- 1Metabolic Medicine Department, Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom
- 2Genetics and Genomic Medicine Department, Great Ormond Street Institute of Child Health, University College London, London, United Kingdom
- 3National Institute of Health Research Great Ormond Street Biomedical Research Centre, London, United Kingdom
Vitamin B12 or cobalamin deficiency is a commonly encountered clinical scenario and most clinicians will have familiarity prescribing Vitamin B12 to treat their patients. Despite the high prevalence of this condition, there is widespread heterogeneity regarding routes, schedules and dosages of vitamin B12 administration. In this review, we summarise the complex metabolic pathway of Vitamin B12, the inherited and acquired causes of Vitamin B12 deficiency and subsequently highlight the disparate international practice of prescribing Vitamin B12 replacement therapy. We describe the evidence base underpinning the novel sublingual, intranasal and subcutaneous modes of B12 replacement in comparison to intramuscular and oral routes, with their respective benefits for patient compliance and cost-saving.
Introduction
In 1934, Whipple, Minot and Murphy were awarded the Nobel Prize for their game-changing discovery that feeding liver meat to patients affected by pernicious anaemia could act as a curative therapy for this previously invariably fatal condition (Whipple et al., 1920; Minot and Murphy, 1926). Subsequent decades of research, including Dorothy Hodgkin’s X-ray crystallography studies (Hodgkin et al., 1956), revealed that Vitamin B12 (otherwise known as cobalamin) was the important therapeutic compound curing these patients. This paved the way to prescribing B12 as a therapeutic compound to reverse cases of B12 deficiency.
Today, Vitamin B12 deficiency is a commonly encountered clinical diagnosis and most clinicians will have prescribed B12 replacement therapy for some of their patients. However, despite the widespread nature of this condition, there are no internationally standardised guidelines regarding dosage regimes, optimal formulation, frequency of administration of B12 therapy and monitoring of therapeutic effect, leading to significantly variable international practice (Andres and Serraj, 2012; Langan and Goodbred, 2017; Sobczyńska-Malefora et al., 2021).
In this review, we aim to summarise the complex pathway by which B12 is absorbed and metabolised and subsequently describe the inherited and acquired causes of B12 deficiency. We then summarise the disparate practice in B12 replacement internationally and highlight the more novel methods of B12 administration, including subcutaneous, intra-nasal and sublingual modes in comparison to intramuscular and oral routes.
Vitamin B12 absorption and metabolism
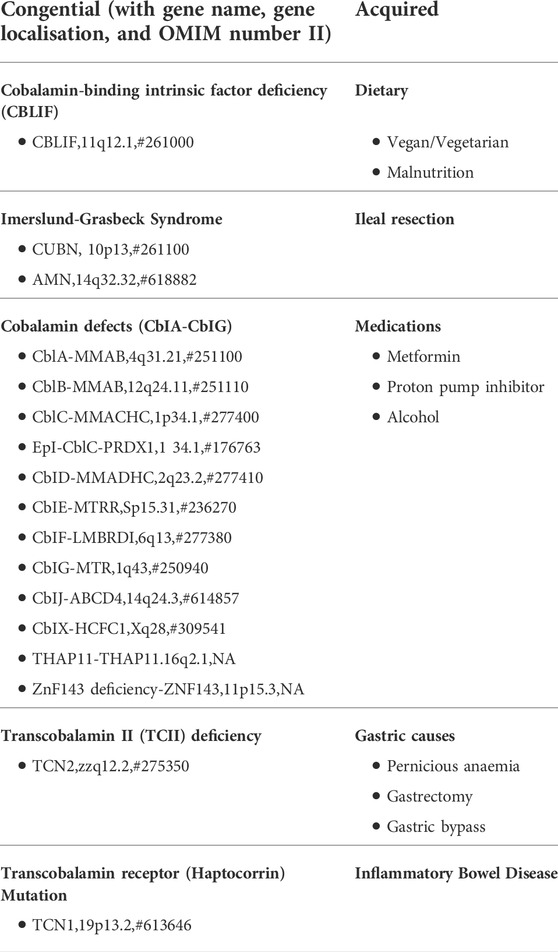
Vitamin B12 is a complex biomolecule that plays a vital part in key biochemical reactions. It is a water-soluble vitamin that is derived from the diet, particularly from eggs, red meat, and dairy. There are two distinct mechanisms by which B12 is absorbed: 1) passive diffusion across the mucous membranes of the gastrointestinal tract (Berlin et al., 1968) and 2) a receptor-mediated absorption process (Figure 1). Only an estimated 1%–2% of oral B12 can be passively absorbed and therefore, high dosages of oral B12 are required to provide therapeutic benefit in cases where the receptor-mediated process malfunctions (Withey et al., 1963).
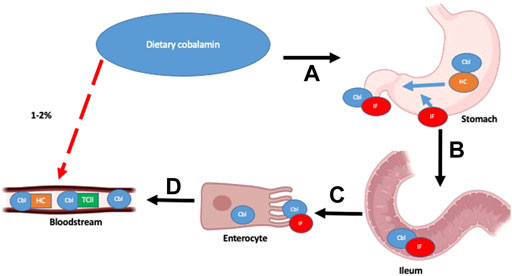
FIGURE 1. Vitamin B12 absorption pathways and main factors and organs involved: Receptor-mediated absorption processes shown as sequential steps (A–D) with black arrows and Passive diffusion process outlined with red, dashed arrow. Detailed description of pathway outlined in text. Adapted from “Human Anatomy, Digestive System,” by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-icons.https://app.biorender.com/biorender-icons
In the receptor-mediated absorptive process, ingested B12 is initially bound to Haptocorrin (HC)/R-factor secreted by salivary glands. Free B12 is once again released following degradation of HC by pancreatic proteases in the duodenum. It subsequently binds to the intrinsic factor (IF), which is secreted by gastric parietal cells. This B12-IF complex reaches the terminal ileum, where it binds to heterodimeric receptors cubam composed of an outer protein cubulin and transmembrane protein amnionless, located on the surface of polarized epithelial enterocytes in the apical brush border (Fyfe et al., 2004). The complex is then endocytosed, and free B12 is released into the bloodstream.
