- 1Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, China
- 2School of Traditional Chinese Medicine, Guangdong Pharmaceutical University, Guangzhou, Guangdong, China
Herbs with a “hot” properties are frequently used to treat cold symptoms in TCM. However, the underlying mechanisms of the herbs with “hot” properties on hypothyroidism have not been investigated. This study aimed to explore four typical “hot” and “cold” property herb on hypothyroidism. Firstly, the difference efficacy between the four typical “hot” property herbs and the four typical “cold” property herbs was assessed by physical signs, thyroid function, and the metabolic profile using multivariate statistical analysis. The influence of the four typical “hot” property herbs on hypothyroidism was validated pathologically. The impact mechanism of the four typical “hot” property herbs on hypothyroidism was investigated through a metabolomics method combined with network analysis. Na+/K+-ATP, ACC1 enzyme, UCP-1, and the PI3K-Akt pathway were used to confirm the metabolite pathways and target-associated metabolites. The results showed that the four typical “hot” property herbs could significantly improve physical signs, thyroid function, and the metabolic profile in hypothyroidism rats, the four typical “cold” property herbs did not show any benefit. Moreover, the four typical “hot” property herbs could improve lipid metabolism, energy metabolism, and thyroid hormone levels by the PI3K-Akt signaling pathway, Ca2+- AMPK signaling pathways, purine metabolism, and tryptophan metabolism. Additionally, the levels of UCP-1, Na+/K + -ATP enzyme, and ACC1 were ameliorated by the four typical “hot” property herbs in hypothyroidism rats. Therefore, a metabolomics strategy combined with network analysis was successfully performed and interpreted the mechanism of the four typical “hot” property herbs on hypothyroidism based on the theory of “cold and hot” properties of TCM well.
Introduction
Hypothyroidism is on of the most common thyroid diseases affecting people worldwide, particularly during pregnancy and childhood (Taylor et al., 2018). Obesity, cold limbs, depression, hyperlipidemia, negative emotions, reduced lipolysis, and gluconeogenesis are the most essential clinical manifestations (Meier and Kaplan, 2002). Currently, thyroid hormone replacement therapy is the main treatment for hypothyroidism (Clarke and Kabadi, 2004). However, during hypothyroidism therapy, the level of thyroid hormones is difficult to regulate, and over-medication was prevalent, increasing the risk of cardiovascular disease (Flynn et al., 2010), osteoporosis (La Vignera et al., 2008), and subclinical liver damage (Beckett et al., 1985). Therefore, there is an urgent need to seek new strategies for the management of hypothyroidism. According to the traditional Chinese medicine (TCM) theory, hypothyroidism is classified as a “cold” syndrome, and “treating cold syndrome with hot herbs and treating heat syndrome with cold herbs” is a fundamental medication principle of Chinese medicine (Xiao et al., 2017). The “cold and hot” properties of TCM refer to the properties that can cause a certain type of reaction in the body, and are used to treat diseases. However, pharmacology is modern science that studies the regularity of interaction between drugs and the body and the mechanism of the drug effect. Although we have reports suggesting that the “hot” drugs have a significant improvement influencing hypothyroidism (Cheng et al., 2016), there are no reports concerning the typical “hot and cold” property herb influencing hypothyroidism. Therefore, it is necessary to further study the effects of the four typical “hot” property herbs and four typical “cold” property herbs on hypothyroidism to provide a basis for treating hypothyroidism, as well as reveal the scientific essence for the medication principle.
TCM has been applied in preventing and treating the diseased for thousands of years (Feng et al., 2006). Complex-component, multi-target, and multi-path are the typical characteristics of Chinese herbs, which can be used to holistically treat diseases (Pan et al., 2020). One of the major challenges facing TCM is explaining the mechanisms underlying the efficacy of medicines used in TCM (Peng et al., 2018). Therefore, a novel research method consistent with TCM characteristics is crucial. Metabolomics is a high-throughput qualitative and quantitative analysis approach focusing on all small endogenous molecule metabolites in the body to reveal the pathological and physiological status at the overall level (Carneiro et al., 2019). Metabolomics with the systematic biological characteristics is consistent with the theory of TCM and provides a powerful tool for the research on TCM (Cheng et al., 2018). Recently, the network pharmacology approach offered a new understanding of Chinese medicine research from a system perspective and at the molecular level by predicting the interactions between multiple targets of compounds in herbs and/or multiple genes related to diseases (Fang et al., 2017). The metabolic process of the body is mainly catalyzed by enzymes, which are inseparable (metabolites are regulated by proteins, and the activity of proteins is also changed by metabolites, such as described in KEGG). Metabolomics can be used to identify specific molecular markers in certain physiological and pathological conditions (Barnes et al., 2016). Network pharmacology emphasizes the regulation of multiple targets by signal pathways to improve the therapeutic effect of drugs and reduce the toxicity and side effects (Yuan et al., 2017), both of them provide insights into the disease at the molecular and systemic levels. These approaches have been widely applied in the assessment of the therapeutic effects and mechanisms of TCM in recent years (Buriani et al., 2012).
Therefore, an integrated metabolomic strategy combined with network analysis methods was applied to reveal the effects of four typical “hot” property herbs and four typical “cold” property herbs on hypothyroidism. According to the records in ancient books of past dynasties, pharmacopoeia of the People’s Republic of China and modern research in the “cold/hot” property, the four typical “hot” property herbs (Jia et al., 2017; Committee, 2020), including Aconitum carmichaeli Debeaux (FZ), Zingiber officinale Roscoe (GJ), Cinnamomum cassia (L.) J. Presl (RG), and Euodia ruticarpa (A. Juss.) Benth. (WZY), were selected. Meanwhile, the four typical “cold” property herbs (Wang et al., 2016; Committee, 2020), including Scutellaria baicalensis Georgi (HQ), Coptis chinensis Franch. (HL), Gardenia jasminoides J. Ellis (ZZ), and Rheum palmatum L. (DH) were also selected as a contrasting experiment to explore the medication principles of the “cold/hot” property. Finally, the results of biochemistry, metabolomics, and the network analysis were integrated to clarify the effect of the four typical “hot” property herbs on hypothyroidism.
Materials and methods
Chemicals and reagents
Acetonitrile (HPLC grade, United States) and formic acid (LC-MS grade, United States) were purchased from Fisher Chemical. The four typical “cold” property herbs: Scutellaria baicalensis Georgi (HQ), Coptis chinensis Franch. (HL), Gardenia jasminoides J. Ellis (ZZ), and Rheum palmatum L. (DH), and the four typical “hot” property herbs: Aconitum carmichaeli Debeaux (FZ), Zingiber officinale Roscoe (GJ), Cinnamomum cassia (L.) J. Presl (RG), and Euodia ruticarpa (A. Juss.) Benth. (WZY), which were purchased from Beijing Tongrentang Co., Ltd (Beijing, China). The herbs were authenticated by Professor Zhenyue Wang (Heilongjiang University of Chinese Medicine, Harbin, China) and all voucher specimens were preserved at Heilongjiang University of Chinese Medicine.
6-propyl-2-thiouracil (PTU) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany, batch Number: BCBR87087), and L-thyroxine (L-T4) was purchased from Aladdin Biochemical Technology Co., Ltd., (Shanghai, China, batch Number: H2014187).
The ELISA kits for rat triiodothyronine (T3, batch Number: C0384010396), thyroxine (T4, batch umber: C0346030349), and thyroid-stimulating hormone (TSH, batch Number: C0373050316) were purchased from Wuhan Huamei Biotechnology Co., Ltd., (Wuhan, China). The ELISA kits for the Na+/K + -ATP enzyme (batch Number: 202109) and Acetyl CoA carboxylase 1 (ACC1, batch Number: 202109) were purchased from Jiangsu Meimian Industry Co., Ltd. (Jiangsu, China).
Herb extraction
The four typical “hot” property herbs (FZ, GJ, RG, and WZY) and the four typical “cold” property herbs (HQ, HL, ZZ, and DH) were extracted by decocting with water three times [mass (g): volume (v) = 10:1, 8:1, and 8:1; 0.5 h each]. Then, the combined decoctions were concentrated and dried in a vacuum to obtain a FZ/GJ/RG/WZY/HQ/HL/ZZ/DH crude extract.
Animals
Sprague–Dawley rats (200 ± 10 g, male) were obtained from Liaoning Changsheng Biotechnology Co., Ltd., [Liaoning, China; Certificate No. SCXK (Liao) 2020–0001]. All the rats were housed under a 12 h/12 h light/dark cycle, at a temperature of 20 ± 3°C, with 60% ± 10% relative humidity. They had free access to standard food and sterile-filtered water. All the animal experiments were approved by the Experimental Animal Ethics Committee of the Heilongjiang University of Chinese Medicine and performed in accordance with relevant guidelines.
