022). The most current algorithms for the treatment and prevention of hypertrophic scars and keloids: A 2020 update of the algorithms published 10 years ago. Plast. Reconstr. Surg. 149 (1), 79e–94e. doi:10.1097/PRS.0000000000008667
Owen, M. C., Gnutt, D., Gao, M., Wärmländer, S. K. T. S., Jarvet, J., Gräslund, A., et al. (2019). Effects of in vivo conditions on amyloid aggregation. Chem. Soc. Rev. 48 (14), 3946–3996. doi:10.1039/C8CS00034D
Podlech, D. (2008). The genusAstragalus L. (Fabaceae) in europe with exclusion of the former soviet union. Feddes Repert. 119 (5-6), 310–387. doi:10.1002/fedr.200811171
Qi, Q., Mao, Y., Yi, J., Li, D., Zhu, K., and Cha, X. (2014). Antifibrotic effects of Astragaloside IV in systemic sclerosis. Cell. Physiol. biochem. 34 (6), 2105–2116. doi:10.1159/000366405
Qi, W., Chen, X., Poronnik, P., and Pollock, C. A. (2008). Transforming growth factor-beta/connective tissue growth factor axis in the kidney. Int. J. Biochem. Cell Biol. 40 (1), 9–13. doi:10.1016/j.biocel.2007.01.006
Qian, W., Cai, X., Qian, Q., Zhang, W., and Wang, D. (2018). Astragaloside IV modulates TGF‐β1‐dependent epithelial‐mesenchymal transition in bleomycin‐induced pulmonary fibrosis. J. Cell. Mol. Med. 22 (9), 4354–4365. doi:10.1111/jcmm.13725
Ramezani, M., Darbandi, N., Khodagholi, F., and Hashemi, A. (2016). Myricetin protects hippocampal CA3 pyramidal neurons and improves learning and memory impairments in rats with Alzheimer's disease. Neural Regen. Res. 11 (12), 1976–1980. doi:10.4103/1673-5374.197141
Ren, S., Zhang, H., Mu, Y., Sun, M., and Liu, P. (2013). Pharmacological effects of astragaloside IV: A literature review. J. Tradit. Chin. Med. 33 (3), 413–416. doi:10.1016/s0254-6272(13)60189-2
Rimar, D., Rosner, I., Nov, Y., Slobodin, G., Rozenbaum, M., Halasz, K., et al. (2014). Brief report: Lysyl oxidase is a potential biomarker of fibrosis in systemic sclerosis. Arthritis Rheumatol. 66 (3), 726–730. doi:10.1002/art.38277
Rui, W., Xie, L., Liu, X., He, S., Wu, C., Zhang, X., et al. (2014). Compound Astragalus and Salvia miltiorrhiza extract suppresses hepatocellular carcinoma progression by inhibiting fibrosis and PAI-1 mRNA transcription. J. Ethnopharmacol. 151 (1), 198–209. doi:10.1016/j.jep.2013.10.022
Sakuma, Y. (2017). Epithelial-to-mesenchymal transition and its role in EGFR-mutant lung adenocarcinoma and idiopathic pulmonary fibrosis. Pathol. Int. 67 (8), 379–388. doi:10.1111/pin.12553
Shan, G., Zhou, X. J., Xia, Y., and Qian, H. J. (2016). Astragalus Membranaceus ameliorates renal interstitial fibrosis by inhibiting tubular epithelial-mesenchymal transition in vivo and in vitro. Exp. Ther. Med. 11 (5), 1611–1616. doi:10.3892/etm.2016.3152
Shao, A., Guo, S., Tu, S., Ammar, A., Tang, J., Hong, Y., et al. (2014). Astragaloside IV alleviates early brain injury following experimental subarachnoid hemorrhage in rats. Int. J. Med. Sci. 11 (10), 1073–1081. doi:10.7150/ijms.9282
Sheng, Z., Jiang, Y., Liu, J., and Yang, B. (2021). UHPLC–MS/MS analysis on flavonoids composition in Astragalus membranaceus and their antioxidant activity. Antioxidants 10 (11), 1852. doi:10.3390/antiox10111852
Shuangcheng, M., Yong, Q., and Rong, A. (2012). Analysis atlas of reference materials of traditional Chinese Medicine in Chinese Pharmacopoeia. Beijing: People's Medical Publishing House.
