
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 14 September 2022
Sec. Renal Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.968980
This article is part of the Research TopicNew Insights into Renal Fibrosis and Therapeutic Effects of Natural Products, Volume IIView all 19 articles
Persistent inflammation associated with recurrent urinary tract infection (rUTI) is a crucial inducement of inflammation-driven renal fibrosis (IDRF). Although continuous low-dose antibiotic therapy (CLAT) is the common treatment for rUTI, its clinical efficacy remains unsatisfactory. Tailin formulation (TLF), a Chinese herbal formulation prescribed for treating rUTI, is effective in alleviating symptoms and reducing recurrence. This study was to evaluate the efficacy and safety of TLF combined with CLAT compared with CLAT used alone in patients with rUTI. In this multicenter, randomized, controlled clinical trial, patients were assigned (1:1) to receive either TLF + CLAT or CLAT for 12 weeks. The primary outcome was the effective rate at week 12 of the treatment. The secondary outcomes were the recurrent rate at week 4 and week 12 post treatment; the post-treatment changes in renal tubular injury markers (urinary N-acetyl-β-d-glucosaminidase (NAG) and β2-microglobulin (β2-MG)), profibrotic factors (urinary monocyte chemoattractant protein-1 (MCP-1) and transforming growth factor beta1 (TGF-β1)), and traditional Chinese medicine (TCM) symptoms, and vital signs indicators and serious adverse events (SAEs) were also monitored throughout the trial. A total of 195 patients were included in the final analysis. The TLF + CLAT group had a higher effective rate and a lower recurrence rate than the CLAT group (p < 0.01). Significant decrease of urinary NAG and β2-MG was observed in the TLF + CLAT group vs. CLAT group (p < 0.01), and similar changes were observed in profibrotic factors (urinary MCP-1 and TGF-β1) (p < 0.05), which indicated that TLF might have potential renal tubular protection and anti-fibrosis effects. Additionally, a positive correlation within a certain range was shown in the correlation analysis of medical history (months) of rUTI patients with urinary MCP-1 (r = 0.50, p < 0.05) and TGF-β1 (r = 0.78, p < 0.01). A significant difference was also observed in TCM symptoms (p < 0.01). There were no obvious adverse reactions that occurred during this study. We conclude that TLF combined with CLAT was superior to CLAT used alone in reducing rUTI recurrence, alleviating the non-infection-related physical symptoms and protecting renal tubular and anti-fibrosis, which suggests this novel therapy might be an available treatment with great promise in treating rUTI.
rUTI is a common clinical refractory disease affecting millions of people across the globe annually (Geerlings, 2016), exerting considerable impact on patient's quality of life (Wagenlehner et al., 2018).
Inflammation of the urinary tract is the critical pathological mechanism of rUTI (Kaur and Kaur, 2021), which might result in renal tubular damage (Lorenzo-Gomez et al., 2020) and renal fibrosis (Lv et al., 2018) in those patients. However, there is insufficient evidence to reflect renal damage and inflammation-driven renal fibrosis (IDRF) as subsequent issues due to the rUTI. Urinary NAG and β2-MG are sensitive markers of tubular injury to predict acute kidney injury (AKI) (Ix and Shlipak, 2021). Elevated urinary NAG and β2-MG values have been described in patients suffering risk factors, including nephrotoxic drugs (Raduly et al., 2021). Therefore, presumably, renal damage attributable to rUTI could be evidenced by the increase of urinary NAG and β2-MG.
Persistent inflammation associated with rUTI is a crucial inducement of IDRF (Humphreys, 2018; Lv et al., 2018), involving complex interactions among multiple inflammatory cells and profibrotic cytokine signaling pathways. Critical profibrotic factors include MCP-1 and TGF-β1. Inflammation might promote fibrosis by inflammatory cells, which is tightly regulated by TGF-β1 (Humphreys, 2018). MCP-1 also plays an important role in the process of inflammation for it could attract other inflammatory factors/cells and has been found to be associated with renal fibrosis (Singh et al., 2021). Given that, changes of critical profibrotic factors (urinary MCP-1 and TGF-β1) in rUTI patients should be paid more attention.
In addition to the high prevalence and economic burden of rUTI, the presumable renal damage and IDRF attributed to rUTI in those patients bring an even greater challenge in the treatment of rUTI.
