- 1School of Clinical Medicine, Chengdu University of TCM, Chengdu, China
- 2Department of colorectal diseases, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Irritable bowel syndrome (IBS) is a gastrointestinal disorder with no structural damage, and its pathogenesis remains unclear. Studies have shown that the brain–gut axis is closely related to the occurrence of IBS. However, studies of IBS related to the brain–gut axis have not been systematically analyzed by bibliometrics and visual analysis. This study is based on 631 publications in the Web of Science Core Collection (WoSCC) to analyze hot spots and trends in this field. The collaborations between different authors, institutions, countries, and keywords were bibliometrically analyzed by CiteSpace software. Meanwhile, VOSviewer analyzed the references. The results show that since 2012, the number of publications has been growing rapidly. According to the collaborative network analysis, the United States, the National University of Ireland, Cork, and J.F. Cryan are the countries, institutions, and authors contributing the most, respectively. Through keywords and literature analysis, mechanisms and therapy associated with IBS and the brain–gut axis have still been a research focus in recent years. Furthermore, the physiological and pathological mechanisms of the brain–gut axis influencing IBS (related to gastrointestinal dysfunction, vagus nerve, visceral pain, intestinal flora, serotonin, tryptophan metabolism, stress, brain-derived neurotrophic factor (BDNF), and malonyldialdehyde) are the future research trends, especially the mechanisms related to intestinal flora. This is the first bibliometric and visualization analysis of IBS and brain–gut axis-related literature to explore research hotspots and trends.
Introduction
IBS is a functional bowel disorder in which abdominal pain or discomfort is associated with defecation or a change in bowel habit and with features of disordered defecation (Longstreth et al., 2006). A systematic review and meta-analysis has demonstrated a global prevalence of IBS of 11% (Lovell & Ford, 2012), and its prevalence varies from ∼7.0 % in Southeast Asian and Middle Eastern studies to 11.8–14.0% in North American, North European, and Australasian studies and to 15.0–21.0% in South European, African, and South American studies (Black & Ford, 2020). Furthermore, IBS poses a significant disease burden, with 20% of adults estimated to develop IBS symptoms in any given year (Zyoud et al., 2021). The annual direct and indirect costs related to IBS are estimated to be up to €8 billion in Europe, ¥123 billion in China, and in excess of US $10 billion in the United States (Ford et al., 2020).
Since publication of the last British Society of Gastroenterology (BSG) guideline in 2007, substantial advances have been made in understanding its complex pathophysiology, resulting in its re-classification as a disorder of gut–brain interaction rather than a functional gastrointestinal disorder (Vasant et al., 2021). However, the mechanism of IBS is not fully clear. Its traditional mechanisms include the gut–brain axis, stress, visceral hypersensitivity, and altered motility (Martin et al., 2018). Since the microbiome has become an indispensable participant in gut–brain communication, researchers proposed the microbiota-gut–brain axis (MGBA) (Collins et al., 2012; Quigley, 2017; Cryan et al., 2019). Preclinical and clinical studies have shown bidirectional interactions within the brain–gut–microbiome axis (Mitrea et al., 2022). Gut microbes communicate with the central nervous system through at least three parallel and interacting channels involving nervous, endocrine, and immune signaling mechanisms (Martin et al., 2018; Mitrea et al., 2022). Evidence suggests a close relationship between the brain–gut axis and depression (Liang et al., 2018), autism (Saurman et al., 2020), Alzheimer’s disease (Kowalski & Mulak, 2019; Socała et al., 2021), Parkinson’s disease (Mulak & Bonaz, 2015; Caputi & Giron, 2018), epilepsy (Ding et al., 2021), hypertension (Yang et al., 2018), chronic kidney disease (Yang et al., 2018), inflammatory bowel disease (Gracie et al., 2019; Peppas et al., 2021), and multiple sclerosis (Parodi & Kerlero De Rosbo, 2021).
The metrological analysis of existing literature shows that the main fields of the MGBA include four research directions: “modeling the MGBA in animal systems,” “interplay between the gut microbiota and the immune system,” “irritable bowel syndrome related to gut microbiota,” and “neurodegenerative diseases related to gut microbiota” (Zyoud et al., 2019). Among them, the brain–gut axis has become the most prolific area for research within IBS and the microbiome globally (Zyoud et al., 2021). However, the association between IBS and the brain–gut axis has not been systematically studied through bibliometric and visualization analysis. Bibliometric analysis refers to use of mathematical and statistical methods to quantitatively analyze all the knowledge carriers of a certain discipline (Ma et al., 2020), playing an important role in reflecting characteristics and future trends. Based on WOSCC, CiteSpace and Vosviewer are used to perform bibliometrics and visualization analysis. This study aims to comprehensively and systematically review the current status of global IBS research related to the brain–gut axis from 2012 to 2021, making up for the lack of bibliometric analysis in this research area.
Methods
Data sources
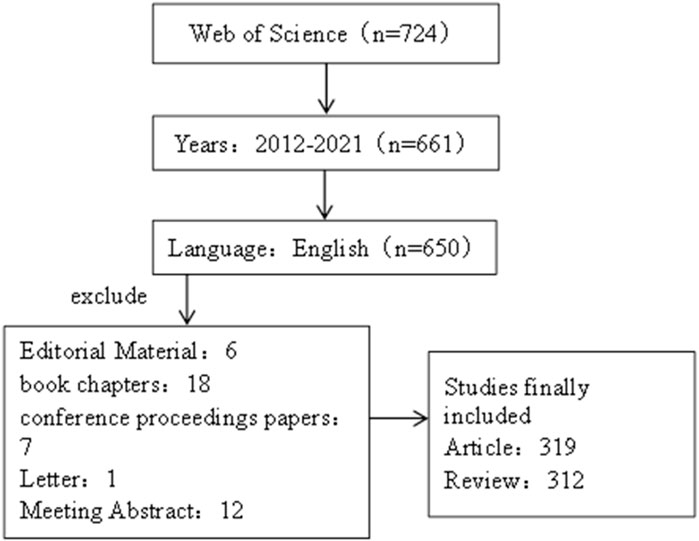
The literature data for this study was obtained from the Web of Science Core Collection (WOSCC), and the retrieval language was set as “English”, and the period was set as “2012-01-01”–“2021-12-31”. At the same time, the retrieval literature type is set as “Article or Review”. The search criteria were subject and free words of irritable bowel syndrome and brain–gut axis. The specific search formula can be seen in the attachment (Search Strategy). Finally, the exported data include “full records and cited references,” and the records are exported as plain text files. The retrieval process was carried out independently by two researchers, and the retrieval date was 20 April 2022. Detailed retrieval results are shown in Figure 1.