In the bloodstream, free B12 may be bound by HC or transcobalamin (TC). B12 bound to HC is not taken up by cells and thus, is biologically inactive (Hunt et al., 2014). However, B12 bound to TC forms a complex known as holoTC, which is the biologically active form of B12, that is taken up into cells via endocytosis using a specific CD320 receptor (Quadros, 2010). Following endocytosis, the HoloTC complex is degraded in lysosomes and free B12 is released into the cytoplasm, via CblF (Rutsch et al., 2009) and CblJ (Coelho et al., 2012).
This free cytoplasmic B12 undergoes a series of intracellular transport and modifications to enable two core enzymatic reactions: 1) adenosyl-B12 acts as a co-factor for the enzyme methylmalonyl-coA mutase that catalyzes the conversion of methylmalonyl-coA to succinyl-coA, thereby enabling restoration of tricarboxylic acid (TCA) cycle intermediates and 2) methyl-B12 enables methionine synthase in the re-methylation of homocysteine to methionine using methyl-tetrahydrofolate (MTHF) as a methyl donor, thereby enabling the vital biochemical pathways of the folate cycle and methionine cycle, facilitating the downstream synthesis of important compounds like DNA and RNA (Figure 2).
FIGURE 2. Summary of intracellular metabolism of transcobalamin. Detailed description of pathway found in text.
Causes of B12 deficiency and criteria for diagnosis
The absorption and metabolic pathway for B12 is highly complex, and defects in any part of this process can lead to therapeutic B12 replacement. The causes of B12 deficiency can be subdivided into congenital and acquired (Table 1).
Congenital causes are rare and are the result of genetic mutations in genes encoding proteins involved in B12 absorption, transport, and intracellular processing. These include 1) congenital pernicious anaemia, where there is a genetic defect in IF synthesis, leading to B12 malabsorption, 2) Imerslund-Gräsbeck Syndrome (IGS) caused by mutations in genes encoding cubulin and amnionless, 3) inherited HC or TCII deficiencies, and 4) intracellular cobalamin defects (CblA-CblG defects). Beyond mutations in the genes coding for these intracellular cobalamin proteins, there are also multiple mutations which affect the transcriptional regulation of MMACHC, leading to a similar phenotype of CblC deficiency (Watkins et al., 2017); In epi-CblC, an epimutation leads to a splicing variant in the adjacent PRDX1 gene causes transcriptional silencing of MMACHC (Guéant et al., 2018). As for CblX deficiency, mutations in the HCFC1 gene affect transcriptional expression of MMACHC (Yu et al., 2013). Finally, THAP11 deficiency (Quintana et al., 2017) and ZNF143 deficiency (Pupavac et al., 2016) also affect the transcriptional regulation of MMACHC. Acquired causes of B12 deficiency can be 1) dietary, especially in vegetarian and vegan populations, 2) autoimmune, as seen in pernicious anaemia, where there is destruction of IF-producing parietal cells mediated by autoantibodies, 3) iatrogenic secondary to disruption of the acid-base status of the stomach (e.g., with proton pump inhibitor use or metformin), and 4) caused by malfunction of the terminal ileum (e.g., inflammatory bowel diseases like Crohn’s disease, gastric bypass or surgical resection).
Regardless of the cause, the diagnosis of B12 deficiency is complex and can present at all ages with a wide spectrum of symptoms. Clinical presenting features of the condition include those relating to anaemia (e.g., pallor, weakness, and fatigue), gastro-intestinal symptoms (e.g., epigastric pain and glossitis), neurological symptoms (e.g., from numbness of extremities and impaired proprioception in subacute combined degeneration of the spinal cord to severe developmental delay, epilepsy, and coma in inherited intracellular cobalamin defects), psychiatric disturbances and neural tube defects in the newborn of a deficient mother (Molloy et al., 2009).
In cases which arouse clinical suspicion, there is no gold standard single test for the diagnosis of this condition (Solomon, 2005; Herrmann and Obeid, 2012), but multiple laboratory investigations can be performed. After all, serum B12 can be within the reference range even in cases of severe B12 deficiency (Devalia et al., 2014). This is also true where the therapeutic effect of B12 supplementation needs to be monitored as after all, as although low B12 levels may indicate deficiency, high B12 levels may not suggest sufficient treatment, especially in the context of persistent symptoms. Possible laboratory investigations which can be performed include serum B12 [i.e., <148 pmol/L cut-off in the UK (Devalia et al., 2014)] and holoTC levels. Confirmatory testing of homocysteine, methionine, and methylmalonic acid levels may also prove useful (Quadros, 2010; Carmel, 2011; Fedosov, 2012). It is important to note that these latter tests are not widely available and the diagnostic cut-off values differ between laboratories (Fedosov, 2012; Aparicio-Ugarriza et al., 2015). Whole exome sequencing or fibroblast complementation studies can be performed in suspected cases of genetic cobalamin defects to identify the disease-causing gene.
