
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 03 October 2022
Sec. Renal Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.969107
This article is part of the Research TopicNew Insights into Renal Fibrosis and Therapeutic Effects of Natural Products, Volume IIView all 19 articles
Lack of effective drugs for acute kidney injury (AKI) grades 1–2 is a crucial challenge in clinic. Our previously single-center clinical studies indicated Chuan Huang Fang (CHF) might have nephroprotection in AKI on chronic kidney disease (CKD) (A on C) patients by preventing oxidant damage and inhibiting inflammation. Reduced glutathione (RG) has recently been shown to increase the clinical effectiveness of high-flux hemodialysis among patients with severe AKI. In this multicenter randomized controlled clinical study, we designed a new protocol to assess the efficacy and safety of CHF combining RG in patients with A on C. We also explored therapeutic mechanisms from renal fibrosis biomarkers. 98 participants were randomly and equally divided into the RG and RG + CHF subgroups. The RG and RG + CHF groups received general treatments with RG and a combination of RG and CHF, respectively. The therapy lasted for 2 weeks. In this study, the primary assessment result was a difference in the slope of serum creatinine (Scr) over the course of 2 weeks. The secondary evaluation outcomes were alterations in blood urea nitrogen (BUN), uric acid (UA), estimated glomerular filtration rate (eGFR), urinary AKI biomarkers, renal fibrosis biomarkers (transforming growth factor-β1 (TGF-β1), connective tissue growth factor (CTGF)), and traditional Chinese medicine (TCM) symptoms. Furthermore, vital signs and adverse events (AEs) were observed. Both groups had a slower renal function decline after treatment than before treatment. Compared with RG group, more reductions of Scr, BUN, UA, and better improvement of eGFR were observed in RG + CHF group (p < 0.05). Additionally, the levels of urinary AKI biomarkers, renal fibrosis biomarkers, and TCM syndromes were decreased in RG + CHF group versus RG group (p < 0.05). No significant between-group differences were observed of AEs. We thus concluded this novel therapy of CHF combining RG might be a useful method for treating A on C patients.
Clinical Trial Registration: http://www.chictr.org.cn, ChiCTR2100043311
Acute kidney injury (AKI) is described as a sudden deterioration in renal function that encompasses both structural failure and loss of functionality (Makris and Spanou, 2016). As a serious complication induced by a variety of critical conditions, AKI might result in significant morbidity and death in both the short and long run (Sawhney and Fraser, 2017). Renal replacement therapy (RRT) ought to be initiated as early as feasible in severe AKI (grade 3), however, there is still a lack of effective drugs for AKI grades 1–2 (Gong et al., 2014; Kellum and Ronco, 2016; Meersch et al., 2018).
Chronic kidney disease (CKD) is a combination of chronic illnesses attributed to a variety of indicators, particularly, inflammation, oxidative stress, and metabolic abnormalities (Hoerger et al., 2015; Yan et al., 2021). AKI and CKD are strongly linked to each other. Furthermore, CKD is an unignorable pathogenic factor for the advancement of AKI (He et al., 2017). Studies have shown that the presence of CKD impaired renal function in individuals with AKI and delayed their recovery after AKI (He et al., 2017; Acosta-Ochoa et al., 2019). It is widely recognized that treating AKI on CKD (A on C) is extremely challenging. Renal fibrosis, which is characterized by glomerulosclerosis and tubulointerstitial fibrosis, is a progressive and chronic condition that affects renal function of CKD patients throughout aging (Bhargava et al., 2021; Li et al., 2021). Renal fibrosis is the most prevalent consequence in practically all instances of progressive CKD, which has few therapeutic choices (Djudjaj and Boor, 2019). With regard to treating renal fibrosis, TCM might be a useful alternative treatment option (Zhou et al., 2020).
According to historical records, Chinese physicians utilized traditional Chinese medicine (TCM) to treat AKI during the era when RRT was absent (Li et al., 2019). In China, TCM has been extensively indicated for the management of renal problems (Norgren and Gong, 2018). Chuan Huang Fang (CHF) is a Chinese herbal formulation synthesized by Professor Xuezhong Gong in Shanghai Municipal Hospital of Traditional Chinese Medicine for the treatment of A on C (Tang et al., 2015; Chen and Gong, 2022b). Our previously single-center clinical studies indicated Chuan Huang Fang (CHF) might have nephroprotection in A on C patients via mechanisms of reducing oxidative damage and decreasing inflammatory reactions (Gong et al., 2014; Gong et al., 2020; Gong et al., 2021). Reduced glutathione (RG) has been shown to be beneficial in controlling local inflammation, reducing accumulation of reactive oxygen species, decreasing inflammatory markers, and lowering oxidative stress in the tissues and organs (Matsubara et al., 2019; Zhang and Bai, 2019; Wang and Wu, 2021). Recently, it has been ascertained that RG could increase the therapeutic efficacy of high-flux hemodialysis among patients with severe AKI (Hu, 2015; Yang et al., 2020). Thus, we subsequently optimized the original protocol by utilizing a new drug treatment method involving the combination of CHF and RG.
We undertook a multicenter randomized controlled trial (RCT) for assessing the clinical efficacy and safety of CHF combing RG against A on C. By completing this trial in a timely manner, a unique pharmacological treatment method for AKI grades 1–2 would be developed.
A multicenter randomized controlled clinical trial was performed in this study. The study duration for this research were ranged from 26 December2016- 13 April2020. Approval for the trial was granted by the international review board and ethical committees of each participating hospital and the Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine (No.2020SHL-KYYS-60). This trial was registered in the Chinese Clinical Trials Register (No. ChiCTR2100043311) before the enrolment of the first participant.