Data analysis
The software used for data analysis in this study is Microsoft Office Excel 2010, CiteSpace.5.8. R3, and Vosviewer1.6.17. Among them, Microsoft Office Excel 2010 is used for publication trend statistics, data sorting, and production of related tables. Also, CiteSpace.5.8 R3 is used to analyze the number of publications, mediating centrality of countries, institutions, and authors in the data and keyword frequency and mediating centrality, keyword outburst, and draw them into visual atlas, while Vosviewer1.6.17 is used to analyze highly cited and co-cited references. The specific parameter settings and result interpretation in CiteSpace have been described and published previously (Zou & Sun, 2021). The time slicing was set from the first day of 2012 to the last day of 2021. Node types include Author, Institution, Country, and Keyword.
Results
Publication trend analysis
After WOSCC retrieval, 631 articles met the retrieval conditions, including 319 articles and 312 reviews. As shown in Figure 2, the number of articles published in 2012 was the lowest (nine articles), while the number of articles published in 2021 was the highest (49 papers). From 2012 to 2021, the number of articles about IBS and the brain–gut axis continued to increase steadily. It indicates that the study of IBS and the brain–gut axis has received more and more attention in the past decade, and more and more related studies have been conducted in this field.
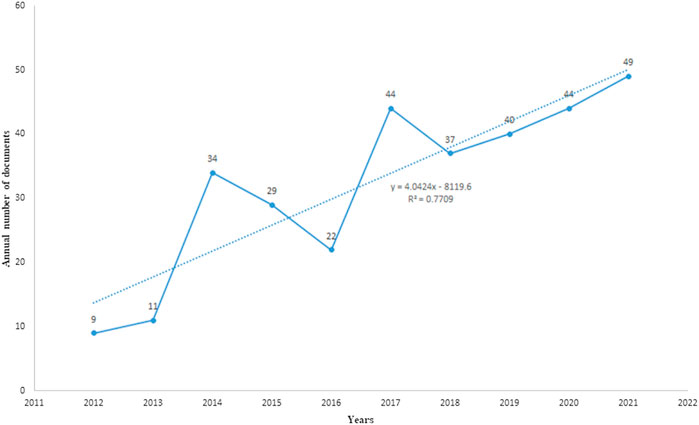
FIGURE 2. Published trend chart concerning IBS and the brain–gut axis. Analysis of countries/regions, institutions, and authors.
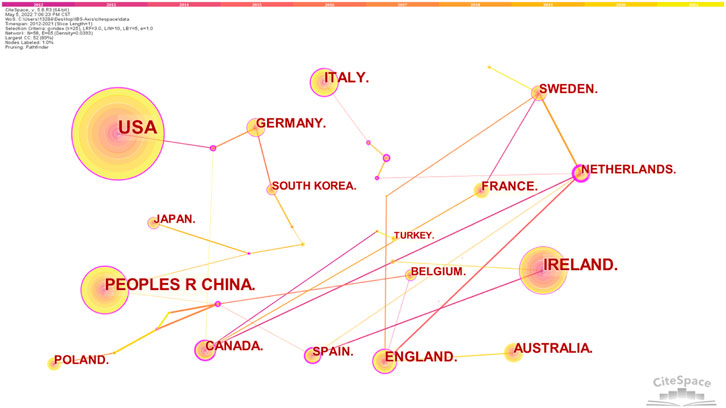
We observed the collaborative network of countries/regions, institutions, and authors for studies related to IBS and the brain–gut axis. The top 10 countries or regions by publication and centrality are shown in Table 1. At the same time, 58 nodes and 65 link lines are generated on the organizational network map (Figure 3). The larger the node is, the more articles are published. The outer circle of the point is represented in purple, with a centrality greater than 0.1. Also, the larger the centrality value, the more cooperation between nodes and other nodes. As shown in Figure 3, the United States contributed the most with the largest number of publications related to IBS and the brain–gut axis, followed by Ireland, People’s Republic of China, Italy, and England, indicating that these five countries are the core forces in the research field of IBS and the brain–gut axis. Also, the top five countries in centrality are the Netherlands, Spain, Brazil, Canada, and the Czech Republic. The Czech Republic only published two articles and the Philippines only published one article, but their centrality was 0.47 and 0.27, respectively. As shown in Table 1, Australia has a large number of documents, but the centrality is 0.00.
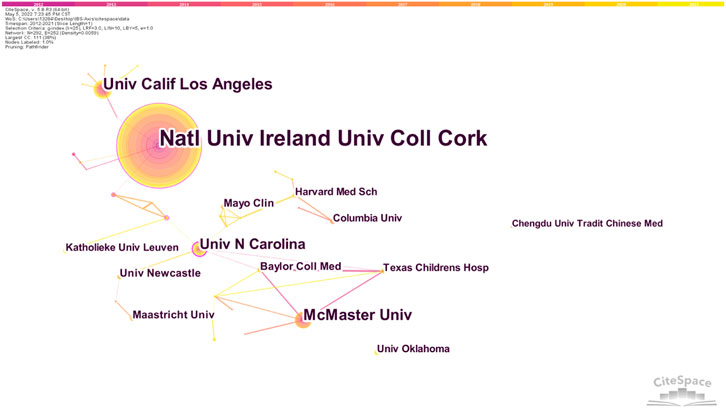
A cooperation network diagram of the published institutions is shown in Figure 4. 292 nodes and 267 link lines are generated on the organizational network map. The five institutions with the most publications are Natl Univ Ireland, Univ Coll Cork, Univ Calif Los Angeles, McMaster Univ, Univ N Carolina, and Univ N Carolina, while Natl Univ Ireland, Univ Coll Cork, Univ Gothenburg, Univ Groningen, and Univ Calif Los Angeles are the four institutions with the strongest centrality.
