- 1Department of Internal Medicine, First Affiliated Hospital of Heilongjiang University of Chinese Medicine, Harbin, China
- 2Department of Internal Medicine, Heilongjiang University of Chinese Medicine, Harbin, China
- 3Department of Obstetrics and Gynecology, Heilongjiang University of Chinese Medicine, Harbin, China
- 4Department of Obstetrics and Gynecology, First Affiliated Hospital of Heilongjiang University of Chinese Medicine, Harbin, China
Objectives: This meta-analysis aimed to assess the effectiveness and safety of Chinese herbal medicine (CHM) in treating chronic fatigue syndrome (CFS).
Methods: Nine electronic databases were searched from inception to May 2022. Two reviewers screened studies, extracted the data, and assessed the risk of bias independently. The meta-analysis was performed using the Stata 12.0 software.
Results: Eighty-four RCTs that explored the efficacy of 69 kinds of Chinese herbal formulas with various dosage forms (decoction, granule, oral liquid, pill, ointment, capsule, and herbal porridge), involving 6,944 participants were identified. This meta-analysis showed that the application of CHM for CFS can decrease Fatigue Scale scores (WMD: –1.77; 95%CI: –1.96 to –1.57; p < 0.001), Fatigue Assessment Instrument scores (WMD: –15.75; 95%CI: –26.89 to –4.61; p < 0.01), Self-Rating Scale of mental state scores (WMD: –9.72; 95%CI:–12.26 to –7.18; p < 0.001), Self-Rating Anxiety Scale scores (WMD: –7.07; 95%CI: –9.96 to –4.19; p < 0.001), Self-Rating Depression Scale scores (WMD: –5.45; 95%CI: –6.82 to –4.08; p < 0.001), and clinical symptom scores (WMD: –5.37; 95%CI: –6.13 to –4.60; p < 0.001) and improve IGA (WMD: 0.30; 95%CI: 0.20–0.41; p < 0.001), IGG (WMD: 1.74; 95%CI: 0.87–2.62; p < 0.001), IGM (WMD: 0.21; 95%CI: 0.14–0.29; p < 0.001), and the effective rate (RR = 1.41; 95%CI: 1.33–1.49; p < 0.001). However, natural killer cell levels did not change significantly. The included studies did not report any serious adverse events. In addition, the methodology quality of the included RCTs was generally not high.
Conclusion: Our study showed that CHM seems to be effective and safe in the treatment of CFS. However, given the poor quality of reports from these studies, the results should be interpreted cautiously. More international multi-centered, double-blinded, well-designed, randomized controlled trials are needed in future research.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022319680], identifier [CRD42022319680].
Introduction
Chronic fatigue syndrome (CFS) is a medically unexplained and debilitating mental and physical condition characterized by persistent fatigue (lasting for at least 6 months) and several other symptoms, including sleep disorders, lengthy malaise after exertion, sore throat, muscle pain, multi-joint pain, tender lymph nodes, headache, impairment of concentration or short-term memory, anxiety, and depression, which lead to severe disability and suffering in patients. Studies have shown that the prevalence of CFS is 0.006%–3% in the general population (Cleare et al., 2015), and 836,000–2.5 million people suffer from CFS in the US alone (Clayton, 2015). In addition, a meta-analysis showed that the overall incidence of CFS is 0.77% and 0.76% in Korea and Japan, respectively (Lim and Son, 2021). If there is no effective treatment, CFS will cause a decline in multi-system function and cause systemic diseases such as immune system, circulatory system, nervous system, digestive system, and visceral dysfunction, thus posing a serious threat to human health.
Although the cause of CFS remains uncertain, popular hypotheses include triggers (viral infections, physical trauma, physical and mental stress, vaccinations, and environmental toxins), microbiome disruption, dysregulated immune response, chronic low-grade inflammation, neuroendocrine abnormalities, oxidative stress, metabolic dysfunction, mitochondrial dysfunction, and genetic predisposition (Brinth et al., 2019; Gregorowski et al., 2019; Noor et al., 2021). These factors can also interact to promote the occurrence and development of CFS. Some studies have suggested that infectious triggers can trigger systemic inflammation by activating the antiviral immune response (Kennedy et al., 2010; Maes et al., 2012; Glassford, 2017; Cortes Rivera et al., 2019). The composition of gut microbes is altered in CFS patients, which might lead to increased intestinal permeability that allows bacterial translocation into the bloodstream, thus increasing systemic inflammation (Deumer et al., 2021). The hypothalamic-pituitary-adrenal (HPA) axis is impaired in patients with CFS, which may result in neuroendocrine abnormalities and metabolic and inflammatory changes (Deumer et al., 2021). In addition, genetic predisposition is associated with autoimmunity (Deumer et al., 2021).
Currently, the treatment of CFS remains suboptimal because there is a lack of an adequate understanding of the mechanisms and etiology of the disease. Current recommendations for the treatment of CFS include cognitive behavioral therapy (CBT), graded exercise therapy (GET), western conventional medicine (WCM), complementary or alternative medicine, and nutritional support therapy. CBT challenges patients’ thoughts to relieve patients’ psychological stress, and this may provide short-term benefits but does not permanently reduce symptoms (Fernie et al., 2016; Geraghty and Blease, 2018). Exercise therapy, including aerobic exercises (e.g., walking, jogging, swimming, and cycling) and anaerobic exercises (e.g., strength and stability exercises), could improve physical function and reduce fatigue (Marques et al., 2015; Larun et al., 2017). However, some patients have expressed disappointment with GET because it can interfere with the outcome of alternative treatments and may indirectly exacerbate symptoms in patients (Goudsmit and Howes, 2017; Geraghty and Blease, 2019). Western conventional medicines such as immune modulators, antivirals, antidepressants, antibiotics, and medications to treat specific symptoms that are used for treating CFS have insufficient evidence for their efficacy and may cause serious adverse effects (Mücke et al., 2015; Smith et al., 2015; Yang et al., 2017). In addition, alternative medicine (e.g., meditation and relaxation response, warm baths, massages, stretching, acupuncture, hydrotherapy, chiropractic, yoga, and Tai Chi), nutritional support therapy, transcutaneous electrical nerve stimulation, physiotherapy, and nerve blocks have all been proposed, but the evidence regarding these treatments is limited and their efficacy is uncertain (Bested and Marshall, 2015; Noor et al., 2021).
Chinese herbal medicine (CHM) has been widely used to treat CFS in China and other parts of the world, such as South Korea and Japan (Wang et al., 2014; Joung et al., 2019; Shin et al., 2021). First, according to the dialectical treatment theory of traditional Chinese medicine, specific formulas consisting of different Chinese herbs are used to treat CFS patients with different symptoms. Such treatment tailored to the patient’s specific needs is urgently needed given the obvious heterogeneity in CFS symptoms. The pathogenesis of CFS in traditional Chinese medicine is the deficiency of qi, blood, and yin and yang, accompanied by the stagnation of qi, fire, phlegm, and blood. The treatment is focused on tonifying deficiencies and relieving bruising. CHM such as Panax ginseng C.A.Mey., Codonopsis pilosula (Franch.) Nannf., and Astragalus mongholicus Bunge can nourish deficiency and improve fatigue and lengthy malaise after exertion in CFS patients, whereas Bupleurum falcatum L. and Citrus × aurantium L., among others, can resolve stagnation and relieve pain, insomnia, swollen lymph nodes, and other symptoms. Therefore, CHM can not only improve the main symptoms, but also relieve the accompanying symptoms in CFS. Second, modern pharmacological research has demonstrated that the modern use of CHM in treating CFS mainly focuses on adjusting immune dysfunction, acting as an antioxidant, improving the energy metabolism disorder, and regulating abnormal activity in the HPA axis (Chen et al., 2010; Chi et al., 2016). Buzhong Yiqi decoction, Kuibi decoction, Danggui Buxue decoction, Young Yum pill, and Renshen Yangrong decoction can regulate the immune function of patients with CFS and relieve fatigue symptoms (Ogawa et al., 1992; Shin et al., 2004; Chen et al., 2010; Yin et al., 2021; Miao et al., 2022). Ginsenoside, Jujube polysaccharide conjugate, Quercetin, Withania somnifera (L.) Dunal, Hypericum perforatum L., and Ginkgo biloba L. can be antioxidants (Logan and Wong, 2001; Singh et al., 2002; Chi et al., 2015). Schisandra Chinensis Polysaccharide (SCP), HEP2-a extracted from Epimedium brevicornum Maxim., can improve energy metabolism and can regulate the abnormal activity of the HPA axis (Chi et al., 2016; Chi et al., 2017). Additionally, multiple randomized controlled trials (RCTs) have reported that CHM significantly improves fatigue, insomnia, and other concomitant symptoms; reduces negative emotions such as anxiety and depression; and clearly improves treatment effectiveness and quality of life compared to exercise therapy and alternative therapy (Wang et al., 2011; Kong, 2012; Wang, 2021). Systematic reviews and meta-analyses comparing CHM with western medicine also confirmed the above views (Peng et al., 2013; Wang et al., 2014). These studies demonstrate the remarkable efficacy and comprehensiveness of CHM for CFS, which is consistent with treatment guidelines emphasizing a holistic, patient-centered approach that considers the patient’s physical, mental, and social well-being (Baker and Shaw, 2007). Finally, CHM has no serious side effects and is relatively safe to treat CFS.
A previous meta-analysis and another systematic review indicated the beneficial role of CHM as a complementary approach for CFS (Peng et al., 2013; Wang et al., 2014). However, those studies were limited in terms of sample size and outcome indicators because the systematic review only assessed 10 RCTs (including 919 patients), and the meta-analysis of 11 RCTs (including 1,049 patients) only assessed clinical efficacy rates and lacked sufficient evidence. In addition, nearly 50 new trials assessing the effects of CHM for CFS have been published since the previous systematic reviews and meta-analyses were published. Therefore, we conducted a larger systematic review and meta-analysis including more outcome indicators (FS-14, FAI, SCL-90, SAS, SDS, clinical symptom scores, IGA, IGG, IGM, NK cell levels, effective rate, and adverse events) to provide a comprehensive update of previously published studies and stronger evidence for the effectiveness of CHM for CFS.
Methods
Protocol and registration
This meta-analysis was reported in compliance with the PRISMA statement, and the protocol was registered on PROSPERO (CRD42022319680). [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022319680]. The full details of the protocol are available on request.
Search strategy
Electronic databases including PubMed, Embase, Cochrane Library, Web of Science, the Chinese National Knowledge Infrastructure (CNKI), Wanfang Database, Chinese VIP Database, the US Clinical Trials Registry, and the Chinese Clinical Trials Registry were systematically searched from their inception to May 2022. There was no restriction on language. The search terms used included “Fatigue Syndrome, Chronic”, “CFS”, “Chronic Fatigue Syndrome”, “Myalgic Encephalomyelitis”, “ME”, “Encephalomyelitis, Myalgic”, “Chronic Fatigue Disorder”, “Fatigue Disorder, Chronic”, “Systemic Exertion Intolerance Disease”, “Chinese herbal medicine”, “Chinese traditional”, “Oriental traditional”, “traditional Chinese medicine”, “traditional Chinese medicinal materials”, “Chinese herb”, “herbal medicine”, “herbal”, “decoction”, “tang”, “pill”, “wan”, “powder”, “formula”, “granule”, “capsule”, “particles”, “ointment”, “prescription”, “receipt”, “placebo”, “random controlled trial”, “random”, and “RCT”. The full details of the search strategy are available (Additional file 1). In addition, we performed manual searches in the reference lists of previously published systematic reviews and meta-analyses on the subject to further look for potentially eligible studies. The search was conducted independently by two authors (YZ and WS).
Eligibility criteria
Types of studies
RCTs assessing the efficacy and safety of CHM in the treatment of CFS were included in our review. We only extracted data from the CHM and control groups when we found relevant studies with three treatment groups.
Types of participants
Trials of participants over the age of 16 were included regardless of gender, culture, or setting. CFS was diagnosed using the Center for Disease Control criteria (1987, 1994, or 1998), the Guiding Principles for Clinical Research of New Chinese Medicines (2002), Chinese medicine internal disease diagnosis and treatment routines, the clinical research guidelines for new Chinese medicines for CFS, Chinese internal medicine diagnoses, or the diagnostic efficacy criteria for Chinese medical evidence. All patients had the primary symptom of unexplained fatigue that lasted at least 6 months accompanied by four or more of the following symptoms: unrefreshing sleep, lengthy malaise after exertion, impairment of concentration or short-term memory, sore throat, tender lymph nodes, multi-joint pain, and headaches.
Types of interventions
The formulations of CHM were included. CHM is defined as medicinal raw materials derived from medicinal plants, minerals, and animal sources, according to the Chinese Pharmacopoeia edited in 2020 (Chinese Pharmacopoeia Commission, 2020). A formulation of CHM is usually made up of two or more herbs to produce a synergistic effect on specific illnesses. These materials are prescribed by doctors based on the individual characteristics of the patient according to the dialectical treatment theory of traditional Chinese medicine (Xiong et al., 2019; Chinese Pharmacopoeia Commission, 2020).
Participants were treated with CHM alone or combined with WCM, GET, or health guidance. We did not place any limits on the formulation of CHM or the duration of treatment, but CHM was required to be taken orally. We did not include experiments combining Chinese herbal medicine with other traditional Chinese medicine treatments.
Types of controls
Patients in the control group used WCM, GET, health guidance, or placebo, with no limit on the duration of treatment. We did not include experiments combining any Chinese medicine therapy.
Types of outcome measures
The primary outcome measures were Fatigue Scale (FS-14) and Fatigue Assessment Instrument (FAI) scores. The secondary outcome measures were Self-Rating Scale of mental state (SCL-90) scores, Self-Rating Anxiety Scale (SAS) scores, Self-Rating Depression Scale (SDS) scores, clinical symptom scores, immunological indicators (IGA, IGG, IGM, and NK cell levels), effective rate, and adverse events.
The clinical symptom scores are used to assess the severity of fatigue. The main symptoms and other symptoms of CFS are scored according to their severity, and a higher cumulative score of all symptoms indicates more severe fatigue symptoms. The effective rate is a measure to assess clinical efficacy. It is assessed at the end of treatment using four grades: clinical cure (the patients’ clinical symptoms were basically cured, and they could live and work normally), markedly effective (the cure rate for major and concomitant clinical symptoms up to 2/3), effective (the cure rate for major and concomitant clinical symptoms is 1/3 to 2/3), and invalid (the cure rate for major and concomitant clinical symptoms <1/3).
Study selection
Study selection was performed independently by two authors (YZ and FJ) according to the inclusion criteria. After eliminating duplicates, they independently scanned the title/abstract and full text to identify eligible studies. Any disagreements were settled by a discussion with a third evaluator (WS).
