
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 03 October 2022
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.954938
The prevention and treatment of cardiovascular diseases (CVDs) have achieved initial results, but the number of CVDs patients will increase rapidly in the next 10 years. Atherosclerosis (AS) is a significant risk factor for CVDs. The impact of lifestyle and daily diet varies considerably between different countries and continents and has been shown to affect the development of various diseases such as diabetes and CVDs. Primary and secondary prevention using alternative supplements and methods to avoid or reduce the use of traditional pharmacological drugs have also become popular. One of the reasons for this is that pharmacological drugs with lipid-lowering, and blood pressure-lowering effects cause many side effects that may negatively impact the quality of life. Patients are now emphasizing reliance on lifestyle changes to reduce cardiovascular risks. Garlic is a medicinal and edible plant that has been used for a long time. In order to reveal garlic application in the prevention and treatment of AS, reviewing the latest domestic and international studies through searching databases. The result shows that the antiatherogenic role of garlic is eximious. And the mechanisms are mainly related to hypolipidemic, antioxidant, antithrombotic, inhibiting angiogenesis, protecting endothelial cells, anti-inflammatory, anti-apoptotic, inhibiting vascular smooth muscle proliferation, and regulating gut microbiota. The main signaling pathways involve AMPK/TLRs, Keap1/Nrf2, PI3K/AKT, PPARγ/LXRα, GEF-H1/RhoA/Rac, etc. The antiatherogenic actions and molecular mechanism of garlic were reviewed in this study to obtain a robust evidence basis for the clinical application and mechanistic study and provide a theoretical basis for further utilization of garlic.
Atherosclerosis is a lipid-driven chronic inflammatory disease and increases morbidity and mortality of CVDs. Inflammation and abnormal lipid metabolism underlie the pathology of AS, mainly involving the inner and middle layers of arteries, resulting in thickening and stiffening of the arterial wall, narrowing of the lumen, and progressive loss of elasticity. Low-density lipoprotein (LDL) accumulates in blood vessels, causing monocytes to phagocytize. The foam cells formed by monocyte phagocytosis of LDL are prone to rupture. The released necrotic products stimulate the proliferation of fibrous tissue, forming fibrous plaques, which continue to develop into atheromatous plaques and eventually lead to AS.
CVDs are the leading cause of death from disease in China (Zhao et al., 2019) and are expected to cause 3 million deaths by 2030 (HALE Collaborators, 2017). The prevalence of CVDs in China is continuously rising, with an estimated 330 million people currently suffering from with CVDs (China Cardiovascular Health and Disease Report Writing Group, 2021). Lately, there has been increased interest and awareness in society regarding the connection between dietary intake and diseases. One of the reasons for this is that pharmacological drugs with lipid-lowering, and blood pressure-lowering effects cause many side effects that may negatively impact the quality of life.
Natural products, such as Chinese herbs, are an ideal source for developing safe and effective drugs for AS (Liu et al., 2015). Herbal or botanical preparations [complementary and alternative medicine (CAM)] have gained tremendous popularity in healthcare maintenance. A large number of populations in both developing and developed countries prefer to use CAM as a treatment and preventive measure for diseases (Qidwai et al., 2003; Frass et al., 2012). The 2007 National Health Interview Survey (NHIS) reported that approximately 38% of United States adults and 12% of children used CAM in the past 12 months; the usage rate of CAM has increased steadily all over the world since 1950 (Barnes et al., 2008; Omeish et al., 2011).
Garlic (Allium sativum) is the underground bulb of Allium sativum L., a member of the broad lily family. The hypolipidemic, antiatherogenic, anticoagulant, antidiabetic, antihypertensive, antimicrobial, anticancer, antioxidant, and immunomodulatory activities of garlic have been fully confirmed in basic and clinical research (HALE Collaborators, 2017). Aged garlic extract (AGE) inhibits coronary artery calcification progression, glucose levels, and blood pressure in patients at increased risk of cardiovascular events in a European cohort (Wlosinska et al., 2020). Phytochemical content in garlic could be a promising therapeutic agent in the future for the treatment of CVDs. Organosulfur compounds are the main active constituents of garlic, although the mechanism remains unclear (Gardner et al., 2007). In this study, the pharmacological effects of garlic and related mechanisms were reviewed to reveal its application in the prevention and treatment of AS.
Four databases of Chinese periodicals and three databases of English are searched comprehensively: China National Knowledge Infrastructure (CNKI, https://www.cnki.net/), Chinese Scientific Journals Full-Text Database (VIP, http://www.cqvip.com/), Wanfang Journal Database (WAN FANG, http://www.wanfangdata.com.cn/index.html) and China Biological Medicine Database (Sinomed, http://www.sinomed.ac.cn/), Pubmed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science (http://isiknowledge.com), and Web of Science (https://www.webofscience.com). Studies on garlic for AS were screened from database construction to May 2022. The complete search strategy is attached (Supplementary Appendix A1). The search was conducted by two searchers using MeSH terms and entry terms. Two evaluators independently screened, evaluated, and cross-checked the literature according to the inclusion/exclusion criteria and consulted a third party to assist in discussing and resolving any disagreements.
The following types of studies were included: The following types of studies were included: 1) experimental studies; 2) clinical trials; 3) not a case report or a review; and 4) medicine identified as garlic or the garlic extract.
The following types of studies were excluded: The following types of studies were excluded: 1) full text not available; and 2) treatments combined with other ingredients.
Based on the screening results, 6870 studies were retrieved, and after excluding duplicate and irrelevant studies, 610 cases remained, and 48 unrelated studies were excluded after reading the titles and abstracts. 219 cases were excluded from reading the full text, of which 126 cases did not meet the inclusion criteria, 93 cases were comments, and finally, 343 cases were included, including 201 in vivo experiments, 63 in vitro experiments, seven in vivo experiments and in vitro experiments, and 72 clinical experiments. The retrieval process is shown in Figure 1.
Garlic is a biennial herb, that is, oblate or short conical in shape, with grayish or light brown membranous scale skin on the outside. Garlic has 6–10 cloves inside the bulbous leaves, borne in whorls around the flowering stem, with a disc-shaped stem base and numerous fibrous roots. Each garlic clove is covered with a membrane, peeled off to reveal white, thick, and juicy scales. It has a strong garlic odor and a pungent taste. Garlic leaves are solid, flat, linear-lanceolate, about 2.5 cm wide, and sheath-like at the base. The flowering stem is erect, about 60 cm high, the spathe has a long beak, 7–10 cm long, and the flowering period is 5–6 months. The bulbs are usually dug in spring and summer and used fresh or dried (Figure 2).
Garlic is widely cultivated in Asia, Africa, and Europe (Tocmo et al., 2017). The Central Asian region is the origin of garlic. There are five species of garlic germplasm in the world, namely, Sativum, Ophioscorodon, Longicuspis, Subtropical, and Pekinense, of which Longicuspis is the most primitive, and other species are differentiated from it (Kamenetsky et al., 2005). Garlic was introduced to mainland China from Xinjiang during the Han Dynasty (Block, 1985). Xinjiang garlic is known as high alliin garlic because of its high active ingredients. The medicinal function of garlic can be traced back to 4000 years ago. At that time, garlic was used as an antiseptic and a stimulant, and the Chinese used garlic to treat exogenous fevers and headaches (Nagini, 2008). Consumption of fresh, cooked garlic is considered safe by the FDA (Rouf et al., 2020). Garlic is known for its wide range of biological activities, including anti-inflammatory (Xie and Du, 2011), antioxidant (Argüello-García et al., 2010; Elosta et al., 2017), anticancer (Petrovic et al., 2018), lipid-lowering (Brandolini et al., 2005), antihypertensive (Liperoti et al., 2017), antiatherogenic (Brown et al., 2015) and cardioprotective role (Bouillaud and Blachier, 2011). Studies have confirmed that garlic plays a role in preventive effects in cardiovascular and cerebrovascular diseases, tumors, diabetes, and others (Cavallito and Bailey, 1944). Some people have difficulty accepting garlic’s pungent and irritating taste but wish to consume garlic for long-term disease prevention and health care. Therefore, garlic products are innovated, and products such as garlic lyophilized powder, allicin, and garlic extract have been developed (Li et al., 2017).
More than 80 monomer compounds have been identified in garlic, mainly divided into volatile and nonvolatile compounds (Figures 3, 4).
Volatile compounds in garlic include sulfur-containing compounds such as lipid-soluble organic sulfides and thiosulfinate (Hu et al., 2019). There are more than 30 kinds of sulfur-containing components in garlic, which is the primary bioactive substance of garlic. The main sulfur-containing compounds in garlic are alliin, allicin, etc. Alliin, chemically named S-Allyl-l-cysteine Sulfoxide (SACS), is an essential sulfur-containing compound in garlic bulbs. The most abundant sulfur-containing amino acid in garlic exists in the cytoplasm of garlic bulb cells (Almatroodi et al., 2019). Alliin inhibits platelet aggregation and may reduce the incidence of AS (Eric, 1985). Sheela et al. found that Alliin effectively reversed the elevation of lipids and lipid peroxides in hypercholesterolemic rats (Kendler, 1987). Alliinase and allinase are endogenous enzymes in garlic. Allinase, also known as Alliin lyase, is a dimeric glycoprotein in the vacuole of garlic scale bud cells and is more sensitive to temperature (Weiner et al., 2009). Allinase activity decreases when garlic is heated and vinegared.
Studies have shown that garlic has played a significant role in medicinal and edible due to the various thiosulfinate. The primary efficacy component is diallyl thiosulfinate (allicin) (Cavallito and Bailey, 1944). Thiosulfinate can inhibit the growth and reproduction of many germs and achieve better roles in anti-inflammatory and sterilizing. It also has antitumor (Bat-Chen et al., 2010), antioxidant (Ilić et al., 2015), lipid-lowering (Elkayam et al., 2013), and glucose-lowering (Arellano-Buendía et al., 2020) effects. After garlic is cut or crushed, alliin contained in the cell meets allinase and splits to produce allicin (Lanzotti, 2006). Allicin is unstable due to its sulfoxide and allyl structure and can be decomposed in a few hours at room temperature in the air. Allicin is the main component of fresh garlic homogenate (Salehi et al., 2019). Allicin can be further decomposed to produce the more stable ajone, dallyl sulfide (DAS), dallyl disulfide (DADS), and a small amount of dallyl trisulfide (DATS), dallyl Tetrasulfide (DATTS), which is the main component of new garlic oil extracted by steam distillation (Cao and Chen, 2008).
Since allicin is unstable, it cannot be prepared directly for use as a medicine. Allicin’s production rate is low because gastric acid inactivates allinase when consuming raw garlic. Therefore, to ensure the stability of the active ingredients of garlic, low-temperature extraction, microwave inactivation, chromatographic separation, ultrafiltration purification, spray drying, and other methods are often used to prepare new garlic preparations with high efficiency. At present, most companies at home and abroad make lyophilized garlic powder and enteric formulations of lyophilized garlic powder to improve the production rate of allicin in vivo (Yang et al., 2021).
DATS is known as a natural broad-spectrum antibiotic. DATS crosses the blood-brain barrier, scavenges free radicals, and achieves antioxidant effects by reducing the production of reactive oxygen species (Yan and Zeng, 2004). DATS has anti-mutagenic and anti-carcinogenic effects (Hong et al., 1992). Methyl allyl trisulfide (MATS) is the most substantial antifungal component in essential garlic oil, which can inhibit platelet aggregation and prevent thromboxane synthesis (Ariga et al., 1981). Ajone was reported as an active substance with a solid antiplatelet aggregation effect (Block, 1985, 1986). Ajone plays an essential role in cell-mediated immunity, humoral immune regulation, and other processes (Lawson et al., 1991).
Another class of compounds in garlic is nonvolatile compounds, mainly including water-soluble organic sulfides, steroidal saponins, saponin elements, flavonoids, phenols, peptides, enzymes, and organoselenium, organogermanium, hemagglutinin, fructans, prostaglandins, etc.
Up to now, 20 saponins have been extracted, isolated, and identified from garlic (Matsuura, 2001). Two new steroidal saponins were isolated from the water-soluble fraction of garlic, one was Proto-iso-eruboside B, and the other was iso-eruboside B. In addition, there were eruboside B and Sativoside C. Further, Sativoside -B2, -B3, -B4, and -B5 could be isolated from eruboside-B (Peng et al., 1996). Spirostanol saponin and Furostanol saponin are the main saponin components of garlic, and both saponins have hypolipidemic, antibacterial, and antitumor effects (Yan and Zeng, 2004). Garlic saponin can significantly inhibit platelet aggregation and prolong blood coagulation time to prevent and delay thrombosis.