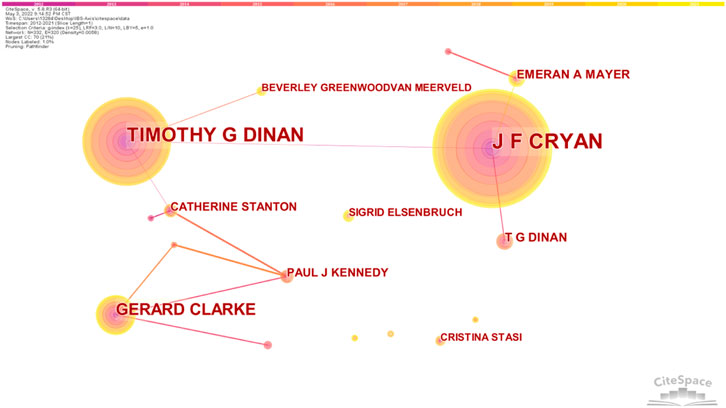
The author’s collaboration network of IBS and the brain–gut axis was observed. According to Figure 5, 332 nodes and 337 link lines are generated on the author’s network map. As shown in Table 1, there are three authors who have published more than 10 articles, with a centrality greater than 0.01. J F Cryan published the most articles (68 articles), followed by Timothy G Dinan (51 articles). The centrality of both of them reached 0.05. J F Cryan, Timothy G Dinan, Gerard Clarke, Emeran A Mayer, and T G Dinan are major contributors in the field of IBS and brain–gut axis research and have significant influence.
Research topic analysis
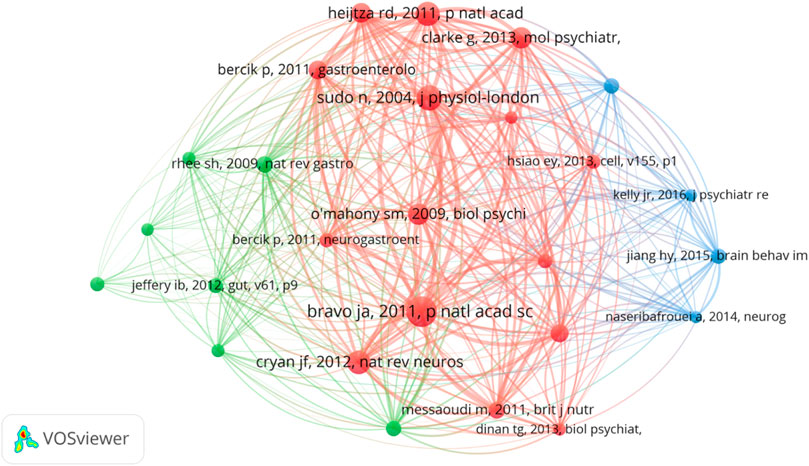
Research basis: analysis of highly co-cited references
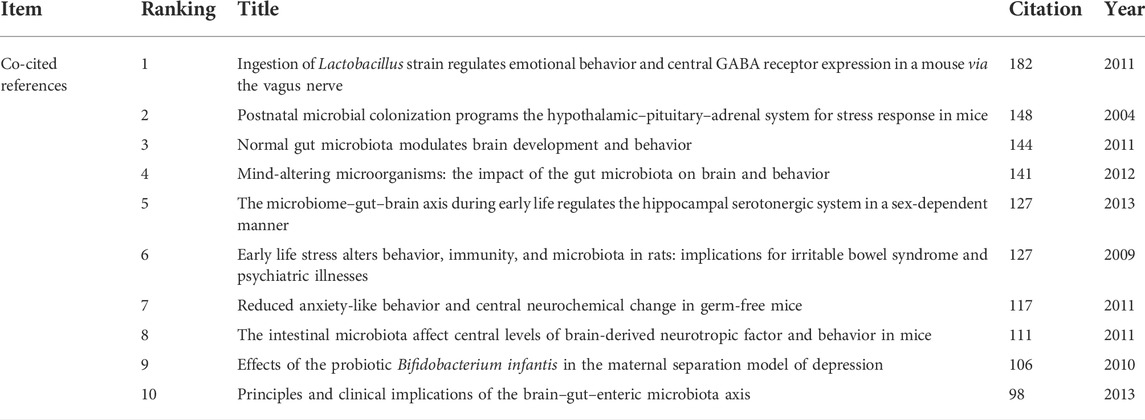
Co-cited references refer to the literature jointly cited by researchers. The purpose of co-citation analysis is to find the research basis of the common field between IBS and the brain–gut axis. We used VOSviewer to draw the co-citation reference graph, and the results showed that 37,025 co-cited references were cited. When the minimum citation time of cited references is set to 70, 26 literatures are left, and the final relational graph is shown in Figure 6. The network graph of highly co-cited references can be divided into three clusters, corresponding to three colors in the graph. The red cluster is mainly concerned with the interaction between intestinal flora and the central nervous system, including the influence of social stress on the immune function of intestinal microbiota (Bailey et al., 2011), the influence of intestinal microbiota on the central level and behavior of brain-derived neurotrophic factor in mice (Bercik et al., 2011), and the regulation of emotional behavior and central GABA receptor expression by Lactobacillus ingestion through the vagus nerve (Bravo et al., 2011). Literatures in the green cluster are more inclined to the relationship between intestinal flora and IBS and the treatment of IBS by probiotics regulating cytokines. Most of the literatures in the blue cluster are about the relationship between intestinal flora and depression. The top 10 literatures with the most co-cited times are shown in Table 2. It could be found that basic studies mainly focused on intestinal flora, emotional behavior, the adrenal system, brain and behavior, the hippocampal serotonergic system, early life stress, and central levels of brain-derived neurotropic factor. Among them, Javier A. Bravo published an article in 2011 that was co-cited the most, which found that lactic acid bacteria (such as Lactobacillus rhamnosus) can directly affect GABA (the main CNS inhibitory neurotransmitter) receptors in the central nervous system of normal healthy animals, thus participating in regulation of many physiological and psychological processes (Bravo et al., 2011). Changes in central GABA receptor expression are associated with pathogenesis of anxiety and depression, as well as with the pathogenesis of functional bowel disease. Lactobacillus rhamnosus (JB-1) reduces stress-induced corticosterone and behaviors associated with anxiety and depression by regulating GABA receptors. In addition, no neurochemical or behavioral effects were observed in mice with the vagus nerve severed, identifying the vagus nerve as the main regulatory component of communication between bacteria exposed to the gut and brain. Their study highlights the important role of bacteria in two-way communication on the brain–gut axis and suggests that certain organisms may prove to be effective adjunctive therapies targeting stress-related disorders such as anxiety and depression. This study lays a foundation for research on the influence of intestinal flora on mental illness and functional bowel disease.
