- 1Department of Pharmacy, The Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
- 2Department of Endocrinology, The Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
- 3Department of Pediatrics, Ningbo Women’s and Children’s Hospital, Ningbo, China
- 4Department of Pediatric Endocrine Genetics and Metabolism, Chengdu Women’s and Children’s Center Hospital, Chengdu, China
- 5Department of Pediatrics, Wuhu First People’s Hospital, Wuhu, China
- 6Department of Pediatrics, First Affiliated Hospital of Army Medical University (Third Military Medical University), Chongqing, China
- 7Department of Pediatrics, The First Affiliated Hospital of Xiamen University, Xiamen, China
- 8Department of Endocrinology and Genetics, Shanghai Children’s Medical Center, Shanghai Jiaotong University School of Medicine, Shanghai, China
- 9Department of Genetics and Endocrinology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
- 10Department of Pediatrics, The Second Affiliated Hospital of Anhui Medical University, Hefei, China
- 11Department of Child Health Care, Liuzhou Maternity and Child Healthcare Hospital, Liuzhou, China
- 12Department of Pediatrics, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
- 13Department of Pediatrics, Zhejiang Provincial Hospital of Chinese Medicine, Hangzhou, China
- 14Department of Pediatrics, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, China
- 15Department of Pediatrics, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 16Department of Pediatrics, Xiangya Hospital, Central South University, Changsha, China
- 17Department of Pediatrics, The Second Xiangya Hospital, Central South University, Changsha, China
- 18Department of Pediatrics, Changchun Children’s Hospital, Changchun, China
- 19Department of Pediatrics, Hangzhou First People’s Hospital, Hangzhou, China
- 20Department of Pediatrics, Shaoxing Second Hospital, Shaoxing, China
- 21Department of Genetics and Endocrinology, Guangzhou Women and Children’s Medical Center, Guangzhou, China
- 22Department of Pediatrics, Qilu Hospital of Shandong University, Jinan, China
- 23Pediatric Research Institute, Qilu Children’s Hospital of Shandong University, Jinan, China
- 24National Clinical Trial Institute, The Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
- 25Research Center for Clinical Pharmacy, Zhejiang University, Hangzhou, China
- 26The Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
Objective: Polyethylene glycol recombinant human growth hormone (PEG-rhGH, Jintrolong®) is the first long-acting rhGH preparation that is approved to treat children with growth hormone deficiency (GHD) in China. Clinical experience with dose selections of PEG-rhGH is scarce. The present study compared the efficacy and safety of a lower dose to increase dosing regimens of PEG-rhGH treatment.
Methods: A multicenter, randomized, open-label, dose-comparison clinical study was conducted to compare the improvements in the height standard deviation score (Ht SDS), height velocity (HV), insulin-like growth factor-1 (IGF-1) SDS, and safety profiles of children with GHD who are treated with 0.2 mg/kg/week of PEG-rhGH dose or 0.14 mg/kg/week for 26 weeks.
Results: Ht SDS, HV, and IGF-1 SDS increased significantly after PEG-rhGH treatment in the two dose groups (p < 0.05). The improvements of Ht SDS, HV, and IGF-1 SDS were more significant in the high-dose group than in the low-dose group (p < 0.05). Ht SDS improvement in low-dose group was not non-inferiority to that in the high-dose group (p = 0.2987). The incidences of adverse events were comparable between the two groups.
Conclusion: The improvements of Ht SDS, HV, and IGF-1 SDS were more significant in the high-dose group than in the low-dose group (p < 0.05). PEG-rhGH at the dose of 0.14 mg/kg/week was effective and safe for children with GHD.
Clinical Trial Registration: clinicaltrials.gov, identifier NCT02908958.
Introduction
Recombinant human growth hormone (rhGH) has been used to treat growth hormone deficiency (GHD) in children for over 30 years with the aim of promoting linear growth (Richmond and Rogol, 2016; Collett-Solberg et al., 2019). The efficacy and safety of rhGH therapy has been demonstrated in many clinical trials (Shih et al., 1994; Peterkova et al., 2007; Slattery et al., 2014; Swerdlow et al., 2015; Rhie et al., 2019; Pfäffle et al., 2020; Backeljauw et al., 2021; Coutant et al., 2021). However, daily rhGH injections lead to poor adherence and decreased effectiveness. A recent meta-analysis reported that up to 71% of patients with GHD and their families were non-adherent to the prescribed treatment (Graham et al., 2018). As a result, several long-acting formulations of rhGH have been developed to reduce the frequency of administrations (Saenger and Mejia-Corletto, 2016; Miller et al., 2020).
Jintrolong® (GeneScience Pharmaceuticals, Changchun, China), a polyethylene glycol rhGH (PEG-rhGH), is the first commercial long-acting rhGH preparation approved in China. Compared to daily rhGH, PEG-rhGH has a longer Tmax and t1/2 and slower plasma clearance, which allows for weekly injection (Hou et al., 2016). Clinical studies have demonstrated the non-inferior efficacy and safety of PEG-rhGH compared to daily rhGH at an equivalent dose for the treatment of GHD (Luo et al., 2017; Qiao et al., 2019; Sun et al., 2021; Wang et al., 2021; Du et al., 2022). Notably, in a Phase III trial, PEG-rhGH treatment at 0.2 mg/kg/week was associated with greater increases in most of the efficacy endpoints, including height velocity (HV), height (Ht) standard deviation score (SDS), and insulin-like growth factor-1 (IGF-1) SDS, compared to daily rhGH dosing of 0.25 mg/kg/week (Luo et al., 2017). IGF-I has an effect on cell proliferation, and its increased serum concentration might be associated with an increased risk of common cancers (Renehan et al., 2004; Pfäffle, 2015). The change in the area under the concentration curve of IGF-1 after 7 days of PEG-rhGH injection at a dose of 0.2 mg/kg was 1.3 folds larger than that of rhGH at 0.25 mg/kg/week in a Phase I clinical trial (p = 0.059) (Hou et al., 2016). These results suggest that PEG-rhGH can be administrated at a lower dose to achieve comparable efficacy and safety.