Data extraction
Two investigators (YZ and XW) independently reviewed and extracted the following information: general information (first author, year of publication, region, and types), characteristics of the participants (sample size, age, gender, and course of disease), details of the intervention and the comparison (type of intervention and duration), and outcomes. Any discrepancies were resolved by discussions or adjudication by a third reviewer (YP).
Quality assessment
The risk of bias in the included studies was evaluated independently by two authors (XW and FJ) using the Cochrane collaboration tool with the following seven domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. Each domain can be classified as “low-risk,” “high-risk,” or “uncertain risk.” Any differences were resolved by discussion with a third evaluator.
Statistical analysis
Statistical analysis was performed using the Stata software (version 12.0; StataCorp, College Station, TX). The weighted mean difference (WMD) for continuous variables and the risk ratio (RR) for dichotomous data with 95% confidence intervals (Cl) were used. Heterogeneity was assessed by the Q test and the I2 statistic. When p ≥ 0.10 and I2 ≤ 50%, the fixed-effect model was used; otherwise, the random effects model was used. p ≤ 0.05 was considered statistically significant. The publication bias was assessed by funnel plots and Egger’s test if the number of trials was sufficient. When heterogeneity was detected, the sensitivity analysis was conducted to assess the stability of the results by excluding individual studies one by one. Subgroup analysis was performed to explore the sources of heterogeneity according to treatment method (CHM vs. WCM, CHM plus WCM vs. WCM, CHM vs. GET, CHM plus GET vs. GET, CHM vs. health guidance, and CHM vs. placebo) and duration of the intervention (≤30 days vs. 31–60 days vs. > 60 days) based on different treatment methods.
Results
Literature search
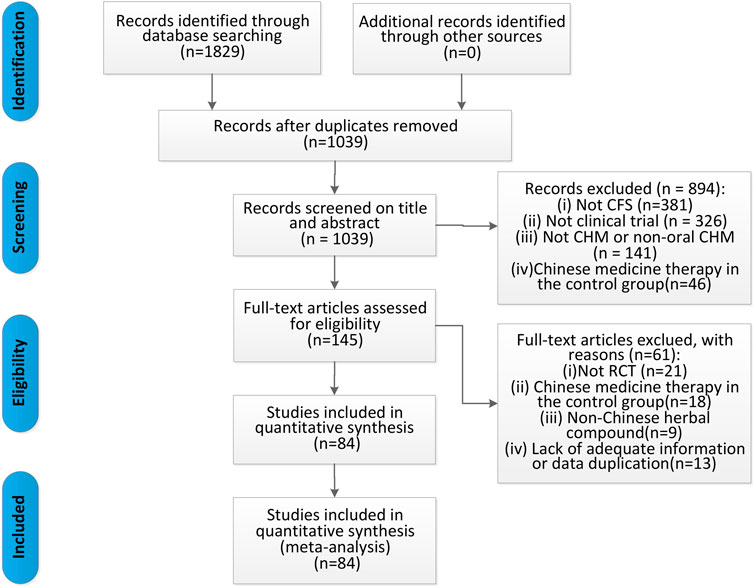
We identified 1,829 articles in the original screening. After eliminating duplicates, 1,039 remained, 894 of which were excluded because they did not meet the inclusion criteria after scanning the titles and abstracts. Moreover, we reviewed the full text of the remaining 145 articles and deleted 61 articles due to the following reasons: 1) non-RCTs, 2) Chinese medicine therapy used in the control group, 3) non-Chinese herbal compounds used, 4) published using repeated data, and 5) missing data. Finally, 84 articles were included in the meta-analysis (Figure 1).
Study characteristics and quality assessment
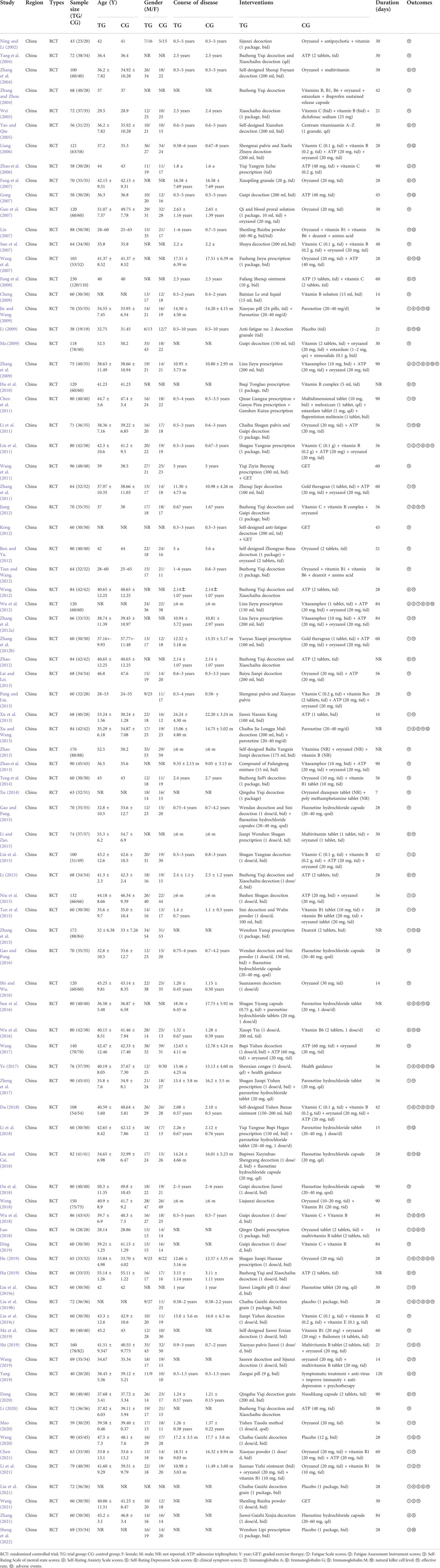
A total of 84 RCTs were included, published from 2002 to 2022, and all studies were conducted in China. The sample sizes in the studies varied from 38 to 230 patients, with a total sample size of 3,552 patients in the treatment groups and 3,392 patients in the control groups. The duration of diseases lasted from 0.5 to 24.27 years. Of the 84 studies, five trials (Li, 2009; Liu J. et al., 2019; Wang, 2020; Liu et al., 2021; Sheng et al., 2022) compared CHM with placebo, whereas comparisons of CHM alone vs. WCM were performed in 63 studies (Ning and Li, 2002; Yang et al., 2004; Zhang et al., 2004; Zhang and Zhou, 2004; Wei, 2005; Yao and Qiu, 2005; Liang, 2006; Zhao et al., 2006; Fang et al., 2007; Gong, 2007; Lin, 2007; Sun et al., 2007; Wang et al., 2007; Fang et al., 2008; Cheng, 2009; Ma, 2009; Zhang et al., 2009; Hu et al., 2010; Chen et al., 2011; Li et al., 2011; Liu et al., 2011; Zhang et al., 2011; Zhang Z. X. et al., 2012; Zhang L. P. et al., 2012; Jiang, 2012; Tian and Wang, 2012; Wang, 2012; Wu et al., 2012; Zhao, 2012; Lai and Lei, 2013; Pang and Liu, 2013; Xu et al., 2013; Zhao, 2013; Zhao et al., 2013; Teng et al., 2014; Xu, 2014; Li, 2015; Li and Zao, 2015; Liu et al., 2015; Niu et al., 2015; Tan et al., 2015; Zhang et al., 2015; Shi and Wu, 2016; Wu et al., 2016; Du, 2018; Luo, 2018; Ou et al., 2018; Weng, 2018; Wu et al., 2018; Liu F. et al., 2019; Liu Y. et al., 2019; Ding, 2019; He, 2019; Hu, 2019; Ma et al., 2019; Shi, 2019; Wang, 2019; Yang, 2019; Dong, 2020; Li, 2020; Mao, 2020; Chen, 2021; Zhang, 2021). CHM plus WCM vs. WCM was compared in 12 studies (Guo et al., 2007; Jie and Wang, 2009; Ren and Yu, 2012; Xu and Wang, 2013; Gao and Pang, 2015; Gao and Pang, 2016; Sun et al., 2016; Wang, 2017; Zheng et al., 2017; Li et al., 2018; Liu and Cai, 2018; Li et al., 2021); CHM vs. GET was compared in one study (Wang, 2021); CHM plus GET vs. GET was compared in two studies (Wang et al., 2011; Kong, 2012); and CHM vs. health guidance was compared in one study (Ye, 2017). The course of treatment ranged from 7 to 120 days. In the outcome indicators, 26 studies (Jie and Wang, 2009; Chen et al., 2011; Li et al., 2011; Liu et al., 2011; Zhang et al., 2011; Zhang L. P. et al., 2012; Xu et al., 2013; Xu and Wang, 2013; Liu et al., 2015; Niu et al., 2015; Tan et al., 2015; Zhang et al., 2015; Ye, 2017; Zheng et al., 2017; Du, 2018; Luo, 2018; Liu F. et al., 2019; Liu J. et al., 2019; He, 2019; Shi, 2019; Mao, 2020; Wang, 2020; Chen, 2021; Li et al., 2021; Liu et al., 2021; Wang, 2021) reported FS-14 scores; nine studies (Zhao et al., 2006; Zhang et al., 2009; Liu et al., 2011; Wu et al., 2012; Liu et al., 2015; Luo, 2018; Wang, 2019; Li et al., 2021; Wang, 2021) reported FAI scores; five studies (Zhang et al., 2009; Zhang Z. X. et al., 2012; Wu et al., 2012; Niu et al., 2015; Sheng et al., 2022) reported SCL-90 scores; seven studies (Jie and Wang, 2009; Xu and Wang, 2013; Sun et al., 2016; Ye, 2017; Yang, 2019; Liu et al., 2021; Zhang, 2021) reported SAS scores; six studies (Jie and Wang, 2009; Xu and Wang, 2013; Sun et al., 2016; Ye, 2017; Liu et al., 2021; Zhang, 2021) reported SDS scores; 24 studies (Zhang et al., 2004; Yao and Qiu, 2005; Fang et al., 2007; Wang et al., 2007; Fang et al., 2008; Li, 2009; Hu et al., 2010; Wang, 2012; Zhao, 2012; Li, 2015; Li and Zao, 2015; Sun et al., 2016; Wu et al., 2016; Ye, 2017; Du, 2018; Liu and Cai, 2018; Luo, 2018; Liu J. et al., 2019; He, 2019; Hu, 2019; Shi, 2019; Dong, 2020; Wang, 2020; Liu et al., 2021) reported clinical symptom scores; eight studies (Zhang et al., 2009; Liu et al., 2011; Jiang, 2012; Wu et al., 2012; Du, 2018; Wu et al., 2018; Liu J. et al., 2019; He, 2019) reported the level of IGA, IGG, and IGM; three studies (Zhang et al., 2009; Wu et al., 2012; Sheng et al., 2022) reported the NK cell levels; and 79 studies (Ning and Li, 2002; Yang et al., 2004; Zhang et al., 2004; Zhang and Zhou, 2004; Wei, 2005; Yao and Qiu, 2005; Liang, 2006; Zhao et al., 2006; Fang et al., 2007; Gong, 2007; Guo et al., 2007; Lin, 2007; Sun et al., 2007; Wang et al., 2007; Fang et al., 2008; Cheng, 2009; Jie and Wang, 2009; Li, 2009; Ma, 2009; Zhang et al., 2009; Hu et al., 2010; Chen et al., 2011; Li et al., 2011; Liu et al., 2011; Wang et al., 2011; Zhang et al., 2011; Jiang, 2012; Kong, 2012; Ren and Yu, 2012; Tian and Wang, 2012; Wang, 2012; Zhang Z. X. et al., 2012; Zhang L. P. et al., 2012; Zhao, 2012; Lai and Lei, 2013; Pang and Liu, 2013; Xu et al., 2013; Xu and Wang, 2013; Zhao, 2013; Zhao et al., 2013; Teng et al., 2014; Xu, 2014; Gao and Pang, 2015; Li and Zao, 2015; Li, 2015; Tan et al., 2015; Zhang et al., 2015; Gao and Pang, 2016; Shi and Wu, 2016; Sun et al., 2016; Wu et al., 2016; Wang, 2017; Ye, 2017; Zheng et al., 2017; Du, 2018; Li et al., 2018; Liu and Cai, 2018; Ou et al., 2018; Weng, 2018; Wu et al., 2018; Luo, 2018; Ding, 2019; He, 2019; Hu, 2019; Liu F. et al., 2019; Liu J. et al., 2019; Liu Y. et al., 2019; Ma et al., 2019; Shi, 2019; Wang, 2019; Yang, 2019; Dong, 2020; Li, 2020; Mao, 2020; Wang, 2020; Chen, 2021; Li et al., 2021; Liu et al., 2021; Wang, 2021) reported effective rate. The occurrence of adverse effects was reported in 14 studies (Liang, 2006; Gong, 2007; Lin, 2007; Wang et al., 2007; Jie and Wang, 2009; Li, 2009; Li et al., 2011; Xu and Wang, 2013; Zhang et al., 2015; Sun et al., 2016; Wu et al., 2016; Ye, 2017; Li et al., 2018; Liu and Cai, 2018). The basic characteristics of the included studies are summarized in Table 1, and components of CHM used in the included studies are presented in Table 2.