Song, L., Li, X., Bai, X., Gao, J., and Wang, C. (2017). Calycosin improves cognitive function in a transgenic mouse model of Alzheimer's disease by activating the protein kinase C pathway. Neural Regen. Res. 12 (11), 1870–1876. doi:10.4103/1673-5374.219049
Soto, C., and Pritzkow, S. (2018). Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 21 (10), 1332–1340. doi:10.1038/s41593-018-0235-9
Sperling, R., Mormino, E., and Johnson, K. (2014). The evolution of preclinical Alzheimer's disease: Implications for prevention trials. Neuron 84 (3), 608–622. doi:10.1016/j.neuron.2014.10.038
Sun, J., Chen, X. L., Zheng, J. Y., Zhou, J. W., and Ma, Z. L. (2016). Astragaloside IV protects new born rats from anesthesia-induced apoptosis in the developing brain. Exp. Ther. Med. 12 (3), 1829–1835. doi:10.3892/etm.2016.3519
Sun, L., Han, R., Guo, F., Chen, H., Wang, W., Chen, Z., et al. (2020a). Antagonistic effects of IL-17 and Astragaloside IV on cortical neurogenesis and cognitive behavior after stroke in adult mice through Akt/GSK-3β pathway. Cell Death Discov. 6 (1), 74. doi:10.1038/s41420-020-00298-8
Sun, L., Zhang, H., Wang, W., Chen, Z., Wang, S., Li, J., et al. (2020b). Astragaloside IV exerts cognitive benefits and promotes hippocampal neurogenesis in stroke mice by downregulating interleukin-17 expression via wnt pathway. Front. Pharmacol. 11, 421. doi:10.3389/fphar.2020.00421
Sun, W., Wei, W., Wu, L., Gui, S., and Wang, H. (2007). Effects and mechanisms of extract from Paeonia lactiflora and Astragalus membranaceus on liver fibrosis induced by carbon tetrachloride in rats. J. Ethnopharmacol. 112 (3), 514–523. doi:10.1016/j.jep.2007.04.005
Sun, W. Y., Wang, L., Liu, H., Li, X., and Wei, W. (2012). A standardized extract from Paeonia lactiflora and Astragalus membranaceus attenuates liver fibrosis induced by porcine serum in rats. Int. J. Mol. Med. 29 (3), 491–498. doi:10.3892/ijmm.2011.844
Suzuki, H., Uchida, K., Nitta, K., and Nihei, H. (2004). Role of mitogen-activated protein kinase in the regulation of transforming growth factor-β-induced fibronectin accumulation in cultured renal interstitial fibroblasts. Clin. Exp. Nephrol. 8 (3), 188–195. doi:10.1007/s10157-004-0297-8
Tahaei Gilan, S. S., Yahya Rayat, D., Ahmed Mustafa, T., Aziz, F. M., Shahpasand, K., Akhtari, K., et al. (2019). Α-synuclein interaction with zero-valent iron nanoparticles accelerates structural rearrangement into amyloid-susceptible structure with increased cytotoxic tendency. Int. J. Nanomedicine 14, 4637–4648. doi:10.2147/IJN.S212387
Tan, Y., Chen, H., and Li, J. (2020). Astragaloside IV: An effective drug for the treatment of cardiovascular diseases. Drug Des. devel. Ther. 14, 3731–3746. doi:10.2147/DDDT.S272355
Tan, Y., Lv, Z. P., Bai, X. C., Liu, X. Y., and Zhang, X. F. (2006). Traditional Chinese medicine Bao Gan Ning increase phosphorylation of CREB in liver fibrosis in vivo and in vitro. J. Ethnopharmacol. 105 (1-2), 69–75. doi:10.1016/j.jep.2005.09.040
Tanjore, H., Xu, X. C., Polosukhin, V. V., Degryse, A. L., Li, B., Han, W., et al. (2009). Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 180 (7), 657–665. doi:10.1164/rccm.200903-0322OC
Tian, H., Lu, J., He, H., Zhang, L., Dong, Y., Yao, H., et al. (2016). The effect of Astragalus as an adjuvant treatment in type 2 diabetes mellitus: A (preliminary) meta-analysis. J. Ethnopharmacol. 191, 206–215. doi:10.1016/j.jep.2016.05.062