Current guidelines for B12 replacement
Vitamin B12 is available in four different formulations—cyanocobalamin (CN-Cbl), hydroxocobalamin (OH-Cbl), methylcobalamin (methyl-Cbl), and adenosylcobalamin (adenosyl-Cbl)—depending on the R residual group attached to the core cobalamin molecule. These formulations have different affinities to key proteins and receptors which facilitate the generation of biologically active intracellular cobalamin and thus, differ in their rates of tissue retention and bioavailability (Paul and Brady, 2017). Additionally, the solvent body in which B12 is dissolved may also influence its overall absorption and bioavailability (Arendt and Nexø, 2011). Regardless of the form of B12 administered, all of them are ultimately reduced to the core cobalamin molecule within the cytoplasm before proceeding to downstream reactions outlined above (Obeid et al., 2015).
The formulation and dosage regimens of B12 used to treat Vitamin B12 deficiency vary widely depending on the aetiology of the deficiency as well as region of medical practice (Table 2). For inherited B12 deficiency, treatment is guided by the genetic condition. Although oral treatment has been trialled with some success (Gangarossa et al., 1996; Bor et al., 2008), the parenteral modality of B12 replacement [particularly intramuscular (IM)], has proven successful in the management of this cluster of rare diseases. In cobalamin-binding intrinsic factor deficiency, retrospective studies of patients with this rare condition have revealed that daily 1 mg intramuscular (IM) injections of OH-Cbl/CN-Cbl are effective and can be gradually spaced out depending on the metabolic and clinical response of patients, with patients eventually becoming stabilised on twice yearly 1 mg intramuscular (IM) injections of OH-Cbl/CN-Cbl (Abdallah et al., 2012). This same treatment regimen has also been used with success in Imerslund-Gräsbeck syndrome (Abdallah et al., 2012), although an alternative treatment regimen which has proven useful in this condition is 1 mg IM OH-Cbl daily for 10 days and then once a month for lifetime (Gräsbeck, 2006). As for cobalamin-related remethylation disorders (CblC or CblJ deficiency), daily 1 mg IM OH-Cbl has proven effective, with frequency of administration individually titrated according to metabolic response (Huemer et al., 2017). Another prospective review of 26 patients with late-onset CblC deficiency with neuropsychiatric manifestations demonstrated efficacy of a different treatment regimen (loading dose of 0.5–1 mg daily IM OH-Cbl or CN-Cbl for 4–8 weeks then maintenance dose of 0.5–1 mg weekly) in improving the metabolic and clinical parameters of these patients (Wang et al., 2019). As for transcobalamin II deficiency, an observational case series of 30 patients revealed that IM administration of 1 mg of OH-Cbl or CN-Cbl weekly for a lifetime appears to be a suitable treatment regimen (Trakadis et al., 2014).

TABLE 2. Formulation and dosage regimes for treatment of vitamin B12 deficiency, subdivided into congenital and acquired causes. Abbreviations: OH-Cbl, hydroxocobalamin; CN-Cbl, cyanocobalamin.
In acquired B12 deficiency, both oral and IM routes have been featured in guidelines to replenish this compound in deficient states. However, there does not appear to be internationally standardised practice in how this is achieved (Wentworth and Copland, 2018), and a non-exhaustive list of varying practice of a few key countries is summarised (Table 2). In Britain, the British National Formulary (BNF) recommends 1 mg IM OH-Cbl 3 times a week for 2 weeks and then maintenance 1 mg IM OH-Cbl every 3 months in patients without neurological involvement; as for patients with neurological improvement, 1 mg IM OH-Cbl on alternate days until there is no clinical improvement and then maintenance 1 mg IM OH-Cbl every 2 months is recommended (NICE, 2022). For asymptomatic, borderline cases, 50
In Canada, advice from the British Columbia Medical Association outlines that 1 mg daily oral CN-Cbl should be used for pernicious anemia or food-bound cobalamin malabsorption (FBCM) whereas in most other cases, a daily dose of 250
In Netherlands, controversy arose when the Dutch Organization of General Practitioners published a 2014 viewpoint that 1 mg daily oral OH-Cbl can be utilised as treatment in cases with a cobalamin count below 148 pmol/L and clinical symptoms, with the Dutch B12 Society disputing the efficacy of this and insisting that the advice from the Dutch Healthcare Institute Pharmacotherapeutic Compass should be followed instead (IM/SC starting dose of 10 injections of 1 mg OH-Cbl at intervals of at least 3 days; maintenance dose once every 2 months or 300
In France, the Strasbourg-based CARE B12 research group recommends differing treatment regimens for pernicious anaemia and other causes of B12 deficiency (Andrès et al., 2018); In pernicious anaemia, the treatment regimens of either 1 mg daily lifelong oral CN-Cbl or 1 mg IM CN-Cbl daily for 1 week then once weekly for 1 month then monthly for life are recommended. However, in cases with moderate to severe neurological manifestations, higher doses of 1–2 mg per day for at least 2–3 months are recommended. In food-bound cobalamin malabsorption, Crohn’s disease, malabsorption, or dietary deficiency, either 1 mg daily oral CN-Cbl daily for 1 month and then 0.125–1 mg daily CN-Cbl until cause is corrected or 1 mg IM daily CN-Cbl for 1 week then once weekly for 1 month then per month for 1–3 months until cause is corrected are recommended. A higher dosage regimen of 1 mg daily for 1–3 months is recommended in cases of severe neurological manifestations (Andrès et al., 2018).
