- 1School of Public Health, Lanzhou University, Lanzhou, China
- 2School of Stomatology, Lanzhou University, Lanzhou, China
- 3Evidence-Based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China
- 4School of Chinese Medicine, Hong Kong Baptist University, Hong Kong, Hong Kong SAR, China
- 5Zhejiang University of Traditional Chinese Medicine, Hangzhou, Zhejiang, China
- 6Wangjing Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 7Evidence Based Social Science Research Center, Health Technology Assessment Center, School of Public Health, Lanzhou University, Lanzhou, China
- 8Key Laboratory of Evidence Based Medicine and Knowledge Translation of Gansu Province, Lanzhou, China
- 9Institute of Health Data Science, Lanzhou University, Lanzhou, China
- 10WHO Collaborating Centre for Guideline Implementation and Knowledge Translation, Lanzhou, China
- 11Guideline International Network Asia, Lanzhou, China
Background: Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory arthropathy. Recommendations for RA, specifically on pharmacotherapy, are essential in clinical practice. However, the direction and strength of recommendations are controversial across current clinical practice guidelines (CPGs) of RA.
Objective: To systematically analyze the consistency of recommendations regarding pharmacotherapy of RA across CPGs.
Methods: 11 electronic databases and websites were comprehensively searched from inception to 14 March 2022, to identify CPGs for diagnosis, therapy, and management of RA. Unambiguous and discrete specifications of the population-intervention-comparison (PIC) framework were used to classify the recommendations. Based on the PIC framework, consistency analyses across CPGs on pharmacotherapy of RA were performed. Two researchers reached a consensus on coding the direction and strength of each recommendation.
Results: Finally, 26 CPGs were included in this study, and 14 of them, which included pharmacotherapy, were performed consistency analysis. 1) 64 recommendations from 14 CPGs were classified into 18 PICs. 2) Seven PICs (38%) were consistent in direction and strength, 10 PICs (56%) were consistent in direction but inconsistent in strength, and one PIC (6%) was inconsistent in direction (hydroxychloroquine, HCQ). 3) Sensitivity analysis tested the robustness, and the inconsistency remained high.
Conclusion: The direction was highly consistent among the recommendations of pharmacotherapy for RA, but the strength was highly inconsistent. Reasons for the inconsistency need to be further investigated, and consistent recommendations could guide the pharmacotherapy of RA in clinical practice.
1 Introduction
Rheumatoid arthritis (RA) is a common chronic disease that manifests as a systemic autoimmune inflammatory type and involves the joints of the hands, wrists, feet, and knees (Zhang et al., 2020). RA primarily causes pain, swelling, stiffness and functional limitation of the joints but also extra-articular manifestations of other organs, including the lungs, heart, blood vessels, skin, and eyes (Smolen et al., 2016). People of all ages could be affected by RA, and the high-risk population ranges from 50 to 59 years old (Smith and Berman, 2022). RA may lead to permanent damage and disability of the joints, and it is a severe public health problem globally and carries an unbearable burden of disease. RA negatively impacts the daily quality of life in patients (Gao et al., 2022). In 2017, RA accounted for 3.4 million (95% uncertainty interval (UI), 2.6–4.4) disability adjusted life years (DALYs) at the global level, with an age-standardized rate of 43.3 (95% UI 33.0–54.5) DALYs per 1,00,000 populations (Safiri et al., 2019). RA is regarded as one of the fifty typical diseases that cause disability globally, and the incidence in women is two to three times that in men (Espinoza et al., 2021; Smith and Berman, 2022).
Clinical Practice Guidelines (CPGs) are used to provide a basis for clinical decision-making and guide clinical practice in the medical and healthcare fields. CPGs play a significant role in regulating healthcare practices, improving patient prognosis, and saving healthcare resources (Zhang et al., 2021). Recommendations formatted for specific issues are the core of the CPGs (Institute of Medicine Committee on Standards for Developing Trustworthy Clinical Practice G Graham et al., 2011). CPGs are developed based on evidence from systematic reviews, balancing the advantages and disadvantages of different interventions to form recommendations. With the growing RA research, CPGs for RA have been developed by a series of academic institutions or organizations, including the American College of Rheumatology (ACR), the European League Against Rheumatism (EULAR), and the Asia-Pacific League of Associations for Rheumatology (APLAR) (Lau et al., 2018; Ho et al., 2019; Park et al., 2020; Smolen et al., 2020). These RA CPGs are updated periodically to improve their applicability and support high-quality clinical care. The common drugs of RA therapy include disease-modifying antirheumatic drugs (DMARDs), non-steroidal anti-inflammatory drugs (NSAIDs), and glucocorticoids (GCs) (Cardiel et al., 2018; Kerschbaumer et al., 2020; Fraenkel et al., 2021). However, controversial pharmacotherapy recommendations are provided in different CPGs. For example, hydroxychloroquine (HCQ) was recommended oppositely in National Institute of Health and Clinical Excellence (NICE) and Hong Kong Society of Rheumatology (HKSR) guidelines (Ho et al., 2019; NICE, 2020). The inconsistency among pharmacotherapy recommendations impedes the practical application of these recommendations and complicates the appropriate selection of CPGs in clinical practice (Erickson et al., 2017; Ge et al., 2018; Yu et al., 2020).
As RA CPGs increased rapidly, there were significant differences and contradictions among the recommendations (Yang et al., 2013; Chen et al., 2020). Therefore, systematic reviews of CPGs were conducted to analyze the consistencies in recommendations among CPGs. Most of the consistency analyses of CPGs were performed to categorize, summarize and explain the differences among recommendations or macroscopically combine the methodology or report quality assessment results (Zhang et al., 2019; Luo et al., 2021; Zhang et al., 2021; Yan et al., 2022). However, compared to traditional analyses on the consistency of CPGs’ recommendations, the population-intervention-comparison (PIC) framework is more scientific and transparent (Alper et al., 2019; Kow et al., 2021). Currently, there is a lack of evidence on the consistency analysis of pharmacotherapy recommendations in RA CPGs (Yu et al., 2020). Therefore, we conducted a consistency analysis for RA pharmacotherapy recommendations to map the various topics covered by current RA CPGs and investigate the consistency in direction and strength among RA pharmacotherapy recommendations.