Clinical experience with dose selections of PEG-rhGH is scarce. A PEG-rhGH dose of 0.14 mg/kg/week is equivalent to a daily rhGH dose of 0.12 IU/kg/d, which is within the recommended dose range for children with GHD. In addition, results of an animal study reported that in rats, a single PEG-rhGH dose of 0.14 mg/kg/week showed the same expected linear growth as a daily rhGH dose of 0.25 mg/kg/week (Zhang et al., 2012). Taken together, the present study aimed to compare the efficacy and safety of PEG-rhGH treatment at a dose of 0.14 mg/kg/week to 0.2 mg/kg/week in children with GHD.
Materials and methods
Study design and participants
This study was a multicenter, randomized, open-label, parallel-group, dose-comparison clinical trial that took place at 22 medical centers in China for 26 weeks. The study protocol was reviewed and approved by the Ethics Committee of the Children’s Hospital, Zhejiang University School of Medicine and other participating centers. The parents or legal guardians of all participating children signed informed consents. The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines.
Eligible participants were prepubertal GHD patients (Tanner stage 1) aged at 3 years or older who had not received any GH treatment for 6 months. GHD was diagnosed using the following criteria: 1) height below -2SD or the third percentile of the normal growth curve for children of the same chronological age (CA) and sex in China (Hui et al., 2009); 2) HV ≤ 5 cm/year; 3) serum GH peak <10 μg/L in two different GH stimulation tests (stimulation with insulin, L-dopa, glucagon, arginine, or clonidine); and 4) bone age (BA) below 10 years for boys and 9 years for girls, with a minimum of a 1 year delay compared to the CA. Key exclusion criteria included renal or hepatic impairment; positive results for hepatitis B virus test, hypersensitivity of the study drug, serious cardiopulmonary, hematologic diseases, systemic infections or immunocompromising disorder, familial history of malignant tumor, diabetes, and other abnormal growth syndromes (i.e., Turners, constitutional delay of puberty, Laron Syndrome, growth hormone receptor deficiency). Those who had participated in other clinical trials within the 3 months prior to enrollment were also excluded.
Randomisation and masking
Patients were randomly assigned in a 1:1 ratio to randomized blocks (6 people per block) using a computer-generated random sequence to receive a PEG-rhGH dose of 0.14 mg/kg/week or 0.2 mg/kg/week. The study medicines and participant numbers were assigned in the forms of block multiples to each center. Each participant was assigned a unique medicine number. The investigators and parents/guardians were not masked to treatment allocation.
Procedures
PEG-rhGH was subcutaneously injected at a fixed time of the day by patients or their parents/guardians, who were able to administrate PEG-rhGH after training. The injection sites could be the lateral upper arm, lateral thigh, or the abdomen except the periumbilical area; the two injection sites were to be more than 2 cm apart. Each administration date and time was carefully recorded on diary cards. The treatments lasted for 26 weeks, and three follow-up visits were scheduled at week 4, 13, and 26 ( ± 5 days) after treatment initiation. At each visit, height and body weight were measured by designated personnel at each center. Blood samples were collected for blood routine tests, blood biochemistry, blood lip, blood glucose, thyroid function, serum IGF-1 concentration and anti-drug antibodies. Pituitary magnetic resonance imaging and electrocardiography were performed at each center. BA radiography was performed using the Tanner-Whitehouse three method at baseline and at week 26. Participants were not to use other medicines that may affect the efficacy of PEG-rhGH, such as gonadotropin-releasing hormone analogs, androgens, anabolic hormones, or other drugs that affect growth and development.
Outcomes
The primary efficacy outcome was the Ht SDS at week 26 after PEG-rhGH treatment. Secondary outcomes included HV and IGF-1 SDS at week 26 after PEG-rhGH treatment. Ht SDS and IGF-1 SDS were defined as the SD scores at each visit, based on the same CA and sex. HV was calculated as the height change per year. Safety was assessed by monitoring the adverse events (AEs), clinical symptoms, and laboratory tests at each visit. AEs were recorded, irrespective of their causal relationship to the treatment.
Statistical analysis
A sample size of at least 191 per group was needed to achieve a power of 90% with an α level of 0.025 for a non-inferiority margin of −20% in Ht SDS change (Sun et al., 2021). We assumed a dropout rate of 20% and to guarantee the robustness of the results, a total of 900 patients were planned to recruit.
Efficacy analysis was performed at week 26 in the modified intention-to-treat (mITT) and per-protocol populations. The mITT population included all randomized patients who received at least one injection of PEG-rhGH and completed at least one follow-up visit. The per-protocol population comprised of all randomized patients from the mITT population who completed all follow-up visits and had no major protocol deviations. Safety analysis was performed on the safety set (SS), which included all randomized patients who received at least one injection and safety record.
Continuous variables are presented as mean ± SD and categorical variables are presented as frequency and percentage. To assess the changes in PEG-rhGH treatment before and after with-in groups, continuous variables were statistically analyzed by paired t-test if they were normally distributed and homogeneous; otherwise, a Wilcoxon rank-sum test was used. Missing data were imputed using the last-observation-carried-forward method. Changes between the two groups were analyzed by covariance (ANCOVA) with baseline as the covariate, taking the center effect into consideration. A chi-squared (χ2) test was used to compare enumeration data and ratios. Results were considered significant at p < 0.05. The non-inferiority of 0.14–0.2 mg/kg/week would be accepted if the lower limit of the two-sided 95% CI for the difference between the two dose groups was greater than the non-inferiority margin. All statistical analyses were performed using SAS (version 9.4, SAS Institute Inc. Cary, NC).
