- 1Beijing Key Laboratory of Pediatric Respiratory Infection Diseases, Key Laboratory of Major Diseases in Children, Ministry of Education, National Clinical Research Center for Respiratory Diseases, National Key Discipline of Pediatrics (Capital Medical University), Beijing Pediatric Research Institute, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China
- 2Department of Pediatrics, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 3Baoding Children’s Hospital, Baoding, China
- 4Children’s Hospital of Hebei Province, Shijiazhuang, China
- 5Children’s Hospital Affiliated to Zhengzhou University, Henan Children’s Hospital, Zhengzhou Children’s Hospital, Zhengzhou, China
- 6Department of Infection Diseases, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China
- 7Department of Neonatology, Sunyi Women’s and Children’s Hospital of Beijing Children’s Hospital, Beijing, China
- 8Department of Clinical Pharmacy, School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, China
- 9NMPA Key Laboratory for Clinical Research and Evaluation of Innovative Drug, Qilu Hospital of Shandong University, Shandong University, Jinan, China
Probability of target attainment is the key factor influencing the outcome of meropenem therapy. The objective of the present study was to evaluate the relationship between the time in which the plasma free concentration of meropenem exceeds the minimum inhibitory concentration of pathogens (fT>MIC) during therapy and the clinical outcome of treatment to optimize meropenem therapy. Critically ill children with infections who had received intravenous meropenem monotherapy were included. The relationship between fT>MIC of meropenem and effectiveness and safety were explored. Data from 53 children (mean age ± standard deviation, 26 months ± 38) were available for final analysis. Children with fT>MIC ≥ 5.6 h (n = 14) had a more significant improvement in antibacterial efficacy in terms of decrease in fever (p = 0.02), white blood cell count (p = 0.014), and C-reactive protein (p = 0.02) compared with children with fT>MIC < 5.6 h (n = 39) after meropenem therapy completed. No drug-related adverse events were shown to have a causal association with meropenem therapy. Our study shows the clinical benefits of sufficient target attainment of meropenem therapy. Meeting a suitable pharmacodynamic target attainment of meropenem is required to ensure better antibacterial efficacy in critically ill infants and children.
Clinical Trial Registration: clinicaltrials.gov, Identifier NCT03643497.
Introduction
Meropenem is the most widely used carbapenem in children for the treatment of severe infections owing to its broad antimicrobial spectrum (including multidrug-resistant bacteria) and favorable safety profile (Thalhammer and Horl, 2000; Cies et al., 2014). Meropenem shows time-dependent antibacterial activity related to the time that the plasma free concentration of meropenem exceeds the minimum inhibitory concentration (MIC) of the pathogen (fT>MIC) (Mathew et al., 2016). Despite its wide use, the standard dosing regimen, administered as 10–40 mg/kg/dose (q8h) infused for 0.5 h, for critically ill infants, children (Kongthavonsakul et al., 2016; Cies et al., 2017; Hassan et al., 2019; Wang et al., 2020) and adults(Alsultan et al., 2021) may fail to meet pharmacodynamic (PD) targets.
Probability of target attainment (PTA) is the key factor influencing the outcome of meropenem therapy. A higher target fT>MIC value ≥70% of the dosage interval has been suggested for patients with severe bacterial infections to ensure bactericidal effectiveness, however, limited data are available in children to support this target (Wang et al., 2020, Cies et al., 2017). Additionally, for some critically ill children without renal impairment, clearance (CL) of therapeutics is augmented thereby decreasing plasma levels, and the volume of distribution (Vd) is increased due to a hyper dynamic state in response to inflammation (Wang et al., 2020; Cies et al., 2017; Rapp et al., 2019). As a result, the probability of target attainment (PTA) of 70% fT>MIC with a standard meropenem dosing regimen is low in critically ill children (Wang et al., 2020). Failure to combat the causative pathogen adequately as a result of a low PTA clearly reduces the probability of successful treatment.
We hypothesized that PTA is the key factor for meropenem treatment success in critically ill children. To optimize meropenem therapy, the relationship between 70% fT>MIC of meropenem [70% × 8 h (the dosage interval) = 5.6 h] and clinical outcome was evaluated in this study.