Antibiotic treatment including CLAT is the most common treatment for rUTI globally (Anger et al., 2019). Although CLAT is effective in inhibiting uropathogenic bacteria and shortening the duration of the infection-related symptoms in episodes, the clinical efficacy and safety of CLAT still remain unsatisfactory due to the presumable side effects and lack of long-term efficacy (Flower et al., 2016; Forbes et al., 2018). Consequently, alternative treatments are being considered, such as Chinese herbal medicine (CHM).
Based on 5,000 years of practice and experience, Chinese medicine is an essential part of healthcare in China. Comparable beneficial outcomes have also been reported for CHM products administered to patients with rUTI (Flower et al., 2019).
TLF (Gong et al., 2006) is a herbal prescription developed by Professor Gong Xuezhong for the treatment of rUTI, consisting of Radix Pseudostellariae (Taizishen), Radix Rehmanniae Recen (Shengdihuang), Sargentodoxa Cuneata (Daxueteng), and Polygonum Cuspidatum (Huzhang). Previous studies by our group have shown the possible roles for TLF in the treatment of rUTI (Gong et al., 2006; Gong et al., 2007; Gong et al., 2010). In animal experiments, we have built rat models of cystitis, acute pyelonephritis (APN), and chronic pyelonephritis (CPN) by E. coli O 111B4 successfully (Gong et al., 2003; Gong et al., 2004) and found that TLF might effectively protect renal tubules from injury, inhibit renal tubular and interstitial inflammation, and cures renal fibrosis in rats with UTI (Gong et al., 2006; Gong et al., 2010). In the clinical trial (Gong et al., 2007), we have found that compared with the levofloxacin group, urine NAG/Cr and β2-MG decreased significantly in the TLF group, suggesting that TLF could protect the renal tubular function of CPN patients.
Since the clinical efficacy of CLAT in the rUTI population is unsatisfactory, this study attempted to determine if there was a more effective alternative in patients with rUTI using LTF combined with CLAT. Moreover, given that persistent inflammation associated with rUTI is a crucial inducement of IDRF, we also explored the possible relationship between the critical profibrotic factors (TGF-β1, MCP-1) and the medical history (months) of rUTI patients in the present study to clarify if renal fibrosis can be associated with rUTI.
Here, we report the results of this study, hoping to provide reliable evidence-based medical evidence for treatment of rUTI and ease the burden for patients with rUTI.
This trial ran from January 2021 to March 2022 and involved three tertiary hospitals (Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine; Shanghai Sixth People’s Hospital, Shanghai Jiaotong University; Shanghai Baoshan District Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine). Patients eligible for the trial in each trial site were assigned a randomized 1:1 ratio to receive LTF + CLAT or CLAT for 12 weeks. This study protocol was approved by the Institutional Ethics Committee (record number: 2020SHL-KY-47) and registered at the Chinese Clinical Trial Registry (registration number: ChiCTR-TRC-10001518).
The sample size was calculated by PASS (version 15.0). Based on the previous trial (Li, 2018), 91 patients per group were needed to achieve 90% power to detect a difference between the group proportions of 0.18. Assuming an attrition rate of 10%, a planned recruitment target of 100 patients per group will be set.
According to the inclusion and exclusion criteria, rUTI patients diagnosed with the qi-yin of spleen and kidney deficiency and damp-heat adhesion syndrome by TCM will be considered potential participants.
Patients would be diagnosed with UTI if
1) urine culture: ≥105 cfu/ml bacteria;
2) urinary sediment: white blood cell (WBC) > 10/HP, or with clinical symptoms of UTI.
rUTI is defined as more than two UTIs in the last 6 months or more than three UTIs in the last 12 months, and the course of the disease is more than 2 years.
Eligibility criteria for inclusion were patients who were aged 18–70 years; history of rUTI (at least two episodes in the last 6 months or more than three episodes in the last 12 months); diagnosed with the qi-yin of spleen and kidney deficiency and damp-heat adhesion syndrome by TCM; no known allergies to the drugs to be prescribed; agreed to take part in the trial and sign informed consent.
Exclusion criteria were pregnancy or lactation; with an in-dwelling catheter; urethral syndrome; chronic kidney disease (CKD) stages IV–V (eGFR<30 ml/min); combined with serious heart and liver function damage or diabetes and other diseases which need immediate treatment; severe central nervous system disease; participating in other drug clinical trials or have participated in other clinical trials in the last 3 months.