After adaptive breeding for 1 week, all rats were randomly divided into 11 groups: control group (control, n = 7), hypothyroidism model group (Hypo, n = 8), positive drug group (Hypo + T4, n = 8), and treatment group by the four typical “hot” property herbs, including Hypo + FZ (n = 8), Hypo + GJ (n = 8), Hypo + RG (n = 8), and Hypo + WZY (n = 8), as well as the contrasting experimental group by the four typical “cold” property herbs, including Hypo + HQ (n = 8), Hypo + HL (n = 8), Hypo + ZZ (n = 8), and Hypo + DH (n = 8). With the exception of the rats in the control group, the hypothyroidism model was established in all rats by intraperitoneal injection PTU (10 mg/kg/d) for 28 days consecutively (Baltaci and Mogulkoc, 2018). The positive drug group was intragastrically administered T4 (0.3 mg/kg/d) from day 14 to the end. The treatment group and contrasting experimental group FZ (7 g/kg/d), GJ (4.7 g/kg/d), RG (2.4 g/kg/d), WZY (2.4 g/kg/d), HQ (4.7 g/kg/d), HL (2.4 g/kg/d), ZZ (4.7 g/kg/d), DH (7 g/kg/d) were respectively continuous intra-gastrically administered for 28 days, respectively (The dose was calculated according to the Chinese Pharmacopoeia).
Sample collection and preparation
After the last administration, the rats were placed alone in metabolic cages and the collection tube in a box with ice was kept to reduce bacterial contamination, for 12 h urine collection, which was centrifuged for 10 min, at 12,000 rpm. The supernatant was stored at −80°C until analysis. After euthanasia with pentobarbital sodium by intraperitoneal injection, the serum was collected by centrifugation at 3,000 rpm for 10 min and stored at −80°C until further analyses. The live, brown adipose tissue (BAT), the thyroid gland were obtained and fixed in 4% paraformaldehyde for histological examinations. Moreover, approximately 0.1 g of the liver tissue was homogenized at a 0.9% saline solution, which was used to detect the Na+/K+-ATPase and ACC l activities and frozen at −80°C before use.
The urine sample was mixed with four-fold cold acetonitrile, vortexed for 2 min, and centrifugation at 4°C at 13,000 rpm for 10 min. After high-speed centrifugation, the prepared supernatant was transferred into a fresh 2 ml LC-MS glass vial. In addition, the quality control (QC) sample was prepared by mixing an equal amount of every sample from an identical experiment group. Finally, 2 μL of the supernatant for each sample was injected into the UPLC-Q-TOF/MS system for metabolomic analysis.
UPLC-Q-TOF/MS analysis
An ACQUITY UPLC system (Waters, Milford, United States) in tandem with a Q-TOF synapt G2-SI mass spectrometer (Waters, Milford, United States) was acquired for LC-MS/MS analyses with an ACQUITY UPLC HSS T3 column (1.8 μm, 2.1 mm × 100 mm, Waters, Milford, United States). The chromatography separation was performed at an ambient temperature of 40°C. The mobile phase included acetonitrile with 0.1 % formic acid (A), and deionized water with 0.1% formic acid (B), and the gradient of eluent was used: 0–15 min, 5%–68 % (A); 15–18 min, 68 %–95 % (A); 18–19 min, 95 %–5 % (A); and 19–20 min, 5 % (A). The flow rate was set at 0.3 ml/min, and the injection volume was 2 µL.
The key parameters of Q-TOF/MS were optimized as follows: scan type: positive and negative, acquire mass over the range 100–1300 Da, scan time: 0.25s, collision energy: 25–35 V, cone voltage: 40 V. the electrospray capillary voltage was 3.2 kV in the positive and negative ionization modes, the ion source temperature was 320°C, and the auxiliary heater temperature was 350°C.
Data processing and metabolism profile analysis
After the UPLC-Q-TOF/MS analysis, the raw data were imported into the Progenesis QI software (version 2.0, Nonlinear Dynamics, Waters, United States) for peak detection, alignment, deconvolution, and normalization. The resultant data matrices were linked to EZ info (version 2.0, Waters Corporation) for the principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA). The list of ions that contributed to the grouping was obtained from loading the S-plot and variable importance plot (VIP). The biomarkers were identified by MS/MS fragment ion and accurate mass by searching reliable online biochemical databases such as the Human metabolome database (HMDB) (http://www.hmdb.ca/) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (https://www.kegg.jp/kegg/) in the Progenesis QI software (Ren et al., 2016).
Hematological analyses, biochemical parameters, and real-time -PCR assay
On day 28, the thyroid tissue was soaked in 4% paraformaldehyde, embedded in paraffin, cut into 5-mm sections, and stained with Hematoxylin and Eosin (H&E). The sections were visualized using light microscopy (Nikon, Tokyo, Japan), and digital images were captured and analyzed.
A commercial ELISA kit (Wuhan Huamei Biotechnology Co., Ltd., Wuhan, China) was used to analyze the levels of T3, T4, and TSH in the serum in accordance with the manufacturer’s protocol. ACC1 and Na+/K+-ATP enzymes in the liver were detected by a commercial ELISA kit (Meimian, Jiangsu, China) in accordance with the manufacturer’s protocols. The rectal temperature of the rats was measured by an electronic standard rectal thermometer and the bodyweight of the rats was weighed by electronic scales at days 1, 7, 14, and 28.
The extracted total RNA from BAT, the primers designed, and the cDNA synthesis were references reported in the literature, which was performed by Wuhan Servicebio Biotechnology Co., Ltd. (Wuhan, China) (Tian et al., 2021). Primers were specific to the target genes, namely: UCP1: 5′- CGGGCTTAAAGAGCGAGAGG- 3’ (forward, accession no. NM_012682.2); GAPHD: 5′- CTGGAGAAACCTGCCAAGTATG- 3’ (forward, accession no. NM_017008.4). Gene expression was evaluated by the DDCT method with GAPDH as a housekeeping gene.
Assay of the Western blot, as previously reported (Zhang et al., 2021), the levels of PI3K (A4992, Abcam, MA, United States), Akt (Ap1208, Abcam, MA, United States), ULK (A8529, Abcam, MA, United States), IL17 (Ao688, Abcam, MA, United States), P-p38 MAPK (AP0526, Abcam, MA, United States), PPAR-γ (A19676, Abcam, MA, United States), UCP-1 (A5857, Abcam, MA, United States), and β-actin (A8227, Abcam, MA, United States) in the liver were examined by using Western blot.
Data collection of network analysis
Firstly, the chemical compositions of the four typical “hot” property herbs from the literature (searched from CNKI, PubMed, Web of Science, and PubChem) and the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, https://tcmsp.com/) database were obtained. The database included a network of chemicals, targets, and drug targets, as well as the pharmacokinetic properties of natural compounds with respect to oral bioavailability, drug similarity, intestinal epithelial permeability, the blood–brain barrier, and water solubility (He et al., 2022). Those meeting certain ADME properties and drug-likeness standards (OB ≥ 0.3, DL≥ 0.18) were chosen for further target prediction analysis (Bickerton et al., 2012). After filtering information, the component and target information of the four typical “hot” property herbs were collected including, FZ (21–28), GJ (5–48), RG (10–113), and WZY (30–454). All the chemical information was then used as a data source for target prediction. And then, the target of hypothyroidism was obtained from OMIM (https://www.omim.org/), Drug bank, and Gene Card (https://www.genecards.org/). Moreover, the intersection of the four typical “hot” property herb targets and hypothyroidism-associated targets were analyzed by a Venn Diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/). And, the PPI network was performed on the STRING database (https://string-db.org/cgi/input.pl). The STRING database is used for searching known and predicted interactions between proteins. In addition to containing experimental data, it contains text mining results from PubMed abstracts, the integration of other database data, and it also uses bioinformatics methods to determine the predicted result. There are already many protein interaction databases, and STRING covers the most species among them. The specific settings in this system are as follows: select “Homo sapiens” in the organism column, select “evidence” in the meaning of network edges column, set the confidence to >0.9, remove the free protein, obtain the correlation data between the targets, and import the acquired data into Cytoscape 3.7.2 for the PPI network (He et al., 2022). The analyses of the GO functional annotation and KEGG signaling pathways were conducted by the Database for the Annotation, Visualization and Integrated Discovery (DAVID) v 6.8 (https://david.ncifcrf.gov/home.jsp) for Gene Ontology (GO) and KEGG pathway enrichment analyses, which were mainly involved in the molecular function (MF), biological process (BP), and cellular components (CC). The cut-off criterion of the DAVID analysis was p < 0.05, which was considered statistically significant (Li et al., 2021; Zhang et al., 2022).
Statistical analysis
All the data were analyzed by using the SPSS 20.0 software (IBM; NY, United States). Statistical significance (p < 0.05) was assessed in comparison with the respective control for each experiment using one-way analysis of variance.