Three hospitals enrolled participants in this study: 1) Shanghai Municipal Hospital of Traditional Chinese Medicine, 2) Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, and 3) Minhang Branch of Yueyang Hospital of Integrative Chinese & Western Medicine Affiliated to Shanghai University of Traditional Chinese Medicine. Overall, 98 participants were classified randomly and equally into the RG and RG + CHF groups. This study enrolled patients who had CKD stages 2–4 complicated with AKI grades 1–2 and toxicity stasis inter-combination syndrome, as well as spleen–kidney qi deficit.
The following were the criteria for participation in the study: 1) patients satisfied all clinical guidelines for chronic kidney disease stages 2–4 and acute kidney injury grades 1–2, 2) patients satisfied the criteria for a diagnosis for the classification of TCM syndromes, 3) 24 h U-pro of patients ≤2.5 g, 4) ages of patients were range from 18 to 70 years, and 5) patients should be volunteered to participate and signed informed consent.
The following were the criteria for excluding participants from the study: 1) patients who were pregnant or lactating, 2) patients with acute primary illnesses of other organs requiring immediate treatment, including active tuberculosis, malignant tumors, or consumption disorders, 3) patients suffering from anorectal disorders who failed to receive enema, 4) patients who had received a kidney transplant, 5) patients who were psychopaths or who had poor compliance, 6) those with an allergic reaction to the therapeutic medication, and 7) those who were enrolled in other clinical trials within the past 3 months.
Based on the Guidelines for Clinical Research of Chinese Medicine (New Drug) (Zheng, 2002)and Diagnosis, Syndrome Differentiation and Efficacy Evaluation of Chronic Renal Failure (Trial Protocol) (He 2006), participants exhibited the following four major symptoms and one to two secondary symptoms, all of which might be indicative of toxicity stasis inter-combination syndrome and spleen–kidney qi deficit. The primary symptoms were: 1) feeling fatigued, having shortness of breath, and being reluctant to talk, 2) a feeling of nausea and vomiting, 3) a dim complexion, and 4) soreness in the knees and waist. The following were the secondary symptoms: 1) discomfort and distention in the abdomen, 2) a loss of appetite, as well as numbness to respond, 3) having skin that is dry and squamous, 4) greasy and thick tongue coating, 5) purple and dark tongue or petechia, and 6) fine tart or slow sunken pulse.
To produce random number sequences, independent biostatisticians used the SPSS (version 21.0) program, which was used to establish these sequences using a simple random approach. The subjects agreed to take part in the research and completed a formal informed consent immediately. Participants also gave their informed permission before being randomly assigned to groups. Participants were assigned a random number and equally classified into two groups by the investigators depending on the order in which they were enrolled in this study: the RG and RG + CHF groups. During the random sampling process, closed envelopes labeled with sequential coding numerals were employed to keep track of allocation information during randomization, and participants, biostatisticians, and investigators were kept blind to the group allocation. Since there was no use of a placebo in the control group, it was not feasible to keep individuals completely unaware of the therapy. It was, however, concealed from all laboratory personnel who were involved in the investigations.
Participants were recruited for the study after providing formal informed permission. They were categorized at random into the RG and the RG + CHF groups. The participants underwent training for study behavior and health to reduce the possibility of loss and ensure that they could follow the experiment without difficulty. Furthermore, they were informed about potential hazards, their rights, and responsibilities, as well as how to deal with an emergency. All of the patients were given a low-protein, high-quality, and low-salt meal plan to follow (protein consumption of 0.6–0.8 g/kg/day). They received basic therapies with the goal of restoring normal water and electrolyte levels as well as acid-base balance abnormalities, controlling blood pressure, improving anemia, and correcting renal bone illnesses. Certain investigators regularly followed up with participants and assisted them to adhere to treatment procedures and undergo tests as per schedule.
The control group received an intravenous injection (IV) of RG 1.8 g mixed with 0.9 percent normal saline or 5 percent glucose in a volume of 250 ml once every day for a period of 2 weeks. The treatment group additionally received 200 ml CHF twice daily for a total of 2 weeks. Simultaneously, they received an enema of the concentrated solution of CHF once a day for 5 days (i.e., 5 times a week), followed by 2 days of rest. RG was obtained from Chongqing Yaoyou Pharmaceutical Co. Ltd. The quality standards of CHF were controlled as follows: 1) All herbal medicines were from Shanghai Municipal Hospital of Traditional Chinese Medicine pharmacy, which were traceable; 2) CHF decoction was produced by the pharmacy; 3) Our team had professional pharmaceutical staff to detect the main monomer content of CHF (emodin and TMP, etc) using fingerprints method regularly. The principal pharmaceutical ingredients of CHF are Prepared rhubarb (Zhidahuang), Ligusticum wallichii (Chuanxiong), Smilacis glabrae (Tufuling), Coptidis rhizome (Huanglian), Codonopsis pilosula (Dangshen), Salviae miltiorrhizae (Danshen), Rhizoma Pinellinae Praeparata (Zhibanxia), Pericarpium citri reticulatae (Chenpi), Cordyceps sinensis (Chongcaojunsi), etc (Gong et al., 2021; Chen and Gong, 2022b). Major composition and action of CHF are demonstrated below (Table 1).
Enema were performed by a professional clinician. A clinician took 100 ml concentrated medicine solution of CHF, waiting for the medicine solution close to almost human temperature, and put it into an enema bag. The patient was told to lie on the side of the operating bed, the clinician inserted enema tube to the patient’s anus 20–30 cm deep and then injected the medicine solution slowly, each dripping time for 20 min. After enema operation, the patient was instructed to lie on his pillow with buttocks rising 10–15 cm. The medical liquid should be maintained in the patient’s intestine for more than 1 h.