Tian, L., Zhao, J., Kang, J., Guo, S., Zhang, N., Shang, L., et al. (2021). Astragaloside IV alleviates the experimental DSS-Induced colitis by remodeling macrophage polarization through STAT signaling. Front. Immunol. 12, 740565. doi:10.3389/fimmu.2021.740565
Wan, Y., Xu, L., Wang, Y., Tuerdi, N., Ye, M., and Qi, R. (2018). Preventive effects of Astragaloside IV and its active sapogenin cycloastragenol on cardiac fibrosis of mice by inhibiting the NLRP3 inflammasome. Eur. J. Pharmacol. 833, 545–554. doi:10.1016/j.ejphar.2018.06.016
Wang, D., Li, R., Wei, S., Gao, S., Xu, Z., Liu, H., et al. (2019). Metabolomics combined with network pharmacology exploration reveals the modulatory properties of Astragali Radix extract in the treatment of liver fibrosis. Chin. Med. 14 (1), 30. doi:10.1186/s13020-019-0251-z
Wang, D., Wang, W., Liang, Q., He, X., Xia, Y., Shen, S., et al. (2018a). DHEA-induced ovarian hyperfibrosis is mediated by TGF-β signaling pathway. J. Ovarian Res. 11 (1), 6. doi:10.1186/s13048-017-0375-7
Wang, L., Chi, Y., Yuan, Z., Zhou, W., Yin, P., Zhang, X., et al. (2014). Astragaloside IV inhibits renal tubulointerstitial fibrosis by blocking TGF-β/Smad signaling pathway in vivo and in vitro. Exp. Biol. Med. 239 (10), 1310–1324. doi:10.1177/1535370214532597
Wang, L., Dong, X., Qin, X., and Li, Z. (2022). Investigating the inter-individual variability of Astragali Radix against cisplatin-induced liver injury via 16S rRNA gene sequencing and LC/MS-based metabolomics. Phytomedicine. 101, 154107. doi:10.1016/j.phymed.2022.154107
Wang, S., and Tsao, P. (2020). Phenotypes of bronchopulmonary dysplasia. Int. J. Mol. Sci. 21 (17), 6112. doi:10.3390/ijms21176112
Wang, X., Gao, Y., Tian, N., Zhu, Z., Wang, T., Xu, J., et al. (2018b). Astragaloside IV represses high glucose-induced mesangial cells activation by enhancing autophagy via SIRT1 deacetylation of NF-κB p65 subunit. Drug Des. devel. Ther. Vol. 12, 2971–2980. doi:10.2147/DDDT.S174058
Wang, X., Gao, Y., Tian, N., Zou, D., Shi, Y., and Zhang, N. (2018c). Astragaloside IV improves renal function and fibrosis via inhibition of miR-21-induced podocyte dedifferentiation and mesangial cell activation in diabetic mice. Drug Des. devel. Ther. Vol. 12, 2431–2442. doi:10.2147/DDDT.S170840
Wang, X. H., and Huang, W. M. (2014). Astragalus polysaccharides exert protective effects in newborn rats with bronchopulmonary dysplasia by upregulating the expression of EGFL7 in lung tissue. Int. J. Mol. Med. 34 (6), 1529–1536. doi:10.3892/ijmm.2014.1951
Wang, X., Wang, Y., Hu, J., Yu, S., Li, B., Cui, Y., et al. (2017). Astragaloside IV, a natural PPARγ agonist, reduces Aβ production in Alzheimer's disease through inhibition of BACE1. Mol. Neurobiol. 54 (4), 2939–2949. doi:10.1007/s12035-016-9874-6
Wang, X., and Zhao, L. (2016). Calycosin ameliorates diabetes-induced cognitive impairments in rats by reducing oxidative stress via the PI3K/Akt/GSK-3β signaling pathway. Biochem. Biophys. Res. Commun. 473 (2), 428–434. doi:10.1016/j.bbrc.2016.03.024
Wang, Y., Li, Y., Zhang, H., Zhu, L., Zhong, J., Zeng, J., et al. (2021). Pharmacokinetics-based comprehensive strategy to identify multiple effective components in Huangqi decoction against liver fibrosis. Phytomedicine. 84, 153513. doi:10.1016/j.phymed.2021.153513
Wang, Z., Zhu, Y., Zhang, Y., Zhang, J., Ji, T., Li, W., et al. (2020). Protective effects of AS-IV on diabetic cardiomyopathy by improving myocardial lipid metabolism in rat models of T2DM. Biomed. Pharmacother. 127, 110081. doi:10.1016/j.biopha.2020.110081