Results
Phenotypical analysis of the four typical “hot and cold” property herbs effect on hypothyroidism model
Bodyweight, levels of food and water intake, and rectal temperature were important phenotypical indexes for hypothyroidism generally being evaluated. As shown in Figure 1, decreased phenotypical indexes were observed in hypothyroidism model rats, which suggested that the disordered metabolism occurred in the body of hypothyroidism rats. Compared with the hypo group, body weight, levels of food and water intake, and rectal temperature were ameliorated by the four typical “hot property” herbs on day 28 and show a similar effect. However, after administration of the four typical “cold” property herbs’ extraction for 4 weeks, the levels of food and water intake was less than the levels of the hypo group (Figures 1C,D), and the bodyweight loss and rectal temperature was not improving, compared with the hypo group (Figures 1A,B).
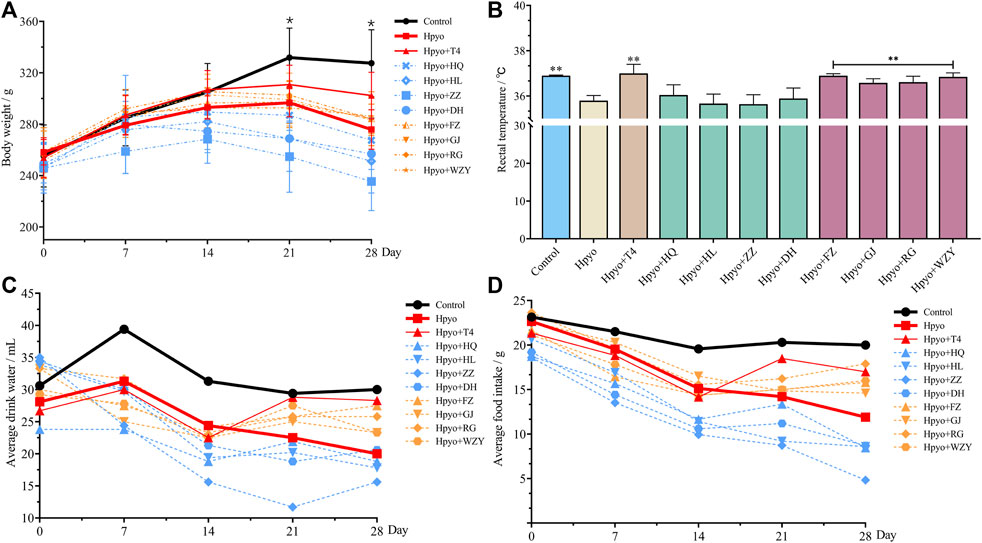
FIGURE 1. The altered trend of body weight (A), body temperature (B) and average water (C)/food (D) intake in each group (n = 8). Note: Compared with the hypothyroidism model group, *p < 0.05, **p < 0.01.
Thyroid function assessment
Firstly, the four typical “cold” property herbs and the four typical “hot” property herbs that affected the thyroid function of hypothyroidism rats were assessed by the levels of T3, T4, and TSH in the serum. As shown in Figure 2, after 4 weeks induced by the PTU, the levels of T3 and T4 were markedly decreased, and the concentration of TSH was obviously increased in the hypo group. However, the low levels of T3 and T4 and the elevated TSH concentration in the serum were significantly ameliorated by the four typical “hot” property herbs and showed similar intervention effects among them. This result suggested that the four typical “hot” property herbs possessed substantial treatment effects on the hypothyroidism rat model. While the four typical “cold” property herbs showed no improvement of the thyroid function in the hypothyroidism model.
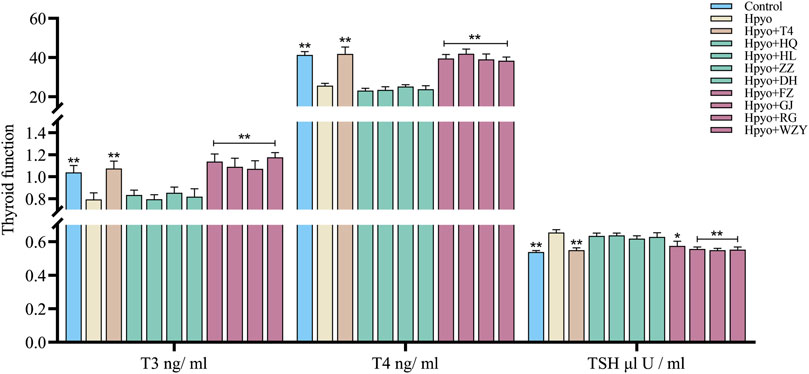
FIGURE 2. Effects of the four typical “hot” property herbs and the four typical “cold” property herbs on the thyroid function of the hypothyroidism model (n = 8). Note: Compared with the model group, *p < 0.05, **p < 0.01.
Metabolic profile analysis of the four “hot and cold” property herb effects on the hypothyroidism model
To maximize collected the metabolism information and fingerprints about the hypothyroidism model, urine samples were acquired both in the positive and negative modes of the ESI (electron spray ionization). Firstly, an unsupervised principal component analysis (PCA) was carried out to explore the influences of the four typical “hot and cold” property herbs on the hypothyroidism model metabolic profile. As the result shows in Figure 3A, a clear difference was observed between the control group and the hypo group, suggesting that the metabolism state of the whole body was changed after 4 weeks induced by the PTU. After administering L-thyroxine and four typical “hot” property herbs, the metabolism state was away from the place of the hypothyroidism model state, and closer likely to the control group metabolism states in the positive ion mode. Meanwhile, the four typical “hot” property herbs also showed different extent improvement effects for hypothyroidism rats in the negative ion mode (Figure 3B). However, the results of the four typical “clod” property herbs affected the hypothyroidism model (as shown in Figures 3C, D), the four typical “cold” property herbs gathered together with the hypo group, or even further away from the control group than the hypothyroidism rats. In addition, the PCA score scatter plots between the control group and each “cold and hot” herb group is shown in the Supplementary Figures S1, S2A–J and all the grouping trends were clear. This result indicated that the “hot” herbs with the same property had similar effects, and herbs with opposite properties had different effects.
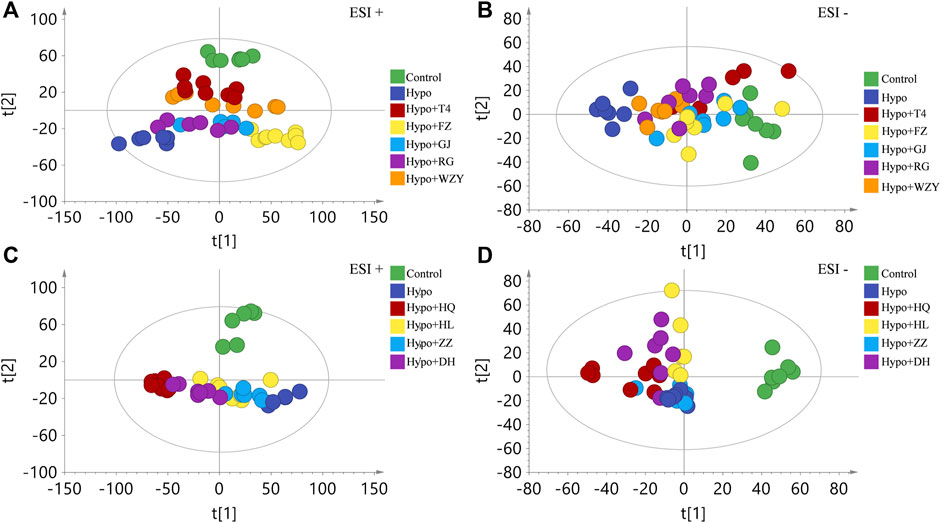
FIGURE 3. The principal component analysis (PCA) analysis of the four typical “hot” property herbs (A,B) represent in the positive and negative ion modes of the ESI, respectively) and the four typical “cold” property herbs (C,D) represent in the positive and negative ion modes of the ESI, respectively) effect on the hypothyroidism rats’ metabolic profile (n = 8).
In the present study, combining the results of physical signs, thyroid function, and metabolic profile, we can conclude that the four typical “cold” property herbs showed no benefit in the hypothyroidism model. Therefore, the four typical “hot” property herbs that affected hypothyroidism were used for further mechanism analysis.
Effects of the four typical “hot” property herbs on the histopathological changes in the thyroid tissue of hypothyroidism rats
The effect of the four typical “hot property” herbs on the histopathological changes in the thyroid tissue of hypothyroidism rats is shown in Figure 4. In the control group, the thyroid gland performed a normal distribution, morphology, and architecture of follicles (Figure 4A). As shown in Figure 4B, clear thyroid gland pathological damage, including follicular luminal obliteration (as indicated by the red arrow), fibroblast proliferation (as indicated by the black arrow), hyperplasia (as indicated by the green arrow), and the follicles appearing with a smaller size (as indicated by the blue arrow), was observed in the hypo group. However, these PTU-induced morphological changes were attenuated by the administration of the extraction of the four typical “hot” property herbs (as shown in Figures 4D–G).