Results
Patient characteristics
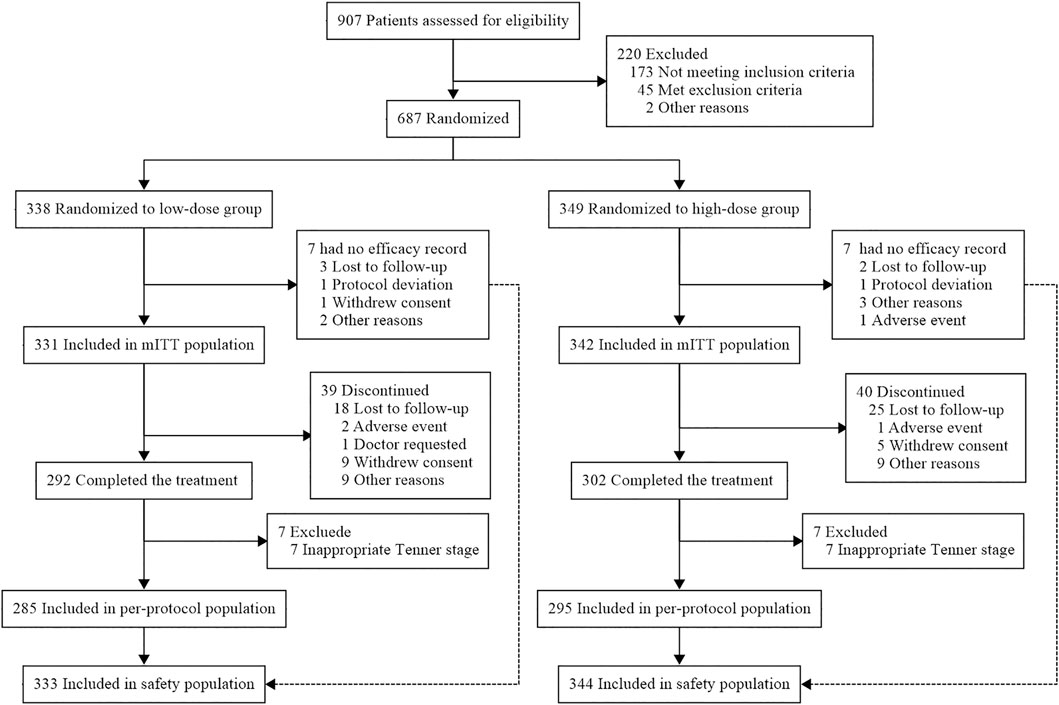
Between October 2014 and December 2017, 907 patients were screened and 687 patients were randomly assigned to receive 0.14 mg/kg/week of PEG-rhGH (n = 338) or 0.2 mg/kg/week (n = 349) (Figure 1). Seven patients in each group had no efficacy records and were not included in the mITT population. A total of 594 patients completed all follow-up visits, and seven patients in each group were excluded during data verification due to puberty. Finally, 285 patients in the low-dose group and 295 in the high-dose group were included in the per-protocol population. Since the results of the efficacy analysis of the per-protocol population were similar to those of the mITT population, only the mITT results are presented.
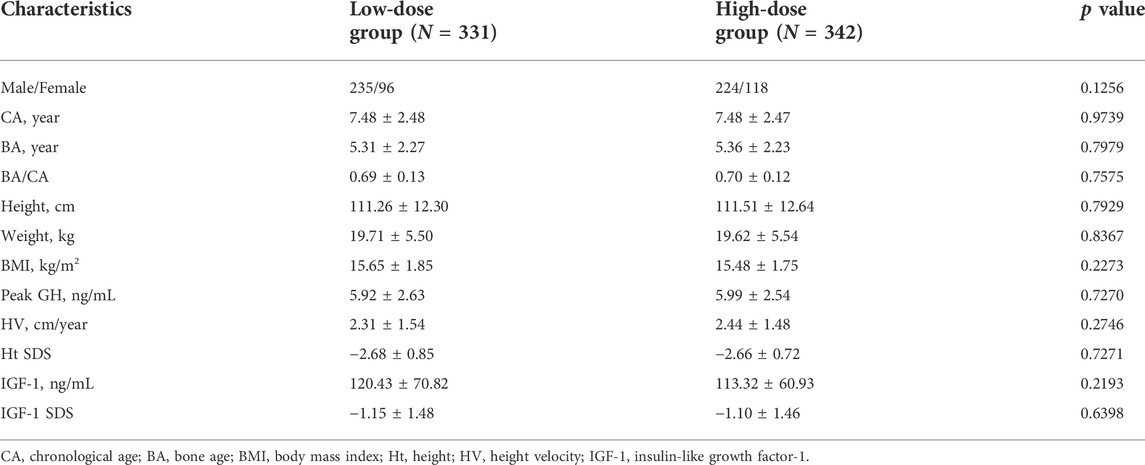
The demographic and baseline characteristics of the study population were comparable between the treatment groups (Table 1). All the patients were preadolescents, and BA/CA indicated retardation of bone maturation. All subjects were negative for Anti-GH antibodies.
Efficacy assessment
After PEG-rhGH treatment, the mean Ht SDS increased significantly in both dose groups at each assessment (Figure 2A). It increased from −2.68 ± 0.85 at baseline to −2.25 ± 0.72 at week 26 (p < 0.0001) in the low-dose group and from −2.66 ± 0.72 to −2.14 ± 0.75 (p < 0.0001) in the high-dose group. At each visit, the mean increments of Ht SDS in the low-dose group and the high-dose group were 0.12 ± 0.12 vs. 0.14 ± 0.13 (p = 0.1302) at week 4, 0.27 ± 0.19 vs. 0.32 ± 0.18 (p = 0.0008) at week 13, and 0.42 ± 0.28 vs. 0.51 ± 0.25 (p < 0.0001) at week 26, respectively. This suggests that the improvement of Ht SDS is dose-dependent, and the high dose had a more significant improvement in linear growth than the low dose. The lower limit of 95% CI of the Ht SDS change difference was −0.13, which was below the margin of −0.10. Thus, non-inferiority of Ht SDS change was not established (p = 0.2987).
