- 1Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Division of Digestive Surgery, Xijing Hospital of Digestive Diseases, Air Force Medical University, Xi’an, China
Background: Significant survival benefit of adjuvant imatinib therapy has been observed in gastrointestinal stromal tumor (GIST). However, the impact of neoadjuvant imatinib on prognosis of GIST remains unclear. This meta-analysis aimed to compare the prognostic impact between upfront surgery and neoadjuvant imatinib plus surgery on GIST.
Methods: A comprehensive literature search was performed to identify eligible studies up to 30 Sep 2021, through PubMed, Embase, Web of Science, and Cochrane Library. Studies compared the impact of upfront surgery and neoadjuvant imatinib plus surgery on disease-free (DFS) or overall survival (OS) in patients with GIST were selected.
Results: Seven eligible studies with 17,171 patients were included. The reduction rates of tumor size in rectal and mixed site GIST were 33% and 29.8%, respectively. Neoadjuvant imatinib was not significantly associated with DFS compared with no-neoadjuvant therapy in rectal GIST (HR: 0.71, 95% CI: 0.35–1.41). The OS of rectal GIST was significantly improved by neoadjuvant imatinib compared with no-neoadjuvant therapy (HR: 0.36, 95% CI: 0.17–0.75).
Conclusion: Neoadjuvant imatinib therapy contributed to tumor shrinkage and R0 resection of rectal GIST. Neoadjuvant imatinib plus surgery significantly improved overall survival of rectal GIST in comparison with upfront surgery.
Introduction
Gastrointestinal stromal tumor is one of the most common mesenchymal tumors arising from the gastrointestinal tract (GI), with an annual incidence of 10 cases per million people globally which accounts for 1–3% of cancers in the entire GI (1, 2). GIST is considered to develop from the gain-of-function mutations of KIT ((Hirota et al., 1998)) and platelet-derived growth factor receptor alpha (PDGFRA) (Heinrich et al., 2003) and can occur anywhere of the GI. The most common site is stomach (60–70%), followed by small intestine (20–30%) and colorectum (5%) (Rubin et al., 2007; Joensuu et al., 2013; Liu et al., 2018).
Surgical resection remains the first choice of curative treatment for primary GIST. Since the first report (Joensuu et al., 2001) of the use of imatinib for metastatic GIST in 2001, various tyrosine kinase inhibitors have been growingly developed and used in clinical treatment of GIST ((Demetri et al., 2002; Demetri et al., 2006; Demetri et al., 2013; Blay et al., 2020; Chen et al., 2020; Heinrich et al., 2020; Chen et al., 2021a)). During this period, significant survival benefit has been observed in those with high-risk GIST who received adjuvant imatinib after surgery (Joensuu et al., 2020). These positive results brought attention to the use of neoadjuvant imatinib for GIST with large size or in special anatomic site. Neoadjuvant therapy has been demonstrated to contribute to the improvement of survival of several malignancies (Das, 2017; Cai et al., 2018; Mittendorf et al., 2020; Chen et al., 2021b). Till now, several retrospective and single-arm studies have reported the feasibility and effectiveness of neoadjuvant therapy on GIST ((Wang et al., 2012; Ling et al., 2021a; Renberg et al., 2022; Wong et al., 2022)). Recent guidelines recommended consideration of neoadjuvant imatinib therapy for patients if the surgical morbidity could be reduced preoperatively (Casali et al., 2018; von Mehren et al., 2020). However, the impact of neoadjuvant imatinib on prognosis of GIST remains unclear due to the absence of strong evidence from randomized controlled trials. Thus, the current meta-analysis aimed to review the relevant literature and provide a comprehensive view of the survival influence of neoadjuvant imatinib on GIST.
Material and methods
Search strategy
A systematic search of literature using keywords as “gastrointestinal stromal tumor,” “GIST,” “neoadjuvant,” “preoperative treatment” and “preoperative therapy,” was carried out by two investigators (ZL and ZZ) through PubMed, Embase, Web of Science and Cochrane Library to identify studies that compared the treatment effect between neoadjuvant therapy and upfront surgery for GIST. The search was updated to 30 Sep 2021. Attempts have been made to get additional eligible studies through searching the references of relevant studies. This study was in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline (Liberati et al., 2009).
Selection criteria
Eligible studies were identified by two investigators (ZL and JS) according to the following criteria: (Miettinen et al., 2003) Participants (P): Patients were diagnosed pathologically and immunohistochemistrically as primary GISTs; (Connolly et al., 2003); Interventions (I) and comparisons (C): Patients received neoadjuvant imatinib followed by surgery and/or adjuvant therapy in research group and upfront surgery and/or adjuvant therapy in control group. The outcomes were compared between research and control groups; (Heinrich et al., 2003); Outcomes (O): Disease-free survival (DFS) and/or overall survival (OS) were/was available or able to be calculated by sufficient data in the studies. When duplicate studies from same center were identified, only the newest or largest study was included. Any discrepancies were resolved by discussion with a third investigator (ZYZ).
Data extraction
The first author, publication year, country, sample size, tumor site, information of neoadjuvant imatinib, surgery, resection margin, adjuvant therapy, follow-up, DFS and OS were extracted independently by two investigators (SWOY and JL). If the hazard ratio (HR) and 95% confidence interval (CI) were not provided in the studies, we either emailed the corresponding author for original results or calculated these data from the Kaplan-Meier survival curves using the methods reported by Tierney et al. (Tierney et al., 2007). A third observer (MWM) engaged in discussions to resolve any controversial issues.
Quality assessment
Two authors (ZL and ZZ) independently assessed the quality of all included studies using the Newcastle-Ottawa Quality Assessment Scale (NOS) (Stang, 2010) with the highest score of nine, and any discrepancies in the scores were resolved by discussion with a third reviewer (JS).