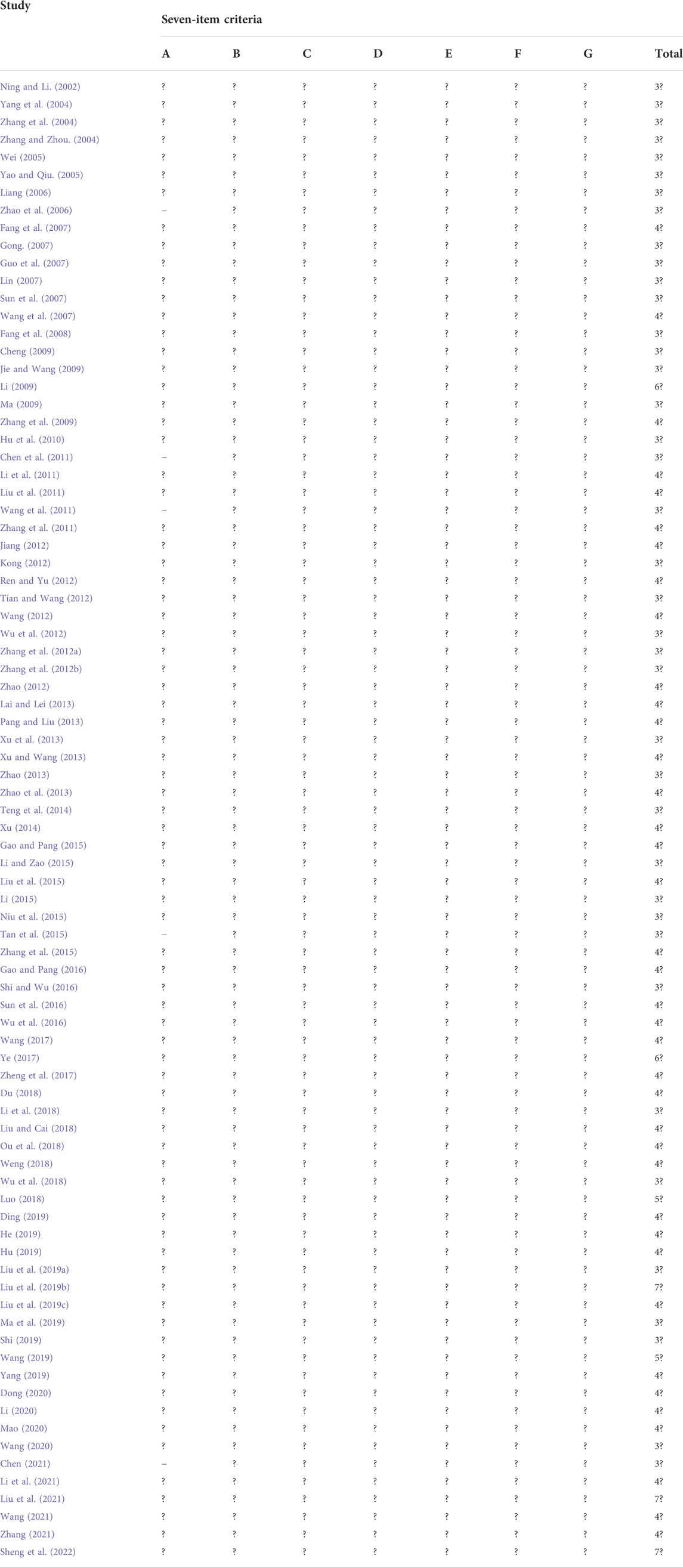
The quality assessment of the included studies is listed in Table 3. The Cochrane score ranged from 3 to 7, and three studies (Liu J. et al., 2019; Liu et al., 2021; Sheng et al., 2022) got 7 points; two studies (Li, 2009; Ye, 2017) got 6 points; two studies (Luo, 2018; Wang, 2019) got 5 points; 38 studies (Fang et al., 2007; Wang et al., 2007; Zhang et al., 2009; Li et al., 2011; Liu et al., 2011; Zhang et al., 2011; Jiang, 2012; Ren and Yu, 2012; Wang, 2012; Zhao, 2012; Lai and Lei, 2013; Pang and Liu, 2013; Xu and Wang, 2013; Zhao et al., 2013; Xu, 2014; Gao and Pang, 2015; Liu et al., 2015; Zhang et al., 2015; Gao and Pang, 2016; Sun et al., 2016; Wu et al., 2016; Wang, 2017; Zheng et al., 2017; Du, 2018; Liu and Cai, 2018; Ou et al., 2018; Weng, 2018; Liu Y. et al., 2019; Ding, 2019; He, 2019; Hu, 2019; Yang, 2019; Dong, 2020; Li, 2020; Mao, 2020; Li et al., 2021; Wang, 2021; Zhang, 2021) got 4 points; and 39 studies (Ning and Li, 2002; Yang et al., 2004; Zhang et al., 2004; Zhang and Zhou, 2004; Wei, 2005; Yao and Qiu, 2005; Liang, 2006; Zhao et al., 2006; Gong, 2007; Guo et al., 2007; Lin, 2007; Sun et al., 2007; Fang et al., 2008; Cheng, 2009; Jie and Wang, 2009; Ma, 2009; Hu et al., 2010; Chen et al., 2011; Wang et al., 2011; Kong, 2012; Tian and Wang, 2012; Wu et al., 2012; Zhang Z. X. et al., 2012; Zhang L. P. et al., 2012; Xu et al., 2013; Zhao, 2013; Teng et al., 2014; Li and Zao, 2015; Li, 2015; Niu et al., 2015; Tan et al., 2015; Shi and Wu, 2016; Li et al., 2018; Wu et al., 2018; Liu F. et al., 2019; Ma et al., 2019; Shi, 2019; Wang, 2020; Chen, 2021) got 3 points. All of the included studies reported random allocation, and 44 studies (Fang et al., 2007; Wang et al., 2007; Li, 2009; Zhang et al., 2009; Li et al., 2011; Liu et al., 2011; Zhang et al., 2011; Jiang, 2012; Ren and Yu, 2012; Wang, 2012; Zhao, 2012; Lai and Lei, 2013; Pang and Liu, 2013; Xu and Wang, 2013; Zhao et al., 2013; Xu, 2014; Gao and Pang, 2015; Liu et al., 2015; Zhang et al., 2015; Gao and Pang, 2016; Sun et al., 2016; Wu et al., 2016; Wang, 2017; Ye, 2017; Zheng et al., 2017; Du, 2018; Liu and Cai, 2018; Luo, 2018; Ou et al., 2018; Weng, 2018; Liu J. et al., 2019; Ding, 2019; He, 2019; Hu, 2019; Wang, 2019; Yang, 2019; Dong, 2020; Li, 2020; Mao, 2020; Li et al., 2021; Liu et al., 2021; Wang, 2021; Zhang, 2021; Sheng et al., 2022) described the method of random sequence generation, whereas the remaining 40 studies (Ning and Li, 2002; Yang et al., 2004; Zhang et al., 2004; Zhang and Zhou, 2004; Wei, 2005; Yao and Qiu, 2005; Liang, 2006; Zhao et al., 2006; Gong, 2007; Guo et al., 2007; Lin, 2007; Sun et al., 2007; Fang et al., 2008; Cheng, 2009; Jie and Wang, 2009; Ma, 2009; Hu et al., 2010; Chen et al., 2011; Wang et al., 2011; Kong, 2012; Tian and Wang, 2012; Wu et al., 2012; Zhang Z. X. et al., 2012; Zhang L. P. et al., 2012; Xu et al., 2013; Zhao, 2013; Teng et al., 2014; Li and Zao, 2015; Li, 2015; Niu et al., 2015; Tan et al., 2015; Shi and Wu, 2016; Li et al., 2018; Wu et al., 2018; Liu F. et al., 2019; Liu Y. et al., 2019; Ma et al., 2019; Shi, 2019; Wang, 2020; Chen, 2021) provided no details. Five studies (Li, 2009; Ye, 2017; Liu J. et al., 2019; Liu et al., 2021; Sheng et al., 2022) mentioned concealment allocation. Three trials (Liu J. et al., 2019; Liu et al., 2021; Sheng et al., 2022) reported double blinding of patients and physicians, and eight trials (Li, 2009; Ye, 2017; Luo, 2018; Liu J. et al., 2019; Liu Y. et al., 2019; Wang, 2019; Liu et al., 2021; Sheng et al., 2022) described blinding of participants. All studies met the criterion of incomplete outcome data as drop-out data, or no drop-out patients were reported specifically. Pre-designed outcomes were reported in all studies, detecting a low risk of reporting bias, and other biases were not found in all included studies.
Results of meta-analysis
Primary outcomes
FS-14 scores
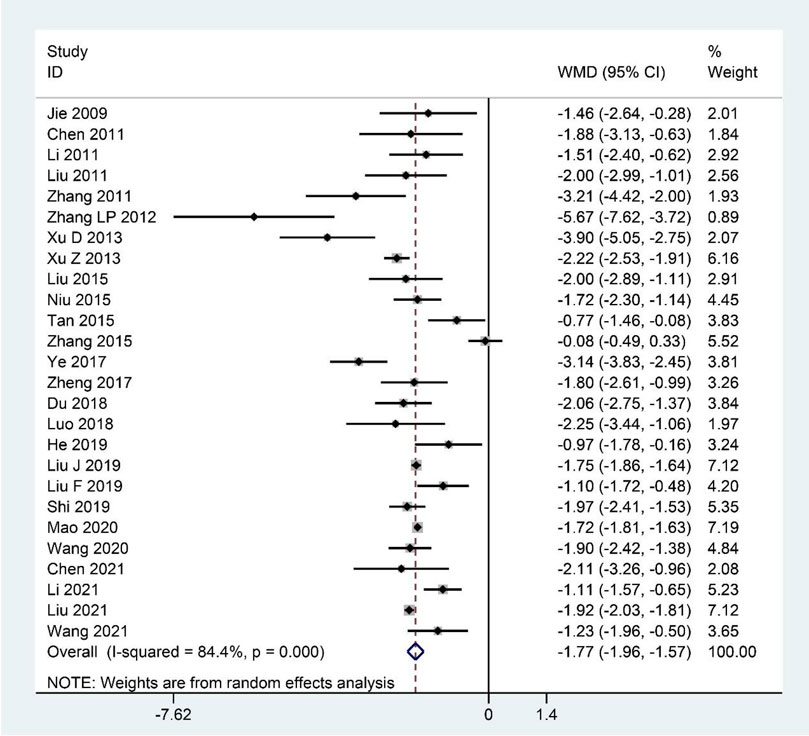
Pooled data from the 26 studies (Jie and Wang, 2009; Chen et al., 2011; Li et al., 2011; Liu et al., 2011; Zhang et al., 2011; Zhang L. P. et al., 2012; Xu et al., 2013; Xu and Wang, 2013; Liu et al., 2015; Niu et al., 2015; Tan et al., 2015; Zhang et al., 2015; Ye, 2017; Zheng et al., 2017; Du, 2018; Luo, 2018; Liu F. et al., 2019; Liu J. et al., 2019; He, 2019; Shi, 2019; Mao, 2020; Wang, 2020; Chen, 2021; Li et al., 2021; Liu et al., 2021; Wang, 2021) reporting the FS-14 scores showed that CHM clearly decreased the FS-14 scores as an adjuvant or monotherapy for CFS compared with the contrast group (WMD: –1.77; 95%CI: –1.96 to –1.57; p < 0.001; p for heterogeneity <0.001; I2 = 84.4%; Figure 2). The subgroup analysis showed similar results (Table 4).
FAI scores
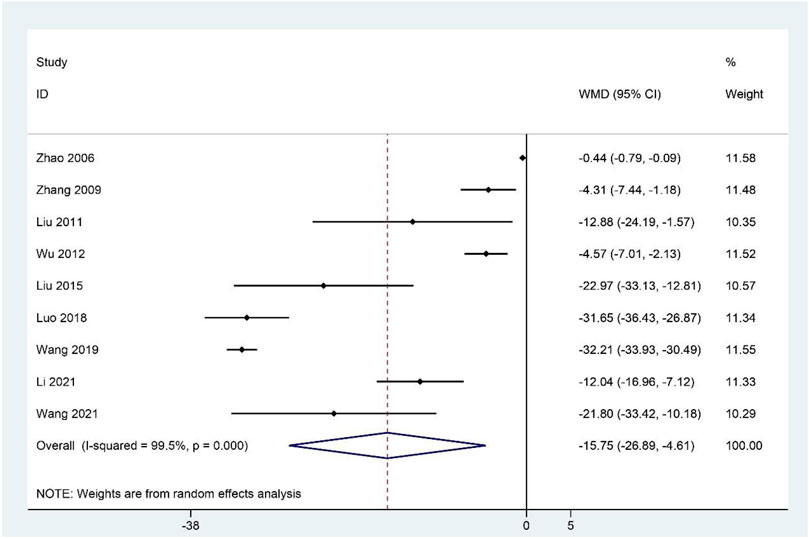
Meta-analysis of the nine studies (Zhao et al., 2006; Zhang et al., 2009; Liu et al., 2011; Wu et al., 2012; Liu et al., 2015; Luo, 2018; Wang, 2019; Li et al., 2021; Wang, 2021) reporting the FAI scores showed that the treatment group had significantly decreased FAI scores compared to the control group (WMD: –15.75; 95%CI: –26.89 to –4.61; p < 0.01; p for heterogeneity <0.001; I2 = 99.5%; Figure 3). Subgroup analysis revealed no significant difference (WMD: –2.88; 95%CI: –6.15 to 0.38; p = 0.084; p for heterogeneity <0.001; I2 = 87.7%) between CHM and WCM groups when the duration of intervention >60 days, whereas the relationship between the CHM treatment group and lower FAI scores remained constant in the other subgroups (Table 4).
Secondary outcomes
SCL-90 scores
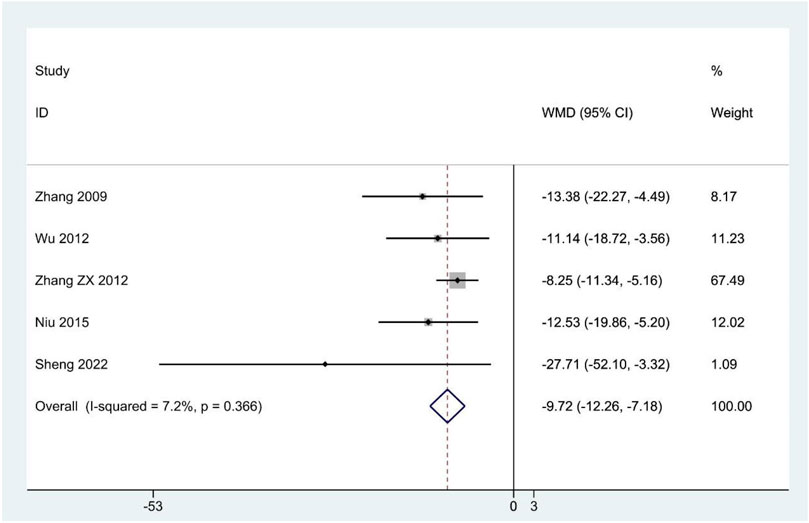
The SCL-90 scores were reported in five studies (Zhang et al., 2009; Zhang Z. X. et al., 2012; Wu et al., 2012; Niu et al., 2015; Sheng et al., 2022). The pooled results suggested that SCL-90 scores were significantly lower in the CHM group compared to the contrast group (WMD: –9.72; 95%CI: –12.26 to –7.18; p < 0.001; p for heterogeneity = 0.366; I2 = 7.2%; Figure 4), and the subgroup analysis showed similar results (Table 4).