2 Materials and methods
2.1 Search strategy
We systematically searched five databases, including PubMed, DynaMed, UTD (UpToDate), CNKI (China National Knowledge Infrastructure), and CBM (China Biology Medicine). Additionally, WHO (World Health Organization), GIN (Guidelines International Network), NICE (National Institute for Health and Care Excellence), SIGN (Scottish Intercollegiate Guidelines Network), ECRI (Emergency Care Research Institute), and Medlive were searched. Our comprehensive search was limited from inception to 14 March 2022. The search strategy consisted of keywords: (“rheumatoid arthritis”) AND (“guidance” OR “guideline” OR “recommendation”). The detailed search strategy was shown in Supplementary Table S1.
2.2 Inclusion and exclusion criteria
Inclusion criteria: 1) guidelines for the diagnosis, treatment, and management of RA; 2) the latest and available version of the guidelines. Exclusion criteria: 1) guidelines published in languages other than Chinese or English; 2) an interpretation version of the guidelines; 3) the protocol of guidelines.
2.3 Literature selection and data extraction
Two researchers independently screened the titles and abstracts to exclude irrelevant literature. The literature, which could not be determined by screening the titles and abstracts, needed to be identified by reviewing the full text. Data extraction was conducted using pre-designed tables in Microsoft Excel 2016. The extracted items included the title, publication year, publication language, development institution/organization, type of guideline, the adaption of other guidelines or not, the method for grading the quality of evidence and strength of recommendations, and counts of recommendations. Data extraction was carried out by two researchers independently. If there were disagreements between literature selection and data extraction, a third researcher was consulted to resolve the disagreements by reaching a consensus.
2.4 Consistency analysis
2.4.1 Inclusion and exclusion criteria of the consistency analysis
We selected guidelines for the consistency analysis from the included studies. Inclusion criteria were 1) guidelines that contained recommendations for pharmacotherapy. Exclusion criteria were 1) guidelines adapted from other guidelines; 2) traditional Chinese medicine (TCM) guidelines; 3) guidelines that the counts of pharmacotherapy recommendations were not stated in the abstracts or full text, which made it impossible to extract.
2.4.2 Recommendation specification
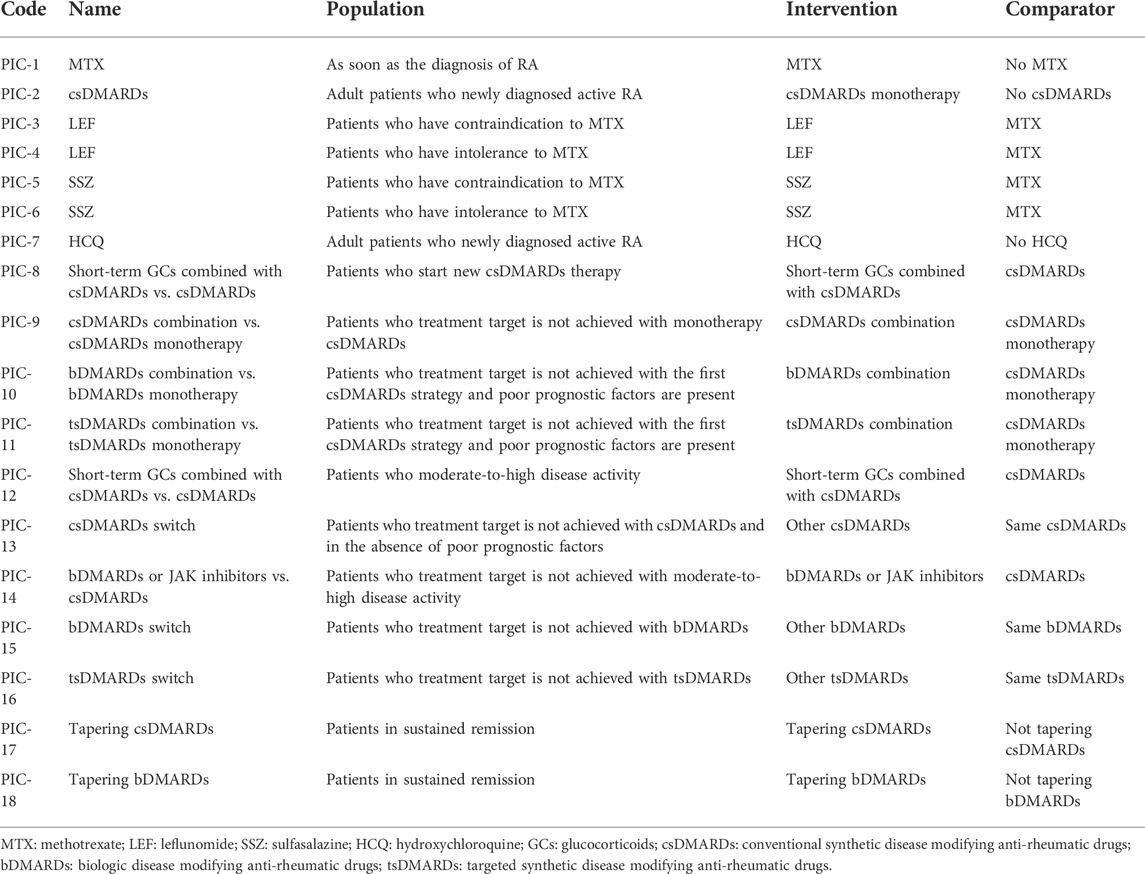
We identified the recommendations regarding pharmacotherapy of RA in the selected CPGs in consistency analysis. Population-intervention-comparison (PIC) concepts precluded differentia among recommendations compared to simple and direct consistency analysis. “P” means population or patient, which should describe the recommendation as appropriate for who or under what circumstances. “I” means intervention, which should describe specifically, such as monotherapy, therapy combination, therapy switch, and dosage reduction. “C” means comparison, which should describe the specific pharmacotherapy compared to the intervention. PIC framework was used to compare the recommendations with the same PIC descriptions. All recommendations about pharmacotherapy of RA were looked through to confirm counts of PIC. Only when two or more recommendations described the same PIC, can the recommendations be compared and formed a PIC. Only one recommendation had an independent PIC, which means no recommendation could compare with it, would be excluded. We included as many comparable recommendations as possible by separating PIC items without changing the original meanings of recommendations. Two researchers reached an agreement in the process. Any disagreements were discussed and solved with a third party. Recommendation specification was a key step that could disambiguate the difference among recommendations.
