- Department of Pharmacy, Obstetrics and Gynecology Hospital of Fudan University, Shanghai, China
Postpartum Depression (PPD) is a serious psychiatric disorder of women within the first year after delivery. It grievously damages women’s physical and mental health. Inflammatory reaction theory is well-established in depression, and also has been reported associated with PPD. This review summarized the inflammatory pathophysiological mechanisms implicated in PPD, including decreased T cell activation, increased proinflammatory cytokines secretion, active kynurenine pathway, and initiated NLRP3 inflammasome. Clinical and preclinical research are both gathered. Potential therapeutical alternatives targeting the inflammatory mechanisms of PPD were introduced. In addition, this review briefly discussed the differences of inflammatory mechanisms between PPD and depression. The research of inflammation in PPD is limited and seems just embarking, which indicates the direction we can further study. As a variety of risky factors contribute to PPD collectively, therapy for women with PPD should be comprehensive, and clinical heterogeneity should be taken into consideration. As PPD has a predictability, early clinical screening and interventions are also needed. This review aims to help readers better understand the inflammatory pathological mechanisms in PPD, so as to identify biomarkers and potential therapeutic targets in the future.
1 Introduction
Postpartum Depression (PPD) is defined as the onset of major depressive disorder (MDD) of women within the first year after delivery (Mughal et al., 2022). It is a serious psychiatric disorder and grievously damages women’s physical and mental health (Balaram and Marwaha, 2022). The clinical symptoms include gloomy mood, reduced interest or pleasure in matters, weariness, insomnia, inappropriate guilt, excessive concern or indifference to infants, and even suicide (Raza and Raza, 2022). The prevalence of PPD is about 15% all over the world (Mughal et al., 2022). Due to the diverse economic levels and screening awareness, the incidence of PPD is different in various regions and may be underestimated (Shorey et al., 2018). PPD not only affects women’s emotion and cognition, but also damages the mother-infant relationship and the growth of young children (Badr et al., 2018; Slomian et al., 2019). It has been reported that child whose mother suffers from PPD is more likely to suffer from depression (Abdollahi et al., 2017; Weissman, 2018; Tainaka et al., 2022). PPD also breaks family harmony and has become a serious social problem (Letourneau et al., 2012). The pathological mechanism of PPD is multifactorial and has not been fully clarified. The potential pathogenesises are as follows (Figure 1): dysfunction of hypothalamic-pituitary-adrenal (HPA) axis, imbalance of hormones (estradiol, progesterone, oxytocin, corticotropin releasing hormone, etc.), imbalance of neurotransmitters (serotonin, dopamine, γ-aminobutyric acid, glutamate, etc.), imbalance of neurosteroids (allopregnanolone, etc.), epigenetics, alterant synaptic connection, neuroinflammation, and so on (Payne and Maguire, 2019; Stewart and Vigod, 2019; Mughal et al., 2022). The therapeutic modalities of PPD mainly include psychotherapy and medical treatment, which are similar with the treatment of conventional depression (Brummelte and Galea, 2016; Stewart and Vigod, 2019). It is worth noting that patients with PPD, who may be breastfeeding, should avoid medications that affect the infants (Becker et al., 2016). Exposure to antidepressants in late pregnancy could lead to neonatal adaptation disorders, such as drowsiness and irritability. According to the available evidence, sertraline and amitriptyline are the preferred antidepressants (Wisner et al., 1996; Hantsoo et al., 2014; Cuomo et al., 2018). Brexanolone, a positive allosteric modulator of γ-aminobutyric acid (GABA) A receptors, is approved as the first drug expressly for treating women with PPD (Kanes et al., 2017; Gunduz-Bruce et al., 2022).
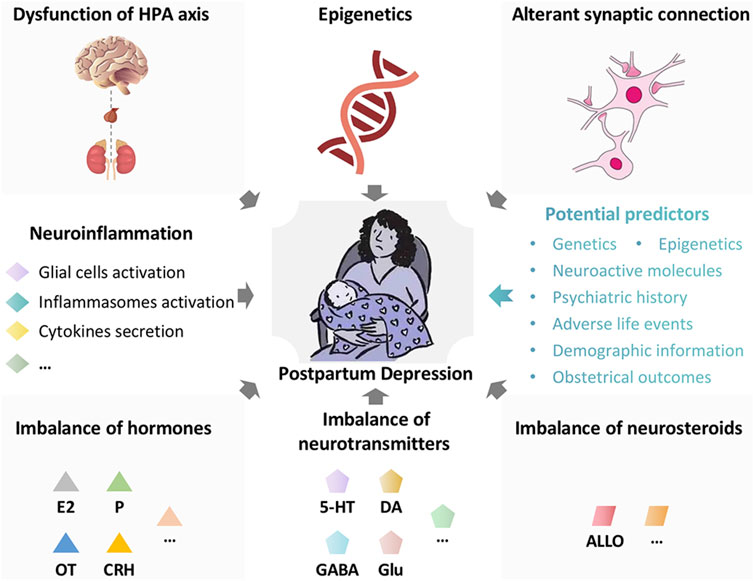
FIGURE 1. The potential pathogenesises and predictors in postpartum depression. The potential pathogenesises in postpartum depression are multiple and as follows: Dysfunction of hypothalamic-pituitary-adrenal (HPA) axis, imbalance of hormones (estradiol, progesterone, oxytocin, corticotropin releasing hormone, etc.), imbalance of neurotransmitters (serotonin, dopamine, γ-aminobutyric acid, glutamate, etc.), imbalance of neurosteroids (allopregnanolone, etc.), epigenetics, alterant synaptic connection, neuroinflammation, and so on. The potential predictors for postpartum depression are as follows: genetics, epigenetics, neuroactive molecules (allopregnanolone, β-endorphin, cortisol, corticotropin-releasing hormone, oxytocin, thyroid function, inflammatory markers, etc.), psychiatric history, adverse life events, demographic information (maternal age, race, socioeconomic status, etc.), and obstetrical outcomes (preterm birth, etc.). HPA axis, hypothalamic-pituitary-adrenal axis; E2, estradiol; P, progesterone; OT, oxytocin; CRH, corticotropin releasing hormone; 5-HT, serotonin; DA, dopamine; GABA, γ-aminobutyric acid; Glu, glutamate; ALLO, allopregnanolone.
Neuroinflammation has been reported associated with depression as evidenced by many studies (Troubat et al., 2021; Won et al., 2021; Craig et al., 2022; Zhou et al., 2022). Peripheral immune cells damage the integrity of blood-brain barrier (BBB) (Van Dyken and Lacoste, 2018). When permeability of the BBB alters, peripheric immune cells infiltrate into the brain (Van Dyken and Lacoste, 2018; Kealy et al., 2020). Microglias are activated and then secrete proinflammatory cytokines (Deng et al., 2020; Jia et al., 2021). Inflammasomes are also activated after the assembly of inflammasomes complex and secrete proinflammatory cytokines (Broz and Dixit, 2016; Deets and Vance, 2021). Astrocytes are stimulated by proinflammatory cytokines, and mediate cascade amplification of inflammatory reaction (Linnerbauer et al., 2020; Jiwaji and Hardingham, 2022). It further impairs the integrity of the BBB(Haruwaka et al., 2019). Thus, a positive circuit of inflammatory response is generated, which aggravates the nerve injury (Linnerbauer et al., 2020). Furthermore, the proinflammatory cytokines activate the HPA axis, which in turn increases the production of cortisol (Noushad et al., 2021). The tryptophan (Trp)-kynurenine (Kyn) pathway is activated as well. Subsequently, the synthesis of quinolinic acid and 3-hydroxykynurenine is increased, which induces oxidative stress and nerve injury (Tattersfield et al., 2004; Mackay et al., 2006). Neuroinflammation is also associated with a variety of neurodegenerative diseases, such as Parkinson’s disease (Tansey et al., 2022), Alzheimer’s disease (Leng and Edison, 2021) and so on (Fontana et al., 2021). PPD is also reported to be closely related to neuroinflammation. In this review, we focused on the potential inflammatory mechanisms to underpin PPD pathophysiology.
2 Inflammatory pathophysiological mechanisms in postpartum depression
Inflammatory responses can occur in the periphery or central nervous system. Pro-inflammatory and anti-inflammatory responses are the two types of inflammatory reactions. Pregnancy is linked to specific immunological responses that protect the fetus from the mother immune system. In order to support immunosuppression, anti-inflammatory cytokines are increased, while pro-inflammatory cytokines are decreased during pregnancy (Al-Azemi et al., 2017; Kwiatek et al., 2021). In response to the physical damage and exertion associated with labor, the anti-inflammatory milieu transforms to a pro-inflammatory state after delivery (Miyoshi et al., 2021). In this section, we will review evidence of inflammatory pathophysiological mechanisms in PPD (Table 1), including roles of T cells, cytokines, kynurenine and inflammasomes.
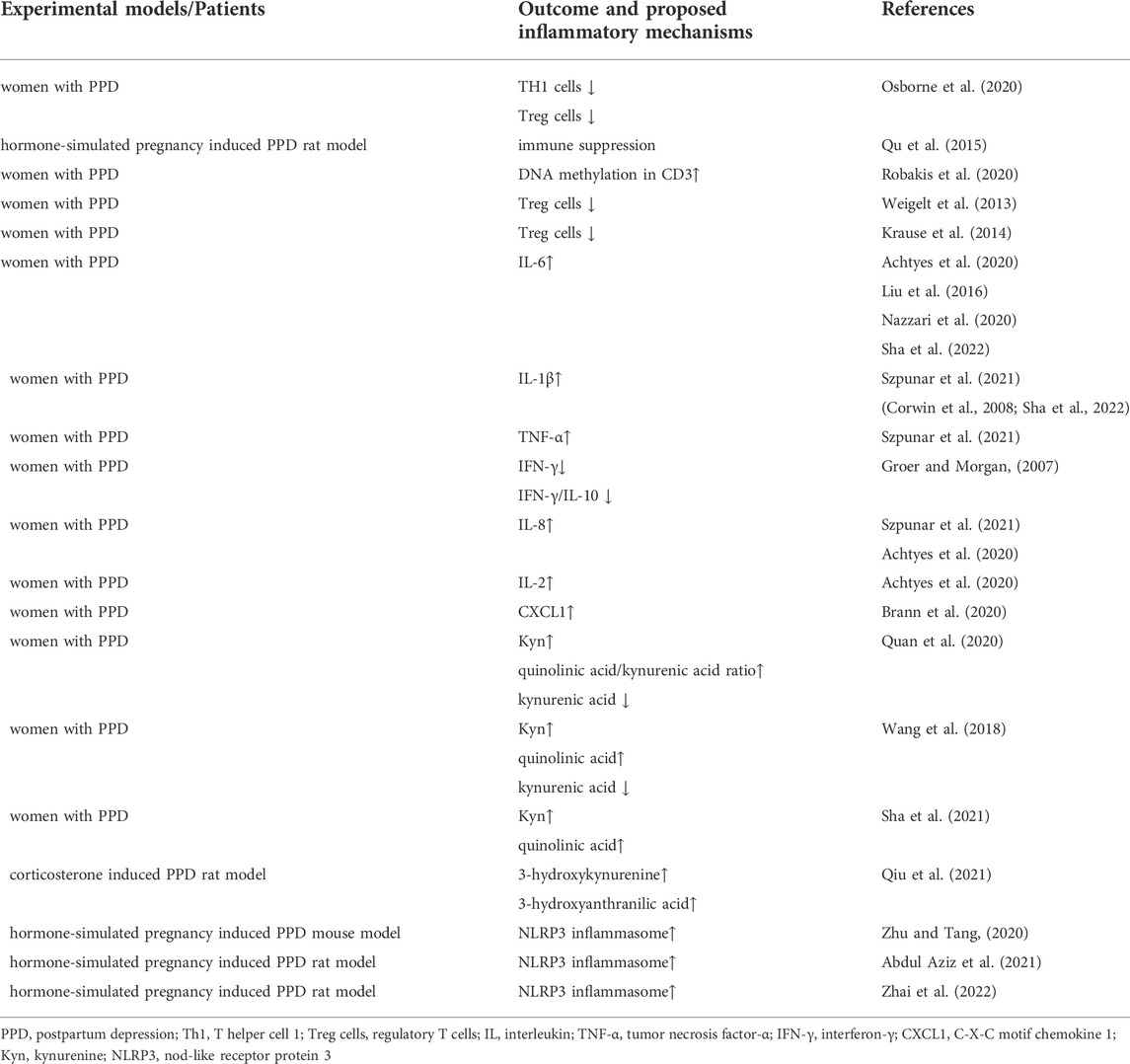
TABLE 1. The summary of inflammatory pathophysiological mechanisms implicated in postpartum depression.