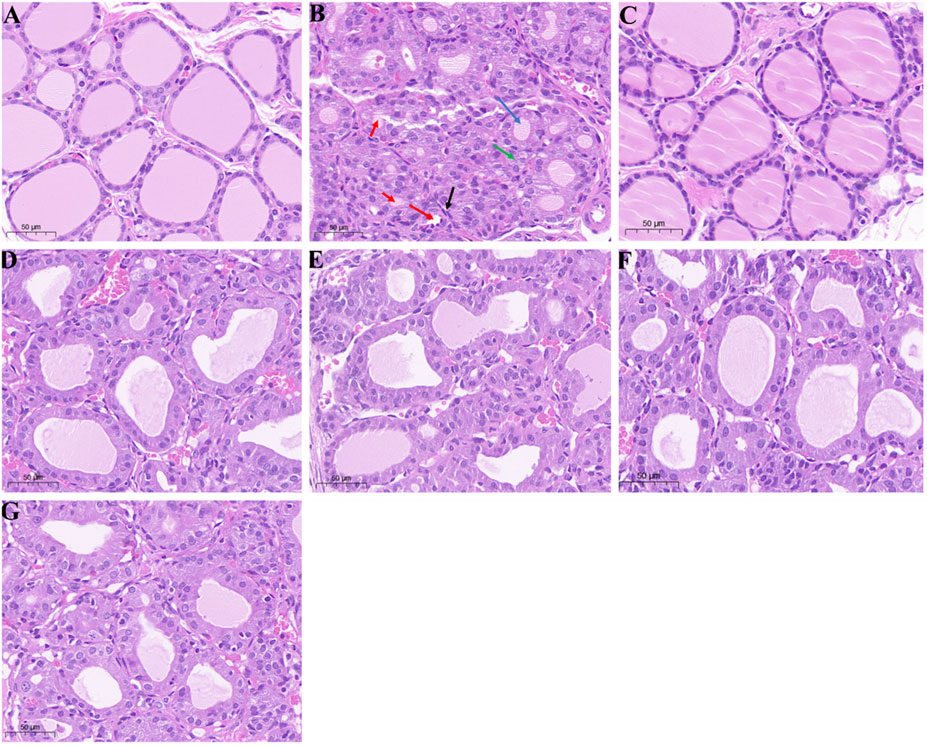
FIGURE 4. Histological examination (H and E) staining of thyroid glands; (A–G) represented the groups of control, hypo, T4, FZ, GJ, RG, and WZY, respectively. Magnification: ×20; Scale bar: 50 μm.
The potential biomarker identification and cluster analysis
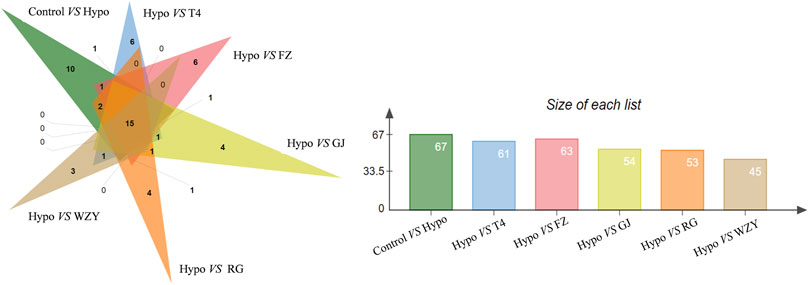
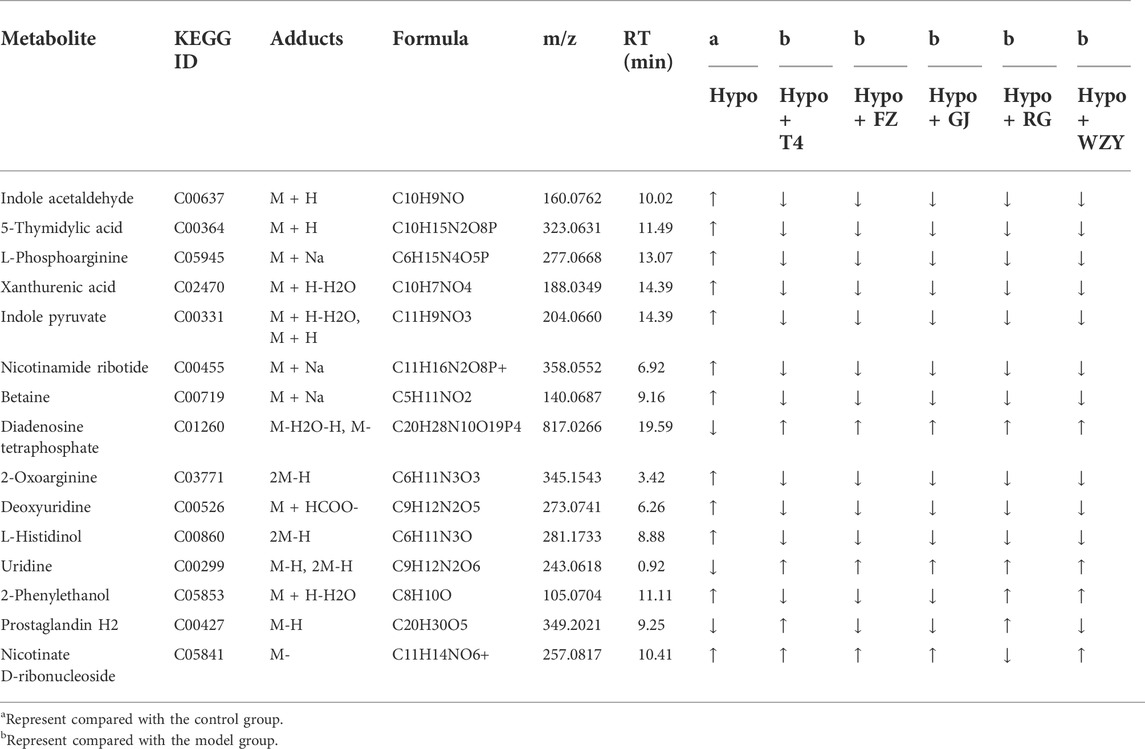
As a supervised learning approach, OPLS_DA could filter out non-essential variables and remarkably improve the accuracy of classification (Zuo et al., 2018). As the results showed, the OPLS_DA score plots between the control group and each hot herb group were significantly separate from the hypothyroidism model (Supplementary Figures S3, S4). Meanwhile, the potential biomarkers were obtained from the OPLS-DA analysis with the predetermined rules (VIP>1; p <0.05). It was suggested that the metabolism state or metabolites have altered. Combined with the results of PCA, those changed metabolites could be the key potential biomarker of working mechanisms. In this study, a total of common 15 specific metabolic biomarkers (Figure 5), related both to hypothyroidism models and the four typical “hot” property herbs, were identified by the QI software (as shown in Table 1).
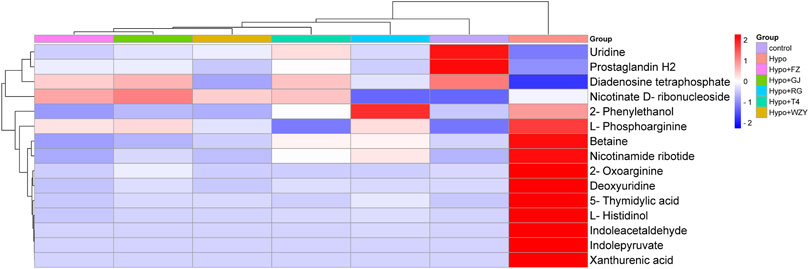
The hot map and cluster analysis results for those metabolites in the control, hypothyroidism model, L-thyroxine (T4), and the four typical “hot” property herb groups not only responded to the importance of metabolites but also validated the previous analysis results. As shown in Figure 6, the result of a cluster analysis shows that the control group is grouped with the L-thyroxine, RG, WZY, GJ, and FZ groups in turn, and is ultimately related to the Hypo group. This result further revealed the improvement effects of the four typical “hot” property herbs on hypothyroidism rats.
The pathway enrichment analysis and metabolic network
In order to further analyze how the four typical “hot” property herbs work on the hypothyroidism model by those different potential biomarkers, the altered metabolites were annotated by the KEGG database, and the pathway was analyzed by the Metabo-Analyst. It was found that 7 perturbed metabolism pathways were involved (Figure 7), mainly including pyrimidine metabolism, nicotinate and nicotinamide metabolisms, D-arginine and D-ornithine metabolisms, tryptophan metabolism, Glycine, serine and threonine metabolisms, arachidonic acid metabolism, and purine metabolism.