Additionally, it can promote fibrinolysis (Peng et al., 1996). Garlic polysaccharide is one of the active ingredients with high content in garlic, accounting for 51% of the dry weight of garlic. Garlic polysaccharide with higher concentration has a more vital scavenging ability for free radicals (Lee et al., 2015). Six flavonoids and two polyphenols have been isolated from garlic, including apigenin, kaempferol, quercetin, luteolin, and N-ferulic acid-based tyramine.
In addition, in garlic, proteins, vitamins, fatty acids, biotin, nicotinic acid, and rare elements such as selenium and germanium have prominent pharmacological activities, such as antibacterial and anti-inflammatory, and cholesterol reduction.
Lipid migration is essential in developing AS (Wu et al., 2018). Oxidative modification of LDL induces phagocytosis and conversion of macrophages to foam cells (Wang H et al., 2017; Sharifi et al., 2019). Lecithin cholesterol acyltransferase (LCAT) catalyzes the esterification of free cholesterol, which promotes the maturation of high-density lipoprotein (HDL) and the reverse transport of cholesterol. Lipoprotein lipase (LPL) is the main enzyme in plasma that catalyzes the esterification of triglyceride-rich lipoproteins. Hepatic lipase (HL) can encourage the liver to take up and remove triglyceride-rich lipoprotein residues. The increased activity of these enzymes will stimulate the metabolism and transformation of lipids and reduce the level of blood lipids.
Kwak et al. (2014) found a reduction in serum total cholesterol (TC) levels in patients with hypercholesterolemia after taking garlic powder by Meta-analysis. Matsumoto et al. (2016) conducted a prospective randomized, double-blind trial of garlic extract for metabolic syndrome. They found that plaque reduction was significantly more significant in the AGE group than in a placebo group and that AGE also could stabilize vulnerable plaque. A meta-analysis of garlic modulation of serum TC conducted by Ried (2016) showed that garlic effectively reduced TC and Low-Density Lipoprotein Cholesterol (LDL-C) concentrations and increased macrophage activity and T and B cell production, with immunomodulatory effects.
Garlic oil could reduce TC and Triglyceride (TG) concentrations in hyperlipidemic mice, probably by promoting the metabolism and conversion of lipoproteins, or inhibiting intestinal cholesterol absorption, slowing down hepatic cholesterol synthesis, and thus accelerating TG breakdown (Zhang et al., 2007; Meng et al., 2016). The expression of Scavenger receptor class A (SR-A) and the cluster of differentiation 36 (CD36) directly affects the lipid deposition in macrophages and has an essential impact on macrophage foaminess (Twigg et al., 2012). C-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38 MAPK) of mitogen-activated protein kinase (MAPK) pathway are critical signal pathways for regulating scavenger receptors and AS (Nikolic et al., 2011). JNK has been reported to regulate AS by regulating scavenger receptor expression, and JNK knockdown macrophages inhibit lipid uptake and foam formation (Sumara et al., 2005; de Nigris et al., 2012). Allicin could significantly inhibit the activation of JNK and p38 MAPK and down-regulate the increase of SR-A and CD36 expression, suggesting that allicin affects macrophage foam formation by regulating the expression of scavenger receptors (Wang L et al., 2017). Allicin promotes cholesterol efflux through upregulation of ATP Binding Cassette Subfamily A Member 1 (ABCA1). It reduces lipid accumulation in THP-1 macrophage-derived foam cells by activating Peroxisome proliferator-activated receptor Gamma (PPARγ)/Liver X receptor α (LXRα) signaling (Lin et al., 2017).
Inhibition of cholesterol synthesis by S-allyl cysteine (SAC), S-ethyl cysteine (SEC), and S-propyl cysteine (SPC) was achieved by inhibiting the activity of hydroxy-3-methyl glutaryl coenzyme A reductase (HMGCR) (Liu and Yeh, 2002). N-acetylcysteine (NAC), SEC, and SPC reduce TG and TC accumulation in the liver by a mechanism associated with the reduction of sterol regulatory element-binding protein-1c (SREBP-1c), sterol regulatory element-binding protein-2 (SREBP-2) (Lin and Yin, 2008). DADS inhibits hepatic TG and relates lipid synthesis by downregulating SREBP-1c expression. In addition, DADS can accelerate TG catabolism and fatty acid oxidative metabolism by upregulating the expression of peroxisome proliferator-activated receptor α (PPARα) in hepatocytes (Wang et al., 2019). SAC activates AMP-activated protein kinase (AMPK) through calcium/calmodulin-dependent kinase (CaMKK), silent information regulator T1, and inhibits sterol regulatory element-binding proteins-1 (SREBP-1)-mediated hepatic adipogenesis (Hwang et al., 2013). Allicin was found to enhance the activity of serum LCAT, LPL, and HL, thereby reducing plasma TG and TC levels (Zhang et al., 2007).
Microsomal triglyceride transfer protein (MTP) is the rate-limiting factor for VLDL apoB secretion. MTP plays an essential role in regulating lipoprotein production in the liver and intestine. Inhibition of MTP activity has been shown to decrease the rate of secretion of apoB-containing lipoproteins in HepG2 cells (Jamil et al., 1996) and intestinal Caco-2 cells (van Greevenbroek et al., 1998) in vitro. Fresh garlic extract (FGE) inhibits the synthesis and secretion of intestinal chylomicrons by suppressing the expression of microsomal MTP genes for lipid-lowering (Lin et al., 2002). Acyl-CoA: cholesterol acyltransferase (ACAT) is the rate-limiting enzyme that catalyzes the formation of cholesteryl esters by linking cholesterol with long-chain fatty acids (Yao et al., 2006). Garlic powder extract (GPE) was able to inhibit the activity of ACAT and enhance the activity of Cholesteryl ester hydrolase (CEH) (Orekhov and Tertov, 1997), thus significantly reducing the accumulation of intracellular cholesteryl esters.
Oxidative stress (OS) is mainly caused by the increase of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which can lead to endothelial remodeling, tissue damage, and eventually AS (Canugovi et al., 2019). In endothelial cells (ECs), vascular smooth muscle cells (VSMCs), and macrophages, mitochondrial dysfunction and nicotinamide adenine dinucleotide phosphate oxidase (NOX) can cause excessive ROS production in vivo and accelerate the development of AS by causing the impaired function of ECs, proliferation, and migration of VSMCs, macrophage foaminess and inflammatory response through various pathways.
A meta-analysis shows that garlic supplementation improves antioxidant status by increasing total antioxidant capacity (T-AOC) and decreasing Malondialdehyde (MDA) levels (Moosavian et al., 2020). SAC inhibits Oxidized Low-Density Lipoprotein (ox-LDL) (Ide and Lau, 2001), inducible nitric oxide synthase (iNOS) activity (Kim et al., 2001), peroxides production, and prevents intracellular glutathione (GSH) depletion in ECs. In addition, it has been shown that garlic polysaccharides can reduce MDA, Nitric Oxide (NO) levels in serum and inhibit lipid peroxidation in mice (Li et al., 2003). DADS activates the Kelch Like ECH Associated Protein 1 (Keap1)/nuclear erythroid 2-related factor 2 (Nrf2) pathway and upregulates NAD. (P)H: quinone oxidoreductase 1 (NQO1), γ-glutamylcysteine synthetase antibody (γ-GCSc), and Superoxide Dismutase 1 (SOD-1) expression levels, and improves hepatic oxidative stress levels in high-fat-fed Wistar rats (Wang et al., 2019). The up-regulation of Sirtuin 1 (SIRT1) activity can reduce ROS production in endothelial cells, thus protecting vascular endothelial cells from ROS damage (Wu et al., 2014). Allicin can promote the phosphorylation of SIRT1, upregulate the activity of SIRT1, and reduce the production of ROS and the expression of plasma activator inhibitor-1 (PAI-1) in cells (Suo et al., 2013; Hu et al., 2016). Garlic sulfide can regulate mitochondrial respiration in cardiac myocytes and produce hydrogen sulfide via myocardial mitochondria, which can diastole contracted vascular smooth muscle (Li et al., 2016) and play the anti-myocardial ischemic effect. Total saponins of garlic (TSG) exerted antioxidant effects by inhibiting MDA content and restoring reduced superoxide dismutase (SOD) activity (Miao et al., 2020). Activation of ox-LDL and nuclear factor kappa-B (NF-κB) is associated with AS, and SAC inhibits ox-LDL activation in macrophages and human umbilical vein endothelial cells (HUVECs) of J774 mice with dose-dependent inhibition of NF-κB activation. It indicated that SAC could slow the progression of AS by inhibiting ox-LDL and interfering with the cascade of oxidative signaling (Ho et al., 2001). Treatment of New Zealand rabbits receiving a high-cholesterol diet with allicin significantly reduces MDA while increasing GSH and SOD levels (El-Sheakh et al., 2016). The antiatherogenic effect of allicin may be related to its ability to scavenge free radicals and restore antioxidant defense systems.
During plaque formation, changes in hemodynamics increase plaque instability and can easily lead to plaque rupture. Once the plaque ruptures, platelet activation in the blood, activation of the coagulation pathway cascade, and multicellular mobilization lead to platelet-rich emboli that block blood vessels and cause malignant clinical events such as ischemic myocardial infarction and stroke (Kobiyama and Ley, 2018). Antithrombotic therapy is an essential part of the treatment of patients with AS. Activated platelets mediate plaque instability involved in chronic AS development by releasing large amounts of inflammatory secretions and expressing multiple membrane immune receptors interacting with different leukocyte subpopulations and endothelial cells (Siegel-Axel et al., 2008; Peter Seizer, 2008; Li et al., 2011).
Possible mechanisms for the antithrombotic activity of garlic include: inhibiting the secretion of Cyclooxygenase-1 (COX1), reducing the synthesis of Thromboxane B2 (TXB2), reducing the secretion of Leukotriene C4 (LTC4C4C) and Prostaglandin E2 (PGE2) (Bordia et al., 1996), reducing the release of arachidonic acid (AA) (Mohammad and Woodward, 1986) from phospholipids, upregulating 5-hydroxy tryptamine (5-HT) and inhibiting the release of coagulation factor IV from platelets (Makheja and Bailey, 1990). Garlic effectively inhibits platelet aggregation induced by the calcium ion aggregate A23187. Therefore, the antiplatelet aggregation effect of garlic may be related to the mobilization of calcium within platelets (Srivastava, 1986).
A double-blind placebo-controlled randomized study showed that treating patients with cerebral atherosclerosis with garlic powder pills (Allicor) for 14 days resulted in a 25% reduction in ADP-induced platelet aggregation and a 22% upregulation in plasma fibrinolytic activity (Sobenin et al., 2010). However, a randomized, double-blind placebo-controlled crossover study showed that garlic oil caused a significant 12% reduction in epinephrine-induced platelet aggregation but had no effect on collagen-induced or ADP-induced platelet aggregation (Wojcikowski et al., 2007). This contradiction suggests that garlic may inhibit platelet aggregation through multiple mechanisms. Clinical studies have confirmed that processed garlic improves the potency and bioavailability of organosulfides and is more likely to exert antithrombotic effects than raw garlic (Lawson and Gardner, 2005). A randomized controlled trial confirmed that garlic oil significantly increased fibrinolytic activity. The standard components of garlic oil, DADS and DATS, inhibited platelet agonist-induced platelet aggregation (PAg) and platelet thromboxane formation (Bordia et al., 1998).
TXB2 and prostaglandin I2 (PGI2) are metabolites of AA. Thromboxane A2 (TXA2) induces vasoconstriction and platelet aggregation and promotes AS formation. In contrast, PGI2 has the function of vasodilation and inhibiting platelet aggregation. The TXA2 and PGI2 are usually determined by measuring their stable metabolites TXB2 and 6-keto-prostaglandin F1α (6-keto-PGF1α). Total saponins of garlic (TSG) reduces the level of TXB2 and increases the level of 6-keto-PGF1α, and the ratio of TXB2 to 6-keto-PGF1α is maintained in a relatively stable dynamic equilibrium to maintain vascular homeostasis (Miao et al., 2020). α-Granules and dense granules enhanced the conduction pathway of platelet activation. A rapid release of ATP characterizes the early phase of platelet activation. Fermented and non-fermented garlic products inhibit ATP release from dense granules and exert antiplatelet effects by inhibiting platelet granule secretion (Irfan et al., 2019). Adhesion molecules such as Vascular Cell Adhesion Molecule 1 (VCAM-1) and intercellular cell adhesion molecule-1 (ICAM-1) promote platelet adhesion and leukocyte recruitment and play an essential role in AS formation (Kim et al., 2018). Garlic powder decreased VCAM-1and ICAM-1 level, significantly increasing activated partial thromboplasting time (APTT). It indicated that garlic might contribute to treating AS by delaying clotting time, altering angiotensin, and decreasing the expression of VCAM-1 and ICAM-1.