2.1 T cells
T cells are essential for the control and clearance of most infections. Major histocompatibility complex (MHC) proteins present short peptide antigens to T cell receptors, and T cells respond to infections in such an antigen-specific way (Kumar, 2018). It plays a key role in adaptive immunity by mediating helper functions to the immune system of the body (Dong, 2021). In healthy women, the postpartum period is a time of increased T cell activation (Osborne et al., 2020). Women with PPD do not have physiologically increased T-cell activity after giving birth. Lauren M Osborne et al. (Osborne et al., 2020) found that T cells were significantly higher in postpartum women without PPD than in healthy non-postpartum controls. Increases of TH1 cells and T regulatory (Treg) cells drove the immunological enhancement in healthy postpartum women, which were absent or muted in women with PPD (Osborne et al., 2020). Similar results were obtained in animal experiments. It was reported that immune suppression occurs 2 weeks after hormone withdrawal in hormone-simulated pregnancy induced PPD rat model (Qu et al., 2015). At present, there are few reports on the possible mechanism of the shift of immune state (from immune suppression to immune activation) before and after childbirth. It was speculated that changes in DNA methylation density in CD3 may be associated to depression during pregnancy (Robakis et al., 2020). Another research implied that lower microRNA-146a expression in monocytes was linked to lower natural Treg cells in PPD (Weigelt et al., 2013). Daniela Krause et al. (2014) declared that Treg cells are reduced both antepartum and postpartum in women with PPD, and the level of Treg cells in pregnancy might be a forecast for PPD. In conclusion, there is much evidence that PPD is accompanied by decreased T cell activation. The compensation of the monocytic system could be a probable result of the T cells-mediated immunosuppression in depressive women (Krause et al., 2014). Monocytes that may pass the BBB, appear to be important in the pathophysiology of depression as contributing to an inflammatory environment in the brain and leading nerve scathe.
2.2 Cytokines
Cytokines include pro-inflammatory and anti-inflammatory types. Proinflammatory cytokines could access the brain through the BBB and participate in many pathophysiologic processes including glial cells activation, neurotransmitter metabolism, and so on (Miller and Raison, 2016; Shi et al., 2022). Among the multiple cytokines, interleukin (IL) -6 has been reported most related to PPD. However, the conclusions are somewhat conflicting. The mainstream view is that serum level of IL-6 in women with PPD is increased, compared to healthy puerperal women (Liu et al., 2016; Payne and Maguire, 2019; Achtyes et al., 2020; Nazzari et al., 2020; Sha et al., 2022; Worthen and Beurel, 2022). Other studies (Ahn and Corwin, 2015; Nagayasu et al., 2021) did not find the correlation between IL-6 levels and the scores of Edinburgh Postpartum Depression Scale (EPDS), depressive symptoms, or stress variables. In an exploratory study among postpartum veterans (Szpunar et al., 2021), the researchers found that elevated IL-1β and tumor necrosis factor-α (TNF-α) might have a positive correlation with the severity of depressive symptom. And the high level of IL-1β was also related to suicidal thoughts during pregnancy (Szpunar et al., 2021). Similarly, other studies showed that uric or plasmic IL-1β was increased in mothers with depressive symptoms or high scores of EPDS (≥13) (Corwin et al., 2008; Sha et al., 2022). In contrast, R Buglione-Corbett’s laboratory (Buglione-Corbett et al., 2018) clarified that serum TNF-α was negatively correlated with EPDS score, and there was no statistically significant associations between depressive symptoms and IL-6 or IL-1β. Serumal interferon-γ (IFN-γ) and the ratio of IFN-γ/IL-10 were decreased in PPD, according to Maureen W Groer et al. (Groer and Morgan, 2007). Besides, the secretion of IL-8 has been reported to increase in the postpartum period (Szpunar et al., 2021). Increased plasma IL-8 or reduced IL-2 was associated with higher risk for PPD (Achtyes et al., 2020). Chemokine is a small molecule cytokine capable of chemotactic cell directional movement. Chemokines and their receptors mediate cell migration, thereby affecting a variety of basic biological processes and disease conditions, such as inflammation and cancer. C-X-C motif chemokine 1 (CXCL1) was reported to significantly elevate in women with PPD (Brann et al., 2020). In general, levels of many cytokines alter in the postpartum period and might potentially become inflammatory biomarkers for PPD.
2.3 Kynurenine
Increased inflammation raises the production of the broadly distributed enzyme indoleamine 2,3-dioxygenase (IDO) (Cervenka et al., 2017). Activation of the HPA axis promotes the hepatic enzyme tryptophan 2,3-dioxygenase (TDO). Both enzymes transform tryptophan (Trp) into kynurenine (Kyn), which is then converted into downstream neurotoxic metabolites, i. e, quinolinic acid and kynurenic acid, to damage neurons (Savitz, 2020). On the other hand, Trp is the precursor of neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). The increased conversion of Trp to Kyn results in less synthesis of 5-HT, which leads to depressive symptoms (Cervenka et al., 2017). Chengxuan Quan et al. (2020) showed that women with PDD had significantly greater Kyn levels 1 day before delivery compared to the control group. Women with PDD had significantly lower kynurenic acid level, higher quinolinic acid level, and higher quinolinic acid/kynurenic acid ratio 3 days after delivery than women without PPD. Similarly, a study (Wang et al., 2018) showed that women with PPD had significantly higher serum Kyn and quinolinic acid concentrations, and lower serum kynurenic acid concentrations 3 days after cesarean section. Qiong Sha et al. (2021) declared that estrogen and progesterone were respectively negatively correlated with Kyn and quinolinic acid in the postpartum period. Animal research (Qiu et al., 2021) also showed that postpartum corticosterone could influence Trp-Kyn pathway, inducing the production of neurotoxic metabolites 3-hydroxykynurenine and 3-hydroxyanthranilic acid. On the contrary, Eric Achtyes et al. (2020) discovered that down-regulation of quinolinic acid was related to high risk for PPD. In general, the activation of Kyn pathway is implicated in PPD as evidenced by many studies (Nazzari et al., 2020). The changes of metabolites of Kyn in postpartum are still conflicting and need to be further researched.
2.4 Inflammasomes
The Nod-like receptor protein (NLRP) inflammasomes are protein complexs that exert important roles in neuroinflammation. Among the various inflammasomes, NLRP3 inflammasome is the most studied one (Huang et al., 2021). When stimulating by risky factors such as adenosine triphosphate, the adapter molecule apoptosis-related speck-like protein (ASC) and pro-caspase-1 are recruited by NLRP3 and form a protein complex (Zhu et al., 2020). Pro-caspase-1 is then transformed into mature caspase-1. Whereafter, caspase-1 mediates the maturation of the proinflammatory cytokines IL-1β and IL-18. The secretion of proinflammatory cytokines leads to downstream inflammatory cascade and cell pyroptotic death (Zhu et al., 2018; Huang et al., 2021). Jialei Zhu et al. (Zhu and Tang, 2020) firstly proposed that astrocytic NLRP3 inflammasome was activated in the hippocampus of PPD mouse model. Another study (Abdul Aziz et al., 2021) also showed that increased NF-кB/NLRP3/caspase-1 activity was detected in the hippocampus of PPD rat model. Similarly, a recent study (Zhai et al., 2022) clarified that NLRP3 inflammasome was activated in the hypothalamus of PPD rat model. Although there have been many reports on the correlation between inflammasomes and the pathological mechanism of depression, the research of inflammasomes in PPD is just embarking. Thus far, there is no study of inflammasome in patients with PPD, and there are only a few animal experiments using hormone-simulated pregnancy induced PPD model. It deserves further study.
3 Potential therapeutical alternatives for postpartum depression targeting the inflammatory mechanisms
At present, the only approved drug specifically used for the treatment of PPD is brexanolone. It is soluble allopregnanolone and targets GABAergic system. It has been reported that injection of allopregnanolone reduced microglial activation and astrocyte proliferation in mouse model (Liao et al., 2009). Another study showed that allopregnanolone synthesis was reduced by IL-6 (Parks et al., 2020). It indicates the potential significance of anti-inflammatory therapy for PPD. In this section, we will review potential therapeutical alternatives for PPD targeting T cells, cytokines, kynurenine or NLRP3 inflammasome in clinical trials.
3.1 T cell-based immunotherapy
T cell-based immunotherapy has received great attention in tumor treatments (Zhang, K. et al., 2022) in recent years. Engineering T cells is a rapidly advancing technology and is a good strategy for stimulating T cells proliferation to effectively target tumors (Belk et al., 2022). However, it may induces serious adverse effects, such as nonspecific inflammation (Belk et al., 2022). Chimeric-antigen receptor (CAR) T cells, as the first commercial products, are approved for hematologic malignancies (Greenbaum et al., 2021). So far, there has been none engineering T cells applicated in the treatment of depression. And it seems be “making a mountain out of a molehill”. The supplementation of some substances regulating T cells in diet or drugs may be better for PPD. In a prospective, randomized-controlled study, trace element selenium (Se) was found to upregulate the activated Treg cells (Hu et al., 2021). Naghmeh Mokhber et al. (2011) conducted a trial to determine the impact of prenatal Se supplementation on women’s levels of PPD. Primigravid pregnant women were randomly assigned to receive Se or placebo every day up until birth. The mean EPDS score in the Se group was markedly lower than that of the control group. It suggests that supplementation with Se during pregnancy would be an effective strategy for the prevention of PPD. Traditional Chinese Medicine and its extracts are also reported to have immunity-enhancing capacity (Wang et al., 2020). Leonurus has the effect of regulating menstruation and plays an auxiliary role in the treatment of gynecological diseases. Leonurine, the extract of Leonurus, was found to regulate Treg/Th17 balance (Du et al., 2020). It exerted antidepressant effects in chronic mild stress-induced depression mouse model (Jia et al., 2017).
