- 1School of Pharmacy, Hunan University of Chinese Medicine, Changsha, China
- 2Center for Standardization and Functional Engineering of Traditional Chinese Medicine in Hunan Province, Changsha, China
- 3Key Laboratory of Modern Research of TCM, Education Department of Hunan Province, Changsha, China
Traditional Chinese medicine (TCM) has attracted a great deal of attention in the treatment of cerebral ischemia is credited with the remarkable neuroprotective effects. However, the imperfect functional mechanism of TCM is a major obstacle to their application. Many studies have been conducted to illustrate the pathophysiology of post-ischemic cerebral ischemia by elucidating the neuronal cell death pathway. Meanwhile, a new type of cell death, ferroptosis, is gradually being recognized in various diseases and is becoming a new pathway of therapeutic intervention strategy to solve many health problems. Especially since ferroptosis has been found to be closely involved into the pathogenesis of cerebral ischemia, it has been considered as a key target in the treatment of cerebral ischemia. Therefore, this paper reviews the latest research findings about the treatment of cerebral ischemia with TCM focused on ferroptosis as a target. Also, in order to explores the possibility of a new approach to treat cerebral ischemia with TCM, we discusses the correlation between ferroptosis and other cell death pathways such as apoptosis and autophagy, which would provide references for the following researches.
Introduction
Cerebral ischemia is a major cerebrovascular disease with high disability and mortality rates, public health and life safety are seriously compromised (Feigin et al., 2015). During the course of cerebral ischemia, a series pathological reactions are triggered, including oxidative stress (Meyers et al., 2012), inflammatory response (Krishnamurthi et al., 2013), excitatory amino acid toxicity (Brott and Bogousslavsky, 2000) and calcium excess (Adams et al., 2007), leading to widespread neuronal death and neurological damage ultimately. Therefore, protecting neurons from damage becomes a neuroprotective strategy (Moskowitz et al., 2010). Currently, multiple programmed cell death (PCD) have been identified to be involved into neuronal death, and it is possible to protect against cerebral ischemic injury by inhibiting PCD-related signaling pathways (Hribljan et al., 2019; Radak et al., 2017; Yu et al., 2019; Liu et al., 2020). It has been established that ferroptosis is a new type of PCD, and the inhibition of ferroptosis could reduce cerebral ischemic injury (Yang et al., 2020; Speer et al., 2013; Li et al., 2018). Naturally, ferroptosis is considered to be a critical therapeutic target for cerebral ischemia.
In TCM theory, Qi-deficiency along with blood-stasis syndrome are the main pathogenesis of cerebral ischemia that is treated by invigorating Qi and removing blood circulation. The classic TCM formula commonly used in cerebral ischemia treatment, such as Buyang Huanwu Decoction (Chen et al., 2020), QiShenYiQi (Wang et al., 2020) and Xinglou Chengqi Decoction (Chen et al., 2017), all conform to the treatment characteristics. With increasing studies on the anti-cerebral ischemia effect of TCM, the neuroprotective effects of TCM have attracted much attention benefit from the remarkable neuroprotective effects (Xia et al., 2014; Tian et al., 2019), so several studies have try to revealed the mechanism of TCM to interven incerebral ischemia via ferroptosis.
This review focuses on ferroptosis, summarizes the existing TCM literature on the regulation of cerebral ischemia by targeting the ferroptosis pathway, and discusses its relation to other cell death pathway. In particular, the importance of ferroptosis in mediating cerebral ischemia is emphasized to further explore specific targets of cerebral ischemia by TCM, which would serve as a reference for potential applications of TCM.
Relevant mechanisms of ferroptosis in cerebral ischemia
Ferroptosis is defined as iron-dependent regulatory programmed cell death caused by decreased activity of glutathione peroxidase 4 (GPX4), accumulation of reactive oxygen species (ROS) and lipid peroxides (Yang and Stockwell, 2015). Iron deposition in the basal ganglia, globus pallidus and white matter regions during critical ischemia/anoxic injury and subsequent resuscitation was reported as early as 1988 (Dietrich and Bradley, 1988). In addition, typical ferroptosis phenomena such as the increased lipid peroxide and the decreased glutathione (GSH) levels were observed after middle cerebral artery occlusion (MCAO) in mice (Ahmad et al., 2014). Furthermore, ferroptosis inhibitors (liproxstatin-1 or ferrostatin-1) were shown to effectively inhibit ischemia-reperfusion injury (Tuo et al., 2017). Thus, targeting ferroptosis have been proven to be an essential neuroprotective strategy against cerebral ischemia.
Up to date, it is widely recognized that there are three related mechanisms for the control of cerebral ischemia by targeting ferroptosis as follows. 1) Iron dyhomeostasis. During cerebral ischemia, large amounts of iron are released from transferrin (Lipscomb et al., 1998), inducing iron, transferrin and transferrin receptor (TFR) accumulates intracellularly (Park et al., 2011; DeGregorio-Rocasolano et al., 2019). Clinical studies have shown that increased hepcidin expression downregulates the iron efflux in brain cells by inducing ferroportin1 (FPN1), leading to iron overload and ferroptosis (Kong et al., 2019; Petrova et al., 2016). 2) Accumulation of lipid peroxide and ROS. In vitro model of cerebral ischemia, increases in acyl-coa synthetase long-chain family member 4 (ACSL4) and 12/15-lipoxygenase (12/15-LOX) have been shown to increase polyunsaturated fatty acids (PUFAs) such as arachidonic acid (AA) and adrenal acid (AdA). It is believed to promote the expression of PUFAs, which in turn stimulates lipid peroxidation and neuronal cell death (Yigitkanli et al., 2017; Cui et al., 2021). In addition, thrombin, a serine protease, instigates ferroptotic signaling by promoting the mobilization and subsequent esterification of AA via the regulation of ACSL4 (Tuo et al., 2022). Iron is also supposed to degrade H2O2 catalytically via the Fenton reaction to produce hydroxyl radicals, leading to the accumulation of ROS. 3) Amino acid axillary toxicity. Glutamate is a major excitatory neurotransmitter in the central nervous system, and its excessive release could cause neurotoxicity. After ischemia, the low expressions of glutamate transporter is observed and the time-dependent increasing of extracellular glutamate inhibits the cystine/glutamate antiporter (system Xc-), which cause the abnormal lipid peroxidation and ferroptosis (Yang et al., 2012; Krzyżanowska, et al., 2017). It has also been reported that HIF 1α decreases system Xc-expression, increases glutamate leakage in ischemic brain injury, and the activation of N-methyl D aspartate receptor (NMDAR) increases high extracellular glutamate concentrations and iron uptake in nerves (Krzyzanpwska et al., 2017; Hsieh et al., 2017; Cheah et al., 2006). The system Xc-is a heterodimeric amino acid transporter comprising a light chain xCT (SLC7A11) and a heavy chain 4F2hc (SLC3A2), which provides the raw meterials needed for GSH synthesis. Furthermore, the levels of SLC7A11 and GPX4 were found to be decreased significantly in MCAO rats compared to sham surgery (Lan et al., 2020), GPX4 inactivation has been attributed to GSH depletion during ischemia-reperfusion (Li et al., 2018), and lipid peroxide-mediated cell death in neurons (Seiler et al., 2008). In addition, tumor protein p53 (TP53, or p53) mediates (either promote or inhibit) ferroptosis via distinct mechanisms (Liu and Gu, 2021), such as p53 can increase the ferroptosis rate via inhibiting SLC7A11 expression (Jiang et al., 2015). Ferritin upregulation can prevent ferroptosis by mediating p53 and SLC7A11 expression in MCAO-induced hippocampal neuronal death (Chen et al., 2021). Additionally, the overexpression or knockdown of p53 significantly modulated GPX4 expression in RBMVECs exposed to the injury induced by OGD combined with hyperglycemic treatment (Chen et al., 2021).
Owning to the description of above, ferroptosis may be induced during cerebral ischemia by pathological conditions such as iron imbalance, lipid peroxidation, aggregation of ROS, and axillary toxicity of amino acids. The specific mechanisms are shown in Figure 1.