Methods
Study design
A multicenter prospective, open-label PD study of meropenem was conducted in Beijing Children’s Hospital, Baoding Children’s Hospital, Henan Children’s Hospital, Hebei Children’s Hospital from 2019 to 2021. Children hospitalized in pediatric intensive care units with bacterial meningitis [National Institute for Health and Care Excellence (NICE), 2018], sepsis (Dellinger et al., 2013) or severe pneumonia (Subspecialty Group of Respiratory Diseases, 2013) who had received meropenem monotherapy (Dainippon Sumitomo Pharma, Osaka, Japan) for a clinically suspected or proven bacterial infection as an intravenous infusion for 0.5–1 h at 20–40 mg/kg/dose (q8h) were included. Exclusion criteria included a history or evidence of chronic pathology of any organ system, receiving meropenem for ≤48 h, non-bacterial infections, and incomplete clinical information. Demographic data, clinical “characteristics,” and response to/adverse events (AE) associated with meropenem therapy were recorded in H6WORLD system (https://www.h6world.cn/home) for the management of clinical data independently.
Calculation of fT>MIC for meropenem
Opportunistic PK samples were collected and plasma concentrations of meropenem were quantified using high-performance liquid chromatography (HPLC) as described in our previous study (Wang et al., 2020). On the basis of the population pharmacokinetic (PK)-PD parameters of meropenem in critically ill infants and children with infections reported (Wang et al., 2020), the fT>MIC for each patient was calculated using the following equation (Dudley and Ambrose, 2000; Bradley et al., 2008; Yun et al., 2010):
where Vd is the volume of distribution, T1/2 is the serum elimination half-life, and Dose is the single dose administered. The information associated with Vd and T1/2 value is detailed in our article (Wang et al., 2020). Meropenem has excellent activity against Gram-positive bacteria, including Streptococcus pneumoniae, and Gram-negative organisms such as Escherichia coli, Haemophilus influenzae, Klebsiella pneumonia, and Neisseria meningitidis; these were the most common bacterial pathogens observed in our study. The MICs for almost all pathogens were ≤1 mg/L (CLSI supplement M100. Wayne, 2019) and the PTA was 18.7% (MIC = 1 mg/L) and 5.8% (MIC = 2 mg/L) at the standard dosing regimen for meropenem under 70% fT>MIC based on our microbiological data (Wang et al., 2020). In order to ensure enough children in the PD target attainment group for efficacy analysis, MIC = 1 mg/L with higher PTA was selected. It should be highlighted that the choice of the 5.6-h cut-off value for meropenem was based on a high PTA at 70% fT>MIC (8-h dose interval). The 70% fT>MIC (defined as the time that the plasma free concentration of meropenem exceeds the MIC) value of meropenem was calculated as 70% × 8 h (the dosage interval) = 5.6 h. Patients with fT>MIC values ≥ 5.6 h were identified as achieving fT>MIC of at least 70% and meeting the target attainment. The relationship between meropenem fT>MIC and effectiveness and safety were explored.
Effectiveness assessment
The assessment of response to meropenem therapy was based on clinical characteristics (signs and symptoms of infection and inflammatory markers) and/or radiological (X-ray or computed tomography scans) findings at first 48–72 h after meropenem treatment and the point when meropenem therapy was completed. Clinical response was defined as improvements in signs and symptoms, laboratory testing values, and infection resolution without worsening of severe pneumonia by chest X-ray examination. Failure was defined as the demonstration of no improvement or deterioration of signs and symptoms, laboratory testing values, or the requirement of additional antibiotics (Schuler, 1995). The effective rates (ER) were calculated using the following equation:
where Ni is the numbers of patients with clinical improvement in children with fT>MIC ≥ 5.6 h or children with fT>MIC < 5.6 h, N is the total numbers of children in the corresponding group above.
Safety assessment
AEs that occurred during meropenem use were recorded and classified by severity (mild, moderate, severe, or life-threatening) and relationship (possibly, probably, certainly, probably not, or certainly not related) to treatment by the investigator independently before and after therapy (Cohen-Wolkowiez et al., 2012; Liu et al., 2018).