Statistical analysis
The pooled survival data were measured using the HR and 95% CI. Some HRs and 95% CIs were extracted from Kaplan-Meier curves using Engauge Digitizer (version 4.1). Statistical heterogeneity was evaluated using the chi-square test and I2 statistics. Subgroup analysis was conducted to identify the source of heterogeneity. The random-effects model was used by default because of the nature of these retrospective studies. The estimated results of the fixed-effects model are also provided for reference. Sensitivity analysis was performed to validate the stability of the model by sequentially omitting each study. The publication bias was not performed as fewer than ten studies were included. Statistical analyses were performed using R software 3.6.1 (R Project for Statistical Computing) with the meta package (4.13-0) (Balduzzi et al., 2019). A two-sided p < 0.05 was considered significant. The GRADE profiler software (version 3.6) was used to estimate the level of evidence (Guyatt et al., 2008).
Results
Eligible studies in the meta-analysis
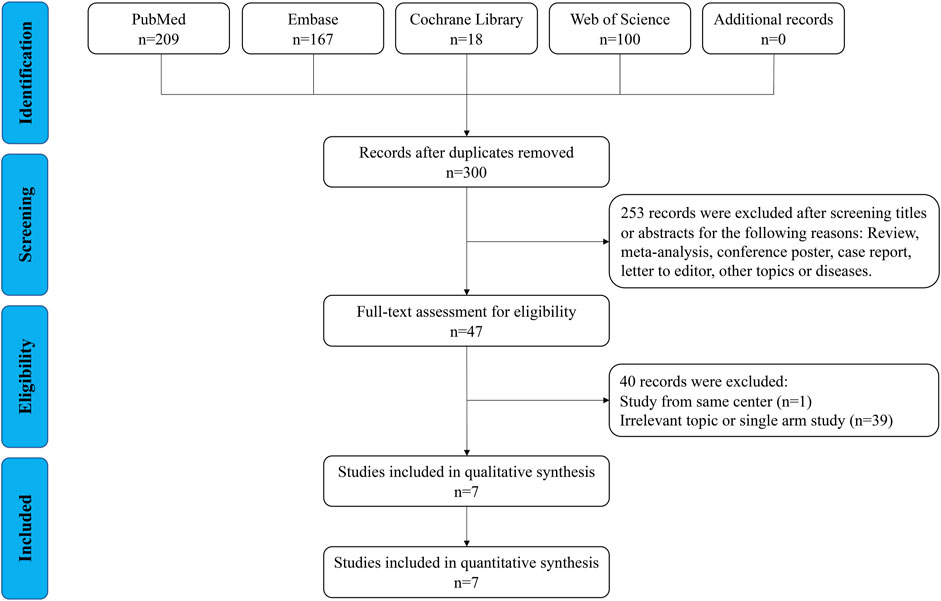
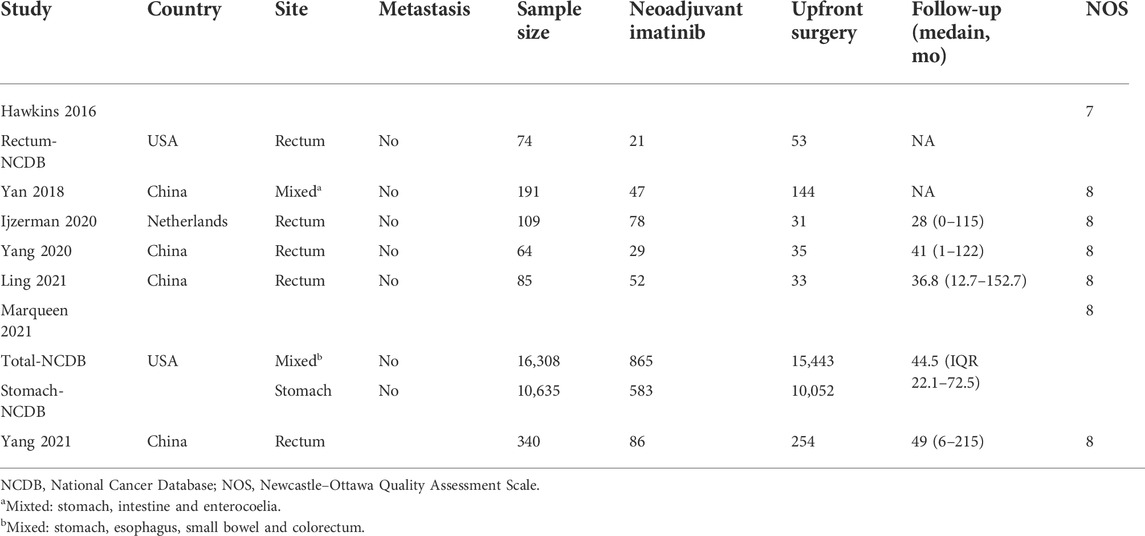
As shown in Figure 1, 494 relevant publications were identified through the literature search. After screening and assessment, seven eligible studies (Hawkins et al., 2017; Yan et al., 2018; NS et al., 2020; Yang et al., 2020; Ling et al., 2021b; Marqueen et al., 2021; Yang et al., 2021) with 17,171 patients were included in this meta-analysis (Table 1 and Supplementary Table S1). There were 1178 patients who received neoadjuvant therapy, and 15,993 patients who received upfront surgery. None of these patients experienced preoperative metastasis. Patients in both groups received adjuvant therapy accordingly. Two studies reported their median reduction rate of tumor size were 29.8% (mixed sites) and 33% (rectum) (Supplementary Table S1), respectively. And in study of Yang 2021 (38), the median tumor size of rectal GIST reduced from 5.8 to 3.8 cm after the use of neoadjuvant imatinib. The NOS scores of the studies ranged from seven to eight, indicating their relatively high quality of methodology. The GRADE evidence profiles of three indicators (resection margin, DFS and OS) were presented in Supplementary Table S2.