FIGURE 1. The current mechanisms of ferroptosis in cerebral ischemia is mainly attributed to: (1) Iron dyhomeostasis leading to iron overload and ferroptosis. (2) Metabolic imbalances of lipids aggravate accumulation of lipid peroxide and ROS. (3) Amino acid axillary toxicity causes system xc-is impaired, which inhibits cystine-glutamate exchange and decreases the generation of the antioxidant GSH and GPX4.
Traditional Chinese medicine targets interventional ferroptosis to alleviate cerebral ischemia
TCM has play a remarkable effect in the treatment of cancer and other serious diseases via ferroptosis signal pathway (Table 1). It is also important to explore the relationship between TCM and ferroptosis in the clinical treatment of cerebral ischemia and drug development.
Active ingredients in traditional chinese medicine
Galangin is a flavonol compound extracted mainly from alpinia officinarum hance and has multiple anti-tumor effects (Feng et al., 2022). Galangin reduced brain cell death in gerbils during cerebral ischemia-reperfusion (I/R), improved learning and memory ability apparently and increased SLC7A11 and GPX4 expression (Guan et al., 2021). Carthamin yellow is a flavonoid extracted from safflower, which is widely used for promoting blood circulation to remove blood stasis and alleviating pain in china (Lu et al., 2019). (Guo et al., 2020) found that carthamin yellow protected rats from ischemic injury by alleviating inflammation and the ferroptosis protection, also it was observed that the inhibition of Fe2+ and ROS accumulation and the reversion protein expression levels of ACSL4, TFR1, GPX4 and FTH1 in the brain after the treatment of carthamin yellow (Guan et al., 2021). Carvacrol is a monoterpenoid found in botanical drugs ang has multiple pharmacological effects (Silva et al., 2018). It has been proven to decrease cell death by increasing GPX4 expression and ferroptosis, realized neuroprotective effects of I/R injury in hippocampal neurons ultimately (Guan et al., 2019). Tanshinone IIA is the main fat-soluble component of Salvia.
Miltiorrhiza bunge (Guan et al., 2021), can inhibit ferroptosis in a cerebral ischemic cell model by regulating iron homeostasis, ROS accumulation and liposomal peroxidation (Xu et al., 2021).
Traditional chinese medicine formula and acupunctur
Naotaifang, a TCM formula consisting of four botanical drugs, which can inhibit the development of ferroptosis by modulating the GPX4-lipid metabolic pathway in the MCAO model (He et al., 2020). In addition, Naotaifang extract can decrease the expression of TFR1 and DMT1 in MCAO rats by improving neurobehavioral scores, meanwhile the accumulation of ROS, MDA and iron were suppressed, and then regulates the expression of ferroptosis markers such as SLC7A11, GPX4, and GSH. It was confirmed that there was a protective effect against ferroptosis (Lan et al., 2020). Furthermore, Naotaifang inhibits neuronal iron absorption by suppressing TFR1 expression via the regulation of HSF1/HSPB1 signal pathway, a series of events such as the increasing expression of FTH1 and ferritin iron stores were detected, and then changes the steady-state balance of iron metabolism which would protect against ROS produced by the Fenton reaction and subsequent peroxidation of lipid-induced neuronal hyperferroptosis in ischemic stroke ultimately (Rao et al., 2021). The acupuncture has been used in China for more than 3,000 years, the modern study on the gave acupuncture stimulation to MCAO rats for seven consecutive days found that the acupuncture group decreased MDA and total iron levels compared to the MCAO group, while superoxide dismutase, GSH and GPX4 gene, FTH1, and FTH1 gene increased, TF, TF gene, TFR1, and TFR1 gene decreased in the MCAO rat model, suggesting that acupuncture exerts a protective effect in the MCAO rat model by modulating oxidative stress and iron-related proteins in the way of ferroptosis pathway. Experiment results have demonstrated that TCM prevents cerebral ischemia by regulating ferroptosis, but the lack of substantial evidence from human samples is the obstacle to our understanding of its potential clinical applications (Li et al., 2021).
The crosstalk between ferroptosis and multiple cell death pathways
Cerebral ischemia, a complex cerebrovascular disorder, is regulated by multiple cell death pathways (Tuo et al., 2022). However, most mechanism studies tend to focus on a single cell death pathway. The individual cell death pathways do not appear to exist independently but have complex biological correlations and play a pivotal role in jointly maintaining the internal balance of various pathological conditions. Bold supposes that the blocking of PCD pathway could be a novel target for the prevention and treatment of various human diseases (Bedoui et al., 2020), and there are several results have been obtained as powerful evidence (Meng et al., 2021). Here, we focus on ferroptosis and discuss the relationship with other PCD to elucidate the crosstalk between multiple PCD pathways, it would be a valuable reference for the potential prevention and treatment of cerebral ischemia.
Ferroptosis and apoptosis
The main pathway of neuronal injury after cerebral ischemia is apoptosis, as a classical cell death pathway, little is known about the potential interaction between ferroptosis and apoptosis in the disease of ischemic. Current studies often combine ferroptosis and apoptosis in anti-cancer therapy rather than cerebral ischemia (Fu et al., 2021; Ye et al., 2021; Zhang et al., 2021). For example, Meng et al. proposed a high-performance pyrite nanoenzyme can kill tumor cells in an anti-apoptotic manner by inducing ferroptosis (Meng et al., 2021); Bao et al. not only verified the self-made nanolongans can carry multiple targeted drugs to the goal sites, but also demonstrated that ferroptosis and apoptosis were both suppression when using a combination of co-targeted anti-cancer therapies (Bao et al., 2019). Logie et al. (2021) showed that cell death by apoptosis and ferroptosis induce different changes of kinase signaling in multiple myeloma cells (Logie et al., 2021). On the other hand, in a related study, apoptosis-dependent ferroptosis was found have occured in leukemia cells (Wang et al., 2020), suggesting a possible interdependence of the two PCD. Ferroptosis inducers also is sensitive to apoptosis by increasing the expression of PUMA, an apoptosis-inducing molecule (Hong et al., 2017). Furthermore, the combination of erastin (ferroptosis inducer) and TRAIL (apoptosis induce ligand) disrupted mitochondrial membrane potential (ΔΨm) effectively, subsequently promoting caspase activation (Lee et al., 2020). In addition, studies have shown that apoptosis/ferroptosis is ROS-dependent (Zhang et al., 2021), and they can be regulated by regulating p53 (Mao et al., 2018; Li et al., 2020). These findings indicate that a combination of ferroptosis and apoptosis is feasible, but the applicability and specific mechanisms of apoptosis and ferroptosis in the pathway of neuronal injury after cerebral ischemia need to be further investigated.
Ferroptosis and autophagy
Autophagy is a dynamic process that depends on the formation and maturation of specific membrane structures, playing a dual role in the regulation of cerebral ischemia. With the intensive researches, ferroptosis was suggested as a type of autophagy-dependent cell death pathway (Zhou et al., 2020). Autophagy is also thought to contribute to iron homeostasis and ROS production, ultimately causing ferroptosis in the cell experiments (Gao et al., 2016). Furthermore, regulatory factors of ferroptosis were found to be transported to lysosomes and exert their functions through autophagy (Zhou et al., 2019). Their molecular mechanisms include NCOA4 (nuclear receptor coactivator 4)-dependent degradation of ferritin by ferritinophagy (Hou et al., 2016) and the inhibition of SLC7A11 activity by the formation of BECN1-SLC7A11 protein complex (Kang et al., 2018). Furthermore, RAB7A, SQSTM1, HSP90, and p53 are also thought to induce ferroptosis by mediating different types of autophagy (Jiao et al., 2021; Liu and Gu, 2022). Recently, it has become clearly that the activation of ferroptosis depends on the induction of autophagy (Kang and Tang., 2017; Torii et al., 2016). For example, the RNA-binding protein ZFP36/TTP is known to protect against ferroptosis by regulating autophagy signaling pathways in hepatic astrocytes (Zhang et al., 2020). However, autophagy markers are not closely related to ferroptosis regulators in the early induction of ferroptosis (Li et al., 2021). Coincidentally, the inhibition of autophagy increases the susceptibility of glioblastoma stem cells by igniting ferroptosis (Buccarelli et al., 2018). In addition, cell death is caused by ferroptosis and autophagy after the treatment of breast cancer cells with siramesine and lapatinib independently (Ma et al., 2017). These complex results may be caused by the differences of disease types and experimental models. There is studies indicate that autophagy and ferroptosis may mediate their interaction to control cerebral ischemia (Liu et al., 2020), but the relationship between the two processes remains a cluster of unknowns for the further research.