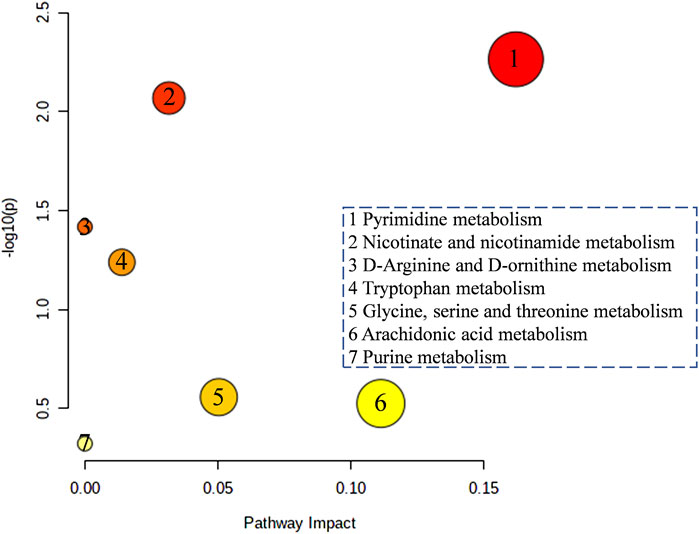
FIGURE 7. The function enrichment analysis of the four typical “hot” property herbs affected by the hypothyroidism model.
Network analysis of the four “hot” property herbs and hypothyroidism model
Then, the potential ingredients and molecular targets of the four typical “hot” property herbs were performed, based on a network pharmacological approach. We identified 214 potential targets associated with the four typical “hot” property herbs and 4,499 targets associated with hypothyroidism, and the common targets of 142 were selected as the key targets (Figure 8A). In combination with high-frequency node analysis in the PPI network (Figure 8B), we found that 25 targets with a circular layout and dark color, including INS (insulin), IL6 (interleukin 6), MAPK (mitogen-activated protein kinase), VEGFA (vascular endothelial growth factor A), TNF (tumor necrosis factor), AKT1 (protein kinase B1), and PPARs (peroxisome proliferator-activated receptors) could the key target proteins. In addition, the GO and KEGG pathway analyses were conducted by the STRING database for the pathway enrichment and function analysis based on the potential targets. The molecular function analysis revealed that these putative targets were mainly involved in the response to the hormones, lipids, and reactive oxygen species, and adjust to the MAPK cascade, inflammatory response, and oxidative stress biological process (as shown in Figure 8D). Moreover, the molecular function analysis revealed that those targets not only modulated the nuclear receptor activity, signaling receptor regulator activity, kinase regulator activity, oxidoreductase activity, peroxidase activity, and monooxygenase activity, but also tuned DNA-binding transcription factor binding, lipid binding, and cytokine receptor binding. Those biological processes and molecular functions related to the pathway mainly included: the PI3K/Akt signaling pathway, IL-17 signaling pathway, MAPK signaling pathway, thyroid hormone signaling pathway, Ca2+ signaling pathway, VEGF signaling pathway, NF-κB signaling pathway, sphingolipid signaling pathway, AMPK signaling pathway, insulin signaling pathway, cAMP signaling pathway, cytochrome P450, and PPAR signaling pathway (as shown in Figure 8C). The network among the key targets and signaling pathways is shown in Figure 8E.
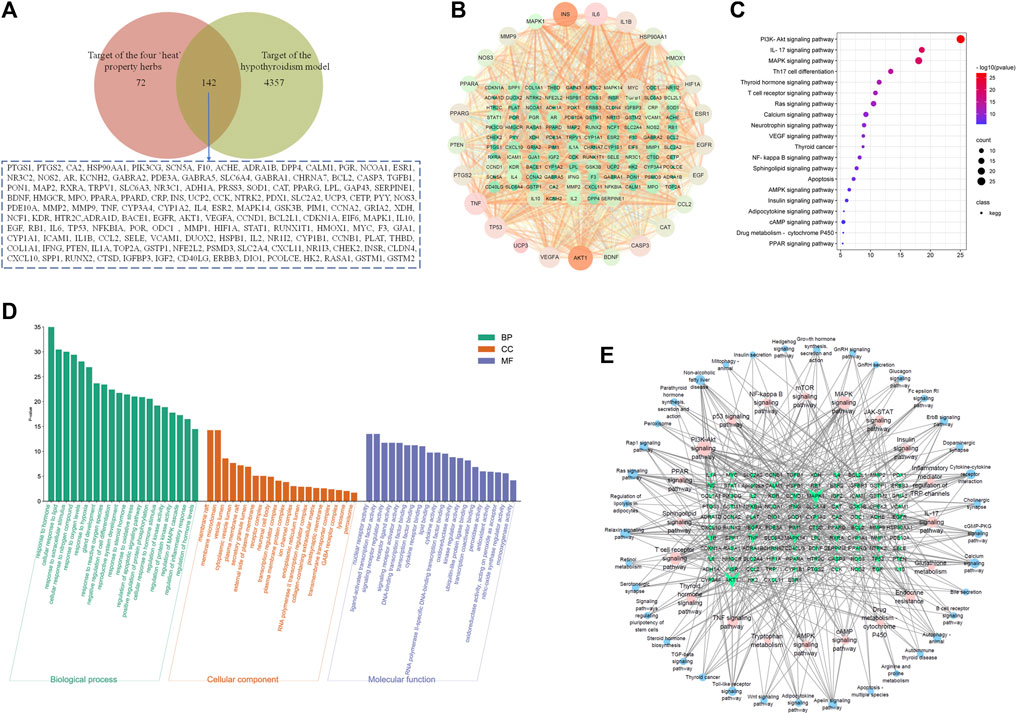
FIGURE 8. The target and network analysis of the four typical “hot” property herbs and the hypothyroidism model. (A): The Venn diagram of the common targets obtained from hypothyroidism and components of the four typical “hot” property herbs; (B): The PPI network with high-frequency node analysis by 142 common targets; (C): The common target pathway enrichment analysis; (D): the GO analysis of the biological processes, cellular components, and molecular functions with common targets; (E): The key targets and signaling pathway network relation-based GO and KEGG analyses.
The metabolomic and integrated analysis and verification
In this study, the perturbed metabolism network, based on the found metabolism pathway by the Metabo-Analyst and the network analysis, was constructed (Figure 9). This clearly illustrated the relationship among disturbed metabolic pathways, recalled metabolites, and key target proteins. As Figure 9 shows, the PI3K- Akt signaling pathway, Ca2+- AMPK signaling pathways are associated with lipid metabolism and energy metabolism; tryptophan metabolism, nicotinate, and nicotinamide metabolism, and arginine and proline metabolisms were associated with energy metabolism and thyroid hormone levels. Moreover, the mRNA level of UCP-1 in BAT, the levels of the Na+/K+-ATP enzyme, and ACC1 in the liver were detected to verify the network analysis and metabolomics results. As shown in Figure 10, decreased levels of UCP-1, the Na+/K+-ATP enzyme, and ACC 1 were observed, which suggested the presence of an energy metabolism imbalance and a disordered lipid metabolism in hypothyroidism rats. And, the four typical “hot” property herbs could ameliorate this disordered energy metabolism and lipid metabolism. Moreover, key proteins (as shown in Figure 10D) in the PI3K/Akt signaling pathway and thyroid hormone signaling pathway were Western blot analyzed and validated, which were restored by the four typical “hot” property herbs included IL17, MAPK, PI3K, Akt, and ULK-1.
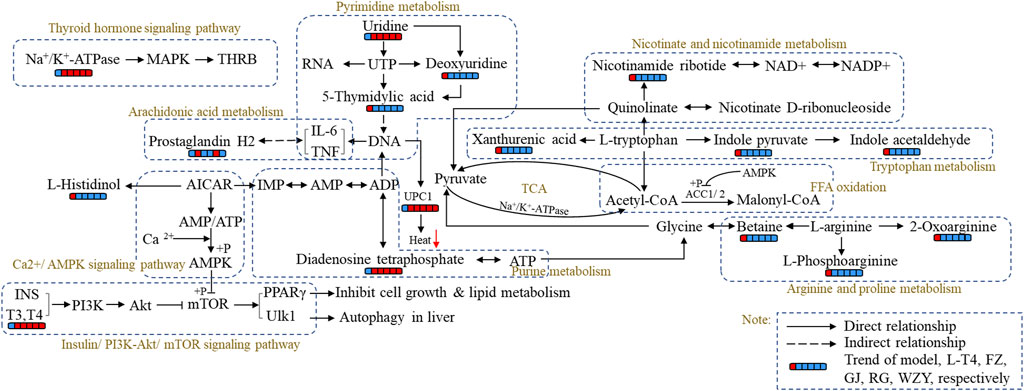
FIGURE 9. The metabolism network of the four typical “hot” property herbs influences the hypothyroidism model based on the hit target of the network analysis and the altered biomarker. Red represents the increased trend; blue represents the decreased trend.