Tissue factor (TF) is a crucial factor in initiating the extrinsic coagulation cascade pathway, which is responsible for producing thrombin from prothrombin via activation of factor VII (Dahlbäck, 2000). Subendothelial TF is also accountable for initiating fibrin formation at sites of vascular injury, and blood-borne TF may be a vital contributor to the propagation of the developing thrombus (Grover and Mackman, 2020). It has been reported that tumor necrosis factor-α (TNF-α) induced TF mRNA expression in HUVECs was suppressed by the inhibition of JNK. Therefore, inhibition of the JNK pathway by DATS may inhibit the induction of TF by TNF-α. DATS inhibited not only TF activity but also TF mRNA and protein expression in vitro (Okue et al., 2022). Garlic is a promising food with anti-thrombotic function, which can suppress both primary and secondary clot formation. The antithrombotic effect of garlic is beneficial for patients who are allergic or intolerant to aspirin and is expected to be an alternative or complementary therapy to antiplatelet therapy.
Plaque angiogenesis is considered to play an essential role in the pathophysiological development of AS. Neointimal angiogenesis is highly related to plaque formation and the risk of plaque rupture. Plaque angiogenesis quickly leads to the formation of plaque and thus increasing the risk of rupture (Sluimer and Daemen, 2009). In the past 30 years, the research mainly focused on the role of new blood vessels in plaque formation and rupture (Kockx et al., 2003; Sluimer et al., 2009), which revealed that there was an expanding network of new blood vessels in plaque in the stenosis near the inflammatory infiltration and necrotic core. Plaque angiogenesis is related to plaque vulnerability and plaque erosion. Many angiogenic factors participate in plaque formation, mainly vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF).
Akt activation is associated with angiogenesis (Morales-Ruiz et al., 2000). In vitro and in vitro experiments proved that Allicin can inhibit angiogenesis and weaken epithelial cell proliferation, tubule formation, actin polymerization, and Akt phosphorylation by reducing VEGF and bFGF expression (Sela et al., 2008). DATS is an effective inhibitor of the angiogenic properties of HUVECs in vitro, for example, inhabiting capillary tube formation and migration. It is related to caspase-dependent induction of apoptosis, inhibition of VEGF secretion, inactivation of vascular endothelial growth factor receptor 2 (VEGF-R2) and Akt kinase, and activation of extracellular regulated protein kinases (ERK1/2) (Xiao et al., 2006).
ECs dysfunction is a crucial factor in the development of AS (Donato et al., 2018). The dysfunction of ECs refers to a decrease in NO-mediated vasodilatory responses in ECs in response to different pathological stimuli. The excessive synthesis of Endothelin-1 (ET1) leads to an increase in vasoconstriction and vascular permeability. This change can lead to the release of pro-inflammatory factors, over-activation of platelets, enhanced oxidation of LDL, and proliferation and migration of vascular smooth muscle cells (Gimbrone and García-Cardeña, 2016; Pi et al., 2018).
Clinical studies have found that allicin reduces ET-1 and C-reaction protein (CRP) levels and elevates NO levels, improves endothelial dysfunction, and reduces the incidence of restenosis in patients after PCI (Wang and Fu, 2009). In patients with coronary artery disease combined with diabetes, oral administration of allicin capsules resulted in a significant improvement in flow-mediated dilation (FMD) and NO levels, and a decline in ICAM-1 level and incidence of major adverse cardiovascular events in the allicin group compared to the control group, which may be related to allicin’s improvement in endothelial function (Nie et al., 2013). A randomized, placebo-controlled, crossover trial suggested that short-term treatment with AGE may improve endothelial function in patients with coronary artery heart disease (CHD) (Williams et al., 2005). Another randomized, double-blind, placebo-controlled trial found that AGE supplementation was beneficial in reducing endothelial biomarkers associated with cardiovascular risks, such as the arterial stiffness index (SI), high-sensitivity C-reactive protein (hsCRP), PAI-1 as well as total antioxidant status (TAS) (Szulińska et al., 2018).
The adhesion of leukocytes/monocytes to endothelium is an early event of AS. Garlic extracts significantly reduce the expression of ICAM-1 and VCAM-1 induced by Interleukin-1 alpha (IL-1a) and considerably inhibit the adhesion of monocytes to endothelial cells stimulated by IL-1a (Rassoul et al., 2006). l-arginine in AGE promotes NO production mediated by Endothelin nitric oxide synthase (eNOS), leading to vasodilation (Nie et al., 2002; Takashima et al., 2017). Oral DADS analogs can reverse L-N G-Nitro arginine methyl ester (l-NAME)-induced systolic blood pressure, oxidative stress, Angiotensin Converting Enzyme (ACE) activity, cyclic guanosine monophosphate (cGMP), and NO levels, which may be related to activation of eNOS (Williams et al., 2005). DAT significantly reduced the levels of MDA and ROS in mitochondria and increased the activities of SOD and Glutathione peroxidase (GSH-Px). DAT protects the vascular endothelium from hyperglycemia-induced damage by reducing oxidative stress in mitochondria (Liu et al., 2014). The calcium-sensing receptor (CaSR) is a member of the G protein-coupled receptor superfamily, and activation of CaSR reduces cell viability and promotes apoptosis (Zhang et al., 2020). Allicin may inhibit cardiomyocyte apoptosis and protect vascular endothelial function by suppressing the expression of CaSR and inhibiting the oxidative stress response (Xu et al., 2020). This suggests that garlic consumption may reduce oxidative damage to endothelial cells and improve vascular function.
Vascular endothelial barrier function is maintained by a cell-to-cell junctional proteins and contributes to vascular homeostasis (Chistiakov et al., 2015). Various risk factors such as inflammation disrupt barrier function through down-regulation of these proteins and promote vascular diseases such as atherosclerosis (Hofmann et al., 2002).
AGE and its primary sulfur-containing constituent, S-1-propenylcysteine (S1PC), reduced hyperpermeability elicited by TNF-α in HUVECs. In addition, S1PC inhibited TNF-α-induced production of myosin light chain (MLC) kinase and inactivation of MLC phosphatase through the suppression of the Rac and Ras homolog gene family, member A (RhoA) signaling pathways, respectively, which resulted in the dephosphorylation of MLC2, a key factor of actin remodeling. Moreover, S1PC inhibited the phosphorylation and activation of guanine nucleotide exchange factor-H1 (GEF-H1), a common upstream key molecule and activator of Rac and RhoA. These effects of S1PC were accompanied by its ability to prevent the disruption of junctional proteins in the cell-cell contact regions and the increase of actin stress fibers induced by TNF-α (Kunimura et al., 2021). The study suggested that AGE and S1PC improve endothelial barrier disruption by inhibiting the GEF-H1/RhoA/Rac pathway.
It is generally known that AS is considered a chronic inflammatory disease because inflammation goes through all AS processes and plays an essential role. When ECs are activated, Inflammatory factors such as monocyte chemotactic protein 1 (MCP-1), interleukin-8 (IL-8), ICAM-1, VCAM-1, Endothelial leukocyte adhesion molecule-1 (ELAM-1), and granular membrane protein 140 (GMP140) attract lymphocytes and monocytes bound to ECs and arterial walls, contributing to inflammation (Zhu et al., 2018). In addition, monocytes differentiate into macrophages which can phagocytize ox-LDL and ultimately transform it into lipid-laden foam cells (Kattoor et al., 2019; Javadifar et al., 2021). During arterial endothelium damage, foam cells form and accumulate and release inflammatory mediators such as MCP-1 and TNF-α (Javadifar et al., 2021). The activation of the NF-κB signaling pathway stimulates the formation of the inflammatory process, which leads ECs to take on AS phenotypes in the carotid sinus (Tabas et al., 2015). In a randomized, double-blind placebo-controlled trial by Martiné Wlosinska et al., 104 AS patients took 2400 mg AGE capsules daily for 12 months. The results showed that AGE could effectively decrease levels of IL-6 (Wlosinska et al., 2020). AGE’s anti-atherosclerosis effects include reducing CRP and TXB2, down-regulating TNF-α and interleukin-1 receptor-activated kinase 4 (IRAK4) productions, and increasing AMPK activity in the liver (Morihara et al., 2017). AGE also regulates the inflammatory process by inducing AMPK activation and down-regulating the Toll-like receptor (TLR) signaling pathway (Miki et al., 2017). Z-Ajone reduces the phosphorylation and nuclear translocation of STAT3, inhibits the activity of Cyclooxygenase-2 (COX2) (Hitchcock et al., 2021), and carries out S-sulfhydrylation of cysteine sulfhydryl groups in two inflammatory proteins, thus producing downstream anti-inflammatory effects.
The innate and adaptive immunity cells play a significant role in atherosclerosis progression. Dynamic change in blood lipid levels could trigger CD4+ T-cells to differentiate into effector cells and produce cytokine during atherogenesis (Tabas and Lichtman, 2017). T helper 1 cell (Th1) is the subset of T lymphocytes mostly found in atherosclerotic lesions based on the cytokine it produces. Th1 cells secrete Interferon-gamma (IFN-g) and TNF-a proinflammatory cytokines to enhance immune response through macrophage activation, smooth muscle cells, and endothelial cells during atherogenesis (Wu et al., 2017). A study confirmed that Single garlic oil could suppress CD4 t-cells activation and NF-κB expression in high-fat diet mice. Furthermore, Single garlic oil plays a role as an athero-protective agent in the High-fat diet condition through the decrease in proinflammatory cytokines such as TNF-a and IFN-g (Lestari et al., 2020). SGO could act as a promising prospect for therapy to improve chronic inflammation in AS.
It has been reported that the development of atherosclerosis alters the ratio of polarized macrophages. M1 macrophages promote the formation of AS plaques by sustaining inflammation, whereas M2 macrophages aid the regression of atherosclerotic (Yang et al., 2020). AGE increased the mRNA or protein levels of arginase1 (Arg1), interleukin-10 (IL-10), CD206, and hypoxia-inducible factor 2α (HIF2α). It decreased that of CD68, HIF1α, and inducible NO synthase in the aorta and spleen of Apo E−/− mice. S1PC increased the level of IL-10-induced Arg1 mRNA and the extent of M2c-like macrophage polarization in vitro. In addition, S1PC increased the population of M2c-like macrophages, suppressing the people of M1-like macrophages and decreasing lipopolysaccharide-induced production of pro-inflammatory cytokines. These effects were accompanied by prolonged phosphorylation of the IL-10 receptor α (IL-10Rα) and signal transducer and activator of transcription 3 (STAT3) that inhibited the interaction between IL-10Rα and Src homology-2-containing inositol 5′-phosphatase 1 (SHIP1) (Miki et al., 2021). These findings suggest that S1PC may help improve atherosclerosis due to its anti-inflammatory effect in promoting IL-10-induced M2c macrophage polarization.
Cell proliferation and apoptosis rates are key indicators of cell viability and apoptosis, further aggravating atherosclerotic plaque’s progression and instability. Allicin significantly increased the cell viability of HUVECs, inhibited apoptosis, and protected against ox-LDL-induced damage in HUVECs by inhibiting caspase-3 and Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-related apoptotic signaling pathways (Chen et al., 2016).
H2O2 can cause apoptosis in vascular ECs through multiple pathways and is recognized as a standard model of oxidative injury. Allicin effectively reduced H2O2-induced apoptosis in HUVECs, probably because allicin stabilized the expression of pro-Caspase-3 protein and decreased the expression of poly adenosine diphosphate-ribose polymerase (PARP) and BCL2-Associated X (Bax) proteins. Allicin can increase SOD, NO, and eNOS and decrease MDA, indicating that allicin can protect HUVECs induced by H2O2 from apoptosis by reducing oxidative stress (Chen et al., 2014).