Ferroptosis and pyroptosis and necroptosis
During cerebral ischemia, neuronal pyroptosis and necroptosis are extensively affected (Xu et al., 2018; Dong et al., 2018). Pyroptosis is thought to be executed by receptor-interacting protein kinase 3 (RIPK3) and a pseudokinase, mixed lineage kinase domain-like protein (MLKL). Previous studies have shown that excessive ROS levels induce gasdamine D (GSDMD)-mediated pyroptosis (Wang et al., 2020). When cecum ligation and puncture mice are treated with Fer-1, pyroapoptotic proteins are simultaneously regulated by GPX4 and PTGS2 (Cao et al., 2022). Furthermore, GPX4 can reduce lipid peroxidation and negatively regulate macrophage pyroapoptosis and polymicrobial sepsis in mice (Kang et al., 2018). Xu et al. also achieved dual induction of ferroptosis/pyroptosis in cancer therapy by aggregation of transferrin and ROS (Xu et al., 2021). Some findings confirmed that pyroptosis and ferroptosis occur in liver injury caused by microplastics (Mu et al., 2022), also found that NLRP3 inflammasome-dominated pyroptosis is involved in the induction of ferroptosis in type 2 diabetes (Chen et al., 2022). Conversely, (Wang et al., 2021), found that the histone deacetylase inhibitor xinostat induced cellular pyroptosis via the caspase-1-related pathway and ferroptosis via the GPX4-related pathway in TSCC cells (Wang et al., 2021). Wang J et al. found that pyroptosis and ferroptosis occur in early and late stages of chronic heart failure, respectively (Wang et al., 2020). These results add complexity and uncertainty to the interpretation of the crosstalk between pyroptosis and ferroptosis.
Necroptosis is a caspase-independent mode of cell death. A growing body of evidence suggests that both ferroptosis and necroptosis are involved. In vivo and in vitro model of neuronal cell death after stroke hemorrhage, the involvement of ferroptosis and necroptosis has been confirmed (Zille et al., 2017). Human neuroblastoma cell lines, a widely used model of Parkinson’s disease, treating with 1-methyl-4-phenylpyridium (MPP+), can induce necroptotic and nonapoptotic cell death, but the progress was been restrained by both treatment of necrostatin-1 and ferrostatin-1 (Ito et al., 2017). Proteomic analysis of the hippocampus revealed the activation of necroptosis and ferroptosis in a mouse model of chronic unpredictable mild stress-induced depression (Cao et al., 2021). The inhibition of mitochondrial complex I induced mitophagy-dependent ROS accumulation via depolarization of mitochondrial membrane potential, leading to the activation of necroptosis along with ferroptosis in melanoma cells (Efferth,2017). And there are crosstalk between ferroptosis and necroptosis were observed apparently in tumor cells and H/R-treated H9c2 cell lines when treating with some agonists. (Feng et al., 2022; Tu et al., 2021). More importantly, exploring the crosstalk between ferroptosis and necroptosis is thought to be a novel therapeutic strategy for cerebral ischemia (Zhou et al., 2021). Mechanistically, GSH degradation by CHAC1 promotes cystine starvation-induced necroptosis and ferroptosis in human triple-negative breast cancer cells via the GCN2-eIF2α-ATF4 pathway (Chen et al., 2017). Also HSP90 defines the regulatory intersection of necroptosis and ferroptosis (Wu et al., 2019). Furthermore, iron excess, one of the mechanisms of ferroptosis, leads to the opening of the mitochondrial permeability transition pore (MPTP), which exacerbates RIP1 phosphorylation and contributes to necroptosis (Zhou et al., 2021).
Crosstalk between multiple PCD is a new target for new drug discovery, and a vast amount of research has shown that the regulation of multiple PCD simultaneously would be a promising strategy for the treatment of disease. Artesunate (Efferth, 2017; Efferth, 2017), a widely prescribed antimalarial drug, has been reported to induce necroptosis and ferroptosis in tumor cells. Saudi Arabia chloride (Mbaveng et al., 2021) also has been reported to induce apoptosis, ferroptosis, necroptosis, and autophagy in malignant tumors, including refractory cancers. Palladium pyrithione complex (Yang et al., 2021) as a kind of broad-spectrum proteasomal deubiquitinase inhibitor, have been reported to inhibit tumor growth by activating caspase-dependent apoptosis and GPX4 degradation-dependent ferroptosis. However, these multifunctional agents are currently under-studied and require further research and development.
Important potential applications of traditional chinese medicine in cerebral ischemia
TCM has unique clinical advantages due to its multiple targets, multiple effects, and multiple pathways (Chen, 2012). Importantly, an increasing number of studies suggest a modulatory effect of TCM on various cell death pathway in experimental models related to cerebral ischemia. Total Saponins of Panax Notoginseng, the main component extracted from Panax Notoginseng, has neuroprotective effects against focal ischemia. It has been reported that it may be associated with the inhibition of apoptosis (Li et al., 2009). Puerariae radix, the familiar kudzu root, is used to protect the brain from cerebral ischemic injury via the inhibition of astrocyte apoptosis (Wang et al., 2014). Curcumin and eugenol, polyphenolic compounds extracted from Curcuma longa, have been noted as being effective against cerebral ischemia-reperfusion by alleviating inflammation and mediating autophagy. (Huang et al., 2018; Sun et al., 2020). Dendrobium alkaloids have been reported to inhibit neuronal cell death by pyroptosis and promote neuronal function after cerebral ischemia-reperfusion injury in vivo and in vitro models (Liu et al., 2020). Tongxinluo, a common TCM prescription, has inhibitory effects on pyroptosis and amyloid-β peptide accumulation after rat cerebral ischemia-reperfusion (Wang et al., 2021). Huwentoxin also maintained the original morphology of mitochondria, decreased the expression of tumor necrosis factor, and protected neurons in rats with cerebral ischemia-reperfusion injury (Wang et al., 2014). In addition, a previous study by our research group have found that Buyang Huanwu Decoction and its similar formulations play the neuroprotective effects by regulating apoptosis and autophagy dynamically in oxidative stress model cells (Liu et al., 2020), and potential regulation of ferroptosis signal pathway with Buyang Huanwu Decoction were explored for the treatment of cerebral ischemia by network pharmacology. (Zhao et al., 2021). Yan She et al. also found that the glycoside of Buyang Huanwu Decoction has a protective effect against pyroptosis after cerebral ischemia-reperfusion injury in rats (She et al., 2019), suggesting that Buyang Huanwu Decoction may regulate apoptosis, autophagy, ferroptosis and pyroptosis during cerebral ischemia. Based on the above, we suppose that TCM can play a neuroprotective effect by regulating various cell death pathways through various molecular mechanisms, which is consistent with the characteristics of multi-target of TCM, and it is expected to reveal the important potential applications of TCM for cerebral ischemia (Figure 2).
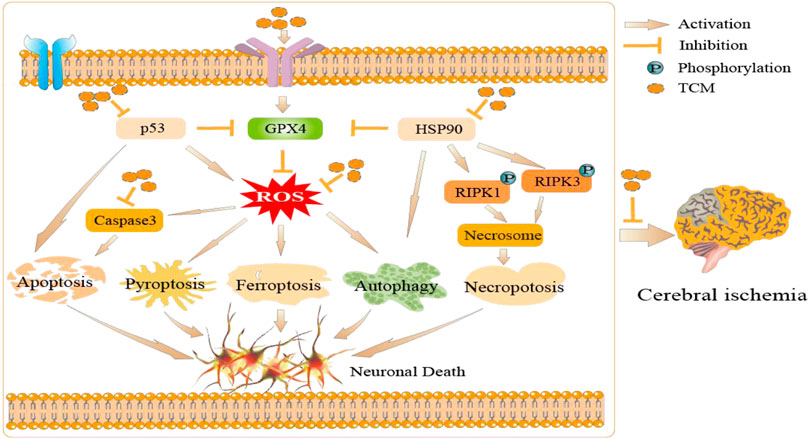
FIGURE 2. The potential applications of TCM in cerebral ischemia: TCM can regulate a variety of cell death pathways through ROS, HSP90, p53, and other molecular mechanisms, and thus play an anti-cerebral ischemia role.