Resection margin
Four studies provided the information of margin resection. Among them, three studies analyzed rectal GIST and one analyzed mixed site GIST (stomach, intestine and enterocoelia). Figure 2A revealed that the R0 resection rate had no significant difference between neoadjuvant imatinib and no-neoadjuvant therapy (HR: 0.54, 95% CI: 0.26–1.10; p = 0.99, I2 = 0%; reference: no-neoadjuvant therapy). In the subgroup analysis of rectal GIST, a trend of higher R0 resection rate ranging from 85.3% to 98.8% was observed in neoadjuvant imatinib group compared with the rate ranging from 74.4% to 92.0% in no-neoadjuvant therapy group (Supplementary Table S1). But the difference was not statistically significant (HR: 0.49, 95% CI: 0.17–1.46; p = 0.99, I2 = 0%; reference: no-neoadjuvant therapy; Figure 2A). Sensitivity analysis was performed by omitting each study sequentially, and the estimated results did not differ significantly, indicating the stability of the model (Figure 2B).
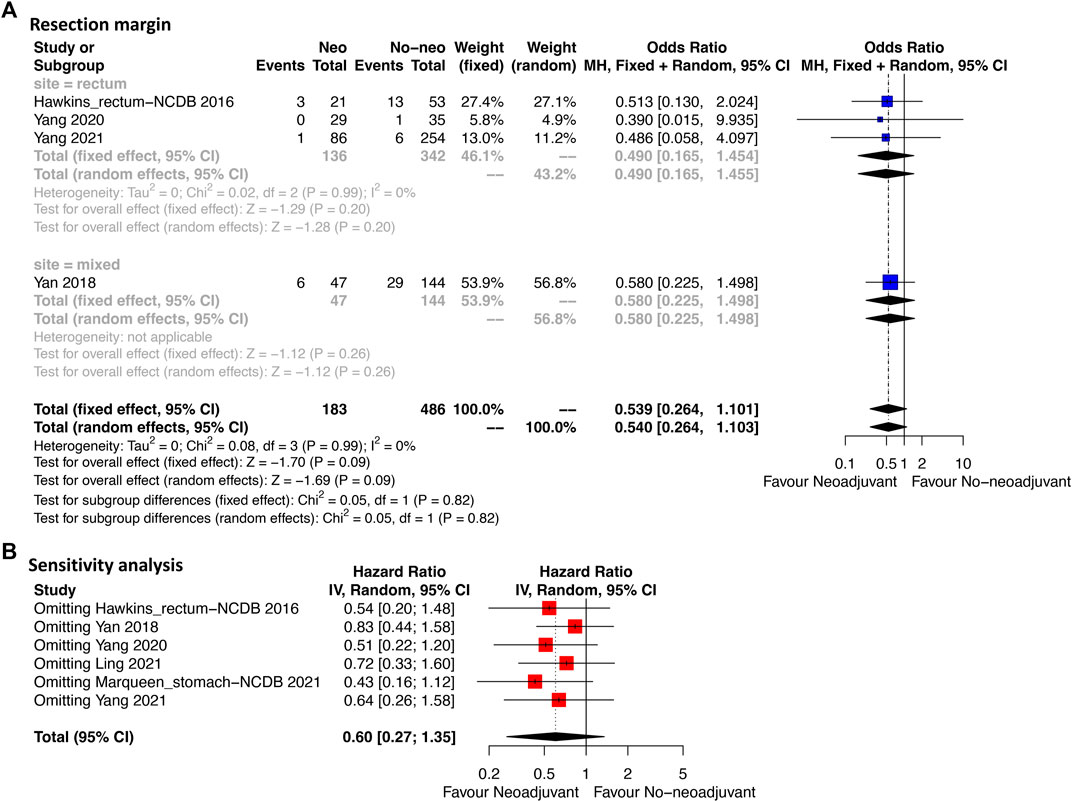
FIGURE 2. Forest plots illustrating resection margin between neoadjuvant and no-neoadjuvant imatinib (A) and sensitivity analysis (B).
Disease-free survival
As shown in Figure 3A, DFS data were available in four studies of which the included cases were all rectal GIST. Neoadjuvant imatinib was not significantly associated with DFS compared with no-neoadjuvant therapy (HR: 0.71, 95% CI: 0.35–1.41; reference: no-neoadjuvant therapy), which was consistent with the estimated results of the fixed-effects model (HR: 0.78, 95% CI: 0.46–1.31; reference: no-neoadjuvant therapy), indicating the absence of heterogeneity among studies (p = 0.21, I2 = 35%). Sensitivity analysis was performed by omitting each study sequentially, and the estimated results did not differ significantly, indicating the stability of the model (Figure 3B).
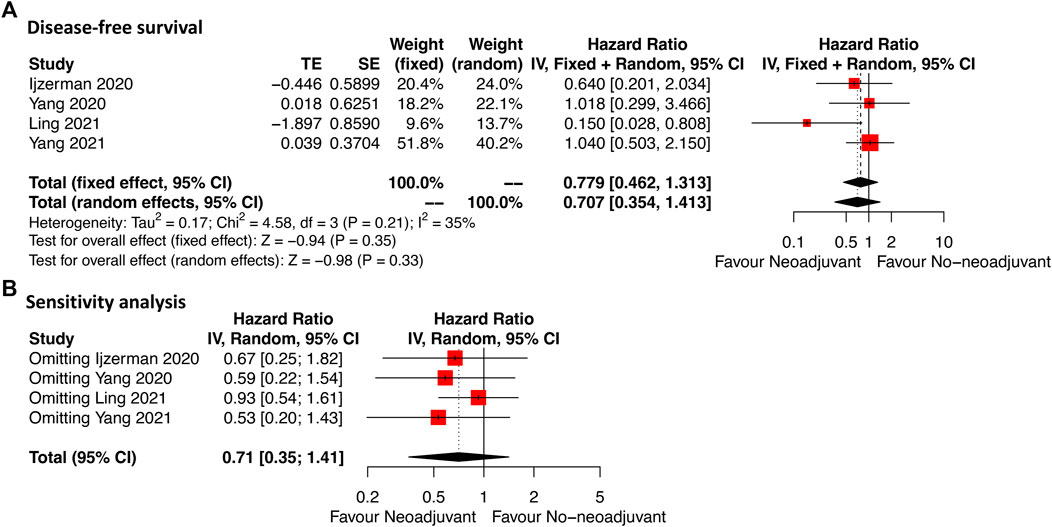
FIGURE 3. Forest plots illustrating disease-free survival between neoadjuvant and no-neoadjuvant imatinib (A) and sensitivity analysis (B).