Statistical analysis
Data are expressed as the means ± standard deviation (SD). Proportions were compared by the χ2 or Fisher’s exact tests, as appropriate. Continuous variables were compared by the t-test or Wilcoxon test, as appropriate. Statistical significance was defined by a two-sided p value of 0.05. All statistical analyses were performed using IBM SPSS software version 25.0.
Results
Patients
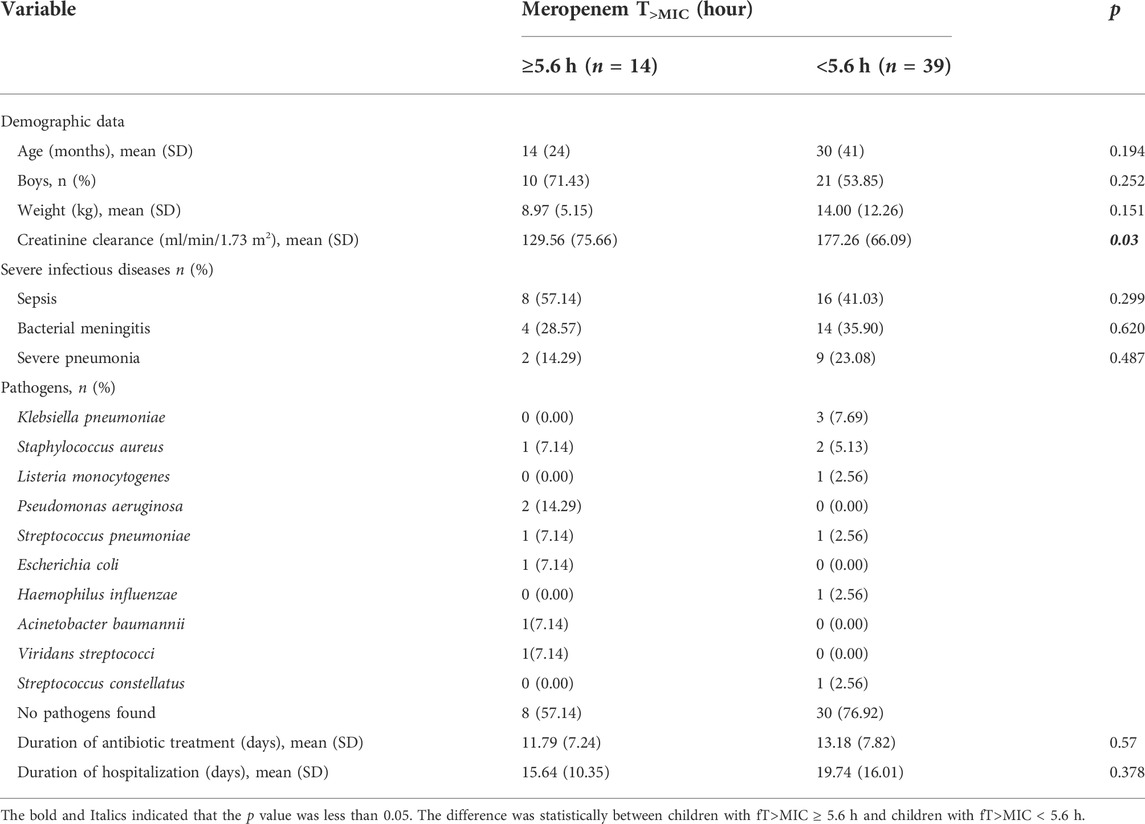
From July 2019 to November 2021, 98 critically ill children with infections who had received intravenous meropenem were screened. There were 53 patients (18 bacterial meningitis, 24 sepsis, and 11 severe pneumonia patients) available for PD analysis after exclusion of 35 patients on two antibiotics (including meropenem), one patient with Kawasaki disease, three patients out of study, three patients with trauma, two patients who had been infected with virus combined and one patient with meropenem treatment for 1 day. The mean age and weight of the 53 children at the time of study were 26 ± 38 months and 12.67 ± 11.07 kg, respectively. There were no significant differences in diseases distribution between the two groups (Table 1).
Calculation of meropenem fT>MIC levels
Meropenem was administrate in the enrolled patients with sepsis, severe pneumonia (20 mg/kg/dose) and bacterial meningitis (40 mg/kg/dose) respectively. The median duration of therapy was 13 days. 111 meropenem concentrations were obtainable. The median number of samples per patients was 2 (range, 1–4). The median meropenem concentration of PK samples was 1.16 (range, 0.2–147.24) μg/ml. The median fT>MIC was 4.38 h (range, 3.48–8.0 h).