Allicin can attenuate apoptosis induced by Lipopolysaccharide (LPS), and its mechanism is related to inhibition of mitochondrial dysfunction, such as inhibition of matrix metalloproteinases (MMPs) collapse, reduction of cytochrome c synthesis, and mitochondrial ATP release (Zhang et al., 2017). Cardiomyocyte apoptosis plays a vital role in the development of AS. High cholesterol diet-induced apoptosis in cardiomyocytes is associated with Fatty acid synthase (Fas)-dependent and mitochondria-dependent apoptotic pathway activity. Mitochondrial-dependent pathway plays an essential role in cell apoptosis by releasing caspase 9. Garlic activates the PI3K-Akt pathway, inhibits TNF-α, Fas, caspase 8, caspase 9, and caspase 3, upregulates the protein level of mitochondrial B-cell lymphoma-2 (Bcl-2), and reduces the protein levels of recombinant human bh3-interacting domain death agonist (B.I.D.) and Bax (Cheng et al., 2013), thus inhibiting myocardial cell apoptosis.
In addition to the above mechanisms, garlic and its extracts can also inhibit the development of AS by inhibiting the proliferation of vascular smooth muscle, regulating gut microbiota and supplement with phytoestrogens.
An essential feature of AS is the transformation of quiescent or differentiated VSMCs into proliferating or dedifferentiated cells, leading to enhanced migration of VSMCs. Ajoene and MATS down-regulate the activities of protein farnesyltransferase (PFTase) and protein geranylgeranyltransferase type I (PGGTase-I), contributing to the inhibition of VSMCs proliferation (Ferri et al., 2003; Golovchenko et al., 2003). AGE reduced aortic fatty streaks and carotid intima-media thickness in cholesterol-fed New Zealand rabbits and acted by reducing tissue cholesterol accumulation and inhibiting smooth muscle proliferation. Thus, AGE may protect against the development of AS (Efendy et al., 1997).
AS is strongly associated with the gut microbiota and its metabolites. A study confirmed that certain beneficial and anti-inflammatory gut commensal bacteria, including Faecalibacterium prausnitzii and Akkermansia spp., were significantly enriched after the 1-week allicin intervention in High-TMAO patients (Panyod et al., 2022). In diet-induced obese (DIO) mice alliin regulates glucose metabolism by reducing Lachnospiraceae and increasing Ruminococcaceae in the intestine, thereby delaying the progression of AS (Zhai et al., 2018). Garlic may be an essential prebiotic, which can induce the growth of beneficial flora (The cardiovascular effects are shown in Table 1 and Figure 5).
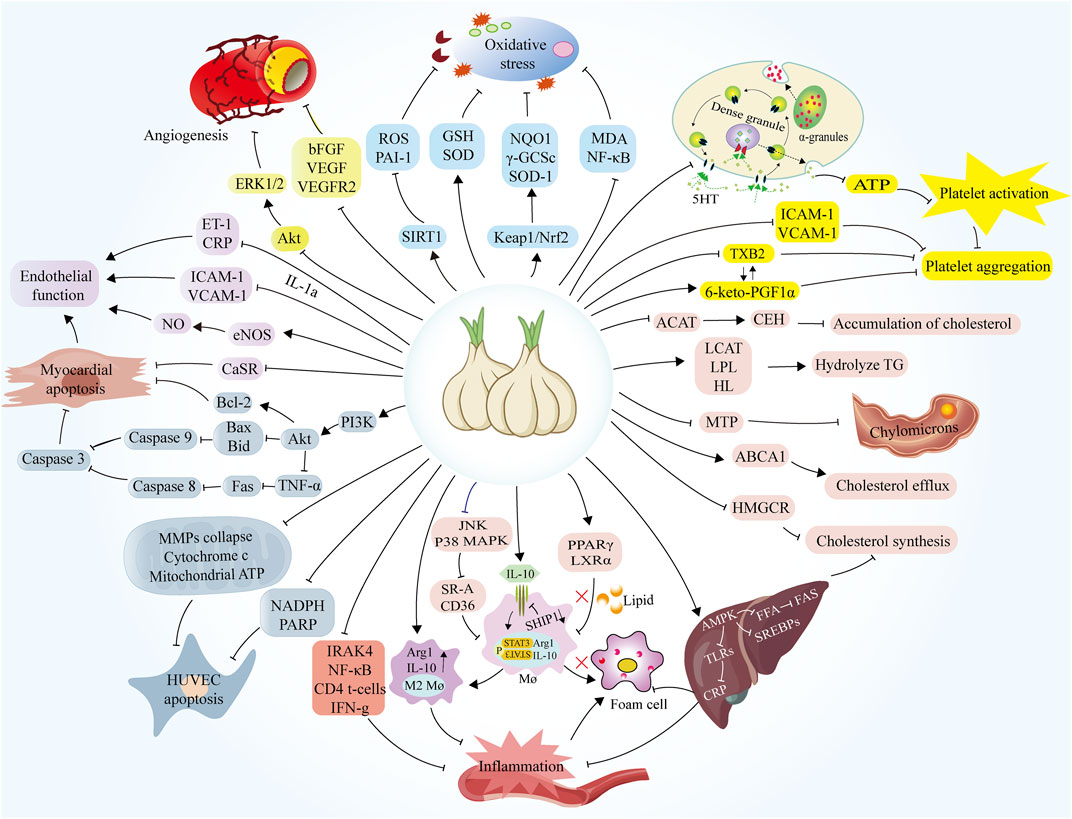
FIGURE 5. Mechanisms of the protective role of garlic in the treatment of atherosclerosis. ACAT, cholesterol acyltransferase; CEH, Cholesteryl ester hydrolase; LCAT, Lecithin cholesterol acyltransferase; LPL, Lipoprotein lipase; HL, Hepatic lipase; MTP, Microsomal triglyceride transfer protein; ABCA1, ATP Binding Cassette Subfamily A Member 1; HMGCR, Hydroxy-3-methyl glutaryl coenzyme A reductase; AMPK, AMP-activated protein kinase; FAS, Fatty acid synthase; SREBP, Sterol regulatory element-binding protein; TLR, Toll-like receptors; CRP, C-reaction protein; PPARγ, Peroxisome proliferator-activated receptor Gamma; LXRα, Liver X receptor α; IL-10, Interleukin-10; SHIP1, Src homology-2-containing inositol 5′-phosphatase 1; STAT3, Signal transducer and activator of transcription 3; Arg1, Arginase1; IRAK4, Interleukin-1 receptor-activated kinase 4; NF-κB, Nuclear factor kappa-B; IFN-g, Interferon gamma; NADPH, Nicotinamide adenine dinucleotide phosphate; PARP, Poly adenosine diphosphate-ribose polymerase; MMPs, Matrix metalloproteinases; TNF-α, Tumor necrosis factor-α; Fas, Fatty acid synthase; Bax, BCL2-Associated X; Bid, Recombinant Human BH3-Interacting Domain Death Agonist; Bcl-2, B-cell lymphoma-2; CaSR, Calcium-sensing receptor; eNOS, Endothelin nitric oxide synthase; ICAM-1, Intercellular cell adhesion molecule-1; VCAM-1, Vascular Cell Adhesion Molecule 1; ERK1/2, Extracellular regulated protein kinases; bFGF, Basic fibroblast growth factor; VEGF, Vascular endothelial growth factor; SIRT1, Sirtuin 1; ROS, Reactive oxygen species; PAI-1, Plasminogen Activator Inhibitor-1; GSH, Glutathione; SOD, Superoxide Dismutase; NQO1, NAD(P)H:quinone oxidoreductase 1; γ-GCSc, γ -glutamylcysteine synthetase antibody; SOD-1, Superoxide Dismutase 1; MDA, Malondialdehyde; TXB2, Thromboxane B2.
The risk of AS progression is significantly increased in postmenopausal women, semmingly retale to decline level of estrogen secretion. The use of the dietary supplement “Karinat”, which is isoflavonoid-rich preparation containing tannins from garlic powder and other herbs, was proved to decrease total cholesterol by 6.3% and lower carotid intima-media thickness progression in postmenopausal women (Myasoedova et al., 2016).
Few pharmacokinetic studies of garlic have been conducted, mainly focusing on alliin, SAC, and DATS. Three groups of rats received 8 mg/kg of alliin and allicin. The absorption and elimination of alliin radioactivity were significantly faster than other garlic components, reaching maximum blood levels within the first 10 min and almost eliminated from the blood after 6 h. Allicin did not reach maximum blood levels until 30–60 min and still existed at the end of the study after 72 h with blood levels >1000 ng-Eq/ml. The mean total urinary and fecal excretion of Allicin after 72 h was 85.5% of the dose, with urinary excretion indicating a minimum absorption rate of 65% (Perez et al., 2014). After injecting 10 mg DATS into the jugular vein of rats, the plasma concentration of DATS reached the peak of 31 μm within 1 min and gradually returned to the baseline level within 24 h. DATS was injected intravenously into rats with microemulsion, and the plasma concentration of DATS reached its peak within 3 h. Following the ingestion of DATS by human subjects, the breakdown product Allyl methyl sulfide (AMS) concentration increased to a peak at 5 h. Furthermore, following ingestion of raw garlic, AMS, allyl methyl disulfide (AMDS), DAS, DADS, DATS, and dimethyl sulfide were detected in the volunteers’ breath. The concentrations of AMDS, DAS, DADS, and DATS peaked within 2–3 h, while the concentrations of the other compounds increased more slowly (Morihara et al., 2017). Further pharmacokinetic studies of garlic are needed to determine its potential to treat AS.
Although garlic is generally considered safe for humans, it can still cause adverse reactions in sensitive individuals when ingested at high doses. A randomized controlled trial was conducted in which ingestion of high doses of raw garlic on an empty stomach caused changes in the intestinal flora, flatulence, and gastrointestinal disturbances to assess the safety of garlic (Rana et al., 2011). In addition, blisters, dermatitis, and burns can be observed by topical application of raw garlic (Piasek et al., 2009). In vivo experiments have shown that prolonged intake of high doses of raw garlic can lead to weight loss and hemolytic anemia. In addition, chronic administration of 50 mg of garlic powder per day produces anti-androgenic effects, leading to reduced sialic acid concentrations in the seminal vesicles, testes, and epididymis and reduced interstitial cell function (Rana et al., 2011). The primary toxicological mechanism of sulfide in garlic is oxidative hemolysis, which is characterized by methemoglobinemia and the formation of Heinz bodies. Early clinical signs include depression, vomiting, loss of appetite, abdominal pain, diarrhea, pale mucous membranes of the fundus, jaundice, increased heart and respiratory rates, weakness, and hemoglobinuria (Salgado et al., 2011). A low dose of garlic is safe, therapeutic dose may cause mild gastrointestinal disorder, while a high dose of garlic may cause liver damage (Rahman et al., 2016). The antithrombotic activity of garlic may interact with oral anticoagulants, so care must be taken when used in concert with oral anticoagulants (Rose et al., 1990). Allicin is a membrane-permeable compound that readily enters cells and interacts with sulfhydryl-containing compounds in cells, such as GSH or cysteine residues in proteins and enzymes containing active cysteine, resulting in cytotoxicity of allicin.
The quality standards of garlic or related species are included in the United States Pharmacopoeia, the European Pharmacopoeia, and the British Pharmacopoeia. The quality standards of garlic were included in the 1977 edition of the Chinese Pharmacopoeia. However, the quality standard for garlic was not found in subsequent editions of the Pharmacopoeia until it was reintroduced in the 2010 edition of the Chinese Pharmacopoeia. Since thiosulfinate and decomposition products are biologically active, and the primary precursor substance is alliin, the leading testing indexes for garlic and garlic-related species internationally are the content of alliin, the activity of allinase or the content of potential allicin. Other indicators are tested for garlic preparations prepared for various purposes and methods. The British, American, and European Pharmacopoeias all contain garlic or related preparations, with alliin or potential allicin as the leading indicator for product quality control. The United States Pharmacopoeia contains the most varieties of garlic, while the British and European Pharmacopoeia only contains garlic powder. Garlic oil is extracted from crushed garlic and includes only the fat-soluble sulfide DAS, DADS, DATS, etc., after the decomposition of allicin, but no water-soluble components and alliin. Garlic extract is extracted by organic solvent, which inhibits allinase activity, and the extract consists of fat-soluble sulfide and allicin without allicin. The preparation prepared by pulverizing garlic cloves into powder contains alliin and a small amount of fat-soluble sulfide (Harauma and Moriguchi, 2006; Corzo-Martínez et al., 2007). Freeze-dried garlic powder and its preparations made from garlic by low-temperature freeze-drying can produce allicin under suitable conditions in vivo due to the retention of alliin and active allinase. Therefore the European Pharmacopoeia and the United States Pharmacopoeia require determining the potential allicin content for the corresponding preparations and raw materials.