A series of related studies were carried out, and data were recorded at baseline and every week throughout the duration of therapy. Patient data was gathered, efficacy-related exams were carried out, and indicators of mechanism and safety, as well as medication administration, were meticulously observed throughout the study.
The primary assessment result was measured and represented as the alteration in the slope of serum creatinine (Scr). The secondary assessment outcomes were post-treatment alterations in blood urea nitrogen (BNU), uric acid (UA), estimated glomerular filtration rate (eGFR), urinary AKI biomarkers including neutrophil gelatinase associated lipocalin (NGAL) and interleukin-18 (IL-18), as well as TCM syndromes. Besides, levels of renal fibrosis biomarkers from serum samples, notably, transforming growth factor-β1 (TGF-β1), and connective tissue growth factor (CTGF) were assessed both before and after treatment. Moreover, 1 week before and 2 weeks following the treatment, safety results and vital signs indices (hemoglobin, serum potassium, urine routine, blood routine, and electrocardiogram) were documented for each participant. In the meantime, adverse events (AEs) were continuously observed, precisely documented, and appropriately addressed by researchers throughout the trial.
Statistical analysis of the improvement in clinical symptoms score was performed in strict compliance with the Guidelines for Clinical Research of Chinese Medicine (New Drug) (Zheng, 2002). The primary and secondary symptoms of toxicity stasis inter-combination syndrome and spleen–kidney qi deficiency were categorized as mild, moderate, and severe with 2, 4, 8 points, and 4, 8, 12 points, respectively. The two values of total scores generated by the measuring scale depending on TCM symptoms for each patient were added together and employed to compute the efficacy indicator (EI).
EI = (Total symptom score before treatment − Total symptom score after treatment)/Total symptom score before treatment × 100%
The treatment efficacy was evaluated using EI. Symptom improvement levels were defined as follows: clinical control (EI ≥ 95%), significant effect (94% > EI ≥ 70%), and effectiveness (69% > EI ≥ 30%) to inefficacy (EI < 30%).
In this study, we used baseline Scr change to estimate sample size. Premised on previous studies (Zhang and Bai, 2019; Gong et al., 2020), we computed the mean Scr value (160.39 μmol/L), and the standard deviation (44.43 μmol/L). Thus, we hypothesized that the Scr level in the RG + CHF group would be obviously lowered (by over 30 μmol/L) as opposed to that in the control group. SPSS (version 21.0) software was utilized to generate a random number table, and the included participants were randomly and equally divided into the control and treatment groups. It was computed with the help of the sample size estimation program PASS (version 15.0.3) to obtain significant difference α = 0.05 (one-sided test) and test efficacy 1–β = 0.90. Premised on a 20% drop-out rate, the predicted sample size for random sampling was 49 cases within every group for 98 cases in the study.
Regarding the Full Analysis Set (FAS), which is made up of individuals who were randomly assigned into two groups, the principal analysis was carried out. Participants who successfully completed the research protocol and had good compliance were defined as the per-protocol sample. In conformity with the Intend-to-Treat (ITT) principle, all subjects receiving test medications were included in a safety analysis.
Continuous variables were presented as mean ± standard deviation or by the median. In this case, categorical variables were displayed in the form of numbers and percentages. Student’s t-tests or one-way ANOVA were applied for continuous data exhibiting normal distribution; in contrast, Pearson’s chi-square test (or Fisher’s exact test for cell count <5 in any cell) was employed for comparisons involving categorical variables. All statistical analyses were conducted using a two-sided design. p < 0.05 was established as a determinant of statistical significance. The analyses of statistical data were carried out by a biostatistician who was not engaged in the research with the aid of the SPSS (version 21.0) software package. There were no further analyses or interim analyses carried out in this study.
After screening 145 patients, 98 participants were enrolled in the study with 21 patients not meeting inclusion criteria, 15 patients declining to participant and 11 patients for other reasons (Figure 1). RG group included 28 male patients and 21 female participants with an average age of (62.9 ± 9.4) years. In RG + CHF group, there were 30 male participants and 19 female participants with an average age of (61.4 ± 10.5) years. The concomitant diseases were also demonstrated below. According to the statistical results, there were no significant difference in gender, age, and concomitant diseases between two groups (p > 0.05). It indicated that the baseline characteristics of two groups were balanced and the efficacy of this clinical study was comparable (Table 2).
Statistical results showed that mean ± SD of the primary outcome Scr after treatment were 207.3 ± 63.4, 177.4 ± 54.6 in RG group and RG + CHF group respectively. Both groups had a significant Scr decline after treatment than before treatment (p < 0.01). Compared with RG group, Scr in RG + CHF group was lower and the difference was statistically significant (p < 0.05) (Table 3; Figure 2).
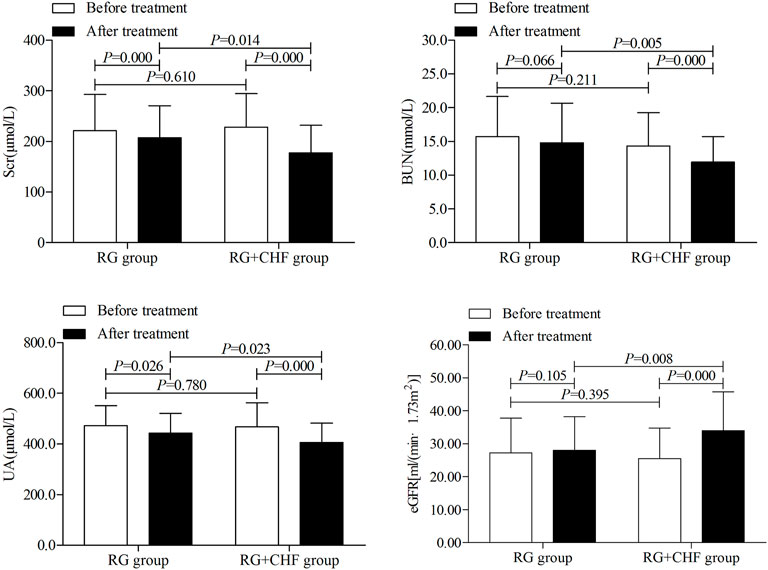
FIGURE 2. The comparison of renal function indicators between two groups before and after treatment.