SAS scores
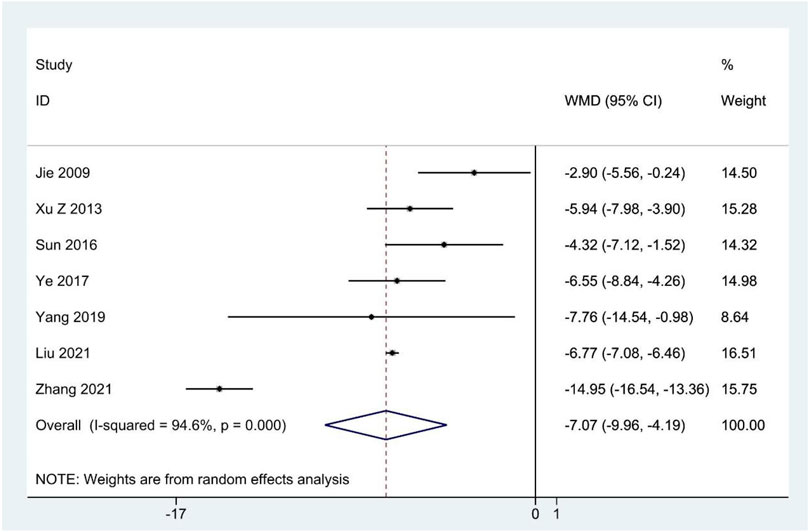
Seven studies (Jie and Wang, 2009; Xu and Wang, 2013; Sun et al., 2016; Ye, 2017; Yang, 2019; Liu et al., 2021; Zhang, 2021) reported the SAS scores, and meta-analysis indicated that CHM therapy clearly decreased SAS scores compared to the contrast group (WMD: –7.07; 95%CI: –9.96 to –4.19; p < 0.001; p for heterogeneity <0.001; I2 = 94.6%; Figure 5), and subgroup analysis showed that the results remained constant (Table 4).
SDS scores
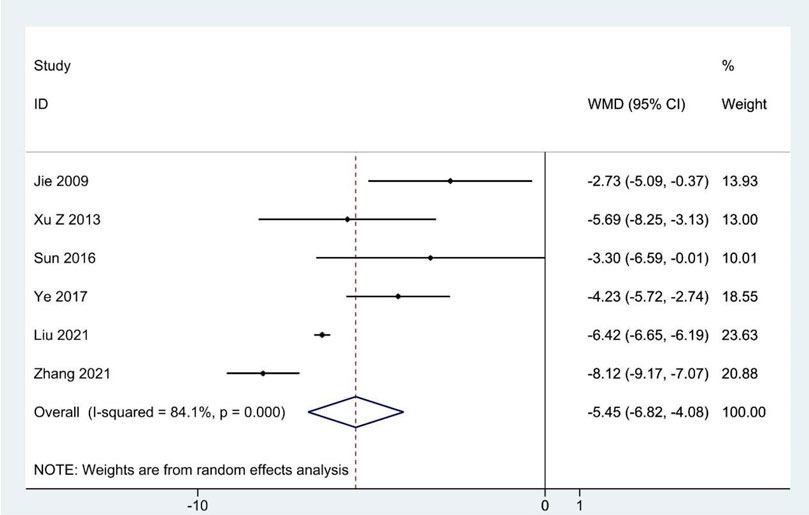
Meta-analysis of six RCTs reporting the SDS scores (Jie and Wang, 2009; Xu and Wang, 2013; Sun et al., 2016; Ye, 2017; Liu et al., 2021; Zhang, 2021) showed that the experimental group had significantly reduced SDS scores compared to the contrast group (WMD: –5.45; 95%CI: –6.82 to –4.08; p < 0.001; p for heterogeneity <0.001; I2 = 84.1%; Figure 6). Subgroup analysis showed that the results remained constant (Table 4).
Clinical symptom scores
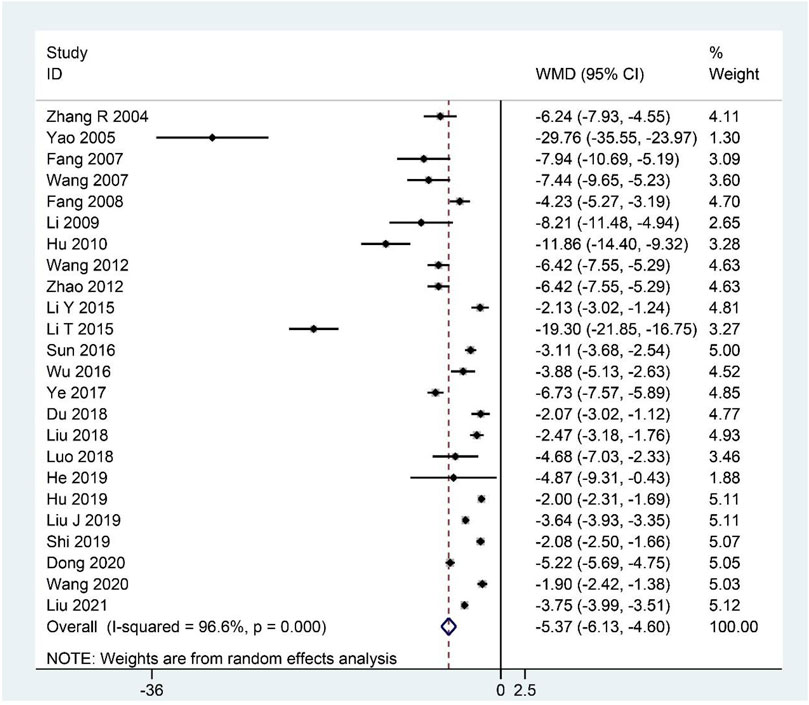
The summary data of 24 studies (Zhang et al., 2004; Yao and Qiu, 2005; Fang et al., 2007; Wang et al., 2007; Fang et al., 2008; Li, 2009; Hu et al., 2010; Wang, 2012; Zhao, 2012; Li, 2015; Li and Zao, 2015; Sun et al., 2016; Wu et al., 2016; Ye, 2017; Du, 2018; Liu and Cai, 2018; Luo, 2018; Liu J. et al., 2019; He, 2019; Hu, 2019; Shi, 2019; Dong, 2020; Wang, 2020; Liu et al., 2021) demonstrated that CHM, as an adjuvant or monotherapy, significantly decreased the clinical symptom scores compared with the control group (WMD: –5.37; 95%CI: –6.13 to –4.60; p < 0.001; p for heterogeneity <0.001; I2 = 96.6%; Figure 7). Subgroup analysis was performed, showing that the conclusion that CHM is relatively effective in treating CFS remained unchanged in each subgroup (Table 4).
Immunological indicators
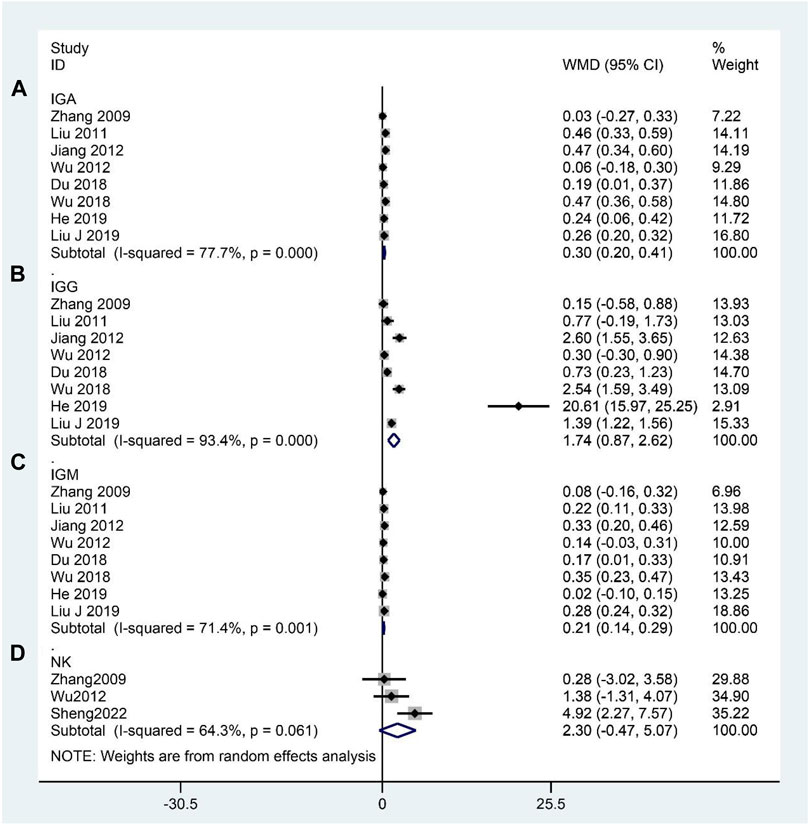
We identified eight RCTs that reported the IGA, IGG, and IGM levels (Zhang et al., 2009; Liu et al., 2011; Jiang, 2012; Wu et al., 2012; Du, 2018; Wu et al., 2018; Liu J. et al., 2019; He, 2019). Meta-analysis indicated that CHM significantly elevated IGA (WMD: 0.30; 95%CI: 0.20–0.41; p < 0.001; p for heterogeneity <0.001; I2 = 77.7%; Figure 8A); IGG (WMD: 1.74; 95%CI: 0.87–2.62; p < 0.001; p for heterogeneity <0.001; I2 = 93.4%; Figure 8B); and IGM (WMD: 0.21; 95%CI: 0.14–0.29; p < 0.001; p for heterogeneity <0.01; I2 = 71.4%; Figure 8C) compared to the contrast group. Three studies (Zhang et al., 2009; Wu et al., 2012; Sheng et al., 2022) reported the NK cell levels, and the meta-analysis indicated no significant difference between the experimental and control groups (WMD: 2.30; 95%CI: –0.47 to 5.07; p = 0.104; p for heterogeneity = 0.061; I2 = 64.3%; Figure 8D). Subgroup analyses of the IGA and IGG revealed no significant difference (WMD: 0.21; 95%CI: –0.12 to 0.53; p = 0.218; p for heterogeneity <0.01; I2 = 86.1%, WMD: 0.95; 95%CI: –0.36 to 2.27; p = 0.154; p for heterogeneity <0.001; I2 = 89.1%, respectively) between the CHM and WCM groups when the duration of intervention >60 days, and the subgroup analysis of the IGM showed no statistical significance (WMD: 0.02; 95%CI: –0.10 to 0.15; p = 0.712; no heterogeneity) between the CHM and WCM groups when the duration of intervention ≤30 days. The rest of the results indicated that the conclusion that CHM can elevate IGA, IGG, and IGM remained constant (Table 4). The subgroup analysis of the NK cell showed no statistical significance (WMD: 0.94; 95%CI: –1.14 to 3.03; p = 0.376; p for heterogeneity = 0.613; I2 = 0.0%) between the CHM and WCM groups, whereas one study comparing CHM with placebo showed that CHM significantly elevated the NK cell levels (WMD: 4.92; 95%CI: 2.27–7.57; p < 0.001; no heterogeneity) (Table 4).
Effective rate
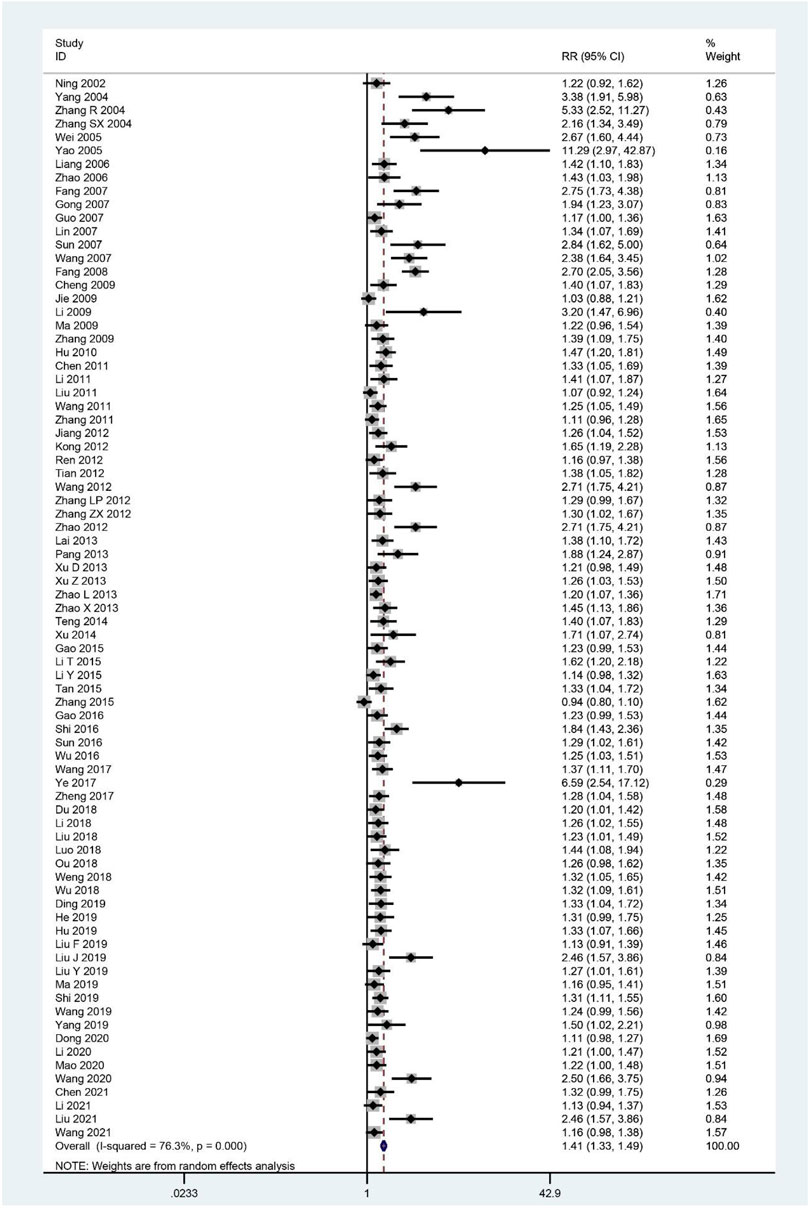
The effective rate was evaluated in 79 trials (Ning and Li, 2002; Yang et al., 2004; Zhang et al., 2004; Zhang and Zhou, 2004; Wei, 2005; Yao and Qiu, 2005; Liang, 2006; Zhao et al., 2006; Fang et al., 2007; Gong, 2007; Guo et al., 2007; Lin, 2007; Sun et al., 2007; Wang et al., 2007; Fang et al., 2008; Cheng, 2009; Jie and Wang, 2009; Li, 2009; Ma, 2009; Zhang et al., 2009; Hu et al., 2010; Chen et al., 2011; Li et al., 2011; Liu et al., 2011; Wang et al., 2011; Zhang et al., 2011; Jiang, 2012; Kong, 2012; Ren and Yu, 2012; Tian and Wang, 2012; Wang, 2012; Zhang Z. X. et al., 2012; Zhang L. P. et al., 2012; Zhao, 2012; Lai and Lei, 2013; Pang and Liu, 2013; Xu et al., 2013; Xu and Wang, 2013; Zhao, 2013; Zhao et al., 2013; Teng et al., 2014; Xu, 2014; Gao and Pang, 2015; Li and Zao, 2015; Li, 2015; Tan et al., 2015; Zhang et al., 2015; Gao and Pang, 2016; Shi and Wu, 2016; Sun et al., 2016; Wu et al., 2016; Wang, 2017; Ye, 2017; Zheng et al., 2017; Du, 2018; Li et al., 2018; Liu and Cai, 2018; Ou et al., 2018; Weng, 2018; Wu et al., 2018; Luo, 2018; Ding, 2019; He, 2019; Hu, 2019; Liu F. et al., 2019; Liu J. et al., 2019; Liu Y. et al., 2019; Ma et al., 2019; Shi, 2019; Wang, 2019; Yang, 2019; Dong, 2020; Li, 2020; Mao, 2020; Wang, 2020; Chen, 2021; Li et al., 2021; Liu et al., 2021; Wang, 2021), and the pooled results showed that it was higher in the treatment group compared to the control group (RR = 1.41; 95%CI: 1.33–1.49; p < 0.001; p for heterogeneity <0.001; I2 = 76.3%; Figure 9). Subgroup analysis showed no significant difference (RR: 1.13; 95%CI: 0.99 to 1.28; p = 0.062; p for heterogeneity = 0.234; I2 = 31.1%) in CHM plus WCM compared with WCM when 30 days < intervention time ≤ 60 days, and only one study compared CHM and GET, showing similar results (RR: 1.16; 95%CI: 0.98–1.38; p = 0.093; no heterogeneity), whereas the rest of the results revealed that effectiveness of CHM for CFS remained constant (Table 4).