Analysis of co-occurring keywords, and burst term
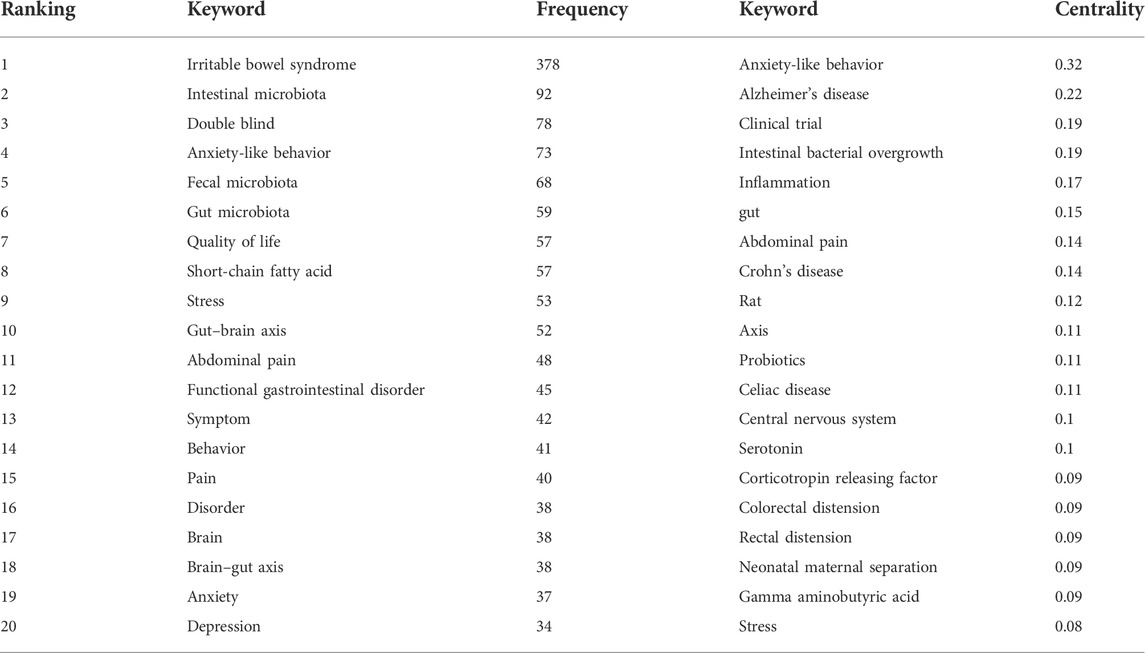
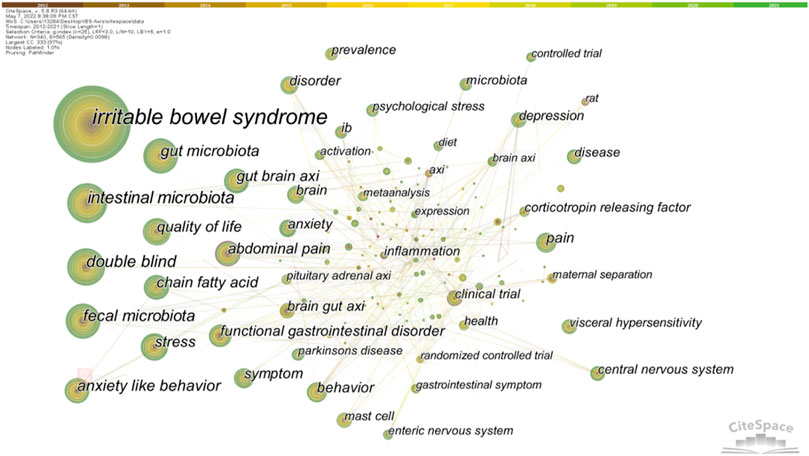
Keyword co-occurrence analysis can trace the research direction of a certain research field and quickly grasp the research topic. We made statistics on keywords related to the brain-gut axis of IBS from 2012 to 2021, and the top 20 keywords in statistical frequency and centrality are shown in Table 3. Obviously, “irritable bowel syndrome” was the most frequently used word, followed by microbiota, which includes the microbiota in the gut and feces. In addition, the frequency of “double blind,” “anxiety-like behavior,” “quality of life,” “chain fatty acid,” “stress,” and “gut brain axis” were all more than 50 times, revealing relevant research topics. Furthermore, the greater the centrality, the greater the bridge function of keywords in connecting two research fields. As shown in Table 3, “anxiety-like behavior” ranked first in centrality, followed by “Alzheimer’s disease,” “clinical trial,” “intestinal bacterial overgrowth,” and “inflammation”. It should be noted that these keywords combine IBS and the brain–gut axis with other studies. The keyword co-occurrence network diagram is shown in Figure 7.
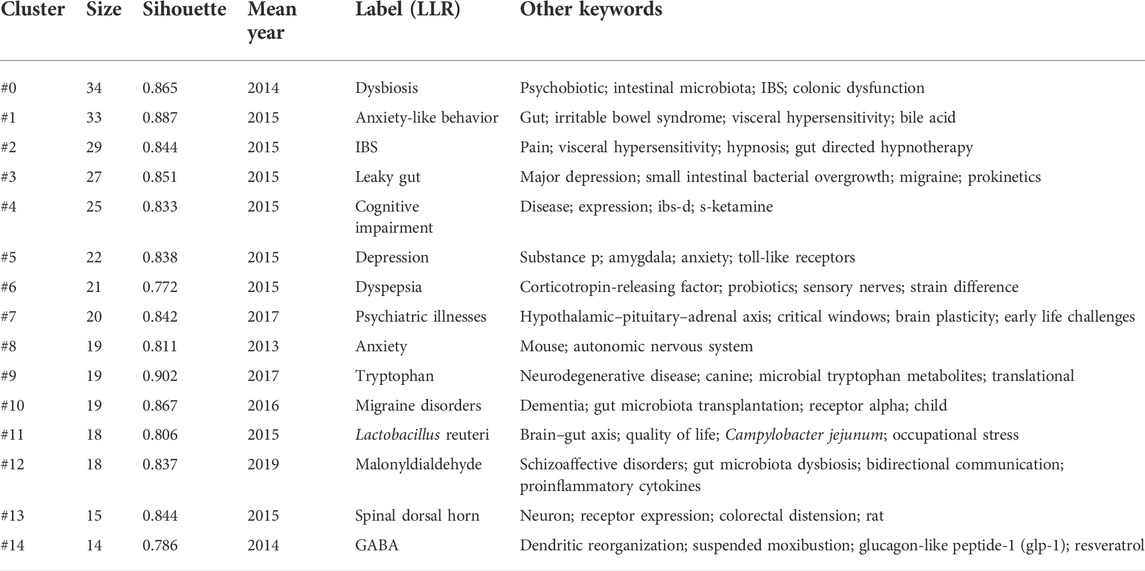
Keyword clustering can classify and summarize research topics. We used CiteSpace to cluster the keywords of IBS and brain–gut axis, resulting in 20 clusters. The first 14 clusters are shown in Table 4. In general, when the silhouette >0.7, the cluster is highly efficient and convincing (Zou & Sun, 2021). The results showed that the clustering average contour value S = 0.8429 (>0.7), indicating that the clustering effect is reasonable.
