- 1Department of Pharmacy, The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China
- 2Guangdong Engineering Technology Research Center for Translational Medicine of Mental Disorders, Guangzhou, China
Objective: To establish a population pharmacokinetic model in Chinese psychiatric patients to characterize escitalopram pharmacokinetic profile to identify factors influencing drug exposure, and through simulation to compare the results with the established therapeutic reference range.
Methods: Demographic information, dosing regimen, CYP2C19 genotype, concomitant medications, and liver and kidney function indicators were retrospectively collected for inpatients taking escitalopram with therapeutic drug monitoring from 2018 to 2021. Nonlinear mixed-effects modeling was used to model the pharmacokinetic characteristics of escitalopram. Goodness-of-fit plots, bootstrapping, and normalized prediction distribution errors were used to evaluate the model. Simulation for different dosing regimens was based on the final estimations.
Results: The study comprised 106 patients and 337 measurements of serum sample. A structural model with one compartment with first-order absorption and elimination described the data adequately. The population-estimated apparent volume of distribution and apparent clearance were 815 and 16.3 L/h, respectively. Age and CYP2C19 phenotype had a significant effect on the apparent clearance (CL/F). CL/F of escitalopram decreased with increased age, and CL/F of poor metabolizer patients was significantly lower than in extensive and immediate metabolizer patients. The final model-based simulation showed that the daily dose of adolescents with poor metabolizer might be as high as 15 mg or 20 mg and referring to the therapeutic range for adults may result in overdose and a high risk of adverse effects in older patients.
Conclusion: A population pharmacokinetics model of escitalopram was successfully created for the Chinese population. Depending on the age of the patients, CYP2C19 genotype and serum drug concentrations throughout treatment are required for adequate individualization of dosing regimens. When developing a regimen for older patients, especially those who are poor metabolizers, vigilance is required.
Introduction
Depression and anxiety disorders affect a large number of people worldwide, which is placing an increasing burden on health services (Hazell, 2021). Nowadays, approaches to treatment include antidepressant and mood-stabilizing drugs, psychotherapy, and physical activity. Escitalopram is still the antidepressant of choice because of its safety, efficacy and tolerability (Cipriani et al., 2009; Sanchez et al., 2014; Cipriani et al., 2018). Escitalopram is highly selective for serotonin transporters and is active against depression (Burke et al., 2002; Wade et al., 2002; Rapaport et al., 2004) and anxiety disorders (Stahl et al., 2003; Davidson et al., 2004).
Escitalopram is an active S-enantiomer of citalopram and is one of the most commonly prescribed selective serotonin reuptake inhibitors (SSRIs). It was launched in the United States in 2002 and China in 2006. The pharmacokinetic profile of escitalopram has been studied extensively in healthy people. The maximum concentration of escitalopram is reached ∼4 h after oral administration of 10–20 mg/day, with an elimination half-life (t1/2) of ∼30 h. This supports the therapeutic plan of a once-daily dose of 10–20 mg, and escitalopram is characterized by oral clearance and volume of distribution of 0.48 L/h/kg and 18.3L/kg, respectively (Søgaard et al., 2005; Rao, 2007). Escitalopram is primarily metabolized in the liver by cytochrome P (CYP)450, particularly CYP2C19, which is a highly polymorphic enzyme that causes interindividual pharmacokinetic differences (Rao, 2007; Pastoor and Gobburu, 2014), and is excreted mainly through the kidneys. The effect of age (Dolder et al., 2010; Yang and Scott, 2010), gender (Montejo et al., 2015), smoking (Oliveira et al., 2017; Scherf-Clavel et al., 2019), CYP2C19 phenotype (Huang et al., 2021), hepatic impairment (Areberg et al., 2006), and renal impairment (Dolder et al., 2010) on the pharmacokinetics of escitalopram have been investigated. The findings of these studies were instrumental in developing specific dosing recommendations for escitalopram for specific populations (Hicks et al., 2015; Brouwer et al., 2021). Escitalopram has been approved for use in China for 16 years, and it is the first-line antidepressant medication in China (Rao, 2007). As a result, it is necessary to investigate the factors that may affect the pharmacokinetics of escitalopram in the Chinese population in order to provide a basis for individualized medication in China.
Population pharmacokinetics (PopPK) modeling is a widely used tool to analyze pharmacokinetic data to individualize dosing regimens. Based on this approach, we can identify potential covariates that influence the pharmacokinetics of escitalopram and establish formulas to describe individual parameters. Compared with traditional pharmacokinetics, the advantage of PopPK is that the sparse blood drug concentrations can be used to quantify the intrinsic and extrinsic factors influencing pharmacokinetics by incorporating different covariates. There have been several studies on the PopPK of escitalopram. The PK parameters have been compared in HIV-infected and uninfected psychiatric patients (Courlet et al., 2019). A PopPK model of escitalopram in patients during the perinatal period has been established (Weisskopf et al., 2020). The effect of age, weight, gender and CYP2C19 genotype on escitalopram exposure has been studied in American and Italian patients. (Jin et al., 2010; Akil et al., 2016; Kim et al., 2021). No systematic PopPK analysis of escitalopram has been established in Chinese psychiatric patients. A PopPK/PD model has been developed in Korean healthy volunteers (Kim et al., 2021). Although the mutation frequency of CYP2C19 genotype in the Chinese population was similar to that in Korean population (Dorji et al., 2019), they did not investigate the effect of CYP2C19 genotype. Additionally, CYP2C19 *2 and *3 have much less mutation frequency in European than in East Asian population, but *17 is higher than in East Asian. Therefore, because of the difference in race and CYP2C19 variant allele frequency, investigation in the Chinese population is curial.
In the present study, we established a PopPK model of escitalopram in Chinese psychiatric patients by retrospectively collecting serum drug concentrations and related information. Compared to previous studies, in addition to the influence of age, sex, weight, height, body mass index (BMI) and CYP2C19 genotype, we included liver and kidney function-related biochemical indicators and combination therapy to complete a comprehensive pharmacokinetic evaluation of escitalopram. Simulations were also conducted to investigate whether patients needed to take different doses of escitalopram under different circumstances. The objective of the current study was to develop a PopPK model for escitalopram in Chinese psychiatric patients to explore the potential factors that contribute to variability in escitalopram pharmacokinetics. Furthermore, the model served to predict average drug exposure under various influencing factors through simulation and compared it with the established therapeutic reference range.
Methods
Subjects and data collection
The data were obtained from psychiatric inpatients in the Affiliated Brain Hospital of Guangzhou Medical University from 2018 to 2021 and monitored drug blood concentrations during this period. Patients were excluded if there was only one blood concentration measurement, and if there was no reliable information about administration and blood sampling times. This study provided an opportunity to evaluate whether age, sex, weight, height, BMI, smoking, drinking, CYP2C19 genotype, alanine aminotransferase (ALT), mitochondrial aspartate aminotransferase (m-AST), total bilirubin (TBIL), albumin (ALB), urea, serum creatinine (Scr), and combination therapy (such as omeprazole and valproic acid) affected the pharmacokinetics of escitalopram. This study was approved by the Institutional Review Board (IRB) in the Affiliated Brain Hospital of Guangzhou Medical University (Approval number: 2021027).
Determination of escitalopram concentrations
Blood samples (three to four ml) were collected into coagulation-promoting tubes and centrifuged at 17,600 g for 3 min. Serum samples (100 µL) were transferred into 2-ml Eppendorf tubes and mixed with 20 µL internal standard (citalopram-d6) and 500 µL acetonitrile. After vortex-mixing for 10 s and centrifugation at 21,130 g for 5 min, ∼100 µL supernatant was removed and transferred to autosampler vials with lining tubing. Escitalopram was measured by HPLC-tandem mass spectrometry (Shimadzu, Kyoto, Japan). Separation was performed on an Agilent Eclipse XDB-C18 column (4.6 × 50 mm, 1.8 µm) with a flow of 0.6 ml/min, and the mobile phase consisted of (A) 75% methanol with 5 mM ammonium formate and (B) methanol for 1.3 min. The injection volume was 1 µL. The linear range was 3–300 ng/ml. This analytical method has been examined by selectivity, specificity, matrix effect, stability, and intra- and inter-batch precision and accuracy.