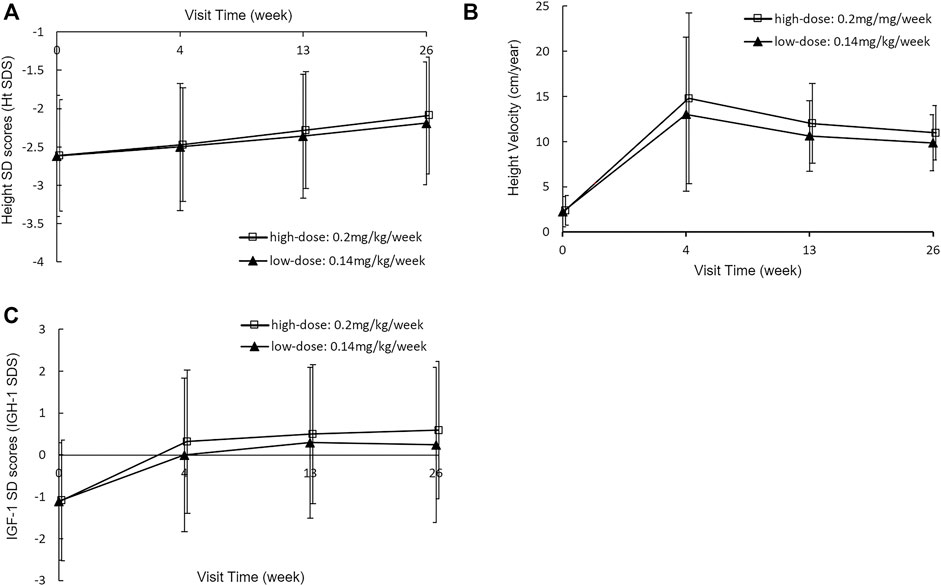
FIGURE 2. Height SDS (A), Height velocity (B) and IGF-1 SDS (C) at baseline and week 4, 13 and 26 with a PEG-rhGH dose of 0.14 mg/kg/week or 0.2 mg/kg/week.
HV increased rapidly in the first 4 weeks of PEG-rhGH treatment in both groups and then decreased slowly (Figure 2B). Similar effects of PEG-rhGH were observed in the HV as was observed in the Ht SDS. HV increased at a rate of 10.89 ± 8.05 cm/year in the low-dose group and 11.91 ± 8.83 cm/year in the high-dose group at week 4 (p = 0.1163). Then the increments decreased to 8.58 ± 4.30 cm/year in the low-dose group and 9.39 ± 8.97 cm/year in the high-dose group at week 13 (p = 0.0108), and 7.73 ± 3.53 cm/year and 8.46 ± 2.99 cm/year at week 26 (p = 0.0042). The low-dose group met the non-inferiority compared with the high-dose group, with a lower limit of 95% CI of −1.22 within the non-inferiority margin (p < 0.0001).
The mean IGF-1 SDS values also increased significantly (Figure 2C). In the low-dose group, it increased from -1.15 ± 1.48 at baseline to 0.06 ± 1.89, 0.20 ± 1.78, and 0.19 ± 1.82 at week 4, 13, and 26, respectively. And in the high-dose group, it increased from -1.10 ± 1.46 at baseline to 0.26 ± 1.74, 0.45 ± 1.68, and 0.57 ± 1.68 at week 4, 13, and 26, respectively. There were no significant increases of IGF-1 SDS at week 13 and 26 from baseline between two groups (p = 0.9508 in the low-dose group and p = 0.3766 in the high-dose group).
Safety
A total of 677 patients were concluded for the Safety analysis: 333 in the low-dose group and 344 in the high-dose group. Anti-GH antibodies were tested for patients at week 13 and week 26 after treatments initiation. No positive anti-drug antibodies were detected in both two groups. There were no statistical differences in the incidence of AEs and SAEs between the two groups (AEs: 50.5% vs. 53.9%, p = 0.3963; SAEs: 1.2% vs. 1.7%, p = 0.7525). The most common AEs were upper respiratory tract infections, followed by cough and fever in both the low-dose group (31.5%, 8.7%, and 7.8%) and the high-dose group (33.5%, 8.9%, and 6.5%). 33 in the low-dose group and 31 in the high-dose group were considered to be PEG-rhGH-related (p = 0.6896). All SAEs were not PEG-rhGH-related except for one case of Henoch-Schonlein purpura in the low-dose group, where the correlation with PEG-rhGH was not identifiable.
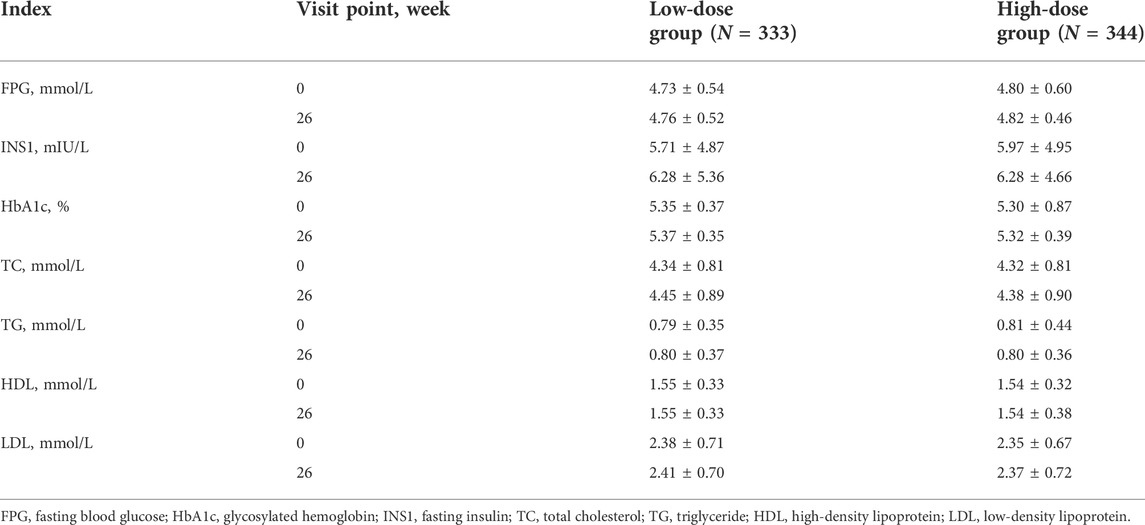
During PEG-rhGH treatment, no statistical changes were found in blood glucose and lipid indexes including fasting blood glucose, fasting insulin, glycosylated hemoglobin, total cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein (p > 0.05) (Table 2).