Wei, Y., Wu, Y., Feng, K., Zhao, Y., Tao, R., Xu, H., et al. (2020). Astragaloside IV inhibits cardiac fibrosis via miR-135a-TRPM7-TGF-β/Smads pathway. J. Ethnopharmacol. 249, 112404. doi:10.1016/j.jep.2019.112404
Wen, W., Chen, J., Ding, L., Luo, X., Zheng, X., Dai, Q., et al. (2018). Astragaloside exerts anti-photoaging effects in UVB-induced premature senescence of rat dermal fibroblasts through enhanced autophagy. Arch. Biochem. Biophys. 657, 31–40. doi:10.1016/j.abb.2018.09.007
Willis, B. C., Liebler, J. M., Luby-Phelps, K., Nicholson, A. G., Crandall, E. D., du Bois, R. M., et al. (2005). Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: Potential role in idiopathic pulmonary fibrosis. Am. J. Pathol. 166 (5), 1321–1332. doi:10.1016/s0002-9440(10)62351-6
Wojcikowski, K., Wohlmuth, H., Johnson, D. W., and Gobe, G. (2010). Effect of Astragalus membranaceus and Angelica sinensis combined with Enalapril in rats with obstructive uropathy. Phytother. Res. 24 (6), 875–884. doi:10.1002/ptr.3038
Wolters, P. J., Collard, H. R., and Jones, K. D. (2014). Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 9 (1), 157–179. doi:10.1146/annurev-pathol-012513-104706
Wu, X., Ding, X., Ding, Z., and Jia, P. (2018). Total flavonoids from leaves of carya cathayensis ameliorate renal fibrosis via the miR-21/smad7 signaling pathway. Cell. Physiol. biochem. 49 (4), 1551–1563. doi:10.1159/000493458
Xu, C., Tang, F., Lu, M., Yang, J., Han, R., Mei, M., et al. (2016). Astragaloside IV improves the isoproterenol-induced vascular dysfunction via attenuating eNOS uncoupling-mediated oxidative stress and inhibiting ROS-NF-κB pathways. Int. Immunopharmacol. 33, 119–127. doi:10.1016/j.intimp.2016.02.009
Xu, D., Xia, Q., Wang, J., and Wang, P. (2008a). Molecular weight and monosaccharide composition of astragalus polysaccharides. Molecules 13 (10), 2408–2415. doi:10.3390/molecules13102408
Xu, X., Chen, X., Ji, H., Li, P., Bian, Y., Yang, D., et al. (2008b). Astragaloside IV improved intracellular calcium handling in Hypoxia-Reoxygenated cardiomyocytes via the sarcoplasmic reticulum ca2+-ATPase. Pharmacology 81 (4), 325–332. doi:10.1159/000121335
Xue, B., Huang, J., Ma, B., Yang, B., Chang, D., and Liu, J. (2019). Astragaloside IV protects primary cerebral cortical neurons from oxygen and glucose Deprivation/Reoxygenation by activating the PKA/CREB pathway. Neuroscience 404, 326–337. doi:10.1016/j.neuroscience.2019.01.040
Yan, H., Chen, Y., Li, L., Jiang, J., Wu, G., Zuo, Y., et al. (2016). Decorin alleviated chronic hydrocephalus via inhibiting TGF-β1/Smad/CTGF pathway after subarachnoid hemorrhage in rats. Brain Res. 1630, 241–253. doi:10.1016/j.brainres.2015.11.004
Yang, J., Wang, H., Zhang, Y., Yang, Y., Lu, M., Zhang, J., et al. (2013). Astragaloside IV attenuates inflammatory cytokines by inhibiting TLR4/NF-кB signaling pathway in isoproterenol-induced myocardial hypertrophy. J. Ethnopharmacol. 150 (3), 1062–1070. doi:10.1016/j.jep.2013.10.017
Yang, L., Dong, X., and Zhang, W. (2020). Astragaloside IV alleviates the brain damage induced by subarachnoid hemorrhage via PI3K/Akt signaling pathway. Neurosci. Lett. 735, 135227. doi:10.1016/j.neulet.2020.135227
Yang, Q., Lu, S., and Sun, H. (2011). Clinical effect of Astragalus granule of different dosages on quality of life in patients with chronic heart failure. Chin. J. Integr. Med. 17 (2), 146–149. doi:10.1007/s11655-011-0647-9