Adverse events
Adverse events were reported in 14 studies (Liang, 2006; Gong, 2007; Lin, 2007; Wang et al., 2007; Jie and Wang, 2009; Li, 2009; Li et al., 2011; Xu and Wang, 2013; Zhang et al., 2015; Sun et al., 2016; Wu et al., 2016; Ye, 2017; Li et al., 2018; Liu and Cai, 2018), and two studies (Sun et al., 2007; Wang, 2017) reported that no adverse events occurred. The rest of the studies did not report the presence or absence of adverse events. The adverse events in the CHM group included mild nausea, dry mouth, indigestion, constipation, and fever. The majority of adverse events were mild, and serious adverse events or deaths were not found in the included studies, which suggests that CHM is relatively safe in patients with CFS.
Sensitivity analysis
We conducted a sensitivity analysis on FS-14, FAI, SAS, SDS, clinical symptom scores, IGA, IGG, IGM, NK cell levels, and effective rate. After we excluded each study one by one, the pooled WMD or RR for the rest of the RCTs did not change significantly, indicating that the result data were robust (Additional file 2).
Publication bias
The funnel plot showed a symmetric distribution of trials on either side of the funnel, and Egger’s test (p = 0.795) was consistent with the funnel plot, indicating that no significant publication bias existed in this meta-analysis (Additional file 3).
Description of the CHM
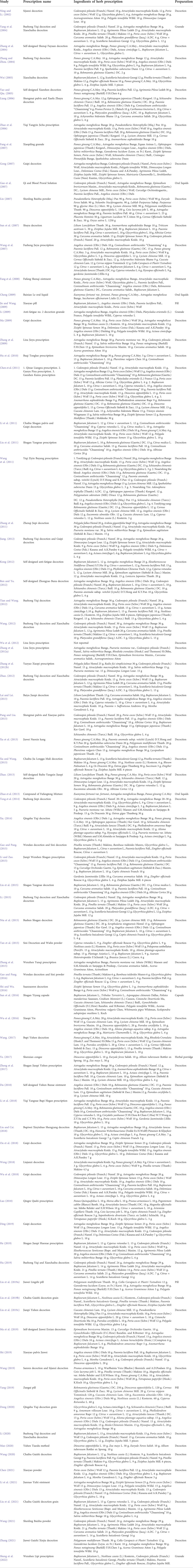
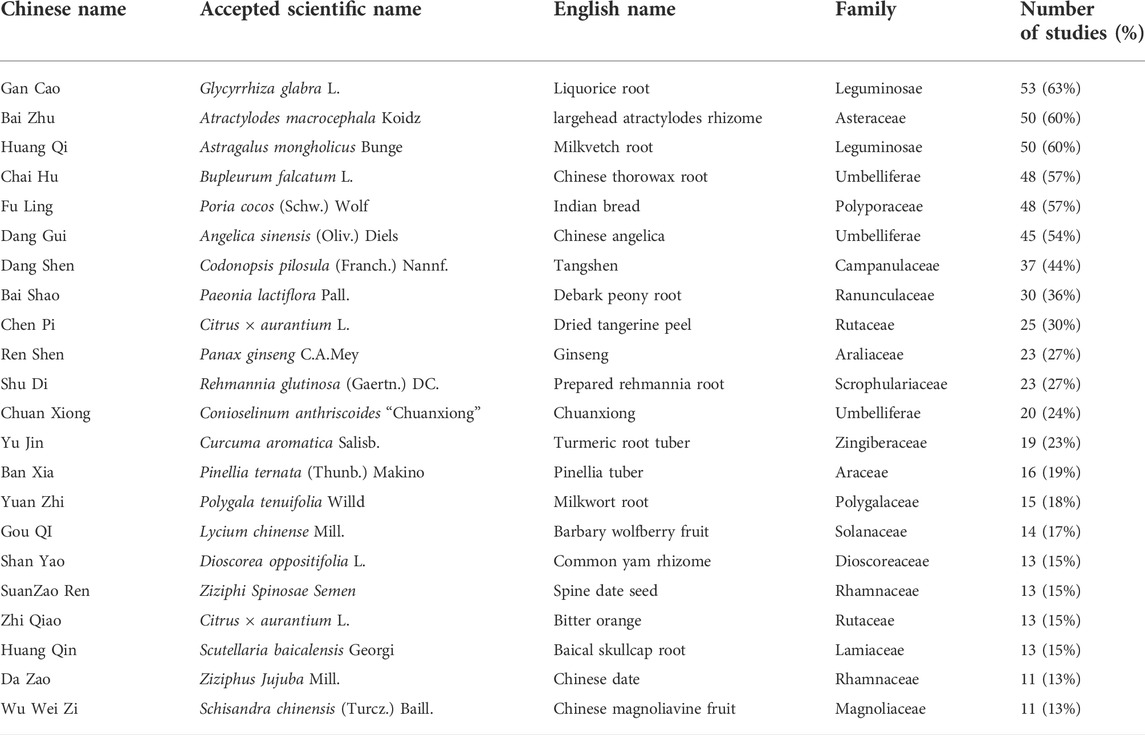
Our study evaluated 69 kinds of Chinese herbal formulas, including 54 decoctions, five granules, three oral liquids, three pills, two ointments, one capsule, and one herbal porridge. The most frequently used herbs in all formulations contained Chai Hu (Bupleurum falcatum L.); Gan Cao (Glycyrrhiza glabra L.); Bai Zhu (Atractylodes macrocephala Koidz.); Dang Gui [Angelica sinensis (Oliv.) Diels]; Huang Qi (Astragalus mongholicus Bunge); Dang Shen [Codonopsis pilosula (Franch.) Nannf.]; Bai Shao (Paeonia lactiflora Pall.); Fu Ling [Poria cocos (Schw.) Wolf]; Chen Pi (Citrus × Aurantium L.); Shu Di (Rehmannia glutinosa (Gaertn.) DC.); Chuan Xiong (Conioselinum anthriscoides “Chuanxiong”); Yu Jin (Curcuma aromatica Salisb.); Shan Yao (Dioscorea oppositifolia L.); Yuan Zhi (Polygala tenuifolia Willd.); Ban Xia [Pinellia ternata (Thunb.) Makino]; Gou QI (Lycium chinense Mill.); Da Zao (Ziziphus jujuba Mill.); Zhi Qiao (Citrus × Aurantium L.); Suanzaoren (Ziziphi Spinosae Semen); Huang Qin (Scutellaria baicalensis Georgi); Ren Shen (Panax ginseng C.A.Mey.); and Wu Wei Zi [Schisandra chinensis (Turcz.) Baill.] (Table 5).
Discussion
Medically unexplained chronic fatigue, including idiopathic chronic fatigue and CFS, is an unexplained adverse condition characterized by fatigue accompanied by behavioral, emotional, social, and cognitive imbalances. Approximately 10% of the general population suffers from chronic fatigue, which significantly reduces their quality of life and their ability to work. This is an important health care issue, presenting major challenges for its sufferers and health services. At present, a clear therapeutic approach is still lacking, but the use of CHM in patients with chronic fatigue is receiving increasing attention from physicians.
Summary of the evidence
A total of 84 RCTs, including 6,944 individuals, were identified for analysis. The findings demonstrated that CHM as adjuvant therapy or monotherapy for CFS could decrease the FS-14, FAI, SCL-90, SAS, SDS, and clinical symptom scores and improve IGA, IGG, IGM, and the effective rate.
Two internationally recognized scales were used to quantitatively assess fatigue. The FS-14 developed by Trudie Chalde et al. in 1993 consists of 14 items, each of which is a fatigue-related question, and it mainly reflects the changes in fatigue symptoms from two different perspectives (physical fatigue and mental fatigue), thus reflecting the real level of fatigue of patients in a more comprehensive way. The FAI includes 27 fatigue-related questions. Subjects rate each item based on their own performance over the previous 2 weeks, which can accurately and quantitatively evaluate the degree and characteristics of fatigue. In this study, CHM treatment significantly reduced FS-14 and FAI scores, indicating that it improved fatigue symptoms.
Patients with CFS commonly suffer from negative emotions such as anxiety, depression, paranoia, and obsessive-compulsive disorder. The degree of negative emotions is mainly assessed by professional mental status assessment scales such as SCL-90, SAS, and SDS. The present meta-analysis shows that CHM treatment can relatively improve negative emotions in patients with chronic fatigue.
The clinical effective rate and clinical symptom scores were used to evaluate the efficacy of CHM in the treatment of CFS because the severity of clinical symptoms is used to determine whether the disease is in remission. The clinical efficiency rate in patients treated with CHM alone or with CHM plus other treatments (e.g., WCM, GET, or health guidance) was 90% (2,961/3,308). The clinical efficiency rate in patients treated only with WCM, GET, health guidance, or placebo was 62% (1,956/3,149). Thus, CHM treatment clearly increased the efficiency rate and reduced the clinical symptom scores compared to WCM, GET, health guidance, or placebo, thus showing that CHM is effective to some extent for CFS.
Numerous studies have revealed that CFS is associated with immune system dysfunction (Matsuda et al., 2009; Guenther et al., 2015; Hornig et al., 2015; Montoya et al., 2017; Sotzny et al., 2018). Most CFS patients are prone to physical weakness and fatigue due to low immune function. Moreover, when the body tissue is in a state of fatigue for a long time, it will consume and destroy the immune system, which will eventually lead to low immune function. Immunoglobulins (IGA, IGG, and IGM) are important parts of humoral immunity. A study found that IGA, IGG, and IGM levels are significantly lower in patients with CFS than in healthy subjects (Hou et al., 2015). Our meta-analysis showed that the treatment group had elevated IGA, IGG, and IGM levels compared to the control group. In addition, immunological indicators also include NK cells and T lymphocyte subsets (CD4+, CD8+, and CD4+/CD8+). Hou’s study showed that NK cell activity, CD4+, and CD8+ were all significantly reduced in CFS patients (Hou et al., 2015). The results of our meta-analysis did not find any obvious effects of CHM on NK cell activity in CFS patients. However, three trials (Zhang et al., 2009; Wu et al., 2012; Sheng et al., 2022) suggested that CFS patients’ NK cell activity was higher in the CHM treatment group. We cannot reject the positive effect of CHM on the NK cell activity of CFS patients based on the negative results of this meta-analysis, which may be due to the lack of appropriate courses of treatment and limited sample sizes. Furthermore, a study showed that CHM dramatically improved NK cell activities, T cell proliferation, CD4 +/CD8 + ratio, and CD4 + counts in CFS rats, suggesting that CHM can improve the immune function of patients with CFS (Chi et al., 2015). Taken together, CHM may prevent CFS by modulating immune function, but further research is needed to confirm this.
Furthermore, only 14 studies referred to minor adverse reactions, and there were no serious adverse events, showing that CHM generally appears safe and effective for treating CFS. Thus, the present evidence supports that CHM can potentially be recommended for use in CFS patients.
Strengths and limitations
Our study included a large number of RCTs and large sample sizes (84 RCTs with 6,944 patients) and used more internationally recognized outcome measures to assess the effectiveness of CHM for CFS from different aspects. These outcome indicators included not only subjective indicators (FS-14, FAI, SCL-90, SAS, SDS, clinical symptom scores, and effective rate), but also the objective immune indicators IGA, IGG, IGM, NK cell levels, and adverse events. In addition, we included many new trials that were not included in the previous reviews and meta-analyses to provide a comprehensive update. Furthermore, the sensitivity analysis demonstrated that the results of the current meta-analysis are relatively robust, and we found no evidence of publication bias in this meta-analysis by funnel plot and Egger’s test.
Some limitations must be considered. First, although we included RCTs, some methodological limitations still existed in most studies. Specifically, 44 trials supplied sufficient information on the randomization process, only five RCTs described allocation concealment, only three trials reported double blinding of patients and physicians, and only eight trials described blinding of participants. These methodological flaws might generate bias, so our results should be interpreted cautiously. Second, there is significant clinical heterogeneity due to the variations in composition and dosage of CHM and different dosage forms of CHM (e.g., decoction, granule, oral liquid, pill, ointment, and capsule). Finally, all trials were conducted in China, which may limit the generalizability of the findings presented here. Therefore, further international multicenter RCTs are needed to popularize the results globally. Furthermore, we conducted subgroup analyses to explore the sources of heterogeneity based on the different intervention duration and measures. The result showed that the heterogeneity was lower after grouping according to the results of subgroup analyses, indicating that differences in intervention duration and measures may also be the underlying source of heterogeneity.
Implications for research
Based on the above limitations, some recommendations are suggested for further studies. First, further rigorously designed trials with high methodological quality are urgently needed. We advise designing and reporting RCTs of CFS strictly according to the CONSORT 2010 statement (Schulz et al., 2010) and the CONSORT Extension for Chinese Herbal Medicine Formulas 2017 (Cheng et al., 2017). Random sequence generation, allocation concealment, and blinding should all be strictly implemented in future studies. Second, an efficacy evaluation system in line with the characteristics of CHM should be set up, and sensitive and practical indicators of CHM should be explored. Third, adverse effects were not reported in many studies. Therefore, the presence or absence of adverse events should be reported in future studies based on the standard format of adverse reactions established by Bian et al. (2010), and clinical trials and studies with longer follow-up times should be conducted to confirm the long-term safety of CHM for CFS.