Discussion and prospects
Studies have suggested that various types of PCD are involved in cerebral ischemia, among which the research and development of drugs targeting ferroptosis has attracted great attention. At present, it remains to be clarified whether compounds targeting ferroptosis regulators have high specificity and adverse reactions in preclinical and clinical environments, while TCM has the advantages of higher clinical value and less side effects due to its clinical origin. However, TCM also has disadvantages such unclear pharmacodynamic substance basis and imperfect functional mechanism. Besides, there are many problems about mechanism to be resolved. For instance, studies have shown that ferroptosis can protect cells from oxidative stress (Fearnhead et al., 2017), suggesting that ferroptosis may also play a dual role in cerebral ischemia. Meanwhile, ferroptosis may also interact with other cell death during cerebral ischemia. Consequently, the resolution of these questions is expected to provide essential directions and targets for the prevention and treatment of cerebral ischemia.
Many existing studies have used the combined therapies to prevent and control diseases. (Zheng et al. (2017)). designed organometallic networks encapsulating p53 plasmids to kill cancer cells by ferroptosis/apoptosis hybridization (Zhang et al., 2021). Weier Bao et al. created nanorongeons capable of carrying multiple targeted drugs for anticancer therapy, the results showed that it can simultaneously intervene ferroptosis and apoptosis (Bao et al., 2019). Although the prevention and treatment of cerebral ischemia by combining various cell death pathways presents exciting possibilities, there are still many problems in current researches. For example, all types of cell death pathways are limited by the development of corresponding inducers and inhibitors. More complicatedly, the dynamics sequence of various cell death events and the relationship between the dual role of cell death in vivo and pathological processes are still unclear. Various problems need to be further studied by comparing the expression of different cell death forms at different times and grouping studies with rational use of various exclusive inhibitors, etc. This paper comprehensively explores the potential pathophysiology of cerebral ischemia, with emphasis on the potential application of TCM to the neuronal cell death pathway, which is helpful to futher promote and improve the relevant research and development of TCM in the treatment of cerebral ischemia.
Author contributions
Writing-original draft: LF. Writing-review and editing:ZFY, PCW, and SY. All authors have agreed on the final version to be published. All authors reviewed and approved the manuscript.
Funding
National Natural Science Foundation of China (81704064); Natural Science Foundation of Hunan Provincial (2022JJ30436); Outstanding Youth Program of Scientific Research of Hunan Provincial Department of Education (20B434); Natural Science Foundation of Changsha City (kq2202259); Scientific Research Project of Hunan Provincial Administration of Traditional Chinese Medicine (202177); Supported by the first-class discipline of traditional Chinese medicine in Hunan Province ([2018]No.3); 2021 Hunan University of Traditional Chinese Medicine Graduate Innovation Project (2021CX53).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TCM, Traditional Chinese medicine; PCD, programmed cell death; TCM, traditional Chinese medicines; GPX4, glutathione peroxidase 4; ROS, reactive oxygen species; GSH, glutathione; MCAO, middle cerebral artery occlusion; TFR, transferrin receptor; FPN1, ferroportin 1; ACSL4, acyl-coa synthetase long-chain family member 4; 12/15-LOX, 12/15-lipoxygenase; PUFAs, polyunsaturated fatty acids; AA, arachidonic acid, AdA, adrenal acid; system Xc-, cystine/glutamate antiporter; NMDAR, N-methyl D aspartate receptor; SLC7A11, suppressing solute carrier 7A11; SLC3A2, solute carrier 3A2; p53, Tumor protein p53; RIPK3, receptor-interacting protein kinase 3.
References
Adams, H. P., del Zoppo, G., Alberts, M. J., Bhatt, D. L., Brass, L., Furlan, A., et al. (2007). Guidelines for the early management of adults with ischemic stroke: A guideline from the American heart association/American stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council, and the atherosclerotic peripheral vascular disease and quality of care outcomes in research interdisciplinary working groups: The American Academy of neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 38 (5), 1655–1711. doi:10.1161/STROKEAHA.107.181486
Ahmad, S., Elsherbiny, N. M., Haque, R., Khan, M. B., Ishrat, T., Shah, Z. A., et al. (2014). Sesamin attenuates neurotoxicity in mouse model of ischemic brain stroke. Neurotoxicology 45, 100–110. doi:10.1016/j.neuro.2014.10.002
Bao, W., Liu, X., Lv, Y., Lu, G. H., Li, F., Zhang, F., et al. (2019). Nanolongan with multiple on-demand conversions for ferroptosis-apoptosis combined anticancer therapy. ACS Nano 13 (1), 260–273. doi:10.1021/acsnano.8b05602
Bedoui, S., Herold, M. J., and Strasser, A. (2020). Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 21 (11), 678–695. doi:10.1038/s41580-020-0270-8
Brott, T., and Bogousslavsky, J. (2000). Treatment of acute ischemic stroke. N. Engl. J. Med. 343 (10), 710–722. doi:10.1056/NEJM200009073431007
Buccarelli, M., Marconi, M., Pacioni, S., De Pascalis, I., D'Alessandris, Q. G., Martini, M., et al. (2018). Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. 9 (8), 841. doi:10.1038/s41419-018-0864-7
Cao, H., Zuo, C., Huang, Y., Zhu, L., Zhao, J., Yang, Y., et al. (2021). Hippocampal proteomic analysis reveals activation of necroptosis and ferroptosis in a mouse model of chronic unpredictable mild stress-induced depression. Behav. Brain Res. 407, 113261. doi:10.1016/j.bbr.2021.113261
Cao, Z., Qin, H., Huang, Y., Zhao, Y., Chen, Z., Hu, J., et al. (2022). Crosstalk of pyroptosis, ferroptosis, and mitochondrial aldehyde dehydrogenase 2-related mechanisms in sepsis-induced lung injury in a mouse model. Bioengineered 13 (3), 4810–4820. doi:10.1080/21655979.2022.2033381
Cheah, J. H., Kim, S. F., Hester, L. D., Clancy, K. W., Patterson, S. E., Papadopoulos, V., et al. (2006). NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron 51 (4), 431–440. doi:10.1016/j.neuron.2006.07.011
Chen, C., Huang, Y., Xia, P., Zhang, F., Li, L., Wang, E., et al. (2021). Long noncoding RNA Meg3 mediates ferroptosis induced by oxygen and glucose deprivation combined with hyperglycemia in rat brain microvascular endothelial cells, through modulating the p53/GPX4 axis. Eur. J. Histochem. 65 (3), 3224. doi:10.4081/ejh.2021.3224