Discussion
Jintrolong® is the first long-acting rhGH preparation approved by the Center for Drug Evaluation of China. Based on the results of the present study, PEG-rhGH is effective and safe at a lower dose of 0.14 mg/kg/week for improving Ht SDS, HV and IGF-1 SDS in children with GHD; non-inferiority of Ht SDS at the dose of 0.14 mg/kg/week was not established after 26 weeks of treatment.
The GH/IGF-1 axis is critical for growth regulation (Balhara et al., 2012). GH induces bone growth by stimulating the production of IGF-1 in the liver, which in turn regulates GH secretion and stimulates longitudinal bone growth in the growth plate (Balhara et al., 2012; Wu et al., 2015). After 26 weeks of PEG-rhGH treatment, significant increases in Ht SDS and HV were observed in both dose groups, as expected. The incremental changes in Ht SDS and HV in the high-dose group at week 26 yielded similar results as were reported in a Phase IV clinical trial and another single-center, nonrandomized cohort study of Jintrolong® at the same dose of 0.2 mg/kg/week (Qiao et al., 2019; Sun et al., 2021), but were less than the results from the Phase III clinical trial of Jintrolong® (Luo et al., 2017). Changes in Ht SDS were negatively correlate with age, baseline IGF-1, and peak GH levels (Sun et al., 2021). The mean peak GH level and mean value of IGF-1 SDS were lower in the Phase III clinical trial, which may explain the differences in growth responses in different clinical trials. Attempts have been made to extend the dosing interval of PEG-rhGH, however, changes of both Ht SDS and HV failed the non-inferiority test to weekly administration of PEG-rhGH or daily administration of rhGH (Sun et al., 2021). There were also some clinical trials that use the HV improvement as the primary efficacy outcome with the non-inferiority margin of −2 cm (Luo et al., 2017; Czepielewski et al., 2019). Although the non-inferiority was not established in terms of improving Ht SDS change, our results demonstrated that the dose of 0.14 mg/kg/week was non-inferior to the dose of 0.2 mg/kg/week in improving HV of children with GHD. Meanwhile, the efficacy of PEG-rhGH treatment at 0.14 mg/kg/week were consistent with the conventional dose of rhGH treatment in previous studies (Xue et al., 2016; Deal et al., 2018; Czepielewski et al., 2019). These results suggest that the PEG-rhGH dose of 0.14 mg/kg/week could be considered as a low dose option to attain an optimistic efficacy, which would reduce both adverse reactions and the treatment costs.
The serum IGF-1 level is an important parameter for monitoring GH treatment. It has been reported that children whose rhGH doses were adjusted maintain serum IGF-1 levels in the upper normal range (+ 1.5- + 2.5 SD) gained better improvement in growth response compared to children with IGF-I levels in the mid-normal range (Cecconi et al., 2004; Cohen et al., 2007; Pfäffle, 2015). Similar to Ht SDS and HV, IGF-1 SDS was significantly elevated during the 26 weeks and showed dose-dependent changes. Notably, the IGF-1 SDS rapidly increased at week four and reached a plateau at week 13 in the low-dose group but continued to increase slightly in the next 5 months in the high-dose group, although no significant differences observed (p > 0.05). The trends were different from the Phase III clinical trial of Jintrolong®, in which IGF-1 SDS reached a plateau at around week 13 and then gradually decreased with the same dose of 0.2 mg/kg/week (Luo et al., 2017). This difference may be attributed to the huge inter-individual variation in IGF-1 levels which are influenced by sex, age, body weight, nutritional status, and puberty stage and so on (Liu et al., 2019; Witkowska-Sędek et al., 2019; Papathanasiou et al., 2021). The previously mentioned cohort study reported that IGF-1 SDS in the PEG-rhGH group reached the upper limit of the normal range (0.96 ± 1.39) during the first 6 months and continued to increase over the next 18 months (Qiao et al., 2019). Considering the risks associated with IGF-1, the question of whether a high dose of PEG-rhGH leads to a supraphysiological level of IGF-1 requires long-term follow-up.
GH activates insulin-sensitive lipase, promotes fat decomposition, inhibits glucose uptake and utilization in skeletal muscles and adipose tissue, reduces glucose consumption, and increases blood glucose levels (Weber et al., 2017). It has been observed that the blood glucose and lipid levels decrease after rhGH treatment (Ciresi et al., 2007; Slattery et al., 2014; Kubo et al., 2017), while other clinical trials have not found significant changes in glucose metabolism after rhGH treatment (Czepielewski et al., 2019). A recent meta-analysis revealed a favorable role of rhGH therapy in lipid metabolism, which might depend on the duration of the intervention; however, the role of rhGH in glucose metabolism was not significant (Yuan et al., 2021). For instance, an increase in HbA1c level was observed after 1 year of rhGH therapy in a retrospective study of 101 pediatric patients with GHD (Pellegrin et al., 2019). For PEG-rhGH, improvements in lipid profiles (Hou et al., 2016) and non-significant changes in lipid metabolism (Wang et al., 2021) have been reported; however, none of them exert an unfavorable effect on glucose metabolism (Hou et al., 2016; Qiao et al., 2019; Wang et al., 2021). In our study, no significant changes were found in glucose or lipid metabolism after 26 weeks of PEG-rhGH treatment in children with GHD, regardless of PEG-rhGH dose. Our metabolomics analysis had revealed a strong association between fatty acids metabolism and the clinical efficacy of PEG-rhGH therapy, which would likely to be involved in fatty acid metabolism and energy metabolism (Li et al., 2022). Long-term follow-up is needed to confirm the effects of PEG-rhGH treatment on glucose and lipid metabolism.