Effectiveness assessment
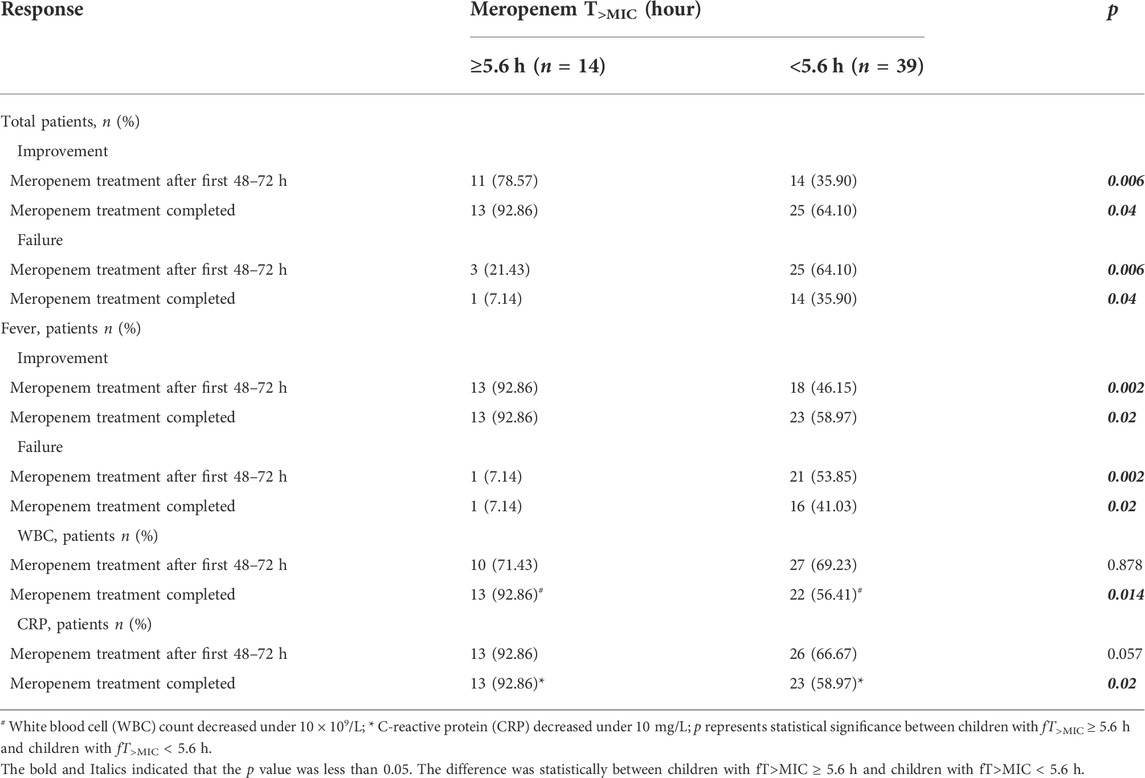
fT>MIC of meropenem was <5.6 h in 39 cases (73.58%) and ≥5.6 h in 14 cases (26.42%). Significant improvement in antibacterial efficacy at first 48–72 h after meropenem treatment (ER: 78.57% v 35.90%, p = 0.006) and the point when meropenem therapy was completed (ER: 92.86% v 64.10%, p = 0.04) was found in children with fT>MIC ≥ 5.6 h compared with children with fT>MIC < 5.6 h. Significantly decreases in fever were present between the two groups at first 48–72 h (p = 0.002) and the point meropenem therapy completed (p = 0.02). Additionally, Clear decreases in white blood cell (WBC) counts (p = 0.014) and C-reactive protein (CRP) (p = 0.02) were also observed between the two groups after meropenem treatment. No significant differences were observed for other effectiveness parameters (Table 2).
fT>MIC of meropenem was <5.6 h in 14 cases (77.78%) and ≥5.6 h in 4 cases (22.22%) in children with bacterial meningitis. More decreases in fever, WBC and CRP were found between the two groups at first 48–72 h and the point meropenem therapy completed. However, only improvement in fever was significant at the early treatment stage(p = 0.023) (Table 3).