Chen, L., Yin, Z., Qin, X., Zhu, X., Chen, X., Ding, G., et al. (2022). CD74 ablation rescues type 2 diabetes mellitus-induced cardiac remodeling and contractile dysfunction through pyroptosis-evoked regulation of ferroptosis. Pharmacol. Res. 176, 106086. doi:10.1016/j.phrs.2022.106086
Chen, M. S., Wang, S. F., Hsu, C. Y., Yin, P. H., Yeh, T. S., Lee, H. C., et al. (2017). CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2α-ATF4 pathway. Oncotarget 8 (70), 114588–114602. doi:10.18632/oncotarget.23055
Chen, P., Ling, L., Ren, Y., Jiang, L., Wu, S., Wang, W., et al. (2017). Efficacy and safety of xinglouchengqi decoction for acute ischemic stroke with constipation: Study protocol for a randomized controlled trial. J. Traditional Chin. Med. 37 (6), 810–818. doi:10.1016/s0254-6272(18)30045-1
Chen, W., Jiang, L., Hu, Y., Tang, N., Liang, N., Li, X. F., et al. (2021). Ferritin reduction is essential for cerebral ischemia-induced hippocampal neuronal death through p53/SLC7A11-mediated ferroptosis. Brain Res. 1, 1752. doi:10.1016/j.brainres.2020.147216
Chen, X., Chen, H., He, Y., Fu, S., Liu, H., Wang, Q., et al. (2020). Proteomics-guided study on Buyang Huanwu decoction for its neuroprotective and neurogenic mechanisms for transient ischemic stroke: Involvements of EGFR/PI3K/Akt/Bad/14-3-3 and jak2/stat3/cyclin D1 signaling cascades. Mol. Neurobiol. 57 (10), 4305–4321. doi:10.1007/s12035-020-02016-y
Chen, Y. F. (2012). Traditional Chinese herbal medicine and cerebral ischemia. Front. Biosci. 4 (3), 809–817. doi:10.2741/E420
Cui, Y., Zhang, Y., Zhao, X., Shao, L., Liu, G., Sun, C., et al. (2021). ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav. Immun. 93, 312–321. doi:10.1016/j.bbi.2021.01.003
DeGregorio-Rocasolano, N., Martí-Sistac, O., and Gasull, T. (2019). Deciphering the iron side of stroke: Neurodegeneration at the crossroads between iron dyshomeostasis, excitotoxicity, and ferroptosis. Front. Neurosci. 13, 85. doi:10.3389/fnins.2019.00085
Dietrich, R. B., and Bradley, W. G. (1988). Iron accumulation in the basal ganglia following severe ischemic-anoxic insults in children. Radiology 168 (1), 203–206. doi:10.1148/radiology.168.1.3380958
Dong, Z., Pan, K., Pan, J., Peng, Q., and Wang, Y. (2018). The possibility and molecular mechanisms of cell pyroptosis after cerebral ischemia. Neurosci. Bull. 34 (6), 1131–1136. doi:10.1007/s12264-018-0294-7
Efferth, T. (2017). Cancer combination therapy of the sesquiterpenoid artesunate and the selective EGFR-tyrosine kinase inhibitor erlotinib. Phytomedicine. 37, 58–61. doi:10.1016/j.phymed.2017.11.003
Efferth, T. (2017). From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 46, 65–83. doi:10.1016/j.semcancer.2017.02.009
Fearnhead, H. O., Vandenabeele, P., and Vanden Berghe, T. (2017). How do we fit ferroptosis in the family of regulated cell death? Cell Death Differ. 24 (12), 1991–1998. doi:10.1038/cdd.2017.149
Feigin, V. L., Mensah, G. A., Norrving, B., Murray, C. J., and Roth, G. A.GBD 2013 Stroke Panel Experts Group (2015). Atlas of the global burden of stroke (1990-2013): The GBD 2013 study. Neuroepidemiology 45 (3), 230–236. doi:10.1159/000441106
Feng, W., Shi, W., Liu, S., Liu, H., Liu, Y., Ge, P., et al. (2022). Fe(III)-Shikonin supramolecular nanomedicine for combined therapy of tumor via ferroptosis and necroptosis. Adv. Healthc. Mat. 11 (2), e2101926. doi:10.1002/adhm.202101926
Fu, J., Li, T., Yang, Y., Jiang, L., Wang, W., Fu, L., et al. (2021). Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. Biomaterials 268, 120537. doi:10.1016/j.biomaterials.2020.120537
Gao, M., Monian, P., Pan, Q., Zhang, W., Xiang, J., and Jiang, X. (2016). Ferroptosis is an autophagic cell death process. Cell Res. 26 (9), 1021–1032. doi:10.1038/cr.2016.95
Greenshields, A. L., Shepherd, T. G., and Hoskin, D. W. (2017). Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate. Mol. Carcinog. 56 (1), 75–93. doi:10.1002/mc.22474
Guan, X., Li, X., Yang, X., Yan, J., Shi, P., Ba, L., et al. (2019). The neuroprotective effects of carvacrol on ischemia/reperfusion-induced hippocampal neuronal impairment by ferroptosis mitigation. Life Sci. 235, 116795. doi:10.1016/j.lfs.2019.116795
Guan, X., Li, Z., Zhu, S., Cheng, M., Ju, Y., Ren, L., et al. (2021). Galangin attenuated cerebral ischemia-reperfusion injury by inhibition of ferroptosis through activating the SLC7A11/GPX4 axis in gerbils. Life Sci. 264, 118660. doi:10.1016/j.lfs.2020.118660
Guo, H., Zhu, L., Tang, P., Chen, D., Li, Y., Li, J., et al. (2021). Carthamin yellow improves cerebral ischemia-reperfusion injury by attenuating inflammation and ferroptosis in rats. Int. J. Mol. Med. 47 (4), 52. doi:10.3892/ijmm.2021.4885
Guo, R., Li, L., Su, J., Li, S., Duncan, S. E., Liu, Z., et al. (2020). Pharmacological activity and mechanism of tanshinone IIA in related diseases. Drug Des. devel. Ther. 14, 4735–4748. doi:10.2147/DDDT.S266911
He, C., Liao, J., Lan, B., Rao, Z, Q., Ge, J, W., and Wang, G, Z. (2020). Effects of Naotaifang on ferroptosis-lipid metabolism pathwang protein expression after cerebral infarction. China J. Traditional Chin. Med. Pharm. 35 (11), 5491–5494.
Hong, S. H., Lee, D. H., Lee, Y. S., Jo, M. J., Jeong, Y. A., Kwon, W. T., et al. (2017). Molecular crosstalk between ferroptosis and apoptosis: Emerging role of ER stress-induced p53-independent PUMA expression. Oncotarget 8 (70), 115164–115178. doi:10.18632/oncotarget.23046
Hou, W., Xie, Y., Song, X., Sun, X., Lotze, M. T., Zeh, H. J., et al. (2016). Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12 (8), 1425–1428. doi:10.1080/15548627.2016.1187366
Hribljan, V., Lisjak, D., Petrović, D. J., and Mitrečić, D. (2019). Necroptosis is one of the modalities of cell death accompanying ischemic brain stroke: From pathogenesis to therapeutic possibilities. Croat. Med. J. 60 (2), 121–126. doi:10.3325/cmj.2019.60.121
Hsieh, C. H., Lin, Y. J., Chen, W. L., Huang, Y. C., Chang, C. W., Cheng, F. C., et al. (2017). HIF-1α triggers long-lasting glutamate excitotoxicity via system xc- in cerebral ischaemia-reperfusion. J. Pathol. 241 (3), 337–349. doi:10.1002/path.4838
Huang, L., Chen, C., Zhang, X., Li, X., Chen, Z., Yang, C., et al. (2018). Neuroprotective effect of curcumin against cerebral ischemia-reperfusion via mediating autophagy and inflammation. J. Mol. Neurosci. 64 (1), 129–139. doi:10.1007/s12031-017-1006-x
Ito, K., Eguchi, Y., Imagawa, Y., Akai, S., Mochizuki, H., and Tsujimoto, Y. (2017). MPP+ induces necrostatin-1- and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells. Cell Death Discov. 3, 17013. doi:10.1038/cddiscovery.2017.13
Jiang, L., Kon, N., Li, T., Wang, S. J., Su, T., Hibshoosh, H., et al. (2015). Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520 (7545), 57–62. doi:10.1038/nature14344