Implications for practice
The evidence available from our study suggested the effectiveness and safety of CHM therapy for CFS. The most commonly used herbs included Bupleurum falcatum L., Glycyrrhiza glabra L., Atractylodes macrocephala Koidz., Angelica sinensis (Oliv.) Diels, Astragalus mongholicus Bunge, Codonopsis pilosula (Franch.) Nannf., Paeonia lactiflora Pall., Poria cocos (Schw.) Wolf, Citrus × aurantium L., Rehmannia glutinosa (Gaertn.) DC., Conioselinum anthriscoides “Chuanxiong,” Curcuma aromatica Salisb., Dioscorea oppositifolia L., Polygala tenuifolia Willd., Pinellia ternata (Thunb.) Makino, Lycium chinense Mill., Ziziphus jujuba Mill., Citrus × aurantium L., Ziziphi Spinosae Semen, Scutellaria baicalensis Georgi, Panax ginseng C.A.Mey., and Schisandra chinensis (Turcz.) Baill. This list can facilitate further exploration of the therapeutic principles of these drugs for CFS in order to further develop herbal prescriptions to improve the efficacy and safety of the treatment of CFS. In addition, the efficacy of CHM depends on the accurate dialectical treatment, and the prescription of CHM should be based on the precise dialectical diagnosis of CFS. Thus, individualized herbal prescriptions can be implemented in future clinical practice by selecting appropriate drugs among the frequently used drugs.
The possible mechanisms of CHM for CFS are as follows. 1) Adjusting the immune dysfunction: a study found that Young Yum Pill, a proprietary herbal drug, could improve immune organ (thymus and spleen) indices, the mitogenic response of lymphocytes, and numbers of T-cell subsets (Yin et al., 2021). Buzhong Yiqi decoction, Kuibi decoction, and Danggui Buxue decoction significantly inhibit tumor necrosis factor-a, IL-6, IL-10, and transforming growth factor-b1 in CFS patients (Shin et al., 2004; Chen et al., 2010; Miao et al., 2022). Furthermore, Renshen Yangrong decoction can ameliorate lower NK cell activity, and extracts of Ginseng can also boost natural killer cell function and the cellular immunity of patients with CFS (Ogawa et al., 1992; See et al., 1997). 2) Antioxidant effects: superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) are two major components of the antioxidative system, and their function is to detoxify reactive oxygen species. Danggui Buxue decoction, Ginsenoside, and Jujube polysaccharide conjugate could improve SOD and GSH-Px activities and decrease MDA levels (Chi et al., 2015; Miao et al., 2022). Additionally, Quercetin, Withania somnifera (L.) Dunal, Hypericum perforatum L., and Ginkgo biloba L. have also been reported to possess beneficial antioxidants for CFS (Logan and Wong., 2001; Singh et al., 2002). 3) Improving metabolic dysfunction: Chi et al.’s study confirmed that SCP treatment affects metabolic pathways, including the TCA cycle and alanine, aspartate, and glutamate metabolism (Chi et al., 2016). Danggui Buxue decoction might regulate serine, glycine, and threonine metabolism to improve energy supply and ameliorate the CFS-weakened immunity (Miao et al., 2022). In addition, HEP2-a increased the creatine level to improve the arginine and proline metabolism (Chi et al., 2017). 4) Regulating the abnormal activity of the HPA axis: Chi inferred that HEP2-a indirectly affected the HPA axis abnormality of CFS by increasing the noradrenaline level (Chi et al., 2017).
Conclusion
In conclusion, the current evidence suggests that CHM, either as adjuvant therapy or monotherapy, decreases FS-14, FAI, SCL-90, SAS, SDS, and clinical symptom scores and enhances IGA, IGG, IGM, and effective rate. However, NK cell levels did not change significantly. In addition, the included studies did not report serious adverse events, suggesting that CHM is relatively safe in patients with CFS. Our findings on commonly used CHM may help investigate their value and further clinical application for CFS. Our study suggests that CHM seems to be effective and safe in the treatment of CFS. However, given the poor quality of the included studies, more international multi-centered, double-blinded, placebo-controlled, well-designed clinical trials are needed in future research.
Author contributions
YZ and WS conceptualized the research question. FJ and YP participated in drafting and writing the review. YZ, FJ, WS, YP, and XW participated in the formulation of retrieval strategies, data acquisition, data analysis, and quality assessment. WS, QJ, and JX participated in the drawing of tables and figures. YZ and WS participated in the critical revision of the manuscript. All authors contributed to the research and approved the final manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81973601), the TCM research projects of Heilongjiang Province (ZHY19-027).
Acknowledgments
We would like to thank YZ and WS for their guidance, which have significantly improved the quality of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.958005/full#supplementary-material
References
Baker, R., and Shaw, E. J. (2007). Diagnosis and Management of Chronic Fatigue Syndrome or Myalgic Encephalomyelitis (Or Encephalopathy): Summary of NICE Guidance. BMJ 335 (7617), 446–448. doi:10.1136/bmj.39302.509005.AE
Bested, A. C., and Marshall, L. M. (2015). Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An Evidence-Based Approach to Diagnosis and Management by Clinicians. Rev. Environ. Health 30 (4), 223–249. doi:10.1515/reveh-2015-0026
Bian, Z. X., Tian, H. Y., Gao, L., Shang, H. C., Wu, T. X., Li, Y. P., et al. (2010). Improving Reporting of Adverse Events and Adverse Drug Reactions Following Injections of Chinese Materia Medica. J. Evid. Based. Med. 3 (1), 5–10. doi:10.1111/j.1756-5391.2010.01055.x
Brinth, L., Nielsen, H., Varming, K., Boonen, S. E., Ebsen, A., Fernández-Guerra, P., et al. (2019). Myalgic Encephalomyelitis or Chronic Fatigue Syndrome. Ugeskr. Laeger 181 (24), V08180570.
Chen, G., Sheng, Z. Y., and Lu, J. (2011). Clinical Observation on 40 Cases of Chronic Fatigue Syndrome Treated by Syndrome Differentiation. Jiangsu J. Tradit. Chin. Med. 43 (3), 46–47.
Chen, R., Moriya, J., Yamakawa, J., Takahashi, T., and Kanda, T. (2010). Traditional Chinese Medicine for Chronic Fatigue Syndrome. Evid. Based. Complement. Altern. Med. 7 (1), 3–10. doi:10.1093/ecam/nen017
Chen, Z. N. (2021). Clinical Observation of Xiaoyao Powder in Treating 33 Cases of Chronic Fatigue Syndrome. Clin. Med. 13 (9), 140–141. doi:10.3969/j.issn.1674-7860.2021.09.05
Cheng, C. W., Wu, T. X., Shang, H. C., Li, Y. P., Altman, D. G., Moher, D., et al. (2017). CONSORT Extension for Chinese Herbal Medicine Formulas 2017: Recommendations, Explanation, and Elaboration (Traditional Chinese Version). Ann. Intern. Med. 167, W7–W20. doi:10.7326/IsTranslatedFrom_M17-2977_1
Cheng, S. H. (2009). Observation on the Efficacy of Fufangteng Mixture in the Treatment of Chronic Fatigue Syndrome. J. Liaoning Univ. Tradit. Chin. Med. 11 (8), 135. doi:10.13194/j.jlunivtcm.2009.08.137.chengshh.018
Chi, A., Kang, C., Zhang, Y., Tang, L., Guo, H., Li, H., et al. (2015). Immunomodulating and Antioxidant Effects of Polysaccharide Conjugates from the Fruits of Ziziphus Jujube on Chronic Fatigue Syndrome Rats. Carbohydr. Polym. 122, 189–196. doi:10.1016/j.carbpol.2014.12.082
Chi, A., Shen, Z., Zhu, W., Sun, Y., Kang, Y., and Guo, F. (2017). Characterization of A Protein-Bound Polysaccharide from Herba Epimedii and its Metabolic Mechanism in Chronic Fatigue Syndrome. J. Ethnopharmacol. 203, 241–251. doi:10.1016/j.jep.2017.03.041
Chi, A., Zhang, Y., Kang, Y., and Shen, Z. (2016). Metabolic Mechanism of A Polysaccharide from Schisandra Chinensis to Relieve Chronic Fatigue Syndrome. Int. J. Biol. Macromol. 93, 322–332. doi:10.1016/j.ijbiomac.2016.08.042
Chinese Pharmacopoeia Commission (2020). The 2020 Edition of Pharmacopoeia of the People’s Republic of China. Beijing, China: Chemical Industry Press.
Clayton, E. W. (2015). Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An IOM Report on Redefining an Illness. JAMA 313 (11), 1101–1102. doi:10.1001/jama.2015.1346
Cleare, A. J., Reid, S., Chalder, T., Hotopf, M., and Wessely, S. (2015). Chronic Fatigue Syndrome. BMJ Clin. Evid. 2015, 1101.
Cortes Rivera, M., Mastronardi, C., Silva-Aldana, C. T., Arcos-Burgos, M., and Lidbury, B. A. (2019). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Comprehensive Review. Diagn. (Basel) 9 (3), 91. doi:10.3390/diagnostics9030091
Deumer, U. S., Varesi, A., Floris, V., Savioli, G., Mantovani, E., López-Carrasco, P., et al. (2021). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): An Overview. J. Clin. Med. 10 (20), 4786. doi:10.3390/jcm10204786
Ding, X. M. (2019). Clinical Analysis of Guipi Decoction in Treating Chronic Fatigue Syndrome of Heart and Spleen Deficiency Type. Chin. Foreign. Med. Treat. 38 (32), 169–171. doi:10.16662/j.cnki.1674-0742.2019.32.169
Dong, L. L. (2020). Qingshu Yiqi Decoction in Treating Spleen Deficiency and Damp-Heat Type Clinical Observation of Chronic Fatigue Syndrome. China’s Naturop. 28 (20), 72–74. doi:10.19621/j.cnki.11-3555/r.2020.203
Du, Y. (2018). Clinical Observation on Self-Made Yishen Buxue Ointment in Treating Chronic Fatigue Syndrome. Guangming Tradit. Chin. Med. 33 (22), 3295–3297. doi:10.3969/j.issn.10038914.2018.22.009
Fang, B., Wang, J. Z., and Zhang, H. F. (2007). Clinical Observation on Chronic Fatigue Syndrome Treated with Anti-fatigue Granule. Chin. J. Integr. Tradit. West. Med. (Chin.) 16 (12), 1622–1623.
Fang, Y. Q., Ren, Y. L., and Wang, G. T. (2008). Clinical Observation on Chronic Fatigue Syndrome Treated with Shenqi Ointment. Northwest Pharm. J. 23 (6), 389–390.
Fernie, B. A., Murphy, G., Wells, A., Nikčević, A. V., and Spada, M. M. (2016). Treatment Outcome and Metacognitive Change in CBT and GET for Chronic Fatigue Syndrome. Behav. Cogn. Psychother. 44 (4), 397–409. doi:10.1017/S135246581500017X
Gao, J., and Pang, M. (2016). Fatigue Syndrome with Liver-Depression and Spleen-Deficiency Type Treated by Wendan Decoction Combined with Sini Powder. Inf. Tradit. Chin. Med. 33 (1), 72–75. doi:10.19656/j.cnki.1002-2406.2016.01.023
Gao, J., and Pang, M. (2015). Treating Chronic Fatigue Syndrome by Draining the Liver and Strengthening the Spleen. Jilin J. Tradit. Chin. Med. 35 (10), 1022–1024. doi:10.13463/j.cnki.jlzyy.2015.10.015
Geraghty, K. J., and Blease, C. (2018). Cognitive Behavioural Therapy in the Treatment of Chronic Fatigue Syndrome: A Narrative Review on Efficacy and Informed Consent. J. Health Psychol. 23 (1), 127–138. doi:10.1177/1359105316667798
Geraghty, K. J., and Blease, C. (2019). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and the Biopsychosocial Model: A Review of Patient Harm and Distress in the Medical Encounter. Disabil. Rehabil. 41 (25), 3092–3102. doi:10.1080/09638288.2018.1481149
Glassford, J. A. (2017). The Neuroinflammatory Etiopathology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front. Physiol. 8, 88. doi:10.3389/fphys.2017.00088
Gong, J. H. (2007). Clinical Observation on 30 Cases of Chronic Fatigue Syndrome Treated with Modified Guipi Decoction. Zhejiang J. Integr. Tradit. Chin. West. Med. 17 (10), 627–628.
Goudsmit, E., and Howes, S. (2017). Bias, Misleading Information and Lack of Respect for Alternative Views Have Distorted Perceptions of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and its Treatment. J. Health Psychol. 22 (9), 1159–1167. doi:10.1177/1359105317707216
Gregorowski, A., Simpson, J., and Segal, T. Y. (2019). Child and Adolescent Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: Where Are We Now? Curr. Opin. Pediatr. 31 (4), 462–468. doi:10.1097/MOP.0000000000000777
Guenther, S., Loebel, M., Mooslechner, A. A., Knops, M., Hanitsch, L. G., Grabowski, P., et al. (2015). Frequent IgG Subclass and Mannose Binding Lectin Deficiency in Patients with Chronic Fatigue Syndrome. Hum. Immunol. 76 (10), 729–735. doi:10.1016/j.humimm.2015.09.028
Guo, J. H., Hu, B., Yao, R. B., Zhao, Z. M., Zhang, Y. W., Zhao, L. J., et al. (2007). A Clinical Research of the Efficacy of Nourishing Both Qi and Blood Peroral Solution in the Treatment of Chronic Fatigue Syndrome. Mil. Med. J. Southeast Chin. 10 (9), 325–327.
He, L. (2019). “Clinical Observation on Chronic Fatigue Syndrome Treated by Shugan Jianpi Huoxue Method,” Hebei, China: Hebei North University.
Hornig, M., Montoya, J. G., Klimas, N. G., Levine, S., Felsenstein, D., Bateman, L., et al. (2015). Distinct Plasma Immune Signatures in ME/CFS Are Present Early in the Course of Illness. Sci. Adv. 1 (1), e1400121. doi:10.1126/sciadv.1400121
Hou, X. Y., Jia, G. P., Tian, L. Y., Gao, F., and Liu, X. W. (2015). Discussion on the Pathogenesis of Chronic Fatigue Syndrome. Hebei Med. J. 37 (16), 2463.
Hu, B., Zhou, Z. D., Zhang, L. T., and Qiu, X. F. (2010). Clinical Observation of Buqi Tongluo Formula in the Treatment of Chronic Fatigue Syndrome. Hubei J. Tradit. Chin. Med. 32 (8), 45.
Hu, H. (2019). Clinical Effect of Buzhong Yiqi Decoction Combined with Xiaochaihu Decoction in the Treatment of Chronic Fatigue Syndrome with Liver Stagnation and Spleen Deficiency Syndrome. Integr. Tradit. Chin. West. Med. 6 (27), 106.