3.2 Cytokine inhibitors
Cytokine inhibitors are commonly used clinically in autoimmune diseases such as rheumatoid arthritis (RA). The role of cytokine inhibitors in depression is still controversial. Tocilizumab is a recombinant humanized anti-human IL-6 receptor monoclonal antibody. A study demonstrated a favourable impact of tocilizumab therapy on anxiety and depression in patients with RA (Tiosano et al., 2020). However, another study showed that blockade of the IL-6 receptor with tocilizumab resulted in significantly more depressive symptoms (Knight et al., 2021). Anti-TNF-α compounds were reported as a potential therapeutic strategy for depression (Uzzan and Azab, 2021). In a randomized controlled trial, TNF antagonism infliximab improved depressive symptoms in patients with high baseline inflammatory biomarkers (Raison et al., 2013). IL-1 receptor antagonist (IL-1RA) is a specific competitive inhibitor of IL-1. It binds to IL-1R and blocks the binding of IL-1α/IL-1β with IL-1R (Maes et al., 2012). A recent study in mouse model demonstrated that the blockade of IL-1R/NF-κB pathway reduced the secretion of complement C3 from astrocyte and regulated synaptic pruning in the prefrontal cortex of depression (Zhang, M.M. et al., 2022). However, a case report showed that IL-1RA anakinra induced depression (Jonville-Bera et al., 2011), which was firstly found to be a new side effect of anakinra. C-X-C motif chemokine receptor 2 (CXCR2) is the receptor of chemokine CXCL1, and the inhibitor of CXCR2 (SB265610) prevented chronic stress-induced depression-like behaviors in mice (Chai et al., 2019). However, there has been no relevant clinical studies. The effect of cytokine inhibitors on depression is mostly carried out in patients with inflammatory diseases (Beurel et al., 2020). It is yet unclear if the improvement is caused, at least in part, by cytokine inhibitor methods’ effects on somatic disorders, but from all of these data, depressed individuals with prominent inflammation benefits from them.
3.3 IDO and TDO inhibitors
One potential strategy for treating depression is to directly target kynurenine synthesis and reduce its harmful downstream metabolites. Therefore, the straightforward process is to suppress IDO and TDO activity in order to stop the accumulation of kynurenine metabolites. The IDO antagonist 1-methyltryptophan (1-MT) has been reported to prevent depressive-like behaviors in many animal experiments (O'Connor et al., 2009; Souza et al., 2017). Clinical trials using 1-MT also have been initiated (Lob et al., 2009). TDO inhibitors include allopurinol, nicotinamide and so on (Badawy, 2019). It has been reported that continued use of low-dose allopurinol was associated with a decreased rate of incident depression (Kessing et al., 2019). The possible pro-longevity effects of nicotinamide adenine dinucleotide precursors have caused further growth of nicotinamide consumption as a dietary supplement (Hwang and Song, 2020). In a randomized, double-blind, and placebo-controlled study, nicotinamide-containing supplements loading between meals in quite low dose can improve depressed mood in young adults with subclinical depression (Tsujita et al., 2019). However, there are potential risks for epigenetic alterations associated with chronic use of nicotinamide at high doses (Hwang and Song, 2020). The possible adverse reactions and their mechanisms are not yet clear, which reminds us to use it cautiously.
3.4 NLRP3 inflammasome inhibitors
In recent years, NLRP3 inflammasome selective inhibitors are under development. Most attempts to inhibit NLRP3 inflammasome focus on compounds that directly bind to NLRP3 and inhibit the assembly of NLRP3 inflammasome complex. MCC950 is a small molecular inhibitor of NLRP3 inflammasome and is reported to exert an anti-depressive role in animal experiments (Li et al., 2022; Liu et al., 2022). At present, there is no clinical trial of MCC950 on depression. OLT1177 is an orally active β-sulfonyl nitrile molecule developed for osteoarthritis, acute gout and heart failure (Marchetti et al., 2018; Aliaga et al., 2021). CY-09 is also an inhibitor of NLRP3 inflammasome potentially used for osteoarthritis, cryopyrin-associated autoinflammatory syndrome (CAPS) and type 2 diabetes (Jiang et al., 2017; Zhang, Y. et al., 2021). In addition, INF39(Shi et al., 2021) and JC-124 (Yin et al., 2018) are inhibitors of NLRP3 inflammasome as well. So far, researchers have not taken OLT1177, CY-09, INF39, and JC-124 into the researches of depression. On the other hand, some medicines have been found to play an antidepressant role by inhibiting NLRP3 inflammasome. A prospective clinical study reveals that pioglitazone metformin complex alleviates psychological distress via inhibiting NLRP3 inflammasome in patients with polycystic ovary syndrome comorbid psychological distress (Guo et al., 2020). Another research identified fluoxetine as a direct NLRP3 inhibitor as it inhibited activation of the NLRP3-ASC inflammasome and inflammatory cytokine release (Ambati et al., 2021).
4 Discussion
As a subtype of depression with a “special period” (puerperium) and “special population” (delivery women), the inflammatory mechanisms of PPD are generally overlaps with that in depression. Meanwhile, some differences exist. In depression, Th17 cells are reported accumulated and the Th17/Treg cell balance was dysregulated (Cui et al., 2021). Similar report has been declared in the study of depression and anxiety during pregnancy (Osborne et al., 2019b). However, this has not been reported in PPD. In a meta-analysis studying inflammatory markers in depression (collecting 5166 patients with depression and 5083 healthy controls) (Osimo et al., 2020), the researchers found that IL-6, TNF-α, IL-12, IL-3, IL-18, and sIL-2R were elevated in depression group. The cytokines upregulated in depression are not exactly same as those in PPD. In terms of inflammasomes, besides NLRP3 inflammasome, depression has also been reported to be associated with the activations of NLRP1 (Song et al., 2020), NLRP2 (Zhang et al., 2020), and AIM2 (Li, Y.K. et al., 2021). In addition to the inflammatory mechanisms mentioned above, recent reports have also shown that depression is related to caspase-gasdermin D-mediated inflammatory programmed cell death, namely pyroptosis (Chai et al., 2022; Li, S. et al., 2021; Yang et al., 2020). It is unknown whether pyroposis also exists in PPD at present, and it is worth exploring in the future. Besides, much evidence suggests an impact of toll-like receptor 4 (TLR4) signaling on depression (Guo et al., 2019; Xu et al., 2020) while it is rarely reported in PPD. On the other hand, microglial M1/M2 polarization plays important roles in mediating the balance between activation and suppression in inflammation (Nakagawa and Chiba, 2014). Many studies have demonstrated that M1 (pro-inflammatory) polarization was related to depression (Kalkman and Feuerbach, 2016; Zhang, L. et al., 2021). It needs more researches to explore whether these pathomechanisms are also relevant to PPD.
Although many antidepressant agents or methods are not specially used for restraining inflammation, they actually play anti-inflammatory roles. In addition to the medicines mentioned in the previous section, some potential agents have also been reported to exert roles through other anti-inflammatory mechanisms. Isoliquiritin (Li, Y. et al., 2021), pinocembrin (Yang et al., 2022), pilose antler peptide (Hu et al., 2022), quercetin (Zhu et al., 2022), etc. ameliorated depression by suppressing pyroptosis in animal models. Arctigenin (Xu et al., 2020), safflower extract (Chen et al., 2021), baicalin (Guo et al., 2019), Xiao-Chai-Hu-Tang (Shao et al., 2021), puerarin (Gao et al., 2021), etc. alleviated depression through TLR4 signaling pathways. Ketamine (Beckett and Niklison-Chirou, 2022; Wu et al., 2022), magnolol (Tao et al., 2021), astragalin (Yao et al., 2022), etc. were reported to attenuate depression and produce anti-inflammatory effects by regulating M2 polarization of microglia. The roles of these agents above in PPD need further animal and clinical trials to explore. Besides, non-steroidal anti-inflammatory drugs (NSAIDs) inhibit the synthesis of prostaglandins in the central nervous system and are commonly used clinically for antipyretic, analgesic, anti-inflammatory and anti-rheumatic effects. In the treatment of conventional depression, anti-inflammatory agents have shown better effects compared to placebo in several randomized controlled trials (Muller et al., 2006; Mohammadinejad et al., 2015; Alamdarsaravi et al., 2017). However, this view is still controversial (Berk et al., 2020; Husain et al., 2020; Baune et al., 2021). More clinical trials and evidence need to confirm its effect. In addition to the agents, some methods may also have an antidepressant effect by anti-inflammation. Acupuncture may achieve treatment effects on depression through suppression of vagal nerve inflammatory responses (Liu et al., 2020). Physical exercise can reduce both depression and inflammation (Paolucci et al., 2018). In addition, microbiome-gut-brain axis shows correlation to depression (Carlessi et al., 2021; Donoso et al., 2022). Though recent systematic reviews (Desai et al., 2021; Trifkovic et al., 2022) demonstrated that there was limited evidence about the effectiveness of probiotics on PPD, probiotics is a promising therapeutical alternative. Correct strain selection should be taken into consideration. And further well-designed, robust clinical trials are needed. All the agents and methods (Table 2) provide new therapeutic ideas for treating PPD.
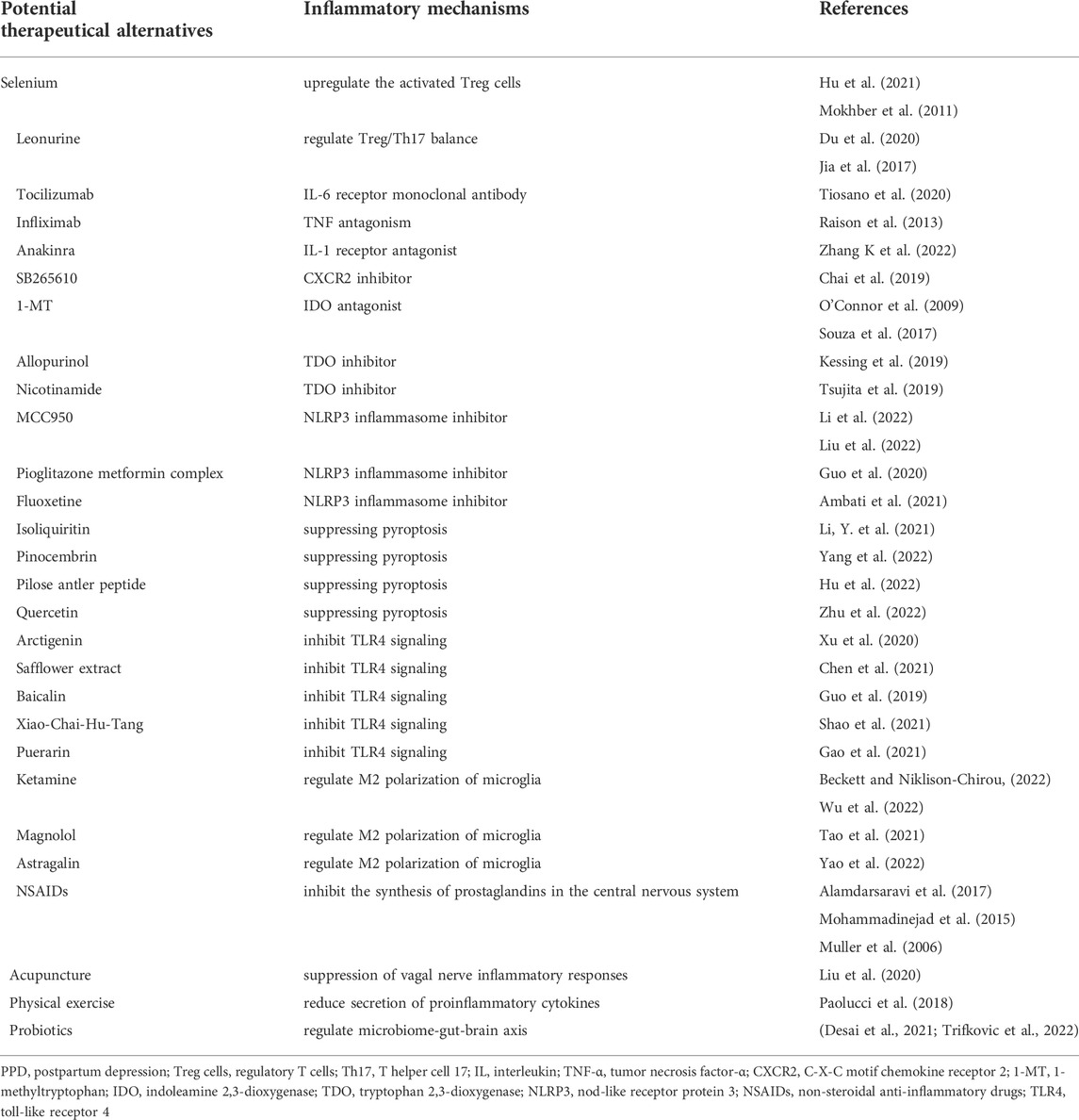
TABLE 2. The summary of potential therapeutical alternatives targeting the inflammatory mechanisms for postpartum depression.