Jiang, T., Cheng, H., Su, J., Wang, X., Wang, Q., Chu, J., et al. (2020). Gastrodin protects against glutamate-induced ferroptosis in HT-22 cells through Nrf2/HO-1 signaling pathway. Toxicol. Vitro. 62, 104715. doi:10.1016/j.tiv.2019.104715
Jiao, H., Yang, H., Yan, Z., Chen, J., Xu, M., Jiang, Y., et al. (2021). Traditional Chinese formula xiaoyaosan alleviates depressive-like behavior in CUMS mice by regulating PEBP1-GPX4-mediated ferroptosis in the Hippocampus. Neuropsychiatr. Dis. Treat. 17, 1001–1019. doi:10.2147/NDT.S302443
Kang, R., and Tang, D. (2017). Autophagy and ferroptosis - what's the connection? Curr. Pathobiol. Rep. 5 (2), 153–159. doi:10.1007/s40139-017-0139-5
Kang, R., Zeng, L., Zhu, S., Xie, Y., Liu, J., Wen, Q., et al. (2018). Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe 24 (1), 97–108.e4. doi:10.1016/j.chom.2018.05.009
Kang, R., Zhu, S., Zeh, H. J., Klionsky, D. J., and Tang, D. (2018). BECN1 is a new driver of ferroptosis. Autophagy 14 (12), 2173–2175. doi:10.1080/15548627.2018.1513758
Kong, D, Y., Huang, Z, H., and Wang, K, H. (2019). Change of serum hepcidin 25 and iron metabolism indicator in acute ischemic stroke patients and its clinical significance. Chin. J. Geriatric Heart Brain Vessel Dis. 17 (05), 455–458. doi:10.3969/j.issn.1009-0126.2015.05.003
Krishnamurthi, R. V., Feigin, V. L., Forouzanfar, M. H., Mensah, G. A., Connor, M., Bennett, D. A., et al. (2013). Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: Findings from the global burden of disease study 2010. Lancet. Glob. Health 1 (5), e259–81. doi:10.1016/S2214-109X(13)70089-5
Krzyżanowska, W., Pomierny, B., Bystrowska, B., Pomierny-Chamioło, L., Filip, M., Budziszewska, B., et al. (2017). Ceftriaxone- and N-acetylcysteine-induced brain tolerance to ischemia: Influence on glutamate levels in focal cerebral ischemia. PLoS One 12 (10), e0186243. doi:10.1371/journal.pone.0186243
Lan, B., Ge, J. W., Cheng, S. W., Zheng, X. L., Liao, J., He, C., et al. (2020). Extract of Naotaifang, a compound Chinese herbal medicine, protects neuron ferroptosis induced by acute cerebral ischemia in rats. J. Integr. Med. 18 (4), 344–350. doi:10.1016/j.joim.2020.01.008
Lee, Y. S., Kalimuthu, K., Park, Y. S., Luo, X., Choudry, M. H. A., Bartlett, D. L., et al. (2020). BAX-dependent mitochondrial pathway mediates the crosstalk between ferroptosis and apoptosis. Apoptosis. 25 (9-10), 625–631. doi:10.1007/s10495-020-01627-z
Li, G., Li, X., Dong, J., and Han, Y. (2021). Electroacupuncture ameliorates cerebral ischemic injury by inhibiting ferroptosis. Front. Neurol. 12, 619043. doi:10.3389/fneur.2021.619043
Li, H., Deng, C. Q., Chen, B. Y., Zhang, S. P., Liang, Y., and Luo, X. G. (2009). Total saponins of Panax notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J. Ethnopharmacol. 121 (3), 412–418. doi:10.1016/j.jep.2008.10.042
Li, J., Liu, J., Xu, Y., Wu, R., Chen, X., Song, X., et al. (2021). Tumor heterogeneity in autophagy-dependent ferroptosis. Autophagy 17 (11), 3361–3374. doi:10.1080/15548627.2021.1872241
Li, L., Hao, Y., Zhao, Y., Wang, H., Zhao, X., Jiang, Y., et al. (2018). Ferroptosis is associated with oxygen-glucose deprivation/reoxygenation-induced Sertoli cell death. Int. J. Mol. Med. 41 (5), 3051–3062. doi:10.3892/ijmm.2018.3469
Li, Y., Cao, Y., Xiao, J., Shang, J., Tan, Q., Ping, F., et al. (2020). Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death Differ. 27 (9), 2635–2650. doi:10.1038/s41418-020-0528-x
Lin, Y., Yang, C, Q., Liao, W, Y., Xiao, C, R., Tan, H, L., Gao, Y, L., et al. (2021). Ophiopogonin D interferes with ferroptosis to reduce the damage of cardiomyocytes induced by Ophiopogonin D. Acta Pharm. Sin. 56 (08), 2241–2247. doi:10.16438/j.0513-4870.2021-0419
Lipscomb, D. C., Gorman, L. G., Traystman, R. J., and Hurn, P. D. (1998). Low molecular weight iron in cerebral ischemic acidosis in vivo. Stroke 29 (2), 487–492. doi:10.1161/01.str.29.2.487
Liu, B., Zhao, C., Li, H., Chen, X., Ding, Y., and Xu, S. (2018). Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem. Biophys. Res. Commun. 497 (1), 233–240. doi:10.1016/j.bbrc.2018.02.061
Liu, D., Dong, Z., Xiang, F., Liu, H., Wang, Y., Wang, Q., et al. (2020). Dendrobium alkaloids promote neural function after cerebral ischemia-reperfusion injury through inhibiting pyroptosis induced neuronal death in both in vivo and in vitro models. Neurochem. Res. 45 (2), 437–454. doi:10.1007/s11064-019-02935-w
Liu, F., Zhu, Y, Z., Zhao, F, Y., Hu, Y, M., Huang, X, J., Fan, R, Y., et al. (2020). Regulation of Buyang Huanwu Decoction extracts on apoptosis and autophagy of PC12 cells models with oxidative stress injury. Chin. Traditional Herb. Drugs 51 (20), 5228–5236. doi:10.7501/j.issn.0253-2670.2020.20.015
Liu, J., Guo, Z. N., Yan, X. L., Huang, S., Ren, J. X., Luo, Y., et al. (2020). Crosstalk between autophagy and ferroptosis and its putative role in ischemic stroke. Front. Cell. Neurosci. 14, 577403. doi:10.3389/fncel.2020.577403
Liu, J., Kuang, F., Kroemer, G., Klionsky, D. J., Kang, R., and Tang, D. (2020). Autophagy-dependent ferroptosis: Machinery and regulation. Cell Chem. Biol. 27 (4), 420–435. doi:10.1016/j.chembiol.2020.02.005
Liu, Y. Q., and Gu, W. (2022). p53 in ferroptosis regulation: the new weapon for the old guardian. Cell Death Differ. 29 (5), 895–910. doi:10.1038/s41418-022-00943-y
Liu, Y. Q., and Gu, W. (2021). The complexity of p53-mediated metabolic regulation in tumor suppression. Semin. Cancer Biol. 27, S1044. doi:10.1016/j.semcancer.2021.03.010
Logie, E., Novo, C. P., Driesen, A., Van Vlierberghe, P., and Vanden Berghe, W. (2021). Phosphocatalytic kinome activity profiling of apoptotic and ferroptotic agents in multiple myeloma cells. Int. J. Mol. Sci. 22 (23), 12731. doi:10.3390/ijms222312731
Lu, Q. Y., Ma, J. Q., Duan, Y. Y., Sun, Y., Yu, S., Li, B., et al. (2019). Carthamin yellow protects the heart against ischemia/reperfusion injury with reduced reactive oxygen species release and inflammatory response. J. Cardiovasc. Pharmacol. 74 (3), 228–234. doi:10.1097/FJC.0000000000000710
Ma, S., Dielschneider, R. F., Henson, E. S., Xiao, W., Choquette, T. R., Blankstein, A. R., et al. (2017). Ferroptosis and autophagy induced cell death occur independently after siramesine and lapatinib treatment in breast cancer cells. PLoS One 12 (8), e0182921. doi:10.1371/journal.pone.0182921
Mao, C., Wang, X., Liu, Y., Wang, M., Yan, B., Jiang, Y., et al. (2018). A G3BP1-interacting lncRNA promotes ferroptosis and apoptosis in cancer via nuclear sequestration of p53. Cancer Res. 78 (13), 3484–3496. doi:10.1158/0008-5472.CAN-17-3454