Determination of CYP2C19 genotype
DNA was extracted utilizing DNA extraction and purification kits from Shanghai BaiO Technology Co. Ltd. The genotype of CYP2C19 was determined using a human CYP2C19 gene detection kit provided by Wuhan Youzhiyou Medical Technology Co. Ltd. DNA amplification was accomplished after extracting DNA and adding the DNA reaction solution. Following the reaction, the Ct values of various channels were calculated using the amplification curves, and the results were determined. With regard to CYP2C19 isoenzymes, patients were divided into three groups according to the predicted phenotypes: extensive metabolizer (EM) if they were homozygous for the wild-type allele *1/*1; intermediate metabolizer (IM) if they carried the *1/*2 or *1/*3 allele; and poor metabolizer (PM) if they carried the *2/*2 or *2/*3 allele.
Modeling strategy and software
The PopPK model of escitalopram was created using the nonlinear mixed-effect modeling program (NONMEM, version 7) with the first-order conditional estimation with inter- and intraindividual variability interaction (FOCE-I) method to estimate population parameters and identify candidate covariates. Pirana (version 2.9.0) was used to document and structure model development. Normalized prediction distribution errors (NPDE) test was performed using the NPDE-add on package in R (version 4.1.1). Perl-speaks-NONMEM (version 3.4.2) was used to conduct bootstrap analysis (n = 1,000). Goodness-of-fit plots were performed using GraphPad Prism (version 9.1.1). Statistical analysis was performed using SPSS (version 25.0).
PopPK model development
A basic model without any covariates was developed initially. The pharmacokinetics were described using a first-compartment model with first-order absorption and first-order elimination in terms of apparent oral clearance (CL/F), apparent volume of distribution (Vd/F), and absorption rate constant (Ka). Due to the paucity of concentration data within a few hours after oral administration, the absorption phase could not be described. We fixed Ka to 0.6 according to an established model in Chinese subjects (Chen et al., 2013). A statistical model was included to describe between-subject and residual variability. The interindividual variabilities of CL/F and Vd/F were evaluated through an exponential error model (Eq 1), and the intraindividual unexplained variability was through a mixed residual error model (Eq 2).
Where Pi represents the estimate of ith individual parameters (Vd/F or CL/F),
Where Y and F denote the model-observed and -predicted escitalopram concentrations, respectively.
The selection of candidate covariates was through the method of stepwise forward selection–backward elimination resulting in the final PopPK model for escitalopram. For concomitant medication, we evaluated the effect of the CYP2C19 inhibitors taken by each patient for that several studies have demonstrated that the magnitude of drug-drug interactions with escitalopram was weak and moderate (Gutierrez et al., 2003; Siccardi et al., 2013) with proton-pump inhibitors having a moderate effect on escitalopram pharmacokinetics (Malling et al., 2005; Gjestad et al., 2015), and we also explored the effect of CYP2C19 inducers. Missing values of weight and height were imputed to the population median value. Covariates would be incorporated into the basic model when their addition reduced the objective function value (OFV) to >6.63 (p < 0.01) and removed from the full model when exclusion of the covariates resulted in an increase <10.83 (p < 0.001). Eqs 3, 4 were applied for continuous (age, height, weight, etc.) and noncontinuous (sex, CYP2C19 genotype, and combination therapy) covariates, and Eqs 5–7 were used to investigate the influence of CYP2C19 genotypes.
The following were continuous covariates:
The following were non-continuous covariates:
Where
Model evaluation
The precision of parameters and the ability of the final covariate model were assessed by goodness-of-fit plots, bootstrapping, and NPDE. At the same time, the plausibility of estimated parameters and relative standard errors, and changes in both inter- and intraindividual variability were also considered. Goodness-of-fit plots were used for the final model quality evaluation, which included: population predicted concentration versus observed concentrations (as known as dependence variables (DV)); individual predicted concentration versus observed concentrations; population predicted concentrations versus conditional weighted residuals (CWRES); and time after last dose versus CWRES. A bootstrap analysis was performed with resampling 1,000 times. The results of bootstrapping were summarized as median, and 95% confidence intervals of each parameter compared with the corresponding parameters obtained with the origin dataset. NPDE is a model evaluation approach based on the fit of each observation and is not easily influenced by experimental design.
Simulation
Simulation can provide escitalopram dosing guidance in Chinese psychiatric patients, and it was conducted under several regimens based on the final estimations to find optimal individualized dosing regimens. We predicted steady-state concentration profiles for the therapeutic doses of 5, 10, 15, and 20 mg qd for adolescents ≥12 and <18 years, adults ≥18 and <65 years, and elderly ≥65 years with different CYP2C19 phenotypes (EM, IM and PM). We performed the simulation to establish: 1) whether the steady-state serum levels in adult patients were in the therapeutic range after administration according to the instructions; 2) whether it was necessary to give older and PM patients half the dose of escitalopram; and 3) whether adolescent patients could be administrated the same dosing regimen as adults. Simulation was performed to ensure that >95% of the trough concentrations were within the therapeutic window during therapy.
Results
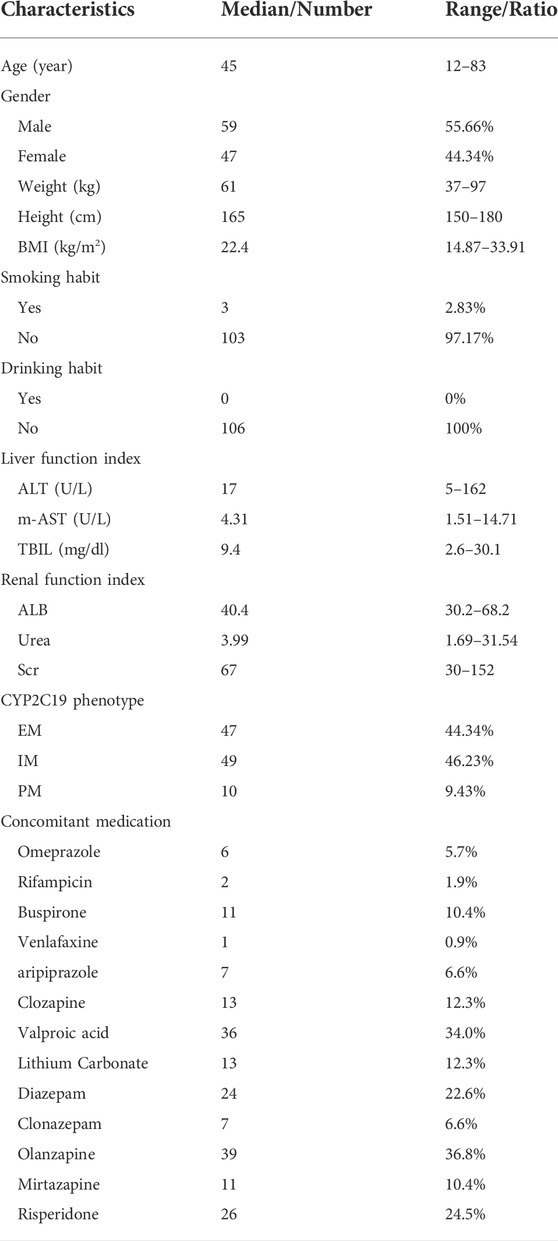
Demographic information
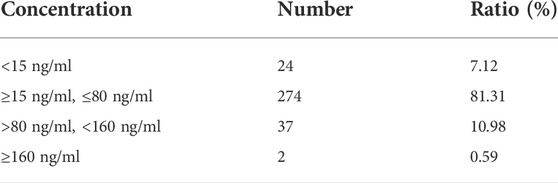
The final dataset for the PopPK model included 106 psychiatric patients and 337 escitalopram measurements in both steady-state and non-steady-state. And the approximate sampling times were most of around trough. All patients were given conventional tablets with 5 mg qd, 10 mg qd, 15 mg qd, 20 mg qd, 5 mg bid, or 10 mg bid. The median dose of escitalopram was 10 mg/day (range 5–30 mg/day). Details on the demographics are summarized in Table 1, and the frequency of CYP2C19 is listed in Table 2. CYP2C19*2, the main mutant and causative allele, was the most common genotype, followed by CYP2C19*3, thus making higher frequencies of *1/*2 and *2/*2 among all test samples. In accordance with the therapeutic drug monitoring guidelines in psychiatry by Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP) in 2017 (Hiemke et al., 2018), we collected information on enrolled patients receiving CYP2C19 inhibitors and inducers during the sampling time, as well as drugs with potential effect. Concomitant medications are shown in Table 1. The therapeutic window and laboratory alert level of escitalopram in AGNP guideline were 15–80 ng/ml and 160 ng/ml, respectively (Hiemke et al., 2018). We summarized the blood drug concentration information in Table 3.