In Australia, the Immigrant Health Service of the Royal Children’s Hospital of Melbourne recommends varying treatment regimens based on age of the patient (Immigrant Health Service, 2013); for infants with neurological involvement or macrocytic anaemia, a 250
The different recommended doses, formulations and treatment schedules both between and within these countries highlight the important disparity in international clinical practice for the treatment of this heterogenous condition. Nevertheless, the IM route of either OH-Cbl or CN-Cbl remains more established and widely recommended for treatment of B12 deficiency.
Oral B12
There is growing popularity for the oral route of B12 replacement, and as outlined above, it is recommended in various countries as a potential treatment modality for B12 deficiency. In fact, it has proven to be the preferred mode of treatment in numerous countries including Sweden, Norway, and Canada (Nilsson et al., 2005). This is largely in view of the high cost savings, ease of administration and reduced pain and injection-associated injury (Wentworth and Copland, 2018).
Three key trials have been comparing IM and oral administration modalities for B12 replacement in small patient groups with varying causes of B12 deficiency. Doses and frequency of administration were variable but ranged anywhere from 0.5 to 2 mg (Kuzminski et al., 1998; Bolaman et al., 2003; Sanz-Cuesta et al., 2020). A recent Cochrane review of these trials, involving 153 patients, found comparable serum B12 levels in patients taking either route (Wang et al., 2018). It is important to highlight that none of these trials looked at the clinical signs and symptoms of these patients nor outcomes relating to their quality of life. Furthermore, observation of patient compliance to daily oral B12 intake is difficult to assess and might affect patient biochemistry and outcomes long-term (Stabler et al., 2013). Finally, none of these studies included intention-to-treat analysis, blinding or sample size calculation, which may have introduced bias in their statistical analysis (Vidal-Alaball et al., 2004; Wentworth and Copland, 2018). Thus, IM administration remains the preferred route of B12 replacement in cases of known malabsorption or severe deficiency.
Sublingual B12
The sublingual route can also be considered for replacement therapy in B12 deficiency. With sublingual administration, the complex B12 absorption process can be bypassed and B12 can directly reach the bloodstream via the sublingual veins. With the relative ease and painless nature of administration, it is especially considered in the paediatric population. Early evidence for the potential efficacy of this route was published in 1999, where 18 patients with B12 deficiency treated exclusively with 7–12 days of 2 mg of sublingual cobalamin daily had 4 times higher B12 levels compared to pre-treatment, reaching therapeutic ranges (Delpre et al., 1999). A further study demonstrated that a dose as small as 0.35 mg once weekly of CN-Cbl over 12 weeks may be sufficient at normalising serum B12 levels (Del Bo et al., 2019).
There have also been further studies comparing treatment outcomes from the sublingual route to the more established IM and oral routes. Sharabi et al. (2003) compared the efficacy of 0.5 mg daily doses of CN-Cbl in either sublingual or oral formulation for 30 subjects with low serum concentrations of B12 and found that B12 levels normalised in 4 weeks for both treatment groups, with no significant difference between the groups. A further Israeli study from 2019 performed a retrospective case note review of >4,000 patients with B12 deficiency treated with sublingual or IM B12 (but without detailing dosages and schedules of administration included in the study) and showed that the mean difference in serum B12 levels before and after treatment was significantly higher in the sublingual than IM group (Bensky et al., 2019).
Tuğba-Kartal and Çağla-Mutlu (2020) also demonstrated in a retrospective review of 129 paediatric patients that a treatment regimen of sublingual B12 (both CN-Cbl and methyl-Cbl) at 1 mg once daily for 7 days then every other day for 3 weeks was as effective as the same dose schedule of IM B12 at normalising serum B12 levels. Finally, a recent 2021 study performed in a paediatric population (0–3 years) in Turkey found that a sublingual methyl-Cbl treatment regimen, comprising 1 mg daily replacement for a week followed by every other day for 2 weeks, 2 days/week for 2 weeks and once a week for 3 months was as effective as similar dose schedules of IM and oral CN-Cbl at normalising B12 levels (Orhan Kiliç et al., 2021).
Although these studies report promising results regarding the sublingual route, it is important to note that almost all of them only include patients with mild, subclinical B12 deficiency, making it difficult to extrapolate their outcomes for patients with severe deficiency or malabsorptive states. These studies often took place for a few week- or month-long period, making it difficult to understand the longer-term potential of sublingual B12 at maintaining serum B12 levels. Sublingual B12 was administered in “laboratory” conditions, where there was no dose omission, making it difficult to extrapolate “real-world” effects, where patient compliance may not be as good. Furthermore, they only look at serum B12 as a biomarker of B12 deficiency and do not consider methylmalonic acid, homocysteine, methionine, haematological parameters, or clinical symptomatology in their assessment of therapeutic effect. Therefore, it remains difficult to recommend with confidence this route of administration for all patient populations.
Intranasal B12
Similar to sublingual B12, intranasal B12 can also bypass the complex B12 absorption process and directly reach the bloodstream. With the ease of administration, it also possesses the same advantages as sublingual therapy for patients and has been shown to be a preferred treatment modality to the IM route (Suzuki et al., 2006). However, evidence of efficacy of this route compared to more established treatment routes remains limited. One study from 1997 recruited six patients with plasma B12 levels of <200 ng/L and administered a 1.5 mg intranasal dose of OH-Cbl at day 0, 14, and 21, subsequently measuring plasma B12 concentrations at timepoints between 1 h and 35 days following administration. This study showed an 8 times increase in the mean cobalamin concentration sustained 1 week following the final dose (Slot et al., 1997). A further small study done recently on 10 paediatric patients similarly found that intranasal OH-Cbl normalised serum B12 levels in deficient patients (Estourgie-van Burk et al., 2020).