2.4.3 Coding direction and strength of recommendations
We assessed the recommendations of each CPG in three steps. Firstly, we assessed whether the CPG contained the recommendation within the range of PICs. If not, we classified the CPG as “out of scope,” and no further coding application was used. Secondly, we assessed the direction of recommendation by comparing the comparison and the intervention. We classified the CPG as “recommended” if it recommended the intervention over the comparison. Conversely, we classified the CPG as “not recommended” if the comparison was recommended over the intervention. Thirdly, the strength of recommendation was assessed. If the recommendation was rated as strong, “A” grade, the highest degree of the strength, or used definitive language to describe the highest degree of obligation or expectations for following the recommendation, we coded the recommendation as “strong strength.” If the recommendation was rated as less than the highest degree of the strength or used indeterminate language to describe a lower degree of obligation or expectations for following the recommendation, we coded the recommendation as “weak strength.” The recommendation without the strength or the CPG without the method for grading the quality of evidence and strength of recommendations, we coded the recommendation as “unspecified strength.”
2.5 Sensitivity analysis
Firstly, recommendations with unspecified strength or guidelines without using a method for grading the quality of evidence and strength of recommendations were excluded from viewing the changes in the direction and strength of recommendations. Secondly, leave-one-out sensitivity analyses were used to assess the robustness of consistency after excluding one guideline at a time, looking at changes in the direction and strength of each PIC in turn. Sensitivity analysis was carried out through these two steps to check whether the scope of included guidelines in the consistency analysis and the strength of the recommendation has a clear impact on the direction and strength of the recommendation and the fluctuation range of the consistency rate.
3 Results
3.1 Characteristics of included clinical practice guidelines
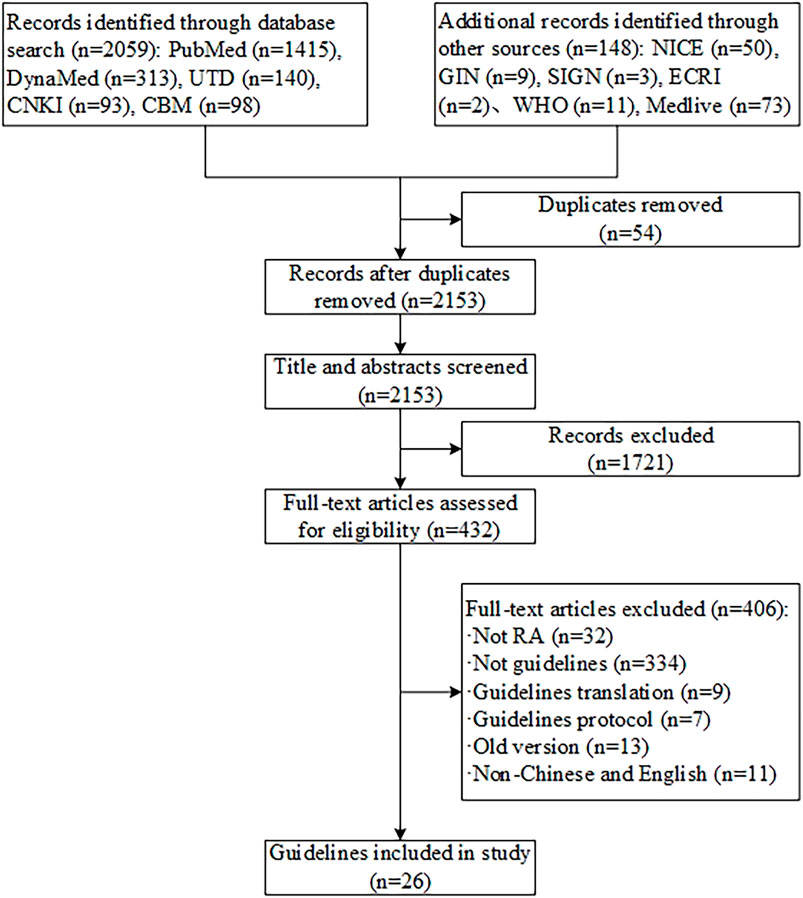
We initially identified 2,207 records, 54 duplicates removed by Endnote software, 1,721 records were excluded after the full-text screening, and 406 records were excluded by reviewing the full text. Ultimately, 26 guidelines met the criteria (see Figure 1). (Lau et al., 2018; Smolen et al., 2020; Ho et al., 2019; Fraenkel et al., 2021; Cardiel et al., 2018; NICE, 2020; Peter et al., 2021; Fang et al., 2020; Alhajeri et al., 2019; Daien et al., 2019; Parisi et al., 2019; Leon et al., 2018; Ataman et al., 2018; Chinese Rheumatology, 2018; Henrique da Mota et al., 2012; Hurkmans et al., 2011; Forestier et al., 2009; Gossec et al., 2006; Jiang et al., 2018; Huang et al., 2011; Jiang, 2019-10; Guidelines for diagnosis and treatment of rheumatoid arthritis, 2010; Luo et al., 2020; Management of Rheumatoid Arthritis, 2019; Management of early rheumatoid arthritis, 2011; Clinical guideline for the diagnosis and management of early rheumatoid arthritis, 2009) Twenty-six guidelines were included in publication years ranging from 2006 to 2021. Eight CPGs were developed in China, three in France, two in the Netherlands, and one in each of the United States, Mexico, the United Kingdom, Europe, Asia Pacific, Italy, Malaysia, Brazil, Scotland, Australia, Spain, Kuwait, and Turkey. The types of included guidelines consisted of diagnosis, treatment, and management. The specific types of therapy were pharmacotherapy, physiotherapy, TCM therapy, psychotherapy, and rehabilitation therapy. The methods for grading the quality of evidence and the strength of recommendations were different. Seven CPGs used the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE), two used the adapted GRADE, three used the Oxford Centre for Evidence-Based Medicine (OCEBM) criteria, one used the Scottish Intercollegiate Guidelines Network (SIGN), one used the Evidence-Based Recommendation Development (EBRO), one used the National Health and Medical Research Council (NHMRC), and four adopted other unnamed standards or tools. The method for grading the quality of evidence and strength of recommendations was not reported in one CPG, and six CPGs not used (see Supplementary Table S2).