PopPK model for escitalopram
The pharmacokinetics of escitalopram were best described by a one-compartment model with first-order absorption and elimination (Courlet et al., 2019; Weisskopf et al., 2020). Owing to the proportional error model that could better describe the present model, we fixed the additive error to 0. The OFV of the basic model was 1923.221. The mean (relative standard error) basic model estimate parameters were 14 L/h (4%) for CL/F and 815 L (16%) for V/F. The between-subject variability was estimated to be 0.146 and 0.216 for CL/F and V/F respectively, and the intraindividual variability was 0.0289 in the proportional error model
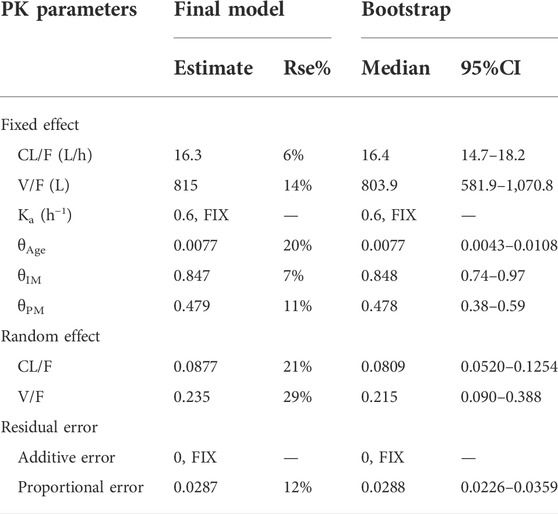
A detailed description of the principal results of covariate analyses is presented. During the forward inclusion process, the CYP2C19 phenotype was a significant covariate for CL/F with the model decreased by 25.58 (p < 0.001) to a final value of 1897.64. Age also had a significant impact on the CL/F of escitalopram with the value of OFV decreasing by 9.928 (p < 0.01) to 1913.293. There were no significant effects of gender, height, weight, BMI, smoking, concomitant medication, and liver or kidney function on CL/F or V/F. When we incorporated age at CL/F forward based on the CYP2C19 phenotype covariate model, the model led to a 21.10 decrease in OFV value to 1876.533, and the full model was developed. The backward elimination step each time removed a covariate from the full model. The values of OFV were increased by 36.76 (p < 0.001) and 21.107 (p < 0.001) for the CYP2C19 phenotype and age to 1913.293 and 1897.64, respectively, which meant CYP2C19 phenotype and age had significant effect on the exposure of escitalopram. And we found no correlation between age and CYP2C19 phenotype. Estimates for PK parameters of the final model are listed in Table 4.
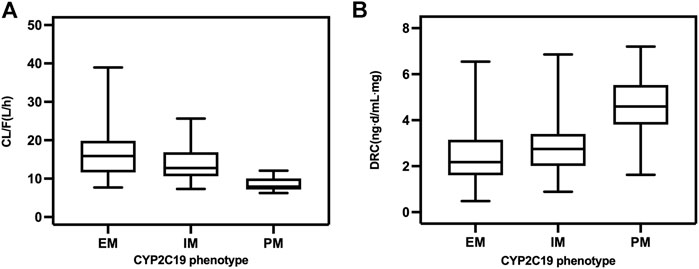
In the final model, there was a decrease in CL/F of escitalopram with increased patient age, and it was also influenced by different CYP2C19 phenotypes. The CL/F was 20.83 L/h in adolescents aged 15 years and 15.84 L/h in adults aged 45 years. In older patients aged 75 years, CL/F decreased to 11.89 L/h. The higher CL/F in EM than in IM and PM patients resulted in the dose-related concentration of IM patients being higher than that in EM patients, while concentration in PM patients was much higher than both IM and EM patients (Figure 1). The estimated population CL/F of escitalopram was 16.73 L/h for EM, 13.96 L/h for IM, and 8.56 L/h for PM patients. CL/F in EM patients was 1.2-fold higher than in IM patients and 1.9-fold higher than in PM patients.
Model validation
Goodness-of-fit plots, NPDE, and bootstrapping illustrated the appropriateness of the covariate model. Figure 2 showed the scatter plots of the observation values versus population (Figure 2A) and individual (Figure 2B) predicted concentrations, which observed a good correlation and were distributed symmetrically around the trend line. This suggested that the final model was a good fit for the observed data. Figure 2C shows the scatter plots of the CWRES from the final PopPK model, with a range between -3.02 and 2.53, which was distributed symmetrically around 0. The plot of time after dose versus CWRES is shown in Figure 2D. The results of bootstrapping are listed in Table 3. All estimated parameters from the final model were within the 95% confidence interval calculated from the bootstrap method, indicating that the model was constructed with good robustness. The results of NPDE are shown in Figure 3.
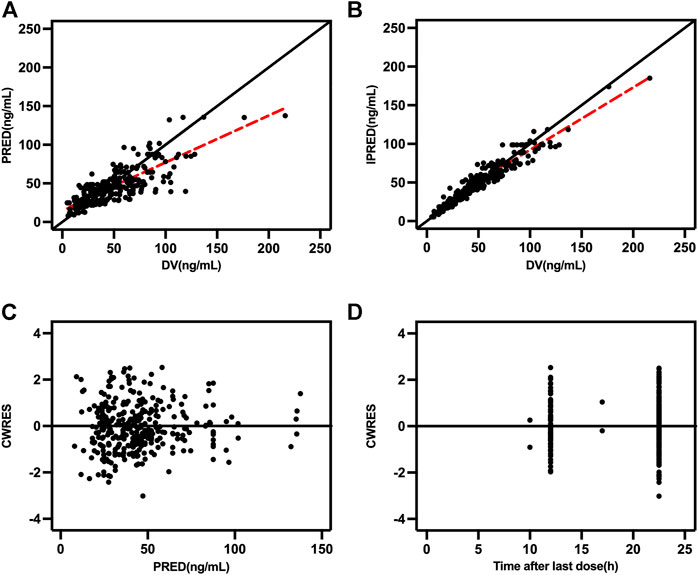
FIGURE 2. Goodness-of-fit plots (A) Population predicted concentration (PRED) versus observed concentrations; (B) individual predicted concentration (IPRED) versus observed concentrations; (C) population predicted concentrations versus conditional weighted residuals (CWRES); and (D) time-after last dose versus CWRES.