Subcutaneous B12
Subcutaneous B12 is occasionally used, especially in patients with inborn errors of cobalamin metabolism, who need high pharmacological doses of B12 to maintain their B12-dependent metabolic pathways and related biochemistry within normal range. Studies looking at the efficacy of the subcutaneous route at replenishing B12 levels in depleted states are limited. Serum and liver vitamin B12 concentrations were found to be significantly elevated for 24 days in lambs treated with a single 2 mg subcutaneous vitamin B12 injection, compared to untreated controls (Grace et al., 1998).
There have also been a few case series published reporting the general efficacy of the subcutaneous route at normalising plasma B12 levels; Lotz-Havla et al. (2021) described the subcutaneous administration of OH-Cbl in four paediatric patients (two patients with CblC deficiency, one patient with CblG deficiency and one patient with CblE deficiency). Total homocysteine levels remained within the target range with this alternative route of administration (vis-à-vis IM B12 which the patients had previously been established on) and was described as a more pain-free and acceptable treatment option for all patients studied. A further case report outlined a subcutaneous port system inserted to deliver daily OH-Cbl for a patient with CblA deficiency—the patient in this case, who previously had been stable on IM B12 injections, had normal methylmalonic acid levels throughout the study period (Maines et al., 2016).
It is critical to note that a rigorous comparison of subcutaneous to IM and oral treatment modalities have not been published yet. This makes it difficult to suggest it as an equal alternative to more established treatment modalities for B12 deficiency.
Potential cost savings
There are numerous cost savings generated when switching from IM B12 to alternative treatment routes. A previous cost minimization analysis from UK prescribing patterns and costs, revealed that switching patients to oral B12 from the IM route could lead to 50% cost savings (Vidal-Alaball et al., 2006). Most savings could come from the reduction of resources associated with home visits. A further study performed in Canada estimated that switching patients established on IM B12 to the oral route could save the single province of Ontario between 1.4 and 9 million US Dollars over a 5-year period (Van Walraven et al., 2001). These cost savings include costs for room rental/upkeep, needle disposal as well as remuneration for attendant health professionals. We hypothesize that consideration of sublingual/intranasal B12 will also lead to similar savings, due to similar ease of administration as oral B12.
Conclusion
B12 deficiency is a common diagnosis in clinical practice. Causes are numerous and the diagnostic process can be complex. The therapeutic management based on B12 supplementation remains heterogeneous both between and within countries on the treatment routes, schedule, and dosage regimens. If oral and intramuscular injections have been proposed historically, alternative treatment routes have been recently proposed with sublingual, intranasal and subcutaneous administrations, which are more patient-friendly than painful intramuscular injections. This is particularly true for paediatric patients. The evidence base requires appropriate clinical trials, with additional endpoints including clinical symptoms of patients and additional biochemical parameters beyond plasma B12 levels alone before these alternative routes can be recommended with confidence in clinical guidelines. It is essential that clinical trials can be developed to effectively compare these routes of administration and that clinicians can share their experiences with these modalities of B12 administration so that they can be popularised and considered as viable alternatives for patients. This need becomes even more pressing in view of the potential cost-savings these alternatives will provide for financially stressed public health services. Nevertheless, in cases of severe deficiency with neurological sequelae, we suggest that IM B12 is used in the first instance to replenish body stores with the treatment regimen, including dosage and formulation, optimised to keep the patient free of symptoms and tailored to the metabolic response of not only plasma B12 levels, but also confirmatory testing of homocysteine, methionine, and methylmalonic acid levels.
Author contributions
RE and JB designed the study. RE wrote the manuscript. All authors reviewed and approved the manuscript.
Funding
JB is supported by funding from the United Kingdom Medical Research Council Clinician Scientist Fellowship MR/T008024/1 and NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of interest
Authors RE and JB were employed by the company Great Ormond Street Hospital for Children NHS Foundation Trust.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdallah, A. B., de Baulny, H. O., Kozyraki, R., Passemard, S., Fenneteau, O., Lebon, S., et al. (2012). How can cobalamin injections be spaced in long-term therapy for inborn errors of vitamin B12 absorption? Mol. Genet. Metab. 107 (1-2), 66–71. doi:10.1016/j.ymgme.2012.07.007
Andres, E., and Serraj, K. (2012). Optimal management of pernicious anemia. J. Blood Med. 3, 97–103. doi:10.2147/JBM.S25620
Andrès, E., Zulfiqar, A-A., Serraj, K., Vogel, T., and Kaltenbach, G. (2018). Systematic review and pragmatic clinical approach to oral and nasal vitamin B12 (cobalamin) treatment in patients with vitamin B12 deficiency related to gastrointestinal disorders. J. Clin. Med. 7 (10), 304. doi:10.3390/jcm7100304
Aparicio-Ugarriza, R., Palacios, G., Alder, M., and González-Gross, M. (2015). A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin. Chem. Lab. Med. 53 (8), 1149–1159. doi:10.1515/cclm-2014-0784
Arendt, J., and Nexø, E. (2011). Treatment response in vitamin B12 deficiency depends on the chosen vitamin B12 preparation. Ugeskr. Laeger 173 (42), 263
Author Anonymous, (2022). Institute of Netherlands. Available at: https://b12-institute.nl/en/letter-to-the-dutch-college-of-general-practitioners-guideline-development-and-science-department-nederlands-huisartsen-genootschap-nhg/.