Overall survival
Six studies providing OS data were included (Figure 4A). For the total cases, patients who received neoadjuvant imatinib had similar OS compared with those who received no-neoadjuvant therapy (HR: 0.52, 95% CI: 0.24–1.14; reference: no-neoadjuvant therapy). However, a moderate heterogeneity was observed (p = 0.01, I2 = 65%). To identify the potential source of heterogeneity, subgroup analysis was performed according to tumor site. A significant decrease of heterogeneity was observed in the subgroup of rectal GIST (p = 0.32, I2 = 15%). In this subgroup, neoadjuvant imatinib was significantly associated with better OS compared with no-neoadjuvant therapy (HR: 0.43, 95% CI: 0.19–1.02), which was consistent with the estimated results of the fixed-effects model (HR: 0.43, 95% CI: 0.21–0.87). Neoadjuvant imatinib significantly improved OS in mixed site GIST (HR: 0.20, 95% CI: 0.05–0.84) but not in gastric GIST (HR: 1.02, 95% CI: 0.94–1.11).
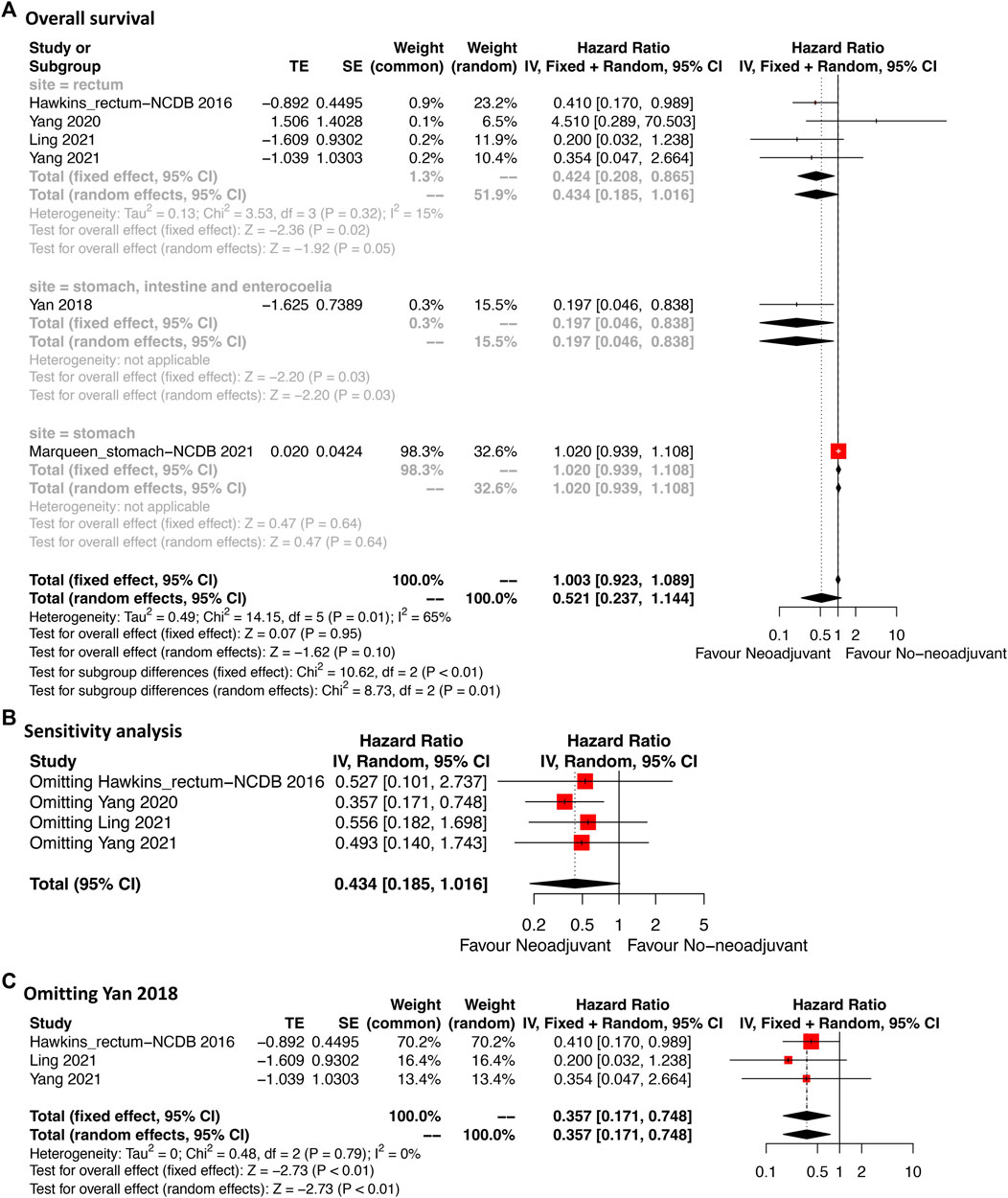
FIGURE 4. Forest plots illustrating overall survival between neoadjuvant and no-neoadjuvant imatinib (A) and sensitivity analyses (B,C).