According to statistical results, it showed that mean ± SD of BUN after treatment were 14.8 ± 5.9,11.9 ± 3.8 in RG group and RG + CHF group respectively. Compared with RG group, BUN in RG + CHF group were lower and the difference was statistically significant (p < 0.01). Besides, UA in RG + CHF group were lower and the difference was statistically significant (p < 0.05), eGFR was improved and the difference was statistically significant (p < 0.01) (Table 4 and Figure 2).
According to statistical results, it showed that mean ± SD of NGAL after treatment were 138.4 ± 82.3, 107.6 ± 42.0 in RG group and RG + CHF group respectively. Compared with RG group, NGAL in RG + CHF group was lower and the difference was statistically significant (p < 0.05). Besides, IL-18 in RG + CHF group was lower and the difference was statistically significant (p < 0.01) (Table 4 and Figure 3).
Statistical results showed that mean ± SD of TGF-β1 after treatment were 115.2 ± 21.6, 143.9 ± 25.3 in RG group and RG + CHF group respectively. Compared with RG group, TGF-β1 in RG + CHF group was lower and the difference was statistically significant (p < 0.05). Besides, CTGF in RG + CHF group was lower and the difference was statistically significant (p < 0.05) (Table 4 and Figure 4).
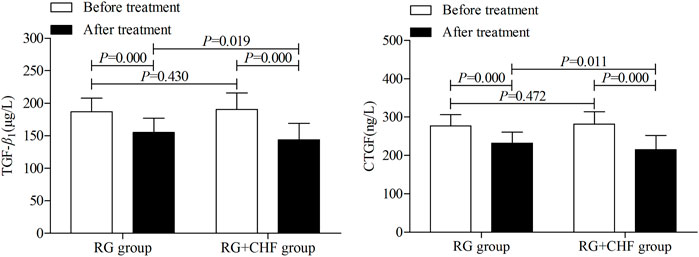
FIGURE 4. The comparison of renal fibrosis biomarkers between two groups before and after treatment.
Statistical results showed that mean ± SD of TCM syndrome scores respectively were 34.2 ± 12.0, 28.7 ± 10.1 in RG group and RG + CHF group after treatment. Compared with RG group, TCM syndrome scores in RG + CHF group were lower and the difference was statistically significant (p < 0.05) (Table 4 and Figure 5).
As shown in the table below, significantly effective rate and effective rate in RG group respectively were 12 (24.5%) and 6 (12.2%). While the significantly effective rate and effective rate were 24 (49.0%) and 13 (26.5%) in RG + CHF group. Total effective rate (75.5%) in RG + CHF group were obviously higher than that (36.7%) in RG group, and there was significant statistically difference between two groups (p < 0.01) (Table 5).
The AEs occurred in the RG and RG + CHF group were demonstrated below (Table 6). There were no SAEs requiring withdrawal reported during the whole treatment period in any group.
Compared with RG group, more reductions of Scr, BUN, UA, and better improvement of eGFR were observed in RG + CHF group. Additionally, the levels of urinary AKI biomarkers, renal fibrosis biomarkers, and TCM syndromes were decreased in RG + CHF group versus RG group. RG + CHF group demonstrated better renal protective effects than RG subgroup.
AKI is a clinical condition hallmarked by a sudden decrease of renal function, whereas CKD is hallmarked by renal functional or structural impairments. As a consequence of AKI, a significant number of oxygen free radicals are produced, endogenous antioxidants are continually depleted, and high levels of inflammatory substances are secreted, all of which contribute to kidney damage progression (Han and Lee, 2019). Moreover, A on C might be caused by the combination of multiple factors such as decreased prostaglandin synthesis, inflammatory reaction, oxidative stress, abnormal hemodynamics, and increases in the production of thromboxane by the kidney cortex (Gong et al., 2012; Gong et al., 2014; Ruedig and Johnson, 2015; Barnett and Cummings, 2018). Despite the fact that the shift from AKI to CKD has previously been shown in multiple studies, researches of A on C is still in its infancy (Sawhney and Fraser, 2017; Cooper et al., 2018; Bagshaw and Wald, 2021).
According to our published papers (Gong et al., 2014; Gong et al., 2020; Gong et al., 2021), the common precipitating factors of A on C include infection, electrolyte disturbance, hypertension, and stress state, etc. But inflammation and oxidative stress play a crucial role in the underlying molecular mechanisms of such an acute kidney damage. Addressing inflammatory reactions is a viable approach in the treatment of both CKD and AKI (Nikolic-Paterson et al., 2021). AKI can potentially elicit reactions comparable to those observed in CKD, including enhanced cytokine production, higher level of inflammatory cell infiltration, epithelial to mesenchymal transition, and activation of fibroblasts (Lv et al., 2018; Black et al., 2019). Oxidative stress is a condition caused by an imbalance between antioxidants and oxidants (Daenen et al., 2019). Mounting evidence has revealed that oxidative stress performs a fundamental function in the advancement of renal disorders and the progression of kidney-related problems (Zhou et al., 2021). Oxidative stress and inflammation are inextricably related, jointly causing and exacerbating the effects of the other (Luo et al., 2021).