Jiang, Q. (2012). Observation on the Efficacy of Buzhong Yiqi Decoction and Guipi Decoction in the Treatment of Chronic Fatigue Syndrome. J. Beijing Univ. Chin. Med. 31 (2), 121–122. doi:10.16025/j.1674-1307.2012.02.016
Jie, R., and Wang, G. P. (2009). Comparative Study of Paroxetine in Combination with Xiaoyao Pill for the Treatment of Chronic Fatigue Syndrome. J. Clin. Psychosom. Dis. 15 (4), 307–313. doi:10.3969/jissn.1672-187X.2009.04.0307-03
Joung, J. Y., Lee, J. S., Cho, J. H., Lee, D. S., Ahn, Y. C., and Son, C. G. (2019). The Efficacy and Safety of Myelophil, an Ethanol Extract Mixture of Astragali Radix and Salviae Radix, for Chronic Fatigue Syndrome: A Randomized Clinical Trial. Front. Pharmacol. 10, 991. doi:10.3389/fphar.2019.00991
Kennedy, G., Khan, F., Hill, A., Underwood, C., and Belch, J. J. (2010). Biochemical and Vascular Aspects of Pediatric Chronic Fatigue Syndrome. Arch. Pediatr. Adolesc. Med. 164 (9), 817–823. doi:10.1001/archpediatrics.2010.157
Kong, F. Y. (2012). Treatment of 30 Cases of Chronic Fatigue Syndrome with Anti-fatigue Decoction Combined with Exercise Therapy. Henan Tradit. Chin. Med. 32 (1), 69–70. doi:10.16367/j.issn.1003-5028.2012.01.029
Lai, J. Z., and Lei, L. M. (2013). Clinical Study of Baiyu Jianpi Decoction in the Treatment of Chronic Fatigue Syndrome. J. Chin. Med. 178 (28), 423–424. doi:10.16368/j.issn.1674-8999.2013.03.040
Larun, L., Brurberg, K. G., Odgaard-Jensen, J., and Price, J. R. (2017). Exercise Therapy for Chronic Fatigue Syndrome. Cochrane Database Syst. Rev. 4 (4), CD003200. doi:10.1002/14651858.CD003200.pub7
Li, C. D., Chen, Z. L., and Huang, N. (2011). The Clinical Study of Soothing Liver and Activating Spleen in Treatment of Chronic Fatigue Syndrome. Liaoning J. Tradit. Chin. Med. 38 (10), 2037–2038. doi:10.13192/j.ljtcm.2011.10.120.lichd.034
Li, J., Chen, X. D., Huang, H. Q., Chen, G. Z., Wang, L., Zhang, H., et al. (2021). Inorganic Nitrate Alleviates Irradiation-Induced Salivary Gland Damage by Inhibiting Pyroptosis. Free Radic. Biol. Med. 13 (17), 130–140. doi:10.1016/j.freeradbiomed.2021.08.227
Li, T., and Zao, Y. Q. (2015). Evaluation on Clinical Effect of Treatment of 37 Female Patients with Chronic Fatigue Syndrome by Chinese Medicines in Invigorating Spleen Warming Kidney and Smoothing. Clin. J. Tradit. Chin. Med. 7 (12), 98–101. doi:10.3969/j.issn.1674-7860.2015.12.049
Li, W., Xu, X. F., Jia, B., Xu, G. J., Chen, H. X., Zheng, T. J., et al. (2018). Exploring the Efficacy of the Formula to Yiqi Yangxue Bupi Hegan Recipe in the Treatment of Chronic Fatigue Syndrome. Chin. J. Mod. Drug. Appl. 12 (15), 208–209. doi:10.14164/j.cnki.cn11-5581/r.2018.15.121
Li, X. (2009). “Clinical Study of Anti-fatigue No. 2 in the Treatment of Sub-healthy Chronic Fatigue of Liver Stagnation, Heart and Spleen Deficiency” (Beijing, China: Chinese Academy of Traditional Chinese Medicine).
Li, Y. (2020). Analysis of Therapeutic Effect and Quality of Life of Buzhong Yiqi Decoction Combined with Xiaochaihu Decoction on Chronic Fatigue Syndrome. Spec. Health 34, 86.
Li, Y. M. (2015). Treatment of Chronic Fatigue Syndrome with Buzhong Yiqi Decoction and Xiaochaihu Decoction Clinical Observation on the Syndrome of Liver Depression and Spleen Deficiency. Asia-Pacific. Tradit. Med. 11 (17), 124–125. doi:10.11954/ytctyy.201517065
Liang, G. (2006). Clinical Observation of the Treatment of 63 Cases of Chronic Fatigue Syndrome by Using Shengmai Decoctionin Combination with Xuefu Zhuyu Decoction. Lishizhen Med. Mat. Med. Res. 17 (5), 801–802.
Lim, E. J., and Son, C. G. (2021). Prevalence of Chronic Fatigue Syndrome (CFS) in Korea and Japan: A Meta-Analysis. J. Clin. Med. 10 (15), 3204. doi:10.3390/jcm10153204
Lin, H. (2007). Treatment of 50 Cases of Chronic Fatigue Syndrome with Modified Shenling Baizhu Powder. New J. Tradit. Chin. Med. 39 (3), 68. doi:10.13457/j.cnki.jncm.2007.03.047
Liu, F., Luo, Q., Luo, Y. H., Luo, J. Y., Luo, H. H., and Deng, Q. (2019a). Clinical Observation on 60 Cases of Chronic Fatigue Syndrome Treated by Jiawei Lingzhi Yishou Pill. Yunnan J. Tradit. Chin. Med. 40 (02), 34–35. doi:10.16254/j.cnki.53-1120/r.2019.02.012
Liu, G. X., and Cai, K. K. (2018). Observation on the Clinical Effect of Bupiwei Xieyinhuo Shengyang Decoction in the Treatment of Chronic Fatigue Syndrome. Guangming Tradit. Chin. Med. 33 (19), 2827–2830. doi:10.3969/j.issn.1003-8914.2018.19.018
Liu, J., Hu, Y. H., Ying, R. J., Shen, J., and Sheng, S. Y. (2019b). Chaihu Guizhi Decoction in the Treatment of Chronic Fatigue Syndrome of Liver-Depression and Spleen-Deficiency Type Clinical Efficacy and Effect on Immune Function of Patients. Lishizhen. Med. Mat. Med. Res. 30 (06), 1414–1416. doi:10.3969/j.issn.1008-0805.2019.06.044
Liu, J., Hu, Y. H., Ying, R. J., and Sheng, Z. Y. (2021). The Clinical Observation of Chaihu Guizhi Decoction in Treating Chronic Fatigue Syndrome and the Influence of Emotional Factors. World. J. Integr. Tradit. West. Med. 16 (10), 1908–1911. doi:10.13935/j.cnki.sjzx.211028
Liu, Y., Peng, Y. Q., Ge, X., Tong, X. H., Yang, T., Zhao, M. C., et al. (2015). The Effect of the Method of Draining the Liver and Nourishing Blood on Fatigue in Patients with Chronic Fatigue Syndrome Degree and Quality of Survival. World. J. Integr. Tradit. West. Med. 10 (09), 1239–1241. doi:10.13935/j.cnki.sjzx.150918
Liu, Y., Peng, Y. Q., Ge, X., Yang, T., and Li, Z. (2019c). Invigorating the Spleen and Nourishing the Kidney for Patients with Chronic Fatigue Syndrome Effects of Free Radical Metabolism. Beijing J. Tradit. Chin. Med. 38 (02), 140–142. doi:10.16025/j.1674-1307.2019.02.013
Liu, Y., Peng, Y. Q., Ge, X., Zhao, M. C., Yang, T., and Zhang, Y. (2011). Clinical Observation of Shugan Yangxue Formula in the Treatment of Chronic Fatigue Syndrome. Chin. J. Integr. Tradit. West. Med. (Chin.) 31 (02), 270–271.
Logan, A. C., and Wong, C. (2001). Chronic Fatigue Syndrome: Oxidative Stress and Dietary Modifications. Altern. Med. Rev. 6 (5), 450–459.
Luo, Y. (2018). “Clinical Observation to the Modified of Clearing Heat and Expelling Damp Decoction in Treating Chronic Fatigue Syndrome of Damp-Heat Syndrome,” Guangdong, China: Guangzhou University of Traditional Chinese Medicine.
Ma, G. X., Zhao, W. H., Wang, H. C., Pan, L. H., Wang, Y., Wang, D. P., et al. (2019). Clinical Observation on Modified Erxian Decoction in Treating Chronic Fatigue Syndrome. Mod. Distance Educ. Chin. Med. 17 (02), 72–74. doi:10.3969/j.issn.1672-2779.2019.02.028
Ma, J. X. (2009). Treatment of 78 Cases of Chronic Fatigue Syndrome with Modified Guipi Decoction. World Health Dig. 6 (19), 23.
Maes, M., Twisk, F. N., Kubera, M., and Ringel, K. (2012). Evidence for Inflammation and Activation of Cell-Mediated Immunity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Increased Interleukin-1, Tumor Necrosis Factor-α, PMN-Elastase, Lysozyme and Neopterin. J. Affect. Disord. 136 (3), 933–939. doi:10.1016/j.jad.2011.09.004
Mao, X. F. (2020). Clinical Discussion and Analysis of Yishen Tiaodu Method in Treating Chronic Fatigue Syndrome of Spleen and Kidney Yang Deficiency Type. World Latest Med. Inf. 20 (A0), 134–135. doi:10.3969/j.issn.1671-3141.2020.100.067
Marques, M. M., De Gucht, V., Gouveia, M. J., Leal, I., and Maes, S. (2015). Differential Effects of Behavioral Interventions with A Graded Physical Activity Component in Patients Suffering from Chronic Fatigue (Syndrome): An Updated Systematic Review and Meta-Analysis. Clin. Psychol. Rev. 40, 123–137. doi:10.1016/j.cpr.2015.05.009
Matsuda, Y., Matsui, T., Kataoka, K., Fukada, R., Fukuda, S., Kuratsune, H., et al. (2009). A Two-Year Follow-Up Study of Chronic Fatigue Syndrome Comorbid with Psychiatric Disorders. Psychiatry Clin. Neurosci. 63 (3), 365–373. doi:10.1111/j.1440-1819.2009.01954.x
Miao, X., Li, S., Xiao, B., Yang, J., and Huang, R. (2022). Metabolomics Study of the Effect of Danggui Buxue Tang on Rats with Chronic Fatigue Syndrome. Biomed. Chromatogr. 36 (7), e5379. doi:10.1002/bmc.5379
Montoya, J. G., Holmes, T. H., Anderson, J. N., Maecker, H. T., Rosenberg-Hasson, Y., Valencia, I. J., et al. (2017). Cytokine Signature Associated with Disease Severity in Chronic Fatigue Syndrome Patients. Proc. Natl. Acad. Sci. U. S. A. 114 (34), E7150–E7158. doi:10.1073/pnas.1710519114
Mücke, M., MochamatCuhls, H., Peuckmann-Post, V., Minton, O., Stone, P., Radbruch, L., et al. (2015). Pharmacological Treatments for Fatigue Associated with Palliative Care. Cochrane Database Syst. Rev. 2015 (5), CD006788. doi:10.1002/14651858.CD006788.pub3
Ning, T. C., and Li, Y. P. (2002). Treatment of 23 Cases of Chronic Fatigue Syndrome with Sijunzi Decoction. Res. Tradit. Chin. Med. 18 (04), 16–17.
Niu, Z. Z., Zhang, X. P., Zhou, H. I., and Wang, X. (2015). “The Observation of Curative Effect of Chronic Fatigue Syndrome Treated by Bushen Shugan Decoctoin,” in Proceedings of the Second Academic Annual Conference of the World Federation of Chinese Medicine Societies Committee on Traditional Chinese Medicine Health Management, 212–214.
Noor, N., Urits, I., Degueure, A., Rando, L., Kata, V., Cornett, E. M., et al. (2021). A Comprehensive Update of the Current Understanding of Chronic Fatigue Syndrome. Anesth. Pain Med. 11 (3), e113629. doi:10.5812/aapm.113629
Ogawa, R., Toyama, S., and Matsumoto, H. (1992). Chronic Fatigue Syndrome-Cases in the Kanebo Memorial Hospital. Nihon Rinsho. 50 (11), 2648–2652.
Ou, Y., Xiao, L., Li, J., Wang, J. H., Liu, W. H., and Yang, G. L. (2018). Clinical Study on the Treatment of Chronic Fatigue Syndrome with Deficiency of Both Heart and Spleen by Guipi Decoction. Inf. Tradit. Chin. Med. 35 (2), 87–90. doi:10.19656/j.cnki.1002-2406.180060
Pang, Y. H., and Liu, j. p. (2013). Therapeutic Effect of Shengmai Powder Plus Modified Xiaoyao Powder for Treatment of Chronic Fatigue Syndrome. J. Guangzhou Univ. Tradit. Chin. Med. 30 (3), 316–318. doi:10.13359/j.cnki.gzxbtcm.2013.03.008
Peng, W., Su, J., Xu, Q., Wang, Q. J., and Jiang, X. J. (2013). Meta-Analysis of Clinical Effect of TCM Intervention on Chronic Fatigue Syndrome. Guangming Tradit. Chin. Med. 28 (07), 1345–1349.
Ren, P., and Yu, X. K. (2012). Chronic Fatigue Syndrome 40 Cases Treated with Buxu Decoction and Rehabilitation Training. Henan Tradit. Chin. Med. 32 (10), 1321. doi:10.16367/j.issn.10035028.2012.10.068
Schulz, K. F., Altman, D. G., and Moher, D.CONSORT Group (2010). CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Ann. Intern. Med. 152 (11), 726–732. doi:10.7326/0003-4819-152-11-201006010-00232
See, D. M., Broumand, N., Sahl, L., and Tilles, J. G. (1997). In Vitro Effects of Echinacea and Ginseng on Natural Killer and Antibody-dependent Cell Cytotoxicity in Healthy Subjects and Chronic Fatigue Syndrome or Acquired Immunodeficiency Syndrome Patients. Immunopharmacology 35 (3), 229–235. doi:10.1016/s0162-3109(96)00125-7
Sheng, S. Y., Shen, j., Chen, Y. Y., Hu, Y. H., Liu, J., and Hu, X. Y. (2022). Treatment of Chronic Fatigue Syndrome of Spleen-Kidney Yang Deficiency by Warming the Kidney and Regulating Fatigue Formula Clinical Efficacy Observation and Effect on Cellular Immunity. Chin. J. Tradit. Med. Sci. Technol. 29 (3), 228–230.