Sensitivity analysis was performed by omitting each study sequentially in rectal GIST subgroup. The result after omitting Yang 2020 was significantly different from that after omitting other three studies which might weaken the credibility of the model (Figure 4B). This might due to the different including criteria in Yang et al.’s study (Yang et al., 2020). Their study mainly compared the transanal and nontransanal surgery for rectal GIST. The prognostic value of neoadjuvant therapy was only analyzed in the multivariate cox model. After omitting the study of Yang 2020 (Figure 4C), the effected result was stable (HR: 0.36, 95% CI: 0.17–0.75) and the heterogeneity additionally decreased (p = 0.79, I2 = 0%) indicating the credibility of the result.
Discussion
The present study compared the clinical effect of neoadjuvant imatinib and upfront surgery on GIST. The benefits of tumor shrinkage as well as improvement of R0 resection rate in rectal GIST were observed after the use of neoadjuvant imatinib therapy. Neoadjuvant imatinib was not significantly associated with DFS compared with no-neoadjuvant therapy in rectal GIST. However, neoadjuvant imatinib significantly improved OS of rectal GIST compared with no-neoadjuvant therapy.
The use of neoadjuvant imatinib has been reported to yield benefits in downstaging to avoid extensive resection in cases of bulky tumors or tumors in particular site, such as rectum (Andtbacka et al., 2007; Fiore et al., 2009; Nishida et al., 2019). A previous observational study (Wilkinson et al., 2015) reported that tumor size and mitotic index significantly reduced after receipt of neoadjuvant imatinib in rectal GIST which allows for less extensive sphincter-preserving surgery. Several studies reported that the sphincter-preserving rate in rectal GIST was 33.3–100% after treatment of neoadjuvant therapy (Kaneko et al., 2019). In the phase II APOLLON trial (Hohenberger et al., 2012), 64% of patients received a less radical surgery after 6 months treatment of neoadjuvant imatinib. In current meta-analysis, two studies reported their median reduction rate of tumor size were 29.8% (mixed sites) and 33% (rectum) (Supplementary Table S1), respectively. And in study of Yang 2021 (38), the median tumor size of rectal GIST reduced from 5.8 to 3.8 cm after neoadjuvant imatinib. The shrinkage of tumor size is considered to contribute to the achievement of R0 resection.
Complete resection is one of the primary concerns in the treatment of GIST ((Schmieder et al., 2016)). The R0 resection rate was previously reported to be 77.3–100% after treatment of neoadjuvant therapy (Kaneko et al., 2019). In the phase II RTOG 0132 study (Wang et al., 2012) including 31 cases of primary GISTs, a 68% rate of R0 resection (21 cases) was reported within the median neoadjuvant therapy duration of 9.9 weeks. Kurokawa et al. (Kurokawa et al., 2017) reported another phase II study with an achievement of 90% R0 resection rate in large gastric GIST treated with neoadjuvant therapy. This high rate of R0 resection was attributed to the long neoadjuvant therapy duration of 6 months. Several studies suggested the best duration of neoadjuvant therapy for maximal tumor response is 6–12 months (Bonvalot et al., 2006; Haller et al., 2007; Kurokawa et al., 2017). Which is in line with the 6 months duration or more recommended by NCCN guidelines (von Mehren et al., 2020).
In current meta-analysis, four studies reported the duration of neoadjuvant imatinib which ranged from 6.3 to 10 months (median) indicating a relatively optimal window for tumor response (Supplementary Table S1). The partial response rate in rectal GIST was reported to be 65.9% and 75% by Ling 2021 (36) and Yang 2021 (38), respectively. What is more, the disease control rate achieved 100% in Ling’s study (Ling et al., 2021b). Yang et al. (Yang et al., 2021) further reported that the effect of neoadjuvant imatinib is dependent on the genetic type and KIT exon 11 mutation responds better than other types which suggested the importance of genetic sequencing. In the rectal GIST subgroup of current study, a trend of higher R0 resection rate ranging from 85.3% to 98.8% was observed in neoadjuvant imatinib group compared with that ranging from 74.4% to 92.0% in no-neoadjuvant therapy group, though the difference was not significant.
Prognosis is another main indication in the evaluation of efficacy of neoadjuvant therapy which has not been sufficiently reported previously. Hawkins et al. (Hawkins et al., 2017) analyzed 333 cases of rectal GIST enrolled in NCDB, and the multivariate analysis showed that neoadjuvant therapy was not related with OS. But in the subgroup of tumors that were larger than 5 cm and received radical resection, neoadjuvant therapy had a significantly higher 5-year OS than no-neoadjuvant therapy (79.2% vs. 51.2%). Ling et al. (Ling et al., 2021b) also demonstrated that neoadjuvant therapy not only reduced tumor size of rectal GIST, but also improved 5-year distant recurrence-free survival and disease-specific survival. But the information of resection margin was not available in their study. In the contrary, a recent multicenter research (Yang et al., 2021) including 340 cases of rectal GISTs from 11 centers in China reported that, the 3-year rates of DFS and OS of those who received neoadjuvant therapy were 95% and 100%, respectively, which were similar in comparison with those of patients who received no-neoadjuvant therapy. Future updated follow-up is warranted for this multicenter study.
In current meta-analysis, DFS was available in four studies which were all focusing on rectal GIST. The pooled results showed that DFS was not significantly associated with neoadjuvant imatinib in rectal GIST. It is reported that positive margin is possibly associated with the recurrence of GIST but this negative impact disappeared in the era of imatinib due to the use of adjuvant imatinib (Liu et al., 2022). This comparable DFS between neoadjuvant imatinib group and no-neoadjuvant therapy group in current meta-analysis might partly due to the balanced rate of R0 resection between the two groups and the proper use of adjuvant imatinib in both groups. However, the OS was significantly improved after receipt of neoadjuvant imatinib in patients with rectal GIST in present study.