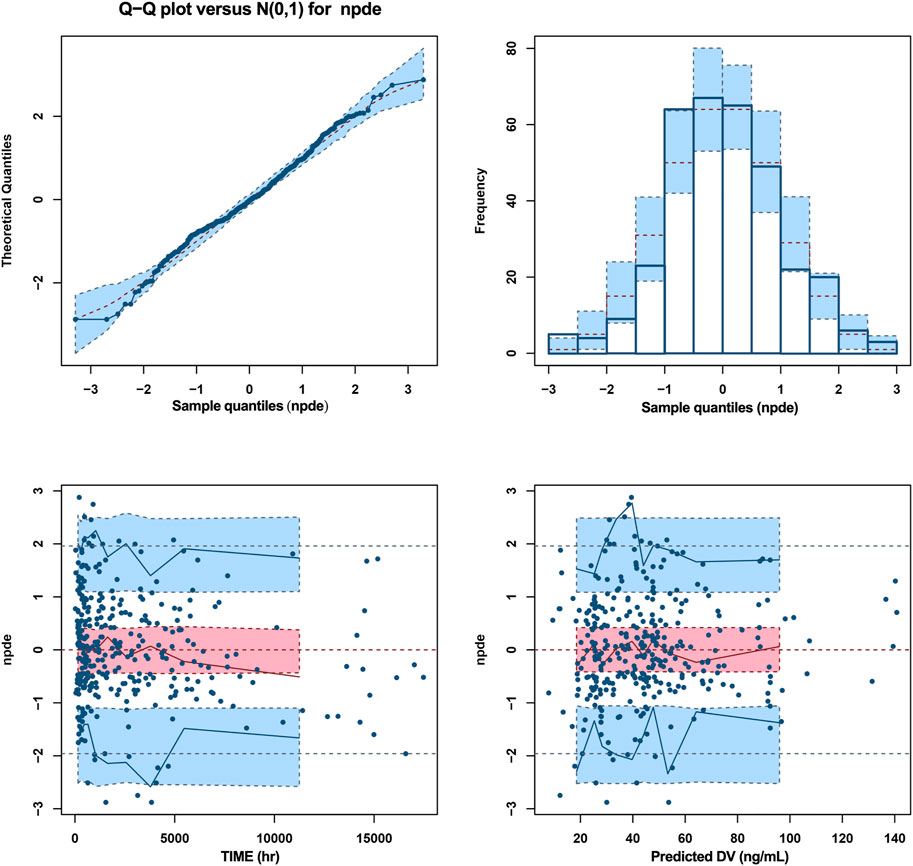
FIGURE 3. NPDE metrics for the PopPK model of escitalopram. The mean of normalized prediction distribution errors (NPDE) was 0.02359, variance was 0.9894, skewness was 0.04414, and kurtosis was 0.3027. The results of t-test and Fisher variance test were 0.664 and 0.911, respectively. The statistical values Shapiro-Wilk (SW) test for normality was 0.0633, and the global adjusted p-value was 0.19.
Dosing simulation for escitalopram dose
Considering the covariates that we selected and common situations in clinical practice, the time courses of escitalopram concentrations in steady state were simulated for different ages and CYP2C19 phenotypes. The therapeutic window of escitalopram is 15–80 ng/ml and the laboratory alert level is 160 ng/ml (Hiemke et al., 2018), and applies to patients aged ≥18 and <65 years. Doses of 10, 15 and 20 mg/day were all within the range of 15–80 ng/ml for EM and IM patients (Figures 4A,B). However, the serum drug concentrations were >80 ng/ml at a daily dose of 20 mg for PM patients (Figure 4C).
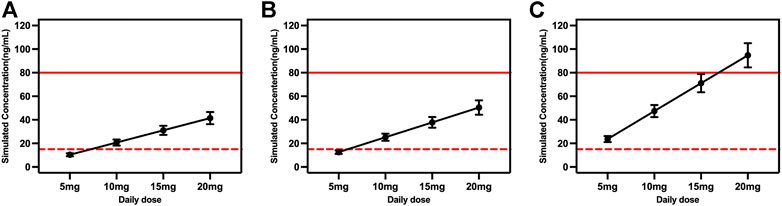
FIGURE 4. Simulated concentrations for ages ≥18 and <65 years in (A) extensive metabolizers; (B) immediate metabolizers, and (C) poor metabolizers at different daily doses. The red dash lines represented 15 ng/ml, and the red solid lines represented 80 ng/ml.
The model-based simulation results in older patients showed that the drug concentration in PM patients was twice as high as that in EM patients under the same dosing regimen (Figure 5). Consistent with the above results, oral administration of 15 or 20 mg/day exceeded 80 ng/ml in PM patients. Accordingly, the recommended dose of escitalopram is no more than 10 mg/day for PM patients.
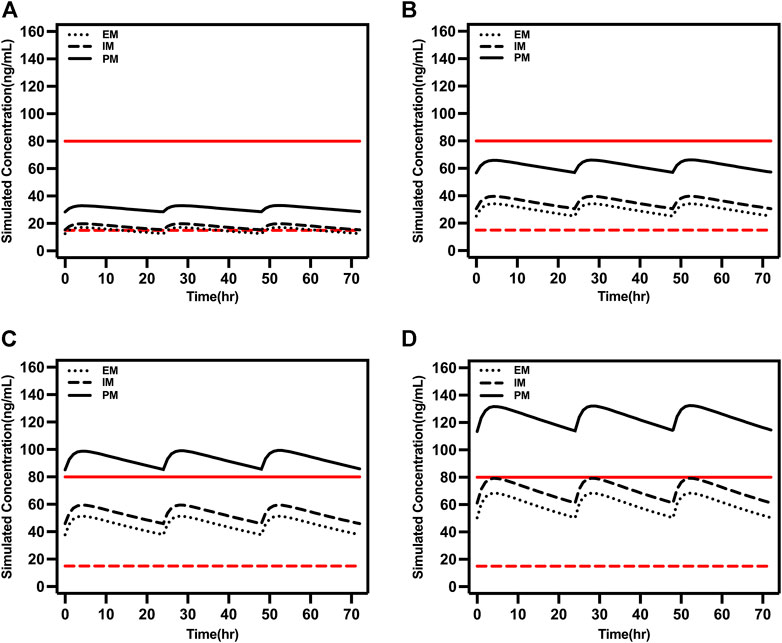
FIGURE 5. Simulated concentrations in EM, IM, and PM older patients (65 years old) at (A) 5 mg/day, (B) 10 mg/day, (C) 15 mg/day, and (D) 20 mg/day. The red dash lines represented 15 ng/ml and the red solid lines represented 80 ng/ml.
Adolescents typically have higher clearance compared to older people, which was reflected in the steady-state trough concentration being within 15–80 ng/ml when the daily dose was 15 or 20 mg in PM adolescents (Figure 6). However, caution is required for PM patients taking daily doses >10 mg.