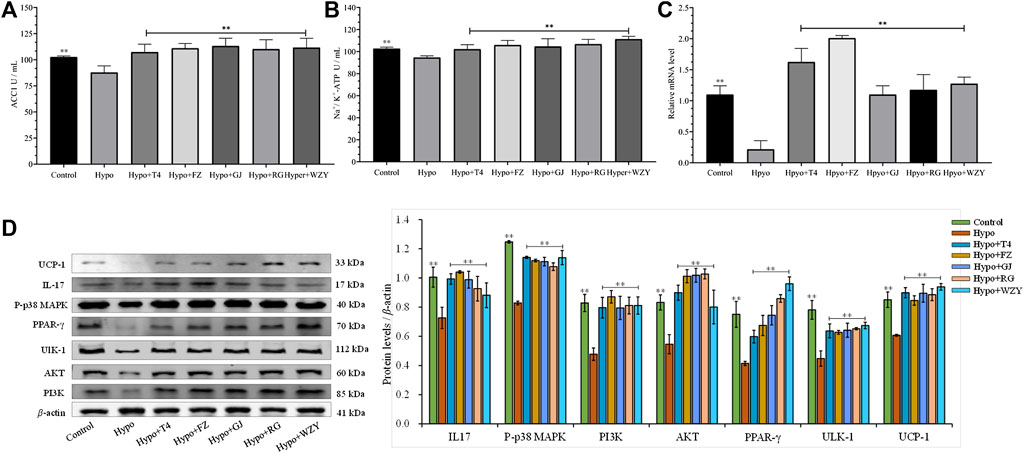
FIGURE 10. The levels of the ACC1 enzyme (A), Na+/K+-ATP enzyme (B) (n = 8) in the liver and the real-time PCR analysis of mRNA levels of UCP-1 in BATs from the hypothyroid model rat using NADPH as the internal control (C); the protein expression levels of IL17, MAPK, PI3K, Akt, and ULK-1 in the liver, and UCP-1 in BATs (D).
Discussion
The curative effect of the four typical “hot” property herbs on the hypothyroidism model
Hypothyroidism, an endocrine disorder disease, is known as the classic cold syndrome category in Chinese medicine (Chen et al., 2021). The medication principle of “treating cold/heat syndrome with hot/cold herbs” is widely used and affirmed in the clinical practice of Chinese medicine. And previous experiments in our laboratory have reported that a typical “hot” herb (Aconiti lateralis radix praeparaia) could improve hypothyroidism in rats with cold syndrome by adjusting the substances and energy metabolism (Xiao et al., 2017). In this study, a series of typical clinical features of hypothyroidism was observed in the hypo group, including huddling up together inactively, struggling weakly during catching, less water and food intake, and a rectal temperature decrement (Zhou et al., 2019). In addition, the decreased levels of T3 and T4, increased TSH concentration, as well as a damaged thyroid tissue also confirmed the success of model building (Ahmed et al., 2010). And the slight weight loss was explained by the lower growth-stimulating effect of thyroid hormones or the loss of appetite due to PTU (Müller and Seitz, 1981). Brown adipose tissue (BAT), as a major thermogenic tissue, is dominated by the thyroid hormones and the nervous system (Obregon, 2014). The uncoupling protein-1 (UCP-1), expressed in the BAT mitochondria, can release energy in the form of heat, rather than storage in the ATP. In this study, a decreased gene expression of UCP1, rectal temperature, and the Na+/K+-ATP enzyme were also observed in the hypothyroidism model (Figure 10). So, the energy metabolism and thermogenics were inhibited in hypothyroidism rats, and this declined the metabolism status that could be improved by the four typical “hot” property herbs.
As shown in the present studies, the results of physical signs, thyroid function, the histological evaluation of thyroid glands, and the metabolism profile analysis clarified that the four typical “hot” property herbs had therapeutic effects on the hypothyroidism model (a cold syndrome). But the four typical “cold” herbs were shown to have no benefit, which was consistent with the TCM theory. In the metabolism profile analysis, the QC sample was closely gathered together in the PCA score scatter plots both in the positive and negative modes, which suggested the great stability of the instrument and the acquiring methods (Supplementary Figures S1A, S2A). Meanwhile, the four typical “hot” property herbs were closely gathered together with the control group, and far away from the hypo group in the PCA score scatter plots (Figures 3A,B) and heat maps (Figure 6) which suggests that the “hot” herbs with the same properties had similar effects and herbs with opposite properties had different effects. In addition, the different curative among the four typical “hot” property herbs mainly contributed to the contents of the metabolites. The metabolism network (Figure 9) and network analysis (Figure 8) further elucidated the inner mechanism.
Influence of the four typical “hot” property herbs on lipid metabolism
Evidence has reported the direct effects of thyroid hormones on hepatic lipid metabolism (Sinha et al., 2018) and the high cardiovascular disease risk in hypothyroidism (Ermantas et al., 2010), which were largely attributed to disordered lipid metabolism. As we all know, the process of converting glucose to fatty acids termed, “de novo lipogenesis,” is induced by the thyroid hormone, and then produces fatty acids, triacylglycerol, and VLDL (Liang et al., 2011). ACC1 is a key enzyme in lipid synthesis, regulated by thyroid hormones (Lu et al., 2011). Studies have reported in hypothyroidism suggesting that lipid synthesis was reduced in the liver, due to the decreased activity of hepatic lipase, which can be recovered with thyroid hormone replacement therapy (Brenta et al., 2016). And a high level of T3 has been used for the treatment of hypercholesterolemia and obesity (Krotkiewski, 2002). In this study, a lower enzymatic activity of ACC1 and a decreased level of acetyl-CoA were observed in hypothyroidism livers (Figure 9), suggesting that the lipid metabolism and energy metabolism were disrupted in hypothyroidism, which is consistent with the previous study. Moreover, the ACC1 and acetyl-CoA were significantly upregulated by the four typical “hot” property herbs. These results suggest that the four typical “hot” property herbs could promote lipid metabolism, which might be one of the mechanisms of “hot” property herbs to improve hypothyroidism.
Current research shows that the mode of action of thyroid hormones mainly include nuclear transcriptional and non-genomic mechanisms (Cao et al., 2005), such as (ⅰ) the transcriptional regulation of target genes via THRs binding to TREs and the recruitment of co-activators to increase RNA polymerase binding to the basal transcriptional protein complex (Sinha et al., 2018). (ⅱ) Thyroid hormones could regulate hepatic lipid metabolism via activating the PI3K - Akt (RACα serine/threonine-protein kinase) signaling pathway, the PKA (cAMP-protein kinase A) and the Ca2+- AMPK signal pathways (Yamauchi et al., 2008), which is a non-genomic regulatory mechanism. In metabolomic and network enrichment analyses, the PPI network and metabolism network analyses (as shown in Figures 8, 9), the PI3K - Akt signaling pathway, and the Ca2+- AMPK signal pathways were closely associated with purine metabolism, pyrimidine metabolism, histamine metabolism, energy metabolism, and lipid metabolism. And there is evidence that purine metabolism is closely related to hypothyroidism (Wu et al., 2013). In addition, a decreased level of diadenosine tetraphosphate in purine metabolism and increased TSH concentration were detected in hypothyroidism rats, which suggests that diadenosine tetraphosphate is involved in the synthesis of TSH. This result was supported by previous studies (Friedrich et al., 2017). So, the four typical “hot” property herbs may ameliorate the hypothyroidism status by the PI3K- Akt signaling pathway, Ca 2+- AMPK signaling pathways for promoting lipid metabolism, energy metabolism through, as well promoting TSH synthesis, purine metabolism.
Influence of the four typical “hot” property herbs on tryptophan metabolism and energy metabolism
Tryptophan is an essential amino acid, involved in a variety of metabolisms, such as energy metabolism (Zhou et al., 2019) and thyroid hormone metabolism (Pereira et al., 2017). It is reported that a dietary tryptophan deficiency can alter the thyroid hormone levels (Vaccari et al., 1990) and lightened depression or anorexia and a poor appetite can be caused by low thyroid hormone levels (Pereira et al., 2017). Moreover, approximately 95% of tryptophan is metabolized via the kynurenine pathway and produced into xanthurenic acid, indole acetaldehyde, and indole pyruvate, which are crucial cofactors in glycolysis and mitochondrial respiration (Zhou et al., 2019), and are involved in the TCA cycle in the form of acetyl-CoA (as shown in Figure 9). So, tryptophan metabolism is closely associated with glycolysis, mitochondrial respiration, and thyroid hormone metabolism. As shown in Figure 9, it was observed in our study that xanthurenic acid, indole acetaldehyde, and indole pyruvate, the downstream metabolic products of tryptophan, clearly increased in the hypo group. The aberrantly increased catabolism of tryptophan may worsen the lack of tryptophan in vivo, and this change was consistent with those previously reported (Wu et al., 2013), and then result in thyroid dysfunction (Kulikov et al., 1999). Meanwhile, the decrease in the acetyl-CoA level detected by the ELISA kit has corroborated this result. However, after the four typical “hot” property herbs were administered to hypothyroidism rats, the levels of xanthurenic acid, indole acetaldehyde, and indole pyruvate returned to normal (Figure 9). Correspondingly, the symptoms of rectal temperature, water and food intake, and the mental state were healed as well. Therefore, the four typical “hot” property herbs could ameliorate the disordered tryptophan metabolism in hypothyroidism rats, thus promoting the lower energy metabolism and thyroid hormone levels.