Our previously single-center clinical studies indicated CHF might have nephroprotection in A on C patients (Gong et al., 2014; Gong et al., 2020; Gong et al., 2021). Besides, we published papers about CHF against AKI in animal and cell models as well. Previous research (Gong et al., 2019b) explored clinical dosage of trivalent arsenic inhibition effect and mechanism of renal toxicity. We found CHF could effectively suppressed clinical dose of trivalent arsenic of the kidney toxicity, and its molecular mechanism were associated with the inhibition of caspase three induced renal tubular epithelial cell apoptosis. We also conducted a research on the mechanism of Zhidahuang-Chuanxiong drug pair on tubular epithelial cell apoptosis in contrast-induced acute kidney injury (CI-AKI) rats (Gong et al., 2013). Thus, it was found that activation of p38MAPK pathway played an important role in pathogenesis of CI-AKI, and Zhidahuang-Chuanxiong drug pair might alleviate renal damage in CI-AKI rats through inhibiting the activation of pathway. Based on this result, we further explored the mechanism of the drug pair from nuclear factor erythroid 2-related factor2/Hemeoxygenase-1(Nrf2/HO-1) pathway (Gong et al., 2017). Nrf2/HO-1 pathway was activated and involved in the process, and Zhidahuang-Chuanxiong drug pair could activate and have renal protective effects of CI-AKI rats by inhibiting this pathway. Tetramethylpyrazine (TMP), an active component in both CHF and the medicinal herbs Ligusticum wallichii (Chuanxiong), has the potential to prevent AKI via a variety of processes, including ameliorating oxidative stress damage, suppressing inflammatory responses, deterring apoptotic cell death of intrinsic renal cells, and modulating autophagy (Gong, 2018; Gong et al., 2019a; Gong, 2020; Chen and Gong, 2022a).
Renal fibrosis is by far the most important mechanism that leads to CKD (Gu et al., 2020). It is originally induced by a variety of biophysiological shocks or inflammatory mediators, and it serves as a protective response in the case of kidney injury. Nevertheless, when kidney injuries are prolonged and overreacted, this reaction becomes pathogenic, ultimately contributing to the emergence of ESRD (Inker et al., 2014). TGF-β1 is the primary profibrotic facilitator in kidney disorders due to its role as a key modulator of fibrosis (Meng et al., 2016). Research findings on the upregulation of active TGF-β1 have additionally validated the profibrotic function of TGF-β1 in the etiology of progressive renal fibrosis in a variety of kidney illnesses (Wong et al., 2017; Humphreys, 2018; Kim et al., 2018). Consequently, it has been hypothesized that TGF-β1 might be a possible treatment target for the clinical management of renal fibrosis. CTGF is an essential component in the onset of renal fibrosis. Although CTGF exists in healthy kidneys as a form of low level, the level is dramatically elevated in a variety of renal illnesses, and it has a substantial impact on the progression of progressive kidney illness (Toda et al., 2018). The gene of CTGF, a matricellular protein, is a straightforward downstream initial response gene of the TGF-β1 (Lee et al., 2015). According to research results, the treatment group could effectively reduce the level of TGF-β1 and CTGF, which might provide clinical evidence for early prevention and treatment of A on C patients.
RRT is predominantly applied to treat severe AKI (grade 3). However, there is currently no recognized medication therapy strategy for AKI grades 1–2 (Gong et al., 2014; Kellum and Ronco, 2016; Meersch et al., 2018; Peters et al., 2018). As indicated by the KDIGO recommendations, the primary therapeutic approach for AKI is to regulate the body’s internal milieu, and the vast majority of therapies are supportive rather than curative. Identifying the underlying cause of AKI, maintaining hemodynamic stability, and dealing with severe consequences are all critical throughout the early stages of the disease (Moore et al., 2018). The maintenance of hemodynamic stability needs to be given extensive consideration in AKI because of the impaired autoregulation mechanisms that are present (Ostermann et al., 2019). As previously stated, RG has the potential to attenuate the damaging peroxide metabolites, decrease oxidative stress, and perform specific metabolic functions in the production of inflammatory components (Matsubara et al., 2019; Zhang and Bai, 2019; Wang and Wu, 2021).
Premised on our previously clinical evidence and recent breakthroughs in clinical therapy of A on C, we designed a new protocol with the combination of CHF and RG. And this study tried to explore the medical mechanism of CHF combining RG treating A on C from the aspect of renal fibrosis. The current research would be beneficial in resolving this clinical dilemma in the future. The combination might successfully minimize renal damage of A on C patients, enhance restoration of kidney functionality, and relieve clinical symptoms in these individuals. As a result, we consider that the clinical application values of this therapy method are satisfactory. Based on the findings of this study, we will continue to explore therapeutic effects of CHF combining RG for patients with A on C to gather more relevant clinical data.
CHF combining RG showed better therapeutic effects than RG alone, and its renal protective effects were associated with reducing Scr, BUN, UA, and improving eGFR, as well as preventing renal fibrosis. We thus concluded that this novel therapy of combining CHF and RG might be a useful method for treating A on C patients.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine. The patients/participants provided their written informed consent to participate in this study.
XG was the chief investigator in charge of this trial program. He designed this study, and coordinated various personnel and material resources to ensure the smooth implementation of the program. In addition, he participated in, instructed, and supervised the whole study. CW and BX were responsible for the enrollment of participants in other two hospitals, and they coordinated related affairs with XG. LC and ZY understood the task of data collection, analysis, and interpretation. Besides, LC mainly drafted the manuscript and assisted the implement of subsequent trial program. DW, JL, and QW assisted to consult relevant literatures, and they contributed many useful advices to this manuscript. The manuscript is critically revised by XG and finally approved by all authors for publication.