During the postpartum period, many women suffer from obesity, sleep deprivation, mastitis, or diabetes, and so on. There is plenty of evidence that these factors have a high risk of inflammation (Pyorala, 2003; Halim and Halim, 2019; Irwin, 2019; Atrooz and Salim, 2020; Berbudi et al., 2020; Miao et al., 2022; Rohm et al., 2022; Shangraw and McFadden, 2022). Raising infants may be physically and financially stressful for women. It has been reported that stress could induce immune dysfunction and is associated with inflammation (Glaser and Kiecolt-Glaser, 2005; Umamaheswaran et al., 2018). In addition, the hormone levels of women change after childbirth. Estrogen (Kovats, 2015; Xu et al., 2016), progesterone (Patel et al., 2017), oxytocin (Tang et al., 2019; Szeto et al., 2020), and corticotropin releasing hormone (Webster et al., 1998; Nakade et al., 2021) all have been reported related to inflammation. Therefore, the potential mechanisms of PPD are highly interrelated. A variety of risky factors contribute to PPD collectively. Therapy for women with PPD will be multifaceted and comprehensive.
On the other hand, retrospective reports and case registry studies indicates significant degrees of consistency in depression throughout pregnancy to postpartum as well as across several years pre-conception to postpartum (Hipwell et al., 2022). It reminds us that the pathophysiological mechanisms implicated in PPD started on (or even before) the pregnancy, and PPD should be considered within a lifespan perspective. It is the successive process, and early clinical screening and interventions are necessary. With existing technology and clinical knowledge, it might be possible to identify a population at risk of getting PPD (Cellini et al., 2022). Plenty of evidence indicates that multiple factors (Figure 1) including genetics (eg. nearly 50% of heritability), epigenetics (eg., DNA methylation at the oxytocin receptor gene), neuroactive molecules (eg., lower levels of allopregnanolone during the second trimester, higher levels of β-endorphin at 25 weeks’ gestation, higher levels of cortisol at day 14 postpartum, higher levels of corticotropin-releasing hormone during pregnancy, lower levels of oxytocin during the third trimester, hypractive thyroid function at delivery, higher levels of inflammatory markers prenatally and at delivery), psychiatric history (antenatal major depressive disorder, anxiety, or other psychiatric disorder), adverse life events (eg., physical, psychological, or sexual abuse), demographic information (eg., younger or older maternal age, black or hispanic race, low socioeconomic status), and obstetrical outcomes (eg. preterm birth), are potential predictors for PPD(Yim et al., 2010; Sylven et al., 2013; Corwin et al., 2015; Guintivano et al., 2018a; Guintivano et al., 2018b; Osborne et al., 2019a; Bauer et al., 2019; Cao and Wei, 2020; Cevik and Alan, 2021; Grippi, 2021; Lapato et al., 2021; Nelson et al., 2022). In terms of inflammatory markers, increase of Treg cells prenatally (Krause et al., 2014), upregulation of IL-6 and high-sensitivity C-reactive protein (Hs-CRP) at delivery (Liu et al., 2016), high DNA methylation at FOXP3 Treg-cell-specific demethylated region (TSDR) prenatally (Sluiter et al., 2020), increase of the IL-8/IL-10 ratio during the third trimester (Corwin et al., 2015) are highly correlated with the occurrence of PPD. In addition, a study has demonstrated that the sum of quinolinic acid, Kyn, 3-OH-kynurenine and 3-OH-anthranilic acid during pregnancy was closely associated with body image dissatisfaction (Roomruangwong et al., 2018). Furthermore, a recent cross-sectional study indicated that maternal and paternal depression were positively associated and served as predictors of one another in the early postnatal period (Zheng et al., 2022). It reminds us that early screening and evaluation (including the partner) is meaningful. In recent years, artificial intelligence is developing rapidly, providing novel methods for perinatal health prediction modeling, diagnostics, early identification, and monitoring (Ramakrishnan et al., 2021). It is hoped that more scientific research and advanced technology will benefit women with PPD in the future.
Collectively, this review summarizes the inflammatory mechanisms implicated in PPD, including decreased T cell activation, up-regulation of proinflammatory cytokines, activation of kynurenine pathway, and activation of NLRP3 inflammasome. The hypothesis diagram and predicted inflammatory markers are shown in Figure 2. At present, some reviews (Payne and Maguire, 2019; Worthen and Beurel, 2022) have reported the roles of inflammation in PPD, but none of them mentioned the effects of inflammasomes. We have further expanded the contents on this basis. There are also some limitations in this review. 1) Some reports have conflicting conclusions, which makes it difficult for us to draw a definite conclusion in the summary. It may be caused by the clinical heterogeneities, including the differences in situations of subjects (race, age, etc.), mode of production (spontaneous delivery, caesarean section, etc.), scoring method (depressive symptom, PPD scale, etc.), the time collecting samples (24 h, 3 days, 3 months, 6 months, etc. after delivery), sampling content (whole blood, serum, plasma, urine, etc.). 2) Overall, there are limited reports about inflammation in PPD. Many experiments have only animal data rather than human data. It may be due to the vulnerability of postpartum population. Further studies are needed. Altogether, this review declares that inflammatory mechanisms play important roles in the pathology of PPD. Furthermore, the inflammatory indicators should be considered possible clinical markers and therapeutic targets in PPD.
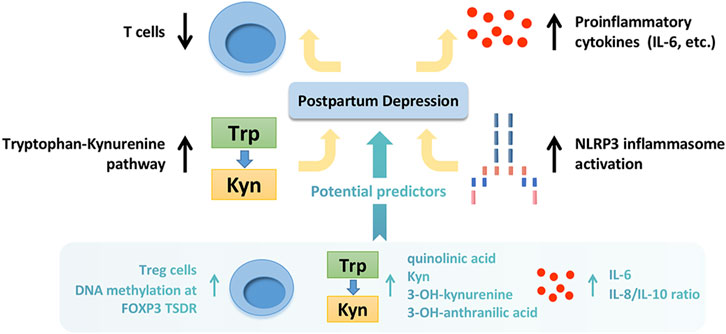
FIGURE 2. The inflammatory mechanisms implicated in postpartum depression and potential predicted markers. The inflammatory mechanisms implicated in postpartum depression include decreased T cell activation, up-regulation of proinflammatory cytokines, activation of kynurenine pathway, and activation of NLRP3 inflammasome. The potential predicted inflammatory markers include increased Treg cells prenatally, upregulation of IL-6 and Hs-CRP at delivery, high DNA methylation at FOXP3 TSDR prenatally, increased IL-8/IL-10 ratio during the third trimester, and high level of quinolinic acid, Kyn, 3-OH-kynurenine and 3-OH-anthranilic acid during pregnancy. Trp, tryptophan; Kyn, kynurenine; Treg cells, regulatory T cells; IL, interleukin; Hs-CRP, high-sensitivity C-reactive protein; DNA, deoxyribonucleic acid; TSDR, Treg-cell-specific demethylated region.
Author contributions
JZ wrote the manuscript. JJ corrected the writing. JT conceptualized and supervised the work, and revised the manuscript.
Funding
This work was supported by the grants from National Natural Science Foundation of China (No. 82104148), Shanghai Sailing Program (No. 21YF1403600), Shanghai “Rising Stars of Medical Talent” Youth Development Program (No. 076478684Q/2022-00033), Project of China Pharmaceutical Association (No. CMEI2022KPYJ00545), Talent Project established by Chinese Pharmaceutical Association Hospital Phamacy department (No. CPA-Z05-ZC-2021-003), and Project of Shanghai Municipal Health Commission on health industry research (No. 201940153).
Acknowledgments
We acknowledge Professor Yue Zhao for suggestions of writing. We thank “Baidu images” (https://image.baidu.com) for the cartoon picture of “postpartum depression” in Figure 1.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdollahi, F., Rezai Abhari, F., and Zarghami, M. (2017). Post-Partum depression effect on child health and development. Acta Med. Iran. 55 (2), 109–114.