Shi, J., and Zha, W. (2019). Predicting Human Pharmacokinetics: Physiologically Based Pharmacokinetic Modeling and In Silico ADME Prediction in Early Drug Discovery. Eur. J. Drug Metab. Pharmacokinet. 4 (1), 135–137. doi:10.1007/s13318-018-0503-9
Shi, Z. H., and Wu, Y. F. (2016). Clinical Observation of Suanzaoren Decoction in the Treatment of Sub-healthy Sleep. Chin. J. Pract. Med. 11 (15), 196–197. doi:10.14163/j.cnki.11-5547/r.2016.15.143
Shin, H. Y., An, N. H., Cha, Y. J., Shin, E. J., Shin, T. Y., Baek, S. H., et al. (2004). Effect of Kuibitang on Lipopolysaccharide-Induced Cytokine Production in Peripheral Blood Mononuclear Cells of Chronic Fatigue Syndrome Patients. J. Ethnopharmacol. 90 (2-3), 253–259. doi:10.1016/j.jep.2003.10.006
Shin, S., Park, S. J., and Hwang, M. (2021). Effectiveness A Herbal Medicine (Sipjeondaebo-Tang) on Adults with Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Integr. Med. Res. 10 (2), 100664. doi:10.1016/j.imr.2020.100664
Singh, A., Naidu, P. S., Gupta, S., and Kulkarni, S. K. (2002). Effect of Natural and Synthetic Antioxidants in A Mouse Model of Chronic Fatigue Syndrome. J. Med. Food 5 (4), 211–220. doi:10.1089/109662002763003366
Smith, M. E., Haney, E., McDonagh, M., Pappas, M., Daeges, M., Wasson, N., et al. (2015). Treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review for A National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med. 162 (12), 841–850. doi:10.7326/M15-0114
Sotzny, F., Blanco, J., Capelli, E., Castro-Marrero, J., Steiner, S., Murovska, M., et al. (2018). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome-Evidence for an Autoimmune Disease. Autoimmun. Rev. 17 (6), 601–609. doi:10.1016/j.autrev.2018.01.009
Sun, H. J., Guo, Y. J., Sun, X. H., Guo, F. C., and Ma, Y. (2016). Clinical Study on Treatment of Chronic Fatigue Syndrome by Shugan Yiyang Cap. J. Tradit. Chin. Med. 213 (31), 272–274. doi:10.16368/j.issn.1674-8999.2016.02.075
Sun, H. J., Zhen, M. H., and Yang, X. W. (2007). The Effect of Shu Yu Formula on the Emotional State and Quality of Life of Patients with Chronic Fatigue Syndrome. Henan Tradit. Chin. Med. 27 (5), 30–32. doi:10.16367/j.issn.1003-5028.2007.05.018
Tan, D. H., Zhang, R., Chen, X. F., and Wu, X. H. (2015). Treatment of 30 Cases of Chronic Fatigue Syndrome by Dredging and Regulating Sanjiao Yuanqi. China J. Chin. Mat. Med. 2015, 1259–1261.
Teng, F. Y., Jiang, Q. Y., and Huang, Y. N. (2014). Observation on the Curative Effect of Buzhong Jiepi Decoction in the Treatment of Chronic Fatigue Syndrome. Guid. J. Tradit. Chin. Med. Pharm. 20 (4), 108–109. doi:10.13862/j.cnki.cn43-1446/r.2014.04.047
Tian, H., and Wang, S. G. (2012). Treatment of 32 Cases of Chronic Fatigue Syndrome with Buzhong Yiqi Decoction. Jiangxi Tradit. Chin. Med. 43 (08), 26–27.
Wang, C. (2019). “Clinical Observation of Sanren Decoction and Sijunzi Decoction in the Treatment of Chronic Fatigue Syndrome of Spleen Deficiency and Dampness Obstruction Syndrome,” Guangzhou, China: Guangzhou University of Chinese Medicine.
Wang, H. (2012). Clinical Study of Buzhong Yiqi Decoction and Xiaochaihu Decoction in Treatment of Chronic Fatigue Syndrome Liver Depression and Spleen Card. Asia-Pacific Tradit. Med. 8 (8), 67–68.
Wang, J. Q. (2020). Observation on the Clinical Effect of Chaihu Guizhi Decoction in the Treatment of Chronic Fatigue Syndrome of Liver Stagnation and Spleen Deficiency. Sci. Regimen. 23 (3), 146–147.
Wang, J. Z., Fang, B., Zhang, H. F., and Wu, C. Y. (2007). Clinical Study on the Treatment of Chronic Fatigue Syndrome with Fuzheng Jieyu Formula. Chin. J. Inf. Tradit. Chin. Med. 14 (9), 75–76.
Wang, M. X. (2021). “Based on the “Gas Monism” to Explore Mechanism of Chronic Fatigue Syndrome (Spleen Deficiency Syndrome) and the Clinical Curative Effect Observation.” Changchun, China: Changchun University of Chinese Medicine.
Wang, R. S. (2017). Randomized Parallel Controlled Study of Bupi Yishen Decoction Combined with Adenosine Triphosphate + Oryzanol in the Treatment of Chronic Fatigue Syndrome of Spleen-Kidney Yang Deficiency. Pract. Tradit. Chin. Intern. Med. 31 (2), 31–33. doi:10.13729/j.issn.1671-7813.2017.02.13
Wang, X. J., Zhang, Y. R., Jian, Q. R., He, Y. X., Wang, X. L., and Lin, L. (2011). Clinical Effective Observation on Treating Chronic Fatigue Syndrome in TCM. Clin. J. Chin. Med. 3 (2), 106–107.
Wang, Y. Y., Li, X. X., Liu, J. P., Luo, H., Ma, L. X., and Alraek, T. (2014). Traditional Chinese Medicine for Chronic Fatigue Syndrome: A Systematic Review of Randomized Clinical Trials. Complement. Ther. Med. 22 (4), 826–833. doi:10.1016/j.ctim.2014.06.004
Wei, L. L. (2005). Clinical Observation of Xiaochaihu Decoction in the Treatment of Chronic Fatigue Syndrome. Chin. Arch. Tradit. Chin. Med. 23 (7), 1315–1316. doi:10.13193/j.archtcm.2005.07.157.weill.078
Weng, Y. N. (2018). Clinical Observation on the Therapeutic Effect of Liujunzi Decoction in Changxia Season in the Chronic Fatigue Syndrome Patients of Spleen Deficiency and Dampness Obstruction. Clin. Res. 235–238. doi:10.26914/c.cnkihy.2018.026256
Wu, J. D., Zhang, X. Q., Zhang, Y., and Zhu, A. S. (2018). Clinical Efficacy of Guipi Decoction in Treating Chronic Fatigue Syndrome with Deficiency of Heart and Spleen. Liaoning J. Tradit. Chin. Med. 45 (2), 305–306. doi:10.13192/j.issn.1000-1719.2018.02.027
Wu, L. L., Zhang, Z. X., and Zhang, Y. (2012). Investigation of Lixu Jieyu Prescription in Treating 120 Cases of Chronic Fatigue Syndrome. Liaoning J. Tradit. Chin. Med. 39 (2), 283–284. doi:10.13192/j.ljtcm.2012.02.96.wull.035
Wu, X. J., Hu, X. Z., Zhao, H. B., Ma, Q. L., and Ma, Z. G. (2016). Clinical Research on Xiaopi - Yin in the Treatment of Spleen - Kidney Deficiency Type Patients of Chronic Fatigue Syndrome. Ningxia Med. J. 38 (10), 922–924. doi:10.13621/j.1001-5949.2016.10.0922
Xiong, X., Yang, X., Li, X., Yue, G., Xing, Y., and Cho, W. C. (2019). Efficacy and Safety of Chinese Herbal Medicine for Patients with Postmenopausal Hypertension: A Systematic Review and Meta-Analysis. Pharmacol. Res. 141, 481–500. doi:10.1016/j.phrs.2019.01.018
Xu, D., Dong, Y. X., and Yang, X. Q. (2013). Observation of Curative Effect of Modified Naoxinkang in Treating 40 Cases of Chronic Fatigue Syndrome. J. Chang. Univ. Tradit. Chin. Med. 29 (02), 281–282. doi:10.13463/j.cnki.cczyy.2013.02.063
Xu, Y. C. (2014). Treatment of Chronic Fatigue Syndrome with Dongyuan Qingshu Yiqi Decoction Combined with Acupuncture. Chin. J. Clin. Res. 27 (4), 485–486. doi:10.13429/j.cnki.cjcr.2014.04.047
Xu, Z. H., and Wang, X. Z. (2013). Chaihu Jia Longgu Muli Decoction in the Treatment of 42 Cases of Chronic Fatigue Syndrome with Liver Depression and Spleen Deficiency. Henan Tradit. Chin. Med. 33 (6), 847–848. doi:10.16367/j.issn.1003-5028.2013.06.012
Yang, D. L. (2019). Clinical Effect of Zuogui Pill on Chronic Fatigue Syndrome of Kidney Deficiency Type. Health Everyone 22, 94–95.
Yang, S. H., Gao, M., Yang, X. W., and Chen, D. Q. (2004). Clinical Observation on Chronic Fatigue Syndrome Treated with Buzhong Yiqi Decoction and Xiaochaihu Decoction. J. Beijing Univ. Chin. Med. 27 (2), 87–89.
Yang, T. T., Wang, L., Deng, X. Y., and Yu, G. (2017). Pharmacological Treatments for Fatigue in Patients with Multiple Sclerosis: A Systematic Review and Meta-Analysis. J. Neurol. Sci. 380, 256–261. doi:10.1016/j.jns.2017.07.042
Yao, R. M., and Qiu, M. Y. (2005). Clinical Observation of Chronic Fatigue Syndrome Treated by Chinese Medicine in Hong Kong. Shanghai J. Tradit. Chin. Med. 39 (6), 12–13. doi:10.16305/j.1007-1334.2005.06.005
Ye, Y. (2017). “The Application Research of Immortal Porridge for Patients with Chronic Fatigue Syndrome Caused by Spleen and Kidney Yang Deficiency,” Chengdu, China: Chengdu University of Chinese Medicine.
Yin, C., Fu, X., Chou, J., Li, J., Chen, Y., Bai, J., et al. (2021). A Proprietary Herbal Drug Young Yum Pill Ameliorates Chronic Fatigue Syndrome in Mice. Phytomedicine. 88, 153602. doi:10.1016/j.phymed.2021.153602
Zhang, J. Y. (2021). Analysis of the Curative Effect of Jiawei Guizhi Xinjia Decoction in the Treatment of Fatigue Syndrome. Heilongjiang J. Tradit. Chin. Med. 50 (06), 104–105.
Zhang, L. P., Dai, B. S., and Xiong, T. X. (2012b). Analysis of Clinical Treatment on Chronic Fatigue Syndrome by Yao Medicine. Chin. J. Exp. Med. Formul. 18 (16), 311–313. doi:10.13422/j.cnki.syfjx.2012.16.006
Zhang, L. P., Xiong, T. X., and Su, C. B. (2011). Effect of Zhenqi Jiepi with Yao Drug Recipe in Treating Patients with Chronic Fatigue Syndrome. J. Liaoning Univ. Tradit. Chin. Med. 13 (5), 177–178. doi:10.13194/j.jlunivtcm.2011.05.179.zhanglp.053
Zhang, R., Li, J., Chen, J., Zhang, Z. J., and Guo, Z. H. (2004). Clinical Observation on the Treatment of Chronic Fatigue Syndrome with Shengqi Fuyuan Decoction. Chin. J. Inf. Tradit. Chin. Med. 11 (2), 148.
Zhang, S. X., and Zhou, W. (2004). Treatment of 40 Cases of Chronic Fatigue Syndrome with Buzhong Yiqi Decoction. Chin. J. Prim. Med. 11 (5), 608.
Zhang, Z. X., Dong, S. G., Huang, Y., Zhang, Y., and Wu, L. L. (2015). Clinical Observation on Chronic Fatigue Syndrome Depression and Anxiety by Wenzhen Yunqi Formula. Liaoning. J. Tradit. Chin. Med. 42 (12), 2346–2349. doi:10.13192/j.issn.1000-1719.2015.12.03
Zhang, Z. X., Wu, L. L., Chen, M., Zhang, W. J., Qiu, L. N., and Xia, X. (2009). Effect of Lixu Jieyu Recipe in Treating 75 Patients with Chronic Fatigue Syndrome. Chin. J. Integr. Tradit. West. Med. (Chin.) 29 (6), 501–505.
Zhang, Z. X., Zhang, Y., Wang, Y., Chen, M., Wu, L. L., Wang, X. J., et al. (2012a). Effect of “Lixu Jieyu Recipe” on Negative Emotion, 5-HT and Cortisol of Patients with Chronic Fatigue Syndrome. Acta Univ. Tradit. Med. Sin. Pharmacol. Shanghai 26 (5), 38–40. doi:10.16306/j.1008-861x.2012.05.021
Zhao, D. F., Duan, H. D., and Zhao, J. W. (2006). Curative Effect Observation of 30 Cases of Chronic Fatigue Syndrome Treated by Nourishing Qi and Nourishing Yin. Tianjin J. Tradit. Chin. Med. 23 (2), 126–127.
Zhao, L. (2013). Self-made Baihe Yangxin Jianpi Decoction in the Treatment of 88 Cases of Fatigue Syndrome (CFS). China Health Vis. 21 (2), 336–337.
Zhao, S. G. (2012). Clinical Study on Buzhong Yiqi Decoction Combined with Xiao Chaihu Decoction in the Treatment of Chronic Fatigue Syndrome with Liver Stagnation and Spleen Deficiency Syndrome. Psychol. Dr. 216, 355–356. doi:10.3969/j.issn.1007-8231.2012.05.408
Zhao, X., He, X. W., and Huang, Q. (2013). Compound of Fufangteng Mixture on CFS with Blood Deficiency Type. Inf. Tradit. Chin. Med. 30 (04), 88–90.
Keywords: herbal medicine, chronic fatigue syndrome, treatment, systematic review, meta-analysis
Citation: Zhang Y, Jin F, Wei X, Jin Q, Xie J, Pan Y and Shen W (2022) Chinese herbal medicine for the treatment of chronic fatigue syndrome: A systematic review and meta-analysis. Front. Pharmacol. 13:958005. doi: 10.3389/fphar.2022.958005
Received: 31 May 2022; Accepted: 01 August 2022;
Published: 29 September 2022.
Edited by:
Jianping Liu, Beijing University of Chinese Medicine, ChinaReviewed by:
Jingbo Zhai, Tianjin University of Traditional Chinese Medicine, ChinaSijia Zhu, Beijing University of Chinese Medicine, China
Copyright © 2022 Zhang, Jin, Wei, Jin, Xie, Pan and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjuan Shen, anVhbndzODFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship.
 Yang Zhang
Yang Zhang Fangfang Jin
Fangfang Jin Xing Wei2
Xing Wei2 Yujia Pan
Yujia Pan Wenjuan Shen
Wenjuan Shen