3.2 Consistency analysis
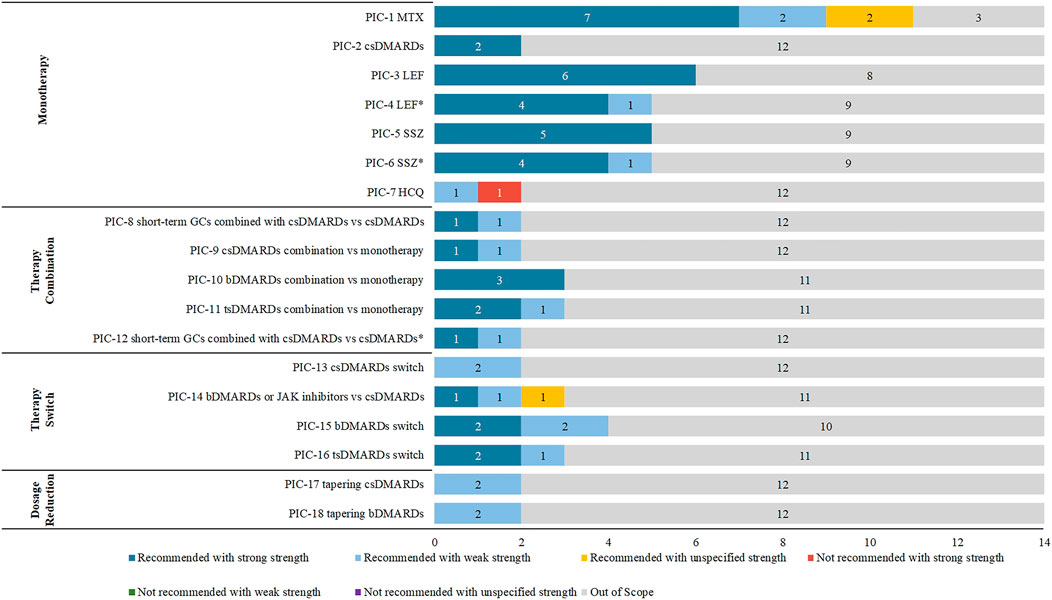
We selected 14 guidelines for consistency analysis from the included guidelines, with the number of recommendations ranging from 10 to 47 (see Supplementary Table S2). The publish year of 11 (78.57%, 11/14) guidelines ranged from 2018 to 2021. Other three guidelines (RACGP, SIGN, and BSR) were published in 2009, 2011, and 2012 respectively. Sixty-four recommendations of the 14 included guidelines were extracted and analyzed. After PIC coding, 18 PICs were obtained (see Table 1), and the details of the direction and strength of the recommendations were shown in Supplementary Table S3. There were 17 (17/18, 94%) PICs with consistent direction, and the only PIC (1/18, 6%) with an inconsistent direction was HCQ monotherapy. There were 7 (7/18, 38%) PICs with consistent direction and strength, including csDMARDs monotherapy, leflunomide (LEF) monotherapy or salazosulfapyridine (SSZ) monotherapy when methotrexate (MTX) was contraindicated, csDMARDs in combination with bDMARDs, and dosage reduction of csDMARDs and bDMARDs (see Figure 2). Among the 18 PICs, the direction of the recommendations was highly consistent, but poor consistency was found in the strength of the recommendations.
3.2.1 Monotherapy
In the monotherapy dimension (PIC1-7), csDMARDs such as MTX, LEF, SSZ, and HCQ were included. Among these PICs, the most recommendations were about MTX and had been included from 11 (11/14, 78.57%) CPGs with the same direction. Further, seven of them had strong strength of recommendation, two had weak strength of recommendation, and two had unspecified strength of recommendation. The two recommendations for csDMARDs monotherapy were consistent in direction and strength. The direction of LEF and SSZ were consistent, but their strengths were diverse when used under different conditions. When MTX was contraindicated, either LEF or SSZ was strongly recommended; when MTX was not tolerated, EULAR, HKSR, SIR, and SFR guidelines gave strong recommendations for LEF or SSZ, and APLAR guidelines gave weak strength of recommendation. The recommendations for HCQ were in different directions. The NICE guideline indicated that HCQ should be considered a first-line treatment (weak strength of recommendation). In contrast, HKSR guideline explained that HCQ should not be used as a first-line treatment for RA (strong strength of recommendation).
3.2.2 Therapy combination
In the therapy combination dimension (PIC8-12), PICs included csDMARDs combined with short-term GCs, csDMARDs combination, bDMARDs combination, or tsDMARDs combination, and their directions were consistent. PIC8 and 12 were both csDMARDs combined with short-term GCs, but the population and strength of the recommendations were inconsistent. The population of PIC8 was patients who started new csDMARDs therapy, which was weak strength of recommendation in NICE guidelines and strong strength of recommendation in EULAR guidelines. In contrast, the population of PIC12 was patients who had moderate-to-high disease activity, which was strong strength of recommendation in SIR guideline and weak strength of recommendation in CRA guideline. The combination of csDMARDs (PIC9) had different strengths. It was strongly recommended in HKSR guideline but weakly in CRA guideline. The direction and strength of the recommendations for csDMARDs combined with bDMARDs (PIC10) were consistent and strong recommendations were given by EULAR, HKSR, and SIR guidelines. In the PIC11, tsDMARDs combination, three CPGs (EULAR, HKSR, and SIR guidelines) gave strong recommendations but only weak CRA guideline.
3.2.3 Therapy switch
In the therapy switch dimension (PIC13-16), the therapy switch was considered when the current treatment strategy failed to achieve the goal, which included the switch about csDMARDs, bDMARDs, and tsDMARDs. The strength of recommendations was inconsistent in all PICs (PIC14-16) except for the PIC13 of another csDMARDs should be considered when the csDMARDs treatment goal failed, which EULAR and SIR guidelines recommended with a weak strength of recommendation. The switch to bDMARDs or JAK inhibitors (PIC14) had included three recommendations from CMR, APLAR, and MOH Malaysia guidelines with different strengths, strong, weak and unspecified. The PIC15, switch to bDMARDs, included four recommendations. Two of them had strong strength of recommendation (EULAR and SFR guidelines), and two had weak strength of recommendation (ACR and HKSR guidelines). The switch to tsDMARDs (PIC16) included three recommendations strongly recommended in EULAR and SFR guidelines but weakly in ACR guideline.
3.2.4 Dosage reduction
In the dosage reduction dimension (PIC17-18), csDMARDs or bDMARDs were considered to reduce the dose when patients were in sustained remission. Two recommendations were included in PIC17 and PIC18, which had the same direction and strength. In dosage reduction of csDMARDs, EULAR, and SIR guidelines were recommended with a weak strength of recommendation. In PIC18, tapering bDMARDs was weakly recommended in HKSR and SIR guidelines.
3.3 Sensitivity analysis
The inconsistency rate remained high after excluding recommendations with unspecified strength (see Table 2; Supplementary Table S4). The leave-one-out sensitivity analysis revealed varying degrees of variation in the consistency rate (see Supplementary Tables S5–S18). The number of coded PICs varied from 13 to 18. The variation in the direction of recommendation consistency rate ranged from -1% to 6%; the variation in the rate for both direction and strength consistency ranged from -15.8% to 14.1% (see Table 2).