The antiatherogenic effects and mechanisms of garlic were discussed in this review, and it was thought that further research should be conducted in the future on the following aspects. Garlic contains multi-bioactive components, such as allicin, DAS, DADS, and DATS, among which allicin is the primary bioactive substance of garlic. DATS has been included in the Chinese Pharmacopoeia (Chinese Pharmacopoeia Commission, 2015), and alliin and allicin have been included in the European Pharmacopoeia (European Pharmacopoeia, 2017). The research progress of Allicin is restricted because of its unstable chemical properties and difficulties in preparation and storage. China has established a patented technology for preparing Allicin, which can extract more stable and higher purity allicin, making it possible to conduct in-depth research. Therefore, there is an urgent need to conduct pharmacological studies on garlic and its active ingredients in the future to clarify the actual active ingredients of garlic as soon as possible.
Recent studies have shown that whole garlic or its components/extracts exhibit multiple preventive and therapeutic effects on AS. The formation of AS is a multifactorial interaction, so it should be studied in depth from various pathways, links, and targets. The biological effects of garlic may meet the need for a multi-targeted therapeutic strategy for AS. However, some contradictory results may be related to the inconsistency between the quality, extraction, preparation, and dosage of garlic and the experimental objects and methods. Therefore, future research on garlic should be deepened in the above aspects. Garlic may exert antiatherogenic effects through hypolipidemic, antioxidant, antithrombotic, inhibiting angiogenesis, protecting endothelial cells, anti-inflammatory, anti-apoptotic, inhibiting vascular smooth muscle proliferation, and regulating gut microbiota. However, the potential mechanisms of absorption, distribution, metabolism, and excretion of garlic and its components/extracts and the synergistic or antagonistic effects between components are unknown, for which further studies should be conducted.
Garlic can treat AS by regulating different signaling pathways, such as AMPK/TLRs, Keap1/Nrf2, PI3K/AKT, PPARγ/LXRα, GEF-H1/RhoA/Rac, etc. However, there is still no molecular mechanism for clinical AS patients. Therefore, the direct AS protection mechanisms of garlic have not been explored. Further studies in animals and humans should evaluate the protective ability of single garlic-derived compounds against AS. Future studies should also focus on the beneficial effects of whole garlic and garlic-derived compounds on AS based on relevant signaling pathways. There have been few clinical trials to monitor garlic’s therapeutic effect in recent years. Therefore, there is an urgent need for large randomized, controlled, and double-blind trials to assess the efficacy and safety of garlic in the treatment of AS from the perspective of clinical practice. In addition, it will make the clinical application of garlic safer and more effective in solving the adverse reactions of garlic by inhibiting oxidized hemolysate and reducing the risk of bleeding.
ML and WY contributed equally to this manuscript. QH designed the study and was the corresponding author. GW and QH drafted the figure. JG and AL drafted the table. ML and WY drafted the initial full manuscript. All authors approved the final version of the manuscript.
This work was funded by the Beijing-Tianjin-Hebei Basic Research Cooperation Project (No. J200020).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.954938/full#supplementary-material
Almatroodi, S. A., Alsahli, M. A., Almatroudi, A., and Rahmani, A. H. (2019). Garlic and its active compounds: A potential candidate in the prevention of cancer by modulating various cell signalling pathways. Anticancer Agents Med. Chem. 19, 1314–1324. doi:10.2174/1871520619666190409100955
Arellano-Buendía, A. S., Castañeda-Lara, L. G., Loredo-Mendoza, M. L., García-Arroyo, F. E., Rojas-Morales, P., Argüello-García, R., et al. fnm (2020). Effects of allicin on pathophysiological mechanisms during the progression of nephropathy associated to diabetes. Antioxidants (Basel, Switz. 9, 1134. doi:10.3390/antiox9111134
Argüello-García, R., Medina-Campos, O. N., Pérez-Hernández, N., Pedraza-Chaverrí, J., and Ortega-Pierres, G. (2010). Hypochlorous acid scavenging activities of thioallyl compounds from garlic. J. Agric. Food Chem. 58, 11226–11233. doi:10.1021/jf102423w
Ariga, T., Oshiba, S., and Tamada, T. (1981). Platelet aggregation inhibitor in garlic. Lancet 1, 150–151. doi:10.1016/s0140-6736(81)90729-7
Barnes, P. M., Bloom, B., and Nahin, R. L. (2008). Complementary and alternative medicine use among adults and children: United States, 2007. Natl. Health Stat. Rep. 10, 1–23. doi:10.1037/e623942009-001
Bat-Chen, W., Golan, T., Peri, I., Ludmer, Z., and Schwartz, B. (2010). Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr. Cancer 62, 947–957. doi:10.1080/01635581.2010.509837
Block, E., Ahmad, S., Catalfamo, J. L., Jain, M. K., and Apitz-Castro, R. (1986). The chemistry of alkyl thiosulfinate esters. 9. Antithrombotic organosulfur compounds from garlic: Structural, mechanistic, and synthetic studies. J. Am. Chem. Soc. 108, 7045–7055. doi:10.1021/ja00282a033
Block, E. (1985). The chemistry of garlic and onions. Sci. Am. 252, 114–119. doi:10.1038/scientificamerican0385-114
Block, E. (1985). The chemistry of garlic and onions. Sci. Am. 252 (3), 114–119. doi:10.1038/scientificamerican0385-114
Bordia, A., Verma, S. K., and Srivastava, K. C. (1998). Effect of garlic (Allium sativum) on blood lipids, blood sugar, fibrinogen and fibrinolytic activity in patients with coronary artery disease. Prostagl. Leukot. Essent. Fat. Acids. 58, 257–263. doi:10.1016/s0952-3278(98)90034-5
Bordia, T., Mohammed, N., Thomson, M., and Ali, M. (1996). An evaluation of garlic and onion as antithrombotic agents. Prostagl. Leukot. Essent. Fat. Acids. 54, 183–186. doi:10.1016/s0952-3278(96)90014-9
Bouillaud, F., and Blachier, F. (2011). Mitochondria and sulfide: A very old story of poisoning, feeding, and signaling. Antioxid. Redox Signal. 15, 379–391. doi:10.1089/ars.2010.3678
Brandolini, V., Tedeschi, P., Cereti, E., Maietti, A., Barile, D., Coïsson, J. D., et al. (2005). Chemical and genomic combined approach applied to the characterization and identification of Italian Allium sativum L. J. Agric. Food Chem. 53, 678–683. doi:10.1021/jf0489623
Brown, D. G., Wilkerson, E. C., and Love, W. E. (2015). A review of traditional and novel oral anticoagulant and antiplatelet therapy for dermatologists and dermatologic surgeons. J. Am. Acad. Dermatol. 72, 524–534. doi:10.1016/j.jaad.2014.10.027
Canugovi, C., Stevenson, M. D., Vendrov, A. E., Hayami, T., Robidoux, J., Xiao, H., et al. (2019). Increased mitochondrial NADPH oxidase 4 (NOX4) expression in aging is a causative factor in aortic stiffening. Redox Biol. 26, 101288. doi:10.1016/j.redox.2019.101288
Cao, H., and Chen, J. (2008). Study of extraction and purification of alliinase from garlic and its clinical application. Proceeding Clin. Med. 17, 83–86. doi:10.3969/j.issn.1671-8631.2008.02.001
Cavallito, C. J., and Bailey, J. H. (1944). Allicin, the antibacterial principle of allium sativum. I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 66, 1950–1951. doi:10.1021/ja01239a048
Chen, S., Tang, Y., Qian, Y., Chen, R., Zhang, L., Wo, L., et al. (2014). Allicin prevents H₂O₂-induced apoptosis of HUVECs by inhibiting an oxidative stress pathway. BMC Complement. Altern. Med. 14, 321. doi:10.1186/1472-6882-14-321
Chen, X., Pang, S., Lin, J., Xia, J., and Wang, Y. (2016). Allicin prevents oxidized low-density lipoprotein-induced endothelial cell injury by inhibiting apoptosis and oxidative stress pathway. BMC Complement. Altern. Med. 16, 133. doi:10.1186/s12906-016-1126-9
Cheng, Y. C., Chang, M. H., Tsai, C. C., Chen, T. S., Fan, C. C., Lin, C. C., et al. (2013). Garlic oil attenuates the cardiac apoptosis in hamster-fed with hypercholesterol diet. Food Chem. 136, 1296–1302. doi:10.1016/j.foodchem.2012.09.076
China Cardiovascular Health and Disease Report Writing Group (2021). China cardiovascular health and disease report 2020. Chin. Circulation J. 6, 885–889. doi:10.3969/j.issn.1007-5062.2021.09.001
Chinese Pharmacopoeia Commission (2015). Pharmacopoeia of the people's Republic of China. Beijing: China Medical Science Press. Part I.
Chistiakov, D. A., Orekhov, A. N., and Bobryshev, Y. V. (2015). Endothelial barrier and its abnormalities in cardiovascular disease. Front. Physiol. 6, 365. doi:10.3389/fphys.2015.00365
Corzo-Martínez, M., Corzo, N., and Villamiel, M. (2007). Biological properties of onions and garlic. Trends Food Sci. Technol. 18, 609–625. doi:10.1016/j.tifs.2007.07.011
de Nigris, F., Rienzo, M., Sessa, M., Infante, T., Cesario, E., Ignarro, L. J., et al. (2012). Glycoxydation promotes vascular damage via MAPK-ERK/JNK pathways. J. Cell. Physiol. 227, 3639–3647. doi:10.1002/jcp.24070
Donato, A. J., Machin, D. R., and Lesniewski, L. A. (2018). Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ. Res. 123, 825–848. doi:10.1161/CIRCRESAHA.118.312563
Efendy, J. L., Simmons, D. L., Campbell, G. R., and Campbell, J. H. (1997). The effect of the aged garlic extract, 'Kyolic', on the development of experimental atherosclerosis. Atherosclerosis 132, 37–42. doi:10.1016/s0021-9150(97)00078-6
El-Sheakh, A. R., Ghoneim, H. A., Suddek, G. M., and Ammar, E. (2016). Attenuation of oxidative stress, inflammation, and endothelial dysfunction in hypercholesterolemic rabbits by allicin. Can. J. Physiol. Pharmacol. 94, 216–224. doi:10.1139/cjpp-2015-0267
Elkayam, A., Peleg, E., Grossman, E., Shabtay, Z., and Sharabi, Y. (2013). Effects of allicin on cardiovascular risk factors in spontaneously hypertensive rats. Israel Med. Assoc. J. IMAJ. 15, 170–173. doi:10.5152/balkanmedj.2012.115
Elosta, A., Slevin, M., Rahman, K., and Ahmed, N. (2017). Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro. Sci. Rep. 7, 39613. doi:10.1038/srep39613
European Pharmacopoeia (2017). Europäisches arzneibuch vol. 9.0. (Stuttgart, Germany: Deutscher Apotheker Verlag), 2359–2360.
Ferri, N., Yokoyama, K., Sadilek, M., Paoletti, R., Apitz-Castro, R., Gelb, M. H., et al. (2003). Ajoene, a garlic compound, inhibits protein prenylation and arterial smooth muscle cell proliferation. Br. J. Pharmacol. 138, 811–818. doi:10.1038/sj.bjp.0705126
Frass, M., Strassl, R. P., Friehs, H., Müllner, M., Kundi, M., and Kaye, A. D. (2012). Use and acceptance of complementary and alternative medicine among the general population and medical personnel: A systematic review. Ochsner J. 12, 45–56.
Gardner, C. D., Lawson, L. D., Block, E., Chatterjee, L. M., Kiazand, A., Balise, R. R., et al. (2007). Effect of raw garlic vs commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia: A randomized clinical trial. Arch. Intern. Med. 167, 346–353. doi:10.1001/archinte.167.4.346
Gimbrone, M. A., and García-Cardeña, G. (2016). Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 118, 620–636. doi:10.1161/CIRCRESAHA.115.306301
Golovchenko, I., Yang, C. H., Goalstone, M. L., and Draznin, B. (2003). Garlic extract methylallyl thiosulfinate blocks insulin potentiation of platelet-derived growth factor-stimulated migration of vascular smooth muscle cells. Metab. Clin. Exp. 52, 254–259. doi:10.1053/meta.2003.50042
Grover, S. P., and Mackman, N. (2020). Tissue factor in atherosclerosis and atherothrombosis. Atherosclerosis 307, 80–86. doi:10.1016/j.atherosclerosis.2020.06.003
HALE Collaborators (2017). Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet 390, 1260–1344. doi:10.1016/S0140-6736(17)32130-X
Harauma, A., and Moriguchi, T. (2006). Aged garlic extract improves blood pressure in spontaneously hypertensive rats more safely than raw garlic. J. Nutr. 136, 769S–773S. doi:10.1093/jn/136.3.769S
Hitchcock, J. K., Mkwanazi, N., Barnett, C., Graham, L. M., Katz, A. A., Hunter, R., et al. (2021). The garlic compound Z-ajoene, S-thiolates COX2 and STAT3 and dampens the inflammatory response in RAW264.7 macrophages. Mol. Nutr. Food Res. 65, e2000854. doi:10.1002/mnfr.202000854
Ho, S. E., Ide, N., and Lau, B. H. (2001). S-allyl cysteine reduces oxidant load in cells involved in the atherogenic process. Phytomedicine 8, 39–46. doi:10.1078/0944-7113-00005
Hofmann, S., Grasberger, H., Jung, P., Bidlingmaier, M., Vlotides, J., Janssen, O. E., et al. (2002). The tumour necrosis factor-alpha induced vascular permeability is associated with a reduction of VE-cadherin expression. Eur. J. Med. Res. 7, 171–176.