This work was supported by grants from National Natural Science Foundation of China (Nos. 82074387 and 81873280), Shanghai Municipal Science and Technology Commission Project (No. 20Y21902200), and Shanghai Municipal Health Commission Project (No. ZY(2021-2023)-0207-01).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acosta-Ochoa, I., Bustamante-Munguira, J., Mendiluce-Herrero, A., Bustamante-Bustamante, J., and Coca-Rojo, A. (2019). Impact on outcomes across KDIGO-2012 AKI criteria according to baseline renal function. J. Clin. Med. 8 (9), E1323. doi:10.3390/jcm8091323
Bagshaw, S. M., and Wald, R. (2021). Starting kidney replacement therapy in critically III patients with acute kidney injury. Crit. Care Clin. 37 (2), 409–432. doi:10.1016/j.ccc.2020.11.005
Barnett, L. M. A., and Cummings, B. S. (2018). Nephrotoxicity and renal pathophysiology: A contemporary perspective. Toxicol. Sci. 164 (2), 379–390. doi:10.1093/toxsci/kfy159
Bhargava, V., Singh, K., Meena, P., and Sanyal, R. (2021). Nephrogenic systemic fibrosis: A frivolous entity. World J. Nephrol. 10 (3), 29–36. doi:10.5527/wjn.v10.i3.29
Black, L. M., Lever, J. M., and Agarwal, A. (2019). Renal inflammation and fibrosis: A double-edged sword. J. Histochem. Cytochem. 67 (9), 663–681. doi:10.1369/0022155419852932
Chen, L., and Gong, X. (2022a). Drug-induced acute kidney injury: Epidemiology, mechanisms, risk factors, and preventive treatment of traditional Chinese medicine. Integr. Med. Nephrol. Androl. 9 (5), 5. doi:10.4103/2773-0387.345767
Chen, L., and Gong, X. (2022b). Efficacy and safety of chuan Huang Fang combining reduced glutathione in treating acute kidney injury (grades 1-2) on chronic kidney disease (stages 2-4): Study protocol for a multicenter randomized controlled clinical trial. Evid. Based. Complement. Altern. Med. 2022, 1099642. doi:10.1155/2022/1099642
Cooper, D. J., Plewes, K., Grigg, M. J., Rajahram, G. S., Piera, K. A., William, T., et al. (2018). The effect of regularly dosed paracetamol versus no paracetamol on renal function in plasmodium knowlesi malaria (PACKNOW): Study protocol for a randomised controlled trial. Trials 19 (1), 250. doi:10.1186/s13063-018-2600-0
Daenen, K., Andries, A., Mekahli, D., Van Schepdael, A., Jouret, F., and Bammens, B. (2019). Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 34 (6), 975–991. doi:10.1007/s00467-018-4005-4
Djudjaj, S., and Boor, P. (2019). Cellular and molecular mechanisms of kidney fibrosis. Mol. Asp. Med. 65, 16–36. doi:10.1016/j.mam.2018.06.002
Gong, X., Duan, Y., Wang, Y., Ye, Z., Zheng, J., Lu, W., et al. (2020). Effects of Chuanhuang Decoction on renal function and oxidative stress in patients of chronic kidney disease at stage 2-4 complicated with acute kidney injury. J. Shanghai Univ. Trad. Chin. Med. Sci. 34 (1), 11–16. doi:10.16306/j.1008-861x.2020.01.002
Gong, X., Duan, Y., Zheng, J., Ye, Z., and Hei, T. K. (2019a). Tetramethylpyrazine prevents contrast-induced nephropathy via modulating tubular cell mitophagy and suppressing mitochondrial fragmentation, CCL2/CCR2-mediated inflammation, and intestinal injury. Oxid. Med. Cell. Longev. 2019, 7096912. doi:10.1155/2019/7096912
Gong, X., Qiu, A., Duan, Y., Ye, Z., Zheng, J., Wang, Q., et al. (2017). Effects of Couplet Medicines of Prepared Radix et Rhizoma Rhei-Rhizoma Ligustici Chuanxiong on Nrf2/HO-1 Signaling Pathway in Renal Tissue of Contrast-induced Nephropathy Rats. J. Shanghai Univ. Trad. Chin. Med. Sci. 31 (06), 58–61. doi:10.16306/j.1008-861x.2017.06.014
Gong, X., Tang, X., Wang, Q., Wang, Y., and Zhou, J. (2014). Observe the clinical efficacy of chuanhuang decoction combined with lipo PGE1 in treating acute kidney injury (AKI) on phase 2∼4 chronic kidney disease (CKD) patients. Chin. J. Integr. Trad. West Med. Nephrol. 15 (9), 784–787.
Gong, X., Wang, Q., Fu, D., Tang, X., Wang, Y., Wang, G., et al. (2013). Research on Radix et Rhizoma Rhei-Rhizoma Ligustici Chuanxiong restraining renal tubular epithelial cell apoptosis in contrast-induced nephropathy rats. Shanghai J. Trad. Chin. Med. 47 (03), 69–71. doi:10.16305/j.1007-1334.2013.03.023
Gong, X., Wang, Y., Fu, D., Wang, Q., Wang, G., Tang, X., et al. (2012). Effects of Zhidahuang - chuanxiong drug pair on tubular epithelial cell apoptosis in contrast - induced nephropathy rats. Chin. J. Integr. Trad. West Med. Nephrol. 13 (8), 675–677.