In summary, the four typical “hot” property herbs improve hypothyroidism in rats possibly through the PI3K-Akt signaling pathway, Ca 2+- AMPK signaling pathways to promote lipid metabolism, energy metabolism, and tryptophan metabolism to improve the declined energy metabolism and thyroid hormone levels.
Conclusion
In this study, we firstly reported that all of the four typical “hot” property herbs could ameliorate the hypothyroidism model to different degrees, whereas no significant amelioration effect was noted in the four typical “cold” property herbs. Moreover, a metabolomic strategy combined with network analysis was successfully performed and well interpreted the mechanism of the four typical “hot” property herbs on hypothyroidism based on the theory of “cold and hot” properties of the TCM. Specifically, the four typical “hot” property herbs might influence hypothyroidism by (ⅰ) acting on the PI3K-Akt signaling pathway, Ca 2+- AMPK signaling pathways to promote lipid metabolism and energy metabolism; (ⅱ) promoting thyroid hormone synthesis through purine metabolism; (ⅲ) acting on the tryptophan metabolism to improve the declined energy metabolism and thyroid hormone levels. These common potential biomarkers might reflect the characteristics of the body after the administration of the four typical “hot” property herbs, in other words, these biomarkers represent the “hot” property of Chinese medicines.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by the Experimental Animal Ethics Committee of Heilongjiang University of Chinese Medicine.
Author contributions
Y-YW: conceptualization, methodology, software, and data curation, writing–original draft preparation. Y-PS: Visualization, investigation. B-YY, Q-HW, and H-XK: writing–reviewing and editing, H-XK: Providing financial support.
Funding
This study was supported by Heilongjiang Touyan Innovation Team Program [Grant Number: (2019) No. 5], Chief Scientist of Qi-Huang Project of National Traditional Chinese Medicine Inheritance and Innovation “One Hundred Million” Talent Project [Grant Number: (2021) No. 7], Qi-Huang Scholar of National Traditional Chinese Medicine Leading Talents Support Program [Grant Number: (2018) No. 284], The Seventh Batch of National Famous Old Traditional Chinese Medicine Experts Experience Heritage Construction Program of National Administration of TCM [Grant Number: (2022) No. 76] and National Famous Old Traditional Chinese Medicine Experts Inheritance Studio Construction Program of National Administration of TCM [Grant Number: (2022) No. 75].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.955905/full#supplementary-material
References
Ahmed, O. M., Abd El-Tawab, S. M., and Ahmed, R. G. (2010). Effects of experimentally induced maternal hypothyroidism and hyperthyroidism on the development of rat offspring: I. The development of the thyroid hormones–neurotransmitters and adenosinergic system interactions. Int. J. Dev. Neurosci. 28 (6), 437–454. doi:10.1016/j.ijdevneu.2010.06.007
Baltaci, A. K., and Mogulkoc, R. (2018). Leptin, neuropeptide Y (NPY), melatonin and zinc levels in experimental hypothyroidism and hyperthyroidism: relation with melatonin and the pineal gland. Horm. Mol. Biol. Clin. Investig. 34(3). doi:10.1515/hmbci-2018-0009
Barnes, S., Benton, H. P., Casazza, K., Cooper, S. J., Cui, X., Du, X., et al. (2016). Training in metabolomics research. I. Designing the experiment, collecting and extracting samples and generating metabolomics data. J. Mass Spectrom. 51, 461–475. doi:10.1002/jms.3782
Beckett, G. J., Kellett, H. A., Gow, S. M., Hussey, A. J., Hayes, J. D., and Toft, A. D. (1985). Raised plasma glutathione S-transferase values in hyperthyroidism and in hypothyroid patients receiving thyroxine replacement: evidence for hepatic damage. Br. Med. J. 291 (6493), 427–431. doi:10.1136/bmj.291.6493.427
Bickerton, G. R., Paolini, G. V., Besnard, J., Muresan, S., and Hopkins, A. L. (2012). Quantifying the chemical beauty of drugs. Nat. Chem. 4 (2), 90–98. doi:10.1038/nchem.1243
Brenta, G., Berg, G., Miksztowicz, V., Lopez, G., Lucero, D., Faingold, C., et al. (2016). Atherogenic lipoproteins in subclinical hypothyroidism and their relationship with hepatic lipase activity: Response to replacement treatment with levothyroxine. Thyroid 26 (3), 365–372. doi:10.1089/thy.2015.0140
Buriani, A., Garcia-Bermejo, M. L., Bosisio, E., Xu, Q., Li, H., Dong, X., et al. (2012). Omic techniques in systems biology approaches to traditional Chinese medicine research: Present and future. J. Ethnopharmacol. 140 (3), 535–544. doi:10.1016/j.jep.2012.01.055
Cao, X., Kambe, F., Moeller, L. C., Refetoff, S., and Seo, H. (2005). Thyroid hormone induces rapid activation of akt/protein kinase B-mammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Mol. Endocrinol. 19 (1), 102–112. doi:10.1210/me.2004-0093
Carneiro, G., Radcenco, A. L., Evaristo, J., and Monnerat, G. (2019). Novel strategies for clinical investigation and biomarker discovery: a guide to applied metabolomics. Horm. Mol. Biol. Clin. Investig. 38(3). doi:10.1515/hmbci-2018-0045
Chen, J., Dou, P., Xiao, H., Dou, D., Han, X., and Kuang, H. (2021). Application of proteomics and metabonomics to reveal the molecular basis of atractylodis macrocephalae rhizome for ameliorating hypothyroidism instead of hyperthyroidism. Front. Pharmacol. 12, 664319. doi:10.3389/fphar.2021.664319
Cheng, J., Lan, W., Zheng, G., and Gao, X. (2018). Metabolomics: A high-throughput Platform for metabolite profile exploration. Methods Mol. Biol. 1754, 265–292. doi:10.1007/978-1-4939-7717-8_16
Cheng, X., Wei, Z., Zhang, G., Shao, X., Li, B., and Gao, R. (2016). A systematic review of combinatorial treatment with warming and invigorating drugs and levothyroxine for hypothyroidism caused by Hashimoto disease. Ann. Transl. Med. 4 (23), 459. doi:10.21037/atm.2016.12.16
Clarke, N., and Kabadi, U. M. (2004). Optimizing treatment of hypothyroidism. Treat. Endocrinol. 3 (4), 217–221. doi:10.2165/00024677-200403040-00003
Committee, S. P. (2020). Pharmacopoeia of the people' rupublic of China. Beijing: Chinese medical science and technology press.
Ermantas, N., Guldiken, S., Demir, M., and Tugrul, A. (2010). Thrombin-activatable fibrinolysis inhibitor (TAFI) antigen and activity assay in patients with primary hypothyroidism. Clin. Appl. Thromb. Hemost. 16 (5), 568–573. doi:10.1177/1076029609350890
Fang, H. Y., Zeng, H. W., Lin, L. M., Chen, X., Shen, X. N., Fu, P., et al. (2017). A network-based method for mechanistic investigation of Shexiang Baoxin Pill’s treatment of cardiovascular diseases. Sci. Rep. 7 (1), 43632. doi:10.1038/srep43632
Feng, Y., Wu, Z., Zhou, X., Zhou, Z., and Fan, W. (2006). Knowledge discovery in traditional Chinese medicine: State of the art and perspectives. Artif. Intell. Med. 38 (3), 219–236. doi:10.1016/j.artmed.2006.07.005
Flynn, R. W., Bonellie, S. R., Jung, R. T., MacDonald, T. M., Morris, A. D., and Leese, G. P. (2010). Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J. Clin. Endocrinol. Metab. 95 (1), 186–193. doi:10.1210/jc.2009-1625
Friedrich, N., Pietzner, M., Cannet, C., Thuesen, B. H., Hansen, T., Wallaschofski, H., et al. (2017). Urinary metabolomics reveals glycemic and coffee associated signatures of thyroid function in two population-based cohorts. PLoS One 12 (3), e0173078. doi:10.1371/journal.pone.0173078
He, S., Wang, T., Shi, C., Wang, Z., and Fu, X. (2022). Network pharmacology-based approach to understand the effect and mechanism of Danshen against anemia. J. Ethnopharmacol. 282, 114615. doi:10.1016/j.jep.2021.114615
Jia, Y., Zhang, Z.-Z., Wei, Y.-H., Xue-Mei, Q., and Li, Z.-Y. (2017). Metabolomics coupled with similarity analysis advances the elucidation of the cold/hot properties of traditional Chinese medicines. Chin. J. Nat. Med. 15 (8), 631–640. doi:10.1016/S1875-5364(17)30091-2
Krotkiewski, M. (2002). Thyroid hormones in the pathogenesis and treatment of obesity. Eur. J. Pharmacol. 440 (2), 85–98. doi:10.1016/S0014-2999(02)01420-6
Kulikov, A., Moreau, X., and Jeanningros, R. (1999). Effects of experimental hypothyroidism on 5-HT1A, 5-HT2A receptors, 5-HT uptake sites and tryptophan hydroxylase activity in mature rat brain. Neuroendocrinology 69 (6), 453–459. doi:10.1159/000054449
La Vignera, S., Vicari, E., Tumino, S., Ciotta, L., Condorelli, R., Vicari, L. O., et al. (2008). L-thyroxin treatment and post-menopausal osteoporosis: relevance of the risk profile present in clinical history. Minerva Ginecol. 60 (6), 475–484.