Although the short-term efficacy and safety of PEG-rhGH treatment has been proven in clinical trials, introduction of a modified PEG molecule may cause new side effects (Lal and Hoffman, 2018). In addition, long-term elevated GH levels produced by PEG-rhGH treatment may induce iatrogenic acromegaly, neoplasia and glucose intolerance (Yuen et al., 2021). Therefore, every centimeter gained from PEG-rhGH treatment comes with a certain amount of risk. Moreover, despite the reduced frequency of injections, the cumulative cost of long-term treatment with PEG-rhGH remains high. Further pharmacoeconomic evaluations are needed to determine the correct cost and risk-benefit ratio.
Our study has some limitations. First, the pharmacokinetic and pharmacodynamic profiles of 0.14 mg/kg/week dosing have not been evaluated in order to explain the differences in the IGF-1 responses between the two PEG-rhGH doses. Second, serum IGF-1 levels increased steadily after PEG-rhGH injection and reached to a peak concentration after 2 days (Hou et al., 2016). However, for the convenience of patients and their parents, the follow-up visit was not strictly set for the second day after dosing according to the study protocol, which might introduce some error in the accuracy of the IGF-1 SDS.
In conclusion, there were significant increases in Ht SDS, HV, and IGF-1 SDS at week 26 after PEG-rhGH treatment in both dose groups. Ht SDS improvement with treatment using 0.14 mg/kg/week of PEG-rhGH was not non-inferiority to that at the standard dose of 0.2 mg/kg/week. Additionally, there were no significant changes in glucose or lipid metabolism after PEG-rhGH treatment at the different doses. Furthermore, a longer follow-up period is needed to assess the long-term efficacy and safety of lower doses of PEG-rhGH to optimize the therapeutic dose of PEG-rhGH.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Children’s Hospital, Zhejiang University School of Medicine, Ningbo Women&Children’s Hospital, Chengdu Women’s and Children’s Center Hospital, Wuhu First People’s Hospital, First Affiliated Hospital of Army Medical University (Third Military Medical University), the First Affiliated Hospital of Xiamen University, Shanghai Children’s Medical Center, Shanghai Jiaotong University School of Medicine, the Second Affiliated Hospital&Yuying Children’s Hospital of Wenzhou Medical University, the Second Affiliated Hospital of Anhui Medical University, Liuzhou Maternity and Child Healthcare Hospital, the Third Affiliated Hospital of Sun Yat-Sen University, Zhejiang Hospital of TCM, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, the First Affiliated Hospital of Guangxi Medical University, Xiangya Hospital, Central South University, the Second Xiangya Hospital, Central South University, Changchun Children’s Hospital, Hangzhou First People’s Hospital, Shaoxing Second Hospital, Guangzhou Women and Children’s Medical Center, Qilu Hospital of Shandong University, Qilu Children’s Hospital of Shandong University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
JF is the principal investigator of the study. XC, JZ, XC, JP, WL, JW, XH, XJ, DL, TZ, SZ, QD, XL, DL, LC, XZ, JL, MD, MZ, LL, JD, and DZ are site investigators and conducted the study in each participating center. JF conceived the study design, managed the study, and coordinated the study. SN helped with study design, management and coordination. ZJ drafted this manuscript. ZJ and XC analyzed the data. ZJ, GD, and YL collected the data. JF provided final approval to the submitted version. All authors contributed to the article and approved the submitted version.
Funding
This research was s supported by the National Natural Science Foundation of China Grant No. 81573516.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AE, adverse event; BA, bone age; CA, chronological age; GH, growth hormone; GHD, growth hormone deficiency; Ht, height; HV, height velocity; IGF-1, insulin-like growth factor-1; mITT, modified intention-to-treat; PEG-rhGH, polyethylene glycol-recombinant human growth hormone; rhGH, recombinant human growth hormone; SD, standard deviation; SDS, standard deviation score; SS, safety set.
References
Backeljauw, P., Miller, B. S., Levy, R., Mccormick, K., Zouater, H., Zabransky, M., et al. (2021). PATRO children, a multi-center, non-interventional study of the safety and effectiveness of Omnitrope(®) (somatropin) treatment in children: Update on the United States cohort. J. Pediatr. Endocrinol. Metab. 34 (4), 431–440. doi:10.1515/jpem-2020-0360
Balhara, B., Misra, M., and Levitsky, L. L. (2012). Recombinant human IGF-1 (insulin-like growth factor) therapy: Where do we stand today? Indian J. Pediatr. 79 (2), 244–249. doi:10.1007/s12098-011-0608-5
Cecconi, E., Gasperi, M., Bogazzi, F., Grasso, L., Genovesi, M., Marcocci, C., et al. (2004). Improvement of growth hormone deficiency in patients with primary hyperparathyroidism after parathyroidectomy: Results of a prospective study. J. Clin. Endocrinol. Metab. 89 (3), 1213–1216. doi:10.1210/jc.2003-031595
Ciresi, A., Amato, M. C., Criscimanna, A., Mattina, A., Vetro, C., Galluzzo, A., et al. (2007). Metabolic parameters and adipokine profile during GH replacement therapy in children with GH deficiency. Eur. J. Endocrinol. 156 (3), 353–360. doi:10.1530/eje.1.02343
Cohen, P., Rogol, A. D., Howard, C. P., Bright, G. M., Kappelgaard, A., Rosenfeld, R. G., et al. (2007). Insulin growth factor-based dosing of growth hormone therapy in children: A randomized, controlled study. J. Clin. Endocrinol. Metab. 92 (7), 2480–2486. doi:10.1210/jc.2007-0204
Collett-Solberg, P. F., Jorge, A., Boguszewski, M., Miller, B. S., Choong, C., Cohen, P., et al. (2019). Growth hormone therapy in children; research and practice - a review. Growth Horm. IGF Res. 44, 20–32. doi:10.1016/j.ghir.2018.12.004
Coutant, R., Bosch Muñoz, J., Dumitrescu, C. P., Schnabel, D., Sert, C., Perrot, V., et al. (2021). Effectiveness and overall safety of NutropinAq® for growth hormone deficiency and other paediatric growth hormone disorders: Completion of the international cooperative growth study, NutropinAq® European registry (iNCGS). Front. Endocrinol. 12, 676083. doi:10.3389/fendo.2021.676083