Limitations existed in current study. Firstly, due to the retrospective nature of these eligible studies, some inherent bias in the study design and process cannot be avoided. Secondly, tumor size and mitotic index as well as their reduction rate after neoadjuvant imatinib were not provided in all eligible studies that an overview of prognostic factors in GIST other than neoadjuvant imatinib was not available. Thirdly, detailed information of tumor rupture and adjuvant therapy were not able to be analyzed which were key factors impacting the prognosis of GIST. Fourthly, five out of the seven studies analyzed rectal GIST so further studies focusing on GISTs in other sites are warranted. Fifthly, a multicenter study including 340 cases of rectal GIST from 11 centers in China were included in this meta-analysis which provided relatively firm results for decision-making of neoadjuvant imatinib therapy. However, randomized controlled trials are still lacking and warranted to clarify the role of neoadjuvant imatinib in treatment of GIST.
Conclusion
Rectal GIST benefits from neoadjuvant imatinib regarding to the achievements of tumor shrinkage and R0 resection. Although neoadjuvant imatinib had no significant advantage on the disease-free survival, patients with rectal GIST who received neoadjuvant imatinib plus surgery had better overall survival than those who received upfront surgery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Study concepts: WK. Study design: FF. Data acquisition: ZL, ZZ, and JS. Quality control of data and algorithms: ZL and ZZ. Data analysis and interpretation: ZL, SO, and MM. Statistical analysis: ZL and JL. Manuscript preparation: ZL. Manuscript editing: XY. Manuscript review: WK and FF. All authors reviewed the manuscript.
Funding
This study was supported in part by grants from 1. Wu Jieping Medical Foundation (320.6750.19020 and 320.6750.2020-08-32); 2. CAMS Innovation Fund for Medical Sciences (2020-I2M-C&T-B-027); 3. Beijing Bethune Charitable Foundation (WCJZL202106); 4. Beijing Xisike Clinical Oncology Research Foundation (Y-HS2019-43).
Acknowledgments
The authors wish to thank the following collaborators who shared original data in a format that was not available in the original publications: Zifeng Yang, Wentai Guo, Rongkang Huang, Minhui Hu, Huaiming Wang, Hui Wang (Ann Transl Med 2020;8(5): 201) and Wenchang Yang, Qian Liu, Guole Lin, Bo Zhang, Hui Cao, Yan Zhao, Lijian Xia, Zhiguo Xiong, Junbo Hu, Yingjiang Ye, Kaixiong Tao, Peng Zhang (J Surg Oncol. 2021;124(7): 1128–1135). We also thank all the researchers and study participants for their contributions. Thanks to Siwen Ouyang for the statistical advice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.966486/full#supplementary-material
Abbreviations
DFS, disease-free survival; GIST, gastrointestinal stromal tumor; GRADE, the Grading of Recommendations Assessment, Development, and Evaluation system; NCCN, National Comprehensive Cancer Network; NOS, Newcastle-Ottawa Quality Assessment Scale; OS, overall survival; R0, microscopically negative resection margin.
References
Andtbacka, R. H., Ng, C. S., Scaife, C. L., Cormier, J. N., Hunt, K. K., Pisters, P. W., et al. (2007). Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Ann. Surg. Oncol. 14, 14–24. doi:10.1245/s10434-006-9034-8
Balduzzi, S., Rucker, G., and Schwarzer, G. (2019). How to perform a meta-analysis with R: A practical tutorial. Evid. Based. Ment. Health 22, 153–160. doi:10.1136/ebmental-2019-300117
Blay, J. Y., Serrano, C., Heinrich, M. C., Zalcberg, J., Bauer, S., Gelderblom, H., et al. (2020). Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. Oncol. 21, 923–934. doi:10.1016/S1470-2045(20)30168-6
Bonvalot, S., Eldweny, H., Pechoux, C. L., Vanel, D., Terrier, P., Cavalcanti, A., et al. (2006). Impact of surgery on advanced gastrointestinal stromal tumors (GIST) in the imatinib era. Ann. Surg. Oncol. 13, 1596–1603. doi:10.1245/s10434-006-9047-3
Cai, Z., Yin, Y., Zhao, Z., Xin, C., Cai, Z., Yin, Y., et al. (2018). Comparative effectiveness of neoadjuvant treatments for resectable gastroesophageal cancer: A Network meta-analysis. Front. Pharmacol. 9, 872. doi:10.3389/fphar.2018.00872
Casali, P. G., Abecassis, N., Aro, H. T., Bauer, S., Biagini, R., Bielack, S., et al. (2018). Gastrointestinal stromal tumours: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29, iv68–iv78. doi:10.1093/annonc/mdy095
Chen, D., Jin, Z., Zhang, J., Xu, C., Zhu, K., Ruan, Y., et al. (2021). Efficacy and safety of neoadjuvant targeted therapy vs. Neoadjuvant chemotherapy for stage iiia EGFR-mutant non-small cell lung cancer: A systematic review and meta-analysis. Front. Surg. 8, 715318. doi:10.3389/fsurg.2021.715318
Chen, T., Ni, N., Yuan, L., Xu, L., Bahri, N., Sun, B., et al. (2021). Proteasome inhibition suppresses KIT-independent gastrointestinal stromal tumors via targeting hippo/YAP/cyclin D1 signaling. Front. Pharmacol. 12, 686874. doi:10.3389/fphar.2021.686874