Patients eligible in each trial site were assigned randomly, using a computer-generated random number sequence. The division of the groups in this study will be blinded to all participants, investigators, and statisticians. The placebo and TLF are the same in the appearance of the drug package.
Participants randomly assigned to the treatment group were administered TLF + CLAT for 12 weeks, and the participants assigned to the control group received CLAT + placebo for 12 weeks. Dosage and duration are shown in Table 1.
The primary outcome was the effective rate at week 12 of treatment. The second outcomes were the recurrence rate at weeks 4 and 12 after treatment; post-treatment changes in urinary NAG/Cr, β2-MG, TGF-β1, and MCP-1; furthermore, traditional Chinese medicine (TCM) symptoms were also scored; meanwhile, vital sign indicators and serious adverse events (SAEs) were monitored throughout the trial.
After treatment was completed, all patients had a follow-up of 12 weeks. Efficacy-related examinations and safety indicators were performed and collected during the treatment and follow-up period. Routine urine examination was conducted at week 0 of the treatment period and every 2 weeks during the treatment period and the follow-up period; cleaning middle urine cultivation was conducted at weeks 0 and 4 and 8 and 12 of the treatment period and weeks 4 and 12 post treatment. Urinary NAG/Cr, β2-MG, TGF-β1, and MCP-1 were measured at weeks 0 and 12 of the treatment period.
Original data were collected and verified by monitors at every visit. Continuous variables of the endpoints were compared with those at the baseline using an analysis of variance or Student’s t-test. After processing the categorical variables, the researchers used Fisher’s exact test, the chi-square test, or the Cochran–Mantel–Haenszel test to analyze the results. The continuous variables conforming to normality will be analyzed using Pearson’s correlation test, and those that do not conform to normality will be analyzed using the Spearman correlation test.
During the recruitment period, patients with uncomplicated rUTI were randomized with a 1:1 ratio into two groups to receive TLF + CLAT or CLAT. Figure 1 shows a CONSORT flow chart. The final data analysis included 98 patients in the TLF + CLAT group and 97 in the CLAT group.
The patients were comparable between the three groups in terms of demographic and disease characteristics.
Table 2 shows that the mean age was 52.24 years (SD 13.92) in the TLF group and 53.01 years (SD 13.90) in the CLAT group; the percentage of females present was 69.39% (68/98) in the TLF group and 68.04% (66/97) in CLAT group; the mean medical history (recorded from the first diagnosis of rUTI) of rUTI patients was 43.85 months (SD 14.24) in the TLF group and 43.06 months (SD 12.76) in the CLAT group; the mean composite TCM syndrome score was 21.15 points (SD 1.89) in the TLF group and 20.71 points (SD 2.32) in the CLAT group.
The primary and secondary outcomes are shown in Table 3. The clinical effective rate of the two groups was calculated at week 12 of treatment. The recurrent rate of the two groups was calculated at weeks 4 and 12 post treatment, separately. Urinary NAG/Cr, β2-MG, TGF-β1, MCP-1, and TCM syndrome of the patients in the two groups were measured at weeks 0 and 12 of the treatment period.
Urinary NAG/Cr was above the normal range before the treatment both in the TLF + CLAT group and CLAT group, with no significant difference (p = 0.116). After 12 weeks of treatment, NAG/Cr decreased in the TLF + CLAT group but not in the CLAT group (mean (SD) 1.74 (1.01) U. mmolCr (−1) vs. 3.18 (1.03) U. mmolCr (−1), respectively; p < 0.01). Similar changes were observed in urinary β2-MG (mean (SD) 0.22 (0.11) U. mmolCr (−1) vs. 0.41 (0.20) U. mmolCr (−1), respectively; p < 0.01).
Urinary TGF-β1 decreased in the TLF + CLAT group but not in the CLAT group (mean (SD) 17.51 (8.38) pgml (−1) vs. 21.11 (8.59) pgml (−1), respectively; p < 0.05). Similar changes were observed in urinary MCP-1 (mean (SD) 6.68 (3.41) ngL (−1) vs. 8.92 (3.46) ngL (−1), respectively; p < 0.05). Additionally, a positive correlation within a certain range was shown in correlation analysis of medical history (months) of rUTI patients with urinary MCP-1 (r = 0.50, p < 0.05) and TGF-β1 (r = 0.78, p < 0.01) (Figures 2, 3).

FIGURE 2. Correlation analysis between medical history (months) of rUTI patients and urinary MCP-1 (week 0).