Abdul Aziz, N. U., Chiroma, S. M., Mohd Moklas, M. A., Adenan, M. I., Ismail, A., Basir, R., et al. (2021). Menhaden fish oil attenuates postpartum depression in rat model via inhibition of NLRP3-inflammasome driven inflammatory pathway. J. Tradit. Complement. Med. 11 (5), 419–426. doi:10.1016/j.jtcme.2021.02.007
Achtyes, E., Keaton, S. A., Smart, L., Burmeister, A. R., Heilman, P. L., Krzyzanowski, S., et al. (2020). Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav. Immun. 83, 239–247. doi:10.1016/j.bbi.2019.10.017
Ahn, S., and Corwin, E. J. (2015). The association between breastfeeding, the stress response, inflammation, and postpartum depression during the postpartum period: Prospective cohort study. Int. J. Nurs. Stud. 52 (10), 1582–1590. doi:10.1016/j.ijnurstu.2015.05.017
Al-Azemi, M., Raghupathy, R., and Azizieh, F. (2017). Pro-inflammatory and anti-inflammatory cytokine profiles in fetal growth restriction. Clin. Exp. Obstet. Gynecol. 44 (1), 98–103. doi:10.12891/ceog3295.2017
Alamdarsaravi, M., Ghajar, A., Noorbala, A. A., Arbabi, M., Emami, A., Shahei, F., et al. (2017). Efficacy and safety of celecoxib monotherapy for mild to moderate depression in patients with colorectal cancer: A randomized double-blind, placebo controlled trial. Psychiatry Res. 255, 59–65. doi:10.1016/j.psychres.2017.05.029
Aliaga, J., Bonaventura, A., Mezzaroma, E., Dhakal, Y., Mauro, A. G., Abbate, A., et al. (2021). Preservation of contractile reserve and diastolic function by inhibiting the NLRP3 inflammasome with OLT1177® (dapansutrile) in a mouse model of severe ischemic cardiomyopathy due to non-reperfused anterior wall myocardial infarction. Molecules 26 (12), 3534. doi:10.3390/molecules26123534
Ambati, M., Apicella, I., Wang, S. B., Narendran, S., Leung, H., Pereira, F., et al. (2021). Identification of fluoxetine as a direct NLRP3 inhibitor to treat atrophic macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 118 (41), e2102975118. doi:10.1073/pnas.2102975118
Atrooz, F., and Salim, S. (2020). Sleep deprivation, oxidative stress and inflammation. Adv. Protein Chem. Struct. Biol. 119, 309–336. doi:10.1016/bs.apcsb.2019.03.001
Badawy, A. A. (2019). Hypothesis: Metabolic targeting of 5-aminolevulinate synthase by tryptophan and inhibitors of heme utilisation by tryptophan 2, 3-dioxygenase as potential therapies of acute hepatic porphyrias. Med. Hypotheses 131, 109314. doi:10.1016/j.mehy.2019.109314
Badr, L. K., Ayvazian, N., Lameh, S., and Charafeddine, L. (2018). Is the effect of postpartum depression on mother-infant bonding universal? Infant Behav. Dev. 51, 15–23. doi:10.1016/j.infbeh.2018.02.003
Bauer, A. E., Liu, X., Byrne, E. M., Sullivan, P. F., Wray, N. R., Agerbo, E., et al. (2019). Genetic risk scores for major psychiatric disorders and the risk of postpartum psychiatric disorders. Transl. Psychiatry 9 (1), 288. doi:10.1038/s41398-019-0629-9
Baune, B. T., Sampson, E., Louise, J., Hori, H., Schubert, K. O., Clark, S. R., et al. (2021). No evidence for clinical efficacy of adjunctive celecoxib with vortioxetine in the treatment of depression: A 6-week double-blind placebo controlled randomized trial. Eur. Neuropsychopharmacol. 53, 34–46. doi:10.1016/j.euroneuro.2021.07.092
Becker, M., Weinberger, T., Chandy, A., and Schmukler, S. (2016). Depression during pregnancy and postpartum. Curr. Psychiatry Rep. 18 (3), 32. doi:10.1007/s11920-016-0664-7
Beckett, C. W., and Niklison-Chirou, M. V. (2022). The role of immunomodulators in treatment-resistant depression: Case studies. Cell Death Discov. 8 (1), 367. doi:10.1038/s41420-022-01147-6
Belk, J. A., Daniel, B., and Satpathy, A. T. (2022). Epigenetic regulation of T cell exhaustion. Nat. Immunol. 23 (6), 848–860. doi:10.1038/s41590-022-01224-z
Berbudi, A., Rahmadika, N., Tjahjadi, A. I., and Ruslami, R. (2020). Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 16 (5), 442–449. doi:10.2174/1573399815666191024085838
Berk, M., Mohebbi, M., Dean, O. M., Cotton, S. M., Chanen, A. M., Dodd, S., et al. (2020). Youth depression alleviation with anti-inflammatory agents (YoDA-A): A randomised clinical trial of rosuvastatin and aspirin. BMC Med. 18 (1), 16. doi:10.1186/s12916-019-1475-6
Beurel, E., Toups, M., and Nemeroff, C. B. (2020). The bidirectional relationship of depression and inflammation: Double trouble. Neuron 107 (2), 234–256. doi:10.1016/j.neuron.2020.06.002
Brann, E., Fransson, E., White, R. A., Papadopoulos, F. C., Edvinsson, A., Kamali-Moghaddam, M., et al. (2020). Inflammatory markers in women with postpartum depressive symptoms. J. Neurosci. Res. 98 (7), 1309–1321. doi:10.1002/jnr.24312
Broz, P., and Dixit, V. M. (2016). Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16 (7), 407–420. doi:10.1038/nri.2016.58
Brummelte, S., and Galea, L. A. (2016). Postpartum depression: Etiology, treatment and consequences for maternal care. Horm. Behav. 77, 153–166. doi:10.1016/j.yhbeh.2015.08.008
Buglione-Corbett, R., Deligiannidis, K. M., Leung, K., Zhang, N., Lee, M., Rosal, M. C., et al. (2018). Expression of inflammatory markers in women with perinatal depressive symptoms. Arch. Womens Ment. Health 21 (6), 671–679. doi:10.1007/s00737-018-0834-1
Cao, S., and Wei, L. (2020). Predictive value of serum CRH/5-HT ratio for postpartum depression. Int. J. Gynaecol. Obstet. 151 (3), 438–442. doi:10.1002/ijgo.13351
Carlessi, A. S., Borba, L. A., Zugno, A. I., Quevedo, J., and Reus, G. Z. (2021). Gut microbiota-brain axis in depression: The role of neuroinflammation. Eur. J. Neurosci. 53 (1), 222–235. doi:10.1111/ejn.14631
Cellini, P., Pigoni, A., Delvecchio, G., Moltrasio, C., and Brambilla, P. (2022). Machine learning in the prediction of postpartum depression: A review. J. Affect. Disord. 309, 350–357. doi:10.1016/j.jad.2022.04.093
Cervenka, I., Agudelo, L. Z., and Ruas, J. L. (2017). Kynurenines: Tryptophan's metabolites in exercise, inflammation, and mental health. Science 357 (6349), eaaf9794. doi:10.1126/science.aaf9794
Cevik, A., and Alan, S. (2021). Are pregnancy and postpartum oxytocin level a predictive biomarker for postpartum depression? J. Obstet. Gynaecol. Res. 47 (12), 4280–4288. doi:10.1111/jog.15023
Chai, H. H., Fu, X. C., Ma, L., Sun, H. T., Chen, G. Z., Song, M. Y., et al. (2019). The chemokine CXCL1 and its receptor CXCR2 contribute to chronic stress-induced depression in mice. FASEB J. 33 (8), 8853–8864. doi:10.1096/fj.201802359RR
Chai, Y., Cai, Y., Fu, Y., Wang, Y., Zhang, Y., Zhang, X., et al. (2022). Salidroside ameliorates depression by suppressing NLRP3-mediated pyroptosis via P2X7/NF-κB/NLRP3 signaling pathway. Front. Pharmacol. 13, 812362. doi:10.3389/fphar.2022.812362
Chen, H., Ma, Y., Chen, M., Chen, J., and Chen, J. (2021). Safflower extract improves depression in mice by inhibiting the TLR4-NLRP3 inflammation signaling pathway. Ann. Palliat. Med. 10 (7), 8015–8023. doi:10.21037/apm-21-1728
Corwin, E. J., Johnston, N., and Pugh, L. (2008). Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biol. Res. Nurs. 10 (2), 128–133. doi:10.1177/1099800408323220
Corwin, E. J., Pajer, K., Paul, S., Lowe, N., Weber, M., and McCarthy, D. O. (2015). Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain Behav. Immun. 49, 86–93. doi:10.1016/j.bbi.2015.04.012
Craig, C. F., Filippone, R. T., Stavely, R., Bornstein, J. C., Apostolopoulos, V., and Nurgali, K. (2022). Neuroinflammation as an etiological trigger for depression comorbid with inflammatory bowel disease. J. Neuroinflammation 19 (1), 4. doi:10.1186/s12974-021-02354-1
Cui, M., Dai, W., Kong, J., and Chen, H. (2021). Th17 cells in depression: Are they crucial for the antidepressant effect of ketamine? Front. Pharmacol. 12, 649144. doi:10.3389/fphar.2021.649144
Cuomo, A., Maina, G., Neal, S. M., De Montis, G., Rosso, G., Scheggi, S., et al. (2018). Using sertraline in postpartum and breastfeeding: Balancing risks and benefits. Expert Opin. Drug Saf. 17 (7), 719–725. doi:10.1080/14740338.2018.1491546
Deets, K. A., and Vance, R. E. (2021). Inflammasomes and adaptive immune responses. Nat. Immunol. 22 (4), 412–422. doi:10.1038/s41590-021-00869-6
Deng, S. L., Chen, J. G., and Wang, F. (2020). Microglia: A central player in depression. Curr. Med. Sci. 40 (3), 391–400. doi:10.1007/s11596-020-2193-1
Desai, V., Kozyrskyj, A. L., Lau, S., Sanni, O., Dennett, L., Walter, J., et al. (2021). Effectiveness of probiotic, prebiotic, and synbiotic supplementation to improve perinatal mental health in mothers: A systematic review and meta-analysis. Front. Psychiatry 12, 622181. doi:10.3389/fpsyt.2021.622181
Dong, C. (2021). Cytokine regulation and function in T cells. Annu. Rev. Immunol. 39, 51–76. doi:10.1146/annurev-immunol-061020-053702
Donoso, F., Cryan, J. F., Olavarria-Ramirez, L., Nolan, Y. M., and Clarke, G. (2022). Inflammation, lifestyle factors, and the microbiome-gut-brain Axis: Relevance to depression and antidepressant action. Clin. Pharmacol. Ther. Epub ahead of print. doi:10.1002/cpt.2581
Du, Y. Y., Chen, Z. X., Liu, M. Y., Liu, Q. P., Lin, C. S., Chu, C. Q., et al. (2020). Leonurine regulates Treg/Th17 balance to attenuate rheumatoid arthritis through inhibition of TAZ expression. Front. Immunol. 11, 556526. doi:10.3389/fimmu.2020.556526
Fontana, L., Ghezzi, L., Cross, A. H., and Piccio, L. (2021). Effects of dietary restriction on neuroinflammation in neurodegenerative diseases. J. Exp. Med. 218 (2), e20190086. doi:10.1084/jem.20190086
Gao, L. N., Yan, M., Zhou, L., Wang, J., Sai, C., Fu, Y., et al. (2021). Puerarin alleviates depression-like behavior induced by high-fat diet combined with chronic unpredictable mild stress via repairing TLR4-induced inflammatory damages and phospholipid metabolism disorders. Front. Pharmacol. 12, 767333. doi:10.3389/fphar.2021.767333
Glaser, R., and Kiecolt-Glaser, J. K. (2005). Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 5 (3), 243–251. doi:10.1038/nri1571
Greenbaum, U., Dumbrava, E. I., Biter, A. B., Haymaker, C. L., and Hong, D. S. (2021). Engineered T-cell receptor T cells for cancer immunotherapy. Cancer Immunol. Res. 9 (11), 1252–1261. doi:10.1158/2326-6066.CIR-21-0269
Grippi, C. (2021). Factors that influence women's symptoms of postpartum depression after discharge of their preterm infants from the NICU. J. Obstet. Gynecol. Neonatal Nurs. 50 (5), 610–620. doi:10.1016/j.jogn.2021.05.003
Groer, M. W., and Morgan, K. (2007). Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology 32 (2), 133–139. doi:10.1016/j.psyneuen.2006.11.007
Guintivano, J., Manuck, T., and Meltzer-Brody, S. (2018a). Predictors of postpartum depression: A comprehensive review of the last decade of evidence. Clin. Obstet. Gynecol. 61 (3), 591–603. doi:10.1097/GRF.0000000000000368