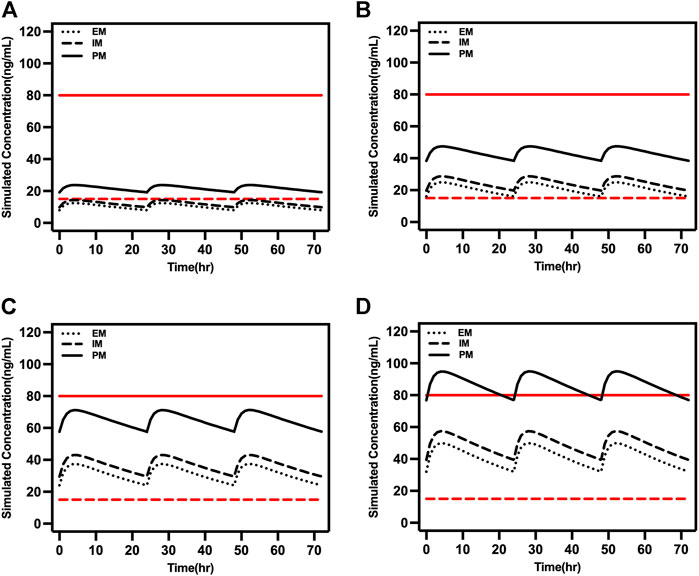
FIGURE 6. Simulated concentrations in EM, IM, and PM adolescents (16 years old) at (A) 5 mg/day, (B) 10 mg/day, (C) 15 mg/day, and (D) 20 mg/day. The red dash lines represented 15 ng/ml and the red solid lines represented 80 ng/ml.
Discussion
PopPK has been utilized extensively in clinical treatment and has become a very useful approach in optimizing individualized dosing regimens, therapeutic drug monitoring, and clinical evaluation of novel drugs. A PopPK model has been created to increase the possibility of meeting suitable pharmacokinetic/pharmacodynamic targets due to the limited therapeutic index of voriconazole and the relatively large systematic interindividual variability (Chen et al., 2019). On the other hand, due to the tendency of order patients to miss doses of medication, they developed a strategy to correct for missed doses through establishing a PopPK model and simulating (Xiao et al., 2021).
In this study, we created a PopPK model for oral administration of escitalopram in Chinese psychiatric patients, while considering demographic, genetic and physiological indicators. The model-predicted covariates of this analysis were in line with several published studies that describe the population pharmacokinetics of escitalopram (Jin et al., 2010; Akil et al., 2016; Courlet et al., 2019). We showed that CL/F of escitalopram varied nearly sevenfold, ranging from 6.26 to 38.93 L/h, which means that the pharmacokinetics of escitalopram in different populations show large interindividual variations. Some intensive sampling designs with escitalopram CL/F of 20–40 L/h, mostly in healthy individuals (Søgaard et al., 2005; Nilausen et al., 2011; Chung et al., 2017), and published PopPK models have a CL/F > 20 or even 30 L/h (van Gorp et al., 2012; Courlet et al., 2019; Kim et al., 2021), while our study showed <20 L/h, which may have been caused by sparse data sampling.
Similar to the previous PopPK studies, our analysis revealed that age and CYP2C19 phenotype contributed differentially to the variability in the pharmacokinetics of escitalopram. Our model results showed a decrease in the CL/F of escitalopram with increasing age. This is in agreement with previously published PopPK models (Jin et al., 2010; Akil et al., 2016). Older patients ≥65 years had a significantly lower CL/F compared with younger healthy volunteers (Fredericson Overø et al., 1985; Bies et al., 2004). As reported previously, older patients had a significantly lower elimination rate than younger patients had (Dhillon et al., 2006), which was confirmed in our study. Actually, our study suggested a 10% decrease in clearance of escitalopram for every 20 years of age, which was less than the previous estimation of a decrease of 30–42% (Jin et al., 2010; Akil et al., 2016). This was consistent with the previously reported decrease in CYP2C19 activity with increasing age (Pollock et al., 1991), and was specifically quantified in our analysis of escitalopram. This might have arisen from the small number of people in each age bracket, although the age ranged from 12 to 83 years. Hence, the dose of escitalopram might need to be adjusted based on age.
In addition, the genetic polymorphism of CYP2C19 had a significant effect on the apparent clearance of escitalopram in previous studies (Areberg et al., 2006; Jin et al., 2010; Chang et al., 2014). Two single-center, randomized, open-label, two-period, two-treatment crossover bioavailability studies with 96 healthy Chinese individuals showed that the exposure of escitalopram in PM subjects and IM subjects increased by 102 and 38% respectively compared with EM, and the efficacy and toxicity of escitalopram varied among individuals with different genotypes (Chang et al., 2014; Jukić et al., 2018). In our study, EM and IM patients with CYP2C19 cleared escitalopram 48.8 and 38.7% faster than PM patients did. This means that metabolism in PMs is greatly reduced, and they experience higher systemic exposure compared with EMs and IMs that have similar clearance. Hence, genetic testing before medication and adjustment of escitalopram dose in PMs should be considered in the clinical treatment of Chinese patients. Moreover, the present findings of the CYP2C19 genotype–phenotype relationship are consistent with the previous study. When breaking the genotype into five categories (*1/*1, *1/*2, *1/*3, *2/*2, and *2/*3), we found that estimations of CL/F for *1/*2 and *1/*3 were similar, as were those for *2/*2 and *2/*3. (Rudberg et al., 2008) showed the effect of CYP2C19*17 on the concentration of escitalopram, and patients with CYP2C19 *17/*17 alleles showed a 42% reduction in concentration. In our study, data for patients with CYP2C19*17 could not be collected because *17 was detected at a low frequency in the Chinese population, and CYP2C19 *1 is the most common allele, followed by *2 and *3 (Chen et al., 2008; Zhou et al., 2009; Tang et al., 2013). We did not consider CYP2D6 and CYP3A4 as covariates because their genetic variation has not been shown to significantly affect serum levels of escitalopram.
We found that sex was not an important factor affecting escitalopram CL/F, although a previous study with a small number of subjects suggested that CL/F of citalopram is higher in men than women (Sidhu et al., 1997). However, sex has not been found to exert a clinically significant effect on pharmacokinetics in healthy volunteers (Søgaard et al., 2005; Dhillon et al., 2006), and (Akil et al., 2016) reported that sex had no effect on escitalopram CL/F. We observed no weight-related difference in escitalopram clearance, although the influence of weight and BMI has been reported previously (Jin et al., 2010; Akil et al., 2016). This may have been caused by incomplete demographic information for some patients enrolled in our study.
Liver and kidney functions affect the metabolism and excretion of drugs. For patients receiving escitalopram with hepatic impairment, the estimated mean area under the curve (AUC) values were 51 and 69% higher for patients with mild and moderate hepatic impairment compared with healthy individuals (Areberg et al., 2006). Although all subjects in the study tolerated the treatment well and no serious adverse events were reported, careful monitoring and dose adjustment during long-term therapy are suggested. There is no conclusive evidence for the role of escitalopram in patients with depression and renal failure; however, pharmacokinetic analysis of citalopram in patients with renal insufficiency revealed that t1/2 increased by 35% and renal clearance decreased by 40% (Joffe et al., 1998). Therefore, caution is recommended in such patients when using escitalopram. The data included a small number of patients with liver and kidney impairment; thus, evaluation of the effect of liver and kidney function in this PopPK analysis was limited.
None of the co-ingested drugs interacted pharmacokinetically with escitalopram in our study. (Malling et al., 2005) found that co-administration with cimetidine or omeprazole caused a moderate increase in exposure and t1/2, and omeprazole and esomeprazole had a wider effect on escitalopram than sertraline and citalopram had (Gjestad et al., 2015). Proton pump inhibitors are predominantly cleared by CYP2C19. Combination of escitalopram with drugs that are also metabolized by CYP2C19 may produce competitive inhibition between two CYP2C19 substrates. However, there were perhaps only seven of our patients treated with combined escitalopram and omeprazole, and no effect of omeprazole on escitalopram was found. Adjunctive treatment with fluvoxamine significantly increases escitalopram concentration (Yasui-Furukori et al., 2016), but there was no co-administration of fluvoxamine in our patients.