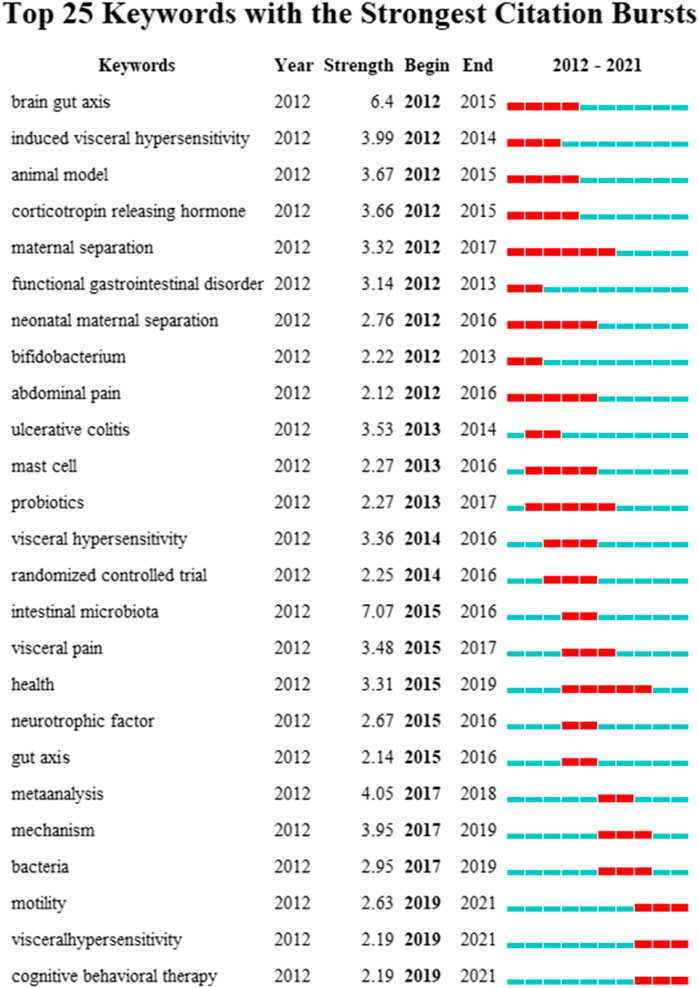
Similarly, keyword bursts can predict potential trends in a research field, implying a sudden increase in research content in a given period of time. Figure 8 shows 25 keywords with the strongest burst strength in this field. The blue line represents the time interval, and the red line represents the duration of the keyword burst. As can be seen, the key topics gradually developed from “induced visceral,” “animal model,” “corticotropin releasing hormone,” and “functional gastrointestinal disorder” to the current dimension of “meta-analysis” and “cognitive behavioral therapy” changes. This indicates that the research on IBS and the brain–gut axis in recent years is being carried out at the level of treatments.
Analysis of highly cited references
We also revealed the top 10 highly cited references related to research on IBS and the brain–gut axis. As shown in Table 5, the most cited article was published in 2012 by J F Cryan et al. (Cryan & Dinan, 2012), which is a review of the impact of gut microbiota (GM) on the brain and behavior, concluding that GM plays a role in regulating anxiety, mood, cognition, and pain and that GM is associated with obesity, autism, and multiple sclerosis. In addition, this study summarizes the mechanism of the microbiome influencing gut–brain signaling, suggesting that the mechanism may include changes in microbial composition, immune activation, vagal nerve signaling, changes in tryptophan metabolism, production of neuroactive metabolites of specific microorganisms, and sugar of the bacterial cell wall. This review not only reflects the research trend but also lays the foundation for the study of the mechanism of IBS based on the brain–gut axis. In summary, the research frontier focuses on the microbiota in the brain–gut axis. These include the relationship between gut flora and brain and behavior, the relationship between the brain–gut axis and serotonin and tryptophan, and the relationship between gut flora and stress-related mental illness or depression, all of which reflect the latest research trends.
Discussion
Publication trend analysis
From 2012 to 2021, the volume of literature on IBS and the brain–gut axis has gradually increased, indicating that this area is still a research hotspot. Clearly, research productivity in this area has increased due to increasing understanding of the role of GM. Among them, the United States is the most productive country in this field, and these findings are consistent with those of previous bibliometric studies (Zyoud et al., 2021). Massive financial support from the strength of the American economy and the motivation of researchers may be important reasons.
Cooperative relationship
Bibliometric analysis can evaluate the cooperation of authors, institutions, and countries in specific research fields (Ma et al., 2020). The centrality value represents the cooperation intensity, and the higher the value, the stronger the cooperation. The Netherlands, Spain, Brazil, Canada, and the Czech Republic rank in the top five in centrality, indicating that they have close cooperation with other countries. However, the centrality of Australia is 0.00, indicating that Australia has little contact with other countries. Research into IBS in Australia may take a step toward promoting collaboration with other countries. In terms of collaboration in institutions, Natl Univ Ireland Univ Coll Cork, Univ Gothenburg, Univ Groningen, and Univ Calif Los Angeles cooperate most closely, with the strongest centrality. This indicates that the United States and Europe have an important influence in the study of IBS and the brain–gut axis. In terms of author collaboration, J F Cryan, Timothy G Dinan, Gerard Clarke, Emeran A Mayer, and T G Dinan work closely with other researchers. Collaboration among these authors may be associated with an interest in the field and substantial financial support for researchers.