Bensky, M. J., Ayalon-Dangur, I., Ayalon-Dangur, R., Naamany, E., Gafter-Gvili, A., Koren, G., et al. (2019). Comparison of sublingual vs. intramuscular administration of vitamin B12 for the treatment of patients with vitamin B12 deficiency. Drug Deliv. Transl. Res. 9 (3), 625–630. doi:10.1007/s13346-018-00613-y
Berlin, H., Berlin, R., and Brante, G. (1968). Oral treatment of pernicious anemia with high doses of vitamin B12 without intrinsic factor. Acta Med. Scand. 184 (1-6), 247–258. doi:10.1111/j.0954-6820.1968.tb02452.x
Bolaman, Z., Kadikoylu, G., Yukselen, V., Yavasoglu, I., Barutca, S., and Senturk, T. (2003). Oral versus intramuscular cobalamin treatment in megaloblastic anemia: A single-center, prospective, randomized, open-label study. Clin. Ther. 25 (12), 3124–3134. doi:10.1016/s0149-2918(03)90096-8
Bor, M. V., Çetin, M., Aytaç, S., Altay, Ç., Ueland, P. M., and Nexo, E. (2008). Long term biweekly 1 mg oral vitamin B12 ensures normal hematological parameters, but does not correct all other markers of vitamin B12 deficiency. A study in patients with inherited vitamin B12 deficiency. Haematologica 93 (11), 1755–1758. doi:10.3324/haematol.13122
British Columbia (2013). Cobalamin (vitamin B12) deficiency - investigation & management. Available at: https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/vitamin-b12.
Carmel, R. (2011). Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: A critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am. J. Clin. Nutr. 94 (1), 348S–358S. doi:10.3945/ajcn.111.013441
Coelho, D., Kim, J. C., Miousse, I. R., Fung, S., du Moulin, M., Buers, I., et al. (2012). Mutations in ABCD4 cause a new inborn error of vitamin B12 metabolism. Nat. Genet. 44 (10), 1152–1155. doi:10.1038/ng.2386
College voor Zorgverzekeringen (2011). Farmacotherapeutisch Kompas, (pharmaceutical reference book) Amstelveen.
Del Bo, C., Riso, P., Gardana, C., Brusamolino, A., Battezzati, A., and Ciappellano, S. (2019). Effect of two different sublingual dosages of vitamin B12 on cobalamin nutritional status in vegans and vegetarians with a marginal deficiency: A randomized controlled trial. Clin. Nutr. 38 (2), 575–583. doi:10.1016/j.clnu.2018.02.008
Delpre, G., Stark, P., and Niv, Y. (1999). Sublingual therapy for cobalamin deficiency as an alternative to oral and parenteral cobalamin supplementation. Lancet 354 (9180), 740–741. doi:10.1016/S0140-6736(99)02479-4
Devalia, V., Hamilton, M. S., and Molloy, A. M. (2014). Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br. J. Haematol. 166 (4), 496–513. doi:10.1111/bjh.12959
Estourgie-van Burk, G. F., van der Kuy, P. H. M., de Meij, T. G., Benninga, M. A., and Kneepkens, C. (2020). Intranasal treatment of vitamin B12 deficiency in children. Eur. J. Pediatr. 179 (2), 349–352. doi:10.1007/s00431-019-03519-0
Fedosov, S. N. (2012). Physiological and molecular aspects of cobalamin transport. Subcell. Biochem. 56, 347–367. doi:10.1007/978-94-007-2199-9_18
Fyfe, J. C., Madsen, M., Højrup, P., Christensen, E. I., Tanner, S. M., de la Chapelle, A., et al. (2004). The functional cobalamin (vitamin B12)–intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood 103 (5), 1573–1579. doi:10.1182/blood-2003-08-2852
Gangarossa, S., Romano, V., and Schiliro', G. (1996). Efficacy of oral administration of high-dose cobamamide in a patient with Imerslund-Grasbeck syndrome. Pediatr. Hematol. Oncol. 13 (4), 387–389. doi:10.3109/08880019609030846
Grace, N., West, D., and Sargison, N. (1998). The efficacy of a subcutaneous injection of soluble Vitamin B12 in lambs. N. Z. Vet. J. 46 (5), 194–196. doi:10.1080/00480169.1998.36089
Gräsbeck, R. (2006). Imerslund-Gräsbeck syndrome (selective vitamin B12 malabsorption with proteinuria). Orphanet J. Rare Dis. 1 (1), 17. doi:10.1186/1750-1172-1-17
Guéant, J-L., Chery, C., Oussalah, A., Nadaf, J., Coelho, D., Josse, T., et al. (2018). A PRDX1 mutant allele causes a MMACHC secondary epimutation in cblC patients. Nat. Commun. 9 (1), 67–12. doi:10.1038/s41467-017-02306-5
Herrmann, W., and Obeid, R. (2012). Cobalamin deficiency. Water Soluble Vitamins, 301. doi:10.1007/978-94-007-2199-9_16
Hodgkin, D. C., Kamper, J., Mackay, M., Pickworth, J., Trueblood, K. N., and White, J. G. (1956). Structure of vitamin B12. Nature 178 (4524), 64–66. doi:10.1038/178064a0
Huemer, M., Diodato, D., Schwahn, B., Schiff, M., Bandeira, A., Benoist, J. F., et al. (2017). Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J. Inherit. Metab. Dis. 40 (1), 21–48. doi:10.1007/s10545-016-9991-4
Hunt, A., Harrington, D., and Robinson, S. (2014). Vitamin B12 deficiency. Bmj 349, g5226. doi:10.1136/bmj.g5226
Immigrant Health Service (2013). Vitamin B12. Available at: https://www.rch.org.au/immigranthealth/clinical/Vitamin_B12/.