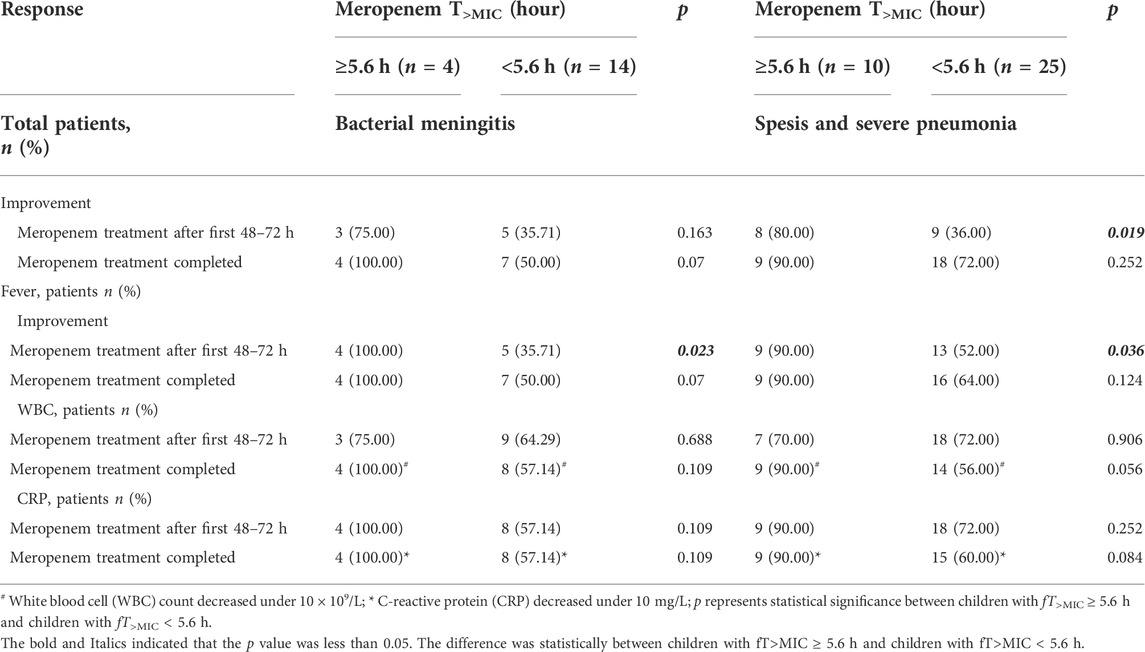
TABLE 3. Clinical response to meropenem therapy in children with bacterial meningitis or spesis and severe pneumonia.
Safety assessment
All 53 patients were assessed for treatment safety. Fourteen mild AEs were reported in ten (18.87%) patients, including granulocytopenia (6), aspartate aminotransferase (5), alanine aminotransferase increase (2), and rash (1). There was no statistically significant difference in meropenem fT>MIC between children with or without AEs (p = 0.715). No patients discontinued meropenem treatment in response to AEs and no drug-related AEs were causally associated with meropenem treatment.
Discussion
Optimizing PK exposure of antibiotics to meet suitable target attainment in critically ill patients could improve infection-related outcomes and reduce bacterial antibiotic resistance. A previous study reported that adjusting doses to achieve a fT>MIC of at least 70% of the dosage interval was more likely to eradicate the causative pathogen (Scaglione et al., 2009). To the best of our knowledge, our study shows the clinical benefits of a high PTA for meropenem therapy in critically ill children.
To ensure better clinical outcome, 70% fT>MIC was selected as the PD target in critically ill infants and children associated with immunodeficient states (Ariano et al., 2005; Scaglione et al., 2009; Franciscus van der Meer et al., 2011). Our study did find that there was significantly clinical improvement in children with fT>MIC ≥ 5.6 h than children with fT>MIC < 5.6 h. We further simulated the clinical efficacy of meropenem with lower fT>MIC (60% of the dosage interval = 4.8 h) as the PD target in these children. However, it was no significant difference between the children with fT>MIC ≥ 4.8 h and children with fT>MIC < 4.8 h (p > 0.05, range 0.141–0.905).