Research basis and hot spot
Bibliometric analysis can also help scholars grasp the development trends of specific research fields. According to the keyword burst analysis, it can be seen that people’s understanding of the influence of the brain–gut axis on IBS has gradually deepened, and the treatment of IBS based on the brain–gut axis is the current research focus. Also, the most commonly used keywords in the last decade can be preferred by researchers to access IBS and the brain–gut axis in this field. In addition, based on the references with high co-citation and high citation, we analyzed the research basis and Frontier related to IBS and the brain–gut axis. Studies have focused on the important role of intestinal flora in the brain–gut axis. Intestinal flora affects not only IBS but also metabolic diseases, central nervous system diseases, and psychological disorders through the brain–gut axis. For example, researchers have shown that ingestion of a Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve (Bravo et al., 2011). Also, colonization by GM impacts mammalian brain development and subsequent adult behavior (Diaz Heijtz et al., 2011). In short, studies of the effects of gut microbiota on the brain and behavior formed the basis of subsequent research.
The relationship among IBS, the brain–gut axis, and gut microbiota
IBS is characterized by recurrent abdominal pain associated with abnormal stool form or frequency (Mearin et al., 2016). The traditional mechanisms of IBS include the brain–gut axis, stress, visceral hypersensitivity, and altered motility (Ford et al., 2020). The most studied part of the brain–gut axis is the GM.
GM is linked to other mechanisms through the brain–gut axis. Many other psychosocial, biological, and environmental factors are associated with IBS and might influence symptom severity. However, it is unclear if these are genuine risk factors because most studies are cross-sectional and do not have the temporal element needed to determine cause and effect.
The human GM is a complex, dynamic, and spatially heterogeneous ecosystem inhabited by a myriad of microorganisms interacting with each other and with the human host, including bacteria, fungi, archae, and viruses (Chen et al., 2021). The human GM plays a key role in a number of metabolic, nutritional, physiological, and immune processes through interactions with human hosts (Ottman et al., 2012). Once GM dysbiosis has adverse health effects on the human body, that will lead to a variety of chronic diseases (Chen et al., 2021).
The relationship between gut microbiota and metabolic diseases
It is worth noting that through the analysis of literature related to IBS and the brain–gut axis, we found that the relationship between GM and metabolic diseases, such as obesity, is one of the research trends. GM and its metabolites can regulate satiety signaling and eating behavior by interacting with intestinal endocrine cells in the distal intestine (Lin et al., 2012). Comparisons of the distal GM of genetically obese mice and their lean littermates, as well as those of obese and lean human volunteers, have revealed that obesity is associated with changes in the relative abundance of the two dominant bacterial divisions, Bacteroidetes and Firmicutes. The obese microbiome has an increased capacity to harvest energy from the diet (Turnbaugh et al., 2006). Furthermore, GM after bariatric surgery (BS) appears to impact appetite regulation and satiety energy balance, thereby affecting weight control and metabolism (Martinou et al., 2022). Moreover, there is growing evidence that several natural herbs can effectively improve dyslipidemia by regulating gut flora (Jia et al., 2021), though their metabolic benefits and mechanisms are still not fully understood.
The relationship between gut microbiota and central nervous system diseases
Intestinal flora is strongly correlated with central nervous system diseases and psychological disorders. Based on studies with experimental animals, significant progress has been made in the past decade in illuminating the role of bidirectional interactions between the GM, the gastrointestinal tract, and the nervous system (Margolis et al., 2021). The most studied interaction paths between the brain and the GM are represented by the endocrine pathway, consisting mainly of the HPA axis and enteric endocrine cells (EECs); the neural pathway, which includes the vagal nerve and the enteric nervous system; and the immune pathway, which is mediated via cytokines (Simon et al., 2021).
On the one hand, the imbalance of intestinal flora promotes neuroinflammation and plays an important role in the progression of Alzheimer’s disease (AD). A sodium oligomannate that has demonstrated solid and consistent cognition improvement in a phase 3 clinical trial in China suppresses gut dysbiosis and the associated phenylalanine/isoleucine accumulation, harnesses neuroinflammation, and reverses cognition impairment (Wang et al., 2019). On the other hand, antidepressants can effectively relieve symptoms in patients with IBS, and psychotherapy seems to be an effective treatment (Ford et al., 2019). These studies suggest that co-morbidity and co-treatment of gastrointestinal diseases, metabolic diseases, central nervous system diseases, and psychological diseases based on the brain–gut axis may be the direction of future research.
In summary, recent studies and clinical evidence have highlighted the importance of the gut microbiome in the pathophysiology of IBS, but the causality and translation of these findings in healthy individuals and patients with gastrointestinal or psychiatric disorders are limited. The specific benefits of using novel therapies such as prebiotics, probiotics, synbiotes, and fecal microbiota transfer (FMT) to regulate the GM in patients with IBS require further research (Simon et al., 2021). The mechanisms of how this broad microbiome affects human intestinal and central nervous system development (Obata & Pachnis, 2016), gastrointestinal motivity (Dimidi et al., 2017), mood (Simpson et al., 2021), cognition, and learning (Gareau, 2016) are still in their infancy, but it undeniably provides a potentially important site for future therapeutic interventions (Osadchiy et al., 2019). It provides directions for future studies of gastrointestinal diseases, metabolic diseases, and neurological diseases.
Limitations
There are some limitations in our study that are similar to those of previous bibliometric studies (Zou & Sun, 2021; Zyoud et al., 2021). First of all, the WOSCC database was selected for our study, and only English articles were selected, which may result in some literature data missing; second, some authors or institutions have different name formats in the WOSCC database, which means their research counts may be scattered; third, this article does not ensure that every publication is completely relevant to the subject that meets the search criteria. Finally, it is not clear how the keywords and centrality were filtered to exclude terms that are not particularly relevant to IBS. For example, “double blind” and “clinical trial” are not particularly related to IBS. However, we believe that the results of our analysis are sufficient to reflect the overall state of the field.