Li, F. H., Guo, S. W., Zhan, T. W., Mo, H. R., Chen, X., Wang, H., et al. (2021). Integrating network pharmacology and experimental evidence to decipher the cardioprotective mechanism of Yiqihuoxue decoction in rats after myocardial infarction. J. Ethnopharmacol. 279, 114062. doi:10.1016/j.jep.2021.114062
Liang, X., Chen, X., Liang, Q., Zhang, H., Hu, P., Wang, Y., et al. (2011). Metabonomic study of Chinese medicine Shuanglong formula as an effective treatment for myocardial infarction in rats. J. Proteome Res. 10 (2), 790–799. doi:10.1021/pr1009299
Lu, X., Xiong, Z., Li, J., Zheng, S., Huo, T., and Li, F. (2011). Metabonomic study on ‘Kidney-Yang Deficiency syndrome’ and intervention effects of Rhizoma Drynariae extracts in rats using ultra performance liquid chromatography coupled with mass spectrometry. Talanta 83 (3), 700–708. doi:10.1016/j.talanta.2010.09.026
Meier, D. A., and Kaplan, M. M. (2002). “Hypothyroidism and thyroiditis,” in Contemporary endocrinology. Totowa, NJ.
Müller, M. J., and Seitz, H. J. (1981). Starvation-induced changes of hepatic glucose metabolism in hypo- and hyperthyroid rats in vivo. J. Nutr. 111 (8), 1370–1379. doi:10.1093/jn/111.8.1370
Obregon, M.-J. (2014). Adipose tissues and thyroid hormones. Front. Physiol. 5, 479. doi:10.3389/fphys.2014.00479
Pan, L., Li, Z., Wang, Y., Zhang, B., Liu, G., and Liu, J. (2020). Network pharmacology and metabolomics study on the intervention of traditional Chinese medicine Huanglian Decoction in rats with type 2 diabetes mellitus. J. Ethnopharmacol. 258, 112842. doi:10.1016/j.jep.2020.112842
Peng, Y., Li, S.-n., Pei, X., and Hao, K. (2018). The multivariate regression statistics strategy to investigate content-effect correlation of multiple components in traditional Chinese medicine based on a partial least squares method. Molecules 23 (3), 545. doi:10.3390/molecules23030545
Pereira, J. C., Pradella Hallinan, M., and Alves, R. C. (2017). Secondary to excessive melatonin synthesis, the consumption of tryptophan from outside the blood-brain barrier and melatonin over-signaling in the pars tuberalis may be central to the pathophysiology of winter depression. Med. Hypotheses 98, 69–75. doi:10.1016/j.mehy.2016.11.020
Ren, X., Zhang, J., Fu, X., Ma, S., Wang, C., Wang, J., et al. (2016). LC-MS based metabolomics identification of novel biomarkers of tobacco smoke-induced chronic bronchitis. Biomed. Chromatogr. 30 (1), 68–74. doi:10.1002/bmc.3620
Sinha, R. A., Singh, B. K., and Yen, P. M. (2018). Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 14 (5), 259–269. doi:10.1038/nrendo.2018.10
Taylor, P. N., Albrecht, D., Scholz, A., Gutierrez-Buey, G., Lazarus, J. H., Dayan, C. M., et al. (2018). Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 14 (5), 301–316. doi:10.1038/nrendo.2018.18
Tian, X., Lu, Q., Zhao, S., Li, J., Luo, Q., Wang, X., et al. (2021). Purple Corn Anthocyanin Affects Lipid Mechanism, Flavor Compound Profiles, and Related Gene Expression of Longissimus Thoracis et Lumborum Muscle in Goats. Animals. 11 (8), 2407. doi:10.3390/ani11082407
Vaccari, A., Rossetti, Z. L., de Montis, G., Stefanini, E., Martino, E., and Gessa, G. L. (1990). Neonatal hypothyroidism induces striatal dopaminergic dysfunction. Neuroscience 35 (3), 699–706. doi:10.1016/0306-4522(90)90340-A
Wang, Y., Zhou, S., Wang, M., Liu, S., Hu, Y., He, C., et al. (2016). UHPLC/Q-TOFMS-based metabolomics for the characterization of cold and hot properties of Chinese materia medica. J. Ethnopharmacol. 179, 234–242. doi:10.1016/j.jep.2015.12.061
Wu, S., Tan, G., Dong, X., Zhu, Z., Li, W., Lou, Z., et al. (2013). Metabolic profiling provides a system understanding of hypothyroidism in rats and its application. PLoS One 8 (2), e55599. doi:10.1371/journal.pone.0055599
Xiao, H., Tan, C., Yang, G., and Dou, D. (2017). The effect of red ginseng and ginseng leaves on the substance and energy metabolism in hypothyroidism rats. J. Ginseng Res. 41 (4), 556–565. doi:10.1016/j.jgr.2016.11.005
Yamauchi, M., Kambe, F., Cao, X., Lu, X., Kozaki, Y., Oiso, Y., et al. (2008). Thyroid hormone activates adenosine 5′-monophosphate-activated protein kinase via intracellular calcium mobilization and activation of calcium/calmodulin-dependent protein kinase kinase-β. Mol. Endocrinol. 22 (4), 893–903. doi:10.1210/me.2007-0249
Yuan, H., Ma, Q., Cui, H., Liu, G., Zhao, X., Li, W., et al. (2017). How can synergism of traditional medicines benefit from network pharmacology? Molecules 22 (7), E1135. doi:10.3390/molecules22071135
Zhang, B., Zeng, M., Li, B., Kan, Y., Wang, S., Cao, B., et al. (2021). Arbutin attenuates LPS-induced acute kidney injury by inhibiting inflammation and apoptosis via the PI3K/Akt/Nrf2 pathway. Phytomedicine. 82, 153466. doi:10.1016/j.phymed.2021.153466
Zhang, B., Zeng, M., Wang, Y., Li, M., Wu, Y., Xu, R., et al. (2022). Oleic acid alleviates LPS-induced acute kidney injury by restraining inflammation and oxidative stress via the Ras/MAPKs/PPAR-γ signaling pathway. Phytomedicine 94, 153818. doi:10.1016/j.phymed.2021.153818
Zhou, N., Yang, Y., Li, K., Ke, Y., Zheng, X., Feng, W., et al. (2019). Integrating strategies of chemistry, biochemistry and metabolomics for characterization of the medication principle of “treating cold/heat syndrome with hot/cold herbs”. J. Ethnopharmacol. 239, 111899. doi:10.1016/j.jep.2019.111899
Keywords: Typical “hot” property herbs, metabolomics, hypothyroidism, network analysis, metabolic mechanism, cold-hot medicine properties
Citation: Wang Y-Y, Sun Y-P, Yang B-Y, Wang Q-H and Kuang H-X (2022) Application of metabolomics and network analysis to reveal the ameliorating effect of four typical “hot” property herbs on hypothyroidism rats. Front. Pharmacol. 13:955905. doi: 10.3389/fphar.2022.955905
Received: 29 May 2022; Accepted: 27 July 2022;
Published: 25 August 2022.
Edited by:
Alexander George Panossian, Phytomed AB, SwedenReviewed by:
Feng Qiu, Tianjin University of Traditional Chinese Medicine, ChinaXuelin Zhou, Capital Medical University, China
Copyright © 2022 Wang, Sun, Yang, Wang and Kuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiu-Hong Wang, cWh3YW5nQGhsanVjbS5uZXQ=; Hai-Xue Kuang, aHhrdWFuZ0BobGp1Y20ubmV0
 Yang-Yang Wang
Yang-Yang Wang Yan-Ping Sun1
Yan-Ping Sun1 Bing-You Yang
Bing-You Yang Hai-Xue Kuang
Hai-Xue Kuang