Czepielewski, M. A., Garret, Q., Vencio, S., Rassi, N., Felicio, J. S., Faria, M. S., et al. (2019). Efficacy and safety of a biosimilar recombinant human growth hormone (r-hGH cristalia) compared with reference r-hGH in children with growth hormone deficiency (ceres study): A randomized, multicentric, investigator-blind, phase 3 trial. Growth Horm. IGF Res. 48-49, 29–35. doi:10.1016/j.ghir.2019.07.003
Deal, C., Kirsch, S., Chanoine, J. P., Lawrence, S., Cummings, E., Rosolowsky, E. T., et al. (2018). Growth hormone treatment of Canadian children: Results from the Genesis phase IV prospective observational study. CMAJ Open 6 (3), E372–E383. doi:10.9778/cmajo.20180020
Du, H., Wu, D., Yi, P., Bai, X., Luo, Y., Yang, H., et al. (2022). Evaluation of efficacy and safety of long-acting PEGylated recombinant human growth hormone (Jintrolong) for patients with growth hormone deficiency. J. Pediatr. Endocrinol. Metab. 35 (4), 511–517. doi:10.1515/jpem-2021-0735
Graham, S., Weinman, J., and Auyeung, V. (2018). Identifying potentially modifiable factors associated with treatment non-adherence in paediatric growth hormone deficiency: A systematic review. Horm. Res. Paediatr. 90 (4), 221–227. doi:10.1159/000493211
Hou, L., Chen, Z. H., Liu, D., Cheng, Y. G., and Luo, X. P. (2016). Comparative pharmacokinetics and pharmacodynamics of a PEGylated recombinant human growth hormone and daily recombinant human growth hormone in growth hormone-deficient children. Drug Des. devel. Ther. 10, 13–21. doi:10.2147/DDDT.S93183
Hui, L., Cheng, J., Xin, Z., and Zhang, Y. Q. (2009). Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Chin. J. PED 47 (7), 487–492.
Kubo, T., Furujo, M., Takahashi, K., Hyodo, Y., Tsuchiya, H., Hattori, M., et al. (2017). Effects of growth hormone treatment on lipid profiles. Indian J. Pediatr. 85 (4), 261–265. doi:10.1007/s12098-017-2509-8
Lal, R. A., and Hoffman, A. R. (2018). Long-acting growth hormone preparations in the treatment of children. Pediatr. Endocrinol. Rev. 16, 162–167. doi:10.17458/per.vol16.2018.lh.longactingghpreparation
Li, J., Pan, W., Qian, J., Ni, Y., Fu, J., and Ni, S. (2022). Metabolomic differential compounds reflecting the clinical efficacy of polyethylene glycol recombinant human growth hormone in the treatment of childhood growth hormone deficiency. Front. Pharmacol. 13, 864058. doi:10.3389/fphar.2022.864058
Liu, H. J., Wang, L. H., and Chen, L. (2019). Evaluation of safety and efficacy of growth hormone therapy by IGF-1 Z score in children with short stature. Adv. Ther. 36 (9), 2374–2383. doi:10.1007/s12325-019-01021-5
Luo, X., Hou, L., Liang, L., Dong, G., Shen, S., Zhao, Z., et al. (2017). Long-acting PEGylated recombinant human growth hormone (Jintrolong) for children with growth hormone deficiency: Phase II and phase III multicenter, randomized studies. Eur. J. Endocrinol. 177 (2), 195–205. doi:10.1530/EJE-16-0905
Miller, B. S., Velazquez, E., and Yuen, K. (2020). Long-acting growth hormone preparations - current status and future considerations. J. Clin. Endocrinol. Metab. 105 (6), e2121–e2133. doi:10.1210/clinem/dgz149
Papathanasiou, T., Agersø, H., Damholt, B. B., Højby, R. M., and Kildemoes, R. J. (2021). Population pharmacokinetics and pharmacodynamics of once-daily growth hormone Norditropin(®) in children and adults. Clin. Pharmacokinet. 60, 1217–1226. doi:10.1007/s40262-021-01011-3
Pellegrin, M. C., Michelon, D., Faleschini, E., Germani, C., Barbi, E., and Tornese, G. (2019). Glucose metabolism evaluated by glycated hemoglobin and insulin sensitivity indices in children treated with recombinant human growth hormone. J. Clin. Res. Pediatr. Endocrinol. 11 (4), 350–357. doi:10.4274/jcrpe.galenos.2019.2019.0281
Peterkova, V., Arslanoglu, I., Bolshova-Zubkovskaya, E., Romer, T., Zdravkovic, D., Kratzsch, J., et al. (2007). A randomized, double-blind study to assess the efficacy and safety of valtropin, a biosimilar growth hormone, in children with growth hormone deficiency. Horm. Res. 68 (6), 288–293. doi:10.1159/000105494
Pfäffle, R., Bidlingmaier, M., Kreitschmann-Andermahr, I., Land, C., Partsch, C. J., Schwab, K. O., et al. (2020). Safety and effectiveness of Omnitrope®, a biosimilar recombinant human growth hormone: More than 10 Years' experience from the PATRO children study. Horm. Res. Paediatr. 93 (3), 154–163. doi:10.1159/000508190
Pfäffle, R. (2015). Hormone replacement therapy in children: The use of growth hormone and IGF-I. Best. Pract. Res. Clin. Endocrinol. Metab. 29 (3), 339–352. doi:10.1016/j.beem.2015.04.009
Qiao, Y., Wang, Z., Han, J., and Li, G. (2019). Use of PEGylated recombinant human growth hormone in Chinese children with growth hormone deficiency: A 24-month follow-up study. Int. J. Endocrinol. 2019, 1–7. doi:10.1155/2019/1438723
Renehan, A. G., Zwahlen, M., Minder, C., O'Dwyer, S. T., Shalet, S. M., and Egger, M. (2004). Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 363 (9418), 1346–1353. doi:10.1016/S0140-6736(04)16044-3
Rhie, Y. J., Yoo, J. H., Choi, J. H., Chae, H. W., Kim, J. H., Chung, S., et al. (2019). Long-term safety and effectiveness of growth hormone therapy in Korean children with growth disorders: 5-year results of LG growth study. PLoS One 14 (5), e0216927. doi:10.1371/journal.pone.0216927
Richmond, E., and Rogol, A. D. (2016). Treatment of growth hormone deficiency in children, adolescents and at the transitional age. Best. Pract. Res. Clin. Endocrinol. Metab. 30 (6), 749–755. doi:10.1016/j.beem.2016.11.005
Saenger, P. H., and Mejia-Corletto, J. (2016). Long-acting growth hormone: An update. Endocr. Dev. 30, 79–97. doi:10.1159/000439333
Shih, K. C., Ho, L. T., Kuo, H. F., Chang, T. C., Liu, P. C., Chen, C. K., et al. (1994). Linear growth response to recombinant human growth hormone in children with growth hormone deficiency. Zhonghua Yi Xue Za Zhi (Taipei) 54(1), 7–13.