Chen, Y., Dong, X., Wang, Q., Liu, Z., Dong, X., Shi, S., et al. (2020). Factors influencing the steady-state plasma concentration of imatinib mesylate in patients with gastrointestinal stromal tumors and chronic myeloid leukemia. Front. Pharmacol. 11, 569843. doi:10.3389/fphar.2020.569843
Connolly, E. M., Gaffney, E., and Reynolds, J. V. (2003). Gastrointestinal stromal tumours. Br. J. Surg. 90, 1178–1186. doi:10.1002/bjs.4352
Das, M. (2017). Neoadjuvant chemotherapy: Survival benefit in gastric cancer. Lancet. Oncol. 18, e307. doi:10.1016/S1470-2045(17)30321-2
Demetri, G. D., Reichardt, P., Kang, Y. K., Blay, J. Y., Rutkowski, P., Gelderblom, H., et al. (2013). Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381, 295–302. doi:10.1016/S0140-6736(12)61857-1
Demetri, G. D., van Oosterom, A. T., Garrett, C. R., Blackstein, M. E., Shah, M. H., Verweij, J., et al. (2006). Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 368, 1329–1338. doi:10.1016/S0140-6736(06)69446-4
Demetri, G. D., von Mehren, M., Blanke, C. D., Van den Abbeele, A. D., Eisenberg, B., Roberts, P. J., et al. (2002). Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 347, 472–480. doi:10.1056/NEJMoa020461
Fiore, M., Palassini, E., Fumagalli, E., Pilotti, S., Tamborini, E., Stacchiotti, S., et al. (2009). Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST). Eur. J. Surg. Oncol. 35, 739–745. doi:10.1016/j.ejso.2008.11.005
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). Grade: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926. doi:10.1136/bmj.39489.470347
Haller, F., Detken, S., Schulten, H. J., Happel, N., Gunawan, B., Kuhlgatz, J., et al. (2007). Surgical management after neoadjuvant imatinib therapy in gastrointestinal stromal tumours (GISTs) with respect to imatinib resistance caused by secondary KIT mutations. Ann. Surg. Oncol. 14, 526–532. doi:10.1245/s10434-006-9228-0
Hawkins, A. T., Wells, K. O., Krishnamurty, D. M., Hunt, S. R., Mutch, M. G., Glasgow, S. C., et al. (2017). Preoperative chemotherapy and survival for large anorectal gastrointestinal stromal tumors: A national analysis of 333 cases. Ann. Surg. Oncol. 24, 1195–1201. doi:10.1245/s10434-016-5706-1
Heinrich, M. C., Corless, C. L., Duensing, A., McGreevey, L., Chen, C. J., Joseph, N., et al. (2003). PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299, 708–710. doi:10.1126/science.1079666
Heinrich, M. C., Jones, R. L., von Mehren, M., Schoffski, P., Serrano, C., Kang, Y. K., et al. (2020). Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): A multicentre, open-label, phase 1 trial. Lancet. Oncol. 21, 935–946. doi:10.1016/S1470-2045(20)30269-2
Hirota, S., Isozaki, K., Moriyama, Y., Hashimoto, K., Nishida, T., Ishiguro, S., et al. (1998). Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279, 577–580. doi:10.1126/science.279.5350.577
Hohenberger, P., Langer, C., Wendtner, C. M., Hohenberger, W., Pustowka, A., Wardelmann, E., et al. (2012). Neoadjuvant treatment of locally advanced GIST: Results of APOLLON, a prospective, open label phase II study in KIT- or PDGFRA-positive tumors. J. Clin. Oncol. 30, 10031. doi:10.1200/jco.2012.30.15_suppl.10031
Joensuu, H., Eriksson, M., Sundby Hall, K., Reichardt, A., Hermes, B., Schutte, J., et al. (2020). Survival outcomes associated with 3 Years vs 1 Year of adjuvant imatinib for patients with high-risk gastrointestinal stromal tumors: An analysis of a randomized clinical trial after 10-year follow-up. JAMA Oncol. 6, 1241–1246. doi:10.1001/jamaoncol.2020.2091
Joensuu, H., Hohenberger, P., and Corless, C. L. (2013). Gastrointestinal stromal tumour. Lancet 382, 973–983. doi:10.1016/S0140-6736(13)60106-3
Joensuu, H., Roberts, P. J., Sarlomo-Rikala, M., Andersson, L. C., Tervahartiala, P., Tuveson, D., et al. (2001). Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N. Engl. J. Med. 344, 1052–1056. doi:10.1056/NEJM200104053441404
Kaneko, M., Emoto, S., Murono, K., Sonoda, H., Hiyoshi, M., Sasaki, K., et al. (2019). Neoadjuvant imatinib therapy in rectal gastrointestinal stromal tumors. Surg. Today 49, 460–466. doi:10.1007/s00595-018-1737-5
Kurokawa, Y., Yang, H. K., Cho, H., Ryu, M. H., Masuzawa, T., Park, S. R., et al. (2017). Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br. J. Cancer 117, 25–32. doi:10.1038/bjc.2017.144
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 339, b2700. doi:10.1136/bmj.b2700
Ling, J. Y., Ding, M. M., Yang, Z. F., Zhao, Y. D., Xie, X. Y., Shi, L. S., et al. (2021). Comparison of outcomes between neoadjuvant imatinib and upfront surgery in patients with localized rectal GIST: An inverse probability of treatment weighting analysis. J. Surg. Oncol. 124, 1442–1450. doi:10.1002/jso.26664
Ling, J. Y., Ding, M. M., Yang, Z. F., Zhao, Y. D., Xie, X. Y., Shi, L. S., et al. (2021). Comparison of outcomes between neoadjuvant imatinib and upfront surgery in patients with localized rectal GIST: An inverse probability of treatment weighting analysis. J. Surg. Oncol. 124, 1442–1450. doi:10.1002/jso.26664
Liu, Z., Zhang, Y., Yin, H., Geng, X., Li, S., Zhao, J., et al. (2022). Comparison of prognosis between microscopically positive and negative surgical margins for primary gastrointestinal stromal tumors: A systematic review and meta-analysis. Front. Oncol. 12, 679115. doi:10.3389/fonc.2022.679115