Guintivano, J., Sullivan, P. F., Stuebe, A. M., Penders, T., Thorp, J., Rubinow, D. R., et al. (2018b). Adverse life events, psychiatric history, and biological predictors of postpartum depression in an ethnically diverse sample of postpartum women. Psychol. Med. 48 (7), 1190–1200. doi:10.1017/S0033291717002641
Gunduz-Bruce, H., Takahashi, K., and Huang, M. Y. (2022). Development of neuroactive steroids for the treatment of postpartum depression. J. Neuroendocrinol. 34 (2), e13019. doi:10.1111/jne.13019
Guo, L. T., Wang, S. Q., Su, J., Xu, L. X., Ji, Z. Y., Zhang, R. Y., et al. (2019). Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflammation 16 (1), 95. doi:10.1186/s12974-019-1474-8
Guo, Q. J., Shan, J., Xu, Y. F., Hu, Y. Y., Huo, C. L., Song, J. Y., et al. (2020). Pioglitazone metformin complex improves polycystic ovary syndrome comorbid psychological distress via inhibiting NLRP3 inflammasome activation: A prospective clinical study. Mediat. Inflamm. 2020, 3050487. doi:10.1155/2020/3050487
Halim, M., and Halim, A. (2019). The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. 13 (2), 1165–1172. doi:10.1016/j.dsx.2019.01.040
Hantsoo, L., Ward-O'Brien, D., Czarkowski, K. A., Gueorguieva, R., Price, L. H., and Epperson, C. N. (2014). A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacol. Berl. 231 (5), 939–948. doi:10.1007/s00213-013-3316-1
Haruwaka, K., Ikegami, A., Tachibana, Y., Ohno, N., Konishi, H., Hashimoto, A., et al. (2019). Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 10 (1), 5816. doi:10.1038/s41467-019-13812-z
Hipwell, A. E., Tung, I., Krafty, R. T., Leong, A. W., Spada, M., Vaccaro, H., et al. (2022). A lifespan perspective on depression in the postpartum period in a racially and socioeconomically diverse sample of young mothers. Psychol. Med. 2022, 1–9. doi:10.1017/S0033291722001210
Hu, Y., Feng, W., Chen, H., Shi, H., Jiang, L., Zheng, X., et al. (2021). Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with hashimoto's thyroiditis: A prospective randomized-controlled trial. Clin. Transl. Sci. 14 (4), 1390–1402. doi:10.1111/cts.12993
Hu, Y., Zhao, M., Zhao, T., Qi, M., Yao, G., and Dong, Y. (2022). The protective effect of pilose antler peptide on CUMS-induced depression through AMPK/Sirt1/NF-κB/NLRP3-Mediated pyroptosis. Front. Pharmacol. 13, 815413. doi:10.3389/fphar.2022.815413
Huang, Y., Xu, W., and Zhou, R. (2021). NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 18 (9), 2114–2127. doi:10.1038/s41423-021-00740-6
Husain, M. I., Chaudhry, I. B., Khoso, A. B., Husain, M. O., Hodsoll, J., Ansari, M. A., et al. (2020). Minocycline and celecoxib as adjunctive treatments for bipolar depression: A multicentre, factorial design randomised controlled trial. Lancet. Psychiatry 7 (6), 515–527. doi:10.1016/S2215-0366(20)30138-3
Hwang, E. S., and Song, S. B. (2020). Possible adverse effects of high-dose nicotinamide: Mechanisms and safety assessment. Biomolecules 10 (5), E687. doi:10.3390/biom10050687
Irwin, M. R. (2019). Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 19 (11), 702–715. doi:10.1038/s41577-019-0190-z
Jia, M., Li, C., Zheng, Y., Ding, X., Chen, M., Ding, J., et al. (2017). Leonurine exerts antidepressant-like effects in the chronic mild stress-induced depression model in mice by inhibiting neuroinflammation. Int. J. Neuropsychopharmacol. 20 (11), 886–895. doi:10.1093/ijnp/pyx062
Jia, X., Gao, Z., and Hu, H. (2021). Microglia in depression: Current perspectives. Sci. China. Life Sci. 64 (6), 911–925. doi:10.1007/s11427-020-1815-6
Jiang, H., He, H., Chen, Y., Huang, W., Cheng, J., Ye, J., et al. (2017). Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 214 (11), 3219–3238. doi:10.1084/jem.20171419
Jiwaji, Z., and Hardingham, G. E. (2022). Good, bad, and neglectful: Astrocyte changes in neurodegenerative disease. Free Radic. Biol. Med. 182, 93–99. doi:10.1016/j.freeradbiomed.2022.02.020
Jonville-Bera, A. P., Guilmot, J. L., Aspe, G., Autret-Leca, E., and Magnant, J. (2011). Is exogenous administration of IL-1ra (anakinra) likely to induce severe depression? Eur. J. Clin. Pharmacol. 67 (2), 213–214. doi:10.1007/s00228-010-0915-1
Kalkman, H. O., and Feuerbach, D. (2016). Antidepressant therapies inhibit inflammation and microglial M1-polarization. Pharmacol. Ther. 163, 82–93. doi:10.1016/j.pharmthera.2016.04.001
Kanes, S., Colquhoun, H., Gunduz-Bruce, H., Raines, S., Arnold, R., Schacterle, A., et al. (2017). Brexanolone (SAGE-547 injection) in post-partum depression: A randomised controlled trial. Lancet 390 (10093), 480–489. doi:10.1016/S0140-6736(17)31264-3
Kealy, J., Greene, C., and Campbell, M. (2020). Blood-brain barrier regulation in psychiatric disorders. Neurosci. Lett. 726, 133664. doi:10.1016/j.neulet.2018.06.033
Kessing, L. V., Rytgaard, H. C., Gerds, T. A., Berk, M., Ekstrom, C. T., and Andersen, P. K. (2019). New drug candidates for depression - a nationwide population-based study. Acta Psychiatr. Scand. 139 (1), 68–77. doi:10.1111/acps.12957
Knight, J. M., Costanzo, E. S., Singh, S., Yin, Z., Szabo, A., Pawar, D. S., et al. (2021). The IL-6 antagonist tocilizumab is associated with worse depression and related symptoms in the medically ill. Transl. Psychiatry 11 (1), 58. doi:10.1038/s41398-020-01164-y
Kovats, S. (2015). Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 294 (2), 63–69. doi:10.1016/j.cellimm.2015.01.018
Krause, D., Jobst, A., Kirchberg, F., Kieper, S., Hartl, K., Kastner, R., et al. (2014). Prenatal immunologic predictors of postpartum depressive symptoms: A prospective study for potential diagnostic markers. Eur. Arch. Psychiatry Clin. Neurosci. 264 (7), 615–624. doi:10.1007/s00406-014-0494-8
Kumar, V. (2018). T cells and their immunometabolism: A novel way to understanding sepsis immunopathogenesis and future therapeutics. Eur. J. Cell Biol. 97 (6), 379–392. doi:10.1016/j.ejcb.2018.05.001
Kwiatek, M., Geca, T., and Kwasniewska, A. (2021). Pro- and anti-inflammatory cytokines in the first trimester-comparison of missed miscarriage and normal pregnancy. Int. J. Environ. Res. Public Health 18 (16), 8538. doi:10.3390/ijerph18168538
Lapato, D. M., Wolf, H. M., Lancaster, E. E., Roberson-Nay, R., and York, T. P. (2021). A primer on DNA methylation and its potential to impact maternal depression risk and assessment during pregnancy and the postpartum. J. Perinat. Neonatal Nurs. 35 (1), 4–7. doi:10.1097/JPN.0000000000000528
Leng, F., and Edison, P. (2021). Neuroinflammation and microglial activation in alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 17 (3), 157–172. doi:10.1038/s41582-020-00435-y
Letourneau, N. L., Dennis, C. L., Benzies, K., Duffett-Leger, L., Stewart, M., Tryphonopoulos, P. D., et al. (2012). Postpartum depression is a family affair: Addressing the impact on mothers, fathers, and children. Issues Ment. Health Nurs. 33 (7), 445–457. doi:10.3109/01612840.2012.673054
Li, H., Guan, Y., Liang, B., Ding, P., Hou, X., Wei, W., et al. (2022). Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome. Eur. J. Pharmacol. 928, 175091. doi:10.1016/j.ejphar.2022.175091
Li S, S., Sun, Y., Song, M., Song, Y., Fang, Y., Zhang, Q., et al. (2021). NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight 6 (23), e146852. doi:10.1172/jci.insight.146852
Li Y, Y., Song, W., Tong, Y., Zhang, X., Zhao, J., Gao, X., et al. (2021). Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J. Neuroinflammation 18 (1), 1. doi:10.1186/s12974-020-02040-8
Li Yk, Y. K., Chen, J. G., and Wang, F. (2021). The emerging roles of absent in melanoma 2 (AIM2) inflammasome in central nervous system disorders. Neurochem. Int. 149, 105122. doi:10.1016/j.neuint.2021.105122
Liao, G., Cheung, S., Galeano, J., Ji, A. X., Qin, Q., and Bi, X. (2009). Allopregnanolone treatment delays cholesterol accumulation and reduces autophagic/lysosomal dysfunction and inflammation in Npc1-/- mouse brain. Brain Res. 1270, 140–151. doi:10.1016/j.brainres.2009.03.027
Linnerbauer, M., Wheeler, M. A., and Quintana, F. J. (2020). Astrocyte crosstalk in CNS inflammation. Neuron 108 (4), 608–622. doi:10.1016/j.neuron.2020.08.012
Liu, C. H., Yang, M. H., Zhang, G. Z., Wang, X. X., Li, B., Li, M., et al. (2020). Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J. Neuroinflammation 17 (1), 54. doi:10.1186/s12974-020-01732-5
Liu, H., Zhang, Y., Gao, Y., and Zhang, Z. (2016). Elevated levels of Hs-CRP and IL-6 after delivery are associated with depression during the 6 months post partum. Psychiatry Res. 243, 43–48. doi:10.1016/j.psychres.2016.02.022
Liu, Q., Zhang, M. M., Guo, M. X., Zhang, Q. P., Li, N. Z., Cheng, J., et al. (2022). Inhibition of microglial NLRP3 with MCC950 attenuates microglial morphology and NLRP3/caspase-1/IL-1β signaling in stress-induced mice. J. Neuroimmune Pharmacol. 2022, 1–12. doi:10.1007/s11481-021-10037-0
Lob, S., Konigsrainer, A., Rammensee, H. G., Opelz, G., and Terness, P. (2009). Inhibitors of indoleamine-2, 3-dioxygenase for cancer therapy: Can we see the wood for the trees? Nat. Rev. Cancer 9 (6), 445–452. doi:10.1038/nrc2639
Mackay, G. M., Forrest, C. M., Stoy, N., Christofides, J., Egerton, M., Stone, T. W., et al. (2006). Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur. J. Neurol. 13 (1), 30–42. doi:10.1111/j.1468-1331.2006.01220.x
Maes, M., Song, C., and Yirmiya, R. (2012). Targeting IL-1 in depression. Expert Opin. Ther. Targets 16 (11), 1097–1112. doi:10.1517/14728222.2012.718331
Marchetti, C., Swartzwelter, B., Gamboni, F., Neff, C. P., Richter, K., Azam, T., et al. (2018). OLT1177, a beta-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. U. S. A. 115 (7), E1530–E1539. doi:10.1073/pnas.1716095115
Miao, P., Ruiqing, T., Yanrong, L., Zhuwen, S., Huan, Y., Qiong, W., et al. (2022). Pyroptosis: A possible link between obesity-related inflammation and inflammatory diseases. J. Cell. Physiol. 237 (2), 1245–1265. doi:10.1002/jcp.30627
Miller, A. H., and Raison, C. L. (2016). The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16 (1), 22–34. doi:10.1038/nri.2015.5
Miyoshi, S., Oda, N., Gion, Y., Taki, T., Mitani, R., Takata, I., et al. (2021). Exacerbation of pulmonary cryptococcosis associated with enhancement of Th2 response in the postpartum period. J. Infect. Chemother. 27 (8), 1248–1250. doi:10.1016/j.jiac.2021.03.025
Mohammadinejad, P., Arya, P., Esfandbod, M., Kaviani, A., Najafi, M., Kashani, L., et al. (2015). Celecoxib versus diclofenac in mild to moderate depression management among breast cancer patients: A double-blind, placebo-controlled, randomized trial. Ann. Pharmacother. 49 (9), 953–961. doi:10.1177/1060028015592215
Mokhber, N., Namjoo, M., Tara, F., Boskabadi, H., Rayman, M. P., Ghayour-Mobarhan, M., et al. (2011). Effect of supplementation with selenium on postpartum depression: A randomized double-blind placebo-controlled trial. J. Matern. Fetal. Neonatal Med. 24 (1), 104–108. doi:10.3109/14767058.2010.482598
Mughal, S., Azhar, Y., and Siddiqui, W. (2022). Postpartum depression. Treasure Island (FL): StatPearls.