The FDA-recommended initial dose of escitalopram is 10 mg qd in adult patients, and 20 mg qd is the maximum dose. Simulation in our study reveals that the standard 5 mg/day regimen in EM and IM patients may lead to trough concentrations below the therapeutic target of 15 ng/ml, with a risk of suboptimal antidepressant efficacy. We also need to consider the effect of different CYP2C19 phenotypes. The Clinical Pharmacogenetics Implementation Consortium guidelines provide escitalopram dosing recommendations for different CYP2C19 genotypes (Hicks et al., 2015). Despite these guidelines being based on studies on the Caucasian population, they may also be suitable for the Chinese adult population. Consistent with our simulation results, EM or IM patients should initiate therapy with the recommended starting dose and maintenance dose up to 20 mg/day. Although IM patients may have elevated serum concentrations of escitalopram, there is little difference compared to EM patients. For PM patients with lower clearance and higher drug serum levels, the starting dose should be reduced by 50% (5 mg/day) and the maximum maintenance dose is 10 mg/day, or selecting drugs not predominantly metabolized by CYP2C19. Simulation results showed that the steady-state trough concentration was within the therapeutic window at a daily dose of 15 mg, but there is a risk of exceeding 80 ng/ml; thus, the maximum dose of 10 mg is recommended for PM patients, which is consistent with the guidelines. When escitalopram does not reach the target clinical efficacy, an increase in dose to 15 mg can be considered, but blood concentrations and adverse effects should be closely monitored.
Older patients are a special population. Although a single-dose clinical study confirmed that the pharmacokinetics of escitalopram were similar between young and older patients, t1/2 and AUC were ∼50% higher than in patients aged 18–35 years. Our study suggests that a daily dose of 10 mg escitalopram gives approximately the same steady-state serum levels in older individuals as a dose of 15 mg in adolescents, and that this is due to the reduced rates of metabolism in the former. Long-term excessive exposure in older people can lead to an increased rate of bradycardia (Barak et al., 2003), falls and fragility fractures (Gorgas et al., 2021); therefore, the starting and maintenance doses need to be fully considered and adjusted by genetic testing and therapeutic drug monitoring. Especially for older PM individuals, trough concentrations are higher than the minimum toxic concentration (80 ng/ml) with 15 or 20 mg/day. The FDA recommends 10 mg as the maximum daily dose for older patients, which implies that referring to the therapeutic range for adults may result in overdose and lead to a high risk of adverse effects. According to the results of the simulation, the therapeutic window on the AGNP guidelines does not extrapolate to people aged ≥65 years and needs to be reformulated. However, it requires to be validated in a large number of clinical trials. The results of the current study can provide a reference for future research.
The FDA approved escitalopram in 2009 for the acute and maintenance treatment of adolescents with major depressive disorder aged 12–17 years. The maximum recommended daily dose for adolescents was 20 mg, which is the same as for adults. Escitalopram was found to be efficacious and well-tolerated in the adolescent population with major depressive disorder when given at a daily dose of 10–20 mg in two clinical trials (Emslie et al., 2009; Findling et al., 2013). The pharmacokinetic differences showed no clinical significance in adolescents compared with adult healthy individuals (Rao, 2007). Furthermore, although the mean t1/2 of escitalopram is shorter in adolescents, there are no differences in maximum concentration and AUC (Bareggi et al., 2007), hence the dose regimen was not affected. Our simulation results in adolescents were mostly consistent with those in adults and not significantly influenced by CYP2C19 genotype which was evidenced by serum blood concentrations within the therapeutic window at 15 mg/day and 20 mg/day for PM subjects. However, the efficacy and tolerance needed further investigation. Nevertheless, the risk of manic conversion during antidepressant treatment is highest in patients aged 10–14 years (Martin et al., 2004). Monitoring for suicidality during pharmacotherapy is necessary, and the frequency of monitoring based on each patient’s particular risk.
There were several limitations that need to be considered. First, the sample size was small and most of the samples were at trough concentrations, which did not sufficiently reflect the absorption and distribution characteristics of escitalopram. Second, the small number of PM patients may have been related to the low frequency of mutations, which needs to be confirmed in further studies. Third, there were few cases of combined medication in our analysis, so it will be necessary to explore other drugs that might affect the pharmacokinetics of escitalopram. Notwithstanding, we obtained systematic data to develop a PopPK model in Chinese psychiatric patients for the first time and performed a simulation. These results provide guidance for making a better therapeutic decision on escitalopram dosing regimen to minimize excessively high exposure to this selective serotonin reuptake inhibitor through incorporating age and CYP2C19 genotype into this assessment.
Conclusion
Our PopPK model demonstrated the influence of age and CYP2C19 phenotype on escitalopram pharmacokinetics in Chinese psychiatric patients. Using a one-compartment model with first-order absorption and elimination achieved good predictive power. According to the simulation results, in contrast to patients ≥18 years, the daily dose for adolescents with PM might be as high as 15 mg or 20 mg and the current therapeutic window of escitalopram might not be suitable for older patients, both of which required further study. Our results emphasized the necessity for genetic testing and therapeutic drug monitoring during treatment for optimal dosage regimen individualization.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by The Affiliated Brain Hospital of Guangzhou Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
All authors contributed to the article. XN, HL, and MZ designed the study. WK, YY, and ZZ collected the data. SL, TX, SS, and XL analyzed the data and established the population pharmacokinetics model. SL wrote the manuscript and DS and YW revised it.
Funding
This study was supported by Science and Technology Plan Project of Guangdong Province (2019B030316001), Guangzhou Municipal Key Discipline in Medicine (2021–2023), and special fund for pharmaceutical research of Guangdong Hospital Association (2021YXMS03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to all participants. And thank International Science Editing for editing this manuscript.