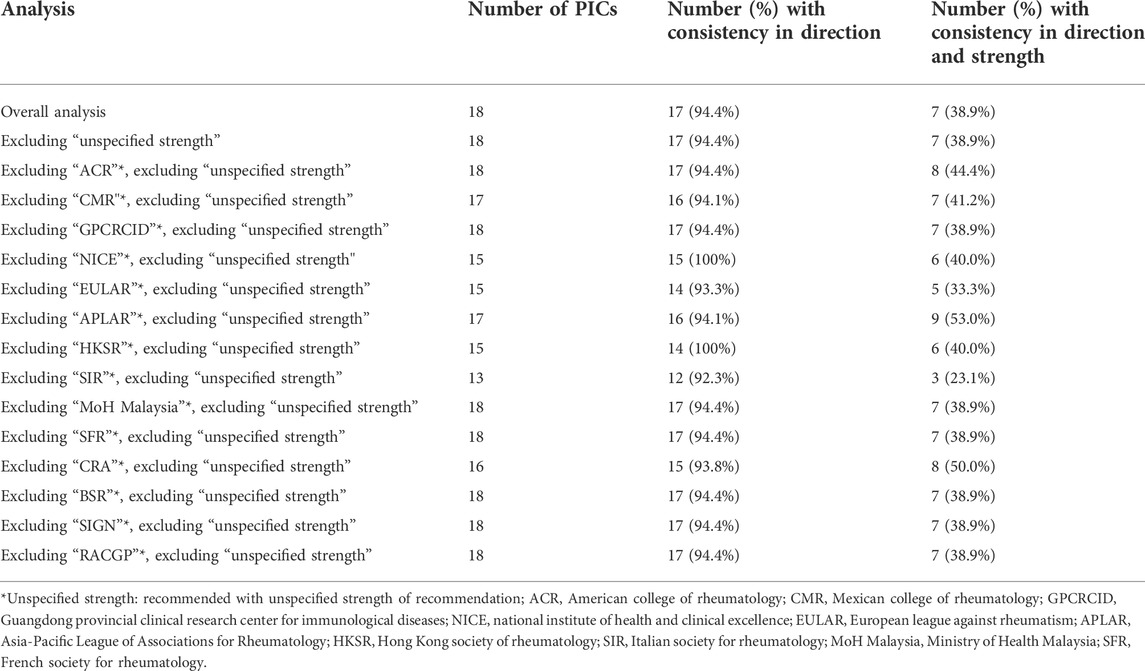
TABLE 2. Consistency of direction and strength across clinical practice guidelines with sensitivity analysis for excluding unspecified strength.
Here we took the CPGs which had the most significant rate changes in the leave-one-out sensitivity analysis as examples to describe. After excluding recommendations with unspecified strength, the direction consistency rate increased to 100% after NICE or HKSR guideline was excluded (see Supplementary Tables S8, S11) and decreased to 92.3% when SIR guideline was excluded (see Supplementary Table S12). After excluding APLAR guideline, the number of recommendations with consistent direction and strength increased to nine, and the consistency rate increased to 53.0% (see Supplementary Table S10). In contrast, after excluding SIR guideline, the number of recommendations with consistent direction and strength remained only three, and the consistency rate decreased to 23.1% (see Supplementary Table S12).
4 Discussion
This study systematically searched the diagnosis, treatment, and management CPGs of RA to map the current topics and pharmacotherapy of RA. Further, the consistency analysis was conducted for the recommendations related to RA pharmacotherapy. The results showed that the pharmacotherapy of RA was consistent in the direction, but the strength was highly inconsistent.
4.1 The significance of consistency analysis of rheumatoid arthritis recommendations in clinical practice guidelines
The recommendations among different CPGs create difficulties in clinical practice and decision-making, specifically when faced with inconsistent directions or various conditions related to recommendations (Mackey and Liang, 2011). In our study, HCQ was recommended in different directions. NICE guideline indicated that HCQ should be considered as a first-line treatment (NICE, 2020), but HKSR stated not to use HCQ because of its slow onset and the availability of more effective csDMARDs (Ho et al., 2019). Recommendations on the HCQ of these two guidelines were formed merely on primary studies, not systematic reviews. Based on a systematic review, HCQ in monotherapy had similar (or even lower) efficacy compared to other csDMARDs. However, the combination of HCQ and other DMARDs would increase the clinical efficacy (Rempenault et al., 2020). Therefore, guideline developers should clearly describe the evidence on which the recommendations are based, the factors considered, and the process and methods used to form the recommendations. Therefore, clinicians might face a dilemma when treating RA with pharmacotherapy. Because following the recommendations in one guideline may violate the recommendations in other guidelines. Clinicians often give special attention to the strength of evidence, but we found that the strength of recommendation in the same PIC was inconsistent. Thus, it is essential to specify the circumstances in which the pharmacotherapy of RA should be used. These issues have complicated the implementation and application of guidelines and resulted in the disconnection between evidence and clinical practice. The inconsistency in CPGs indicated that the clinical questions were still unspecific and unclear, which needed to be supported by more studies. Therefore, we advocated paying more attention to controversial recommendations and updating the guidelines in time to improve the consistency.
4.2 Potential reasons for poor consistency of rheumatoid arthritis recommendations
There are many underlying reasons for the poor consistency among recommendations. First, the time span of the guideline development may cause heterogeneity because the emergence of new evidence may overturn old concepts or perspectives (Kow et al., 2021; Zhang et al., 2021). Therefore, the differences among the guidelines could be observed according to the publication year. Second, the lack of high-quality evidence may lead to formulating recommendations based on expert opinions rather than evidence. Further, the selection and interpretation of evidence may be inconsistent among guidelines. Third, different countries and regions consider local conditions, factors, values, preferences, and types and degrees of stakeholder participation differently. Fourth, different countries and regions may have quite different populations and population composition, and drug policies, which might impact the development of clinical recommendations. RA imposes a substantial economic burden and the expenditures for RA pharmacotherapy increase with severity (Santos-Moreno et al., 2021). Drug policy, clinical recommendations, and consensus conferences of different countries or regions should be considered judiciously during specific applications of innovative and expensive pharmacology. Fifth, different methods for grading the quality of evidence and strength of recommendations may form inconsistent recommendations based on the same evidence (Jolliffe et al., 2018). These reasons might lead to high-inconsistent recommendations. Further research should determine the reasons for the inconsistency.