Muller, N., Schwarz, M. J., Dehning, S., Douhe, A., Cerovecki, A., Goldstein-Muller, B., et al. (2006). The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: Results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol. Psychiatry 11 (7), 680–684. doi:10.1038/sj.mp.4001805
Nagayasu, Y., Fujita, D., Daimon, A., Nunode, M., Sawada, M., Sano, T., et al. (2021). Possible prevention of post-partum depression by intake of omega-3 polyunsaturated fatty acids and its relationship with interleukin 6. J. Obstet. Gynaecol. Res. 47 (4), 1371–1379. doi:10.1111/jog.14592
Nakade, Y., Kitano, R., Yamauchi, T., Kimoto, S., Sakamoto, K., Inoue, T., et al. (2021). Effect of central corticotropin-releasing factor on hepatic lipid metabolism and inflammation-related gene expression in rats. Int. J. Mol. Sci. 22 (8), 3940. doi:10.3390/ijms22083940
Nakagawa, Y., and Chiba, K. (2014). Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharm. (Basel) 7 (12), 1028–1048. doi:10.3390/ph7121028
Nazzari, S., Molteni, M., Valtorta, F., Comai, S., and Frigerio, A. (2020). Prenatal IL-6 levels and activation of the tryptophan to kynurenine pathway are associated with depressive but not anxiety symptoms across the perinatal and the post-partum period in a low-risk sample. Brain Behav. Immun. 89, 175–183. doi:10.1016/j.bbi.2020.06.015
Nelson, T., Ernst, S. C., and Watson-Singleton, N. N. (2022). Perinatal complications, poor hospital treatment, and positive screen for postpartum depressive symptoms among black women. J. Racial Ethn. Health Disparities 2022, 1–8. doi:10.1007/s40615-022-01322-6
Noushad, S., Ahmed, S., Ansari, B., Mustafa, U. H., Saleem, Y., and Hazrat, H. (2021). Physiological biomarkers of chronic stress: A systematic review. Int. J. Health Sci. 15 (5), 46–59.
O'Connor, J. C., Lawson, M. A., Andre, C., Moreau, M., Lestage, J., Castanon, N., et al. (2009). Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2, 3-dioxygenase activation in mice. Mol. Psychiatry 14 (5), 511–522. doi:10.1038/sj.mp.4002148
Osborne, L. M., Betz, J. F., Yenokyan, G., Standeven, L. R., and Payne, J. L. (2019a). The role of allopregnanolone in pregnancy in predicting postpartum anxiety symptoms. Front. Psychol. 10, 1033. doi:10.3389/fpsyg.2019.01033
Osborne, L. M., Brar, A., and Klein, S. L. (2019b). The role of Th17 cells in the pathophysiology of pregnancy and perinatal mood and anxiety disorders. Brain Behav. Immun. 76, 7–16. doi:10.1016/j.bbi.2018.11.015
Osborne, L. M., Gilden, J., Kamperman, A. M., Hoogendijk, W. J. G., Spicer, J., Drexhage, H. A., et al. (2020). T-cell defects and postpartum depression. Brain Behav. Immun. 87, 397–403. doi:10.1016/j.bbi.2020.01.007
Osimo, E. F., Pillinger, T., Rodriguez, I. M., Khandaker, G. M., Pariante, C. M., and Howes, O. D. (2020). Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5, 166 patients and 5, 083 controls. Brain Behav. Immun. 87, 901–909. doi:10.1016/j.bbi.2020.02.010
Paolucci, E. M., Loukov, D., Bowdish, D. M. E., and Heisz, J. J. (2018). Exercise reduces depression and inflammation but intensity matters. Biol. Psychol. 133, 79–84. doi:10.1016/j.biopsycho.2018.01.015
Parks, E. E., Logan, S., Yeganeh, A., Farley, J. A., Owen, D. B., and Sonntag, W. E. (2020). Interleukin 6 reduces allopregnanolone synthesis in the brain and contributes to age-related cognitive decline in mice. J. Lipid Res. 61 (10), 1308–1319. doi:10.1194/jlr.RA119000479
Patel, B. G., Rudnicki, M., Yu, J., Shu, Y., and Taylor, R. N. (2017). Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet. Gynecol. Scand. 96 (6), 623–632. doi:10.1111/aogs.13156
Payne, J. L., and Maguire, J. (2019). Pathophysiological mechanisms implicated in postpartum depression. Front. Neuroendocrinol. 52, 165–180. doi:10.1016/j.yfrne.2018.12.001
Pyorala, S. (2003). Indicators of inflammation in the diagnosis of mastitis. Vet. Res. 34 (5), 565–578. doi:10.1051/vetres:2003026
Qiu, W., Go, K. A., Lamers, Y., and Galea, L. A. M. (2021). Postpartum corticosterone and fluoxetine shift the tryptophan-kynurenine pathway in dams. Psychoneuroendocrinology 130, 105273. doi:10.1016/j.psyneuen.2021.105273
Qu, M., Tang, Q., Li, X., Zhao, R., Li, J., Xu, H., et al. (2015). Shen-Qi-Jie-Yu-Fang has antidepressant effects in a rodent model of postpartum depression by regulating the immune organs and subsets of T lymphocytes. Neuropsychiatr. Dis. Treat. 11, 1523–1540. doi:10.2147/NDT.S83964
Quan, C., Wang, S., Duan, K., Ma, J., Yu, H., Yang, M., et al. (2020). The role of kynurenine pathway and kynurenic aminotransferase alleles in postpartum depression following cesarean section in Chinese women. Brain Behav. 10 (4), e01566. doi:10.1002/brb3.1566
Raison, C. L., Rutherford, R. E., Woolwine, B. J., Shuo, C., Schettler, P., Drake, D. F., et al. (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry 70 (1), 31–41. doi:10.1001/2013.jamapsychiatry.4
Ramakrishnan, R., Rao, S., and He, J. R. (2021). Perinatal health predictors using artificial intelligence: A review. Womens Health 17, 17455065211046132. doi:10.1177/17455065211046132
Robakis, T. K., Lee, S., Werner, E., Liu, G., Miller, M., Wylie, D., et al. (2020). DNA methylation patterns in T lymphocytes are generally stable in human pregnancies but CD3 methylation is associated with perinatal psychiatric symptoms. Brain Behav. Immun. Health 3, 100044. doi:10.1016/j.bbih.2020.100044
Rohm, T. V., Meier, D. T., Olefsky, J. M., and Donath, M. Y. (2022). Inflammation in obesity, diabetes, and related disorders. Immunity 55 (1), 31–55. doi:10.1016/j.immuni.2021.12.013
Roomruangwong, C., Kanchanatawan, B., Carvalho, A. F., Sirivichayakul, S., Duleu, S., Geffard, M., et al. (2018). Body image dissatisfaction in pregnant and non-pregnant females is strongly predicted by immune activation and mucosa-derived activation of the tryptophan catabolite (TRYCAT) pathway. World J. Biol. Psychiatry 19 (3), 200–209. doi:10.1080/15622975.2016.1213881
Savitz, J. (2020). The kynurenine pathway: A finger in every pie. Mol. Psychiatry 25 (1), 131–147. doi:10.1038/s41380-019-0414-4
Sha, Q., Achtyes, E., Nagalla, M., Keaton, S., Smart, L., Leach, R., et al. (2021). Associations between estrogen and progesterone, the kynurenine pathway, and inflammation in the post-partum. J. Affect. Disord. 281, 9–12. doi:10.1016/j.jad.2020.10.052
Sha, Q., Madaj, Z., Keaton, S., Escobar Galvis, M. L., Smart, L., Krzyzanowski, S., et al. (2022). Cytokines and tryptophan metabolites can predict depressive symptoms in pregnancy. Transl. Psychiatry 12 (1), 35. doi:10.1038/s41398-022-01801-8
Shangraw, E. M., and McFadden, T. B. (2022). Graduate Student Literature Review: Systemic mediators of inflammation during mastitis and the search for mechanisms underlying impaired lactation. J. Dairy Sci. 105 (3), 2718–2727. doi:10.3168/jds.2021-20776
Shao, S., Jia, R., Zhao, L., Zhang, Y., Guan, Y., Wen, H., et al. (2021). Xiao-Chai-Hu-Tang ameliorates tumor growth in cancer comorbid depressive symptoms via modulating gut microbiota-mediated TLR4/MyD88/NF-κB signaling pathway. Phytomedicine. 88, 153606. doi:10.1016/j.phymed.2021.153606
Shi, S., Chen, T., and Zhao, M. (2022). The crosstalk between neurons and glia in methamphetamine-induced neuroinflammation. Neurochem. Res. 47 (4), 872–884. doi:10.1007/s11064-021-03513-9
Shi, Y., Lv, Q., Zheng, M., Sun, H., and Shi, F. (2021). NLRP3 inflammasome inhibitor INF39 attenuated NLRP3 assembly in macrophages. Int. Immunopharmacol. 92, 107358. doi:10.1016/j.intimp.2020.107358
Shorey, S., Chee, C. Y. I., Ng, E. D., Chan, Y. H., Tam, W. W. S., and Chong, Y. S. (2018). Prevalence and incidence of postpartum depression among healthy mothers: A systematic review and meta-analysis. J. Psychiatr. Res. 104, 235–248. doi:10.1016/j.jpsychires.2018.08.001
Slomian, J., Honvo, G., Emonts, P., Reginster, J. Y., and Bruyere, O. (2019). Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Womens Health 15, 1745506519844044. doi:10.1177/1745506519844044
Sluiter, F., Incollingo Rodriguez, A. C., Nephew, B. C., Cali, R., Murgatroyd, C., and Santos, H. P. (2020). Pregnancy associated epigenetic markers of inflammation predict depression and anxiety symptoms in response to discrimination. Neurobiol. Stress 13, 100273. doi:10.1016/j.ynstr.2020.100273
Song, A. Q., Gao, B., Fan, J. J., Zhu, Y. J., Zhou, J., Wang, Y. L., et al. (2020). NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. J. Neuroinflammation 17 (1), 178. doi:10.1186/s12974-020-01848-8
Souza, L. C., Jesse, C. R., de Gomes, M. G., Del Fabbro, L., Goes, A. T. R., Donato, F., et al. (2017). Activation of brain indoleamine-2, 3-dioxygenase contributes to depressive-like behavior induced by an intracerebroventricular injection of streptozotocin in mice. Neurochem. Res. 42 (10), 2982–2995. doi:10.1007/s11064-017-2329-2
Stewart, D. E., and Vigod, S. N. (2019). Postpartum depression: Pathophysiology, treatment, and emerging therapeutics. Annu. Rev. Med. 70, 183–196. doi:10.1146/annurev-med-041217-011106
Sylven, S. M., Elenis, E., Michelakos, T., Larsson, A., Olovsson, M., Poromaa, I. S., et al. (2013). Thyroid function tests at delivery and risk for postpartum depressive symptoms. Psychoneuroendocrinology 38 (7), 1007–1013. doi:10.1016/j.psyneuen.2012.10.004
Szeto, A., Cecati, M., Ahmed, R., McCabe, P. M., and Mendez, A. J. (2020). Oxytocin reduces adipose tissue inflammation in obese mice. Lipids Health Dis. 19 (1), 188. doi:10.1186/s12944-020-01364-x
Szpunar, M. J., Malaktaris, A., Baca, S. A., Hauger, R. L., and Lang, A. J. (2021). Are alterations in estradiol, cortisol, and inflammatory cytokines associated with depression during pregnancy and postpartum? An exploratory study. Brain Behav. Immun. Health 16, 100309. doi:10.1016/j.bbih.2021.100309
Tainaka, H., Takahashi, N., Nishimura, T., Okumura, A., Harada, T., Iwabuchi, T., et al. (2022). Long-term effect of persistent postpartum depression on children's psychological problems in childhood. J. Affect. Disord. 305, 71–76. doi:10.1016/j.jad.2022.02.061