According to the instructions, meropenem was administered at 40 or 20 mg/kg in children with bacterial meningitis or other infections, respectively. There was no difference in disease distribution between two groups (p = 0.299–0.620). Therefore minor effect under different doses for meropenem was related with our conclusion. In addition, our study showed the better clinical benefits (fever, WBC and CRP) of a high PTA of meropenem therapy in children with bacterial meningitis (n = 18). Unfortunately, most of the differences were not significant which associated with the limited patients enrolled. Similar results were also found in children (n = 35) with severe pneumonia (n = 11) and sepsis (n = 24) (Table 3). When the analysis was performed target the whole patients, the differences between the two groups were dramatical as the total population increased (Table 2).
In our study, clinical signs improved in 71.70% cases, which is similar to what Punpanich et al. (2012) reported (68.1%) with a standard dosing regimen for meropenem. However, their regimen could not reach the PD target for susceptible and multi-resistant organisms in critically ill children (Wang et al., 2020). As shown here, optimization of the dose regimen for meropenem is a viable means of improving its efficacy. Children with a high PTA had a significant improvement in antibacterial efficacy compared with children with low target attainment at both the early and final stages of meropenem treatment. Length of hospitalization also decreased in children with a high PTA, although the difference between the two groups was not statistically significant, which is possibly attributed to the limited number of patients. Furthermore, the proportion of patients with decreased inflammatory markers in the group with fT>MIC ≥ 5.6 h was lower than that in the group with fT>MIC < 5.6 h, but this was also not significant. However, for the patients with inflammatory markers reduced to normal levels, there were significant differences between the two groups. Taken together, a high PTA observably increases the probability of recovery.
We further found that the children with fT>MIC < 5.6 h had higher creatinine clearance than children with fT>MIC ≥ 5.6 h (177.26 v129.56 ml/min/1.73 m2), which resulted in rapid excretion of meropenem and lower fT>MIC. To determine the optimal dosage regimens, we evaluated treatment frequency and infusion time, showing that 40 mg/kg/dose (q8h) with 4-h infusion and 110 mg/kg/day with continuous infusion can achieve PTA for bacteria with a low MIC (≤2 mg/L) and high MIC (2–8 mg/L), respectively (Wang et al., 2020). Multicenter random clinical trials associated with the efficacy and safety of these recommended regimens are under way.
There are several limitations in our study. Firstly, this study was limited by the small number of children enrolled. Secondly, the selection of the cut-off value (70% fT>MIC = 5.6 h, MIC = 1 mg/L) for PTA of meropenem was mainly based on our microbiological and PK-PD data. Different values and MICs might be targeted with bacterial pathogens with higher MICs. Finally, only patients on meropenem monotherapy were included in this study. Because combined antimicrobial therapy might introduce additional complicating factors in the interpretation of the relationship between PTA of meropenem and clinical outcome.
Conclusion
Our study demonstrates the clinical benefits of a high PTA with meropenem therapy in critically ill children with infections. Children with meropenem fT>MIC ≥ 5.6 h showed better antibacterial efficacy. Prospective trials to confirm this discovery in a larger pediatric population will be conducted in future studies.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of each participating hospital (2019-k-185, 2020-6-1) and registered on clinicaltrials.gov (ID: NCT03643497). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
ZW, JB, and DY contributed equally to this paper. ZW, WZ, and AS conceived and designed the study. ZW, JB, DY, GL, YT, JY, ZJ, TJ, XT, HQ, LeD, LiD, and QZ contributed to patient recruitment, data collection, data analysis and data interpretation. ZW wrote the first draft of the manuscript. JB, DY, WZ, and AS provided administrative, technical, or material support. WZ and AS supervised the study. ZW, WZ, and AS contributed to the critical revision of the manuscript for important intellectual content. WZ and AS contributed equally to this work. All authors reviewed and approved the final version of the manuscript.
Funding
The study was funded by the Special Research Project of Capital Health Development (2020-1-1991) and Beijing Natural Science Foundation (J200005). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We would like to acknowledge the support of Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Baoding Children’s Hospital, Hebei Children’s Hospital, Henan Children’s Hospital and Beijing Friendship Hospital, Capital Medical University.