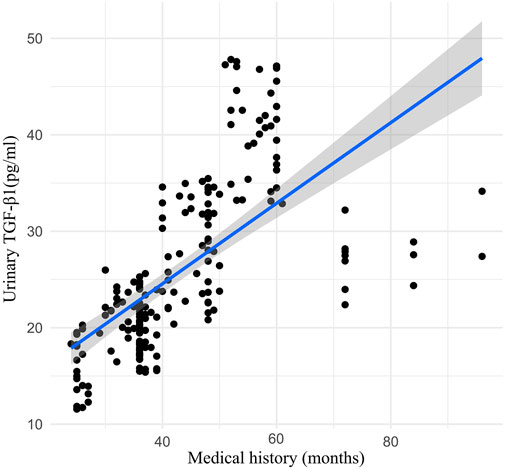
FIGURE 3. Correlation analysis between medical history (months) of rUTI patients and urinary TGF-β1 (week 0).
Compared with patients treated with CLAT alone, TLF combined with CLAT resulted in a higher clinical effective rate and lower recurrence rate and was sustained thereafter. For long-term clinical efficacy, the total effective rates were 90.82% (89/98) in the TLF + CLAT group and 74.23% (72/97) in the CLAT group, p < 0.05. For long-term clinical efficacy, the recurrent rate at week 4 post treatment was 14.29% (14/98) in the TLF + CLAT group and 36.08% (36/97) in the CLAT group; the recurrent rate at week 12 post treatment was 19.39% (19/98) in the TLF + CLAT group and 47.42% (46/97) in the CLAT group. Clinical efficacy is evaluated by the disappearance or improvement of clinical symptoms and uropathogenic clearance. More details could be found in Table 4.
Groups were similar in TCM syndrome scores before the treatment, with a mean composite symptom score of 21.15 points (SD 1.89) in the TLF + CLAT group and 20.71 points (SD 2.32) in the CLAT group. After 12 weeks of treatment, groups showed significant differences, with a mean composite symptom score of 6.22 points (SD 4.09) in the TLF + CLAT group and 11.44 points (SD 5.52) in the CLAT group, p < 0.01.
There were no significant changes in the safety results, including blood routine, liver and kidney function, and electrocardiogram before and after treatment in the two groups, and no obvious adverse reactions occurred. CLAT-related laboratory abnormalities were not observed during this trial.
In this study, we found that compared with CLAT, TLF combined with CLAT resulted in a significantly higher clinical effective rate and lower recurrence rate. Notably, additional benefits in protecting renal tubule, inhibiting renal fibrosis, and alleviating the non-infection-related physical signs and subjective symptoms were also observed in patients treated with TLF + CLAT. There were no obvious adverse reactions that occurred during this study.
CHM has been shown to be either used alone or combined with antibiotics, which may be more effective than antibiotics alone in treating (Yu et al., 2018). Equally, our previous studies showed relatively good clinical efficacy of TLF compared with CLAT in the treatment of rUTI (Gong et al., 2003; Gong et al., 2004; Gong et al., 2006; Gong et al., 2007; Gong et al., 2010). In the present study, we combined TLF with CLAT to treat rUTI and evaluated the clinical efficacy of this novel therapy. Compared with patients administrated with CLAT alone, a higher clinical effective rate was observed in TLF + CLAT group at week 12 of treatment. Furthermore, recurrent rates were monitored respectively at week 4 and week 12 post treatment to observe the long-term efficacy of the two treatments. Our results showed that TLF combined with CLAT has a longer and more stable effect; indirectly, it also suggested that TLF might have benefits to enhance the long-term efficacy.
RUTI involves inflammation of the urinary tract, which might result in renal tubular damage and ultimately kidney failure in those patients (Ichino et al., 2009; Lorenzo-Gomez et al., 2020). Urinary NAG and β2-MG are sensitive markers of tubular injury (Ix and Shlipak, 2021). The study has shown that abnormal expression of the urinary kidney injury marker (NAG) was also observed in rUTI patients (Lorenzo-Gomez et al., 2020). Therefore, quantitative detection of urinary renal tubular injury markers in patients with rUTI should be paid more attention, which might be helpful for early prevention of renal damage attributable to rUTI. In the present study, increased urinary renal tubular injury markers (NAG/Cr and β2-MG) were observed in rUTI patients and were reduced more after TLF + CLAT treatment compared with CLAT. Accordingly, TLF + CLAT might have an effect on renal tubular protection in rUTI patients.