Mbaveng, A. T., Noulala, C. G. T., Samba, A. R. M., Tankeo, S. B., Abdelfatah, S., Fotso, G. W., et al. (2021). The alkaloid, soyauxinium chloride, displays remarkable cytotoxic effects towards a panel of cancer cells, inducing apoptosis, ferroptosis and necroptosis. Chem. Biol. Interact. 333, 109334. doi:10.1016/j.cbi.2020.109334
Meng, X., Li, D., Chen, L., He, H., Wang, Q., Hong, C., et al. (2021). High-performance self-cascade pyrite nanozymes for apoptosis-ferroptosis synergistic tumor therapy. ACS Nano 15 (3), 5735–5751. doi:10.1021/acsnano.1c01248
Meyers, P. M., Schumacher, H. C., Alexander, M. J., Derdeyn, C. P., Furlan, A. J., Higashida, R. T., et al. (2012). Performance and training standards for endovascular acute ischemic stroke treatment. Neurology 79 (13), S234–S238. doi:10.1212/WNL.0b013e318269595b
Moskowitz, M. A., Lo, E. H., and Iadecola, C. (2010). The science of stroke: Mechanisms in search of treatments. Neuron 67 (2), 181–198. doi:10.1016/j.neuron.2010.07.002
Mu, Y., Sun, J., Li, Z., Zhang, W., Liu, Z., Li, C., et al. (2022). Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere 291 (2), 132944. doi:10.1016/j.chemosphere.2021.132944
Park, U. J., Lee, Y. A., Won, S. M., Lee, J. H., Kang, S. H., Springer, J. E., et al. (2011). Blood-derived iron mediates free radical production and neuronal death in the hippocampal CA1 area following transient forebrain ischemia in rat. Acta Neuropathol. 121 (4), 459–473. doi:10.1007/s00401-010-0785-8
Petrova, J., Manolov, V., Vasilev, V., Tzatchev, K., and Marinov, B. (2016). Ischemic stroke, inflammation, iron overload - connection to a hepcidin. Int. J. Stroke 11 (1), NP16–7. doi:10.1177/1747493015607509
Probst, L., Dächert, J., Schenk, B., and Fulda, S. (2017). Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem. Pharmacol. 140, 41–52. doi:10.1016/j.bcp.2017.06.112
Radak, D., Katsiki, N., Resanovic, I., Jovanovic, A., Sudar-Milovanovic, E., Zafirovic, S., et al. (2017). Apoptosis and acute brain ischemia in ischemic stroke. Curr. Vasc. Pharmacol. 15 (2), 115–122. doi:10.2174/1570161115666161104095522
Rao, Z, Q., Mei, Z, G., Ge, J, W., Yang, M., Mi, Z, D., Lan, B., et al. (2021). Mechanism of Naotaifang on ischemic stroke through regulating cellular iron transport and inhibiting ferroptosis. Chin. Traditional Herb. Drugs 52 (21), 6552–6560. doi:10.7501/j.issn.0253-2670.2021.21.013
Roh, J. L., Kim, E. H., Jang, H., and Shin, D. (2017). Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 11, 254–262. doi:10.1016/j.redox.2016.12.010
Seiler, A., Schneider, M., Förster, H., Roth, S., Wirth, E. K., Culmsee, C., et al. (2008). Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8 (3), 237–248. doi:10.1016/j.cmet.2008.07.005
She, Y., Shao, L., Zhang, Y., Hao, Y., Cai, Y., Cheng, Z., et al. (2019). Neuroprotective effect of glycosides in Buyang Huanwu Decoction on pyroptosis following cerebral ischemia-reperfusion injury in rats. J. Ethnopharmacol. 242, 112051. doi:10.1016/j.jep.2019.112051
Silva, E. R., Carvalho, F. O., Teixeira, L. G. B., Santos, N. G. L., Felipe, F. A., Santana, H. S. R., et al. (2018). Pharmacological effects of carvacrol in in vitro studies: A review. Curr. Pharm. Des. 24 (29), 3454–3465. doi:10.2174/1381612824666181003123400
Speer, R. E., Karuppagounder, S. S., Basso, M., Sleiman, S. F., Kumar, A., Brand, D., et al. (2013). Hypoxia-inducible factor prolyl hydroxylases as targets for neuroprotection by "antioxidant" metal chelators: From ferroptosis to stroke. Free Radic. Biol. Med. 62, 26–36. doi:10.1016/j.freeradbiomed.2013.01.026
Sun, X., Wang, D., Zhang, T., Lu, X., Duan, F., Ju, L., et al. (2020). Eugenol attenuates cerebral ischemia-reperfusion injury by enhancing autophagy via AMPK-mTOR-P70S6K pathway. Front. Pharmacol. 11, 84. doi:10.3389/fphar.2020.00084
Tian, Z., Tang, C., and Wang, Z. (2019). Neuroprotective effect of ginkgetin in experimental cerebral ischemia/reperfusion via apoptosis inhibition and PI3K/Akt/mTOR signaling pathway activation. J. Cell. Biochem. 120 (10), 18487–18495. doi:10.1002/jcb.29169
Torii, S., Shintoku, R., Kubota, C., Yaegashi, M., Torii, R., Sasaki, M., et al. (2016). An essential role for functional lysosomes in ferroptosis of cancer cells. Biochem. J. 473 (6), 769–777. doi:10.1042/BJ20150658
Tu, H., Zhou, Y. J., Tang, L. J., Xiong, X. M., Zhang, X. J., Ali Sheikh, M. S., et al. (2021). Combination of ponatinib with deferoxamine synergistically mitigates ischemic heart injury via simultaneous prevention of necroptosis and ferroptosis. Eur. J. Pharmacol. 898, 173999. doi:10.1016/j.ejphar.2021.173999
Tuo, Q. Z., Lei, P., Jackman, K. A., Li, X. L., Xiong, H., Li, X. L., et al. (2017). Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatry 22 (11), 1520–1530. doi:10.1038/mp.2017.171
Tuo, Q. Z., Liu, Y., Xiang, Z., Yan, H. F., Zou, T., Shu, Y., et al. (2022). Thrombin induces ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion. Signal Transduct. Target. Ther. 7 (1), 59. doi:10.1038/s41392-022-00917-z
Tuo, Q. Z., Zhang, S. T., and Lei, P. (2022). Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med. Res. Rev. 42 (1), 259–305. doi:10.1002/med.21817
Wang, B., Lyu, Z., Chan, Y., Li, Q., Zhang, L., Liu, K., et al. (2021). Tongxinluo exerts inhibitory effects on pyroptosis and amyloid-β peptide accumulation after cerebral ischemia/reperfusion in rats. Evid. Based. Complement. Altern. Med. 2021, 5788602. doi:10.1155/2021/5788602
Wang, C., Yuan, W., Hu, A., Lin, J., Xia, Z., Yang, C. F., et al. (2020). Dexmedetomidine alleviated sepsis-induced myocardial ferroptosis and septic heart injury. Mol. Med. Rep. 22 (1), 175–184. doi:10.3892/mmr.2020.11114
Wang, J., Deng, B., Liu, Q., Huang, Y., Chen, W., Li, J., et al. (2020). Pyroptosis and ferroptosis induced by mixed lineage kinase 3 (MLK3) signaling in cardiomyocytes are essential for myocardial fibrosis in response to pressure overload. Cell Death Dis. 11 (7), 574. doi:10.1038/s41419-020-02777-3
Wang, J., Jia, L, Q., Song, N., Wu, Y., Chen, S., Cao, Y., et al. (2021). Sijunzi decoction improves iipid deposition in atherosclerotic mice via iron death pathway. Prog. Anatomical Sci. 27 (1), 75–78. doi:10.16695/j.cnki.1006-2947.2021.01.020
Wang, N., Zhang, Y., Wu, L., Wang, Y., Cao, Y., He, L., et al. (2014). Puerarin protected the brain from cerebral ischemia injury via astrocyte apoptosis inhibition. Neuropharmacology 79, 282–289. doi:10.1016/j.neuropharm.2013.12.004
Wang, X., Liu, K., Gong, H., Li, D., Chu, W., Zhao, D., et al. (2021). Death by histone deacetylase inhibitor quisinostat in tongue squamous cell carcinoma via apoptosis, pyroptosis, and ferroptosis. Toxicol. Appl. Pharmacol. 410, 115363. doi:10.1016/j.taap.2020.115363