Conflict of interest
The reviewer HH declared a shared parent affiliation with the authors ZW, GL, XT, HQ, and AS to the handling editor at the time of review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alsultan, Abdullah, Dasuqi, Shereen A., Aljamaan, Fadi, Omran, Rasha A., Ali Syed, Saeed, AlJaloud, Turki, et al. (2021). Pharmacokinetics of meropenem in critically ill patients in Saudi Arabia. Saudi Pharm. J. 29, 1272–1277. doi:10.1016/j.jsps.2021.09.017
Ariano, Robert E., Anna, Nyhlén, Donnelly, J. Peter, Sitar, Daniel S., Harding, Godfrey K. M., and Zelenitsky, Sheryl A. (2005). Pharmacokinetics and pharmacodynamics of meropenem in febrile neutropenic patients with bacteremia. Ann. Pharmacother. 39, 32–38. doi:10.1345/aph.1E271
Author Anonymous (2018). 2018 surveillance of meningitis (bacterial) and meningococcal septicaemia in under 16s: Recognition, diagnosis and management. London: NICE guideline CG102.
Bradley, John S., Sauberan, Jason B., Ambrose, Paul G., Bhavnani, Sujata M., Rasmussen, Maynard R., and Capparelli, Edmund V. (2008). Meropenem pharmacokinetics, pharmacodynamics, and Monte Carlo simulation in the neonate. Pediatr. Infect. Dis. J. 27, 794–799. doi:10.1097/INF.0b013e318170f8d2
Cies, J. J., Moore, W. S., Enache, A., and Chopra, A. (2017). Population pharmacokinetics and pharmacodynamic target attainment of meropenem in critically ill young children. J. Pediatr. Pharmacol. Ther. 22, 276–285. doi:10.5863/1551-6776-22.4.276
Cies, Jeffrey J., Moore, Wayne S., J Dickerman, Mindy, Small, Christine, Carella, Dominick, Chopra, Arun, et al. (2014). Pharmacokinetics of continuous-infusion meropenem in a pediatric patient receiving extracorporeal life support. Pharmacotherapy 34, e175–e179. doi:10.1002/phar.1476
Cohen-Wolkowiez, Michael, Poindexter, Brenda, Bidegain, Margarita, Schelonka, Robert L., Randolph, David A., Ward, Robert M., et al. (2012). Safety and effectiveness of meropenem in infants with suspected or complicated intra-abdominal infections. Clin. Infect. Dis. 55, 1495–1502. doi:10.1093/cid/cis758
Dellinger, R. Phillip, Levy, Mitchell M., Rhodes, Andrew, Annane, Djillali, Gerlach, Herwig, Opal, Steven M., et al. (2013). Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 41, 580–637. doi:10.1097/CCM.0b013e31827e83af
Dudley, M. N., and Ambrose, P. G. (2000). Pharmacodynamics in the study of drug resistance and establishing in vitro susceptibility breakpoints: Ready for prime time. Curr. Opin. Microbiol. 3, 515–521. doi:10.1016/s1369-5274(00)00132-6
Franciscus van der Meer, A., Marcus, Marco A. E., DaniëlTouw, J., JohannesProost, H., and Neef, Cees (2011). Optimal sampling strategy development methodology using maximum a posteriori Bayesian estimation. Ther. Drug Monit. 33, 133–146. doi:10.1097/FTD.0b013e31820f40f8
Hassan, Hazem E., Ivaturi, Vijay, Gobburu, Jogarao, and Green, Thomas P. (2019). Dosage regimens for meropenem in children with Pseudomonas infections do not meet serum concentration targets. Clin. Transl. Sci. 13, 301–308. doi:10.1111/cts.12710
Kongthavonsakul, Kritsana, Lucksiri, Aroonrut, Eakanunkul, Suntara, Roongjang, Somjing, and Oberdorfer, Peninnah (2016). Pharmacokinetics and pharmacodynamics of meropenem in children with severe infection. Int. J. Antimicrob. Agents 48, 151–157. doi:10.1016/j.ijantimicag.2016.04.025
Liu, Shuping, Zheng, Yi, Wu, Xirong, Xu, Baoping, Liu, Xiuyun, Feng, Guoshuang, et al. (2018). Early target attainment of azithromycin therapy in children with lower respiratory tract infections. J. Antimicrob. Chemother. 73, 2846–2850. doi:10.1093/jac/dky273
Mathew, S. K., Mathew, B. S., Neely, M. N., Naik, G. S., Prabha, Ratna, Jacob, G. G., et al. (2016). A nonparametric pharmacokinetic approach to determine the optimal dosing regimen for 30-minute and 3-hour meropenem infusions in critically ill patients. Ther. Drug Monit. 38, 593–599. doi:10.1097/FTD.0000000000000323
Punpanich, Warunee, Srisarang, Suchada, and Prachantasen, Uraiwan (2012). Therapeutic effectiveness of the generic preparation of meropenem (Mapenem) in the treatment of moderate to severe infection in children. J. Med. Assoc. Thai 95, 895–902.