Kuzminski, A. M., Del Giacco, E. J., Allen, R. H., Stabler, S. P., and Lindenbaum, J. (1998). Effective treatment of cobalamin deficiency with oral cobalamin. Blood 92 (4), 1191–1198. doi:10.1182/blood.v92.4.1191.416k15_1191_1198
Langan, R. C., and Goodbred, A. J. (2017). Vitamin B12 deficiency: Recognition and management. Am. Fam. Physician 96 (6), 384
Lotz-Havla, A. S., Weiß, K. J., Schiergens, K. A., Brunet, T., Kohlhase, J., Regenauer-Vandewiele, S., et al. (2021). Subcutaneous vitamin B12 administration using a portable infusion pump in cobalamin-related remethylation disorders: A gentle and easy to use alternative to intramuscular injections. Orphanet J. Rare Dis. 16 (1), 215–218. doi:10.1186/s13023-021-01847-9
Maines, E., Morandi, G., Gugelmo, G., Ion-Popa, F., Campostrini, N., Pasini, A., et al. (2016). Vitamin B 12 administration by subcutaneous catheter device in a cobalamin A (cblA) patient. JIMD Rep. 35, 29–31. doi:10.1007/8904_2016_20
Minot, G. R., and Murphy, W. P. (1926). Treatment of pernicious anemia by a special diet. J. Am. Med. Assoc. 87 (7), 470–476. doi:10.1001/jama.1926.02680070016005
Molloy, A. M., Kirke, P. N., Troendle, J. F., Burke, H., Sutton, M., Brody, L. C., et al. (2009). Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics 123 (3), 917–923. doi:10.1542/peds.2008-1173
NICE (2022). BNF via NICE is only available in the UK. Available at: https://bnf.nice.org.uk/drugs/hydroxocobalamin/.
Nilsson, M., Norberg, B., Hultdin, J., Sandström, H., Westman, G., and Lökk, J. (2005). Medical intelligence in Sweden. Vitamin B12: Oral compared with parenteral? Postgrad. Med. J. 81 (953), 191–193. doi:10.1136/pgmj.2004.020057
Obeid, R., Fedosov, S. N., and Nexo, E. (2015). Cobalamin coenzyme forms are not likely to be superior to cyano-and hydroxyl-cobalamin in prevention or treatment of cobalamin deficiency. Mol. Nutr. Food Res. 59 (7), 1364–1372. doi:10.1002/mnfr.201500019
Orhan Kiliç, B., Kiliç, S., Şahin Eroğlu, E., Gül, E., and Belen Apak, F. B. (2021). Sublingual methylcobalamin treatment is as effective as intramuscular and peroral cyanocobalamin in children age 0–3 years. Hematology 26 (1), 1013–1017. doi:10.1080/16078454.2021.2010877
Paul, C., and Brady, D. M. (2017). Comparative bioavailability and utilization of particular forms of B12 supplements with potential to mitigate B12-related genetic polymorphisms. Integr. Med. 16 (1), 42–49.
Pupavac, M., Watkins, D., Petrella, F., Fahiminiya, S., Janer, A., Cheung, W., et al. (2016). Inborn error of cobalamin metabolism associated with the intracellular accumulation of transcobalamin-bound cobalamin and mutations in ZNF143, which codes for a transcriptional activator. Hum. Mutat. 37 (9), 976–982. doi:10.1002/humu.23037
Quadros, E. V. (2010). Advances in the understanding of cobalamin assimilation and metabolism. Br. J. Haematol. 148 (2), 195–204. doi:10.1111/j.1365-2141.2009.07937.x
Quintana, A. M., Yu, H-C., Brebner, A., Pupavac, M., Geiger, E. A., Watson, A., et al. (2017). Mutations in THAP11 cause an inborn error of cobalamin metabolism and developmental abnormalities. Hum. Mol. Genet. 26 (15), 2838–2849. doi:10.1093/hmg/ddx157
Rutsch, F., Gailus, S., Miousse, I. R., Suormala, T., Sagne, C., Toliat, M. R., et al. (2009). Identification of a putative lysosomal cobalamin exporter altered in the cblF defect of vitamin B 12 metabolism. Nat. Genet. 41 (2), 234–239. doi:10.1038/ng.294
Sanz-Cuesta, T., Escortell-Mayor, E., Cura-Gonzalez, I., Martin-Fernandez, J., Riesgo-Fuertes, R., Garrido-Elustondo, S., et al. (2020). Oral versus intramuscular administration of vitamin B12 for vitamin B12 deficiency in primary care: A pragmatic, randomised, non-inferiority clinical trial (OB12). BMJ open 10 (8), e033687. doi:10.1136/bmjopen-2019-033687
Sharabi, A., Cohen, E., Sulkes, J., and Garty, M. (2003). Replacement therapy for vitamin B12 deficiency: Comparison between the sublingual and oral route. Br. J. Clin. Pharmacol. 56 (6), 635–638. doi:10.1046/j.1365-2125.2003.01907.x
Slot, W. B., Merkus, F., Van Deventer, S., and Tytgat, G. (1997). Normalization of plasma vitamin B12 concentration by intranasal hydroxocobalamin in vitamin B12-deficient patients. Gastroenterology 113 (2), 430–433. doi:10.1053/gast.1997.v113.pm9247460
Sobczyńska-Malefora, A., Delvin, E., McCaddon, A., Ahmadi, K. R., and Harrington, D. J. (2021). Vitamin B12 status in health and disease: A critical review. Diagnosis of deficiency and insufficiency–clinical and laboratory pitfalls. Crit. Rev. Clin. Lab. Sci. 58 (6), 399–429. doi:10.1080/10408363.2021.1885339
Solomon, L. R. (2005). Cobalamin-responsive disorders in the ambulatory care setting: Unreliability of cobalamin, methylmalonic acid, and homocysteine testing. Blood 105 (3), 978–985. doi:10.1182/blood-2004-04-1641
Stabler, S. P., Korson, M., Jethva, R., Allen, R. H., Kraus, J. P., Spector, E. B., et al. (2013). Metabolic profiling of total homocysteine and related compounds in hyperhomocysteinemia: Utility and limitations in diagnosing the cause of puzzling thrombophilia in a family. JIMD Rep. 368 (2), 149–163. doi:10.1007/8904_2013_235
Suzuki, D. M., Alagiakrishnan, K., Masaki, K. H., Okada, A., and Carethers, M. (2006). Patient Acceptance of intranasal cobalamin gel for vitamin B12 replacement therapy. Hawaii Med. J. 65 (11), 311–314.