Liu, Z., Zheng, G., Liu, J., Liu, S., Xu, G., Wang, Q., et al. (2018). Clinicopathological features, surgical strategy and prognosis of duodenal gastrointestinal stromal tumors: A series of 300 patients. BMC Cancer 18, 563. doi:10.1186/s12885-018-4485-4
Marqueen, K. E., Moshier, E., Buckstein, M., and Ang, C. (2021). Neoadjuvant therapy for gastrointestinal stromal tumors: A propensity score-weighted analysis. Int. J. Cancer 149, 177–185. doi:10.1002/ijc.33536
Miettinen, M., Kopczynski, J., Makhlouf, H. R., Sarlomo-Rikala, M., Gyorffy, H., Burke, A., et al. (2003). Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: A clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am. J. Surg. Pathol. 27, 625–641. doi:10.1097/00000478-200305000-00006
Mittendorf, E. A., Zhang, H., Barrios, C. H., Saji, S., Jung, K. H., Hegg, R., et al. (2020). Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 396, 1090–1100. doi:10.1016/S0140-6736(20)31953-X
Nishida, T., Holmebakk, T., Raut, C. P., and Rutkowski, P. (2019). Defining tumor rupture in gastrointestinal stromal tumor. Ann. Surg. Oncol. 26, 1669–1675. doi:10.1245/s10434-019-07297-9
Ns, I. J., Mohammadi, M., Tzanis, D., Gelderblom, H., Fiore, M., Fumagalli, E., et al. (2020). Quality of treatment and surgical approach for rectal gastrointestinal stromal tumour (GIST) in a large European cohort. Eur. J. Surg. Oncol. 46, 1124–1130. doi:10.1016/j.ejso.2020.02.033
Renberg, S., Zhang, Y., Karlsson, F., Branstrom, R., Ahlen, J., Jalmsell, L., et al. (2022). The role of neoadjuvant imatinib in gastrointestinal stromal tumor patients: 20 years of experience from a tertial referral center. Int. J. Cancer 151, 906–913. doi:10.1002/ijc.34052
Rubin, B. P., Heinrich, M. C., and Corless, C. L. (2007). Gastrointestinal stromal tumour. Lancet 369, 1731–1741. doi:10.1016/S0140-6736(07)60780-6
Schmieder, M., Henne-Bruns, D., Mayer, B., Knippschild, U., Rolke, C., Schwab, M., et al. (2016). Comparison of different risk classification systems in 558 patients with gastrointestinal stromal tumors after R0-resection. Front. Pharmacol. 7, 504. doi:10.3389/fphar.2016.00504
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi:10.1007/s10654-010-9491-z
Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S., and Sydes, M. R. (2007). Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16. doi:10.1186/1745-6215-8-16
von Mehren, M., Kane, J. M., Bui, M. M., Choy, E., Connelly, M., Dry, S., et al. (2020). NCCN guidelines insights: Soft tissue sarcoma, version 1.2021. J. Natl. Compr. Canc. Netw. 18, 1604–1612. doi:10.6004/jnccn.2020.0058
Wang, D., Zhang, Q., Blanke, C. D., Demetri, G. D., Heinrich, M. C., Watson, J. C., et al. (2012). Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: Long-term follow-up results of radiation therapy Oncology group 0132. Ann. Surg. Oncol. 19, 1074–1080. doi:10.1245/s10434-011-2190-5
Wilkinson, M. J., Fitzgerald, J. E., Strauss, D. C., Hayes, A. J., Thomas, J. M., Messiou, C., et al. (2015). Surgical treatment of gastrointestinal stromal tumour of the rectum in the era of imatinib. Br. J. Surg. 102, 965–971. doi:10.1002/bjs.9818
Wong, L. H., Sutton, T. L., Sheppard, B. C., Corless, C. L., Heinrich, M. C., and Mayo, S. C. (2022). Neoadjuvant tyrosine kinase inhibitor therapy for patients with gastrointestinal stromal tumor: A propensity-matched analysis. Am. J. Surg. 224, 624–628. doi:10.1016/j.amjsurg.2022.03.045
Yan, L. H., Chen, Z. N., Li, C. J., Chen, J., Qin, Y. Z., Chen, J. S., et al. (2018). Prolonging Gastrointestinal-Stromal-Tumor-free life, an optimal suggestion of imatinib intervention ahead of operation. J. Cancer 9, 3850–3857. doi:10.7150/jca.25263
Yang, W., Liu, Q., Lin, G., Zhang, B., Cao, H., Zhao, Y., et al. (2021). The effect of neoadjuvant imatinib therapy on outcome and survival in rectal gastrointestinal stromal tumors: A multiinstitutional study. J. Surg. Oncol. 124, 1128–1135. doi:10.1002/jso.26628
Keywords: gastrointestinal stromal tumor, neoadjuvant imatinib, upfront surgery, R0, prognosis, meta-analysis
Citation: Liu Z, Zhang Z, Sun J, Li J, Zeng Z, Ma M, Ye X, Feng F and Kang W (2022) Comparison of prognosis between neoadjuvant imatinib and upfront surgery for GIST: A systematic review and meta-analysis. Front. Pharmacol. 13:966486. doi: 10.3389/fphar.2022.966486
Received: 11 June 2022; Accepted: 19 July 2022;
Published: 29 August 2022.
Edited by:
Adina Turcu-Stiolica, University of Medicine and Pharmacy of Craiova, RomaniaReviewed by:
Daniela Calina, University of Medicine and Pharmacy of Craiova, RomaniaDaniele Pironi, Sapienza University of Rome, Italy
Copyright © 2022 Liu, Zhang, Sun, Li, Zeng, Ma, Ye, Feng and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Feng, c3VyZ2VvbmZlbmdmYW5AMTYzLmNvbQ==; Weiming Kang, a2FuZ3dlaW1pbmdAMTYzLmNvbQ==
 Zhen Liu
Zhen Liu Zimu Zhang1
Zimu Zhang1 Juan Sun
Juan Sun Jie Li
Jie Li Ziyang Zeng
Ziyang Zeng Mingwei Ma
Mingwei Ma Weiming Kang
Weiming Kang