References
Akil, A., Bies, R. R., Pollock, B. G., Avramopoulos, D., Devanand, D. P., Mintzer, J. E., et al. (2016). A population pharmacokinetic model for R- and S-citalopram and desmethylcitalopram in Alzheimer's disease patients with agitation. J. Pharmacokinet. Pharmacodyn. 43(1), 99–109. doi:10.1007/s10928-015-9457-6
Areberg, J., Christophersen, J. S., Poulsen, M. N., Larsen, F., and Molz, K. H. (2006). The pharmacokinetics of escitalopram in patients with hepatic impairment. Aaps J. 8(1), E14–E19. doi:10.1208/aapsj080102
Barak, Y., Swartz, M., Levy, D., and Weizman, R. (2003). Age-related differences in the side effect profile of citalopram. Prog. Neuropsychopharmacol. Biol. Psychiatry 27(3), 545–548. doi:10.1016/s0278-5846(03)00041-1
Bareggi, S. R., Mundo, E., Dell'Osso, B., and Altamura, A. C. (2007). The use of escitalopram beyond major depression: Pharmacological aspects, efficacy and tolerability in anxiety disorders. Expert Opin. Drug Metab. Toxicol. 3(5), 741–753. doi:10.1517/17425255.3.5.741
Bies, R. R., Feng, Y., Lotrich, F. E., Kirshner, M. A., Roose, S., Kupfer, D. J., et al. (2004). Utility of sparse concentration sampling for citalopram in elderly clinical trial subjects. J. Clin. Pharmacol. 44(12), 1352–1359. doi:10.1177/0091270004269647
Brouwer, J., Nijenhuis, M., Soree, B., Guchelaar, H. J., Swen, J. J., van Schaik, R. H. N., et al. (2021). Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2C19 and CYP2D6 and SSRIs. Eur. J. Hum. Genet., 1–7. doi:10.1038/s41431-021-01004-7
Burke, W. J., Gergel, I., and Bose, A. (2002). Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. J. Clin. Psychiatry 63(4), 331–336. doi:10.4088/jcp.v63n0410
Chang, M., Tybring, G., Dahl, M. L., and Lindh, J. D. (2014). Impact of cytochrome P450 2C19 polymorphisms on citalopram/escitalopram exposure: A systematic review and meta-analysis. Clin. Pharmacokinet. 53(9), 801–811. doi:10.1007/s40262-014-0162-1
Chen, B., Xu, Y., Jiang, T., Feng, R., Sun, J., Zhang, W., et al. (2013). Estimation of CYP2D6*10 genotypes on citalopram disposition in Chinese subjects by population pharmacokinetic assay. J. Clin. Pharm. Ther. 38(6), 504–511. doi:10.1111/jcpt.12029
Chen, C., Yang, T., Li, X., Ma, L., Liu, Y., Zhou, Y., et al. (2019). Population pharmacokinetics of voriconazole in Chinese patients with hematopoietic stem cell transplantation. Eur. J. Drug Metab. Pharmacokinet. 44(5), 659–668. doi:10.1007/s13318-019-00556-w
Chen, L., Qin, S., Xie, J., Tang, J., Yang, L., Shen, W., et al. (2008). Genetic polymorphism analysis of CYP2C19 in Chinese Han populations from different geographic areas of mainland China. Pharmacogenomics 9(6), 691–702. doi:10.2217/14622416.9.6.691
Chung, H., Kim, A., Lim, K. S., Park, S. I., Yu, K. S., Yoon, S. H., et al. (2017). Pharmacokinetics and effect on the corrected QT interval of single-dose escitalopram in healthy elderly compared with younger adults. Int. Clin. Psychopharmacol. 32(1), 20–26. doi:10.1097/yic.0000000000000149
Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., et al. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 391(10128), 1357–1366. doi:10.1016/s0140-6736(17)32802-7
Cipriani, A., Santilli, C., Furukawa, T. A., Signoretti, A., Nakagawa, A., McGuire, H., et al. (2009). Escitalopram versus other antidepressive agents for depression. Cochrane Database Syst. Rev. 2, Cd006532. doi:10.1002/14651858.CD006532.pub2
Courlet, P., Guidi, M., Glatard, A., Alves Saldanha, S., Cavassini, M., Buclin, T., et al. (2019). Escitalopram population pharmacokinetics in people living with human immunodeficiency virus and in the psychiatric population: Drug-drug interactions and probability of target attainment. Br. J. Clin. Pharmacol. 85(9), 2022–2032. doi:10.1111/bcp.13994
Davidson, J. R., Bose, A., Korotzer, A., and Zheng, H. (2004). Escitalopram in the treatment of generalized anxiety disorder: Double-blind, placebo controlled, flexible-dose study. Depress. Anxiety 19(4), 234–240. doi:10.1002/da.10146
Dhillon, S., Scott, L. J., and Plosker, G. L. (2006). Escitalopram: A review of its use in the management of anxiety disorders. CNS Drugs 20(9), 763–790. doi:10.2165/00023210-200620090-00010
Dolder, C., Nelson, M., and Stump, A. (2010). Pharmacological and clinical profile of newer antidepressants: Implications for the treatment of elderly patients. Drugs Aging 27(8), 625–640. doi:10.2165/11537140-000000000-00000
Dorji, P. W., Tshering, G., and Na-Bangchang, K. (2019). CYP2C9, CYP2C19, CYP2D6 and CYP3A5 polymorphisms in south-east and East Asian populations: A systematic review. J. Clin. Pharm. Ther. 44(4), 508–524. doi:10.1111/jcpt.12835
Emslie, G. J., Ventura, D., Korotzer, A., and Tourkodimitris, S. (2009). Escitalopram in the treatment of adolescent depression: A randomized placebo-controlled multisite trial. J. Am. Acad. Child. Adolesc. Psychiatry 48(7), 721–729. doi:10.1097/CHI.0b013e3181a2b304
Findling, R. L., Robb, A., and Bose, A. (2013). Escitalopram in the treatment of adolescent depression: A randomized, double-blind, placebo-controlled extension trial. J. Child. Adolesc. Psychopharmacol. 23(7), 468–480. doi:10.1089/cap.2012.0023
Fredericson Overø, K., Toft, B., Christophersen, L., and Gylding-Sabroe, J. P. (1985). Kinetics of citalopram in elderly patients. Psychopharmacol. Berl. 86(3), 253–257. doi:10.1007/bf00432209
Gjestad, C., Westin, A. A., Skogvoll, E., and Spigset, O. (2015). Effect of proton pump inhibitors on the serum concentrations of the selective serotonin reuptake inhibitors citalopram, escitalopram, and sertraline. Ther. Drug Monit. 37(1), 90–97. doi:10.1097/ftd.0000000000000101
Gorgas, M. Q., Torres, F., Vives, R., Lopez-Rico, I., Capella, D., Pontes, C., et al. (2021). Effects of selective serotonin reuptake inhibitors and other antidepressant drugs on the risk of hip fracture: A case-control study in an elderly mediterranean population. Eur. J. Hosp. Pharm. 28(1), 28–32. doi:10.1136/ejhpharm-2019-001893
Gutierrez, M.M., Rosenberg, J., and Abramowitz, W. (2003). An evaluation of the potential for pharmacokinetic interaction between escitalopram and the cytochrome P450 3A4 inhibitor ritonavir. Clin. Ther. 25(4), 1200–1210. doi:10.1016/s0149-2918(03)80076-0
Hazell, P. (2021). Updates in treatment of depression in children and adolescents. Curr. Opin. Psychiatry 34(6), 593–599. doi:10.1097/yco.0000000000000749
Hicks, J. K., Bishop, J. R., Sangkuhl, K., Müller, D. J., Ji, Y., Leckband, S. G., et al. (2015). Clinical Pharmacogenetics implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 98(2), 127–134. doi:10.1002/cpt.147
Hiemke, C., Bergemann, N., Clement, H. W., Conca, A., Deckert, J., Domschke, K., et al. (2018). Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: Update 2017. Pharmacopsychiatry 51(1-02), e1. doi:10.1055/s-0037-1600991
Huang, X., Li, C., Li, C., Li, Z., Li, X., Liao, J., et al. (2021). CYP2C19 genotyping may provide a better treatment strategy when administering escitalopram in Chinese population. Front. Pharmacol. 12, 730461. doi:10.3389/fphar.2021.730461
Jin, Y., Pollock, B. G., Frank, E., Cassano, G. B., Rucci, P., Müller, D. J., et al. (2010). Effect of age, weight, and CYP2C19 genotype on escitalopram exposure. J. Clin. Pharmacol. 50(1), 62–72. doi:10.1177/0091270009337946