Yang, W. T., Zheng, X. W., Chen, S., Shan, C. S., Xu, Q. Q., Zhu, J. Z., et al. (2017). Chinese herbal medicine for Alzheimer's disease: Clinical evidence and possible mechanism of neurogenesis. Biochem. Pharmacol. 141, 143–155. doi:10.1016/j.bcp.2017.07.002
Yasuoka, H., Yamaguchi, Y., and Feghali-Bostwick, C. A. (2014). The membrane-associated adaptor protein DOK5 is upregulated in systemic sclerosis and associated with IGFBP-5-induced fibrosis. PLoS One 9 (2), e87754. doi:10.1371/journal.pone.0087754
Yu, K., Li, Q., Shi, G., and Li, N. (2018). Involvement of epithelial-mesenchymal transition in liver fibrosis. Saudi J. Gastroenterol. 24 (1), 5–11. doi:10.4103/sjg.SJG_297_17
Yu, W. N., Sun, L. F., and Yang, H. (2016). Inhibitory effects of astragaloside IV on Bleomycin-Induced pulmonary fibrosis in rats via attenuation of oxidative stress and inflammation. Inflammation 39 (5), 1835–1841. doi:10.1007/s10753-016-0420-5
Zhang, D., Bian, Z., Xu, J., Wu, H., Gu, C., Zhou, B., et al. (2012). Astragaloside IV alleviates hypoxia/reoxygenation-induced neonatal rat cardiomyocyte injury via the protein kinase a pathway. Pharmacology 90 (1-2), 95–101. doi:10.1159/000339476
Zhang, L., Li, Z., He, W., Xu, L., Wang, J., Shi, J., et al. (2015a). Effects of astragaloside IV against the TGF-β1-Induced Epithelial-to-Mesenchymal transition in peritoneal mesothelial cells by promoting smad 7 expression. Cell. Physiol. biochem. 37 (1), 43–54. doi:10.1159/000430332
Zhang, L., Xie, R., Yang, J., Zhao, Y., Qi, C., Bian, G., et al. (2019a). Chronic pain induces nociceptive neurogenesis in dorsal root ganglia from Sox2-positive satellite cells. Glia 67 (6), 1062–1075. doi:10.1002/glia.23588
Zhang, S., Tang, F., Yang, Y., Lu, M., Luan, A., Zhang, J., et al. (2015b). Astragaloside IV protects against Isoproterenol-Induced cardiac hypertrophy by regulating NF-κB/PGC-1α signaling mediated energy biosynthesis. PLoS One 10 (3), e0118759. doi:10.1371/journal.pone.0118759
Zhang, Y., Tao, C., Xuan, C., Jiang, J., and Cao, W. (2020). Transcriptomic analysis reveals the protection of Astragaloside IV against diabetic nephropathy by modulating inflammation. Oxid. Med. Cell. Longev. 2020, 9542165. doi:10.1155/2020/9542165
Zhang, Y., Zhang, Y., Jin, X., Zhou, X., Dong, X., Yu, W., et al. (2019b). The role of astragaloside IV against cerebral ischemia/reperfusion injury: Suppression of apoptosis via promotion of P62-LC3-autophagy. Molecules 24 (9), 1838. doi:10.3390/molecules24091838
Zhang, Z., Jiang, J., Li, Z., and Wan, W. (2022). The change of cytokines and gut microbiome in preterm infants for bronchopulmonary dysplasia. Front. Microbiol. 13, 804887. doi:10.3389/fmicb.2022.804887
Zhao, J., Yang, P., Li, F., Tao, L., Ding, H., Rui, Y., et al. (2012). Therapeutic effects of astragaloside IV on myocardial injuries: Multi-target identification and network analysis. PLoS One 7 (9), e44938. doi:10.1371/journal.pone.0044938
Zheng, S., Powell, D. W., Zheng, F., Kantharidis, P., and Gnudi, L. (2016). Diabetic nephropathy: Proteinuria, inflammation, and fibrosis. J. Diabetes Res. 2016, 5241549. doi:10.1155/2016/5241549
Zhou, X., Sun, X., Gong, X., Yang, Y., Chen, C., Shan, G., et al. (2017). Astragaloside IV from Astragalus membranaceus ameliorates renal interstitial fibrosis by inhibiting inflammation via TLR4/NF-кB in vivo and in vitro. Int. Immunopharmacol. 42, 18–24. doi:10.1016/j.intimp.2016.11.006