Hong, J. Y., Wang, Z. Y., Smith, T. J., Zhou, S., Shi, S., Pan, J., et al. (1992). Inhibitory effects of diallyl sulfide on the metabolism and tumorigenicity of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A/J mouse lung. Carcinogenesis 13, 901–904. doi:10.1093/carcin/13.5.901
Hu, B., Kuang, H., Xin, Y., Wang, Y., Wang, Z., Jiang, H., et al. (2019). Lipid-lowering activity and mechanism of allii sativi bulbus. Chin. J. Exp. Traditional Med. Formulae 25, 181–186. doi:10.13422/j.cnki.syfjx.20190808
Hu, H., Pan, Y., Fan, X., Hu, X., Zou, W., and Lin, X. (2016). Allicin inhibits H_20_2-induced senescence in human umbilical vein endothelial cells through activation of sirt1. Chin. J. Biochem. Mol. Biol. 32, 536–543. doi:10.13865/j.cnki.cjbmb.2016.05.09
Hwang, Y. P., Kim, H. G., Choi, J. H., Do, M. T., Chung, Y. C., Jeong, T. C., et al. (2013). S-allyl cysteine attenuates free fatty acid-induced lipogenesis in human HepG2 cells through activation of the AMP-activated protein kinase-dependent pathway. J. Nutr. Biochem. 24, 1469–1478. doi:10.1016/j.jnutbio.2012.12.006
Ide, N., and Lau, B. H. (2001). Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-kappa b activation. J. Nutr. 131, 1020S–6S. doi:10.1093/jn/131.3.1020S
Ilić, D. P., Stojanović, S., Najman, S., Nikolić, V. D., Stanojević, L. P., Tačić, A., et al. (2015). Biological evaluation of synthesized allicin and its transformation products obtained by microwaves in methanol: Antioxidant activity and effect on cell growth. Biotechnol. Biotechnol. Equip. 29, 189–194. doi:10.1080/13102818.2014.994267
Irfan, M., Kim, M., Kim, K. S., Kim, T. H., Kim, S. D., Hong, S. B., et al. (2019). Fermented garlic ameliorates hypercholesterolemia and inhibits platelet activation. Evid. Based Complement. Altern. Med. 2019, 3030967. doi:10.1155/2019/3030967
Jamil, H., Gordon, D. A., Eustice, D. C., Brooks, C. M., Dickson, J. K., Chen, Y., et al. (1996). An inhibitor of the microsomal triglyceride transfer protein inhibits apoB secretion from HepG2 cells. Proc. Natl. Acad. Sci. U. S. A. 93, 11991–11995. doi:10.1073/pnas.93.21.11991
Javadifar, A., Rastgoo, S., Banach, M., Jamialahmadi, T., Johnston, T. P., and Sahebkar, A. (2021). Foam cells as therapeutic targets in atherosclerosis with a focus on the regulatory roles of non-coding RNAs. Int. J. Mol. Sci. 22, 2529. doi:10.3390/ijms22052529
Kamenetsky, R., Shafir, I. L., Khassanov, F., Kik, C., Heusden, A., Ginkel, V. V., et al. (2005). Diversity in fertility potential and organo-sulphur compounds among garlics from Central Asia. Biodivers. Conservation 14, 281–295. doi:10.1007/s10531-004-5050-9
Kattoor, A. J., Kanuri, S. H., and Mehta, J. L. (2019). Role of ox-LDL and LOX-1 in atherogenesis. Curr. Med. Chem. 26, 1693–1700. doi:10.2174/0929867325666180508100950
Kendler, B. S. (1987). Garlic (allium sativum) and onion (Allium cepa): A review of their relationship to cardiovascular disease. Prev. Med. 16, 670–685. doi:10.1016/0091-7435(87)90050-8
Kim, K. M., Chun, S. B., Koo, M. S., Choi, W. J., Kim, T. W., Kwon, Y. G., et al. (2001). Differential regulation of NO availability from macrophages and endothelial cells by the garlic component S-allyl cysteine. Free Radic. Biol. Med. 30, 747–756. doi:10.1016/s0891-5849(01)00460-9
Kim, L., Lim, Y., Park, S. Y., Kim, Y. J., Kwon, O., Lee, J. H., et al. (2018). A comparative study of the antithrombotic effect through activated endothelium of garlic powder and tomato extracts using a rodent model of collagen and epinephrine induced thrombosis. Food Sci. Biotechnol. 27, 1513–1518. doi:10.1007/s10068-018-0469-z
Kobiyama, K., and Ley, K. (2018). Atherosclerosis: A chronic inflammatory disease with an autoimmune. Compon. Circ. Res. 123, 1118–1120. doi:10.1161/CIRCRESAHA.118.313816
Kockx, M. M., Cromheeke, K. M., Knaapen, M. W., Bosmans, J. M., De Meyer, G. R., Herman, A. G., et al. (2003). Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23, 440–446. doi:10.1161/01.ATV.0000057807.28754.7F
Kunimura, K., Miki, S., Takashima, M., and Suzuki, J. I. (2021). S-1-propenylcysteine improves TNF-α-induced vascular endothelial barrier dysfunction by suppressing the GEF-H1/RhoA/Rac pathway. Cell Commun. Signal 19, 17. doi:10.1186/s12964-020-00692-w
Kwak, J. S., Kim, J. Y., Paek, J. E., Lee, Y. J., Kim, H. R., Park, D. S., et al. (2014). Garlic powder intake and cardiovascular risk factors: A meta-analysis of randomized controlled clinical trials. Nutr. Res. Pract. 8, 644–654. doi:10.4162/nrp.2014.8.6.644
Lanzotti, V. (2006). The analysis of onion and garlic. J. Chromatogr. A 1112, 3–22. doi:10.1016/j.chroma.2005.12.016
Lawson, L. D., and Gardner, C. D. (2005). Composition, stability, and bioavailability of garlic products used in a clinical trial. J. Agric. Food Chem. 53, 6254–6261. doi:10.1021/jf050536+
Lawson, L. D., Wang, Z. J., and Hughes, B. G. (1991). Identification and HPLC quantitation of the sulfides and dialk(en)yl thiosulfinates in commercial garlic products. Planta Med. 57, 363–370. doi:10.1055/s-2006-960119
Lee, H. H., Han, M. H., Hwang, H. J., Kim, G. Y., Moon, S. K., Hyun, J. W., et al. (2015). Diallyl trisulfide exerts anti-inflammatory effects in lipopolysaccharide-stimulated RAW 264.7 macrophages by suppressing the Toll-like receptor 4/nuclear factor-κB pathway. Int. J. Mol. Med. 35, 487–495. doi:10.3892/ijmm.2014.2036
Lestari, S. R., Atho'illah, M. F., Christina, Y. I., and Rifa'i, M. (2020). Single garlic oil modulates T cells activation and proinflammatory cytokine in mice with high fat diet. J. Ayurveda Integr. Med. 11, 414–420. doi:10.1016/j.jaim.2020.06.009
Li, C., Cai, F., Wu, J., and Chen, J. (2003). Effect of Garlic polysaccharide on lipid peroxidation and nitric oxide in mice with viral myocarditis. Mod. J. Integr. Traditional Chin. West. Med. 12, 2636–2638. doi:10.3969/j.issn.1008-8849.2003.24.006
Li, F., Li, Q., Wu, S., and Tan, Z. (2017). Salting-out extraction of allicin from garlic (Allium sativum L.) based on ethanol/ammonium sulfate in laboratory and pilot scale. Food Chem. 217, 91–97. doi:10.1016/j.foodchem.2016.08.092
Li, F., Zhang, J., He, P., and Li, Y. (2016). Prospects for garlic sulfur compounds developing into hydrogen sulfide donor drugs. Traditional Chin. Drug Res. Clin. Pharmacol. 27, 588–592. doi:10.19378/j.issn.1003-9783.2016.04.025
Li, Z., Yang, F., Dunn, S., Gross, A. K., and Smyth, S. S. (2011). Platelets as immune mediators: Their role in host defense responses and sepsis. Thromb. Res. 127, 184–188. doi:10.1016/j.thromres.2010.10.010
Lin, C. C., and Yin, M. C. (2008). Effects of cysteine-containing compounds on biosynthesis of triacylglycerol and cholesterol and anti-oxidative protection in liver from mice consuming a high-fat diet. Br. J. Nutr. 99, 37–43. doi:10.1017/S0007114507793881
Lin, M. C., Wang, E. J., Lee, C., Chin, K. T., Liu, D., Chiu, J. F., et al. (2002). Garlic inhibits microsomal triglyceride transfer protein gene expression in human liver and intestinal cell lines and in rat intestine. J. Nutr. 132, 1165–1168. doi:10.1093/jn/132.6.1165
Lin, X. L., Hu, H. J., Liu, Y. B., Hu, X. M., Fan, X. J., Zou, W. W., et al. (2017). Allicin induces the upregulation of ABCA1 expression via PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells. Int. J. Mol. Med. 39, 1452–1460. doi:10.3892/ijmm.2017.2949
Liperoti, R., Vetrano, D. L., Bernabei, R., and Onder, G. (2017). Herbal medications in cardiovascular medicine. J. Am. Coll. Cardiol. 69, 1188–1199. doi:10.1016/j.jacc.2016.11.078
Liu, L. L., Yan, L., Chen, Y. H., Zeng, G. H., Zhou, Y., Chen, H. P., et al. (2014). A role for diallyl trisulfide in mitochondrial antioxidative stress contributes to its protective effects against vascular endothelial impairment. Eur. J. Pharmacol. 725, 23–31. doi:10.1016/j.ejphar.2014.01.010
Liu, L., and Yeh, Y. Y. (2002). S-alk(en)yl cysteines of garlic inhibit cholesterol synthesis by deactivating HMG-CoA reductase in cultured rat hepatocytes. J. Nutr. 132, 1129–1134. doi:10.1093/jn/132.6.1129
Liu, Q., Li, J., Hartstone-Rose, A., Wang, J., Li, J., Janicki, J. S., et al. (2015). Chinese herbal compounds for the prevention and treatment of atherosclerosis: Experimental evidence and mechanisms. Evid. Based Complement. Altern. Med. 2015, 752610. doi:10.1155/2015/752610
Makheja, A. N., and Bailey, J. M. (1990). Antiplatelet constituents of garlic and onion. Agents actions 29, 360–363. doi:10.1007/BF01966468
Matsumoto, S., Nakanishi, R., Li, D., Alani, A., Rezaeian, P., Prabhu, S., et al. (2016). Aged garlic extract reduces low attenuation plaque in coronary arteries of patients with metabolic syndrome in a prospective randomized double-blind study. J. Nutr. 146, 427S–432S. doi:10.3945/jn.114.202424
Matsuura, H. (2001). Saponins in garlic as modifiers of the risk of cardiovascular disease. J. Nutr. 131, 1000S–5S. doi:10.1093/jn/131.3.1000S
Meng, L., Zhang, J., Song, X., Zhou, B., and Gao, F. (2016). Study on the function of lowering blood lipid of garlic oil. Chin. J. Public Health Eng. 15, 247–249.