Rapp, Mélanie, Urien, Saïk, Foissac, Frantz, Béranger, Agathe, Bouazza, Naïm, Benaboud, Sihem, et al. (2019). Population pharmacokinetics of meropenem in critically ill children with different renal functions. Eur. J. Clin. Pharmacol. 76, 61–71. doi:10.1007/s00228-019-02761-7
Scaglione, F., Esposito, S., Leone, S., Lucini, V., Pannacci, M., Ma, L., et al. (2009). Feedback dose alteration significantly affects probability of pathogen eradication in nosocomial pneumonia. Eur. Respir. J. 34, 394–400. doi:10.1183/09031936.00149508
Schuler, D. (1995). Safety and efficacy of meropenem in hospitalised children: Randomised comparison with cefotaxime, alone and combined with metronidazole or amikacin. Meropenem paediatric study group. J. Antimicrob. Chemother. 36, 99–108. doi:10.1093/jac/36.suppl_a.99
Subspecialty Group of Respiratory Diseases (2013). The society of pediatrics, Chinese medical association, editorial board, Chinese journal of pediatrics[guidelines for management of community acquired pneumonia in children (the revised edition of 2013) (I)]. Zhonghua Er Ke Za Zhi 51, 745–752.
Thalhammer, F., and Hörl, W. H. (2000). Pharmacokinetics of meropenem in patients with renal failure and patients receiving renal replacement therapy. Clin. Pharmacokinet. 39, 271–279. doi:10.2165/00003088-200039040-00003
Wang, Ze-Ming, Chen, Xiao-Yu, Jing, Bi, Wang, Mei-Ying, Xu, Bao-Ping, Tang, Bo-Hao, et al. (2020). Reappraisal of the optimal dose of meropenem in critically ill infants and children: A developmental pharmacokinetic-pharmacodynamic analysis. Antimicrob. Agents Chemother. 64, e00760. doi:10.1128/AAC.00760-20
Wayne, P. A. (2019). Performance standards for antimicrobial susceptibility testing. 29th ed. Wayne, PA: Clinical and Laboratory Standards InstituteCLSI supplement M100.
Keywords: fT>MIC, meropenem, children, critically ill, pharmacodynamic target attainment
Citation: Wang Z, Bi J, You D, Tang Y, Liu G, Yu J, Jin Z, Jiang T, Tian X, Qi H, Dong L, Dong L, Zhang Q, Zhao W and Shen A (2022) Improving the efficacy for meropenem therapy requires a high probability of target attainment in critically ill infants and children. Front. Pharmacol. 13:961863. doi: 10.3389/fphar.2022.961863
Received: 05 June 2022; Accepted: 09 September 2022;
Published: 05 October 2022.
Edited by:
Jian Gao, Shanghai Children’s Medical Center, ChinaReviewed by:
Hairong Huang, Beijing Chest Hospital, Capital Medical University, ChinaLeping Ye, First Hospital, Peking University, China
Copyright © 2022 Wang, Bi, You, Tang, Liu, Yu, Jin, Jiang, Tian, Qi, Dong, Dong, Zhang, Zhao and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhao, emhhbzR3ZWkyQGhvdG1haWwuY29t; Adong Shen, c2hlbmFkb25nQGJjaC5jb20uY24=
†These authors have contributed equally to this work
 Zeming Wang1,2†
Zeming Wang1,2† Hui Qi
Hui Qi Wei Zhao
Wei Zhao Adong Shen
Adong Shen