Gong, X., Ye, Z., Xu, X., Chen, L., Xu, Y., Yuan, D., et al. (2021). Effects of Chuanhuang Formula combined with prostaglandin E1 in treating patients of chronic kidney disease complicated with acute kidney injury and its influence on NLRP3. J. Shanghai Univ. Trad. Chin. Med. Sci. 35 (06), 12–16. doi:10.16306/j.1008-861x.2021.06.002
Gong, X. Z. (2020). Chinese medicine might Be A promising way for A solution to arsenic nephrotoxicity. Chin. J. Integr. Med. 26 (2), 83–87. doi:10.1007/s11655-019-3210-8
Gong, X., Zheng, J., Duan, Y., and Ye, Z. (2019b). Effect of Chuanhuang Fang on apoptosis of renal tubular epithelial cells in rats with trivalent arsenic nephrotoxicity. Beijing Med. J. 41, 1089–1093. doi:10.15932/j.0253-9713.2019.12.009
Gong, X. Z. (2018). Recent advances in Chinese medicine for contrast-induced nephropathy. Chin. J. Integr. Med. 24 (1), 6–9. doi:10.1007/s11655-017-2906-x
Gu, Y. Y., Liu, X. S., Huang, X. R., Yu, X. Q., and Lan, H. Y. (2020). TGF-Β in renal fibrosis: Triumphs and challenges. Future Med. Chem. 12 (9), 853–866. doi:10.4155/fmc-2020-0005
Han, S. J., and Lee, H. T. (2019). Mechanisms and therapeutic targets of ischemic acute kidney injury. Kidney Res. Clin. Pract. 38 (4), 427–440. doi:10.23876/j.krcp.19.062
He, L. Q. (2006). Diagnosis, syndrome differentiation and efficacy evaluation of chronic renal failure (trial protocol). Shanghai J. Tradit. Chin. Med. 40 (8), 8–9.
He, L., Wei, Q., Liu, J., Yi, M., Liu, Y., Liu, H., et al. (2017). AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 92 (5), 1071–1083. doi:10.1016/j.kint.2017.06.030
Hoerger, T. J., Simpson, S. A., Yarnoff, B. O., Pavkov, M. E., Ríos Burrows, N., Saydah, S. H., et al. (2015). The future burden of CKD in the United States: A simulation model for the CDC CKD initiative. Am. J. Kidney Dis. 65 (3), 403–411. doi:10.1053/j.ajkd.2014.09.023
Hu, D. J. (2015). Reduced glutathione with high flux hemodialysis therapy of acute renal injury in clinical study. J. Clin. Nephrol. 15 (1), 39–41.
Humphreys, B. D. (2018). Mechanisms of renal fibrosis. Annu. Rev. Physiol. 80, 309–326. doi:10.1146/annurev-physiol-022516-034227
Inker, L. A., Astor, B. C., Fox, C. H., Isakova, T., Lash, J. P., Peralta, C. A., et al. (2014). KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 63 (5), 713–735. doi:10.1053/j.ajkd.2014.01.416
Kellum, J. A., and Ronco, C. (2016). The 17th acute disease quality initiative international consensus conference: Introducing precision renal replacement therapy. Blood Purif. 42 (3), 221–223. doi:10.1159/000448500
Kim, K. K., Sheppard, D., and Chapman, H. A. (2018). TGF-β1 signaling and tissue fibrosis. Cold Spring Harb. Perspect. Biol. 10 (4), a022293. doi:10.1101/cshperspect.a022293
Lee, S. Y., Kim, S. I., and Choi, M. E. (2015). Therapeutic targets for treating fibrotic kidney diseases. Transl. Res. 165 (4), 512–530. doi:10.1016/j.trsl.2014.07.010
Li, H. D., Meng, X. M., Huang, C., Zhang, L., Lv, X. W., and Li, J. (2019). Application of herbal traditional Chinese medicine in the treatment of acute kidney injury. Front. Pharmacol. 10, 376. doi:10.3389/fphar.2019.00376
Li, S. S., Sun, Q., Hua, M. R., Suo, P., Chen, J. R., Yu, X. Y., et al. (2021). Targeting the wnt/β-catenin signaling pathway as a potential therapeutic strategy in renal tubulointerstitial fibrosis. Front. Pharmacol. 12, 719880. doi:10.3389/fphar.2021.719880
Luo, L. P., Suo, P., Ren, L. L., Liu, H. J., Zhang, Y., and Zhao, Y. Y. (2021). Shenkang injection and its three anthraquinones ameliorates renal fibrosis by simultaneous targeting IƙB/NF-ƙB and keap1/nrf2 signaling pathways. Front. Pharmacol. 12, 800522. doi:10.3389/fphar.2021.800522
Lv, W., Booz, G. W., Wang, Y., Fan, F., and Roman, R. J. (2018). Inflammation and renal fibrosis: Recent developments on key signaling molecules as potential therapeutic targets. Eur. J. Pharmacol. 820, 65–76. doi:10.1016/j.ejphar.2017.12.016
Makris, K., and Spanou, L. (2016). Acute kidney injury: Definition, pathophysiology and clinical phenotypes. Clin. Biochem. Rev. 37 (2), 85–98.