4.3 Study strengths and limitations
The strengths of this study were as follows. First, to our knowledge, this is the first study that used the PIC framework to analyze the consistency of RA pharmacotherapy recommendations among CPGs. Second, we used a robust scientific PIC framework to encode the recommendations and analyzed the consistency among the recommendations in detail. Third, we focused on commonly-used and essential pharmacotherapy in the clinic. We also visualized the overall consistency among these recommendations, providing concise and detailed information for clinicians and researchers who may benefit from these findings while noting the existing gaps.
This study had some limitations as well. There may be some bias as the methods for grading the quality of evidence and strength of recommendations were different. The strength of recommendations was divided into strong and weak, and the highest degree of strength was coded as strong. The strength less than the highest degree was coded as weak according to the strength given in the guidelines. Local circumstances and drug policies vary among countries and should also be noticed, which may make recommendations different and create bias.
4.4 Suggestions for the future rheumatoid arthritis research and clinical practice guidelines
Developers need to refer to internationally recognized methods and standards for guideline development. First, developers should write a protocol and register before the guideline development work is carried out (Ge et al., 2018). Second, forming recommendations should adopt the currently recognized and more applicable methods for grading the quality of evidence and strength of recommendations, such as GRADE (Group, 2012; Dijkers, 2013). Further, the recommendations should be based on the highest quality of evidence, and their usage conditions should be clarified (Brignardello-Petersen et al., 2021). Accordingly, CPGs could be better implemented to provide evidence-based support for clinical practice and decision-making. However, the communications between CPG developers may be confused or hindered if the grading systems of evidence are different (Brozek et al., 2009; Sun et al., 2019). Third, high-quality evidence for RA should be produced via original research and systematic reviews in the future to develop RA guidelines and support the recommendations. Many factors need to be considered and balanced during the development of guidelines, so it is hard to maintain a high degree of consistency in the strength of the recommendations. Forth, systematic reviews of guidelines are suggested to conduct, indicating research gaps. Future studies on the consistency of recommendations among CPGs should pay attention to the following points. First, with the CPGs updated quickly, the publish time period of CPGs need to be considered. Second, whether CPGs are national or just developed by the single institution (or organization) and should be checked. It is inappropriate to analyze these CPGs together. Third, the availability of new drugs in the nations should be scrutinized. Because the local circumstances and drug policies are various. With the continuous revising and updating of the CPGs, we believe that high-quality guidelines could be developed in the future to form clear, definite, and consistent recommendations for the pharmacotherapy of RA.
5 Conclusion
Our study found that current recommendations for RA pharmacotherapy were almost consistent in direction but were highly inconsistent in strength. CPGs should be standardized and scientifically developed further. High-quality original research and systematic reviews on RA pharmacotherapy with inconsistent direction and strength are needed to fill the research gaps.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
XS and YC developed the research design. YH, YZH, and YM analyzed the data and interpreted the results. XS, YH, YZH, SF, XW, and XF drafted manuscript. XL, YM, YX, NY, and XH did literature screening and data extraction. All of the authors contributed to drafting the manuscript. CW and WC made great contributions to the revision and discussion of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
The work was supported by National Key R&D Program of China (2018YFC1705503) and Science and Technology Program of Gansu Province (20JR5RA262).
Acknowledgments
We would like to thank the authors, organizations and institutions of the included RA guidelines for their contributions to our consistency analysis. We would like to thank MogoEdit (https://www.mogoedit.com) for its English editing during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.967787/full#supplementary-material
References
Alhajeri, H., Abutiban, F., Al-Adsani, W., Al-Awadhi, A., Aldei, A., AlEnizi, A., et al. (2019). Kuwait association of rheumatology 2018 treatment recommendations for patients with rheumatoid arthritis. Rheumatol. Int. 39 (9), 1483–1497. doi:10.1007/s00296-019-04372-y
Alper, B. S., Price, A., van Zuuren, E. J., Fedorowicz, Z., Shaughnessy, A. F., Oettgen, P., et al. (2019). Consistency of recommendations for evaluation and management of hypertension. JAMA Netw. Open 2 (11), e1915975. doi:10.1001/jamanetworkopen.2019.15975
Ataman, S., Sunar, I., Yilmaz, G., Bodur, H., Nas, K., Ayhan, F. F., et al. (2018). Turkish League against rheumatism (TLAR) recommendations for the pharmacological management of rheumatoid arthritis: 2018 update under guidance of current recommendations. Arch. Rheumatol. 33 (3), 251–271. doi:10.5606/ArchRheumatol.2018.6911
Brignardello-Petersen, R., Carrasco-Labra, A., and Guyatt, G. H. (2021). How to interpret and use a clinical practice guideline or recommendation users' guides to the medical literature. Jama-Journal Am. Med. Assoc. 326 (15), 1516–1523. doi:10.1001/jama.2021.15319
Brozek, J. L., Akl, E. A., Alonso-Coello, P., Lang, D., Jaeschke, R., Williams, J. W., et al. (2009). Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy 64 (5), 669–677. doi:10.1111/j.1398-9995.2009.01973.x
Cardiel, M. H., Carrillo, S., Perez, M., Andrade, L., Pacheco Tena, C., Silveira, L. H., et al. (2018). Update of the Mexican College of rheumatology guidelines for the pharmacological treatment of rheumatoid arthritis. Reumatol. Clin. 17 (4), 215–228. doi:10.1016/j.reuma.2019.04.002
Chen, Y., Zhang, J. Y., and Zhang, T. S. (2020). Systematic evaluation of the guidelines: What, why, how. Med. J. Peking Union Med. Coll. Hosp. 11 (03), 320–324.
Chinese Rheumatology, A. (2018). [2018 Chinese guideline for the diagnosis and treatment of rheumatoid arthritis]. Zhonghua Nei Ke Za Zhi 57 (4), 242–251. doi:10.3760/cma.j.issn.0578-1426.2018.04.004
Clinical guideline for the diagnosis and management of early rheumatoid arthritis (2009). Clinical guideline for the diagnosis and management of early rheumatoid arthritis. South Melbourne, Victoria, Australia: The Royal Australian College of General Practitioners.
Daien, C., Hua, C., Gaujoux-Viala, C., Cantagrel, A., Dubremetz, M., Dougados, M., et al. (2019). Update of French society for rheumatology recommendations for managing rheumatoid arthritis. Jt. Bone Spine 86 (2), 135–150. doi:10.1016/j.jbspin.2018.10.002
Dijkers, M. (2013). Introducing GRADE: A systematic approach to rating evidence in systematic reviews and to guideline development. KT Update 1 (5), 1–9.