Persistent inflammation associated with rUTI is a crucial inducement of IDRF, which is a complicated process involving proinflammatory and profibrotic paracrine mediators, such as growth factors, TGF-β1, MCP-1, and cytokines (Humphreys, 2018; Gu et al., 2020; Singh et al., 2021). As the critical profibrotic factors, urinary MCP-1 and TGF-β1 were monitored to evaluate the anti-fibrosis effects of the two treatments in the present study. Urinary TGF-β1 and MCP-1 were tested at week 0 and week 12 of treatment. Correlation analysis showed that the medical history (months) of rUTI patients was positively correlated with the level of profibrotic factors at week 0, suggesting that rUTI might be a risk factor for IDRF. Also, after 12 weeks of treatment, compared with CLAT, the expression of urinary TGF-β1 and MCP-1 decreased significantly in the TLF + CLAT group, which showed that TLF combined with CLAT has benefits in protecting against renal fibrosis. Therefore, attention should be paid to early prevention of renal fibrosis in the treatment of patients with rUTI. However, the specificity and sensitivity of urinary TGF-β1 and MCP-1 in the diagnosis of renal fibrosis attributable to rUTI still need more support from evidence-based clinical research, which already became a trigger for our subsequent experiments.
Furthermore, benefits in alleviating the non-infection-related physical signs and subjective symptoms were also observed in rUTI patients treated with TLF + CLAT. In clinical practice, we find that symptoms such as abdominal distention and fatigue would persist for a considerable time even after the infection is under control, which would result in significant inconvenience to those patients. The guidelines for rUTI showed that, since 2011, the non-infection-related physical signs and subjective symptoms have been recognized as part of the diagnosis criteria, along with “self-diagnosis of UTI” (Dason et al., 2011; Anger et al., 2019). This suggests that treatment of RUTI should not only focus on the negative result of urine culture but also pay attention to eradicate or alleviate the non-infection-related physical signs and subjective symptoms of rUTI patients. Therefore, this novel treatment is also a promising strategy helping to improve the health-related quality of life of rUTI patients.
In conjunction with the previous evidences (Gong et al., 2006; Gong et al., 2007; Gong et al., 2010; Lorenzo-Gomez et al., 2020), our results suggest that renal injury and IDRF might be the consequences due to rUTI in those patients, which involves complex interactions among multiple inflammatory cells and profibrotic cytokine signaling pathways. Given that, it is becoming more and more challenging to treat rUTI since it may not be possible to treat this disease by targeting one single molecular pathway. In other words, a multi-pharmacological approach involving several anti-inflammatory and antifibrotic molecules may need to be employed.
Chinese herbal is a natural medicine, contains a variety of effective ingredients, and has long been used to treat rUTI (Flower et al., 2015). TLF is an effective herbal prescription developed for treating rUTI. With the characteristics of the multi-ingredients, the mechanism of action and targets of Chinese herbal prescription TLF in patients with rUTI are also diverse. Studies by our group have shown the possible roles for TLF in the treatment of rUTI, including effects on reducing recurrence rate, alleviating symptoms, protecting renal tubular function, and inhibiting renal fibrosis (Gong et al., 2003; Gong et al., 2004; Gong et al., 2006; Gong et al., 2007; Gong et al., 2010). Consequently, TLF might be the multi-pharmacological approach with great promise in treating rUTI. However, further studies are still needed to investigate the mechanism of TLF against rUTI.
This study was designed as a double-blind RCT, with strict quality control processes for manufacturing TLF and placebo. The major contribution of our research is to demonstrate that rUTI is a significant threat that could cause renal damage and fibrosis, and TLF might be a multi-pharmacological approach involving anti-inflammatory and antifibrotic molecules and with great promise in treating rUTI. Since our study duration was relatively short (24 weeks), further investigation with longer follow-up is warranted to validate the role of urinary TGF-β1 and MCP-1 in the IDRF diagnosis and the impact of TLF in renal damage and fibrosis attributable to rUTI.
TLF combined with CLAT was superior to CLAT used alone in rUTIs in alleviating symptoms and decreasing recurrence and might have renal tubular protection and anti-fibrosis effects, which suggests TLF combined with CLAT might be an available treatment for rUTI. Moreover, TLF might be a multi-pharmacological approach involving anti-inflammatory and antifibrotic molecules and with great promise in treating rUTI.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine. The patients/participants provided their written informed consent to participate in this study.