Trakadis, Y., Alfares, A., Bodamer, O., Christodoulou, J., Connor, P., Nordwall, M., et al. (2014). Update on transcobalamin deficiency: Clinical presentation, treatment and outcome. J. Inherit. Metab. Dis. 37 (3), 461–473. doi:10.1007/s10545-013-9664-5
Tuğba-Kartal, A., and Çağla-Mutlu, Z. (2020). Comparison of sublingual and intramuscular administration of vitamin B12 for the treatment of vitamin B12 deficiency in children. Rev. Invest. Clin. 72 (6), 380–385. doi:10.24875/RIC.20000208
Van Walraven, C., Austin, P., and Naylor, C. D. (2001). Vitamin B12 injections versus oral supplements. How much money could be saved by switching from injections to pills? Can. Fam. Physician 47 (1), 79
Vidal-Alaball, J., Butler, C., Hood, K., Cannings, R., McCaddon, A., and Papaioannou, A. (2004). Oral vitamin B12 versus parenteral vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst. Rev. 1. doi:10.1002/14651858.cd004655
Vidal-Alaball, J., Butler, C. C., and Potter, C. C. (2006). Comparing costs of intramuscular and oral vitamin B12 administration in primary care: A cost-minimization analysis. Eur. J. Gen. Pract. 12 (4), 169–173. doi:10.1080/14017430601049449
Wang, H., Li, L., Qin, L. L., Song, Y., Vidal-Alaball, J., and Liu, T. H. (2018). Oral vitamin B 12 versus intramuscular vitamin B 12 for vitamin B 12 deficiency. Cochrane Database Syst. Rev. 3 (33), CD004655. doi:10.1002/14651858.cd004655.pub3
Wang, S-J., Yan, C-Z., Wen, B., and Zhao, Y-Y. (2019). Clinical feature and outcome of late-onset cobalamin C disease patients with neuropsychiatric presentations: A Chinese case series. Neuropsychiatr. Dis. Treat. 15, 549–555. doi:10.2147/NDT.S196924
Watkins, D., and Rosenblatt, D. S. (2017). “Inherited defects of cobalamin metabolism,” in Vitamin B12: Advances and insights. Editor R. Obeid (Florida, United States: CRC Press), 94
Wentworth, B. J., and Copland, A. P. (2018). Revisiting vitamin B12 deficiency: A clinician’s guide for the 21st century. Pract. Gastroenterol. 182, 29. https://www.practicalgastro-digital.com/practicalgastro/december_2018/MobilePagedArticle.action?articleId=1458298.
Whipple, G. H., Robscheit, F., and Hooper, C. (1920). Blood regeneration following simple anemia: IV. Influence of meat, liver and various extractives, alone or combined with standard diets. Am. J. Physiology-Legacy Content 53 (2), 236–262. doi:10.1152/ajplegacy.1920.53.2.236
Withey, J., Jones, J., and Kilpatrick, G. (1963). Long-term trial of oral treatment of pernicious anaemia with vitamin-B12–peptide. Br. Med. J. 1 (5345), 1583–1585. doi:10.1136/bmj.1.5345.1583
Keywords: cobalamin, hydroxocobalamin, cyanocobalamin, vitamin B12, metabolic
Citation: Elangovan R and Baruteau J (2022) Inherited and acquired vitamin B12 deficiencies: Which administration route to choose for supplementation?. Front. Pharmacol. 13:972468. doi: 10.3389/fphar.2022.972468
Received: 18 June 2022; Accepted: 23 August 2022;
Published: 29 September 2022.
Edited by:
Miguel Gonzalez-Muñoz, University Hospital La Paz, SpainReviewed by:
Emmanuel Andrès, Hôpitaux Universitaires de Strasbourg, FranceMalvina Hoxha, Catholic University Our Lady of Good Counsel, Albania
Copyright © 2022 Elangovan and Baruteau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julien Baruteau, ai5iYXJ1dGVhdUB1Y2wuYWMudWs=
 Ramyia Elangovan
Ramyia Elangovan Julien Baruteau
Julien Baruteau