Erickson, J., Sadeghirad, B., Lytvyn, L., Slavin, J., and Johnston, B. C. (2017). The scientific basis of guideline recommendations on sugar intake: A systematic review. Ann. Intern. Med. 166 (4), 257–267. doi:10.7326/M16-2020
Espinoza, G., Maldonado, G., Narvaez, J., Guerrero, R., Citera, G., and Rios, C. (2021). Beyond rheumatoid arthritis evaluation: What are we missing? Open Access Rheumatol. 13, 45–55. doi:10.2147/OARRR.S298393
Fang, L. K., Huang, C. H., Xie, Y., Liu, Q., Wang, X. Q., He, D. Y., et al. (2020). Practice guidelines for patients with rheumatoid arthritis. Zhonghua Nei Ke Za Zhi 59 (10), 772–780. doi:10.3760/cma.j.cn112138-20200807-00734
Forestier, R., Andre-Vert, J., Guillez, P., Coudeyre, E., Lefevre-Colau, M. M., Combe, B., et al. (2009). Non-drug treatment (excluding surgery) in rheumatoid arthritis: Clinical practice guidelines. Jt. Bone Spine 76 (6), 691–698. doi:10.1016/j.jbspin.2009.01.017
Fraenkel, L., Bathon, J. M., England, B. R., St Clair, E. W., Arayssi, T., Carandang, K., et al. (2021). 2021 American College of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 73 (7), 1108–1123. doi:10.1002/art.41752
Gao, R-C., Wu, Z-G., Wu, Z-Z., and Hao, M. (2022). Frailty in rheumatoid arthritis: A systematic review and meta-analysis. Jt. Bone Spine 89 (4), 105343. doi:10.1016/j.jbspin.2022.105343
Ge, L., Tian, J. H., Li, Y. N., Pan, J. X., Li, G., Wei, D., et al. (2018). Association between prospective registration and overall reporting and methodological quality of systematic reviews: A meta-epidemiological study. J. Clin. Epidemiol. 93, 45–55. doi:10.1016/j.jclinepi.2017.10.012
Gossec, L., Pavy, S., Pham, T., Constantin, A., Poiraudeau, S., Combe, B., et al. (2006). Nonpharmacological treatments in early rheumatoid arthritis: Clinical practice guidelines based on published evidence and expert opinion. Jt. Bone Spine 73 (4), 396–402. doi:10.1016/j.jbspin.2006.01.008
Institute of Medicine Committee on Standards for Developing Trustworthy Clinical Practice G (2011). “Institute of medicine committee on standards for developing Trustworthy clinical practice G,” in Clinical practice guidelines we can trust. Editors R. Graham, M. Mancher, D. Miller Wolman, S. Greenfield, and E. Steinberg (Washington (DC): National Academies Press (US) Copyright 2011 by the National Academy of Sciences). All rights reserved.
Guidelines for diagnosis and treatment of rheumatoid arthritis (2010). Guidelines for diagnosis and treatment of rheumatoid arthritis. Chin. J. Rheumatology 14 (04), 265–270.
Henrique da Mota, L. M., Cruz, B. A., Brenol, C. V., Pereira, I. A., Rezende-Fronza, L. S., Bertolo, M. B., et al. (2012). Consenso 2012 da Sociedade Brasileira de Reumatologia para o tratamento da artrite reumatoide. Rev. Bras. Reumatol. 52 (2), 152–174. doi:10.1590/s0482-50042012000200002
Ho, C. T. K., Mok, C. C., Cheung, T. T., Kwok, K. Y., and Yip, R. M. L. (2019). Management of rheumatoid arthritis: 2019 updated consensus recommendations from the Hong Kong society of rheumatology. Clin. Rheumatol. 38 (12), 3331–3350. doi:10.1007/s10067-019-04761-5
Huang, Y., Wang, C., and Chen, W. (2011). Guidelines for diagnosis and treatment of rheumatoid arthritis. Chin. Med. Mod. Distance Educ. China 11 (20), 1673–7202.
Hurkmans, E. J., van der Giesen, F. J., Bloo, H., Boonman, D. C., van der EschM., , FluitM., , et al. (2011). Physiotherapy in rheumatoid arthritis: Development of a practice guideline. Acta Reumatol. Port. 36 (2), 146–158.
Jiang, Q., Wang, H., and Gong, X. (2018). Rheumatoid arthritis disease evidence combined treatment guidelines. J. Tradit. Chin. Med. 59 (20), 1794–1800.
Jiang, Q. (20192020). International clinical practice guidelines in traditional Chinese medicine rheumatoid arthritis. World Chin. Med. 15 (20), 3160–3168.
Jolliffe, L., Lannin, N. A., Cadilhac, D. A., and Hoffmann, T. (2018). Systematic review of clinical practice guidelines to identify recommendations for rehabilitation after stroke and other acquired brain injuries. Bmj Open 8 (2), e018791. doi:10.1136/bmjopen-2017-018791
Kerschbaumer, A., Sepriano, A., Smolen, J. S., van der Heijde, D., Dougados, M., van Vollenhoven, R., et al. (2020). Efficacy of pharmacological treatment in rheumatoid arthritis: A systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 79 (6), 744–759. doi:10.1136/annrheumdis-2019-216656
Kow, C. S., Capstick, T., Zaidi, S. T. R., and Hasan, S. S. (2021). Consistency of recommendations from clinical practice guidelines for the management of critically ill COVID-19 patients. Eur. J. Hosp. Pharm. 28 (1), 42–46. doi:10.1136/ejhpharm-2020-002388
Lau, C. S., Chia, F., Dans, L., Harrison, A., Hsieh, T. Y., Jain, R., et al. (2018). 2018 update of the APLAR recommendations for treatment of rheumatoid arthritis. Int. J. Rheum. Dis. 22 (3), 357–375. doi:10.1111/1756-185X.13513
Leon, L., Redondo, M., Fernandez-Nebro, A., Gomez, S., Loza, E., Montoro, M., et al. (2018). Expert recommendations on the psychological needs of patients with rheumatoid arthritis. Rheumatol. Int. 38 (12), 2167–2182. doi:10.1007/s00296-018-4057-6
Luo, J., Lan, P., and Hongyu, C. (2020). Clinical practice guidelines in traditional Chinese medicine rehabilitation—rheumatoid arthritis. Rehabil. Med. 30 (01), 16–25.