XG is the chief investigator of the trial and contributed to conceive the research, organize, and supervise the study. TL carried out the trial registration, drafted the manuscript, and participated in the data collection. GJ and WL contributed in enrolling patients at sub-centers of this research; YX and GY participated in the clinical data monitoring and the organization of study visits. All authors revised the protocol critically for important intellectual content and approved the final version of the manuscript.
This trial is funded by the National Natural Science Foundation of China (No. 82074387 and No. 81873280) and Shanghai Municipal Education Commission Gaofeng Gaoyuan Discipline Construction Project (No. 02.ZY05.191311N). The funders had no role in study design and conduction, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors thank GJ and WL for their efforts in enrolling patients at Shanghai Sixth People’s Hospital, Shanghai Baoshan District Hospital of Integrated Traditional Chinese and Western Medicine, respectively. We are also thankful to all of the patients who participated in the study and the staff of the participating hospitals for their support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anger, J., Lee, U., Ackerman, A. L., Chou, R., Chughtai, B., Clemens, J. Q., et al. (2019). Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J. Urol. 202 (2), 282–289. doi:10.1097/JU.0000000000000296
Dason, S., Dason, J. T., and Kapoor, A. (2011). Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can. Urol. Assoc. J. 5 (5), 316–322. doi:10.5489/cuaj.11214
Flower, A., Harman, K., Lewith, G., Moore, M., Bishop, F. L., Stuart, B., et al. (2016). Standardised Chinese herbal treatment delivered by GPs compared with individualised treatment administered by practitioners of Chinese herbal medicine for women with recurrent urinary tract infections (RUTI): Study protocol for a randomised controlled trial. Trials 17, 358. doi:10.1186/s13063-016-1471-5
Flower, A., Harman, K., Willcox, M., Stuart, B., and Moore, M. (2019). The RUTI trial: A feasibility study exploring Chinese herbal medicine for the treatment of recurrent urinary tract infections. J. Ethnopharmacol. 243, 111935. doi:10.1016/j.jep.2019.111935
Flower, A., Wang, L. Q., Lewith, G., Liu, J. P., and Li, Q. (2015). Chinese herbal medicine for treating recurrent urinary tract infections in women. Cochrane Database Syst. Rev. 6, CD010446. doi:10.1002/14651858.CD010446.pub2
Forbes, R., Ali, A., Abouhajar, A., Brennand, C., Brown, H., Carnell, S., et al. (2018). ALternatives to prophylactic antibiotics for the treatment of recurrent urinary tract infection in women (ALTAR): Study protocol for a multicentre, pragmatic, patient-randomised, non-inferiority trial. Trials 19 (1), 616. doi:10.1186/s13063-018-2998-4
Geerlings, S. E. (2016). Clinical presentations and epidemiology of urinary tract infections. Microbiol. Spectr. 4 (5), 1–11. doi:10.1128/microbiolspec.UTI-0002-2012
Gong, X. Z., Yang, J., Meng, Q., Zheng, P. D., and He, L. Q. (2003). Comparision of pathological morphology in two rat model of acute pyelonephritis. Shenzhen J. Integr. Traditional Chin. West. Med. 13 (2), 74–77. doi:10.16458/j.cnki
Gong, X. Z., Zheng, P. D., Yang, J., and Feng, Z. (2007). Effect of the Qi-tonifying Kidney-nourishing and Damp-heat-relieving Method on the tubular function in patients with chronic pyelonephritis. Jiangsu J. Traditional Chin. Med. 39 (8), 19–21. doi:10.3969/j.issn.1672-397X.2007.08.014
Gong, X. Z., Zheng, P. D., Yang, J., and Meng, Q. (2004). Building rat model of chronic pyelonephritis. Beijing Med. Journal(Chin) 26 (6), 391–394. doi:10.15932/j.0253
Gong, X. Z., Zheng, P. D., Yang, J., Meng, Q., and Li, H. Y. (2006). Effect of tailinfang on renal tubular function of chronic pyelonephritis rats. Chin. J. Integr. Traditional West. Nephrol. 7 (3), 138–140.