Wang, Y. R., Mao, H. F., and Chen, J. Q. (2014). Effects of huwentoxin on tumor necrosis factor apoptotic pathway in the hippocampus of a rat model of cerebral ischemia[J]. Zhongguo Zuzhi Gongcheng Yanjiu 18 (36), 5813–5818. doi:10.3969/j.issn.2095-4344.2014.36.013
Wang, Y., Xiao, G., He, S., Liu, X., Zhu, L., Yang, X., et al. (2020). Protection against acute cerebral ischemia/reperfusion injury by QiShenYiQi via neuroinflammatory network mobilization. Biomed. Pharmacother. 125, 109945. doi:10.1016/j.biopha.2020.109945
Wang, Z., Chen, X., Liu, N., Shi, Y., Liu, Y., Ouyang, L., et al. (2021). A nuclear long non-coding RNA LINC00618 accelerates ferroptosis in a manner dependent upon apoptosis. Mol. Ther. 29 (1), 263–274. doi:10.1016/j.ymthe.2020.09.024
Wu, Z., Geng, Y., Lu, X., Shi, Y., Wu, G., Zhang, M., et al. (2019). Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 116 (8), 2996–3005. doi:10.1073/pnas.1819728116
Xia, X. H., Li, Q., and Liu, M. (2014). Neuroprotective effect of a formula, moschus combined with borneolum synthcticum, from traditional Chinese medicine on ischemia stroke in rats. Evid. Based. Complement. Altern. Med. 2014, 157938. doi:10.1155/2014/157938
Xie, Y., Song, X., Sun, X., Huang, J., Zhong, M., Lotze, M. T., et al. (2016). Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem. Biophys. Res. Commun. 473 (4), 775–780. doi:10.1016/j.bbrc.2016.03.052
Xu, L., and Tang, Q. Q. (2019). Research on the mechanism of tanshinone IIA inhibiting ferroptosis in HT22 hippocampus cells. Acta Univ. Med. Anhui 54 (06), 833–839. doi:10.19405/j.cnki.issn1000-1492.2019.06.001
Xu, R., Yang, J., Qian, Y., Deng, H., Wang, Z., Ma, S., et al. (2021). Ferroptosis/pyroptosis dual-inductive combinational anti-cancer therapy achieved by transferrin decorated nanoMOF. Nanoscale Horiz. 6 (4), 348–356. doi:10.1039/d0nh00674b
Xu, Y., Zhang, J., Ma, L., Zhao, S., Li, S., Huang, T., et al. (2018). The pathogenesis of necroptosis-dependent signaling pathway in cerebral ischemic disease. Behav. Neurol. 2018, 6814393. doi:10.1155/2018/6814393
Yamaguchi, Y., Kasukabe, T., and Kumakura, S. (2018). Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int. J. Oncol. 52 (3), 1011–1022. doi:10.3892/ijo.2018.4259
Yang, L., Chen, X., Yang, Q., Chen, J., Huang, Q., Yao, L., et al. (2020). Broad spectrum deubiquitinase inhibition induces both apoptosis and ferroptosis in cancer cells. Front. Oncol. 10, 949. doi:10.3389/fonc.2020.00949
Yang, W., Liu, X., Song, C., Ji, S., Yang, J., Liu, Y., et al. (2021). Structure-activity relationship studies of phenothiazine derivatives as a new class of ferroptosis inhibitors together with the therapeutic effect in an ischemic stroke model. Eur. J. Med. Chem. 209, 112842. doi:10.1016/j.ejmech.2020.112842
Yang, W. S., and Stockwell, B. R. (2016). Ferroptosis: Death by lipid peroxidation. Trends Cell Biol. 26 (3), 165–176. doi:10.1016/j.tcb.2015.10.014
Yang, X., He, Z., Zhang, Q., Wu, Y., Hu, Y., Wang, X., et al. (2012). Pre-ischemic treadmill training for prevention of ischemic brain injury via regulation of glutamate and its transporter GLT-1. Int. J. Mol. Sci. 13 (8), 9447–9459. doi:10.3390/ijms13089447
Ye, Z., Zhuo, Q., Hu, Q., Xu, X., Mengqi, Liu., Zhang, Z., et al. (2021). FBW7-NRA41-SCD1 axis synchronously regulates apoptosis and ferroptosis in pancreatic cancer cells. Redox Biol. 38, 101807. doi:10.1016/j.redox.2020.101807
Yigitkanli, K., Zheng, Y., Pekcec, A., Lo, E. H., and van Leyen, K. (2017). Increased 12/15-lipoxygenase leads to widespread brain injury following global cerebral ischemia. Transl. Stroke Res. 8 (2), 194–202. doi:10.1007/s12975-016-0509-z
Yu, S., Yu, M., He, X., Wen, L., Bu, Z., and Feng, J. (2019). KCNQ1OT1 promotes autophagy by regulating miR-200a/FOXO3/ATG7 pathway in cerebral ischemic stroke. Aging Cell 18 (3), e12940. doi:10.1111/acel.12940
Yu, Y., Xie, Y., Cao, L., Yang, L., Yang, M., Lotze, M. T., et al. (2015). The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol. Cell. Oncol. 2 (4), e1054549. doi:10.1080/23723556.2015.1054549
Zhang, M., Zhang, T., Song, C., Qu, J., Gu, Y., Liu, S., et al. (2021). Guizhi Fuling Capsule ameliorates endometrial hyperplasia through promoting p62-Keap1-NRF2-mediated ferroptosis. J. Ethnopharmacol. 274, 114064. doi:10.1016/j.jep.2021.114064
Zhang, Q., Yi, H., Yao, H., Lu, L., He, G., Wu, M., et al. (2021). Artemisinin derivatives inhibit non-small cell lung cancer cells through induction of ROS-dependent apoptosis/ferroptosis. J. Cancer 12 (13), 4075–4085. doi:10.7150/jca.57054
Zhang, Z., Guo, M., Li, Y., Shen, M., Kong, D., Shao, J., et al. (2020). RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy 16 (8), 1482–1505. doi:10.1080/15548627.2019.1687985
Zhao, F, Y., Yang, H. L., Zhu, Y, Z., Lin, M, Y., and Liu, F. (2021). Exploring the mechanism of buyan Huanwu decoction regulating on cerebral ischemic stroke through ferroptosis based on network pharmacology. J. Hunan Univ. Chin. Med. 41 (07), 1065–1072. doi:10.3969/j.issn.1674-070X.2021.07.016
Zheng, D. W., Lei, Q., Zhu, J. Y., Fan, J. X., Li, C. X., Li, C., et al. (2017). Switching apoptosis to ferroptosis: Metal-organic network for high-efficiency anticancer therapy. Nano Lett. 17 (1), 284–291. doi:10.1021/acs.nanolett.6b04060
Zhou, B., Liu, J., Kang, R., Klionsky, D. J., Kroemer, G., and Tang, D. (2020). Ferroptosis is a type of autophagy-dependent cell death. Semin. Cancer Biol. 66, 89–100. doi:10.1016/j.semcancer.2019.03.002
Zhou, Y., Liao, J., Mei, Z., Liu, X., and Ge, J. (2021). Insight into crosstalk between ferroptosis and necroptosis: Novel therapeutics in ischemic stroke. Oxid. Med. Cell. Longev. 2021, 9991001. doi:10.1155/2021/9991001
Zhou, Y., Shen, Y., Chen, C., Sui, X., Yang, J., Wang, L., et al. (2019). The crosstalk between autophagy and ferroptosis: What can we learn to target drug resistance in cancer? Cancer Biol. Med. 16 (4), 630–646. doi:10.20892/j.issn.2095-3941.2019.0158
Keywords: traditional Chinese medicine(TCM), cerebral ischemia, ferroptosis, apoptosis, autophagy
Citation: Zhao F, Peng C, Sun Y, Li H, Du K and Liu F (2022) Potential application of traditional Chinese medicine in cerebral ischemia—Focusing on ferroptosis. Front. Pharmacol. 13:963179. doi: 10.3389/fphar.2022.963179
Received: 07 June 2022; Accepted: 26 July 2022;
Published: 23 September 2022.
Edited by:
Yanqing Liu, Columbia University, United StatesReviewed by:
Yi Wang, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, ChinaXiaolu Zhu, Columbia University, United States
Copyright © 2022 Zhao, Peng, Sun, Li, Du and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Liu, ZmxpdTA4MjVAMTI2LmNvbQ==
 Fengyan Zhao
Fengyan Zhao Caiwang Peng
Caiwang Peng Yang Sun
Yang Sun Hengli Li1,2,3
Hengli Li1,2,3