Slattery, M., Bredella, M. A., Stanley, T., Torriani, M., and Misra, M. (2014). Effects of recombinant human growth hormone (rhGH) administration on body composition and cardiovascular risk factors in obese adolescent girls. Int. J. Pediatr. Endocrinol. 2014 (1), 22. doi:10.1186/1687-9856-2014-22
Sun, C., Lu, B., Liu, Y., Zhang, Y., Wei, H., Hu, X., et al. (2021). Reduced effectiveness and comparable safety in biweekly vs. Weekly PEGylated recombinant human growth hormone for children with growth hormone deficiency: A phase IV non-inferiority threshold targeted trial. Front. Endocrinol. 12, 779365. doi:10.3389/fendo.2021.779365
Swerdlow, A. J., Cooke, R., Albertsson-Wikland, K., Borgstrom, B., Butler, G., Cianfarani, S., et al. (2015). Description of the SAGhE cohort: A large European study of mortality and cancer incidence risks after childhood treatment with recombinant growth hormone. Horm. Res. Paediatr. 84 (3), 172–183. doi:10.1159/000435856
Wang, C., Huang, H., Zhao, C., Zhao, J., Xiong, R., Jin, R., et al. (2021). The impact of pegylated recombinant human growth hormone replacement therapy on glucose and lipid metabolism in children with growth hormone deficiency. Ann. Palliat. Med. 10 (2), 1809–1814. doi:10.21037/apm-20-871
Weber, M. M., Biller, B. M., Pedersen, B. T., Pournara, E., Christiansen, J. S., Hoybye, C., et al. (2017). The effect of growth hormone (GH) replacement on blood glucose homeostasis in adult nondiabetic patients with GH deficiency: Real-life data from the NordiNet® international outcome study. Clin. Endocrinol. 86 (2), 192–198. doi:10.1111/cen.13256
Witkowska-Sędek, E., Rumińska, M., Majcher, A., and Pyrżak, B. (2019). Gender-dependent growth and insulin-like growth factor-1 responses to growth hormone therapy in prepubertal growth hormone-deficient children. Adv. Exp. Med. Biol. 1133, 65–73. doi:10.1007/5584_2018_284
Wu, S., Yang, W., and De Luca, F. (2015). Insulin-like growth factor-independent effects of growth hormone on growth plate chondrogenesis and longitudinal bone growth. Endocrinology 156 (7), 2541–2551. doi:10.1210/en.2014-1983
Xue, Y., Gao, Y., Wang, S., and Wang, P. (2016). An examination of the effects of different doses of recombinant human growth hormone on children with growth hormone deficiency. Exp. Ther. Med. 11 (5), 1647–1652. doi:10.3892/etm.2016.3091
Yuan, Y., Zhou, B., Liu, S., Wang, Y., Wang, K., Zhang, Z., et al. (2021). Meta-analysis of metabolic changes in children with idiopathic growth hormone deficiency after recombinant human growth hormone replacement therapy. Endocrine 71 (1), 35–46. doi:10.1007/s12020-020-02435-w
Yuen, K., Miller, B. S., Boguszewski, C. L., and Hoffman, A. R. (2021). Usefulness and potential pitfalls of long-acting growth hormone analogs. Front. Endocrinol. 12, 637209. doi:10.3389/fendo.2021.637209
Keywords: PEG-rhGH, GHD, IGF-1, dose, children
Citation: Jiang Z, Chen X, Dong G, Lou Y, Zhang J, Cheng X, Pan J, Liao W, Wu J, Huang X, Jin X, Liu D, Zeng T, Zhu S, Dong Q, Luo X, Lan D, Cao L, Zhang X, Liu J, Dai M, Zhang M, Liu L, Dong J, Zhao D, Ni S and Fu J (2022) Short-term efficacy and safety of a lower dose of polyethylene glycol recombinant human growth hormone in children with growth hormone deficiency: A randomized, dose-comparison study. Front. Pharmacol. 13:955809. doi: 10.3389/fphar.2022.955809
Received: 29 May 2022; Accepted: 13 July 2022;
Published: 11 August 2022.
Edited by:
Yang Zhou, Brown University, United StatesReviewed by:
Zhixin Zhang, China-Japan Friendship Hospital, ChinaHuiwen Zhang, Xinhua Hospital, China
Copyright © 2022 Jiang, Chen, Dong, Lou, Zhang, Cheng, Pan, Liao, Wu, Huang, Jin, Liu, Zeng, Zhu, Dong, Luo, Lan, Cao, Zhang, Liu, Dai, Zhang, Liu, Dong, Zhao, Ni and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junfen Fu, ZmpmNjhAemp1LmVkdS5jbg==; Shaoqing Ni, Y2huc3FAemp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Zhouhong Jiang
Zhouhong Jiang Xuefeng Chen2†
Xuefeng Chen2† Wei Liao
Wei Liao Jinzhun Wu
Jinzhun Wu Junfen Fu
Junfen Fu