Conclusion
This study is based on bibliometrics and visualization analysis to assess and quantify a global overview of IBS and brain–gut axis-related research productivity from 631 publications on the Web of Science from 2012 to 2021. According to the analysis of the publications and references, the number of literature on IBS related to the brain–gut axis has continued to increase over the last decade. The United States, Ireland, China, Italy, and the United Kingdom are the countries that produce the most articles. Furthermore, it provides a comprehensive review of the progress of IBS and brain–gut axis research, identifies hot topics in the field, and points out potential directions for future research. According to the analysis of keywords and cited references, it can be found that the mechanism and treatment related to IBS and the brain–gut axis are still the focus of research in recent years. In addition, future research trends may include physiological and pathological mechanisms related to the brain–gut axis affecting IBS (related to gastrointestinal dysfunction, vagus nerve, visceral pain, intestinal flora, serotonin, tryptophan metabolism, stress, BDNF, and malonyldialdehyde), especially the mechanisms related to intestinal flora; moreover, this study found that the brain–gut axis is not only closely associated with IBS but also affects metabolic diseases, central nervous system diseases, and psychological diseases, such as obesity, Alzheimer’s disease, and depression, through intestinal flora. It suggests that future research can no longer be limited to studying the influence of the brain–gut axis on a single disease but to link diseases with shared mechanisms, explore deeper mechanisms, and find new directions for disease treatment. Furthermore, there are few literatures on different subtypes of IBS. In the future, studies on the relationship between the brain–gut axis and IBS can be divided into different subtypes. This timely review analyzes the results of IBS in relation to the brain–gut axis and may advance this field and lay the foundation for future research.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
P-NW and SX jointly determined the theme; P-NW wrote the draft, and SX supplemented. PZ and W-QY helped make charts and tables. MC revised the manuscript. T-CT is responsible for further analysis of the article. All of them contributed to the manuscript and agreed to submit the final version of the manuscript.
Funding
Funding was provided by the National Natural Science Foundation of China (number: 81774321) and the National Key Research and Development Program of China (number: 2019YFC1709004).
Acknowledgments
Thanks for the fund support provided by the National Natural Science Foundation of China and the National Key Research and Development Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.956204/full#supplementary-material
References
Bailey, M. T., Dowd, S. E., Galley, J. D., Hufnagle, A. R., Allen, R. G., and Lyte, M. (2011). Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 25 (3), 397–407. doi:10.1016/j.bbi.2010.10.023
Bercik, P., Park, A. J., Sinclair, D., Khoshdel, A., Lu, J., Huang, X., et al. (2011). The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 23 (12), 1132–1139. doi:10.1111/j.1365-2982.2011.01796.x
Black, C. J., and Ford, A. C. (2020). Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 17 (8), 473–486. doi:10.1038/s41575-020-0286-8
Bravo, J. A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., et al. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A. 108 (38), 16050–16055. doi:10.1073/pnas.1102999108
Caputi, V., and Giron, M. C. (2018). Microbiome-gut-brain Axis and toll-like receptors in Parkinson's disease. Int. J. Mol. Sci. 19 (6), E1689. doi:10.3390/ijms19061689
Chen, Y., Zhou, J., and Wang, L. (2021). Role and mechanism of gut microbiota in human disease. Front. Cell. Infect. Microbiol. 11, 625913. doi:10.3389/fcimb.2021.625913
Collins, S. M., Surette, M., and Bercik, P. (2012). The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 11, 735–742. doi:10.1038/nrmicro2876
Cryan, J. F., and Dinan, T. G. (2012). Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13 (10), 701–712. doi:10.1038/nrn3346
Cryan, J. F., O'riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The microbiota-gut-brain Axis. Physiol. Rev. 99 (4), 1877–2013. Physiological reviews99. doi:10.1152/physrev.00018.2018
Diaz Heijtz, R., Wang, S., Anuar, F., Qian, Y., Björkholm, B., Samuelsson, A., et al. (2011). Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. U. S. A. 108 (7), 3047–3052. doi:10.1073/pnas.1010529108
Dimidi, E., Christodoulides, S., Scott, S. M., and Whelan, K. (2017). Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Adv. Nutr. 8 (3), 484–494. doi:10.3945/an.116.014407
Ding, M., Lang, Y., Shu, H., Shao, J., and Cui, L. (2021). Microbiota-gut-brain Axis and epilepsy: A review on mechanisms and potential therapeutics. Front. Immunol. 12, 742449. doi:10.3389/fimmu.2021.742449
Ford, A. C., Lacy, B. E., Harris, L. A., Quigley, E. M. M., and Moayyedi, P. (2019). Effect of antidepressants and psychological therapies in irritable bowel syndrome: An updated systematic review and meta-analysis. Am. J. Gastroenterol. 114 (1), 21–39. doi:10.1038/s41395-018-0222-5
Ford, A. C., Sperber, A. D., Corsetti, M., and Camilleri, M. (2020). Irritable bowel syndrome. Lancet (London, Engl. 396 (10263), 1675–1688. doi:10.1016/s0140-6736(20)31548-8
Gareau, M. G. (2016). Cognitive function and the microbiome. Int. Rev. Neurobiol. 131, 227–246. doi:10.1016/bs.irn.2016.08.001
Gracie, D. J., Hamlin, P. J., and Ford, A. C. (2019). The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment. Lancet. Gastroenterol. Hepatol. 4 (8), 632–642. doi:10.1016/s2468-1253(19)30089-5
Jia, X., Xu, W., Zhang, L., Li, X., Wang, R., and Wu, S. (2021). Impact of gut microbiota and microbiota-related metabolites on hyperlipidemia. Front. Cell. Infect. Microbiol. 11, 634780. doi:10.3389/fcimb.2021.634780
Kowalski, K., and Mulak, A. (2019). Brain-gut-microbiota Axis in Alzheimer's disease. J. Neurogastroenterol. Motil. 25 (1), 48–60. doi:10.5056/jnm18087
Liang, S., Wu, X., Hu, X., Wang, T., and Jin, F. (2018). Recognizing depression from the Microbiota⁻Gut⁻Brain Axis. Int. J. Mol. Sci. 19 (6), E1592. doi:10.3390/ijms19061592