Luo, X., Liu, Y., Ren, M., Zhang, X., Janne, E., Lv, M., et al. (2021). Consistency of recommendations and methodological quality of guidelines for the diagnosis and treatment of COVID-19. J. Evid. Based. Med. 14 (1), 40–55. doi:10.1111/jebm.12419
Mackey, T. K., and Liang, B. A. (2011). The role of practice guidelines in medical malpractice litigation. Virtual Mentor 13 (1), 36–41. doi:10.1001/virtualmentor.2011.13.1.hlaw1-1101
Management of early rheumatoid arthritis (2011). Management of early rheumatoid arthritis. Scottish Intercollegiate Guidelines Network.
Management of Rheumatoid Arthritis (2019). Management of rheumatoid arthritis. Putrajaya, Malaysia: Malaysia Health Technology Assessment Section (MaHTAS), Medical Development Division, Ministry of Health Malaysia.
NICE (2020). Rheumatoid arthritis in adults: Management (NG100). London, UK: National Institute for Health and Care Excellence.
Parisi, S., Bortoluzzi, A., Sebastiani, G. D., Conti, F., Caporali, R., Ughi, N., et al. (2019). The Italian Society for Rheumatology clinical practice guidelines for rheumatoid arthritis. Reumatismo 71 (S1), 22–49. doi:10.4081/reumatismo.2019.1202
Park, E-J., Kim, H., Jung, S. M., Sung, Y. K., Baek, H. J., and Lee, J. (2020). The use of biological disease-modifying antirheumatic drugs for inflammatory arthritis in korea: Results of a Korean expert consensus. J. Rheum. Dis. 27 (1), 4–21. doi:10.4078/jrd.2020.27.1.4
Peter, W. F., Swart, N. M., Meerhoff, G. A., and Vliet Vlieland, T. P. M. (2021). Clinical practice guideline for physical therapist management of people with rheumatoid arthritis. Phys. Ther. 101 (8), pzab127. doi:10.1093/ptj/pzab127
Rempenault, C., Combe, B., Barnetche, T., Gaujoux-Viala, C., Lukas, C., Morel, J., et al. (2020). Clinical and structural efficacy of hydroxychloroquine in rheumatoid arthritis: A systematic review. Arthritis Care Res. 72 (1), 36–40. doi:10.1002/acr.23826
Safiri, S., Kolahi, A. A., Hoy, D., Smith, E., Bettampadi, D., Mansournia, M. A., et al. (2019). Global, regional and national burden of rheumatoid arthritis 1990-2017: A systematic analysis of the global burden of disease study 2017. Ann. Rheum. Dis. 78 (11), 1463–1471. doi:10.1136/annrheumdis-2019-215920
Santos-Moreno, P., Alvis-Zakzuk, N. J., Villarreal-Peralta, L., Carrasquilla-Sotomayor, M., de la Hoz-Restrepo, F., and Alvis-Guzman, N. (2021). Centers of excellence implementation for treating rheumatoid arthritis in Colombia: A cost-analysis. Clin. Outcomes Res. 13, 583–591. doi:10.2147/ceor.s308024
Smith, M. H., and Berman, J. R. (2022). What is rheumatoid arthritis? JAMA 327 (12), 1194. doi:10.1001/jama.2022.0786
Smolen, J. S., Aletaha, D., and McInnes, I. B. (2016). Rheumatoid arthritis. Lancet 388 (10055), 2023–2038. doi:10.1016/S0140-6736(16)30173-8
Smolen, J. S., Landewe, R. B. M., Bijlsma, J. W. J., Burmester, G. R., Dougados, M., Kerschbaumer, A., et al. (2020). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 79 (6), 685–699. doi:10.1136/annrheumdis-2019-216655
Sun, Y., Gao, Y., Chen, J., Sun, H., Cai, Y. T., Ge, L., et al. (2019). Evidence mapping of recommendations on diagnosis and therapeutic strategies for diabetes foot: An international review of 22 guidelines. Metabolism. 100, 153956. doi:10.1016/j.metabol.2019.153956
Yan, M., Chen, L., Yang, M., Zhang, L., Niu, M., Wu, F., et al. (2022). Evidence mapping of clinical practice guidelines recommendations and quality for depression in children and adolescents. Eur. Child. Adolesc. Psychiatry 1, 1. doi:10.1007/s00787-022-01958-z
Yang, K., Chen, Y., Li, Y., and Schunemann, H. J. (2013). Can China master the guideline challenge? Health Res. Policy Syst. 11 (1), 1–3. doi:10.1186/1478-4505-11-1
Yu, Y., Wang, D., Zhou, Q., Wang, C., Ma, X., Gao, Y., et al. (2020). Recommendations in clinical practice guidelines on gout: Systematic review and consistency analysis. Clin. Exp. Rheumatol. 38 (5), 964–972.
Zhang, M., Zhou, Y., Zhong, J., Wang, K., and Ding, Y. (2019). Current guidelines on the management of gestational diabetes mellitus: A content analysis and appraisal. BMC Pregnancy Childbirth 19, 200. doi:10.1186/s12884-019-2343-2
Zhang, Q., Liu, Q., Lin, C., Baima, Y., Li, H., Gong, H., et al. (2020). The prevalence of rheumatoid arthritis in middle-aged and elderly people living in Naqu City, Tibet, Autonomous Region of China. J. Orthop. Surg. Res. 15 (1), 338. doi:10.1186/s13018-020-01883-4
Keywords: rheumatoid arthritis, guidelines, recommendation, consistency, strength of recommendation
Citation: Hu Y, Han Y, Ma Y, Fan S, Wang X, Fu X, Hu X, Luo X, Ma Y, Xun Y, Yang N, Wen C, Cao W, Song X and Chen Y (2022) Consistency of recommendations for pharmacotherapy of rheumatoid arthritis. Front. Pharmacol. 13:967787. doi: 10.3389/fphar.2022.967787
Received: 13 June 2022; Accepted: 10 October 2022;
Published: 25 October 2022.
Edited by:
Dario Roccatello, University of Turin, ItalyReviewed by:
Xiaowei Sherry Yan, Sutter Health, United StatesLu Liangjing, Shanghai Jiao Tong University, China
Copyright © 2022 Hu, Han, Ma, Fan, Wang, Fu, Hu, Luo, Ma, Xun, Yang, Wen, Cao, Song and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuping Song, c29uZ3hwQGx6dS5lZHUuY24=; Yaolong Chen, Y2hlbnlhb2xvbmdAdmlwLjE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Yue Hu1†
Yue Hu1† Xinyu Fu
Xinyu Fu Xufei Luo
Xufei Luo Yanfang Ma
Yanfang Ma Yangqin Xun
Yangqin Xun Nan Yang
Nan Yang Xuping Song
Xuping Song Yaolong Chen
Yaolong Chen