Gong, X. Z., Zheng, P. D., Yang, J., Zhou, J. J., and He, L. Q. (2010). Effects of “Tailin Decoction” on renal scarring suppression in chronic pyelonephrits rats. Shanghai J. Traditional Chin. Med. 44 (4), 72–75. doi:10.16305/j.1007
Gu, Y., Liu, X., Huang, X., Yu, X., and Lan, H. (2020). TGF-β in renal fibrosis triumphs and challenges. Future Med. Chem. 12 (9), 853–866. doi:10.4155/fmc-2020-0005
Humphreys, B. D. (2018). Mechanisms of renal fibrosis. Annu. Rev. Physiol. 80, 309–326. doi:10.1146/annurev-physiol-022516-034227
Ichino, M., Kuroyanagi, Y., Kusaka, M., Mori, T., Ishikawa, K., Shiroki, R., et al. (2009). Increased urinary neutrophil gelatinase associated lipocalin levels in a rat model of upper urinary tract infection. J. Urol. 181 (5), 2326–2331. doi:10.1016/j.juro.2009.01.010
Ix, J. H., and Shlipak, M. G. (2021). The promise of tubule biomarkers in kidney disease: A review. Am. J. Kidney Dis. 78 (5), 719–727. doi:10.1053/j.ajkd.2021.03.026
Kaur, R., and Kaur, R. (2021). Symptoms, risk factors, diagnosis and treatment of urinary tract infections. Postgrad. Med. J. 97 (1154), 803–812. doi:10.1136/postgradmedj-2020-139090
Li, L. w. (2018). Clinical observation on the treatment of recurrent urinary tract infection by Bushen Tonglin prescription combined with antimicrobial drugs. China: Liaoning University of TCM.
Lorenzo-Gomez, M. F., Flores-Fraile, M. C., Marquez-Sanchez, M., Flores-Fraile, J., Gonzalez-Casado, I., Padilla-Fernandez, B., et al. (2020). Increased urinary markers of kidney damage in the institutionalized frail elderly due to recurrent urinary tract infections. Ther. Adv. Urol. 12, 1756287220974133. doi:10.1177/1756287220974133
Lv, W., Booz, G. W., Wang, Y., Fan, F., and Roman, R. J. (2018). Inflammation and renal fibrosis: Recent developments on key signaling molecules as potential therapeutic targets. Eur. J. Pharmacol. 820, 65–76. doi:10.1016/j.ejphar.2017.12.016
Raduly, Z., Price, R. G., Dockrell, M. E. C., Csernoch, L., and Pocsi, I. (2021). Urinary biomarkers of mycotoxin induced nephrotoxicity-current status and expected future trends. Toxins (Basel) 13 (12), 848. doi:10.3390/toxins13120848
Singh, S., Anshita, D., and Ravichandiran, V. (2021). MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 101, 107598. doi:10.1016/j.intimp.2021.107598
Wagenlehner, F., Wullt, B., Ballarini, S., Zingg, D., and Naber, K. G. (2018). Social and economic burden of recurrent urinary tract infections and quality of life: A patient web-based study (GESPRIT). Expert Rev. pharmacoecon. Outcomes Res. 18 (1), 107–117. doi:10.1080/14737167.2017.1359543
Yu, G. Y., Xie, Y. M., Gao, N., Sun, Y., Miao, R. P., Han, S. J., et al. (2018). Clinical application evaluation of clinical practice guideline on traditional Chinese medicine therapy alone or combined with antibiotics for uncomplicated lower urinary tract infection. Zhongguo Zhong Yao Za Zhi(Chin) 43 (24), 4746–4752. doi:10.19540/j.cnki.cjcmm.2018.0126
Keywords: recurrent urinary tract infection, renal fibrosis, tailin formulation, continuous low-dose antibiotic therapy, traditional Chinese medicine, randomized controlled trial
Citation: Li T, Xu Y, Yuan G, Lu W, Jian G and Gong X (2022) Efficacy and safety of tailin formulation combined with continuous low-dose antibiotic therapy in patients with recurrent urinary tract infection: A multicenter, randomized, controlled clinical trial. Front. Pharmacol. 13:968980. doi: 10.3389/fphar.2022.968980
Received: 14 June 2022; Accepted: 22 August 2022;
Published: 14 September 2022.
Edited by:
Dan-Qian Chen, Northwest University, ChinaReviewed by:
Senyan Liu, Shanghai Changzheng Hospital, ChinaCopyright © 2022 Li, Xu, Yuan, Lu, Jian and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuezhong Gong, c2huYW5zaGFuQHllYWgubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.