Miao, Q., Wang, R., Bai, D., Xue, X., Xu, J., Sun, X., et al. (2020). Antiatherosclerosis properties of total saponins of garlic in rats. Evid. Based Complement. Altern. Med. 2020, 3683659. doi:10.1155/2020/3683659
Miki, S., Inokuma, K. I., Takashima, M., Nishida, M., Sasaki, Y., Ushijima, M., et al. (2017). Aged garlic extract suppresses the increase of plasma glycated albumin level and enhances the AMP-activated protein kinase in adipose tissue in TSOD mice. Mol. Nutr. Food Res. 61, 1. doi:10.1002/mnfr.201600797
Miki, S., Suzuki, J. I., Takashima, M., Ishida, M., Kokubo, H., and Yoshizumi, M. (2021). S-1-Propenylcysteine promotes IL-10-induced M2c macrophage polarization through prolonged activation of IL-10R/STAT3 signaling. Sci. Rep. 11, 22469. doi:10.1038/s41598-021-01866-3
Mohammad, S. F., and Woodward, S. C. (1986). Characterization of a potent inhibitor of platelet aggregation and release reaction isolated from allium sativum (garlic). Thromb. Res. 44, 793–806. doi:10.1016/0049-3848(86)90025-3
Moosavian, S. P., Arab, A., Paknahad, Z., and Moradi, S. (2020). The effects of garlic supplementation on oxidative stress markers: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 50, 102385. doi:10.1016/j.ctim.2020.102385
Morales-Ruiz, M., Fulton, D., Sowa, G., Languino, L. R., Fujio, Y., Walsh, K., et al. (2000). Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ. Res. 86, 892–896. doi:10.1161/01.res.86.8.892
Morihara, N., Hino, A., Miki, S., Takashima, M., and Suzuki, J. I. (2017). Aged garlic extract suppresses inflammation in apolipoprotein E-knockout mice. Mol. Nutr. Food Res. 61, 1. doi:10.1002/mnfr.201700308
Myasoedova, V. A., Kirichenko, T. V., Melnichenko, A. A., Orekhova, V. A., Ravani, A., Poggio, P., et al. (2016). Anti-atherosclerotic effects of a phytoestrogen-rich herbal preparation in postmenopausal women. Int. J. Mol. Sci. 17 (8), 1318. doi:10.3390/ijms17081318
Nagini, S. (2008). Cancer chemoprevention by garlic and its organosulfur compounds-panacea or promise. Anticancer Agents Med. Chem. 8, 313–321. doi:10.2174/187152008783961879
Nie, X., Li, G., and Jiang, H. (2002). Influence of allitridi on plasm GMP-140, D-dimer, NO and NOS in treating angina Pectoris. Shanghai J. Traditional Chin. Med. 36, 7–8. doi:10.3969/j.issn.1007-1334.2002.04.003
Nie, X., Zhao, Y., Shi, D., Liu, Y., Zhou, Z., Su, L., et al. (2013). Effect of allicin capsules on prognosis and endothelial function after PCI in patients with coronary artery disease combined with diabetes mellitus. Natl. Med. J. China 93, 2052–2055. doi:10.3760/cma.j.issn.0376-2491.2013.26.010
Nikolic, D., Calderon, L., Du, L., and Post, S. R. (2011). SR-A ligand and M-CSF dynamically regulate SR-A expression and function in primary macrophages via p38 MAPK activation. BMC Immunol. 12, 37. doi:10.1186/1471-2172-12-37
Okue, S., Yaguchi, M., Miura, A., Ozaki-Masuzawa, Y., Hosono, T., and Seki, T. (2022). The garlic-derived organosulfur compound diallyl trisulphide suppresses tissue factor function. Food Funct. 13, 1246–1255. doi:10.1039/d1fo02206g
Omeish, A. F., Abbadi, W., Ghanma, I. M., Drabaa, Z., Botoosh, F. A., Seif, A., et al. (2011). Hospital-based study on the use of herbal medicine in patients with coronary artery disease in Jordan. JPMA. J. Pak. Med. Assoc. 61, 683–687.
Orekhov, A. N., and Tertov, V. V. (1997). In vitro effect of garlic powder extract on lipid content in normal and atherosclerotic human aortic cells. Lipids 32, 1055–1060. doi:10.1007/s11745-997-0136-7
Panyod, S., Wu, W. K., Chen, P. C., Chong, K. V., Yang, Y. T., Chuang, H. L., et al. (2022). Atherosclerosis amelioration by allicin in raw garlic through gut microbiota and trimethylamine-N-oxide modulation. NPJ Biofilms Microbiomes 8, 4. doi:10.1038/s41522-022-00266-3
Peng, J., Chen, H., Qiao, Y., Ma, L., Takao, N., Suzuki, H., et al. (1996). Two new steroidal saponin components from garlic and their effects on blood coagulation. Acta Pharm. Sin. 31, 607–612. doi:10.3321/j.issn:0513-4870.1996.08.009
Perez, A., Gonzalez-Manzano, S., Jimenez, R., Perez-Abud, R., Haro, J. M., Osuna, A., et al. (2014). The flavonoid quercetin induces acute vasodilator effects in healthy volunteers: Correlation with beta-glucuronidase activity. Pharmacol. Res. 89, 11–18. doi:10.1016/j.phrs.2014.07.005
Petrovic, V., Nepal, A., Olaisen, C., Bachke, S., Hira, J., Søgaard, C. K., et al. (2018). Anti-Cancer potential of homemade fresh garlic extract is related to increased endoplasmic reticulum stress. Nutrients 10, 450. doi:10.3390/nu10040450
Pi, X., Xie, L., and Patterson, C. (2018). Emerging roles of vascular endothelium in metabolic homeostasis. Circ. Res. 123, 477–494. doi:10.1161/CIRCRESAHA.118.313237
Piasek, A., Bartoszek, A., and Namieśnik, J. (2009). Phytochemicals that counteract the cardiotoxic side effects of cancer chemotherapy. Postepy Hig. Med. Dosw (Online). 63, 142–158.
Qidwai, W., Alim, S. R., Dhanani, R. H., Jehangir, S., Nasrullah, A., and Raza, A. (2003). Use of home remedies among patients presenting to family physicians. J. Coll. Physicians Surg. Pak 13, 62–63.
Rahman, K., Lowe, G. M., and Smith, S. (2016). Aged garlic extract inhibits human platelet aggregation by altering intracellular signaling and platelet shape change. J. Nutr. 146, 410S–415S. doi:10.3945/jn.114.202408
Rana, S. V., Pal, R., Vaiphei, K., Sharma, S. K., and Ola, R. P. (2011). Garlic in health and disease. Nutr. Res. Rev. 24, 60–71. doi:10.1017/S0954422410000338
Rassoul, F., Salvetter, J., Reissig, D., Schneider, W., Thiery, J., and Richter, V. (2006). The influence of garlic (Allium sativum) extract on interleukin 1alpha-induced expression of endothelial intercellular adhesion molecule-1 and vascular cell adhesion molecule-1. Phytomedicine 13, 230–235. doi:10.1016/j.phymed.2005.01.010
Ried, K. (2016). Garlic lowers blood pressure in hypertensive individuals, regulates serum cholesterol, and stimulates immunity: An updated meta-analysis and review. J. Nutr. 146, 389S–396S. doi:10.3945/jn.114.202192
Rose, K. D., Croissant, P. D., Parliament, C. F., and Levin, M. B. (1990). Spontaneous spinal epidural hematoma with associated platelet dysfunction from excessive garlic ingestion: A case report. Neurosurgery 26, 880–882. doi:10.1097/00006123-199005000-00026
Rouf, R., Uddin, S. J., Sarker, D. K., Islam, M. T., Ali, E. S., Shilpi, J. A., et al. (2020). Antiviral potential of garlic (allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 104, 219–234. doi:10.1016/j.tifs.2020.08.006
Salehi, B., Zucca, P., Orhan, I. E., Azzini, E., Adetunji, C. O., Mohammed, S. A., et al. (2019). Allicin and health: A comprehensive review. Trends Food Sci. Technol. 1, 1. doi:10.1016/j.tifs.2019.03.003
Salgado, B., Monteiro, L., and Rocha, N. (2011). Allium species poisoning in dogs and cats. J. Venom. Anim. Toxins Incl. Trop. Dis. 17, 4–11. doi:10.1590/S1678-91992011000100002
Sela, U., Brill, A., Kalchenko, V., Dashevsky, O., and Hershkoviz, R. (2008). Allicin inhibits blood vessel growth and downregulates Akt phosphorylation and actin polymerization. Nutr. Cancer 60, 412–420. doi:10.1080/01635580701733083
Sharifi, M., Futema, M., Nair, D., and Humphries, S. E. (2019). Polygenic hypercholesterolemia and cardiovascular disease risk. Curr. Cardiol. Rep. 21, 43. doi:10.1007/s11886-019-1130-z
Siegel-Axel, D., Daub, K., Seizer, P., Lindemann, S., and Gawaz, M. (2008). Platelet lipoprotein interplay: Trigger of foam cell formation and driver of atherosclerosis. Cardiovasc. Res. 78, 8–17. doi:10.1093/cvr/cvn015
Sluimer, J. C., and Daemen, M. J. (2009). Novel concepts in atherogenesis: Angiogenesis and hypoxia in atherosclerosis. J. Pathol. 218, 7–29. doi:10.1002/path.2518
Sluimer, J. C., Kolodgie, F. D., Bijnens, A. P., Maxfield, K., Pacheco, E., Kutys, B., et al. (2009). Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J. Am. Coll. Cardiol. 53, 1517–1527. doi:10.1016/j.jacc.2008.12.056
Sobenin, I. A., Pryanishnikov, V. V., Kunnova, L. M., Rabinovich, Y. A., Martirosyan, D. M., and Orekhov, A. N. (2010). The effects of time-released garlic powder tablets on multifunctional cardiovascular risk in patients with coronary artery disease. Lipids Health Dis. 9, 119. doi:10.1186/1476-511X-9-119
Srivastava, K. C. (1986). Evidence for the mechanism by which garlic inhibits platelet aggregation. Prostagl. Leukot. Med. 22, 313–321. doi:10.1016/0262-1746(86)90142-3
Sumara, G., Belwal, M., and Ricci, R. (2005). Jnking" atherosclerosis. Cell. Mol. Life Sci. 62, 2487–2494. doi:10.1007/s00018-005-5253-6
Suo, R., Zhao, Z. Z., Tang, Z. H., Ren, Z., Liu, X., Liu, L. S., et al. (2013). Hydrogen sulfide prevents H₂O₂-induced senescence in human umbilical vein endothelial cells through SIRT1 activation. Mol. Med. Rep. 7, 1865–1870. doi:10.3892/mmr.2013.1417
Szulińska, M., Kręgielska-Narożna, M., Świątek, J., Styś, P., Kuźnar-Kamińska, B., Jakubowski, H., et al. (2018). Garlic extract favorably modifies markers of endothelial function in obese patients -randomized double blind placebo-controlled nutritional intervention. Biomed. Pharmacother. = Biomedecine Pharmacother. 102, 792–797. doi:10.1016/j.biopha.2018.03.131
Tabas, I., García-Cardeña, G., and Owens, G. K. (2015). Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 209, 13–22. doi:10.1083/jcb.201412052
Tabas, I., and Lichtman, A. H. (2017). Monocyte-Macrophages and T Cells in atherosclerosis. Immunity 47, 621–634. doi:10.1016/j.immuni.2017.09.008
Takashima, M., Kanamori, Y., Kodera, Y., Morihara, N., and Tamura, K. (2017). Aged garlic extract exerts endothelium-dependent vasorelaxant effect on rat aorta by increasing nitric oxide production. Phytomedicine 24, 56–61. doi:10.1016/j.phymed.2016.11.016
Tocmo, R., Wu, Y., Liang, D., Fogliano, V., and Huang, D. (2017). Boiling enriches the linear polysulfides and the hydrogen sulfide-releasing activity of garlic. Food Chem. 221, 1867–1873. doi:10.1016/j.foodchem.2016.10.076
Twigg, M. W., Freestone, K., Homer-Vanniasinkam, S., and Ponnambalam, S. (2012). The LOX-1 scavenger receptor and its implications in the treatment of vascular disease. Cardiol. Res. Pract. 2012, 632408. doi:10.1155/2012/632408
van Greevenbroek, M. M., Robertus-Teunissen, M. G., Erkelens, D. W., and de Bruin, T. W. (1998). Participation of the microsomal triglyceride transfer protein in lipoprotein assembly in caco-2 cells: Interaction with saturated and unsaturated dietary fatty acids. J. Lipid Res. 39, 173–185. doi:10.1016/s0022-2275(20)34213-9
Wang, H. H., Garruti, G., Liu, M., Portincasa, P., and Wang, D. Q. (2017). Cholesterol and lipoprotein metabolism and atherosclerosis: Recent advances in reverse cholesterol transport. Ann. Hepatol. 16, s27–s42. doi:10.5604/01.3001.0010.5495
Wang, K., and Fu, N. (2009). Efficacy of allicin for restenosis of geriatric myocardial infarction after percutaneous coronary intervention. China Pharm. 20, 2141–2143. doi:10.1097/mca.0b013e32832e5c61
Wang, L., Zhang, J., Hu, Y., and Zhang, H. (2017). Effects and mechanisms of allicin on atherosclerosis in high-fat diet fed mice. Chin. J. Arteriosclerosis 25, 140–144.