Joffe, P., Larsen, F. S., Pedersen, V., Ring-Larsen, H., Aaes-Jørgensen, T., Sidhu, J., et al. (1998). Single-dose pharmacokinetics of citalopram in patients with moderate renal insufficiency or hepatic cirrhosis compared with healthy subjects. Eur. J. Clin. Pharmacol. 54(3), 237–242. doi:10.1007/s002280050452
Jukić, M. M., Haslemo, T., Molden, E., and Ingelman-Sundberg, M. (2018). Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: A retrospective study based on 2, 087 patients. Am. J. Psychiatry 175(5), 463–470. doi:10.1176/appi.ajp.2017.17050550
Kim, Y., Kim, A., and Chung, J. Y. (2021). Population pharmacokinetic/pharmacodynamic modeling of delayed effect of escitalopram-induced QT prolongation. J. Affect. Disord. 285, 120–126. doi:10.1016/j.jad.2021.02.048
Malling, D., Poulsen, M. N., and Søgaard, B. (2005). The effect of cimetidine or omeprazole on the pharmacokinetics of escitalopram in healthy subjects. Br. J. Clin. Pharmacol. 60(3), 287–290. doi:10.1111/j.1365-2125.2005.02423.x
Martin, A., Young, C., Leckman, J. F., Mukonoweshuro, C., Rosenheck, R., Leslie, D., et al. (2004). Age effects on antidepressant-induced manic conversion. Arch. Pediatr. Adolesc. Med. 158(8), 773–780. doi:10.1001/archpedi.158.8.773
Montejo, A. L., Deakin, J. F., Gaillard, R., Harmer, C., Meyniel, F., Jabourian, A., et al. (2015). Better sexual acceptability of agomelatine (25 and 50 mg) compared to escitalopram (20 mg) in healthy volunteers. A 9-week, placebo-controlled study using the PRSexDQ scale. J. Psychopharmacol. 29(10), 1119–1128. doi:10.1177/0269881115599385
Nilausen, D., Zuiker, R. G., and van Gerven, J. (2011). The perception and pharmacokinetics of a 20-mg dose of escitalopram orodispersible tablets in a relative bioavailability study in healthy men. Clin. Ther. 33(10), 1492–1502. doi:10.1016/j.clinthera.2011.09.012
Oliveira, P., Ribeiro, J., Donato, H., and Madeira, N. (2017). Smoking and antidepressants pharmacokinetics: A systematic review. Ann. Gen. Psychiatry 16, 17. doi:10.1186/s12991-017-0140-8
Pastoor, D., and Gobburu, J. (2014). Clinical pharmacology review of escitalopram for the treatment of depression. Expert Opin. Drug Metab. Toxicol. 10(1), 121–128. doi:10.1517/17425255.2014.863873
Pollock, B. G., Perel, J. M., Kirshner, M., Altieri, L. P., Yeager, A. L., Reynolds, C. F., et al. (1991). S-mephenytoin 4-hydroxylation in older Americans. Eur. J. Clin. Pharmacol. 40(6), 609–611. doi:10.1007/bf00279979
Rao, N. (2007). The clinical pharmacokinetics of escitalopram. Clin. Pharmacokinet. 46(4), 281–290. doi:10.2165/00003088-200746040-00002
Rapaport, M. H., Bose, A., and Zheng, H. (2004). Escitalopram continuation treatment prevents relapse of depressive episodes. J. Clin. Psychiatry 65(1), 44–49. doi:10.4088/jcp.v65n0107
Rudberg, I., Mohebi, B., Hermann, M., Refsum, H., and Molden, E. (2008). Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clin. Pharmacol. Ther. 83(2), 322–327. doi:10.1038/sj.clpt.6100291
Sanchez, C., Reines, E. H., and Montgomery, S. A. (2014). A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike? Int. Clin. Psychopharmacol. 29(4), 185–196. doi:10.1097/yic.0000000000000023
Scherf-Clavel, M., Deckert, J., Menke, A., and Unterecker, S. (2019). Smoking is associated with lower dose-corrected serum concentrations of escitalopram. J. Clin. Psychopharmacol. 39(5), 485–488. doi:10.1097/jcp.0000000000001080
Siccardi, M., Marzolini, C., Seden, K., Almond, L., Kirov, A., Khoo, S., et al. (2013). Prediction of drug-drug interactions between various antidepressants and efavirenz or boosted protease inhibitors using a physiologically based pharmacokinetic modelling approach. Clin. Pharmacokinet 52(7), 583–592. doi:10.1007/s40262-013-0056-7
Sidhu, J., Priskorn, M., Poulsen, M., Segonzac, A., Grollier, G., and Larsen, F. (1997). Steady-state pharmacokinetics of the enantiomers of citalopram and its metabolites in humans. Chirality 9(7), 686–692. doi:10.1002/(SICI)1520-636X(1997)9:7<686::AID-CHIR9>3.0.CO;2-5
Søgaard, B., Mengel, H., Rao, N., and Larsen, F. (2005). The pharmacokinetics of escitalopram after oral and intravenous administration of single and multiple doses to healthy subjects. J. Clin. Pharmacol. 45(12), 1400–1406. doi:10.1177/0091270005280860
Stahl, S. M., Gergel, I., and Li, D. (2003). Escitalopram in the treatment of panic disorder: A randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 64(11), 1322–1327. doi:10.4088/jcp.v64n1107
Tang, X. F., Wang, J., Zhang, J. H., Meng, X. M., Xu, B., Qiao, S. B., et al. (2013). Effect of the CYP2C19 2 and 3 genotypes, ABCB1 C3435T and PON1 Q192R alleles on the pharmacodynamics and adverse clinical events of clopidogrel in Chinese People after percutaneous coronary intervention. Eur. J. Clin. Pharmacol. 69(5), 1103–1112. doi:10.1007/s00228-012-1446-8
van Gorp, F., Duffull, S., Hackett, L. P., and Isbister, G. K. (2012). Population pharmacokinetics and pharmacodynamics of escitalopram in overdose and the effect of activated charcoal. Br. J. Clin. Pharmacol. 73(3), 402–410. doi:10.1111/j.1365-2125.2011.04091.x
Wade, A., Michael Lemming, O., and Bang Hedegaard, K. (2002). Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. Int. Clin. Psychopharmacol. 17(3), 95–102. doi:10.1097/00004850-200205000-00001
Weisskopf, E., Guidi, M., Fischer, C. J., Bickle Graz, M., Beaufils, E., Nguyen, K. A., et al. (2020). A population pharmacokinetic model for escitalopram and its major metabolite in depressive patients during the perinatal period: Prediction of infant drug exposure through breast milk. Br. J. Clin. Pharmacol. 86(8), 1642–1653. doi:10.1111/bcp.14278
Xiao, T., Wang, Z., Li, G., Huang, S., Zhu, X., Liu, S., et al. (2021). What to do about missed doses? A retrospective study of olanzapine in the elderly. Drug Des. devel. Ther. 15, 3411–3423. doi:10.2147/dddt.S316110
Yang, L. P., and Scott, L. J. (2010). Escitalopram: In the treatment of major depressive disorder in adolescent patients. Paediatr. Drugs 12(3), 155–163. doi:10.2165/11204340-000000000-00000
Yasui-Furukori, N., Tsuchimine, S., Kubo, K., Ishioka, M., Nakamura, K., Inoue, Y., et al. (2016). The effects of fluvoxamine on the steady-state plasma concentrations of escitalopram and desmethylescitalopram in depressed Japanese patients. Ther. Drug Monit. 38(4), 483–486. doi:10.1097/ftd.0000000000000303
Keywords: CYP2C19 genotype, elderly, escitalopam, adolescent, population pharmacokinetics
Citation: Liu S, Xiao T, Huang S, Li X, Kong W, Yang Y, Zhang Z, Ni X, Lu H, Zhang M, Shang D and Wen Y (2022) Population pharmacokinetics model for escitalopram in Chinese psychiatric patients: effect of CYP2C19 and age. Front. Pharmacol. 13:964758. doi: 10.3389/fphar.2022.964758
Received: 09 June 2022; Accepted: 28 June 2022;
Published: 18 July 2022.
Edited by:
Miao Yan, Central South University, ChinaReviewed by:
Akhmad Kharis Nugroho, Faculty of Pharmacy Universitas Gadjah Mada, IndonesiaHwi-Yeol Yun, Chungnam National University, South Korea
Copyright © 2022 Liu, Xiao, Huang, Li, Kong, Yang, Zhang, Ni, Lu, Zhang, Shang and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dewei Shang, c2hhbmdfZGV3ZWlAMTYzLmNvbQ==; Yuguan Wen, d2VueXVndWFuZGVkZUAxNjMuY29t
 Shujing Liu
Shujing Liu Tao Xiao
Tao Xiao Shanqing Huang
Shanqing Huang Xiaolin Li
Xiaolin Li Wan Kong
Wan Kong Ye Yang
Ye Yang Zi Zhang1,2
Zi Zhang1,2 Haoyang Lu
Haoyang Lu Dewei Shang
Dewei Shang Yuguan Wen
Yuguan Wen