Tang, Y., Shi, Y., Gao, Y., Xu, X., Han, T., Li, J., et al. (2019). Oxytocin system alleviates intestinal inflammation by regulating macrophages polarization in experimental colitis. Clin. Sci. 133 (18), 1977–1992. doi:10.1042/CS20190756
Tansey, M. G., Wallings, R. L., Houser, M. C., Herrick, M. K., Keating, C. E., and Joers, V. (2022). Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol., 1–17. doi:10.1038/s41577-022-00684-6
Tao, W., Hu, Y., Chen, Z., Dai, Y., Hu, Y., and Qi, M. (2021). Magnolol attenuates depressive-like behaviors by polarizing microglia towards the M2 phenotype through the regulation of Nrf2/HO-1/NLRP3 signaling pathway. Phytomedicine. 91, 153692. doi:10.1016/j.phymed.2021.153692
Tattersfield, A. S., Croon, R. J., Liu, Y. W., Kells, A. P., Faull, R. L., and Connor, B. (2004). Neurogenesis in the striatum of the quinolinic acid lesion model of Huntington's disease. Neuroscience 127 (2), 319–332. doi:10.1016/j.neuroscience.2004.04.061
Tiosano, S., Yavne, Y., Watad, A., Langevitz, P., Lidar, M., Feld, J., et al. (2020). The impact of tocilizumab on anxiety and depression in patients with rheumatoid arthritis. Eur. J. Clin. Invest. 50 (9), e13268. doi:10.1111/eci.13268
Trifkovic, K. C., Micetic-Turk, D., Kmetec, S., Strauss, M., Dahlen, H. G., Foster, J. P., et al. (2022). Efficacy of direct or indirect use of probiotics for the improvement of maternal depression during pregnancy and in the postnatal period: A systematic review and meta-analysis. Healthc. (Basel) 10 (6), 970. doi:10.3390/healthcare10060970
Troubat, R., Barone, P., Leman, S., Desmidt, T., Cressant, A., Atanasova, B., et al. (2021). Neuroinflammation and depression: A review. Eur. J. Neurosci. 53 (1), 151–171. doi:10.1111/ejn.14720
Tsujita, N., Akamatsu, Y., Nishida, M. M., Hayashi, T., and Moritani, T. (2019). Effect of tryptophan, vitamin B6, and nicotinamide-containing supplement loading between meals on mood and autonomic nervous system Activity in young adults with subclinical depression: A randomized, double-blind, and placebo-controlled study. J. Nutr. Sci. Vitaminol. 65 (6), 507–514. doi:10.3177/jnsv.65.507
Umamaheswaran, S., Dasari, S. K., Yang, P., Lutgendorf, S. K., and Sood, A. K. (2018). Stress, inflammation, and eicosanoids: An emerging perspective. Cancer Metastasis Rev. 37 (2-3), 203–211. doi:10.1007/s10555-018-9741-1
Uzzan, S., and Azab, A. N. (2021). Anti-TNF-alpha compounds as a treatment for depression. Molecules 26 (8), 2368. doi:10.3390/molecules26082368
Van Dyken, P., and Lacoste, B. (2018). Impact of metabolic syndrome on neuroinflammation and the blood-brain barrier. Front. Neurosci. 12, 930. doi:10.3389/fnins.2018.00930
Wang, S., Quan, C., Tan, Y., Wen, S., Zhang, J., and Duan, K. (2018). Correlation between kynurenine metabolites and postpartum depression. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 43 (7), 725–731. doi:10.11817/j.issn.1672-7347.2018.07.005
Wang, Y., Zhang, Q., Chen, Y., Liang, C. L., Liu, H., Qiu, F., et al. (2020). Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed. Pharmacother. 121, 109570. doi:10.1016/j.biopha.2019.109570
Webster, E. L., Torpy, D. J., Elenkov, I. J., and Chrousos, G. P. (1998). Corticotropin-releasing hormone and inflammation. Ann. N. Y. Acad. Sci. 840, 21–32. doi:10.1111/j.1749-6632.1998.tb09545.x
Weigelt, K., Bergink, V., Burgerhout, K. M., Pescatori, M., Wijkhuijs, A., and Drexhage, H. A. (2013). Down-regulation of inflammation-protective microRNAs 146a and 212 in monocytes of patients with postpartum psychosis. Brain Behav. Immun. 29, 147–155. doi:10.1016/j.bbi.2012.12.018
Weissman, M. M. (2018). Postpartum depression and its long-term impact on children: Many new questions. JAMA Psychiatry 75 (3), 227–228. doi:10.1001/jamapsychiatry.2017.4265
Wisner, K. L., Perel, J. M., and Findling, R. L. (1996). Antidepressant treatment during breast-feeding. Am. J. Psychiatry 153 (9), 1132–1137. doi:10.1176/ajp.153.9.1132
Won, E., Na, K. S., and Kim, Y. K. (2021). Neuroinflammation-associated alterations of the brain as potential neural biomarkers in anxiety disorders. Int. J. Mol. Sci. 23 (1), E6546. doi:10.3390/ijms21186546
Worthen, R. J., and Beurel, E. (2022). Inflammatory and neurodegenerative pathophysiology implicated in postpartum depression. Neurobiol. Dis. 165, 105646. doi:10.1016/j.nbd.2022.105646
Wu, M., Zhao, L., Wang, Y., Guo, Q., An, Q., Geng, J., et al. (2022). Ketamine regulates the autophagy flux and polarization of microglia through the HMGB1-RAGE Axis and exerts antidepressant effects in mice. J. Neuropathol. Exp. Neurol. 81, 931–942. doi:10.1093/jnen/nlac035
Xu, X., Piao, H. N., Aosai, F., Zeng, X. Y., Cheng, J. H., Cui, Y. X., et al. (2020). Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br. J. Pharmacol. 177 (22), 5224–5245. doi:10.1111/bph.15261
Xu, Y., Sheng, H., Bao, Q., Wang, Y., Lu, J., and Ni, X. (2016). NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav. Immun. 56, 175–186. doi:10.1016/j.bbi.2016.02.022
Yang, F., Zhu, W., Cai, X., Zhang, W., Yu, Z., Li, X., et al. (2020). Minocycline alleviates NLRP3 inflammasome-dependent pyroptosis in monosodium glutamate-induced depressive rats. Biochem. Biophys. Res. Commun. 526 (3), 553–559. doi:10.1016/j.bbrc.2020.02.149
Yang, R., Yang, J., Li, Z., Su, R., Zou, L., Li, L., et al. (2022). Pinocembrin inhibits P2X4 receptor-mediated pyroptosis in Hippocampus to alleviate the behaviours of chronic pain and depression comorbidity in rats. Mol. Neurobiol. Epub ahead of print. doi:10.1007/s12035-022-03023-x
Yao, G., Bai, Z., Niu, J., Zhang, R., Lu, Y., Gao, T., et al. (2022). Astragalin attenuates depression-like behaviors and memory deficits and promotes M2 microglia polarization by regulating IL-4R/JAK1/STAT6 signaling pathway in a murine model of perimenopausal depression. Psychopharmacol. Berl. 239 (8), 2421–2443. doi:10.1007/s00213-022-06133-5
Yim, I. S., Glynn, L. M., Schetter, C. D., Hobel, C. J., Chicz-Demet, A., and Sandman, C. A. (2010). Prenatal beta-endorphin as an early predictor of postpartum depressive symptoms in euthymic women. J. Affect. Disord. 125 (1-3), 128–133. doi:10.1016/j.jad.2009.12.009
Yin, J., Zhao, F., Chojnacki, J. E., Fulp, J., Klein, W. L., Zhang, S., et al. (2018). NLRP3 inflammasome inhibitor ameliorates amyloid pathology in a mouse model of alzheimer's disease. Mol. Neurobiol. 55 (3), 1977–1987. doi:10.1007/s12035-017-0467-9
Zhai, X., Chen, Y., Han, X., Zhu, Y., Li, X., Zhang, Y., et al. (2022). The protective effect of hypericin on postpartum depression rat model by inhibiting the NLRP3 inflammasome activation and regulating glucocorticoid metabolism. Int. Immunopharmacol. 105, 108560. doi:10.1016/j.intimp.2022.108560
Zhang K, K., Ma, Y., Wang, D., Liu, J., An, J., Li, Y., et al. (2022). In vivo activation of T-cell proliferation by regulating cell surface receptor clustering using a pH-driven interlocked DNA nano-spring. Nano Lett. 22 (5), 1937–1945. doi:10.1021/acs.nanolett.1c04562
Zhang L, L., Zhang, L., and Sui, R. (2021). Ganoderic acid A-mediated modulation of microglial polarization is involved in depressive-like behaviors and neuroinflammation in a rat model of post-stroke depression. Neuropsychiatr. Dis. Treat. 17, 2671–2681. doi:10.2147/NDT.S317207
Zhang MM, M. M., Guo, M. X., Zhang, Q. P., Chen, X. Q., Li, N. Z., Liu, Q., et al. (2022). IL-1R/C3aR signaling regulates synaptic pruning in the prefrontal cortex of depression. Cell Biosci. 12 (1), 90. doi:10.1186/s13578-022-00832-4
Zhang, Q., Sun, Y., He, Z., Xu, Y., Li, X., Ding, J., et al. (2020). Kynurenine regulates NLRP2 inflammasome in astrocytes and its implications in depression. Brain Behav. Immun. 88, 471–481. doi:10.1016/j.bbi.2020.04.016
Zhang Y, Y., Lin, Z., Chen, D., and He, Y. (2021). CY-09 attenuates the progression of osteoarthritis via inhibiting NLRP3 inflammasome-mediated pyroptosis. Biochem. Biophys. Res. Commun. 553, 119–125. doi:10.1016/j.bbrc.2021.03.055
Zheng, J., Sun, K., Aili, S., Yang, X., and Gao, L. (2022). Predictors of postpartum depression among Chinese mothers and fathers in the early postnatal period: A cross-sectional study. Midwifery 105, 103233. doi:10.1016/j.midw.2021.103233
Zhou, S., Chen, R., She, Y., Liu, X., Zhao, H., Li, C., et al. (2022). A new perspective on depression and neuroinflammation: Non-coding RNA. J. Psychiatr. Res. 148, 293–306. doi:10.1016/j.jpsychires.2022.02.007
Zhu, J., Hu, Z., Han, X., Wang, D., Jiang, Q., Ding, J., et al. (2018). Dopamine D2 receptor restricts astrocytic NLRP3 inflammasome activation via enhancing the interaction of beta-arrestin2 and NLRP3. Cell Death Differ. 25 (11), 2037–2049. doi:10.1038/s41418-018-0127-2
Zhu, J., Sun, T., Zhang, J., Liu, Y., Wang, D., Zhu, H., et al. (2020). Drd2 biased agonist prevents neurodegeneration against NLRP3 inflammasome in Parkinson's disease model via a beta-arrestin2-biased mechanism. Brain Behav. Immun. 90, 259–271. doi:10.1016/j.bbi.2020.08.025
Zhu, J., and Tang, J. (2020). LncRNA Gm14205 induces astrocytic NLRP3 inflammasome activation via inhibiting oxytocin receptor in postpartum depression. Biosci. Rep. 40 (8), BSR20200672. doi:10.1042/BSR20200672
Keywords: postpartum depression, inflammation, T cell, cytokine, kynurenine, inflammasome
Citation: Zhu J, Jin J and Tang J (2022) Inflammatory pathophysiological mechanisms implicated in postpartum depression. Front. Pharmacol. 13:955672. doi: 10.3389/fphar.2022.955672
Received: 29 May 2022; Accepted: 24 October 2022;
Published: 03 November 2022.
Edited by:
Tao Xu, Anhui Medical University, ChinaReviewed by:
Malvina Hoxha, Catholic University Our Lady of Good Counsel, AlbaniaChutima Roomruangwong, Chulalongkorn University, Thailand
Copyright © 2022 Zhu, Jin and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Tang, MTgxN0BmY2t5eS5vcmcuY24=
†These authors have contributed equally to this work and share first authorship
 Jialei Zhu
Jialei Zhu Jing Jin
Jing Jin Jing Tang
Jing Tang