Matsubara, A., Oda, S., Jia, R., and Yokoi, T. (2019). Acute kidney injury model established by systemic glutathione depletion in mice. J. Appl. Toxicol. 39 (6), 919–930. doi:10.1002/jat.3780
Meersch, M., Küllmar, M., Schmidt, C., Gerss, J., Weinhage, T., Margraf, A., et al. (2018). Long-term clinical outcomes after early initiation of RRT in critically ill patients with AKI. J. Am. Soc. Nephrol. 29 (3), 1011–1019. doi:10.1681/asn.2017060694
Meng, X. M., Nikolic-Paterson, D. J., and Lan, H. Y. (2016). TGF-Β: The master regulator of fibrosis. Nat. Rev. Nephrol. 12 (6), 325–338. doi:10.1038/nrneph.2016.48
Moore, P., Hsu, R., and Liu, K. (2018). Management of acute kidney injury: Core curriculum 2018. Am. J. Kidney Dis. 72, 136–148. doi:10.1053/j.ajkd.2017.11.021
Nikolic-Paterson, D. J. G. K., and Ma, F. Y. (2021). JUN amino (2021). JUN amino terminal kinase in cell death and inflammation in acute and chronic kidney disease. Integr. Med. Nephrol. Androl. 8 (10). doi:10.4103/imna.imna_35_21
Norgren, S., and Gong, X. Z. (2018). Contrast-induced nephropathy-time for Western medicine and Chinese medicine to team up. Chin. J. Integr. Med. 24 (1), 3–5. doi:10.1007/s11655-017-2905-y
Ostermann, M., Liu, K., and Kashani, K. (2019). Fluid management in acute kidney injury. Chest 156, 594–603. doi:10.1016/j.chest.2019.04.004
Peters, E., Antonelli, M., Wittebole, X., Nanchal, R., François, B., Sakr, Y., et al. (2018). A worldwide multicentre evaluation of the influence of deterioration or improvement of acute kidney injury on clinical outcome in critically ill patients with and without sepsis at ICU admission: Results from the intensive care over nations audit. Crit. Care 22 (1), 188. doi:10.1186/s13054-018-2112-z
Ruedig, E., and Johnson, T. E. (2015). An evaluation of health risk to the public as a consequence of in situ uranium mining in Wyoming, USA. J. Environ. Radioact. 150, 170–178. doi:10.1016/j.jenvrad.2015.08.004
Sawhney, S., and Fraser, S. D. (2017). Epidemiology of AKI: Utilizing large databases to determine the burden of AKI. Adv. Chronic Kidney Dis. 24 (4), 194–204. doi:10.1053/j.ackd.2017.05.001
Tang, X., Gong, X., and Wang, Q. (2015). Experiences of professor Gong Xuezhong in treating acute kidney injury on chronic kidney disease based on toxicity and blood stasis. Shenzhen J. Integr. Trad. Chin. West Med. 25 (17), 40–42. doi:10.16458/j.cnki.1007-0893.2015.17.021
Toda, N., Mukoyama, M., Yanagita, M., and Yokoi, H. (2018). CTGF in kidney fibrosis and glomerulonephritis. Inflamm. Regen. 38, 14. doi:10.1186/s41232-018-0070-0
Wang, W. M., and Wu, Y. (2021). Clinical effect of reduced glutathione combined with jinshuibao in the treatment of acute renal injury. Guide China Med. 19 (24), 7–9.
Wong, E. B., Ndung'u, T., and Kasprowicz, V. O. (2017). The role of mucosal-associated invariant T cells in infectious diseases. Immunology 150 (1), 45–54. doi:10.1111/imm.12673
Yan, H., Xu, J., Xu, Z., Yang, B., Luo, P., and He, Q. (2021). Defining therapeutic targets for renal fibrosis: Exploiting the biology of pathogenesis. Biomed. Pharmacother. 143, 112115. doi:10.1016/j.biopha.2021.112115
Yang, F., Tong, J., Li, H., Chen, J., and Dai, L. (2020). Effects of reduced glutathione combined with high-flux hemodialysis on serum CysC, KIM-1 and Scr in patients with acute kidney injury. Med. J. West China 32 (6), 863–867.
Zhang, L., and Bai, L. (2019). Therapeutic effect of Reduced Glutathione on acute kidney injury in patients with sepsis. Chin. Mode Med. 26 (9), 54–60.
Zheng, X. (2002). Guidelines for clinical research of Chinese medicine (new drug). Beijing: Chinese Medicine and Science Publication House.
Zhou, F., Zou, X., Zhang, J., Wang, Z., Yang, Y., and Wang, D. (2021). Jian-pi-yi-shen formula ameliorates oxidative stress, inflammation, and apoptosis by activating the Nrf2 signaling in 5/6 nephrectomized rats. Front. Pharmacol. 12, 630210. doi:10.3389/fphar.2021.630210
Zhou, S., Ai, Z., Li, W., You, P., Wu, C., Li, L., et al. (2020). Deciphering the pharmacological mechanisms of taohe-chengqi decoction extract against renal fibrosis through integrating network Pharmacology and experimental validation in vitro and in vivo. Front. Pharmacol. 11, 425. doi:10.3389/fphar.2020.00425
Keywords: acute kidney injury, chuan huang fang, chronic kidney disease, renal fibrosis, reduced glutathione, traditional Chinese medicine
Citation: Chen L, Ye Z, Wang D, Liu J, Wang Q, Wang C, Xu B and Gong X (2022) Chuan Huang Fang combining reduced glutathione in treating acute kidney injury (grades 1–2) on chronic kidney disease (stages 2–4): A multicenter randomized controlled clinical trial. Front. Pharmacol. 13:969107. doi: 10.3389/fphar.2022.969107
Received: 14 June 2022; Accepted: 12 September 2022;
Published: 03 October 2022.
Edited by:
Dan-Qian Chen, Northwest University, ChinaCopyright © 2022 Chen, Ye, Wang, Liu, Wang, Wang, Xu and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuezhong Gong, c2huYW5zaGFuQHllYWgubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.