Wang, Q., Ma, X., Li, X., Li, M., Yu, T., Liu, Z., et al. (2019). Hypolipidemic effect and antioxidation mechanism of diallyl disulfide in rats with hyperlipidemia. J. Toxicol. 33, 55–62.
Weiner, L., Shin, I., Shimon, L. J., Miron, T., Wilchek, M., Mirelman, D., et al. (2009). Thiol-disulfide organization in alliin lyase (alliinase) from garlic (Allium sativum). Protein Sci. 18, 196–205. doi:10.1002/pro.10
Williams, M. J., Sutherland, W. H., McCormick, M. P., Yeoman, D. J., and de Jong, S. A. (2005). Aged garlic extract improves endothelial function in men with coronary artery disease. Phytother. Res. 19, 314–319. doi:10.1002/ptr.1663
Wlosinska, M., Nilsson, A. C., Hlebowicz, J., Hauggaard, A., Kjellin, M., Fakhro, M., et al. (2020). The effect of aged garlic extract on the atherosclerotic process - a randomized double-blind placebo-controlled trial. BMC Complement. Med. Ther. 20, 132. doi:10.1186/s12906-020-02932-5
Wojcikowski, K., Myers, S., and Brooks, L. (2007). Effects of garlic oil on platelet aggregation: A double-blind placebo-controlled crossover study. Platelets 18, 29–34. doi:10.1080/09537100600800636
Wu, J., Xia, S., Kalionis, B., Wan, W., and Sun, T. (2014). The role of oxidative stress and inflammation in cardiovascular aging. Biomed. Res. Int. 2014, 615312. doi:10.1155/2014/615312
Wu, M. Y., Li, C. J., Hou, M. F., and Chu, P. Y. (2017). New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int. J. Mol. Sci. 18, 1. doi:10.3390/ijms18102034
Wu, T. T., Gao, Y., Zheng, Y. Y., Ma, Y. T., and Xie, X. (2018). Atherogenic index of plasma (AIP): A novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 17, 197. doi:10.1186/s12944-018-0828-z
Xiao, D., Li, M., Herman-Antosiewicz, A., Antosiewicz, J., Xiao, H., Lew, K. L., et al. (2006). Diallyl trisulfide inhibits angiogenic features of human umbilical vein endothelial cells by causing Akt inactivation and down-regulation of VEGF and VEGF-R2. Nutr. Cancer 55, 94–107. doi:10.1207/s15327914nc5501_12
Xie, W., and Du, L. (2011). Diabetes is an inflammatory disease: Evidence from traditional Chinese medicines. Diabetes Obes. Metab. 13, 289–301. doi:10.1111/j.1463-1326.2010.01336.x
Xu, W., Shi, Y., Meng, X., Zhang, W., and Gao, T. (2020). Protective effect of allicin on heart and its influence on aortic morphology, cardiomyocyte calcium sensing receptor and apoptotic index in rats with coronary heart disease. Chin. J. Evidence-Based Cardiovasc. Med. 12, 1236–1239. doi:10.3969/j.issn.1674-4055.2020.10.19
Yan, C. K., and Zeng, F. D. (2004). Recent advances in research of garlic's chemical constituents and theirs pharmacological effects. Chin. New Drugs J. 13, 688–691. doi:10.3321/j.issn:1003-3734.2004.08.005
Yang, L., H, X., S, B., Guan, M., and Li, X. (2021). Study on the reaction of garlic and uterine in the UPLC-MS/MS of garlic sausol. Life Sci. Instrum. 19, 66–71. doi:10.11967/2021191210
Yang, S., Yuan, H. Q., Hao, Y. M., Ren, Z., Qu, S. L., Liu, L. S., et al. (2020). Macrophage polarization in atherosclerosis. Clin. Chim. Acta 501, 142–146. doi:10.1016/j.cca.2019.10.034
Yao, X. M., Song, B. L., Wang, C. H., Zhang, W. J., Lin, C. X., and Li, B. L. (2006). Human acyl-coenzyme A: Cholesterol aacyltransferase(ACAT). J. Shanghai Jiaot. Univ. Sci. 24, 108–115. doi:10.3969/j.issn.1671-9964.2006.01.023
Zhai, B., Zhang, C., Sheng, Y., Zhao, C., He, X., Xu, W., et al. (2018). Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci. Rep. 8, 3527. doi:10.1038/s41598-018-21421-x
Zhang, M., Pan, H., Xu, Y., Wang, X., Qiu, Z., and Jiang, L. (2017). Allicin decreases lipopolysaccharide-induced oxidative stress and inflammation in human umbilical vein endothelial cells through suppression of mitochondrial dysfunction and activation of Nrf2. Cell. Physiol. biochem. 41, 2255–2267. doi:10.1159/000475640
Zhang, T., Tong, X., and Liu, X. (2007). Study on the antihyperlipidemia effect of allicin and the mechanism. Chin. J. Exp. Traditional Med. Formulae 13, 32–35. doi:10.3969/j.issn.1005-9903.2007.02.012
Zhang, W., Zhu, T., Chen, L., Luo, W., and Chao, J. (2020). MCP-1 mediates ischemia-reperfusion-induced cardiomyocyte apoptosis via MCPIP1 and CaSR. Am. J. Physiol. Heart Circ. Physiol. 318, H59–H71. doi:10.1152/ajpheart.00308.2019
Zhao, D., Liu, J., Wang, M., Zhang, X., and Zhou, M. (2019). Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 16, 203–212. doi:10.1038/s41569-018-0119-4
Zhu, Y., Xian, X., Wang, Z., Bi, Y., Chen, Q., Han, X., et al. (2018). Research progress on the relationship between atherosclerosis and inflammation. Biomolecules 8. doi:10.3390/biom8030080
5-HT 5-hydroxy tryptamine
6-keto-PGF1α 6-keto-prostaglandin F1α
AA Arachidonic acid
ABCA1 ATP Binding Cassette Subfamily A Member 1
ACAT Acyl-CoA:cholesterol acyltransferase
ACE Angiotensin-Converting Enzyme
AGE Aged garlic extract
AMPK AMP-activated protein kinase
APTT Activated partial thromboplasting time
Arg1 Arginase1
AS Atherosclerosis
Bax BCL2-Associated X
Bcl-2 B-cell lymphoma-2
bFGF Basic fibroblast growth factor
Bid Recombinant Human BH3-Interacting Domain Death Agonist
CAM Complementary and alternate medicine
CaMKK Calcium/calmodulin-dependent kinase kinase
CaSR Calcium-sensing receptor
CD36 Cluster of differentiation 36
CEH Cholesteryl ester hydrolase
cGMP Cyclic guanosine monophosphate
CHD Coronary artery heart disease
COX1 Cyclooxygenase-1
COX2 Cyclooxygenase-2
CRP C-reaction protein
CVDs Cardiovascular diseases
DADS Diallyl disulfide
DAS Diallyl sulfide
DATS Diallyl trisulfide
DATTS Diallyl Tetrasulfide
DIO Diet-induced obese
ECs Endothelial cells
EDHF Endothelium-derived hyperpolarizing factor
ELAM-1 Endothelial leukocyte adhesion molecule-1
eNOS Endothelin nitric oxide synthase
ERK1/2 Extracellular regulated protein kinases
ET1 Endothelin-1
FAS Fatty acid synthase
FGE Fresh garlic extract
FMD Flow-mediated dilation
G6PDH Glucose-6-Phosphate Dehydrogenase
GEF-H1 Guanine nucleotide exchange factor-H1
GMP140 Granular membrane protein 140
GPE Garlic powder extract
GSH Glutathione
GSH-Px Glutathione peroxidase
HDL High-density lipoprotein
HIF2α Hypoxia-inducible factor 2α
HL Hepatic lipase
HMGCR Hydroxy-3-methyl glutaryl coenzyme A reductase
hsCRP High-sensitivity C-reactive protein
HUVECs Human umbilical vein endothelial cells
ICAM-1 Intercellular cell adhesion molecule-1
IFN-g Interferon gamma
IL-10 Interleukin-10
IL-10Rα IL-10 receptor α
IL-12β Interleukin-12 beta
IL-18 Interleukin 18
IL-1a Interleukin-1 alpha
IL-1β Interleukin-1 beta
IL-6 Interleukin- 6
IL-8 Interleukin-8
iNOS Inducible nitric oxide synthase
IRAK4 Interleukin-1 receptor-activated kinase 4
JAK3 Janus Kinase 3
JNK C-Jun N-terminal kinase
LCAT Lecithin cholesterol acyltransferase
LDL Low-density lipoprotein
LDL-C Low-density lipoprotein cholesterol
L-NAME L-N G-Nitro arginine methyl ester
LPL Lipoprotein lipase
LPS Lipopolysaccharide
LTC4C Leukotriene C4
LXRα Liver X receptor α
MAPK Mitogen-activated protein kinase
MATS Methyl allyl trisulfide
MCP-1 Monocyte chemotactic protein 1
MDA Malondialdehyde
MLC Myosin light chain
MMPs Matrix metalloproteinases
MTTP Microsomal triglyceride transfer protein
Mø Macrophages
NAC N-acetylcysteine
NADPH Nicotinamide adenine dinucleotide phosphate
NF-κB Nuclear factor kappa-B
NO Nitric oxide
NOS Nitric oxide synthase
NQO1 NAD(P)H:quinone oxidoreductase 1
Nrf2 Nuclear erythroid 2-related factor 2
OS Oxidative stress
ox-LDL LDL oxidation
p38 MAPK P38 mitogen-activated protein kinase
PAI-1 Plasminogen Activator Inhibitor-1
PARP Poly adenosine diphosphate-ribose polymerase
PDGF Platelet-Derived Growth Factor
PGE2 Prostaglandin E2
PGGTase-I Protein geranylgeranyltransferase type I
PGI2 Prostaglandin I2
PPARα Peroxisome proliferator-activated receptor alpha
PPARγ Peroxisome proliferator-activated receptor Gamma
RhoA Ras homolog gene family, member A
RNS Reactive nitrogen species
ROS Reactive oxygen species
S1PC S-1-propenylcysteine
SAC S-allyl cysteine
SACS S-allyl cysteine sulfoxide
SEC S-acetylcysteine
SHIP1 Src homology-2-containing inositol 5′-phosphatase 1
SI Stiffness index
SIRT1 Sirtuin 1
STAT3 Signal transducer and activator of transcription 3
SOD Superoxide Dismutase
SOD-1 Superoxide Dismutase 1
SPC S-propylcysteine
SR-A Scavenger receptor class A
SREBP-1 Sterol regulatory element-binding proteins-1
SREBP-1c Sterol regulatory element-binding protein-1c
SREBP-2 Sterol regulatory element-binding protein-2
T-AOC Total antioxidant capacit
TAS Total antioxidant status
TC Total cholesterol
TF Tissue factor
Th1 Thelper 1 cell
TLR Toll-like receptors
TNF-α Tumor necrosis factor-α
TSG Total Saponins of Garlic
TSOD Tsumura Suzuki Obese Diabetes
TXA2 Thromboxane A2
TXB2 Thromboxane B2
VCAM-1 Vascular Cell Adhesion Molecule 1
VEGF Vascular endothelial growth factor
VEGF R2 Vascular endothelial growth factor receptor 2
VSMCs Vascular smooth muscle cells
γ-GCSc γ -glutamylcysteine synthetase antibody
Keywords: garlic, Allium sativum, atherosclerosis, oxidative stress, inflammation, endothelial dysfunction
Citation: Li M, Yun W, Wang G, Li A, Gao J and He Q (2022) Roles and mechanisms of garlic and its extracts on atherosclerosis: A review. Front. Pharmacol. 13:954938. doi: 10.3389/fphar.2022.954938
Received: 27 May 2022; Accepted: 13 September 2022;
Published: 03 October 2022.
Edited by:
Mariarosaria Bucci, University of Naples Federico II, ItalyReviewed by:
Veronika Myasoedova, Monzino Cardiology Center (IRCCS), ItalyCopyright © 2022 Li, Yun, Wang, Li, Gao and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingyong He, aGVxaW5neW9uZ2dAMTYzLmNvbQ==
†These authors have contributed equally to this work and share the first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.