Lin, H. V., Frassetto, A., Kowalik, E. J., Nawrocki, A. R., Lu, M. M., Kosinski, J. R., et al. (2012). Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 7 (4), e35240. doi:10.1371/journal.pone.0035240
Longstreth, G. F., Thompson, W. G., Chey, W. D., Houghton, L. A., Mearin, F., and Spiller, R. C. (2006). Functional bowel disorders. Gastroenterology 130 (5), 1480–1491. doi:10.1053/j.gastro.2005.11.061
Lovell, R. M., and Ford, A. C. (2012). Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 10 (7), 712–721.e4. e714. doi:10.1016/j.cgh.2012.02.029
Ma, C., Su, H., and Li, H. (2020). Global research trends on prostate diseases and erectile dysfunction: A bibliometric and visualized study. Front. Oncol. 10, 627891. doi:10.3389/fonc.2020.627891
Margolis, K. G., Cryan, J. F., and Mayer, E. A. (2021). The microbiota-gut-brain Axis: From motility to mood. Gastroenterology 160 (5), 1486–1501. doi:10.1053/j.gastro.2020.10.066
Martin, C. R., Osadchiy, V., Kalani, A., and Mayer, E. A. (2018). The brain-gut-microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 6 (2), 133–148. doi:10.1016/j.jcmgh.2018.04.003
Martinou, E., Stefanova, I., Iosif, E., and Angelidi, A. M. (2022). Neurohormonal changes in the gut-brain Axis and underlying neuroendocrine mechanisms following bariatric surgery. Int. J. Mol. Sci. 23 (6), 3339. doi:10.3390/ijms23063339
Mearin, F., Lacy, B. E., Chang, L., Chey, W. D., Lembo, A. J., Simren, M., et al. (2016). Bowel disorders. Gastroenterology 150, 1393–1407.e5. doi:10.1053/j.gastro.2016.02.031
Mitrea, L., Nemeş, S. A., Szabo, K., Teleky, B. E., and Vodnar, D. C. (2022). Guts imbalance imbalances the brain: A review of gut microbiota association with neurological and psychiatric disorders. Front. Med. 9, 813204. doi:10.3389/fmed.2022.813204
Mulak, A., and Bonaz, B. (2015). Brain-gut-microbiota axis in Parkinson's disease. World J. Gastroenterol. 21 (37), 10609–10620. doi:10.3748/wjg.v21.i37.10609
Obata, Y., and Pachnis, V. (2016). The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology 151 (5), 836–844. doi:10.1053/j.gastro.2016.07.044
Osadchiy, V., Martin, C. R., and Mayer, E. A. (2019). The gut-brain Axis and the microbiome: Mechanisms and clinical implications. Clin. Gastroenterol. Hepatol. 17 (2), 322–332. doi:10.1016/j.cgh.2018.10.002
Ottman, N., Smidt, H., De Vos, W. M., and Belzer, C. (2012). The function of our microbiota: Who is out there and what do they do? Front. Cell. Infect. Microbiol. 2, 104. doi:10.3389/fcimb.2012.00104
Parodi, B., and Kerlero De Rosbo, N. (2021). The gut-brain Axis in multiple sclerosis. Is its dysfunction a pathological trigger or a consequence of the disease? Front. Immunol. 12, 718220. doi:10.3389/fimmu.2021.718220
Peppas, S., Pansieri, C., Piovani, D., Danese, S., Peyrin-Biroulet, L., Tsantes, A. G., et al. (2021). The brain-gut Axis: Psychological functioning and inflammatory bowel diseases. J. Clin. Med. 10 (3), 377. doi:10.3390/jcm10030377
Quigley, E. M. M. (2017). Microbiota-brain-gut Axis and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 17 (12), 94. doi:10.1007/s11910-017-0802-6
Saurman, V., Margolis, K. G., and Luna, R. A. (2020). Autism spectrum disorder as a brain-gut-microbiome Axis disorder. Dig. Dis. Sci. 65 (3), 818–828. doi:10.1007/s10620-020-06133-5
Simon, E., Călinoiu, L. F., Mitrea, L., and Vodnar, D. C. (2021). Probiotics, prebiotics, and synbiotics: Implications and beneficial effects against irritable bowel syndrome. Nutrients 13 (6), 2112. doi:10.3390/nu13062112
Simpson, C. A., Diaz-Arteche, C., Eliby, D., Schwartz, O. S., Simmons, J. G., and Cowan, C. S. M. (2021). The gut microbiota in anxiety and depression - a systematic review. Clin. Psychol. Rev. 83, 101943. doi:10.1016/j.cpr.2020.101943
Socała, K., Doboszewska, U., Szopa, A., Serefko, A., Włodarczyk, M., Zielińska, A., et al. (2021). The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 172, 105840. doi:10.1016/j.phrs.2021.105840
Turnbaugh, P. J., Ley, R. E., Mahowald, M. A., Magrini, V., Mardis, E. R., and Gordon, J. I. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature Nature444 (7122), 1027–1031. doi:10.1038/nature05414
Vasant, D. H., Paine, P. A., Black, C. J., Houghton, L. A., Everitt, H. A., Corsetti, M., et al. (2021). British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 70 (7), 1214–1240. doi:10.1136/gutjnl-2021-324598
Wang, X., Sun, G., Feng, T., Zhang, J., Huang, X., Wang, T., et al. (2019). Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell. Res. 29 (10), 787–803. doi:10.1038/s41422-019-0216-x
Yang, T., Richards, E. M., Pepine, C. J., and Raizada, M. K. (2018). The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 14 (7), 442–456. doi:10.1038/s41581-018-0018-2
Zou, X., and Sun, Y. (2021). Bibliometrics analysis of the research status and trends of the association between depression and insulin from 2010 to 2020. Front. psychiatry 12, 683474. doi:10.3389/fpsyt.2021.683474
Zyoud, S. H., Smale, S., Waring, W. S., Sweileh, W., and Al-Jabi, S. W. (2021). Global research trends in the microbiome related to irritable bowel syndrome: A bibliometric and visualized study. World J. Gastroenterol. 27 (13), 1341–1353. doi:10.3748/wjg.v27.i13.1341
Keywords: irritable bowel syndrome, brain–gut axis, microbiome, bibliometrics, visualization analysis
Citation: Wu P-N, Xiong S, Zhong P, Yang W-Q, Chen M and Tang T-C (2022) Global trends in research on irritable bowel syndrome and the brain–gut axis: Bibliometrics and visualization analysis. Front. Pharmacol. 13:956204. doi: 10.3389/fphar.2022.956204
Received: 30 May 2022; Accepted: 16 August 2022;
Published: 08 September 2022.
Edited by:
Raquel Abalo, Rey Juan Carlos University, SpainReviewed by:
Swapna Mahurkar-Joshi, University of California, Los Angeles, United StatesCalinoiu Florina Lavinia, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Xiaoyi Hou, Sichuan University, China
Copyright © 2022 Wu, Xiong, Zhong, Yang, Chen and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tai-Chun Tang, dGFuZ3RhaWNodW5AY2R1dGNtLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Peng-Ning Wu
Peng-Ning Wu Shuai Xiong
Shuai Xiong Peng Zhong
Peng Zhong Wan-Qing Yang
Wan-Qing Yang Min Chen
Min Chen Tai-Chun Tang
Tai-Chun Tang