Zhou, X., Zou, J., Ao, C., Gong, D., Chen, X., and Ma, Y. (2020). Renal protective effects of astragaloside IV, in diabetes mellitus kidney damage animal models: A systematic review, meta-analysis. Pharmacol. Res. 160, 105192. doi:10.1016/j.phrs.2020.105192
Zhou, Y., Tong, X., Ren, S., Wang, X., Chen, J., Mu, Y., et al. (2016). Synergistic anti-liver fibrosis actions of total astragalus saponins and glycyrrhizic acid via TGF-β1/Smads signaling pathway modulation. J. Ethnopharmacol. 190, 83–90. doi:10.1016/j.jep.2016.06.011
Zuo, C., Xie, X. S., Qiu, H. Y., Deng, Y., Zhu, D., and Fan, J. M. (2009). Astragalus mongholicus ameliorates renal fibrosis by modulating HGF and TGF-β in rats with unilateral ureteral obstruction. J. Zhejiang Univ. Sci. B 10 (5), 380–390. doi:10.1631/jzus.B0820230
Zwickey, H., Brush, J., Iacullo, C. M., Connelly, E., Gregory, W. L., Soumyanath, A., et al. (2007). The effect of echinacea purpurea, Astragalus membranaceus and Glycyrrhiza glabra on CD25 expression in humans: A pilot study. Phytother. Res. 21 (11), 1109–1112. doi:10.1002/ptr.2207
Glossary
AD Alzheimer’s disease;
AKT protein kinase B
AM Astragalus mongholicus
APS Astragalus polysaccharide
AS-IV astragaloside IV
BPD bronchopulmonary dysplasia
CASE compound Astragalus and Salvia miltiorrhiza extract
CAG cycloastragalus alcohol
CG calycosin-7-O-β-D-glucoside
DM diabetes mellitus
DN diabetic nephropathy
ECM extracellular matrix
EGFL7 epidermal growth factor-like domain 7
EMT epithelial–mesenchymal transformation
ERK extracellular regulated protein kinase
FRD fibrosis-related diseases
GAS5 growth arrest specific 5
GSK-3β glycogen synthase kinase-3β
GST-P glutathione S-traferase placental type
HGF hepatocyte growth factor
HIF hypoxic inducible factor
HO-1 heme oxygenase-1
HSCs hepatic stellate cells
IL-17 interleukin
LPO lipid peroxidation
MAPK mitogen-activated protein kinase
MCP-1 Monocyte Chemoattractant Protein
MMP matrix metalloproteinase
mTORC1 mammalian target of rapamycin complex 1
NF-κB nuclear factor-kappa B
Nrf2 nuclear factor-erythroid 2-related factor 2
OGD oxygen-glucose deprivation
p70S6K ribosomal protein S6 kinaseβ-1
PAE Paeonia lactiflora and Astragalus Mongholicus extract
PAI-1 plasminogen activatorinhibitor-1
PD peritoneal dialysis
PDGFR-β platelet-derived growth factor receptor beta
PGC-1α peroxisome proliferator-activated receptor-γ coactivator 1α
PI3K phosphatidylinositol 3-hydroxy kinase
PS porcine-serum
SAH subarachnoid hemorrhage
SIKE The suppressor of IKKε
SSc systemic sclerosis
T2DM type-2 diabetes mellitus
TBK1 TANK-binding kinase 1
TCM traditional Chinese medicine
TGF-β1 transforming growth factor β1
TLR4 toll-like receptor 4
Keywords: Astragalus mongholicus, fibrosis-related diseases, inflammation, oxidative stress, metabolic regulation
Citation: Gong F, Qu R, Li Y, Lv Y and Dai J (2022) Astragalus Mongholicus: A review of its anti-fibrosis properties. Front. Pharmacol. 13:976561. doi: 10.3389/fphar.2022.976561
Received: 23 June 2022; Accepted: 18 August 2022;
Published: 07 September 2022.
Edited by:
Mohammad Reza Khazdair, Birjand University of Medical Sciences, IranCopyright © 2022 Gong, Qu, Li, Lv and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Lv, bHZ5aW5nMTk2NkBzbXUuZWR1LmNu; Jingxing Dai, ZGFpanhAc211LmVkdS5jbg==
 Fengying Gong1
Fengying Gong1 Jingxing Dai
Jingxing Dai